Abstract
Eosinophilic myocarditis (EM) is a rare disease associated with significant morbidity and mortality. This case series follows the clinical courses of 3 patients with EM. The use of mepolizumab, an anti–interleukin-5 monoclonal antibody, as an adjunctive treatment was associated with stabilization of cardiac function and improved long-term outcomes.
Key Words: eosinophilic myocarditis, interleukin-5, myocarditis, reduced ejection fraction, steroid-sparing
Graphical abstract
Eosinophilic myocarditis (EM) is a rare form of myocardial inflammation characterized by eosinophilic infiltration. Two-thirds of EM cases have underlying causes such as hypereosinophilic syndrome (HES), eosinophilic granulomatosis with polyangiitis (EPGA), and hypersensitivity reactions.1 Eosinophils mediate cellular damage in a distinctive pattern causing myocardial dysfunction with a variety of clinical manifestations.2 Early diagnosis and treatment are critical because EM is associated with significant morbidity and mortality.1
Learning Objectives
-
•
To recognize the challenges of treating recurrent EM.
-
•
To understand evidence supporting the use of mepolizumab in EM treatment.
-
•
To identify a role for mepolizumab as maintenance therapy for EM.
Currently, no evidence-based guidelines for management of EM exist. The criterion standard treatment is an extended course of high-dose corticosteroids, which are associated with numerous adverse health effects.3 Mepolizumab, an anti–interleukin (IL)-5 monoclonal antibody, has proven to be effective in lowering eosinophil counts and reducing steroid dependence and is FDA approved for the treatment of HES and EGPA.4, 5, 6 In this case series, we describe 3 patients with EM who were treated with mepolizumab.
Cases
Patient 1
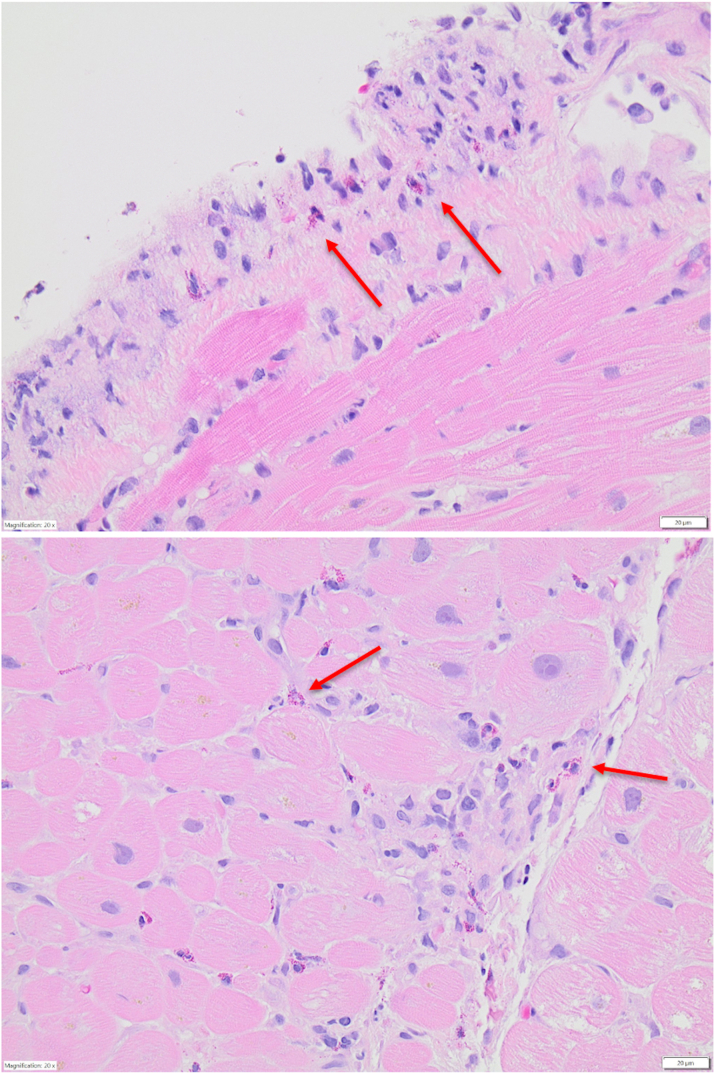
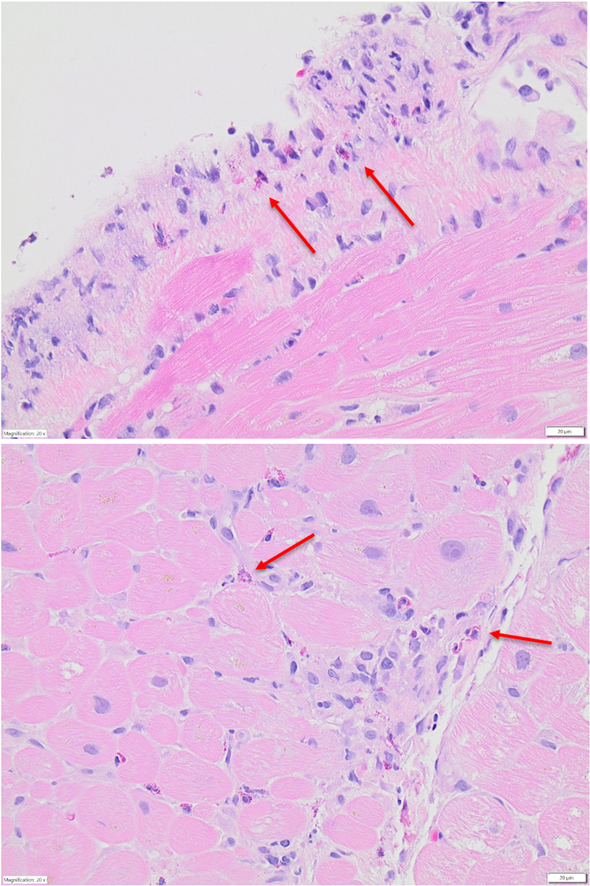
