Abstract
In this investigation, cellulose-degrading fungi and bacteria were isolated from different partially decomposed cellulose-rich substrates, such as groundnut residues, rice straw, and rotten wood, following dilution plating techniques on carboxymethyl cellulose agar media and screening for potential cellulose degradation ability. The development of a clear halo zone surrounding the microbial colonies during the initial screening process using the Congo red test (20 isolates) suggested cellulose hydrolysis, and the highest cellulase production activity was implied by the isolates with the largest clear zone ratio (9 isolates). Using both macroscopic and microscopic examinations, as well as standard biochemical tests outlined in Bergey’s Manual of Determinative Bacteriology, the genus-level identification of fungi and bacteria was accomplished. In order to molecularly identify the 4 isolated fungal and bacterial strains at the species level after being ultimately selected for cellulase production potential under in vitro studies, fungal and bacterial DNA was extracted and amplified by PCR using the universal primers ITS1 and ITS4 for fungi (ITS rRNA, 5.8S rRNA) and 8F and 1492R for bacterial isolates (16S rRNA). After sequencing, the PCR results were compared to other comparable sequences in GenBank (NCBI). Based on the available NCBI data, phylogenetic analysis of their ribosomal gene partial sequences revealed that DAJ2 (PP086700) shares 100% homology with Aspergillus foetidus, DTJ4 (PP086699) shares 99.74% similarity with Trichoderma atrobrunnium, DBJ6 (PP082584) shares 100% identity with Priestia megaterium, and DMB9 (PP082585) shares 99.88% homology with Micrococcus yunnanensis. The cellulolytic potential of Phanerochaete chrysosporium is well established. Therefore, it was considered a standard culture for comparison and was collected from the MTCC, Chandigarh, India. Overall, all 4 selected isolates and the check organism were mutually compatible or synergistic with each other, and their consortium is useful for the accelerated decomposition of organic constituents during rapid composting.
Keywords: Cellulose degradation, Consortium, In vitro, Isolation, Microbes, Sequencing
Introduction
The most prevalent agricultural waste and source of biomass on the terrain is cellulose (Tomme et al. 1995). It is composed of glucose units linked together in a linear polymerase chain by β–1,4 glycosidic bonds (Heck et al. 2002). The photosynthetic process results in the production of cellulose waste, which is a significant renewable bioresource (Jarvis 2003; Zhang and Lynd 2004). Approximately 1011 tons of dry plant matter are produced annually by the fixation of CO2 through photosynthetic processes; cellulose accounts for more than half of this substance (Eriksson et al. 1990). By using these resources, new biomaterials such as fuels, chemicals, fertilizers, animal feeds, and soil conditioners can be produced (Sims et al. 2010; Gong et al. 2020; Agrawal et al. 2021). However, inadequate use or poor management of these biomasses pollutes the Earth’s surface, endangering human health in a number of ways (Du et al. 2015). Plant lignocellulosic material is efficiently degraded by the enzyme or multienzyme complex involved in the hydrolysis of cellulose, known as cellulase (Lynd et al. 2002; Jahangeer et al. 2005). Cellulolytic fungi and bacteria also play a significant role in the biodegradation process in nature because they are able to break down cellulose by producing cellulase enzymes (Li et al. 2023; Datta 2024). Bacteria use these cellulosic substrates as a source of energy by reducing cellulose, which leads to the synthesis of monomeric carbohydrates, antibiotics, and single-cell proteins, although they do not have enough cellulase production compared with fungi. Despite bacteria having a higher rate of multiplication, there has been more attention and investigations focused on cellulolytic fungal species than on bacterial cellulose-degrading species. In most cases, bacterial cellulases function as powerful and extremely effective catalysts (Gautam et al. 2010). Multiple enzymes are secreted by soil-isolated fungi and bacteria that breakdown lignocellulosic biomass (Bruce et al. 2010; Walters et al. 2022). To hydrolyze cellulosic biomass, three different types of enzymes operate sequentially: cellobio-hydrolase or exoglucanases, the endoglucanase carboxymethyl cellulase (CMCase), and β-glucosidases (cellobiases) (Bhat 2000). These enzymes are frequently produced by a number of bacterial genera, including Cellulomonas sp., Pseudomonas sp. (Nakamura and Kppamura 1982), Bacillus sp. and Micrococcus sp. (Hussain et al. 2017), as well as fungi (Shin et al. 2000), which are now extensively used in industrial applications. Maximum endoglucanase activity was detected in Cellulomonas sp., Bacillus sp., and Micrococcus sp. at 40 °C and neutral pH, according to Immanuel et al. (2006). As a result of their capacity to break down cellulose, several bacterial and fungal species have been studied extensively to learn more about their physico-biochemical characteristics and potential use in biotechnological processes (Petre et al. 1999). One of the most diverse types of microorganisms is the fungus, which lives in a variety of environments, including soil, water, food sources, and plant components such as leaves, roots, and fruits (Rebecca et al. 2012; Sartori et al. 2013). Although the exact quantity of fungi involved is still unknown, only 5–13% of the total fungal species assessed globally have been characterized. As a result, it is still crucial to isolate and identify fungal strains from various environmental sources to view and recognize more species, providing strains for ecological remediation, biological control, industrial applications, scientific classification editing, and assessing their effects in nature (Blackwell 2011). The level of enzymes generated by filamentous fungi is greater than that generated by yeast and bacteria, making them the preferred choice for commercial enzyme synthesis (Bakri et al. 2003). It is widely known that Aspergillus sp. and Trichoderma sp. effectively produce cellulases (van Peij et al. 1998; Bhat 2000; Lynd et al. 2002), and they are the most prevalent cellulolytic fungi found in composting materials (Ashraf et al. 2007). The Aspergillus genus has the ability to dominate the enzyme industry because practically all of its members produce cellulase. Four hypercellulolytic fungal cultures, Trichoderma viride, Aspergillus nidulans, Aspergillus awamori, and Phanerochaete chrysosporium, were combined to decompose paddy straw in perforated pits at IARI, New Delhi (Roy et al. 2022). Although a wide variety of microorganisms are able to break down the components of plant cell walls, only a few of them produce considerable amounts of cell-free hydrolases that can effectively breakdown complicated lignocelluloses in vitro. Phanerochaete chrysosporium secretes two distinct enzymes—cell-bound enzymes and extracellular enzymes (Wood and McCrae 1978; Bhat and Bhat 1997)—and is considered one of the most significant and widely used fungi in composting (Varma et al. 2015). Phanerochaete chrysosporium and Trichoderma reesei can increase temperatures during composting and promote cellulose decomposition during composting (Irigoyen et al. 2011).
As cellulose has a high degree of crystallinity and a complicated structure (Kaur et al. 2021), the rate-limiting step in the creation of compost is its breakdown (Agrawal et al. 2018). In general, single microbial strains are typically not very successful at breaking down cellulose, and inducing genes to produce more cellulase is not a cost-effective method of doing so. However, microbial consortia composed of several different microbial strains frequently exhibit stronger cellulase activity than single strains and are therefore garnering increasing interest (Zhang and Dong 2022). According to Jiang et al. (2021), the cellulose-degrading efficiency of a five-member fungal consortium was noticeably greater than that of each member acting alone. Nevertheless, the cellulose-degrading efficiency of a consortium could be worse than that of its individual members if it consists of competing or rival members (Boddy 2000). Therefore, this study aimed to screen and identify potential cellulolytic fungal and bacterial strains isolated from partially decomposed cellulose-rich compounds under in vitro conditions to develop an efficient consortium followed by a compatibility test for the rapid decomposition of organic materials during composting.
