Abstract
Immunization strategies using plasmid DNA can potentially improve humoral and cellular immune responses that protect against cancer and infectious diseases. The chicken anemia virus-derived Apoptin protein exhibits remarkable specificity in its ability to induce apoptosis in tumor cells, but not in normal diploid cells. Interleukin-18 (IL-18) is a Th1-type cytokine that has demonstrated potential as a biological adjuvant in murine tumor models. In this study, we analyzed the anti-tumor potential and mechanism of action of simultaneous Apoptin and IL-18 gene transfer in C57BL/6 mice bearing Lewis lung carcinoma (LLC). Here we report that the growth of established tumors in mice immunized with pAPOPTIN in conjunction with pIL-18 was significantly inhibited compared with the growth of tumors in mice immunized with the empty vector (EV) or pAPOPTIN alone. Furthermore, the immunization of mice with pAPOPTIN in conjunction with pIL-18 elicited strong natural killer activity and LLC tumor-specific cytotoxic T lymphocyte (CTL) responses in vitro. In addition, T cells from lymph nodes of mice vaccinated with pIL-18 or pAPOPTIN + pIL-18 secreted high levels of the Th1 cytokine IL-2 and IFN-γ, indicating that the regression of tumor cells is related to a Th1-type dominant immune response. These results demonstrate that vaccination with Apoptin together with IL-18 may be a novel and powerful strategy for cancer immunotherapy.
Keywords: IL-18, Apoptin, Tumor regression, Anti-tumor immunity
Introduction
Lung cancer is the most common form of malignancy and cause of cancer-related deaths worldwide. It is the second commonest cancer in the UK (excluding non-melanoma skin cancer); about 34,000 people in the UK die every year from lung cancer. Each year, an estimated 37,500 people will be diagnosed with lung cancer in the UK [1]. Lung cancer also presents serious health-care concerns in various other countries, including China. Although chemotherapy remains the standard treatment for lung cancer, and, indeed, chemotherapy significantly improves the symptoms and the quality of life of afflicted patients, only a modest increase in survival rate can be achieved [2–5].
Apoptosis is frequently impaired in many human tumors, and is also an important mechanism in chemotherapy-induced tumor cell death. Therefore, modulation of apoptosis by targeting pro-apoptotic and anti-apoptotic proteins may be a powerful and effective method for treating cancer [6]. Although the use of drugs that induce apoptosis has been introduced in cancer therapy, non-tumor cytotoxic effects still occur in normal cells because of the similarity between genes that evoke apoptosis in normal cells and tumor cells. Thus, the development of more selective treatments that stimulate apoptosis is needed to minimize side effects and maximize treatment efficacy. Apoptin, a protein derived from chicken anemia virus (CAV), selectively induces apoptosis in a wide variety of transformed cells but not in primary cells [7–9]. Apoptin’s cellular localization is crucial for its selective toxicity towards cancer cells. In primary cells, it remains in the cytoplasm, whereas in cancer cells it migrates into the nucleus and ultimately kills the cell by the activation of the mitochondrial death pathway, in a Nur77-dependent manner [10]. These findings indicate that Apoptin presents a promising candidate for anti-tumor therapy.
Recent advances in cancer therapy have focused on treatments with cytokine-mediated anti-tumor mechanisms [11]. These approaches are based on the increased understanding that the mixture of cytokines produced in the tumor microenvironment plays an important role in cancer pathogenesis. Specifically, cytokines released in response to infection, inflammation, and immunity can inhibit tumor development and progression. Interleukin-18 (IL-18) is a member of the IL-1 family of pro-inflammatory cytokines, and was originally identified for its ability to induce high levels of IFN-γ secretion from both natural killer (NK) and T cells [12]. IL-18 is produced by activated macrophages and dendritic cells, and appears to play an important role in driving Th1-dominated immune responses [13]. Systemic administration of recombinant IL-18 has been shown to induce significant anti-tumor effects [13, 14], and as such, IL-18 has been studied as a vaccine adjuvant and as an immunomodulatory molecule.
Recently, IL-18 has been demonstrated to have potential as a biological adjuvant in murine tumor models [14, 15]. However, rIL-18 administration has resulted in severe “septic shock-like” toxicity, particularly when combined with rIL-12 [16], which may ultimately prevent the widespread clinical application of this recombinant protein. To overcome these systemic toxic reactions, we examined, in a mouse tumor model, the effectiveness of therapeutic immunization with a pIL-18 plasmid alone or with co-administration of the pAPOPTIN plasmid. We report that either pIL-18 alone or in combination with pAPOPTIN not only elicits strong NK activity and tumor-specific cytotoxic T lymphocyte (CTL) responses, but also induces Th1 cytokine production in C57BL/6 mice bearing Lewis lung carcinoma (LLC). These findings suggest, for the first time to our knowledge, that treatment with Apoptin in combination with IL-18 significantly improves the development of the anti-tumor immune responses and inhibits tumor growth in LLC-bearing C57BL/6 mice.
Materials and methods
Plasmids, tumor cell lines, and animals
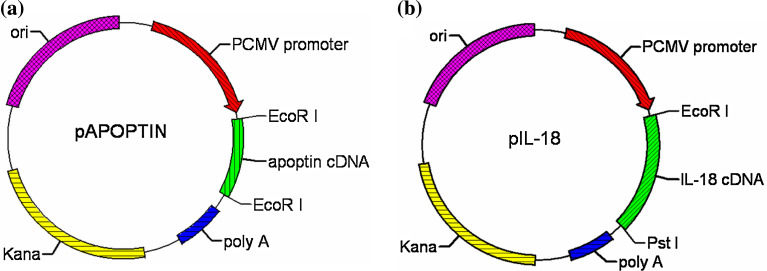
All plasmids contained a eukaryotic cistron with the human cytomegalovirus immediate–early promoter for high-level expression in a wide range of mammalian cells, and the bovine growth hormone polyadenylation signal for efficient transcription termination and polyadenylation of mRNA. The empty vector (EV) plasmid pVAX1 (Invitrogen, Carlsbad, USA), which does not encode any eukaryotic genes, was used as a negative control. Apoptin gene derived from the Cux-1 strain of CAV, as described before [17], was a kind gift from Dr. Yanbo Xu (Academy of Military Medical Sciences, Changchun, China). The pAPOPTIN plasmid (Fig. 1a) encoding and expressing the Apoptin gene has been described before [18]. The pIL-18 plasmid encodes and expresses mouse IL-18 (Fig. 1b). All plasmids for DNA immunizations were grown in Escherichia coli DH5α™ strain (Invitrogen), and large-scale preparation of the plasmid DNA was carried out by alkaline lysis using Endofree Qiagen Plasmid-Giga kits (Qiagen, Chatsworth, CA) according to the manufacturer’s instructions. DNA was then precipitated, suspended in sterile phosphate-buffered saline at a concentration of 1 mg/ml, and stored in aliquots at −20°C for subsequent use in immunization protocols.
Fig. 1.
Schematic representation of pAPOPTIN and pIL-18 recombinant plasmids
The LLC cell line, B16, a melanoma cell line derived from C57BL/6 mice, and YAC-1, an NK-sensitive lymphoma cell line of A/S(H-2a) origin, were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA) and maintained in culture in RPMI-1640 medium or Dulbecco’s modified Eagle medium (DMEM) supplemented with glucose, l-glutamine, HEPES buffer, Pen-strep, fetal bovine serum at 37°C, and 5% CO2.
Animal care was provided in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (The Ministry of Science and Technology Publication). Female C57BL/6 mice (6–8 weeks, 25 g) were purchased from Gao Xin Experimental Animal Center (Changchun, Jilin, China). The animals were provided food and water ad libitum and housed in an environment of controlled temperature and humidity with 12:12 h light:dark cycles. Animals were allowed at least 48 h to acclimate prior to any procedures.
Transfection of LLC cells with pIL-18 and pAPOPTIN
Transfections using lipofectamine (Invitrogen) were carried out as recommended by the manufacturer. Briefly, LLC cells were plated 3 × 105 per well in a 6-well plate. Twenty hours later, the cells were transfected. For each well, mixtures of recombinant pIL-18 and pAPOPTIN in 0.1 ml of DMEM without serum and 10 μl of transfection reagent in 0.1 ml of DMEM without serum were incubated at room temperature for 40 min, diluted with 0.8 ml of DMEM without serum, and added to a plate previously washed twice with 2 ml of DMEM without serum. Cells were incubated for 5 h, medium was removed, and 2 ml of supplemented DMEM was added. Transfection mixtures for the negative control contained 1 μg of empty plasmid/well.
