Abstract
Blockade of CTLA-4 by monoclonal antibodies (mAb) can mediate regression of tumors and increase the efficacy of tumor antigen specific vaccines. Blockade of CTLA-4 has also been shown to significantly increase the avidity of antigen-specific T cells after immunization with live recombinant viral vector based vaccine. Here, we demonstrate a biological synergy between CTLA-4 blockade and active vaccine therapy consisting of recombinant vaccinia and avipox viruses expressing carcinoembryonic antigen (CEA) and three T cell costimulatory molecules to enhance antitumor effects. However, this synergy was very much dependent on the temporal relationship of scheduling of the two agents. We evaluated the strategies in both a foreign antigen model using β-galactosidase as immunogen, and in a “self” antigen model using CEA as immunogen. For antitumor activity the model used consisted of mice transgenic for human CEA and a murine carcinoma cell line transfected with CEA. The enhanced antitumor activity after vaccine and CTLA-4 blockade did not result in any signs of autoimmunity. These studies form a rational basis for the use of vector-based vaccines with anti-CTLA-4 and demonstrate that both enhancement of positive costimulatory signals and inhibition of negative costimulatory signals can be simultaneously exploited. These studies also underscore the importance of “drug” scheduling in vaccine combination therapies.
Keywords: CTLA-4, Costimulation, Vaccine
Introduction
Successful T-cell based immunotherapy is mainly dependent upon the appropriate activation and persistence of antigen-specific antitumor T cell responses.
As tumorigenesis represents a pathogenic process, a prolonged antigen-specific T cell response is always desirable. One innovative approach has been to block a crucial inhibitory arm of the costimulatory pathway using monoclonal antibodies against the CTLA-4 molecule expressed by Ag-primed activated T cells [1]. The clinical use of CTLA-4 in tumor patients has yielded conflicting results.
The administration of anti-CTLA-4 monoclonal antibody (mAb) as a single agent in preclinical models has been demonstrated to increase the antitumor activity in unvaccinated tumor-bearing hosts [2–4]. Tumor therapy using anti-CTLA-4 mAb alone is mostly effective in mice bearing moderately to highly immunogenic tumors and typically at the earlier time points of tumor growth. On the contrary, little or no tumor rejection occurs in mice when challenged with weakly immunogenic or non-immunogenic tumors [5, 6]. The failure of anti-CTLA-4 mAb as a single agent against poorly immunogenic tumors led to the exploration of its use in combination with other therapies.
Most defined tumor antigens recognized by T cells are self-antigens (Ags). Because high avidity, self-reactive T cells are likely deleted in the thymus during development, any residual T cells existing in the periphery may therefore be low avidity and unresponsive due to peripheral tolerance mechanisms [7–9]. Activation of these residual T cells is critical for targeting tumors by immunotherapy. We have previously reported that the use of anti-CTLA-4 mAb in combination with poxviral-based vaccines not only increased the quantity of Ag-specific T cells but also their avidity [10]. In a foreign Ag model using β-galactosidase (β-gal) as immunogen, CTLA-4 blockade increased the avidity of T cells by 16-fold compared with the vaccine along with GM-CSF. In a self-Ag model using carcinoembryonic antigen (CEA) as immunogen in CEA transgenic (Tg) mice, CTLA-4 blockade increased the functional avidity of T cells by 30-fold compared with the rV-CEA/TRICOM vaccine along with GM-CSF [10]. Because little is currently known about using anti-CTLA-4 mAb in conjunction with vaccinia- or fowlpox-based vaccines, here we sought to determine the optimal sequence for the use of anti-CTLA-4 mAb along with vaccinia- or fowlpox-based vaccine containing β-gal as antigen. We then extended the study for tumor therapy utilizing a tumor-associated antigen (TAA)-specific vaccine regimen to induce and potentiate T cell responses against CEA in combination with anti-CTLA-4 mAb administration. CEA expression is prevalent among diverse human carcinomas, namely, colorectal, gastric, pancreatic, breast and non-small cell lung malignancies [11]. Transgenic mice, which express the human CEA gene [12] as “self” in tissues in a manner similar to that expressed in humans, were utilized as a more relevant preclinical model to investigate various experimental immunotherapies and potential autoimmune consequences. These mice contain the human CEA transgene under the control of the endogenous human CEA promoter. Moreover, these CEA-Tg mice contain serum CEA protein in levels (5–100 ng/ml) similar to those found in patients with CEA-expressing carcinomas [13]. Previous studies have demonstrated that these mice are tolerant to CEA by their inability to mount either CEA-specific T cell responses or CEA-specific antibody responses after multiple vaccinations with CEA protein in adjuvant. These mice thus provide a model for peripheral tolerance to a “self” TAA [13]. To evaluate the effectiveness, mechanism, and potential coincidental autoimmunity of different vaccine regimens directed against a “self” TAA, we have employed this model. CEA-Tg mice were vaccinated with a poxvirus-based diversified prime and boost regimen: recombinant vaccinia prime (rV) followed by recombinant fowlpox boosts (rF). These recombinant viruses encoded the CEA gene as well as genes for a TRIad of COstimulatory Molecules (B7-1, ICAM-1, LFA-3; designated TRICOM). The advantages of the diversified prime and boost regimen have been described [14], as well as the use of costimulation by inserting multiple costimulatory molecules into these vectors [15]. GM-CSF is known to function as a biological vaccine adjuvant because of its actions as a major stimulatory cytokine for Langerhans and dendritic cells. It has also been demonstrated that GM-CSF produced by recombinant avian poxviruses enriches the regional lymph nodes with antigen-presenting cells (APC) and acts as an immunoadjuvant [16]. The rV-CEA-TRICOM/rF-CEA-TRICOM vaccine regimen was used in combination with rF-GM-CSF and anti-CTLA-4 mAb. We selected the optimum time schedule to treat the weakly immunogenic murine adenocarcinoma cell line MC38-CEA+, which was implanted subcutaneously (s.c.).
Overall, these results revealed for the first time that the sequence of anti-CTLA-4 mAb administration is crucial for its use along with vaccinia virus to achieve optimal T cell responses. This is the first paper to demonstrate that the combination of both positive costimulations using poxviral-based vaccine and blocking of negative signals by using anti-CTLA-4 mAb can be used for effective tumor therapy. Furthermore, blocking of CTLA-4 by using anti-CTLA-4 mAb concurrent with vaccine significantly improved the therapeutic efficacy of a recombinant anticancer vaccine regimen without inducing any symptoms of autoimmunity.
Materials and methods
Tumor cells
For in vivo studies, murine colon carcinoma MC38 cells (H-2b) expressing human CEA were generated by retroviral transduction with CEA cDNA [17], and are designated MC38-CEA+. For cytotoxicity assays, the target tumor cell line EL-4 (H-2b, thymoma) was obtained from American Type Culture Collection (Manassas, VA, USA).
