Abstract
Context
An association of thyroid function with mood disorders has been widely suggested, but very few studies have examined this association longitudinally.
Objective
We assessed the cross-sectional and longitudinal association between thyroid function and depression in a population-based cohort.
Methods
A total of 9471 individuals were included in cross-sectional analyses, of whom 8366 had longitudinal data. At baseline, we assessed thyroid function using serum samples (thyrotropin [TSH], free thyroxine (FT4), and thyroid peroxidase antibodies) and depressive symptoms using the Centre for Epidemiologic Studies Depression (CES-D) scale. Incident depressive events (n = 1366) were continuously followed up with the CES-D and clinical interviews. We analyzed the cross-sectional association of thyroid function and thyroid disease with depressive symptoms using linear and logistic regression, and the longitudinal association with Cox proportional hazard models for depressive events.
Results
Lower TSH levels and lower and higher FT4 levels were cross-sectionally associated with more depressive symptoms with a B value of −0.07 per 1 unit increase of natural log-transformed TSH (95% CI −0.11; −0.04). Furthermore, hypothyroidism was cross-sectionally associated with less depressive symptoms and hyperthyroidism with more depressive symptoms. Longitudinally, there was a U-shaped association between FT4 and incident depressive events but only in euthyroid participants.
Conclusion
We show a cross-sectional association between thyroid (dys)function with depressive symptoms, and a U-shaped association between FT4 and incident depressive events in euthyroid individuals. Our findings suggest an association of thyroid function with the risk of developing depression, albeit small. Reverse causation and additional underlying factors may also contribute to the association.
Keywords: TSH, FT4, thyroid disease, depression, cross-sectional, prospective
Severe alterations in thyroid function have been related to neurological deficits and psychiatric diseases such as major depressive disorder (1, 2). People with overt hypothyroidism and hyperthyroidism can present with depressive symptoms and disorders (3, 4), and patients with hypothyroidism have been described to present with features such as energy loss and fatigue, which are also diagnostic criteria for depressive disorders (5). However, most patients with thyroid disorders present with only mild overt or even subclinical thyroid disease (5) and the evidence for the link between mild thyroid dysfunction and depression is unclear. Thyroid function is related to short- and long-term brain changes, including neuronal plasticity processes, angiogenesis, and neurogenesis in adults (6), and these alterations are related to depression (2, 7). Thus, we hypothesized a short- and long-term effect of thyroid function on depression. In a smaller subset of the Rotterdam Study, we previously reported that lower levels of thyrotropin (TSH) in the normal range are related to higher occurrence of depressive symptoms in cross-sectional analyses and greater risk of incident depressive syndromes in longitudinal analyses in a population-based sample of euthyroid participants (8), but free thyroxine (FT4) levels were not available at that time. Similarly, Varella et al (9) showed a positive association between low TSH and incident depression in women, and this association remained even when restricting analyses to euthyroid participants. In contrast, a cross-sectional study by Delitala et al showed no association between TSH and depression but described a U-shaped relation between FT4 and depression where low and high levels of FT4 were related to more depressive symptoms (10). The link between thyroid autoimmunity and depression has been suggested before, with some studies reporting a higher prevalence of lifetime diagnosis of depression in individuals with thyroid autoimmunity, although not consistently (11, 12). Furthermore, autoimmunity per se has been suggested to be related to depression (13), and thyroid peroxidase antibody (TPOAb) as a marker of autoimmunity is not always related to alterations in TSH and/or thyroid hormones, suggesting that thyroid function is not enough to evaluate the effect of thyroid autoimmunity (14, 15). Overall, the evidence on the association between thyroid function and depression is still inconclusive. Most of the previous studies were cross-sectional, providing limited information on temporality, and those that examined the association in longitudinal analyses had small to moderate sample sizes (eg, n = 44) (16), only included participants with major depressive disorder (16), or only assessed euthyroid participants (8).
In this population-based cohort study, we examined the association of thyroid function parameters (TSH, FT4, and TPOAb) with depressive symptoms in cross-sectional analyses and with incident depressive events in longitudinal analyses. We hypothesized that participants with thyroid disease and participants with low TSH levels would present more depressive symptoms cross-sectionally, and would have a higher risk of developing incident depressive events.
Materials and Methods
The present analyses were performed using data from the Rotterdam Study. The Rotterdam Study is a population-based prospective study located in the district of Ommoord in the city of Rotterdam in The Netherlands (17). Objectives, design and aim have been described previously (17). We included 3 independent cohorts from the Rotterdam Study. The first cohort includes 7983 participants aged 55 years or older and it started in 1989. The second cohort includes 3011 participants aged 55 years and older, and started in 2000, and the third cohort is composed of 3932 participants who are 45 years or older and started in 2006. By 2008 the Rotterdam Study consisted of 14 926 participants. In total, there were 9684 participants with thyroid function measurements available at baseline, collected between 1997 and 2008 (third visit of the first cohort, first visit of the second cohort, and first visit of the third cohort). From these, 9471 also had information on depressive symptoms at baseline and were included in the cross-sectional analyses. For the longitudinal analyses, we additionally excluded participants who did not have information on depression collected prospectively (available from follow-up research visits or medical records, n = 5) or who had a depressive event before or at baseline (baseline defined as the date of blood sample collection, n = 1100). In total, we included 8366 participants in the longitudinal analyses (Fig. S1 (18)). Additionally, in these analyses, participants were censored if they moved house, died, or at the end of the study (January 1, 2012 for the first and second cohorts, and January 1, 2019 for the third cohort), whichever came first.
The Rotterdam Study was approved by the Medical Ethics Committee of the Erasmus MC (registration number MEC 02.1015) and by the Dutch Ministry of Health, Welfare and Sport (Population Screening Act WBO, license number 1071272-159521-PG). The Rotterdam Study Personal Registration Data collection is filed with the Erasmus MC Data Protection Officer under registration number EMC1712001. The Rotterdam Study has been entered into the Dutch Trial Register (NTR; https://onderzoekmetmensen.nl) and into the WHO International Clinical Trials Registry Platform (ICTRP https://www.who.int/clinical-trials-registry-platform, search portal https://trialsearch.who.int/) under shared catalogue number NL6645/NTR6831. All participants provided written informed consent to participate in the study and to have their information obtained from treating physicians.
Thyroid Function Measurements
Thyroid function measurements (TSH, FT4, and TPOAb) were determined with the electrochemiluminescence immunoassay “ECLIA”, Roche using serum samples collected between 1997 and 2008 and stored at −80 °C (19). Reference ranges were defined for TSH as 0.40 to 4.0 mIU/L and for FT4 as 11 to 25 pmol/L (19). TPOAb levels with a value >35 kU/mL were considered positive according to the recommendations of the manufacturer. Euthyroidism was defined based on reference values of TSH and FT4, no thyroid-altering medication use at baseline, and TPOAb lower or equal to 35 kU/mL. Hypothyroidism and hyperthyroidism, including subclinical thyroid dysfunction, were defined based on TSH levels (hypothyroidism was defined as TSH >4.0 mIU/L and hyperthyroidism was defined as TSH <0.4 mIU/L) and no thyroid-altering medication use at baseline. Participants who were taking thyroid-altering medication at baseline were included in analyses when examining the association in the full range of thyroid function, but were excluded in analyses in euthyroid participants and when studying thyroid disease.
