Abstract
Autoimmune encephalitis is a relatively novel nosological entity characterized by an immune-mediated damage of the central nervous system. While originally described as a paraneoplastic inflammatory phenomenon affecting limbic structures, numerous instances of non-paraneoplastic pathogenesis, as well as extra-limbic involvement, have been characterized. Given the wide spectrum of insidious clinical presentations ranging from cognitive impairment to psychiatric symptoms or seizures, it is crucial to raise awareness about this disease category. In fact, an early diagnosis can be dramatically beneficial for the prognosis both to achieve an early therapeutic intervention and to detect a potential underlying malignancy. In this scenario, the radiologist can be the first to pose the hypothesis of autoimmune encephalitis and refer the patient to a comprehensive diagnostic work-up – including clinical, serological, and neurophysiological assessments.
In this article, we illustrate the main radiological characteristics of autoimmune encephalitis and its subtypes, including the typical limbic presentation, the features of extra-limbic involvement, and also peculiar imaging findings. In addition, we review the most relevant alternative diagnoses that should be considered, ranging from other encephalitides to neoplasms, vascular conditions, and post-seizure alterations. Finally, we discuss the most appropriate imaging diagnostic work-up, also proposing a suggested MRI protocol.
Keywords: Magnetic resonance imaging, Encephalitis, Limbic encephalitis, Autoimmune encephalitis, Autoimmune diseases of the nervous system
Introduction
Autoimmune encephalitis (AE) refers to a spectrum of disorders characterized by inflammatory processes affecting the brain tissue and originating from an immune-mediated pathophysiological mechanism targeting neurons [1, 2]. The reported incidence ranges from 3/million to 8/million person-years in epidemiologic studies in the western world [3, 4], but many authors argue that this disease category is still under-diagnosed and misdiagnosed [5, 6].
AE was originally described as a paraneoplastic phenomenon affecting limbic structures, and causing subacute onset of behavior and memory disturbances, along with seizures, in the presence of an underlying neoplasms [7–9]. Subsequent evidence led to the detection of autoantibodies linked with AE [10, 11], and contributed to the recognition that non-paraneoplastic instances are relatively common [2]. Currently, AE includes a number of subtypes, that can be classified based on the associated autoantibody (Ab) found in the serum and/or CSF. AE Ab target either intracellular (Group I – such as anti-Hu, anti-Ma/Ta, anti-GAD, anti-Yo, and anti-CV2/CRMP5), or cell surface neuronal antigens (Group II – including anti-LGI1, anti-CASPR2, anti-GABAAR, anti-GABABR, anti-NMDAR, and anti-AMPAR). This distinction bears significant clinical, prognostic, and pathophysiological implications, a general rule being that Group I AE are associated with a worse prognosis than Group II AE [1, 12]. Group I AE are more likely associated with an underlying malignancy (Group I Ab are also referred to as ‘onconeural antibodies’) [13]. For instance, anti-Hu AE – the most common paraneoplastic AE with a reported incidence of 0.4/million person-years [4] – is associated with tumors in 75–80% of cases (mainly small cell lung carcinoma, SCLC) [14, 15]. On the other hand, Group II AE are more commonly non-paraneoplastic conditions that can affect patients belonging to a wide age range, including younger and sometimes pediatric patients [2, 14]. Anti-NMDAR (N-methyl D-aspartate receptor) AE and anti-LGI1 (leucine-rich glioma inactivated) AE – the most common Group II AE, with a reported incidence of 0.5/million and 0.4/million person-years, respectively [4] – show an association with neoplasms in 30%-40% and in 10% of cases, respectively [14, 16]. Despite the abovementioned rule of thumb for neoplastic associations is overall valid, some AE subtypes represent notable exceptions. For instance, GABABR (gamma-amino butyric acid B receptor) antibodies (group II) are frequently associated with SCLC, while GAD (glutamic acid decarboxylase) antibodies (targeting intracellular antigens) are typically associated with non-paraneoplastic AE, and they are more often associated with systemic autoimmune conditions (e.g. type 1 diabetes mellitus) rather than with neoplasms (< 10% of cases) [14]. More in general, different antibodies have a different likelihood of tumor associations, and the updated 2021 diagnostic criteria for AE [17] propose to stratify AE-related antibodies in low-, intermediate- and high-risk, based on the likelihood of tumor associations. In addition to a worse prognosis due to the underlying malignancies, Group I AE are generally characterized by a worse treatment response and are more likely to induce irreversible tissue damage [1]. Such differences are related to the underpinning pathophysiological mechanisms. While in Group II the autoantibodies are believed to serve a pathogenetic function, in Group I the immune-mediated damage is sustained by CD8 + T-cells and the extent of the antibody contribution to the neural damage is still being evaluated [13]. As a general rule, in Group I antitumoral immune response cross-reacts with neural antigens causing lymphocyte-mediated neuronal killing. Conversely, in Group II autoantibodies bind to cell-surface epitopes, typically belonging to ion-channels, and result in disease-causing synaptic function interference [1, 2, 16]. Therefore, in Group II AE antibody-depleting therapies (such as IVIG or plasma exchange/immunoadsorption) [16] are usually more effective and immune-mediated insults are more often reversible.
From a clinical perspective, most AE patients present with a subacute onset and progression (< 3 months) of various symptoms, mainly related to limbic disfunction, including memory deficits, psychiatric symptoms, seizures, and altered mental status – as reported in the diagnostic criteria for ‘possible AE’ [18]. When AE involves extratemporal structures presentations may also include movement disorders, ataxia, autonomic or sleep disorders, for instance. Although limbic encephalitis (LE) is one of the most common forms of AE, different AE subtypes are related to peculiar clinical findings. Anti-Hu AE often causes a sensory neuropathy and a cerebellar syndrome, in addition to or independently from the classic limbic involvement [15, 17]. While anti-GAD AE is usually a typical LE featuring prominent seizures, anti-Ma/Ta AE patients exhibit pure LE symptoms in a minority of cases, but often shows additional diencephalic or brainstem disfunction – e.g., gaze palsy [12, 19]. Anti-NMDAR AE causes the most defined clinical syndrome – leading to the proposal of specific and unique diagnostic criteria [18] – with a typical progression from viral-like prodromes to prominent psychiatric/behavioral symptoms, rapidly followed by memory deficits, language impairment, seizures, dyskinesias, altered state of consciousness and finally autonomic disfunction or central hypoventilation [16]. Anti-LGI1 AE also presents characteristic clinical features, such as faciobrachial dystonic seizures (FBDS), hyponatremia, and sleep disturbance [14, 20]. FBDS are briefs jerks involving in most cases the facial muscles associated with elevation and dystonic posturing of the ipsilateral upper limb (and more rarely the lower limb). These events can involve both sides and occur at a high frequency (even > 200 episodes/day). Anti-CASPR2 AE may have a wide clinical presentation, from pure limbic encephalitis, cerebellar dysfunction or peripheral nerve hyperexcitability to the well-defined Morvan syndrome where peripheral nerve hyperexcitability coexists with i) cognitive symptoms or seizures and ii) central autonomic disfunction or insomnia [14, 16, 21]. Anti-AMPAR (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor) AE often shows a psychiatric syndrome in the context of a limbic encephalitis [12, 22]. When paraneoplastic, tumor types that are most commonly associated with AE include: SCLC (anti-Hu and multiple other Group I AE), ovarian tumors (anti-NMDAR and anti-Yo), testicular tumors (anti-Ma/Ta), Hodgkin lymphoma (Tr/DNER and multiple other Group II AE) [14, 16]. A more extensive overview of clinical syndromes and tumor types related to AE subtypes are provided in some recent articles [14, 16].
The Graus criteria [18] also allow for the diagnosis of AE in the absence of neuronal antibodies. Antibody-negative AE mostly presents with a limbic phenotype (seronegative definite LE) [23]. In addition, patients not fulfilling criteria for LE can be diagnosed in a specific subgroup named “antibody-negative but probable AE” (ANPRA), which remains heterogeneous and poorly defined [23]. Finally, a category named “possible AE” exists in the Graus criteria, but it should be intended as a “work-in-progress” category identifying patients that should undergo a thorough work-up for a definite diagnosis, including CSF testing. Overall, the existence of seronegative AE cases is due to a number of reasons, including some Ab not being detected by commercial assays (but exclusively by means of in-house assays in reference centers) [5], new AE-associated Ab being constantly discovered, and some cell-mediated AE potential lacking associated Ab [14, 24]. Challenges in diagnosis of antibody negative AE have been recently reviewed [25].
In this complex and constantly evolving scenario where the clinical onset may be insidious and the Ab detection is not always a decisive diagnostic tool, radiologists should be capable of recognizing the typical imaging features of limbic encephalitis (Section “Typical Limbic Encephalitis, imaging findings”) in order to pose the suspicion of AE in patients presenting with seizures, cognitive or psychiatric symptoms, and altered state of consciousness. In addition, the radiologist should be aware that AE may also present with peculiar features and/or extra-limbic involvement (Section “Imaging patterns of extra-limbic involvements”). The recognition of a potential AE is crucial, as it initiates a multidisciplinary effort – including accurate neurological evaluation, electrophysiological assessment, CSF and serum examination – aimed at a timely diagnosis. This is pivotal for prognosis, as it allows an early initiation of therapy and an immediate screening for potential underlying malignancies.
Furthermore, we provide an overview of the alternative diagnostic hypotheses (Section “Differential diagnosis”) – including infectious conditions, neoplasms, vascular and post-seizure alterations – that should be carefully ruled out since they require a different diagnostic and therapeutical management. Finally, in the light of the latest findings regarding AE radiological features, we suggest a dedicated imaging protocol for the diagnosis (Section “Suggested imaging diagnostic work-up”).
There are different approaches that can be used to classify AE [26]. These include a serological classification based on the type of antibodies, an etiological classification that distinguishes idiopathic AE, paraneoplastic AE, post infectious AE, and iatrogenic AE (associated for instance with immunomodulatory/suppressive drugs and immune check points inhibitors). Among the possible classification concepts, this review adopts an anatomical classification, the most useful for the radiologist. As a complementary approach, Tables 1 and 2 present a synopsis of clinical and radiologic findings associated with the main antibodies, also providing a brief overview of antibodies not discussed in detail in the text, along with the corresponding references. We purposefully did not include in this review MOG-IgG associated disorder (MOGAD) as, even though some of these patients can present with encephalitic manifestations, such as ADEM or cortical encephalitis, most patients present a demyelinating phenotype. Recently, radiological features of MOGAD have been reviewed elsewhere [27].
Table 1.
