Abstract
Purpose: As prognosis of advanced pancreatic cancer remains gloomy, novel therapeutic modalities have to be developed. Immunotherapy, which targets tumor-associated antigens of tumor cells or tumor stroma, is currently under investigation. As survivin is expressed by neoplastic and tumor endothelial cells, but rarely by normal cells, this antigen appears as an intriguing target molecule. Methods: A 72-year old patient, suffering from pancreatic cancer refractory to gemcitabine therapy, received the survivin-based peptide vaccinations consisting of 100 μg of a modified HLA-A2 restricted survivin96–104 epitope in Montanide®. Each visit the patient was assessed for adverse events, quality of life and immunological response. Immunemonitoring was performed by IFN-γ-ELISPOT analysis of peripheral blood lymphocytes. Clinical outcome was evaluated by repetitive computed tomography. Results: Under vaccination with survivin peptides the patient initially underwent partial remission of liver metastasis which proceeded after 6 months into a complete remission with a duration of 8 months. Immunological monitoring revealed strong vaccine-induced immune-reactivity against survivin. Unfortunately, after the patient was weaned from vaccination in state of no evidence of disease, he developed recurrent disease. Conclusion: T-cell responses against survivin-expressing cells of the tumor itself and tumor endothelium should impact tumor growth and metastasis. The presented patient with pancreatic cancer is the first example of a successful application of a survivin-based vaccination in the clinical setting. An ongoing phase I/II trial with HLA-A1, -A2 and -B35 restricted survivin peptides for patients with advanced cancer will provide further information towards this notion.
Keywords: Pancreatic cancer, Tumor angiogenesis, Survivin, Peptide vaccination
Introduction
The treatment of pancreatic carcinoma, especially at an advanced stage, remains a great challenge. Despite improvement in clinical management, this malignancy is rarely curable. Standard chemotherapeutic regimens with gemcitabine, 5-fluorouracil or irinotecan achieve an objective response rate between 0 and 30%, but rarely longlasting complete responses, and the overall 5-year survival rate is less than 5% [9]. We present here a patient with advanced pancreatic cancer who achieved first a partial response for 6 months followed by a complete remission which was maintained for 8 months under immunotherapy with HLA-A2 restricted survivin peptides together with the adjuvant Montanide. To our knowledge, this is the first demonstration that the induction of an immune response against the universal tumor antigen survivin is successful in fighting human cancer in the clinical setting.
Case report
A 72-year old man presented to a surgical unit with progressive weight loss during the last few months and lack of appetite. The patient had a long history of alcohol abuse with subsequent chronic pancreatitis persisting for several years with no fever and no night sweats.
On physical examination the abdomen was tender with normal bowel sounds but moderate painful in the upper abdomen. General examination disclosed no further abnormalities. Laboratory testing showed an elevation of the cholestatic parameters, i.e., alkaline phosphatase to 1,107 U l−1, γ-glutamine transferase to 476 U l−1 but normal level of total bilirubin. Further routine laboratory testing revealed results within normal limits. Striking, however, was a nearly threefold elevation of the tumor markers CEA (8.4 μg l−1) and CA 19-9 (122 U l−1). Computed tomography (CT) scans of the abdomen showed a cystic mass in projection on the head of the pancreas and a dilated intra- and extrahepatic biliary tract. This cystic tumor of the pancreas was subsequently confirmed by an esophagogastroduodenoscopy and a MRCP. As pancreatic cancer was suspected, the patient underwent a Whipple operation. Histopathological examination of the specimen revealed a low grade mucinous adenocarcinoma of the pancreas with total destruction of the papilla and invasion of the common bile duct as well as the adjacent duodenal wall (Fig. 1a). No lymph node metastases in 12 analyzed gastric and pancreatic nodes were detected. Due to the extensive and invasive growth of the tumor, only a R2-resection was achieved, with macroscopic tumor residues in the dorsal part of the excised tissue. Additional CT scans of the chest and the abdomen did not show any metastases; hence the tumor was classified as T3 N0 M0 G1 R2.
