Abstract
Chokeberry, Aronia melanocarpa, is an indigenous fruit from North America used as food and to prevent chronic disease by Indigenous Peoples. The objective of this study was to test anti-inflammatory effects of anthocyanin on palmitic acid (PA)-induced IL-6 gene expression, IL-6 DNA methylation, and histone (H3) acetylation. Additionally, we examined effects of anthocyanins Cyanidin-3-O-galactoside (C3Gal) and Cyanidin-3-glucoside (C3G) on IL-6 gene expression. Human primary pre-adipocytes were treated with chokeberry juice extract (CBE), C3Gal or C3G in the presence or absence of PA or lipopolysaccharide (LPS). CBE inhibited LPS- and PA-induced IL-6 mRNA expression (p < 0.0001), while C3G and C3Gal had smaller effects. Human IL-6 promoter DNA methylation was increased (p = 0.0256) in CBE treated cells compared to control. Histone H3 acetylations were not affected by CBE or PA treatment. These data indicate that CBE epigenetically reduced PA-induced inflammation by regulating IL-6 DNA methylation without affecting histone modifications in human preadipocyte cells.
Keywords: Aronia melanocarpa, Anthocyanin, Inflammation, DNA methylation, IL-6 promoter, Human preadipocyte
1. Introduction
Increased systemic and localized inflammation are associated with the development of insulin resistance (Rehman et al., 2017), type 2 diabetes mellitus (T2DM) (Raghav et al., 2018), arthritis (Prasad et al., 2021), cardiovascular diseases (Cao et al., 2016), and cancer (Goh et al., 2016; Nost et al., 2021). Obesity (Kunz et al., 2021), excess adipose tissue (Greenberg and Obin, 2006), and excessive energy intake promote systemic inflammation. Dietary bioactive flavonoids reduce systemic inflammation and associated chronic disease prevalence (Zhang et al., 2020; Hang et al., 2018; Maleki et al., 2019), thereby making flavonoids potentially important food factors for preventing chronic disease. Chokeberry (Aronia melanocarpa) is one of the highest flavonoid-containing fruits exceeding that of both blueberries (Mendelova et al., 2022) and blackberries (Zapolska-Downar et al., 2012). Chokeberry has been historically distributed around the Great Lakes and the eastern regions of North America from Newfoundland, Canada to the southern portion of the Appalachian mountains (Hardin, 1973). Through careful observation and study Indigenous Peoples recognized and utilized chokeberry as both food and a means of preventing chronic diseases (Kokotkiewicz et al., 2010). On account of a high prevalence of chronic disease among Indigenous Peoples (Williams et al., 2019; O’Connell et al., 2021), attainable, culturally connected, and sustainable dietary solutions for disease prevention need to be determined.
Inflammation results from a lesion or factor (i.e., palmitic acid) (Roe, 2021) that activates an immune response. Immune cells and adipocyte/preadipocytes secrete proinflammatory cytokines and chemokines via an autocrine, paracrine, or endocrine manner to elicit local inflammation (Roe, 2021). Adipocytes respond to proinflammatory signaling molecules that act via cell surface receptors to promote systemic inflammation (Tanaka et al., 2014). It is known that adipose tissue and white adipocytes are one of the major tissue and cell type sources of pro-inflammatory cytokine IL-6 (Tanaka et al., 2014) that stimulates the inflammatory and auto-immune processes in type 2 diabetes mellitus (Rehman et al., 2017), atherosclerosis (Zheng et al., 2022) and rheumatoid arthritis (Larsson et al., 2023). Chokeberry extract reduces inflammation as measured by IL-6 and IL-8 concentrations (Naruszewicz et al., 2007; Iwashima et al., 2019; Appel et al., 2015). Furthermore, consumption of whole chokeberry (Pei et al., 2019), chokeberry juice (Appel et al., 2015) and isolated bioactive compounds (e.g., anthocyanin) found in chokeberry (Goh et al., 2016) all have anti-inflammatory effects in vivo and in vitro.
Chokeberry contains procyanidins and anthocyanins (Kokotkiewicz et al., 2010). Anthocyanins make up 25–57% of the polyphenols in chokeberry with cyanidin-3-galactoside (C3Gal) being the most abundant and cyanidin-3-glucosicde (C3G) being the least abundant (Oszmianski and Wojdylo, 2005; Tian et al., 2017). These polyphenols and other bioactive compounds have anti-inflammatory effects via regulation of epigenetic pathways that are known to generally affect epigenetic modification of genes, and gene expression (Kim et al., 2017; Guo et al., 2015; Zhao et al., 2018). Epigenetics changes are reversable and heritable modifications that control gene expression through alterations of DNA, histones, chromatin, and RNAi without changing the DNA sequence (Perkins et al., 2016). Understanding the epigenetic mechanisms that regulate chronic low-grade inflammation is important for reducing development of metabolic diseases (Russo et al., 2017). Although there has been ample research in understanding the physiological pathways associated with inflammation, there is still much to be elucidated about role of epigenetic effect on those pathways. Additionally understanding how dietary foods as a whole or individual bioactive compounds can epigenetically affect gene expression provides insight on how our foods can reduce inflammation and inflammation induced chronic illnesses (Russo et al., 2017).
Inflammatory cytokine synthesis, including IL-6, is epigenetically regulated (Cai et al., 2020; Takahashi et al., 2015). Increased methylation at the IL-6 promoter coincided with increased expression of IL-6 mRNA in obese women (Na et al., 2015). IL-6 gene expression increases when induced by LPS via increased DNA methylation at the IL-6 promoter in bovine endometrial cells (Wang et al., 2018). Alpinetin, a flavonoid found in Alpinia katsumadai Hayata plant, inhibited IL-6 gene expression in murine macrophages by increasing methylation at CpG islands in the IL-6 promoter region (Hu et al., 2020). Therefore, IL-6 is epigenetically regulated in the promoter region and by flavonoid treatments.
The current study extends previous research in this area by testing the anti-inflammatory effects of flavonoids found in chokeberry extract on human primary pre-adipocyte cells epigenetically regulated. Previous work has shown that flavonoids and foods with high flavonoid content affect inflammation pathways in human tissues and that isolated flavonoids (i.e., fisetin and C3G) regulate transcription factors (NF-kB) (Peng et al., 2018) and decrease inflammatory genes expression (IL-6) (Sun and Li, 2018). Cell culture research utilizing chokeberry extract per se has found reductions in expression of IL-1B, IL-6 and IL-8 mRNA in human endothelial cells (Iwashima et al., 2019). A clinical trial utilizing another flavonoid-rich extract demonstrated that consuming tart cherry juice reduced inflammation markers chemokine MCP-1 and cytokine TNF-alpha monocytes (Martin et al., 2018). Research has also examined flavonoid effects on epigenetic regulation of inflammation in human cell lines. In human monocytes (−)-epicatechin reduced inflammation by epigenetic regulation of H3K9 and dimethylation of H3K4 (Cordero-Herrera et al., 2017), while Genistein reduced inflammation in human umbilical vein endothelial cells by repression of the NF-kB signaling pathway through microRNA-155/SOCS1 (Zhang et al., 2017).
Thus, the objective of this study was to determine the effects of whole fruit chokeberry juice extract (CBE) on IL-6 DNA methylation, histone (H3) acetylation, and IL-6 mRNA expression in primary human pre-adipocyte cells (HPA) in the presence and absence of PA induced inflammation. Additionally, we examined C3Gal and C3G effects on IL-6 expression in HPA in order to determine whether anti-inflammatory potential differs between CBE and isolated polyphenolic compounds, C3G and C3Gal. We hypothesized that CBE increases H3 acetylation to decrease IL-6 mRNA expression. We further hypothesized that CBE would decrease methylation in the IL-6 promoter region resulting in decreased IL-6 mRNA expression. In this study new evidence is presented that CBE reduces IL-6 mRNA expression induced by palmitic acid via regulating IL-6 promotor DNA methylation without affecting global histone H3 acetylation of lysine residues K9, K14, and K18 in human preadipocyte cells.
2. Material and methods
2.1. Cell Treatment: Chokeberry extract and palmitic acid
Experiment 1 reported in Results 3.1, 3.6 and 3.7, primary subcutaneous human preadipocytes (American Type Culture Collection (ATCC), Manassas VA, PCS-210-010) were seeded at density of 150,000 cells/well in complete fibroblast medium (cFBM, ATCC, PCS-201-030) with fibroblast growth kit-low serum (ATCC, PCS-201-401), gentamicin, and phenol red. The cells were pre-treated with CBE (4 μl/ml equivalent of 2 μM C3G) for three hours, washed, and treated for additional three hours with one of four treatments: 0.17 mM bovine serum albumin (BSA) for control; 2 μM CBE; 1.0 mM PA + 2 μM CBE; and 1.0 mM PA. Experiments 2–5, reported in Results 3.2 to 3.5 with C3G, C3Gal or CBE; and PA or lipopolysaccharide (LPS; 1 mg/ml) were done seeded in 12 well plates, at a density of 80,000 cells per well. Cells were harvested and stored at −80C° freezer for isolation of genomic DNA, total RNA and protein using AllPrep DNA/RNA/Protein Mini Kit (Qiagen, Valencia, CA).