Clinical and radiologic features of autoimmune encephalitides associated with autoantibodies targeting intracellular antigens
| Abs target [reference] |
Clinical syndrome | Ig subclasses | CSF findings | Tumor association | Neuroradiological pattern | Immunotherapy response |
|---|---|---|---|---|---|---|
| Hu (ANNA-1) [4, 15, 28] | Limbic encephalitis, cerebellar ataxia and/or sensory neuropathy | - | > 80% abnormal |
75% lung cancer |
10% abnormal; temporal T2 hyperintensity | Poor/absent |
| Yo (PCA-1) [17, 29, 30] | Subacute cerebellar ataxia | IgG1 | > 90% abnormal |
> 90% pelvic or breast tumor |
Acute MRI mostly normal; cerebellar atrophy at follow-up | Poor/absent |
|
CV2/CRMP5 |
Striatal Encephalitis, cerebellar ataxia, myelopathy, chorea, peripheral and/or cranial neuropathy |
- | > 90% abnormal |
> 80% SCLC and thymoma |
Striatal T2 hyperintensity | Poor/absent |
| Ma2 [14, 19, 33, 34] | Limbic, midbrain and/or brainstem encephalitis | - | 80% abnormal |
> 80% testicular tumors |
70% abnormal; temporal and/or midbrain T2 hyperintensity with 50% contrast enhancement |
30% improvement |
|
GAD65 |
Limbic encephalitis, seizures, cerebellar ataxia and/or stiff person syndrome | - | 30–100% abnormal |
< 10% thymoma |
Temporal T2 hyperintensity, 30% develop medial temporal sclerosis at follow-up |
Highly variable, from no to complete response |
| Ri (ANNA-2) [37] | Cerebellar ataxia, isolated tremor and/or oculomotor alteration | - | > 95% abnormal |
92% breast cancer |
20% abnormal; brainstem T2 hyperintensity | Poor |
|
Amphiphysin [38] |
Myelopathy, Stiff-man syndrome, cerebellar ataxia, encephalopathy and/or limbic encephalitis | - | 61% abnormal |
79% lung and breast cancer |
Normal or temporal T2 hyperintensities when limbic encephalitis | Poor |
| MAP1B (PCA-2) [39] | Peripheral neuropathy, myelopathy, cerebellar ataxia and/or cognitive decline/encephalopathy | - | 74% abnormal |
79% lung cancer |
40–50% abnormal; spinal cord T2 hyperintensity with contrast enhancement, cerebral atrophy | 58% improvement |
| SOX1 (AGNA) [40–42] | Lambert-Eaton myasthenic syndrome, cerebellar degeneration and/or encephalitis | - | > 60% abnormal |
94% lung cancer |
40–50% abnormal; temporal T2 hyperintensity, cerebellar atrophy | Variable but generally poor |
| KHLH 11 [43, 44] | Cerebellar ataxia and/or limbic encephalitis | - | 100% abnormal |
70% testicular seminoma |
70% abnormal; midbrain/temporal/cerebellar T2 hyperintensity, mild cerebellar atrophy | 69% improvement or stabilized |
|
NIF [45] |
Cerebellar ataxia, encephalopathy, myelopathy, limbic encephalitis and/or cranial neuropathies | - | 70% abnormal | 77% neuroendocrine tumors | 40–50% abnormal; cerebellar atrophy, temporal T2 hyperintensity, cranial nerve enhancement) | 70% improvement |
|
PDE10A [46] |
Movement disorders | - | 100% abnormal | 85% | 80% abnormal; basal ganglia T2 hyperintensity, leptomeningeal enhancement | Poor |
| DACH1 (ANNA-3) [47] | Neuropathy, encephalitis and/or cerebellar ataxia | - | 66% abnormal | 90% neuroendocrine tumors | NA | 70% improvement |
|
Tr/DNER [48] |
Subacute cerebellar ataxia and/or encephalopathy | IgG1 and IgG3 | 59% abnormal |
89% Hodgkin lymphoma |
Cerebellar atrophy | Poor |
| Trim46 [49, 50] | Subacute cerebellar ataxia, encephalopathy, movement disorder, limbic encephalitis, myelopathy and/or progressive dementia | - | 64% abnormal |
82% neuroendocrine tumors |
50% abnormal; temporal T2 hyperintensity, cerebellar atrophy | Poor/absent |
| AP3B2 [51] | Cerebellar ataxia, spinocerebellar ataxia, myeloneuropathy and/or neuropathy | - | 100% abnormal |
10% renal cell carcinoma |
75% abnormal; cerebellar/spinal cord atrophy | Stabilization, no improvement |
| RGS8 [52] | Cerebellar ataxia | IgG1 | May be abnormal | lymphoma | At follow-up cerebellar atrophy | Variable, possible improvement |
| AK5 [53] | Limbic encephalitis, rapidly progressive amnesia | > IgG1 | 100% abnormal | < 5% | 90% abnormal at onset; temporal T2 hyperintensity, 90% abnormal at follow-up; severe atrophy | 20% improvement |
| AGO [54] | Limbic encephalitis, sensory neuronopathy and/or cerebellar ataxia | > IgG1 | 50% abnormal |
20% miscellaneous |
> 90 abnormal; temporal T2/FLAIR hyperintensity, cerebellar atrophy | 60% improvement, 20% stabilization |
| GFAP [55] | Encephalitis, meningitis, myelitis and/or papillitis | - | > 80% abnormal |
30% ovarian teratoma in presence of concomitant NSAbs |
> 60% abnormal; brain linear perivascular radial enhancement | > 80 improvement |
| Zic4 [56, 57] | Subacute cerebellar ataxia | - | May be normal |
90% lung cancer |
Cerebellar atrophy | Poor |
|
Septin 5 and 7 [58] |
Cerebellar ataxia, encephalopathy, eye movement alteration and/or myelopathy | IgG1 and IgG2 | 73% abnormal | 15% |
30% abnormal in Septin-5; cerebellar atrophy - 60–70% abnormal in Septin-7; temporal T2/FLAIR hyperintensity, cerebral atrophy |
80% improvement |
ANNA: anti-neuronal nuclear antibody, PCA: anti-Purkinje cell cytoplasmic antibody, CRMP5: collapsin response mediator protein 5, GAD: glutamic acid decarboxylase, MAP1B: microtubule-associated protein 1B, SOX: Sry-like high mobility group box, AGNA: anti-glial nuclear antibody, KLHL11: Kelch-like protein 11, NIF: neuronal intermediate filament, PDE: phosphodiesterase, DACH1: Dachshund-homolog, DNER: Delta/Notch-like epidermal growth factor-related receptor, Trim: tripartite motif-containing protein, AP3B2: AP-3 complex subunit beta-2, RGS: regulator of G-protein signaling, AK: adenylate kinase, AGO: Argonaute, GFAP: glial fibrillary acidic protein, Zic: Zinc-finger protein
Table 2.
Clinical and radiologic features of autoimmune encephalitides associated with autoantibodies targeting neuronal surface antigens
| Abs target [reference] |
Clinical syndrome | Ig subclasses | CSF findings | Tumor association | Neuroradiological pattern | Immunotherapy response |
|---|---|---|---|---|---|---|
| NMDAR [59–62] | Encephalitis, neuropsychiatric manifestations, movement disorders, dysautonomia and/or coma | IgG1 | 80% abnormal |
> 40% ovarian teratoma |
Mostly normal. In a minority temporal lobe T2 hyperintensity | 80% improvement; a second line is frequently required |
| LGI1 [20, 63] | Limbic encephalitis, facio-brachial dystonic seizures and/or hyponatremia | > IgG4 but also IgG1-IgG2 | 25% abnormal | < 5% thymoma | 70% abnormal; temporal T2 hyperintensity, less commonly basal ganglia T2/FLAIR hyperintensity | 60–70% improvement |
|
CASPR2 |
Limbic encephalitis, dysautonomia, insomnia, peripheral nerve hyperexcitability and/or neuropathic pain | > IgG4 but also IgG1 | 35% abnormal | 15% thymoma | 30% abnormal; temporal T2 hyperintensity | > 80% improvement |
| GABAA R [65–67] | Encephalitis, status epilepticus, behavior alteration and/or movement disorders | IgG1 | 60% abnormal | 25% thymoma | 90% abnormal; multifocal temporal and frontal T2 hyperintensity (GABAA R pattern) | > 80% improvement |
| GABAB R [68, 69] | Limbic encephalitis | IgG1 | > 90% abnormal |
50% small cell lung cancer |
60% abnormal; temporal T2 hyperintensity | 75% improvement |
| AMPA R [22, 70] | Limbic encephalitis, memory loss, psychosis and/or motor deficits | IgG1 | 70% abnormal |
65% lung cancer and thymoma |
70% abnormal; temporal T2 hyperintensity, less commonly multifocal T2/FLAIR hyperintensity | 70% improvement |
| Neurexin-3α [71] | Encephalitis, decreased level of consciousness and/or movement disorders | - | > 90% abnormal | < 5% | 20% abnormal; temporal T2 hyperintensity | 60% improvement |
| IgLON5 [72, 73] | Brainstem encephalitis, obstructive sleep apnoea and/or chorea. ALS like syndrome | > IgG4 but also IgG1 | 60% abnormal | < 5% | > 90% normal | 50% improvement but not sustained |
|
DPPX |
Encephalitis with prodromal weigh loss or diarrhea followed by cognitive dysfunction, CNS hyperexcitability, cerebellar and/or brainstem dysfunction | IgG1 and IgG4 | 50–60% abnormal | < 5% | > 90% normal or aspecific findings | 60% improvement |
|
D2R [77] |
Basal ganglia encephalitis and/or psychiatric dysfunction | NA | 75% abnormal | < 5% | 50% abnormal; basal ganglia T2 hyperintensity | Apparently good |
| mGluR5 [78] | Encephalitis, psychiatric and cognitive symptoms, movement disorders, sleep dysfunction | IgG1 but also IgG2-IgG3 | > 90% abnormal |
50% Hodgkin lymphoma |
50% abnormal; extralimbic regions T2 hyperintensity | > 80% improvement |
| mGluR1 [79] | Cerebellar ataxia and behavioural/cognitive symptoms | IgG1 | > 80% abnormal | 10% lymphoma | 40% abnormal; cerebellum T2 hyperintensity, less commonly subcortical lesions/leptomeningeal enhancement, 80% cerebellar atrophy at follow-up | 40% improvement |
| GluK2 [80] | Cerebellar ataxia | IgG1 | > 90% abnormal | Rare but high in presence of concomitant NSAbs | > 75% abnormal; cerebellum T2 hyperintensity or atrophy | 50–60% improvement |
| SEZ6L2 [81] | Cerebellar ataxia and mild extrapyramidal symptoms | IgG4 | 25% abnormal | < 5% | > 90% normal | Poor |
NMDAR: N-methyl D-aspartate receptor, LGI: leucine-rich glioma inactivated, CASPR2: contactin-associated protein-like 2, GABAR: gamma-amino butyric acid receptor, AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, IgLON5: immunoglobulin-like cell adhesion molecule 5, DPPX: dipeptidyl aminopeptidase-like protein, D2R: dopamine receptor 2, mGluR: metabotropic glutamate receptor, GluK2: glutamate kainate receptor subunit 2, seizure-related 6 homolog like 2
Typical Limbic Encephalitis, imaging findings
Limbic encephalitis (LE) represents the typical presentation of AE, and was first described in the 1960s as a clinico-pathological entity [7, 82] with medial temporal lobe (MTL) symptoms caused by an inflammatory process involving structures of the limbic system, including the hippocampus, amygdala, hypothalamus, cingulate gyrus and limbic cortex. Reports on the MRI correlates of this neuropathology were published at the end of the 1980s [83]. Although imaging findings are often not confined to these areas, the identification of bilateral involvement of the MTL on T2-weighted MRI is a key diagnostic feature in the typical pattern of LE and this MRI pattern is one of the four diagnostic criteria which are necessary for a diagnosis of definite LE according to Graus et al. [18]. Importantly, this MRI finding enables a diagnosis of definite AE in the pertinent clinical scenario even in the absence of neuronal antibodies. Conversely, in the presence of a negative MRI, unilateral medial temporal lobe anomalies or other MRI patterns (cortical/subcortical, striatal, diencephalic, brainstem, encephalomyelitis, and meningoencephalitis) only a diagnosis of possible or probable AE can be formulated unless there is evidence of neuronal antibodies. The role of MRI is obviously also to rule out other non-immune disorders that may have unilateral involvement such as seizures, herpes simplex virus encephalitis or gliomas. FDG-PET can be useful in identifying metabolic alterations in temporo-medial regions when the MRI is negative and thus serves as a substitute of the MRI abnormalities to fulfill diagnostic criteria for definite LE [18].
AE subtypes
The MRI pattern of anatomical involvement of the limbic lobe has been associated with numerous auto-antibodies, and, despite a certain variability across-studies, some AE subtypes seem to more likely present with LE. Anti-LGI1 AE, among the most frequent types of AE overall, is also considered one the most frequent cause of non-paraneoplastic LE [84, 85] – although a non-negligible portion of anti-LGI1 AE cases is paraneoplastic, and specifically associated with thymomas [86]. Across studies, anti-LGI1 AE is reported to show the typical LE pattern in 73–83% of the cases [63, 87]. For instance, in the cohort described by van Sonderen and colleagues [88], LE was present in 34 out of 38 patients, and the typical hippocampal T2-hyperintensity was observed in 79% of the patients. In another study on 76 patients with anti-LGI1 associated cognitive impairment, Ariño et al. [63] found a typical LE pattern in 83% of cases, while the remaining cases showed either non-LE (4%) or encephalopathy (13%, with no MRI and CSF anomalies). Along with anti-LGI1, anti-CASPR2 (contactin-associated protein-like 2) antibodies are part of the voltage-gated potassium channel-complex (VGKC) antibodies. For anti-CASPR2 AE, despite LE is considered a common presentation [89], typical MRI findings are reported to be less frequent. In a cohort of 33 patients van Sonderen et al. [21] reported medial temporal lobe T2-hyperintensity in only 8 subjects (24%) – bilateral in all cases. Binks and colleagues [90] reported up to 50% of cases of anti-LGI1 and anti-CASPR2 being MRI-negative, therefore a potential diagnosis of these AE subtypes should not be ruled out in the absence of suspicious MRI findings. Other AE subtypes consistently associated with typical medial temporal lobe MRI alterations include anti-GABABR AE (ranging ~ 50–60% of cases across studies) [68, 91] and anti-AMPAR AE (~ 55% of cases) [22]. Anti-GAD antibodies, too, tend to present as a typical LE when causing encephalitis (reportedly, in 59% of cases) [92]. Other AE subtypes, such as anti-Hu and anti-Ma/Ta are not exclusively associated with LE findings [12], while anti-CV2/CRMP5 (collapsin response mediator protein 5) AE are classically extra-limbic and present as striatal encephalitis (see Section “Imaging patterns of extra-limbic involvement”), rarely showing temporal involvement [85]. Finally, it is worth remembering that MRI can be unremarkable in patients with clinical features of LE. Among MRI-negative AE, it is worth mentioning anti-NMDAR AE. While not strictly considered LE, NMDAR can also present with limbic symptoms, and very frequently MRI negative (in ~ 70–90% of cases across studies) [61, 93, 94]. Indeed, for the dedicated 2016 diagnostic criteria for NMDAR-AE [18], MRI findings are not taken into consideration.
T2/FLAIR findings
LE is characterized by a typical MTL involvement that can be evaluated on T2-weighted and T2-weighted FLAIR images. For brevity, T2-weighted images will be referred to as “T2” and T2-weighted FLAIR images will be referred to as “FLAIR”, while “T2/FLAIR” will refer to findings that are appreciable on T2 and/or FLAIR. The hallmarks of MTL AE are T2/FLAIR hyperintensity and swelling of the hippocampi and amygdalae. In 2006, based on MRI findings from a historic case series, Urbach et al. [95] summarized the typical essential features and progression pattern of LE as follows: unilateral or bilateral hyperintensity and swelling of MTL structures within ~ 3 months from the clinical onset, regression of the swelling within ~ 9 months, and gradual volume loss (i.e. atrophy) starting within ~ 1 year. However, it is to note that, even in their cohort, the timing of these findings was highly variable across patients, with some cases of absent swelling, swelling persisting for over one year, or atrophy appearing within few months. In a more recent study enrolling patients with LE [96], quantitative volumetric analyses of MTL demonstrated a bilateral volume increase in amygdala at baseline, followed by a regression of the swelling at the follow-up scan (~ 6 months), and by a volume loss due to atrophy at the third scan (~ 1 year). Notably, as opposed to anti-GAD AE, in anti-VGKC AE the volume alterations also involved the hippocampus, and were more pronounced. Overall, MTL swelling is reported to occur in 63–78% of anti-VGKC AE [97, 98]. Further evidence demonstrated that the volume increase of MTL structures is more prominent in the early phases of the disease, that may be asymmetric, and that different AE subtypes may present specific volumetric alteration patterns [99].
