Abstract
Since immunity is generally suppressed by immunoregulatory factors, such as transforming growth factor-beta (TGF-β), interleukin (IL)-10, and vascular endothelial growth factor (VEGF), produced by tumor cells or stromal cells surrounding tumor cells, various kinds of cancer immunotherapy mostly fail to elicit potent antitumor immunity. Herein, we tested whether neutralization of TGF-β can elicit strong antitumor immune responses and tumor regression in tumor-bearing mice. A plasmid DNA, pcDNA-sTGFβR/huIg, encoding a fusion protein consisting of the extracellular domain of TGF-β type II receptor (TGFβRII) and the Fc portion of human IgG heavy chain, was injected through different routes into B6 mice carrying established tumors of E.G7 cells, which consist of the poorly immunogenic tumor cells EL4, transfected with the ovalbumin (OVA) gene. The frequency of OVA-specific cytotoxic T lymphocytes (CTL), in the treated mice. increased resulting in the tumor eradication and relapse-free survival in around 70% of the E.G7-bearing mice. In contrast, administration of mock DNA into E.G7-bearing mice did not elicit tumor-specific immune responses. Therefore, administration of DNA encoding TGFβRII allowed tumor-bearing hosts to elicit sufficiently potent antitumor immune responses without requirement of further active antigen-immunization. This strategy seems to be applicable to clinical therapy against cancer, because it is low-cost, safe, and easy to manipulate.
Keywords: Soluble TGFβRII DNA, Experimental gene therapy, Immunomodulation, Immune responses to cancer
Introduction
The outcome of many clinical trials of cancer immunotherapy, such as cytokine administration, effector cell transfer, tumor cell or peptide vaccines, and dendritic cell (DC) vaccines, have not been satisfactory [15]. One of the reasons for the poor efficacy is that immunity is generally suppressed by immunoregulatory factors produced by tumor cells and stromal cells surrounding tumor, i.e., VEGF, TGF-β, and IL-10, as the stage of disease advances in patients with advanced and metastatic cancer [2, 13]. Due to these factors, maturation processes of antigen-presenting cells (APC), commitment of helper T (Th) precursor lymphocytes to type-1 Th lymphocytes and recruitment, and activation of tumor-specific CTLs are impaired in the patients, resulting in unresponsiveness of the immune system to tumor cells [1, 4, 5, 7, 8, 10, 11, 17, 18, 20]. To induce potent antitumor immune responses capable of eliminating tumor cells in cancer-bearing hosts, blockade or neutralization of the immunosuppressive factors seems to be essential.
TGF-β has been reported to be produced by many tumor cells and suppress antitumor immunity in hosts. On the other hand, TGF-β also has been shown to promote tumor angiogenesis [16]. Thus, TGF-β is considered to be profoundly involved in cancer progression. Several groups have tried to neutralize TGF-β to enhance antitumor immunity. Inoculation of EL4 mouse thymoma that was genetically engineered to secrete soluble TGFβRII (sTGFβRII) prevented the growth of other tumors [19]. Neutralization by antibody against TGF-β prevented tumor growth in the presence of activated DC, but the administration of anti-TGF-β alone showed no significant antitumor effect [9]. Another group reported that transgenic mice expressing dominant-negative TGFβRII on T cells did not accept tumor cell growth and that RAG1−/− mice reconstituted by these T cells survived after tumor challenge. However, tumor prevention was only effective for the first 3 days after reconstitution of the T cells expressing dominant-negative TGFβRII, and it has not been exactly determined if blockade of TGF-β signaling induces antitumor immunity in mice carrying fully established tumors [6]. Consequently, although neutralization of TGF-β is promising, the method still contains obstacles to be overcome before it can be applied in cancer therapy.
In this study, we inoculated a plasmid DNA encoding soluble TGFβRII into tumor-bearing mice for neutralization of tumor-producing TGF-β. Because of easy administration, low price, and stable expression, administration of the DNA is thought to be more rational than periodical and repeated administrations of the neutralizing antibodies or soluble TGFβRII proteins. By inoculation of sTGFβRII DNA, potent cytotoxic activity specific for immunogenic tumors was spontaneously elicited, and the established tumors were eradicated without any further active antigen sensitization. Further, this treatment does not require determination of MHC alleles of patients or tumor-associated antigen (TAA) peptides. Therefore, this strategy seems to be applicable in human cancer therapy.
Materials and methods
Cells and mice
A 6- to 8-week-old female C57BL/6 mice were purchased from CLEA Japan Inc. (Tokyo, Japan) and maintained under specific-pathogen-free conditions.
The murine lymphoma cell lines, EL4 and its derivative, E.G7, which was generated by transducing the chicken ovalbumin (OVA) gene into EL4 cells; a melanoma cell line, B16F1; and a natural killer (NK) cell-sensitive cell line, YAC-1 were all purchased from American Type Culture Collection (Rockville, MD, USA).