Depression Measurements
At baseline, depressive symptoms were assessed with the Dutch version of the Centre for Epidemiologic Studies Depression (CES-D) scale (20, 21). This validated self-report 20-item instrument for research in individuals from the general population assesses the occurrence of depressive symptoms in the past week (20). The possible response values for each question range from 0 to 3 and the total sum score from 0 to 60. A score of 16 or higher was defined as clinically relevant depressive symptoms (21, 22). In our cross-sectional analyses, we included as outcomes of interest the depressive symptoms score and the clinically relevant depressive symptoms.
Incident depressive events were collected in 3 ways. First, the CES-D was assessed at each follow-up visit. Second, participants with a score of 16 or higher were interviewed by a trained research assistant (23) using a semistructured interview (Schedules for Clinical Assessment in Neuropsychiatry, SCAN) (24, 25) to determine any depressive episode following the diagnostic criteria of the DSM-IV-TR (Diagnostic and Statistical Manual of Mental Disorders, 4th edition revised). Third, medical records were continuously followed up for depressive symptoms and disorders (26). This systematic follow-up was performed by trained research assistants who assessed the medical records of GPs for information that indicated potential depression, such as depressive symptoms, medication, and major life events (26). Afterwards, 2 trained research assistants or health care professionals with mental health expertise evaluated the information and categorized each potential event based on a pre-established protocol (26); disagreements were resolved with help of a psychiatrist or psychologist. For the current study, we defined our main outcome in longitudinal analyses as any incident depressive event (any DSM-IV TR mood disorder [except bipolar episode or disorder] or clinically relevant depressive symptoms). Depressive syndrome refers to dysthymia (based on DSM-IV criteria and diagnosed by a mental health professional), depression diagnosed by a physician, or self-reported when the participant consulted a GP or a mental health professional, and minor depression based on DSM-IV criteria (26). Two secondary outcomes were included in additional analyses. First, we examined incident major depressive disorder, to assess a more clinically relevant outcome, and, second, incident depressive syndromes (major depressive disorder or depressive syndrome) to allow comparability with the previous study performed in this cohort (8). For the definitions of the outcomes in longitudinal analyses, an event was considered as the first episode of each outcome occurring in participants with no events at baseline. Participants with bipolar disorder were included as part of the control group.
Covariates
Covariates used in this study include age, sex, cohort, education, body mass index (BMI), physical activity, smoking, alcohol consumption, and diet. The cohort categorical covariate states whether the participants were part of the first, second, or third cohort of the Rotterdam Study. Education was self-reported and classified into 4 categories: primary (primary education), lower (lower/intermediate general education or lower vocational education), intermediate (intermediate vocational education or higher general education), and high (higher vocational education or university) (27). BMI (kg/m2) was calculated from height in meters and weight in kilograms of the participant. Height and weight were measured at the research center using a stadiometer and a digital scale, respectively (28). Physical activity was evaluated based on the Zutphen Physical Activity Questionnaire (29) and the LASA Physical Activity Questionnaire (30) and presented as metabolic equivalent of task (MET) hours per week to measure the intensity of physical activity. We used a standardized measure of physical activity in analyses. Smoking information was collected in the home interview and was defined as never, former, or current. Alcohol consumption was defined as total alcohol intake in grams per day. As described in previous studies (27), information on dietary intake was collected using the Food Frequency Questionnaire (the assessment in Rotterdam Study cohort 1 [RS1] and Rotterdam Study cohort 2 [RS2] used a slightly different approach from Rotterdam Study cohort 3 [RS3] (27)), and a score indicating the adherence to the Dutch dietary guidelines was calculated. Covariates were collected at the study baseline (except for education and diet in RS1, which were collected before this study's baseline, between 1989 and 1993).
Statistical Analyses
We studied the association between TSH, FT4, TPOAb status, and thyroid disease (hypothyroidism and hyperthyroidism) with depressive symptoms and clinically relevant depressive symptoms cross-sectionally and with incident depressive events longitudinally. TSH and the depressive symptoms score were transformed due to skewness of the residuals (TSH with the natural logarithm transformation and the depressive symptoms score with the square root transformation). Two analytical models were used to study the association of interest. A first, minimally adjusted model (Model 1) included as covariates age, sex, and cohort, and a second model (Model 2) additionally included the adjustment for BMI, physical activity, smoking, alcohol consumption, diet, and education. Cross-sectional analyses were performed with the depressive symptoms score (continuous CES-D score) as outcome using linear regression models, and with the clinically relevant depressive symptoms (CES-D ≥ 16 vs CES-D < 16) in logistic regression analyses. Longitudinal analyses addressed the incident depressive events, based on Cox proportional hazard models. We evaluated the proportional hazards assumption statistically by using the Schoenfeld test and assessing the Schoenfeld plot. The proportional hazard assumption was met in the analyses of the associations between thyroid (dys)function and depression outcomes after including in all Cox models the stratifying factor for the cohort covariate. The presence of nonlinearity in the analyses was assessed by including restricted cubic splines with 3 knots.
Considering that the association between thyroid function and depression might be different in euthyroid individuals compared with those outside of the reference range (31), we also examined the association between thyroid function and depressive outcomes in euthyroid participants as part of our main analyses. In this subsample (n = 7174 in cross-sectional analyses, n = 6383 in longitudinal analyses) we examined the association of continuous TPOAb levels with our outcomes.
Various sensitivity analyses were performed. First, to explore the longitudinal association of thyroid function with incident depressive events at different follow-up times, we repeated our analyses using 2 and 5 years as the follow-up time. Second, we assessed the association between thyroid function and incident major depressive disorder, and between thyroid function and incident depressive syndromes (in analyses with the incident depressive syndromes, events were defined as major depressive disorder or depressive syndromes). For the longitudinal analyses with the depressive syndromes as outcome, we first excluded the participants with depressive symptoms, using the same definition as the previous study in this cohort (8), and, second, we repeated the analyses but including those individuals with depressive symptoms as part of the controls, given that they are free of the outcome under study (32). Third, we additionally performed the main analyses excluding participants with bipolar disorder (n = 8).
Missing data were accounted for with multiple imputation (the maximum missing values were in the variables of diet 25.3%, and alcohol 17.5%) using 30 imputed datasets and the mice package version 3.15.0 (33). All analyses were performed using R statistical software version 4.2.1 (34). The Cox proportional hazard models were performed with the survival R-package version 3.4-0 (35).
Results
Baseline characteristics are presented in Table 1. The mean age of the participants was 64.9 (standard deviation (SD) 9.7) years at baseline, and 57% were women. The median TSH was 1.9 mIU/L with an interquartile range (IQR) of 1.3 to 2.8, and FT4 was of 15.7 pmol/L (SD 2.3) on average. The median depressive symptoms score was 2.0 with an IQR of 0.0 to 7.0 and the percentage of participants with clinically relevant depressive symptoms at baseline was 8.7%. The median follow-up period was 10.7 years (IQR 7.2-11.8). In this period, we observed a total of 1366 incident depressive events, with an incidence rate of 17.5 cases per 1000 person-years. The sample characteristics presented for those with and without clinically relevant depressive symptoms are shown in the Supplement (Table S1 (18)).