Even though LE is unilateral in a number of cases (up to ~ 50%, according to a recent case series [98], many authors agree that the typical presentation is with a bilateral involvement of MTL, sometimes asymmetric [100]. Conversely, in the presence of unilateral MTL alterations, the hypothesis of Herpes Simplex Encephalitis (HSE) should be carefully taken into consideration [100]. Consistently, according to the 2016 criteria, in the absence of positive neuronal antibodies bilateral MTL involvement is a necessary finding for the diagnosis of “definite” LE [18].
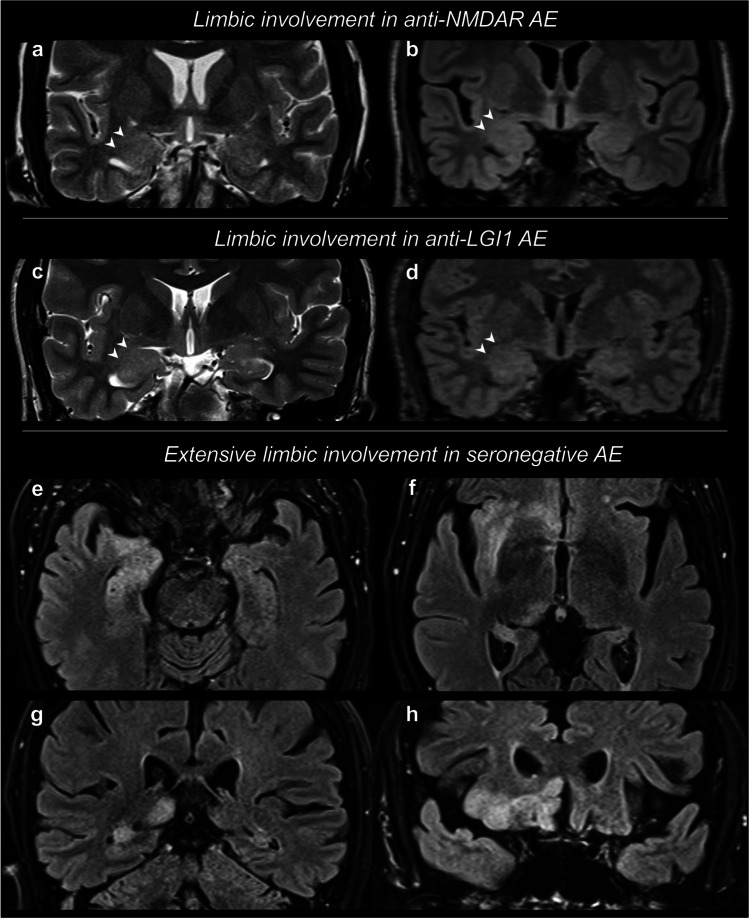
Regarding T2/FLAIR alterations, it is worth mentioning that the identification of subtle signal and volume changes in MTL structures may be challenging and reader-dependent, especially in patients with a mild disease. Figure 1a–d shows two cases of LE with subtle radiologic findings. For instance, in a recent study by Schievelkamp and colleagues [101], the authors reported an unsatisfactory diagnostic accuracy of the readers in distinguishing LE patients from age-matched healthy controls (accuracy: 64–74%), and a rather low inter-reader agreement for the identification of MTL signal and volume abnormalities. To address this issue, some authors proposed automated quantitative approaches to objectively identify MTL T2-signal alterations and aid the diagnosis of LE [102].
Fig. 1.
Limbic encephalitis. Limbic encephalitis (LE) can present with subtle radiologic findings, such as a swelling of the amygdala, with associated subtle signal hyperintensity, as visible (arrowheads) on coronal T2-weighted (a, c) and T2-weighted FLAIR (b, d) in two distinct cases of anti-NMDAR AE (a, b) and anti-LGI1 AE (c, d). Other patients may demonstrate more extensive alterations of the limbic structures. In this case (e–h), LE extensively involved the right limbic system, including amygdala (e), head of the hippocampus (e), body and tail of the hippocampus (e, f, g), insular cortex (f), thalamus/pulvinar (f), basal frontal gyri (f, h), and cingulate gyrus (h). White matter alterations are also noted, involving the external and extreme capsule (f). Following a thorough diagnostic work-up, a diagnosis of seronegative AE was posed
In association with MTL involvement, the typical LE pattern sometimes shows a more wide-spread limbic involvement, with a T2-signal abnormality and grey matter swelling also in the insula, in the lateral aspects of the temporal lobe, in the basal aspects of the frontal lobe, and in the cingulate gyrus; in addition, basal ganglia and thalamus involvement is not infrequent [100, 103]. Figure 1e–h displays a case of LE with extensive involvement of the limbic system structures.
Hypointense foci on T2*-weighted images, consistent with the presence of blood degradation products, are rare in AE [104].
Contrast Enhancement (CE) and Diffusion Weighted Imaging (DWI)
CE and/or DWI restriction in the involved brain regions can occur in some cases, but is not considered typical of LE [18, 105]. On the contrary, prominent CE and a clear DWI restriction are often considered hallmarks of HSE [105, 106].
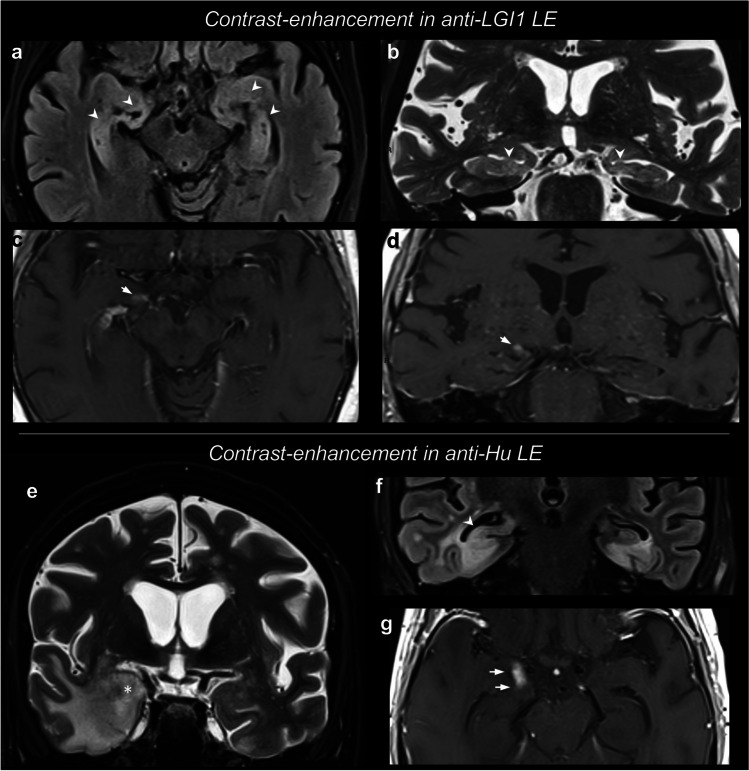
In a recent case series, Kotsenas and colleagues [98] described a mild MTL CE in 28% of cases, and MTL DWI restriction in 43% of cases. Notably, in this cohort, CE and DWI restriction were significantly associated with the development of mesial temporal sclerosis (MTS) in the follow-up scans. Since CE was also previously reported in 15–25% of cases of LE [107], the presence of mild, patchy or poorly-delimited enhancing areas can be considered as a possible finding in a minority of LE cases. A peculiar instance is represented by anti-Ma AE, for which CE is reportedly more frequent (up to ~ 38%) [19]. Figure 2 shows two cases of LE with contrast-enhancement, both with bilateral yet asymmetric findings.
Fig. 2.
Limbic encephalitis with contrast-enhancement. Both cases presented with an encephalitic bilateral involvement of the medial temporal lobe (MTL) structures, and unilateral areas of contrast-enhancement in the right amygdala (arrows). The arrowheads show the bilateral areas of T2/FLAIR hyperintensity in the MTL. The anti-LGI1 AE case shows more symmetric involvement of the amygdalae and hippocampi on T2/FLAIR. The anti-Hu AE case shows an asymmetric pattern, with a more predominant swelling and hyperintensity of the right amygdala (asterisk in e) and hyperintensity of the right body of the hippocampus (arrowheads in f). These alterations are associated with MTL grey and white matter hyperintensity, including edema with finger-like appearance
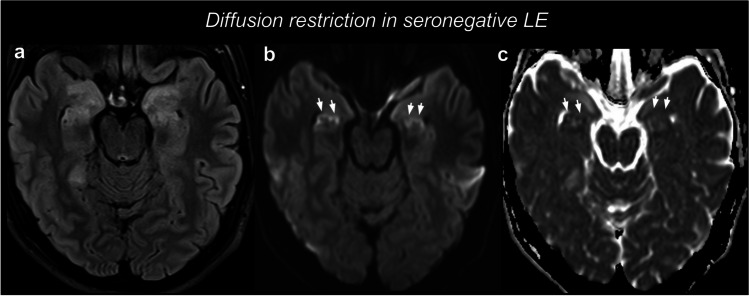
Conversely, the occurrence of DWI restriction is more controversial. In other cohorts of LE, MTL lesions showed either subtle DWI changes only in few cases [108], or no DWI changes in any cases [109]. In addition, many articles reporting DWI changes [98, 100] did not evaluate ADC maps, and a recent study demonstrated that anti-LGI1 AE is characterized by DWI hyperintensity without ADC decrease [103]. Therefore, in many cases DWI changes may be ascribable to the “shine-through” effect, which was extensively described for AE [12, 110]. In fact, in a recent paper, only 9% of patients with LE showed reduced ADC values demonstrating an actual DWI restriction [111]. Overall, we can state that in AE cortical involvement with restricted diffusion on DWI is uncommon unless related to cytotoxic edema associated with epileptic activity. Outside this instance, cortical involvement with restricted diffusion should lead to consider other pathological entities in the differential diagnosis, for instance prion diseases as described in the section of the differential diagnosis. However, diffusion restriction can be seen in AE, and Fig. 3 represents an example of LE with symmetric and bilateral diffusion restriction.
Fig. 3.
Limbic encephalitis with diffusion restriction. In this seronegative case, T2-weighted FLAIR images (a) showed a marked symmetric swelling and hyperintensity of the amygdala and head of the hippocampus. DWI (b) and ADC (c) revealed a gyriform diffusion restriction in the hippocampus (arrows), with ADC values as low as 0.58 × 103 mm2/s (right) and 0.72 × 103 mm2/s (left). After a diagnostic work-up, a diagnosis of seronegative AE was posed
Atrophy and Mesial Temporal Sclerosis (MTS)
As already mentioned, LE may result in MTL volume loss due to atrophy in follow-up scans ~ 1 year after the disease onset, even though atrophy appears earlier in some cases [95, 96]. In a subset of patients, such atrophy displays the characteristic imaging features of MTS. MTS, also referred to as hippocampal sclerosis, consists in chronic gliosis and neural loss. On T2/FLAIR images, MTS can be identified as a signal increase and volume loss of hippocampus [112]. The degree of atrophy can be assessed on coronal reconstructions of 3D T1-weighted imaging that highlight a reduction in volume of the hippocampus and amygdala with relative dimensional increase of the temporal horn of the lateral ventricle and choroidal fissure. The hyperintensity is usually easier to visualize on T2/FLAIR images, but needs to be confirmed with T2 weighted images, that are considered more reliable and less prone to false positive findings [112]. While historically considered as both a cause and a consequence of temporal lobe epilepsy [113], the radiological appearance of MTS has been more recently identified as a possible sequela of encephalitis [12, 114, 115]. The prevalence of MTS as a sequela of LE is reported to be ~ 43–50% in anti-LGI1 AE cases across series [88, 98, 103], and ~ 33% in anti-GAD AE [36]. Notably, in a series including anti-VGKC AE patients, anti-LGI1 but not anti-CASPR AE evolved in MTS and DWI changes and CE at baseline predicted MTS [98].
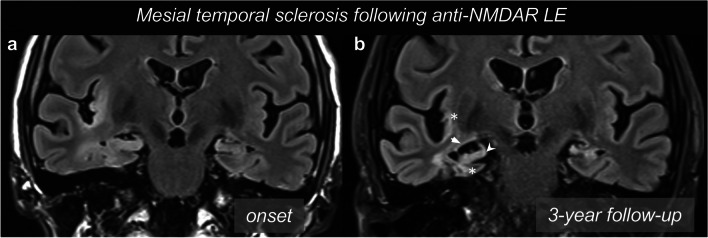
Interestingly, evidence from the literature suggests that hippocampal volumetric and morphological anomalies after AE may be characterized by peculiar features. A recent article [36] applied advanced volumetric and shape analyses and demonstrated that chronic alterations in anti-GAD AE consist in subtle shape abnormalities of selected areas in the hippocampus head, rather than a clear hippocampal atrophy, while other areas (including CA3 and hippocampal fissure) seem to be spared by the atrophy. Conversely, a cohort of patients with non-immune temporal lobe epilepsy presented a more widespread volume loss and deformation of the hippocampi, as well as a higher occurrence of hippocampal sclerosis. Figure 4 features images of a LE which resulted in mesial temporal sclerosis.
Fig. 4.
