Abstract
Experimental evidences supporting the epidermal growth factor receptor (EGFR) as an important molecule for tumor metastasis had been accumulated. Currently, anti-EGFR monoclonal antibodies (mAbs) constitute a promising approach for the treatment of patients with metastatic tumors. However, the mechanisms associated with the potent anti-metastatic effect of these mAbs have not been completely elucidated due to the lack of appropriate syngeneic preclinical models. In this paper, we have investigated the effects of 7A7, an antibody specific to murine EGFR, on the metastatic properties of D122 murine lung carcinoma. 7A7 mAb significantly impaired metastatic spread of D122 cells in C57BL/6 mice by direct anti-proliferative and pro-apoptotic effects on tumor metastasis. 7A7 mAb capacity to inhibit EGFR activation on D122 cells could contribute to its anti-metastatic effect. In addition, 7A7 mAb was able to induce in vitro antibody-dependent cell-mediated cytotoxicity on D122 cells. Interestingly, 7A7 mAb treatment increased the number of natural killer cells, T lymphocytes and dendritic cells infiltrating the metastatic sites. More strikingly, depletion of CD8+ and CD4+ T cells in vivo completely abrogated the 7A7 mAb anti-metastatic activity whereas function of natural killer cells was irrelevant. This study supports an in vivo role for T cell response in the mechanism of action of anti-EGFR mAbs, suggesting the induction of an adjuvant effect.
Keywords: EGFR, Monoclonal antibodies, Cancer immunotherapy, Metastasis, T cell response
Introduction
Although cancer represents a worldwide health care problem with approximately ten million new cases diagnosed annually, the overall mortality of patients has been not reduced significantly over recent decades. Indeed, the major cause of death from cancer, the metastatic spread of the primary tumor, usually is rather resistant to conventional chemotherapy and other treatments. Among a variety of agents able to stimulate the metastatic process, epidermal growth factor (EGF) has been included. In addition to its classical effect on cell differentiation and proliferation, EGF promotes motility and in vitro invasion in different tumor cells [1–5]. Moreover, increased expression of EGFR has been associated with a growing number of epithelial malignancies [6] and an enhancement of their metastatic potential [7, 8].
Several strategies have focused on the treatment of patients with metastatic cancer, for whom anti-EGFR mAbs currently represent a promising approach [9]. However, the precise mechanisms involved in the anti-metastatic capacity of anti-EGFR mAbs have not been sufficiently explored. In vivo experiments [10–12] studying the anti-metastatic effect of anti-EGFR mAbs are quite limited because they have been conducted with xenografts in nude mice. The nude mice model is rather inconvenient considering that in these animals conventional T cell repertories are absent. In this regard, a recent report demonstrated that passive immunotherapy with Rituximab (chimeric CD20 mAb) can further promote such a kind of “secondary” active specific anti-tumor immunity [13]. In fact, some studies have indicated that maximal clinical and molecular responses to anti-EGFR mAb therapy may take several months [14, 15], suggesting that an adaptative immune response could be involved. Thus, the use of immunologically normal individuals in a complete autologous scenario would be a more useful preclinical strategy for the in vivo evaluation of the anti-metastatic capacity of anti-EGFR mAbs. In order to address this idea, we have generated the 7A7, the only anti-extracellular domain of murine EGFR mAb reported. This mAb was able to recognize the murine EGFR present in normal and tumor cells by different techniques, such as Western blot, Flow cytometry and Immunohistochemistry [16].
In this paper, we provide the first report of anti-metastatic activity for an anti-EGFR mAb in immunocompetent mice. 7A7 mAb showed an impressive anti-metastatic effect on D122 tumor. Interestingly, in addition to the conventional mechanisms associated with anti-EGFR mAb action, we found an indispensable contribution of T cells to the anti-metastatic properties of 7A7 mAb.
Materials and methods
Antibodies and reagents
7A7 mAb [16] and isotype control 3B11 [17] were obtained at the Center of Molecular Immunology (Havana, Cuba). Antibodies to poly (ADP-ribose) polymerase (PARP), P-EGFR (Tyr992), PP-ERK1/2 (Thr202/Tyr204), P-STAT 3 (signal transducer and activator of transcription 3) (Tyr705), P-Akt (Ser473) and antibodies to total ERK1/2, STAT 3 and Akt were used for Western blot experiments and obtained from Cell Signaling Technology, Inc. (Beverly, MA, USA). Antibodies specific to molecules CD4, CD8, CD11c conjugated to FITC (fluorescein iso-thiocyanate) and NK 1.1 conjugated to PE (phycoerytrin) were used for Flow cytometry analysis and purchased from BD Biosciences (Palo Alto, CA, USA). The synthetic retinoid fenretinide, reported as an inductor of apoptosis in cancer cells [18], was generous gifts from Dr. Marco Corazzari (Lab Cell Biology, National Institute for Infectious Diseases “L. Spallanzani”, Rome). The murine EGF and the isoflavone genistein were purchased from Upstate Biotechnology, Inc. (Lake Placid, NY, USA) and Sigma (St. Louis, MO, USA), respectively.
Tumor cell line
The C57BL/6-derived D122 metastatic clone of the Lewis lung carcinoma [19] was cultured in RPMI medium (Life Technologies, Inc., Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin (Life Technologies).
Cell proliferation assay
D122 cell proliferation was measured by MTT assay [20]. D122 cells (1 × 105) were seeded in RPMI 10% FBS in flat-bottomed 96-well plates (Costar, Cambridge, MA, USA). After 12 h, the cells were switched to 1% FBS, and the mAbs (0.5–10 μg/ml) were added; the cells were incubated for additional 12, 24 or 48 h. Subsequently, MTT (1 mg/ml; Sigma) was added and incubated for 4 h at 37°C. Next, the formed formazan crystals were dissolved with DMSO. The absorbance difference 540–620 nm was determined using a Microwell System reader (Organon Teknika Inc., Salzburg, Austria).