Table 1.
Baseline characteristics of participants
| Characteristics | Sample for cross-sectional analyses (n = 9471) | Sample for longitudinal analyses (n = 8366) |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Women, n (%) | 5375 (56.8%) | 4576 (54.7%) |
| Age (years) | 64.9 (9.7) | 64.7 (9.6) |
| Grams alcohol per day, median (IQR) | 6.2 (0.5-15.0) | 6.4 (0.5-15.0) |
| Smoking status, n (%) | ||
| Current | 2124 (22.4%) | 1832 (21.9%) |
| Former | 4445 (46.9%) | 4006 (47.9%) |
| Never | 2901 (30.6%) | 2527 (30.2%) |
| BMI (kg/m2) | 27.3 (4.2) | 27.2 (4.1) |
| Participants' education, n (%) | ||
| Primary | 1162 (12.3%) | 962 (11.5%) |
| Lower | 3832 (40.5%) | 3366 (40.2%) |
| Intermediate | 2768 (29.2%) | 2471 (29.5%) |
| High | 1708 (18.0%) | 1567 (18.7%) |
| TSH (mIU/L), median (IQR) | 1.9 (1.3-2.8) | 1.9 (1.3-2.8) |
| FT4 (pmol/L) | 15.7 (2.3) | 15.7 (2.3) |
| TPOAb positive, n (%) | 1249 (13.2%) | 1091 (13.1%) |
| Incident cases of any depressive event, n (%) | — | 1366 (16.3%) |
| Follow-up (years), median (IQR) | — | 10.7 (7.2-11.8) |
| Depressive symptoms score at baseline, median (IQR) | 2.0 (0.0-7.0) | — |
| Clinically relevant depressive symptoms at baseline, n (%) | 828 (8.7%) | — |
Values are mean and standard deviation unless otherwise specified.
The cutoff for TPOAb positivity was 35 kU/mL.
Incident cases defined as cases with no events before the date of thyroid measurement and with at least one event occurring during follow-up.
Depressive symptoms score was not transformed for descriptives.
Abbreviations: BMI, body mass index; FT4, free thyroxin; IQR, interquartile range; TSH, thyroid stimulating hormone; TPOAb, thyroid peroxidase antibody.
Thyroid Function and Depression Outcomes
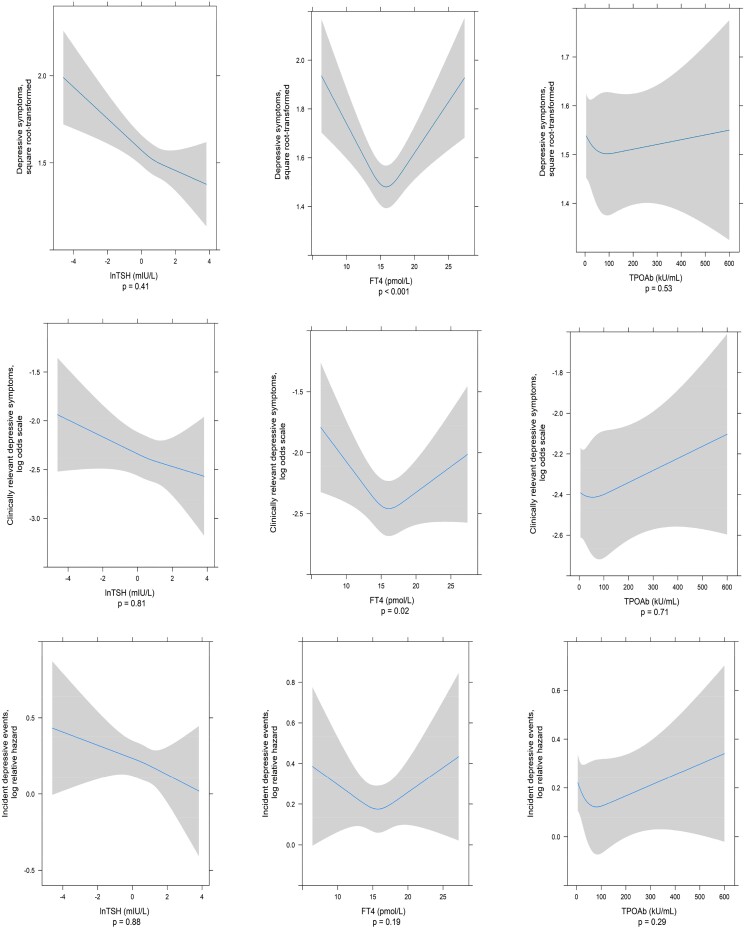
The cross-sectional association between thyroid function and depressive symptoms and clinically relevant depressive symptoms is presented in Table 2 and Fig. 1. In Model 1, an increase in TSH was related to fewer depressive symptoms (B value [per 1 unit increase of natural log-transformed TSH] = −0.09, 95% CI −0.12; −0.05), and this association remained after adjustment for covariates in Model 2 (B = −0.07, 95% CI −0.11; −0.04). Similarly, higher TSH levels were associated with fewer clinically relevant depressive symptoms (vs no clinically relevant depressive symptoms), with an odds ratio (OR) of 0.91 (95% CI 0.84; 0.98), which did not materially change in the fully adjusted model (OR 0.92, 95% CI 0.85; 1.00). A nonlinear association was observed between FT4 and depressive symptoms (Fig. 1) (P for nonlinearity < .001, P for association < .001), in which low and high FT4 levels were related to more depressive symptoms compared with median FT4 levels. TPOAb positivity showed no association with depressive symptoms (B = −0.01, 95% CI −0.10; 0.08). As a post hoc analysis, we repeated the main analyses excluding those taking thyroid-altering medication at baseline. The results remained largely unchanged (Table S2 (18)).
Table 2.
