Abstract
The Thomsen-Friedenreich disaccharide (TFα) is a promising antigen for tumor immunotargeting, since it is almost exclusively expressed on carcinoma tissues. So far, an obstacle preventing the exploitation of TF for immunotargeting has been the lack of suitable (non-IgM) antibodies with high affinity and specificity. Recently we reported on a novel strategy for generating antibodies toward small uncharged carbohydrates and the generation of recombinant antibodies toward TF. Among them, two multivalent scFv antibodies showed sub-micromolar functional affinities and appeared well suited for immunotargeting. In the present study, the trimeric scFv(1aa) and the tetrameric scFv(0aa) have been further developed for radioimmunotargeting. The scFvs were radiolabeled with 111In using DTPA as chelator without losing binding activity or molecular stoichiometry. Binding affinities as high as 1 × 10−7 M toward TF displayed on living cells were determined. Antibody biodistribution and tumor targeting efficacy were studied in TF-positive human breast cancer (ZR-75-1) bearing mice. TF was successfully targeted in vivo with tumor uptakes of ∼11 and 8% ID/g after 24 h for the trimeric and tetrameric scFv, respectively. These results validate TF as a potent antigen for tumor targeting. The biodistribution of the scFvs was comparable to that reported for IgGs. In contrast to the IgGs, the serum clearance of the scFvs was very fast, which could be an advantage in a therapeutic setting. Furthermore, kidney uptake, which is often critical for small recombinant antibodies labeled with radio-metals, was low with the tetramer (11% ID/g). We conclude that the multimeric anti-TF scFvs are promising candidates to be further developed toward therapeutic application.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-007-0292-5) contains supplementary material, which is available to authorized users.
Keywords: Single chain fragment variable (scFv), Thomsen-Friedenreich antigen (TF), Multivalent scFv, Biodistribution, Tumor targeting
Introduction
Among tumor-associated carbohydrate antigens, the Thomsen-Friedenreich antigen (TF, Galβ1-3GalNAcα1-O-Ser/Thr, synonymous with CD176, TFα, or the core-1 structure) seems particularly promising for an immunotherapeutic strategy due to its outstanding tumor-specificity [9, 25, 26]. As an oncofetal antigen, TF is cryptic in healthy adults [5] but is displayed on mucins and other membrane glycoproteins on tumor cells as a result of incomplete O-glycosylation [13, 18]. TF has been found on many carcinomas, of which breast, gastrointestinal and prostate carcinomas as well as liver metastasis from colorectal carcinomas are particularly promising targets for a TF-based immunotherapy [4, 24, 26].
We have recently reported on the generation of the first recombinant antibodies with TF-specificity [22]. A series of multivalent scFvs were selected from phage display libraries by alternating panning on asialoglycophorin and TF conjugated to polyacrylamide. The most promising scFvs encoded identical VH and VL genes except for minor variations in the primer encoding regions. The multimerisation grade of the selected scFvs was shown to depend on the length of the linker between the VH and the VL in agreement with previous reports on scFv multimerisation [11, 28]. Shortening the linker down to 2 residues resulted in the formation of dimeric scFvs, whereas the scFv with an 1-residue linker (scFv(1aa)) formed trimeric complexes, and the scFv in which the linker was omitted (scFv(0aa)) formed tetrameric complexes.
Compared to the monovalent scFv(18aa), all multimeric scFvs bound TF with considerably higher functional affinities [22]. This was expected, and the observation supports the hypothesis that multivalency is the important factor for high affinity binding of carbohydrates [20, 22]. The tetrameric scFv(0aa) and trimeric scFv(1aa) were found to have the highest functional affinities and were therefore chosen as antibodies to evaluate TF as an antigen for tumor targeting in vivo.
For radioimmunotherapeutic strategies in vivo tumor-specific targeting is a prerequisite in order to deliver the majority of the radioactive payload to the tumor. Labeling of antibodies with radio-metals (e.g. 90Y or 111In) is achieved via chelator groups that are conjugated to the antibodies. The therapeutic efficacy of a radiometal-labeled antibody—besides a high tumor uptake and a limited uptake in normal organs—depends on a range of additional factors. The in vivo stability of the labeled antibody, the density of the antigen on the tumor, the tumor penetration of the antibody, binding of the antibody to circulating tumor antigens, and other parameters are all involved and determine the success. Many of these parameters are influenced by antibody size and functional affinity, which can be modulated by antibody engineering. During the last two decades, a variety of recombinant antibodies have been generated and evaluated for these factors. In general, small recombinant antibodies were expected to exhibit superior pharmacokinetics due to their better tissue penetration, which is assumed to provide better access to the tumor. However, in reality the monovalent scFvs have disappointed mainly due to their weak binding and fast clearance via the kidney [12]. The kidney uptake has evolved as the dose-limiting factor for radioimmunotherapeutic strategies with small recombinant antibodies combined with radiometals such as 90Y, because the radio-labeled metabolites remain in the kidney and cause damage to the organ [23, 30]. From radioimmunotherapeutic trials with 90Y-labeled IgGs, which are larger (150 kDa), other critical organs such as liver and bone marrow have been reported. This is presumably caused by the longer serum half-lives of IgGs.
A solution could come from multimeric scFvs. The trimeric scFv(1aa) (84 kDa) and the tetrameric scFv(0aa) (112 kDa) developed by us [22] are bigger than the filtration cut-off in the kidney, and should not be taken up in the kidney to the same extent as single scFvs.
We report here on the first successful tumor targeting using TF as antigen and our multimeric (trimeric and tetrameric) scFvs. Moreover, this is the first report on biodistribution comparing trimeric and tetrameric scFvs labeled with a radiometal, 111In, which shows fast serum clearance combined with reduced kidney uptake particularly of the tetrameric scFv.
Materials and methods
Antigens and cell lines
Asialoglycophorin and glycophorin from human blood type MN were purchased from Sigma-Aldrich (Taufkirchen, Germany), and were used as positive and negative control antigens. Glycophorin A contains 16 O-glycans and a single N-glycan [29]. Almost all O-glycans are TFα structures masked with sialic acid. Most frequently, TF is di-sialylated (78%), but also mono-sialylated TF (17%) and tri-sialylated TF (5%) are found [7]. Upon treatment with neuraminidase, the sialic acids are cleaved off resulting in the exposure of up to 16 TF structures per molecule at defined, different distances. Oligosaccharides conjugated to poly[N-(2-hydroxyethyl)acrylamide] (PAA) and TF sepharose were purchased from Syntesome (Munich, Germany). Monomeric TF was obtained from BioCarb (Lund, Sweden). ZR-75-1 is a human breast carcinoma cell line (ATCC CRL-1500). K-562 is a human chronic myelogenous leukemia cell line (ATCC CCL-243). KG-1 is a human acute myelogenous leukemia cell line (ATCC CCL-246). NM-D4 is a proprietary glyco-engineered cell line derived from human chronic myelogenous leukaemia (Glycotope, Berlin, Germany).
Production and purification of the scFv(0aa) and scFv(1aa)
The scFvs were expressed in E. coli and purified by immobilized metal affinity chromatography (IMAC) as follows. Electrocompetent Rosetta(DE3)pLysS cells (Novagen, Germany) were transformed with pET11a(PelB) plasmid encoding the scFv(1aa), and an overnight culture inoculated in 2 × TY (16 g tryptone, 10 g yeast extract, 5 g NaCl per l) supplemented with ampicillin (100 μg/ml), chloramphenicol (33 μg/ml) and 1% glucose. The overnight culture was diluted 1:100 and incubated at 25°C in 2 × TY with an additional supplement of 50 mM sucrose. At an OD600 nm of ∼0.6 the culture was induced with 500 μM isopropyl-β-d-thiogalactopyranoside (IPTG) (Sigma–Aldrich) and incubated overnight at 25°C. Bacteria were harvested by centrifugation, and a TES fraction prepared by osmotic shock treatment of the bacteria. This was done by resuspension in ice-cold TES buffer (50 mM Tris–HCl, 1 mM EDTA, 20% sucrose, pH 8, 1/20 culture volume) followed by stirring at 4°C for 30 min. Subsequently, the same volume of ice-cold 5 mM MgSO4 was added and the mixture maintained at 4°C for another 30 min. This is an efficient method for lysing the Rosetta cells because they express T7 lysozyme. Finally, the TES fraction was cleared by centrifugation (4,600 rpm for 1 h) and dialysed overnight against a 10 mM imidazole phosphate buffer (50 mM phosphate, 300 mM NaCl, 10 mM imidazole, pH 8.0). The scFvs were purified from the TES fraction by IMAC applying an open column with 5 ml Chelating SepharoseTM Fast Flow (Amersham Biosciences, Freiburg, Germany) loaded with NiSO4. After loading, the column was washed with 100 ml phosphate buffer supplemented with 50 mM imidazole (50 mM phosphate, 300 mM NaCl, 50 mM imidazole, pH 8.0) followed by 10 ml phosphate buffer with 70 mM imidazole. The protein was eluted with phosphate buffer containing 300 mM imidazole, and dialysed against PBS overnight at 4°C.
