Abstract
Background:
Although the incidence of hospital-associated respiratory virus infection (HARVI) is well recognized, the risk factors for infection and impact on patient outcomes are not well characterized.
Methods:
We identified a cohort of all inpatient admissions ≥24 hours duration at a single academic medical center from 2017 to 2020. HARVI were defined as respiratory virus detected in a test ordered after the 95th percentile of the virus-specific incubation period. Risk factors for HARVI were assessed using Cox proportional hazards models of the competing outcomes of HARVI and discharge. The associations between time-varying HARVI status and the rates of ICU admission, discharge, and in-hospital death were estimated using Cox-proportional hazards models in a competing risk framework.
Results:
HARVI incidences were 8.8 and 3.0 per 10,000 admission days for pediatric and adult patients, respectively. For adults, congestive heart failure, renal disease, and cancer increased HARVI risk independent of their associations with length of stay. HARVI risk was also elevated for patients admitted in September–June relative to July admissions. For pediatric patients, cardiovascular and respiratory conditions, cancer, medical device dependence, and admission in December increased HARVI risk. Lengths of stay were longer for adults with HARVI compared to those without, and hospital-associated influenza A was associated with increased risk of death. Rates of ICU admission were increased in the 5 days after HARVI identification for adult and pediatric patients. HARVI was not associated with length of stay or death among pediatric patients.
Conclusions:
HARVI is associated chronic health conditions and increases morbidity and mortality.
Respiratory viruses are common causes of adult and pediatric hospitalization due to community-acquired pneumonia, exacerbation of chronic conditions, and other sequelae of infection.1–4 Although influenza, respiratory syncytial virus (RSV), and now SARS-CoV-2 are notable causes of severe illness, a large proportion of respiratory virus-associated hospitalizations are caused by other species.5 Healthcare-associated outbreaks have been described for influenza, RSV, common-cold coronaviruses (ccCoV), adenovirus, parainfluenza, rhinovirus, and human metapneumovirus (hMPV).6 The total burden of hospital-associated respiratory virus infections (HARVI), not just those related to sizeable outbreaks, has increasingly been recognized given the availability and utilization of multiplex respiratory pathogen panels.7–10
Although characterization of HARVI incidence has improved, risk factors for infection have not been well characterized, and the impact of HARVI on hospitalized patient outcomes is not entirely clear.11–13 Analysis of risk factors for and outcomes of hospital-acquired infections, in general, is difficult because of methodologic challenges such as competing risks, time-dependent bias, and confounding by baseline health status.14,15 Although statistical methods are well developed to address these challenges, they have not been applied to HARVI. Studies of HARVI outcomes have typically been descriptive: reporting average lengths of stay, and numbers of intensive care unit (ICU) admissions and deaths.7,16 These descriptive analyses are useful, but they do not provide evidence for the extent to which these adverse outcomes are attributable to HARVI.
In this retrospective cohort study, we sought (1) to estimate the overall and species-specific incidences of HARVI, (2) to identify risk factors for acquisition of HARVI, and (3) to determine whether HARVI is associated with increased length of stay, ICU admission, and in-hospital mortality.
Methods
Study population and data sources
We identified a cohort of all inpatient admissions of ≥24 hours duration to University of Michigan adult and pediatric hospitals over 3 study periods. The first 2 periods (2017–2018 and 2018–2019) were defined from July 1 through June 30 of each year. The third period (2019–2020) was truncated at the start of the COVID-19 pandemic and was defined from July 1, 2019 through February 28, 2020. All analyses were performed separately for adult (≥18 years) and pediatric (<18 years) patients. This research was approved by the University of Michigan Medical School Institutional Review Board under a waiver of informed consent.
We obtained the following information from the electronic health record: demographics including age, sex, and race and ethnicity; inpatient encounter data including admission and discharge dates, and International Classification of Disease, Tenth Revision (ICD-10) diagnosis codes; vital signs including temperature, heart rate, and respiratory rate; and dates and results of white blood cell counts and respiratory virus diagnostic tests. Charlson comorbidity index (CCI) and pediatric complex chronic condition (CCC) scores were calculated for adult and pediatric patients, respectively, using all available inpatient ICD-10 diagnosis codes in a given study period.17,18 Patients were also categorized based on whether or not they had a diagnosis from each chronic condition category used in the calculation of CCI (acute myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, liver disease, diabetes, hemiplegia or paraplegia, renal disease, and cancer) and CCC scores (cardiovascular disease, respiratory disease, neurologic or neuromuscular disease, renal disease, gastrointestinal disease, hematologic or immunologic disease, metabolic disease, congenital or genetic disease, malignancy, neonatal conditions (eg, premature, low birth weight, etc), medical device dependence (eg, ventilator, gastrostomy, wheel chair, etc), and history of transplantation. Adults were defined as having systemic inflammatory response syndrome (SIRS) if they met ≥2 of the following criteria within 48 hours of admission: body temperature >38°C or <36°C, respiratory rate >20 breaths per minute, heart rate >90 beats per minute, or white blood cell count >12,000 or <4,000 per mm3. Missing SIRS criteria data were imputed to the mean for each criterion (ie, the criterion was not met).
HARVI case definition
Respiratory virus infection was defined as a positive result from either (1) influenza or RSV-specific reverse-transcription polymerase chain reaction or (2) molecular respiratory virus panel (FilmArray respiratory panel) testing for the following: adenovirus; ccCoV 229E, HKU1, NL63, and OC43; hMPV; human rhinovirus-enterovirus; influenza A(H1N1pdm09), A(H3N2), and B; parainfluenza viruses 1, 2, 3, and 4; and RSV.
For each virus, those first identified in a test ordered more than a threshold number of days after hospital admission were defined as HARVIs. Those before the threshold were defined as community acquired. Virus-specific thresholds were defined based on the 50th, 75th, and 95th percentiles of the virus-specific incubation periods estimated in Lessler et al.19 Because the incubation period of hMPV is not well characterized, hMPV thresholds were imputed as those of RSV given the similarity of the 2 viruses.19 Adenovirus, ccCoV, and parainfluenza viruses did not have 95th percentile incubation periods estimated; for these viruses, the 95th percentile threshold was imputed as the 75th percentile plus one day. Virus-specific incidence is reported for each of the 50th, 75th, and 95th percentile thresholds. The 95th percentile threshold is used in subsequent risk-factor and outcomes analysis.
Statistical methods
Overall and virus-specific incidence was calculated as the number of HARVIs per 10,000 patient admission days. Individual patients could contribute multiple admissions to the analysis. For virus-specific analyses, readmissions within 180 days of a community- or hospital-associated viral infection were not considered at risk for reinfection with the same virus and were excluded. Binomial 95% confidence intervals (95% CI) were calculated using the Wilson method in the “Hmisc” package in R software (R Foundation for Statistical Computing, Vienna, Austria). Patients were characterized in terms of age, sex, race and ethnicity, CCI conditions and score (adults), CCC conditions and score (children), meeting ≥2 SIRS criteria (adults), study period, and month of admission. Patient characteristics were compared by HARVI status using χ2 tests for categorical variables and Wilcoxon rank-sum tests for continuous variables.
Direct and indirect effects of patient characteristics on the rate of HARVI were assessed by estimating the cause-specific relative hazards of the competing outcomes of HARVI and hospital discharge in Cox proportional hazards models.20 Patient characteristics significantly associated with increased or decreased rates of HARVI outcomes were considered direct risk or protective factors. Characteristics significantly associated with increased or decreased length of stay were considered indirect risk or protective factors for HARVI because they modify the time at risk of infection in the hospital. The combined direct and indirect effect of patient characteristics on the rate of incident HARVI was assessed by estimating the subdistribution relative hazard in Fine-Gray models.21 Age, sex, race and ethnicity, CCI conditions and score (adults), CCC conditions and score (children), meeting ≥2 SIRS criteria (adults), and month of admission were assessed as risk factors in multivariable models.