Cell migration assay
Experiments assessing D122 cell motility were performed using 24-well chemotaxis chambers (Nunc, Naperville, IL, USA). D122 cells were cultured for 24 h in RPMI 1% FBS; the cell suspensions were pretreated 30 min with mAbs (0.5–10 μg/ml) and seeded (5 × 104/100 μl) on the upper compartment of the chamber. The murine EGF (50 ng/ml) was added on the lower chamber as chemoattractant. After an incubation period of 12 h, the filters were fixed, and cells that had migrated to the lower surface were manually counted under a microscope (×200). Measurement of D122 cells migration in absence of EGF was used as negative control.
Western blot analysis
D122 cells were grown to 70% of confluence in 6-well plates (Costar) and incubated with serum-free medium for 12 h. The medium was changed to fresh serum-free medium with 7A7 mAb (0.5–10 μg/ml) or genistein (50 μM) for 30 min. Next, the cells were incubated with murine EGF (100 ng/ml) for 5 min. The cell lysates were prepared in RIPA buffer (PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) with 50 mM NaF, 1 mM Na3VO4, 5 mM EDTA and 1 mM phenylmethylsulphonylfluoride that were freshly added to the lysis solution before each experiment. Protein concentrations were determined according to bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA). Cell extracts were applied to 7.5% SDS-PAGE gels and transferred to polyvinylidine difluoride membranes (Gelman, Ann Arbor, MI, USA). Polyvinylidine difluoride membranes were blocked with NEGT buffer (0.15 M NaCl, 5 mM EDTA, 500 mM Tris-HCl (pH 7.5), 0.02% Tween 20, 0.04% Gelatin) and incubated with the primary antibodies described above. The protein content was visualized using horseradish peroxidase-conjugated secondary antibodies (BD Biosciences) followed by Chemiluminescent Substrate (Pierce).
Cell cycle analysis and apoptosis measurement
D122 cells were plated (5 × 105) in RPMI 10% FBS in 6-well plates. Twenty-four hours later, the mAbs (1 μg/ml) or fenretinide (15 μM) were added in RPMI 1% FBS and the cells were incubated for additional 12, 24 and 48 h. To analyze cell cycle and DNA fragmentation, the cells were fixed with ice-cold methanol/acetone (4:1) and stained by incubation with a solution containing 100 μg/ml propidium iodide (PI; Sigma) and 50 μg/ml RNase (Sigma). To measure phosphatidil serine exposition an Annexin V-PI double staining was carried out according to the manufacturer’s protocol (Bender MedSystems, Vienna, Austria). All analyses were performed on a FACScan flow cytometer (BD Biosciences) by collecting a minimum of 20,000 events and analyzed using the WinMDI 2.8 and ModFit 3.0 software packages. The PARP fragmentation in D122 cells was determined by Western blot analysis with anti-PARP antibody as described above.
ADCC assay
To measure antibody-dependent cell-mediated cytotoxicity (ADCC) a fluorometric assay was used, similar to the one described for the determination of lymphocyte antigen-specific lysis [21]. D122 cells (6 × 104) were stained with CFSE (Molecular Probes, Eugene, OR, USA) and incubated with mAbs (1 μg/ml) and splenocytes mice for 4 h at 37°C. CFSE-stained D122 cells without treatment were used as control. Flow cytometry analysis of the D122 cell population was performed in 60 s acquisition setting. The percentage of dead cells by ADCC was determined using the following formula: [(number of control CFSE-stained D122 cells − number of treated CFSE-stained D122 cells)/number of control CFSE-stained D122 cells] × 100.
Experimental metastasis experiments
All animal studies described in this paper were done according to protocols approved by Institutional Animal Care and Use Committee of the Center of Molecular Immunology. D122 cells were grown to 70–85% confluence before being harvested for cell counting. Cells (2.5 × 105) were injected into lateral tail veins of 6- to 8-week-old female C57BL/6 mice (Center for Laboratory Animal Production, Havana, Cuba). To measure the effect of mAb treatment on D122 metastasis, mAbs (56 μg/inoculation) were administered the day before tumor challenge and three doses the following weeks (prophylactic schedule) or the mAbs administration began at day 6 after tumor challenge and continued three doses per week (therapeutic schedule). Three weeks after tumor injection, the mice were euthanized, and the lungs were removed. The number of D122 lung metastasis was counted or the lungs were subjected to histologic examination as described below.
In vivo depletion experiments
Depletion of natural killer (NK) cells, CD8+ or CD4+ cells in D122 tumor-bearing mice was achieved by ip injections of 0.1 mg of anti-NK 1.1, anti-CD8+ or anti-CD4+ mAbs (obtained from rat anti-mouse hybridomas PK136, YTS169 or YTS191 (American Type Culture Collection, Manassas, VA, USA) and purified as previously described [22]) on days 5, 9, 13 and 17 after tumor challenge. The efficiency of depletions was assessed by Flow cytometry using naive mice. In all cases, the levels of depletions were higher than 95%.
Histological analysis
Formalin-fixed lungs were processed using the paraffin technique, stained with hematoxylin and eosin, and metastatic tumor foci were analyzed based on morphological criteria (×40). Apoptotic tumor cells were characterized by cell shrinkage, small dense nuclei and nuclear debris, and eosinophilic cytoplasm [23]. The number of apoptotic cells and the total cell number per high-power microscopic field (×400) in tumor foci were counted, and a minimum of 500 cells and 5 high-powered fields were analyzed. The apoptotic index was expressed as the percentage of apoptotic cells of the total tumor cells counted. Complementary, the mitotic index was also calculated.