To deplete agents potentially interfering with the chelating reaction or the chelator, different secondary purification procedures were applied for the two multimers.
The tetrameric scFv(0aa) was finally purified and concentrated on a TF-PAA-sepharose affinity column (Syntesome). This column had a TF distribution particularly suited for the enrichment of tetrameric complexes of scFv(0aa). The column was loaded with IMAC-purified scFv(0aa) in PBS at 1 ml/min, and eluted with PBS (pH 11).
The trimeric scFv(1aa) was finally purified by ion exchange chromatography using a 5 ml Hitrap Q HP column (Pharmacia, Upsala, Sweden). The IMAC purified scFv(1aa) was diluted tenfold in carbonate buffer to yield final concentrations of 50 mM Na2CO3, pH 8.7 and 50 mM NaCl. The protein was loaded on a 5 ml HiTrap Q HP column (Pharmacia) at a high flow-rate (5 ml/min). The column was washed with 15 ml of 50 mM Na2CO3, pH 8.7, containing 50 mM NaCl, before eluting by increasing the NaCl concentration to 300 mM. Eluted protein was re-buffered into PBS using Centricon spin-tubes (Amicon, Witten, Germany), and equilibrated to 500 μg/ml before storing at −80°C. These conditions selectively enriched for trimeric complexes of the scFvs as revealed by size exclusion chromatography.
Surface plasmon resonance
Measurements were performed on a BIAcore 2000 instrument (Pharmacia). Asialoglycophorin was immobilised non-covalently on the dextran matrix of a CM5 sensor chip using the standard amine immobilisation procedure according to BIAcore protocols. The scFv(1aa) was bound to the chip as described previously [22], and the dissociation analysed in PBS or in PBS with TF-positive substances. The flow rate was 5 μl/min at 25°C.
Immunohistochemistry and immunofluorescence
Cryostat sections (4 μm) were prepared from human tumors or xenotransplant tumors (human primary colorectal carcinomas grown subcutaneously on NCR:nu/nu mice) and stained according to standard procedures with some modifications. Xenotransplant tumor sections were treated with 3% H2O2 in PBS to inhibit endogeous peroxidase activity, and blocked with 1:5 diluted normal goat serum. Incubation with the purified multibody (10 μg/ml) was done at +4°C for 1 h. Visualization of the multibody was achieved with the mouse mAb 9B11 (Cell Signaling Technology, Frankfurt/M, Germany, 1:1000, 30 min) recognizing the Myc tag, followed by a POD-labeled F(ab´)2 fragment from goat anti-mouse immunoglobulin serum (Jackson Laboratories, via Dianova, Hamburg, Germany, 1:200), and finally by diaminobenzidine (DAB). Human tumor sections were fixed with acetone and treated with 0.6% H2O2 in methanol. Endogenous biotin was blocked using the biotin blocking system (Dako Cytomation, Cambridgeshire, UK) prior to the detection of TF with the scFvs and the ABC mouse IgG kit (Vector Laboratories, Peterborough, UK) in combination with the 9B11 anti-myc antibody. Staining was performed with DAB. Counterstaining was done with haematoxylin. Washings were done 3 times with PBS. Slides were embedded with Entellan (Merck, Darmstadt, Germany).
Immunofluorescence staining was performed with cells grown on multiwell slides (10 wells) for 2 h. Thereafter the medium was carefully removed (sucked off) from the edge of the wells as far as possible, and the cells on the slide air-dried. After fixation with 4% formaldehyde in PBS (5 min, RT) the slides were incubated with a drop of about 50 μl per well of purified multibody as above, followed by 9B11, and finally by a Cy3-labeled anti-mouse Ig antiserum (Jackson Laboratories, 1:100). Counterstaining was done with DAPI (0.5 μg/ml, 1 min). Slides were embedded with PBS/glycerol (1:1) containing a trace of p-phenylenediamine.
The slides were examined with an Axioplan 2 imaging microscope (Zeiss, Jena, Germany) equipped with an AxioCam camera system.
Antibody conjugation to DTPA
Chelating was achieved by adding 15 molar excess p-SCN-Bz-DTPA (p-isothiocyanatobenzyl-diethylene-triaminepentaacetic acid, Macrocyclics, Dallas, USA) to the scFvs (0.5 mg/ml) in 50 mM carbonate buffer, pH 8.7, 50 mM NaCl, and incubating the mixture at room temperature (RT) for 1 h. Subsequently, residual free DTPA was depleted by extensive spin filtration (Centricon YM-10, Amicon) before the scFvs were aliquoted and stored at −80°C in 300 mM acetate buffer, pH 4.3, which was used for radiolabeling.
The multimerisation grade of the DTPA-conjugated scFvs was analysed by size exclusion chromatography using a custom-made G-200 column equilibrated in PBS and run at 0.1 ml/min as described previously [22]. The binding specificity of the DTPA-conjugated scFvs was investigated in ELISA by probing for binding toward a representative panel of relevant test antigens (asialoglycophorin, glycophorin, TFα-PAA, TFβ-PAA, sia-TFα-PAA; and BSA). The DTPA-conjugated scFvs were also compared quantitatively with the non-conjugated scFvs applying serial dilutions in an ELISA with coated asialoglycophorin.
Radiolabeling of antibodies with 111In
Labeling of the DTPA-conjugated scFvs was performed at pH 4.3 simply by adding 111InCl3 (specific activity 415.8 mCi/μg, Perkin Elmer, Boston, USA) and allowing incorporation at RT for 2 h. Labeling efficiencies achieved were over 90%. Subsequently, residual free 111InCl3 was removed and the protein re-buffered into PBS by spin-filtration (YM-30, Amicon). The labeling efficiency, purity, and stability of labeled antibodies were assayed by thin layer chromatography (TLC) and SDS-PAGE followed by autoradiography. Finally, the multimerisation grade of the scFvs was controlled by size exclusion chromatography. For radioimmunoassays and cell binding experiments the specific activity of the antibodies applied were ∼0.03 μCi/μg, and ∼1 μCi/μg for the in vivo biodistribution experiments.
Radioimmunoassays (RIA) and cell binding assays
Asialoglycophorin, glycophorin and the TFα-PAA conjugate were coated in microtiter plates. Following blocking, 100 μl of 111In-labeled scFv in serial dilutions were incubated in the wells for 60 min at RT to enable binding. After washing the wells three times with 200 μl PBS, bound scFv was retrieved in 200 μl 500 mM NaOH, 1% SDS, and the radioactivity was measured.
The cell binding assays were performed with 2 × 106 cells in fixed volumes of 200 μl in microtiter plates. 111In-labeled scFv in serial dilutions (0.5–10 μg) were incubated with the cells for 60 min to enable binding. Incubations were performed at 4°C to minimise potential internalisation and metabolism of the antibodies. Cells were retrieved by centrifugation, washed twice in PBS, and the radioactivity in the cell pellet was measured. For both assays, the data were processed in Scatchard plots. In some cases, consecutive cell binding experiments were carried out with an initial antibody concentration of 2 μg in 200 μl, and then applying the supernatant to subsequent cell binding experiments. This was done in four consecutive rounds.