The association between HARVI and the competing outcomes of discharge and death was estimated in covariate-adjusted Cox-proportional hazards models with HARVI status modeled as a time-varying covariate. Death was defined as occurring within 30 days of first HARVI identification or within 2 days of hospital discharge for those without HARVI identification. The association between HARVI and first intensive care unit (ICU) admission was similarly estimated in covariate-adjusted Cox-proportional hazards models with time-varying HARVI status (categorically defined as: uninfected [referent], and HARVI identified 0–5, 6–10, and >10 days ago) and competing outcome of discharge (alive or deceased). Outcome models were adjusted for age, sex, non-Hispanic Black race and ethnicity, CCI score (adults), CCC score (children), meeting ≥2 SIRS criteria (adults), and peak season admission (December, January, February, or March).
Results
The 2017–2018 study period included 41,616 adult and 12,445 pediatric inpatient encounters; the 2018–2019 study period included 42,277 adult and 12,386 pediatric encounters; and the abbreviated 2019–2020 period included 27,622 adult and 8,365 pediatric encounters (Supplementary Fig. 1 online). In each study period, 10% of adult encounters received respiratory virus testing. For pediatric patients, the proportion of encounters with testing was higher and increased over time (12%, 13%, and 16%).
For adults, overall incidence of HARVI across the 3 periods was 4.52, 3.85, and 3.04 infections per 10,000 admission days based on the 50th, 75th, and 95th incubation period percentile thresholds, respectively (Table 1). For pediatric patients, HARVI incidence was ~3 times higher than for adults with each case definition: 13.17, 10.69, and 8.75 infections per 10,000 admission days, respectively. Rhinovirus-enterovirus and influenza A were the most common viruses identified in adults, accounting for >50% of all HARVIs. Among pediatric patients, rhinoviruses-enteroviruses were identified in 56% of HARVI cases using the 95th incubation period percentile definition. Compared to adults, pediatric patients were more likely to have HARVIs with adenovirus, ccCoV, parainfluenza, and rhinovirus-enterovirus. The confidence intervals of incidence estimates for all other viruses overlapped.
Table 1.
Incidence of HARVI as Defined by Time From Hospital Admission to the Order Date of the First Positive Test Being Longer Than the 50th, 75th, or 95th Percentile of the Virus-Specific Incubation Perioda
| Virus | Virus-Specific Incubation Period Quantile Used to Define Hospital-Associated Infections | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 50th Percentile | 75th Percentile | 95th Perentileb | |||||||
| Threshold, d | No. | Incidencec | Threshold, d | No. | Incidencec | Threshold, d | No. | Incidencec | |
| Adult | |||||||||
| Adenovirus | 5.6 | 9 | 0.12 (0.06–0.22) | 6.5 | 7 | 0.09 (0.04–0.19) | 7.5 | 5 | 0.06 (0.03–0.15) |
| Coronavirus | 3.2 | 33 | 0.42 (0.30–0.60) | 3.5 | 31 | 0.40 (0.28–0.57) | 4.5 | 29 | 0.37 (0.26–0.54) |
| Human metapneumovirusd | 4.4 | 29 | 0.37 (0.26–0.54) | 5.1 | 26 | 0.34 (0.23–0.49) | 6.3 | 21 | 0.27 (0.18–0.41) |
| Influenza A | 1.4 | 73 | 0.95 (0.75–1.19) | 1.9 | 60 | 0.78 (0.60–1.00) | 2.8 | 50 | 0.65 (0.49–0.85) |
| Influenza B | 1.4 | 15 | 0.19 (0.12–0.32) | 1.9 | 10 | 0.13 (0.07–0.24) | 2.8 | 9 | 0.12 (0.06–0.22) |
| Parainfluenza | 2.6 | 36 | 0.46 (0.33–0.64) | 3.2 | 32 | 0.41 (0.29–0.58) | 4.2 | 26 | 0.33 (0.23–0.49) |
| Respiratory syncytial virus | 4.4 | 27 | 0.35 (0.24–0.51) | 5.1 | 26 | 0.34 (0.23–0.49) | 6.3 | 24 | 0.31 (0.21–0.46) |
| Rhinovirus-enterovirus | 1.9 | 144 | 1.89 (1.60–2.22) | 2.7 | 122 | 1.60 (1.34–1.91) | 4.5 | 84 | 1.10 (0.89–1.36) |
| Totale | … | 351 | 4.52 (4.07–5.02) | … | 299 | 3.85 (3.44–4.31) | … | 236 | 3.04 (2.67–3.45) |
| Pediatric | |||||||||
| Adenovirus | 5.6 | 28 | 1.28 (0.88–1.85) | 6.5 | 21 | 0.96 (0.63–1.46) | 7.5 | 19 | 0.87 (0.55–1.35) |
| Coronavirus | 3.2 | 32 | 1.44 (1.02–2.04) | 3.5 | 31 | 1.40 (0.98–1.98) | 4.5 | 29 | 1.31 (0.91–1.88) |
| Human metapneumovirusd | 4.4 | 9 | 0.41 (0.22–0.78) | 5.1 | 9 | 0.41 (0.22–0.78) | 6.3 | 8 | 0.36 (0.18–0.72) |
| Influenza A | 1.4 | 16 | 0.73 (0.45–1.18) | 1.9 | 10 | 0.45 (0.25–0.84) | 2.8 | 9 | 0.41 (0.22–0.78) |
| Influenza B | 1.4 | 11 | 0.50 (0.28–0.89) | 1.9 | 7 | 0.32 (0.15–0.65) | 2.8 | 6 | 0.27 (0.12–0.59) |
| Parainfluenza | 2.6 | 32 | 1.44 (1.02–2.04) | 3.2 | 30 | 1.35 (0.95–1.93) | 4.2 | 24 | 1.08 (0.73–1.61) |
| Respiratory syncytial virus | 4.4 | 19 | 0.88 (0.56–1.37) | 5.1 | 18 | 0.83 (0.53–1.31) | 6.3 | 16 | 0.74 (0.45–1.20) |
| Rhinovirus-enterovirus | 1.9 | 182 | 8.87 (7.67–10.25) | 2.7 | 141 | 6.87 (5.83–8.11) | 4.5 | 108 | 5.27 (4.36–6.36) |
| Totale | … | 292 | 13.17 (11.74–14.77) | … | 237 | 10.69 (9.41–12.14) | … | 194 | 8.75 (7.60–10.07) |
Note. HARVI; hospital-associated respiratory virus infection; CI, confidence interval.
Virus specific incubation periods taken from Lessler et al.19
Estimates of the 95th percentile of the incubation period were unavailable for adenovirus, coronavirus, and parainfluenza. For this analysis, the 95th percentile for these viruses was taken as the 75th percentile plus 1 day.
Events per 10,000 admission days (95% CI).
The incubation period for human metapneumovirus was not estimated by Lessler et al19 because of limited data. For this analysis, the incubation period of human metapneumovirus was considered consistent with that of respiratory syncytial virus given concordance between the limited data and similarities between the viruses.
Virus specific incidence does not add to the total because some patients had codetection of multiple respiratory viruses in the same test or serial detection of multiple viruses throughout the same hospital admission. Codetection or serial detection events were counted individually within each virus specific row but counted as 1 event in the total row.
The median time from hospital admission to HARVI identification for adults was 9 (interquartile range, 6–16) days (Fig. 1). In bivariate analyses, adult HARVI cases were older, more likely to be male, to have higher CCI scores, to meet ≥SIRS criteria within 48 hours of admission, and to have been admitted during December–March compared to patients who did not have a HARVI (Table 2). Adult HARVI patients were also more likely to have a comorbidity from each CCI category, except for rheumatoid disease and hemiplegia or paraplegia. Only influenza A-associated HARVI cases were significantly older than uninfected patients, and only hMPV- and rhinovirus-enterovirus–associated HARVI cases were more likely to be male. Significant differences in incidence by study period were only observed for influenza A; parainfluenza and rhinovirus-enterovirus were notably not associated with month of admission consistent with increased transmission in the spring, summer, and early fall relative to other viruses. With the exception of influenza B, patients with all virus species were more likely to have higher CCI scores compared to patients without HARVI. However, the patterns of association varied by virus for specific comorbidity categories.
Figure 1.