Analysis of lung mononuclear cells
To evaluate the lung-infiltrating immune cells, D122 metastasis-bearing mice treated with mAbs in therapeutic setting were euthanized the days 8, 12 and 17 after tumor injection. Lung mononuclear cells were isolated as described previously [24]. Briefly, the lungs were cut into small pieces followed by incubation for 30 min at 37°C in RPMI containing collagenase (1 mg/ml; Worthington Biochemical Corp., Lakewood, NJ, USA) and DNAse (0.5 mg/ml; Sigma). The resultant single suspensions were passed through a PreSeparation Filter (Miltenyi Biotec, Bergich Gladbach, Germany), and subsequently centrifuged over Ficoll-Paque Plus (1.077 g/ml; StemCell Technologies, Vancouver, British Columbia, Canada). The mononuclear cells were collected, and the expression of CD11c, CD4, CD8 and NK 1.1 molecules on these cells was determined by Flow cytometry using specific antibodies described above.
Statistical analyses
The variables of two groups were compared using the independent samples t test. For comparisons between three or more groups, an analysis of variance (ANOVA) was performed, followed by multiple comparisons of means by Tukey post-test. When the variables studied were not normally distributed, nonparametric statistical methods were applied. The Mann–Whitney U test was used to compare variables between two groups. When three or more groups were compared, the Kruskall–Wallis test with Dunn post-test was used. Data were considered significant when P < 0.05.
Results
7A7 mAb treatment induces an effective elimination of D122 lung metastasis
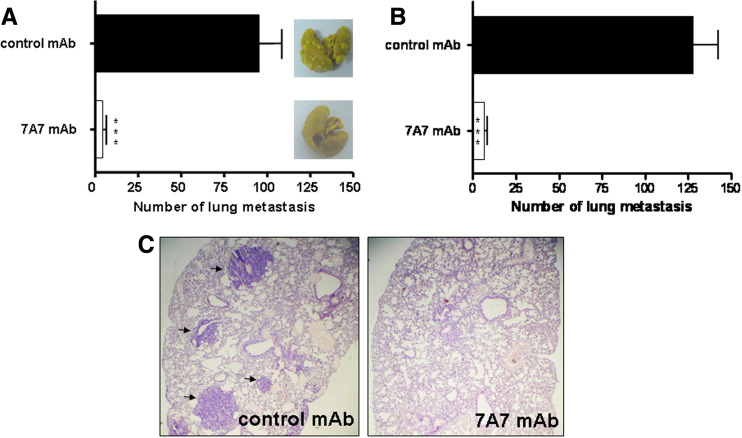
The anti-metastatic effect of 7A7 mAb on D122 murine lung carcinoma was evaluated using an experimental metastasis assay. Administration of 7A7 mAb previous to tumor challenge significantly reduced the number of D122 lung metastasis compared with a control mAb (Fig. 1a). To determine whether 7A7 mAb anti-metastatic effect observed in the prophylactic setting could be extended to the therapeutic setting, we carried out a similar experiment in which mAb was administered 6 days after tumor cell inoculation. At that moment, a substantial number of D122 lung micrometastasis could already been detected in challenged mice (data not shown). The delay in 7A7 mAb treatment did not affect its anti-metastatic effect against D122 cells, obtaining also a significant decrease in the metastasis number (Fig. 1b). Histopathological studies performed on tissue slides from lung lobes revealed multiple, large metastatic nodules of a poorly differentiated carcinoma in control mice. In contrast, 7A7 mAb-treated animals showed a normal lung tissue with only occasional micrometastatic foci (Fig. 1c).
Fig. 1.
7A7 mAb anti-metastatic effect on D122 tumor. a Prophylactic administration of 7A7 mAb effectively reduces D122 lung metastasis. D122 cells were injected into lateral tail veins of mice (10 per group) (day 0). mAbs (56 μg/inoculation) were administered the day before tumor challenge and three doses the following weeks. Mice were killed at day 21, and the number of D122 lung metastasis was counted. A representative lung from each group is shown. b Therapeutic administration of 7A7 mAb is as efficient to eliminate D122 metastasis as the prophylactic treatment. Treatment began at day 6 and continued as described in a. One representative experiment of three is shown in each case and analyzed according to Mann–Whitney U test. 7A7 mAb versus control mAb: ***P < 0.001. Error bars indicate the SD. c Lung sections of mice treated as in b were stained with hematoxylin and eosin on day 21. Black arrows show metastatic foci
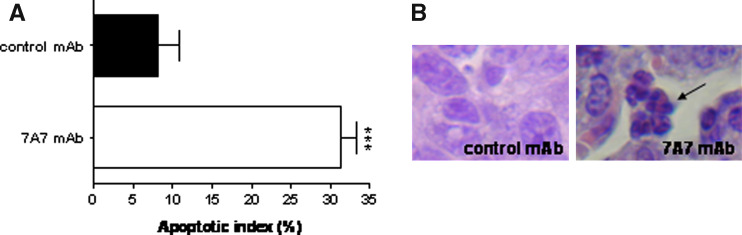
Moreover, this histological analysis of lung samples revealed that 7A7 mAb treatment induced anti-proliferative and pro-apoptotic effects in the metastatic sites. Lung tumor foci showed a normal mitotic index (1.93 ± 0.7%) in control mice, while no mitotic activity was detected in 7A7 mAb-treated mice. Also, it was obtained a significant increase in apoptotic index in the micrometastasis remaining after treatment with 7A7 mAb (31.24 ± 4.7%), when compared with the value observed in control mAb (8.1 ± 6.61%) (Fig. 2a). These results demonstrate that repeated 7A7 mAb treatment induces mitotic arrest, followed by condensation of the nuclear material and appearance of multiple apoptotic bodies (Fig. 2b).