Animals
For in vivo studies, 6- to 8-week-old female C57BL/6 or CEA-Tg mice were used. Female C57BL/6 mice were obtained from the National Cancer Institute, Frederick Cancer Research Facility (Frederick, MD, USA). C57/BL6 mice, transgenic for human CEA (designated CEA-Tg) were originally obtained from a breeding pair provided by Dr. John Thompson (Institute of Immunobiology, University of Freiburg, Freiburg, Germany). The generation and characterization of the CEA-Tg mouse has been previously described [12]. Mice were housed and maintained under pathogen-free conditions in micro-isolator cages.
Antibodies
Anti-CTLA-4 mAb (9H10) [18], a kind gift from J. Allison (University of California, Berkley, CA, USA), was purified at Protein Expression Laboratory, NCI/Frederick. Control Purified Syrian Hamster IgG2 kappa was obtained from BD Biosciences (San Jose, CA, USA).
Recombinant poxvirus vaccines
The recombinant vaccinia and fowlpox viruses containing the human CEA gene and the murine B7-1, ICAM-1, and LFA-3 genes (designated rV-CEA/TRICOM and rF-CEA/TRICOM, respectively) have been described [15]. The recombinant vaccinia viruses designated rV-LacZ/TRICOM was constructed in a similar manner and contain the LacZ gene encoding β-gal as described [24]. The recombinant fowlpox virus containing the gene for murine GM-CSF under control of the 40 k promoter has been described [16]. Drs. D. Panicali, G. Mazzara, and L. Gritz of Therion Biologics Corporation (Cambridge, MA, USA) kindly provided all Orthopox viruses as part of an CRADA between the NCI/NIH and Therion Biologics Corporation (Cambridge, MA, USA).
Lymphocyte proliferation assay
To evaluate CD4+ T cell responses mice were vaccinated s.c once either with 1 × 108 pfu rV-Lac Z/TRICOM (Figs. 1, 2) or rF-LacZ/TRICOM (Figs. 3, 4) admixed with 1 × 107 pfu rF-GM-CSF and 21 days later splenic T cells were tested for proliferation in response to protein antigens as previously described [19]. Briefly, pooled splenic T cells (1.5 × 105 cells/well) were cultured in 96 well flat-bottomed plates with irradiated naïve syngeneic splenocytes as APC (5 × 105 cells/well) and β-gal protein or HSA (3–100 μg/ml). As a positive control, cells were stimulated with the T cell mitogen Concanavalin A (Con-A 2.5 μg/ml, Sigma, St. Louis, MO, USA). T cells and APC were cultured without stimulation as controls. Cells were cultured for 5 days and 3H-thymidine (1 μCi/well) was added to the wells 18–24 h before harvesting using a Tomtec cell harvester (Wallac Inc., Gaithersburg, MD, USA). The incorporated radioactivity was measured using a liquid scintillation counter (Wallac 1205 Betaplate, Wallac Inc.).
Fig. 1.
Determination of optimal timing of anti-CTLA-4 regimen to enhance CD4+ T cell responses induced by recombinant vaccinia vaccination (rV-LacZ-TRICOM). C57BL/6 mice were vaccinated subcutaneously with 1 × 108 pfu rV-LacZ-TRICOM in 100 μl volume or as a control 100 μl of HBSS instead of rV-LacZ-TRICOM (inset) on day 0. Anti-CTLA-4 antibody was administered intraperitoneally three times at doses of 100, 50, and 50 μg, respectively, over a 6-day period as indicated. Lymphoproliferation of T cells in response to β-gal protein. Results are means ± SE of triplicate cultures and are representative of three experiments
Fig. 2.
Effect of timing of anti-CTLA-4 antibody regimen on CD8+ T cell responses induced by rV-LacZ-TRICOM vaccination. C57BL/6 mice were vaccinated with rV-LacZ-TRICOM in the presence or absence of CTLA-4 antibody. a β-gal-specific lysis of tumor cells by CTL assay. b IFN-γ production from CD8+ T cells of vaccinated C57BL/6 mice stimulated with β-gal as a positive or OVA as a negative peptide for 24 h. Lysis is reported from an E:T ratio of 60:1. Similar results were obtained in three individual experiments. *Significant as compared with vaccine alone (P < 0.05)
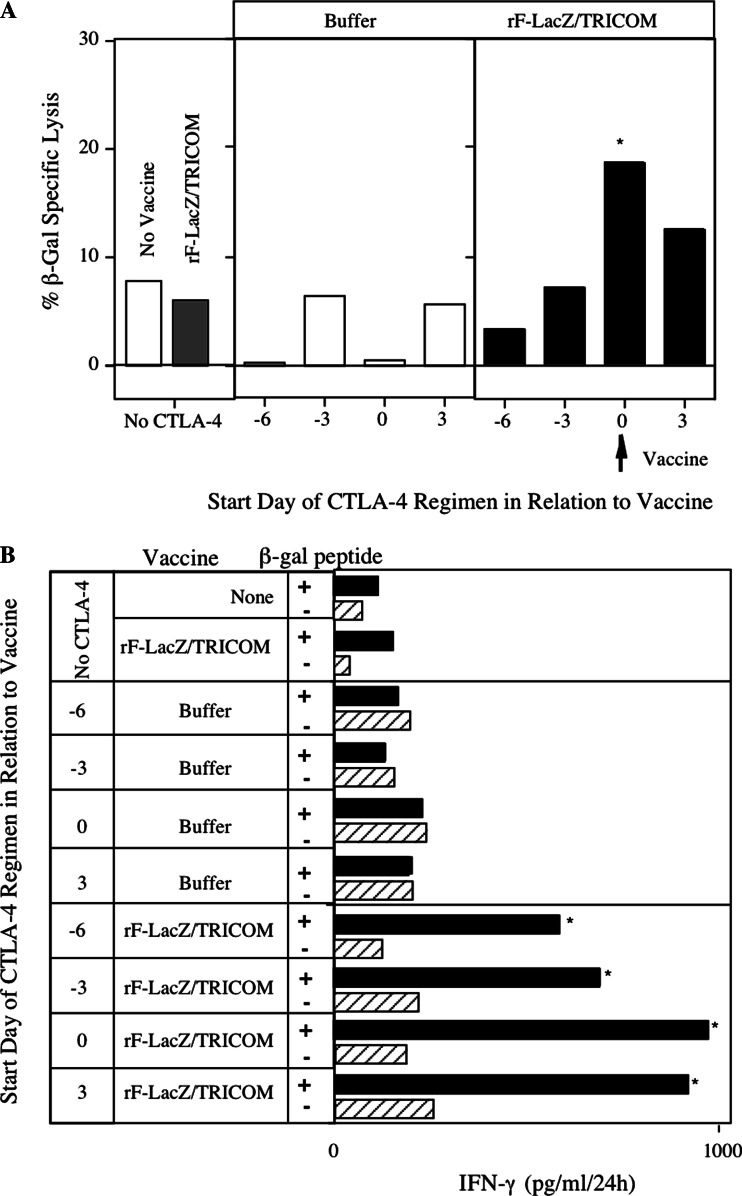
Fig. 3.