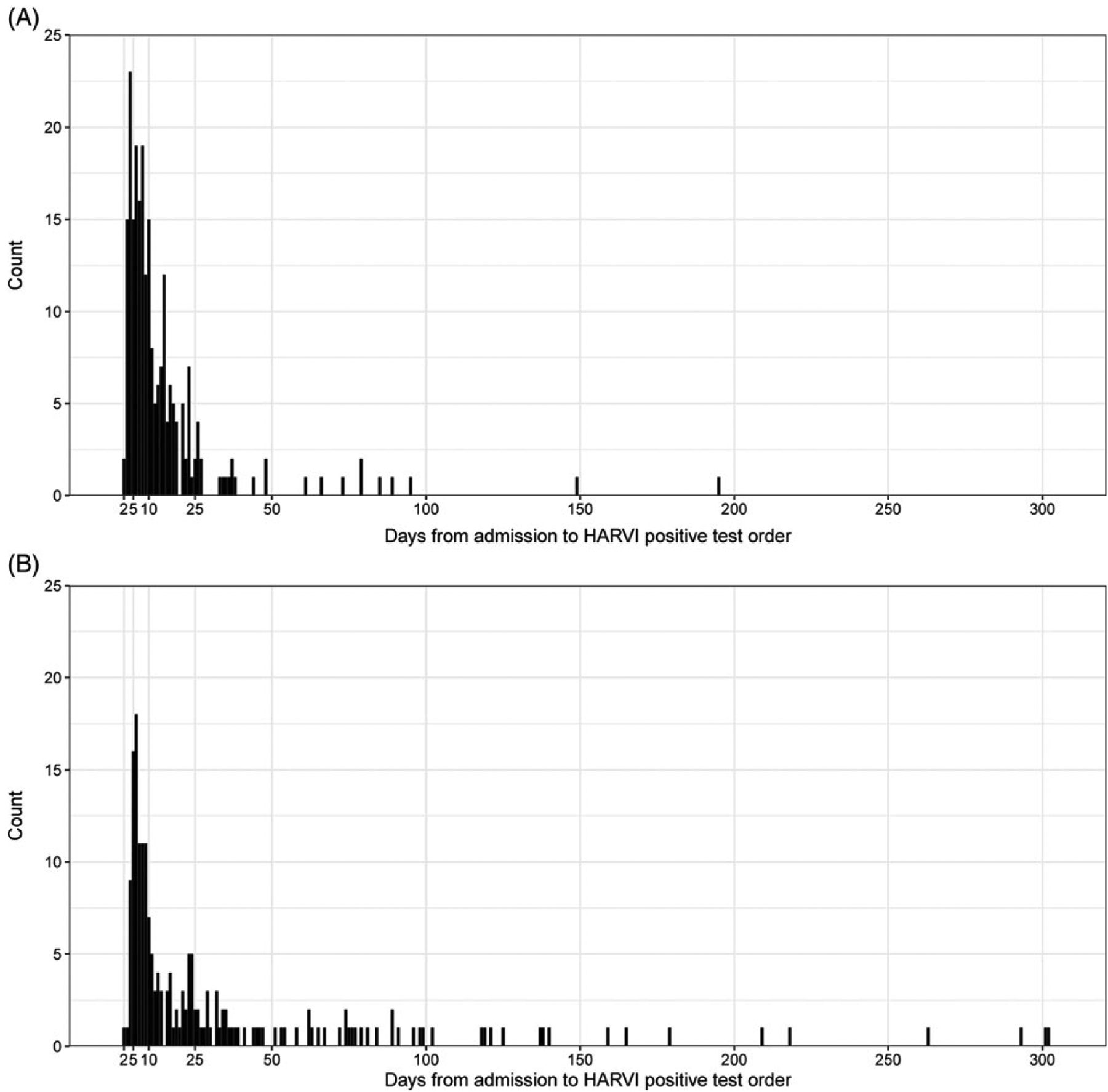
Days from hospital admission to the order date of the first positive respiratory virus test among adult (A) and pediatric (B) patients with hospital-associated respiratory virus infections (HARVI) defined by the 95th percentile of the virus specific incubation period threshold.
Table 2.
Characteristics of Hospitalized Adult Patients Admitted Between July 1, 2017, and February 28, 2020, by HARVI Statusa
| Variable | w/o HARVI (N=111,279), No. (%)b | Any HARVI (N=236), No. (%)b | Adenovirus (N=5), No. (%)b | ccCoV (N=29), No. (%)b | hMPV (N=21), No. (%)b | Influenza A (N=50), No. (%)b | Influenza B (N=9), No. (%)b | PIV (N=26), No. (%)b | RSV (N=24), No. (%)b | hRV/EV (N=84), No. (%)b |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, median y (IQR) | 57 (36–69) | 60 (43–71)* | 48 (46–61) | 60 (44–71) | 62 (43–71) | 65 (53–76)** | 51 (40–61) | 60 (36–74) | 60 (52–70) | 57 (36–68) |
| Sex | *** | * | *** | |||||||
| Female | 60,930 (55) | 103 (44) | 3 (60) | 14 (48) | 6 (29) | 24 (48) | 6 (67) | 13 (50) | 10 (42) | 31 (37) |
| Male | 50,349 (45) | 133 (56) | 2 (40) | 15 (52) | 15 (71) | 26 (52) | 3 (33) | 13 (50) | 14 (58) | 53 (63) |
| Race | ||||||||||
| NH Asian | 3,060 (3) | 2 (1) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| NH Black | 13,246 (12) | 27 (11) | 2 (40) | 2 (7) | 5 (24) | 5 (10) | 1 (11) | 1 (4) | 4 (17) | 9 (11) |
| NH White | 88,628 (80) | 191 (81) | 3 (60) | 23 (79) | 15 (71) | 42 (84) | 7 (78) | 24 (92) | 20 (83) | 66 (79) |
| Other/Unknown/Missing | 6,345 (6) | 16 (7) | 0 (0) | 4 (14) | 1 (5) | 2 (4) | 1 (11) | 1 (4) | 0 (0) | 8 (10) |
| Charlson score, median (IQR) | 2 (0–6) | 5 (3–8)*** | 9 (9–10)** | 4 (2–8)** | 7 (4–10)*** | 6 (3–8)*** | 2 (2–3) | 4 (3–6)* | 6 (4–9)*** | 4 (2–6)*** |
| Chronic condition | ||||||||||
| Myocardial infarction | 14,262 (13) | 46 (19)** | 2 (40) | 6 (21) | 6 (29)* | 10 (20) | 1 (11) | 6 (23) | 6 (25) | 12 (14) |
| Congestive heart failure | 24,680 (22) | 107 (45)*** | 1 (20) | 12 (41)* | 15 (71)*** | 27 (54)*** | 4 (44) | 13 (50)*** | 11 (46)** | 30 (36)** |
| Peripheral vascular disease | 18,331 (16) | 56 (24)** | 2 (40) | 2 (7) | 9 (43)** | 15 (30)** | 0 (0) | 5 (19) | 8 (33)* | 17 (20) |
| Cerebrovascular disease | 12,260 (11) | 51 (22)*** | 3 (60)* | 6 (21) | 7 (33)** | 15 (30)*** | 0 (0) | 4 (15) | 5 (21) | 14 (17) |
| Chronic pulmonary disease | 28,899 (26) | 85 (36)*** | 3 (60) | 9 (31) | 11 (52)** | 19 (38) | 6 (67)* | 8 (31) | 8 (33) | 27 (32) |
| Rheumatoid disease | 5,622 (5) | 18 (8) | 1 (20) | 4 (14) | 1 (5) | 3 (6) | 1 (11) | 0 (0) | 3 (12) | 6 (7) |
| Peptic ulcer disease | 4,160 (4) | 18 (8)** | 1 (20) | 4 (14)* | 1 (5) | 4 (8) | 0 (0) | 3 (12) | 1 (4) | 7 (8)* |
| Liver disease | 13,658 (12) | 42 (18)** | 2 (40) | 5 (17) | 6 (29)* | 10 (20) | 1 (11) | 3 (12) | 8 (33)** | 11 (13) |
| Diabetes | 27,799 (25) | 86 (36)*** | 3 (60) | 11 (38) | 9 (43) | 23 (46)*** | 2 (22) | 10 (38) | 10 (42) | 21 (25) |
| Hemiplegia or paraplegia | 5,430 (5) | 16 (7) | 1 (20) | 1 (3) | 1 (5) | 2 (4) | 0 (0) | 2 (8) | 6 (25)*** | 5 (6) |
| Renal disease | 26,179 (24) | 103 (44)*** | 4 (80)* | 11 (38) | 16 (76)*** | 22 (44)*** | 3 (33) | 11 (42)* | 9 (38) | 34 (40)*** |
| Cancer | 27,401 (25) | 110 (47)*** | 3 (60) | 15 (52)*** | 9 (43) | 15 (30) | 4 (44) | 13 (50)** | 11 (46)* | 45 (54)*** |
| ≥2 SIRS criteria | 27,079 (24) | 83 (35)*** | 2 (40) | 14 (48)** | 7 (33) | 16 (32) | 2 (22) | 10 (38) | 8 (33) | 28 (33)* |
| Study period | * | |||||||||
| 2017–2018 | 41,519 (37) | 97 (41) | 3 (60) | 14 (48) | 9 (43) | 23 (46) | 6 (67) | 12 (46) | 8 (33) | 30 (36) |
| 2018–2019 | 42,199 (38) | 78 (33) | 1 (20) | 12 (41) | 8 (38) | 9 (18) | 1 (11) | 9 (35) | 9 (38) | 31 (37) |
| 2019–2020 | 27,561 (25) | 61 (26) | 1 (20) | 3 (10) | 4 (19) | 18 (36) | 2 (22) | 5 (19) | 7 (29) | 23 (27) |
| Admission Month | *** | ** | ** | *** | *** | *** | ||||
| Jul | 10,590 (10) | 5 (2) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 4 (5) |
| Aug | 10,976 (10) | 7 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (12) | 0 (0) | 4 (5) |
| Sep | 10,230 (9) | 20 (8) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 3 (12) | 0 (0) | 16 (19) |
| Oct | 10,997 (10) | 15 (6) | 0 (0) | 1 (3) | 1 (5) | 1 (2) | 0 (0) | 1 (4) | 1 (4) | 11 (13) |
| Nov | 10,194 (9) | 16 (7) | 1 (20) | 3 (10) | 3 (14) | 1 (2) | 0 (0) | 2 (8) | 2 (8) | 7 (8) |
| Dec | 10,126 (9) | 34 (14) | 0 (0) | 6 (21) | 4 (19) | 5 (10) | 2 (22) | 4 (15) | 8 (33) | 6 (7) |