Methylthiazoldiphenyl tetrazoliumbromide assay
Cytotoxicity was evaluated by the inhibition of cell growth or reduction of cell viability. The amount of viable cells was detected using a colorimetric methylthiazoldiphenyl tetrazoliumbromide (MTT) assay (Sigma-Aldrich BRL, St Louis, USA) as described [17]. Briefly, cells were seeded at a concentration of 1.5 × 104 cells/ml in a 96-well plate. After overnight incubation, cells were transfected with pIL-18 and pAPOPTIN for various periods of time. Cells were then incubated in a humidified atmosphere with 5% CO2 for 3 days, after which the MTT reagent (at a final concentration of 0.5 mg/ml) was added to the culture medium and incubated at 37°C for 4 h. The medium was removed and formazan was dissolved in DMSO, and the optical density was measured at 590 nm using a Bio-assay plate reader (BioRad, USA). The proportion of growth inhibition was determined using the formula: Growth inhibition = (control OD - sample OD)/control OD.
Cell viability assay
Cells were seeded on 24-well tissue culture plates. After overnight incubation, cells were transfected with pIL-18 and pAPOPTIN for various periods of time. The cultures were maintained at 37°C in a tissue culture incubator containing 5% CO2 for 3 days. The cells were then collected by trypsinization and stained with trypan blue. The dead cells and the total cells were counted. The percentage of viable cells (%) was calculated as [(total cells − dead cells)/total cells] × 100% [19].
Acridine orange/ethidium bromide staining
Eighty microliters of acridine orange/ethidium bromide (AO/EB) cocktail mixed with 1 ml of DMEM was added to the culture plates. Fields of stained cells were selected and focused using fluorescence microscopy (Olympus, Japan). Viable cells stained only with AO were bright green with intact cellular structures; early apoptotic cells stained with AO contained bright green nuclei. Late apoptotic cells stained by AO and EB appeared reddish-orange in color from the condensation of chromatin. These cells also presented with reduced cell size [20].
Tumor model development
The in vivo anti-tumor effects of the pIL-18 and pAPOPTIN plasmids were evaluated in the LLC solid tumor model. To initiate subcutaneous tumors, the right hind flank region of all C57BL/6 mice was shaved and disinfected. Approximately, 1 × 106 LLC cells suspended in 100–200 μl in sterile Hank’s balanced salt solution was injected subcutaneously into the exposed flank region of ether-anesthetized mice. Following tumor inoculation, the animals were monitored daily until subcutaneous tumors were palpable and approximately 10 × 15 mm in diameter. During the time of tumor development and subsequent administration of the control and test formulations, the health and body weight of the animals were monitored regularly. The greatest length of a tumor mass (a) and the width perpendicular to it (b) were measured at least twice per week and the tumor size was reported as a × b.
DNA immunizations
LLC-bearing C57BL/6 mice were divided into four experimental groups (n = 8 per group): (1) The control group received a 100-μg dose of the EV plasmid pVAX1 intratumorally by direct injection into the center of the tumor mass. (2) The pIL-18 group received a 100-μg dose of the pIL-18 plasmid intramuscularly. (3) The pAPOPTIN group received a 100-μg dose of the pAPOPTIN plasmid intratumorally. (4) The pIL-18 + pAPOPTIN group received a 100-μg dose of the pAPOPTIN plasmid intratumorally and a 100-μg dose of the pIL-18 plasmid intramuscularly. All immunizations were carried out using 25-gauge needles. DNA immunizations were performed at biweekly intervals, for up to three immunizations. One week after the last immunization, the animals were sacrificed by CO2 inhalation; spleens and sera were harvested for analysis.
To investigate the anti-tumor activity of combined administration of IL-18 and Apoptin for survival analyses, LLC-bearing C57BL/6 mice were immunized thrice as described above with EV, pIL-18, pAPOPTIN, or pIL-18 + pAPOPTIN. This experiment was performed twice with n = 8 mice per group.
Histopathological analysis
Mice were inoculated with tumors and administered treatment as described above. One week after the last immunization, mice were sacrificed as described above. Tumor specimens were then obtained for histopathological analysis. Tumor specimens were fixed in 10% neutral-buffered formalin overnight. Paraffin-embedded sections were prepared by routine methods and stained with hematoxylin and eosin.
T cell phenotype assay by immunofluorescence
We isolated splenocytes from four mice from each group 7 days after the last vaccination as described in the figure legends. Briefly, spleen tissue was crushed and passed through a 70-Å m nylon mesh. Cells were collected in a culture medium. Following two washes in the culture medium, cells were incubated for 10 min in RBC lysis buffer. Levels of CD3, CD4, and CD8 molecules were assessed by direct immunofluorescence assays using FITC-labeled anti-CD3, and PE-labeled anti-CD4 and anti-CD8 mAbs (all from eBioscience, San Diego, USA) and analyzed as previously described [21].
Cytotoxicity assay
Cytotoxic assays of CTL and NK cells were carried out using CytoTox 96® Non-Radioactive Cytotoxicity Assay kits (Promega, Madison, WI, USA), which rely on the detection of lactate dehydrogenase (LDH) released from dead cells to determine non-specific and tumor-specific cytotoxicity, respectively. One week after the last immunization, spleens were removed for the preparation of single-cell suspensions and spleen tissue was pressed against fine nylon mesh. Erythrocytes were depleted from the tissue with RBC lysis buffer, and macrophages were removed by the adherence of splenocytes on plastic plates for 2 h. Non-adherent lymphocytes were directly used as NK effector cells. YAC-1 cells were used as target cells. Spleen lymphocytes were co-cultured with irradiated LLC cells (6,000 rad) at a ratio of 25:1, with 5 × 106 lymphocytes and 2 × 105 irradiated LLC cells in 2 ml of DMEM, plus 10% FCS in each well of a 24-well plate. Five days later, T cells were harvested and purified from the cultures using Ficoll-Paque density gradient centrifugation. These T cells were used as CTL effector cells in an LDH-release assay against LLC and irrelevant B16 target cells. Ten thousand target cells per well were mixed with effector cells at various effector/target cell ratios in quadrisection and were incubated for 6 h. The percentage of specific lysis was calculated as: [(experimental CPM − spontaneous CPM)/(maximal CPM − spontaneous CPM)] × 100. Spontaneous CPM released in the absence of effector cells was less than 10% of specific lysis. The maximal CPM was released by adding 1% Triton X-100 to wells in the experiment.
Quantitation of cytokine secretion
One week after the last immunization, regional lymph nodes were removed for harvesting T lymphocytes. These T cells were co-cultured with irradiated LLC cells (6,000 rad) at a ratio of 2:1, with 0.5 × 106 T cells and 0.25 × 106 irradiated LLC cells per well in a 96-well plate. Co-culture was carried out in quadruplicate, and the supernatants were harvested and pooled at 24 h and at day 3 for IL-2, IFN-γ, IL-4, and IL-10 quantification, respectively. Secreted cytokines were quantified using eBioscience Mouse Th1/Th2 ELISA kits (eBioscience). The results were normalized to known standard curves.
Statistical analyses
Statistical differences between groups were evaluated by Student’s t tests. Findings were considered significant when two-tailed P < 0.05.