Medial temporal sclerosis as a sequela of limbic encephalitis. In this case of anti-NMDAR AE, T2-weighted FLAIR images (a) exhibited signal hyperintensity in the right MTL structures and insular cortex. The encephalitic insult evolved with atrophic changes over time. After three years, T2-weighted FLAIR images (b) showed medial temporal sclerosis, with volume loss of amygdala and hippocampus (arrowhead), as well as atrophy of the parahippocampus and insular cortex (asterisks), associated with an enlargement of the temporal horn of the lateral ventricle and choroidal fissure (arrow)
Imaging patterns of extra-limbic involvement
Aside from the typical limbic pattern, AE can involve different extra-limbic anatomical structures. For the purpose of this review, we have grouped AE which may present with extra-limbic involvement in: cortical AE, perivascular involvement, striatal AE, diencephalic AE, AE with involvement of the rhombencephalon (brainstem and/or cerebellum). Combined involvement of different structures can be seen, including combined limbic and extra-limbic patterns. Of note, extra-limbic PET alterations both in terms of hypo- and hypermetabolism have been reported for AE [116].
Cortical involvement
Anatomical involvement of cortex can be seen mainly in anti-NMDAR, anti-MOG (myelin oligodendrocyte glycoprotein), and anti-GABAAR AE. In anti-NMDAR AE Zhan et al. found MRI abnormalities in 49% of patients, 72% of which showed involvement of cortex of different lobes, with or without a combined hippocampal involvement [117]. Antibodies to the glial protein MOG can be associated with acute disseminated encephalomyelitis (more frequent in the pediatric population) and cerebral cortical encephalitis (more frequent in adults), and, as such, have been included into the spectrum of AE [17]. The cortical encephalitis is characterized by a unilateral [118] or bilateral frontal cortex involvement [119]. In these patients, who typically present with focal seizures, often evolving to bilateral tonic–clonic seizures, brain MRI demonstrates unilateral or bilateral cerebral cortical hyperintensities on T2/FLAIR sequences, with swelling of the cortex. Anti GABAAR AE affects mostly children and young adults, presenting most commonly with seizures (88%), cognitive impairment (67%), behavioral changes (46%), alterations of consciousness (42%), or abnormal movements (35%). T2/FLAIR MRI can demonstrate multifocal bilateral or unilateral cortical and subcortical areas of hyperintensity predominantly occurring in the temporal and frontal lobes, and less frequently in the parietal or occipital lobes. Basal ganglia and cerebellum can also be involved [66]. Interestingly, these multifocal T2/FLAIR changes can be asynchronous, with some appearing while others are disappearing along the disease course. On post-contrast T1-weighted images these lesions do not show enhancement although cases with gyriform leptomeningeal enhancement have been described. Typically, lesions are not characterized by restricted diffusion on DWI.
Perivascular involvement
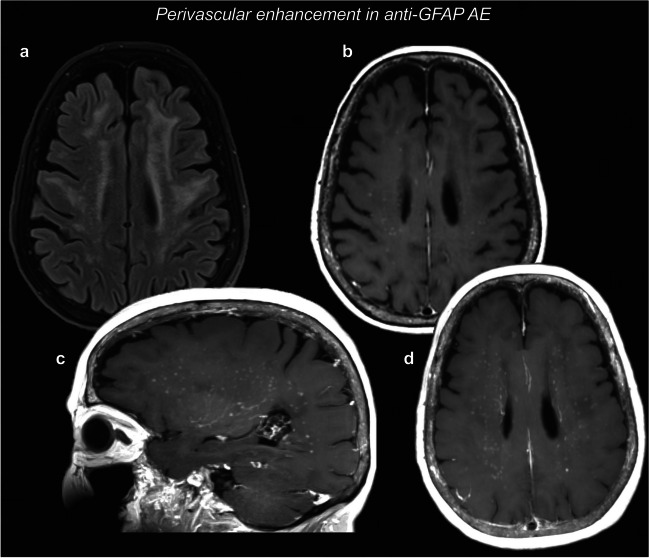
A very peculiar case is represented by anti-GFAP (glial fibrillary acidic protein) AE. The most distinctive finding is a perivascular radial contrast-enhancement in the centrum semiovale, perpendicular to the lateral ventricles, which can be found in ~ 50% of the cases, and can co-localize with white matter T2/FLAIR hyperintense areas [55]. These findings are clearly displayed in the representative case in Fig. 5. Alternatively, this radial pattern can be seen in the cerebellum in a minority of cases. Other MRI abnormalities include leptomeningeal or ependymal enhancement, serpentine enhancement, and/or accompanying long-segment spinal cord alterations [55, 120]. Given the characteristic radiologic appearance of radial contrast-enhancement in anti-GFAP AE, the radiologist is sometimes the first physician to pose the suspicion of this type of encephalitis.
Fig. 5.
Perivascular enhancement in the centrum semiovale. T2-weighted FLAIR images (a) showed widespread signal alterations in the bi-hemispheric white matter. Post-contrast T1-weighted images (b) showed numerous spots of perivascular enhancement in the centrum semiovale, which can be more easily appreciated with maximum intensity projection (MIPs) images (c, d). Anti-GFAP autoantibodies were positive in CSF and serum
Basal ganglia involvement
Anatomical involvement of basal ganglia can be seen in AE with anti-CV2/CRMP5, anti-D2R (dopamine receptor 2), anti-NMDAR, and sometimes anti-LGI1 Ab. Anti-CV2/CRMP5 IgG target an intracellular antigen (collapsin response mediator protein) and have been reported in the setting of various paraneoplastic syndromes, including peripheral neuropathy, cranial neuropathy, gastroparesis, encephalitis, cerebellar ataxia, myelopathy, and chorea. Patients with anti-CV2/CRMP5 present with chorea or involuntary movements, and striatal involvement on MRI T2-weighted sequences, without diffusion restriction. Striatal involvement associated with spinal cord signal alterations has been reported [31]. Anti-CV2/CRMP5 has a strong cancer association, in particular with small-cell lung cancer or thymoma [121]. Another antibody recently described in patients with renal cancer and lung cancer is phosphodiesterase 10A IgG (PDE10A). Half of the patients described by Zekeridou et al. [46] had chorea or ballismus. It is interesting to note that in two of these patients the onset of movement disorders was described after the use of immune checkpoint inhibitor. MRI showed T2/FLAIR hyperintensities in the basal ganglia [46]. Differential diagnosis should include more common toxic and metabolic disorders with basal ganglia involvement and lack of restricted diffusion is helpful in the differential diagnosis with Creutzfeldt-Jakob disease (CJD). There are sporadic cases of anti-NMDAR AE with basal ganglia involvement. In some reported pediatric cases, basal ganglia involvement was characterized by restricted diffusion on DWI [122, 123] and, exceptionally, also a thalamic involvement was described [17]. In the context of anti-LGI1 AE some patients with faciobrachial dystonic seizures may show T1 and/or T2 hyperintensity (alone or combined) in the basal ganglia, with T1 hyperintensities persisting longer than the T2 hyperintensities (median 11 weeks vs 1 week) [124].
Diencephalic involvement
In AE diencephalic-hypothalamic dysfunction can present with endocrine abnormalities, hyperthermia, hyperphagia, somnolence but also other severe dysautonomia symptoms. MRI T2/FLAIR hyperintensities can be detected in thalamus, geniculate bodies, hypothalamus and subthalamic nuclei, usually with bilateral and relatively symmetric involvement. In anti-Ma2 AE diencephalic involvement is usually seen in combination with abnormalities in the limbic system, although isolated diencephalic involvement has been reported [34]. Enhancement can be seen in approximately half of the patients in at least one of the areas abnormal on T2/FLAIR [19].
Brainstem involvement
In rhombencephalitis the inflammatory process involves the brainstem with variable involvement of the cerebellum. In the acute phase MRI may be unremarkable or demonstrate T2/FLAIR hyperintensity within the brainstem, with or without cerebellar abnormalities. In the chronic phase parenchymal volume loss may ensue. Various antibodies are associated with brainstem involvement, the most common being anti-Ma2, anti-Ri, and anti-KLHL11 (Kelch-like protein 11). Patients with anti-Ma2 AE and brainstem encephalitis most often have ophthalmoplegia [19]. In anti-Ma2 AE encephalitis MRI is abnormal in up to 74% of cases. Limbic encephalitis is the most common pattern, but this AE may present with different combinations of limbic, diencephalic, or brain stem encephalitis. Brainstem encephalitis at MRI is more often associated with lesions in the midbrain and less commonly in the pons and/or medulla oblungata. Anti-Ri AE was initially described in association with opsoclonus–myoclonus syndrome and cerebellar ataxia in women with breast cancer but it can present with a wider spectrum of neurologic involvement, the cerebellum and the brainstem being most commonly affected. Brain MRI has been reported as pathological in 18% of cases, most commonly with T2 signal changes in the brainstem [37]. In anti-Hu AE Dalmau et al. [28] reported occurrence of symptoms associated with brainstem dysfunction in 31%. MR imaging findings usually correlate with clinical features and typically include T2/FLAIR hyperintense lesions in the medial temporal lobes with variable involvement of the cerebellum and brain stem. It is noteworthy that MRI detectable lesions reflecting clinical features in brainstem syndromes are frequently absent. In a series of 14 patients with anti-Hu associated brainstem encephalitis MRI was always normal [125]. Anti-KLHL11 encephalitis is a relatively recent pathological entity, most commonly presenting with a rhombencephalitis phenotype with ataxia, diplopia, dysarthria, vertigo, hearing loss, and tinnitus. A recent study described a strong association with testicular tumors [126]. In this cohort [126], MRI has been reported as abnormal in 76% (n = 28), showing T2/FLAIR hyperintensity, which were most commonly seen in the temporal lobe (n = 12), followed by cerebellum (n = 9), and more rarely in the brainstem (n = 3) and diencephalon (n = 3). In one patient there was also spinal cord central gray matter involvement. In a few instances there was associated enhancement (n = 3) and in single case leptomeningeal and cranial nerve V enhancement. At follow-up, cerebellar atrophy or medial temporal lobe atrophy was reported. Interestingly, three patients had hypertrophic olivary degeneration.
Cerebellar involvement
Cerebellar ataxia has been described as a typical feature in the setting of paraneoplastic syndromes, and onconeural Ab positivity has been reported in a large number of patients with paraneoplastic cerebellar degeneration. Some of the antibodies most commonly associated with cerebellar involvement are anti-Yo/PCA11 (38%) and anti-Hu/ANNA-1 (32%) [28]. The ataxic syndrome associated with anti‐Yo antibody, or anti-PCA1 (Purkinje cell cytoplasmic antibody type 1), is the most common among the forms of paraneoplastic cerebellar degeneration (PCD). It typically presents with subacute development of pancerebellar deficits reaching clinical plateau within 6 months. The majority of cases have been reported in women in association with pelvic or breast tumors. There can be clinical manifestations of cerebellar dysfunction also in anti-Ma and anti-CV2/CRMP5 AE, and in anti-NMDAR AE cerebellar symptoms have been described in the pediatric population, while rare in adults [127–129]. In these cases of autoimmune cerebellitis, MRI is often unremarkable at presentation, even though T2/FLAIR hyperintensity of cerebellar hemispheres can be seen, while a more typical imaging finding is paraneoplastic cerebellar degeneration (PCD), consisting in cerebellar atrophy at follow-up, manifesting months to years later [29]. Finally, it is worth mentioning anti-GluK2 (glutamate kainate receptor subunit 2) AE, which can cause cerebellitis and can acknowledge obstructive hydrocephalus as a complication [80].
Differential Diagnosis
Depending on anatomical sites involved and MRI signal features, AE can present with radiological characteristics resembling other diseases, including other encephalitides, neoplasms, vascular conditions, and post-seizure alterations. A correct differential diagnosis is crucial for patient management, in particular to promptly initiate an effective therapeutic strategy.
Herpes Simplex Encephalitis (HSE)
HSE is the most important alternative diagnosis to consider when suspecting limbic encephalitis, since both entities tend to involve the mesial temporal structures and HSE is rather common, accounting for ~ 20% of limbic encephalitides overall [85]. Both clinical and imaging presentations can aid distinguishing between these two conditions.
From a clinical standpoint, HSE is more prone to present abruptly and with fever [85, 130], and is more typically associated with abnormal CSF findings [85]. Conversely, psychiatric manifestations point towards AE [131]. For instance, in a study comparing HSE and AE patients, psychiatric symptoms were exclusively seen in AE [100]. Additional clinical features more frequent in AE include memory deficits, seizures, and involuntary movements [132].
The limbic system involvement on MRI is more typically bilateral in AE, whereas in HSE is generally unilateral (or bilateral and asymmetric in some cases) [100]. In addition, the presence of striatal or thalamic MRI abnormalities advocates for a diagnosis of AE [85, 132]. In a recent study enrolling 95 patients with infectious or autoimmune encephalitis [132], the hippocampal involvement was significantly more frequent in AE (42% vs 22% of cases), and thalamic and basal ganglia anomalies were also slightly more frequent. However, it must be pointed out that HSE accounted for only ~ 14% of the infectious cohort in this study. Basal ganglia involvement was also reported as a key diagnostic clue by Oyanguren et al. [100], as this sign alone had sensitivity/specificity 0.82/1 in distinguishing AE from HSE in their cohort. In addition, in their cohort insular involvement was more common in HSE. Another study [130] on 251 cases focused on distinguishing temporal lobe HSE from its mimics, including AE and other infectious/autoimmune conditions. In a multivariate model, the bilateral involvement of MTL and the presence of extra-limbic alterations were associated with a lower probability of HSE (odds ratio 0.38 and 0.37, respectively).