A 34-year-old woman with a history of eczema and asthma presented to the emergency department with transient amnesia and was diagnosed with acute stroke with the use of brain imaging. She was also found to have a globally hypokinetic left ventricle with ejection fraction (LVEF) of 40% and a moderate-size pericardial effusion with signs of early tamponade on echocardiography. Urgent pericardiocentesis with pericardial fluid analysis demonstrated a predominance of eosinophils. Laboratory studies revealed severe peripheral eosinophilia with an absolute eosinophil count (AEC) of 5.26 × 103/μL. Troponin I and B-type natriuretic peptide levels were elevated. Cardiac magnetic resonance imaging (CMR) showed LVEF of 31% as well as evidence of myopericarditis, endomyocardial fibrosis, and left ventricular mural thrombus. The patient was treated with high-dose glucocorticoids for suspected myocarditis. Endomyocardial biopsy confirmed EM (Figure 1), which was attributed to HES as hematologic and rheumatologic evaluations were unrevealing. The patient improved clinically with complete resolution of peripheral eosinophilia. She was discharged with a high-dose prednisone taper, metoprolol succinate, lisinopril, and warfarin.
Figure 1.
Patient 1 Endomyocardial Biopsy
Endomyocardial biopsy demonstrating eosinophil infiltration of the myocardium (red arrows indicate eosinophils).
Repeated CMR 8 months later revealed improved LVEF of 46% and resolving myocardial inflammation according to T2-weighted imaging. Given clinical improvement, prednisone was stopped. Within 2 months, however, she developed relapsing symptoms and rising eosinophils again, requiring treatment with a prolonged prednisone taper. To target eosinophil activity, she was started on 300 mg mepolizumab subcutaneously every 28 days, which she tolerated well. Prednisone was tapered off within 1 month. At 16 months after mepolizumab initiation, she had no symptom recurrence and had not required rehospitalization. Notably, repeated CMR showed complete resolution of inflammation and stable LVEF (Figure 2).