2.2. Chokeberry extract, C3G, and C3Gal
Frozen fresh Chokeberry was purchased from Northwest Wild Foods (Burlington, Washington) and stored at −20C°. Extract from the frozen berries was attained from 100 g of berries, blended, and centrifuged at 15,000 RPM, 5 min at 4C°. The juice extract was aliquoted and stored at −80C° freezer. Based on quantitative data published in a study (Olivas-Aguirre et al., 2016), we estimated that 20 mg of C3G was contained in 100 g of chokeberry. A dose study was conducted to determine an optimal CBE treatment concentration (e.g., 2 μM C3G equivalent or 4 μl/ml) used for treatments. Similarly, 2 μM concentration for both C3G and C3Gal (Sigma-Aldrich, Burlington, MA, USA) were used.
2.3. Conjugation of fatty acid
68.5 mg sodium palmitate (PA) (Sigma, Burlington, MA) was dissolved in 10 ml Dulbexxo’s phosphate buffered saline (dPBS) (ATCC, Manassas, VA) in a 70C° water bath. 4.534 g of fatty acid free bovine serum albumin (FAFBSA) (Sigma, Burlington, MA) was dissolved in 17 ml (37C°) dPBS and placed inside a 37C° incubator. dPBS was added to FAFBSA to bring to a volume of 20 ml. Ten milliliters of FAFBSA were transferred to a flask inside the 37C° incubator, and 6 ml PA was added. After 20 min of conjugation, an additional 2 ml PA were added and conjugated for 40 min. The conjugated solution was brought up to a 20 ml volume with dPBS for a concentration of 1.7 mM FAFBSA 1.0 mM PA. The remaining 10 ml FAFBSA was diluted with 10 ml dPBS for a concentration of 1.7 mM FAFBSA. Phenol red was added to FAFBSA:PA and FAFBSA solutions. Solutions were filtered with a Luer-Lok 20 ml syringe (Becton, Dickenson and Co., Franklin Lakes, NJ) and Acrodisc 0.2 μM Supor membrane, low protein binding, non-pyrogenic filter (Paul Corp., Ann Arbor, MI) in a safety cabinet, aliquoted, and stored at −20C°.
2.4. RNA, DNA and protein isolation
Experiment 1 cells were homogenized (Retsch MM200 homogenizer, Retsch, Newtown, PA) and total RNA, DNA and protein were isolated using AllPrep DNA/RNA/Protein Mini Kit (Cat No 80004, Qiagen, Valencia, CA, USA). Total RNA extraction for experiments 2–5 from human pre-adipocytes was done according to the protocol of Qiagen’s RNeasy lipid tissue mini kit (Qiagen, Valencia, CA, USA) and using Qiacube (Qiagen, Valencia, CA, USA).
2.5. RT-qPCR analysis
RT-qPCR analysis was performed using methods from our previously published study (Claycombe-Larson et al., 2022). cDNA was synthesized using the Quantitect Reverse Transcriptase kit (Qiagen, Valencia, CA, USA). Gene expression was measured by qPCR (ABI Prism 7500 PCR System, Applied Biosystems, Foster City, CA USA) using Rox FastStart Universal Probe Master mix (Roche, Indianapolis, IN, USA), Hs-IL6 primers were purchased from Integrated DNA Technology (IDT, Coralville, IA, USA): forward, 5′-GCAGATGAGTACAAAAGTCCTGA-3′; reverse, 5-TTCTGTGCCTGCAGCTTC-3′; and probe, 5′-56-FAM/CAACCACAA/ZEN/ATGCCAGCCTGCT/3IABkFQ/-3′. The endogenous control (18S rRNA) was purchased from Applied Biosystems (Foster City, CA, USA). The mRNA expression was normalized to 18S rRNA. The ΔΔCT method was used to calculate fold changes in IL-6 mRNA expression.
2.6. DNA methylation analysis
200 ng of DNA extracted from experiment 1 was treated with sodium bisulfite using Bisulphite Kit (Qiagen, Valencia, CA, USA). Pyro-sequencing of the DNA was conducted using 20 ng of bisulphite treated DNA using PyroMark Q48 (Qiagen, Valencia, CA, USA). IL-6 primers (Qiagen Valencia, CA, USA) (GenBank AF372214.2) were:
Forward (position −268 to −243): 5′-GGGAAAAAAGAAAGTAAAGGAAGAGT-3′
Reverse (position −81 to −54): (Biotin)5′-AAAACTCATAAAAAAATCCCACATTTAA-3′
Sequencing Primer (position −255 to −240): 5′-GTAAAGGAAGAGTGGTT-3′
The target CpG island containing region of the IL-6 promoter includes:
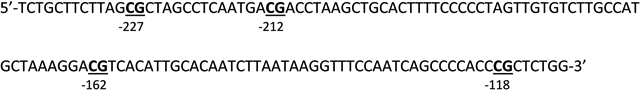
2.7. Western Blot
Western Blot was analyzed on protein extracted from experiment 1 using method from our previously published study (Claycombe-Larson et al., 2022). Proteins (25 μg) were resolved on Novex WedgeWell 4–20 % Tris-Glycine Gel (Invitrogen, Thermo Fisher Scientific, Carlsbad, CA, USA) and then transferred to a 0.45 μM Immobilon-FL transfer membrane (Millipore, Burlington, MA, USA) and incubated in Li-Cor Intercept Blocking Buffer (Li-Cor Biosciences, Lincoln, NE, USA) with primary antibodies anti-Histone H3 (acetyl K9) antibody ab10812 (Abcam, Cambridge, MA, USA), anti-acetyl-Histone H3 (Lys14) antibody 07–353 (Millipore, Burlington, MA, USA), and anti-acetyl-Histone H3 (Lys18) antibody 07–354 (Millipore, Burlington, MA, USA) with anti-histone H3 antibody ab10799 (Abcam, Cambridge, MA, USA) as an endogenous control. Images were developed with the Odyssey M (Li-Cor Biosciences, Lincoln, NE, USA) and analyzed with Empiria Studio Software (Li-Cor Biosciences, Lincoln, NE, USA).
2.8. Statistical analysis
GraphPad Prism 9 was used to determine significance of the difference between conditions using one-way ANOVA for the qPCR, Western Blot and DNA methylation data followed by a Tukey’s Post-hoc test to determine which conditions were significantly different at p < 0.05. The qPCR data are presented as the mean ± standard error of the mean (SEM) with the control group acting as the unit of measure and the expression of treatments relative to the control group as fold-change. The Western Blot and DNA methylation data are presented as mean ± SEM.
3. Results
3.1. Effect of PA, CBE and anthocyanins on IL-6 mRNA expression
As shown in Fig. 1, IL-6 expression is presented as fold change ± SEM with values for Control 1.00 ± 0.92 fold, CBE 1.06 ± 0.94 fold, CBE + PA 21.57 ± 0.91 fold and PA 627.71 ± 1.17-fold. No increase (p = 0.9998) in IL-6 mRNA expression was detected in HPA cells treated with CBE (FAFBSA + CBE) compared to the control group. Treatment of HPA cells with PA resulted in an increase of IL-6 mRNA expression compared to the Control (FAFBSA) (p < 0.0001), CBE (p < 0.0001) and CBE + PA (p = 0.0004) conditions. Treatment of HPA cells with CBE + PA increased IL-6 expression compared to the Control (p = 0.0011) and CBE (p = 0.002) conditions; but showed a decrease (p = 0.0004) in expression compared to PA treatment.
Fig. 1.
