Abstract
MuS110 is a BiTE antibody bispecific for murine EpCAM (CD326) and murine CD3. A recent study has shown that muS110 has significant anti tumor activity at well-tolerated doses as low as 5 μg/kg in orthotopic breast and lung cancer models (Amann et al. in Cancer Res 68:143–151, 2008). Here, we have explored the safety profile of muS110 at higher doses. Escalation to 50 μg/kg muS110 caused in mice transient loss of body weight, and transient piloerection, hypomotility, hypothermia and diarrhoea. These clinical signs coincided with serum peaks of TNF-α, IL-6, IL-2, IFN-γ and IL-4, and an increase of surface markers for T cell activation. Because activation of T cells in response to BiTE antibodies is typically dependent on target cells, we analyzed mouse blood for the presence of EpCAM+ cells. Various mouse strains presented with a subpopulation of 2–3% EpCAM+ blood cells, mostly B and T lymphocytes, which was not detected in human blood samples. In vitro experiments in which the number of EpCAM+ cells in blood samples was either reduced or increased suggested that both T cell activation and cytokine release in response to muS110 was dependent on the number of target-expressing cells. In support for a role of EpCAM+ lymphocytes in the observed side effects, reduction of EpCAM+ blood cells in mice via a low-dose pre treatment with muS110 dramatically increased the tolerability of animals up to at least 500 μg/kg of the BiTE antibody. This high tolerability to muS110 occurred in the presence of non-compromised T cells. No damage to EpCAM+ epithelial tissues was evident from histopathological examination of animals daily injected with 100 μg/kg muS110 for 28 days. In summary, these observations suggest that side effects of muS110 in mice were largely caused by an acute T cell activation that was triggered by a subpopulation of EpCAM+ lymphocytes. Because humans have extremely low numbers of EpCAM+ cells in blood, this toxicity of an EpCAM-specific BiTE may be specific for mice.
Keywords: Chinese Hamster Ovarian Cell, Cytokine Release, Cytometric Bead Array, EpCAM Expression, Activation Marker CD69
Introduction
Targeted therapies are beginning to revolutionize the treatment of malignant diseases. One approach is recombinant monoclonal antibodies targeting cell surface antigens of tumor cells, or soluble factors required for tumor growth. Antibodies rituximab, trastuzumab, bevacizumab and cetuximab have advanced to become a standard of care in non-Hodgkin lymphoma, HER-2-positive breast cancer and colon carcinoma, respectively [42]. Antibody-based therapies for treatment of cancer patients are typically combined with conventional therapies that support their action by debulking tumor mass, sensitization for apoptosis, or increasing antibody access to tumor cells. Numerous approaches are being explored that aim at increasing the efficacy of tumor cell-binding antibodies. For instance, antibodies are being conjugated with toxic payloads such as radioisotopes, bacterial or plant toxins, pro-drugs, and chemotherapies [17, 31]. Alternatively, the antibody’s Fc portions are being mutated for increased performance of antibody-dependent cellular cytotoxicity (ADCC) [17, 21].
Another promising approach to increase the potency of antibody-based therapeutics is the conjugation of antibodies with a second binding specificity for recognition of cytotoxic T cells [29, 44]. This way, antibodies can engage the most potent killer cells of the human organism. Ideally, by such T cell-recruiting, bispecific antibodies transiently connect a tumor cell with a resting T cell leading to formation of a proper cytolytic synapse, delivery of granzymes and perforin into target cells, and serial lysis of target cells by newly activated T cells. This should occur independently of T cell receptor specificity, antigen presentation and costimulation. One class of bispecific T cell engaging antibodies that fulfil these criteria are called BiTE antibodies [6, 47]. BiTE antibodies consist of two flexibly linked single-chain antibodies––one specific for the invariant CD3 complex of T cell receptors, the other one for a selected surface antigen on cancer cells. A first BiTE antibody specific for CD19, called MT103/MEDI-538, has shown high potency and clinical activity with confirmed complete and partial responses in therapy-refractory non-Hodgkin lymphoma patients [8, 9]. A second BiTE antibody called MT110 [15], which is targeting epithelial cell adhesion molecule (EpCAM, or CD326), has started clinical testing in late-stage lung, gastric and colorectal cancer patients. MT110 and other EpCAM-specific BiTE antibodies have shown high activity against human xenografts in various mouse models [15, 39, 40].
EpCAM is not only a very frequently and highly expressed tumor-associated antigen of most human adenocarcinoma [7, 45], but is also expressed on a variety of normal epithelial tissues, including pancreas, lung, bile ducts and intestine [4, 23, 34]. In order to assess whether BiTE antibodies can distinguish between EpCAM expressed on tumor and normal tissues, we generated a murine EpCAM/murine CD3-bispecific BiTE antibody called muS110, which is similar to MT110 in terms of structure, binding affinities, in vitro cytotoxic activity, and in vivo efficacy [4]. MuS110 has shown significant anti-tumor activity in both an orthotopic breast cancer and lung metastasis mouse model at well tolerated daily intravenous (i.v.) doses of as low as 2 μg/kg/day. EpCAM expression on human and mouse tissues was found to be largely overlapping. Here, we investigated i.v. doses of muS110 exceeding those needed for efficacy in order to explore potential side effects and dose-limiting toxicities of muS110. In theory, these can be either related to overt T cell activation, damage to EpCAM-expressing normal tissues by redirected T cells, or to both.
Here we report that the dose-limiting toxicities of muS110 are related to effects of transiently released pro-inflammatory cytokines, which are reminiscent of those seen with anti-CD3 monoclonal antibodies in mice. Both, in vitro and in vivo experiments suggest that T cell activation in response to bolus i.v. infusion of muS110 is dependent on the presence of a subpopulation of EpCAM-expressing T and B lymphocytes. Upon depletion of this cell population by a low-dose pre treatment with the BiTE antibody, the tolerability of muS110 in mice was dramatically increased and, in no instance, damage to EpCAM-expressing normal epithelial tissues of mice was observed. Of note, EpCAM+ lymphocytes were not found in human blood samples, indicating that side effects seen in mice may have been species-specific.
Materials and methods
Cell lines and cultivation
Kato III gastric carcinoma cells were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany) and were grown in RPMI (Invitrogen, Gaithersburg, MD) supplemented with 10% fetal calf serum (Invitrogen, Gaithersburg, MD). DHFR deficient Chinese hamster ovarian (CHO) cells and CTLL-2 cytotoxic T lymphocytes were purchased from the American Type Cell Culture Collection (ATCC, Manassas, USA). DHFR deficient CHO cells were transfected with a plasmid encoding murine EpCAM. CHO/muEpCAM cells were grown in suspension with HyQ medium (HyClone, Logan, USA), supplemented with 20 nM methotrexate (Sigma-Aldrich, Steinheim, Germany) for selection. CTLL-2 cells were grown in ISCOVE medium (Biochrom AG, Berlin, Germany) supplemented with 10% fetal calf serum (Invitrogen, Gaithersburg, MD), 0.1 mg/l penicillin/ streptomycin (Sigma-Aldrich, Steinheim, Germany), 50 nM 2-mercaptoethanol (Invitrogen, Gaithersburg, MD) and 5.5 ng/l proleukin (Chiron Corp. Lim, Uxbridge, UK). All in vitro assays were performed in RPMI (Invitrogen, Gaithersburg, MD, USA) supplemented with 10% fetal calf serum (Invitrogen, Gaithersburg, MD, USA).
Construction and production of MT110, muS110 and control BiTE antibody
MT110 was engineered by recombinant DNA technology using an antibody specific for human EpCAM (5-10) and human CD3 (diL2K) [15]. To generate muS110, an antibody specific for murine CD3 (mCD3-1) and an antibody specific for murine EpCAM (G8.8) were used. To generate the control BiTE antibody, antibodies specific for murine CD3 (mCD3-1) and human EpCAM (5–10) were used. The hybridoma-producing rat α-murine EpCAM monoclonal antibody (mAB) (G8.8) was obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD and maintained by the Department of Biological Sciences (University of Iowa, Iowa City, IA, USA). The murine variable domains recognizing human EpCAM (scFv, 5–10) were obtained by phage display. Human T helper cell epitopes and MHC class II anchor amino acids were removed from diL2K antibody resulting in a deimmunized antibody (diL2K) [38]. Sequences encoding the variable domains of the respective antibodies were amplified and modified as described [32]. Expression plasmids were transfected into DHFR-deficient CHO cells and protein expression was performed as described by Kaufman et al. [27]. MT110 was purified from cell culture supernatants as described earlier [30].
Antibodies
Staining of cell surface markers was carried out using directly conjugated mABs against human EpCAM (EBA-1), human CD4 (SK3), human CD8 (SK1), human CD19 (HD37; Dako, Hamburg, Germany), human CD25 (CD25-3G10; Invitrogen, Gaithersburg, MD), murine CD3 (145-2C11), murine CD4 (H129.19), murine CD8 (53–6.7), murine CD69 (H1.2F3), murine CD25 (3C7), murine CD45 (30-F11), murine CD19 (1D3), murine EpCAM (G8.8), murine CD49b (DX5) and murine Ly6G (RB6-8C5). Directly conjugated non-specific rat IgG2a (R35-95) or mouse IgG1 (MOPC-31C) mABs were used as isotype controls. All antibodies were obtained from BD Bioscience, Heidelberg, Germany, if not otherwise stated.
Mouse strains and animal studies
In vivo experiments were performed in 6–12-week old BALB/c or C57BL/6 mice bred in the Institute for Immunology (Munich, Germany). Evaluation of EpCAM+ lymphocyte numbers was carried out with 8-week old female and male BALB/c, C57BL/6 N, C3H/HEN and CD-1 mice obtained from Charles River (Sulzfeld, Germany). All experiments were performed in accordance to the German Animal Protection Law with permission from the local authorities.