| Jan | 10,550 (9) | 41 (17) | 2 (40) | 1 (3) | 2 (10) | 21 (42) | 0 (0) | 2 (8) | 6 (25) | 8 (10) |
| Feb | 9,561 (9) | 30 (13) | 0 (0) | 5 (17) | 0 (0) | 13 (26) | 1 (11) | 0 (0) | 4 (17) | 7 (8) |
| Mar | 7,022 (6) | 28 (12) | 1 (20) | 5 (17) | 5 (24) | 6 (12) | 4 (44) | 1 (4) | 3 (12) | 5 (6) |
| Apr | 7,062 (6) | 19 (8) | 0 (0) | 5 (17) | 4 (19) | 0 (0) | 2 (22) | 2 (8) | 0 (0) | 7 (8) |
| May | 7,146 (6) | 10 (4) | 1 (20) | 2 (7) | 0 (0) | 3 (6) | 0 (0) | 3 (12) | 0 (0) | 3 (4) |
| Jun | 6,825 (6) | 11 (5) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 4 (15) | 0 (0) | 6 (7) |
Note. ccCoV, common-cold coronavirus; HARVI; hospital-associated respiratory virus infection; hMPV, human metapneumovirus; hRV/EV, human rhinovirus–enterovirus; IQR, interquartile range; NH, non-Hispanic; PIV, parainfluenza; RSV, respiratory syncytial virus; SIRS, systemic inflammatory response syndrome.
Numbers in the table represent inpatient admissions; individual patients may have been admitted to the hospital multiple times during the study period.
Data are no. (%) unless otherwise specified.
P < .05 statistically significant difference relative to admissions without HARVI.
P < .01 statistically significant difference relative to admissions without HARVI.
P < .001 statistically significant difference relative to admissions without HARVI.
Among pediatric patients, the median time from hospital admission to HARVI identification was 14 (interquartile range, 7–37) days (Fig. 1). Non-Hispanic black patients were over-represented among pediatric HARVI cases compared to those without HARVI (Table 3). Pediatric HARVI cases were more likely to have any of the chronic conditions that comprise the CCC score and had higher median CCC scores (5 vs 0) than patients without HARVI. In contrast to adults, the incidence of pediatric HARVI did vary significantly by study period with HARVI cases overrepresented in the 2018–2019 season. Also unlike adults, overall HARVI status was not associated with month of admission for pediatric patients. However, HARVI with ccCoV, influenza A, and RSV identification was significantly more likely among patients admitted during the winter months. Patterns of association were otherwise fairly similar by virus species among pediatric patients.
Table 3.
Characteristics of Hospitalized Pediatric Patients Admitted Between July 1, 2017, and February 28, 2020, by HARVI Statusa
| Variable | w/o HARVI (N=33,002), No. (%)b | Any HARVI (N=194), No. (%)b | Adenovirus (N=19), No. (%)b | ccCov (N=29), No. (%)b | hMPV (N=8), No. (%)b | Influenza A (N=9), No. (%)b | Influenza B (N=6), No. (%)b | PIV (N=24), No. (%)b | RSV (N=16), No. (%)b | hRV/EV (N=108), No. (%)a |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, median y (IQR) | 0 (0–8) | 1 (0–6) | 3 (0–7) | 1 (0–7) | 2 (1–3) | 4 (1–5) | 5 (1–8) | 3 (1–6) | 0 (0–2) | 0 (0–6) |
| Sex | * | |||||||||
| Female | 15,903 (48) | 82 (42) | 8 (42) | 15 (52) | 2 (25) | 2 (22) | 2 (33) | 13 (54) | 3 (19) | 48 (44) |
| Male | 17,099 (52) | 112 (58) | 11 (58) | 14 (48) | 6 (75) | 7 (78) | 4 (67) | 11 (46) | 13 (81) | 60 (56) |
| Race | * | * | ||||||||
| NH Asian | 1,595 (5) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| NH Black | 4,464 (14) | 33 (17) | 5 (26) | 4 (14) | 0 (0) | 2 (22) | 1 (17) | 5 (21) | 1 (6) | 21 (19) |
| NH White | 23,280 (71) | 136 (70) | 11 (58) | 25 (86) | 7 (88) | 7 (78) | 4 (67) | 19 (79) | 11 (69) | 70 (65) |
| Other/Unknown/Missing | 3,663 (11) | 24 (12) | 3 (16) | 0 (0) | 1 (12) | 0 (0) | 1 (17) | 0 (0) | 4 (25) | 16 (15) |
| CCC score, median (IQR) | 0 (0–2) | 5 (3–6)*** | 6 (5–7)*** | 4 (3–6)*** | 6 (4–6)*** | 1 (0–4) | 4 (2–6)* | 4 (2–7)*** | 4 (1–6)*** | 5 (3–6)*** |
| Chronic condition | ||||||||||
| Neurologic and neuromuscular | 4,246 (13) | 68 (35)*** | 7 (37)** | 9 (31)** | 4 (50)* | 1 (11) | 1 (17) | 10 (42)*** | 2 (12) | 43 (40)*** |
| Cardiovascular | 6,592 (20) | 136 (70)*** | 16 (84)*** | 21 (72)*** | 6 (75)** | 4 (44) | 4 (67)* | 15 (62)*** | 11 (69)*** | 78 (72)*** |
| Respiratory | 2,909 (9) | 97 (50)*** | 11 (58)*** | 17 (59)*** | 4 (50)** | 2 (22) | 2 (33) | 8 (33)*** | 6 (38)** | 57 (53)*** |
| Renal and urologic | 2,235 (7) | 37 (19)*** | 6 (32)** | 3 (10) | 2 (25) | 1 (11) | 1 (17) | 4 (17) | 3 (19) | 22 (20)*** |
| Gastrointestinal | 4,280 (13) | 100 (52)*** | 14 (74)*** | 11 (38)*** | 5 (62)** | 3 (33) | 1 (17) | 12 (50)*** | 9 (56)*** | 57 (53)*** |