Materials and methods
Isolation and screening of efficient cellulose degrading fungal and bacterial strains
Sample collection, serial dilutions and spread plating methods
Two categories of inoculants, fungus and bacterium, were used for bioaugmentation of the groundnut residues. The cellulolytic fungi and bacteria were isolated from different partially decomposed cellulose-rich substrates, such as groundnut residues, rice straw, and rotten wood, following dilution plating techniques on carboxymethyl cellulose (CMC) agar media (composition per liter—KCl: 0.2 g, NH4H2PO4: 1 g, MgSO4·7H2O: 1 g, yeast extract: 1 g, carboxymethyl cellulose: 26 g, agar: 20 g) through the spread plate method by using antibacterial and antifungal agents, respectively, to obtain the desired single microbial colony. One gram of sample was added to 9 ml of sterilized saline solution (0.85% NaCl) to make a 1:10 dilution (10–1 concentration), and the mixture was firmly shaken. It was further diluted to a factor of 10–6. Following the dilutions, 1 ml of inoculum from the serially diluted samples (up to a concentration of 10–6) was spread on CMC agar media plates, and the plates were then incubated at 30 ± 2 °C and 37 °C for 6–7 days and 1–2 days, respectively, for fungi and bacteria (Ahmad et al. 2013; Rathore et al. 2014).
Primary screening by the congo red test
After incubation, the media plates were flooded for 15 min with 0.1% (w/v) Congo red dye (Congo red assay). After that, the plates were washed by flooding with 1 M NaCl solution for 10 min to remove the dye. The development of a clear halo zone surrounding the microbial colony, which indicates cellulose hydrolysis, was recorded on a 0 to 4 rating scale as follows: 0 indicates no change; 1 indicates a positive result; 2 denotes a halo zone of 1–3 mm; 3 denotes a halo zone of 4–6 mm; and 4 denotes a halo zone of 7 mm or more (Hendricks et al. 1995; Gopalakrishnan et al. 2015). The isolates with the highest zone diameter-to-colony diameter ratio were chosen for further experiments. The largest clear zone ratio implied the highest cellulase production activity (Rathore et al. 2014). The identified and marked colonies were subcultured again on CMC agar media for preservation and further use.
Identification of selected fungal isolates through morphological, microscopic and biochemical characterization
The fungal morphology was studied macroscopically by observing the color of the colony, shape of the colony, texture of the colony, color of the spores, and microscopically by observing the type of spores, production of chlamydospores, presence of filaments or hyphae, and type of hyphae under the EVOS® FL Color Imaging System (Life Technologies TM, USA) using the codes and descriptions of Barnett and Hunter (1972); Devanathan et al. (2007); Gaddeyya et al. (2012); Sharma and Singh (2014) for fungal genera and species, followed by biochemical tests such as growth on glucose and citric acid, following standard procedures.
Identification of selected bacterial isolates through morphological, microscopic and biochemical characterization
Bacterial isolates were screened for their Gram reaction, colony morphology (color, shape, texture, transparency, presence of odor, etc.) (Garrity et al. 2005), and microscopic characteristics, such as the shape of the bacteria and spore formation, under the EVOS® FL Color Imaging System (Life Technologies TM, USA). Different biochemical tests, such as the catalase test, citrate utilization test, oxidase test, indole test, urease test, methyl red (MR) test, voges-proskauer (VP) test, starch hydrolysis, gelatin hydrolysis, and carbohydrate utilization tests, such as glucose, sucrose, lactose, mannose, and mannitol, were performed to identify the isolates provisionally up to the genus level, as described in Bergey’s Manual of Determinative Bacteriology (Buchanan and Gibbons 1974; Holt et al. 1994).
Collection of fungal culture as a standard check
Cultured Phanerochaete chrysosporium strain CBS129.27 was obtained from the Microbial Type Culture Collection and Gene Bank (MTCC), Chandigarh, India (Collection Acc. No: 4955). The fungus Phanerochaete chrysosporium is the most potent producer of cellulase, which was well explained by Bhat and Bhat (1997). Therefore, it is considered a standard culture for comparison and for making efficient consortia.
Rescreening of efficient strains based on cellulase production potentials under in vitro studies
For cellulase production under in vitro conditions, each microorganism was grown in 25 ml of Mandels and Andreotti’s broth (Mandels and Andreotti 1978) supplemented with carboxymethyl cellulose (GRM329, HiMedia, India) as the sole source of carbon in 100 ml Erlenmeyer flasks at 28 ± 2 °C on a rotary shaker (120 rpm) for 12 days. Five millilitres of growth solution was centrifuged at 15,000 rpm using a refrigerated benchtop centrifuge (Eppendorf 5810R) for 30 min, and the resulting supernatant was analyzed for cellulase activity (endo–β–1,4–glucanase CMCase) (Dey et al. 2002). An uninoculated control was also used. The assay mixture containing 0.5 ml of enzyme, 1.0 ml of citrate buffer, and 0.5 ml of carboxymethyl cellulose (1% w/v in 0.05 M sodium citrate buffer, pH 4.8) as a substrate was incubated for 30 min at 50 °C (Gomes et al. 2006), and 3 ml of di-nitro salicylic acid (DNS) reagent was added to stop the reaction. The absorbance of the supernatant was measured at 540 nm and compared to that of the blank. The quantity of reducing sugars (glucose) liberated in 30 min was determined colorimetrically by the DNSA method (Miller 1959), and the cellulase activity (IU ml−1 broth) was expressed as 1 μmol of glucose liberated per minute under standard assay conditions. Each microorganism was tested in triplicate, and the average value was recorded. The obtained data were statistically analyzed for one–way analysis of variance and standard deviation using SPSS (version 20.0, SPSS Inc., Chicago, IL, USA) software. By employing Duncan’s multiple range test (DMRT) (Duncan 1955), the significant (at the P = 0.05 level) variation between the treatment data (mean ± SE) was determined using SPSS. The isolates with the highest activity were selected for optimization of cellulase production.
Note: Under standard conditions, 1 IU ml−1 = 1 μmol of glucose liberated min−1 (i.e., 0.18 mg of glucose min−1). 1 mg glucose = 1/(0.18 × 0.5 × 30) = 0.37 μmol ml−1 min−1 (for 30 min or 0.5 h). Therefore, the CMC unit (IU ml−1) = mg of glucose released × 0.37 (Wood and Bhat 1998; Eveleigh et al. 2009; Ahmad et al. 2013).
PCR analysis of genomic DNA for the identification and validation of selected strains
DNA extraction and PCR amplification
Partial sequencing of the 16S rRNA gene was performed to characterize each selected bacterial strain up to the species level. In the case of fungi, sequencing of the ITS rRNA region (ITS1, ITS2) and 5.8S rRNA gene was performed to identify the isolates up to the species level. The genomic DNA of the bacterial and fungal isolates was extracted and purified following the manufacturer’s protocol for Promega (Promega, Inc.). The 16S rRNA of the bacterial isolates was amplified using 8F (5ʹAGAGTTTGATCCTGGCTCAG3ʹ) and 1492R (5ʹACGGCTACCTTGTTACGACTT3ʹ) universal primers with amplicon sizes of ∼852 bp (Micrococcus sp.) and ∼946 bp (Bacillus sp.). The universal primers used for the ribosomal amplification of the fungal isolates were ITS1 (5ʹTCCGTAGGTGAACCTGCGG3ʹ), which hybridizes at the beginning, and ITS4 (5ʹTCCTCCGCTTATTGATATGC3ʹ), which hybridizes at the end, with an amplicon size of ∼240 bp for Aspergillus sp. and ∼392 bp for Trichoderma sp. The amplification was determined via 1% agarose gel electrophoresis.
Sequencing and analysis
The PCR products were sent for sequencing to Macrogen, Inc. (Seoul, Korea). The obtained assembled and trimmed nucleotide sequences were compared with other related sequences in GenBank (NCBI, India) through BLAST searches using the BLASTN program (http://www.ncbi.nlm.nih.gov/blast/) (Liu et al. 2000; Landeweert et al. 2003; Javadi et al. 2012), and identity was ascertained at both the genus and species levels. ClustalW software (BioEdit 5.0) was used for alignment (Thompson et al. 1997). The phylogenetic trees were constructed by the neighbor-joining method as described by Saitou and Nei (1987) using MEGA 11 software (Tamura et al. 2021). To evaluate the topology or structure of the phylogenetic tree, Felsenstein’s (1985) bootstrap resampling method was employed with 1000 replicates.