Pharmacokinetic and toxicological analysis was contracted Huntingdon Life Sciences Ltd (Alconbury, Huntingdon, Cambridgeshire, UK). Female BALB/c mice muS110 received by intravenous injection either muS110 (5, 15 and 50 μg/kg), or control BiTE antibody (500 μg/kg). Body temperature and overall appearance of mice were assessed before and 0.5, 1, 2, 4, 6, 8, 10 and 24 h after BiTE injection. At each time point, blood samples were drawn from four animals and processed to serum for pharmacokinetic analysis of muS110 and determination of serum cytokine levels.
For in vivo depletion of EpCAM+ lymphocytes, female BALB/c mice received by intravenous injection 10 μg/kg/day muS110 for seven consecutive days. Control animals received a control BiTE antibody instead. Depleted and control animals were challenged thereafter by daily intravenous injection of muS110 at 100, 300 and 500 μg/kg for up to 10 days. Body temperature and overall appearance of mice was assessed before and 1, 2, 4, 8 and 24 hours after injection of high BiTE doses.
Histological and pathological studies were contracted to Huntingdon Life Sciences Ltd (Alconbury, Huntingdon, Cambridgeshire, UK). Sixteen male and 16 female BALB/c mice per group received intravenous injection of 10 μg/kg/day muS110 or vehicle for 7 days followed by 100 μg/kg/day muS110 or vehicle for 28 days.
Determination of muS110-mediated T cell activation in vivo
T cell activation in vivo was analyzed by dissection of mesenteric lymph nodes before and 1, 2, 4, 10, 24 h after BiTE antibody administration [40]. Isolated cells were stained with fluorescent conjugated α-murine CD4, α-murine CD8 and α-murine CD69 or α-murine CD25 mABs and expression of CD69 or CD25 was monitored by flow cytometry.
Determination of muS110 serum concentrations
MuS110 serum concentrations were quantified by a bioassay measuring the TDA release of BATDA-labeled target cells lysed through muS110 redirected T cells. According to manufacturers instructions exponentially growing CHO/muEpCAM cells were labeled with the DELFIA® BATDA reagent (Wallac Oy, Turku, Finland). Serum samples, BATDA-labeled target cells (2.5 × 103/well) and CTLL-2 effector cells (1.5 × 105/well) were incubated together to allow redirected lysis. The supernatant containing TDA released by dead cells was incubated with DELFIA® Europium solution (Wallac Oy, Turku, Finland) to allow stable formation of a highly fluorescent Europium-TDA chelate (EuTDA). The amount of EuTDA was quantified by analysis of the relative fluorescence intensity (FLsample) at 520 nm with a Victor Multilabel Counter (Wallac Oy, Turku, Finland). Fluorescence intensity of background lysis (FLBlank) was determined in a sample without muS110. Fluorescence intensity of maximal lysis (FLmax) was determined using DELFIA® lysis buffer (Wallac Oy, Turku, Finland) according to manufactures instructions. The percentage of specific cell lysis was calculated as 100 × (FLsample − FLBlank)/(FLmax − FLblank). Standard samples were generated by a fourfold serial dilution of muS110 (2 μg/ml–7.6 pg/ml) and specific cell lysis was plotted against muS110 concentrations. The limit of quantification was determined as 1 ng/ml.
Pharmacokinetic analysis
Pharmacokinetic calculations of muS110 were performed by the pharmacokinetic software package WinNonlin Professional 4.1 (Pharsight Corporation, Mountain View, CA). Parameters were determined by non-compartmental analysis based on model 201 (intravenous bolus injection). The distribution half-life (t1/2 alpha) was calculated using a log-linear regression of the first three sampling time points, whereas the terminal elimination half-life (t1/2 beta) was calculated by the last five sampling time points with a measurable concentration of muS110.
Determination of blood cell numbers
Female C57BL/6 mice were intravenously injected with 15 μg/kg muS110 or control BiTE antibody and blood was collected from animals before and 2, 4, 8, 24 h after BiTE antibody administration in heparinized tubes. Blood cell numbers were determined using BD TruCOUNT tubes (BD, Heidelberg, Germany) in accordance with the manufacturer. Briefly, 50 μl of fresh blood were stained with fluorescent labeled α-murine CD45, α-murine CD19, α-murine CD49b, α-murine CD4, α-murine CD8, α-murine CD3 and α-murine Ly6G mABs in BD TruCOUNT tubes containing a defined number of fluorescent beads. Erythrocytes were lysed by incubation with BD FACS lysing solution (BD, Heidelberg, Germany) and cell suspensions were analyzed by flow cytometry. CD45 positive cells were considered as leukocytes and subpopulations were defined by co-expression of surface markers CD19 (B cells) or CD3 (T cells). Total blood cell numbers were determined by (number of double positive events /number of events in bead region) × (number of beads per test/ test volume). Additionally the hematokrit was determined to rule out injection induced volume effects upon blood cell count.
EpCAM protein expression in murine and human immune cells
The presence of EpCAM+ lymphocytes in human and murine blood was evaluated by isolation of PBMC from human and mouse blood (BALB/c, CD-1, C57BL/6 N, C3H/HEN). Lymphocytes of spleen, mesenteric and cervical lymph nodes were isolated from BALB/c mice as described recently [40]. Human PBMC were stained for CD19 and EpCAM, and murine lymphocytes were stained for CD45, CD19, CD49b, CD4, CD8, CD3, Ly6G and EpCAM using fluorescent coupled mAbs or the corresponding isotype controls. Cells were blocked by incubation with α-murine CD16/32 FcγII/III receptor (2.KG2; BD Bioscience, Heidelberg, Germany) for 15 min at 4°C. EpCAM+ leukocytes were quantified by flow cytometry.
Depletion of EpCAM+ cells from mouse splenocytes and PBMC
In vitro depletion of EpCAM+ cells was achieved using Dynabeads® (Invitrogen, Gaithersburg, MD) followed by fluorescence activated cell sorting (FACS). Briefly, PBMC were incubated with α-murine EpCAM mAB (G8.8; 1 μg/ml) followed by α-rat IgG (Invitrogen, Karlsruhe, Germany) coated magnetic Dynabeads®. EpCAM+ cells were repeatedly depleted from cell preparations using the provided magnet. PBMC (1.5 × 106 cells/ml) depleted from EpCAM+ cells were stained with PE-conjugated α-murine EpCAM mAB (0.5 μg/ml) and sorted by a MoFlo High Performance Cell Sorter (DAKO, Hamburg, Germany). In vivo depletion of EpCAM+ lymphocytes was achieved by intravenous administration of 10 μg/kg/day muS110 for 7 days.
In vitro activation assay
BiTE antibody induced T cell activation in vitro was analyzed by incubation of human PBMC or murine splenocytes with MT110 and muS110, respectively in the presence or absence of EpCAM+ cells. The control BiTE antibody, which binds to murine CD3 but not to murine EpCAM, was used as negative control as murine splenocytes could not completely be depleted of EpCAM+ cells. Murine splenocytes and splenocytes partially depleted in vitro from EpCAM+ cells were incubated with a tenfold serial dilution of muS110 or control BiTE antibody (1 pg/ml–1 μg/ml) for 48 h. Human PBMC were incubated in the presence or absence of 0.2 and 2% natively EpCAM expressing human KatoIII cells, respectively, and a tenfold serial dilution of MT110 (1 pg/ml–1 μg/ml) for 40 h. Splenocytes isolated from in vivo EpCAM+ cell-depleted animals were incubated with 0.5 μg/ml muS110 for 20 h in the presence or absence of 10% murine EpCAM expressing CHO cells.
Activation of CD4+ and CD8+ T cells was determined by flow cytometry using antibodies against murine and human CD4, CD8 and CD25 or CD69. All assays were performed in triplicates and sigmoidal dose response curves with R 2 values > 0.9 were obtained by Prism Software (Graph Pad Software, San Diego, CA, USA).
Determination of cytokine concentration in mouse serum and cell culture supernatants
Cytokine concentrations of cell culture supernatants and murine serum were determined by the human and murine Th1/Th2 cytometric bead array (CBA) kit, the murine inflammatory CBA kit or the CBA Flex set system (BD Bioscience, Heidelberg, Germany), respectively, in accordance to the manufactures protocol.
Histopathology
Histological and pathological studies were contracted to Huntingdon Life Sciences Ltd (Alconbury, Huntingdon, Cambridgeshire, UK). Eyes were fixed in Davidson’s fluid, testes and epididymides were fixed in Bouin’s solution prior to transfer to 70% industrial methylated spirit. All other samples were fixed in 10% neutral buffered formalin (adrenals, brain, caecum, colon, duodenum, femurs, gall bladder, harderian glands, heart, ileum, jejunum, kidney, lachrymal glands, larynx, liver, lungs, lymph nodes mandibular and mesenteric, mammary area (caudal), oesophagus, optic nerves, ovaries, pancreas, pituitary, prostate, rectum, salivary glands, seminal vesicles, skeletal muscle, spinal cord, spleen, stomach, thymus, thyroid with parathyroids, tongue, ureters, urinary bladder, uterus and cervix, vagina). Tissue samples were dehydrated, embedded in paraffin wax, sectioned at approximately 4–5 µm thickness and stained with haematoxylin and eosin. A reviewing pathologist undertook a peer review of the microscopic findings.