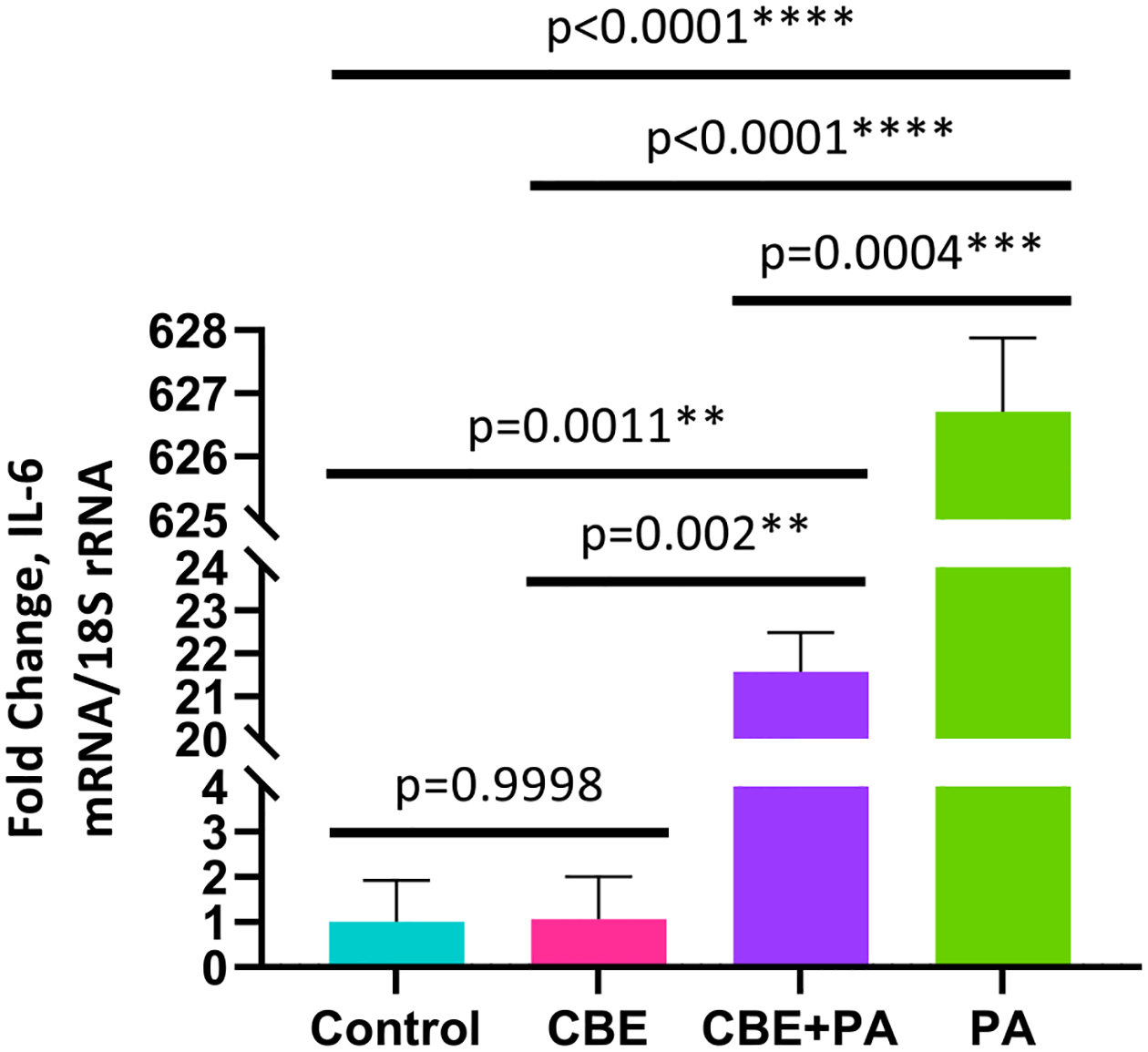
Effects of treatment of CBE on inflammation induced by PA as determined by IL-6 expression from AllPrep extraction. RT-qPCR validation results for IL-6 expression in human primary preadipocytes presented as fold-change ± SEM. Fold-change is relative to the control. (A) PA and CBE treatment results. Significant (P < 0.05) effects identified through a one-way ANOVA and Tukey’s Post Hoc. RNA extracted from same source as Figs. 3 and 4.
3.2. Effects of LPS, and cyanidin 3-glucoside on IL-6 mRNA expression
As shown in Fig. 2A, IL-6 expression is presented as fold change ± SEM with values for Control 1 ± 0.33 fold, C3G 0.06 ± 1.05 fold, C3G + LPS 6.43 ± 0.59 fold and LPS 16.28 ± 0.52 fold. The control had greater (p = 0.0006) expression than C3G and lower expression than C3G + LPS (p = 0.0258) and LPS (p = 0.005). The C3G condition was also lower in expression than the C3G + LPS (p < 0.0001) and LPS (p < 0.0001) conditions. There was no difference (p = 0.4537) between C3G + LPS and LPS conditions.
Fig. 2. Effects of treatments of C3G, C3Gal, and CBE on inflammation induced by PA or LPS as determined by IL-6 expression.
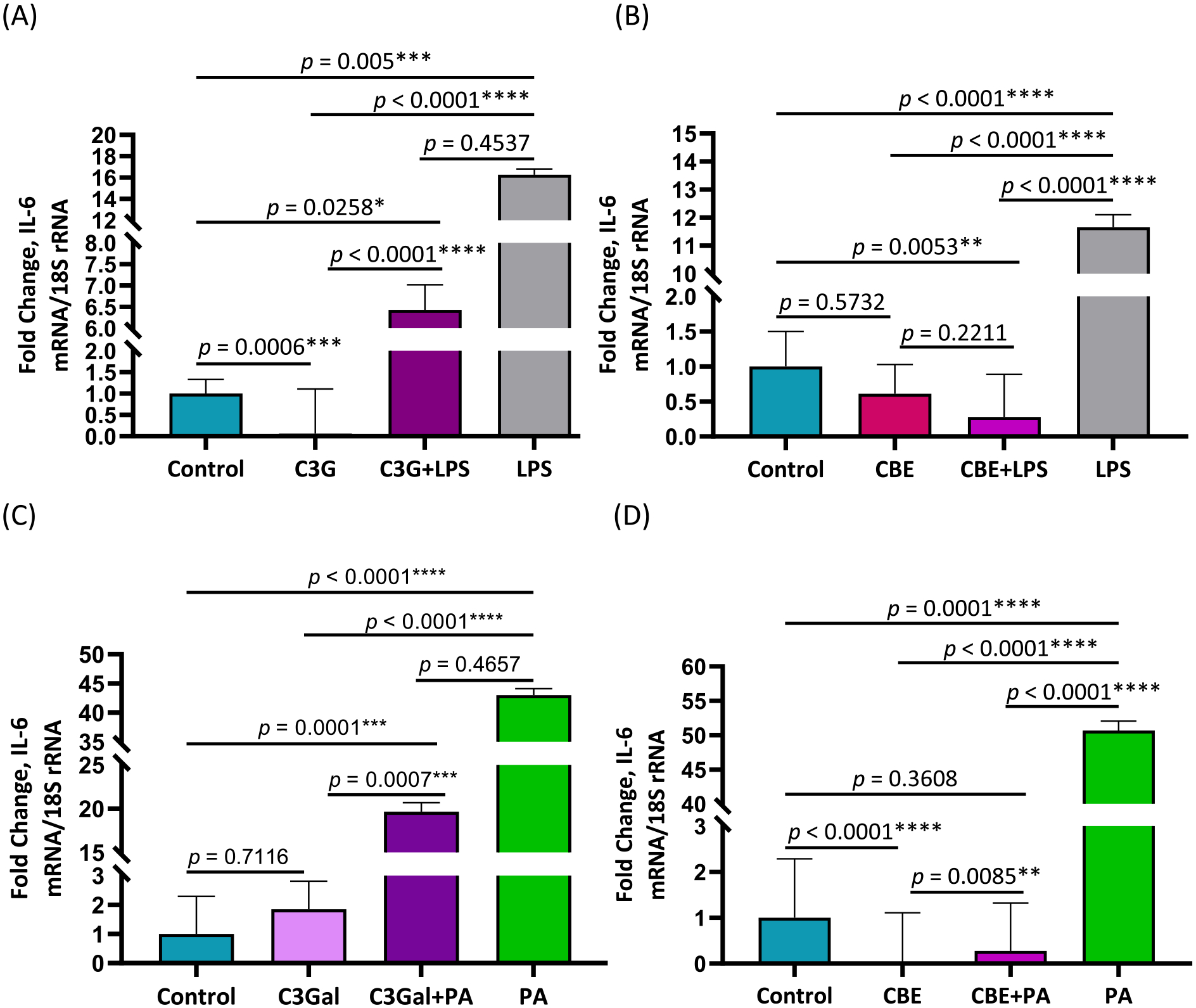
RT-qPCR validation results for IL-6 expression in human primary preadipocytes presented as fold-change ± SEM. Fold-change is relative to the control. (A) LPS and C3G treatments results (B) LPS and CBE treatment results (C) PA and C3Gal treatment results (D) PA and CBE treatment results. Significant (P < 0.05) effects identified through a one-way ANOVA and Tukey’s Post Hoc.
3.3. Effect of LPS, CBE and anthocyanins on IL-6 mRNA expression
As shown in Fig. 2B, IL-6 expression is presented as fold change ± SEM with values for Control 1.00 ± 0.50 fold, CBE 0.61 ± 0.42 fold, CBE + LPS 0.28 ± 0.61 fold and LPS 11.66 ± 0.44 fold. The control group was not significantly different than CBE (p = 0.5732) and significantly higher than CBE + LPS (p = 0.0053) and lower than LPS (p < 0.0001). CBE treatment is not significantly different than the CBE + LPS (p = 0.2211), but significantly lower than LPS (p < 0.0001). CBE + LPS treatment was significantly lower than LPS (p < 0.0001).
3.4. Effect of PA, and cyanidin 3-galactocide on IL-6 mRNA expression
As shown in Fig. 2C, IL-6 expression is presented as fold change ± SEM with values for Control 1 ± 1.29 fold, C3Gal 1.85 ± 0.96 fold, C3Gal + PA 19.66 ± 1.03 fold and PA 43.01 ± 1.09 fold. The control condition did not differ (p = 0.7116) from C3Gal and was lower than C3Gal + PA (p = 0.0001) and PA (p < 0.0001) conditions. C3Gal condition was also lower than the C3Gal + PA (p = 0.0007) and PA (p < 0.0001) conditions. There was no difference (p = 0.4657) between the C3Gal + PA and PA conditions.