Association between TSH, FT4, and TPOAb positivity with depressive symptoms and incident depressive events
| TSH (mIU/L) | FT4 (pmol/L) | TPOAb positivity | |||||
|---|---|---|---|---|---|---|---|
| Cross-sectional analyses | n | Total = 9471 | Total = 9471 | Total = 9462 (positive = 1249) | |||
| Depressive symptoms score | B (95% CI) | P value | B (95% CI) | P value | B (95% CI) | P value | |
| Model 1 | −0.09 (−0.12; −0.05) | <.001 | — | — | −0.01 (−0.09; 0.08) | .89 | |
| Model 2 | −0.07 (−0.11; −0.04) | < .001 | — | — | −0.01 (−0.10; 0.08) | .80 | |
| Clinically relevant depressive symptoms | n | OR (95% CI) (Dep, Yes = 828) | P value | OR (95% CI) (Dep, Yes = 828) | P value | OR (95% CI) (Dep, Yes = 827)a | P value |
|---|---|---|---|---|---|---|---|
| Model 1 | 0.91 (0.84; 0.98) | .02 | — | — | 1.04 (0.85; 1.27) | .73 | |
| Model 2 | 0.92 (0.85; 1.00) | .06 | — | — | 1.03 (0.84; 1.26) | .79 |
| Longitudinal analyses | n | Total = 8366 | Total = 8366 | Total = 8358 (positive = 1091) | |||
|---|---|---|---|---|---|---|---|
| Incident depressive events | HR (95% CI) (Dep, yes = 1366) |
P value | HR (95% CI) (Dep, yes = 1366) |
P value | HR (95% CI) (Dep, yes = 1365)b |
P value | |
| Model 1 | 0.94 (0.89; 1.00) | .07 | 1.00 (0.98; 1.03) | .72 | 1.01 (0.86; 1.17) | .94 | |
| Model 2 | 0.95 (0.90; 1.01) | .12 | 1.00 (0.98; 1.03) | .84 | 1.00 (0.86; 1.16) | .97 |
Table presents the effect estimate for linear regression analyses with the depressive symptoms score, the OR for analyses with the clinically relevant depressive symptoms, and the HR for analyses with the incident depressive events (stratified Cox model using cohort as stratifying factor). HR in the analyses with TSH represents the hazard ratio per each log-transformed unit of TSH. The cutoff for TPOAb positivity was 35 kU/mL. TSH was log-transformed. The depressive symptoms score was square-root transformed.
Model 1: Adjusted for age, sex and cohort.
Model 2: Model 1 + BMI, physical activity, smoking, alcohol, diet, and education.
Abbreviations: Dep, depression outcomes; FT4, free thyroxine; HR, hazard ratio; OR, odds ratio; TPOAb, thyroid peroxidase antibody; TSH, thyroid stimulating hormone.
a Among subjects with TPOAb positive, cases with clinically relevant depressive symptoms = 128.
b Among subjects with TPOAb positive, incident depressive events = 199.
Figure 1.
Plots of the association between thyroid function and depressive symptoms and incident depressive events based on Model 2. TSH was log-transformed. The depressive symptoms score was square-root transformed. All plots were performed using ordinary least squares regression or logistic regression or Cox proportional hazard models, with the accompanying 95% CI and using restricted cubic splines with 3 knots for the thyroid measures. P denotes the P value for nonlinearity.
In the longitudinal analyses, our results for the association between thyroid function and the incidence of depressive events did not reach statistical significance (eg, TSH: hazard ratio (HR) 0.95, 95% CI 0.90; 1.01). TPOAb positivity was not associated with incident depressive events (HR 1.00, 95% CI 0.86; 1.16) (Table 2). In post hoc analyses, we excluded participants taking thyroid-altering medication at baseline and showed that higher TSH levels were related to incident depressive events (HR 0.92, 95% CI 0.86; 0.99) (Table S2 (18)).
Regarding thyroid disease, we showed a cross-sectional association with depressive symptoms, such that hypothyroidism was related to less depressive symptoms (B = −0.10, 95% CI −0.21; 0.00) and hyperthyroidism to more depressive symptoms (B = 0.29, 95% CI 0.10; 0.48). In contrast, there was no association in the cross-sectional analyses of thyroid disease status with the presence of clinically relevant depressive symptoms nor in the longitudinal analyses with incident depressive events (Table 3).
Table 3.
Association between thyroid disease and depressive symptoms and incident depressive events
| Hypothyroidism | Hyperthyroidism | ||||
|---|---|---|---|---|---|
| Cross-sectional analyses | n | Total n = 8802, hypothyroid = 864 | Total n = 8158, hyperthyroid = 220 | ||
| Depressive symptoms score | B (95% CI) | P value | B (95% CI) | P value | |
| Model 1 | −0.12 (−0.22; −0.02) | .02 | 0.30 (0.10; 0.49) | .003 | |
| Model 2 | −0.10 (−0.21; 0.00) | .04 | 0.29 (0.10; 0.48) | .003 | |
| Clinically relevant depressive symptoms | n | OR (95% CI) (Dep, yes = 741)a | P value | OR (95% CI) (Dep, yes = 690)b | P value |
|---|---|---|---|---|---|
| Model 1 | 0.88 (0.68; 1.13) | .31 | 1.10 (0.69; 1.75) | .69 | |
| Model 2 | 0.90 (0.70; 1.17) | .44 | 1.09 (0.68; 1.73) | .73 |
| Longitudinal analyses | Total n = 7810, hypothyroid = 765 | Total n = 7233, hyperthyroid = 188 | |||
|---|---|---|---|---|---|
| Incident depressive events | n | HR (95% CI) (Dep, yes = 1232)c |
P value | HR (95% CI) (Dep, yes = 1143)d |
P value |
| Model 1 | 0.99 (0.83; 1.19) | .93 | 1.30 (0.95; 1.79) | .10 | |
| Model 2 | 1.00 (0.83; 1.20) | 1.00 | 1.33 (0.97; 1.83) | .08 | |
Table presents the effect estimate for linear regression analyses with the depressive symptoms score, the OR for analyses with the clinically relevant depressive symptoms, and the HR for analyses with the incident depressive events (stratified Cox model using cohort as stratifying factor). Thyroid disease was defined based on TSH reference levels and participants taking thyroid-altering medication at baseline were excluded.
The depressive symptoms score was square-root transformed.
Model 1: Adjusted for age, sex and cohort.
Model 2: Model 1 + BMI, physical activity, smoking, alcohol, diet, and education.
Abbreviations: BMI, body mass index; Dep, depression outcomes; OR, odds ratio; HR, hazard ratio.
a Among subjects with hypothyroidism, cases with clinically relevant depressive symptoms = 72.
b Among subjects with hyperthyroidism, cases with clinically relevant depressive symptoms = 21.
c Among subjects with hypothyroidism, incident depressive events = 129.
d Among subjects with hyperthyroidism, incident depressive events = 40.
Analyses in participants with thyroid function within the reference range are described in Table 4 and in Supplement (Fig. S2 (18)). Higher TSH levels were associated with fewer depressive symptoms (B = −0.09, 95% CI −0.16; −0.02), and there was a U-shaped association between FT4 and TPOAb with depressive symptoms (FT4: P for nonlinearity = .001, P for association = .002; TPOAb: P for nonlinearity = .01, P for association = .04). Additionally, the U-shaped association for FT4 was also observed when examining clinically relevant depressive symptoms (P for nonlinearity = .03, P for association = .05). In the longitudinal analyses in euthyroid participants, there was an inverse U-shaped association between TSH and incident depressive events, and a U-shaped association between FT4 and incident depressive events (TSH P for nonlinearity = .03, P for association = .03; and FT4 P for nonlinearity = .02, P for association = .02).
Table 4.
