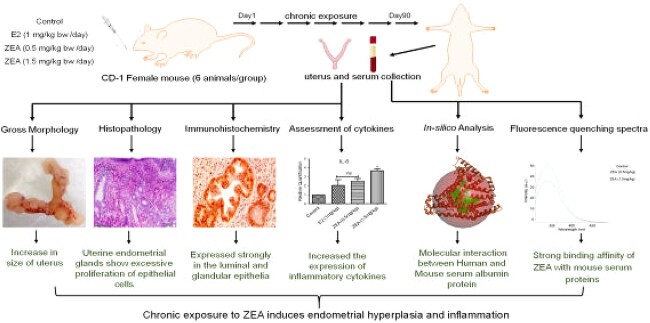
Abstract
Background
Zearalenone (ZEA), a natural food contaminant, is reported to act as a mycoestrogen due to its estrogen-mimicking properties. According to studies, ZEA has a greater potential for estrogenic activity compared to any other naturally occurring non-steroidal estrogen. ZEA has been found in the endometrium of individuals with reproductive problems and the serum of children facing early puberty. These studies suggested a possible link between ZEA exposure and endometrial toxicity; nonetheless, no thorough research has been done. This study assessed the endometrium’s response to chronic ZEA exposure.
Methods
Four groups of CD-1 female mice were exposed to control, estradiol (E2), and two different doses of ZEA for 90 days. At the end of treatment, blood and uterus were collected, and samples were used for inflammatory cytokines level, immunochemical, histopathological, and biophysical analysis.
Results
Our data indicated that the uterus showed a change in body/organ weight ratio, while other organs did not have any notable changes. Immunochemical and histological studies showed hyperplasia and a higher number of glands in the endometrium after ZEA and E2 exposure. Similarly, proliferation markers such as proliferative cell nuclear antigen (PCNA), Ki-67, and inflammatory cytokines such as interleukin 6 (IL-6), interleukin 8 (IL-8), and interferon-gamma (IFN-?) levels were found to be higher in the E2 and ZEA-exposed groups.
Conclusion
Our finding conclude that ZEA targets the uterus and cause inflammation due to increased levels of inflammatory cytokines and proliferation mediators, as well as systemic toxicity denoted by a strong binding affinity with serum proteins.
Keywords: Zearalenone, Mycoestrogen, Endocrine disruptors, Endometrium, Toxicology
Graphical Abstract
Graphical Abstract.
Introduction
Mycotoxin contamination is currently one of the most challenging concerns in food safety. According to recent research, staple foods like cereals, nuts, fruits, and vegetables have been contaminated with mycotoxins. These occurrences create a scenario of prolonged exposure, sometimes at high levels, leading to adverse effects. Consumption of mycotoxin—contaminated food/feed leads to several adverse health effects due to their teratogenic, hepatotoxic, haematotoxic, immunotoxic, and genotoxic potential.1
In addition, mycotoxins also play a significant role in alterations of inflammatory responses. Several studies have showed that exposure to mycotoxins can initiate or enhance inflammation.2,3 Further, these mycotoxins-induced inflammatory responses are found to play a crucial role in the development and progression of various diseases, including chronic inflammatory diseases, respiratory conditions like asthma and chronic obstructive pulmonary disease, gastrointestinal disorders, autoimmune disorders, and the promotion of various types of cancer.4,5 Additionally, inflammatory mediators such as cytokines can stimulate cell proliferation, survival, angiogenesis, invasion, and migration. Different mycotoxins may affect different organs, and their inflammatory effects can vary accordingly.6
Nevertheless, mycotoxin-related adverse health consequences have not yet been widely recognised as a concern from a public health perspective in low-income nations, including India.
Zearalenone (ZEA), an estrogenic mycotoxin, is frequently found to contaminate crops such as oats, corn, wheat, and rice, ultimately affecting animal feed and human food. Occurrences of ZEA at high levels have been detected in different cereals and foodstuffs from various pockets of the globe, such as Europe, Asia, and Africa.7
Despite such an alarming situation, only sixteen countries across the globe have food safety regulations concerned with ZEA contamination.8 The maximum permissible limit allowed by the European Union (EU) legislation for ZEA is 75 μg/kg for processed cereals, 100-200 μg/kg for unprocessed cereals, 50 μg/kg in snacks made of cereals, and 20 μg/kg in processed cereal-based foods.9 However, surprisingly, several surveillance studies conducted over time indicated that the entire globe is facing a serious problem of ZEA contamination.10 Another study has found that cereals like beans, corn, grains, maize, and feed mixtures for fattening pigs were found to be contaminated with ZEA, and out of the total analysed samples, 75% samples (range 15-866 μg/kg) were exceeding the EU limits.11 Similarly, a surveillance study from India found that staple foods are contaminated with ZEA, as the author found 80% of the total samples were positive for ZEA, of which 33% exceeded the EU limits.12 In the same study, the author also concluded that ZEA contamination was ~17 fold higher in rice and ~ 8 fold higher in wheat samples than the prescribed tolerable daily intake of ZEA as specified by the European Food Safety Authority (EFSA).12 These findings raised a serious health concern for a population that consumed cereal as the main meal twice a day.
In addition to the high prevalence of ZEA in food commodities, accumulating studies show that it possesses estrogenic properties. The resorcyclic acid lactone moiety of its chemical structure enables ZEA to mimic as E2, (endogenous hormone) by interacting with estrogen receptors (ERs) and subsequently regulating the expression of estrogen-responsive genes.11 Moreover, Wentao’s in vivo research revealed that ZEA directly contributes to intestinal inflammation, accompanied by alterations in cytokine levels.13 In another study Zearalenone caused severe haemorrhagic inflammation of the endometrium, affecting embryo implantation.14 In silico studies have demonstrated a strong binding of ZEA with estrogen receptors, suggesting agonist behaviour of ZEA towards estrogen receptors.15–17 In addition, several in vitro studies have also reported the estrogenic effect, oxidative imbalance, and cellular and genotoxicity caused by ZEA.18,19 In concordance, ZEA has been associated with several reproductive dysfunctions in animals, such as infertility, ovarian changes or dysfunction, miscarriage, reduced ovulation, false estrus, vulvovaginitis, lesions, embryotoxic effects, abnormal birth, and stillbirth.20 Along with in silico, in-vitro, and in vivo studies, the levels of ZEA and its metabolites have also been detected in the serum of children with idiopathic puberty and the endometrium tissues of women diagnosed with endometrial cancer.21–23 A recent exposure study detected ZEA and correlated its presence with the risk of inflammation, cancer, and disorders of the reproductive and endocrine systems.10 Thus, these studies strongly suggest a possible link between ZEA and endometrial toxicity.