Results
Side effects of muS110 administration in BALB/c mice
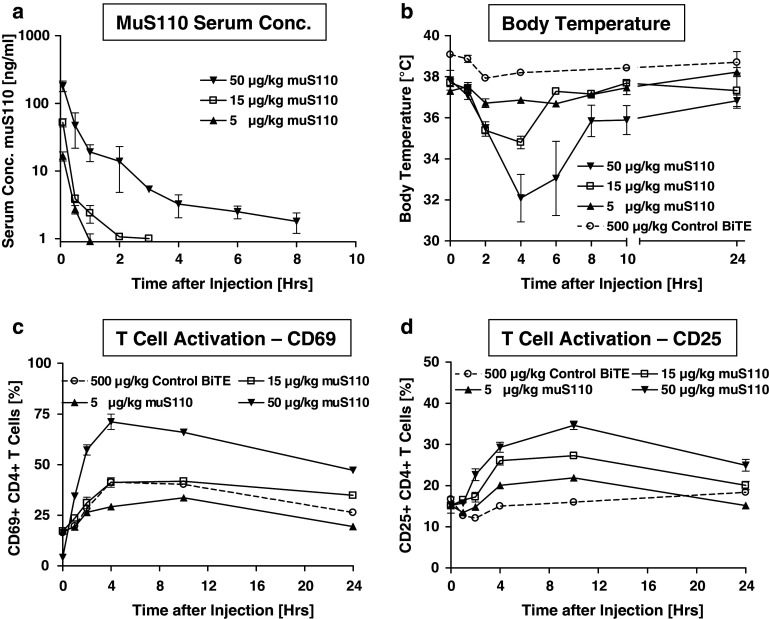
The safety profile of muS110 was analyzed following intravenous infusion of 5, 15 and 50 μg/kg mus110, and control BiTE antibody (500 μg/kg) to 20 female BALB/c mice per treatment group. At the indicated time points (Fig. 1a), serum concentrations of mus110 were determined in order to evaluate muS110 exposure and pharmacokinetic parameters. MuS110 exhibited a 3-phasic curve progression with an early distribution phase between 0 and 1 h, and a first and second elimination phase. Serum concentrations fell below the limit of quantification within 1–2 h in animals, which had received 5 and 15 μg/kg muS110. Therefore, the terminal serum half-life of 4 hours could only be determined in the highest dose group receiving 50 μg/kg muS110. Overall, dose-dependent muS110 exposure could be demonstrated.
Fig. 1.
Pharmacokinetic profile, body temperature and T cell activation markers after single intravenous injection of muS110. Female BALB/c mice (n = 20) received injection of 5, 15 or 50 μg/kg muS110 or 500 μg/kg control BiTE antibody. a Serum concentration of muS110 (n = 4); b body temperature (n = 20) as determined before and 0.5, 1, 2, 4, 6, 8, 10, 24 h after BiTE antibody injection. c Activation of T cells isolated from mesenteric lymph nodes before and 1, 2, 4, 10, 24 h after muS110 administration was determined by analysis of c CD69, or d CD25 expression using flow cytometry (n = 4). Error bars indicate standard error of the mean. The dashed lines are results with a control BiTE antibody solely sharing the CD3-binding moiety with muS110
A time- and dose-dependent onset of side effects was observed up to 24 h after administration of muS110 (Table 1; Fig. 1b). The most prominent side effects were hypomotility, piloerection, body weight reduction, hypothermia and diarrhoea. These symptoms were mild or absent in animals treated with the solely CD3-binding control BiTE antibody given at a concentration of 500 μg/kg. While infusion of 5 μg/kg muS110 resulted in hardly any side effects, 15 μg/kg muS110 induced moderate to strong hypomotility, piloerection, diarrhea and a decrease in body temperature by approximately 3°C within the first 4 h, which was quickly reversible. Animals showed a hunched posture, indicating discomfort, but recovered quickly thereafter. Administration of 50 μg/kg muS110 induced more pronounced clinical signs, e.g., a pronounced drop in body temperature of 5.7°C persisting for at least 10 h after treatment (Fig. 1b). Bolus intravenous doses of ≥500 μg/kg were lethal in all animals within 24 h.
Table 1.
Clinical signs after single intravenous injection of muS110
| muS110 μg/kg | Hypomotility | Piloerection | Diarrhea | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–4 h | 4–10 h | 10–24 h | 0–4 h | 4–10 h | 10 –24 h | 0–4 h | 4–10 h | 10–24 h | |
| 5 | ± | − | − | − | − | − | − | − | − |
| 15 | + | − | − | + | − | − | + | − | − |
| 50 | + | ± | − | + | ± | − | + | ± | − |
Female BALB/c mice (n = 20) received intravenous injection of 5, 15 or 50 μg/kg muS110. Clinical signs like piloerection, diarrhoea and hypomotility were monitored 1, 2, 4, 6, 8, 10 and 24 h after injection
− No effects, ± weak effects,+ moderate to strong effects
Flow cytometric analysis for activated CD25+ or CD69+ T cells isolated from blood, spleen, and from mesenterial, Inguinalis superficiales and cervical lymph nodes revealed a dose-dependent systemic T cell activation at the indicated time points after muS110 administration (Fig. 1c–d). The onset of T cell activation was slightly faster for CD8+ (data not shown) than for CD4+ cells (Fig. 1c, d). Time- and dose-dependent upregulation of CD69 and CD25 expression on T cells peaked 4 and 10 h after intravenous injection of muS110, respectively, and sustained thereafter. Administration of the control BiTE antibody at 500 μg/kg induced a less pronounced upregulation of the early activation marker CD69, while expression of CD25 was hardly altered. Binding of a BiTE antibody solely to CD3 was therefore insufficient to induce a strong and sustained T cell activation, even at a high dose.
MuS110 induces transient increase of cytokines
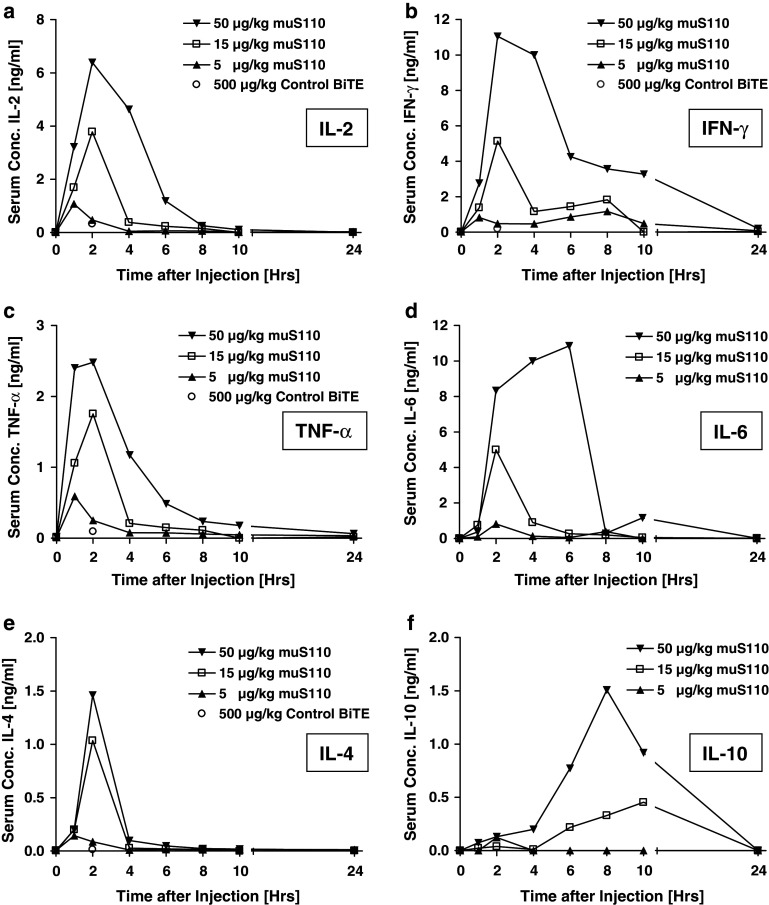
Stimulation of the CD3/TcR complex by bivalent anti-CD3 mABs like KT3 or 145-2C11 resulted in systemic T cell activation followed by a release of pro inflammatory cytokines [1–3, 46]. This was accompanied by the acute onset of several clinical symptoms termed “cytokine release syndrome”, including hypomotility, hypothermia, piloerection and diarrhoea. We therefore analyzed muS110-treated mice for serum cytokine concentrations of IL-2, IFN-γ, TNF-α, IL-4, IL-6, IL-10 (Fig. 2a–f) as well as IL-12/p70, IL-5, IL-3, MCP-1 and GM-CSF (data not shown) at various time points after BiTE antibody administrations. MuS110 infusion induced a dose-dependent and transient release of IL-2, IFN-γ, TNF-α, IL-4, IL-6, IL-10 (Fig. 2), and IL-12/p70, MCP-1 and IL-5 (data not shown). Treatment with 5 μg/kg muS110 resulted in a very limited cytokine release, whereas the 50 μg/kg induced a strong cytokine surge. For most cytokines, the onset of release was fast, and peak values were reached 2 h after intravenous bolus infusion of muS110. IL-5, IL-10 and IL-6 reached peak levels not before 6–8 h.
Fig. 2.
Serum cytokine profiles after intravenous administration of muS110. Female BALB/c mice (n = 20) received intravenous bolus injection of 5, 15 or 50 μg/kg muS110 or 500 μg/kg control BiTE antibody (dashed lines), respectively. Animals (n = 4) were bled before and 1, 2, 4, 6, 8, 10, 24 h after BiTE antibody administration. Serum samples were pooled and analyzed for a IL-2, b IFN-γ, c TNF-α, d IL-6, e IL-4 and f IL-10 using the mouse Th1/Th2 or inflammatory CBA Kit (BD Bioscience, Heidelberg, Germany). For the control BiTE antibody only peak values of IL-2, IFN-γ, TNF-α and IL-4 2 h after injection are shown as open circles
Levels of most cytokines were back to normal within 4–10 h (Fig. 2). The cytokine release induced by muS110 was faster than reported for anti-CD3 mAbs 145-2C11 or KT3 (2 vs. 4 h), but released the same set of inflammatory cytokines (data not shown). Cytokine peak levels as well as serum concentrations versus time profiles seemed to coincide with the intensity and the course of clinical symptoms observed at different dose levels.