Fig. 2.
7A7 mAb induces pro-apoptotic effects on D122 lung metastasis. Lung sections of mice treated as in Fig. 1b stained with hematoxylin and eosin on day 21. a Apoptotic cells were counted. The data were analyzed according to t test. 7A7 mAb versus control mAb: ***P < 0.001. The bars represent the mean of apoptotic index for five to six fields counted. The error bars indicate the SD. b Black arrow shows apoptotic bodies
7A7 mAb inhibits cellular functions associated with EGFR signaling
The anti-EGFR mAbs are able to inhibit the receptor activation, blocking a number of key cellular functions regulated by the EGFR that could explain their anti-metastatic effects. To examine 7A7 mAb effect on EGFR signaling, we determined whether this mAb inhibits EGFR phosphorylation. In D122 cells, EGFR tyrosine phosphorylation was induced by EGF treatment and completely inhibited by 7A7 mAb (Fig. 3a). Moreover, this mAb was able to reduce signal transduction pathways induced by EGFR activation related to cell division and survival, such as mitogen-activated protein kinase (MAPK), phosphotidylinositol-3 kinase (PI3K) and STAT 3 pathways (Fig. 3b). Cell incubation with the isoflavone genistein was used as positive control for inhibition of EGFR phosphorylation (Fig. 3c).
Fig. 3.
7A7 mAb inhibits EGF-induced EGFR signaling in D122 cells. a Phosphorylation of EGFR is abolished in 7A7 mAb-treated D122 cells. Immunoblot for phosphorylated and total EGFR were performed for serum-starved D122 cells after a 30 min treatment with increased concentrations of 7A7 mAb. b 7A7 mAb induces a reduction in the activation of downstream EGFR signaling transduction pathways. Immunoblots for phosphorylated and total ERK1/2, STAT 3, Akt were performed as in a. c Serum-starved D122 cells treated with genistein were used as positive control. All experiments were performed at least three times with similar results. Representative data are shown
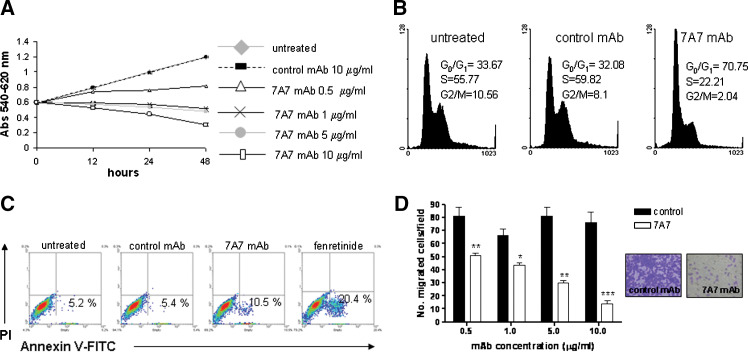
Consequently, we explored whether the processes associated with EGFR activation were inhibited by 7A7 mAb in D122 cells. We first determined 7A7 mAb effect on D122 cell proliferation, cell growth kinetics were examined in response to varying doses of 7A7 mAb using MTT assay. With 0.5 μg/ml of this mAb, inhibition of growth was achieved, in contrast with untreated or control mAb-treated cells (Fig. 4a). Moreover, an increase in the proportion of cells in G0-G1 phase with the corresponding decrease of cells in S and G2-M phases was observed after treatment with 7A7 mAb (Fig. 4b). When 7A7 mAb concentrations were increased in the proliferation experiments, cell numbers were reduced to levels lower than initial cell number before treatment (Fig. 4a), indicating that D122 cells were killed in response to 7A7 mAb treatment. To examine the contribution of apoptosis to 7A7 mAb-induced cytotoxicity in D122 cells, several known markers of this cell death process were evaluated. We analyzed the exposure of phosphatidyl serine, a phospholipid that is normally confined to the inner leaflet of the plasma membrane and is externalized upon apoptosis induction, by staining with Annexin V-FITC. After treatment of D122 cells with 7A7 mAb, an increase of cells stained with Annexin V-FITC was detected (Fig. 4c). In untreated and control mAb-treated cells, an increase of phosphatidyl serine externalization was not observed, corresponding to the lack of a cytotoxic effect in these cells. Also, we found that 7A7 mAb induced a significant DNA fragmentation and PARP cleavage, a nuclear polymerase target of caspase-3, in D122 cells compared with control mAb (data not shown). The fenretinide was used as positive control for these experiments. Moreover, the effect of 7A7 mAb on the chemotaxis of D122 cells was assessed using a transwell cell culture chamber. The motility of these cells was significantly decreased after 7A7 mAb treatment (Fig. 4d). Taken together, these results indicate that the blocking of EGFR activation by an anti-EGFR mAb can contribute to its anti-metastatic effect through several ways.
Fig. 4.