Endogenous estrogens are physiologically essential; however, the presence of unopposed/high levels of xenoestrogen(s) may lead to aberrant ER signalling, which alters physiological function and, in adverse conditions, may enhance the risk of hormone-sensitive cancer development.24 Since ZEA has estrogenic properties similar to those of the endogenous hormone estradiol, it is possible that it can cause unwanted estrogenic effects in the body after exposure. Moreover, surveillance studies have also shown the high levels of ZEA in food, hence, it is quite likely that people are getting exposed to ZEA, thus, delineation of chronic exposure effects is highly warranted. In this study, the chronic effect of ZEA was evaluated using a CD-1 female mouse as an animal model. Mice were exposed to control, E2, and two different dosages of ZEA for 90 days. There were no significant changes in weight gain and mortality observed during the experiment. However, both dosages of ZEA and E2 caused an increase in weight and hyperplasia in the uterus, higher expression of proliferative markers, and levels of cytokines associated with inflammation, i.e. IL-6, IL-8, and IFN-γ. In addition, molecular docking and fluorescence spectroscopic analysis showed a strong binding affinity of ZEA with mouse serum proteins, suggesting a possible cause for the systemic toxicity of ZEA.
Materials and methods
Chemicals
Zearalenone (ZEA) (Cat no. Z2125-10MG), 17-β estradiol (E2) (Cat no. E8875-1G), and 3,3′-diaminobenzidine (DAB) (Cat no. D3939), dibutylphthalatepolystyrenexylene (DPX) mountant for histology (Cat no. 06522) was procured from Sigma Aldrich (Co. St. Louis, MO, USA). Primary antibodies of PCNA (Cat no. D3H8P), Ki-67 (Cat no. D3B5) and secondary antibody anti rabbit IgG, (Cat no. 7074P2) were procured from Cell Signalling Technology (Danvers, Massachusetts, USA). ABC peroxidase staining kit (Cat no. 32020) was purchased from ThermoScientific (Waltham, MA, USA). BD vacutainer serum tubes (Cat no. 367812) were obtained from BD India Pvt. Ltd (BD, USA). Other chemicals and reagents/solvents were procured from the best available commercial sources, ensuring the highest purity.
Animal’s housing, accommodation and diet
Female CD-1 mice (4 to 6 weeks old) were received from, the animal breeding facility of CSIR-IITR, Lucknow, India. After acclimatization for one week, mice were divided into four groups of six weight-matched mice in each group. Mice were housed in a polypropylene cage as per standard laboratory conditions (12 h light/dark cycle; 22 ± 2 °C; 50%–60% humidity). Mice were provided ad libitum reverse osmosis water and an estrogen-free pellet diet (Cat no. 1324, M/s Altromin, Inc., Germany). Mice were cared for and maintained as recommended by the committee for the purpose of the control and supervision of experiments on animals (CPCSEA), Government of India. The study protocol was reviewed and approved by the Institutional Animal Ethics Committee of CSIR-IITR, Lucknow, India (approval no. IITR/IAEC/03/20).
Experimental design
Dosage preparation
Before treatment of the mice, dose formulations for control, E2, and ZEA were prepared. E2 and ZEA were first pre-dissolved in a small amount of DMSO and then diluted with corn oil to achieve the required concentrations. The DMSO concentration was not more than 0.4% maximum in all the dose formulations. Tolerable daily intake (TDI), established by the European Food Safety Authority (EFSA) for ZEA is 0.25 μg/kg bw/day for humans, which has been taken into consideration when choosing the doses of ZEA. The doses were prepared every week and pre-dissolved ZEA and E2 administered to each animal according to body weight gain.
Animal treatment
The body weight of each individual mouse was recorded after acclimatization. Subsequently, mice were split into four groups based on body weight. The control group mice received 100 μl of diluted 0.4% DMSO in corn oil. Another group of mice received E2 (1 mg/kg bw). Similar to this, another two groups of mice received ZEA at doses of 0.5 and 1.5 mg/kg bw respectively. During the treatment, body weight of mice was recorded weekly and any signs and symptoms of toxic manifestations were also observed.
Histology
The uterus was isolated, rinsed with chilled normal saline solution, fixed, and preserved in 10% buffered formalin. Further fixed tissues were embedded in paraffin, and blocks were prepared after processing. Uterus sections of 5 μm thickness were cut using a rotary microtome (Leica RM2155, Leica, GmbH, Nussloch, Germany) and then stained with haematoxylin and eosin for microscopic examination of histopathological changes. After staining, the photomicrograph using an Olympus BX53 upright microscope (Olympus, Shinjuku, Tokyo, Japan), the sections were examined. The changes were evaluated as per guidelines by nomenclature and diagnostic criteria of the Society of Toxicological Pathologist.
Morphometrical studies
In each of the H & E-stained uterine sections, myometrium widths (MW), glandular epithelium heights (GEH), luminal epithelium heights (LEH), and myometrium widths (MW) were quantified. Cell Sens standard imaging software (Olympus, Shinjuku, Tokyo, Japan) was used for all measurements. Measurements of LEH, GEH, and MW were expressed as means with a standard error of the mean (SEM).
Immunohistochemistry (IHC)
Paraffin was removed by immersing in absolute xylene for 15 min. Further, the sections were sequentially rehydrated by gradually decreasing concentrations of ethanol (100%; 95%; 70%) and ddH2O. The deparaffinization steps were followed by heat antigen retrieval, where sections were boiled for 30 min in sodium citrate buffer (10 mM; pH 6.0) and then rinsed with PBS. Next, the endogenous peroxidase activity of sections were inhibited using 3% hydrogen peroxide (H2O2) and blocked with 5% BSA in TBS for 60 min incubation at room temperature. Following blocking, sections were again incubated with primary antibodies of PCNA and Ki-67 (1:250 dilution) for overnight at 4°C. Next morning, following washing with TBS, sections were incubated with secondary antibody (anti-rabbit; HRP conjugated; 1:1000 dilution) for 60 min and then stained with DAB reagent. Finally, counterstaining of sections was done with haematoxylin, and DPX-mounted slides were visualised and photomicrograph using a light microscope, the Olympus BX53 upright microscope (Olympus, Shinjuku, Tokyo, Japan). Quantification of PCNA and Ki-67 expression was performed by counting positive cells (brown deposits in the nucleus) in the randomly selected five non-overlapping fields from a minimum of three different slides.
mRNA expression of IFN-γ, IL-6, IL-1α, IL-10 and IL-8, IL-2 by real time PCR
The mRNA expression of inflammatory markers was measured using a qPCR assay. One hundred mg of uterine tissues were homogenised and total RNA was isolated using Trizol (Qiagen, Valencia, CA, USA), cDNA was prepared using the reverse transcription kit as recommended by the manufacturer (Applied Biosystems, CA, USA). PCR Master Mix Kit (Qiagen, Valencia, CA, USA) and SYBR Green master mix (Qiagen, Valencia, CA, USA) were used for qRT-PCR experiments. The Quant Studio 6 Flex apparatus and software (Thermo, Waltham, MA, USA) were used to carry out the qRT-PCR experiments. Relative Quantity (RQ) and changes in mRNA expression were determined using the following equation, RQ = 2-∆∆CT, where ∆∆CT stood for the differences in the ∆CT value of treated sets compared to control. GAPDH was used as the housekeeping gene. The sequence of forward (5′–3′) and reverse (5′–3′) primer sets for selected targeted genes is mentioned in Table 1.
Table 1.