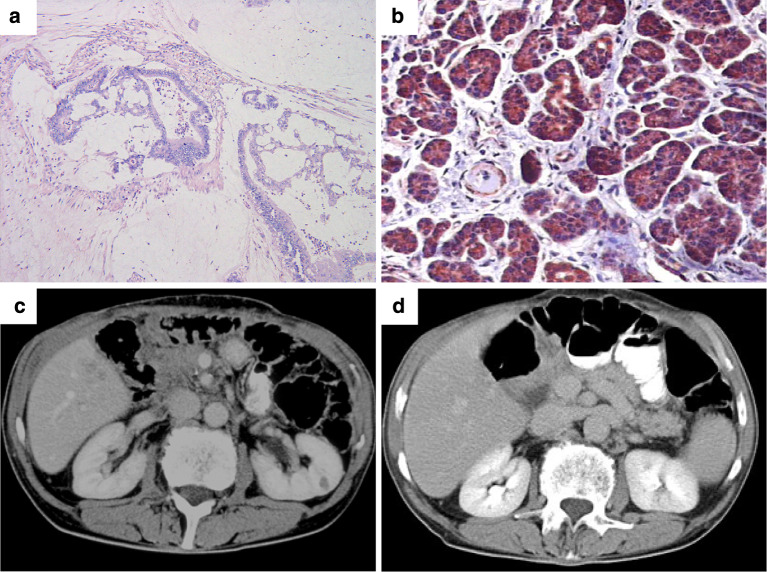
Fig. 1.
a Hematoxylin–eosin stain, showing a diffuse infiltration of a mucinous carcinoma. b Immunhistochemical stain of survivin-expressing cells in pancreatic carcinoma. Positive are neoplastic and endothelial cells. c Helical dual-phase, contrast-enhanced CT-scans of the abdomen. Marginal hypodense focus in the liver with enhancement in the early portovenous contrast medium phase. d No remaining sign of liver metastasis
After an uncomplicated postoperative recovery of 2 months an adjuvant monochemotherapy with gemcitabine (1,000 mg m−2 of body surface) at day 1, 8, 15 was initiated. After 1 year of therapy with a total of 12 cycles gemcitabine, CT scans of the abdomen revealed a newly developed 2.5×1.5 cm2 large, hypodense lesion in segment 5 of the liver suspicious for hepatic metastasis (Fig. 1c). At the primary tumor site, no signs of recurrent disease was detected despite the fact that initially only a R2-resection was possible.
Since no established standard therapeutic regimens exist for this condition, the patient was offered participation in an experimental trial. This study evaluates the therapeutic impact on clinical outcome of a peptide vaccination with HLA-A1, -A2 and -B35 restricted epitopes derived from the universal tumor antigen survivin against solid tumors expressing survivin (Fig. 1b). HLA-typing of the patient revealed an HLA-A2 phenotype and the patient was enroled upon informed consent.
The vaccine, i.e., 100 μg of a modified HLA-A2 restricted survivin96–104 peptide emulgated in Montanide® ISA-51 (Seppic, France), was administered as a deep subcutaneous injection alternately into the left and right thigh in time intervals of 1, 3 and finally 4, 12 and 24 weeks. Toxicity was monitored by direct questioning, clinical examination and blood analysis. No major side effects were observed and a good performance status was maintained for 1 year of ongoing vaccinations. The only side effect of the vaccine was a red, slightly painful induration at the vaccination site, e.g., a sign of local, immunological reaction (Fig. 2a), which corresponded to NCI CTC toxicity grade 1–2. This reaction was accompanied by a distinct, indolent lymph node swelling in the respective groins. No abnormal laboratory testing was observed.