Biodistribution analyses with the scFv(0aa) and the scFv(1aa)
A mouse model was established based on xenotransplants of the human breast cancer cell line ZR-75-1 in nude mice by subcutaneous injection of 107 cells and grown for 3–5 weeks until palpable. TF expression in the tumors was confirmed by immunohistochemistry using a TF-specific human chimeric IgG1 generated with the variable genes from the scFvs. 111In-labeled scFv(0aa) and scFv(1aa) were injected into the tail vein in 200 μl doses (5 μg scFv in PBS supplemented with 0.1% FCS). Injection, sacrifice and organ resection were performed at EPO GmbH (Berlin, Germany). Serum, brain, bone marrow, heart, lung, liver, spleen, and kidney were isolated 24 h after injection and the organ weights determined. Radioactivity was measured and the data processed as % ID/g (percent injected dose per gram organ).
Results
Characterisation of unlabeled and DTPA-conjugated multivalent scFvs
The multivalent single chain antibodies were expressed in E. coli, and the proteins purified via their hexaHis tag by Immobilised Metal Affinity Chromatograpy (IMAC) as described [22]. The stringent washing conditions (up to 70 mM imidazole) ensured a fairly pure preparation of the scFvs. The trimeric scFv(1aa) gave yields of 1.3 mg/l in shake flask cultures (N = 74), whereas the more complex tetrameric scFv(0aa) yielded merely 0.25 mg/l (N = 22). In comparison, the less complex, dimeric scFv(2aa) were expressed quite well with an average yield of 2.5 mg/l (N = 3). The multimerisation grade of the three scFv constructs was analysed by size exclusion chromatography. The scFv(0aa) formed tetrameric complexes, the scFv(1aa) formed trimeric complexes (Fig. 1a), and the scFv(2aa) formed dimeric complexes (not shown).
Fig. 1.
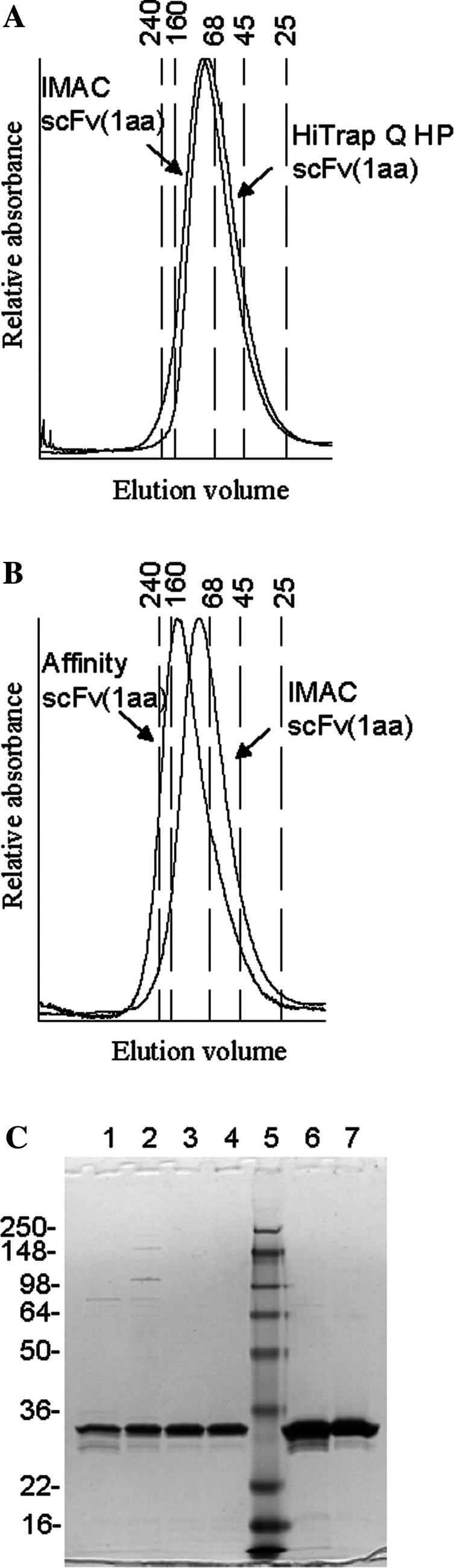
Characterization of scFv preparations. a, b Size exclusion chromatography of scFv(1aa) multibodies purified by different methods. IMAC with subsequent HiTrap Q HP purification (a) yielded trimers, whereas IMAC followed by affinity chromatography selected tetramers (b), which were a small part of the original scFv(1aa) preparation. Numbers on top of the graph indicate the M r of marker proteins; some of them were drawn as vertical lines. c SDS-PAGE of scFv(1aa) during various purification steps. Note that multimers disintegrate into monomers under these conditions. Lanes 1 and 2 IMAC-purified scFv(0aa) and scFv(1aa), respectively; 3 affinity-purified scFv(0aa); 4 HiTrap Q HP-purified scFv(1aa); 5 molecular markers; 6 and 7 DTPA-conjugated scFv(0aa) and scFv(1aa), respectively
The scFv(0aa) was further purified on a TF affinity column which enriched for the tetrameric complexes, wheras the scFv(1aa) was further purifed by ion-exchange chromatography under conditions which enriched for trimeric complexes as seen in Fig. 1b. Affinity purification of the scFv(1aa) resulted in the selective enrichment of a minor fraction of tetrameric scFv complexes of about 5% (Fig. 1b). All constructs were stable. Their profile was not changed after 2 weeks at 4°C or 24 h at 37°C as analysed by size exclusion chromatography (data not shown). The same was true for their binding activity (see below). SDS gels of the different purification steps showed in each case a single band (Fig. 1c for scFv(1aa); note that multimeric scFvs disintegrate into monomers under the SDS conditions).
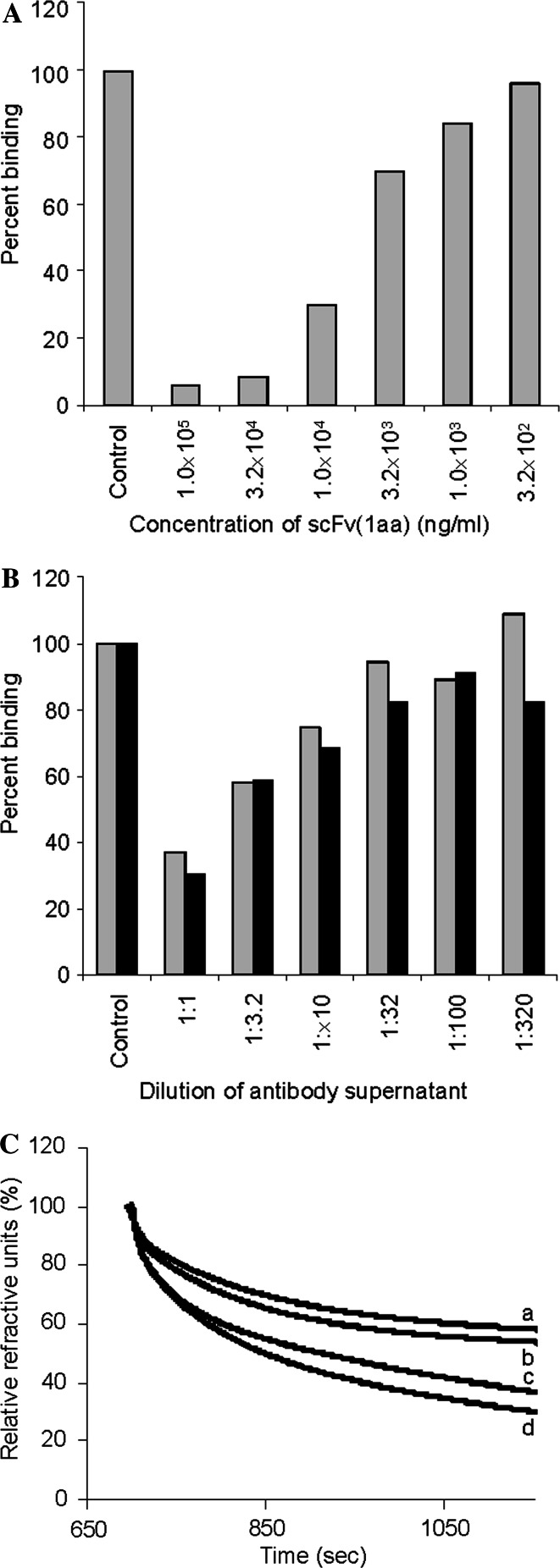
The binding specificity of the anti-TF scFvs has already been extensively studied [22]. Out of a panel of more than 80 mono- and oligosaccharides only TFα and to a much lesser extent the immunologically similar core-2 trisaccharide (the extended TFα) was recognized. This specificity is shared with almost all mouse anti-TF antibodies (own unpublished data). To further validate TFα as the epitope of the scFv, a competition ELISA was performed with the TF-specific antibody A78-G/A7 showing that the scFv(1aa) could compete with the binding of A78-G/A7 to the TFα-carrying protein asialoglycophorin (Fig. 2a). Vice versa, the binding of the scFv(1aa) could be competed with A78-G/A7 as well as with another anti-TF antibody (Nemod TF2) (Fig. 2b). Furthermore, a displacement assay performed by surface plasmon resonance (BIAcore) showed that monomeric TFα did not have a big effect, whereas multiple TFα moieties displayed on either PAA or asialoglycophorin showed a pronounced displacement effect (Fig. 2c). This is also in accord with the multimeric nature of the scFv preparations. In addition, we have performed immunocytochemistry and immunohistochemistry as a final proof of specificity (shown for scFv(1aa) in Fig. 3).