List of primers used in qRT-PCR.
| Target Gene | Forward primer 5 ′–3 ′ | Reverse primer 5 ′–3 ′ |
|---|---|---|
| IL-8 | AAAATTTTCGTTATATTTCG | TCCGATAACTTTTTATATCAT |
| IL-6 | GCTACCAAACTGGATATAATCAGG | CCAGGTAGCTATGGTACTCCAGAA |
| IL-2 | ACTCTGATATTGCTGATGAA | GATGAACTTGGACCTCTG |
| IL-10 | AGCAGGTGAAGAGTGATT | GCAGTTGATGAAGATGTC |
| IL-1α | GGCAGTGTTGCTCCATGTAA | TCAGCCACATTATGGCAAAG |
| IFN-γ | TTAACTCAAGTGGCATAG | TGATTCAATGACGCTTAT |
| GAPDH | AGTGGCAAAGTGGAGATT | GTGGAGTCATACTGGAACA |
In silico analysis
The three-dimensional protein structure of human serum albumin (hsa) (PDB ID: 1ao6) was retrieved from the Protein Data Bank (https://www.rcsb.org/). Heteroatoms, water molecules, and ions were removed from the protein molecule using the Discovery Studio (DS) software. Further, the protein modelling of mouse serum albumin (msa) structure was derived from by use of SWISS-MODEL (https://swissmodel.expasy.org) The binding sites of both targets were determined by the DS. The PubChem database (https://pubchem.ncbi.nlm.nih.gov/) was adopted to curate the SDF format of ZEA.
Molecular docking was carried out to investigate the molecular interaction among the selected targets and ligands to determine binding energies and interaction patterns using Autodock Vina. Before initiation of the docking study, the target and ligand files were converted into pdbqt file format, along with grid box was generation based on the identified binding site. The grid values were set up to the protein’s active sites. In addition, the grid box values of human serum albumin (100x98x124, with centers of x = 30.369, y = 32.577, and z = 23.5) were specified in the configuration file. Similarly, in the following configuration file, the grid box values for mouse serum albumin (104×108×112, with centres of x = 41.246, y = 17.42, z = 69.031) were specified. Autodock Vina run was finally started with the specified commands to generate the output files. The Discovery Studio Visualizer was used to examine the docked conformations of the complex.
Spectrophotometric spectral analysis for mouse serum-Zearalenone interaction
Fluorescence spectra analysis of ZEA-treated mouse serum was performed using an Eclipse spectrofluorometer (MY19010001; Agilent Technologies, Santa Clara, CA, USA). The excitation and emission slits of the spectroflourometer were set at 5 nm and the scanning speed was kept at medium mode; 25 ± 0.1 °C in 1 cm path length cuvette. The Tyrosine and tryptophan fluorescence of control and treated mice’s serum was noted in the wavelength range of 300-500 nm after excitation at 280 nm. An interaction between different ZEA (0.5 and 1.5 mg/kg bw) and animal serum was performed on fluorometric titration and qualitative analysis was done. The average of three independent scans were represented as fluorescence spectra while were recorded with 50-fold diluted mouse serum albumin. Fluorescence quenching was estimated using following formula:
 |
Where FI implies fluorescence intensity.
Statistical analysis
For statistical comparisons, the means ± standard error were calculated for all the parameters assessed compared to that of the control group using one-way ANOVA, followed by Dunnett’s multiple comparisons test and GraphPad Prism Software Version 9 (La Jolla, CA). Value of (*p< 0.05, **p< 0.01 and ***p< 0.001) was considered as statistically significant.
Results
Chronic exposure of Zearalenone does not cause body weight changes or general toxicity
In the present 90-days study repeated-dose oral toxicity study, all the mice survived until the scheduled termination. There were no significant changes in weight gain, and no mortality was observed during the treatment (Data not shown).
Chronic exposure of Zearalenone causes gross morphological changes in the uterus
During necropsy, the morphology of the isolated uterus was assessed. Compared to controls, both doses of ZEA and E2 exposure caused prominent changes in the morphology of the uterus, such as an increase in size and swelling in the uterine horn (Fig. 1a–d). However as shown in Fig. 1e, quantitative analysis of the relative weight of uteri of mice from control, E2, and different doses of ZEA treated group was also done. The uterine weight of the control group mice was 6.37 ± 0.47 mg, while uterine weight of 0.5 mg/kg bw and 1.5 mg/kg bw ZEA treated groups were 8.09 ± 0.53 mg and 11.95 ± 1.23 mg, respectively. The E2-treated group also showed an increase in the uterine weight of 10.80 ± 0.70 mg compared to controls. Quantifications suggest that both doses of ZEA and E2 treatment cause a 1.2, 1.88 and 1.7-fold increase compared to the control group mice. The change in uterine weight of E2 and ZEA (1.5 mg/kg bw) treated group mice were significant, whereas, uterine weight increases in mice treated with ZEA (0.5 mg/kg) was non-significant.
Fig. 1.
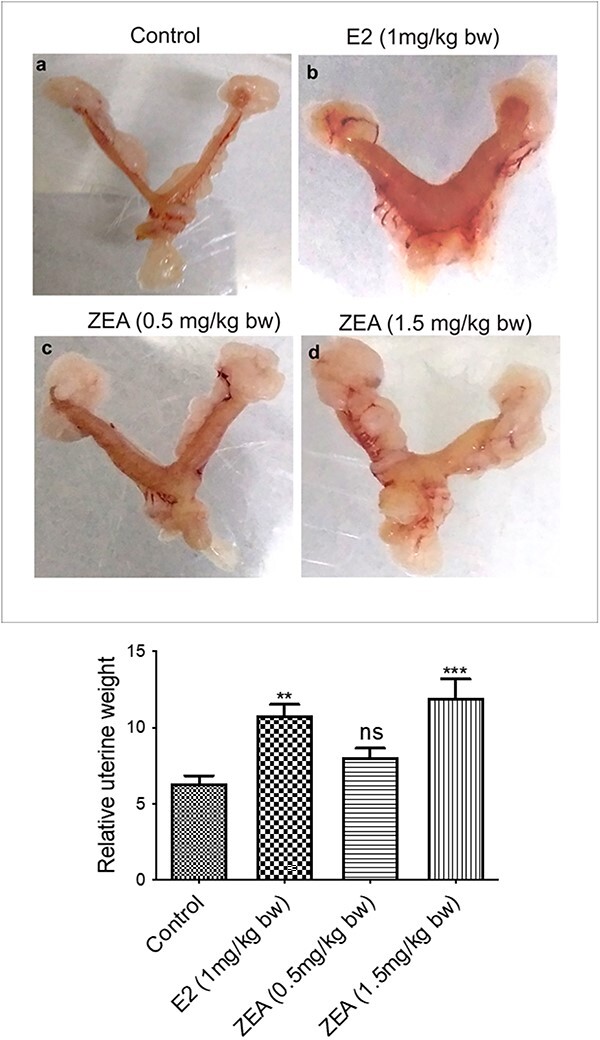
Effect of E2 and ZEA on uterine morphology in CD-1 mice. Gross images of the uterus from 90-day exposure CD-1 mice (a) control, (b) E2 (1 mg/kg bw), (c) ZEA (0.5 mg/kg bw), and (d) ZEA (1.5 mg/kg bw), (E) relative uterine weight, which are expressed as mean + SEM of six animals. *p< 0.05; **p< 0.01, ***p< 0.001 significant with respect to the control group, n.s. = not significant.