Association between TSH and TPOAb (continuous) with depressive symptoms and incident depressive events in euthyroid participants
| Cross-sectional analyses | TSH (mIU/L) | TPOAb | |||
|---|---|---|---|---|---|
| Depressive symptoms score | n | Total = 7174 | Total = 7174 | ||
| B (95% CI) | P value | B (95% CI) | P value | ||
| Model 1 | −0.12 (−0.19; −0.05) | .001 | — | — | |
| Model 2 | −0.09 (−0.16; −0.02) | .01 | — | — | |
| Clinically relevant depressive symptoms | n | OR (95% CI) (Dep, yes = 586) |
P value | OR (95% CI) (Dep, yes = 586) |
P value |
|---|---|---|---|---|---|
| Model 1 | 0.75 (0.63; 0.90) | .002 | 1.01 (1.00; 1.03) | .10 | |
| Model 2 | 0.79 (0.66; 0.95) | .01 | 1.01 (0.99; 1.02) | .31 |
| Longitudinal analyses | n | Total = 6383 | Total = 6383 | ||
|---|---|---|---|---|---|
| Incident depressive events | HR (95% CI) (Dep, yes = 978) |
P value | HR (95% CI) (Dep, yes = 978) |
P value | |
| Model 1 | — | — | 1.00 (0.98; 1.01) | .58 | |
| Model 2 | — | — | 0.99 (0.98; 1.00) | .15 |
Table presents the effect estimate for linear regression analyses with the depressive symptoms score, the OR for analyses with the clinically relevant depressive symptoms, and the HR for analyses with the incident depressive events (stratified Cox model using cohort as stratifying factor). TSH was log-transformed. The depressive symptoms score was square-root transformed. TPOAb was used as a continuous variable in the analyses.
Model 1: Adjusted for age, sex and cohort.
Model 2: Model 1 + BMI, physical activity, smoking, alcohol, diet and education.
Euthyroidism defined as TSH reference levels (0.4-4.0 mIU/L), FT4 reference levels (11-25 pmol/L), no thyroid-altering medication use at baseline, and TPOAb lower or equal to 35 kU/mL.
The results for TSH, FT4, and TPOAb that are not presented in this table are nonlinear associations and can be found in Fig. S2.
Abbreviations: Dep, depression outcomes; HR, hazard ratio; OR, odds ratio; TSH, thyroid-stimulating hormone; TPOAb, thyroid peroxidase antibody.
Sensitivity Analyses
We explored the prospective association between thyroid function and incident depressive events occurring within 2 and within 5 years of follow-up, and the lack of association observed in the main analyses between TSH, FT4, and TPOAb positivity with incident depressive events remained unchanged (Table 5). Also, there was no association between thyroid function and incident major depressive disorder or incident depressive syndromes (Table 6; Table S3 (18)). Finally, the exclusion of participants with bipolar disorder did not change our results (Table 6).
Table 5.
Association between TSH, FT4, and TPOAb positivity with incident depressive events, across different follow-up periods
| TSH (mIU/L) | FT4 (pmol/L) | TPOAb positivity | |||||
|---|---|---|---|---|---|---|---|
| n | Total = 8366 | Total = 8366 | Total = 8358 (positive = 1091) | ||||
| Incident depressive events within 2 years of follow-up | HR (95% CI) (Dep, yes = 206) |
P value | HR (95% CI) (Dep, yes = 206) |
P value | HR (95% CI) (Dep, yes = 206)a |
P value | |
| 1.03 (0.87; 1.24) | .71 | 0.98 (0.92; 1.04) | .43 | 0.98 (0.66; 1.47) | .94 | ||
| n | Total = 8366 | Total = 8366 | Total = 8358 (positive = 1091) | ||||
|---|---|---|---|---|---|---|---|
| Incident depressive events within 5 years of follow-up | HR (95% CI) (Dep, yes = 755) |
P value | HR (95% CI) (Dep, yes = 755) |
P value | HR (95% CI) (Dep, yes = 755)b |
P-value | |
| 0.95 (0.87; 1.03) | .23 | 1.00 (0.97; 1.03) | .91 | 0.95 (0.77; 1.17) | .66 |
HR for analyses with the incident depressive events (stratified Cox model using cohort as stratifying factor).
The cutoff for TPOAb positivity was 35 kU/mL. TSH was log-transformed.
Model: Adjusted for age, sex, cohort, BMI, physical activity, smoking, alcohol, diet and education.
Median (IQR) follow-up time in total sample n = 8366: in analyses of 2 years: 2.00 years (2.00, 2.00); in 5 years: 5.00 years (5.00, 5.00).
Abbreviations: Dep, depression outcomes; FT4, free thyroxine; HR, hazard ratio; TPOAb, thyroid peroxidase antibody; TSH, thyroid stimulating hormone.
a Among subjects with TPOAb positive, incident depressive events = 28.
b Among subjects with TPOAb positive, incident depressive events = 105.
Table 6.
Association between TSH, FT4, and TPOAb positivity with incident major depressive disorder, incident depressive syndromes, and incident depressive events (excluding bipolar disorder from analyses)
| Longitudinal analyses | TSH (mIU/L) | FT4 (pmol/L) | TPOAb positivity | ||||
|---|---|---|---|---|---|---|---|
| n | Total = 9304 | Total = 9304 | Total = 9295 (positive = 1225) | ||||
| Incident major depressive disorder | HR (95% CI) (Dep, yes = 188) |
P value | HR (95% CI) (Dep, yes = 188) |
P value | HR (95% CI) (Dep, yes = 188)a |
P value | |
| Model 1 | 0.95 (0.81; 1.11) | .50 | 1.01 (0.95; 1.08) | .69 | 0.67 (0.42; 1.07) | .10 | |
| Model 2 | 0.95 (0.81; 1.12) | .53 | 1.01 (0.95; 1.08) | .71 | 0.67 (0.42; 1.07) | .09 | |
| n | Total = 9199 | Total = 9199 | Total = 9190 (Positive = 1209) | ||||
|---|---|---|---|---|---|---|---|
| Incident depressive syndrome | HR (95% CI) (Dep, yes = 391) |
P value | HR (95% CI) (Dep, yes = 391) |
P value | HR (95% CI) (Dep, yes = 391)b |
P value | |
| Model 1 | 1.03 (0.91; 1.17) | .62 | 0.97 (0.93; 1.02) | .20 | 0.84 (0.62; 1.13) | .25 | |
| Model 2 | 1.05 (0.93; 1.18) | .47 | 0.97 (0.93; 1.01) | .17 | 0.83 (0.61; 1.12) | .23 | |
| n | Total = 8358 | Total = 8358 | Total = 8350 (positive = 1091) | ||||
|---|---|---|---|---|---|---|---|
| Incident depressive events (excluding bipolar disorder from analyses) |
HR (95% CI)
(Dep, yes = 1366) |
P value |
HR (95% CI)
(Dep, yes = 1366) |
P value |
HR (95% CI)
(Dep, yes = 1365) c |
P value | |
| Model 1 | 0.94 (0.89; 1.00) | .07 | 1.00 (0.98; 1.03) | .74 | 1.00 (0.86; 1.17) | .95 | |
| Model 2 | 0.95 (0.90; 1.01) | .13 | 1.00 (0.98; 1.03) | .86 | 1.00 (0.86; 1.16) | .96 | |
Table presents the HR for analyses with the incident major depressive disorder, the incident depressive syndromes, and incident depressive events (excluding bipolar disorder from analyses) (stratified Cox model using cohort as stratifying factor).