Fig. 2.
Competition experiments. a, b Competition ELISAs with immobilized asialoglycophorin (aGP). a Binding of mouse anti-TF antibody A78-G/A7 was inhibited by different amounts of purified scFv(1aa). Bound A78-G/A7 was detected with POD-labeled anti-mouse Ig antiserum. b Binding of scFv(1aa) was inhibited by dilutions of supernatants from two mouse anti-TF antibodies, A78-G/A7 (grey bars) or NEMOD-TF2 (black bars). Binding of scFv(1aa) was detected with rabbit anti-myc antiserum followed by POD-labeled anti-rabbit Ig antiserum. c Analysis of binding kinetics by surface plasmon resonance on a BIAcore biosensor instrument under competition conditions. Overlay plot of sensorgrams of the dissociation of scFv(1aa) from aGP coupled to a dextran chip as influenced by different TF-carrying molecules: a control (PBS); b monomeric TFα (200 mM); c aGP (50 μM); d TFα coupled to PAA (multimeric, 50 μM)
Fig. 3.
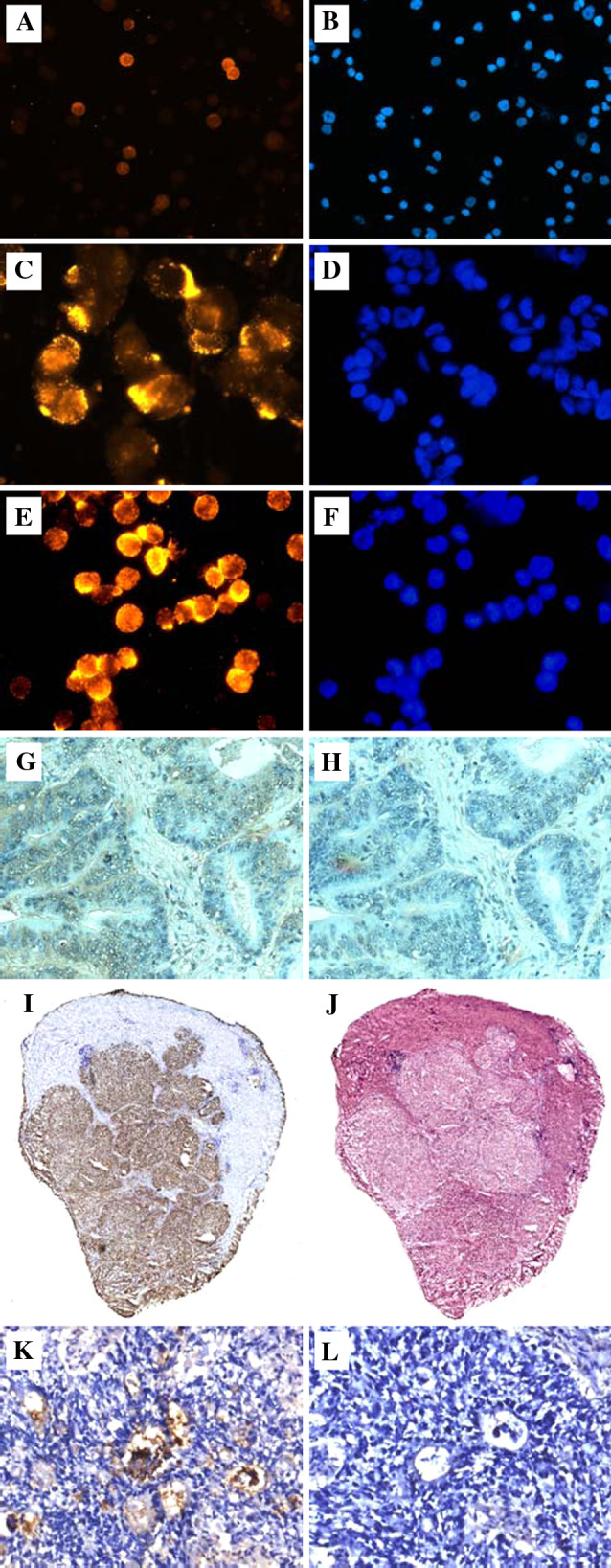
Immunostaining of human tumor cells and tissues with the trimeric anti-TF multibody scFv(1aa). a, b KG-1 (AML cell line); c, d ZR-75-1 (breast cancer cell line); e, f NM-D4 (glycoengineered CML cell line); a, c, e stained in immunofluorescence (IF); b, d, f counterstaining of nuclei with DAPI (identical fields as in IF). In a, around ten out of 80 cells (12.5%) are membrane-stained by the multibody with different intensities. This pattern is identical to that seen with the mouse anti-TF antibody A78-G/A7 (not shown). g Cryosection of human colon carcinoma stained in immunohistochemistry (IHC), h adjacent section to g as omission control. i Whole-size cryosection of human hepatocellular carcinoma stained in IHC, j adjacent section stained with hematoxylin/eosin. k cryosection of a primary human colon carcinoma transplanted s.c. into an NCR:nu/nu mouse stained in IHC, l omission control
Prior to conjugation of diethylenetriamine pentaacetic acid (DTPA) to the scFvs, we investigated the stability of the scFvs under the different buffer and temperature conditions employed. An ELISA-based binding activity assay was employed to investigate the effect of the 3 buffers employed for the conjugation and purification (supplementary Fig. 1A–C). As expected, the scFvs were stable in PBS of pH 7.2 at temperatures between 4 and 37°C, but lost some activity after long incubation at 45°C (t 1/2 ∼ 27 h). In contrast, the scFvs lost their binding activity quickly after incubation in the carbonate buffer pH 8.7 used for conjugation, especially at higher temperatures (t 1/2 ∼ 27.5 h, 8 h, 3 h and 7 min at 21, 30, 37, and 45°C, respectively). The impact of this finding was that the chelating reaction should be carried out at RT or lower and within a limited incubation time. The impact of the chelator-to-scFv ratio was investigated at 25, 30, and 37°C revealing no damaging effect on binding activity using a higher molar ratio of the chelator (up to 15-fold excess). Consequently, the scFvs were chelated using a 15-fold molar excess of chelator. Finally, it was found that the scFvs were very stable in the acetate buffer (pH 4.5) used for 111In labeling. Of particular interest was the finding that the scFvs could be conveniently stored at −80°C in this buffer without any detectable loss of binding activity. The antigen specificity of the DTPA-conjugated scFv(1aa) was determined in ELISA against a selection of test antigens (data not shown). The TFα-specific binding pattern was retained as anticipated since the scFvs bound asialoglycophorin, but not glycophorin (in which the TF epitopes are masked with sialic acids) or BSA. Furthermore, the scFvs bound TFα conjugated to PAA, but not TFβ or sialyl-TFα conjugated to PAA. The multimerisation grade of the DTPA-conjugated scFvs was analysed by size exclusion chromatography. The DTPA-conjugated scFvs had roughly the same elution profile as the non-conjugated scFvs, as shown for the scFv(1aa) in the supplementary Fig. 1d. Finally, the binding activities of the scFvs were tested in ELISA against asialoglycophorin. Dilution series of the DTPA-conjugated scFv(1aa) demonstrated a similar binding activity as the non-conjugated scFv(1aa) (supplementary Fig. 1E).