As for MRI signal evaluation, HSE can present with CE areas and areas of DWI restriction [133], that are rather infrequent in AE (as previously discussed). In addition, hemorrhagic spots on T2*-weighted images and necrotic areas can be seen in HSE [131, 133]. However, it is to note that the traditional necrotic-hemorrhagic appearance of HSE is seen in the late stages of the disease, and these days patients are often evaluated and imaged before these features may reveal [133].
As emerges from these studies, distinguishing AE and HSE is often a challenging task, even though fever, neuropsychiatric manifestations, lesion site, and lesion signal features may point towards one or the other. In general, if HSE is suspected based on one or more characteristics, anti-viral treatment should be started immediately. Finally, it is worth mentioning that multiple pieces of evidence demonstrated that HSE itself can be a trigger for subsequently developing AE, and specifically anti-NMDAR AE [85, 134, 135]. Figure 6a–c shows a case of HSE with contrast-enhancement and hemorrhagic foci.
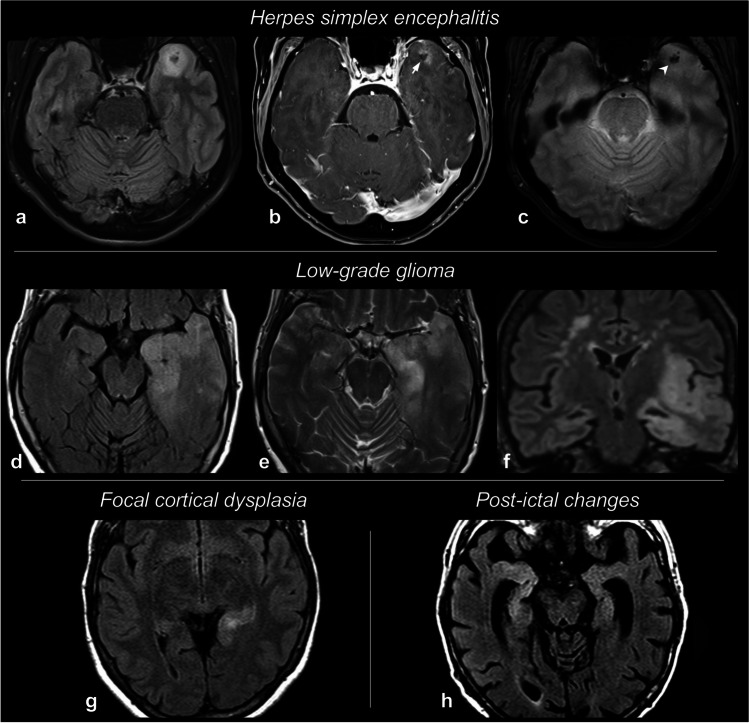
Fig. 6.
Differential diagnoses of autoimmune encephalitis. Some examples of conditions mimicking limbic autoimmune encephalitis are collected here. In a typical case of herpes simplex encephalitis (HSE) (a–c), unilateral T2-weighted FLAIR hyperintensity of the left anterior temporal areas (a) is associated with post-contrast T1 focal enhancement, with a central non-enhancing and hypointense spot (b, arrow), corresponding to hemorrhagic foci on T2*-weighted gradient-echo images (c, arrowhead). In a patient with low-grade glioma (LGG) (d–f), T2-weighted FLAIR (d, f) and T2-weighted TSE (e) exhibit a marked swelling and hyperintensity of the left anterior temporal lobe and insula, with a widespread thickening of the cortex and mass effect on the sulci. An example of focal cortical dysplasia (FCD) located in the tail of the left hippocampus is shown on T2-weighted FLAIR images (g). Finally, T2-weighted FLAIR images (h) obtained after an epileptic seizure demonstrate a MTL signal hyperintensity due to post-ictal changes
Steroid-responsive encephalopathy associated with autoimmune thyroiditis (SREAT)
Previously known as Hashimoto’s encephalitis (HE), SREAT is a condition associated with autoantibodies against thyroid antigens, for which the pathogenetic role is still controversial [136]. SREAT can mimic AE both by presenting with T2/FLAIR hyperintensities in the MTL structures, and by causing memory deficits, psychiatric symptoms and seizures [12, 136]. Moreover, as seen in AE, SREAT may also be MRI-negative (~ 50% of the cases) [136]. However, SREAT is characterized by a peculiar “migratory pattern”, consisting in the disappearance of some signal abnormalities over time, while new signal alterations appear in new sites [12, 136]. Additionally, SREAT often presents a preferential white matter involvement: leukoencephalopathy with confluent T2/FLAIR alterations [12, 136]. These two MRI findings aid the distinction between SREAT and AE. In addition, approximately 1/3 of SREAT cases occur in patients with a known thyroid disfunction, according to a revision of 251 cases [136]. It is worth noting that the definition of SREAT/HE is evolving. For instance, evidence from a recent study has challenged SREAT/HE definition, by highlighting that in a cohort of patients fulfilling criteria for these conditions, these criteria are not able to actually predict response to steroids [137].
Other infectious and immune-mediated conditions
AE with a striatal involvement, especially anti-CV2/CRMP5, may resemble other conditions affecting the basal ganglia, and particularly Creutzfeldt-Jacob disease (CJD). In the suspicion of CJD, seeking the typical patterns of DWI restriction in the basal ganglia and/or in the cortex is crucial [138].
As already discussed, some Group I AE (e.g. anti-Ma/Ta, anti-Hu) can involve the brainstem and the cerebellum. In these cases, alternative causes of rhombencephalitis should be considered, especially Listeria encephalitis. In Listeria encephalitis, enhancement is frequent and supratentorial involvement is rare. In addition, CSF examination and CSF and blood cultures may aid the diagnosis [139]. It should be noted that AE is a relatively rare cause of rhombencephalitis, as other bacterial (Listeria), viral (Herpesviridae, Epstein-Barr), and immune-mediated (Behçet) etiologies are more common [139].
Rarely, primary CNS vasculitis may resemble AE. In those cases, acquiring angiographic images to demonstrate abnormal vascular structures may be helpful. In addition, DWI is useful in case of vasculitis-induced micro-infarctions [85].
Lesions in diencephalon can also be related to neuromyelitis optica spectrum disorders (NMOSD); this involvement is characteristic but not pathognomonic for NMOSD and is considered one of features in the 2015 revised diagnostic criteria [140]. Typically, diencephalic lesions involve the peri-ependymal surfaces of the midbrain adjacent to the cerebral aqueduct and thalamus or hypothalamus adjacent to the third ventricle and the pattern of enhancement is more diffuse (cloud-like, ring-like, leptomeningeal) [28]. Other diagnostic considerations when basal ganglia are involved include sarcoidosis, IgG-4 disease-related syndromes, histiocytosis, Behçet’s Disease, Granulomatosis with Polyangiitis, ADEM, tuberculosis, fungal and bacterial infection [141].
As for the perivascular radial contrast-enhancement pattern involving the supratentorial white matter seen in anti-GFAP AE, it is worth remembering that a similar finding can be seen in neurosarcoidosis [142, 143] and sometimes in lymphomatoid granulomatosis (LYG) [144]. When this presentation is encountered, the next step in management should be dosing the serological levels of anti-GFAP to confirm or rule out anti-GFAP AE.
Finally, even COVID-19-associated encephalitis was reported to involve limbic structures (hippocampus, amygdala, MTL, cingulate gyrus) in some cases, and should be therefore be considered in the differential [145, 146], at least in SARS-CoV-2 positive patients.
Neoplasms
Gliomas involving MTL structures can mimic limbic encephalitis, especially in cases that do not present with a striking mass effect and CE. Most of these cases are low-grade gliomas (LGG), with an infiltrative growth pattern. In these cases, typically LGG may show indistinct margins, mass effect, and extra-limbic extension (perhaps with white matter involvement), which would not be expected in AE [85]. In addition, LGG are classically unifocal and unilateral, while bilateral alterations would point to AE. However, on very rare occasions also high-grade gliomas can present with atypical imaging features and can be mistaken for LE. In a recent paper on the topic [147], the authors report that this misdiagnosis can occur in ~ 2% of suspected AE. After reviewing 13 cases of glioblastoma mimicking AE, they argue that in these cases both clinical manifestations and conventional MRI may be misleading, as patients often had memory deficits, psychiatric symptoms, and seizures, and their scans showed non-enhancing T2/FLAIR limbic alterations, bilateral in more than half of the cases. In these challenging cases, the authors recommend to perform spectroscopy and perfusion imaging, in order to detect suspicious findings advocating for glioblastoma (high Cho/Cr ratio, elevated perfusion metrics). A case of LGG mimicking LE is represented in Fig. 6d–f.
Seizure-related alterations
Seizure-induced T2/FLAIR alterations, related to post-ictal edematous changes, may resemble LE, too [85]. Recently, Budhram et al. [111] suggested that in the presence of specific hippocampal diffusion restriction patterns (gyriform and/or diffuse) the diagnosis of post-ictal changes should be favored, as opposed to AE. ADC reduction following seizures are considered the result of post-ictal cytotoxic edema. Follow-up imaging, as well as electrophysiology studies, may also be useful to differentiate these two conditions. Figure 6h shows a case of post-ictal MTL alterations.
Suggested imaging diagnostic work-up
When clinical history and neurological and electrophysiologic (i.e. EEG) examination evoke the suspicion of AE an extensive workup is necessary to confirm the diagnosis and to exclude other pathological entities as reported in the previous chapters.
Neuroimaging
Neuroimaging plays an important role in the work up. Brain CT is frequently the first imaging modality used, particularly with subacute presentations when the patients are admitted in the ER. It must be stressed that CT is not sensitive for the identification of brain abnormalities in AE and MRI should be always acquired whenever possible. In fact, brain MRI is key in identifying the presence of brain abnormalities in AE and to characterize the anatomical pattern of involvement (i.e. limbic or extra-limbic). It is worth noting that in the acute phase patients can be uncooperative and MRI under sedation could be required.
MRI should be acquired with and without gadolinium-based contrast agents and the acquisition protocol ideally should include high resolution 3D-T1, T2-FLAIR, TSE T2, DWI, SWI and post-contrast T1 [148–150]. 3D T1-weighted images provide more anatomical detail to identify enhancing areas, and also are helpful to monitor atrophic changes over time. 3D turbo spin-echo (TSE) is superior to 3D inversion-recovery gradient-recalled echo (IR-GRE) in the identification of small foci of contrast-enhancement (higher lesion conspicuity), as proven on brain metastasis studies [151, 152]. If IR-GRE is used, it may be advisable to also obtain additional 2D spin-echo T1 images after contrast, which may improve the detection of small enhancing foci, as already recommended for the identification of small brain metastases [151, 152]. As for T2-weighted and/or T2-weighted FLAIR images, it is advisable to acquire also coronal images to evaluate the volumes and symmetry of medial temporal lobes structures. As for DWI, classic clinical sequences with b = 1000 and ≥ 3 directions are generally sufficient. Recent case series suggest the role of perfusion studies, including arterial spin labeling (ASL) in the pediatric population [122].
Ideally, brain MRI should be obtained before the lumbar puncture to avoid difficulties in the interpretation of post contrast imaging, with pachymeningeal thickening and enhancement, frequently detected in the setting of post lumbar puncture CSF hypotension. However, when encephalitis is suspected, guidelines recommend timely collection of CSF in order to assess viral etiologies and start empiric antiviral treatment. CSF collection in encephalitis is also crucial to test for anti-neuronal antibodies, that are pivotal to diagnose AE. The appropriate timing of brain MRI should be evaluated in each patient, carefully considering the likelihood of an underlying viral etiology that would prioritize lumbar puncture over brain imaging [153]. Brain FDG-PET also plays an important role since it can provide additional information of brain involvement also in patients with negative MRI. Specific metabolic patterns were shown to correlate with antibody types – e.g., occipito-parietal hypometabolism axnd anti-NMDAR, MTL hypometabolism with anti-LGI1 and with onconeural antibodies [154].
Whole body imaging
Computed tomography (CT) of the chest, abdomen, and pelvis with contrast is recommended as the first screening for associated malignancies in those cases where a paraneoplastic etiology is suspected. Importantly, the type of neuronal antibody detected is crucial to stratify cancer risk and to orient towards specific cancer subtypes. Additionally, some clinical phenotypes are also associated with a higher risk for accompanying cancer [17].
The main limitation of the CT evaluation is its low sensitivity to early breast and testicular cancers; if these tumors are suspected a mammogram or testicular ultrasound should be performed [155]. Breast MRI should then be obtained if mammography is negative but breast cancer suspicion remains high. Young male patients (< 50 years) with a diagnosis of anti-Ma2 AE may have microscopic testicular neoplasms even in the absence of positive ultrasound findings [156]. In case of a negative testicular ultrasound, suspicious clinical signs (e.g., testicular enlargement) and risk factors (e.g., cryptorchidism) should be investigated, and these patients should be closely monitored with repeat ultrasound in order to identify the potential appearance of microcalcifications at later follow-ups [156].
Transvaginal ultrasound or pelvic MRI to assess the presence of ovarian teratoma or adenocarcinoma must be considered in female patients, especially in the case of young and middle-aged women with anti-NMDAR encephalitis.
Whole body FDG-PET has a higher sensitivity when compared to CT for the detection of occult neoplasms, and should be obtained if the initial screening is negative and the suspicion of cancer is high, or in patients with carcinoma unknown primary (CUP) syndrome.
In patients with paraneoplastic neurological syndromes (PNS) and paraneoplastic antibodies if primary screening is negative, it is recommended to repeat screening after 3–6 months and then screen every 6 months up till 4 years [155].