Results
Apoptin and IL-18 inhibited cancer cell growth in vitro
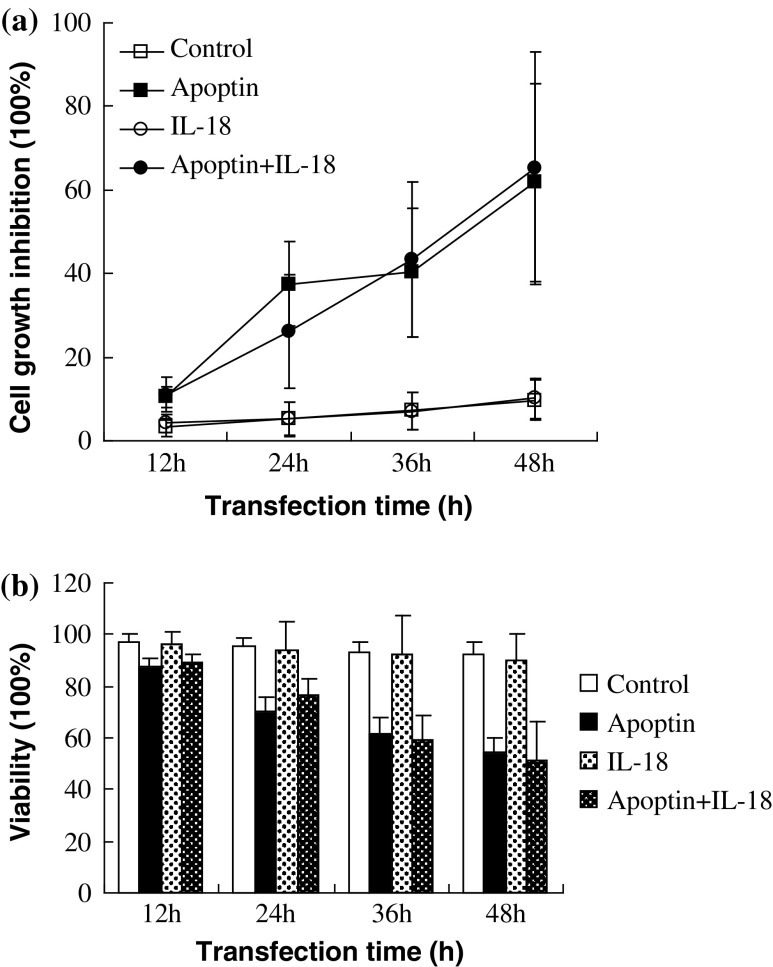
To evaluate the viability of LLC tumor cells after exposure to Apoptin and IL-18, two different methodological approaches were employed: one that reflects mitochondrial function (MTT) and one that gauges plasma membrane permeability (trypan blue exclusion). Figure 2a, b illustrates the effects of Apoptin and IL-18 on tumor cell growth in vitro using two standard cell viability measures. EV controls were included for each transfection period, and pIL-18 and pAPOPTIN treatment values were compared statistically to their corresponding time-dependent controls.
Fig. 2.
pIL-18 and pAPOPTIN extracts inhibit growth of LLC cells. a Cells were transfected with pIL-18 and pAPOPTIN for different periods of time and cell viability was determined by MTT assay. Growth inhibition was calculated as the percent inhibition relative to control. b Cells were transfected with pIL-18 and pAPOPTIN for different periods of time. The cells were harvested by trypsinization, stained with trypan blue, and the viable and dead cells were counted. Viable cells (%) = [(total cells − dead cells)/total cells] × 100%
For cultured cells, in the control group, there was a downward trend over time in trypan blue exclusion of 97, 95, and 92% at 12, 24, and 48 h, respectively. Introduction of pAPOPTIN alone and pIL-18 + pAPOPTIN further decreased trypan blue exclusion to 61, 54, and 59, 51% of the corresponding controls (P < 0.01) after 36 and 48 h of treatment, respectively. However, there was no statistical difference between the pIL-18 alone and control group (P > 0.05).
The MTT assay is commonly used as an estimate of cell viability based on the activity of mitochondrial succinate dehydrogenase. Recombinant plasmid pAPOPTIN alone and pIL-18 + pAPOPTIN inhibited the cell proliferation by 61.7 and 65.2%, respectively, after 48 h of treatment (P < 0.01 for both comparisons). As with cell permeability, there was no statistical difference between the pIL-18 alone and control group in mitochondrial function (P > 0.05). These results strongly suggest that in vitro cytotoxicity is primarily mediated by pAPOPTIN, but not by pIL-18.
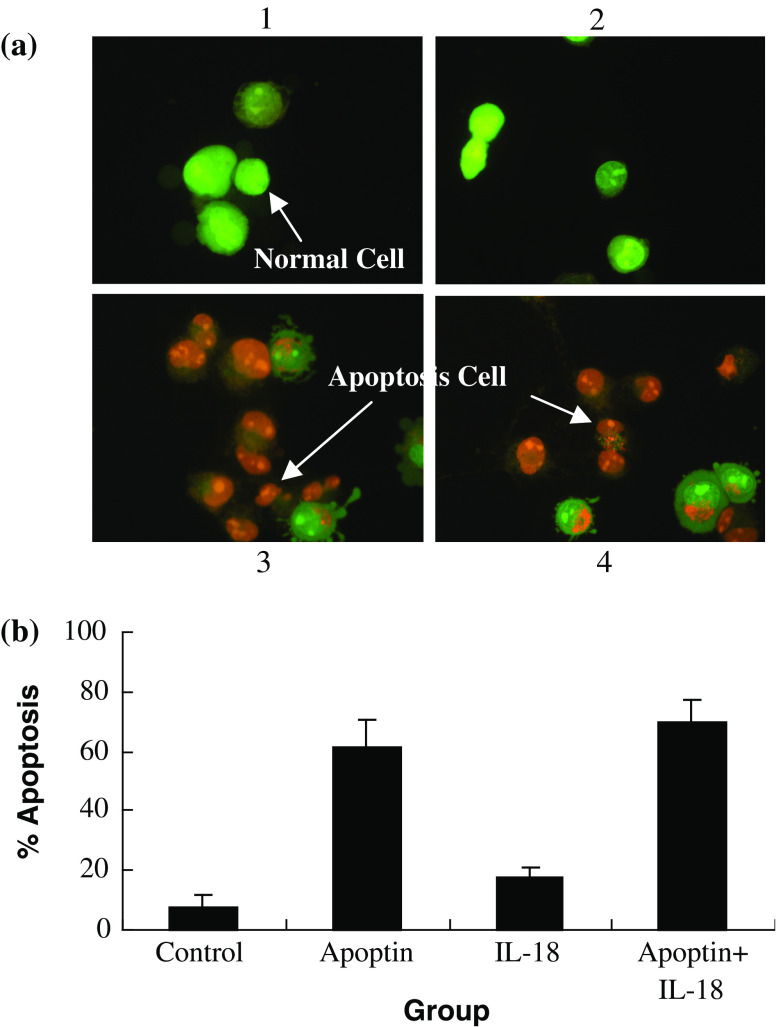
Morphological change of LLC cells
To examine whether pAPOPTIN and/or pIL-18 is capable of inducing LLC cells apoptosis in vitro, LLC cells were stained with AO/EB. Figure 3 shows photomicrographs of LLC cells 48 h after control, pIL-18, and/or pAPOPTIN treatment. The morphology of control cells consisted of green nuclei and intact cellular structure (Fig. 3a1). When treated with pIL-18 alone for 48 h, there were no observable morphological alterations in LLC cells as compared with the control cells (Fig. 3a2). However, when treated with pAPOPTIN alone or pIL-18 + APOPTIN for 48 h (Fig. 3a3, 4, respectively), cells exhibited cell shrinkage, membrane blebbing, chromatin condensation, and formation of apoptotic bodies. Although only 7% of the cells exhibited structural alterations consistent with apoptosis, in the control group, this proportion increased to 61 and 69%, respectively, after treatment with pAPOPTIN alone or pIL-18 + pAPOPTIN (P < 0.01).
Fig. 3.