3.5. Effect of PA, CBE and anthocyanins on IL-6 mRNA expression
As shown in Fig. 2D, IL-6 expression is presented as fold change ± SEM with values for Control 1 ± 01.29 fold, CBE 0.02 ± 1.09 fold, CBE + PA 0.28 ± 1.04 fold and PA 50.68 ± 1.36 fold. The control condition was greater (p < 0.0001) than CBE and lower (p = 0.0008) than PA, but not different (p = 0.3608) than CBE + PA. CBE was lower than the CBE + PA (p = 0.0085), and PA (p < 0.0001) conditions. CBE + PA was lower (p < 0.0001) than the PA condition.
3.6. Effects of PA, CBE, and anthocyanins on IL-6 DNA methylation
As shown in Fig. 3, DNA methylation in four CpG sites in the promoter region of IL-6 were analyzed and are reported as overall percentage methylation calculated as percentage methylated CpG divided by percentage methylated CpG and unmethylated CpG. Fig. 3A, IL-6 promoter CpG site percent methylation at position 1 (−227 cytosine) were 7.00 ± 1.2 for Control, 8.14 ± 1.64 for CBE, 5.78 ± 0.94 for CBE + PA, and for 8.78 ± 0.66 for PA treatments. Fig. 3B, IL-6 promoter CpG site percent methylation at position 2 (−212 cytosine) were 5.88 ± 0.97 for Control, 6.43 ± 1.40 for CBE, 4.22 ± 0.10 for CBE + PA, and 5.22 ± 0.80 for PA treatments. Fig. 3C, IL-6 promoter CpG site percent methylation at position 3 (−162 cytosine) were 14.13 ± 1.46 for Control, 14.86 ± 3.50 for CBE, 11.89 ± 2.31 for CBE + PA, and 14.11 ± 1.45 for PA treatments. Fig. 4C, IL-6 promoter CpG site percent methylation at position 4 (−118 cytosine) were 9.63 ± 2.23 for Control, 18.29 ± 2.88 for CBE, 10.44 ± 1.32 for CBE + PA, and 13.11 ± 1.39 for PA treatments. ANOVA showed no differences between treatments for methylation of the DNA at CpG position 1–3: Position 1 (p = 0.2196), Position 2 (p = 0.4919), Position 3 (p = 0.7916). Position 4 ANOVA showed a difference (p = 0.0229) which is attributed to the CBE treatment. The IL-6 promoter region for CpG position 4 was more methylated than the control (p = 0.0256) and CBE + PA (p = 0.0418), but not PA (p = 0.2710) conditions.
Fig. 3.
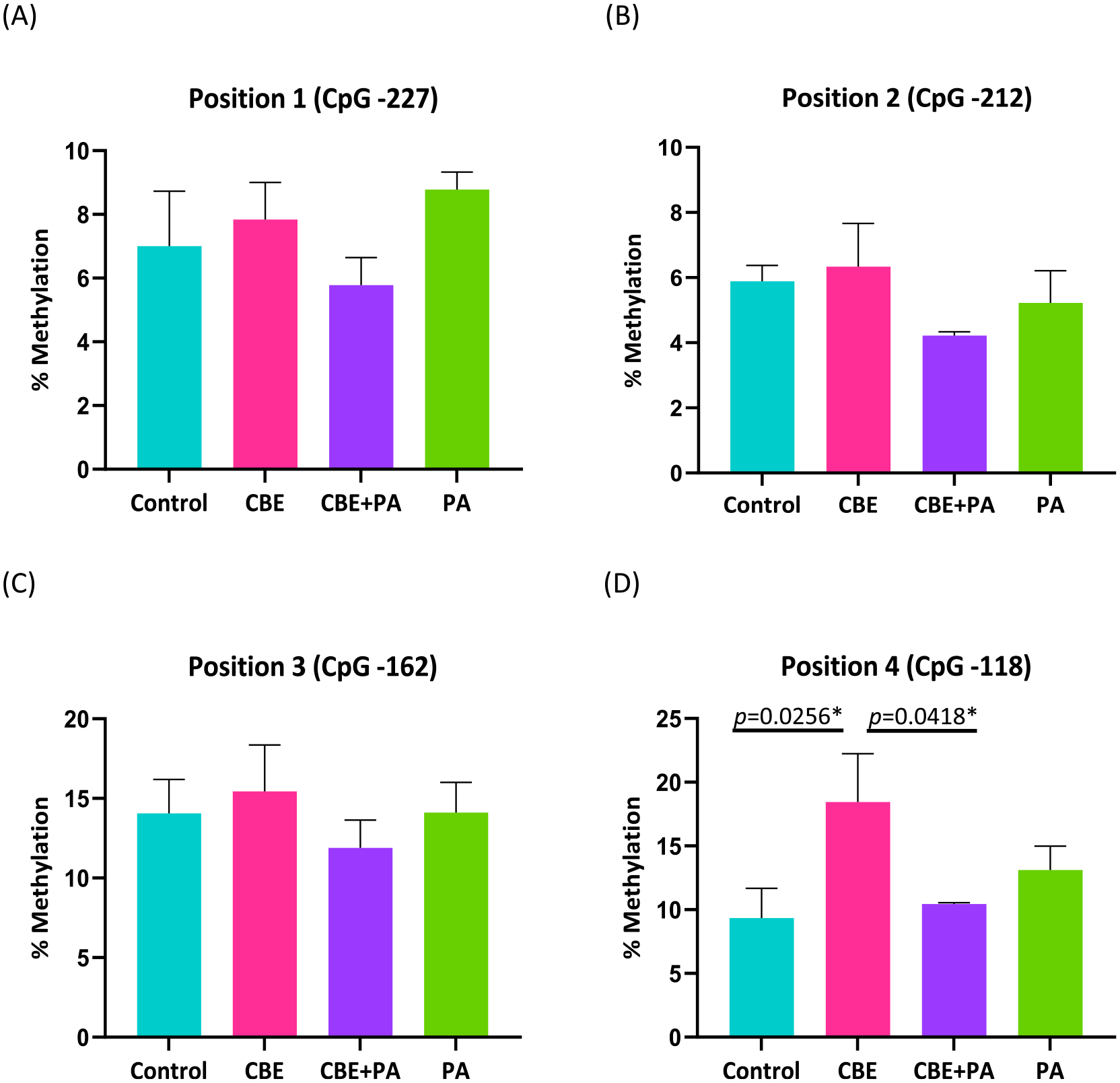
Effects of treatment of CBE and PA on methylation of DNA in the IL-6 Promoter region from AllPrep extraction. Methylation analysis of four CpG islands from pyrosequenced DNA of IL-6 promoter region. (A) Position 1 (CpG −227); (B) Position 2 (CpG −212); (C) Position 3 (CpG −162); (D) Position 4 (CpG −118). Significant (P < 0.05) effects identified through a one-way ANOVA and Tukey’s Post Hoc. DNA extracted from same source as Figs. 1 and 4.
Fig. 4.
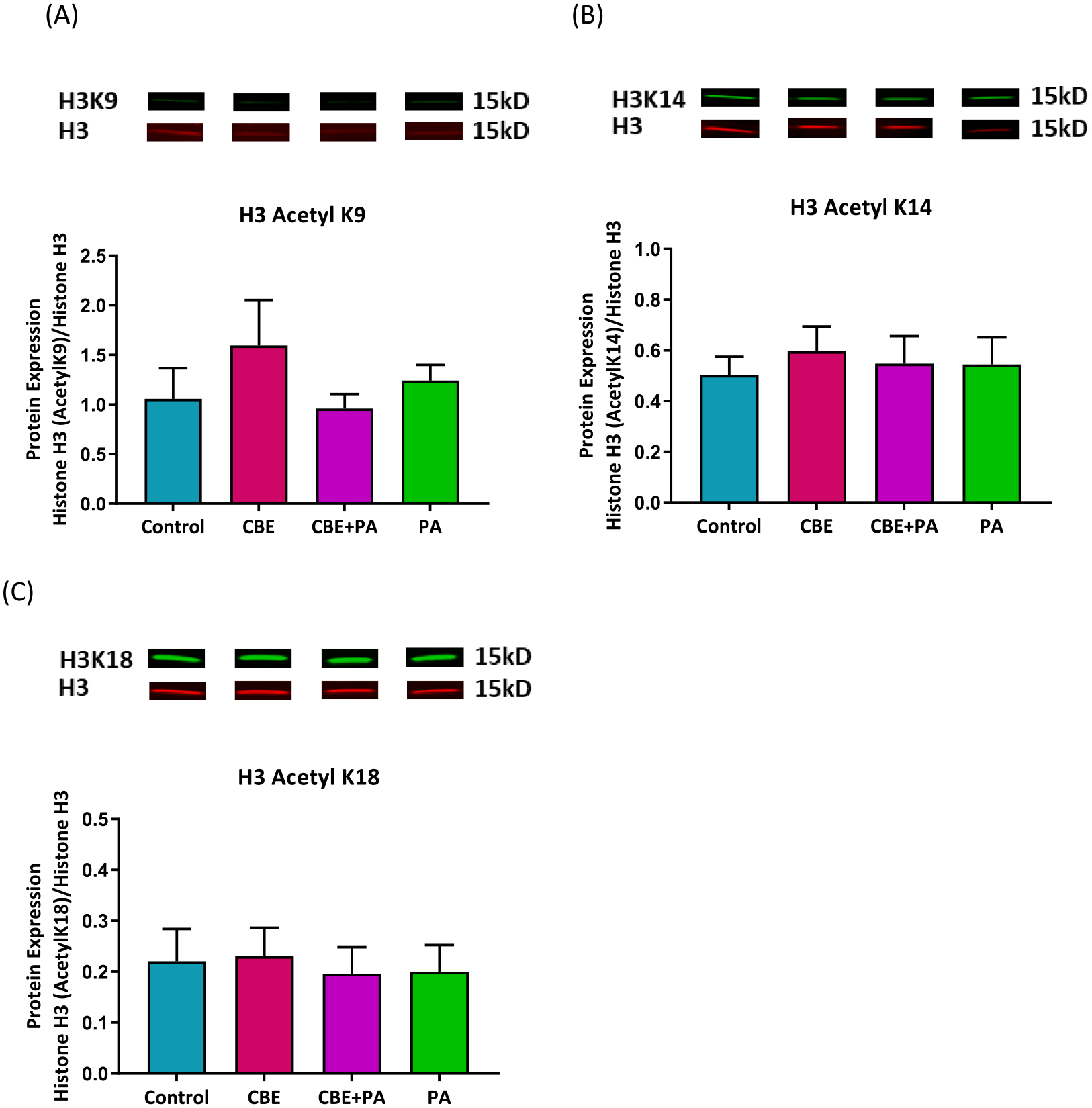
Effects of treatment of CBE and PA on Histone H3 acetylation from AllPrep extraction. Histone H3 acetylation of Lysine residues 9, 14, and 18 as determine by protein expression relative to the H3 Histone. (A) Acetylation of H3K9 (B) Acetylation of H3K14 (C) Acetylation of H3K18. Significant (P < 0.05) effects identified through a one-way ANOVA and Tukey’s Post Hoc. Protein extracted from same source as Figs. 1 and 3.