Conclusions
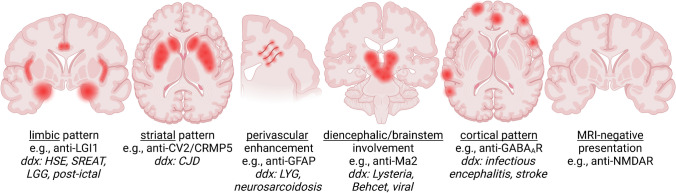
Autoimmune encephalitis (AE) is a challenging diagnosis, as it includes a variety of subtypes that differ in clinical presentation, radiologic appearance, and serologic findings. Additionally, it is often misdiagnosed, since it can mimic other conditions. The radiologist should be aware of the main imaging findings associated with these entities, including limbic and extra-limbic patterns (Fig. 7), as well as the useful clues for differential diagnosis, and an overall understanding of the clinical and biological underpinnings of this disease. A prompt diagnosis of AE can improve the prognosis both by expediting immunosuppressive treatment and allowing for the screening for an underlying malignancy.
Fig. 7.
Schematic overview of the main radiographic patterns in AE, along with examples of associated autoantibodies and with the main differential diagnoses. Note that more than one presentation can be seen for each autoantibody, and that sometimes more than one pattern can coexist (e.g., limbic plus brainstem) HSE = Herpes simplex encephalitis, SREAT = steroid-responsive encephalopathy associated with autoimmune thyroiditis, LGG = low-grade glioma, CJD = Creutzfeldt-Jakob Disease, LYG = lymphomatoid granulomatosis. Created with BioRender.com
Acknowledgements
The authors are thankful to Benjamin M. Ellingson for helping with the schematic representation of AE radiographic patterns (Figure 7).
Abbreviations
- AE
Autoimmune encephalitis
- AMPAR
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- CASPR2
Contactin-associated protein-like 2
- CRMP5
Collapsin response mediator protein 5
- D2R
Dopamine receptor 2
- GABAR
Gamma-Amino butyric acid receptor
- GAD
Glutamic acid decarboxylase
- GFAP
Glial fibrillary acidic protein
- Gluk2
Glutamate kainate receptor 2
- HSE
Herpes Simplex Encephalitis
- KLHL11
Kelch-like protein 11
- LE
Limbic encephalitis
- LGI1
Leucine-rich glioma inactivated
- MOG
Myelin oligodendrocyte glycoprotein
- NMDAR
N-Methyl D-Aspartate Receptor
- PCA2
Purkinje Cell Cytoplasmic Ab Type 2
- PERM
Progressive encephalomyelitis with rigidity and myoclonus
- SPS
Stiff person syndrome
- VGCC
Voltage gated calcium channel
Funding
Open access funding provided by Università degli Studi di Pavia within the CRUI-CARE Agreement. The present study was supported by the Italian Ministry of Health, “Ricerca Corrente 2022–2024” grant to the IRCCS Mondino Foundation. FM and CA are supported by #nextgenerationEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006) - A Multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022).
Declarations
Editorial Board Members and Editors
The authors have no editorial positions in this journal.
Employment
The authors have no relevant employment to disclose.
Conflict of Interest / Competing interests
FM received speaker honorarium from Roche Diagnostic S.p.A. MG received honoraria for the participation to advisory boards from Roche and UCB. The other authors have no relevant financial interests to disclose.
The authors have no relevant non-financial interests to disclose.
Ethics approval
The images shown were obtained from patients who gave written informed consent to have their data used for research purposes. No images that can lead to subject identification are included in this article.
Informed consent
Patients gave written informed consent to have their data used for research purposes.
Footnotes
Francesco Sanvito and Anna Pichiecchio share first authorship.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lancaster E. The diagnosis and treatment of autoimmune encephalitis. J Clin Neurol. 2016;12:1–13. doi: 10.3988/jcn.2016.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodfellow JA, Mackay GA. Autoimmune encephalitis. J R Coll Physicians Edinb. 2019;49:287–294. doi: 10.4997/JRCPE.2019.407. [DOI] [PubMed] [Google Scholar]
- 3.Dubey D, Pittock SJ, Kelly CR, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83:166–177. doi: 10.1002/ana.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hébert J, Riche B, Vogrig A, et al (2020) Epidemiology of paraneoplastic neurologic syndromes and autoimmune encephalitides in France. Neurol Neuroimmunol Neuroinflammation 7:. 10.1212/NXI.0000000000000883 [DOI] [PMC free article] [PubMed]
- 5.Zuliani L, Zoccarato M. Open issues in antibody-mediated encephalitis. Future Neurol. 2020;15:2–5. doi: 10.2217/fnl-2019-0026. [DOI] [Google Scholar]
- 6.Flanagan EP, Geschwind MD, Lopez-Chiriboga AS, et al. Autoimmune Encephalitis Misdiagnosis in Adults. JAMA Neurol. 2023;80:30–39. doi: 10.1001/jamaneurol.2022.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corsellis JA, Goldberg GJ, Norton AR. “Limbic encephalitis” and its association with carcinoma. Brain. 1968;91:481–496. doi: 10.1093/brain/91.3.481. [DOI] [PubMed] [Google Scholar]
- 8.Markham M, Abeloff MD. Small-cell lung cancer and limbic encephalitis. Ann Intern Med. 1982;96:785. doi: 10.7326/0003-4819-96-6-785_1. [DOI] [PubMed] [Google Scholar]
- 9.Gultekin SH, Rosenfeld MR, Voltz R, et al (2000) Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain 123 ( Pt 7:1481–1494. 10.1093/brain/123.7.1481 [DOI] [PubMed]
- 10.Lai M, Huijbers MGM, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9:776–785. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain. 2010;133:2734–2748. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelley BP, Patel SC, Marin HL, et al. Autoimmune encephalitis: Pathophysiology and imaging review of an overlooked diagnosis. Am J Neuroradiol. 2017;38:1070–1078. doi: 10.3174/ajnr.A5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lancaster E, Dalmau J. Neuronal autoantigens-pathogenesis, associated disorders and antibody testing. Nat Rev Neurol. 2012;8:380–390. doi: 10.1038/nrneurol.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sechi E, Flanagan EP. Antibody-Mediated Autoimmune Diseases of the CNS: Challenges and Approaches to Diagnosis and Management. Front Neurol. 2021;12:1–18. doi: 10.3389/fneur.2021.673339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graus F, Keime-Guibert F, Reñe R, et al. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain. 2001;124:1138–1148. doi: 10.1093/brain/124.6.1138. [DOI] [PubMed] [Google Scholar]
- 16.Hermetter C, Fazekas F, Hochmeister S (2018) Systematic review: Syndromes, early diagnosis, and treatment in autoimmune encephalitis. Front Neurol 9:. 10.3389/fneur.2018.00706 [DOI] [PMC free article] [PubMed]
- 17.Graus F, Vogrig A, Muñiz-Castrillo S, et al (2021) Updated Diagnostic Criteria for Paraneoplastic Neurologic Syndromes. Neurol Neuroimmunol neuroinflammation 8:. 10.1212/NXI.0000000000001014 [DOI] [PMC free article] [PubMed]
- 18.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol - NIH. 2016;15:391–404. doi: 10.1016/S1474-4422(15)00401-9.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain. 2004;127:1831–1844. doi: 10.1093/brain/awh203. [DOI] [PubMed] [Google Scholar]
- 20.Sonderen A Van, Coenders EC, Sanchez E, et al (2016) Anti-LGI1 encephalitis 10.1212/WNL.0000000000003173
- 21.van Sonderen A, Ariño H, Petit-Pedrol M, et al. The clinical spectrum of Caspr2 antibody-associated disease. Neurology. 2016;87:521–528. doi: 10.1212/WNL.0000000000002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Höftberger R, van Sonderen A, Leypoldt F, et al. Encephalitis and AMPA receptor antibodies: Novel findings in a case series of 22 patients. Neurology. 2015;84:2403–2412. doi: 10.1212/WNL.0000000000001682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee W-J, Lee H-S, Kim D-Y, et al. Seronegative autoimmune encephalitis: clinical characteristics and factors associated with outcomes. Brain. 2022;145:3509–3521. doi: 10.1093/brain/awac166. [DOI] [PubMed] [Google Scholar]
- 24.Graus F, Escudero D, Oleaga L, et al. Syndrome and outcome of antibody-negative limbic encephalitis. Eur J Neurol. 2018;25:1011–1016. doi: 10.1111/ene.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalmau J, Graus F. Diagnostic criteria for autoimmune encephalitis: utility and pitfalls for antibody-negative disease. Lancet Neurol. 2023;22:529–540. doi: 10.1016/S1474-4422(23)00083-2. [DOI] [PubMed] [Google Scholar]
- 26.Abboud H, Probasco J, Irani SR, et al. Autoimmune encephalitis: proposed recommendations for symptomatic and long-term management. J Neurol Neurosurg Psychiatry. 2021;92:897–907. doi: 10.1136/jnnp-2020-325302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cacciaguerra L, Redenbaugh V, Chen JJ, et al. Timing and Predictors of T2-Lesion Resolution in Patients With Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease. Neurology. 2023;101:e1376–e1381. doi: 10.1212/WNL.0000000000207478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalmau J, Graus F, Rosenblum MK, Posner JB. Anti-Hu–associated paraneoplastic encephalomyelitis/sensory neuronopathy. A clinical study of 71 patients. Medicine (Baltimore) 1992;71:59–72. doi: 10.1097/00005792-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Gilmore CP, Elliott I, Auer D, Maddison P. Diffuse cerebellar MR imaging changes in anti-Yo positive paraneoplastic cerebellar degeneration. J Neurol. 2010;257:490–491. doi: 10.1007/s00415-009-5407-9. [DOI] [PubMed] [Google Scholar]
- 30.Venkatraman A, Opal P. Paraneoplastic cerebellar degeneration with anti-Yo antibodies - a review. Ann Clin Transl Neurol. 2016;3:655–663. doi: 10.1002/acn3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dericioglu N, Gocmen R, Tan E. Paraneoplastic striatal encephalitis and myelitis associated with anti-CV2/CRMP-5 antibodies in a patient with small cell lung cancer. Clin Neurol Neurosurg. 2018;170:117–119. doi: 10.1016/j.clineuro.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Psimaras D, Carpentier AF, Rossi C. Cerebrospinal fluid study in paraneoplastic syndromes. J Neurol Neurosurg Psychiatry. 2010;81:42–45. doi: 10.1136/jnnp.2008.159483. [DOI] [PubMed] [Google Scholar]
- 33.Honnorat J, Plazat LO. Autoimmune encephalitis and psychiatric disorders. Rev Neurol (Paris) 2018;174:228–236. doi: 10.1016/j.neurol.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Neto ADP, de Aquino BCV, de Morais Brito PS, et al. ANTI-MA2 encephalitis mimicking diencephalic demyelinating syndrome. Interdiscip Neurosurg. 2021;23:100980 . doi: 10.1016/j.inat.2020.100980. [DOI] [Google Scholar]
- 35.119. Dade M, Berzero G, Izquierdo C, et al (2020) Neurological Syndromes Associated with Anti-GAD Antibodies. Int J Mol Sci 21:. 10.3390/ijms21103701 [DOI] [PMC free article] [PubMed]
- 36.Conde-Blanco E, Pascual-Diaz S, Carreño M, et al. Volumetric and shape analysis of the hippocampus in temporal lobe epilepsy with GAD65 antibodies compared with non-immune epilepsy. Sci Rep. 2021;11:10199. doi: 10.1038/s41598-021-89010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simard C, Vogrig A, Joubert B, et al (2020) Clinical spectrum and diagnostic pitfalls of neurologic syndromes with Ri antibodies. Neurol Neuroimmunol neuroinflammation 7:. 10.1212/NXI.0000000000000699 [DOI] [PMC free article] [PubMed]
- 38.Pittock SJ, Lucchinetti CF, Parisi JE, et al. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann Neurol. 2005;58:96–107. doi: 10.1002/ana.20529. [DOI] [PubMed] [Google Scholar]
- 39.Gadoth A, Kryzer TJ, Fryer J, et al. Microtubule-associated protein 1B: Novel paraneoplastic biomarker. Ann Neurol. 2017;81:266–277. doi: 10.1002/ana.24872. [DOI] [PubMed] [Google Scholar]
- 40.Sun X, Tan J, Sun H, et al. Anti-SOX1 Antibodies in Paraneoplastic Neurological Syndrome. J Clin Neurol. 2020;16:530–546. doi: 10.3988/jcn.2020.16.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stich O, Klages E, Bischler P, et al. SOX1 antibodies in sera from patients with paraneoplastic neurological syndromes. Acta Neurol Scand. 2012;125:326–331. doi: 10.1111/j.1600-0404.2011.01572.x. [DOI] [PubMed] [Google Scholar]
- 42.Vabanesi M, Pinto A-L, Vogrig A, et al. SOX1 antibody-related paraneoplastic neurological syndromes: clinical correlates and assessment of laboratory diagnostic techniques. J Neurol. 2023;270:1691–1701. doi: 10.1007/s00415-022-11523-y. [DOI] [PubMed] [Google Scholar]