LLC cell death induced by pIL-18 and pAPOPTIN. a Cells at 1 × 106 cells/well were transfected with pIL-18 and pAPOPTIN for 48 h, stained with AO/EB, and their morphology was assessed immediately using florescence microscopy (×400). (1) Control group: viable cells with green nuclei and intact structure. (2) pIL-18 group: pIL-18 treatment for 48 h. There were no visible apoptosis-associated morphological alternations in LLC cells. (3) pAPOPTIN group: pAPOPTIN treatment for 48 h. Cells in late apoptosis were present, characterized by dense orange condensation of chromatin in nuclei and reduced cell size (arrows). (4) pIL-18 + pAPOPTIN group: pIL-18 + pAPOPTIN treatment for 48 h. As above, cells in late apoptosis were present, characterized by dense orange condensation of chromatin in nuclei and reduced cell size (arrows). b This procedure was used to quantify the number of apoptotic cells after treatment with pIL-18 and pAPOPTIN
Combined administration of IL-18 and Apoptin synergistically enhances the regression of established tumors
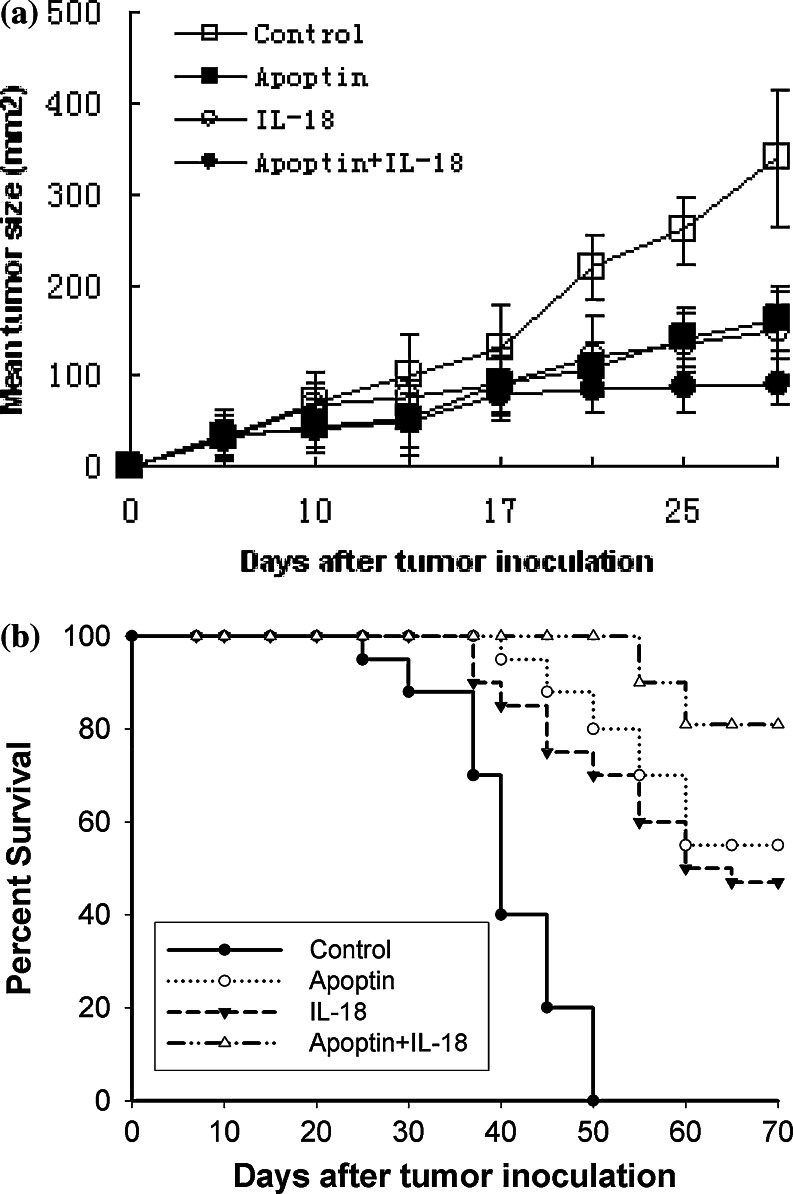
We next examined whether IL-18 gene transfer enhances the therapeutic potential of pAPOPTIN in the LLC tumor model. C57BL/6 mice were injected s.c. with 1 × 106 LLC cells. On day 7, these tumors exhibited a mean tumor area of 20–30 mm2. Tumor-bearing mice were then immunized with a 100-μg dose of the pIL-18 and/or pAPOPTIN plasmid(s). In control mice, tumors were injected with a 100-μg dose of the EV plasmid pVAX1.
As shown in Fig. 4a, the growth of LLC tumors in pAPOPTIN-alone-treated and pIL-18-alone-treated mice was significantly inhibited compared with that seen in control mice treated with the EV plasmid pVAX1 (P < 0.05 for all comparisons at 17, 21, 25, and 28 days). However, there was no statistical difference between the pIL-18-alone and pAPOPTIN-alone groups (P > 0.05 for all comparisons at 14, 17, 21, 25, and 28 days).
Fig. 4.
Therapeutic effects of vaccination with pIL-18 and pAPOPTIN. Seven days after tumor inoculation, C57BL/6 mice bearing established LLC tumors were treated with the pIL-18 and pAPOPTIN plasmid therapies. a Tumor growth was measured as the product (a × b) of the maximal diameter (a) and the perpendicular diameter (b). Bars represent the standard deviation of tumor size with five mice per group. Each data point represents the mean tumor size ± SD. This experiment was performed twice and essentially identical results were obtained. b Survival of mice in the respective treatment groups. Mice surviving at the last follow-up were tumor-free unless otherwise noted. This experiment was performed twice and essentially identical results were obtained
Although immunization with pIL-18 + pAPOPTIN did not lead to a complete regression of the established tumors, tumor growth was significantly inhibited compared with that seen in mice treated with the other protocols (P < 0.01 for all comparisons at 21, 25, and 28 days vs. control; P < 0.05 for all comparisons at 21, 25, and 28 days vs. pIL-18 alone or pAPOPTIN alone).
Of 16 mice, 13 (81%) treated with IL-18 plus Apoptin achieved curative responses compared with 9 of 16 mice (56%) treated with Apoptin alone, 7 of 16 mice (45%) treated with IL-18 alone, and 0 of 16 control mice (0%) treated with vehicle alone (Fig. 4b; P < 0.05, IL-18 plus Apoptin vs. IL-18 alone, but P > 0.05, IL-18 plus Apoptin vs. Apoptin alone). These results demonstrate that IL-18 gene transfer enhances the therapeutic potential of the pAPOPTIN plasmids in the LLC tumor model.
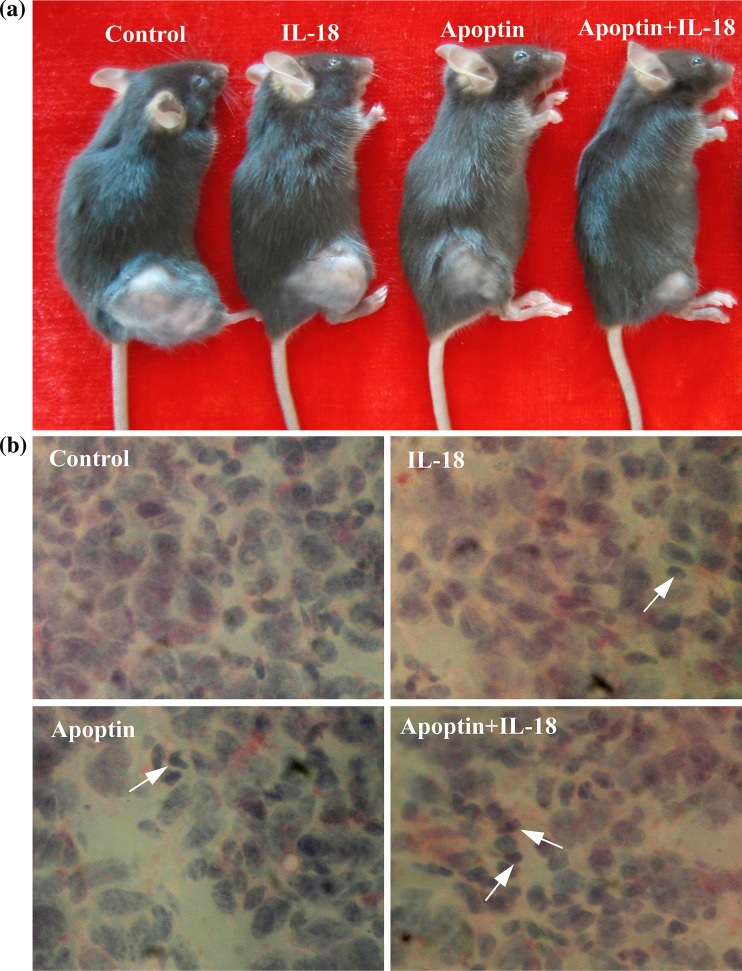
One week after the last immunization, histological examination of tumors by hematoxylin and eosin (H and E) staining showed that tumor areas after Apoptin and IL-18 treatment exhibited aberrant morphology characterized by loss of tissue integrity, increase in interstitial space, and visible remnants of disintegrated cells (Fig. 5b). Such morphological aberrations were not found in the control tumors (Fig. 5b). These data demonstrate an Apoptin-induced and IL-18 induced anti-tumor effect at a cellular level in vivo.