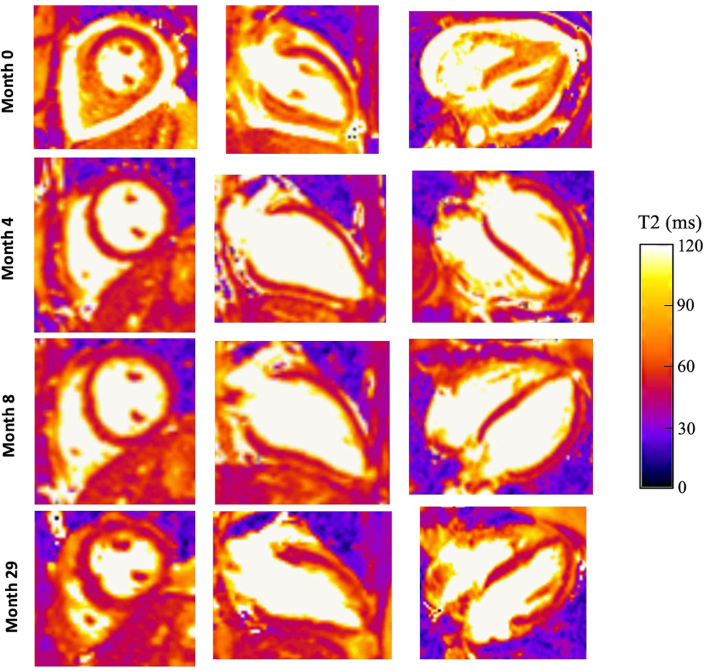
Figure 2.
Patient 1 Serial T-2 Weighted CMR Imaging
Comparison of serial T2-weighted cardiac magnetic resonance (CMR) images for patient 1. At time of diagnosis (month 0), CMR imaging demonstrated diffusely elevated T2 signal throughout the myocardium. After corticosteroid initiation, CMR imaging (months 4 and 8) showed resolution of elevated T2 signal. Sixteen months after initiation of mepolizumab and cessation of maintenance corticosteroids, T2-weighted CMR imaging (month 29) showed no evidence of myocardial inflammation or edema.
Patient 2
A 65-year-old man with a history of asthma was diagnosed with myocarditis with the use of CMR after several hospitalizations for chest pain. Laboratory studies demonstrated an AEC of 0.5 K/μL at initial hospitalization. He was treated with colchicine and his symptoms improved. Eight years after the initial diagnosis of myocarditis, he was hospitalized again for recurrent, severe chest pain and significant eosinophilia (AEC 0.99 × 103/μL). Repeated left heart catheterization redemonstrated nonobstructive coronary artery disease (CAD), and CMR showed acute on chronic myocarditis with LVEF of 50%. Colchicine once more improved his symptoms.
This patient’s history of asthma, significant peripheral eosinophilia, and recurrent myocarditis confirmed with the use of CMR are consistent with EGPA-related EM. Given its efficacy treating EGPA, 300 mg mepolizumab subcutaneously every 28 days was initiated with significant relief from chest pain and asthma symptoms. One year later, he had presented to the emergency department once for chest pain. At the time, his cardiac and hematologic tests were unremarkable and he was discharged. Repeated CMR demonstrated no significant changes and stable LVEF. At follow-up, the patient endorsed greatly improved functional status without any cardiac symptoms.
Patient 3
A 61-year-old woman with history of CAD, recent left-side ischemic stroke, hyperlipidemia, hypertension, asthma, and allergic rhinitis was hospitalized for acute right-side ischemic stroke. Days before, she was admitted for treatment of antigen-positive Legionella pneumonia and had resolution of her bilateral infiltrates according to chest x-ray by the time of discharge. Work-up for recurrent stroke revealed a newly reduced LVEF of 40%-45% with inferior and inferolateral wall hypokinesis on echocardiography. Myocardial perfusion imaging was not suggestive of ischemia. She was hospitalized again 2 months later for acute sensorimotor deficits and dyspnea and was found to have left basal ganglia intraparenchymal hemorrhage. Computed tomography of the chest demonstrated diffuse infiltrates. Diagnostic work-up demonstrated a white blood cell count of 21.38 × 103/μL with an AEC of 11.3 × 103/μL and elevated troponin. Bronchoalveolar lavage was hypocellular with no eosinophils present and unrevealing regarding infectious etiology. Echocardiography redemonstrated a mildly reduced LVEF with regional wall motion abnormalities. She was discharged on an empiric prednisone taper for her pulmonary symptoms.