- 43.Mandel-Brehm C, Dubey D, Kryzer TJ, et al. Kelch-like Protein 11 Antibodies in Seminoma-Associated Paraneoplastic Encephalitis. N Engl J Med. 2019;381:47–54. doi: 10.1056/NEJMoa1816721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogrig A, Péricart S, Pinto A-L, et al. Immunopathogenesis and proposed clinical score for identifying Kelch-like protein-11 encephalitis. Brain Commun. 2021;3:fcab185. doi: 10.1093/braincomms/fcab185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basal E, Zalewski N, Kryzer TJ, et al. Paraneoplastic neuronal intermediate filament autoimmunity. Neurology. 2018;91:e1677–e1689. doi: 10.1212/WNL.0000000000006435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zekeridou A, Kryzer T, Guo Y, et al. Phosphodiesterase 10A IgG: A novel biomarker of paraneoplastic neurologic autoimmunity. Neurology. 2019;93:e815–e822. doi: 10.1212/WNL.0000000000007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zekeridou A, Yang B, Lennon VA, et al. Anti-Neuronal Nuclear Antibody 3 Autoimmunity Targets Dachshund Homolog 1. Ann Neurol. 2022;91:670–675. doi: 10.1002/ana.26320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernal F, Shams’ili S, Rojas I, , et al. Anti-Tr antibodies as markers of paraneoplastic cerebellar degeneration and Hodgkin’s disease. Neurology. 2003;60:230–234. doi: 10.1212/01.wnl.0000041495.87539.98. [DOI] [PubMed] [Google Scholar]
- 49.van Coevorden-Hameete MH, van Beuningen SFB, Perrenoud M, et al. Antibodies to TRIM46 are associated with paraneoplastic neurological syndromes. Ann Clin Transl Neurol. 2017;4:680–686. doi: 10.1002/acn3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valencia-Sanchez C, Knight AM, Hammami MB, et al. Characterisation of TRIM46 autoantibody-associated paraneoplastic neurological syndrome. J Neurol Neurosurg Psychiatry. 2022;93:196–200. doi: 10.1136/jnnp-2021-326656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Honorat JA, Lopez-Chiriboga AS, Kryzer TJ, et al. Autoimmune gait disturbance accompanying adaptor protein-3B2-IgG. Neurology. 2019;93:e954–e963. doi: 10.1212/WNL.0000000000008061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miske R, Scharf M, Stark P, et al (2021) Autoantibodies Against the Purkinje Cell Protein RGS8 in Paraneoplastic Cerebellar Syndrome. Neurol Neuroimmunol neuroinflammation 8:. 10.1212/NXI.0000000000000987 [DOI] [PMC free article] [PubMed]
- 53.Muñiz-Castrillo S, Hedou JJ, Ambati A, et al. Distinctive clinical presentation and pathogenic specificities of anti-AK5 encephalitis. Brain. 2021;144:2709–2721. doi: 10.1093/brain/awab153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Do L-D, Moritz CP, Muñiz-Castrillo S, et al (2021) Argonaute Autoantibodies as Biomarkers in Autoimmune Neurologic Diseases. Neurol Neuroimmunol neuroinflammation 8:. 10.1212/NXI.0000000000001032 [DOI] [PMC free article] [PubMed]
- 55.Flanagan EP, Hinson SR, Lennon VA, et al. Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: Analysis of 102 patients. Ann Neurol. 2017;81:298–309. doi: 10.1002/ana.24881. [DOI] [PubMed] [Google Scholar]
- 56.Bataller L, Wade DF, Graus F, et al. Antibodies to Zic4 in paraneoplastic neurologic disorders and small-cell lung cancer. Neurology. 2004;62:778–782. doi: 10.1212/01.wnl.0000113749.77217.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pozas J, Albarrán-Fernández V, González-Campo L, et al. Anti-Zic4 paraneoplastic cerebellar degeneration in a patient with EGFR-mutated NSCLC: a case report. Transl lung cancer Res. 2022;11:1497–1502. doi: 10.21037/tlcr-21-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hinson SR, Honorat JA, Grund EM, et al. Septin-5 and -7-IgGs: Neurologic, Serologic, and Pathophysiologic Characteristics. Ann Neurol. 2022;92:1090–1101. doi: 10.1002/ana.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13:167–177. doi: 10.1016/S1474-4422(13)70282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al-Diwani A, Handel A, Townsend L, et al. The psychopathology of NMDAR-antibody encephalitis in adults: a systematic review and phenotypic analysis of individual patient data. The lancet Psychiatry. 2019;6:235–246. doi: 10.1016/S2215-0366(19)30001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ariño H, Armangué T, Petit-Pedrol M, et al. Anti-LGI1-associated cognitive impairment: Presentation and long-term outcome. Neurology. 2016;87:759–765. doi: 10.1212/WNL.0000000000003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Irani SR, Pettingill P, Kleopa KA, et al. Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol. 2012;72:241–255. doi: 10.1002/ana.23577. [DOI] [PubMed] [Google Scholar]
- 65.Petit-Pedrol M, Armangue T, Peng X, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014;13:276–286. doi: 10.1016/S1474-4422(13)70299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spatola M, Petit-Pedrol M, Simabukuro MM, et al. Investigations in GABA(A) receptor antibody-associated encephalitis. Neurology. 2017;88:1012–1020. doi: 10.1212/WNL.0000000000003713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deng B, Cai M, Qiu Y, et al (2022) MRI Characteristics of Autoimmune Encephalitis With Autoantibodies to GABAA Receptor: A Case Series. Neurol Neuroimmunol neuroinflammation 9:. 10.1212/NXI.0000000000001158 [DOI] [PMC free article] [PubMed]
- 68.Höftberger R, Titulaer MJ, Sabater L, et al. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology. 2013;81:1500–1506. doi: 10.1212/WNL.0b013e3182a9585f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9:67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laurido-Soto O, Brier MR, Simon LE, et al. Patient characteristics and outcome associations in AMPA receptor encephalitis. J Neurol. 2019;266:450–460. doi: 10.1007/s00415-018-9153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gresa-Arribas N, Planagumà J, Petit-Pedrol M, et al. Human neurexin-3α antibodies associate with encephalitis and alter synapse development. Neurology. 2016;86:2235–2242. doi: 10.1212/WNL.0000000000002775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sabater L, Gaig C, Gelpi E, et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014;13:575–586. doi: 10.1016/S1474-4422(14)70051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gaig C, Graus F, Compta Y, et al. Clinical manifestations of the anti-IgLON5 disease. Neurology. 2017;88:1736–1743. doi: 10.1212/WNL.0000000000003887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hara M, Ariño H, Petit-Pedrol M, et al. DPPX antibody-associated encephalitis: Main syndrome and antibody effects. Neurology. 2017;88:1340–1348. doi: 10.1212/WNL.0000000000003796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boronat A, Gelfand JM, Gresa-Arribas N, et al. Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv4.2 potassium channels. Ann Neurol. 2013;73:120–128. doi: 10.1002/ana.23756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Piepgras J, Höltje M, Michel K, et al. Anti-DPPX encephalitis: pathogenic effects of antibodies on gut and brain neurons. Neurology. 2015;85:890–897. doi: 10.1212/WNL.0000000000001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dale RC, Merheb V, Pillai S, et al. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain. 2012;135:3453–3468. doi: 10.1093/brain/aws256. [DOI] [PubMed] [Google Scholar]
- 78.Spatola M, Sabater L, Planagumà J, et al. Encephalitis with mGluR5 antibodies: Symptoms and antibody effects. Neurology. 2018;90:e1964–e1972. doi: 10.1212/WNL.0000000000005614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spatola M, Petit Pedrol M, Maudes E, et al. Clinical features, prognostic factors, and antibody effects in anti-mGluR1 encephalitis. Neurology. 2020;95:e3012–e3025. doi: 10.1212/WNL.0000000000010854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Landa J, Guasp M, Míguez-Cabello F, et al. Encephalitis with Autoantibodies against the Glutamate Kainate Receptors GluK2. Ann Neurol. 2021;90:101–117. doi: 10.1002/ana.26098. [DOI] [PubMed] [Google Scholar]
- 81.Landa J, Guasp M, Petit-Pedrol M, et al (2021) Seizure-related 6 homolog like 2 autoimmunity: Neurologic syndrome and antibody effects. Neurol Neuroimmunol neuroinflammation 8:. 10.1212/NXI.0000000000000916 [DOI] [PMC free article] [PubMed]
- 82.Brierly JB, Corsellis JAN, Hierons R, Nevin S. Subacute encephalitis of later adult life mainly affecting the limbic areas. Brain. 1960;83:357–368. doi: 10.1093/brain/83.3.357. [DOI] [Google Scholar]
- 83.Kohler J, Hufschmidt A, Hermle L, et al. Limbic encephalitis: two cases. J Neuroimmunol. 1988;20:177–178. doi: 10.1016/0165-5728(88)90157-9. [DOI] [PubMed] [Google Scholar]
- 84.Dalmau J, Rosenfeld MR. Autoimmune encephalitis update. Neuro Oncol. 2014;16:771–778. doi: 10.1093/neuonc/nou030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Da Rocha AJ, Nunes RH, Maia ACM, Do Amaral LLF. Recognizing autoimmune-mediated encephalitis in the differential diagnosis of limbic disorders. Am J Neuroradiol. 2015;36:2196–2205. doi: 10.3174/ajnr.A4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gadoth A, Pittock SJ, Dubey D, et al. Expanded phenotypes and outcomes among 256 LGI1/CASPR2-IgG-positive patients. Ann Neurol. 2017;82:79–92. doi: 10.1002/ana.24979. [DOI] [PubMed] [Google Scholar]
- 87.Finke C, Prüss H, Heine J, et al. Evaluation of cognitive deficits and structural hippocampal damage in encephalitis with leucine-rich, glioma-inactivated 1 antibodies. JAMA Neurol. 2017;74:50–59. doi: 10.1001/jamaneurol.2016.4226. [DOI] [PubMed] [Google Scholar]
- 88.van Sonderen A, Thijs RD, Coenders EC, et al. Anti-LGI1 encephalitis: Clinical syndrome and long-term follow-up. Neurology. 2016;87:1449–1456. doi: 10.1212/WNL.0000000000003173. [DOI] [PubMed] [Google Scholar]
- 89.Ghimire P, Khanal UP, Gajurel BP, et al (2020) Anti-LGI1, anti-GABABR, and Anti-CASPR2 encephalitides in Asia: A systematic review. Brain Behav 10:. 10.1002/brb3.1793 [DOI] [PMC free article] [PubMed]
- 90.Binks SNM, Klein CJ, Waters P, et al. LGI1, CASPR2 and related antibodies: a molecular evolution of the phenotypes. J Neurol Neurosurg Psychiatry. 2018;89:526–534. doi: 10.1136/jnnp-2017-315720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu F, Shan W, Lv R, et al. Clinical Characteristics of Anti-GABA-B Receptor Encephalitis. Front Neurol. 2020;11:403. doi: 10.3389/fneur.2020.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gagnon M-M, Savard M. Limbic Encephalitis Associated With GAD65 Antibodies: Brief Review of the Relevant literature. Can J Neurol Sci Le J Can des Sci Neurol. 2016;43:486–493. doi: 10.1017/cjn.2016.13. [DOI] [PubMed] [Google Scholar]
- 93.Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133:1655–1667. doi: 10.1093/brain/awq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Florance NR, Davis RL, Lam C, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66:11–18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Urbach H, Soeder BM, Jeub M, et al. Serial MRI of limbic encephalitis. Neuroradiology. 2006;48:380–386. doi: 10.1007/s00234-006-0069-0. [DOI] [PubMed] [Google Scholar]
- 96.Wagner J, Witt J-A, Helmstaedter C, et al. Automated volumetry of the mesiotemporal structures in antibody-associated limbic encephalitis. J Neurol Neurosurg Psychiatry. 2015;86:735–742. doi: 10.1136/jnnp-2014-307875. [DOI] [PubMed] [Google Scholar]
- 97.Guerin J, Watson RE, Carr CM, et al (2019) Autoimmune epilepsy: Findings on MRi and FDG-Pet. Br J Radiol 92:. 10.1259/bjr.20170869 [DOI] [PMC free article] [PubMed]
- 98.Kotsenas AL, Watson RE, Pittock SJ, et al. MRI findings in autoimmune voltage-gated potassium channel complex encephalitis with seizures: one potential etiology for mesial temporal sclerosis. AJNR Am J Neuroradiol. 2014;35:84–89. doi: 10.3174/ajnr.A3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ernst L, David B, Gaubatz J, et al. Volumetry of Mesiotemporal Structures Reflects Serostatus in Patients with Limbic Encephalitis. AJNR Am J Neuroradiol. 2019;40:2081–2089. doi: 10.3174/ajnr.A6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oyanguren B, Sánchez V, González FJ, et al. Limbic encephalitis: a clinical-radiological comparison between herpetic and autoimmune etiologies. Eur J Neurol. 2013;20:1566–1570. doi: 10.1111/ene.12249. [DOI] [PubMed] [Google Scholar]
- 101.Schievelkamp A-H, Jurcoane A, Rüber T, et al. Limbic Encephalitis in Patients with Epilepsy-is Quantitative MRI Diagnostic? Clin Neuroradiol. 2019;29:623–630. doi: 10.1007/s00062-018-0705-1. [DOI] [PubMed] [Google Scholar]
- 102.Wagner J, Schoene-Bake J-C, Malter MP, et al. Quantitative FLAIR analysis indicates predominant affection of the amygdala in antibody-associated limbic encephalitis. Epilepsia. 2013;54:1679–1687. doi: 10.1111/epi.12320. [DOI] [PubMed] [Google Scholar]