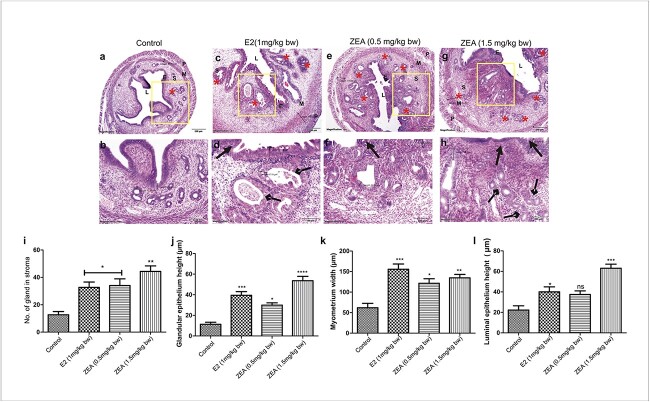
Chronic exposure of Zearalenone causes hyperplasia in the uterus of CD-1 mice
Histological evaluation of the mouse uterus after 90 days of E2 and ZEA exposure revealed proliferative endometrium with an increased number of glands, myometrium width (MW), glandular epithelium height (GEH), and luminal epithelium height (LEH) (Fig. 2a-h). The chronic exposure produced a remarkable difference in the variable density of proliferative endometrial glands (indicated by arrows by rhombus heads) and the “irregular” distribution of glands throughout the endometrium, which was intriguing (Fig. 2a,c,e,g). Quantitative analysis showed that control group mice have only 12.7 ± 2.33 mean number of glands, while two doses of ZEA and E2 have 34 ± 3.9, 44.3 ± 4.05, and 32.6 ± 4.9 numbers of glands, respectively. The numbers of glands in the ZEA and E2 treated groups are statistically significant compared to control group (Fig. 2i–l).
Fig. 2.
Effect of E2 and ZEA on uterine histopathology in CD-1 mice. Haematoxylin and eosin staining of uterine sections from the following groups; (a, b) control group showing the normal histoarchitecture; (c, d) E2 (1mg/kg bw), (e, f) ZEA (0.5 mg/kg bw); and (g, h) ZEA (1.5 mg/kg bw) showing increased endometrium thickness with hyperplasia and intraluminal papillary protections (indicated by arrows with triangle heads) and increased number of glands (indicated by*) and changes in gland architecture (indicated by arrows with rhombus heads. (i) Graphical representation of changes in the number of glands in the uterus. (j) Morphometric analysis was also performed, and quantitative analyses of glandular epithelium height (GEH) (k); myometrium height (MH) (l) and luminal epithelium height (LEH) are shown. Magnification: 4X; 10X. Each value represents the mean ± SEM of six animals. * < 0.05; **p< 0.01; ***p< 0.001 significant with respect to control group.
Further, mean measurements of LEH, GEH and MW were done in uterus sections for morphometric analysis. As shown in Fig. 2b,d,f,h, E2 and both doses of ZEA exposure led to an increase in the height of LEH and GEH as well as the width of MW. LEH in high dose ZEA and E2 was 63.00 ± 4.04 and 40.07 ± 4.8 μm, respectively, compared to control (22.24 ± 4.21 μm). A significant increase in the LEH in E2 and a high dose of ZEA were observed (Fig. 2j). Moreover, a similar pattern was found in the measurements of MW and GEH. MW in high doses of ZEA and E2 was 134.8 ± 8.290 and 155.5 ± 12.82 μm, which is significantly high compared to control (61.89 ± 10.63 μm) (Fig. 2k). Interestingly, the GEH of E2 and ZEA was also significantly increased compared to control. GEH in both doses of ZEA-treated groups was 29.92 ± 2.22 and 53.62 ± 4.23 μm. The E2 treated group also depicted an increase in the GEH, which was 39.45 ± 3.6 μm (Fig. 2l).
Chronic Zearalenone exposure caused uterus proliferation
To determine the proliferative effect of ZEA on the uterus, the expression of Ki-67 and PCNA was measured in treated as well as control tissues. Immunohistochemical (IHC) analysis of uterus sections showed prominent PCNA expression in the ZEA and E2 treated groups (Fig. 3a-h). Quantification of PCNA positive cells showed that control had 5.67 ± 1.45, while two different doses of ZEA and E2 had 26.33 ± 2.33, 38.33 ± 2.40 and 28.33 ± 4.4% PCNA positive cells in comparison to control (Fig. 3i). PCNA positive cells in the endometrial lining are indicated by arrows with triangle heads (Fig. 3d,f,h).
Fig. 3.
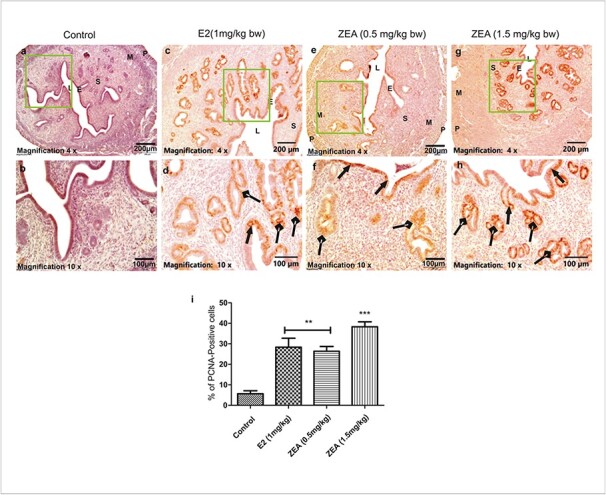
Immunohistochemical analysis of PCNA in uterine tissues. A representative photomicrograph shows the expression of PCNA in (a, b) control; (c, d) E2 (1 mg/kg bw); (e, f) ZEA (0.5 mg/kg bw) and (g, h); ZEA (1.5 mg/kg bw) treated groups. (i) Graphical representation of percent PCNA positive cells in each group. Scale bars: 200 and 100 μm. S, stroma; LE, luminal epithelium; GE, glandular epithelium. Each value represents the mean ± SEM of Six animals. **p< 0.01; ***p< 0.001 significant with respect to control group.
Further, similar changes were observed in the levels of Ki-67 around the endometrial and glandular lining upon ZEA and E2 exposure (Fig. 4a-h). Quantitative analysis showed that the expression of Ki-67 was increased by 28.33% in the E2-treated group and by 19.50, 2.10, and 29.00% in both doses of ZEA, respectively, compared to the control group (Fig. 4i). Quantification of PCNA and Ki-67 expression was performed by counting positive cells (brown deposits in the nucleus) in the randomly selected five non-overlapping fields from a minimum of three different slides. The percentage of PCNA/Ki-67 + ve was calculated by following the methods described earlier. Both PCNA and Ki-67 are effective markers for evaluating cell proliferation in endometrial hyperplasia. PCNA reflects the DNA synthesis phase (involved in DNA replication and repair) of the cell cycle and is expressed throughout the entire cycle, while Ki-67 (ribosomal RNA transcription and cell division) is expressed specifically through the active phases of the cell cycle (G1, S, G2, and mitosis) and is absent in resting cells (G0). Evaluating both markers provide a more comprehensive understanding of the different stages of the cell cycle and the proliferative potential of cells and assesses both DNA replication and cell division, providing a more comprehensive assessment of cell proliferation.
Fig. 4.