Compatibility test
Five millimeter discs of fungal strains (7 days old) were placed separately on opposite sides of each other on PDA plates (MH096, HiMedia, India) one cm away from the edge, following the dual culture plating technique outlined by Dennis and Webster (1971), and their growth was checked after incubation for 6–7 days at 30 ± 2 °C. The compatibility between bacterial strains was determined using nutrient agar media (M001, HiMedia, India) under in vitro conditions following the method of Fukui et al. (1994). The bacterial strains were cross-streaked, which means that they were streaked both vertically and horizontally to each other, and the plates were incubated for 72 h at room temperature (30 ± 2 °C) to observe the inhibitory zone. When there is no inhibition zone, the bacterial strains are compatible, and when there is an inhibition zone, the strains are incompatible. Similarly, fungal and bacterial strains were tested for their compatibility with each other using the dual culture plate method described by Siddiqui and Shaukat (2003). Accordingly, an overnight culture of bacteria grown on CMC agar media was streaked on one side of a PDA plate (1 cm away from the edge). The other side of the Petri dish (1 cm from the edge) was inoculated with a 5 mm fungal culture disc (9 days old). The plates were then incubated at 30 ± 2 °C for 6–7 days, after which the zone of inhibition was determined. Every set of combinations was tested three times.
Preparation of the fungal and bacterial consortium
The fungal and bacterial cultures were grown separately in liquid CMC media (CMC broth) for 7 days. Efficient microbial cultures of fungi and bacteria consortium or the inoculation liquid was prepared by culturing 1 ml of broth from each of 5 microorganisms in a 250 ml Erlenmeyer flask containing 100 ml of CMC broth and incubating at 30 ± 2 °C for 3–4 days in a shaker (200 rpm). The corresponding population of each microorganism was measured at their unit volume (CFU ml−1 of broth) before it was used for making the consortium.
Results and discussion
Isolation and screening of efficient cellulose decomposing fungal and bacterial strains
Cellulase breaks down cellulose in the plant cell wall, and carboxymethyl cellulose (CMC) agar medium was used to measure the production of cellulase by fungi and bacteria. Septiani et al. (2019) stated that this medium is appropriate because CMC is a water-soluble derivative of pure cellulose that enables microorganisms to transform cellulose into glucose through the action of cellulase enzymes. Following dilution plating techniques on CMC agar medium through the spread plate method with antibacterial and antifungal agents, to obtain a single microbial colony and proceed with the Congo red assay, the cellulolytic fungi and bacteria were screened out and isolated from the collected samples. The 20 acquired isolates in total were subsequently examined for qualitative cellulose-degrading activity using the Congo red assay.
Congo red assay test for cellulose hydrolysis
Only 9 of the 20 isolates were chosen based on their capacity to hydrolyze cellulose. The presence of cellulase, which is responsible for the hydrolysis of cellulose, was demonstrated by the clear halo zone that formed around the microbial colonies on the Congo red agar plates. Among these, 4 fungal isolates, DAJ1, DAJ2, DTJ3, and DTJ4, showed obvious hallow zones at an observed scale of 4, with an average Zd/Cd ratio of 2.93; however, DAJ2 had the highest ratio (3.27), followed by DTJ4 (3.20). Another 2 isolates, DAJ1 and DTJ3, displayed the next highest ratios of 2.75 and 2.50, respectively. Only 5 bacterial isolates (DBJ5, DBJ6, DBJ7, DMB8, and DMB9) showed hallow zones that ranged in size from 5 mm (scale 3) to 14 mm (4 scale). The average ratio of zone to colony diameter was 2.53, with maximum and minimum ratios of 3.50 (DBJ6) and 1.66 (DMB8), respectively. Two of the bacterial isolates, DBJ6 and DMB9, had the highest levels of cellulolytic activity (scale 4) and Zd/Cd ratios (3.50 and 3.00, respectively), followed by DBJ7 (scale 4 and ratio 2.50), DBJ5, and DMB8 (scale 3; ratios 2.00 and 1.66, respectively). These findings showed that the isolated bacteria and fungi have a modest to significant capacity to produce cellulase. These 9 cellulolytic isolates were considered efficient isolates and were selected for further quantitative analysis based on their relatively high hallow zone expression and Zd/Cd ratios (Table 1).
Table 1.
Cellulose degradation capability of selected fungal and bacterial strains isolated from partially decomposed cellulose-rich substrates
| Serial number | Isolates | Rating | Zone diameter (mm) | Colony diameter (mm) | Ratio Zd/Cd | |
|---|---|---|---|---|---|---|
| 1 | Fungal isolates | DAJ1 | 4 | 33 | 12 | 2.75 |
| 2 | DAJ2 | 4 | 36 | 11 | 3.27 | |
| 3 | DTJ3 | 4 | 30 | 12 | 2.50 | |
| 4 | DTJ4 | 4 | 32 | 10 | 3.20 | |
| 5 | Bacterial isolates | DBJ5 | 3 | 6 | 3 | 2.00 |
| 6 | DBJ6 | 4 | 14 | 4 | 3.50 | |
| 7 | DBJ7 | 4 | 10 | 4 | 2.50 | |
| 8 | DMB8 | 3 | 5 | 3 | 1.66 | |
| 9 | DMB9 | 4 | 12 | 4 | 3.00 | |
Observations were recorded on a 0 to 4 rating scale as follows: 0 indicates no change; 1 indicates a positive result; 2 denotes a halo zone of 1–3 mm; 3 denotes a halo zone of 4–6 mm; and 4 denotes a halo zone of 7 mm and above
Morphological, microscopic and biochemical identification of the fungal isolates
The morphological, microscopic, and biochemical characteristics of the fungal isolates are summarized in Tables 2 and 3. The diverse morphological, microscopic, and biochemical characteristics of each fungal isolate served as evidence to distinguish them among many fungal genera. The colony colors of the isolates DAJ1 and DAJ2 were initially white and later black, and the reverse of the colony was pale yellow or buff colored, whereas the other 2 isolates, DTJ3 and DTJ4, were initially cottony white (mycelial color) and later light yellowish to green with a colorless and dull yellowish color on the reverse side, respectively (Fig. 1). The DTJ3 and DTJ4 isolates formed two to three concentric rings, but DAJ1 and DAJ2 formed flat circular and flask-shaped colonies, respectively. All of the fungal isolates produced smooth walled conidiospores and septate hyphae, but only some of them, DAJ1 and DAJ2, expressed filamentous hyphae, while the other 2 isolates, DTJ3 and DTJ4, exhibited highly branched hyphae. DAJ1 and DAJ2 produced black and blackish brown conidia, respectively, while DTJ3 and DTJ4 formed bright green and dark green conidia, respectively. Two isolates (DTJ3 and DTJ4) had purely woolly textures, while DAJ1 and DAJ2 had cottony and velvety textures, respectively. All of them showed evidence of positive growth on both glucose and citric acid. Therefore, based on the morphological, microscopic, and biochemical characterizations described above, it can be stated that the isolates DAJ1 and DAJ2 belong to the genus Aspergillus (Silva et al. 2011; Mrudula and Murugammal 2011; Alsohaili and Bani-Hasan 2018), while the isolates DTJ3 and DTJ4 belong to the genus Trichoderma (Shah and Afiya 2019; Kumar et al. 2020).
Table 2.
Morphological and microscopic characteristics of the fungal isolates
| Characteristics | DAJ1 | DAJ2 | DTJ3 | DTJ4 |
|---|---|---|---|---|
| Color of colony | Initially, white, later black, reverse of the colony pale yellow or buff colored | Initially, white, later black, reverse of the colony pale yellow or buff colored | Initially, cottony white, later light yellowish to green, reverse of the colony colorless | Initially, cottony white, later light yellowish to green, reverse of the colony dull yellowish in color |
| Shape of colony | Flat, circular | Flask shaped | Concentric ring | Concentric ring |
| Texture of colony | Cottony | Velvety | Woolly | Woolly |
| Type of spores | Asexual spores, i.e., conidia | Asexual spores, i.e., conidia | Asexual spores, i.e., conidia | Asexual spores, i.e., conidia |
| Color of spores (Conidial color) | Black | Blackish brown | Bright green | Dark green |
| Conidial wall | Smooth | Smooth | Smooth | Smooth |
| Production of chlamydospores | Present | Present | Present, but in least number | Present, but in least number |
| Presence of filaments/hyphae | Yes | Yes | Yes | Yes |
| Type of hyphae | Septate and filamented hyphae | Septate and filamented hyphae | Septate and highly branched hyphae | Septate and highly branched hyphae |
| Result | Aspergillus sp. | Aspergillus sp. | Trichoderma sp. | Trichoderma sp. |
Table 3.