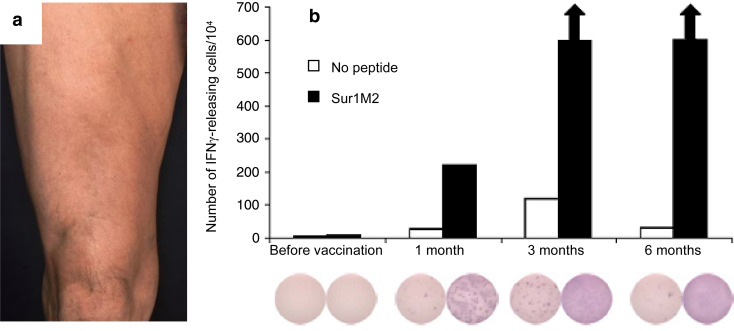
Fig. 2.
a Vaccination site of the right thigh. Erythematous, palpable, slightly painful indurations without local hyperthermia. b Immunological monitoring during vaccination therapy with survivin peptide. The IFNγ-ELISPOT analysis of peripheral blood lymphocytes (PBL) shows a significant increase in survivin reactive cells over the time of 6 months
After four vaccinations the first tumor staging was performed. CT scans of the chest and the abdomen showed an unchanged, solitary liver metastasis (2.5×1.5 cm2) without any sign of further disease progression. Three months later, after six vaccinations, the hepatic metastasis had slightly regressed to a size of 2.0×1.5 cm2 as seen in helical, dual-phase contrast-enhanced CT scans of the abdomen. After a total of 9 months of ongoing vaccinations only residual hepatic liver metastases, corresponding to partial remission, were documented. Moreover, normal values of tumor markers (CEA 2.4 μg l−1 and CA 19-9 4.4 U l−1) and routine laboratory testing were obtained at this time. Histological confirmation of the hepatic metastases, however, was not obtained since the risk of the necessary procedure outweighed the possible gain for the patient. The diagnosis of progressive and regressive disease was therefore based on clinical and laboratory findings, i.e., normalization of elevated tumor markers, as well as a thorough radiological workup which was confirmed by two independent radiologists. They particularly took into account, that solitary liver metastases of pancreatic carcinoma are not common but more often occur in multiple locations. Notably, after 14 months since vaccination start, no focal intrahepatic lesions and no sign of local tumor recurrence could be found in sectional images (Fig. 1d). At this time of complete remission the patient was weaned from immunological therapy, i.e., vaccination intervals were increased to finally 6 months. Parallel to radiological and clinical staging examinations, immunological monitoring by IFNγ-ELISPOT analysis of peripheral blood lymphocytes was performed. Notably, the objective clinical response to vaccination correlated with a significant increase in survivin reactive T-lymphocytes (Fig. 2b).
The patient remained with no evidence of disease over a time period of 8 months of ongoing vaccinations. He developed, however, a reduced performance status due to a chronic, painful spondylodiscitis. After being hospitalized for a complicated pneumothorax and peripheral lung embolism, recurrent disease with newly emerged liver metastases was diagnosed on radiological images. Before frequent survivin peptide vaccinations could be reinitiated to re-boost the immunological responses, the patient developed a pneumonia and died from global respiratory failure. For ethical reasons we renounced to obtain additional blood for immunological monitoring at the time of deterioration.
Discussion
Immunotherapy of cancer is currently at its crossroads [15]. The lack of clinical efficacy, however, may not necessarily be due to the inefficiency of the immune system itself, but to the wrong targets it has been directed to attack [7]. We recently hypothesized that inhibitors of apoptosis may be more suitable targets for immunotherapy [2]. In this respect, the potential of survivin had been scrutinized by us and others. Survivin is a structurally unique member of the inhibitor of apoptosis protein (IAP) family, which is highly upregulated in most human cancers, e.g., pancreatic carcinoma [16], and correlates with an unfavorable clinical outcome [8]. It both inhibits apoptosis and controls cell division [6]. It should be noted that survivin is expressed both by the neoplastic cells themselves and by the tumor endothelial cells which build up the essential vascular network (Fig. 1b) [18]. With few exceptions, including activated endothelial cells in response to injury, survivin is undetectable in differentiated adult tissues [1].
Targeting survivin is a promising approach to treat cancer [3]. Inhibiting survivin or other members of the IAP-family both radiosensitizes cancer cells and facilitates the cytotoxic activity of chemotherapeutic agents [11]. Abrogating the function of survivin does not only limit the proliferative potential and viability of tumor cells directly [2], but also inhibits tumor angiogenesis [10]. As survivin is preferentially expressed in tumor versus normal tissue, adverse effects on normal, differentiated cells are unlikely [12]. Indeed, the presented patient did not develop any adverse events related to immunotherapy other than a local reaction at the vaccination site, despite induction of impressive immune reactivity against the survivin peptide as measured by IFNγ-ELISPOT.