Determination of optimal timing of anti-CTLA-4 regimen to enhance CD4+ T cell responses induced by recombinant fowlpox vaccination (rF-LacZ-TRICOM). C57BL/6 mice were vaccinated subcutaneously with 1 × 108 pfu rF-LacZ-TRICOM in 100 μl volumes or as a control 100 μl of HBSS instead of rF-LacZ-TRICOM (inset) on day 0. Anti-CTLA-4 antibody was administered intraperitoneally three times at doses of 100, 50, and 50 μg, respectively, over a 6-day period as indicated. Lymphoproliferation of T cells in response to β-gal protein. Results are means ± SE of triplicate cultures and are representative of three experiments
Fig. 4.
Effect of timing of anti-CTLA-4 antibody regimen on CD8+ T cell responses induced by rF-LacZ-TRICOM vaccination. C57BL/6 mice were vaccinated with rF-LacZ-TRICOM in the presence or absence of CTLA-4 antibody. a β-gal-specific lysis of tumor cells by CTL assay. b IFN-γ production from CD8+ T cells of vaccinated C57BL/6 mice stimulated with β-gal as a positive or OVA as a negative peptide for 24 h. Similar results were obtained in three individual experiments. *Significant as compared with vaccine alone (P < 0.05)
Cytotoxicity assay
To evaluate CD8+ T cell responses, splenic T cells from vaccinated mice were tested for lysis of EL-4 cells pulsed with β-gal peptide or ovalbumin peptide. Spleen cells were stimulated with H-2Kb-restricted β-gal peptide (10 μg/ml, DAPIYTNV) for 6 days and then lymphocytes were separated by centrifugation through a Ficoll–Hypaque gradient. EL-4 tumor cells were prepared for use as targets in a standard cytotoxicity assay using 51Cr, as previously described [20]. These radio-labeled cells (5 × 103 cells/well) were co-incubated with 10 μg/ml β-gal peptide or ovalbumin257–254 (SIINFEKL) peptide as control and peptide stimulated T cells from vaccinated mice at various effectors: target ratios for 4 h at 37°C with 5% CO2. After incubation, supernatants were collected using a Supernatant Collection System (Skantron, Sterling, VA, USA), and radioactivity was quantitated using a gamma counter (Cobra Auto-gamma; Packard, Downers Grove, IL, USA). The percentage of specific release of 51Cr was determined by the standard equation:
 |
Cytokine production assay
To evaluate CD8+ T cell responses, splenic T cells from vaccinated mice were tested for cytokine production. Spleen cells were stimulated with H-2Kb-restricted peptide β-gal (10 μg/ml) and 6 days later lymphocytes were separated by centrifugation through a Ficoll–Hypaque gradient. The recovered lymphocytes (5 × 106 cells/well) were restimulated with β-gal peptide or control ovalbumin peptide at the same concentrations along with irradiated naive splenocytes (5 × 106 cells/well) for 24 h in 24 well plates. Supernatants were collected and IFN-γ was quantitated by Cytokine Bead Array Kit (BD Pharmingen, San Jose, CA, USA) [21].
Determination of CD8+ T cell avidity: tetramer dissociation
T cell avidity was determined by tetramer dilution analysis as described by Ercolini et al. [22]. Briefly, to determine the relative avidity of β-gal-specific CTL in C57BL/6 mice (n = 3/group) after vaccination, splenocytes were stained with FITC-conjugated anti-CD3e mAb (Pharmingen), and CyChrome-conjugated anti-CD8 mAb (Pharmingen). After 30 min, cells were washed, and PE-conjugated β-gal/H-2Kb-tetramer (NIH Tetramer Facility, NIAID, Bethesda, MD, USA) was added at varying dilutions and incubated for 30 min at 8–12°C. Cells were washed twice and immediately fixed with fresh 1% Para formaldehyde in PBS. Tetramer staining was analyzed by gating only on CD8 cells. Results were expressed as the percentage of tetramer positive cells versus tetramer dilution. To normalize groups within each experiment, data were also expressed as the percentage of maximum tetramer binding versus dilution. Finally, the natural logarithm of the normalized data was plotted against dilution. The relative avidity of each T cell population was derived from the reciprocal of the natural logarithm of the normalized data plotted against dilution [23].
Tumor therapy studies
Six-week-old female CEA-Tg mice were injected by s.c. route in the right flank area with 3 × 105 MC38-CEA+ cells. Four days following tumor transplant, mice were vaccinated s.c. once with 1 × 108 pfu rV-CEA-TRICOM admixed with 1 × 107 pfu rF-GM-CSF. On day 11, mice were boosted with 1 × 108 pfu rF-CEA-TRICOM admixed with 1 × 107 pfu rF-GM-CSF. This booster vaccination regimen was repeated two additional times at 7-day intervals. Anti-CTLA-4 mAb was administered by intraperitoneal (i.p.) route on day 4 (100 μg), day 7 (50 μg), and day 10 (50 μg) following tumor transplant as described earlier [3, 5]. Tumors were measured twice a week by digital caliper in two dimensions, and the volumes were calculated as previously described [15].
Autoimmune assays
Mice that were successfully treated by vaccine therapy alone or the combination of vaccine therapy and CTLA-4 mAb were monitored for 5 months. Control mice consisted of age-matched CEA-Tg mice that did not receive tumor and were not vaccinated. Upon euthanization, serum was analyzed for auto-antibodies. Antibody levels to antinuclear antibodies (ANA), rheumatoid factor, nuclear ribonuclear protein, histone, SCL-70 (DNA topoisomerase I), dsDNA, ssDNA, and circulating immune complexes were determined in a qualitative or semi-quantitative manner (Alpha Diagnostic International, San Antonio, TX, USA) according to the manufacturer’s instructions.
Statistical analysis of the data
Where indicated, the results of tests of significance are reported as P values and are derived from Student’s t test using a two-tailed distribution. P values were calculated at 95% using Statview 4.1 software package (Abacus Concepts Inc., Berkeley, CA, USA).
Results
Recombinant vaccinia vaccine in combination with anti-CTLA-4 mAb
Studies were conducted to determine the effect of anti-CTLA-4 mAb on the T cell responses induced by rV-LacZ-TRICOM using β-gal as a model system. To reveal the optimum time for administration of anti-CTLA-4 mAb along with a live replicating recombinant vaccinia vector, C57BL/6 mice were vaccinated with rV-LacZ-TRICOM and anti-CTLA-4 mAb was injected at various times in relation to the vaccine. As seen in Fig. 1, administration of anti-CTLA-4 mAb starting at the same day as rV-LacZ-TRICOM (days 0, 3, 6 schedule) resulted in significantly (P = 0.001) enhanced β-gal-specific CD4+ T cell responses. Using this sequence, CD4+ T cells showed almost a three-fold higher proliferation at all concentrations of β-gal protein. Administration of anti-CTLA-4 mAb 3 days before or 3 days after vaccination also enhanced the Ag-specific CD4+ T cell response to some extent; however, administration of mAb starting at 6 days prior (days −6, −3, 0 schedule) to vaccine resulted in no enhancement of proliferation.