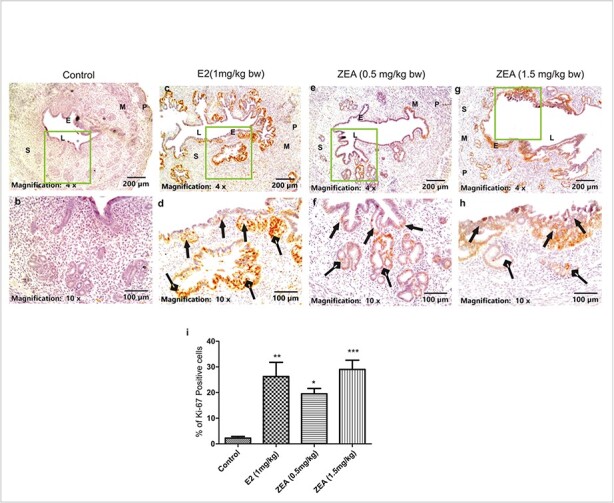
Immunohistochemical analysis of Ki-67 in uterine tissue. A representative photomicrograph shows the expression of Ki-67 in (a, b) control; (c, d) E2 (1 mg/kg bw); (e, f) ZEA (0.5 mg/kg bw); and (g, h) ZEA (1.5 mg/kg bw) treated groups. (i) quantitative analysis in terms of the number of Ki-67 positive cells in each group is shown. ZEA and E2 exposed mice suggest endometrial hyperplasia with higher Ki-67 expressions (indicated by arrows with triangle heads), benign proliferative epithelium in glandular regions (indicated by arrows with rhombus heads). Scale bars: 200 and 100 μm. S, stroma; LE, luminal epithelium; GE, glandular epithelium. Each value represents the mean ± SEM of Six animals. *p< 0.05; **p< 0.01; ***p< 0.001 significant with respect to the control group.
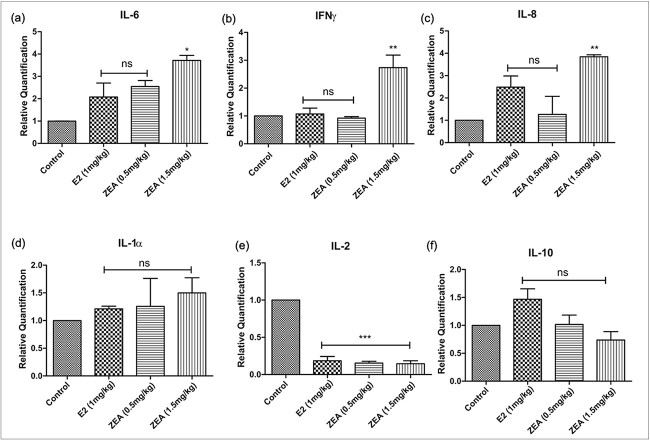
Effect of Zearalenone exposure on the expression of inflammatory markers at the mRNA level
Real-time PCR was used to assess the expression of six cytokines (IL-6, IL-1α, IL-8, IFN-γ, IL-2, and IL-10). In comparison to the control, ZEA treatment significantly increased the expression of IL-6 (4-fold), IL-8 (4-fold), IL-1α (1.6-fold), and IFN-γ (2.7-fold) compared to the control (Fig. 5a-d). Oppositely, the expression of IL-10 remained constant, whereas IL-2 levels were markedly reduced after ZEA exposure (Fig. 5e,f).
Fig. 5.
The effect of E2 and ZEA treatment on the mRNA expression of (a) IL-6; (b) IFN γ; (c) IL-8; (d) IL-1α; (e) IL-2; (f) IL-10 in the uterine tissue. Each value represents the mean ± SEM of six animals. *p < 0.05; **p< 0.01, ***p 0.001significant with respect to the control group, n.s. = not significant.
Studies on the interactions between serum proteins and Zearalenone
The uterus is one of the target organs of ZEA-mediated damage, as evidenced by its histopathology and high levels of inflammatory and proliferative molecular markers. In silico binding prediction and an in vitro assay depicting the binding of ZEA with serum albumin were carried out to assess this possibility and investigate the interaction of ZEA with serum albumin.
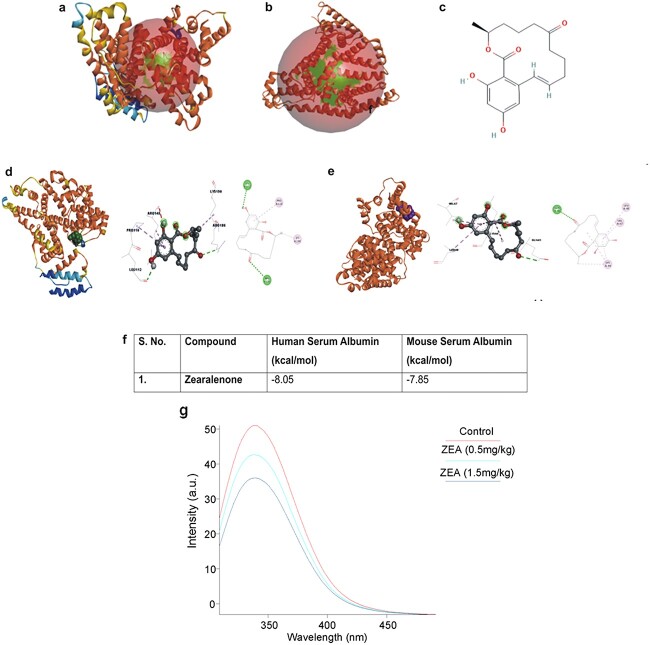
Molecular docking studies predict binding of Zearalenone with human and mouse serum proteins
Molecular interaction between human and mouse serum proteins with ZEA was studied (Fig. 6a-e). Autodock Vina was used to perform docking analyses of the specific mentioned ligands and proteins by employing a particular dimension of the grid described in the methodology section. ZEA interacts with human serum albumin, forming two hydrogen bonds with residues ARG186 and LEU112 as well as four hydrophobic interactions, including a Pi-Sigma bond with ARG145, one Alkyl bond with LYS190, two Pi-Alkyl bonds with PRO110 and AGR145 (Fig. 6f).
Fig. 6.
Interaction studies between serum proteins and ZEA. The study was performed using in silico tools and an in vitro serum binding assay. 3D structures of selected protein targets andtheir binding sites (a) human serum albumin (PDB ID: 1ao6) and (b) mouse serum albumin (modelled protein); (c) 2-dimensional chemical structures of ZEA; (d) human serum albumin (hsa) interaction with ZEA; (e) mouse serum albumin (msa) interaction with ZEA; (f) binding energy upon interaction with hsa and msa; (g) spectrophotometric spectral analysis for mouse serum-ZEA interaction; (h) In vitro serum binding assay with animal serum (CD-1 mice): Intrinsic fluorescence emission spectra of CD-1 mice serum (1.0 × 10−5 M) excited at 280 nm in the presence of 0.5 mg/kg bw and 1.5 mg/kg bw dose of ZEA. The quenching of the intrinsic fluorescence intensity of CD-1 mouse serum suggests the binding of ZEA to serum proteins in a dose dependent manner.