Fig. 5.
Macroscopic and microscopic appearances after treatment of C57BL/6 mice bearing LLC. a Macroscopic appearance in control and pIL-18 and/or pAPOPTIN-treated mice. Note the significant decrease in tumor volume associated with pIL-18 + pAPOPTIN treatment. b Hematoxylin–eosin staining of the tumor from control and pIL-18 and/or pAPOPTIN-treated mice. Note the significant aberrant morphology characterized by loss of tissue integrity, increase in interstitial space, and visible remnants of disintegrated cells in tumor areas after Apoptin and IL-18 treatment. Arrows indicate tumor cells containing condensed dark nuclei. (original magnification: ×400)
Flow cytometric analysis of lymphocytes from the spleen
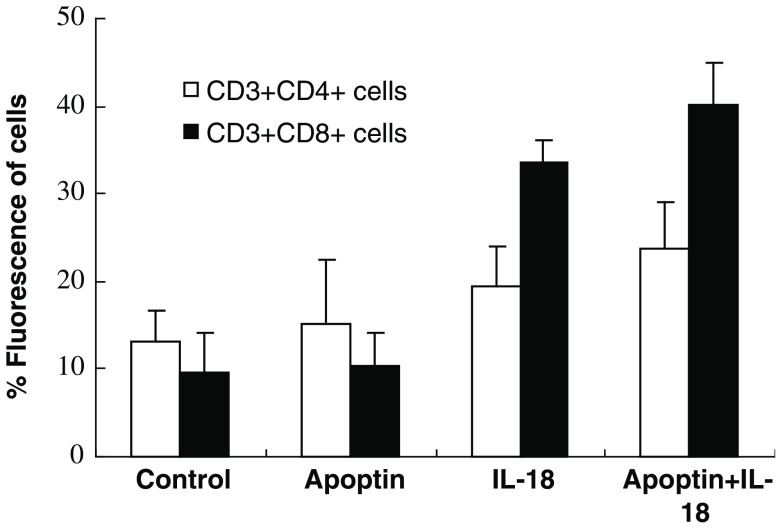
To investigate the potential mechanism of anti-tumor effect in C57BL/6 mice bearing LLC with Apoptin and IL-18, the suspended lymphocytes were harvested from the spleen and the number of CD3+, CD4+, and CD8+ cells was analyzed with FITC-labeled or PE-labeled monoclonal antibodies by FACS analysis. The percentage of CD3+CD4+ cells in the spleen of mice treated with pIL-18 alone and pIL-18 + pAPOPTIN was greater than 1.5-fold (P < 0.05, 19.4 vs. 13.1%) and 1.7-fold (P < 0.05, 23.7 vs. 13.1%) the percentage of the mice treated with the EV plasmid (Fig. 6), respectively. The percentage of CD3+CD8+ cells in the spleen of mice treated with pIL-18 alone and pIL-18+pAPOPTIN plasmid was more than 3.6-fold (P < 0.01, 33.7 vs. 9.5%) and 4.2-fold (P < 0.01, 40.2 vs. 9.5%) the percentage of the mice treated with the EV plasmid (Fig. 6), respectively. However, there was no statistical difference in the number of CD3+CD4+ or CD3+CD8+ cells between the pAPOPTIN-alone and control group (P > 0.05). Taken together, these data indicate that pIL-18 immunization resulted in in vivo clonal expansion of both CD4+ and CD8+ T cell populations, significantly enhancing the development of the immune response.
Fig. 6.
Increased number of T cells in splenocytes in mice vaccinated with pIL-18 and pAPOPTIN. Immune cells obtained from the spleens of individual mice were assayed. Each group contained four mice. Cells were stained with FITC-conjugated anti-mouse CD3 and PE-conjugated CD4 or CD8 antibodies (bicolor). Isotype-matched PE-conjugated and FITC-conjugated irrelevant antibodies were used in each case as negative controls. The percentage of CD3+CD4+ or CD3+CD8+ T cells was significantly higher in spleen of both the pIL-18 alone and the pIL-18 + pAPOPTIN groups compared with the EV control group. This experiment was repeated two more times with similar results
Enhanced NK and CTL responses induced by pIL-18 and pAPOPTIN plasmids
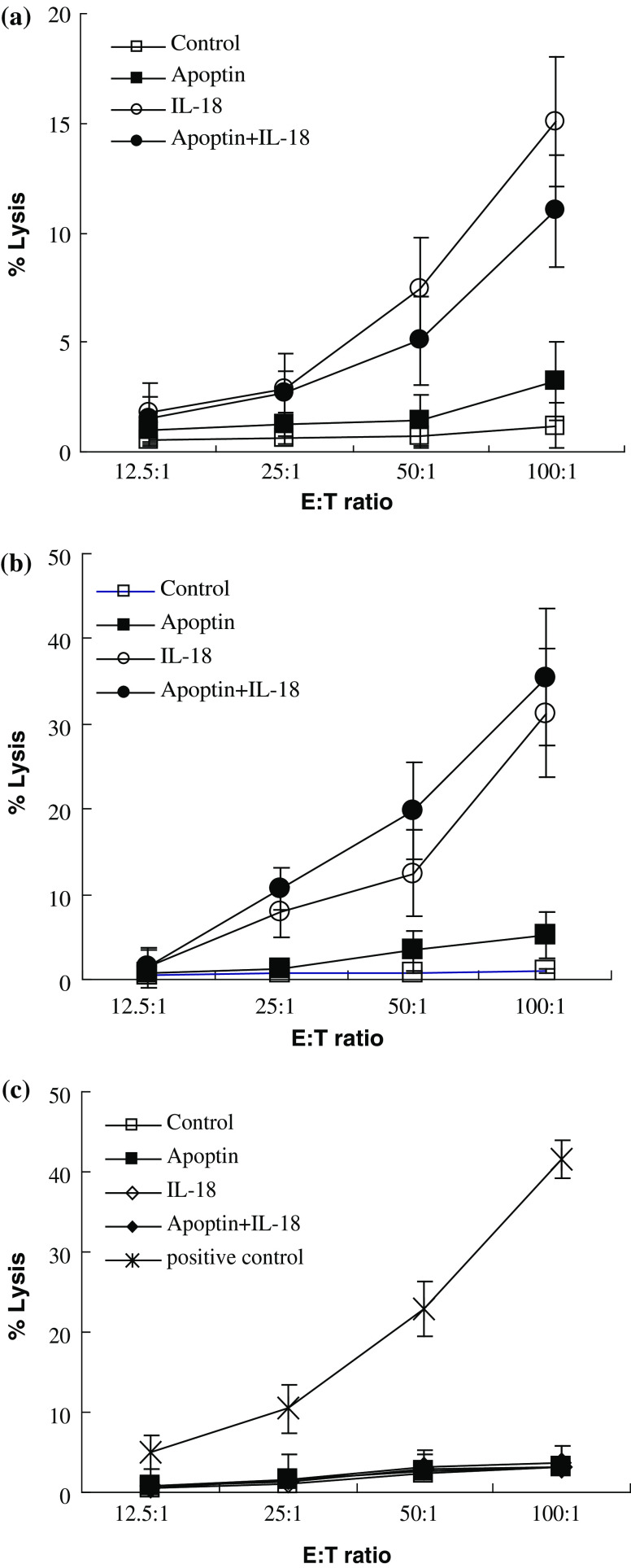
We next employed an LDH-release assay to address the non-specific and specific anti-tumor effector functions induced by vaccination with the pIL-18 and pAPOPTIN plasmids. As shown in Fig. 7a, NK activity in mice vaccinated with pIL-18 alone and pIL-18 + pAPOPTIN increased compared with NK activity in mice vaccinated with the EV plasmid. Notably, the splenic NK activity increased significantly after immunization with pIL-18 alone (P < 0.01 compared to control values). It is unclear that what leads to the phenomenon, however we do not exclude the possibility that it is associated with a poor response to Apoptin in vivo.