The effect of timing of anti-CTLA-4 mAb administration on Ag-specific CD8+ T cells was also evaluated by β-gal-specific CTL activity and by β-gal-specific IFN-γ production in response to peptide. As seen in Fig. 2a, administration of anti-CTLA-4 mAb starting at the same day as rV-LacZ-TRICOM (days 0, 3, 6 schedule) showed statistically significant (P = 0.001) enhanced lysis by CD8+ T cells (rV-LacZ-TRICOM, 16% vs. 38% with rV-LacZ-TRICOM + anti-CTLA-4 mAb starting on day 0). Administration of anti-CTLA-4 mAb 3 days after vaccine (days 3, 6, 9 schedule) also resulted in increased lysis (28%) by T cells compared with vaccine alone. Administration of anti-CTLA-4 mAb starting 3 or 6 days prior to rV-LacZ-TRICOM did not have any effect on T cell-mediated lysis.
Next we evaluated Ag-specific CD8+ T cell response by measuring β-gal-specific IFN-γ production after vaccinating with rV-LacZ-TRICOM in combination with anti-CTLA-4 mAb on different days. As seen in Fig. 2b, IFN-γ production by CD8+ T cells in response to β-gal peptide was highest (3,400 pg/ml/24 h) at day 0 of CTLA-4 mAb administration compared with 1,900 pg/ml/24 h with rV-LacZ-TRICOM alone. Although administration of anti-CTLA-4 mAb starting 3 days (days −3, 0, 3 schedule) prior to rV-LacZ-TRICOM did not have any boosting effect on T cell-mediated lysis, when β-gal-specific IFN-γ was measured it secreted almost an equal amount of IFN-γ to that of day 0 (days 0, 3, 6 schedule) administration; these results are statistically significant compared with the use of rV-LacZ/TRICOM alone. The administration of anti-CTLA-4 mAb 3 days later or 6 days before vaccination, however, had minimal effect in comparison with vaccination alone (Fig. 2b).
Recombinant fowlpox vaccine in combination with anti-CTLA-4 mAb
The initial studies described above used a replication competent vaccinia virus. Replication defective fowlpox viruses have been used in many vaccine strategies [14, 24, 25] as a boosting reagent. We therefore conducted studies to determine whether the optimal timing of anti-CTLA-4 mAb administration along with rF-CEA-TRICOM would be similar to that with vaccinia. The administration of anti-CTLA-4 mAb on the same day (days 0, 3, 6) as vaccination showed the best proliferation (P = 0.001) compared with the other days (Fig. 3). Although CD8+ T cells after vaccination alone were unable to mediate, β-gal-specific CTL activity was significantly (P = 0.001) enhanced (6 vs. 18%) after administration of anti-CTLA-4 mAb on the same day as vaccination (Fig. 4a). The additive effect of anti-CTLA-4 mAb on fowlpox vaccination was observed when the β-gal-specific IFN-γ production was measured, reaching its maximum on the days 0, 3, 6 schedule, regardless of the day of administration of anti-CTLA-4 mAb. Taken together, however, these data indicate that for both vaccinia and fowlpox vectors the optimal timing of anti-CTLA-4 mAb administration is concurrent with vaccine.
Effect of positive versus negative costimulatory regulation on T cell avidity
Employing the optimal dose scheduling, we next compared positive costimulation (rV-LacZ/B7-1, Fig. 5a) versus negative costimulatory signal regulation (rV-LacZ + anti-CTLA-4, Fig. 5b) on the avidity of the Ag-specific T cells using rV-LacZ as a control. Both vaccination strategies induced similar higher avidity T cells in vaccinated mice than the use of rV-LacZ alone (Fig. 5a, b). We then asked if the combined use of positive and negative costimulatory strategies would enhance T cell avidity. Toward this aim, mice were vaccinated with rV-LacZ/B7-1 ± anti-CTLA-4 mAb. As seen in Fig. 5c, there was a 22-fold increase in T cell avidity with the addition of anti-CTLA-4 mAb to rV-LacZ/B7-1.
Fig. 5.
The role of positive costimulation using multiple costimulatory molecules in combination with the modulation of a negative signal on T cell avidity. a–e C57BL6 mice were vaccinated with rV-LacZ (squares), rV-LacZ/B7–1 (diamonds), or rV-LacZ/TRICOM (circles). Groups were administered buffer (open symbols) or CTLA-4 mAb (closed symbols) in conjunction with the indicated vaccine. After 14 days, spleens were harvested and β-gal-specific T cell avidity was determined by tetramer dilution assay. f Avidity of antigen-specific T cells as determined by tetramer dilution. The relative avidity of each T cell population was derived from the reciprocal of the natural logarithm of the normalized data plotted against dilution. The avidity of β-gal-specific T cells from mice vaccinated with rV-LacZ was designated 1X and avidity of all other T cell groups was compared to 1X
As B7-1 acts on the CD28 molecules on T cells and anti-CTLA-4 blocks the effect of negative signals via CTLA-4, one could hypothesize that there would be no need for additional costimulatory modalities. To examine this, we immunized mice with rV-LacZ/TRICOM ± anti-CTLA-4 mAb. As seen in Fig. 5d, there was a 44-fold increase in the avidity of β-gal-specific T cells compared to rV-LacZ alone. However, the addition of anti-CTLA-4 mAb to rV-LacZ resulted in a profound difference (611 fold) in the avidity of β-gal-specific T cells (Fig. 5e).
Blocking CTLA-4 enhances efficiency of vaccine therapy
To determine whether blocking CTLA-4 by administering mAb can improve the tumor therapy induced by a recombinant anticancer vaccine regimen, we vaccinated CEA-Tg mice with a diversified prime and boost regimen with CEA-TRICOM vectors in combination with anti-CTLA-4 mAb administration. The vaccine regimen consisted of priming mice with rV-CEA-TRICOM admixed with rF-GM-CSF and boosting with rF-CEA-TRICOM admixed with rF-GM-CSF on days 11, 18, and 25. MC38CEA+ tumor cells were injected s.c. in the right flank of CEA-Tg mice. Four days following tumor transplant, groups of mice were then divided into those that received (a) no treatment; (b) vaccine alone; (c) CTLA-4 mAb alone; and (d) the combination of vaccination and CTLA-4 mAb administration (Fig. 6). The fowlpox vectors have been shown to express the recombinant antigens for up to 21 days [16, 24]; therefore, it could be hypothesized that the administration of anti-CTLA-4 mAb might be used any time later than the administration of rF-CEA-TRICOM. However, we used the anti-CTLA-4 mAb only during the priming of mice. Well aware of the importance of the timing of CTLA-4 blockade by mAb, we administered the CTLA-4 mAb starting at the same time as priming of the mice. Tumors of mice that did not receive any treatment grew progressively, ultimately causing the death of the animals (100% by day 30, Fig. 6a). Therapy of tumors with rV-CEA-TRICOM priming on day 4 followed by rF-CEA-TRICOM boosting on days 11, 18, and 25 (Fig. 6b) did not significantly inhibit tumor growth (P = 0.1425 as compared with no treatment). Treatment of mice with CTLA-4 mAb alone (on days 0, 3, and 6) ultimately failed to significantly affect the extent of tumor growth in these mice (P = 0.128 as compared with no treatment). Therapy of tumors with the combination of the vaccine regimen and anti-CTLA-4 mAb, however, resulted in a marked and significant decrease in tumor growth rate, and tumor volume (P = 0.0001 vs. no treatment; P = 0.0003 vs. CTLA-4 mAb alone; P = 0.0001 vs. vaccine alone; Fig. 6d). In addition, 20% of the mice treated with the combination of CTLA-4 mAb and vaccine therapy resolved their tumor mass and remained tumor free for the duration of the experiment (150 days).