3.7. Effects of PA, CBE and anthocyanins on histone H3 acetylation
Treatment with CBE, CBE + PA or PA did not show a difference in acetylation in comparison to the control or each other. The ANOVA indicated that there were no differences between treatments for acetylation of H3K9 (p = 0.5001), H3K14 (p = 0.9163) and H3K18 (p = 0.9658).
4. Discussion
Chokeberry is an indigenous fruit that grows in North America which was used in pemmican and as tea by Indigenous Peoples who benefited from the high flavonoid content that is known to alleviate symptoms of diet related disease (Kokotkiewicz et al., 2010). Chronic systemic inflammation is associated with diseases such as obesity, cancer, diabetes, and arthritis (Rehman et al., 2017; Prasad et al., 2021; Nost et al., 2021; Kunz et al., 2021) with a greater prevalence in Indigenous Peoples of North America (Williams et al., 2019). Reducing chronic inflammation may be key for preventing onset of these chronic diseases. Understanding the physiologic, genetic, and epigenetic mechanisms that regulate chronic low-grade inflammation may shed an important light on reducing chronic disease risk in venerable/at risk populations. The current study demonstrated, for the first time, that chokeberry juice extract reduces PA-induced inflammation by regulating IL-6 DNA methylation without affecting histone modifications in human pre-adipocyte cells.
In our current study, hypermethylation of Position 4 CpG −118 site (induced by CBE treatment; Fig. 3D) decreased IL-6 gene expression when compared to the control. Although not observed in the experiment in which the DNA methylation was analyzed (Fig. 1), treatment with CBE reduced IL-6 expression in the two other experiments (Fig. 2B and D). Our results agree with a human lung cancer cell model study in which the four CpG sites between −240 and −80 region of IL-6 gene were hypermethylated and lower IL-6 expression compared to non-cancer cells (Tekpli et al., 2013). In addition, T-cells treated with 5-azacytidine from people with systemic lupus erythematosus (SLE) and from healthy adults showed an increase in IL-6 mRNA expression and a decrease in methylation at CpG islands between −419 and −115 (Mi and Zeng, 2008). SK-N-BE human neuroblastoma cells treated with alpha-lipoic acid, an anti-inflammatory molecule, resulted in a decrease in IL-6 mRNA expression and hypermethylation of CpG cites in IL-6 gene regions 1446 to 1648; and upstream IL-6 promoter region of −245 to −43 (Dinicola et al., 2017). In toto, these findings indicate that CpGs in this region of the IL-6 promoter may suppress transcription when hypermethylated and that CBE, in this study, may be providing a protective effect against IL-6 expression.
This protective effect is observed in the difference of IL-6 mRNA expression between CBE and CBE + PA treatments (Fig. 1). Pre-treatment of CBE in both would result in the hypermethylation of Position 4. The subsequent treatment of CBE + PA results in the PA demethylating Position 4 to control levels (Fig. 3D) and significantly reducing IL-6 mRNA expression in comparison to PA treatment alone (Fig. 1).
However, CpG site in Position 4 was slightly methylated in the PA treatment, which may indicate that the increase in methylation increases transcription, and that CBE has a volatile constituent resulting in the increase methylation (Kokotkiewicz et al., 2010). Although as stated above, in the presence of PA, Position 4 is demethylated. Therefore, if increased methylation of Position 4 was the result of a volatile constituent in CBE, then there should be greater methylation percentage in the CBE + PA treatment compared to either CBE or PA instead of the decrease observed. The methylation caused by CBE may act on a different pathway than that of the PA.
For example, LPS induced IL-6 transcription by activating nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) pathway in RAW 264.7 macrophages, while subsequent treatment with chokeberry concentrate inhibited transcription factor NF-kB and reduced IL-6 expression in a dose dependent manner (Appel et al., 2015). PA increase the IL-6 expression in 3T3-L1 adipocytes by activating the NF-kB pathway and that pretreatment with anthocyanin extract inhibited the PA induced NF-kB pathway (Muscara et al., 2019). Transcription factor p65 along with other transcription factors in the NF-kB pathway bind to the promoter region of IL-6 to increase expression (Kobayashi et al., 2016). The activation of the nuclear factor E2-related factor (Nrf2) pathway decreases IL-6 expression by Nrf2 binding in close proximity to IL-6 resulting in an inhibitory effect by interfering with P65 activation of IL-6 and also disrupting RNA Polymerase II recruitment, but its mechanisms haven’t been elucidated (Kobayashi et al., 2016). Chokeberry may activate the Nrf2 pathway with flavonoids C3G (Fratantonio et al., 2015; Ferrari et al., 2016), and C3Gal (Cui et al., 2021) which have been reported to increase Nrf2 expression. Therefore, methylation of Position 4 may be one of the mechanisms that is used by Nrf2 pathway to protect against PA induced IL-6 transcription by inhibiting transcription factors and possibly specificity protein 1(SP1), which binds between nucleotide −123 to −119 (Tanaka et al., 2014).
The flavonoids C3G and C3Gal are among many found in CBE (Oszmianski and Wojdylo, 2005) which may in combination with other flavonoids in CBE exert a significant inhibition on IL-6 expression of which our data supports. We determined that 2 μM C3G partially reduced IL-6 expression caused by LPS treatment (Fig. 2A). It was also determined that 2 μM C3Gal partially reduced IL-6 expression caused by PA (Fig. 2C). Previous research has shown that 5 μM and 10 μM treatment of C3G showed a dose dependent inhibition of expression of IL-6 in 3T3-L1 adipocyte cells induced by PA with a reduction in expression of approximately 1.7 fold for 5 μM and 2.2 fold for 10uM than PA treatment (Molonia et al., 2020). Mouse macrophage cells exposed to particulate matter (<10 μM) induced IL-6 expression with a subsequent treatment of C3Gal resulting in a decrease in expression observed in the greater concentrations (200 and 400 μg/ml), but not lower concentration (100 μg/ml) (Cui et al., 2021). The result of no effect of 2uM doses of C3G and C3Gal on IL-6 mRNA expression in the current study aligns with the results of previous studies that there is a low threshold for effectiveness, but further research is necessary to determine this lower threshold. However, the expression of IL-6 in C3G + LPS was 2.5 fold lower in comparison to LPS alone and C3Gal + PA was 2.2 fold lower in comparison to PA alone, which are comparable to those reported for 5 μM (1.7 fold lower) and 10 μM (2.1 fold lower). This may be due to a greater variance in our data. It may be worth testing if C3G and C3Gal have an additive effect at reducing IL-6 expression in future research and in combination with other anthocyanins found in chokeberry.
Global histone H3K9 and H3K14 acetylation has been shown to be decreased by treatment with lipoteichoic acid and peptidoglycan in bovine mammary epithelial cells (MAC-T) (Wu et al., 2020). Global histone H3K18 hyperacetylation has been induced in human lymphoma cells (HBL-2) treated with alkylating agents. However, in this study the global acetylation of Histone H3 at lysine residues H3K9, H3K14, and H3K18 were not significantly different between treatments and a more specific analysis that targets the Histones specific to the IL-6 region (Fig. 4) may be needed. Research using Chromatin Immunoprecipitation (ChIP) has been used to determine acetylation of histone H3 specific to the IL-6 promoter (Wada et al., 2014; Hu et al., 2017; Zhang et al., 2015). In mouse macrophages treated with LPS, ChIP analysis indicated H3 and H4 acetylation were positively correlated with IL-6 expression (Zhang et al., 2015). In mouse macrophages treated with paraquot, ChIP-qPCR showed increase levels of H3K4me3 and H3K9ac at the IL-6 promoter coinciding with increased expression of IL-6 (Hu et al., 2017). And in human rheumatoid arthritis synovial fibroblasts treated with curcumin, a ChIP assay determined that increased acetylation of histone H3 results in increased IL-6 production (Wada et al., 2014). Therefore, in future research ChIP assay should be used to further determine the effects of chokeberry on histone H3 acetylation and methylation in correlation to IL-6 expression.
