Abstract
The aim of this study was to determine the immunogenicity and antitumor activity of autologous, tumor-derived heat shock protein gp96-peptide complex vaccine (HSPPC-96; Oncophage®) given with GM-CSF and IFN-α in pre-treated metastatic (AJCC stage IV) melanoma patients. Patients underwent surgical resection of metastatic lesions for HSPPC-96 production. HSPPC-96 was administered subcutaneously (s.c.) in four weekly intervals (first cycle). Patients with more available vaccine and absence of progressive disease received four additional injections in 2-week intervals (second cycle) or more. GM-CSF was given s.c. at the same site at days –1, 0 and +1, while IFN-α (3 MU) was administered s.c. at a different site at days +4 and +6. Antigen-specific anti-melanoma T and NK lymphocyte response was assessed by enzyme-linked immunospot assay on peripheral blood mononuclear cells obtained before and after vaccination. Thirty-eight patients were enrolled, 20 received at least four injections (one cycle) of HSPPC-96 and were considered assessable. Toxicity was mild and most treatment-related adverse events were local erythema and induration at the injection site. Patients receiving at least four injections of HSPPC-96 were considered evaluable for clinical response: of the 18 patients with measurable disease post surgery, 11 showed stable disease (SD). The ELISPOT assay revealed an increased class I HLA-restricted T and NK cell-mediated post-vaccination response in 5 out of 17 and 12 out of the 18 patients tested, respectively. Four of the five class I HLA-restricted T cell responses fall in the group of SD patients. Vaccination with autologous HSPPC-96 together with GM-CSF and IFN-α is feasible and accompanied by mild local and systemic toxicity. Both tumor-specific T cell-mediated and NK cell responses were generated in a proportion of patients. Clinical activity was limited to SD. However, both immunological and clinical responses were not improved as compared with those recorded in a previous study investigating HSPPC-96 monotherapy.
Keywords: Vaccination, Metastatic melanoma, Heat shock proteins, GM-CSF, IFN-α, Phase II trial
Introduction
We have previously shown that approximately 50% of therapy refractory stage IV (American Joint Commission on Cancer, AJCC) metastatic melanoma patients vaccinated after surgery with autologous tumor-derived heat shock protein peptide complexes gp96 (HSPPC-96)‡ developed class I HLA-restricted tumor-specific T cell immunity. Clinical responses and long-term disease stabilization were noted in this study [1]. Results of this and other similar studies have been recently discussed [2].
Since the local administration of GM-CSF has been reported to recruit inflammatory-like cells, to activate dendritic cells and to increase the immunogenicity of vaccines both in animal models and humans [3–5], in an attempt to potentially enhance the immune and clinical response of the HSPPC-96 vaccine, we designed this protocol in which GM-CSF was given at the site of the HSPPC-96 injection. Moreover, in order to up-regulate the expression of the target HLA/peptide complex recognizable by T cells, which is often down-regulated in metastatic melanoma cells [6], patients also received low dose IFN-α twice a week after vaccine administration. Such a dose has been previously shown to up-regulate the expression of class I HLA in peripheral blood cells of melanoma patients [7].
Here we report the results of this trial which confirms the in vivo immunogenicity of HSPPC-96 even in combination with GM-CSF and IFN-α at least in a fraction of patients with advanced melanoma and suggests a clinical effect in terms of disease stabilization. However, this study fails to demonstrate a noticeable improvement in the frequency and intensity of both immunologic and clinical responses as compared with our previous study of vaccination of clinically similar patients receiving HSPPC-96 monotherapy [1].
Patients and treatments
Between March 2002 and February 2003, 38 pre-treated stage IV AJCC metastatic melanoma patients were enrolled in the study and had surgical resection of tumor tissue for vaccine production. Patients were enrolled at two Italian centers: the Istituto Nazionale Tumori (n=31) and the Regional Oncology Center of Aviano (n=7). Of these patients, 27 started treatment and 20 received at least one cycle of four vaccinations and were considered assessable. Eligibility criteria for study participation included the following: (1) histologically verified melanoma with resectability of at least one lesion that provided the necessary amount of non-necrotic neoplastic tissue (5 g, four of which to be used for vaccine preparation and one for immunological assays); (2) performance status (Zubrod) of two or less; (3) life expectancy of at least 5 months; (4) normal WBC and platelets counts, and hemoglobin level greater than 10 g/l; (5) bilirubin less than 1.5 times normal, ALT less than four times normal, and adequate renal function with serum creatinine less than twice normal; (6) fully recovered from prior anticancer therapy with at least a 4-week interval from the last administration of prior anticancer treatment.
All patients underwent clinical and radiographic staging before treatment. Patients were excluded from the study if they had active brain metastases, had concomitant autoimmune or malignant diseases or were receiving concurrent anticancer therapy, required immunosuppressive drugs, or had a history of serious intercurrent medical illness. Women of childbearing potential had a negative serum pregnancy test before entry into the study and agreed to use an effective method of contraception while on treatment. All patients gave written informed consent to participate in the study.
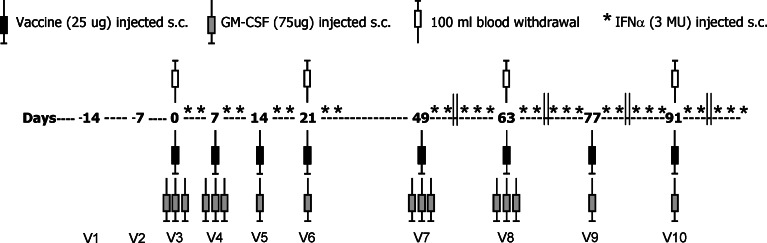
The clinical protocol was approved by the Internal Scientific Review Board and by the Independent Ethics Committee of the Istituto Nazionale Tumori of Milan and of the other participating center. Patients were vaccinated with autologous HSPPC-96 starting 5–8 weeks after resection of tumor metastases; they received 25 μg of vaccine given subcutaneously (s.c.) at weekly intervals for 4 weeks (first cycle). After 4 weeks of rest and if no progression occurred, patients received four additional injections every 2 weeks (second cycle) if the vaccine was available. Similarly, additional vaccinations were given after two cycles where available. The cytokine GM-CSF (Leukomax, Novartis Farma, Origgio, VA, Italy) was administered s.c. in the amount of 75 μg the day before, the same day of the vaccination and the following day during the first two vaccinations of the first and second cycle, and at the same site like HSPPC-96 vaccinations. During the other vaccinations, GM-CSF was administered only once, along with HSPPC-96. IFN-α 2b (Schering-Plough, Milan, Italy) was given as 3 MU s.c. twice weekly, 1 and 3 days after the last administration of GM-CSF during the first cycle and at a site different from that of GM-CSF. During the second cycle, IFN-α was given three times a week during the weeks in between the vaccination weeks (see Figure 1).