Biochemical characteristics of the fungal isolates
| Characteristics | DAJ1 | DAJ2 | DTJ3 | DTJ4 |
|---|---|---|---|---|
| Growth on glucose | + | + | + | + |
| Growth on citric acid | + | + | + | + |
| Result | Aspergillus sp. | Aspergillus sp. | Trichoderma sp. | Trichoderma sp. |
‘+’: Positive, ‘−’: Negative
Fig. 1.
Morphological and microscopic characterization of the selected isolates. A Aspergillus sp. colony on CMC agar media–Top side, B Aspergillus sp. colony on CMC agar media–Reverse side, C Trichoderma sp. on CMC agar media–Early stage, D Trichoderma sp. on CMC agar media–Mature stage, E Single colony of Bacillus sp. on CMC agar media, F Single colony of Micrococcus sp. on CMC agar media, G and H Microscopic view of Aspergillus sp., I and J Microscopic view of Trichoderma sp., K Microscopic view of Micrococcus sp., L Rod shaped vegetative cells and endospore-stained view of Bacillus sp. under the microscope, M Phanerochaete chrysosporium on CMC agar media, N Streaking of Bacillus sp., O Streaking of Micrococcus sp
Morphological, microscopic and biochemical identification of the bacterial isolates
The demonstrated bacterial isolates, i.e., DBJ5, DBJ6, DBJ7, DMB8, and DMB9, were examined macroscopically by observing colony features (color, shape, texture, transparency, etc.) and microscopically (endospore formation, shape of bacteria, etc.), as depicted in Table 4. All of the bacterial isolates were gram-positive, odorless, and opaque in transparency. Except for DMB8 and DMB9, which were cocci or spherical shaped, the rest were rod shaped. The isolates DBJ5, DBJ6, and DBJ7 showed a variety of colony shapes and textures, including large, circular, convex, rough and dry colonies with jagged edges, whereas the isolates DMB8 and DMB9 displayed smooth, mucoid, bright yellow pigmented medium, flat, circular colonies with entire margins (Fig. 1). While DMB8 and DMB9 did not produce any spores, the isolates DBJ5, DBJ6, and DBJ7 produced endospores.
Table 4.
Morphological and microscopic characteristics of the bacterial isolates
| Characteristics | DBJ5 | DBJ6 | DBJ7 | DMB8 | DMB9 |
|---|---|---|---|---|---|
| Gram staining | Positive | Positive | Positive | Positive | Positive |
| Shape of bacteria | Rod | Rod | Rod | Cocci or spherical | Cocci or spherical |
| Shape of colony | Large, circular, flat colony with jagged edges | Large, circular, convex colony with jagged edges | Large, circular, convex colony with jagged edges | Medium, flat, circular colony with entire margins | Medium, flat, circular colony with entire margins |
| Color of colony | Milky white | White to slightly yellow | White to slightly yellow | Bright yellow pigmentation | Bright yellow pigmentation |
| Texture of colony | Rough and dry | Rough and dry | Rough and dry | Smooth and mucoid | Smooth and mucoid |
| Transparency | Opaque | Opaque | Opaque | Opaque | Opaque |
| Spore formation | Endospore forming | Endospore forming | Endospore forming | Non endospore forming | Non endospore forming |
| Presence of odor | Absent | Absent | Absent | Absent | Absent |
| Result | Bacillus sp. | Bacillus sp. | Bacillus sp. | Micrococcus sp. | Micrococcus sp. |
To further evaluate the characteristics of these bacterial isolates, biochemical tests were performed (Table 5). All of the bacterial isolates showed negative results in the citrate utilization test and indole production but positive results in the catalase test, gelatin hydrolysis, and growth on glucose and sucrose. The oxidase test was positive for DMB8 and DMB9 and negative for the remaining isolates, whereas starch hydrolysis was positive for DBJ5, DBJ6, and DBJ7 and negative for DMB8 and DMB9. In the case of the VP test, all of the isolates gave a negative result except DBJ5, but the reverse was observed for the urease test, in which DBJ5 gave a negative result, while the others showed a positive result. The methyl red test was positive for DBJ6 and DBJ7 and negative for DBJ5, DMB8, and DMB9. Therefore, it can be concluded that the isolates DBJ5, DBJ6, and DBJ7 belong to the genus Bacillus (Beesley et al. 2010; Hussain et al. 2017), while the isolates DMB8 and DMB9 belong to the genus Micrococcus (Annamalai et al. 2010; Marzan et al. 2017) based on the abovementioned morphological, microscopic, and biochemical reactions.
Table 5.
Biochemical characteristics of the bacterial isolates
| Characteristics | DBJ5 | DBJ6 | DBJ7 | DMB8 | DMB9 |
|---|---|---|---|---|---|
| Catalase test | + | + | + | + | + |
| Citrate utilization test | − | − | − | − | − |
| Oxidase test | − | − | − | + | + |
| Indole test | − | − | − | − | − |
| Urease test | − | + | + | + | + |
| Methyl red (MR) test | − | + | + | − | − |
| Voges-proskauer (VP) test | + | − | − | − | − |
| Starch hydrolysis | + | + | + | − | − |
| Gelatin hydrolysis | + | + | + | + | + |
| Carbohydrate utilization test | |||||
| Glucose | + | + | + | + | + |
| Sucrose | + | + | + | + | + |
| Lactose | − | − | − | − | − |
| Mannose | + | + | − | − | − |
| Mannitol | − | − | − | + | + |
| Result | Bacillus sp. | Bacillus sp. | Bacillus sp. | Micrococcus sp. | Micrococcus sp. |
Cellulase production potentials of selected isolates under in vitro studies
All of the selected strains of bacteria (Bacillus sp. and Micrococcus sp.) and fungi (Aspergillus sp. and Trichoderma sp.) were explored for their reducing sugar content and cellulose production activity to support further investigation, which was assumed to be due to their in vitro cellulase production potentials (Table 6). According to in vitro studies, all of the isolated strains of fungi and bacteria had a detectable range of cellulase production potentials that were effectively correlated with the reducing sugar content. Among them, the isolate DBJ6 (Bacillus sp.) was the most potent producer of cellulase (0.899 ± 0.0041 IU ml−1 broth), which was very close (0.893 ± 0.0018 IU ml−1 broth) to that of DAJ2 (Aspergillus sp.), and the least (0.520 ± 0.0018 IU ml−1 broth) was found in the case of the isolate DMB8 (Micrococcus sp.). The cellulase activity values of the isolates DAJ2 (Aspergillus sp.) (0.893 ± 0.0018 IU ml−1 broth), DTJ4 (Trichoderma sp.) (0.872 ± 0.0013 IU ml−1 broth), DBJ6 (Bacillus sp.) (0.899 ± 0.0041 IU ml−1 broth), and DMB9 (Micrococcus sp.) (0.822 ± 0.0026 IU ml−1 broth) were comparable and exceeded that of Phanerochaete chrysosporium (0.779 ± 0.0026 IU ml−1 broth), which is considered a standard culture. However, the other isolates had lower abundances than did the control organism (Phanerochaete chrysosporium). Therefore, these 4 isolates were considered for further study to develop an efficient consortium.
Table 6.