On follow-up, her presentation of marked peripheral eosinophilia, recurrent strokes, pulmonary infiltrates, and myocarditis was deemed to be most likely secondary to HES. CMR showed a worsened LVEF of 35%, as well as active myocardial inflammation and prominent subendocardial fibrosis consistent with EM. Given persistent dyspnea, poor functional status, and myocardial inflammation, her prednisone dose was increased, and guideline-directed medical therapy (GDMT) was optimized. Three months after initiation of 300 mg subcutaneous mepolizumab every 28 days for treatment of HES, she experienced improvement in symptoms, functional capacity, and eosinophil count. Maintenance prednisone was successfully tapered without worsening of symptoms. Repeated CMR showed stable LVEF and myocardial inflammation (Figure 3), so reduced-dose prednisone was continued. On a regimen of GDMT, low-dose prednisone, and mepolizumab, she continued to report stable functional capacity and symptoms. Her eosinophil counts remained undetectable. Furthermore, LVEF improved to 40%-45% on echocardiography, and she has not required hospitalization for any cardiac indication.
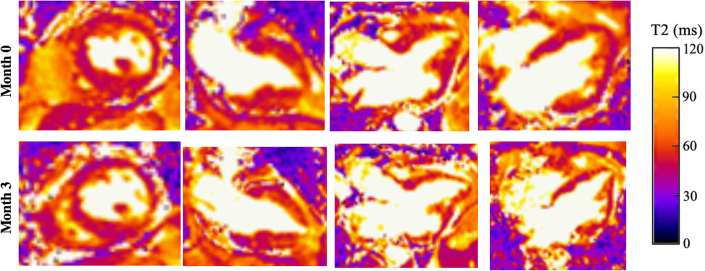
Figure 3.
Patient 3 Serial T-2 Weighted CMR Imaging
Comparison of serial T2-weighted cardiac magnetic resonance (CMR) images for patient 3. Before initiation of mepolizumab and after initial steroid taper (month 0), CMR imaging demonstrated diffusely elevated T2 signal throughout the myocardium. After the initiation of mepolizumab in combination with a reduced prednisone dose, CMR imaging (month 3) showed stability of elevated T2 signal in the setting of clinical symptom improvement.
Discussion
EM is a rare cause of myocardial inflammation, with high rates of morbidity and mortality. Although a small percentage of EM cases have disease-specific treatments, most require treatment with long-term corticosteroids to prevent relapsing symptoms.1, 2, 3 Mepolizumab, an anti–IL-5 monoclonal antibody, is a promising steroid-sparing adjunctive treatment for EM.
The present case series describes the clinical courses of 3 patients with recurrent HES or EPGA-related EM. Mepolizumab was selected for treatment, instead of other immunosuppressives, because it directly targets eosinophils with fewer adverse effects and has proven efficacy treating HES and EGPA.4, 5, 6 All patients demonstrated stabilization or improvement in myocardial inflammation and LVEF on serial cardiac imaging, and no patients required rehospitalization for cardiac symptoms. Patients 1 and 3 had long-term corticosteroid dependence, yet both significantly reduced or discontinued steroid use after starting mepolizumab. In addition, all patients endorsed significant improvement in functional status and symptoms once mepolizumab was initiated. Because no long-term studies exist, mepolizumab will be continued indefinitely and reevaluated based on individualized risk-benefit discussions. Clinical courses are summarized in Table 1.