Model 1: Adjusted for age, sex and cohort.
Model 2: Model 1 + BMI, physical activity, smoking, alcohol, diet, and education.
The cutoff for TPOAb positivity was 35 kU/mL. TSH was log-transformed.
Abbreviations: Dep, depression; FT4, free thyroxine; HR, hazard ratio; TPOAb, thyroid peroxidase antibody; TSH, thyroid stimulating hormone.
a Among subjects with TPOAb positive, incident major depressive disorder cases = 20.
b Among subjects with TPOAb positive, incident depressive syndromes cases = 49.
c Among subjects with TPOAb positive, incident depressive events = 199.
As a post hoc analysis and following our lack of longitudinal associations between hypothyroidism and incident depressive events, we further explored whether participants who had subclinical hypothyroidism at baseline may develop overt hypothyroidism and, subsequently, incident depressive events over time. We excluded the first 2 years of follow-up, and we showed no association between subclinical hypothyroidism and incident depressive events in analyses fully adjusted for confounders (HR 1.02, 95% CI 0.83; 1.25)) (total sample n = 7355; n subclinical hypothyroid participants = 663; n of participants with depressive event in total sample = 1030; n of participants with an incident depressive event among those with subclinical hypothyroidism = 102).
Discussion
In the present study using a large population-based cohort, we show a cross-sectional association between thyroid function and depressive symptoms as well as clinically relevant depressive symptoms. Low TSH levels and low and high FT4 levels were associated with more depressive symptoms, and hypothyroidism was associated with fewer depressive symptoms while hyperthyroidism was related to more depressive symptoms. Also, in euthyroid participants, there was an inverse U-shaped and a U-shaped association between TSH and FT4, respectively, with incident depressive events. We additionally observed an association between low TSH levels and incident depressive events when excluding thyroid medication at baseline in analyses using the full range of thyroid function.
In our study, we described a relation between low and high FT4 and low TSH with more depression. These counterintuitive results are also described in previous literature. For instance, Panicker et al examined a sample of more than 30 000 participants and showed that in males (n = 9319) not receiving thyroid supplementation, TSH had a cross-sectional inverse association with depression score (measured with the Hospital Anxiety and Depression Scale) (36, 37). Similarly, Medici et al described that lower values of TSH in the reference range were related to more depressive symptoms in the cross-sectional analyses using a smaller subsample from the current study cohort (n = 943) (8). Regarding FT4, a similar U-shaped association between FT4 and depressive symptoms was described in a cross-sectional study that evaluated 3138 adult participants from a population-based sample in Italy (10). Overall, previous research supports a cross-sectional relation between thyroid hormones and depressive symptoms (8, 10), which we confirmed in our analyses. In the presence of chronic or severe diseases, there is an alteration in thyroid parameters, known as nonthyroidal illness, commonly presenting with low TSH and low FT4 levels (38). In this study, low TSH and low FT4 levels were related to depression, and these results can be partially explained by nonthyroidal illness. However, based on the longitudinal associations observed, a possibly complementary explanation is the effect of thyroid function on depression, for instance with the effect of high FT4 levels on depression resulting from hyperthyroidism or thyrotoxicosis via various biological mechanisms, such as alterations in neurotransmitters (39).
Some studies have addressed the association between thyroid disease and depression. Individuals with hyperthyroidism have been shown to present a higher prevalence of major depressive disorder (4) and features characteristic of depression such as insomnia and weight loss (40). We confirmed this association in the current study. Regarding hypothyroidism, we described a relation with fewer depressive symptoms in our study, differing from the often hypothesized association between hypothyroidism and more depressive symptoms (3). However, the literature on this association is still inconclusive with studies reporting null, positive, and negative associations. Specifically, a recent meta-analysis addressing hypothyroidism and depression in 25 studies showed a small positive association (41). In contrast, a recent study described that LT4 (levothyroxine) supplementation did not help in the prevention of depressive symptoms in subclinical hypothyroidism (42). Finally, Panicker et al described that higher levels of TSH were related to lower depression scores in the HUNT study, when examining participants without LT4 supplementation. As explained by the authors, this exclusion implies a potential selection bias, since most symptomatic hypothyroid individuals might already be receiving treatment and would be excluded from analyses (37).
In contrast to the previous study performed in a small subsample of the current cohort (8), there was no association of TSH with incident depressive events in our longitudinal analysis, except for an inverse U-shaped association only seen in euthyroid participants and an association between low TSH and incident depressive events in participants who were not taking thyroid-altering medication at baseline. Overall, there are mixed findings among ours and previous longitudinal studies (8, 43), suggesting that any effect of TSH/FT4 on depression is likely to be small and that the association in euthyroid individuals might be different compared with those outside of the reference range (31). Also, discrepancies across studies might be due to sample size difference, as smaller samples are related to higher sampling variability (44), and to follow-up time differences (eg, 2 years (43), 8 years (8)). Thus, more studies combining larger sample sizes and repeated measures across a long follow-up are needed.
Although the possible mechanisms underlying the presence of depression in thyroid dysfunction are not well understood, a dysregulation of neurotransmitters, an alteration of the blood–brain barrier, and a dysfunctional gene expression have been described (45). For instance, our findings of a U-shaped association between FT4 and incident depressive events in euthyroid individuals could be partly explained by alterations in neurotransmitters. Low thyroid hormone levels have been related to a decrease in the γ-aminobutyric acid (GABA) levels in the brain and changes in GABA have been related to neuropsychiatric disorders, such as depression (46). On the other hand, high levels of FT4 are suggested to cause an exhaustion of the noradrenergic system, which leads to the occurrence of depression (39). Additionally, given that the U-shaped association between FT4 and depressive symptoms was found in all cross-sectional analyses, we cannot rule out the presence of other factors that may underlie the association, the possibility of a bidirectional effect, or that the association between thyroid function and depression is mainly limited to a short-term relation, such as that observed for vitamin D and depression (22). Regarding the bidirectional effect, a recent study used bidirectional Mendelian randomization analyses and showed no evidence for a causal relationship between thyroid function in the reference range and the risk for major depressive disorders or depressive symptoms either, as well as no evidence for a causal effect of major depressive disorder on thyroid function (nonthyroidal illness (47)) (48). However, the low percentage of currently explained variance for thyroid function likely limits the power of these type of studies (49). Also, there is little evidence from cohort studies on the longitudinal relationship between depression at baseline and alterations of thyroid function in the long-term.
This study offers a comprehensive insight into the relation between thyroid function and depression, as we used a large population-based sample, with a reliable and validated depression measure and a long follow-up to assess incident cases of depressive events, in which the measure of incident depressive events was based on various information sources and the occurrence of depressive events was constantly monitored (22). While our results in the cross-sectional analyses are in line with previous studies (8, 10), longitudinal evidence is more mixed (8, 9). Studies have suggested a biological mechanism underlying the association between thyroid function and depression outcomes (10, 50); however, more research is needed to understand the underlying pathways and whether differences across studies could be related to sample characteristics or whether a prospective association between thyroid function and incident depressive events is specific to certain subgroups in the population. Importantly, it is worth noting that any potential effect of thyroid (dys)function on depression is likely to be smaller than generally expected (41).