Antibodies
Anti-OVA polyclonal antibody was provided by Cortex Biochem Inc. (San Leandro, CA, USA). Horseradish peroxidase (HRP)-conjugated antirabbit, antimouse, and antihuman immunoglobulin antibodies were purchased from Valeant Pharmaceuticals International (Bryan, OH, USA). Fluorescent isothiocyanate (FITC)-conjugated antimouse CD8 monoclonal antibody was purchased from BD Biosciences Pharmingen (San Diego, CA, USA).
Plasmid DNA
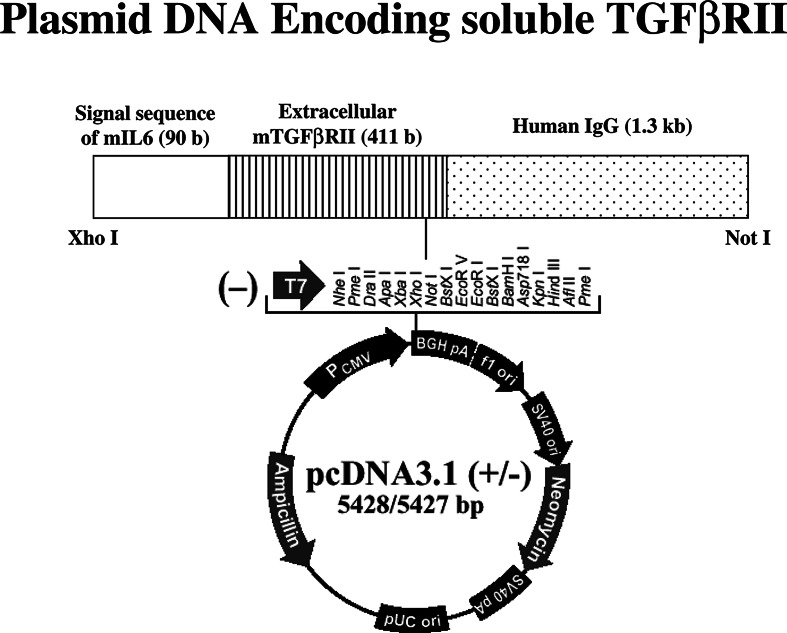
An expression plasmid vector, pcDNA-OVA, was prepared by cloning the full-length OVA cDNA, obtained by digesting pAc-neo-OVA (a gift from Dr. Michael J. Bevan, Department of Immunology, University of Washington, Seattle, WA, USA) with EcoRI (TOYOBO Inc., Osaka, Japan), into pcDNA3.1 (Invitrogen, Carlsbad, CA, USA). To prepare DNA encoding, the chimeric protein comprising soluble TGFβRII fused to the human IgG heavy chain, total RNA was isolated from mouse CTL clone 4G3 (generously provided by Dr. Keiko Udaka, Department of Immunology, Kochi Medical School) to synthesize cDNA using oligo-dT and random primers (Invitrogen). The extracellular domain of TGFβRII was amplified by PCR using 5′-GAGCTCGGTCTATGACGACCGA-3′ and 5′-GGATCCAACAGGTCGGGACTGCTGGTGGT-3′. The PCR product was cloned into pCDM8-human IgG1 [12] (generously provided by Dr. Toshimitsu Uede, Division of Molecular Immunology, Hakkaido University) following digestion with XhoI and BamHI. Its original signal sequence was replaced with that derived from mouse IL-6, and subsequently the sTGFβ R/huIgG1 gene (1.8 kb) was isolated by digestion with XhoI and NotI followed by cloning into pcDNA3.1. All recombinant constructs were confirmed by sequence analysis on a Perkin–Elmer ABI PRISM 310 DNA sequencer (Branchburg, NJ, USA), and the resulting plasmid was designated as pcDNA-sTGFβR/huIg (Fig. 1).
Fig. 1.
Plasmid DNA construct. The DNA fragment (1.8 kb) encoding the chimeric protein comprising a signal sequence derived from mouse IL-6, the extracellular domain of TGFβRII and the human IgG1 heavy chain was cloned into pcDNA3.1
DNA transfection and immunocytochemical study
The B16F1 cells were cultured in a 6-well culture plate until they had reached semi-confluent density. The cells were transduced with pcDNA-sTGFβR/huIg or mock DNA using a polycationic reagent (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Forty-eight hours after transfection, the cells were washed in PBS, fixed in 3% paraformaldehyde in PBS at 4°C for 30 min followed by incubation with 100% methanol at −20°C for 10 min. Thereafter, the cells were washed in PBS, incubated with 5 μg/ml rat antihuman immunoglobulin antibody (ICN Pharmaceutical Inc., Aurora, OH, USA), washed in PBS and then incubated with biotinylated antirat IgG (Valeant Pharmaceuticals International, CA, USA) diluted at 1/2000. The cells were then incubated in horseradish peroxidase (HRP)-conjugated avidin solution (Vector Laboratories Inc., Burlingame, CA, USA) at room temperature for 90 min and subsequently colored by incubation in 0.4 mg/ml 3.3′-diaminobenzidine (Nakalai Tesque, Kyoto, Japan) for 10 min at room temperature. Adding PBS to each well stopped the reaction. Approximately, 20% of the tumor cells were positively stained 48 h after transfection, whereas, none of cells transfected with control empty pcDNA vector produced the fusion protein. Thus, the cells that were transfected with pcDNA-sTGFβR/huIg apparently produced the fusion protein.