Administration of the control BiTE antibody at a dose of 500 μg/kg did not result in elevated cytokine levels, suggesting that solely CD3 binding by the BiTE antibody was not sufficient to induce cytokine release by T cells but may require conjugation of T cells with EpCAM+ target cells, as was previously shown for MT110 [14].
MuS110-mediated lymphocyte migration in mice
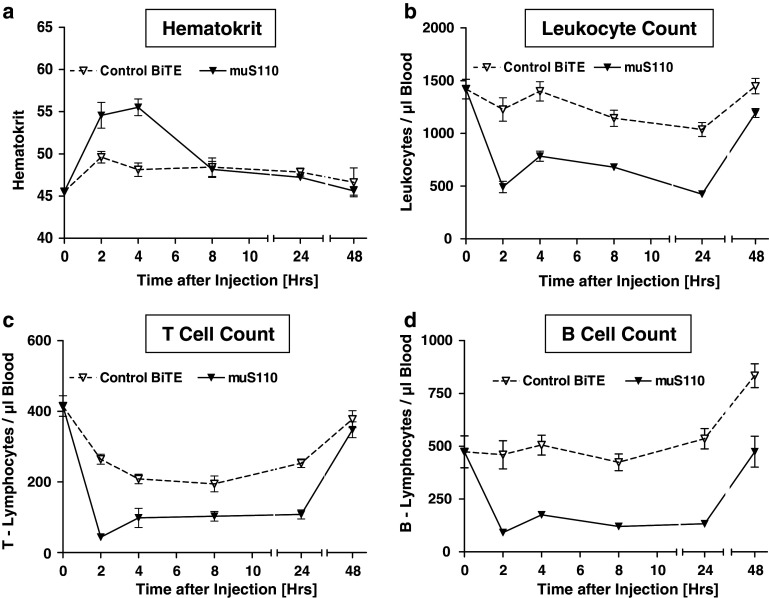
Anti-CD3 mABs are known to induce peripheral lymphocytopenia in mice [19]. Early stages of lymphocytopenia are characterized by upregulation of adhesion molecules on leukocytes and concomitant redistribution [16]. In order to study effects of muS110 on redistribution of blood cells, mice were treated by i.v. injection of 15 μg/kg muS110 or control BiTE antibody. Total numbers of leukocytes and T and B cell numbers were determined at various time points after BiTE administration (Fig. 3). Hematokrit was determined to investigate effects on blood volume. A significant increase of the hematokrit was observed between 2 and 4 h after muS110 injection, while it was barely increased after injection of control BiTE antibody (Fig. 3a). This is most likely a consequence of hemoconcentration, as can be caused by cytokine-induced vascular leakage [34], and may lead to an overestimation of blood cell numbers in animals receiving muS110.
Fig. 3.
The effect of intravenous administration of muS110 on leukocyte numbers. Female C57BL/6 mice (n = 30) received intravenous injection of 15 μg/kg muS110 or control BiTE antibody. a Hematokrit, b total numbers of leukocytes, c T cells, and d B cells were determined before and 2, 4, 8, 24 h after BiTE antibody administration (n = 5) using antibodies directed against murine CD45, CD3, and CD19 with TruCOUNT Tubes (BD Bioscience, Heidelberg, Germany). Error bars indicate standard error of the mean
Treatment with 15 μg/kg muS110 led to a rapid decrease in absolute leukocyte numbers, which was observed 2 h after injection, lasted for more than 24 h, and returned to baseline after 48 h (Fig. 3b). In response to muS110, the number of T cells decreased approximately eightfold and that of B cells fivefold (Fig. 3c–d). MuS110 did not significantly affect absolute numbers of NK cells or monocytes (data not shown). Unlike bivalent anti-CD3 mAbs [26], muS110 treatment did not significantly down-modulate T cell receptor on T cells in vivo (data not shown).
Administration of the control BiTE antibody at 500 μg/kg had only a minor effect on absolute leukocyte or B cell numbers whereas absolute numbers of T cells decreased twofold and were back to normal levels after 48 h (Fig. 3b–d). This indicates that even a high dose of the solely CD3-binding control BiTE had a comparatively weak effect on lymphocyte/leukocyte migration.
Mouse but not human lymphocytes express EpCAM
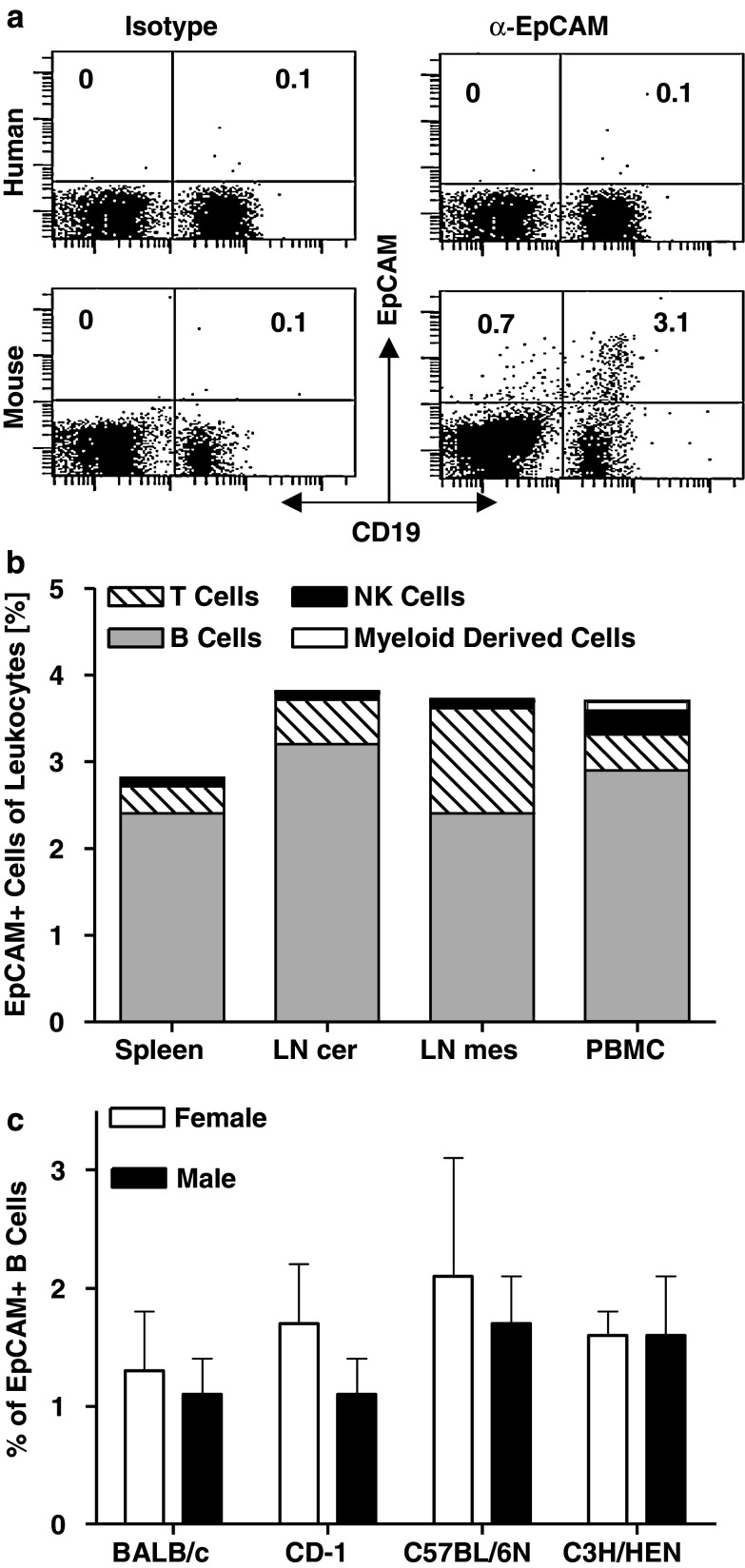
T cell activation by EpCAM- and CD19-specific BiTE antibodies was shown to be strictly dependent on the presence of appropriate target cells [14]. Contrary to the human-specific BiTE antibodies, we observed T cell activation in vitro by muS110 in the absence of added EpCAM+ target cells. We therefore suspected that EpCAM+ target cells may have been present in mouse blood. Murine PBMC and lymphocytes isolated from spleen, mesenteric and cervical lymph nodes were analyzed for EpCAM expression by FACS. Indeed, 3.1% of CD19+ B cells were found to express EpCAM in mouse blood (Fig. 4a, lower panel). Essentially no EpCAM+ cells were found in human blood above the background of the isotype control antibody (<0.1%; upper panel; [34]).
Fig. 4.