7A7 mAb induces anti-proliferative, pro-apoptotic and anti-migratory effects on D122 cells. a Dose-dependent cell growth inhibition by 7A7 mAb. D122 cells were treated with mAbs for 12, 24 or 48 h. Cell proliferation, expressed as Abs 540–620 nm, was measured by MTT assay. Each point represents the mean of triplicate wells. b Arrest in G0-G1 phase of the cell cycle by 7A7 mAb. D122 cells stained with PI (100 μg/ml) after 48 h exposure to mAbs (0.5 μg/ml) were analyzed by Flow cytometry. Percentages of cell populations in the different phases of the cell cycle are included. c 7A7 mAb treatment induces apoptosis. D122 cells treated with mAbs (1 μg/ml) or fenretinide (15 μM) for 12 h were double-tained with Annexin V-FITC and PI and analyzed by Flow cytometry. d Cell migration was significantly inhibited by 7A7 mAb treatment. D122 cell migration in response to EGF (50 ng/ml) was determined using a chemotaxis chamber. The bars represent the mean of five fields counted. One representative experiment out of three is shown and analyzed using ANOVA and Tukey post-test. 7A7 mAb versus control mAb: ***P < 0.001, **P < 0.01, *P < 0.05. Representative microscope images of the filters lower surface of Mabs-treated cells (10 μg/ml) are shown. The error bars in a and d indicate the SD
7A7 mAb anti-metastatic effect on D122 tumor is abrogated by CD8+ and CD4+ T cells depletion
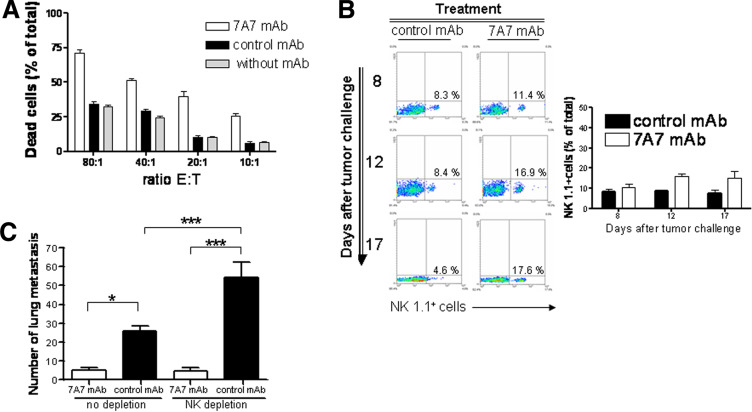
Considering the effectiveness of anti-EGFR mAbs in the clinic, immunological mechanisms are likely to be involved in their effects. We measured 7A7 mAb capacity to induce ADCC activity on CFSE-stained D122 cells by Flow cytometry. 7A7 mAb mediated a potent D122 cell lysis for all ratios of mouse splenocytes:target cells assayed (Fig. 5a). Next, we verified the relevance of this mechanism to 7A7 mAb anti-metastatic effect on D122 tumor. First, we evaluated the infiltration of NK cells, a cell population that could mediate ADCC response, in the lungs of D122 metastasis-bearing mice treated with 7A7 mAb. 7A7 mAb treatment provoked an increase of NK cell numbers in the metastatic site compared with control for all days measured (Fig. 5b). However, depletion experiments revealed that NK cells were not required to 7A7 mAb anti-metastatic effect against D122 tumor. Moreover, 7A7 mAb effects overcame the pro-metastatic effect of the NK depletion (Fig. 5c). Interestingly, the 7A7 mAb treatment also produced a considerable increase in the percentage of DCs (CD11c+), CD4+ and CD8+ cells in the lungs of D122 metastasis-bearing mice (Fig. 6a). To determine whether CD8+ and CD4+ cells were involved in 7A7 mAb effect on D122 metastasis, each cell population was individually depleted. Both depletions caused a total abrogation of 7A7 mAb anti-metastatic effect (Fig. 6b). These data indicate that the T cell response plays a crucial role in the anti-metastatic effect of 7A7 mAb.
Fig. 5.
7A7 mAb anti-metastatic effect on D122 cells is independent of NK cell activity. a 7A7 mAb induces specific ADCC. CFSE-stained D122 cells were incubated for 4 h at 37°C with mAbs (1 μg/ml) and mice splenocytes. The lysis of target cells was determined by Flow cytometry. The bars represent the mean of triplicate wells. Results shown are representative of two experiments. b Increase of lung-infiltrating NK cells by 7A7 mAb treatment. An experiment as described in Fig. 1b (10 per group) was carried out. At the indicated days, one representative mouse in each group was killed, and lung mononuclear cells were isolated. The percentage of NK 1.1+ cells was analyzed by Flow cytometry using a specific antibody. The graph represents the results of three representative mice. c NK cell depletion does not affect 7A7 mAb anti-metastatic effect. An experiment as described in Fig. 1b (10 per group) was carried out; depletion of NK cells by a specific antibody (0.1 mg/inoculation) began before 7A7 mAb treatment (day 5) and continued until the end of the assay. This experiment was repeated twice, with similar results. Statistical analyses were performed using Kruskal–Wallis test and Dunn post-test. *P < 0.05, ***P < 0.001. The error bars in a and c indicate the SD
Fig. 6.
T cell response is required for 7A7 mAb anti-metastatic effect on D122 tumor. a Increase of lung-infiltrating immune cells by 7A7 mAb treatment. An experiment as described in Fig. 1b (10 per group) was carried out. At the indicated days, one representative mouse in each group was killed, and lung mononuclear cells were isolated. The percentages of CD11c+, CD4+ and CD8+ cells were analyzed by Flow cytometry. For each cell population, the graphs represent the results of three representative mice. b 7A7 mAb anti-metastatic effect on D122 cells is abrogated by depletion of CD8+ and CD4+ cells. An experiment as described in Fig. 1b (10 per group) was carried out; depletion of CD4+ or CD8+ cells by a specific antibody (0.1 mg/inoculation) began before 7A7 mAb treatment (day 5) and continued until the end of assay. One representative experiment out of two is shown and analyzed according to Kruskal–Wallis test and Dunn post-test. **P < 0.01; ***P < 0.001. The error bars indicate the SD
Discussion
Agents targeting the EGFR pathway hold particular promise for the treatment of patients with advanced disease, for whom standard chemotherapy is generally palliative. Expression of EGFR on numerous types of solid tumors [6] and the association of EGFR activation with tumorigenic process, including proliferation, anti-apoptosis and metastatic spread [25], make this pathway a particularly compelling target for rational drug design. In fact, mAbs directed at the ligand-biding extracellular domain of EGFR are currently in late-stage clinical testing of metastatic patients with encouraging results [9]. However, important issues remain to be addressed. These include precise mechanisms involved in the anti-metastatic effect of these mAbs.