DNA administration
A 30 μg of pcDNA-sTGFβR/huIg was incorporated into the envelope of Hemagglutinating Virus of Japan (HVJ) (Ishihara Sangyo Kaisha Ltd., Kusatsu, Japan) according to the manufacturer’s instructions, dissolved in 50 μl of PBS and injected in the peritoneal cavity, in the tumor, or in the hind-leg quadriceps muscle of female C57BL/6 mice.
Tumor challenge experiment
Female C57BL/6 mice were inoculated subcutaneously in the right flank with 2×105 EL4 or E.G7 cells. The mice were injected with 30 μg of pcDNA-sTGFβR/huIg or control pcDNA3.1 with or without pcDNA-OVA vaccination 10–12 days after tumor challenge, when tumors of 8–12 mm in diameter were detected. The DNA injection was repeated every other week. The sizes of tumors were monitored twice a week, and the volume of the tumor was calculated using the following formula: (length) × (width)2/2.
Enzyme-linked immunosorbent assay (ELISA)
Mice were inoculated with plasmid DNA twice at a 2-week interval. One week after the last injection, the mice were bled and serum antibody levels were tested. Ninety-six-well ELISA plates (Nalgene Nunc International, Roskilde, Denmark) were coated with 50-μl/well of 20-μg/ml OVA (Sigma, St. Louis, MO, USA), and then 50 μl of serially diluted immune sera was added to the wells. The plates were incubated at 37°C for 60 min, washed with PBS, and then incubated with HRP-conjugated antimouse immunoglobulin diluted at 1/1,000 for 60 min at 37°C. After three washes with PBS, color was developed by incubation with a substrate solution consisting of 50 μl of 0.05 M o-phenylenediamine and H2O2 (Nacalai Tesque, Kyoto, Japan). The reaction was stopped by adding 50 μl of 4 N HCl, and absorbance at 492 nm was measured using a microplate reader.
Cellular staining with MHC tetramers
Tumor-challenged mice were killed 7 days after the second DNA administration and spleen cells from the mice were cultured in RPMI containing 10% FCS and 5 μg/ml OVA257–264 peptides for 3 days. The cells were then incubated with FITC-conjugated anti-CD8 monoclonal antibody and PE-conjugated H-2Kb /OVA257–264 complex (MBL Co., Ltd., Nagoya, Japan) at room temperature for 30 min. After two washes with PBS, cells were examined to quantify OVA-specific CTLs by flow cytometry. Flow cytometric analyses were performed using a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, CA, USA). Data were presented as dot plots using CellQuest software (Becton Dickinson).
Cytotoxicity assay
Spleen cells from the treated mice were cultured in RPMI 1640 containing 10% FCS, 50 μM 2-mercaptoethanol, 2 μg/ml OVA-Kb peptides and 1 ng/ml recombinant mouse IL-2 (Wako Pure Chemical Industries Ltd., Osaka, Japan) for 6 days. After in vitro stimulation, the spleen cells were co-cultured with 1×104 EL4 or E.G7 cells that were labeled with Na512 CrO4(Perkin Elmer Life Science, MA, USA) at various effector/target (E/T) ratios in 96-well culture plates for 4 or 5 h. The amount of 51 Cr released from lysed target cells in the cell supernatants was estimated using an LKB-Wallac 1275 Minigamma counter (EG&G Wallac, Turku, Finland). The ratio (%) of chromium release was calculated using the following formula: % specific lysis=[(experimental release−spontaneous release)/(maximal release−spontaneous release)]×100.
Statistical analysis
Reactivity of mouse sera to antigens in ELISA and cytotoxic responses of mouse spleen cells to targets, and the numbers of CTLs in the mouse spleens were compared by Student‘s t test. A P value below 0.05 was considered statistically significant. Kaplan–Meier curves were generated for time of survival of the mice. Curves for two groups were compared with the log-rank test for equality of survivor functions.