Fig. 1.
Scheme of treatment and of time of blood withdrawal of stage IV melanoma patients
Patients were monitored for toxicity, including complete clinical evaluation, standard blood tests including differential blood counts, serum chemistry, urinalysis, cardiac, liver and renal functions, and autoimmune reactions (anti-microsomal and anti-thyroglobulin antibodies, T3, free T4, and anti-nucleoprotein antibodies). Ophthalmologic examinations were carried out at 4, 8 and 12 weeks to assess possible autoimmune reactions caused by melanoma/retina cross-reacting differentiation antigens.
Tumor evaluation was performed before resection of metastatic melanoma, at pre-treatment visits 1 or 2 (V1 or V2) (baseline evaluation), at week 4 (V6) of the study, and thereafter every 3 months and as clinically indicated. All time points of response were recorded from the time of surgery. This was done by the same sequential diagnostic imaging method. The size of tumor lesion was evaluated as the sum of the longest perpendicular diameters of the relevant target lesions and recorded. Clinical responses were defined according to (RECIST) response evaluation criteria in solid tumors [8]. Time to progression (TTP) was calculated from the day of surgery until first documented progression or last documented evaluation without progression. Overall survival was calculated from the day of surgery until the last contact date or the date of death. Skin punch biopsies were carried out at the site of vaccination whenever possible.
Immunological evaluation
Eighty to 100 ml of heparinized blood was obtained by preparation of a buffy-coat from each patient before vaccination (V1 or 2) and at V6 (fourth vaccination), V7 (fifth vaccination, 4 weeks afterward) and at V10 (eighth vaccination). In the case of patients who received additional vaccinations, blood was obtained also during the subsequent follow-up visit at V11 or V12. PBMCs were isolated by Ficoll gradient centrifugation and frozen in aliquots in liquid nitrogen. The immune tests (see below) were all performed at the Istituto Nazionale Tumori to assess the patients‘ anti-melanoma immune response. Peripheral blood mononuclear cells (PBMCs) of 17 out of 19 evaluable subjects could be analyzed; patient 01-022 was not assessed since he was found positive for HCV.
Preparation of vaccine
HSPPC-96 vaccine was manufactured from autologous tumor samples of each patient by Antigenics Inc. (Lexington, MA, USA) as reported previously [1] with minor modifications. After dissection and freezing, each sample was shipped to Antigenics facility in Lexington, MA, and processed under good manufacturing practice conditions.
Evaluation of the immune response
HLA typing
Serological typing for HLA-A, -B, -C was performed by the standard two-stage complement-dependent microcytotoxicity assay. For molecular typing, genomic DNA was purified from protease-treated PBMCs with QIAmp DNA (Qiagen, Hilden, Germany), and HLA typing was performed by amplification with sequence-specific primers (Dynal, Bromborough, UK).
Delayed-type hypersensitivity (DTH)
Skin reactions at the site of vaccination were assessed 1 h after every injection of the vaccine and/or GM-CSF by the attending physician, and after 24 and 48 h by the physician or the instructed patient. DTH was carried out by injection of 5×105 irradiated (100 Gy) autologous melanoma cells (when available) derived from melanoma lesions by mechanical processing from patients 01-001 to 01-0019 and of 1×106 from patients 01-020 to 01-0031 because of lack of positive DTH reaction with the lower dose. These cells were tested for sterility by routine microbiological assays. Cells were suspended in 10% human AB serum, aliquoted, stored in liquid nitrogen and thawed before injection. Autologous irradiated (30 Gy) PBMCs, obtained from blood by Ficoll gradient (Pharmacia Biosystem, Uppsala, Sweden) centrifugation, were also injected as negative control immediately before the first, fourth and fifth vaccination. All injections were done in 0.5 ml volume intra-dermally at sites distant from that of vaccine and cytokine administration.
Enzyme-linked immunospot assay
This assay allows for the direct testing of antigen recognition by patient lymphocytes (T or NK) and has been used in several trials of vaccination, including those with HSPPC-96 [1, 9–13]. The enzyme-linked immunospot (ELISPOT) assay was carried out as recently described [1, 13]. Briefly, 96-well nitrocellulose plates (Mlititer, Millipore, Bedford, MA, USA) were coated with 50 μl/well of mouse anti-human IFN-γ mAb (Mabtech, Nacka, Sweden) in sterile buffer and incubated overnight at 4°C then washed to remove unbound antibody. Previously thawed PBMCs were suspended in 10% FCS RPMI and added to the plates at the concentration of 1.67×105 per well (in three or six replicates for each target) in 100 μl/well volume. After 30 min incubation at 37°C, targets were added at the concentration of 1.67×104 per well. Non-specific total control release of IFN-γ was obtained by pokeweed mitogen (1 μg/ml, Sigma, Munich, Germany) stimulation. Blocking experiments were performed by pre-incubating target cells for 30 min at 37°C with the anti-class I or anti-class II mAb W6.32 or L243, respectively. To control the ELISPOT assay, the anti-Melan-A/MART-1 T cell clone A42 in the presence or absence of the cognate antigen (T2 cells pulsed with Melan-A/MART-127–35 peptide or the Melan-A/MART-1+ melanoma 501) were always included at the concentration of 400 cells/well. After 20 h of incubation at 37°C, the plates were washed and wells incubated for 2 h at room temperature with 100 μl/well of biotinylated anti-human IFN-γ mAb (Mabtech). Afterward, wells were washed again and 50 μl/well of streptavidin alkaline phosphatase (Mabtech) added. After 1 h incubation at room temperature, plates were washed extensively and added to 100 μl/well of the substrate (Biorad Laboratories, Hercules, CA, USA). Color development was stopped by washing in tap water when dark spots emerged. Plates were then left to dry overnight and spots counted by a computer-assisted ELISPOT reader (Bioline, AID, Turin, Italy). Statistical analysis of the difference in ELISPOT data (after background subtraction) obtained before and after vaccination, was performed by using the Student’s t test for unpaired samples. Standard deviation was evaluated in three or six well replicates. A P<0.05 was considered statistically significant.