| Hematologic and immunologic | 3,233 (10) | 62 (32)*** | 10 (53)*** | 10 (34)*** | 4 (50)** | 2 (22) | 2 (33) | 11 (46)*** | 4 (25) | 29 (27)*** |
| Metabolic | 4,777 (14) | 80 (41)*** | 13 (68)*** | 9 (31)* | 4 (50)* | 2 (22) | 3 (50)* | 10 (42)** | 7 (44)** | 41 (38)*** |
| Other congenital or genetic | 3,160 (10) | 59 (30)*** | 6 (32)** | 10 (34)*** | 3 (38)* | 0 (0) | 0 (0) | 9 (38)*** | 6 (38)** | 33 (31)*** |
| Malignancy | 2,785 (8) | 48 (25)*** | 6 (32)** | 8 (28)** | 1 (12) | 1 (11) | 1 (17) | 7 (29)** | 2 (12) | 27 (25)*** |
| Premature and neonatal | 2,389 (7) | 47 (24)*** | 2 (11) | 4 (14) | 3 (38)* | 0 (0) | 1 (17) | 2 (8) | 3 (19) | 37 (34)*** |
| Medical device dependence | 5,188 (16) | 136 (70)*** | 17 (89)*** | 21 (72)*** | 7 (88)*** | 3 (33) | 4 (67)** | 13 (54)*** | 10 (62)*** | 79 (73)*** |
| Transplantation | 395 (1) | 18 (9)*** | 5 (26)*** | 1 (3) | 1 (12) | 1 (11) | 1 (17) | 4 (17)*** | 0 (0) | 8 (7)*** |
| Study period | * | *** | * | * | ||||||
| 2017–2018 | 12,389 (38) | 56 (29) | 0 (0) | 10 (34) | 4 (50) | 3 (33) | 2 (33) | 5 (21) | 6 (38) | 30 (28) |
| 2018–2019 | 12,303 (37) | 83 (43) | 12 (63) | 13 (45) | 1 (12) | 3 (33) | 0 (0) | 14 (58) | 2 (12) | 48 (44) |
| 2019–2020 | 8,310 (25) | 55 (28) | 7 (37) | 6 (21) | 3 (38) | 3 (33) | 4 (67) | 5 (21) | 8 (50) | 30 (28) |
| Admission month | ** | * | ** | |||||||
| Jul | 3,083 (9) | 15 (8) | 2 (11) | 1 (3) | 1 (12) | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 11 (10) |
| Aug | 3,182 (10) | 17 (9) | 1 (5) | 2 (7) | 0 (0) | 0 (0) | 1 (17) | 1 (4) | 0 (0) | 13 (12) |
| Sep | 3,072 (9) | 17 (9) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (8) | 1 (6) | 14 (13) |
| Oct | 3,205 (10) | 15 (8) | 1 (5) | 0 (0) | 0 (0) | 1 (11) | 0 (0) | 3 (12) | 2 (12) | 10 (9) |
| Nov | 3,001 (9) | 17 (9) | 3 (16) | 0 (0) | 1 (12) | 0 (0) | 1 (17) | 6 (25) | 1 (6) | 8 (7) |
| Dec | 3,068 (9) | 30 (15) | 2 (11) | 7 (24) | 2 (25) | 2 (22) | 2 (33) | 3 (12) | 8 (50) | 11 (10) |
| Jan | 3,098 (9) | 17 (9) | 4 (21) | 6 (21) | 0 (0) | 4 (44) | 0 (0) | 0 (0) | 2 (12) | 2 (2) |
| Feb | 2,829 (9) | 17 (9) | 1 (5) | 3 (10) | 2 (25) | 2 (22) | 0 (0) | 0 (0) | 1 (6) | 10 (9) |
| Mar | 2,140 (6) | 11 (6) | 0 (0) | 2 (7) | 0 (0) | 0 (0) | 2 (33) | 0 (0) | 1 (6) | 7 (6) |
| Apr | 2,081 (6) | 11 (6) | 0 (0) | 3 (10) | 1 (12) | 0 (0) | 0 (0) | 3 (12) | 0 (0) | 5 (5) |
| May | 2,146 (7) | 16 (8) | 2 (11) | 3 (10) | 1 (12) | 0 (0) | 0 (0) | 2 (8) | 0 (0) | 11 (10) |
| Jun | 2,097 (6) | 11 (6) | 2 (11) | 2 (7) | 0 (0) | 0 (0) | 0 (0) | 3 (12) | 0 (0) | 6 (6) |
Note. CCC, pediatric complex chronic condition; ccCoV, common-cold coronavirus; HARVI; hospital-associated respiratory virus infection; hMPV, human metapneumovirus; hRV/EV, human rhinovirus/enterovirus; IQR, interquartile range; NH, non-Hispanic; PIV, parainfluenza; RSV, respiratory syncytial virus; SIRS, systemic inflammatory response syndrome.
Numbers in the table represent inpatient admissions; individual patients may have been admitted to the hospital multiple times during the study period.
Data are no. (%) unless otherwise specified.
P < .05 statistically significant difference relative to admissions without HARVI.
P < .01 statistically significant difference relative to admissions without HARVI.
P < .001 statistically significant difccCoV, common-col ference relative to admissions without HARVI.
We used competing risks models to estimate direct and indirect risk factors for HARVI. In adults, congestive heart failure, renal disease, and cancer comorbidities were estimated to directly increase the rate of HARVI by 65%, 45%, and 124%, respectively (Fig. 2 and Supplementary Table 1 online). Risk of HARVI was also elevated for patients admitted September through June relative to July admissions with hazard ratios above 5 from December to April. Men, non-Hispanic black patients, those with all comorbidities except myocardial infarction, and those meeting ≥2 SIRS criteria had significantly lower rates of discharge (ie, longer hospital stays), an indirect risk factor for HARVI. Fine-Gray model estimates combining these direct and indirect effects indicated that congestive heart failure, cerebrovascular disease, renal disease, and cancer comorbidities increased the overall risk of HARVI. Patients meeting ≥2 SIRS criteria also had higher overall risk of HARVI, and admission in December–April remained a strong overall risk factor.
Figure 2.