Results
Suppression of tumor growth and prolonged survival of tumor-bearing mice by administration of pcDNA-sTGFβR/huIg
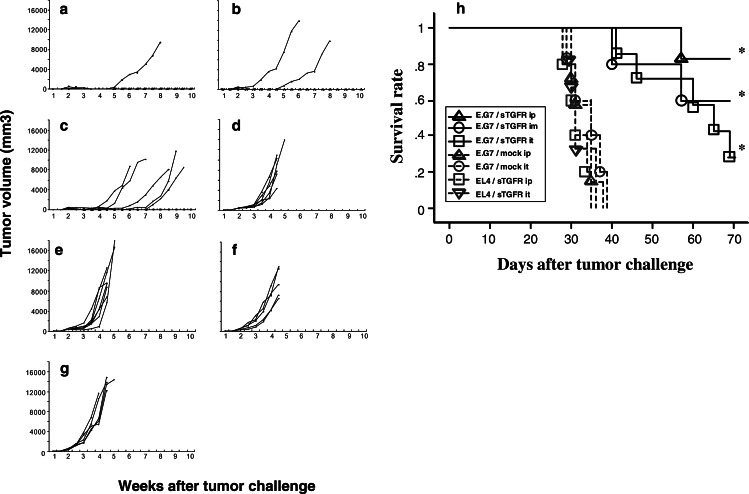
After E.G7 or EL4 tumors grew to 8–12 mm diameter in B6 mice, they were inoculated with pcDNA-sTGFβR/huIg or control DNA at intervals of 2 weeks. The established E.G7-tumors were completely eradicated in 83 or 60% of the treated mice by intraperitoneal or intramuscular injections of pcDNA-sTGFβR/huIg, respectively (Fig. 2a, b). In contrast, intraperitoneal or intramuscular administration of the control DNA vector into B6 mice carrying E.G7-tumors or administration of pcDNA-sTGFβR/huIg into B6 mice carrying EL4-tumors demonstrated no suppressive effects on tumor growth (Fig. 2d–g). Interestingly, intratumoral injection of pcDNA-sTGFβR/huIg led to temporary complete regression of E.G7-tumors in six of the seven mice (1.5–5 weeks), but thereafter, the tumors relapsed in four of the six mice (Fig. 2c). We performed the same experiment three times and acquired nearly the same results. Overall, E.G7 tumors were eradicated in 14 of 18 mice (77%) or 10 of 16 mice (63%) after intraperitoneal or intramuscular inoculation, respectively, of pcDNA-sTGFβR/huIg. In contrast, none of 16 mice showed tumor eradication after control DNA inoculation.
Fig. 2.
Suppression of tumor growth and prolonged survival by administration of pcDNA-sTGFβR/huIg in tumor-bearing mice. The C57BL/6 mice were inoculated with 2×105 EL4 or E.G7 cells. The mice were injected with 30 μg of pcDNA-sTGFβR/huIg (sTGFR) or control pcDNA3.1 (mock) through different routes 10–12 days after tumor inoculation. The DNA injection was repeated every other week. The sizes of the tumors were monitored twice a week. The EG.7-bearing mice received pcDNA-sTGFβR/huIg intraperitonieally (ip) (a; n=6), intramuscularly (im) (b; n=5) or intratumorally (it) (c; n=7). Control pcDNA3.1 was inoculated intraperitonieally (d; n=6) or intratumorally (e; n=7). The EL4-bearing mice received pcDNA-sTGFβR/huIg intraperitonieally (f; n=5) or intratumorally (g; n=5). Kaplan–Meier curves were generated for survival time of the mice (h). Experiments were repeated three times and the results are representative of the experiments. *P<0.004
Next, we examined survival of the tumor-bearing mice after the various treatments. Administration of pcDNA-sTGFR/huIg prolonged survival of E.G7-bearing mice. All groups of E.G7-bearing mice that were inoculated with pcDNA-sTGFR/huIg survived statistically longer than E.G7-bearing mice that received control DNA (P<0.004) (Fig. 3h). Intraperitoneal or intramuscular administration of pcDNA-sTGFR/huIg seems more appropriate than intratumoral administration for therapeutic purposes (survival ratios were 83, 60, and 29%, respectively), especially intraperitoneal administration is significant comparing with intratumoral administration (P<0.05). These survival ratios corresponded to the tumor regression ratios described above. This result was nearly identical in three experiments. Finally, 29–83% of the mice were tumor-free after administration of pcDNA-sTGFR/huIg, and tumors had not relapsed for at least 100 days in these mice (data not shown).
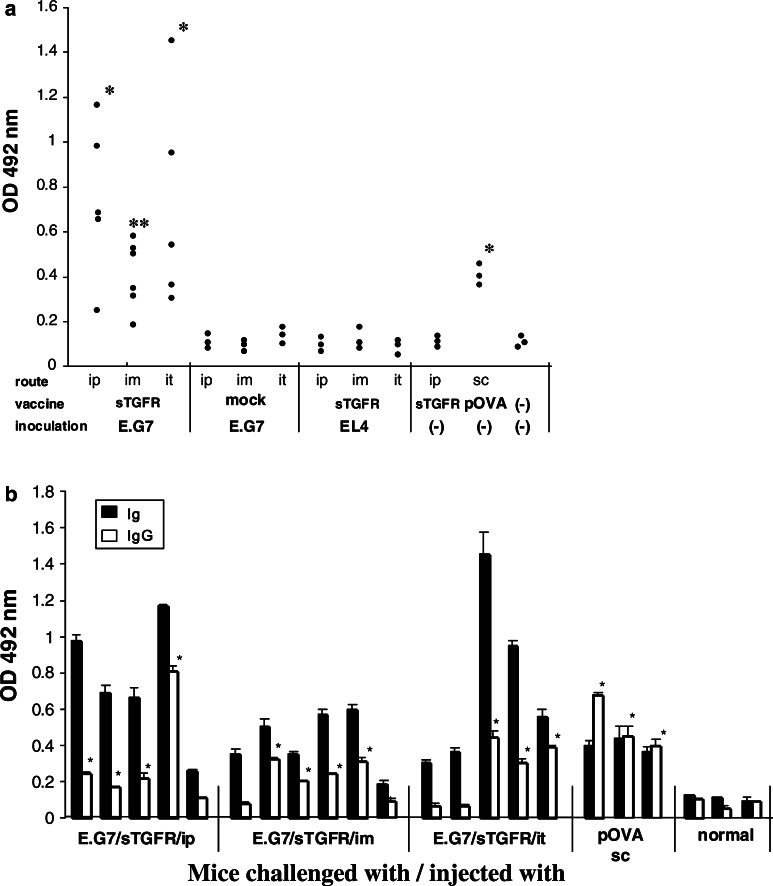
Fig. 3.