- 103.Shao X, Fan S, Luo H, et al. Brain Magnetic Resonance Imaging Characteristics of Anti-Leucine-Rich Glioma-Inactivated 1 Encephalitis and Their Clinical Relevance: A Single-Center Study in China. Front Neurol. 2021;11:1–10. doi: 10.3389/fneur.2020.618109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gillon S, Chan M, Chen J, et al. MR Imaging Findings in a Large Population of Autoimmune Encephalitis. AJNR Am J Neuroradiol. 2023;44:799–806. doi: 10.3174/ajnr.A7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ball C, Fisicaro R, Morris L, 3rd, et al. Brain on fire: an imaging-based review of autoimmune encephalitis. Clin Imaging. 2022;84:1–30. doi: 10.1016/j.clinimag.2021.12.011. [DOI] [PubMed] [Google Scholar]
- 106.Renard D, Nerrant E, Lechiche C. DWI and FLAIR imaging in herpes simplex encephalitis: a comparative and topographical analysis. J Neurol. 2015;262:2101–2105. doi: 10.1007/s00415-015-7818-0. [DOI] [PubMed] [Google Scholar]
- 107.Heine J, Prüss H, Bartsch T, et al. Imaging of autoimmune encephalitis - Relevance for clinical practice and hippocampal function. Neuroscience. 2015;309:68–83. doi: 10.1016/j.neuroscience.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 108.Elkhider H, Sharma R, Kapoor N, et al. Autoimmune encephalitis and seizures, cerebrospinal fluid, imaging, and EEG findings: a case series. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2022;43:2669–2680. doi: 10.1007/s10072-021-05617-0. [DOI] [PubMed] [Google Scholar]
- 109.Baumgartner A, Rauer S, Mader I, Meyer PT. Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J Neurol. 2013;260:2744–2753. doi: 10.1007/s00415-013-7048-2. [DOI] [PubMed] [Google Scholar]
- 110.Sener RN. MRI and diffusion MRI in nonparaneoplastic limbic encephalitis. Comput Med imaging Graph Off J Comput Med Imaging Soc. 2002;26:339–342. doi: 10.1016/s0895-6111(02)00022-8. [DOI] [PubMed] [Google Scholar]
- 111.Budhram A, Britton JW, Liebo GB, et al. Use of diffusion-weighted imaging to distinguish seizure-related change from limbic encephalitis. J Neurol. 2020;267:3337–3342. doi: 10.1007/s00415-020-10007-1. [DOI] [PubMed] [Google Scholar]
- 112.Malmgren K, Thom M. Hippocampal sclerosis–origins and imaging. Epilepsia. 2012;53(Suppl 4):19–33. doi: 10.1111/j.1528-1167.2012.03610.x. [DOI] [PubMed] [Google Scholar]
- 113.Liu Z, Mikati M, Holmes GL. Mesial temporal sclerosis: pathogenesis and significance. Pediatr Neurol. 1995;12:5–16. doi: 10.1016/0887-8994(94)00122-i. [DOI] [PubMed] [Google Scholar]
- 114.Popkirov S, Ismail FS, Grönheit W, et al. Progressive hippocampal sclerosis after viral encephalitis: Potential role of NMDA receptor antibodies. Seizure. 2017;51:6–8. doi: 10.1016/j.seizure.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 115.Nóbrega-Jr AW, Gregory CP, Schlindwein-Zanini R, et al. Mesial temporal lobe epilepsy with hippocampal sclerosis is infrequently associated with neuronal autoantibodies. Epilepsia. 2018;59:e152–e156. doi: 10.1111/epi.14534. [DOI] [PubMed] [Google Scholar]
- 116.Bordonne M, Chawki MB, Doyen M, et al. Brain (18)F-FDG PET for the diagnosis of autoimmune encephalitis: a systematic review and a meta-analysis. Eur J Nucl Med Mol Imaging. 2021;48:3847–3858. doi: 10.1007/s00259-021-05299-y. [DOI] [PubMed] [Google Scholar]
- 117.Zhang T, Duan Y, Ye J, et al. Brain MRI Characteristics of Patients with Anti-N-Methyl-D-Aspartate Receptor Encephalitis and Their Associations with 2-Year Clinical Outcome. AJNR Am J Neuroradiol. 2018;39:824–829. doi: 10.3174/ajnr.A5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ogawa R, Nakashima I, Takahashi T, et al. MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol Neuroinflammation. 2017;4:e322 . doi: 10.1212/NXI.0000000000000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fujimori J, Takai Y, Nakashima I, et al. Bilateral frontal cortex encephalitis and paraparesis in a patient with anti-MOG antibodies. J Neurol Neurosurg Psychiatry. 2017;88:534–536. doi: 10.1136/jnnp-2016-315094. [DOI] [PubMed] [Google Scholar]
- 120.Fang B, McKeon A, Hinson SR, et al. Autoimmune glial fibrillary acidic protein astrocytopathy: A novel meningoencephalomyelitis. JAMA Neurol. 2016;73:1297–1307. doi: 10.1001/jamaneurol.2016.2549. [DOI] [PubMed] [Google Scholar]
- 121.Yu Z, Kryzer TJ, Griesmann GE, et al. CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunity. Ann Neurol. 2001;49:146–154. doi: 10.1002/1531-8249(20010201)49:2<146::AID-ANA34>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 122.Sachs JR, Zapadka ME, Popli GS, Burdette JH. Arterial spin labeling perfusion imaging demonstrates cerebral hyperperfusion in anti-NMDAR encephalitis. Radiol Case Reports. 2017;12:833–837. doi: 10.1016/j.radcr.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gorman MP, Gombolay GY, Mehan WAJ, Thibert RL. Case 27–2018: A 3-Year-Old Boy with Seizures. N Engl J Med. 2018;379:870–878. doi: 10.1056/NEJMcpc1802824. [DOI] [PubMed] [Google Scholar]
- 124.Flanagan EP, Kotsenas AL, Britton JW, et al (2015) Basal ganglia T1 hyperintensity in LGI1-autoantibody faciobrachial dystonic Seizures. Neurol Neuroimmunol NeuroInflammation 2:. 10.1212/NXI.0000000000000161 [DOI] [PMC free article] [PubMed]
- 125.Saiz A, Bruna J, Stourac P, et al. Anti-Hu-associated brainstem encephalitis. J Neurol Neurosurg Psychiatry. 2009;80:404–407. doi: 10.1136/jnnp.2008.158246. [DOI] [PubMed] [Google Scholar]
- 126.Dubey D, Wilson MR, Clarkson B, et al. Expanded Clinical Phenotype, Oncological Associations, and Immunopathologic Insights of Paraneoplastic Kelch-like Protein-11 Encephalitis. JAMA Neurol. 2020;77:1420–1429. doi: 10.1001/jamaneurol.2020.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vitaliani R, Mason W, Ances B, et al. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58:594–604. doi: 10.1002/ana.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maqbool M, Oleske DA, Huq AHM, et al. Novel FDG-PET findings in anti-NMDA receptor encephalitis: a case based report. J Child Neurol. 2011;26:1325–1328. doi: 10.1177/0883073811405199. [DOI] [PubMed] [Google Scholar]
- 129.Hacohen Y, Deiva K, Pettingill P, et al. N-methyl-D-aspartate receptor antibodies in post-herpes simplex virus encephalitis neurological relapse. Mov Disord. 2014;29:90–96. doi: 10.1002/mds.25626. [DOI] [PubMed] [Google Scholar]
- 130.Chow FC, Glaser CA, Sheriff H, et al. Use of clinical and neuroimaging characteristics to distinguish temporal lobe herpes simplex encephalitis from its mimics. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2015;60:1377–1383. doi: 10.1093/cid/civ051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Armangue T, Leypoldt F, Dalmau J. Autoimmune encephalitis as differential diagnosis of infectious encephalitis. Curr Opin Neurol. 2014;27:361–368. doi: 10.1097/WCO.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huang CN, Tian XB, Jiang SM, et al. Comparisons between infectious and autoimmune encephalitis: Clinical signs, biochemistry, blood counts, and imaging findings. Neuropsychiatr Dis Treat. 2020;16:2649–2660. doi: 10.2147/NDT.S274487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Venkatesan A, Jagdish B. Imaging in Encephalitis. Semin Neurol. 2019;39:312–321. doi: 10.1055/s-0039-1687838. [DOI] [PubMed] [Google Scholar]
- 134.Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17:760–772. doi: 10.1016/S1474-4422(18)30244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nissen MS, Ørvik MS, Nilsson AC, et al. NMDA-receptor encephalitis in Denmark from 2009 to 2019: a national cohort study. J Neurol. 2022;269:1618–1630. doi: 10.1007/s00415-021-10738-9. [DOI] [PubMed] [Google Scholar]
- 136.Laurent C, Capron J, Quillerou B, et al. Steroid-responsive encephalopathy associated with autoimmune thyroiditis (SREAT): Characteristics, treatment and outcome in 251 cases from the literature. Autoimmun Rev. 2016;15:1129–1133. doi: 10.1016/j.autrev.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 137.Mattozzi S, Sabater L, Escudero D, et al. Hashimoto encephalopathy in the 21st century. Neurology. 2020;94:e217–e224. doi: 10.1212/WNL.0000000000008785. [DOI] [PubMed] [Google Scholar]
- 138.Bozluolcay M, Elmali AD, Menku SF, et al. Magnetic resonance imaging findings in probable Creutzfeld-Jacob disease: comparison with electroencephalography and cerebrospinal fluid characteristics. Acta Radiol short reports. 2014;3:2047981614552218. doi: 10.1177/2047981614552218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jubelt B, Mihai C, Li TM, Veerapaneni P. Rhombencephalitis / brainstem encephalitis. Curr Neurol Neurosci Rep. 2011;11:543–552. doi: 10.1007/s11910-011-0228-5. [DOI] [PubMed] [Google Scholar]
- 140.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Saleem SN, Said A-HM, Lee DH (2007) Lesions of the hypothalamus: MR imaging diagnostic features. Radiogr a Rev Publ Radiol Soc North Am Inc 27:1087–1108. 10.1148/rg.274065123 [DOI] [PubMed]
- 142.Costa A, Silva C, Taipa R, et al. Teaching NeuroImage: Perivascular Radial Enhancement in Neurosarcoidosis. Neurology. 2023;101:e1948–e1949. doi: 10.1212/WNL.0000000000207830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bathla G, Watal P, Gupta S, et al. Cerebrovascular manifestations in neurosarcoidosis: how common are they and does perivascular enhancement matter? Clin Radiol. 2018;73:907.e15–907.e23. doi: 10.1016/j.crad.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 144.Tateishi U, Terae S, Ogata A, et al. MR imaging of the brain in lymphomatoid granulomatosis. AJNR Am J Neuroradiol. 2001;22:1283–1290. [PMC free article] [PubMed] [Google Scholar]
- 145.Efe IE, Aydin OU, Alabulut A, et al. COVID-19-Associated Encephalitis Mimicking Glial Tumor. World Neurosurg. 2020;140:46–48. doi: 10.1016/j.wneu.2020.05.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stoian A, Stoian M, Bajko Z, et al (2022) Autoimmune Encephalitis in COVID-19 Infection: Our Experience and Systematic Review of the Literature. Biomedicines 10:. 10.3390/biomedicines10040774 [DOI] [PMC free article] [PubMed]
- 147.Vogrig A, Joubert B, Ducray F, et al. Glioblastoma as differential diagnosis of autoimmune encephalitis. J Neurol. 2018;265:669–677. doi: 10.1007/s00415-018-8767-1. [DOI] [PubMed] [Google Scholar]
- 148.Van Steenhoven RW, de Vries JM, Bruijstens AL, et al (2023) Mimics of Autoimmune Encephalitis: Validation of the 2016 Clinical Autoimmune Encephalitis Criteria. Neurol Neuroimmunol neuroinflammation 10:. 10.1212/NXI.0000000000200148 [DOI] [PMC free article] [PubMed]
- 149.Abboud H, Probasco JC, Irani S, et al. Autoimmune encephalitis: proposed best practice recommendations for diagnosis and acute management. J Neurol Neurosurg Psychiatry. 2021;92:757–768. doi: 10.1136/jnnp-2020-325300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Britton PN, Eastwood K, Paterson B, et al. Consensus guidelines for the investigation and management of encephalitis in adults and children in Australia and New Zealand. Intern Med J. 2015;45:563–576. doi: 10.1111/imj.12749. [DOI] [PubMed] [Google Scholar]
- 151.Suh CH, Jung SC, Kim KW, Pyo J. The detectability of brain metastases using contrast-enhanced spin-echo or gradient-echo images: a systematic review and meta-analysis. J Neurooncol. 2016;129:363–371. doi: 10.1007/s11060-016-2185-y. [DOI] [PubMed] [Google Scholar]
- 152.Kaufmann TJ, Smits M, Boxerman J, et al. Consensus recommendations for a standardized brain tumor imaging protocol for clinical trials in brain metastases. Neuro Oncol. 2020;22:757–772. doi: 10.1093/neuonc/noaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Solomon T, Michael BD, Smith PE, et al. Management of suspected viral encephalitis in adults–Association of British Neurologists and British Infection Association National Guidelines. J Infect. 2012;64:347–373. doi: 10.1016/j.jinf.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 154.Morbelli S, Zoccarato M, Bauckneht M, et al. 18F-FDG-PET and MRI in autoimmune encephalitis: a systematic review of brain findings. Clin Transl Imaging. 2018;6:151–168. doi: 10.1007/s40336-018-0275-x. [DOI] [Google Scholar]
- 155.Titulaer MJ, Soffietti R, Dalmau J, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur J Neurol. 2011;18:19–e3. doi: 10.1111/j.1468-1331.2010.03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mathew RM, Vandenberghe R, Garcia-Merino A, et al. Orchiectomy for suspected microscopic tumor in patients with anti-Ma2-associated encephalitis. Neurology. 2007;68:900–905. doi: 10.1212/01.wnl.0000252379.81933.80. [DOI] [PMC free article] [PubMed] [Google Scholar]