Over the past few years it has become obvious that cancer immunotherapies designed for direct attacks on tumor cells have their limitation due to the heterogeneity and instability of the transformed cells as well as the complex, modulating interaction between neoplastic and stromal cells. Since the pioneering work of Folkman [5] in 1971, the essential role of angiogenesis for tumor growth and distant spread has become a central focus of basic and translational cancer research. To this end, we recently demonstrated in a murine model that targeting survivin by an immunological approach leads to eradication of pulmonary metastases by a combinational effect of inducing tumor cell apoptosis and suppressing tumor angiogenesis [19]. The synergistic effects of targeting both neoplastic and endothelial cells may explain the remission of hepatic metastasis of pancreatic carcinoma after vaccination with a HLA-A2 restricted survivin peptide. Liver metastases of pancreatic carcinoma are often highly vascularized and vascular density correlates with prognosis [17]. Several clinical trials have uncovered the impact of antiangiogenic strategies on growth control of pancreatic cancer, with a significantly stronger effect if combined with chemo- or radiotherapy.
The strong immunological response against survivin peptides at the time of tumor response underscores, that the observed tumor regression was indeed by vaccination [14]. In this respect, we have recently demonstrated that in sanguis and in situ analysis of vaccine-induced immune responses may be correlated to a larger extent than initially anticipated [4]. Moreover, the effect of cessation of therapy, albeit unfortunate, underscores the effect of the vaccination. When vaccination was weaned in a state of no evidence of disease, disease recurred with newly developed liver metastasis and the patient deteriorated rapidly. This relapse may be attributed to a diminished reactivity of CD8+ lymphocytes against survivin due to vaccination weaning [14]. However, due to the rapid deterioration of the patient’s condition at time of tumor relapse we did not obtain material for further immunological monitoring because of ethical reasons. Thus, other immune-escape mechanisms, such as loss of survivin or MHC class I expression, have to be taken into account as well [13].
This case report of a complete remission of metastasized pancreatic carcinoma may very well be regarded as an anecdotal spontaneous response. However, even “if one swallow does not make a summer”, the clinical response together with the vaccination-induced immunological response suggests that targeting cells which support tumor growth in combination with a direct attack of neoplastic cells is a promising approach to treat solid tumors and worth further testing. Indeed, an ongoing clinical trial using a survivin vaccine as a second line therapy will provide additional information (http://www.clinicaltrials.gov).
Materials and methods
Vaccine
The vaccine consists of 100 μg of a modified HLA-A2 survivin96–104 epitope (LMLGEFLKL) in Montanide® ISA-51. The patient received the vaccination as a deep subcutaneous injection every month.
Immunemonitoring
IFN-γ-ELISPOT analysis of peripheral blood lymphocytes, obtained at different time points during ongoing vaccination, was performed according to standard procedures as described elsewhere [12].
Acknowledgments
The authors are grateful to Prof. Dr. S. Delorme, Department of Radiology, University of Heidelberg, Germany, for his critical review of the radiological images. We are further indebted to Marianne Berwick and Per thor Straten for helpful discussions during preparation of the manuscript as well as Brigitte Bauer for her excellent data managing. The clinical trial was supported in part by the Deutsche Forschungsgemeinschaft (DFG) grant KFO-124/1-1.
Footnotes
The clinical trial was supported in part by the Deutsche Forschungsgemeinschaft (DFG) grant KFO-124/1-1. The clinical trial was approved by the local ethical committee and informed consent was obtained from each patient. Human investigations were performed in accordance with an assurance filed with and approved by the Department of Health and Human Services.