Cellulase production potentials of selected fungal and bacterial isolates on the 6th day of fermentation (in vitro, ml−1 broth)
| Isolates | Reducing sugars (mg ml−1) | Cellulase (IU ml−1)## |
|---|---|---|
| Aspergillus sp. (DAJ1) | 0.736 ± 0.0018f | 0.2723 ± 0.00068 |
| Aspergillus sp. (DAJ2)** | 0.893 ± 0.0018b | 0.3304 ± 0.00069 |
| Trichoderma sp. (DTJ3) | 0.721 ± 0.0018 h | 0.2668 ± 0.00068 |
| Trichoderma sp. (DTJ4)** | 0.872 ± 0.0013c | 0.3226 ± 0.00047 |
| Bacillus sp. (DBJ5) | 0.551 ± 0.0022i | 0.2039 ± 0.00083 |
| Bacillus sp. (DBJ6)** | 0.899 ± 0.0041a | 0.3326 ± 0.00015 |
| Bacillus sp. (DBJ7) | 0.727 ± 0.0018 g | 0.2690 ± 0.00066 |
| Micrococcus sp. (DMB8) | 0.520 ± 0.0018j | 0.1924 ± 0.00066 |
| Micrococcus sp. (DMB9)** | 0.822 ± 0.0026d | 0.3041 ± 0.00098 |
| Control | 0.032 ± 0.0017 k | 0.0118 ± 0.00064 |
| Collected fungi from MTCC (Check organism) | ||
| Phanerochaete chrysosporium | 0.779 ± 0.0026e | 0.2882 ± 0.00094 |
Each microorganism was tested in triplicate, and the average value was recorded
##1 mg glucose = 1/(0.18 × 0.5 × 30) = 0.37 μmole min−1 ml−1 (for 30 min or 0.5 h)
Therefore, CMC unit (IU ml−1) = mg of glucose released × 0.37
**The isolates DAJ2, DTJ4, DBJ6, and DMB9 were considered for further study to develop an efficient consortium
The different letters following the data (mean ± SE) indicate significant (at the P = 0.05 level) differences based on Duncan’s multiple range test (DMRT) (Duncan 1955)
The production of reducing sugars from in vitro studies revealed that the 6th day (144 h) was the peak of fermentation (Table 6), after which cellulase activity declined rapidly. There was a decrease in the synthesis of hydrolytic cellulase enzymes after 144 h of cultivation and fermentation. The principal metabolite (glucose) acquired from the breakdown of cellulose (carboxymethyl cellulose) by the microorganisms necessary for their development and proliferation may have run out, which could account for this decrease. The results of the production of cellulolytic enzymes by these efficient isolates during the breakdown of carboxymethyl cellulose corroborate the findings of Kachlishvili et al. (2012), who reported that the optimal time for certain basidiomycetes to produce these enzymes during the fermentation of food wastes was 144 h. However, it has been observed that certain fungi produce cellulolytic enzymes in less than 120 h of incubation, while others take longer, up to 144 h (Tao et al. 2010; Nathan et al. 2014).
Molecular identification and validation of the selected efficient strains
The 2 cellulase-producing bacterial strains were identified through 16S rRNA gene partial sequencing, and 2 fungal isolates were recognized through sequencing of the ITS rRNA region (ITS1, ITS2) and 5.8S rRNA gene. Previous research has indicated that ITS rRNA region, 5.8S rRNA gene and 16S rRNA gene sequencing are reliable techniques for identifying both fungal and bacterial species and differentiating between closely related species (Hussain et al. 2017). Therefore, the selected isolates were further analyzed, and the sequencing results were submitted to GenBank under the following accession numbers: PP086700 (DAJ2), PP086699 (DTJ4), PP082584 (DBJ6), and PP082585 (DMB9). The isolates were identified via phylogenetic analysis and nucleotide homology based on their strong resemblance to the recognized species. Based on the available NCBI data, phylogenetic analysis of their ribosomal gene (rRNA) partial sequences revealed that DAJ2 shares 100% homology with Aspergillus foetidus, DTJ4 shares 99.74% similarity with Trichoderma atrobrunnium, DBJ6 shares 100% identity with Priestia megaterium (previously known as Bacillus megaterium; Gupta et al. 2020), and DMB9 shares 99.88% homology with Micrococcus yunnanensis (Table 7). The phylogenetic trees of the isolated strains are presented in Figs. 2a, b and 3a, b.
Table 7.
Molecular identification of the selected efficient strains
| Isolates/strains | Source | Scientific name/identity | Genbank accession number | Similarity percentage |
|---|---|---|---|---|
| DAJ2 | Rotten wood | Aspergillus foetidus | PP086700 | 100% |
| DTJ4 | Rotten wood | Trichoderma atrobrunnium | PP086699 | 99.74% |
| DBJ6 | Partially decomposed rice straw | Priestia megaterium* | PP082584 | 100% |
| DMB9 | Partially decomposed rice straw | Micrococcus yunnanensis | PP082585 | 99.88% |
*Priestia megaterium was previously known as Bacillus megaterium (Gupta et al. 2020)
Fig. 2.
Phylogenetic link between the representative species and the selected cellulolytic fungal isolates based on the ITS rRNA region and 5.8S rRNA gene sequences created with the neighbor-joining technique. a DAJ2 (Aspergillus foetidus), b DTJ4 (Trichoderma atrobrunnium)
Fig. 3.
Phylogenetic link between the representative species and the selected cellulolytic bacterial isolates based on 16S rRNA gene partial sequences created with the neighbor-joining technique. a DBJ6 (Priestia megaterium), b DMB9 (Micrococcus yunnanensis)
Compatibility test
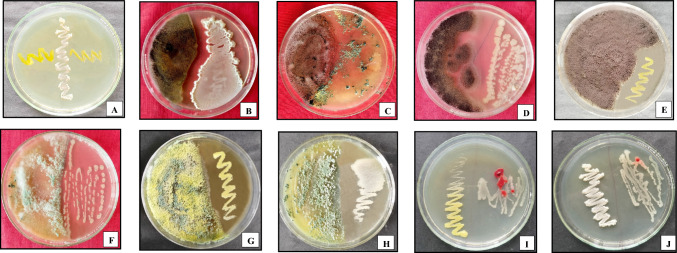
The results of the compatibility test from in vitro studies showed that all the isolates were mutually compatible or synergistic with each other and did not exhibit any antagonistic effects, indicating that there was no inhibition zone around the growing microbial colonies. All the tested isolates also showed a positive compatibility with the check organism Phanerochaete chrysosporium (Table 8, Fig. 4). Based on the results of the compatibility test, it can be concluded that all the tested potential cellulolytic microbial strains can be grown on the same media or substrates as they work synergistically in one container (petri plate) and can be used as a mixed culture (consortium) for the decomposition of organic materials in composting. Nevertheless, the cellulose-degrading efficiency of a consortium could be worse than that of its individual members if it consists of competing or rival members (Boddy 2000).
Table 8.
Compatibility test among 4 isolated strains and Phanerochaete chrysosporium
| Sl. No | Potential cellulolytic microbial strains | Compatibility test result |
|---|---|---|
| 1 | DAJ2 > < DTJ4 | + |
| 2 | DAJ2 > < Phanerochaete chrysosporium | + |
| 3 | DTJ4 > < Phanerochaete chrysosporium | + |
| 4 | DBJ6 > < DMB9 | + |
| 5 | DAJ2 > < DBJ6 | + |
| 6 | DAJ2 > < DMB9 | + |
| 7 | DTJ4 > < DBJ6 | + |
| 8 | DTJ4 > < DMB9 | + |
| 9 | DBJ6 > < Phanerochaete chrysosporium | + |
| 10 | DMB9 > < Phanerochaete chrysosporium | + |
Description: (+) = Positive or compatible test results
DAJ2: Aspergillus foetidus strain DAJ2 with a similarity level of 100%
DTJ4: Trichoderma atrobrunnium strain DTJ4 with a similarity level of 99.74%
DBJ6: Priestia megaterium strain DBJ6 with a similarity level of 100%
DMB9: Micrococcus yunnanensis strain DMB9 with a similarity level of 99.88%
Phanerochaete chrysosporium strain CBS129.27 was obtained from MTCC, Chandigarh, India (Collection Acc. No: 4955)
Fig. 4.