Fig. 6.
Administration of CTLA-4 antibody enhances the efficacy of vaccine therapy. CEA-Tg mice were injected with MC38CEA+ tumor cells s.c. Mice were vaccinated with rV-CEA-TRICOM in the presence or absence of anti-CTLA-4 antibody and tumor volume was measured. a Mice received no treatment. b Mice were vaccinated with rV-CEA-TRICOM on day 4, followed by boosting with rF-CEA-TRICOM on days 11, 18, and 25. c Mice received CTLA-4 antibody on days 4 (100 μg), 7 (50 μg), and 10 (50 μg). d Mice were vaccinated with rV-CEA-TRICOM on day 4, followed by boosting with rF-CEA-TRICOM on days 11, 18, and 25 along with CTLA-4 antibody on days 4 (100 μg), 7 (50 μg), and 10 (50 μg). Tumor growth was monitored for 40 days
Therapeutic effect induced by the combination therapy of vaccination with CTLA-4 mAb did not induce autoimmunity
CTLA-4 blockade in selective murine models enhanced immune-mediated tumor rejection [26] but coincided with autoimmunity such as depigmentation in a melanoma model [27]. It has also been reported that in clinical trials of patients with stage IV metastatic melanoma treated with humanized CTLA-4 antibody (MDX-010; Medarex Inc., Princeton, NJ, USA) along with gp100 peptide, three of 14 in that trial experienced objective tumor regression but six of 14 patients experienced grade III/IV autoimmunity [28, 29]. As previously stated, CEA is a self-antigen expressed in normal gastrointestinal epithelium in CEA-Tg mice at levels similar to those in humans and CEA protein is found in the serum of CEA-Tg mice at levels seen in human colon carcinoma patients. Studies were conducted to determine whether there was any evidence of autoimmunity observed in mice cured of their tumor following the combination therapy (CEA-TRICOM plus GM-CSF plus CTLA-4 mAb) (Table 1). Those mice did not show any sign of weight loss or any other clinical sign of autoimmunity. Mice cured of tumor (Fig. 6b, d) were followed for 5 months and control mice consisted of age-matched CEA-Tg mice that did not receive tumor and were not treated. At 5 months, sera were examined for levels of ANA, as well as antibodies specific for rheumatoid factor, nuclear ribonuclear protein, histone, topoisomerase-1 (scl-70), dsDNA, ssDNA, or circulating immune complexes. Cured mice developed anti-CEA and anti-vaccinia antibody response. Those tumor-free mice, however, did not develop any detectable antibody responses against B7-1, ICAM-1, LFA-3, or GM-CSF compared with the control mice. Those mice also did not develop any detectable antibodies to histone, dsDNA, ssDNA, and SCL-70. These results indicated no evidence of autoimmunity in mice that received the CEA-TRICOM vaccine regimen with rF-GM-CSF and CTLA-4 mAb (Table 1).
Table 1.
Serum immunological parameters of CEA-Tg mice that received tumor therapy with CEA/TRICOM vectors ± CTLA-4 mAb
| Test | Normal | aV/F-CEA/TRICOM + rF-GM-CSF (n = 1) | bV/F-CEA/TRICOM + rf-GM-CSF + anti-CTLA-4 (n = 4) | Age-matched control groupc (n = 5) |
|---|---|---|---|---|
| CEA Abd | <50 | 1,640 ± 250 | 1,780 ± 250 | <50 |
| Vaccinia Abd | Negative | 300 | 200 | <50 |
| Fowlpox Abd | Negative | >250 | >250 | <50 |
| B7-1 Abe | Negative | <100 | <100 | <100 |
| ICAM-1 Abe | Negative | <100 | <100 | <100 |
| LFA-3 Abe | Negative | <100 | <100 | <100 |
| GM-CSF-Abf | Negative | Negative | Negative | Negative |
| nRNP Abg | Negative | Negative | Negative | Negative |
| Histone Abg | Negative | Negative | Negative | Negative |
| Scl-70 Abg | Negative | Negative | Negative | Negative |
| dsDNA Abg | Negative | Negative | Negative | Negative |
| ssDNA Abg | Negative | Negative | Negative | Negative |
| CICg | Negative | Negative | Negative | Negative |
aTwelve-week-old CEA-Tg mice bearing 4d SC MC32a tumors were vaccinated once with 1 × 108 pfu rV-CEA/TRICOM admixed with rF-GM-CSF (1 × 107 pfu). Seven days following the primary vaccination, mice were boosted with 1 × 108 pfu rF-CEA/TRICOM admixed with 1 × 107 pfu rF-GM-CSF. This booster vaccination regimen was repeated two additional times at 7-day intervals. Serum samples were taken after 5 months
bTwelve-week-old CEA-Tg mice bearing 4d SC MC32a tumors were vaccinated once with 1 × 108 pfu rV-CEA/TRICOM admixed with rF-GM-CSF (1 × 107 pfu). Mice were administered 100 μg anti-CTLA-4 IP on day 0, and 50 μg IP on days 3 and 6. Seven days following the primary vaccination, mice were boosted with 1 × 108 pfu rF-CEA/TRICOM admixed with 1 × 107 pfu rF-GM-CSF. This booster vaccination regimen was repeated two additional times at 7-day intervals. Serum samples were taken after 5 months
cSerum samples were taken from age-matched CEA-Tg mice (8 months)
dAb titer reported as the highest reciprocal serum dilution yielding positive signal
eAnti-costimulatory molecule Ab titers reported as the highest reciprocal serum dilution yielding positive signal. Detection limits anti-B7-1 0.49 ng/ml; anti-ICAM-1 7.8 ng/ml; anti-LFA-3 15.6 ng/ml
fAnti-GM-CSF Ab titers determined by capture ELISA. Detection limit 3 ng/ml
gAntinuclear antibodies (ANA): nRNP, nuclear ribonuclear protein; SCL-70 (DNA topoisomerase 1); dsDNA, double-stranded DNA; ssDNA, single-stranded DNA; CIC, circulating immune complexes. Results are semi-quantitative and are expressed as 0 to 4+
Discussion
A fundamental issue in the CD28-CTLA-4 system is how a process regulated by the integration of positive and negative signals, generated by receptors that bind the same ligand, can ever be activated when the affinity of the inhibitory receptor for the ligand greatly exceeds that of the stimulatory receptor. The answer is likely to involve the temporal and spatial separation of expression. CD28 is constitutively expressed on the T cell surface, whereas CTLA-4 expression is upregulated after T cell activation and reaches a maximum after 2–3 days [30]. This initially suggested symmetrical functions for the molecules: CD28 in the initiation of T cell activation and CTLA-4 in the termination of the response. However, the finding that CTLA-4 mRNA can be detected as early as 1 h after activation and that CTLA-4 colligation affects interleukin 2 (IL-2) mRNA accumulation as early as 4 h after activation suggested that CTLA-4 might be functionally relevant much earlier than would be expected from cell surface expression data. Because naïve T cells require many hours of contact with an APC to become fully activated and extensively proliferate, there appears to be sufficient time for the transcription, translation, and processing of CTLA-4 before this process is completed [26]. It is therefore crucial to understand the appropriate