Binding characteristics of the 111In-labeled scFvs
The scFvs were labeled with 111In as described in Materials and methods. The quality analysis done in thin layer chromatography, SDS-PAGE and size exclusion chromatography convincingly revealed the purity of the radiolabeled multibodies (shown for scFv(1aa) in the supplementary Fig. 2). Specific binding was shown in RIAs using immobilized asialoglycophorin versus glycophorin and in cell binding experiments with the TF expressing cell line NM-D4 and the TF-negative cell line K562 as control, respectively. K-562 revealed a very low percentage of bound scFv(1aa) [less than 3% compared to the specific binding (Table 2)]. Scatchard plot analyses of two sets of RIA experiments obtained with the scFv(0aa) and shown in Fig. 4a yielded linear trendlines with similar slopes, from which dissociation constants (K d) could be calculated. This resulted in K d values for the scFv(0aa) of 2.7 × 10−8 M and 3.9 × 10−8 M for asialoglycophorin coated at 0.5 and 1.0 μg/ml, respectively. Similar experiments were carried out with the scFv(1aa). The calculated K d values are summarized in Table 1. In general, the values were in good agreement with the K d values of unlabeled scFvs determined by surface plasmon resonance.
Table 2.
Scatchard analysis of cell binding assays
| N a | Cell line | Binding sitesb | % Bindingc | K d (M) | |
|---|---|---|---|---|---|
| ScFv(0aa) | 4 | NM-D4 | 4.8 × 106 ± 3.3 × 106 | 37.8 ± 6.2 | 1.0 × 10−7 ± 4.6 × 10−8 |
| 3 | ZR-75−1 | 2.5 × 106 ± 2.0 × 105 | 10.3 ± 2.9 | 4.2 × 10−7 ± 1.6 × 10−7 | |
| ScFv(1aa) | 9 | NM-D4 | 8.1 × 106 ± 5.5 × 106 | 17.2 ± 7.5 | 5.3 × 10−7 ± 1.8 × 10−7 |
| 3 | ZR-75-1 | 2.2 × 106 ± 1.2 × 106 | 2.3 ± 0.6 | 1.5 × 10−6 ± 7.4 × 10−7 | |
| 2 | K-562 | – | 0.8 | – |
aNumber of individual experiments
bNumber of binding sites per cell. Determined by extending the trend line of the Scatchard plot to the abscissa
cPercent of the antibodies bound to the cell pellet
Fig. 4.
Binding characteristics of labeled multivalent scFvs. a Scatchard plot analysis of two sets of data from RIA experiments with scFv(1aa) using immobilised asialoglycophorin at two concentrations. From the slope of the trendline the functional affinity was calculated (Table 2). b Scatchard plot analysis of data from cell binding experiments with NM-D4 cells using scFv(0aa). The antibody affinity is calculated from the slope of the line, and the number of antibody binding sites per cell from the intercept with the x axis. c Lindmo plot of data from a series of cell binding experiments using serial dilutions of NM-D4 cells (in the range 1.0 × 106 to 3.2 × 107 cells) and the antibody scFv(1aa). Applying the law of mass action on the equation for the association constant, and correlating for the fraction of active antibody (r) yields an equation [17], from which an “r” value of 48% can be calculated
Table 1.
Dissociation constants (K d) of III In-labeled (RIA) and unlabeled (Biacore) scFv
| RIA | Biacorea | ||
|---|---|---|---|
| 0.5 μg/ml | 1.0 μg/ml | ||
| scFv(0aa) | 2.7 × 10−8 M | 3.9 × 10−8 M | 8.8 × 10−8 M |
| scFv(1aa) | 1.0 × 10−7 M | 1.7 × 10−7 M | 2.2 × 10−7 M |
aTaken from [22]
From a targeting perspective, however, it was more relevant to investigate the binding characteristics of the 111In-labeled scFvs toward the TF epitope displayed on living cells. Besides giving a dissociation constant for the binding, this type of assay can provide information about the number of binding sites per cell and the percentage of antibody binding in the experiment. Data from cell binding assays performed with two TF-positive cell lines, NM-D4 and ZR-75-1, were processed in Scatchard plots, as illustrated for the scFv(1aa) in Fig. 4b. Dissociation constants for the two antibodies using both cell lines are presented in Table 2. For both antibodies, the percentage of bound antibodies was higher with NM-D4 cells compared to ZR-75-1 cells. Furthermore, the percentage of bound antibody in cell binding experiments with the scFv(1aa) was considerably lower compared to the scFv(0aa). To illustrate that the percentage of bound antibody in the cell binding experiments does not correspond to the maximal binding capacity of the 111In-labeled scFv preparations, two different strategies were applied. First, cell binding experiments were performed in which the antibody supernatants were re-applied in four consecutive rounds. The results showed that supernatants from earlier rounds of cell binding still contained active antibodies, demonstrating an overall active fraction of over 60% (data not shown). This was also the case with an enhanced cell concentration (107 cells per 200 μl). Lindmo et al. [17] applied another strategy to elucidate the same aspect. According to his strategy, dilution series of the cell concentration can be processed to determine the active fraction of the antibody preparation. Applying the equation derived by Lindmo and co-workers ([AbTotal]/[AbBound] = 1/r + 1/rKa[AgFree]), we calculated an active fraction (r value) of 48% (see Fig. 4c and legend for details). This is a lower value compared to that obtained with the first method, where an active fraction of over 60% was reached.
Another parameter to be determined was the potential loss of binding activity of the scFvs after incubation in serum at 37°C. The result was that the cell binding activity remained unchanged for at least 24 h under these conditions (data not shown).
Biodistribution in ZR-75-1 nude mice xenografts
The TF-positive breast cancer cell line ZR-75-1 (Fig. 3c) was further validated as a choice for a suitable mouse model in the cell binding assays (Table 2).
The biodistribution results after 24 h (Fig. 5a, b) demonstrated a tumor uptake of ∼11% ID/g for the trimeric scFv(1aa) and of ∼8% ID/g for the tetrameric scFv(0aa). Uptake in organs was considerably lower except in the kidney. Interestingly, the kidney uptake for the trimer was high (∼57% ID/g), but much lower for the tetramer (∼11% ID/g).
Fig. 5.
Biodistribution of multivalent scFvs in xenografted mice. a Biodistribution of 111In-labeled scFv(1aa) (triabody) after 24 h. b Biodistribution of 111In-labeled scFv(0aa) (tetrabody) after 24 h. Specific activity was ∼1 μCi/μg protein. Se serum, Tu tumor, Br brain, Bo bone marrow, He heart, Lu lung, Li liver, Sp spleen, Ki kidney. Means from groups of four mice each are expressed as % ID/g. cAmount of 111In-labeled scFv(1aa) (dotted line) and scFv(0aa) (solid line) in the serum of xenografted mice over time
The serum clearance of the scFv(0aa) was comparable to that of the scFv(1aa) (Fig. 5c), which agrees well with their similar tumor targeting ability. However, it does not explain the difference in kidney uptake. One strategy for reducing the kidney uptake has reportedly been the co-administration of lysine [2]; however, in the case of our scFv(1aa) antibody co-administration of 50 mg lysine had no effect on either kidney uptake, tumor uptake, or serum clearance (data not shown).
Discussion
We set out to investigate the tumor targeting potential of two new recombinant multivalent antibodies recognising the tumor-specific Thomsen-Friedenreich carbohydrate antigen [22]. They reveal sub-micromolar functional affinities, high specificity for TF, and applicability in ELISA and immunocytochemistry [22].