Numerous studies conducted in cell culture and in mouse xenograft models of human cancer have demonstrated that anti-EGFR mAbs block EGFR activation, inhibiting many mechanisms that could contribute to tumor metastasis. The blockade of EGFR activation by anti-EGFR antibodies leads to cell cycle arrest and apoptosis of different human tumor cell lines [26, 27]. Several in vitro studies have demonstrated down-regulation of the pro-angiogenic factors following treatment of EGFR expressing cells with anti-EGFR mAbs [10, 28]. Similar observations have been made in xenograft tumor models, where down-regulation of angiogenesis factors by these mAbs was accompanied by a reduction in the number of new blood vessels [10, 11]. Moreover, Huang et al. [29] showed that cetuximab, an anti-human EGFR mAb, can suppress tumor cell migration, matrix metalloproteinase-9 expression and local invasion in a head and neck squamous cell carcinoma, and similar results have been obtained in other tumor cell lines [2]. Also, the involvement of ADCC in the overall target cell destruction after anti-EGFR antibodies application had been studied. Previous reports have shown that anti-EGFR mAbs potentially support this immune system effector function [30, 31], but its contribution to the in vivo anti-tumor effect of these antibodies has not been elucidated.
In the studies describe in this paper, we used the 7A7 mAb, specific to murine EGFR, to study the anti-metastatic effect of anti-EGFR mAbs in immunocompetent mice. The tumor model selected was D122 metastatic clone of the Lewis lung carcinoma; previous studies have shown that this tumor model is EGFR-positive and sensitive to anti-EGFR-based immunotherapy [16, 32]. Our experiments demonstrate that 7A7 mAb used as a single agent and in equivalent doses to those used in patients (56 μg in mice represent 200 mg in humans) is capable of eradicating established D122 metastatic nodules. In the remaining metastatic sites of 7A7 mAb-treated mice, we detected apoptosis levels similar to the reported in murine carcinomas treated in vivo with chemotherapeutic agents [33]. Our in vitro studies indicate that 7A7 mAb display similar anti-tumor mechanisms to those described for anti-human EGFR mAbs. This mAb was able to inhibit EGFR activation inducing G1 phase arrest, apoptotic response and inhibition of migration in D122 cells. Moreover, 7A7 mAb induced a specific in vitro ADCC activity in these cells.
Some studies have indicated that maximal clinical and molecular responses to anti-EGFR mAb therapy may take several months [14, 15], suggesting that short-term mechanism such as EGFR signaling inhibition and ADCC are not the only ones involved. Interestingly, this is not a unique property of anti-EGFR mAbs because it has also been observed in lymphoma therapy with Rituximab [34]. This raises the question of whether certain mAbs work in two ways and induce a second cellular “control mechanism” after initial response which is not anymore directly mediated by the mAb. The results of a study reported by Selenko et al. [13] suggest that CTL might be critical in the control of non-Hodgkin’s lymphomas by Rituximab. Up to date the involvement of CTL response in the anti-metastasic action of anti-EGFR mAbs has not been elucidated due to in vivo experiments studying their anti-metastatic effect have been conducted with xenografts in nude mice [10–12]. Our results using a syngeneic preclinical model demonstrate that 7A7 mAb treatment increases the number of various immune cells in metastatic site that could be implicated in the development of an anti-tumor-specific immune response, such as T lymphocytes and DCs. Indeed, when we carried out in vivo depletion experiment of CD4+ and CD8+ cells, we observed that anti-metastatic effect of 7A7 mAb is completely dependent of both T cell populations. The presence of an increased number of NK cells in tumor infiltrated after 7A7 mAb treatment was also detected, but these cells are not involved in the anti-metastatic action of this mAb.
In summary, we describe a novel anti-metastatic mechanism of anti-EGFR mAbs based on the induction of a T cell response. Additional studies are required to determine the mechanisms involved in the generation of T cells by 7A7 mAb, as well as their specificity. Recent data have changed the way we understand how dying tumor cells, particularly those undergoing apoptosis, engage with anti-tumor immune responses. Although the original definition of apoptosis excludes inflammation, it is now clear that innate immunity can be targeted by apoptosis [35], and there has been much speculation about the equivalent of all forms of apoptosis [36]. It has indeed been postulated that apoptosis occurring during development or tissue turnover is immunological “soft”. However, when massive apoptosis occurs, the tolerogenic system might be overwhelmed, resulting in “secondary necrosis” and release of endogenous pro-inflammatory mediators [37, 38]. Thus, it is possible that the apoptotic process induced by 7A7 mAb through blocking of EGFR signaling causes an “inflammatory microenvironment” in the metastatic site, with subsequent maturation of DCs that had phagocyted apoptotic bodies from tumor cells. Another important question would be whether specific response induced by anti-EGFR mAbs is an advantage with respect to tyrosine kinase inhibitors. Thus, a next step will be to define the contribution of T cell response in the treatment of EGFR positive tumors with specific tyrosine kinase inhibitors.
Acknowledgments
We thank Armando López (Center of Molecular Immunology) for their excellent technical assistance with animal experiments.