timing to block this inhibitory signal to achieve maximum Ag-specific T cell responses. The vaccine used in this study induced the expression of the ligand for CD28 (B7-1 in TRICOM) that could also influence the need for blocking the negative signaling by CTLA-4. Indeed, the findings in our experiments suggest that administration of CTLA-4 mAb concurrent with priming of T cells by vaccination results in significantly increased antigen-specific CD4+ and CD8+ T cell responses compared with its administration 3 days before or 3 and 6 days after vaccination (Figs. 1–4). Our finding supports the data of Espenschied et al. [31], in which anti-CTLA-4 mAb was used during priming of mice using rMVA-p53 vaccine. But the optimal timing of anti-CTLA-4 mAb administration is different when it was used along with DNA vaccine or peptide vaccine. During DNA vaccination, CTLA-4 mAb was administered during each booster immunization of the mice [32] and it was used 1 day after peptide vaccination [33].
The fundamental basis of effective immunotherapy and the capacity to overcome a potential diversity of tumor escape mechanisms will likely require a synergism between both quantitative and qualitative aspects of the T lymphocyte-mediated immune response. The mechanism underlying the ability of CTLA-4 mAb blockade to increase antitumor immunity remains to be clarified. Investigations by Egen et al. [30] revealed that CTLA-4 traffics to the immunologic synapse in response to T cell activation, thereby delivering an attenuating signal. In immunized mice, tumor reactive memory or effector T cells encountering antigen loaded dendritic cells in the periphery or secondary lymphoid tissues may be the targets for CTLA-4 mAb blockade. Alternatively, this antibody may modulate the activities of regulatory T cells that constitutively express surface CTLA-4 [34].
Numerous preclinical studies have demonstrated anti-tumor effects in some rodent models with the use of anti-CTLA-4 antibody [3–6]. Several theories have been put forth [26, 35–37] as to the role of anti-CTLA-4 mAb, but the mechanism has not been fully elucidated. Data have been presented [38] that the antibody inhibits the activity of CD4+CD25+CTLA-4+ Treg cells, while other data refute this [34]. Another theory states, “Preferential inhibition of these T cells by CTLA-4 could slow antigen-driven selection, thereby preventing high-affinity T cell clones from dominating the response during its early stages. Thus, the attenuation model would predict that in the absence of CTLA-4-mediated inhibition, higher affinity T cell clones would out-compete weaker clones ...” [26]. The studies reported here are the first to show that the exploitation of the negative regulation by administration of anti-CTLA-4 mAb in combination with positive costimulation by vector-based vaccine will actually increase the avidity of antigen-specific T cells (Fig. 5).
In discussing the possible mechanism of T cell activation, it is worth mentioning that under conditions where CD28-B7 signaling is not limiting, the scenario in which CTLA-4 abrogates full activation by raising the threshold for CD28 costimulation does not apply [26]. Thus, under conditions where B7 is not limiting, TCR and CD28 may be primarily responsible for determining whether a cell enters the cell cycle, whereas CTLA-4 regulates the extent of subsequent division. By preferentially restricting the expansion of cells that receive stronger TCR signals, CTLA-4 would prevent T cells bearing higher affinity TCRs from dominating the response. Thus, in the absence of CTLA-4, the distribution of cells within the responding population would shift toward higher affinity TCRs. Because our vaccine contains B7-1, we favor the hypothesis that anti-CTLA-4 mAb preferentially expands high-avidity T cells that recognize CEA. It has also previously been shown that anti-CTLA-4 mAb activates high avidity antigen-specific T cells after vaccination with the TRICOM vaccine [30]. Our findings presented here suggest that appropriate blocking of CTLA-4 function results in the activation of a defined number of Ag-specific T cells with higher avidity and provides protection to poorly accessible s.c. tumors, which is in accordance with the hypothesis of other authors [26]. In addition, these observations are in agreement with previous studies which reported that the use of anti-CTLA-4 mAb in combination with pox viral-based vaccines not only increased the quantity but also the avidity of the CEA-specific CD8+ T cells [10].
Our data in this model indicate a synergy between CTLA-4 blockades and a vaccinia virus or fowlpox-based cancer vaccine along with rF-GM-CSF. CEA-Tg mice treated with either the vaccine or antibody alone had no reduction in tumor growth, whereas the combination of both resulted in a significant reduction in tumor growth. This suggests that an additional source of antigen along with the costimulatory molecules from the virus-based vaccine contribute to T cell priming, which is even enhanced by the blockade of CTLA-4/B7 interactions. In a recent publication, we evaluated different vaccines and vaccine strategies for their ability to enhance the quantity and avidity of CTL responses [10]. CD8+ T cell quantity was measured by tetramer staining and avidity was determined by tetramer dissociation as well as by cytolytic function. There, the combination of GM-CSF and CTLA-4 mAb along with pox viral-based vaccine was synergistic in terms of increasing the avidity of Ag-specific CD8+ T cells. Furthermore, it was shown that the use of anti-CTLA-4 mAb to block the inhibitory role of CTLA-4 can increase significantly the precursor frequency of the Ag-specific T cells compared with rV-CEA-TRICOM plus rF-GM-CSF vaccine. The cytolytic function is also 30-fold higher than rV-CEA-TRICOM plus rF-GM-CSF vaccination [10]. Therefore, it can be hypothesized that the high affinity T cells generated after the appropriate blocking of negative regulator using anti-CTLA-4 mAb could bring about the better antitumor response. Although the addition of CTLA-4 mAb has significantly (P = 0.0001) increased the therapeutic efficacy of the TRICOM vaccine, it did not have any effect on 40% of the mice administered the combination therapy of CTLA-4 mAb and TRICOM vaccine. This result probably could be improved by the addition of other modalities of tumor therapy such as sublethal radiation [21], intratumoral injection [39] and the inhibition of angiogenesis [40]. Those other modalities are known to initiate the antigenic cascade and change the microenvironment of the tumor.