EpCAM+ cells are present in blood and lymph nodes of mice. a Human PBMC (upper panel) and murine splenocytes (lower panel) were double-stained for CD19 and EpCAM, or with corresponding isotype control antibodies. b Cells isolated from spleen, cervical (LN cer) and mesenteric lymph nodes (LN mes) as well as from peripheral blood (PBMC) of two male BALB/c mice were stained for CD3 (T cells), CD19 (B cells), CD49b (NK cells) and Ly6G (myeloid derived cells) in combination with an anti-murine EpCAM mAb, or the corresponding isotype control. Percentage of cells positive for EpCAM and the respective markers are shown. c PBMC of six female and male BALB/c, C57BL/6 N, C3H/HEN and CD-1 mice were stained for CD19 and EpCAM and analyzed by flow cytometry. Sex and strain specific percentage of CD19/EpCAM double positive cells are shown. Error bars indicate standard error of the mean
EpCAM expression was analyzed in more detail on murine lymphocytes (CD45, CD3, and CD19), natural killers (CD49b) and myeloid cells (Ly6G) using respective cell type-specific antibodies for FACS analysis. The majority of EpCAM+ cells in mouse blood co-expressed CD19 or CD45/RB220, showing their B cell origin (Fig. 4b). EpCAM+ B cells made up for 2–3% of leukocytes found in various lymphoid organs and blood. The second most frequent subpopulation of EpCAM+ cells in lymphoid organs of mice were T cells.
To rule out mouse strain specificity, PBMC from strains CD-1, C57BL/6 N, C3H/HEN and BALB/c were analyzed for EpCAM+/CD19+ double-positive B cells. As shown in Fig. 4c, the percentage of EpCAM+ B cells did not significantly differ between mouse strains and gender, and had a mean incidence of 1–3%. No significant age-related differences were observed between the mice strains tested (data not shown).
MuS110-induced T cell activation and cytokine release depends on EpCAM+ lymphocytes in murine blood
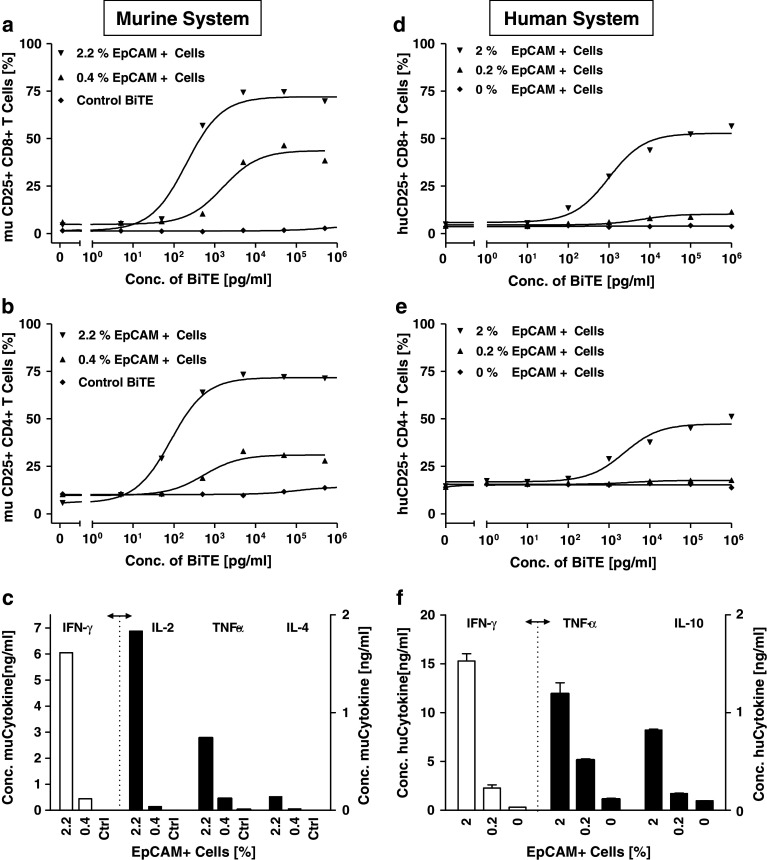
The capacity of small numbers of EpCAM+ cells to induce BiTE antibody-mediated T cell activation and cytokine release was evaluated in vitro. A complete depletion of EpCAM+ cells from murine PBMC could not be achieved, even when magnetic bead- and FACS-based approaches were combined. However, reduction of EpCAM+ cells in murine PBMC from 2.2 to 0.4% was found to substantially reduce T cell activation by muS110 of both CD8+ and CD4+ cells, as determined by expression of the late activation marker CD25 (Fig. 5a, b). More impressively, release of cytokines IFN-γ, IL-2, TNF-α, and IL-4 was almost completely abrogated by reduction of EpCAM+ cells from 2 to 0.4%, with cytokine levels coming close to those seen with the control BiTE antibody (Fig. 5c).
Fig. 5.
EpCAM+ cells are needed for T cell activation by muS110 and MT110. Murine splenocytes (2.2% EpCAM+ cells) or splenocytes partially depleted from EpCAM+ cells (0.4% EpCAM+ cells) were incubated for 48 h with the indicated concentrations of muS110, or with control BiTE antibody (left panel; murine system). Human PBMC were incubated for 40 h in the absence or presence of 0.2 or 2% KatoIII cells with the indicated concentrations of MT110 (right panel; human system). Thereafter CD8+ and CD4+ lymphocytes were analyzed for CD25 expression by flow cytometry. a, d Percentage of CD8+CD25+ and b, e percentage of CD4+CD25+ cell are shown. Supernatants from cells treated with 0.1 μg/ml MT110, muS110 and control BiTE antibody, respectively were analyzed for cytokine levels using the c mouse CBA Th1/Th2 detection kit (BD Bioscience, Heidelberg, Germany) or the f human CBA Flex set (BD Bioscience, Heidelberg, Germany). Representative results of three independent experiments are shown. Error bars indicate standard error of the mean. The dashed lines separate measurements shown at different scale
Using BiTE antibody MT110, the effect of adding human EpCAM+ cells to human PBMC was analyzed (Fig. 5d–f). Human PBMC, which are essentially devoid of EpCAM+ cells (Fig. 4a), were co cultivated for 48 h with different amounts of added EpCAM+ human carcinoma Kato III cells. As previously described [14], no activation of CD8+ or CD4+ T cell could be detected when increasing concentrations of MT110 were incubated with PBMC in the absence of EpCAM+ target cells (Fig. 5d, e). However, addition of 2% EpCAM+ cells to human PBMC led to activation of approximately 50% of CD8+ and 25% of CD4+ T cells in a MT110 dose-dependent manner. The upregulation of CD25 was accompanied by release of IFN-γ, TNF-α and IL-10 from human PBMC to similar levels as found with murine PBMC in response to muS110 (Fig. 5f; compare to 5c). Addition of only 0.2% EpCAM+ cells to human PBMC no longer strongly induced CD25 expression on CD4+ and CD8+ human T cells by MT110. However, the presence of 0.2% EpCAM+ cells still caused a robust release of TNF-α and a small response of IFN-γ in response to MT110.
These data suggest that EpCAM+ B cells in the murine leukocyte compartment could well have been responsible in mice for the profound and fast T cell activation, robust cytokine release and, ultimately, for the acute dose limiting side effects of muS110 infusion.
Depletion of EpCAM+ lymphocytes in mice dramatically increases tolerability to muS110
In an effort to demonstrate that EpCAM+ lymphocytes in blood contributed to the acute toxicity of muS110 in mice, we sought for means to selectively deplete or reduce both EpCAM+ B and T cell subpopulations in animals. Based on the in vitro data above, the temporary absence of such cells is expected to reduce T cell activation and thereby increase the tolerability of mice to much higher doses of muS110, which otherwise would be toxic.
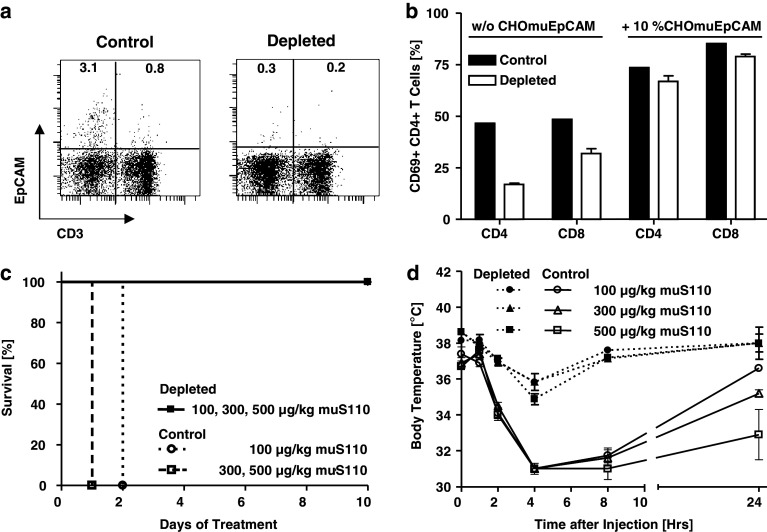
In a series of experiments, we found that a low-dose pre-treatment for 7 days with a daily i.v. dose of 10 μg/kg muS110 was suitable for substantial redirected lysis of EpCAM+ B and T cells in mice (Fig. 6a). This low-dose regimen only caused mild and swiftly reversible side effects (data not shown). Reduction of cells in peripheral blood of mice was approximately from 3.1 to 0.3% for EpCAM+ B cells and from 0.8 to 0.2% for EpCAM+ T cells, still leaving a total of 0.5% EpCAM+ lymphocytes. Control animals treated with either daily i.v. doses of 10 μg/kg control BiTE antibody or PBS vehicle had no change in their percentage of EpCAM+ lymhocytes (data not shown).
Fig. 6.