Fig. 7.
Cytotoxicity assays. a NK cytotoxicity assays. NK cells that were obtained from splenocytes of mice 7 days after the last immunization with pIL-18 and pAPOPTIN plasmids were used as effector cells in an LDH-release assay. In this assay, YAC-1 cells were used as target cells. b CTL cytotoxicity assays. T cells that were derived from splenocytes of mice immunized with pIL-18 and pAPOPTIN plasmids were used as effector cells in an LDH-release assay, in which LLC tumor cells were used as target cells. c To confirm that T cell cytotoxicity was LLC tumor-specific, we also included another tumor cell line of C57BL/6 origin, B16, as a target control in this experiment. B16-specific CD8+ T cells were used as positive controls. Values represent the mean of triplicate from two experiments. Standard deviation (SD) of each point is less than 5% of the mean value
To evaluate the specific killing activity of the LLC-reactive T cells, a CTL cytotoxicity assay was performed. As shown in Fig. 7b, LLC-specific CTL activity in mice vaccinated with pIL-18 alone and pIL-18 + pAPOPTIN increased significantly compared with the CTL activity in mice vaccinated with the EV plasmid. Interestingly, LLC-specific CTL activity increased significantly after immunization with the pIL-18 + pAPOPTIN plasmid (P < 0.01 compared to control values). In this experiment, no T cells except positive control exhibited cytotoxicity towards the B16 tumor cells (Fig. 7c). These results demonstrate that NK and tumor-specific CTL activity are markedly increased in mice immunized with the pIL-18 alone and pIL-18 + pAPOPTIN plasmids.
Increased Th1 cytokine secretion after immunization with pIL-18 and pAPOPTIN
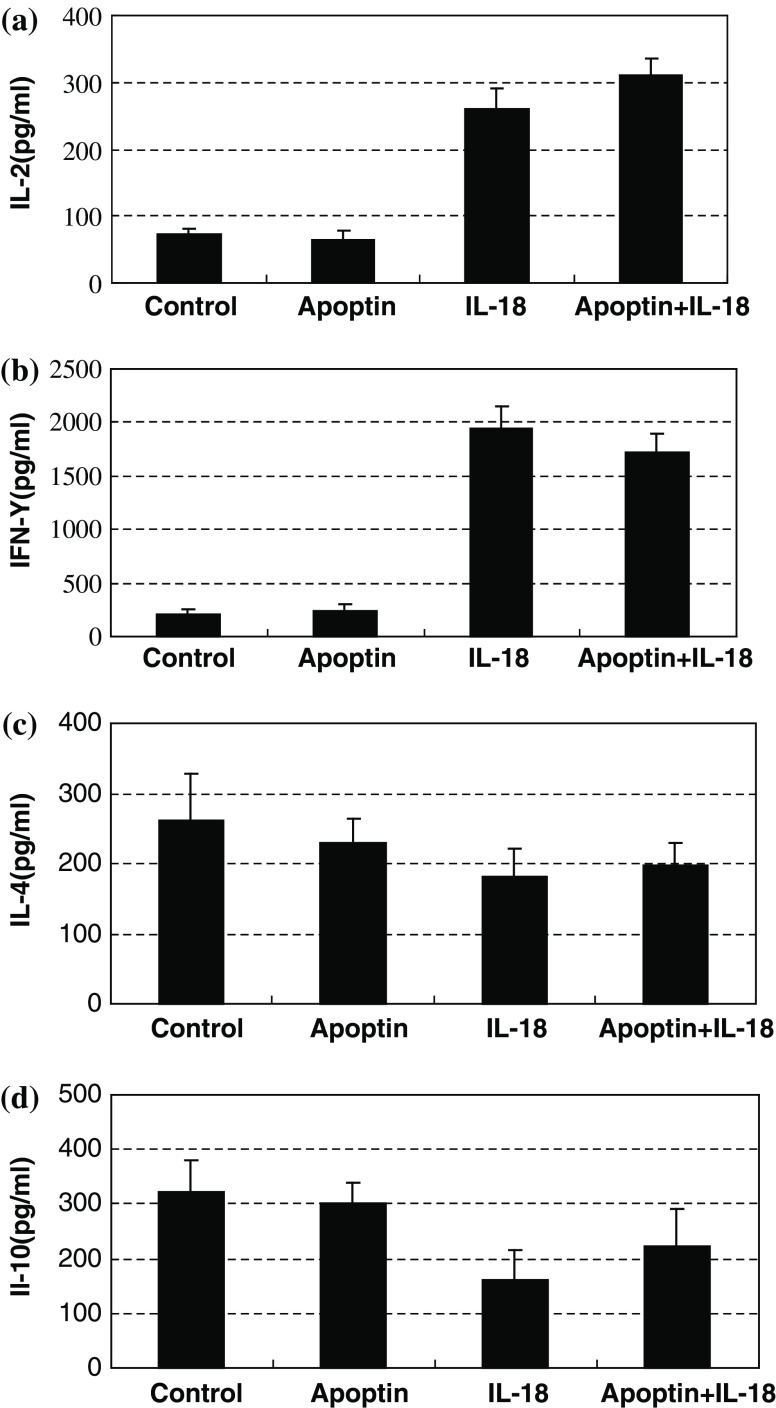
The above results prompted us to examine whether IL-18 skews immune response toward Th1. Therefore, the phenotype of LLC-reactive T cells were further evaluated by examining the secretion of the cytokines IL-2, IFN-γ, IL-4, and IL-10 via ELISA. One week following the final vaccination with the pIL-18 and/or pAPOPTIN plasmids, T cells from regional lymph nodes were harvested. Cells were co-cultured with irradiated LLC cells at a ratio of 2:1 in a total volume of 100 μl at 37 °C in 5% CO2. After 1–3 days, supernatants were harvested to analyze IL-2, IFN-γ, IL-4, and IL-10 levels. T cells from the mice immunized with pIL-18 alone or pIL-18 + pAPOPTIN secreted a significantly higher level of IL-2 (P < 0.01) and IFN-γ (P < 0.01) than did T cells from mice immunized with the EV plasmid or pAPOPTIN alone (Fig. 8). However, in contrast to the secretion of Th1 cytokines, low levels of IL-4 and IL-10 were seen from T cells from pIL-18-alone-treated or pIL-18 + pAPOPTIN-treated mice. Moreover, the IL-4 and IL-10 secretion was slightly decreased in the presence of IL-18. This profile of cytokine secretion suggests that IL-18 enhances the induction of anti-tumor immune responses by promoting a Th1-dominant response.
Fig. 8.
Effects of pIL-18 and pAPOPTIN on cytokine secretion of T cells from regional lymph nodes. Mice were immunized with pIL-18 and pAPOPTIN plasmids, and 7 days after the last immunization, T cells were harvested from regional nodes in the immunized mice and co-cultured with irradiated LLC cells. Supernatants were harvested for measurement of IL-2, IFN-γ, IL-4, and IL-10 secretion levels 1 and 3 days after co-culturing. Values represent the mean ± SD pg/ml/0.5 × 106 cells of triplicate samples
Discussion
The clinical usefulness of novel cancer therapies is determined not only by their efficiency in eliminating tumor cells, but also by their cytotoxic specificity. Tumor cell specificity is an important prerequisite for successful cancer therapy [22]. Specificity for malignant cells within a tumor allows for a greater range of treatment dose, potentially enhancing efficacy. Recently, in vitro studies with the CAV-derived protein Apoptin have demonstrated that this 121 amino acid protein has intrinsic tumor cell specificity. Specifically, Apoptin can induce apoptosis in cell lines derived from a great variety of human tumors, for example, hepatoma, lymphoma, cholangiocarcinoma, melanoma, breast and lung tumor, and colon carcinoma. In contrast, Apoptin does not induce apoptosis in normal, non-transformed cells such as fibroblasts, keratinocytes, or smooth muscle cells [8]. Apoptin has shown efficacy in treating human xenografted tumors in mice and is currently being evaluated as a gene therapy agent to selectively destroy cancer cells [23]. The molecular mechanism by which Apoptin induces apoptosis is largely unknown. Several studies have demonstrated that nuclear localization of Apoptin is required for induction of apoptosis [7, 24]. In a preliminary experiment, a single intratumoral injection of AdMLPvp3 into a xenogeneic tumor (HepG2 cells in Balb/Cnu/nu mice) resulted in a significant reduction of tumor growth [25]. These findings suggest that Apoptin may used to selectively eliminate malignant cells, provided that it can be delivered to target cells in vivo in sufficient amounts.