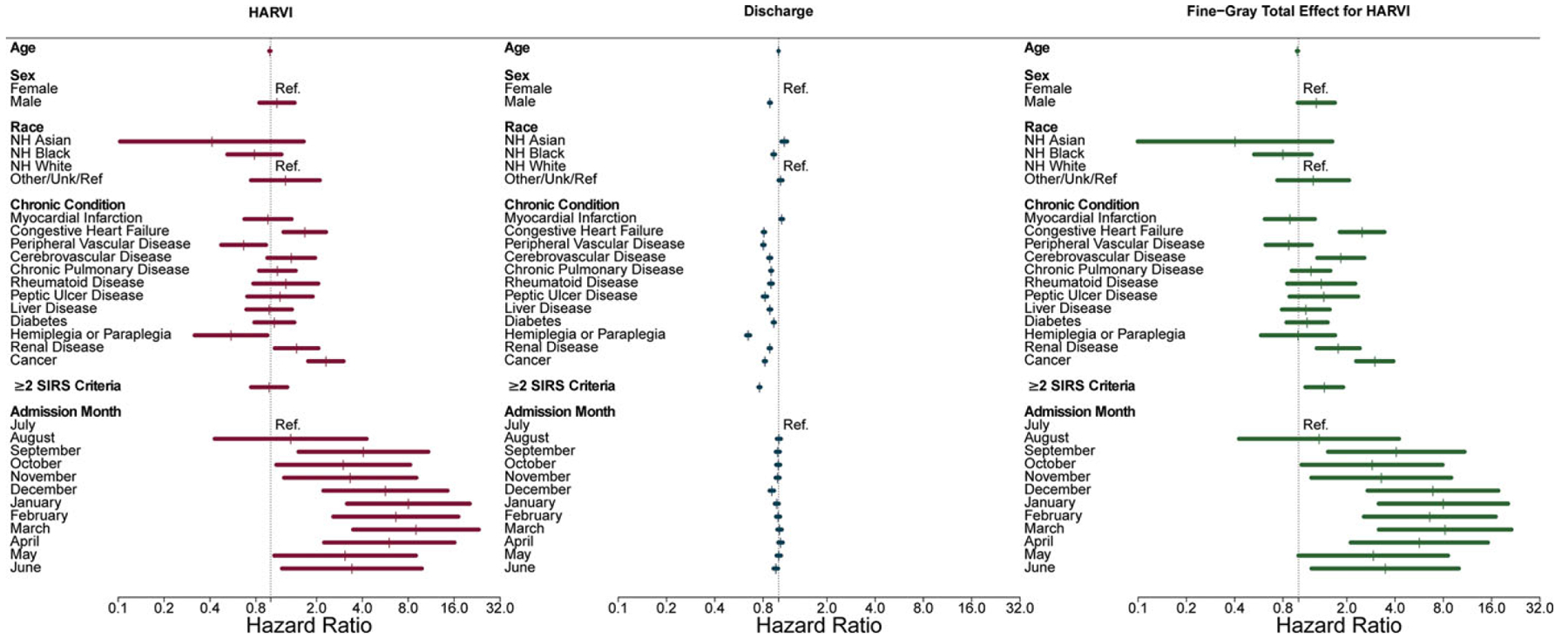
Risk factors for hospital-associated respiratory virus infection (HARVI) among adult inpatients. Competing risks analysis estimates direct and indirect (through length of stay) effects of factors on risk of HARVI; Fine-Gray analysis estimates absolute effects (combining direct and indirect effects) of factors on risk of HARVI.
Chronic conditions including cardiovascular disease, respiratory disease, cancer, and medical device dependence were directly associated with increased rates of HARVI among pediatric patients (Fig. 3 and Supplementary Table 2 online). The direct hazard of HARVI decreased with increasing age and was lower among pediatric patients with neonatal conditions. In contrast to adults, the direct hazard of HARVI was only elevated for children admitted in the month of December. Increasing age, non-Hispanic black race and ethnicity, all chronic conditions (except renal disease and transplantation), and admission between October and February were all associated with decreased hazard of discharge (ie, longer hospital stays). Combining direct and indirect hazards of HARVI, chronic conditions including cardiovascular disease, respiratory disease, metabolic disease, cancer, medical device dependence, and transplantation all increased the overall hazard of HARVI as did admission in the month of December. Increasing age decreased the overall hazard of HARVI in pediatric patients.
Figure 3.
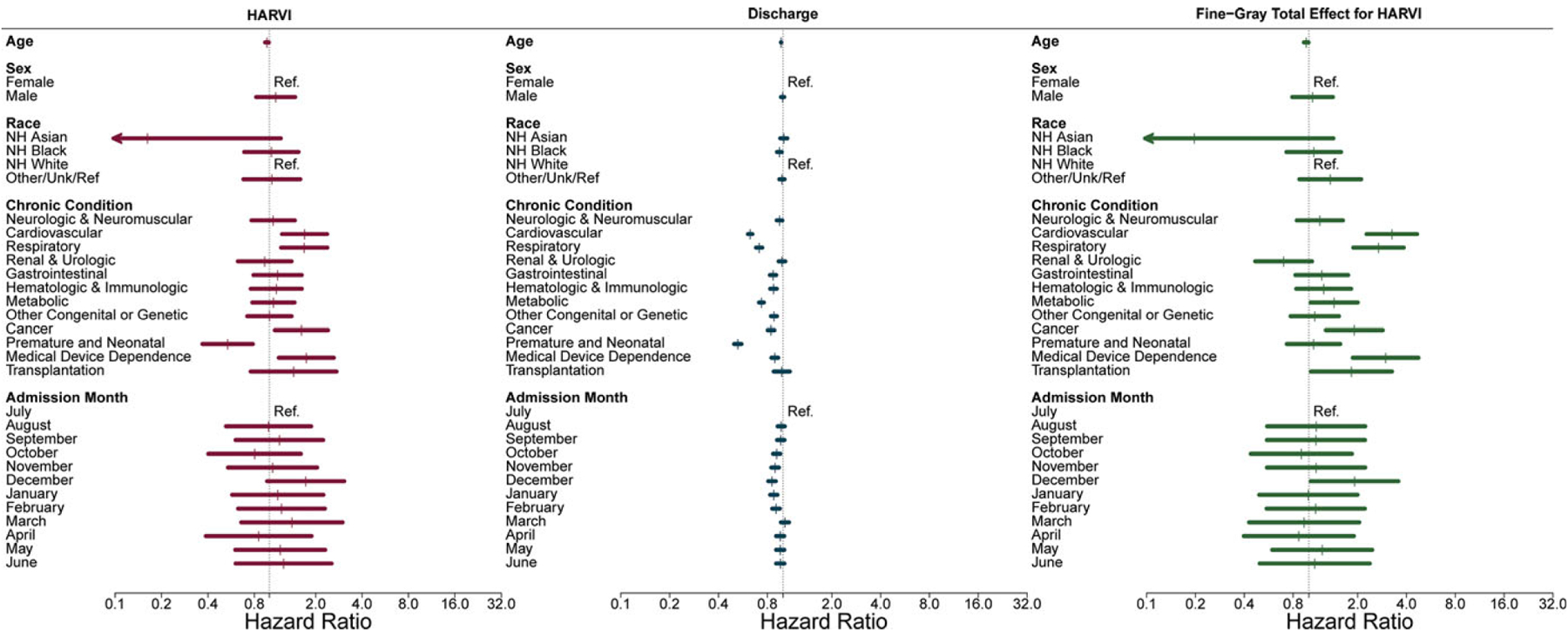
Risk factors for hospital-associated respiratory virus infection (HARVI) among pediatric inpatients. Competing risks analysis estimates direct and indirect (through length of stay) effects of factors on risk of HARVI; Fine-Gray analysis estimates absolute effects (combining direct and indirect effects) of factors on risk of HARVI.
Overall, HARVI reduced the daily hazard of discharge by 30% significantly increasing lengths of stay among adult inpatients (Table 4). All viruses had hazard ratios <1 for discharge (range, 0.66–0.97); however, longer lengths of stay were only statistically significant for ccCoV, influenza A, influenza B, and rhinovirus-enterovirus. HARVI was associated with a 55% increase in the hazard of death in adults within 30 days of infection overall. In virus-specific analyses, only influenza A was significantly associated with an increased hazard of death relative to patients without HARVI (135% increase). Moreover, 10 deaths (20%) occurred among the 50 influenza HARVI cases. The hazard ratios for first ICU admission were 2.16 (95% CI, 1.20–3.89) 0 to 5 days, 1.93 (95% CI, 0.81–4.60) 6 to 10 days, and 2.39 (95% CI, 1.05–5.46) >10 days post-HARVI identification relative to patients without HARVI. Species-specific hazard ratios for first ICU admission could not be estimated due to small sample sizes.
Table 4.