Biophysical studies confirm the binding of Zearalenone with mouse serum proteins
Florescence spectral titrations were carried out using ZEA and mouse serum. The serum from control mice showed an excitation at 280 nm and emission at 339 nm, but the fluorescence intensity decreased with the serum from mice that had been given ZEA. We found that the amount of ZEA administered has an inverse relationship with the decrease in fluorescence intensity. The fluorescence intensity was quenched by 24.19% in the (0.5 mg/kg bw) ZEA- treated mice serum and by 34.81% in the (1.5 mg/kg bw) ZEA-treated mice serum (Fig. 6g).
Discussion
ZEA, an environmental estrogen, frequently contaminates cereals, cereal- based foods, and animal feed, enabling its entry into the food supply chain. The situation is quite concerning due to ZEA’s frequent presence in food items and its estrogenic properties. Therefore, this study is the first step towards understanding the adverse effects of chronic exposure of ZEA using a mouse model. Two doses of ZEA (0.5 mg/kg bw) and (1.5 mg/kg bw) were given to CD-1 female mice for 90 days. In the toxicity assessment, changes in body weight are often consider as a sensitive indicator of toxicity in experiments involving animals. However, in this study neither E2 nor ZEA at both doses resulted any significant change in body weight of the experimental animals. Thus, there was no overt toxicity from E2 or either dose of ZEA. However, to further gain an understanding of the toxicity effects of ZEA, morphological, histopathological, and biochemical examinations were done.
However, interestingly, E2 and ZEA treatment groups of mice showed an increased in the size of uterus when compared to the control group and histological evaluation of enlarged uterus showed a significantly increased number of glands, and a higher glandular to stromal ratio. Our findings are supported by others, where ZEA caused an increase in total uterine weight, myometrial hypertrophy, and as well as an increase in endometrial gland development.25 ZEA-caused enlargement of the uterus provided a strong hint about the involvement of inflammatory and proliferative activities. Therefore, immunohistochemical analysis of the well-established proliferation markers, PCNA and Ki-67, was performed. Their expression was increased in ZEA treated groups compared to control. Ki-67 plays a crucial role in cell division (mitosis) and is present during all phases of the cell cycle except for the G0 (quiescent) phase. In addition, reports also suggested that accumulation of Ki-67 is relatively high in aggressive forms of tumours with poor prognosis and overall survival.26,27 Importantly, similar to KI-67 expression, a higher expression of PCNA was also found in the uteri after action of ZEA and E2-treated mice. PCNA, is a protein that plays a role in DNA replication and repair, acts as a processivity factor for DNA polymerase during DNA synthesis and is found to be involved in various cellular processes, including proliferation. Thus, alterations in the expression of both proliferative markers suggested that ZEA caused a proliferative change in the uterus and leads to hyperplasia.
The tissue-specific proliferative changes are accompanied by inflammatory responses, which are often governed by cytokines.28 ZEA has been found to act as a double-edged sword in the context of the inflammatory cytokine response. ZEA is has been observed to increase the levels of pro-inflammatory cytokines such as TGF-β1, IL-6, and IL-1 in the serum and kidney fractions of pregnant rats.29 However, contradictory to this, ZEA has also been found to suppress pro-inflammatory cytokines showing an inflammation-repelling phenomenon.30 Therefore, the present investigation delt with the crucial question of how ZEA would affect the cytokine profile upon chronic exposure. mRNA expression levels of IL-6, IL-8 and IFN-γ were found to be significantly increased in uterine tissue after ZEA exposure. However, IL-1α showed a tendential increase, while a decrease in IL-10 was noticed after ZEA exposure. Our findings suggested that ZEA exposure caused an induction in the inflammatory cytokines (IL-6, IL-8, IL-1α, and IFN-γ) but a suppression in the anti-inflammatory cytokine, IL-10. This imbalance of pro- and anti-inflammatory cytokines may promote inflammation leading to hyperplasia in the endometrium of the uterus after ZEA exposure. In further in support of our observation, similar alterations in cytokine levels have been reported after exposure to xenobiotics which caused reproductive toxicities.31 Elevated levels of IL-1 and IFN-γ have been found in the uterine fluid of patients suffering from infertility and miscarriage.28,32 There are reports that suggest that some mycotoxins, such as aflatoxin B1 increased the production of proinflammatory cytokines, IL-1β, IL-6, and TNF-α leading to hepatic and renal injuries.33 In addition, studies also report that microglial cells elevates the pro-inflammatory cytokines (IL-6,TNF-α) in response to Ochratoxin A (OTA) and contributes to inflammation induced neurotoxicity.34 Similarly, another mycotoxin Deoxynivalenol (DON)-induce injury in intestinal tissues of piglets, where IL-1β,IL-6 and TNF-α levels were elevated in the intestinal tissue.35
The mechanism of action of ZEA is primarily estrogenic; however, other toxic manifestations apart from reproductive disorders are also reported.36 The presence of ZEA has been noticed in the serum of children with idiopathic puberty.37 In addition, researchers have also reported a possible interaction of ZEA with human serum albumin.38 These reports raised the possibility that apart from its estrogenic mode of action, ZEA may have the potential through binding with serum proteins. Hence, to ascertain this possibility, in silico and in vitro binding studies were undertaken to study the interaction between ZEA and serum albumin. This is the first attempt to evaluate the possible interaction of ZEA with serum proteins, which is occurring under in vivo conditions. In this study, the first possible interaction of ZEA with serum of mice was evaluated and compared with serum of control group mouse. In this experiment, ZEA showed a decent amount of binding energy stabilised by the presence of hydrogen bond interactions. The ability of ZEA to interact with serum protein was confirmed by fluorescence quenching assay using mouse serum. The inhibition in fluorescence with increasing doses of ZEA further establishes that ZEA can form positive interactions with serum proteins, if available in blood. Thus, this unique property of ZEA may lead to its long presence in blood and may cause systemic toxicity and unwanted estrogenic effects.
Conclusion
This study revealed that ZEA targets the uterus and can cause inflammation due to increased levels of inflammatory cytokines and proliferation mediators, as well as systemic toxicity denoted by a strong binding affinity with serum proteins. However, additional studies in animal models and clinical settings are needed to validate these findings, and to understand the adverse effects after chronic exposure. Further, more in-depth study is needed to develop the appropriate regulations and guidelines to ensure the safety of the food supply chain and to improve public health policies.
Acknowledgments
We express our gratitude to Director of our institute for his interest and support towards this study. VS is grateful for the award of Senior Research Fellowship by the Council of Scientific and Industrial Research (CSIR). VS conveys her gratitude to Academy of Scientific &Innovative Research (AcSIR), New Delhi. This research work is part of the Ph.D thesis of VS. The institutional manuscript communication number is IITR/SEC/MS/2023/41. English language correction by Prof. Susan M Fischer is also acknowledged.
Contributor Information
Varsha Singh, Food Toxicology Laboratory, Food, Drug and Chemical Toxicology Group, CSIR-Indian Institute of Toxicology Research, Vishvigyan Bhawan, 31 Mahatma Gandhi Marg, Lucknow, 226001, Uttar Pradesh, India; Academy of Scientific and Innovative Research (AcSIR) Kamla Nehru Nagar, Ghaziabad, 201002, Uttar Pradesh, India.