Since scFv lack the Fc part of antibody molecules, they are not expected to be beneficial in a tumor patient by themselves, but may serve as carriers for toxic molecules. Chemotoxic drugs are not an option in this case since our anti-TF antibodies are not internalized (own unpublished data). Therefore, radioactive labeling was chosen. In any case, certain preconditions have to be fulfilled by the antibody such as high tumor specificity, high affinity, high antigen density on the tumor, and low binding of the antibody to normal tissues. With small scFv antibodies some of these parameters like tumor penetration, tumor retention, and serum half life have been determined. Among the radiometals available, 111In and 90Y are already in clinical application for imaging and therapy, and are therefore of particular interest. Both are easily attached to antibodies via chelators that previously have been conjugated to the antibodies [8, 32]. However, for therapeutic strategies, a problematically high kidney uptake has been observed for small recombinant antibodies labeled with radiometals this way [23, 30]. Thus, our multimeric scFvs are interesting because they are bigger than the cut-off Mr for kidney filtration, and should therefore not be taken up in the kidney to the same extent as reported for monovalent scFvs.
An interesting aspect during the purification was the fact that affinity chromatography with TFα-PAA-Sephadex resulted in the preferred binding of the tetrameric as compared to the trimeric scFv. This may result simply from a higher functional affinity of the tetramer or may be caused by a more tetramer-compatible TF density of the immunosorbent (TF-PAA), which was 20% on a molar basis (data by Syntesome). Several lines of evidence indicate a decisive role of the type of TF clustering in the recognition of “natural” TF by anti-TF antibodies (unpublished data). The advantage of asialoglycophorin as a “universal TF antigen” is the fact that it contains an array of TFα moieties at different distances and densities.
The conjugation of the chelator to the scFvs may interfere with the antibody activity. Therefore, it was essential to ensure sustained antibody integrity and binding activity after DTPA conjugation and 111In labeling. We were able to show that the DTPA-conjugated scFvs were identical to the non-conjugated scFvs in all assays. As expected, the scFvs were also efficiently labeled with 111In without affecting the antibody integrity as judged by the elution of a single peak in size exclusion chromatography. The functional affinities of the radiolabeled scFvs determined by RIA were in good agreement with the functional affinities of the non-conjugated antibodies measured by surface plasmon resonance. Both methods consistently showed a fourfold higher functional affinity of the tetrameric scFv(0aa) compared to the trimeric scFv(1aa).
TF is part of the protein glycosylation and may be displayed on different membrane glycoproteins (e.g., MUC1 or CD44v6), resulting in considerable variation in the distribution of TF on cells. This is obviously different from TF displayed on immobilized asialoglycophorin. With respect to the potential applicability of the anti-TF scFvs in immunotargeting, it was important to establish that the scFvs did indeed bind to TF displayed on living tumor cells with high affinity. The functional affinities of the antibodies toward TF displayed on living cells were therefore determined with the radioactivemultibodies, yielding submicromolar affinities for both the trimer and the tetramer, whereby the latter displayed about fourfold higher affinities than the trimer, thus confirming the RIA data. The impact of the affinity difference combined with the number of binding sites as determined for both cell types is evident in the fraction of the antibodies bound to the cells (Table 2, % Binding). The highest percentage of binding under the standardized conditions was observed for the tetramer (scFv(0aa)) on NM-D4 cells (∼37.5%). The trimer (scFv(1aa)) displayed a ∼5-fold lower affinity to NM-D4 cells, but an ∼1.5-fold higher number of binding sites per cell, and accordingly a reduced binding percentage (∼17.2%). Similar comparisons can be made for the ZR-75-1 cell binding experiments. The affinity of the scFv(0aa) was ∼4-fold higher than that of the scFv(1aa), but a similar number of binding sites per cell resulted in an about fourfold difference in the proportion of bound antibody (∼10.3% versus ∼ 2.3%, respectively). This shows that the functional affinity of the antibodies is modified by the antigen presentation on the cells. It also shows that the percentage of bound antibody observed in cell binding experiments should not be mistaken for the immunoreactive fraction of the antibody preparation. To illustrate this, consecutive cell binding experiments were performed in which the antibody supernatants were re-applied in successive incubations. Although a plateau was not yet reached after four consecutive incubations, the results clearly demonstrated that the active fraction of the antibody preparation was higher than the fraction bound during a single incubation.
The Lindmo plot yielded an active fraction of 48% for the scFv(1aa) using NM-D4 cells, which is higher than the binding percentage in the individual cell binding experiments, but less than the cumulative binding percentage in the consecutive cell binding experiments. Lindmo et al. investigated this phenomenon in detail and demonstrated that the active fraction (r) was modified by experimental settings such as incubation time, temperature, cell concentration, and antibody concentration and therefore dependent on the kinetics of the binding [17]. The 48% value in our case is thus to be understood as the highest binding percentage achievable with the scFv(1aa) under our standard incubation volume, time, and temperature, and with increased cell number (>2 × 107).
Based on the consecutive cell binding experiments, the SDS-PAGE radiograph, and the single peak elution in size exclusion chromatography, we have no reason to assume that our antibody preparations should contain significant amounts of inactive or degraded antibody. High antibody stability was confirmed by the conserved binding activity and integrity after incubation in the presence of serum at 37°C for at least 24 h.
The impact of antibody affinity on in vivo tumor targeting has been discussed and analyzed extensively. In the 1980s, the heterogeneous distribution of antibodies in tumors [14] gave rise to models describing the effect of an elevated interstitial pressure in the tumor on the accumulation in the tumor [15]. Extension of these models led to the formulation of the “binding site barrier” hypothesis, the idea that macromolecular ligands could be prevented from penetrating tumors by their successful binding to the target antigen already at the tumor periphery [16, 31, 33]. However, recently Graff and Wittrup have argued that the “binding site barrier” is merely a transition state in antibody penetration process [10]. The antibody will be driven into the tumor as long as there is an antibody concentration gradient between the tumor and the serum. Still, all the models have indicated an optimal K d of 1 nM for tumor immunotargeting, which is in agreement with the experimental finding of Adams et al. [1]. It should be noted that the affinities reported by Adams et al. were not measured in cell binding experiments. On the other hand, the humanised LewisY IgG antibody, hu3S193, labeled with 111In, displayed a functional affinity toward LewisY on cells of 1.0 × 10−7 M, and showed very promising biodistributions in tumor models (∼24% ID/g after 24 h) [6]. The comparison with hu3S193 is particularly interesting because it is a well characterized carbohydrate-binding antibody. The 4.6 × 106 binding sites per cell (MCF-7) found for hu3S193 are in the same range of the number of binding sites found for our scFvs on ZR-75-1 cells.
Taken together, our in vitro binding affinities of the scFvs to tumor cells were very promising, and encouraged us to perform in vivo biodistribution experiments. Using the ZR-75-1 cells xenograft model, we measured a tumor uptake of ∼8 and 11% ID/g after 24 h for the scFv(0aa) and scFv(1aa), respectively. This is the first report on in vivo tumor targeting of TF, and thus establishes TF as a potential antigen for therapeutic antibody strategies. The tumor uptake was good compared to small recombinant antibodies, but modest compared to IgGs as for example hu3S193. The difference to hu3S193 may partly be accounted for by the different affinity and number of binding sites in our scFv/ZR-75-1 system.
Another important factor is the serum clearance. Both the scFv(1aa) and the scFv(0aa) show fast serum clearance with less than 2% ID/g remaining after 24 h. In comparison, the serum levels for chimeric or humanized IgGs after 24 h normally range from 10 to 15% ID/g [6, 19]. The fast serum clearance of our trimeric scFv(1aa) and tetrameric scFv(0aa) is a finding that can be beneficial in therapeutic strategies, where a relatively slow clearance can be problematic due to unintended damage of normal organs, and can cause hematopoetic toxicity [12, 21].