In this study, we have presented a novel approach for cancer gene therapy, based on the tumor-specific activity of Apoptin. We first observed that gene transfer of Apoptin into LLC tumor cells inhibited tumor growth and induced apoptosis of LLC tumor cells. To further investigate the therapeutic benefit of Apoptin for LLC tumors, we then treated well-established tumors with intratumoral or intramuscular injections over a period of 28 days. With this approach, we were able to achieve partial regression of tumors treated with the pAPOPTIN plasmid, supporting the potential use of Apoptin as an anticancer agent.
Gene therapy with Apoptin offers unique advantages over current approaches for cancer therapy. For example, resistance to cancer therapies is often caused by the inactivation of important apoptotic pathways which occurs in the majority of malignant tumors [26–28]. Gene therapy approaches based on restoring part of the apoptotic pathway, for instance, transduction of p53, p16, bax, and bcl-x, are expected to be successful in a limited subset of tumors, depending on the particular mutations in their apoptotic machinery. The fact that Apoptin does not require a functional p53 pathway, is not hindered by inhibition of apoptosis by Bcl-2 or Bcr-abl expression, and apparently acts downstream of most decision factors, suggests that it will be applicable to a wide range of tumors. These characteristics suggest that Apoptin represents a promising candidate for anti-tumor therapy.
The introduction of cytokine genes into tumor cells has become a widely used technique for gene therapy treatment of malignant diseases and, indeed, various cytokine genes introduced into mouse tumor cells have demonstrated anti-tumor potential. IL-18 is a potent IFN-γ-inducing cytokine [12, 29] with substantial preclinical anti-tumor activity [13, 14, 30, 31]. Tumor cells engineered to produce IL-18 are significantly less tumorigenic in vivo [14, 30], and systemic administration of the IL-18 protein has demonstrated therapeutic activity in several murine tumor models [13, 14, 31]. In this study, we evaluated, for the first time to our knowledge, the anti-tumor potential and mechanism of action of simultaneous Apoptin and IL-18 gene transfer in C57BL/6 mice bearing LLC. Our results demonstrate that the growth of established tumors in mice immunized with Apoptin in conjunction with the IL-18 plasmid is significantly inhibited compared with the growth of tumors in mice immunized with EV or Apoptin alone. These findings suggest that the immunization phase of the combinational therapy plays an essential role in “priming” for subsequently broadened anti-tumor immune responses.
A number of studies [21, 32–34] showed that CD4+ and CD8+ T lymphocytes are essential for the induction of an anti-tumor immune response with cytokine-based cancer vaccines. In this study, the numbers of CD4+ and CD8+ T lymphocytes were higher in the mice vaccinated with IL-18 compared with the mice vaccinated with the EV control. Importantly, the number of CD8+ T lymphocytes in the tumors was greater when Apoptin, in conjunction with IL-18, was used as the vaccine. Of particular significance is the finding that that there were greater numbers of both CD4+ and CD8+ T lymphocytes in mice treated with Apoptin in conjunction with IL-18 compared to those treated with IL-18 alone. Some previous results [35] suggest that apoptotic tumor cell death may serve as a source of Ag to be efficiently acquired by dendritic cells to promote anti-tumor immune responses.
Previous reports have demonstrated that both CTLs and NK cells play important roles in the anti-tumor responses induced by systemic administration of IL-18 in murine tumor models [13, 14]. Osaki et al. [14] reported that direct injection of an IL-18 adenovirus into tumors combined with systemic administration of IL-12 exerted anti-tumor effects mediated primarily by NK cells, and partially by both CD4+ and CD8+ lymphocytes. Our results indicate that NK and tumor-specific CTL activity are markedly increased in mice immunized with pAPOPTIN in conjunction with pIL-18 in the LLC model. This finding is consistent with the results of other immune response studies using IL-18 plasmids administered as adjuvants in DNA vaccines [2, 36–38]. Our results further report that T cells from the lymph nodes of mice vaccinated with IL-18 secreted high levels of the Th1 cytokines IL-2 and IFN-γ, indicating that the regression of tumor cells is related to a Th1-type dominant immune response.
In conclusion, here we have demonstrated a synergistic anti-tumor effect as a result of combinatorial gene therapy with Apoptin and IL-18. In combination with IL-18, the unique action of Apoptin may provide a novel, promising candidate for cancer gene therapy.
Acknowledgments
We thank Dr. Ying Li, University of Virginia, for her valuable assistance and suggestions. We also thank ELIXIGEN Co. for excellent technical assistance. This work was supported by a grant (No. G1999011902) from National Key Foundation Project, The Ministry of Science and Technology, the People’s Republic of China.
References
- 1.(2002) Chemotherapy and non-small-cell lung cancer. Drug Ther Bull 40:9–11 [DOI] [PubMed]
- 2.Bonomi P, Kim K, Fairclough D, Cella D, Kugler J, Rowinsky E, Jiroutek M, Johnson D. Comparison of survival and quality of life in advanced non-small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2000;18:623–631. doi: 10.1200/JCO.2000.18.3.623. [DOI] [PubMed] [Google Scholar]
- 3.Rana SN, Li X, Chaudry IH, Bland KI, Choudhry MA. Inhibition of IL-18 reduces myeloperoxidase activity and prevents edema in intestine following alcohol and burn injury. J Leukoc Biol. 2005;77:719–728. doi: 10.1189/jlb.0704396. [DOI] [PubMed] [Google Scholar]
- 4.Sandler AB, Nemunaitis J, Denham C, von Pawel J, Cormier Y, Gatzemeier U, Mattson K, Manegold C, Palmer MC, Gregor A, Nguyen B, Niyikiza C, Einhorn LH. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2000;18:122–130. doi: 10.1200/JCO.2000.18.1.122. [DOI] [PubMed] [Google Scholar]
- 5.ten Bokkel Huinink WW, Bergman B, Chemaissani A, Dornoff W, Drings P, Kellokumpu-Lehtinen PL, Liippo K, Mattson K, von Pawel J, Ricci S, Sederholm C, Stahel RA, Wagenius G, Walree NV, Manegold C. Single-agent gemcitabine: an active and better tolerated alternative to standard cisplatin-based chemotherapy in locally advanced or metastatic non-small cell lung cancer. Lung Cancer. 1999;26:85–94. doi: 10.1016/S0169-5002(99)00067-7. [DOI] [PubMed] [Google Scholar]
- 6.Hu W, Kavanagh JJ. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol. 2003;4:721–729. doi: 10.1016/S1470-2045(03)01277-4. [DOI] [PubMed] [Google Scholar]
- 7.Danen-Van Oorschot AA, Zhang YH, Leliveld SR, Rohn JL, Seelen MC, Bolk MW, Van Zon A, Erkeland SJ, Abrahams JP, Mumberg D, Noteborn MH. Importance of nuclear localization of apoptin for tumor-specific induction of apoptosis. J Biol Chem. 2003;278:27729–27736. doi: 10.1074/jbc.M303114200. [DOI] [PubMed] [Google Scholar]
- 8.Oro C, Jans DA. The tumour specific pro-apoptotic factor apoptin (Vp3) from chicken anaemia virus. Curr Drug Targets. 2004;5:179–190. doi: 10.2174/1389450043490631. [DOI] [PubMed] [Google Scholar]