Anti-OVA antibody elicited by administration of pcDNA-sTGFβR/huIg in mice. Mice with or without challenged tumors received pcDNA-sTGFβR/huIg or pcDNA3.1 twice through different routes at a 2-week interval. Similarly, OVA protein (pOVA) was injected twice subcutaneously with a 2-week interval. One week after the last injection, the mice were bled and their sera were tested by ELISA for reactivity with OVA. The absorbance at a dilution of 1:40 was plotted in each group (a). Both anti-OVA IgM and IgG were detected by this assay in which antimouse immunoglobulin was used as secondary antibody. *P<0.001; **P=0.0054. The sera shown in a were examined for the reactivity of IgG with OVA (b). *P<0.05
Elicitation of anti-OVA antibody by administration of pcDNA-sTGFβR/huIg in E.G7-bearing mice
The B6 mice carrying E.G7 or EL4 tumors were inoculated with pcDNA-sTGFβR/huIg or control pcDNA3.1 twice with an interval of 2 weeks. The treated mice were bled 7 days after the last injection and the sera were examined for the reactivity with OVA protein by ELISA. The OVA-specific antibodies, including IgG and IgM, were significantly elicited in the E.G7-bearing mice intraperitoneally, intramuscularly, or intratumorally inoculated with pcDNA-sTGFβR/huIg, compared with normal mice (the mean absorbance=0.1337) (Fig. 3a). In contrast, neither pcDNA-sTGFβR/huIg administration into EL4-bearing mice nor control DNA administration to E.G7-bearing mice induced anti-OVA antibodies. Similarly, OVA-specific IgG was significantly higher in 60–80% of the E.G7-bearing mice inoculated with pcDNA-sTGFβR/huIg through various routes compared with normal mice (the mean absorbance of normal sera=0.119) (Fig. 3b). The level of IgG directed against OVA in sTGFβRII DNA-inoculated mice was almost comparable to that in OVA protein (pOVA)-immunized mice.
Cytotoxic activity of spleen cells from treated mice against tumor cells
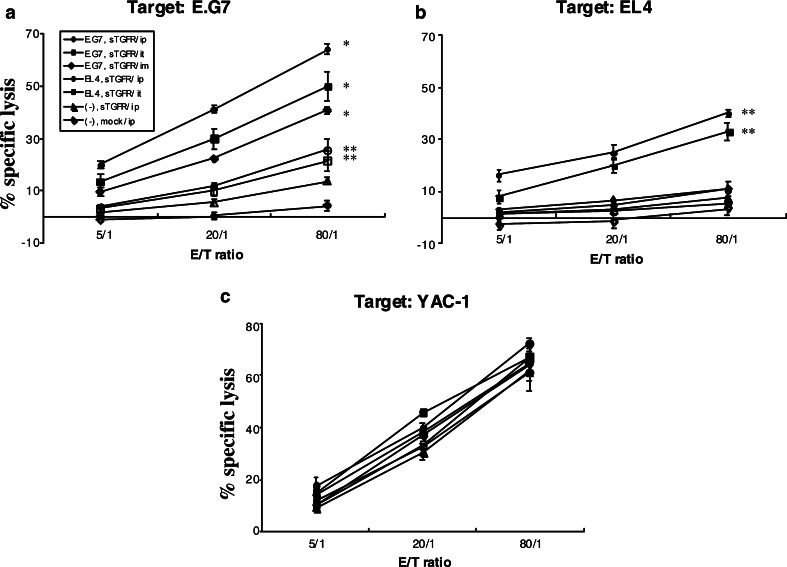
Spleen cells from E.G7-bearing mice, intramuscularly (i.m.), intratumorally (i.t.), or intraperitoneally (i.p.) inoculated with pcDNA-sTGFβR/huIg, showed 38, 47, or 67%, respectively, specific cytotoxic activity against E.G7 cells at an 80:1 E/T ratio (Fig. 4a). On the other hand, spleen cells from EL4-carrying mice inoculated with pcDNA-sTGFβR/huIg only demonstrated modest but detectable levels of cytotoxicity against E.G7 cells, compared with no tumor-carrying mice inoculated with control DNA. Spleen cells from mice bearing E.G7 cells inoculated with control DNA showed slight CTL activity. However, this activity seems to be non-specific, because no CTL responses specific for OVA 257–264 + Kb were detected (Fig. 5b). In addition, spleen cells from mice carrying E.G7 cells inoculated with pcDNA-sTGFR/huIg also showed strong cytotoxic activity against EL4 cells (approximately 30% specific lysis at an 80:1 E/T ratio, Fig. 4b). The NK cell activity was not affected by DNA administration, as shown in Fig. 4c.