Compatibility test within the selected microbial strains. A Priestia megaterium and Micrococcus yunnanensis, B Aspergillus foetidus and Priestia megaterium, C Aspergillus foetidus and Trichoderma atrobrunnium, D Aspergillus foetidus and Phanerochaete chrysosporium, E Aspergillus foetidus and Micrococcus yunnanensis, F Trichoderma atrobrunnium and Phanerochaete chrysosporium, G Trichoderma atrobrunnium and Micrococcus yunnanensis, H Trichoderma atrobrunnium and Priestia megaterium, I Micrococcus yunnanensis and Phanerochaete chrysosporium, J Priestia megaterium and Phanerochaete chrysosporium
Preparation of the microbial consortium for cellulose degradation
Efficient microbial cultures of fungi and bacteria consortium or the inoculation liquid was prepared at a ratio of 1:1:1:1:1 (v/v) of each of the 5 microorganisms (please refer to the Materials and Methods section). The corresponding population of each microorganism was measured at their unit volume (CFU ml−1 of broth) before the consortium was generated. The microbial population of each of the 4 isolated strains was greater than that of the check organism Phanerochaete chrysosporium (Table 9). Generally, single microbial strains are not very successful at breaking down cellulose, and gene induction is not a cost-effective method for boosting cellulase production. On the other hand, microbial consortia composed of several different microbial strains are becoming increasingly popular since they frequently exhibit stronger cellulase activity than single strains (Zhang and Dong 2022). According to Jiang et al. (2021), the cellulose-degrading efficiency of a five-member fungal consortium was noticeably greater than that of each member acting alone. Mixing of two or more species has better degradation ability than that of a single species (Kanaly et al. 2000). Furthermore, each consortium member has various requirements for achieving its own maximum production of cellulase. It is critical that the conditions they share when working as a team (consortium) guarantee maximum cellulase production of the consortium rather than of a single member (Zhang and Dong 2022).
Table 9.
Corresponding population of the selected strains at their unit volume at the time of preparation of the consortium
| Strains | Aspergillus foetidus (DAJ2) | Trichoderma atrobrunnium (DTJ4) | Priestia megaterium (DBJ6) | Micrococcus yunnanensis (DMB9) | Phanerochaete chrysosporium (CBS129.27) |
|---|---|---|---|---|---|
| Population (CFU ml−1) | 4.0 × 105 | 3.7 × 105 | 3.6 × 109 | 3.4 × 109 | 3.2 × 105 |
Conclusion
This research aimed to screen and identify potential cellulolytic fungal and bacterial strains isolated from partially decomposed cellulose-rich compounds under in vitro conditions to develop an efficient consortium for the rapid decomposition of organic materials during composting. After a series of examinations for screening (primary screening by the Congo red test and rescreening based on cellulase production potentials under in vitro studies) and identification (morphological, microscopic, biochemical, and molecular identification) of potential cellulolytic microbial strains, Aspergillus foetidus strain DAJ2, with a similarity level of 100% (PP086700); Trichoderma atrobrunnium strain DTJ4, with a similarity level of 99.74% (PP086699); Priestia megaterium strain DBJ6, with a similarity level of 100% (PP082584); and Micrococcus yunnanensis strain DMB9, with a similarity level of 99.88% (PP082585), were considered efficient strains for making consortium. Cultured Phanerochaete chrysosporium strain CBS129.27 was obtained from MTCC, Chandigarh, India (Collection Acc. No: 4955), for comparison, and an efficient consortium with cellulolytic potential is well established. Finally, all of the 4 selected isolated strains and the check organism were mutually compatible or synergistic with each other, and their consortium is useful for the accelerated decomposition of organic constituents in rapid composting.
Acknowledgements
The first author is thankful to the Department of Science and Technology (DST), Ministry of Science and Technology, Government of India for supporting with DST-INSPIRE Fellowship (IF210025). All the authors are grateful to Vice-Chancellor, Bidhan Chandra Krishi Viswavidyalaya for providing the infrastructural facilities to carry out this work. The authors would like to thank Microbial Type Culture Collection and Gene Bank (MTCC), Chandigarh, India for the provision of supplying the microbial strains (Phanerochaete chrysosporium) used in this research. The first author would like to thank Mr. Sourish Chakraborty for his continuous help during the sample collection and isolation procedure.
Author contributions
DR and SKG-designed the research; DR-performed methodology, investigation, data curation, formal analysis, software, also wrote, reviewed and edited the original draft of the article; SKG-performed supervision, data analysis, software, validation, and also reviewed and edited the original draft. KKP-performed the GenBank (NCBI) registration process of the molecularly identified fungal and bacterial isolates, also reviewed and edited the original draft. All the authors read and approved the final manuscript.
Funding
This research is funded by the Department of Science and Technology (DST), Ministry of Science and Technology, Government of India (DST-INSPIRE Fellowship/IF210025).
Data availability
All the data generated or analysed during this study are included in this published article.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Accession numbers
GenBank Accession number: PP086700; PP086699; PP082584; PP082585.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
All the authors gave their consent for publication of the results.
References
- Agrawal R, Satlewal A, Mathur AS, Gupta RP, Raj T, Kumar R, Tuli DK. Kinetic and enzyme recycling studies of immobilized β-glucosidase for lignocellulosic biomass hydrolysis. Environ Eng Manag J. 2018;17:1385–1398. doi: 10.30638/eemj.2018.137. [DOI] [Google Scholar]
- Agrawal R, Verma A, Singhania RR, Varjani S, Di Dong C, Patel AK. Current understanding of the inhibition factors and their mechanism of action for the lignocellulosic biomass hydrolysis. Bioresour Technol. 2021;332:125042. doi: 10.1016/j.biortech.2021.125042. [DOI] [PubMed] [Google Scholar]
- Ahmad B, Nigar S, Shah SSA, Bashir S, Ali J, Yousaf S, Bangash JA. Isolation and identification of cellulose degrading bacteria from municipal waste and their screening for potential antimicrobial activity. World Appl Sci J. 2013;27:1420–1426. doi: 10.5829/idosi.wasj.2013.27.11.81162. [DOI] [Google Scholar]
- Alsohaili SA, Bani-Hasan BM. Morphological and molecular identification of fungi isolated from different environmental sources in the northern eastern desert of jordan. Jordan J Biol Sci. 2018;11:329–337. [Google Scholar]
- Annamalai N, Giji S, Arumugam M, Balasubramanian T. Purification and characterization of chitinase from micrococcus sp. AG84 isolated from marine environment. Afr J Microbiol Res. 2010;4:2822–2827. [Google Scholar]
- Ashraf R, Shahid F, Ali AD. Association of fungi, bacteria and actinomycetes with different composts. Pak J Bot. 2007;39:2141–2151. [Google Scholar]
- Bakri YP, Jacques P, Thonart P. Xylanase production by Penicillum canescens 10–10c in solid-state fermentation. Appl Biochem Biotechnol. 2003;105–108:737–748. doi: 10.1385/ABAB:108:1-3:737. [DOI] [PubMed] [Google Scholar]
- Barnett HL, Hunter BB. Illustrated genera of imperfect fungi. In: Illustrated genera of imperfect fungi. 3. Minneapolis, EE, UU: Burgess Publishing Co.; 1972. p. 241. [Google Scholar]