Melanoma and other cell lines
Tumor cell suspensions were obtained by mechanical processing of melanoma lesions after surgery; in some cases enzymatic digestion was also used (see Ref. [1]. Tumor cells were then aliquoted, frozen, and stored in liquid nitrogen). The melanoma line 501mel and 624.38mel were used as HLA-A2*0201 target cells [14]. T2 is a TAP-deficient lymphoma line that can express pulsed HLA-A*0201-restricted peptides and present them to T lymphocytes. Tumor cell lines were maintained in RPMI 1640 with 10% FCS unless otherwise indicated.
Immunopathology of resected melanoma metastases
Routine histologic diagnosis and immunohistochemistry (IHC) evaluation were performed for all tumor samples used for vaccine production and for some recurrent lesions detected during treatment. Classification of the metastatic foci on the basis of the “brisk/non-brisk” system for tumor-infiltrating lymphocytes [15] was performed on routine hematoxylin and eosin-stained slides. In addition, this was correlated with IHC analysis using the routine immunoperoxidase technique. Tissue sections were stained with the following antibodies: CD3 and CD4 (Novocastra Labs, Newcastle on Tyne, UK), CD8 (DAKO A/S, Glostrup, Denmark), CD45R0 (DAKO A/S), CD56 N M (Sigma), HMB45 (DAKO A/S), S-100 (DAKO A/S), Melan-A/MART-1 (clone A103, Novocastra Labs), HLA-DR (clone LN3, Biotest AG, Dreieich, Germany), anti-HLA-A,-B,-C framework mAb HC-10 (kindly provided by Dr Soldano Ferrone, Buffalo, USA). Previously well-documented melanoma cases were used as positive controls for such melanoma antigens. If all tumor-infiltrating lymphocytes were of T phenotype, the CD3 population was considered to be 100%. This was used as a baseline for a semi-quantitative count of CD4, CD8 and CD45 cells that were estimated as a percentage of the CD3+ counterparts. HLA-A, -B, -C and melanoma antigens (gp100 and Melan-A/MART-1) were arbitrarily considered as down-regulated when less than 20% of the tumor cells were positive and highly expressed when at least 50% the of tumor cells were positive after staining with the given antibody.
Results
Patients and vaccination
Of the 38 patients (see Table 1 for demographics and clinical data) who underwent surgery for removal of melanoma lesions, 20 received at least one or two (n=11) complete cycles of vaccination (four vaccinations per cycle). Others (n=18) could not receive a complete cycle of vaccination for reasons listed in Table 2. Nine patients (# 01-002, 01-007, 01-008, 01-010, 01-012, 01-013, 01-014, 01-016, 02-005) received 1–5 injections of HSPPC-96 beyond the second cycle.
Table 1.
Characteristcs of the 20 assessable patients
| Characteristic | No. of patients |
|---|---|
| Sex | |
| Male | 12 |
| Female | 8 |
| Performance status | |
| 0 | 19 |
| 1 | 1 |
| 2 | 0 |
| Age | |
| Median (years) | 49.6 |
| Range (years) | 24.0–70.8 |
| Site of metastasis | |
| Single metastasis | 3 |
| Cutaneous/subcutaneous | 2 |
| Lymph nodes | 1 |
| Multiple metastases | 17 |
| Subcutaneous and lymph nodes | 8 |
| Subcutaneous and/or lymph nodes + visceral | 9 |
| Previous treatments | |
| Surgery only | 5 |
| IFN-α | 0 |
| Surgery + chemotherapy/radiotherapy + IFN-α ± IL-2 | 15 |
Table 2.
Frequency and causes of drop-out occurring during vaccine production and before the conclusion of the first vaccination cycle of 38 eligible melanoma patients
| Cause | No. of patients dropped out | Percentage |
|---|---|---|
| Disease progression | 10/38 | 26.3 |
| Withdrawn consent | 1/38 | 2.6 |
| Drug depletion | 5/38 | 13.2 |
| No drug | 2/38 | 5.3 |
| TOTAL | 18/38 | 47.4 |
Of the 20 patients who completed at least one or two cycles of vaccination, five were pre-treated with surgery alone while the remaining patients were pre-treated with surgery, chemotherapy, radiotherapy and/or cytokine (IL-2/IFN-α)-therapy (Table 1).
Toxicity
Common treatment-related AEs in 20 evaluable patients were erythema (n=16) and induration (n=13) when the vaccine was given in combination with GM-CSF. In five patients treated with additional HSPPC-96 without accompanying cytokine administration after the initial two vaccination cycles, such local reactions were not observed. There were few other systemic and loco-regional side-effects, possibly related to treatment, that were observed only in a minority of patients: nausea (n=4), vertigo (n=2), fatigue (n=2), vomiting (n=1), malaise (n=1), fever (n=3), pain (n=1) and itching (n=2) at injection site.
Clinical response
Table 3 shows the clinical response to vaccination. The time point of response evaluation was at V6, i.e., after the first cycle of vaccination. Of the 18 assessable patients with measurable disease, 11 had stable disease (SD), which lasted from 130 to 320 days. Two of these patients who had SD for 320 (patient 01-007) and 281 (patient 01-023) days after depletion of their HSPPC-96 vials went off protocol and received two different peptide-based vaccines. Patient 01-007 underwent surgery for a small subcutaneous lesion after the end of the second vaccination cycle and was still disease-free at 446 days.
Table 3.