Abbreviations
- EGFR
Epidermal growth factor receptor
- mAb
Monoclonal antibody
- EGF
Epidermal growth factor
- PARP
Poly (ADP-ribose) polymerase
- STAT 3
Signal transducer and activator of transcription 3
- FBS
Fetal bovine serum
- PI
Propidium iodide
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- MAPK
Mitogen-activated protein kinase
- PI3–K
Phosphotidylinositol-3 kinase
- NK
Natural killer
- DC
Dendritic cell
Footnotes
This work was supported by the Cuban Government.
References
- 1.Khazaie K, Schirrmacher V, Lichtner RB. EGF receptor in neoplasia and metastasis. Cancer Metastasis Rev. 1993;12:255–274. doi: 10.1007/BF00665957. [DOI] [PubMed] [Google Scholar]
- 2.Verbeek BS, Adriaansen-Slot SS, Vroom TM, Beckers T, Rijksen G. Overexpression of EGFR and c-erbB2 causes enhanced cell migration in human breast cancer cells and NIH3T3 fibroblasts. FEBS Lett. 1998;425:145–150. doi: 10.1016/S0014-5793(98)00224-5. [DOI] [PubMed] [Google Scholar]
- 3.O-Charoenrat P, Rhys-Evans P, Modjtahedi H, Court W, Box G, Eccles S. Overexpression of epidermal growth factor receptor in human head and neck squamous carcinoma cell lines correlates with matrix metalloproteinase-9 expression and in vitro invasion. Int J Cancer. 2000;86:307–317. doi: 10.1002/(SICI)1097-0215(20000501)86:3<307::AID-IJC2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 4.Radinsky R, Risin S, Fan D, Dong Z, Bielenberg D, Bucana CD, Fidler IJ. Level and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cells. Clin Cancer Res. 1995;1:19–31. [PubMed] [Google Scholar]
- 5.Price JT, Wilson HM, Haites NE. EGF increases in vitro invasion, motility and adhesion interactions of the primary renal carcinoma cell line, A704. Eur J Cancer. 1996;32A:1977–1982. doi: 10.1016/0959-8049(96)00207-9. [DOI] [PubMed] [Google Scholar]
- 6.Salomon D, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-I. [DOI] [PubMed] [Google Scholar]
- 7.Xue C, Wyckoff J, Liang F, Sidani M, Violini S, Tsai K, Zhang Y, Sahai E, Condeelis J, Segall J. EGFR overexpression results in increased tumor cell motility in vivo coordinately with enhanced intravasation and metastasis. Cancer Res. 2006;66:192–197. doi: 10.1158/0008-5472.CAN-05-1242. [DOI] [PubMed] [Google Scholar]
- 8.Turner T, Chen P, Goodly L, Wells A. EGFR signaling enhances in vivo invasiveness of DU-145 human prostate carcinoma cells. Clin Exp Metastasis. 1996;14:409–418. doi: 10.1007/BF00123400. [DOI] [PubMed] [Google Scholar]
- 9.Pal SK, Pegram M. Epidermal growth factor receptor and signal transduction: potential targets for anti-cancer therapy. Anticancer Drugs. 2005;16:483–494. doi: 10.1097/00001813-200506000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Perrotte P, Matsumoto T, Inoue K, Kuniyasu H, Eve BY, Hicklin DJ, Radinsky R, Dinney CP. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 1999;5:257–265. [PubMed] [Google Scholar]
- 11.Bruns CJ, Harbison MT, Davis DW, Portera CA, Tsan R, McConkey DJ, Evans DB, Abbruzzese JL, Hicklin DJ, Radinsky R. Epidermal growth factor receptor blockade with C225 plus gemcitabine results in regression of human pancreatic carcinoma growing orthotopically in nude mice by antiangiogenic mechanisms. Clin Cancer Res. 2000;6:1936–1948. [PubMed] [Google Scholar]
- 12.Prewett M, Rothman M, Waksal H, Feldman M, Bander NH, Hicklin DJ. Mouse-human chimeric anti-epidermal growth factor receptor antibody C225 inhibits the growth of human renal cell carcinoma xenografts in nude mice. Clin Cancer Res. 1998;4:2957–2966. [PubMed] [Google Scholar]
- 13.Selenko N, Maidic O, Draxier S, Berer A, Jager U, Knapp W, Stockl J. CD20 antibody (C2B8)-induced apoptosis of lymphoma cells promotes phagocytosis by dendritic cells and cross-priming of CD8+ cytotoxic T cells. Leukemia. 2001;15:1619–1626. doi: 10.1038/sj.leu.2402226. [DOI] [PubMed] [Google Scholar]
- 14.Crombet T, Osorio M, Cruz T, Roca C, del Castillo R, Mon R, Iznaga-Escobar N, Figueredo R, Koropatnick J, Renginfo E, Fernandez E, Alvarez D, Torres O, Ramos M, Leonard I, Perez R, Lage A. Use of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patients. J Clin Oncol. 2004;22:1646–1654. doi: 10.1200/JCO.2004.03.089. [DOI] [PubMed] [Google Scholar]
- 15.Crombet T, Figueredo J, Catala M, González S, Selva J, Cruz T, Toledo C, Silva S, Pestano5 Y, Ramos1 M, Leonard1 I, Torres1 O, Marinello6 P, Pérez1 R, Lage A. Treatment of high-grade glioma patients with the humanized anti-epidermal growth factor receptor (EGFR) antibody h-R3. Cancer Biol Therapy. 2006;5:375–379. doi: 10.4161/cbt.5.4.2522. [DOI] [PubMed] [Google Scholar]
- 16.Garrido G, Sánchez B, Rodriguez HM, Menna PL, Alonso D, Fernandez LE. 7A7 MAb: a new tool for the pre-clinical evaluation EGFR-based therapies. Hybrid Hybridomics. 2004;23:168–175. doi: 10.1089/1536859041224280. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez AM, Perez A, Hernandez AM, Macias A, Alfonso M, Bombino G, Perez R. Syngeneic anti-idiotypic monoclonal antibodies to an anti-NeuGc-containing ganglioside monoclonal antibody. Hybrid Hybridomics. 1998;17:527–534. doi: 10.1089/hyb.1998.17.527. [DOI] [PubMed] [Google Scholar]