Fig. 4.
Cytotoxic activity of spleen cells from pcDNA-sTGFβR/huIg-injected mice against tumor cells. Mice with or without challenged tumors received administration of pcDNA-sTGFβR/huIg or pcDNA3.1 twice through different routes with a 2-week interval. One week after the last injection, mice were sacrificed and their spleen cells were tested for cytotoxic activity against E.G7 (a), EL4 (b), or YAC-1 (c) target cells after in vitro stimulation with OVA-Kb peptides for 6 days. * P<0.001; ** P<0.02. Experiments were repeated three times and the results are representative of the experiments
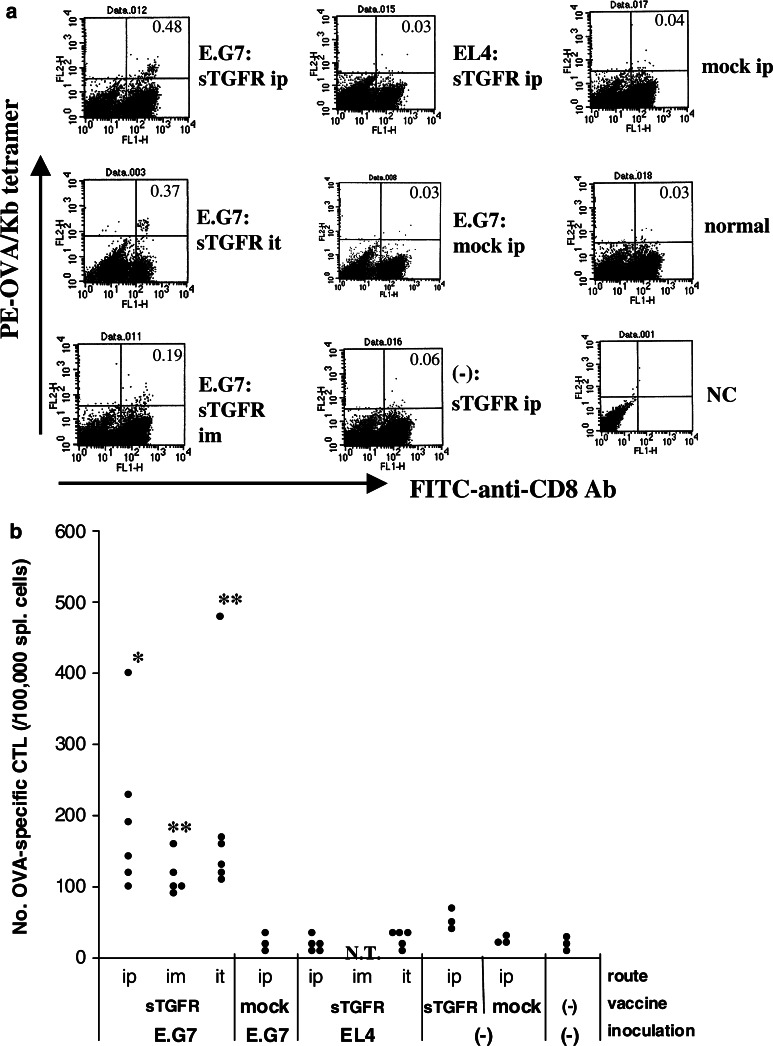
Fig. 5.
Frequency of CTLs reactive with OVA in pcDNA-sTGFβR/huIg-injected mice. Mice with or without challenged tumors received administration of pcDNA-sTGFβR/huIg or pcDNA3.1 twice through different routes with a 2-week interval. One week after the last injection, mice were sacrificed and their spleen cells were stained with FITC-anti-CD8 antibody and PE-OVA/Kb peptide tetramers after in vitro stimulation with OVA-Kb peptides for 6 days. The stained cells were analyzed by flow cytometry (a). The number of CD8-positive T cells reactive with OVA-Kb peptides in 105 spleen cells was plotted for each group of mice (b). Experiments were repeated three times and results are representative of the experiments. * P<0.02; ** P<0.01; NT not tested
Frequency of OVA-specific CTLs in treated mice
Next, we examined the frequency of CTLs specific for OVA 257–264 + Kb molecule in spleens of the pcDNA-sTGFR/huIg-treated mice. Increased frequencies of CTLs reactive with OVA257–264 epitopes were observed in all of the E.G7-bearing mice that had received pcDNA-sTGFR/huIg, regardless of injection route (Fig. 5a, b). These frequencies were correlated with killing activity. Namely, the order of killing activity (i.p. > i.t. > i.m.) was the same as the order of OVA-specific CTL frequencies. None of the E.G7-bearing mice that were inoculated with control DNA or the EL4-bearing mice that had received pcDNA-sTGFR/huIg demonstrated increased frequency of OVA-specific CTLs in their spleens.