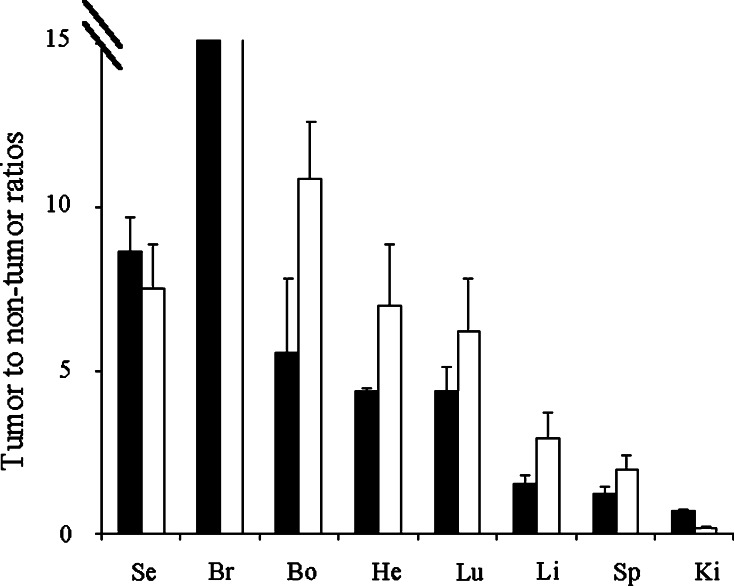
Figure 6 shows the tumor to non-tumor ratios for the scFv(0aa) and the scFv(1aa) in various organs illustrating the potential of these antibody constructs. In general, the ratios for the scFvs are very similar to the ratios seen for 111In-labeled IgGs, also with respect to liver, kidney and spleen. However, due to the faster blood-clearance, the tumor to serum ratios for the scFvs are better than what has been found for IgGs such as for example hu3S193 and Herceptin [6, 19].
Fig. 6.
Tumor to non-tumor ratios for various organs calculated from the data presented in Fig. 5. Filled columns scFv(0aa) (tetrabodies), open columns scFv(1aa) (triabodies); Se serum, Br brain, Bo bone marrow, He heart, Lu lung, Li liver, Sp spleen, Ki kidney
The kidney uptake of very small radiometal-labeled antibodies has turned out to be one of the problematic aspects of radioimmunotargeting because the radiolabeled metabolites tend to be trapped in the kidney following antibody internalisation [23, 30]. Compared to the kidney uptake of monomeric scFvs labeled with radiometals (as for example the 119, 117 and 300% ID/g reported for scFvs in [34, 35]), the larger constructs reported so far have performed better as was expected. In general, the reported biodistributions have indicated a correlation between kidney uptake and the kidney filtration cut-off value (∼70–90 kDa).
In comparison to diabodies with a M r of ∼60 kDa and kidney uptake of ∼126 and ∼65% ID/g as reported by Yazaki et al. [36] and Tahtis et al. [27], respectively, the kidney uptake of our trimeric antibody (84 kDa, ∼57% ID/g) is within the expected range. Our tetrameric scFv(0aa) (112 kDa), with a kidney uptake of only ∼11% ID/g, would support the “filtration cut-off” interpretation of kidney uptake rates. Other factors may also be involved. One of these factors is the net charge of the antibody. A different strategy to overcome the nephrotoxicity of radiometal-labeled immunoconjugates is co-injection of lysine [2, 3, 30, 36]. However, in our hands a supplementation with lysine had no effect on the renal accumulation of our scFv(1aa). Furthermore, since the scFv(0aa) is not taken up in the kidney to the same extent, but carries the same net charge as the scFv(1aa), we assume that the antibody size rather than the net charge is crucial for the kidney uptake in our case.
Thus the overall biodistribution of the minibody construct reported by Yazaki et al. [36] and the tetrameric scFv construct reported here are unique, making them particularly attractive for the development of immunotherapeutics.
In summary, we demonstrate here for the first time the potential of TF as a target for an antibody immunotherapy using two novel antibody formats, the trimeric scFv(1aa) and the tetrameric scFv(0aa). The in vitro characterization indicated an affinity advantage of the scFv(0aa) compared to the scFv(1aa), but this was not reflected on the tumor uptake in vivo. The main difference between to two constructs was the significantly lower kidney uptake of the tetrameric antibody, scFv(0aa). We believe that our data justify the further development of the multimeric anti-TF scFvs for therapeutic application.
Electronic supplementary material
Supplementary Fig. 1 DTPA conjugation conditions and quality analysis after conjugation. a–c Binding activity of the scFv(1aa) after incubation in three different buffers used during the DTPA conjugation at different temperatures (N = 2). d Size exclusion chromatography of the chelated versus the nonchelated scFv(1aa). e Binding activity of the chelated versus the nonchelated scFv(1aa) to asialoglycophorin
Supplementary Fig. 2 Quality analysis of 111In labeling. a Thin layer chromatography of labeled protein. Lanes: 1, free 111In; 2, 111In-labeled unbound DTPA (111In in excess); 3, 111In-labeled scFv(1aa). b Radiography of SDS-PAGE. 111In-labeled scFv(1aa) was loaded on SDS-PAGE and visualised via radiography. c Size exclusion chromatography. 111In-labeled scFv(1aa) and an 111In-labeled control mouse IgG were examined in size exclusion chromatography
Acknowledgments
The work was supported by the Danish Research Training Council (Forskeruddannelsesrådet), NEMOD Biotherapeutics GmbH & Co.KG, and Glycotope GmbH.
Abbreviations
- aa
Amino acid
- DTPA
Diethylenetriamine pentaacetic acid
- PAA
Polyacrylamide
- RIA
Radioimmunoassay
- scFv
Single chain fragment variable
- TF
Thomsen-Friedenreich antigen
- TLC
Thin layer chromatography
- VH and VL
Variable domain of heavy chain and light chain, respectively
- % ID/g
Per cent injected dose per gram organ
- N
Number of individual experiments
References
- 1.Adams GP, Schier R, McCall AM, Simmons HH, Horak EM, Alpaugh RK, Marks JD, Weiner LM. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res. 2001;61:4750–4755. [PubMed] [Google Scholar]
- 2.Behr TM, Sharkey RM, Juweid ME, Blumenthal RD, Dunn RM, Griffiths GL, Bair HJ, Wolf FG, Becker WS, Goldenberg DM. Reduction of the renal uptake of radiolabeled monoclonal antibody fragments by cationic amino acids and their derivatives. Cancer Res. 1995;55:3825–3834. [PubMed] [Google Scholar]
- 3.Behr TM, Sharkey RM, Sgouros G, Blumenthal RD, Dunn RM, Kolbert K, Griffiths GL, Siegel JA, Becker WS, Goldenberg DM. Overcoming the nephrotoxicity of radiometal-labeled immunoconjugates: improved cancer therapy administered to a nude mouse model in relation to the internal radiation dosimetry. Cancer. 1997;80:2591–2610. doi: 10.1002/(SICI)1097-0142(19971215)80:12+<2591::AID-CNCR35>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Cao Y, Karsten U, Liebrich W, Haensch W, Springer GF, Schlag PM. Expression of Thomsen-Friedenreich-related antigens in primary and metastatic colorectal carcinomas: a reevaluation. Cancer. 1995;76:1700–1708. doi: 10.1002/1097-0142(19951115)76:10<1700::AID-CNCR2820761005>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y, Stosiek P, Springer GF, Karsten U. Thomsen-Friedenreich-related carbohydrate antigens in normal adult human tissues: a systematic and comparative study. Histochem Cell Biol. 1996;106:197–207. doi: 10.1007/BF02484401. [DOI] [PubMed] [Google Scholar]
- 6.Clarke K, Lee FT, Brechbiel MW, Smyth FE, Old LJ, Scott AM. In vivo biodistribution of a humanized anti-Lewis Y monoclonal antibody (hu3S193) in MCF-7 xenografted BALB/c nude mice. Cancer Res. 2000;60:4804–4811. [PubMed] [Google Scholar]
- 7.Fukuda M, Lauffenburger M, Sasaki H, Rogers ME, Dell A. Structures of novel sialylated O-linked oligosaccharides isolated from human erythrocyte glycophorins. J Biol Chem. 1987;262:11952–11957. [PubMed] [Google Scholar]
- 8.Goldenberg DM. Advancing role of radiolabeled antibodies in the therapy of cancer. Cancer Immunol Immunother. 2003;52:281–296. doi: 10.1007/s00262-002-0348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goletz S, Cao Y, Danielczyk A, Ravn P, Schoeber U, Karsten U. Thomsen-Friedenreich antigen: the “hidden” tumor antigen. Adv Exp Med Biol. 2003;535:147–162. doi: 10.1007/978-1-4615-0065-0_10. [DOI] [PubMed] [Google Scholar]