Payal Mandal, Food Toxicology Laboratory, Food, Drug and Chemical Toxicology Group, CSIR-Indian Institute of Toxicology Research, Vishvigyan Bhawan, 31 Mahatma Gandhi Marg, Lucknow, 226001, Uttar Pradesh, India.
Shweta Singh Chauhan, Academy of Scientific and Innovative Research (AcSIR) Kamla Nehru Nagar, Ghaziabad, 201002, Uttar Pradesh, India; Computational Toxicology Facility, Toxicoinformatics and Industrial Research, CSIR-Indian Institute of Toxicology Research, Vishvigyan Bhawan, 31 Mahatma Gandhi Marg, Lucknow, 226001, Uttar Pradesh, India.
Ishrat Jahan Saifi, Food Toxicology Laboratory, Food, Drug and Chemical Toxicology Group, CSIR-Indian Institute of Toxicology Research, Vishvigyan Bhawan, 31 Mahatma Gandhi Marg, Lucknow, 226001, Uttar Pradesh, India; Academy of Scientific and Innovative Research (AcSIR) Kamla Nehru Nagar, Ghaziabad, 201002, Uttar Pradesh, India.
Marhaba, Food Toxicology Laboratory, Food, Drug and Chemical Toxicology Group, CSIR-Indian Institute of Toxicology Research, Vishvigyan Bhawan, 31 Mahatma Gandhi Marg, Lucknow, 226001, Uttar Pradesh, India; Academy of Scientific and Innovative Research (AcSIR) Kamla Nehru Nagar, Ghaziabad, 201002, Uttar Pradesh, India.
P V Sandeep, Food Toxicology Laboratory, Food, Drug and Chemical Toxicology Group, CSIR-Indian Institute of Toxicology Research, Vishvigyan Bhawan, 31 Mahatma Gandhi Marg, Lucknow, 226001, Uttar Pradesh, India.
Pankaj Jagdale, Central Pathology Facility, CSIR-Indian Institute of Toxicology Research (CSIR-IITR), Vishvigyan Bhawan, 31, Mahatma Gandhi Marg, Lucknow, 226001, Uttar Pradesh, India.
Anjaneya Ayanur, Central Pathology Facility, CSIR-Indian Institute of Toxicology Research (CSIR-IITR), Vishvigyan Bhawan, 31, Mahatma Gandhi Marg, Lucknow, 226001, Uttar Pradesh, India.
Kausar Mahmood Ansari, Food Toxicology Laboratory, Food, Drug and Chemical Toxicology Group, CSIR-Indian Institute of Toxicology Research, Vishvigyan Bhawan, 31 Mahatma Gandhi Marg, Lucknow, 226001, Uttar Pradesh, India; Academy of Scientific and Innovative Research (AcSIR) Kamla Nehru Nagar, Ghaziabad, 201002, Uttar Pradesh, India.
Author contributions
KMA; designed the study and wrote the manuscript, VS; performed the experiments, analyze the data, and wrote the first draft of manuscript. PM; analyzed and interpreted data, SSC performed and analyzed the in silico data, IJS and PVS; performed, analyzed and interpretated the serum binding assay studies, M; helped in animal dosing studies; PJ; performed histopathology processing, AA; analyzed andinterpreted histopathology and immunohistochemistry data.
Funding
The financial assistance of Indian Council of Medical Research (ICMR) (Sanction No.F.N.5/9/1318/2020-Nut) is gratefully acknowledged.
Conflict of interest statement: There are no conflicts of interest to declare.
Data availability statement
There is no other data is available for this article. All the data have been used in the figures.
References
- 1. Alshannaq A, Yu J-H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int J Environ Res Public Health. 2017:14(6):632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown R, Priest E, Naglik JR, Richardson JP. Fungal toxins and host immune responses. Front Microbiol. 2021:12:643639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Payros D, Dobrindt U, Martin P, Secher T, Bracarense APFL, Boury M, Laffitte J, Pinton P, Oswald E, Oswald IP. The food contaminant Deoxynivalenol exacerbates the Genotoxicity of gut microbiota. MBio. 2017:8(2):10–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vignal C, Djouina M, Pichavant M, Caboche S, Waxin C, Beury D, Hot D, Gower-Rousseau C, Body-Malapel M. Chronic ingestion of deoxynivalenol at human dietary levels impairs intestinal homeostasis and gut microbiota in mice. Arch Toxicol. 2018:92(7):2327–2338. [DOI] [PubMed] [Google Scholar]
- 5. Vincent M, Percier P, de Prins S, Huygen K, Potemberg G, Muraille E, Romano M, Michel O, Denis O. Investigation of inflammatory and allergic responses to common mold species: results from in vitro experiments, from a mouse model of asthma, and from a group of asthmatic patients. Indoor Air. 2017:27(5):933–945. [DOI] [PubMed] [Google Scholar]
- 6. Sun Y, Song Y, Long M, Yang S. Immunotoxicity of three environmental mycotoxins and their risks of increasing pathogen infections. Toxins (Basel). 2023:15(3):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu H, Zhang J, Chen Y, Zhu J. Zearalenone and its masked forms in cereals and cereal-derived products: a review of the characteristics, incidence, and fate in food processing. J Fungi. 2022:8(9):976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Han X, Huangfu B, Xu T, Xu W, Asakiya C, Huang K, He X. Research progress of safety of Zearalenone: a review. Toxins (Basel). 2022:14(6):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Agriopoulou S, Stamatelopoulou E, Varzakas T. Control strategies : prevention and detoxification in foods. Food Secur. 2020:86(2):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li F, Zhao X, Jiao Y, Duan X, Yu L, Zheng F, Wang X, Wang L, Wang JS, Zhao X, et al. Exposure assessment of aflatoxins and zearalenone in edible vegetable oils in Shandong, China: health risks posed by mycotoxin immunotoxicity and reproductive toxicity in children. Environ Sci Pollut Res. 2023:30(2):3743–3758. [DOI] [PubMed] [Google Scholar]
- 11. Ropejko K, Twarużek M. Zearalenone and its metabolites—general overview, occurrence, and toxicity. Toxins (Basel). 2021:13(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rai A, Dixit S, Singh SP, Gautam NK, das M, Tripathi A. Presence of Zearalenone in cereal grains and its exposure risk assessment in Indian population. J Food Sci. 2018:83(12):3126–3133. [DOI] [PubMed] [Google Scholar]
- 13. Fan W, Lv Y, Ren S, Shao M, Shen T, Huang K, Zhou J, Yan L, Song S. Zearalenone (ZEA)-induced intestinal inflammation is mediated by the NLRP3 inflammasome. Chemosphere. 2018:190:272–279. [DOI] [PubMed] [Google Scholar]
- 14. Kang J, Li Y, Ma Z, Wang Y, Zhu W, Jiang G. Protective effects of lycopene against zearalenone-induced reproductive toxicity in early pregnancy through anti-inflammatory, antioxidant and anti-apoptotic effects. Food Chem Toxicol. 2023:179:113936. [DOI] [PubMed] [Google Scholar]