Discussion
The TGF-β is one of the most potent immunosuppressive factors produced by many tumor cells. This cytokine exerts regulatory effects on various kinds of immune responses, such as maturation blockade of DCs, imbalance of Th function, inhibition of cytokine production responsible for priming T cells and impaired CTL activation [3, 9, 14, 19]. Therefore, antitumor immunity is generally suppressed in tumor-bearing animals or cancer patients. In this study, plasmid DNA encoding soluble TGFβRII was inoculated into tumor-bearing mice to neutralize TGF-β. Administration of sTGFβRII DNA into mice carrying OVA-expressing tumors elicited higher titers of anti-OVA antibodies than did inoculation with control DNA (Fig. 3a). However, titers of IgG directed against OVA in pOVA-immunized mice were comparable to those in sTGFβRII DNA-inoculated mice (Fig. 3b). These findings suggest no correlation between anti-OVA humoral immunity and E.G7 tumor regression, because immunization with pOVA failed to suppress growth of established E.G7 tumors (data not shown). Rather, OVA-specific cytotoxic activity and suppressive effects on tumor growth were correlated, which was demonstrated in E.G7 tumor-carrying mice after inoculation with pcDNA-TGFR/huIg (Figs. 2, 4).
Spleen cells from EL4-bearing mice that had received pcDNA-TGFβR/huIg showed weak but detectable levels of cytotoxic activity against E.G7 cells (Fig. 4a). Furthermore, spleen cells from E.G7-bearing mice that were inoculated with pcDNA-TGFβR/huIg showed significant levels of cytotoxic activity against EL4 cells (Fig. 4b). These findings suggest that some CTLs recognizing less immunogenic antigens shared between EL4 and E.G7 cells are activated after administration of pcDNA-TGFβR/huIg. The NK cell activity was not involved in the cytolysis because cytolytic activity against YAC-1 cells was not affected by any of the treatments, as shown in Fig. 4c. Since EL4 cells are thought to be poorly immunogenic tumor cells, spontaneously elicited antitumor cellular immunity by administration of pcDNA-sTGFβR/huIg may not be strong enough to suppress EL4 tumor growth. Thus, when tumors possess potent immunogenicity, neutralization of TGF-β alone enhances antitumor immunity that has been induced in vivo in the presence of tumor, resulting in tumor regression. On the other hand, even poorly immunogenic tumors might be eradicated, if pcDNA-sTGFβR/huIg was injected in the presence of treatment enhancing immunogenicity, e.g., administration of DCs with tumor-associated antigen peptide.
In this study, pcDNA-sTGFβR/huIg was administered by three different routes, i.p., i.m., or i.t. The cytotoxic activities against E.G7 cells and the frequencies of OVA-specific CTLs were not different between mice inoculated with pcDNA-sTGFβR/huIg i.p.or i.t. However, E.G7 tumors relapsed after temporary complete regression (1.5–6 weeks) in four of the six mice receiving intratumoral administration of pcDNA-sTGFβR/huIg (Fig. 2c). In these mice, it is likely that there were no sTGFβRII-producing cells after disappearance of tumor cells and that relapsed tumors, therefore lacked pcDNA-sTGFβR/huIg production. Products of sTGFR/huIg were not detected in sera from these four mice in which E.G7 tumors had relapsed, whereas, intraperitoneal and intramuscular administrations of pcDNA-sTGFβR/huIg were able to maintain high levels of sTGFβRII in mouse sera, ranging from 1,200 to 2,600 pg/ml (data not shown). Alternatively, tumors reappearing after regression may have lost OVA antigen, with the result that OVA-specific CTLs could not kill the tumors, which therefore grew. In any case, for therapeutic purposes, pcDNA-sTGFβR/huIg should be administered either i.p. or i.m.
A previous report by Kobie et al. [9] demonstrated that neutralization of TGF-β by administration of anti-TGF-β antibody enhanced the efficacy of a DC-based vaccine. Thus, we examined whether simultaneous inoculation with pcDNA-sTGFβR/huIg and pcDNA-OVA elicited a more effective antitumor immune response capable of eradicating tumors than did inoculation with pcDNA-sTGFβR/huIg alone. In contrast to our expectation, simultaneous inoculation with pcDNA-sTGFβR/huIg and pcDNA-OVA did not elicit an additive effective antitumor immune response (data not shown), compared with the inoculation of pcDNA-sTGFβR/huIg alone. Because inoculation with pcDNA-sTGFβR/huIg does not require examination of patients’ MHC alleles or histological types or origins of the particular cancer to be treated, and because it is low-cost, safe, and easy to manipulate, it seems applicable to clinical therapy against cancer. However, for the treatment of poorly immunogenic tumors, this strategy requires an improvement to enhance antigenicity of the tumors.
Acknowledgments
This work was founded in part by Grant-in-aid for scientific research from the Ministry of Education, Science, Sports and Culture, Japan (Nos. 10671249, 13671380, 14571262, and 15591340).