- 10.Graff CP, Wittrup KD. Theoretical analysis of antibody targeting of tumor spheroids: importance of dosage for penetration, and affinity for retention. Cancer Res. 2003;63:1288–1296. [PubMed] [Google Scholar]
- 11.Holliger P, Prospero T, Winter G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci USA. 1993;90:6444–6448. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudson PJ, Souriau C. Engineered antibodies. Nat Med. 2003;9:129–134. doi: 10.1038/nm0103-129. [DOI] [PubMed] [Google Scholar]
- 13.Hull SR, Carraway KL. Mechanism of expression of Thomsen-Friedenreich (T) antigen at the cell surface of a mammary adenocarcinoma. FASEB J. 1988;2:2380–2384. doi: 10.1096/fasebj.2.8.3360239. [DOI] [PubMed] [Google Scholar]
- 14.Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47:3039–3051. [PubMed] [Google Scholar]
- 15.Jain RK, Baxter LT. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res. 1988;48:7022–7032. [PubMed] [Google Scholar]
- 16.Juweid M, Neumann R, Paik C, Perez-Bacete MJ, Sato J, van Osdol W, Weinstein JN. Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res. 1992;52:5144–5153. [PubMed] [Google Scholar]
- 17.Lindmo T, Boven E, Cuttitta F, Fedorko J, Bunn PA. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods. 1984;72:77–89. doi: 10.1016/0022-1759(84)90435-6. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd KO, Burchell J, Kudryashov V, Yin BW, Taylor-Papadimitriou J. Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. Demonstration of simpler and fewer glycan chains in tumor cells. J Biol Chem. 1996;271:33325–33334. doi: 10.1074/jbc.271.52.33317. [DOI] [PubMed] [Google Scholar]
- 19.Lub-de Hooge MN, Kosterink JG, Perik PJ, Nijnuis H, Tran L, Bart J, Suurmeijer AJ, de Jong S, Jager PL, de Vries EG. Preclinical characterisation of 111In-DTPA-trastuzumab. Br J Pharmacol. 2004;143:99–106. doi: 10.1038/sj.bjp.0705915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackenzie R, To R. The role of valency in the selection of anti-carbohydrate single-chain Fvs from phage display libraries. J Immunol Methods. 1998;220:39–49. doi: 10.1016/S0022-1759(98)00143-4. [DOI] [PubMed] [Google Scholar]
- 21.Potamianos S, Varvarigou AD, Archimandritis SC. Radioimmunoscintigraphy and radioimmunotherapy in cancer: principles and application. Anticancer Res. 2000;20:925–948. [PubMed] [Google Scholar]
- 22.Ravn P, Danielczyk A, Jensen KB, Kristensen P, Christensen PA, Larsen M, Karsten U, Goletz S. Multivalent scFv display of phagemid repertoires for the selection of carbohydrate-specific antibodies and its application to the Thomsen-Friedenreich antigen. J Mol Biol. 2004;343:985–996. doi: 10.1016/j.jmb.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 23.Rogers BE, Franano FN, Duncan JR, Edwards WB, Anderson CJ, Connett JM, Welch MJ. Identification of metabolites of 111In-diethylenetriaminepentaacetic acid-monoclonal antibodies and antibody fragments in vivo. Cancer Res. 1995;55:5714s–5720s. [PubMed] [Google Scholar]
- 24.Slovin SF, Ragupathi G, Musselli C, Fernandez C, Diani M, Verbel D, Danishefsky S, Livingston P, Scher HI. Thomsen-Friedenreich (TF) antigen as a target for prostate cancer vaccine: clinical trial results with TF cluster (c)-KLH plus QS21 conjugate vaccine in patients with biochemically relapsed prostate cancer. Cancer Immunol Immunother. 2005;54:694–702. doi: 10.1007/s00262-004-0598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984;224:1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- 26.Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med. 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 27.Tahtis K, Lee FT, Smyth FE, Power BE, Renner C, Brechbiel MW, Old LJ, Hudson PJ, Scott AM. Biodistribution properties of (111)indium-labeled C-functionalized trans-cyclohexyl diethylenetriaminepentaacetic acid humanized 3S193 diabody and F(ab’)(2) constructs in a breast carcinoma xenograft model. Clin Cancer Res. 2001;7:1061–1072. [PubMed] [Google Scholar]
- 28.Todorovska A, Roovers RC, Dolezal O, Kortt AA, Hoogenboom HR, Hudson PJ. Design and application of diabodies, triabodies and tetrabodies for cancer targeting. J Immunol Methods. 2001;248:47–66. doi: 10.1016/S0022-1759(00)00342-2. [DOI] [PubMed] [Google Scholar]
- 29.Pisano A, Redmond JW, Williams KL, Gooley AA. Glycosylation sites identified by solid-phase Edman degradation: O-linked glycosylation motifs on human glycophorin A. Glycobiology. 1993;3:429–435. doi: 10.1093/glycob/3.5.429. [DOI] [PubMed] [Google Scholar]
- 30.Tsai SW, Li L, Williams LE, Anderson AL, Raubitschek AA, Shively JE. Metabolism and renal clearance of 111In-labeled DOTA-conjugated antibody fragments. Bioconjug Chem. 2001;12:264–270. doi: 10.1021/bc0000987. [DOI] [PubMed] [Google Scholar]
- 31.van Osdol W, Fujimori K, Weinstein JN. An analysis of monoclonal antibody distribution in microscopic tumor nodules: consequences of a “binding site barrier”. Cancer Res. 1991;51:4776–4784. [PubMed] [Google Scholar]
- 32.von Mehren M, Adams GP, Weiner LM. Monoclonal antibody therapy for cancer. Annu Rev Med. 2003;54:343–369. doi: 10.1146/annurev.med.54.101601.152442. [DOI] [PubMed] [Google Scholar]
- 33.Weinstein JN, van OsdolW Early intervention in cancer using monoclonal antibodies and other biological ligands: micropharmacology and the “binding site barrier”. Cancer Res. 1992;52:2747s–2751s. [PubMed] [Google Scholar]
- 34.Willuda J, Honegger A, Waibel R, Schubiger PA, Stahel R, Zangemeister-Wittke U, Pluckthun A. High thermal stability is essential for tumor targeting of antibody fragments: engineering of a humanized anti-epithelial glycoprotein-2 (epithelial cell adhesion molecule) single-chain Fv fragment. Cancer Res. 1999;59:5758–5767. [PubMed] [Google Scholar]
- 35.Willuda J, Kubetzko S, Waibel R, Schubiger PA, Zangemeister-Wittke U, Pluckthun A. Tumor targeting of mono-, di-, and tetravalent anti-p185(HER-2) miniantibodies multimerized by self-associating peptides. J Biol Chem. 2001;276:14385–14392. doi: 10.1074/jbc.M011669200. [DOI] [PubMed] [Google Scholar]
- 36.Yazaki PJ, Wu AM, Tsai SW, Williams LE, Ikler DN, Wong JY, Shively JE, Raubitschek AA. Tumor targeting of radiometal labeled anti-CEA recombinant T84.66 diabody and t84.66 minibody: comparison to radioiodinated fragments. Bioconjug Chem. 2001;12:220–228. doi: 10.1021/bc000092h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 DTPA conjugation conditions and quality analysis after conjugation. a–c Binding activity of the scFv(1aa) after incubation in three different buffers used during the DTPA conjugation at different temperatures (N = 2). d Size exclusion chromatography of the chelated versus the nonchelated scFv(1aa). e Binding activity of the chelated versus the nonchelated scFv(1aa) to asialoglycophorin
Supplementary Fig. 2 Quality analysis of 111In labeling. a Thin layer chromatography of labeled protein. Lanes: 1, free 111In; 2, 111In-labeled unbound DTPA (111In in excess); 3, 111In-labeled scFv(1aa). b Radiography of SDS-PAGE. 111In-labeled scFv(1aa) was loaded on SDS-PAGE and visualised via radiography. c Size exclusion chromatography. 111In-labeled scFv(1aa) and an 111In-labeled control mouse IgG were examined in size exclusion chromatography