The results here support previous research that chokeberry reduces inflammation by lowering IL-6 mRNA expression (Iwashima et al., 2019; Appel et al., 2015). In addition, our results align with those reporting that alpinetin regulates IL-6 mRNA expression by DNA methylation of the IL-6 promoter region in RAW246.7 murine macrophages (Hu et al., 2017). Alpinetin increased methylation at two CpG islands containing multiple CpG sites in the IL-6 promotor region −500 to −2500 (Hu et al., 2017), while our results indicated that chokeberry increase methylation at one CpG site −118 at Position 4 in human preadipocytes. Chokeberry effects on IL-6 promoter methylation and IL-6 mRNA expression in human preadipocytes build on our understanding of its anti-inflammatory properties.
A proposed mechanism (Fig. 5) for the interaction between CBE and PA on both CpG methylation of Position 4 (−118) and IL-6 mRNA expression involves the activation of both the Nrf2 and NF-kB pathways, respectively. During pretreatment with CBE, the Nrf2 pathway is activated by flavonoids: C3G, C3Gal and/or by another flavonoid in the group of polyphenols. Nrf2 pathway activities increase methylation at Position 4 which then inhibits binding of SP1 transcription factor, as well as, disrupting RNA Polymerase II and other transcription factors as previously reported, resulting in a decrease in IL-6 expression (Kobayashi et al., 2016). We postulate that when PA is added during treatment it induces the NF-kB pathway and IL-6 transcription while reducing methylation at Position 4. When both CBE and PA are present, each compounds induce their pathways which both effect the IL-6 methylation and mRNA expression antagonistically resulting in a reduction in methylation at Position 4 and decreased expression IL-6 compared to PA treatment alone.
Fig. 5. Chokeberry Extract and PA interaction.
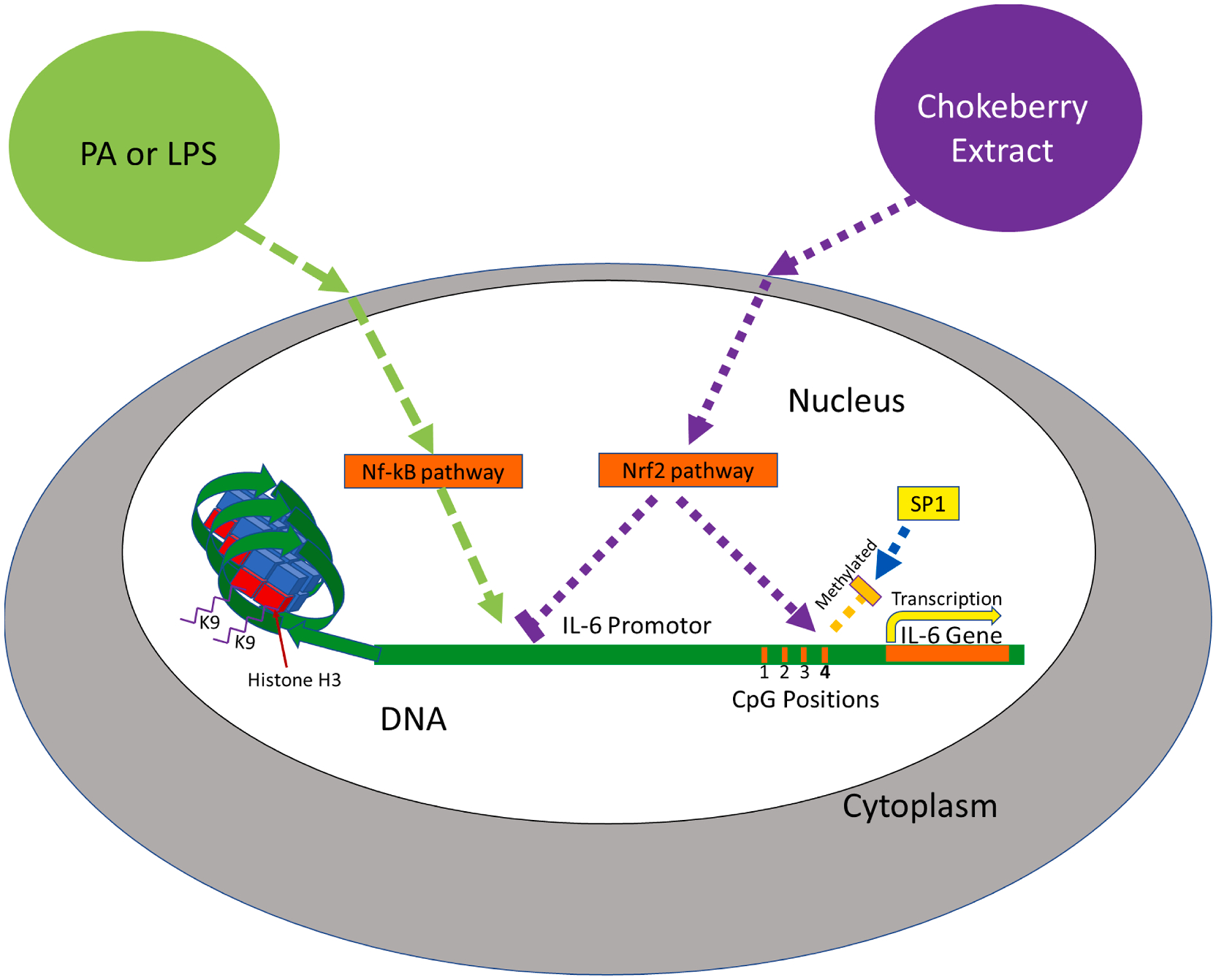
Chokeberry Extract treatment induces transcription of the Nrf2 pathway, hinders NF-kB pathway, and increases methylation of CpG Position 4. Methylation of Position 4 inhibits SP1 binding. PA treatment induces transcription of the IL-6 gene by the NF-kB pathway, but in the presence of CBE IL-6 expression is reduced.
There are some limitations to this study. This study was done in vitro and there may be different physiological factors affecting the interactions in vivo. Although methylation of IL-6 promoter was observed, the additional specific epigenetic regulators that induces those effects still needs to be elucidated. Additionally, the exact mechanism underlying CBE modulate both the NF-kB and Nrf2 pathways in human pre-adipocyte need to be further investigated.
In conclusion, the health benefits that chokeberry may have had on indigenous peoples in staving off chronic illness may be attributed to its anti-inflammatory effects. The epigenetic changes on inflammatory genes like IL-6 may maintain that protection between meals that include chokeberry. In this research we have demonstrated that Chokeberry extract is effective at alleviating inflammation caused by PA or LPS as indicated by IL-6 mRNA expression in primary human preadipocyte cells. However, C3G and C3Gal only partially inhibit IL-6 expression at the 2 μM concentration indicating other bioactive components in CBE may be involved in reducing IL-6 gene expression. Also, global histone H3 acetylation of lysine residues K9, K14, and K18 does not indicate that either PA or CBE effect IL-6 gene expression, and more specific analysis is needed. The data here indicate that CBE has epigenetic modifying effects of the IL-6 promoter region in human preadipocyte cells which may contribute to lowering PA induced IL-6 expression. Although, increased methylation at CpG −118 still needs to be further clarified as to how it is acting as a protective effect against PA treatment. The epigenetic effects of CBE and PA in relation to each other still needs further elucidation. As a result, increased consumption of chokeberry juice may be a feasible dietary solution to reducing inflammation that can cause chronic disease.
Acknowledgments
This work was supported by U.S. Department of Agriculture, Agricultural Research Service project 3062-51000-054-00D and NIGMS Grand Number: 5P20GM139759-03. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. The ITRRC is supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM139759. This research was also supported in part by an appointment to the Agricultural Research Service (ARS) Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the U.S. Department of Agriculture (USDA). ORISE is managed by ORAU under DOE contract number DE-SC0014664. All opinions expressed in this paper are the author’s and do not necessarily reflect the policies and views of USDA, DOE, or ORAU/ORISE.
Footnotes
Ethics Statement
This article does not contain any studies with human or animal participants.