The effect of reducing EpCAM+ lymphocytes in mice on the tolerability of high doses of muS110. Female BALB/c mice (n = 5) received daily i.v. doses of 10 μg/kg muS110 or of control BiTE antibody/ PBS for seven consecutive days. a Depletion of EpCAM+ lymphocytes in mice. Splenocytes isolated from PBS control and low-dose muS110 pre-treated mice were stained for CD3 and EpCAM by specific mAbs in order to monitor the in vivo depletion of EpCAM+ B (left gate) and T cells (right gate) by FACS analysis. The percentage of EpCAM+ cells in the respective gates is given relative to the total number of lymphocytes analyzed by FACS. b CD69 expression on T cells from control and muS100-pre-treated mice. Splenocytes isolated from control (PBS) and low-dose muS110 pre-treated animals were incubated for 20 h in the absence or presence of 10% muEpCAM+ CHO cells with 0.5 μg/ml muS110. Thereafter CD8+ and CD4+ lymphocytes were analyzed for CD69 expression by flow cytometry. c Survival of mice challenged with high doses of muS110. EpCAM+ lymphocyte-depleted and control BiTE antibody-treated animals were challenged by daily i.v. infusion with high doses of muS110 (100, 300, 500 μg/kg/day) for ten consecutive days. d The effect of low-dose muS110 treatment on side effects of mice. Body temperature (n = 5) profiles were determined for low-dose muS110 adapted and control mice following 1, 2, 4, 8, 24 h after high-dose BiTE antibody challenge. SEM is shown
In vitro, muS110 still induced the upregulation of T cell activation marker CD69 on splenocytes isolated from EpCAM+ lymphocyte-depleted mice. Compared to control aninmals, the percentage of activated CD4+ and CD8+ T cells was, however, significantly reduced in splenocytes from EpCAM+ lymphocyte-depleted animals (Fig. 6b). This was most likely due to the reduced number of EpCAM+ lymphocytes, because addition of 10% EpCAM-expressing CHO cells restored comparable CD69 expression on a high percentage of T cells from muS110 pre treated to the level of control animals. This also showed that T cells from low-dose muS110 pre treated mice could still be fully activated if appropriate target cells for muS110 are presented.
Mice with substantially reduced EpCAM+ lymphocytes and control animals were challenged with daily i.v. doses of muS110 of 100, 300 and 500 μg/kg for a total of 10 days. Animals receiving 10 μg/kg of the control BiTE antibody showed severe side effects upon high-dose muS110 challenge such that all had to be euthanized within 48 h due to bad overall appearance (Fig. 6c). In contrast, all animals pre treated with a low-dose of muS110 survived doses of 100, 300 and even 500 μg/kg of muS110 given daily i.v. for 10 days. Only mild and reversible side effects were noted in these animals such as a modest and transient reduction of body temperature (Fig. 6d) and of body weight (data not shown). These remaining side effects could be due to a limited T cell activation triggered by the remaining 0.5% EpCAM+ lymphocytes (see Fig. 6a), consistent with the in vitro data from splenocytes (see Fig. 6b). Of note, low-dose pre-treatment with muS110 did not impair the cytotoxic or proliferative capacity of T cells, which was indistinguishable from that of control mice (see Fig. 6b; data not shown; Amann et al., submitted).
MuS110 does not induce EpCAM-related tissue lesions
Apart from cytokine release by activated T cells, another conceivable adverse reaction for an EpCAMxCD3-bispecific BiTE antibody is damage to EpCAM-expressing normal epithelial tissues by redirected cytotoxic T cells. The expression level and distribution of EpCAM was analyzed on 32 different mouse tissues in a tissue cross-reactivity study performed according to regulatory guidelines [4]. Many tissues of mice showed expression of EpCAM at various intensities, as determined by immunohistochemical staining of fixed tissue slices with muS110 (and the parental anti-EpCAM mAb) (Table 2). Despite expression of EpCAM on organs such as kidney (score = 1), lung (=1), pancreas (=3), pituitary (=2–3), parathyroid (=3, 4), colon (=4), or prostate (=3), daily dosing of mice with muS110 for 35 days revealed no evidence for lesions in normal tissues expressing the EpCAM target antigen. In this vehicle-controlled study, muS110 was administered to 30 animals by daily bolus intravenous infusion for up to 35 days and up to dose levels of 100 μg/kg/day for 28 days. As described in Fig. 6, these animals were adapted by low-dose muS110 treatment in order to tolerate daily repeated doses of 100 μg/kg. Essentially, all histopathological findings noted with EpCAM+ tissues in 30 muS110-treated animals were observed at a similar frequency in the 32 vehicle control animals. Most findings were of rather low frequency in both groups and therefore not considered to be specific for muS110. In summary, there was no evidence that muS110 caused any specific histopathologically detectable damage to EpCAM-expressing normal tissues (Table 2).
Table 2.
Histopathological examination of EpCAM-positive tissues from mice treated with muS110
| EpCAM positive tissue | Score of EpCAM expression | Number of analyzed animals vehicle | Number of analyzed animals MuS110 | MuS110 specific histopathological findings |
|---|---|---|---|---|
| Caecum | 2 | 32 | 30 | None |
| Colon | 4 | 32 | 30 | None |
| Eyes | 2 | 32 | 30 | None |
| GI tract other | 2–4 | 32 | 30 | None |
| Kidneys | 1 | 32 | 30 | None |
| Liver | 1 | 32 | 30 | None |
| Lung and bronchi | 1 | 32 | 30 | None |
| Lymph nodes mandibular | 1 | 32 | 30 | None |
| Lymph nodes mesenteric | 1 | 32 | 30 | None |
| Oesophagus | 2–4 | 32 | 30 | None |
| Pancreas | 3 | 32 | 30 | None |
| Pituitary | 2–3 | 32 | 30 | None |
| Salivary glands | 2–4 | 32 | 30 | None |
| Thymus | 1 | 32 | 30 | None |
| Parathyroids | 3–4 | 23 | 22 | None |
| Ureters | 2 | 32 | 30 | None |
| Urinary bladder | 2 | 32 | 30 | None |
| Male reproductive system | ||||
| Prostata | 3 | 16 | 16 | None |
| Testes | 1 | 16 | 16 | None |
| Female reproductive system | ||||
| Mammary area (caudal) | 2 | 16 | 14 | None |
| Uterus | 2–4 | 16 | 14 | None |
| Uterine cervix | 2 | 16 | 14 | None |
| Vagina | 2 | 16 | 14 | None |
Sixteen male and female BALB/c mice per group received daily intravenous injections of vehicle for 35 days or of 10 μg/kg/day muS110 for 7 days followed by 100 μg/kg/day muS110 for 28 days. Samples of tissues were fixed, dehydrated, embedded in paraffin wax, sectioned at approximately 4–5 µm thickness, and stained with H&E. All slides were inspected and evaluated by a certified pathologist. MuS110 specific findings for EpCAM positive tissues (score of stain ≥ 1) are shown. The scoring of tissues for relative intensity and frequency by immunohistochemical analysis for EpCAM expression is described in Amann et al. [4]. The EpCAM expression score from 1–4 is as follows: 1 light staining and/or low percentage of cells stained; 2 moderate staining and/or moderate percentage of cells stained; 3 medium-strong staining and/or or medium percentage of cells stained; 4 intense staining and/or or high percentage of cells stained
GI tract other includes duodenum, ileum and jejunum
Discussion
A previous study has shown that anti tumor activities of the mouse-specific BiTE antibody muS110 were not accompanied by detectable side effects, indicating a therapeutic window [4]. Here, we studied higher doses of muS110 to explore its dose-limiting toxicity. The maximum tolerated dose (MTD) of muS110 in mice was approximately 50 μg/kg muS110, corresponding to a therapeutic index (TI) of approximately 10. Dose-dependent side effects of muS110 were primarily caused by a transient release of inflammatory cytokines through activated T cells. First, T cells in blood, spleen and lymph nodes of mice were found to newly express activation markers CD69 and CD25 in response to muS110 infusions. Second, the levels of a host of inflammatory cytokines were found to increase in blood shortly after infusion of muS110. Third, coinciding with cytokine peaks, mice showed clinical signs as previously described in mice after direct administration of inflammatory cytokines [11, 36, 43], and in response to agonistic anti-CD3 monoclonal antibodies [1, 2, 18, 19, 22]. Fourth, glucocorticoids reduced T cell activation and cytokine release, which considerably increased the tolerability of mice for muS110 (data not shown; Amann et al., submitted). Last, muS110 induced a fast redistribution of T cells. This may be due to upregulation of adhesion molecules such as CD18 and has also been reported to occur in mice after infusion of anti-CD3 mAbs [16]. Cytokine release may further contribute to lymphocyte redistribution phenomena as described in response to TNF-α and IL-2, both of which are known to boost lymphocyte adhesion and migration [5, 24].
Side effects of muS110 with relationship to T cell activation and cytokine release appeared to rely on a bispecific binding of the BiTE antibody to both CD3 on T cells and to EpCAM on blood-borne cells. The dependency of side effects of muS110 on bispecific target binding is supported by the following observations. A control BiTE antibody sharing with muS110 the CD3-binding domain did not show side effects or significant cytokine release even at a concentration of 500 μg/kg, i.e., ten times the MTD of muS110. Monovalent binding to CD3 was therefore not sufficient in mice to elicit a comprehensive T cell activation and related side effects. Lack of T cell activation in the absence of target cells has also been observed for BiTE antibodies MT103 and MT110 [14]. T cell activation by muS110 in mice must therefore have relied on simultaneous access of the BiTE antibody to both T cells and EpCAM-expressing cells. One possible source for the latter is epithelial cells, which frequently and highly express EpCAM in mice. However, the absence of any significant damage of normal EpCAM-expressing epithelia by muS110-redirected T cells suggests that EpCAM on normal epithelial tissues is not well accessible to BiTE or T cells, or both. A number of reasons for this have recently been discussed in detail [6, 7] including complexation of EpCAM with other proteins and sequestration of EpCAM in intercellular boundaries of epithelia.