Effects of Hospital-Associated Respiratory Virus Infection (HARVI) on Time to Discharge and In-Hospital Deatha
| Variable | Discharge | Death | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Admissions No. (days w/o HARVI) | HARVI No. (days after HARVI) | Unadjusted Hazard Ratio (95% CI) | Adjusteda Hazard Ratio (95% CI) | Deaths w/o HARVI | Deaths after HARVI | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI)b | |
| Adult | ||||||||
| Any HARVI | 111,515 (776,530) | 236 (4,101) | 0.68 (0.61–0.76) | 0.70 (0.62–0.79) | 2,738 | 28 | 1.52 (1.04–2.24) | 1.55 (1.04–2.29) |
| Adenovirus | 111,436 (779,502) | 5 (173) | 0.63 (0.25–1.59) | 0.85 (0.33–2.18) | 2,765 | 0 | … | … |
| ccCoV | 111,515 (780,132) | 29 (471) | 0.67 (0.53–0.85) | 0.68 (0.52–0.89) | 2,768 | 3 | 1.26 (0.41–3.88) | 1.26 (0.42–3.80) |
| hMPV | 111,192 (777,287) | 21 (404) | 0.77 (0.50–1.19) | 0.97 (0.63–1.48) | 2,756 | 4 | 2.38 (0.90–6.27) | 1.95 (0.67–5.66) |
| Influenza A | 110,905 (774,988) | 50 (854) | 0.64 (0.52–0.80) | 0.71 (0.57–0.88) | 2,738 | 10 | 2.84 (1.47–5.49) | 2.35 (1.12–4.92) |
| Influenza B | 111,384 (779,407) | 9 (130) | 0.82 (0.60–1.12) | 0.71 (0.52–0.97) | 2,765 | 0 | ||
| PIV | 111,515 (780,127) | 26 (481) | 0.72 (0.48–1.07) | 0.66 (0.41–1.07) | 2,769 | 2 | 0.89 (0.22–3.55) | 1.29 (0.33–5.00) |
| RSV | 111,112 (776,480) | 24 (588) | 0.72 (0.54–0.97) | 0.83 (0.64–1.08) | 2,735 | 1 | 0.43 (0.06–3.07) | 0.37 (0.05–2.90) |
| hRV/EV | 109,917 (765,093) | 84 (1,393) | 0.69 (0.55–0.85) | 0.68 (0.55–0.83) | 2,693 | 8 | 1.20 (0.58–2.48) | 1.41 (0.70–2.83) |
| Pediatric | ||||||||
| Any HARVI | 33,196 (221,496) | 194 (6,748) | 0.81 (0.71–0.92) | 1.02 (0.88–1.18) | 247 | 5 | 0.59 (0.22–1.58) | 0.52 (0.20–1.36) |
| Adenovirus | 32,925 (224,764) | 19 (739) | 0.77 (0.57–1.03) | 1.22 (0.86–1.74) | 253 | 1 | 1.06 (0.14–8.16) | 0.88 (0.11–6.91) |
| ccCoV | 33,196 (227,583) | 29 (658) | 0.90 (0.65–1.25) | 1.14 (0.81–1.62) | 256 | 1 | 1.15 (0.15–8.52) | 1.17 (0.16–8.52) |
| hMPV | 32,992 (226,172) | 8 (268) | 0.95 (0.64–1.39) | 1.46 (0.97–2.20) | 257 | 0 | … | … |
| Influenza A | 33,019 (226,565) | 9 (320) | 0.92 (0.59–1.43) | 0.70 (0.45–1.11) | 256 | 0 | … | … |
| Influenza B | 33,122 (227,043) | 6 (260) | 0.74 (0.48–1.13) | 0.61 (0.28–1.32) | 256 | 0 | … | … |
| PIV | 33,196 (227,573) | 24 (668) | 0.84 (0.59–1.19) | 1.08 (0.73–1.59) | 256 | 0 | … | … |
| RSV | 32,553 (222,803) | 16 (462) | 0.87 (0.62–1.22) | 0.76 (0.43–1.36) | 255 | 1 | 1.45 (0.19–10.79) | 1.53 (0.22–10.75) |
| hRV/EV | 31,347 (206,305) | 108 (4,241) | 0.82 (0.68–0.98) | 1.09 (0.90–1.31) | 237 | 2 | 0.37 (0.08–1.68) | 0.30 (0.07–1.38) |
Note. ccCoV, common-cold coronavirus; hMPV, human metapneumovirus; hRV/EV, human rhinovirus/enterovirus; IQR, interquartile range; PIV, parainfluenza; RSV, respiratory syncytial virus; SIRS, systemic inflammatory response syndrome.
Competing risks of discharge and death were modeled in Cox proportional hazards models with time-varying HARVI status.
Adjusted models included the following covariates: Age (years), sex, non-Hispanic Black race/ethnicity, CCI score (adults), CCC score (children), meeting ≥2 SIRS criteria (adults), and peak season admission (December, January, February, or March).
No HARVIs were associated with increased length of stay or risk of death for pediatric patients. However, pediatric patients were at increased risk of first ICU admission shortly after HARVI identification. Relative to pediatric patients without a HARVI, the hazard ratios for first ICU admission were 5.76 (95% CI, 2.84–11.65) for 0–5 days after HARVI identification; 1.49 (95% CI, 0.21–10.87) for 6–10 days after HARVI identification; and 0.94 (95% CI, 0.26–3.39) for >10 days after HARVI identification.
Discussion
HARVI incidence varied by viral species and was higher among pediatric patients, similar to previous studies.7–10,22,23 For adults, HARVI increased the length of hospital stays and hazard of ICU admission after infection. Hospital-associated influenza infections were also associated with increased risk of in-hospital death for adults. HARVIs were not associated with increased length of stay or mortality in pediatric patients, but the rate of ICU admission in the first 5 days after HARVI identification was >5-fold higher compared to patients without HARVI. Relative to adults, pediatric patients were infected later in their hospitalization; 25% of HARVIs were identified >37 days after admission. It may be that the absence of an observed effect of HARVI on increased length of stay in pediatric patients was because those infected were likely to have long lengths of stay regardless of infection. Overall, these results suggest that HARVIs are a significant source of morbidity and mortality for hospitalized patients.
Lengths of stay were longer for adults with hospital-associated influenza A and B, ccCoV, and rhinovirus–enterovirus compared to those who were not infected. Although this is not a surprising outcome for influenza, ccCoV and rhinovirus–enterovirus infections are mainly associated with common-cold symptoms.24,25 Despite the perceived lack of severity, rhinoviruses and ccCoV have been associated with severe outcomes in published health-care-associated outbreaks, and rhinoviruses have been reported to be the common cause of community-acquired pneumonia.1,2,26–29 Both rhinoviruses and ccCoV, as well as other respiratory viruses, have also been demonstrated to have the potential to cause severe illness in adults and children following community-acquired infection.5,12,30
In adults, the rate of HARVI was higher among those with congestive heart failure, renal disease, and cancer. Similarly among children, cardiovascular and respiratory conditions, cancer, and medical device dependence were all associated with increased rate of HARVI. The impact of these conditions was more pronounced and additional risk factors were identified when considering the indirect effect that chronic conditions had on HARVI risk through extended duration of hospital stays, and thus time at risk. These findings highlight the importance of accounting for competing risks when evaluating risk factors for and outcomes associated with hospital-acquired infections.31 We also observed strong seasonal time dependence in the rates of HARVI among adults, with the highest rates among those admitted during the winter months. Interestingly, time dependence was not observed for pediatric patients. We hypothesize that this is because rhinoviruses comprised a larger proportion of HARVIs among pediatric patients than adults, and rhinovirus seasonality is less pronounced than that of other respiratory viruses.9,24
This study had several limitations. Sample sizes were small, particularly for analyses of specific viruses and ICU outcomes, thereby increasing the risk that we may have missed detecting true associations. In contrast, we did not adjust for multiple testing; thus, we may have detected chance associations. We focused on a single health system over 3 years prior to the COVID-19 pandemic. Our results may not be completely generalizable to other settings or other periods. The COVID-19 pandemic led to dramatic changes in healthcare utilization and delivery, and infection prevention strategies have continued to evolve.32–36 Also, we did not review patient charts to assess the timing of onset of new symptoms relative to HARVI detection. This may have resulted in overestimation of HARVI incidence if diagnosis of community-acquired infections was delayed. However, our use of virus-specific 95th percentile incubation period thresholds to define HARVI is more conservative than most previous studies.10 In contrast, we may have missed HARVI cases if clinical testing was not performed. Finally, other risk factors for infection and severe outcomes may exist that were not assessed, such as smoking status.