- Beesley CA, Vanner CL, Helsel LO, Gee JE, Hoffmaster AR. Identification and characterization of clinical bacillus spp. isolates phenotypically similar to bacillus anthracis. FEMS Microbiol Lett. 2010;313:47–53. doi: 10.1111/j.1574-6968.2010.02120.x. [DOI] [PubMed] [Google Scholar]
- Bhat MK. Cellulases and related enzymes in biotechnology. Biotechnol Adv. 2000;18:355–383. doi: 10.1016/S0734-9750(00)00041-0. [DOI] [PubMed] [Google Scholar]
- Bhat MK, Bhat S. Cellulose degrading enzymes and their potential industrial applications. Biotechnol Adv. 1997;15:583–620. doi: 10.1016/S0734-9750(97)00006-2. [DOI] [PubMed] [Google Scholar]
- Blackwell M. The fungi: 1, 2, 3 … 5.1 million species? Am J Bot. 2011;98:426–438. doi: 10.3732/ajb.1000298. [DOI] [PubMed] [Google Scholar]
- Boddy L. Interspecific combative interactions between wood-decaying basidiomycetes. FEMS Microbiol Ecol. 2000;31:185–194. doi: 10.1111/j.1574-6941.2000.tb00683.x. [DOI] [PubMed] [Google Scholar]
- Bruce T, Martinez IB, Neto OM, Vicente ACP, Kruger RH, Thompson FL. Bacterial community diversity in the brazilian atlantic forest soils. Microb Ecol. 2010;60:840–849. doi: 10.1007/s00248-010-9750-2. [DOI] [PubMed] [Google Scholar]
- Buchanan RE, Gibbons NE. Bergey’s Manual of Determinative Bacteriology. 8. Baltimore: Williams & Wilkins Co; 1974. p. 1268. [Google Scholar]
- Datta R. Enzymatic degradation of cellulose in soil: a review. Heliyon. 2024;10:e24022. doi: 10.1016/j.heliyon.2024.e24022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis C, Webster J. Antagonistic properties of species-groups of trichoderma: III. Hyphal Interaction Trans Br Mycol Soc. 1971;57:363–369. doi: 10.1016/S0007-1536(71)80050-5. [DOI] [Google Scholar]
- Devanathan G, Shanmugan A, Balasubramanian T, Manivannan S. Cellulase production by aspergillus niger isolated from coastal mangrove debris. Trend Appl Sci Res. 2007;2:23–27. doi: 10.3923/tasr.2007.23.27. [DOI] [Google Scholar]
- Dey R, Pal KK, Chauhan SM, Bhatt DM, Misra JB. Groundnut shell decomposition potential of some cellulolytic microorganisms. Indian J Microbiol. 2002;42:165–167. [Google Scholar]
- Du W, Sun C, Liang J, Han Y, Yu J, Liang Z. Improvement of laccase production and its characterization by mutagenesis. J Food Biochem. 2015;39:101–108. doi: 10.1111/jfbc.12111. [DOI] [Google Scholar]
- Duncan DB. Multiple range and multiple F test. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- Eriksson KEL, Blanchette RA, Ander P. Microbial and enzymatic degradation of wood and wood components. Berlin: Springer-Verlag. Springer Series in Wood Science; 1990. p. 63. [Google Scholar]
- Eveleigh DE, Mandels M, Andreotti R, Roche C. Measurement of saccharifying cellulase. Biotechnol Biofuels. 2009;2:21. doi: 10.1186/1754-6834-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Fukui R, Schroth MN, Hendson M, Hancock JG. Interaction between strains of pseudomonas in sugar beet spermospheres and their relationship to pericarp colonization by pythium ultimum in soil. Phytopathol. 1994;84:1322–1330. doi: 10.1094/Phyto-84-1322. [DOI] [Google Scholar]
- Gaddeyya G, Niharika PS, Bharathi P, Kumar PKR. Isolation and identification of soil mycoflora in different crop fields at salur mandal. Adv Appl Sci Res. 2012;3:2020–2026. [Google Scholar]
- Garrity GM, Brenner DJ, Krieg NR, Staley JT. Bergey’s Manual of Systematic Bacteriology. 2. USA: Springer; 2005. pp. 323–359. [Google Scholar]
- Gautam S, Bundela PS, Pandaey AK, Jamaluddin AMK, Sarsaiya S. Cellulase production by pseudomonas spp. isolated from municipal waste. Int J Acad Res. 2010;2:330–333. [Google Scholar]
- Gomes I, Shaheen M, Rahman SR, Gomes DJ. Comparative studies on production of cell wall-degrading hydrolases by Trichoderma reesei and T. viride in submerged and solid-state cultivations. Bangladesh J Microbiol. 2006;23:149–155. doi: 10.3329/bjm.v23i2.882. [DOI] [Google Scholar]
- Gong X, Zou H, Qian C, Yu Y, Hao Y, Li L, Wang Q, Jiang Y, Ma J. Construction of in situ degradation bacteria of corn straw and analysis of its degradation efficiency. Ann Microbiol. 2020;70:1–15. doi: 10.1186/s13213-020-01601-9. [DOI] [Google Scholar]
- Gopalakrishnan S, Srinivas V, Alekhya G, Prakash B, Kudapa H, Rathore A, Varshney RK. The extent of grain yield and plant growth enhancement by plant growth-promoting broad-spectrum streptomyces sp. in chickpea. Springerplus. 2015;4:31. doi: 10.1186/s40064-015-0811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RS, Patel S, Saini N, Chen S. Robust demarcation of 17 distinct bacillus species clades, proposed as novel bacillaceae genera, by phylogenomics and comparative genomic analyses: description of robertmurraya kyonggiensis sp. nov. and proposal for an emended genus bacillus limiting it only to the members of the subtilis and cereus clades of species. Int J Syst Evol Microbiol. 2020;70:5753–5798. doi: 10.1099/ijsem.0.004475. [DOI] [PubMed] [Google Scholar]
- Heck JX, Hertz PF, Ayub MAZ. Cellulase and xylanase productions by isolated Amazon Bacillus strains using soybean industrial residue based solid-state cultivation. Braz J Microbiol. 2002;33:213–218. doi: 10.1590/S1517-83822002000300005. [DOI] [Google Scholar]
- Hendricks CW, Doyle JD, Hugley B. A new solid medium for enumerating cellulose-utilizing bacteria in soil. Appl Environ Microbiol. 1995;61:2016–2019. doi: 10.1128/aem.61.5.2016-2019.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JG, Krieg NG, Sneathm PHA, Staley JT, Williams ST. Bergey’s Manual of Determinative Bacteriology. 9. Baltimore: Williams and Wilikins; 1994. pp. 786–788. [Google Scholar]
- Hussain AA, Abdel-Salam MS, Abo-Ghalia HH, Hegazy WK, Hafez SS. Optimization and molecular identification of novel cellulose degrading bacteria isolated from egyptian environment. J Genet Eng Biotechnol. 2017;15:77–85. doi: 10.1016/j.jgeb.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immanuel G, Dhanusha R, Prema P, Palavesam AJIJ. Effect of different growth parameters on endoglucanase enzyme activity by bacteria isolated from coir retting effluents of estuarine environment. Int J Environ Sci Technol. 2006;3:25–34. doi: 10.1007/BF03325904. [DOI] [Google Scholar]
- Irigoyen, Pachec F, Sesma M. Turning, microbial inoculation and use of eisenia foetida in household organic waste composting. J Agric Sci Technol. 2011;1:734–738. [Google Scholar]
- Jahangeer S, Khan N, Jahangeer S, Sohail M, Shahzad S, Khan S. Screening and characterization of fungal cellulases isolated from the native environmental source. Pak J Bot. 2005;37:739–748. [Google Scholar]
- Jarvis M. Chemistry: cellulose stacks up. Nature. 2003;426:611–612. doi: 10.1038/426611a. [DOI] [PubMed] [Google Scholar]
- Javadi MA, Ghanbary MAT, Tazick Z. Isolation and molecular identification of soil inhabitant Penicillia. Ann Biol Res. 2012;3:5758–5761. [Google Scholar]
- Jiang GF, Yang TJ, Zheng HP, Wei Z, Wang SM, Fan XT, Shen QR, Xu YC. Construction and evaluation of fungal consortia effect on maize straw degradation. J Plant Nutr Fertil. 2021;27:284–292. doi: 10.11674/zwyf.20363. [DOI] [Google Scholar]
- Kachlishvili E, Khardziani T, Meterveli E, Kobakhidze A, Elisashvili V. Screening of novel basidiomycetes for the production of lignocellulolytic enzymes during fermentation of food wastes. J Waste Convers Bioprod Biotechnol. 2012;1:9–15. doi: 10.5147/jpgs.2012.0078. [DOI] [Google Scholar]
- Kanaly RA, Bartha R, Watanabe K, Harayama S. Rapid mineralization of benzo[a]pyrene by a microbial consortium growing on diesel fuel. Appl Environ Microbiol. 2000;66:4205–4211. doi: 10.1128/AEM.66.10.4205-4211.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Sharma N, Munagala M, Rajkhowa R, Aallardyce B, Shastri Y, Agrawal R. Nanocellulose: resources, physio-chemical properties, current uses and future applications. Front Nanotechnol. 2021;3:747329. doi: 10.3389/fnano.2021.747329. [DOI] [Google Scholar]