Tumor response to vaccination
| No. of evaluable patients | 20 |
| Median time to progressiona (N=20) | 145 days |
| 95% CI=97 to 188 days | |
| Median overall survival at the end of studya (N=20) | 583 days |
| 95% CI=291 to N/A days | |
| No. of patients with stable disease after one cycle | 11/20 (55%) |
| No. of patients with progression after one cycle | 8/20 (40%) |
| No. of patients disease-free after one cycle | 1/20 (5%)b |
aMedian time to progression and median survival times were calculated by Kaplan–Meier method
bPatient 01–013 was made disease free by surgery for 414 days
Two patients (# 01-013 and 01-029) were vaccinated in an adjuvant setting, i.e., after being rendered disease-free by surgery for vaccine procurement (Table 4). While patient 01-029 showed progression at the end of the first cycle, patient 01-013 had a long-lasting disease-free interval (414 days), after which tumor recurred and two consecutive debulking surgical procedures were needed to return to the disease-free status.
Table 4.
Clinical and immunologic response in the 20 vaccinated patients
| Patient # | Clinical responsea | HLA-restricted T cell recognition | |
|---|---|---|---|
| Auto-Me | Allo-Me | ||
| Fs2001-002 | PRO | + | ND |
| 01-003 | PRO | − | − |
| 01-007 | SD | + | + |
| 01-008 | SD | + | − |
| 01-010 | SD | − | − |
| 01-011 | PRO | NDb | − |
| 01-012 | SD | − | − |
| 01-013 | Disease freec,d, | + | − |
| 01-014 | SD | + | − |
| 01-016 | SD | + | ND |
| 01-017 | PRO | − | − |
| 01-022 | SD | ND | ND |
| 01-023 | SD | ND | + |
| rid 01-026 | PRO | ND | ND |
| 01-028 | PRO | ND | + |
| 01-029 | PROd | ND | − |
| 01-031 | SD | + | − |
| 02-004 | SD | − | + |
| 02-005 | SD | − | − |
| 02-007 | PRO | ND | ND |
The values are evaluated by ELISPOT in the presence of autologous or HLA-A, -B-compatible allogeneic melanoma cells and of anti-HLA class I mAb performed either at V6 and/or V7
aAs assessed at V7 as overall best
bNot done
cDisease-free after surgery and during the two cycles of vaccination for three additional boostings
dThese patients were vaccinated in an adjuvant setting, i.e., after being rendered disease-free by surgery for vaccine procurement
The median survival time of these patients (n=20) was 583 days (as of March 2004) and median time to progression was 145 days. It is of note that two patients (# 01-010 and 01-012) with SD, received nine vaccinations (two cycles plus one injection) had, after receiving CVD-based chemotherapy off protocol, a durable partial response (PR) and a continuing stabilization, respectively.
Immunological response
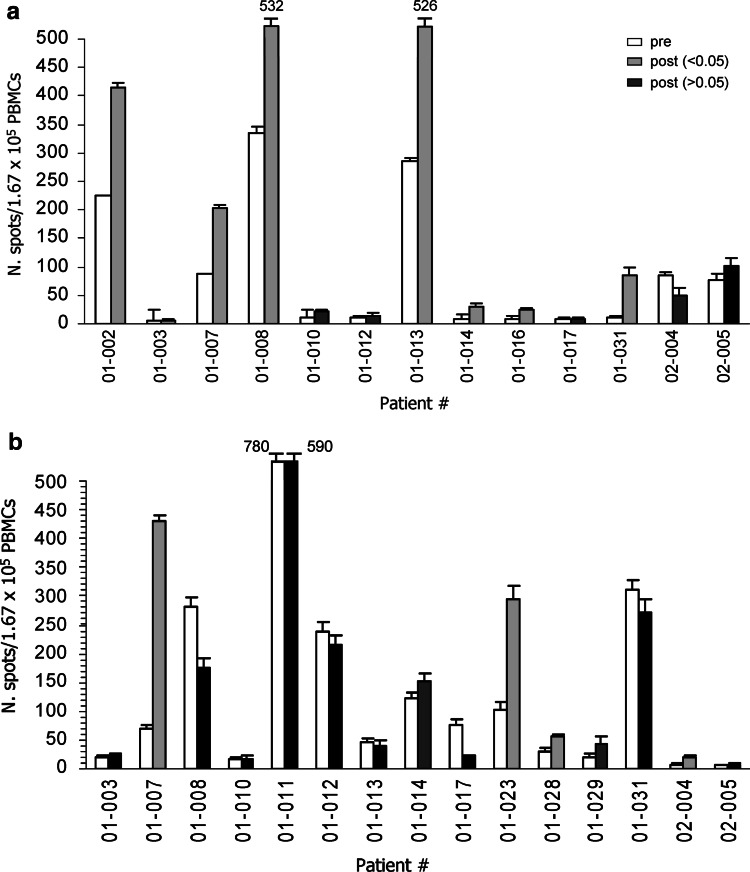
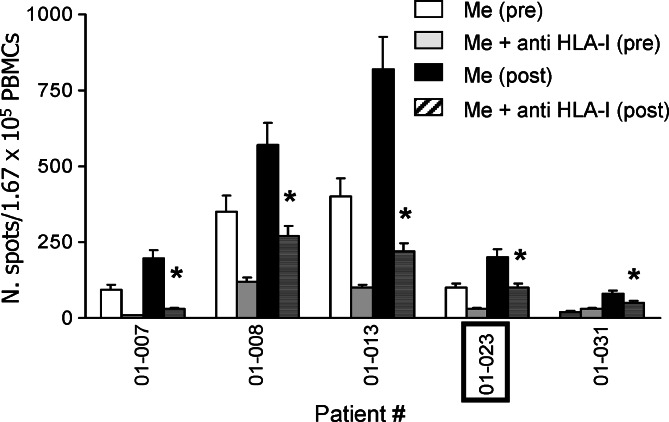
Patients were monitored at V2 (before vaccination), V6 (day of the fourth vaccination), V7 (day of onset of the second cycle) and at V10 (day of the end of the vaccination) to determine whether HSPPC-96 vaccine could increase the frequency of melanoma-specific T and of NK lymphocytes in peripheral blood. Fresh PBMCs were obtained before and at different times after vaccination, and ELISPOT assay for IFN-γ was performed using melanoma cells as targets, i.e., when either autologous and/or allogeneic but HLA-A/B compatible melanoma cells were available which occurred in 17 patients. Figure 2a shows that an increased T cell reaction against autologous melanoma cells could be detected in seven of 13 patients tested and for whom autologous melanoma cells were available (patients # 01-002, −007, −008, −013, −014, −016, and −031) whereas 4 of 15 patients tested showed an increased T cell recognition of allogeneic HLA-A and/or -B compatible melanoma lines after vaccination (patients # 01-007, −023, −028 and 02-004) (Fig. 2b and Table 4). For five of these patients (# 