Here, we found that a low percentage of murine lymphocytes express EpCAM. These were mostly peripheral B cells and a lower extent T cells. EpCAM expression on mouse lymphoid cells has been noted earlier, but thus far only dendritic cells [12], plasma cells [37], thymocytes and thymic epithelial cells [35] were reported to express EpCAM. We observed that reduction of EpCAM+ B and T cells in peripheral blood samples by column removal or via redirected lysis in animals can significantly reduce T cell activation by muS110. Moreover, a human cell culture system allowed demonstration that spiking of only 2% EpCAM+ cells can trigger T cell activation and cytokine release by MT110, which was comparable in intensity to what has been observed with muS110 in the mouse cell culture system. Of note, MT110 did not induce any T cell activation in human blood samples––unless EpCAM+ cells are added. Together, these findings support the notion that muS110-mediated activation of T cells was triggered by EpCAM+ lymphocytes in blood and lymphoid organs of mice, and may have been responsible for the acute side effects seen after infusion of muS110.
T cell activation via EpCAM+ lymphocytes may also explain the transient nature of side effects in mice. With the substantial reduction of EpCAM+ lymphocytes in mice by muS110-redirected T cells, the initial T cell activating stimulus for muS110 would be gradually decreased, resulting in a reduction of side effects from and recovery from the systemic T cell response. Together, these findings support the notion that muS110-mediated activation of T cells was triggered by EpCAM+ lymphocytes in blood and lymphoid organs of mice, and may have been responsible for the acute side effects seen after infusion of muS110. T cells isolated from mice that had their EpCAM+ lymphocytes reduced retained their potential to be activated by a CD3 stimulus (see Fig. 6b) and their capacity for redirected lysis of EpCAM+ target cells (Amann et al., in preparation). Toleration to high muS110 doses by low-dose pre-treatment with muS110 is thus unlikely to have resulted from a T cell anergy.
The absence of any significant damage to normal EpCAM-expressing epithelia by muS110-redirected T cells in mice treated with very high doses of muS110 suggests that EpCAM+ solid epithelial tissues in mice could not substitute for EpCAM+ lymphocytes for activation of T cells in the presence of BiTE antibody muS110. This may indicate that EpCAM expressed on normal tissue is not accessible to either muS110 or T cells, or both. This is in contrast to EpCAM on tumor cells where anti-tumor activity was already evident in mice at doses as low as 2 μg/kg muS110 [4].
A number of mechanisms may contribute to the therapeutic window of muS110 in mice [47]. First, an association of EpCAM in normal epithelial cells with other protein partners, including tetraspanins, CD44 and claudin-7, may obscure binding sites for muS110. For a therapeutic window, such protein partner need to be reduced or absent from tumor cells, which has actually been shown for tetraspanin CD9 and claudins. Second, much of EpCAM on normal tissue appears to be sequestered within intercellular boundaries, as is evident from immunohistochemical analysis. Intercellular boundaries in epithelia are very dense and highly organized structures that serve as diffusion barriers, which may compromise access of muS110 to at least part of the EpCAM target antigen on normal epithelial cells. These dense epithelial structures are largely absent from tumor tissue. Third, it is known that normal epithelial tissues are embedded within extracellular matrix and covered by a basal membrane, while the highly proteolytic environment of cancer tissue is substantially breaking down such mechanical barriers. Hence, several factors may contribute to a differential accessibility of EpCAM on normal versus tumor tissue potentially explaining the absence of damage to EpCAM+ tissues in high-dose muS110-treated mice.
The situation with muS110 in the murine system is reminiscent of bolus i.v. administered human CD19-specific BiTE antibody MT103 (also called MEDI-538) in primates. With the latter BiTE antibody, T cells also encounter target-expressing lymphocytes in the blood stream right after i.v. infusion. Rapid redistribution of lymphocytes and cytokine release was observed upon repeated 2 h i.v. infusions of MT103 to chimpanzees [41]. In a phase I clinical trial,, BiTE antibody MT103 showed no significant cytokine release at the dose levels reported [8, 9, 28]. This may have been mitigated by tapered steroid hormone administration and continuous infusion of MT103.
Our new study supports the notion that muS110 could well discriminate between EpCAM found exposed on circulating lymphocytes and the EpCAM embedded in normal epithelial tissues. Should the human EpCAM-specific BiTE antibody MT110 likewise make this distinction, it may have a larger therapeutic window than muS110 in mice. This is because in humans EpCAM+ lymphocytes are largely absent, such that T cells may only be locally activated if they encounter MT110 that is bound to accessible EpCAM antigen on tumor cells.
Footnotes
M. Amann and M. Friedrich contributed equally to this work.
References
- 1.Alegre M, Depierreux M, Florquin S, Najdovski T, Vandenabeele P, Abramowicz D, Leo O, Deschodt-Lanckman M, Goldman M. Acute toxicity of anti-CD3 monoclonal antibody in mice: a model for OKT3 first dose reactions. Transplant Proc. 1990;22:1920–1921. [PubMed] [Google Scholar]
- 2.Alegre M, Vandenabeele P, Flamand V, Moser M, Leo O, Abramowicz D, Urbain J, Fiers W, Goldman M. Hypothermia and hypoglycemia induced by anti-CD3 monoclonal antibody in mice: role of tumor necrosis factor. Eur J Immunol. 1990;20:707–710. doi: 10.1002/eji.1830200337. [DOI] [PubMed] [Google Scholar]
- 3.Alegre ML, Vandenabeele P, Depierreux M, Florquin S, Deschodt-Lanckman M, Flamand V, Moser M, Leo O, Urbain J, Fiers W, et al. Cytokine release syndrome induced by the 145-2C11 anti-CD3 monoclonal antibody in mice: prevention by high doses of methylprednisolone. J Immunol. 1991;146:1184–1191. [PubMed] [Google Scholar]
- 4.Amann M, Brischwein K, Lutterbuese P, Parr L, Petersen L, Lorenczewski G, Krinner E, Bruckmeier S, Lippold S, Kischel R, Lutterbuese R, Kufer P, Baeuerle PA, Schlereth B. Therapeutic window of MuS110, a single-chain antibody construct bispecific for murine EpCAM and murine CD3. Cancer Res. 2008;68:143–151. doi: 10.1158/0008-5472.CAN-07-2182. [DOI] [PubMed] [Google Scholar]
- 5.Ariel A, Yavin EJ, Hershkoviz R, Avron A, Franitza S, Hardan I, Cahalon L, Fridkin M, Lider O. IL-2 induces T cell adherence to extracellular matrix: inhibition of adherence and migration by IL-2 peptides generated by leukocyte elastase. J Immunol. 1998;161:2465–2472. [PubMed] [Google Scholar]
- 6.Baeuerle PA, Gires O. EpCAM (CD326) finding its role in cancer. Br J Cancer. 2007;96:417–423. doi: 10.1038/sj.bjc.6603494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baeuerle PA, Reinhardt C, Kufer P. BiTE: a new class of antibodies that recruit T-cells. Drugs Future. 2008;33(2):1–11. doi: 10.1358/dof.2008.033.02.1172578. [DOI] [Google Scholar]
- 8.Bargou R, Noppeney R, Schuler M, Hess G, Gerecke C, Zettl F, Birkmann J, Viardot A, Kirchinger P, Baeuerle P, Reinhardt C, Klinger M, Libicher M, Goebeler M, Einsele H, Kufer P, Zugmaier G, Leo E. The bi-specific T-cell enhancer (BiTE) MT103 (MEDI-538) induces clinical responses in heavily pre-treated NHL patients: update from the ongoing phase I study MT103-104. Blood. 2006;108:693. [Google Scholar]
- 9.Bargou RC, Kufer P, Goebeler M, Knop S, Einsele H, Noppeney R, Viardot A, Hess G, Riethmueller G, Reitsma D, Leo E, Reinhardt C, Zugmaier G. The anti-CD19 bispecific T-cell engager (BiTE) MT103 (MEDI-538), induces dose-dependent complete and partial responses in relapsed Non-Hodgkin Lymphoma (NHL): phase I study MT103–104. ASH Annu Meet Abstr. 2007;110:2557. [Google Scholar]
- 10.Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 11.Blackwell TS, Christman JW. Sepsis and cytokines: current status. Br J Anaesth. 1996;77:110–117. doi: 10.1093/bja/77.1.110. [DOI] [PubMed] [Google Scholar]
- 12.Borkowski TA, Nelson AJ, Farr AG, Udey MC. Expression of gp40, the murine homologue of human epithelial cell adhesion molecule (Ep-CAM), by murine dendritic cells. Eur J Immunol. 1996;26:110–114. doi: 10.1002/eji.1830260117. [DOI] [PubMed] [Google Scholar]