There are some limitations that need to be taken into account in our study. First, we do not have repeated measures of thyroid function that would allow us to test the possibility of reverse causation. Second, the individuals from our sample are mainly Caucasian, limiting the generalizability of our findings to other populations. Third, there was a small number of subjects who had both thyroid disease (defined based on TSH cutoff) and depressive outcomes (clinically relevant depressive symptoms and incident depressive events), which could influence the power of these specific analyses. However, we also performed analyses with the continuous TSH variable, and results were similar to those with thyroid disease.
In this population-based sample of 9471 participants, we showed that lower levels of TSH and low and high FT4 levels were associated with more depressive symptoms cross-sectionally. We also described a U-shaped, albeit weak, association of FT4 with incident depressive events specifically in euthyroid participants, and an association between low TSH and incident depressive events in participants not taking thyroid-altering medication, suggesting that thyroid function may have only a small causal role in the long-term incidence of depression in the general population. Future studies should determine whether reverse causation due to nonthyroidal illness, physiological co-occurrence, or additional specific factors could explain the robust cross-sectional link between thyroid function and depressive symptoms. Furthermore, given that the link between thyroid function and depression is not fully understood, more research is also needed to determine the influence of thyroid hormone supplementation on depressive symptoms in patients with subclinical thyroid disease.
Acknowledgments
The authors are grateful to the study participants, the staff from the Rotterdam Study and the participating general practitioners and pharmacists.
Abbreviations
- BMI
body mass index
- CES-D
Centre for Epidemiologic Studies Depression
- FT4
free thyroxine
- TPOAb
thyroid peroxidase antibody
- TSH
thyrotropin
Contributor Information
Oscar Hernando Roa Dueñas, Department of Epidemiology, Erasmus University Medical Center, 3000 CA Rotterdam, The Netherlands.
Amy Hofman, Department of Epidemiology, Erasmus University Medical Center, 3000 CA Rotterdam, The Netherlands.
Annemarie I Luik, Department of Epidemiology, Erasmus University Medical Center, 3000 CA Rotterdam, The Netherlands; Trimbos Institute—The Netherlands Institute of Mental Health and Addiction, 3521 VS Utrecht, The Netherlands.
Marco Medici, Academic Center for Thyroid Diseases, Erasmus University Medical Center, 3000 CA Rotterdam, The Netherlands; Department of Internal Medicine, Erasmus University Medical Center, 3000 CA Rotterdam, The Netherlands.
Robin P Peeters, Academic Center for Thyroid Diseases, Erasmus University Medical Center, 3000 CA Rotterdam, The Netherlands; Department of Internal Medicine, Erasmus University Medical Center, 3000 CA Rotterdam, The Netherlands.
Layal Chaker, Department of Epidemiology, Erasmus University Medical Center, 3000 CA Rotterdam, The Netherlands; Academic Center for Thyroid Diseases, Erasmus University Medical Center, 3000 CA Rotterdam, The Netherlands; Department of Internal Medicine, Erasmus University Medical Center, 3000 CA Rotterdam, The Netherlands.
Funding
The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam.
Disclosures
None.
Data Availability
Data can be obtained upon request. Requests should be directed towards the management team of the Rotterdam Study (datamanagement.ergo@erasmusmc.nl), which has a protocol for approving data requests.
References
- 1. Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metabol. 2007;3(3):249‐259. [DOI] [PubMed] [Google Scholar]
- 2. Montero-Pedrazuela A, Venero C, Lavado-Autric R, et al. Modulation of adult hippocampal neurogenesis by thyroid hormones: implications in depressive-like behavior. Mol Psychiatry. 2006;11(4):361‐371. [DOI] [PubMed] [Google Scholar]
- 3. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550‐1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shoib S, Ahmad J, Wani MA, et al. Depression and anxiety among hyperthyroid female patients and impact of treatment. Middle East Curr Psychiatry. 2021;28(1):26. [Google Scholar]
- 5. Chaker L, Razvi S, Bensenor IM, Azizi F, Pearce EN, Peeters RP. Hypothyroidism. Nat Rev Dis Primers. 2022;8(1):30. [DOI] [PubMed] [Google Scholar]
- 6. Talhada D, Santos CRA, Gonçalves I, Ruscher K. Thyroid hormones in the brain and their impact in recovery mechanisms after stroke. Front Neurol. 2019;10:1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsuno H, Tsuchimine S, O’Hashi K, et al. Association between vascular endothelial growth factor-mediated blood–brain barrier dysfunction and stress-induced depression. Mol Psychiatry. 2022;27(9):3822‐3832. [DOI] [PubMed] [Google Scholar]
- 8. Medici M, Direk N, Visser WE, et al. Thyroid function within the normal range and the risk of depression: a population-based cohort study. J Clin Endocrinol Metab. 2014;99(4):1213‐1219. [DOI] [PubMed] [Google Scholar]
- 9. Varella AC, Benseñor IM, Janovsky CCPS, et al. Thyroid-stimulating hormone levels and incident depression: results from the ELSA-Brasil study. Clin Endocrinol (Oxf). 2021;94(5):858‐865. [DOI] [PubMed] [Google Scholar]
- 10. Delitala AP, Terracciano A, Fiorillo E, Orru V, Schlessinger D, Cucca F. Depressive symptoms, thyroid hormone and autoimmunity in a population-based cohort from Sardinia. J Affect Disord. 2016;191:82‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Engum A, Bjøro T, Mykletun A, Dahl AA. Thyroid autoimmunity, depression and anxiety; are there any connections? An epidemiological study of a large population. J Psychosom Res. 2005;59(5):263‐268. [DOI] [PubMed] [Google Scholar]
- 12. van de Ven AC, Muntjewerff JW, Netea-Maier RT, et al. Association between thyroid function, thyroid autoimmunity, and state and trait factors of depression. Acta Psychiatr Scand. 2012;126(5):377‐384. [DOI] [PubMed] [Google Scholar]
- 13. Glanville KP, Coleman JRI, O’Reilly PF, Galloway J, Lewis CM. Investigating pleiotropy between depression and autoimmune diseases using the UK biobank. Biol Psychiatry Global Open Sci. 2021;1(1):48‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Degner D, Haust M, Meller J, Rüther E, Reulbach U. Association between autoimmune thyroiditis and depressive disorder in psychiatric outpatients. Eur Arch Psychiatry Clin Neurosci. 2015;265(1):67‐72. [DOI] [PubMed] [Google Scholar]
- 15. Feng X-Z, Wang K, Li Z, et al. Association between thyroid autoimmunity and clinical characteristics in first-episode and drug-naive depressed patients with suicide attempts. Gen Hosp Psychiatry. 2023;83:156‐163. [DOI] [PubMed] [Google Scholar]