01-007, −008, −013, −023, −031) showing a statistically significant increase in T cell response after vaccination either on autologous (patients # 01-007, −008, −013, −031) or allogeneic (patient # 01-023) melanoma cell lines, the increased T cell response was class I HLA-restricted with at least two subjects (patient # 01-008, −013) displaying a class I HLA-restricted reaction against the tumor already before vaccination (Fig. 3). The addition of anti-class II HLA Ab L243 never inhibited the T cell reactions (data not shown). Table 4 shows that the four subjects with residual disease after surgery for vaccine procurement (patient # 01-007, −008, −023, −031) as well as the patient vaccinated in an adjuvant setting (patient # 01-013), had SD or a significantly prolonged disease-free interval, respectively, indicating a possible association between T cell and clinical response, as already suggested in the previous study of HSPPC-96 vaccination [1]. Moreover, it is of note that in three cases (patients # 01-008, −013 and −031) the treatment was associated with an increased T cell recognition of autologous, but not of allogeneic, HLA-compatible melanoma cells, suggesting recognition of individual antigens (Table 4).
Fig. 2.
Recognition of autologous (a) or allogeneic HLA-A/-B compatible (b) melanoma lines by PBMCs of vaccinated patients. PBMCs were obtained from the blood of each patient before (V2) and after (V6 or 7) treatment and tested by IFN-γ-ELISPOT assay. Gray columns indicate statistically significant differences at P< 0.05
Fig. 3.
Class I HLA-restricted release by recognition of melanoma cells as evaluated by inhibition of IFN-γ anti-class I HLA mAb (ELISPOT assay). In all but one (01-023, boxed) case target cells were autologous (01-007, 01-008, 01-013, 01-031) melanoma cells. * indicates statistically significant inhibition
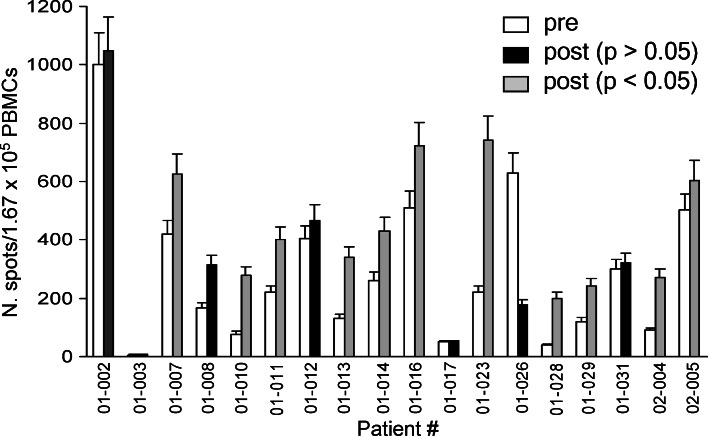
The activity of patients’ PBMCs was also evaluated in the presence of NK-sensitive K562 target cells. As shown in Fig. 4, NK activity was already present before vaccination in several patients and significantly increased in 11 of them (patients # 01-007, −010, −011, −013, −014, −016, −023, −028, −029; 02-004, 02-005) after vaccination. In five cases (patients # 01-002, −010, −014, −016; 02-005), ex vivo NK activity on autologous melanoma cells was further boosted in vitro in the presence of anti-HLA-A,-B,-C mAb, a finding which can be explained by the masking of HLA molecules on tumor cells that then allows the triggering of NK-activating receptors (data not shown) [16]. In six patients typed as HLA-A*0201 (n=3) or as HLA-A*0301 (n=3), known melanoma peptides (Melan-A/MART1 and gp100/209) were used as target in the ELISPOT assay but none of the patients’ PBMCs recognized the peptides (data not shown).
Fig. 4.
NK activity of patient PBMCs as evaluated by ELISPOT assay before and after treatment. Gray columns indicate statistically significant differences at P< 0.05–0.01
Time course of the T cell response during vaccination
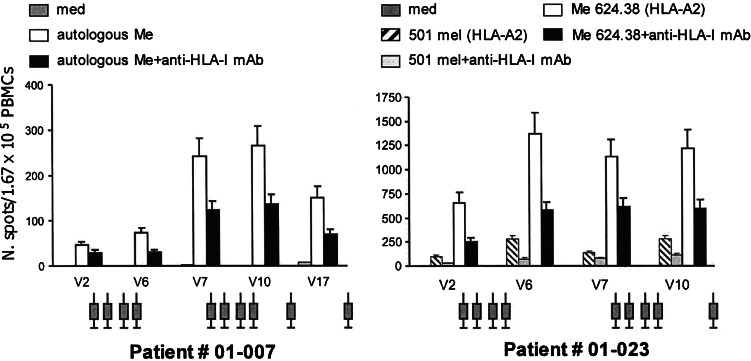
In order to assess the kinetics of the immune response to the vaccine during treatment, PBMCs drawn at different times from selected patients were tested repeatedly by ELISPOT. Figure 5 shows the results of two patients tested on autologous (# 01-007) and on allogeneic (# 01-023) melanoma lines. Patient 01-007 started to develop a class I HLA-restricted T cell response on the autologous melanoma line at the end of the first cycle (V6) that further increased at the end of the second cycle (V10) and even after resection of a small subcutaneous nodule (V17) while receiving additional boosts of HSPCC-96. A similar pattern was seen for patient 01-023 though he could not be tested after V10.