References
- 1.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 2.Chouaib S, Asselin-Paturel C, Mami-Chouaib F, Caignard A, Blay JY. The host-tumor immune conflict: from immunosuppression to resistance and destruction. Immunol Today. 1997;18:493–497. doi: 10.1016/S0167-5699(97)01115-8. [DOI] [PubMed] [Google Scholar]
- 3.Fargeas C, Wu CY, Nakajima T, Cox D, Nutman T, Delespesse G. Differential effect of transforming growth factor beta on the synthesis of Th1-like and Th2-like lymphokines by human T lymphocytes. Eur J Immunol. 1992;22:2173–2176. doi: 10.1002/eji.1830220833. [DOI] [PubMed] [Google Scholar]
- 4.Gabrilovich DI, Corak J, Ciernik IF, Kavanaugh D, Carbone DP. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res. 1997;3:483–490. [PubMed] [Google Scholar]
- 5.Ghosh AK, Moore M. Tumour-infiltrating lymphocytes in cervical carcinoma. Eur J Cancer. 1992;28:1910–1916. doi: 10.1016/0959-8049(92)90034-Y. [DOI] [PubMed] [Google Scholar]
- 6.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7:1118–1122. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 7.Hilders CG, Ras L, van Eendenburg JD, Nooyen Y, Fleuren GJ. Isolation and characterization of tumor-infiltrating lymphocytes from cervical carcinoma. Int J Cancer. 1994;57:805–813. doi: 10.1002/ijc.2910570608. [DOI] [PubMed] [Google Scholar]
- 8.Kiertscher SM, Steven JL, Dubinett M, Roth MD. Tumors promote altered maturation and early apoptosis of monocyte-derived dendritic cells. J Immunol. 2000;164:1269–1276. doi: 10.4049/jimmunol.164.3.1269. [DOI] [PubMed] [Google Scholar]
- 9.Kobie JJ, Wu RS, Kurt RA, Lou S, Adelman MK, Whitesell LJ, Ramanathapuram LV, Arteaga CL, Akporiaye ET. Transforming growth factor β inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res. 2003;63:1860–1864. [PubMed] [Google Scholar]
- 10.Mehrotra S, Stevens R, Zengou R, Chakraborty NG, Butterfield LH, Economou JS, Dorsky DI, Mukherji B. Regulation of melanoma epitope-specific cytolytic T lymphocyte response by immature and activated dendritic cells, in vitro. Cancer Res. 2003;63:5607–5614. [PubMed] [Google Scholar]
- 11.Merogi AJ, Marrogi AJ, Ramesh R, Robinson WR, Fermin CD, Freeman SM. Tumor-host interaction: analysis of cytokines, growth factors, and tumor-infiltrating lymphocytes in ovarian carcinoma. Hum Pathol. 1997;28:321–331. doi: 10.1016/S0046-8177(97)90131-3. [DOI] [PubMed] [Google Scholar]
- 12.Murakami M, Takahashi Y, Isashi Y, Kon S, Jia WY, Inobe M, Abe R, Uede T. Identification and characterization of an alternative cytotoxic T lymphocyte-associated protein 4 binding molecule on B cells. Proc Natl Acad Sci USA. 1996;93:7838–7842. doi: 10.1073/pnas.93.15.7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohm JE, Carbone DP. VEGF as a mediator of tumor-associate immunodeficiency. Immunol Res. 2001;23:263–272. doi: 10.1385/IR:23:2-3:263. [DOI] [PubMed] [Google Scholar]
- 14.Palladino MA, Morris RE, Starnes HF, Levinson AD. The transforming growth factor-betas. A new family of immunoregulatory molecules. Ann NY Acad Sci. 1990;593:181–189. doi: 10.1111/j.1749-6632.1990.tb16110.x. [DOI] [PubMed] [Google Scholar]
- 15.Pardoll D, Allison J. Cancer immunotherapy: breaking the barriers to harvest the crop. Nat Med. 2004;10:887–892. doi: 10.1038/nm0904-887. [DOI] [PubMed] [Google Scholar]
- 16.Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, Fauci AS. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheu BC, Lin RH, Lien HC, Ho HN, Hsu SM, Huang SC. Predominant Th2/Tc2 polarity of tumor-infiltrating lymphocytes in human cervical cancer. J Immunol. 2001;167:2972–2978. doi: 10.4049/jimmunol.167.5.2972. [DOI] [PubMed] [Google Scholar]
- 18.Steinbrink K, Graulich E, Kubsch S, Knop J, Enk AH. CD4(+) and CD8(+) anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468–2476. doi: 10.1182/blood.V99.7.2468. [DOI] [PubMed] [Google Scholar]
- 19.Won J, Kim H, Park EJ, Hong Y, Kim SJ, Yun T. Tumorigenicity of mouse thymoma is suppressed by soluble type II transforming growth factor ß receptor therapy. Cancer Res. 1999;59:1273–1277. [PubMed] [Google Scholar]
- 20.Yang AS, Lattime EC. Tumor-induced interleukin 10 suppresses the ability of splenic dendritic cells to stimulate CD4 and CD8 T-cell responses. Cancer Res. 2003;63:2150–2157. [PubMed] [Google Scholar]