- 16. Berent D, Zboralski K, Orzechowska A, Gałecki P. Thyroid hormones association with depression severity and clinical outcome in patients with major depressive disorder. Mol Biol Rep. 2014;41(4):2419‐2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ikram MA, Brusselle G, Ghanbari M, et al. Objectives, design and main findings until 2020 from the Rotterdam Study. Eur J Epidemiol. 2020;35(5):483‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roa Dueñas OH, Hofman A, Luik AI, Medici M, Peeters RP, Chaker L. Data from: supplement for the cross-sectional and longitudinal association between thyroid function and depression: a population-based study. 2023. 10.6084/m9.figshare.23532012. [DOI] [PMC free article] [PubMed]
- 19. Chaker L, van den Berg ME, Niemeijer MN, et al. Thyroid function and sudden cardiac death. Circulation. 2016;134(10):713‐722. [DOI] [PubMed] [Google Scholar]
- 20. Radloff LS. The CES-D scale:A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385‐401. [Google Scholar]
- 21. Beekman ATF, Deeg DJH, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. BRIEF COMMUNICATION.: criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med. 1997;27(1):231‐235. [DOI] [PubMed] [Google Scholar]
- 22. Jovanova O, Aarts N, Noordam R, Carola-Zillikens M, Hofman A, Tiemeier H. Vitamin D serum levels are cross-sectionally but not prospectively associated with late-life depression. Acta Psychiatr Scand. 2017;135(3):185‐194. [DOI] [PubMed] [Google Scholar]
- 23. Zijlmans JL, Vernooij MW, Ikram MA, Luik AI. The role of cognitive and brain reserve in late-life depressive events: the rotterdam study. J Affect Disord. 2023;320:211‐217. [DOI] [PubMed] [Google Scholar]
- 24. Wing JK, Babor T, Brugha T, et al. SCAN: schedules for clinical assessment in neuropsychiatry. Arch Gen Psychiatry. 1990;47(6):589‐593. [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization . Schedules for Clinical Assessment in Neuropsychiatry, Version 2.1. 2nd ed. World Health Organization; 1997. [Google Scholar]
- 26. Luijendijk HJ, van den Berg JF, Dekker MJ, et al. Incidence and recurrence of late-life depression. Arch Gen Psychiatry. 2008;65(12):1394‐1401. [DOI] [PubMed] [Google Scholar]
- 27. Voortman T, Kiefte-de Jong JC, Ikram MA, et al. Adherence to the 2015 Dutch dietary guidelines and risk of non-communicable diseases and mortality in the Rotterdam Study. Eur J Epidemiol. 2017;32(11):993‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen Z, Schoufour JD, Rivadeneira F, et al. Plant-based diet and adiposity over time in a middle-aged and elderly population: the rotterdam study. Epidemiology. 2019;30(2):303‐310. [DOI] [PubMed] [Google Scholar]
- 29. Caspersen CJ, Bloemberg BPM, Saris WHM, Merritt RK, Kromhout D. The prevalence of selected physical activities and their relation with coronary heart disease risk factors in elderly men: the zutphen study, 1985. Am J Epidemiol. 1991;133(11):1078‐1092. [DOI] [PubMed] [Google Scholar]
- 30. Stel VS, Smit JH, Pluijm SM, Visser M, Deeg DJ, Lips P. Comparison of the LASA physical activity questionnaire with a 7-day diary and pedometer. J Clin Epidemiol. 2004;57(3):252‐258. [DOI] [PubMed] [Google Scholar]
- 31. Williams MD, Harris R, Dayan CM, Evans J, Gallacher J, Ben-Shlomo Y. Thyroid function and the natural history of depression: findings from the Caerphilly Prospective Study (CaPS) and a meta-analysis. Clin Endocrinol (Oxf). 2009;70(3):484‐492. [DOI] [PubMed] [Google Scholar]
- 32. Grimes DA, Schulz KF. Compared to what? Finding controls for case-control studies. Lancet. 2005;365(9468):1429‐1433. [DOI] [PubMed] [Google Scholar]
- 33. van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1‐67. [Google Scholar]
- 34. R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020. [Google Scholar]
- 35. Therneau TM. A package for survival analysis in R. 2021.
- 36. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361‐370. [DOI] [PubMed] [Google Scholar]
- 37. Panicker V, Evans J, Bjøro T, Åsvold BO, Dayan CM, Bjerkeset O. A paradoxical difference in relationship between anxiety, depression and thyroid function in subjects on and not on T4: findings from the HUNT study. Clin Endocrinol (Oxf). 2009;71(4):574‐580. [DOI] [PubMed] [Google Scholar]
- 38. Guo J, Hong Y, Wang Z, Li Y. Analysis of the incidence of euthyroid sick syndrome in comprehensive intensive care units and related risk factors. Front Endocrinol (Lausanne). 2021;12:656641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bunevicius R, Prange AJ, Jr. Thyroid disease and mental disorders: cause and effect or only comorbidity? Curr Opin Psychiatry. 2010;23(4):363‐368. [DOI] [PubMed] [Google Scholar]
- 40. Demet MM, Ozmen B, Deveci A, Boyvada S, Adiguzel H, Aydemir O. Depression and anxiety in hyperthyroidism. Arch Med Res. 2002;33(6):552‐556. [DOI] [PubMed] [Google Scholar]
- 41. Bode H, Ivens B, Bschor T, Schwarzer G, Henssler J, Baethge C. Association of hypothyroidism and clinical depression: A systematic review and meta-analysis. JAMA Psychiatry. 2021;78(12):1375‐1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wildisen L, Feller M, Del Giovane C, et al. Effect of levothyroxine therapy on the development of depressive symptoms in older adults with subclinical hypothyroidism: an ancillary study of a randomized clinical trial. JAMA Network Open. 2021;4(2):e2036645‐e2036645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim JS, Zhang Y, Chang Y, et al. Subclinical hypothyroidism and incident depression in young and middle-age adults. J Clin Endocrinol & Metabol. 2018;103(5):1827‐1833. [DOI] [PubMed] [Google Scholar]
- 44. Marek S, Tervo-Clemmens B, Calabro FJ, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603(7902):654‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jurado-Flores M, Warda F, Mooradian A. Pathophysiology and clinical features of neuropsychiatric manifestations of thyroid disease. J Endocr Soc. 2022;6(2):bvab194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu B, Yang H, Gao F, et al. Investigation of brain GABA+ in primary hypothyroidism using edited proton MR spectroscopy. Clin Endocrinol (Oxf). 2017;86(2):256‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fliers E, Boelen A. An update on non-thyroidal illness syndrome. J Endocrinol Invest. 2021;44(8):1597‐1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kuś A, Kjaergaard AD, Marouli E MFDG, et al. Thyroid function and mood disorders: A Mendelian randomization study. Thyroid. 2021;31(8):1171‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Teumer A, Chaker L, Groeneweg S, et al. Genome-wide analyses identify a role for SLC17A4 and AADAT in thyroid hormone regulation. Nat Commun. 2018;9(1):4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dayan CM, Panicker V. Hypothyroidism and depression. Eur Thyroid J. 2013;2(3):168‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be obtained upon request. Requests should be directed towards the management team of the Rotterdam Study (datamanagement.ergo@erasmusmc.nl), which has a protocol for approving data requests.