- Kumar G, Singh A, Pandey S, Singh J, Chauhan SS, Srivastava M. Morpho-molecular identification of trichoderma sp. and their mycoparasitic activity against soil borne pathogens. Int J Bioresour Stress Manag. 2020;11:613–627. doi: 10.23910/1.2020.2131. [DOI] [Google Scholar]
- Landeweert R, Leeflang P, Kuyper TW, Hoffland E, Rosling A, Wernars K, Smit E. Molecular identification of ectomycorrhizal mycelium in soil horizons. Appl Environ Microbiol. 2003;69:327–333. doi: 10.1128/AEM.69.1.327-333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Miao N, Liu S. Habitats shape root-associated fungal and bacterial communities of minjiang fir saplings. J For Res. 2023;34:1491–1502. doi: 10.1007/s11676-023-01609-2. [DOI] [Google Scholar]
- Liu D, Coloe S, Baird R, Pedersen J. Application of PCR to the identification of dermatophyte fungi. J Med Microbiol. 2000;49:493–497. doi: 10.1099/0022-1317-49-6-493. [DOI] [PubMed] [Google Scholar]
- Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev. 2002;66:506–577. doi: 10.1128/mmbr.66.3.506-577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandels M, Andreotti RE. Problems and challenges in cellulose and cellulose fermentation. Process Biochem. 1978;13:6–13. [Google Scholar]
- Marzan LW, Hossain M, Mina SA, Akter Y, Chowdhury AMA. Isolation and biochemical characterization of heavy-metal resistant bacteria from tannery effluent in chittagong city, bangladesh: bioremediation viewpoint. Egypt J Aquat Res. 2017;43:65–74. doi: 10.1016/j.ejar.2016.11.002. [DOI] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Mrudula S, Murugammal R. Production of cellulase by aspergillus niger under submerged and solid-state fermentation using coir waste as a substrate. Braz J Microbiol. 2011;42:1119–1127. doi: 10.1590/S1517-838220110003000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kppamura K. Isolation and identification of crystalline cellulose hydrolyzing bacterium and its enzymatic properties. J Ferment Technol. 1982;60:343–348. [Google Scholar]
- Nathan VK, Esther Rani M, Rathinasamy G, Dhiraviam KN, Jayavel S. Process optimization and production kinetics for cellulase production by Trichoderma viride VKF3. Springerplus. 2014;3:1–12. doi: 10.1186/2193-1801-3-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petre M, Zarnea G, Adrian P, Gheorghiu E. Biodegradation and bioconversion of cellulose wastes using bacterial and fungal cells immobilized in radio polymerized hydrogels. Resour Conserv Recycl. 1999;27:309–332. doi: 10.1016/S0921-3449(99)00028-2. [DOI] [Google Scholar]
- Rathore SS, Mannivannan A, Narendhirakannan RT. Screening of cellulase producing microorganisms from lake area containing water hyacinth for enzymatic hydrolysis of cellulose. J Adv Sci Res. 2014;5:23–30. [Google Scholar]
- Rebecca LJ, Dhanalakshmi V, Sharmila S, Susithra G, Kumar S, Bala S. Isolation, identification and characterization of fungi from rhizosphere soil of Barleria cristata. Int J Hortic Crop Sci Res. 2012;2:1–6. [Google Scholar]
- Roy D, Gunri SK, Neogi S, Ali O, Sharma J, Bhadu A, Singh B. Effect of microbes in enhancing the composting process: a review. Int J Plant Soil Sci. 2022;34:630–641. doi: 10.9734/ijpss/2022/v34i232469. [DOI] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sartori FG, Leandro LF, Montanari LB, de Souza MGM, Pires RH, Sato DN, Leite CQF, de Andrade PK, Martins CHG. Isolation and identification of environmental mycobacteria in the waters of a hemodialysis center. Curr Microbiol. 2013;67:107–111. doi: 10.1007/s00284-013-0341-6. [DOI] [PubMed] [Google Scholar]
- Septiani D, Suryadi H, Mun’im A, Mangunwardoyo W. Production of cellulase from aspergillus niger and trichoderma reesei mixed culture in carboxymethylcellulose medium as sole carbon. Biodiversitas. 2019;20:3539–3544. doi: 10.13057/biodiv/d201211. [DOI] [Google Scholar]
- Shah MM, Afiya H. Introductory chapter: Identification and isolation of Trichoderma spp.–their significance in agriculture, human health, industrial and environmental application. In book: Trichoderma–The Most Widely Used Fungicide. 5 Princes Gate Court, London, SW7 2QJ, United Kingdom: Intech Open publishers; 2019. pp. 1–12. [Google Scholar]
- Sharma KK, Singh US. Cultural and morphological characterization of rhizospheric isolates of fungal antagonist trichoderma. J Appl Nat Sci. 2014;6:451–456. [Google Scholar]
- Shin CS, Lee JP, Lee JS, Park SC. Enzyme production of Trichoderma reesei rut C-30 on various lignocellulosic substrates. Appl Biochem Biotechnol. 2000;84:237–245. doi: 10.1385/ABAB:84-86:1-9:237. [DOI] [PubMed] [Google Scholar]
- Siddiqui IA, Shaukat SS. Combination of pseudomonas aeruginosa and pochonia chlamydosporia for control of root infecting fungi in tomato. J Phytopathol. 2003;151:215–222. doi: 10.1046/j.1439-0434.2003.00708.x. [DOI] [Google Scholar]
- Silva DM, Batista LR, Rezende EF, Fungaro MHP, Sartori D, Alves E. Identification of fungi of the genus aspergillus section nigri using polyphasic taxonomy. Braz J Microbiol. 2011;42:761–773. doi: 10.1590/S1517-83822011000200044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims REH, Mabee W, Saddler JN, Taylor M. An overview of second-generation biofuel technologies. Bioresour Technol. 2010;101:1570–1580. doi: 10.1016/j.biortech.2009.11.046. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Kumar S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YM, Zhu XZ, Huang JZ, Ma SJ, Wu XB, Long MN, Chen QX. Purification and properties of endoglucanase from a sugarcane bagasse hydrolyzing strain, aspergillus glaucus XC9. J Agric Food Chem. 2010;58:6126–6130. doi: 10.1021/jf1003896. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: fexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomme P, Warren RAJ, Gilkes NR. Cellulose hydrolysis by bacteria and fungi. Adv Microb Physiol. 1995;37:1–81. doi: 10.1016/S0065-2911(08)60143-5. [DOI] [PubMed] [Google Scholar]
- van Peij NNME, Gielkens MMC, de Veies RP, Visser J, de Graff LH. The transcriptional activator XlnR regulates both xylanolytic endoglucanase gene expression in aspergillus niger. Appl Environ Microbiol. 1998;64:3615–3617. doi: 10.1128/AEM.64.10.3615-3619.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma VS, Ramu K, Kalamdhad AS. Carbon decomposition by inoculating Phanerochaete chrysosporium during drum composting of agricultural waste. Environ Sci Pollut Res. 2015;22:7851–7858. doi: 10.1007/s11356-014-3989-y. [DOI] [PubMed] [Google Scholar]
- Walters KE, Capocchi JK, Albright MB, Hao Z, Brodie EL, Martiny JB. Routes and rates of bacterial dispersal impact surface soil microbiome composition and functioning. ISME J. 2022;16:2295–2304. doi: 10.1038/s41396-022-01269-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood TM, Bhat KM. Method for measuring cellulase activities Methods in Enzymology. New York, NY, USA: Academic Press; 1998. pp. 87–112. [Google Scholar]
- Wood TM, McCrae SI. The cellulase of Trichoderma koningii. Purification and properties of some endoglucanase components with special reference to their action on cellulose when acting alone and in synergism with the cellobiohydrolase. Biochem J. 1978;171:61–72. doi: 10.1042/bj1710061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Dong Y. Design and application of an efficient cellulose-degrading microbial consortium and carboxymethyl cellulase production optimization. Front Microbiol. 2022;13:957444. doi: 10.3389/fmicb.2022.957444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YHP, Lynd LR. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: noncomplexed cellulase systems. Biotechnol Bioeng. 2004;88:797–824. doi: 10.1002/bit.20282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data generated or analysed during this study are included in this published article.