CTLA-4 mAb administration in preclinical models has been shown to accelerate and exacerbate clinical signs of several T cell-mediated experimental autoimmune diseases [32], including autoimmune encephalomyelitis (EAE) [41], neuritis [42], diabetes [43] and a B cell-mediated autoimmune disease, experimental autoimmune myasthenia gravis (EAMG) [44]. A large percentage of mice cured of melanoma lesions by anti-CTLA-4 therapy developed vitiligo, starting at the site of vaccination and in most cases progressing to a distant location [5]. In addition, in a prostate cancer model, treatment with CTLA-4 resulted in marked prostatitis accompanied by the destruction of epithelium [6]. The underlying mechanism of action of CTLA-4 mAb in these animal models was the enhanced production of proinflammatory cytokines such as IL-2, IFN-γ, and TNF-α. In addition, CTLA-4 was reported to be essential for the peripheral tolerance of CD4+ T cells. These results are in contrast to those reported here, where antitumor therapy is associated with no autoimmune phenomenon. The disparity between the results reported here and those above could be attributable to the targeted tissue (prostate or melanoma vs. colon). In a clinical trial, T cell responses against cognate gp100 peptides were detected in vitro in all 11 patients with stage IV metastatic melanoma treated with humanized CTLA-4 antibody (MDX-010; Medarex Inc., Princeton, NJ, USA). In addition to objective tumor regression, 6 of 14 patients experienced grade III/IV autoimmunity [29]. Other than vitiligo, severe autoimmune pathologies were also demonstrable, including dermatitis, colitis and hepatitis. In contrast, the first clinical trial with fully humanized CTLA-4 mAb did not induce any symptoms of autoimmunity [45]. Thus, CTLA-4 mAb treatment could break peripheral tolerance to auto-antigens, leading to the induction of autoimmunity. In a Phase I trial using anti-CTLA-4 mAb, dose-related autoimmune events, predominantly skin and GI toxicities, were in evidence and patients also mounted an antigen-specific immune response [46]. In examining these two studies, the dosage of CTLA-4 antibody could be a major determinant in mounting autoimmunity. Hurwitz et al. [47] showed that the synergy of CTLA-4 blockade with GM-CSF induced enhancement of Ag presentation to Ag-specific T cells and converted an autoimmune response to a self-antigen into an antitumor response. In our study [10] we have also demonstrated that the addition of rF-GM-CSF can increase the Ag-specific CTL-mediated lysis 20-fold compared with rV-CEA-TRICOM along with CTLA-4 mAb. In our study, mice cured of tumor after receiving the CEA-TRICOM vaccine regimen along with rF-GM-CSF and CTLA-4 mAb showed no evidence of autoimmunity. Having used vaccines containing the B7-1 molecule, we can hypothesize that after CD28 engagement, CTLA-4 would rise faster and higher than if we used vaccines without B7-1. Thus, the role of anti-CTLA-4 mAb may be different for our vaccine compared with other vaccines used in other studies.
CTLA-4 blockade is emerging as an important immunological adjuvant in cancer immunotherapy, and will be tested in combination with a variety of vaccines in clinical trials over the next few years. In other preclinical and clinical trials, CTLA-4 antibody has been used either with peptide vaccination or DNA vaccination. Here, we have demonstrated that CTLA-4 mAb can be used with live viral vector-based vaccines containing costimulatory molecules and GM-CSF. These studies form a rational basis for the use of vector-based vaccines with anti-CTLA-4 and demonstrate that both enhancement of positive costimulatory signals and inhibition of negative costimulatory signals can be simultaneously exploited. These studies also underscore the importance of “drug” scheduling in vaccine combination therapies.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center of Cancer Research. The authors thank Dr. Scott Abrams and Dr. Connie Rogers for helpful review of this material and Debra Weingarten for her editorial assistance in the preparation of this paper.
References
- 1.Allison JP, Krummel MF. The Yin and Yang of T cell costimulation. Science. 1995;270(5238):932–933. doi: 10.1126/science.270.5238.932. [DOI] [PubMed] [Google Scholar]
- 2.Hilburger Ryan M, Abrams SI. Characterization of CD8+ cytotoxic T lymphocyte/tumor cell interactions reflecting recognition of an endogenously expressed murine wild-type p53 determinant. Cancer Immunol Immunother. 2001;49(11):603–612. doi: 10.1007/s002620000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 4.Yang YF, Zou JP, Mu J, Wijesuriya R, Ono S, Walunas T, et al. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997;57(18):4036–4041. [PubMed] [Google Scholar]
- 5.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190(3):355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60(9):2444–2448. [PubMed] [Google Scholar]
- 7.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 8.Engelhard VH, Bullock TN, Colella TA, Sheasley SL, Mullins DW. Antigens derived from melanocyte differentiation proteins: self-tolerance, autoimmunity, and use for cancer immunotherapy. Immunol Rev. 2002;188:136–146. doi: 10.1034/j.1600-065X.2002.18812.x. [DOI] [PubMed] [Google Scholar]
- 9.Guevara-Patino JA, Turk MJ, Wolchok JD, Houghton AN. Immunity to cancer through immune recognition of altered self: studies with melanoma. Adv Cancer Res. 2003;90:157–177. doi: 10.1016/S0065-230X(03)90005-4. [DOI] [PubMed] [Google Scholar]
- 10.Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, Schlom J. Multiple costimulatory modalities enhance CTL avidity. J Immunol. 2005;174(10):5994–6004. doi: 10.4049/jimmunol.174.10.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlom J, Tsang KY, Kantor JA, Abrams SI, Zaremba S, Greiner J, et al. Strategies in the development of recombinant vaccines for colon cancer. Semin Oncol. 1999;26(6):672–682. [PubMed] [Google Scholar]
- 12.Eades-Perner AM, van der Putten H, Hirth A, Thompson J, Neumaier M, von Kleist S, et al. Mice transgenic for the human carcinoembryonic antigen gene maintain its spatiotemporal expression pattern. Cancer Res. 1994;54(15):4169–4176. [PubMed] [Google Scholar]
- 13.Kass E, Schlom J, Thompson J, Guadagni F, Graziano P, Greiner JW. Induction of protective host immunity to carcinoembryonic antigen (CEA), a self-antigen in CEA transgenic mice, by immunizing with a recombinant vaccinia-CEA virus. Cancer Res. 1999;59(3):676–683. [PubMed] [Google Scholar]
- 14.Hodge JW, McLaughlin JP, Kantor JA, Schlom J. Diversified prime and boost protocols using recombinant vaccinia virus and recombinant non-replicating avian pox virus to enhance T-cell immunity and antitumor responses. Vaccine. 1997;15(6–7):759–768. doi: 10.1016/S0264-410X(96)00238-1. [DOI] [PubMed] [Google Scholar]
- 15.Hodge JW, Sabzevari H, Yafal AG, Gritz L, Lorenz MG, Schlom J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59(22):5800–5877. [PubMed] [Google Scholar]