References
- 1.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 2.Andersen MH, Becker JC, Straten PT. Regulators of apoptosis: suitable targets for immune therapy of cancer. Nat Rev Drug Discov. 2005;4:399–409. doi: 10.1038/nrd1717. [DOI] [PubMed] [Google Scholar]
- 3.Andersen MH, Pedersen LO, Capeller B, Brocker EB, Becker JC, Straten PT. Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res. 2001;61:5964–5968. [PubMed] [Google Scholar]
- 4.Berger TG, Haendle I, Schrama D, Luftl M, Bauer N, Pedersen LO, Schuler-Thurner B, Hohenberger W, Straten PT, Schuler G, Becker JC. Circulation and homing of melanoma-reactive T cells to both cutaneous and visceral metastases after vaccination with monocyte-derived dendritic cells. Int J Cancer. 2004;20(111):229–237. doi: 10.1002/ijc.20238. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197108122850711. [DOI] [PubMed] [Google Scholar]
- 6.Hirokawa N. Microtubule organization and dynamics dependent on microtubule-associated proteins. Curr OpinCell Biol. 1994;6:74–81. doi: 10.1016/0955-0674(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 7.Hofmeister V, Vetter C, Schrama D, Brocker EB, Becker JC (2005) Tumor stroma-associated antigens for anti-cancer immunotherapy. Cancer Immunol Immunother pp 1–14 [DOI] [PMC free article] [PubMed]
- 8.Islam A, Kageyama H, Takada N, Kawamoto T, Takayasu H, Isogai E, Ohira M, Hashizume K, Kobayashi H, Kaneko Y, Nakagawara A. High expression of Survivin, mapped to 17q25, is significantly associated with poor prognostic factors and promotes cell survival in human neuroblastoma. Oncogene. 2000;19:617–623. doi: 10.1038/sj.onc.1203358. [DOI] [PubMed] [Google Scholar]
- 9.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 10.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 11.Lu B, Mu Y, Cao C, Zeng F, Schneider S, Tan J, Price J, Chen J, Freeman M, Hallahan DE. Survivin as a therapeutic target for radiation sensitization in lung cancer. Cancer Res. 2004;64:2840–2845. doi: 10.1158/0008-5472.CAN-03-3547. [DOI] [PubMed] [Google Scholar]
- 12.Otto K, Andersen MH, Eggert A, Keikavoussi P, Pedersen LO, Rath JC, Bock M, Brocker EB, Straten PT, Kampgen E, Becker JC. Lack of toxicity of therapy-induced T cell responses against the universal tumour antigen survivin. Vaccine. 2005;23:884–889. doi: 10.1016/j.vaccine.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Pawelec G. Tumour escape from the immune response. Cancer Immunol Immunother. 2004;53:843. doi: 10.1007/s00262-004-0531-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez-Diez A, Spiess PJ, Restifo NP, Matzinger P, Marincola FM. Intensity of the vaccine-elicited immune response determines tumor clearance. J Immunol. 2002;168:338–347. doi: 10.4049/jimmunol.168.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg SA. Shedding light on immunotherapy for cancer. N Engl J Med. 2004;350:1461–1463. doi: 10.1056/NEJMcibr045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92:271–278. doi: 10.1002/1097-0142(20010715)92:2<271::AID-CNCR1319>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Shishido T, Yasoshima T, Denno R, Mukaiya M, Sato N, Hirata K. Inhibition of liver metastasis of human pancreatic carcinoma by angiogenesis inhibitor TNP-470 in combination with cisplatin. Jpn J Cancer Res. 1998;89:963–969. doi: 10.1111/j.1349-7006.1998.tb00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran J, Rak J, Sheehan C, Saibil SD, LaCasse E, Korneluk RG, Kerbel RS. Marked induction of the IAP family antiapoptotic proteins survivin and XIAP by VEGF in vascular endothelial cells. Biochem Biophys Res Commun. 1999;264:781–788. doi: 10.1006/bbrc.1999.1589. [DOI] [PubMed] [Google Scholar]
- 19.Xiang R, Mizutani N, Luo Y, Chiodoni C, Zhou H, Mizutani M, Ba Y, Becker JC, Reisfeld RA. A DNA vaccine targeting survivin combines apoptosis with suppression of angiogenesis in lung tumor eradication. Cancer Res. 2005;65:553–561. [PubMed] [Google Scholar]