- 9.Poon IK, Oro C, Dias MM, Zhang J, Jans DA. Apoptin nuclear accumulation is modulated by a CRM1-recognized nuclear export signal that is active in normal but not in tumor cells. Cancer Res. 2005;65:7059–7064. doi: 10.1158/0008-5472.CAN-05-1370. [DOI] [PubMed] [Google Scholar]
- 10.Maddika S, Booy EP, Johar D, Gibson SB, Ghavami S, Los M. Cancer-specific toxicity of apoptin is independent of death receptors but involves the loss of mitochondrial membrane potential and the release of mitochondrial cell-death mediators by a Nur77-dependent pathway. J Cell Sci. 2005;118:4485–4493. doi: 10.1242/jcs.02580. [DOI] [PubMed] [Google Scholar]
- 11.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 12.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 13.Micallef MJ, Yoshida K, Kawai S, Hanaya T, Kohno K, Arai S, Tanimoto T, Torigoe K, Fujii M, Ikeda M, Kurimoto M. In vivo antitumor effects of murine interferon-gamma-inducing factor/interleukin-18 in mice bearing syngeneic Meth A sarcoma malignant ascites. Cancer Immunol Immunother. 1997;43:361–367. doi: 10.1007/s002620050345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osaki T, Peron JM, Cai Q, Okamura H, Robbins PD, Kurimoto M, Lotze MT, Tahara H. IFN-gamma-inducing factor/IL-18 administration mediates IFN-gamma- and IL-12-independent antitumor effects. J Immunol. 1998;160:1742–1749. [PubMed] [Google Scholar]
- 15.Marshall DJ, Rudnick KA, McCarthy SG, Mateo LR, Harris MC, McCauley C, Snyder LA. Interleukin-18 enhances Th1 immunity and tumor protection of a DNA vaccine. Vaccine. 2006;24:244–253. doi: 10.1016/j.vaccine.2005.07.087. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura S, Otani T, Ijiri Y, Motoda R, Kurimoto M, Orita K. IFN-gamma-dependent and -independent mechanisms in adverse effects caused by concomitant administration of IL-18 and IL-12. J Immunol. 2000;164:3330–3336. doi: 10.4049/jimmunol.164.6.3330. [DOI] [PubMed] [Google Scholar]
- 17.Tan F, Zhang Y, Xu Y, Liu W, Zhang Y, Ouyang H. Amplification and cloning of genomic DNA of apoptin. Chin J Vet Sci. 1998;18:421–423. [Google Scholar]
- 18.Mi Z, Jin N, Gong W, Xue L, Lian H, Xie L, Li P. Construction and expression of the nucleic acid vaccine pVVP3 and pVHN and its effect on tumor cell. Chin J Biochem Mol Biol. 2003;19:704–708. [Google Scholar]
- 19.Wu J, Wu Y, Yang BB. Anticancer activity of Hemsleya amabilis extract. Life Sci. 2002;71:2161–2170. doi: 10.1016/S0024-3205(02)02013-1. [DOI] [PubMed] [Google Scholar]
- 20.Kiechle FL, Malinski T. Nitric oxide: biochemistry, pathophysiology, and detection. Am J Clin Pathol. 1993;100:567–575. doi: 10.1093/ajcp/100.5.567. [DOI] [PubMed] [Google Scholar]
- 21.Dasgupta S, Tripathi PK, Bhattacharya-Chatterjee M, O’Malley BW, Jr, Chatterjee SK. Recombinant vaccinia virus expressing IL-2 generates effective anti-tumor responses in an orthotopic murine model of head and neck carcinoma. Mol Ther. 2003;8:238–248. doi: 10.1016/S1525-0016(03)00148-5. [DOI] [PubMed] [Google Scholar]
- 22.Fischer U, Schulze-Osthoff K. New approaches and therapeutics targeting apoptosis in disease. Pharmacol Rev. 2005;57:187–215. doi: 10.1124/pr.57.2.6. [DOI] [PubMed] [Google Scholar]
- 23.van der Eb MM, Pietersen AM, Speetjens FM, Kuppen PJ, van de Velde CJ, Noteborn MH, Hoeben RC. Gene therapy with apoptin induces regression of xenografted human hepatomas. Cancer Gene Ther. 2002;9:53–61. doi: 10.1038/sj.cgt.7700397. [DOI] [PubMed] [Google Scholar]
- 24.Guelen L, Paterson H, Gaken J, Meyers M, Farzaneh F, Tavassoli M. TAT-apoptin is efficiently delivered and induces apoptosis in cancer cells. Oncogene. 2004;23:1153–1165. doi: 10.1038/sj.onc.1207224. [DOI] [PubMed] [Google Scholar]
- 25.Pietersen AM, van der Eb MM, Rademaker HJ, van den Wollenberg DJ, Rabelink MJ, Kuppen PJ, van Dierendonck JH, van Ormondt H, Masman D, van de Velde CJ, van der Eb AJ, Hoeben RC, Noteborn MH. Specific tumor-cell killing with adenovirus vectors containing the apoptin gene. Gene Ther. 1999;6:882–892. doi: 10.1038/sj.gt.3300876. [DOI] [PubMed] [Google Scholar]
- 26.Wallace-Brodeur RR, Lowe SW. Clinical implications of p53 mutations. Cell Mol Life Sci. 1999;55:64–75. doi: 10.1007/s000180050343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed JC. Bcl-2: prevention of apoptosis as a mechanism of drug resistance. Hematol Oncol Clin North Am. 1995;9:451–473. [PubMed] [Google Scholar]
- 28.Hickman JA, Potten CS, Merritt AJ, Fisher TC. Apoptosis and cancer chemotherapy. Philos Trans R Soc Lond B Biol Sci. 1994;345:319–325. doi: 10.1098/rstb.1994.0112. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura K, Okamura H, Wada M, Nagata K, Tamura T. Endotoxin-induced serum factor that stimulates gamma interferon production. Infect Immun. 1989;57:590–595. doi: 10.1128/iai.57.2.590-595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukumoto H, Nishio M, Nishio K, Heike Y, Arioka H, Kurokawa H, Ishida T, Fukuoka K, Nomoto T, Ohe Y, Saijo N. Interferon-gamma-inducing factor gene transfection into Lewis lung carcinoma cells reduces tumorigenicity in vivo. Jpn J Cancer Res. 1997;88:501–505. doi: 10.1111/j.1349-7006.1997.tb00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto W, Osaki T, Okamura H, Robbins PD, Kurimoto M, Nagata S, Lotze MT, Tahara H. Differential antitumor effects of administration of recombinant IL-18 or recombinant IL-12 are mediated primarily by Fas–Fas ligand- and perforin-induced tumor apoptosis, respectively. J Immunol. 1999;163:583–589. [PubMed] [Google Scholar]
- 32.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/S1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 33.Smyth MJ, Taniguchi M, Street SE. The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J Immunol. 2000;165:2665–2670. doi: 10.4049/jimmunol.165.5.2665. [DOI] [PubMed] [Google Scholar]
- 34.Dasgupta S, Bhattacharya-Chatterjee M, O’Malley BW, Jr, Chatterjee SK. Recombinant vaccinia virus expressing interleukin-2 invokes anti-tumor cellular immunity in an orthotopic murine model of head and neck squamous cell carcinoma. Mol Ther. 2006;13:183–193. doi: 10.1016/j.ymthe.2005.06.481. [DOI] [PubMed] [Google Scholar]
- 35.Russo V, Tanzarella S, Dalerba P, Rigatti D, Rovere P, Villa A, Bordignon C, Traversari C. Dendritic cells acquire the MAGE-3 human tumor antigen from apoptotic cells and induce a class I-restricted T cell response. Proc Natl Acad Sci USA. 2000;97:2185–2190. doi: 10.1073/pnas.040540197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sin JI, Kim JJ, Boyer JD, Ciccarelli RB, Higgins TJ, Weiner DB. In vivo modulation of vaccine-induced immune responses toward a Th1 phenotype increases potency and vaccine effectiveness in a herpes simplex virus type 2 mouse model. J Virol. 1999;73:501–509. doi: 10.1128/jvi.73.1.501-509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woldbaek PR, Tonnessen T, Henriksen UL, Florholmen G, Lunde PK, Lyberg T, Christensen G. Increased cardiac IL-18 mRNA, pro-IL-18 and plasma IL-18 after myocardial infarction in the mouse: a potential role in cardiac dysfunction. Cardiovasc Res. 2003;59:122–131. doi: 10.1016/S0008-6363(03)00339-0. [DOI] [PubMed] [Google Scholar]
- 38.Triccas JA, Sun L, Palendira U, Britton WJ. Comparative effects of plasmid-encoded interleukin 12 and interleukin 18 on the protective efficacy of DNA vaccination against Mycobacterium tuberculosis. Immunol Cell Biol. 2002;80:346–350. doi: 10.1046/j.1440-1711.2002.01087.x. [DOI] [PubMed] [Google Scholar]