- 18.Corazzari M, Lovat PE, Oliverio S, Di Sano F, Donnorso RP, Redfern CP, Piacentini M. Fenretinide: a p53-independent way to kill cancer cells. Biochem Biophys Res Commun. 2005;331:810–815. doi: 10.1016/j.bbrc.2005.03.184. [DOI] [PubMed] [Google Scholar]
- 19.Eisenbach L, Hollander N, Greenfeld L, Yakor H, Segal S, Feldman M. The differential expression of H-2K versus H-2D antigens, distinguishing high-metastatic from low-metastatic clones, is correlated with the immunogenic properties of the tumor cells. Int J Cancer. 1984;34:567–573. doi: 10.1002/ijc.2910340421. [DOI] [PubMed] [Google Scholar]
- 20.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of radiosensitivity. Cancer Res. 1987;47:943–946. [PubMed] [Google Scholar]
- 21.Sheehy ME, McDermott AB, Furlan SN, Klenerman P, Nixon DF. A novel technique for the fluorometric assessment of T lymphocyte antigen specific lysis. J Immunol Methods. 2001;249:99–110. doi: 10.1016/S0022-1759(00)00329-X. [DOI] [PubMed] [Google Scholar]
- 22.Lasarte JJ, Sarobe P, Prieto J, Borras-Cuesta F. In vivo cytotoxic T-lymphocyte induction may take place via CD8 T helper lymphocytes. Res Immunol. 1995;146:35. doi: 10.1016/0923-2494(96)80238-0. [DOI] [PubMed] [Google Scholar]
- 23.Liao J, Yang GY, Park ES, Meng X, Sun Y, Jia D, Seril DN, Yang CS. Inhibition of lung carcinogenesis and effects on angiogenesis and apoptosis in A/J mice by oral administration of green tea. Nutr Cancer. 2004;48:44–53. doi: 10.1207/s15327914nc4801_7. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe T, Kawamura T, Kawamura H, Haga M, Shirai K, Watanabe H, Eguchi S, Abo T. Intermediate TCR cells in mouse lung: their effector function to induce pneumonitis in mice with autoimmune-like graft-versus-host disease. J Immunol. 1997;158:5805–5814. [PubMed] [Google Scholar]
- 25.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787–2799. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 26.Janmaat ML, Kruyt FA, Rodriguez JA, Giaccone G. Response to epidermal growth factor receptor inhibitors in non-small cell lung cancer cells: limited antiproliferative effects and absence of apoptosis associated with persistent activity of extracellular signal-regulated kinase or Akt kinase pathways. Clin Cancer Res. 2003;9:2316–2326. [PubMed] [Google Scholar]
- 27.Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:1935–1940. [PubMed] [Google Scholar]
- 28.Yang XD, Wang P, Fredlin P, Davis CG (2002) ABX-EGF, a fully human anti-EGF receptor monoclonal antibody: inhibition of prostate cancer in vitro and in vivo. Proc Am Soc Clin Oncol; Abstract 2454
- 29.Huang SM, Li J, Harari PM. Molecular inhibition of angiogenesis and metastatic potential in human squamous cell carcinomas after epidermal growth factor receptor blockade. Mol Cancer Ther. 2002;1:507–514. [PubMed] [Google Scholar]
- 30.Naramura M, Gillies SD, Mendelsohn J, Reisfeld RA, Mueller BM. Therapeutic potential of chimeric and murine anti-(epidermal growth factor receptor) antibodies in a metastasis model for human melanoma. Cancer Immunol Immunother. 1993;37:343–349. doi: 10.1007/BF01518458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bier H, Hoffmann T, Haas I, van Lierop A. Anti-(epidermal growth factor) receptor monoclonal antibodies for the induction of antibody-dependent cell-mediated cytotoxicity against squamous cell carcinoma lines of the head and neck. Cancer Immunol Immunother. 1998;46:167–173. doi: 10.1007/s002620050475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sánchez B, Suárez E, Garrido G, Hernández T, Pérez R, Ullrich A, Fernández L. Active anti-metastatic immunotherapy in Lewis Lung Carcinoma with self EGFR extra cellular domain protein in VSSP adjuvant. Int J Cancer. 2006;119:2190–2199. doi: 10.1002/ijc.21851. [DOI] [PubMed] [Google Scholar]
- 33.Macluskey M, Baillie R, Chandrachud LM, Pendleton N, Schor AM. High levels of apoptosis are associated with improved survival in non-small cell lung cancer. Anticancer Res. 2000;20:2123–2128. [PubMed] [Google Scholar]
- 34.Cartron G, Watier H, Golay J, Solal-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood. 2004;104:2635–2639. doi: 10.1182/blood-2004-03-1110. [DOI] [PubMed] [Google Scholar]
- 35.Restifo N. Building better vaccines: how apoptotic cell death can induce inflammation and activate innate and adaptative immunity. Curr Opin Immunol. 2000;12:597–603. doi: 10.1016/S0952-7915(00)00148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lake RA, Robinson BWS. Immunotherapy and chemotherapy-a practical partnership. Nat Rev. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 37.Fen H, Zeng Y, Graner MW, Katsanis E. Stressed apoptotic tumor cells stimulate dendritic cells and induce specific cytotoxic T cells. Blood. 2002;100:4108–4115. doi: 10.1182/blood-2002-05-1389. [DOI] [PubMed] [Google Scholar]
- 38.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]