Fig. 5.
Time course of the HLA-A-restricted T cell-mediated immune response against melanoma during vaccination in two patients (# 01-007 and 01-023 tested on autologous and allogeneic melanoma cells, respectively) selected because of their showing a long stable disease. V2 visit 2, before starting vaccination; V6 visit 6, end of the first cycle of vaccination; V7 onset of the second cycle; V10 end of the second cycle, V17 additional infusions after the second cycle. Syringes refer to injection of the vaccine
The time course of the anti-melanoma-specific T cell response in the tested patients reveals a rapid development of the reaction which is sustained over the period of vaccination while fading away when immunization is interrupted. Similar findings have been reported in our previous study [1].
Immunopathology of resected metastases and recurrences
In seven patients both the metastatic tumor from which the vaccine was manufactured and a resected recurrent lesion could be studied by IHC assessing the nature of the infiltrate and the expression of HLA and melanoma antigens by tumor cells. Since IFN-α was administered after the vaccine in an attempt to up-regulate the HLA-I/peptide complexes by residual tumor cells, it was of interest to see whether in two recurring lesions (patient # 01-002, 01-029) obtained as early as 5–6 days after IFN-α administration any increase in the expression of these molecules could be detected. The two metastatic lesions obtained after IFN-α treatment failed to show an increase in the expression of class I HLA since case # 01-002 actually displayed a clear reduction of HLA expression as compared with the first metastasis (from 100 to 50% of the tumor cells testing positive) whereas the second case 01-029 remained completely negative for the expression of these molecules.
Three cases of biopsies at the vaccination site and of autologous distant normal skin were performed. Vaccination site contained mild lymphocyte infiltrates in the peri-vascular location of the dermis. CD4+ T lymphocytes accounted for 60% and CD8+ cells for the remaining 40% of the infiltrate (data not shown).
Clinical response, T cell response and HLA/melanoma antigen expression by the original tumor
A possible association between immune the response elicited by vaccination, expression of HLA and at least one of the melanoma antigens studied (MART-1 or Gp100) by the metastatic tumor, and clinical outcome, is presented in Table 5. It is evident that ten out of 16 patients listed (# 1-023 tumor could not be examined) showed an association between HLA/antigen expression and clinical outcome (7/16 a positive association, i.e. high HLA/antigen and SD, and 3/16 a negative association, i.e., low HLA/antigen and PRO). In two cases (# 1-022, 1-023), SD was associated either to high HLA/antigen or to T cell response only, due to the lack of T cell response and HLA/antigen evaluation, respectively. Considering the two groups of responders (ten SD) and progressors (seven patients), there was no correlation between HLA class I expression and T cell response. However, a clear correlation is evident between clinical outcome and T cell response since, among the ten SD patients, seven had developed a T cell reaction while only one of six progressors did so.
Table 5.
Association between clinical response, T cell response and HLA/melanoma antigen expression by the original tumor
| Patient # | Clinical response | T cell responsea | Percentage of cell expressingb | ||
|---|---|---|---|---|---|
| MART1 | Gp100 | HLA-I | |||
| 003 | PRO | − | 90 | 20 | 100 |
| 007c | SD | + | 100 | 100 | 80 |
| 008c | SD | + | 40 | 0 | 100 |
| 010c | SD | − | 60 | 60 | 100 |
| 011c | PRO | − | < 5 | < 5 | < 5 |
| 012 | SD | − | 50 | 80 | 10 |
| 013 | DF | + | 30 | 5 | 70 |
| 014c | SD | + | 100 | 100 | 100 |
| 016c | SD | + | 40 | 90 | 100 |
| 017 | PRO | − | 60 | 10 | 100 |
| 022c | SD | ND | 90 | 20 | 100 |
| 023 | SD | + | ND | ND | ND |
| 026 | PRO | ND | 100 | 100 | 100 |
| 028c | PRO | + | 70 | 100 | 20 |
| 029c | PRO | − | 100 | 100 | 0 |
| 031c | SD | + | 100 | 60 | 100 |
aOnly HLA class I-restricted T cell responses were considered, including those directed against autologous and/or allogeneic melanoma cells (see Table 4)
bAs evaluated by immunohistochemistry
cCorrelation between clinical response and HLA/MAA expression
Discussion
In this trial stage IV, pre-treated melanoma patients received autologous tumor-derived HSPPC-96 along with GM-CSF and IFN-α in an attempt to potentially enhance the immunologic and clinical response obtained in a previous phase II study conducted with similar subjects receiving HSPPC-96 monotherapy [1]. The combination treatment in this study was well tolerated with few episodes of low-grade local or systemic toxicity caused by the administered cytokines. However, the clinical response was limited to a high frequency of SD (11 out of 20 patients treated) though three patients experienced a prolonged clinical benefit after HSPPC-96 vaccination. One patient (# 01-013), vaccinated without evidence of disease, had a long lasting disease-free interval (414 days). Two additional patients (# 01-010 and 01-012) who received chemotherapy off protocol after HSPPC-96 vaccination, showed one (# 01-010) had a long-lasting (602 days) PR, while the other (# 01-012) had a long-term stabilization of the disease.
This work shows that the HSPPC-96 autologous vaccine administered with GM-CSF could elicit an anti-melanoma, class I HLA-restricted T cell-mediated immune reaction in a proportion of patients treated but that neither the frequency of responding subjects (5 out of 13) nor the strength of the reaction was noticeably higher than that obtained in a previous study of similar patients vaccinated with the HSPPC-96 monotherapy [1].
Of some interest is the observation made in the present study, but not in the previous one, that three patients developed an increase in class I HLA-restricted recognition of autologous but not of allogeneic class I HLA-compatible melanoma cells, suggesting a recognition of individual type of tumor antigens as described in animal models [17].