CRediT authorship contribution statement
Dale C. Brunelle: Writing – original draft, Visualization, Investigation, Formal analysis, Data curation. Kate J. Larson: Methodology, Writing – review & editing, Visualization, Investigation, Supervision, Validation, Resources, Project Administration, Funding acquisition, Conceptualization. Amy Bundy: Writing – review & editing, Visualization, Investigation, Formal analysis, Data curation. James N. Roemmich: Writing – review & editing, Funding acquisition. Donald Warne: Writing – review & editing, Conceptualization. Nicole Redvers: Writing – review & editing, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- Appel K, Meiser P, Millan E, Collado JA, Rose T, Gras CC, … Munoz E (2015). Chokeberry (Aronia melanocarpa (Michx.) Elliot) concentrate inhibits NF-kappaB and synergizes with selenium to inhibit the release of pro-inflammatory mediators in macrophages. Fitoterapia, 105, 73–82. 10.1016/j.fitote.2015.06.009 [DOI] [PubMed] [Google Scholar]
- Cai L, Zhan M, Li Q, Li D, & Xu Q (2020). DNA methyltransferase DNMT1 inhibits lipopolysaccharide-induced inflammatory response in human dental pulp cells involving the methylation changes of IL-6 and TRAF6. Molecular Medicine Reports, 21 (2), 959–968. 10.3892/mmr.2019.10860 [DOI] [PubMed] [Google Scholar]
- Cao SS, Luo KL, & Shi L (2016). Endoplasmic Reticulum Stress Interacts With Inflammation in Human Diseases. Journal of Cellular Physiology, 231(2), 288–294. 10.1002/jcp.25098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycombe-Larson KJ, Bundy A, Lance EB, Darland DC, Casperson SL, & Roemmich JN (2022). Postnatal exercise protects offspring from high-fat diet-induced reductions in subcutaneous adipocyte beiging in C57Bl6/J mice. The Journal of Nutritional Biochemistry, 99, Article 108853. 10.1016/j.jnutbio.2021.108853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycombe-Larson KJ, Bundy AN, Kuntz T, Hur J, Yeater KM, Casperson S, … Roemmich JN (2022). Effect of a maternal high-fat diet with vegetable substitution on fetal brain transcriptome. The Journal of Nutritional Biochemistry, 108, Article 109088. 10.1016/j.jnutbio.2022.109088 [DOI] [PubMed] [Google Scholar]
- Cordero-Herrera I, Chen X, Ramos S, & Devaraj S (2017). (−)-Epicatechin attenuates high-glucose-induced inflammation by epigenetic modulation in human monocytes. European Journal of Nutrition, 56(3), 1369–1373. 10.1007/s00394-015-1136-2 [DOI] [PubMed] [Google Scholar]
- Cui YM, Lin Y, Meng XJ, Ma JX, Deng HT, Liu X, … Zhao J (2021). Cyanidin-3-galactoside from Aronia melanocarpa ameliorates PM10 induced pulmonary injury by modulating M1/M2 macrophage polarization and NRF2/Sirt1 MAPK signaling. Journal of Functional Foods, 78. 10.1016/j.jff.2021.104363 [DOI] [Google Scholar]
- Dinicola S, Proietti S, Cucina A, Bizzarri M, & Fuso A (2017). Alpha-Lipoic Acid Downregulates IL-1beta and IL-6 by DNA Hypermethylation in SK-N-BE Neuroblastoma Cells. Antioxidants (Basel), 6(4). 10.3390/antiox6040074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Speciale A, Cristani M, Fratantonio D, Molonia MS, Ranaldi G, … Cimino F (2016). Cyanidin-3-O-glucoside inhibits NF-kB signalling in intestinal epithelial cells exposed to TNF-alpha and exerts protective effects via Nrf2 pathway activation. Toxicology Letters, 264, 51–58. 10.1016/j.toxlet.2016.10.014 [DOI] [PubMed] [Google Scholar]
- Fratantonio D, Speciale A, Ferrari D, Cristani M, Saija A, & Cimino F (2015). Palmitate-induced endothelial dysfunction is attenuated by cyanidin-3-O-glucoside through modulation of Nrf2/Bach1 and NF-kappaB pathways. Toxicology Letters, 239 (3), 152–160. 10.1016/j.toxlet.2015.09.020 [DOI] [PubMed] [Google Scholar]
- Goh AR, Youn GS, Yoo KY, Won MH, Han SZ, Lim SS, … Park J (2016). Aronia melanocarpa Concentrate Ameliorates Pro-Inflammatory Responses in HaCaT Keratinocytes and 12-O-Tetradecanoylphorbol-13-Acetate-Induced Ear Edema in Mice. Journal of Medicinal Food, 19(7), 654–662. 10.1089/jmf.2015.3624 [DOI] [PubMed] [Google Scholar]
- Greenberg AS, & Obin MS (2006). Obesity and the role of adipose tissue in inflammation and metabolism. The American Journal of Clinical Nutrition, 83(2), 461S–465S. 10.1093/ajcn/83.2.461S [DOI] [PubMed] [Google Scholar]
- Guo Y, Shu L, Zhang C, Su ZY, & Kong AN (2015). Curcumin inhibits anchorage-independent growth of HT29 human colon cancer cells by targeting epigenetic restoration of the tumor suppressor gene DLEC1. Biochemical Pharmacology, 94(2), 69–78. 10.1016/j.bcp.2015.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang Y, Qin X, Ren T, & Cao J (2018). Baicalin reduces blood lipids and inflammation in patients with coronary artery disease and rheumatoid arthritis: A randomized, double-blind, placebo-controlled trial. Lipids in Health and Disease, 17 (1), 146. 10.1186/s12944-018-0797-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin JW (1973). The enigmatic chokeberries (Aronia, Rosaceae). Bulletin of the Torrey Botanical Club, 100(No. 3(May-Jun)), 178–184. [Google Scholar]
- Hu K, Li Y, Liang M, Liu L, Chen Y, Huang M, … Yin H (2020). Inhibitory effect of alpinetin on IL-6 expression by promoting cytosine methylation in CpG islands in the IL-6 promoter region. Molecular Genetics & Genomic Medicine, 8(1), Article e993. 10.1002/mgg3.993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Yu Y, Huang H, Fan H, Hu L, Yin C, … Chen F (2017). Epigenetic regulation of interleukin 6 by histone acetylation in macrophages and its role in Paraquat-induced pulmonary fibrosis. Frontiers in Immunology, 7, 696. 10.3389/fimmu.2016.00696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashima T, Kudome Y, Kishimoto Y, Saita E, Tanaka M, Taguchi C, … Iida K (2019). Aronia berry extract inhibits TNF-alpha-induced vascular endothelial inflammation through the regulation of STAT3. Food & Nutrition Research, 63. 10.29219/fnr.v63.3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Na H, Kasai H, Kawai K, Li YS, & Yang M (2017). Comparison of Blueberry (Vaccinium spp.) and Vitamin C via Antioxidative and Epigenetic Effects in Human. Journal of Cancer Prevention, 22(3), 174–181. 10.15430/JCP.2017.22.3.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, … Yamamoto M (2016). Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nature Communications, 7, 11624. 10.1038/ncomms11624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokotkiewicz A, Jaramicz Z, & Luczkiewicz M (2010). Aronia Plants: A Review of Traditional Use, Biological Activities, and Perspectives for Modern Medicin. Journal of Medicinal Food, 13(2), 255–269. 10.1089/jmf.2009.0062 [DOI] [PubMed] [Google Scholar]
- Kunz HE, Hart CR, Gries KJ, Parvizi M, Laurenti M, Dalla Man C, … Lanza IR (2021). Adipose tissue macrophage populations and inflammation are associated with systemic inflammation and insulin resistance in obesity. American Journal of Physiology. Endocrinology and Metabolism, 321(1), E105–E121. 10.1152/ajpendo.00070.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson C, Andersson KME, Nadali M, Silfversward ST, Bokarewa MI, & Erlandsson MC (2023). MicroRNA and interleukin 6 interplay in the adipose tissue of rheumatoid arthritis patients. Clinical and Experimental Rheumatology, 41(1), 32–40. 10.55563/clinexprheumatol/f4vlvt [DOI] [PubMed] [Google Scholar]
- Maleki SJ, Crespo JF, & Cabanillas B (2019). Anti-inflammatory effects of flavonoids. Food Chemistry, 299, Article 125124. 10.1016/j.foodchem.2019.125124 [DOI] [PubMed] [Google Scholar]
- Martin KR, Burrell L, & Bopp J (2018). Authentic tart cherry juice reduces markers of inflammation in overweight and obese subjects: A randomized, crossover pilot study. Food & Function, 9(10), 5290–5300. 10.1039/c8fo01492b [DOI] [PubMed] [Google Scholar]
- Mendelova A, Mendel L, Solgajova M, Kolesarova A, Marecek J, & Zelenakova L (2022). Comparison of the influence of different fruit drying methods on the content of selected bioactive substances. The Journal of Microbiology, Biotechnology and Food Sciences, 12(special issue). 10.55251/jmbfs.9223. Special Issue. [DOI] [Google Scholar]
- Mi XB, & Zeng FQ (2008). Hypomethylation of interleukin-4 and −6 promoters in T cells from systemic lupus erythematosus patients. Acta Pharmacologica Sinica, 29(1), 105–112. 10.1111/j.1745-7254.2008.00739.x [DOI] [PubMed] [Google Scholar]