- 13.Brandl C, Haas C, d‘Argouges S, Fisch T, Kufer P, Brischwein K, Prang N, Bargou R, Suzich J, Baeuerle PA, Hofmeister R. The effect of dexamethasone on polyclonal T cell activation and redirected target cell lysis as induced by a CD19/CD3-bispecific single-chain antibody construct. Cancer Immunol Immunother. 2007;56:1551–1563. doi: 10.1007/s00262-007-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brischwein K, Schlereth B, Guller B, Steiger C, Wolf A, Lutterbuese R, Offner S, Locher M, Urbig T, Raum T, Kleindienst P, Wimberger P, Kimmig R, Fichtner I, Kufer P, Hofmeister R, da Silva AJ, Baeuerle PA. MT110: a novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol Immunol. 2006;43:1129–1143. doi: 10.1016/j.molimm.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 15.Brischwein K, Parr L, Pflanz S, Volkland J, Lumsden J, Klinger M, Locher M, Hammond SA, Kiener P, Kufer P, Schlereth B, Baeuerle PA. Strictly target cell-dependent activation of T cells by bispecific single-chain antibody constructs of the BiTE class. J Immunother. 2007;30:798–807. doi: 10.1097/CJI.0b013e318156750c. [DOI] [PubMed] [Google Scholar]
- 16.Buysmann S, Bemelman FJ, Schellekens PT, van Kooyk Y, Figdor CG, ten Berge IJ. Activation and increased expression of adhesion molecules on peripheral blood lymphocytes is a mechanism for the immediate lymphocytopenia after administration of OKT3. Blood. 1996;87:404–411. [PubMed] [Google Scholar]
- 17.Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 18.Charpentier B, Hiesse C, Ferran C, Lantz O, Fries D, Bach JF, Chatenoud L. Acute clinical syndrome associated with OKT3 administration. Prevention by single injection of an anti-human TNF monoclonal antibody. Presse Med. 1991;20:2009–2011. [PubMed] [Google Scholar]
- 19.Chatenoud L, Ferran C, Bach JF. The anti-CD3-induced syndrome: a consequence of massive in vivo cell activation. Curr Top Microbiol Immunol. 1991;174:121–134. doi: 10.1007/978-3-642-50998-8_9. [DOI] [PubMed] [Google Scholar]
- 20.Chatenoud L, Legendre C, Ferran C, Bach JF, Kreis H. Corticosteroid inhibition of the OKT3-induced cytokine-related syndrome–dosage and kinetics prerequisites. Transplantation. 1991;51:334–338. doi: 10.1097/00007890-199102000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Desjarlais JR, Lazar GA, Zhukovsky EA, Chu SY. Optimizing engagement of the immune system by anti-tumor antibodies: an engineer’s perspective. Drug Discov Today. 2007;12:898–910. doi: 10.1016/j.drudis.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Ferran C, Dy M, Sheehan K, Merite S, Schreiber R, Landais P, Grau G, Bluestone J, Bach JF, Chatenoud L. Inter-mouse strain differences in the in vivo anti-CD3 induced cytokine release. Clin Exp Immunol. 1991;86:537–543. doi: 10.1111/j.1365-2249.1991.tb02966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gottlinger H, Johnson J, Riethmuller G. Biochemical and epitope analysis of the 17–1A membrane antigen. Hybridoma. 1986;5(Suppl 1):S29–S37. [PubMed] [Google Scholar]
- 24.Green DM, Trial J, Birdsall HH. TNF-alpha released by comigrating monocytes promotes transendothelial migration of activated lymphocytes. J Immunol. 1998;161:2481–2489. [PubMed] [Google Scholar]
- 25.Hayashi R, Wada H, Ito K, Adcock IM. Effects of glucocorticoids on gene transcription. Eur J Pharmacol. 2004;500:51–62. doi: 10.1016/j.ejphar.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch R, Gress RE, Pluznik DH, Eckhaus M, Bluestone JA. Effects of in vivo administration of anti-CD3 monoclonal antibody on T cell function in mice. II. In vivo activation of T cells. J Immunol. 1989;142:737–743. [PubMed] [Google Scholar]
- 27.Kaufman RJ. Selection and coamplification of heterologous genes in mammalian cells. Methods Enzymol. 1990;185:537–566. doi: 10.1016/0076-6879(90)85044-O. [DOI] [PubMed] [Google Scholar]
- 28.Klinger M, Kufer P, Kirchinger P, Lutterbuse R, Leo E, Reinhardt C, Bauerle P, Bargou R. T cell responses during long-term continuous infusion of MT103 (MEDI-538; anti-CD19 BiTE) in patients with relapsed B-NHL: data from dose-escalation study MT103-104. Blood. 2006;108:2725. [Google Scholar]
- 29.Kontermann RE. Recombinant bispecific antibodies for cancer therapy. Acta Pharmacol Sin. 2005;26:1–9. doi: 10.1111/j.1745-7254.2005.00008.x. [DOI] [PubMed] [Google Scholar]
- 30.Kufer P, Zettl F, Borschert K, Lutterbuse R, Kischel R, Riethmuller G. Minimal costimulatory requirements for T cell priming and TH1 differentiation: activation of naive human T lymphocytes by tumor cells armed with bifunctional antibody constructs. Cancer Immunol. 2001;1:10. [PubMed] [Google Scholar]
- 31.Lambert JM. Drug-conjugated monoclonal antibodies for the treatment of cancer. Curr Opin Pharmacol. 2005;5:543–549. doi: 10.1016/j.coph.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Mack M, Riethmuller G, Kufer P. A small bispecific antibody construct expressed as a functional single-chain molecule with high tumor cell cytotoxicity. Proc Natl Acad Sci USA. 1995;92:7021–7025. doi: 10.1073/pnas.92.15.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyamoto T, Fujinaga T, Yamashita K, Hagio M. Changes of serum cytokine activities and other parameters in dogs with experimentally induced endotoxic shock. Jpn J Vet Res. 1996;44:107–118. [PubMed] [Google Scholar]
- 34.Moldenhauer G, Momburg F, Moller P, Schwartz R, Hammerling GJ. Epithelium-specific surface glycoprotein of Mr 34, 000 is a widely distributed human carcinoma marker. Br J Cancer. 1987;56:714–721. doi: 10.1038/bjc.1987.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson AJ, Dunn RJ, Peach R, Aruffo A, Farr AG. The murine homolog of human Ep-CAM, a homotypic adhesion molecule, is expressed by thymocytes and thymic epithelial cells. Eur J Immunol. 1996;26:401–408. doi: 10.1002/eji.1830260220. [DOI] [PubMed] [Google Scholar]
- 36.Panelli MC, White R, Foster M, Martin B, Wang E, Smith K, Marincola FM. Forecasting the cytokine storm following systemic interleukin (IL)-2 administration. J Transl Med. 2004;2:17. doi: 10.1186/1479-5876-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao CG, Chianese D, Doyle GV, Miller MC, Russell T, Sanders RA, Jr, Terstappen LW. Expression of epithelial cell adhesion molecule in carcinoma cells present in blood and primary and metastatic tumors. Int J Oncol. 2005;27:49–57. [PubMed] [Google Scholar]
- 38.Raum T, Gruber R, Riethmuller G, Kufer P. Anti-self antibodies selected from a human IgD heavy chain repertoire: a novel approach to generate therapeutic human antibodies against tumor-associated differentiation antigens. Cancer Immunol Immunother. 2001;50:141–150. doi: 10.1007/PL00006684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlereth B, Fichtner I, Lorenczewski G, Kleindienst P, Brischwein K, da Silva A, Kufer P, Lutterbuese R, Junghahn I, Kasimir-Bauer S, Wimberger P, Kimmig R, Baeuerle PA. Eradication of tumors from a human colon cancer cell line and from ovarian cancer metastases in immunodeficient mice by a single-chain Ep-CAM-/CD3-bispecific antibody construct. Cancer Res. 2005;65:2882–2889. doi: 10.1158/0008-5472.CAN-04-2637. [DOI] [PubMed] [Google Scholar]
- 40.Schlereth B, Kleindienst P, Fichtner I, Lorenczewski G, Brischwein K, Lippold S, da Silva A, Locher M, Kischel R, Lutterbuse R, Kufer P, Baeuerle PA. Potent inhibition of local and disseminated tumor growth in immunocompetent mouse models by a bispecific antibody construct specific for Murine CD3. Cancer Immunol Immunother. 2006;55:785–796. doi: 10.1007/s00262-005-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlereth B, Quadt C, Dreier T, Kufer P, Lorenczewski G, Prang N, Brandl C, Lippold S, Cobb K, Brasky K, Leo E, Bargou R, Murthy K, Baeuerle PA. T-cell activation and B-cell depletion in chimpanzees treated with a bispecific anti-CD19/anti-CD3 single-chain antibody construct. Cancer Immunol Immunother. 2006;55:503–514. doi: 10.1007/s00262-005-0001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stern M, Herrmann R. Overview of monoclonal antibodies in cancer therapy: present and promise. Crit Rev Oncol Hematol. 2005;54:11–29. doi: 10.1016/j.critrevonc.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Talmadge JE, Bowersox O, Tribble H, Lee SH, Shepard HM, Liggitt D. Toxicity of tumor necrosis factor is synergistic with gamma-interferon and can be reduced with cyclooxygenase inhibitors. Am J Pathol. 1987;128:410–425. [PMC free article] [PubMed] [Google Scholar]
- 44.Weiner LM. Bispecific antibodies in cancer therapy. Cancer J. 2000;6(Suppl 3):S265–S271. [PubMed] [Google Scholar]
- 45.Went P, Vasei M, Bubendorf L, Terracciano L, Tornillo L, Riede U, Kononen J, Simon R, Sauter G, Baeuerle PA. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br J Cancer. 2006;94:128–135. doi: 10.1038/sj.bjc.6602924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wissing KM, Desalle F, Abramowicz D, Willems F, Leo O, Goldman M, Alegre ML. Down-regulation of interleukin-2 and interferon-gamma and maintenance of interleukin-4 and interleukin-10 production after administration of an anti-CD3 monoclonal antibody in mice. Transplantation. 1999;68:677–684. doi: 10.1097/00007890-199909150-00014. [DOI] [PubMed] [Google Scholar]
- 47.Wolf E, Hofmeister R, Kufer P, Schlereth B, Baeuerle PA. BiTEs: bispecific antibody constructs with unique anti-tumor activity. Drug Discov Today. 2005;10:1237–1244. doi: 10.1016/S1359-6446(05)03554-3. [DOI] [PubMed] [Google Scholar]