- 16.Kass E, Panicali DL, Mazzara G, Schlom J, Greiner JW. Granulocyte/macrophage-colony stimulating factor produced by recombinant avian poxviruses enriches the regional lymph nodes with antigen-presenting cells and acts as an immunoadjuvant. Cancer Res. 2001;61(1):206–214. [PubMed] [Google Scholar]
- 17.Robbins PF, Kantor JA, Salgaller M, Hand PH, Fernsten PD, Schlom J. Transduction and expression of the human carcinoembryonic antigen gene in a murine colon carcinoma cell line. Cancer Res. 1991;51(14):3657–3662. [PubMed] [Google Scholar]
- 18.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182(2):459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantor J, Irvine K, Abrams S, Kaufman H, DiPietro J, Schlom J. Antitumor activity and immune responses induced by a recombinant carcinoembryonic antigen-vaccinia virus vaccine. J Natl Cancer Inst. 1992;84(14):1084–1091. doi: 10.1093/jnci/84.14.1084. [DOI] [PubMed] [Google Scholar]
- 20.Bergmann-Leitner ES, Abrams SI. Treatment of human colon carcinoma cell lines with anti-neoplastic agents enhances their lytic sensitivity to antigen-specific CD8+ cytotoxic T lymphocytes. Cancer Immunol Immunother. 2001;50(9):445–455. doi: 10.1007/s002620100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64(12):4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 22.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, et al. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201(10):1591–1602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmberg K, Mariathasan S, Ohteki T, Ohashi PS, Gascoigne NR. TCR binding kinetics measured with MHC class I tetramers reveal a positive selecting peptide with relatively high affinity for TCR. J Immunol. 2003;171(5):2427–2434. doi: 10.4049/jimmunol.171.5.2427. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Bronte V, Chen PW, Gritz L, Panicali D, Rosenberg SA, et al. Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen. J Immunol. 1995;154(9):4685–4692. [PMC free article] [PubMed] [Google Scholar]
- 25.Aarts WM, Schlom J, Hodge JW. Vector-based vaccine/cytokine combination therapy to enhance induction of immune responses to a self-antigen and antitumor activity. Cancer Res. 2002;62(20):5770–5777. [PubMed] [Google Scholar]
- 26.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3(7):611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 27.van Elsas A, Sutmuller RP, Hurwitz AA, Ziskin J, Villasenor J, Medema JP, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194(4):481–489. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100(14):8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phan GQ, Touloukian CE, Yang JC, Restifo NP, Sherry RM, Hwu P, et al. Immunization of patients with metastatic melanoma using both class I- and class II-restricted peptides from melanoma-associated antigens. J Immunother. 2003;26(4):349–356. doi: 10.1097/00002371-200307000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16(1):23–35. doi: 10.1016/S1074-7613(01)00259-X. [DOI] [PubMed] [Google Scholar]
- 31.Espenschied J, Lamont J, Longmate J, Pendas S, Wang Z, Diamond DJ, et al. CTLA-4 blockade enhances the therapeutic effect of an attenuated poxvirus vaccine targeting p53 in an established murine tumor model. J Immunol. 2003;170(6):3401–3407. doi: 10.4049/jimmunol.170.6.3401. [DOI] [PubMed] [Google Scholar]
- 32.Gregor PD, Wolchok JD, Ferrone CR, Buchinshky H, Guevara-Patino JA, Perales MA, et al. CTLA-4 blockade in combination with xenogeneic DNA vaccines enhances T-cell responses, tumor immunity and autoimmunity to self antigens in animal and cellular model systems. Vaccine. 2004;22(13–14):1700–1708. doi: 10.1016/j.vaccine.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 33.Davila E, Kennedy R, Celis E. Generation of antitumor immunity by cytotoxic T lymphocyte epitope peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4 blockade. Cancer Res. 2003;63(12):3281–3288. [PubMed] [Google Scholar]
- 34.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194(6):823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernandez J, Ko A, Sherman LA. CTLA-4 blockade enhances the CTL responses to the p53 self-tumor antigen. J Immunol. 2001;166(6):3908–3914. doi: 10.4049/jimmunol.166.6.3908. [DOI] [PubMed] [Google Scholar]
- 36.Hurwitz AA, Yu TF, Leach DR, Allison JP. CTLA-4 blockade synergies with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci USA. 1998;95(17):10067–10071. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allison JP, Chambers C, Hurwitz A, Sullivan T, Boitel B, Fournier S, et al. A role for CTLA-4-mediated inhibitory signals in peripheral T cell tolerance? Novartis Found Symp. 1998;215:92–8. doi: 10.1002/9780470515525.ch7. [DOI] [PubMed] [Google Scholar]
- 38.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192(2):295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kudo-Saito C, Schlom J, Hodge JW. Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clin Cancer Res. 2005;11(6):2416–2426. doi: 10.1158/1078-0432.CCR-04-1380. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen AE, Buus S, Claesson MH. Treatment of transplanted CT26 tumour with dendritic cell vaccine in combination with blockade of vascular endothelial growth factor receptor 2 and CTLA-4. Cancer Lett. 2005;235(2):229–238. doi: 10.1016/j.canlet.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Perrin PJ, Maldonado JH, Davis TA, June CH, Racke MK. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J Immunol. 1996;157(4):1333–1336. [PubMed] [Google Scholar]
- 42.Zhu J, Zou L, Zhu S, Mix E, Shi F, Wang H, et al. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade enhances incidence and severity of experimental autoimmune neuritis in resistant mice. J Neuroimmunol. 2001;115(1–2):111–117. doi: 10.1016/S0165-5728(01)00255-7. [DOI] [PubMed] [Google Scholar]
- 43.Luhder F, Hoglund P, Allison JP, Benoist C, Mathis D. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med. 1998;187(3):427–432. doi: 10.1084/jem.187.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang HB, Shi FD, Li H, Chambers BJ, Link H, Ljunggren HG. Anti-CTLA-4 antibody treatment triggers determinant spreading and enhances murine myasthenia gravis. J Immunol. 2001;166(10):6430–6436. doi: 10.4049/jimmunol.166.10.6430. [DOI] [PubMed] [Google Scholar]
- 45.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100(8):4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanderson K, Scotland R, Lee P, Liu D, Groshen S, Snively J, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23(4):741–750. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 47.Ji Q, Gondek D, Hurwitz AA. Provision of granulocyte-macrophage colony-stimulating factor converts an autoimmune response to a self-antigen into an antitumor response. J Immunol. 2005;175(3):1456–1463. doi: 10.4049/jimmunol.175.3.1456. [DOI] [PubMed] [Google Scholar]