As for the effect of HSPPC-96 on the NK activity, we found that 12 of 18 patients that could be tested developed a statistically significant increased reaction against K562 after vaccination, a finding indicating the ability of the vaccine to stimulate even this component of the innate immune system. We cannot exclude that IFN-α may have contributed to such an increase of NK activity though in a previous trial in patients with liver metastases of colorectal carcinoma given HSPPC-96 monotherapy a similar in vivo activation of NK activity was observed [9]. Moreover, this has been previously reported in animal models where NK cells were shown to be required for therapeutic anti-tumor activity mediated by HSPs [18] and in a small group of cancer patients as well [19]. It is of interest also that HSPs expressed on the tumor cell surface may stimulate not only the cytolytic activity of NK cells but also their chemotaxis [20]. We have found that even gp96 can stimulate in vitro human NK cells to release cytokine and to kill tumor lines thus potentially explaining their increased activity upon HSPPC-96 vaccination [18] though the role of NK in anti-tumor activity in our clinical setting remains to be determined.
It is of interest that the observations made in this study do not indicate a positive effect of GM-CSF or IFN-α at the regimen given on the immune response or clinical activity in this patient group studied. GM-CSF has been described to possibly increase the immunogenicity of different peptide-based melanoma vaccines [5, 21, 22] and of a recombinant Ep-CAM vaccine against colorectal cancers though in a small number of patients [23]. However, at least one study has shown a lack or even a suppressive effect of GM-CSF on the induction of CEA-specific T cell response [24] and in the only randomized clinical trial in which the same antigen (irradiated autologous tumor cells) was given either with IFN-α or GM-CSF as adjuvants no difference in immunologic and clinical response rate was found [25].
In addition, IFN-α at the dose administered did not seem to up-regulate significantly the HLA class I expression. However, this could only be investigated in two subjects where two different metastatic lesions could be examined within a week after the first cytokine administration. Moreover, the lesions analyzed were resected from clinically progressing tumors, which could have become resistant to IFN-α effect of HLA class I up-regulation. In fact, it is known that melanoma cells can be defective in terms of IFN signaling, a feature which may prevent even the cytotoxic activity of this cytokine on melanoma cells [26].
Altogether, possible explanations of these observations could be as follows. Firstly, the combination of two immunomodulatory cytokines with a personalized vaccine is complex and may require a carefully developed schedule of administration for which this study may present the first step. In this regard, the data from this trial first investigating the combination in humans may serve as the basis for modifications of the treatment regimen toward a potentially more active schedule. Secondly, the population of pre-treated patients with metastatic melanoma may not be the best group to study because of rapid progression in many patients not allowing for sufficient time for an immune response and subsequent clinical response to develop. Thirdly, the sample size of this study, as in most trials of this nature was rather small and may not allow for smaller differences to be detected, particularly in the context of a comparison with historical controls.
We conclude that the addition of low-dose local GM-CSF, plus s.c. IFN-α to HSPPC-96 vaccine and in a low amount, did not noticeably increase the frequency of patients mounting a T cell immune response in comparison with a previous study in which patients received HSPPC-96 monotherapy. As possible reasons for these observations different treatment schedules for the triple combination in a large, less advanced patient population may need to be explored.
However, this study confirmed the T cell immunogenicity of HSPPC-96 and appears to be associated with a high frequency of disease stabilization in this heavily pre-treated, advanced patient population.
Acknowledgements
This study was supported by Antigenics Inc. (Lexington, MA, USA). The authors wish to thanks Drs Claudia Lombardo and Fernando Ravagnani for HLA typing, Mr Giulio Pezzaglia and Mr Gianluigi Rigamonti for excellent nursing work, and Ms Grazia Barp for editorial assistance.
Abbreviations
- AEs
adverse events
- DTH
delayed-type hypersensitivity
- ELISPOT
enzyme-linked immunospot
- GM-CSF
granulocyte/macrophage-colony stimulating factor
- HSP
heat shock proteins
- HSPPC-96
heat shock proteins peptide complexes gp96
- IFN
interferon
- IHC
immunohistochemistry
- IL
interleukin
- NK
natural killer
- PBMC
peripheral blood mononuclear cells
- RECIST
response evaluation criteria in solid tumors
- s.c.
subcutaneously
Footnotes
L.P. and R.P. have equally contributed to the work.
References
- 1.Belli F, Testori A, Rivoltini L, Maio M, Andreola G, Sertoli MR, Gallino P, Piris A, Cattelan A, Lazzari I, Carrabba M, Scita G, et al. Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: Clinical and immunologic findings. J Clin Oncol. 2002;20:4169–4180. doi: 10.1200/JCO.2002.09.134. [DOI] [PubMed] [Google Scholar]
- 2.Parmiani G, Testori A, Maio M, Castelli C, Rivoltini L, Pilla L, Belli F, Mazzaferro V, Coppa J, Patuzzo R, Sertoli MR, Hoos A, et al. Heat-shock proteins and their use as anti-cancer vaccines. Clin Cancer Res. 2004;10:8142–8146. doi: 10.1158/1078-0432.CCR-04-1194. [DOI] [PubMed] [Google Scholar]
- 3.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte-macrophage colony stimulating factor. J Exp Med. 1992;176:1693–1699. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soiffer R, Lynch T, Mihm M, Jung K, Rhuda C, Schmollinger JC, Hodi FS, Liebster L, Lam P, Mentzer S, Singer S, Tanabe KK, et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage-colony stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci USA. 1998;95:13141–13146. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber J, Sondak VK, Scotland R, Phillip R, Wang F, Rubio V, Stuge TB, Groshen SG, Gee C, Jeffery GG, Sian S, Lee PP. Granulocyte-macrophage-colony stimulating factor added to a multipeptide vaccine for resected stage II melanoma. Cancer. 2003;97:186–200. doi: 10.1002/cncr.11045. [DOI] [PubMed] [Google Scholar]