- Molonia MS, Occhiuto C, Muscara C, Speciale A, Bashllari R, Villarroya F, … Cristani M (2020). Cyanidin-3-O-glucoside restores insulin signaling and reduces inflammation in hypertrophic adipocytes. Archives of Biochemistry and Biophysics, 691, Article 108488. 10.1016/j.abb.2020.108488 [DOI] [PubMed] [Google Scholar]
- Muscara C, Molonia MS, Speciale A, Bashllari R, Cimino F, Occhiuto C, … Cristani M (2019). Anthocyanins ameliorate palmitate-induced inflammation and insulin resistance in 3T3-L1 adipocytes. Phytotherapy Research, 33(7), 1888–1897. 10.1002/ptr.6379 [DOI] [PubMed] [Google Scholar]
- Na YK, Hong HS, Lee WK, Kim YH, & Kim DS (2015). Increased methylation of interleukin 6 gene is associated with obesity in Korean women. Molecules and Cells, 38(5), 452–456. 10.14348/molcells.2015.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruszewicz M, Laniewska I, Millo B, & Dluzniewski M (2007). Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infraction (MI). Atherosclerosis, 194(2), e179–e184. 10.1016/j.atherosclerosis.2006.12.032 [DOI] [PubMed] [Google Scholar]
- Nost TH, Alcala K, Urbarova I, Byrne KS, Guida F, Sandanger TM, & Johansson M (2021). Systemic inflammation markers and cancer incidence in the UK Biobank. European Journal of Epidemiology, 36(8), 841–848. 10.1007/s10654-021-00752-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell JM, Rockell JE, Ouellet JC, Yoder S, Lind KE, Wilson C, … Manson SM (2021). The Prevalence of Cardiovascular Disease and Other Comorbidities Among American Indian and Alaska Native Adults with Diabetes. EC Journal of Endocrinology and Metabolism Research, 6(2), 5–20. https://www.ncbi.nlm.nih.gov/pubmed/34766170. [PMC free article] [PubMed] [Google Scholar]
- Olivas-Aguirre FJ, Rodrigo-Garcia J, Martinez-Ruiz ND, Cardenas-Robles AI, Mendoza-Diaz SO, Alvarez-Parrilla E, … Wall-Medrano A (2016). Cyanidin-3-O-glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules, 21(9). 10.3390/molecules21091264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oszmianski J, & Wojdylo A (2005). Aronia melanocarpa phenolics and their antioxidant activity. European Food Research and Technology, 221(6), 809–813. 10.1007/s00217-005-0002-5 [DOI] [Google Scholar]
- Pei R, Liu J, Martin DA, Valdez JC, Jeffery J, Barrett-Wilt GA, … Bolling BW (2019). Aronia Berry Supplementation Mitigates Inflammation in T Cell Transfer-Induced Colitis by Decreasing Oxidative Stress. Nutrients, 11(6). 10.3390/nu11061316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng HL, Huang WC, Cheng SC, & Liou CJ (2018). Fisetin inhibits the generation of inflammatory mediators in interleukin-1beta-induced human lung epithelial cells by suppressing the NF-kappaB and ERK1/2 pathways. International Immunopharmacology, 60, 202–210. 10.1016/j.intimp.2018.05.004 [DOI] [PubMed] [Google Scholar]
- Perkins DJ, Patel MC, Blanco JC, & Vogel SN (2016). Epigenetic Mechanisms Governing Innate Inflammatory Responses. Journal of Interferon & Cytokine Research, 36(7), 454–461. 10.1089/jir.2016.0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Kulshreshtha A, Lall R, & Gupta SC (2021). Inflammation and ROS in arthritis: Management by Ayurvedic medicinal plants. Food & Function, 12(18), 8227–8247. 10.1039/d1fo01078f [DOI] [PubMed] [Google Scholar]
- Raghav A, Ahmad J, & Alam K (2018). Preferential recognition of advanced glycation end products by serum antibodies and low-grade systemic inflammation in diabetes mellitus and its complications. International Journal of Biological Macromolecules, 118 (Pt B), 1884–1891. 10.1016/j.ijbiomac.2018.07.033 [DOI] [PubMed] [Google Scholar]
- Rehman K, Akash MSH, Liaqat A, Kamal S, Qadir MI, & Rasul A (2017). Role of Interleukin-6 in Development of Insulin Resistance and Type 2 Diabetes Mellitus. Critical Reviews in Eukaryotic Gene Expression, 27(3), 229–236. 10.1615/CritRevEukaryotGeneExpr.2017019712 [DOI] [PubMed] [Google Scholar]
- Roe K (2021). An inflammation classification system using cytokine parameters. Scandinavian Journal of Immunology, 93(2), Article e12970. 10.1111/sji.12970 [DOI] [PubMed] [Google Scholar]
- Russo GL, Vastolo V, Ciccarelli M, Albano L, Macchia PE, & Ungaro P (2017). Dietary polyphenols and chromatin remodeling. Critical Reviews in Food Science and Nutrition, 57(12), 2589–2599. 10.1080/10408398.2015.1062353 [DOI] [PubMed] [Google Scholar]
- Sun Y, & Li L (2018). Cyanidin-3-glucoside inhibits inflammatory activities in human fibroblast-like synoviocytes and in mice with collagen-induced arthritis. Clinical and Experimental Pharmacology & Physiology, 45(10), 1038–1045. 10.1111/1440-1681.12970 [DOI] [PubMed] [Google Scholar]
- Takahashi A, de Andres MC, Hashimoto K, Itoi E, & Oreffo RO (2015). Epigenetic regulation of interleukin-8, an inflammatory chemokine, in osteoarthritis. Osteoarthritis Cartilage, 23(11), 1946–1954. 10.1016/j.joca.2015.02.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Narazaki M, & Kishimoto T (2014). IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspectives in Biology, 6(10), Article a016295. 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekpli X, Landvik NE, Anmarkud KH, Skaug V, Haugen A, & Zienolddiny S (2013). DNA methylation at promoter regions of interleukin 1B, interleukin 6, and interleukin 8 in non-small cell lung cancer. Cancer Immunology, Immunotherapy, 62 (2), 337–345. 10.1007/s00262-012-1340-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Liimatainen J, Alanne AL, Lindstedt A, Liu P, Sinkkonen J, … Yang B (2017). Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chemistry, 220, 266–281. 10.1016/j.foodchem.2016.09.145 [DOI] [PubMed] [Google Scholar]
- Wada TT, Araki Y, Sato K, Aizaki Y, Yokota K, Kim YT, … Mimura T (2014). Aberrant histone acetylation contributes to elevated interleukin-6 production in rheumatoid arthritis synovial fibroblasts. Biochemical and Biophysical Research Communications, 444(4), 682–686. 10.1016/j.bbrc.2014.01.195 [DOI] [PubMed] [Google Scholar]
- Wang J, Yan X, Nesengani LT, Ding H, Yang L, & Lu W (2018). LPS-induces IL-6 and IL-8 gene expression in bovine endometrial cells “through DNA methylation”. Gene, 677, 266–272. 10.1016/j.gene.2018.07.074 [DOI] [PubMed] [Google Scholar]
- Williams SL, Kaigler A, Armistad A, Espey DK, & Struminger BB (2019). Creating a Public Health Community of Practice to Support American Indian and Alaska Native Communities in Addressing Chronic Disease. Preventing Chronic Disease, 16, E109. 10.5888/pcd16.190193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Chen J, Sun Y, Dong X, Wang Z, Chen J, & Dong G (2020). PGN and LTA from Staphylococcus aureus Induced Inflammation and Decreased Lactation through Regulating DNA Methylation and Histone H3 Acetylation in Bovine Mammary Epithelial Cells. Toxins (Basel), 12(4). 10.3390/toxins12040238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapolska-Downar D, Bryk D, Malecki M, Hajdukiewicz K, & Sitkiewicz D (2012). Aronia melanocarpa fruit extract exhibits anti-inflammatory activity in human aortic endothelial cells. European Journal of Nutrition, 51(5), 563–572. 10.1007/s00394-011-0240-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Xu Z, Zhao H, Wang X, Pang J, Li Q, … Ling W (2020). Anthocyanin supplementation improves anti-oxidative and anti-inflammatory capacity in a dose-response manner in subjects with dyslipidemia. Redox Biology, 32, Article 101474. 10.1016/j.redox.2020.101474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhao Z, Pang X, Yang J, Yu H, Zhang Y, … Zhao J (2017). Genistein Protects Against Ox-LDL-Induced Inflammation Through MicroRNA-155/SOCS1-Mediated Repression of NF-kB Signaling Pathway in HUVECs. Inflammation, 40(4), 1450–1459. 10.1007/s10753-017-0588-3 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhao K, Shen Q, Han Y, Gu Y, Li X, … Cao X (2015). Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature, 525 (7569), 389–393. 10.1038/nature15252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Zhang J, & Chang N (2018). Epigenetic modification of Nrf2 by sulforaphane increases the antioxidative and anti-inflammatory capacity in a cellular model of Alzheimer’s disease. European Journal of Pharmacology, 824, 1–10. 10.1016/j.ejphar.2018.01.046 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Li Y, Ran X, Wang D, Zheng X, Zhang M, … Wu J (2022). Mettl14 mediates the inflammatory response of macrophages in atherosclerosis through the NF-kappaB/IL-6 signaling pathway. Cellular and Molecular Life Sciences, 79(6), 311. 10.1007/s00018-022-04331-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