- 15. Cozzini P, Dellafiora L. In silico approach to evaluate molecular interaction between mycotoxins and the estrogen receptors ligand binding domain: a case study on zearalenone and its metabolites. Toxicol Lett. 2012:214(1):81–85. [DOI] [PubMed] [Google Scholar]
- 16. Ehrlich V. Hazard assessment through hybrid in vitro / in silico approach: the case of zearalenone. ALTEX. 2015:32(4):275–286. [DOI] [PubMed] [Google Scholar]
- 17. Dellafiora L, Ruotolo R, Perotti A, Cirlini M, Galaverna G, Cozzini P, Buschini A, Dall’Asta C. Molecular insights on xenoestrogenic potential of zearalenone-14-glucoside through a mixed in vitro/in silico approach. Food Chem Toxicol. 2017:108(Pt A):257–266. [DOI] [PubMed] [Google Scholar]
- 18. Agahi F, Álvarez-Ortega N, Font G, Juan-García A, Juan C. Oxidative stress, glutathione, and gene expression as key indicators in SH-SY5Y cells exposed to zearalenone metabolites and beauvericin. Toxicol Lett. 2020:334:44–52. [DOI] [PubMed] [Google Scholar]
- 19. Frizzell C, Ndossi D, Verhaegen S, Dahl E, Eriksen G, Sørlie M, Ropstad E, Muller M, Elliott CT, Connolly L. Endocrine disrupting effects of zearalenone, alpha- and beta-zearalenol at the level of nuclear receptor binding and steroidogenesis. Toxicol Lett. 2011:206(2):210–217. [DOI] [PubMed] [Google Scholar]
- 20. Zhou J, Zhao L, Huang S, Liu Q, Ao X, Lei Y, Ji C, Ma Q. Zearalenone toxicosis on reproduction as estrogen receptor selective modulator and alleviation of zearalenone biodegradative agent in pregnant sows. J Anim Sci Biotechnol. 2022:13(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Massart F, Meucci V, Saggese G, Soldani G. High growth rate of girls with precocious puberty exposed to estrogenic mycotoxins. J Pediatr. 2008:152(5):690–695. [DOI] [PubMed] [Google Scholar]
- 22. Pajewska M, Łojko M, Cendrowski K, Sawicki W, Kowalkowski T, Buszewski B, Gadzała-Kopciuch R. The determination of zearalenone and its major metabolites in endometrial cancer tissues. Anal Bioanal Chem. 2018:410(5):1571–1582. [DOI] [PubMed] [Google Scholar]
- 23. Yang R, Wang Y-M, Zhang L, Zhao ZM, Zhao J, Peng SQ. Prepubertal exposure to an oestrogenic mycotoxin zearalenone induces central precocious puberty in immature female rats through the mechanism of premature activation of hypothalamic kisspeptin-GPR54 signaling. Mol Cell Endocrinol. 2016:437:62–74. [DOI] [PubMed] [Google Scholar]
- 24. Wang X, Ha D, Yoshitake R, Chan YS, Sadava D, Chen S. Exploring the biological activity and mechanism of Xenoestrogens and phytoestrogens in cancers: emerging methods and concepts. Int J Mol Sci. 2021:22(16):8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang L, Liao W, Dong J, Chen X, Huang L, Yang W, Jiang S. Zearalenone promotes uterine hypertrophy through AMPK/mTOR mediated autophagy. Toxins (Basel). 2024:16(2):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kitson S, Sivalingam VN, Bolton J, McVey R, Nickkho-Amiry M, Powell ME, Leary A, Nijman HW, Nout RA, Bosse T, et al. Ki-67 in endometrial cancer: scoring optimization and prognostic relevance for window studies. Mod Pathol. 2017:30(3):459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pan Y, Yuan Y, Liu G, Wei Y. P53 and Ki-67 as prognostic markers in triple-negative breast cancer patients. PLoS One. 2017:12(2):e0172324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fitzgerald HC, Salamonsen LA, Rombauts LJR, Vollenhoven BJ, Edgell TA. The proliferative phase underpins endometrial development: altered cytokine profiles in uterine lavage fluid of women with idiopathic infertility. Cytokine. 2016:88:12–19. [DOI] [PubMed] [Google Scholar]
- 29. Jia Z, Liu M, Qu Z, Zhang Y, Yin S, Shan A. Toxic effects of zearalenone on oxidative stress, inflammatory cytokines, biochemical and pathological changes induced by this toxin in the kidney of pregnant rats. Environ Toxicol Pharmacol. 2014:37(2):580–591. [DOI] [PubMed] [Google Scholar]
- 30. Yan W-K, Liu Y-N, Song S-S, Kang JW, Zhang Y, Lu L, Wei SW, Xu QX, Zhang WQ, Liu XZ, et al. Zearalenone affects the growth of endometriosis via estrogen signaling and inflammatory pathways. Ecotoxicol Environ Saf. 2022:241:113826. [DOI] [PubMed] [Google Scholar]
- 31. Tohamy HG, Lebda MA, Sadek KM, Elfeky MS, el-Sayed YS, Samak DH, Hamed HS, Abouzed TK. Biochemical, molecular and cytological impacts of alpha-lipoic acid and Ginkgo biloba in ameliorating testicular dysfunctions induced by silver nanoparticles in rats. Environ Sci Pollut Res. 2022:29(25):38198–38211. [DOI] [PubMed] [Google Scholar]
- 32. Tyagi P, Alharthi N. Evaluation of pro-inflammatory cytokine level in cases of idiopathic recurrent spontaneous miscarriage in Saudi Arabia. Biomed Biotechnol Res J. 2020:4(3):225. [Google Scholar]
- 33. Aleissa MS, Alkahtani S, Abd Eldaim MA, Ahmed AM, Bungău SG, Almutairi B, Bin-Jumah M, AlKahtane AA, Alyousif MS, Abdel-Daim MM. Fucoidan ameliorates oxidative stress, inflammation, DNA damage, and Hepatorenal injuries in diabetic rats intoxicated with aflatoxin B 1. Oxidative Med Cell Longev. 2020:2020:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chansawhang A, Phochantachinda S, Temviriyanukul P, Chantong B. Corticosterone potentiates ochratoxin A-induced microglial activation. Biomol Concepts. 2022:13(1):230–241. [DOI] [PubMed] [Google Scholar]
- 35. Wang X-C, Zhang Y-F, Cao L, Zhu L, Huang YY, Chen XF, Chu XY, Zhu DF, Ur Rahman S, Feng SB, et al. Deoxynivalenol induces intestinal damage and inflammatory response through the nuclear factor-κB Signaling pathway in piglets. Toxins (Basel). 2019:11(11):663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu X-L, Wu R-Y, Sun X-F, Cheng SF, Zhang RQ, Zhang TY, Zhang XF, Zhao Y, Shen W, Li L. Mycotoxin zearalenone exposure impairs genomic stability of swine follicular granulosa cells in vitro. Int J Biol Sci. 2018:14(3):294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Asci A, Durmaz E, Erkekoglu P, Pasli D, Bircan I, Kocer-Gumusel B. Urinary zearalenone levels in girls with premature thelarche and idiopathic central precocious puberty. Minerva Pediatr. 2014:66(6):571–578. [PubMed] [Google Scholar]
- 38. Poór M, Kunsági-Máté S, Bálint M, Hetényi C, Gerner Z, Lemli B. Interaction of mycotoxin zearalenone with human serum albumin. J Photochem Photobiol B Biol. 2017:170:16–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no other data is available for this article. All the data have been used in the figures.