- 6.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/S0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 7.Giacomini M, Fraioli R, Calabro AM, DiFilippo F, Natali PG. Class I major histocompatibility complex enhancement by recombinant leukocyte interferon in the peripheral blood mononuclear cells and plasma of melanoma patients. Cancer Res. 1991;51:652–656. [PubMed] [Google Scholar]
- 8.Therasse P, Arbuck SG, Eisenhauer EA. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 9.Mazzaferro V, Coppa J, Carrabba MG, Rivoltini L, Schiavo M, Regalia E, Mariani L, Camerini T, Marchianò A, Andreola S, Camerini R, Corsi M, et al. Vaccination with autologous tumor-derived heat-shock protein Gp96 after liver resection for metastatic colorectal cancer. Clin Cancer Res. 2003;9:3235–3245. [PubMed] [Google Scholar]
- 10.Pass HA, Schwarz SL, Wunderlich JR, Rosenberg SA. Immunization of patients with melanoma peptide vaccines: Immunologic assessment using the ELISPOT assay. Cancer J Sci Am. 1998;4:316–323. [PubMed] [Google Scholar]
- 11.Lewis JJ, Janetzki S, Schaed S, Panageas KS, Wang S, Williams L, Meyers M, Butterworth L, Livingston PO, Chapman PB, Houghton AN. Evaluation of CD8+ T-cell frequency by the ELISPOT assay in healthy individuals and in patients with metastatic melanoma immunized with tyrosinase peptide. Int J Cancer. 2000;87:391–398. doi: 10.1002/1097-0215(20000801)87:3<391::AID-IJC13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 12.Scheibenbogen C, Romero P, Rivoltini L, Herr W, Schmittel A, Cerottini JC, Woelfel T, Eggermont AMM, Keilholz U. Quantification of antigen-reactive T cells in peripheral blood by IFN-γ-ELISPOT and chromium release assay: a four centre comparative trial. J Immunol Methods. 2000;244:81–89. doi: 10.1016/S0022-1759(00)00257-X. [DOI] [PubMed] [Google Scholar]
- 13.Rivoltini L, Castelli C, Carrabba M, Mazzaferro V, Pilla L, Huber V, Coppa J, Gallino G, Scheibenbogen C, Squarcina P, Cova A, Camerini R, et al. Human tumor-derived heat shock protein 96 mediates in vitro activation and in vivo expansion of melanoma-and colon carcinoma-specific T cells. J Immunol. 2003;171:3467–3474. doi: 10.4049/jimmunol.171.7.3467. [DOI] [PubMed] [Google Scholar]
- 14.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL, Miki T, Rosenbergs SA. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci USA. 1994;91:3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mihm MC, Jr, Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Invest. 1996;74:43–47. [PubMed] [Google Scholar]
- 16.Moretta L, Bottino C, Pende D, Mingari MC, Biassoni R, Moretta A. Human natural killer cells: their origin, receptors and function. Eur J Immunol. 2002;32:1205–1211. doi: 10.1002/1521-4141(200205)32:5<1205::AID-IMMU1205>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 17.Tamura Y, Peng P, Liu K, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 18.Pilla L, Squarcina P, Coppa J, Mazzaferro V, Huber V, Pende D, Maccalli C, Sovena G, Mariani L, Castelli C, Parmiani G, Rivoltini L. Natural killer and NK-Like T-cell activation in colorectal carcinoma patients treated with autologous tumor-derived heat shock protein 96. Cancer Res. 2005;65:3942–3949. doi: 10.1158/0008-5472.CAN-04-3493. [DOI] [PubMed] [Google Scholar]
- 19.Janetzki S, Palla D, Rosenhauer V, Lochs H, Lewis JJ, Srivastava PK. Immunization of cancer patients with autologous cancer-derived heat shock protein gp96 preparations: a pilot study. Int J Cancer. 2000;88:232–238. doi: 10.1002/1097-0215(20001015)88:2<232::AID-IJC14>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 20.Gastpar R, Gross C, Rossbacher L, Ellwart J, Riegger J, Multhoff G. The cell surface-localized heat shock protein 70 epitope TKD induces migration and cytolytic activity selectively in human NK cells. J Immunol. 2004;172:972–980. doi: 10.4049/jimmunol.172.2.972. [DOI] [PubMed] [Google Scholar]
- 21.Scheibenbogen C, Schmittel A, Keilhjolz U, Allgauer T, Hofmann U, Max R, Thiel E, Schadendorf D. Phase 2 trial of vaccination with tyrosinase peptides and granulocyte-macrophage colony-stimulating factor in patients with metastatic melanoma. J Immunother. 2000;23:275–281. doi: 10.1097/00002371-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Slingluff CL, Petroni GR, Yamshikov GV, Barnd DL, Eastham S, Galavotti H, Patterson JW, Deacon DH, Hibbitts S, Teates D, Neese PY, Grosh WW, et al. Clinical and immunological results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony stimulating factor in adjuvant or pulsed on dendritic cells. J Clin Oncol. 2003;21:4016–4026. doi: 10.1200/JCO.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Ullenhag GJ, Frodin J-E, Mosolitis S, Kiaii S, Hassan M, Bonnet MC, Moingeon P, Mellstedt H, Rabbani H. Immunization of colorectal carcinoma patients with a recombinant canarypox virus expressing the tumor antigen Ep-CAM/KSA (ALVAC-KSA) and granulocyte macrophage colony-stimulating factor induced a tumor-specific cellular immune response. Clin Cancer Res. 2003;9:2447–2456. [PubMed] [Google Scholar]
- 24.Von Meheren M, Arlen P, Gulley J, Rogatko A, Cooper HS, Meropol NJ, Alpaugh RK, Davey M, McLaughlin S, Beard MT, Tsang KY, Schlom J, Weiner LM. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma. Clin Cancer Res. 2001;7:1181–1191. [PubMed] [Google Scholar]
- 25.Dillman RO, Wiemann M, Nayak SK, deLeon C, Hood K, DePriest C. Interferon-gamma or granulocyte-macrophage colony-stimulating factor administered as adjuvants with a vaccine of irradiated autologous tumor cells from short-term cell line cultures: a randomized phase 2 trial of the cancer biotherapy research group. J Immunother. 2003;26:367–373. doi: 10.1097/00002371-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Pansky A, Hildebrand P, Fasler-Kan E, Beglinger C, Heim MH. Defective JAK-STAT signal transduction pathway in melanoma cell resistant to growth inhibition by interferon-α. Int J Cancer. 2000;85:720–725. doi: 10.1002/(SICI)1097-0215(20000301)85:5<720::AID-IJC20>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]