Institutional policies during this study period aligned with CDC infection prevention guidance, including placing individuals with acute respiratory illness under droplet precautions, a preference for private rooms for patients with acute respiratory illness, mandatory staff influenza vaccination, and symptom screening of visitors during the influenza season. HARVI still occurred despite these policies, and our results suggest that these infections increased patient morbidity and mortality. Much research has been carried out in recent years aimed at identifying HARVI transmission pathways and interventions to reduce transmission to patients from healthcare workers, other patients, and visitors to the hospital.37 However, more research is needed to optimize infection prevention strategies and use of diagnostics to accurately evaluate and reduce the burden of HARVI.
Supplementary Material
Acknowledgments.
Financial support.
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant no. K01AI141579 to J.G.P). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing interests.
J.G.P. reports grant funding and consulting fees from CSL Seqirus, outside of the submitted work. R.M. reports no conflicts of interest. A.S.L. reports consulting fees from Roche, outside of the submitted work. K.S.K reports consulting fees from Merck, Shionogi, AbbVie, Entasis, Qpex, Allecra, Carb-X, VenatoRx, MicuRx, and GSK outside of the submitted work.
Footnotes
Supplementary material. To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2023.263
PREVIOUS PRESENTATION. These data were presented in poster 640 at the Society for Healthcare Epidemiology of America Spring Conference on April 14, 2023, in Seattle, Washington.
References
- 1.Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med 2015;373:415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among US children. N Engl J Med 2015;372: 835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark TW, Medina M, Batham S, Curran MD, Parmar S, Nicholson KG. Adults hospitalised with acute respiratory illness rarely have detectable bacteria in the absence of COPD or pneumonia; viral infection predominates in a large prospective UK sample. J Infect 2014;69:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez A Respiratory virus surveillance among children with acute respiratory illnesses—new vaccine surveillance network, United States, 2016–2021. Morb Mortal Wkly Rep 2022;71:1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmerman RK, Balasubramani GK, D’Agostino HEA, et al. Population-based hospitalization burden estimates for respiratory viruses, 2015–2019. Influenza Other Respir Viruses 2022;16:1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrie JG, Talbot TR. Healthcare-acquired viral respiratory diseases. Infect Dis Clin N Am 2021;35:1055–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow EJ, Mermel LA. Hospital-acquired respiratory viral infections: incidence, morbidity, and mortality in pediatric and adult patients. Open Forum Infect Dis 2017;4:ofx006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi H-S, Kim M-N, Sung H, et al. Laboratory-based surveillance of hospital-acquired respiratory virus infection in a tertiary-care hospital. Am J Infect Control 2017;45:e45–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrie JG, Lauring AS, Martin ET, Kaye KS. Hospital-associated respiratory virus infection in children and adults: it does not just occur during cold and flu season. Open Forum Infect Dis 2020;7:ofaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Most ZM, Jackson P, Sebert M, Perl TM. Contrasting definitions and incidence of healthcare-associated respiratory viral infections in a pediatric hospital. Infect Control Hosp Epidemiol 2023;44:55–61. [DOI] [PubMed] [Google Scholar]
- 11.Shi T, McLean K, Campbell H, Nair H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: a systematic review and meta-analysis. J Glob Health 2015;5:010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Self WH, Williams DJ, Zhu Y, et al. Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J Infect Dis 2016;213:584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi T, Arnott A, Semogas I, et al. The etiological role of common respiratory viruses in acute respiratory infections in older adults: a systematic review and meta-analysis. J Infect Dis 2020;222:S563–S569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolkewitz M, Allignol A, Harbarth S, de Angelis G, Schumacher M, Beyersmann J. Time-dependent study entries and exposures in cohort studies can easily be sources of different and avoidable types of bias. J Clin Epidemiol 2012;65:1171–1180. [DOI] [PubMed] [Google Scholar]
- 15.Schumacher M, Allignol A, Beyersmann J, Binder N, Wolkewitz M. Hospital-acquired infections—appropriate statistical treatment is urgently needed! Int J Epidemiol 2013;42:1502–1508. [DOI] [PubMed] [Google Scholar]
- 16.Manchal N, Mohamed MRS, Ting M, et al. Hospital-acquired viral respiratory tract infections: an underrecognized nosocomial infection. Infect Dis Health 2020;25:175–180. [DOI] [PubMed] [Google Scholar]
- 17.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 18.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr 2014;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lessler J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, Cummings DAT. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis 2009;9:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol 2009;170:244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A Proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc 1999;94:496–509. [Google Scholar]
- 22.Valenti WM, Menegus MA, Hall CB, Pincus PH, Douglas RG. Nosocomial viral infections: I. Epidemiology and significance. Infect Control 1980; 1:33–37. [DOI] [PubMed] [Google Scholar]
- 23.Quach C, Shah R, Rubin LG. Burden of healthcare-associated viral respiratory infections in children’s hospitals. J Pediatr Infect Dis 2018;7: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs SE, Lamson DM, St. George K, Walsh TJ. Human rhinoviruses. Clin Microbiol Rev 2013;26:135–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McIntosh K, Perlman S, Monto A, Englund JA. A proposal to refer to four coronaviruses of limited human virulence ‘common cold coronaviruses.’ J Infect Dis 2022;226:2047–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louie JK, Yagi S, Nelson FA, et al. Rhinovirus outbreak in a long-term care facility for elderly persons associated with unusually high mortality. Clin Infect Dis 2005;41:262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zinna S, Lakshmanan A, Tan S, et al. Outcomes of nosocomial viral respiratory infections in high-risk neonates. Pediatrics 2016;138:e20161675. [DOI] [PubMed] [Google Scholar]
- 28.Hand J, Rose EB, Salinas A, et al. Severe respiratory illness outbreak associated with human coronavirus NL63 in a long-term care facility. Emerg Infect Dis 2018;24:1964–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beury D, Fléchon L, Maurier F, et al. Use of whole-genome sequencing in the molecular investigation of care-associated HCoV-OC43 infections in a hematopoietic stem cell transplant unit. J Clin Virol 2020;122:104206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veiga ABG da, Martins LG, Riediger I, Mazetto A, Debur M do C, Gregianini TS. More than just a common cold: endemic coronaviruses OC43, HKU1, NL63, and 229E associated with severe acute respiratory infection and fatality cases among healthy adults. J Med Virol 2021;93: 1002–1007. [DOI] [PubMed] [Google Scholar]
- 31.Wolkewitz M, Cooper BS, Bonten MJM, Barnett AG, Schumacher M. Interpreting and comparing risks in the presence of competing events. BMJ 2014;349:g5060. [DOI] [PubMed] [Google Scholar]
- 32.WHO statement on the fifteenth meeting of the IHR (2005) Emergency Committee on the COVID-19 Pandemic. World Health Organization; website. https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic. Accessed May 8, 2023. [Google Scholar]
- 33.Talbot TR, Hayden MK, Yokoe DS, et al. Asymptomatic screening for severe acute respiratory coronavirus virus 2 (SARS-CoV-2) as an infection prevention measure in healthcare facilities: challenges and considerations. Infect Control Hosp Epidemiol 2023;44:2–7. [DOI] [PubMed] [Google Scholar]
- 34.Chiu SS, Cowling BJ, Peiris JSM, Chan ELY, Wong WHS, Lee KP. Effects of nonpharmaceutical COVID-19 interventions on pediatric hospitalizations for other respiratory virus infections, Hong Kong. Emerg Infect Dis 2022;28:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wee LE, Conceicao EP, Sim XYJ, Ko KKK, Ling ML, Venkatachalam I. Reduction in healthcare-associated respiratory viral infections during a COVID-19 outbreak. Clin Microbiol Infect 2020;26:1579–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong S-C, Lam GK-M, AuYeung CH-Y, et al. Absence of nosocomial influenza and respiratory syncytial virus infection in the coronavirus disease 2019 (COVID-19) era: implication of universal masking in hospitals. Infect Control Hosp Epidemiol 2021;42:218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Most ZM. Beyond personal protective equipment: adjunctive methods for control of healthcare-associated respiratory viral infections. Curr Opin Infect Dis 2020;33:312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