Table 1.
Summary of Clinical Courses
| Patient # | Eosinophilia Diagnosis | Presentation |
Follow-Up |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms/Diagnosis | Imaging | Active Inflammation | Histology | Steroid Treatment | Other Therapies | Imaging | Steroid Dependence | Cardiac-Related Readmissions | ||
| 1 | HES | Transient amnesia, CVA, moderate pericardial effusion with early tamponade | TTE LVEF 35%-40%; CMR LVEF 31% |
Yes | Eosinophils on myocardial, bone marrow, and pleural fluid samples | 60 mg prednisone followed by taper | 300 mg mepolizumab monthly | TTE LVEF At rest 45%-50%, stress 60%-65% | No | 0 |
| 2 | EGPA | Chest pain, myopericarditis | CMR LVEF 50% | Yes | None | None | Colchicine, 300 mg mepolizumab monthly | TTE LVEF 50%-55% | No | 0 |
| 3 | HES | Basal ganglia IPH | TTE LVEF 45%-50% | Unknown | None | 40 mg prednisone daily for 14 days then every other day | 300 mg mepolizumab monthly | TTE LVEF 40%-45% | Yes, at reduced dose | 0 |
CMR = cardiac magnetic resonance imaging; CVA = cerebrovascular accident; EGPA = eosinophilic granulomatosis with polyangiitis; LVEF = left ventricular ejection fraction; HES = hypereosinophilic syndrome; IPH = intraparenchymal hemorrhage; TTE = transthoracic echocardiography.
Although there are no studies evaluating the efficacy of mepolizumab in EM, previous case reports demonstrate similar improvement in cardiac symptoms and testing with mepolizumab.7,8 In comparison, the present publication establishes the sustained benefit of mepolizumab over a longer surveillance period and suggests a potential role for mepolizumab as an adjunctive maintenance therapy for EM.
Our findings are intriguing, but interpretation is complicated by variability in treatment regimens, lack of standardization for LVEF evaluation, and subjectivity of symptom assessment. Nonetheless, this series adds to the growing body of evidence supporting the use of mepolizumab in EM. Future prospective randomized studies would be useful in quantifying the relationship between mepolizumab and cardiac outcomes.
Conclusions
Patients with EM who were treated with mepolizumab had stabilization of LVEF, decreased steroid dependence, and improved cardiac symptoms. This case series adds to the growing body of evidence supporting the use of mepolizumab in treatment of EM. These findings encourage further studies in investigating a role for mepolizumab in routine management of EM.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
Endomyocardial biopsy images were supplied by courtesy of Drs Sergey Brodsky and Stephanie Laus from the Ohio State University Wexner Medical Center Department of Pathology, and CMR images and cine images by courtesy of Dr Karolina Zareba from the Ohio State University Wexner Medical Center Division of Cardiovascular Medicine.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Brambatti M., Matassini M.V., Adler E.D., et al. Eosinophilic myocarditis: characteristics, treatment, and outcomes. J Am Coll Cardiol. 2017;70(19):2364–2375. doi: 10.1016/j.jacc.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 2.Cheung C., Constantine M., Ahmadi A., et al. Eosinophilic myocarditis. Am J Med Sci. 2017;354(5):486–492. doi: 10.1016/j.amjms.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Kuchynka P., Palecek T., Masek M., et al. Current diagnostic and therapeutic aspects of eosinophilic myocarditis. Biomed Res Int. 2016;2016 doi: 10.1155/2016/2829583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothenberg M.E., Klion A.D., Roufosse F.E., et al. Treatment of patients with hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358:1215–1228. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 5.Roufosse F., Kahn J.E., Rothenberg M.E., et al. HES Mepolizumab Study Group Efficacy and safety of mepolizumab in hypereosinophilic syndrome: a phase III, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;146(6):1397–1405. doi: 10.1016/j.jaci.2020.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wechsler M.E., Akuthota P., Jayne D., et al. EGPA Mepolizumab Study Team Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376(20):1921–1932. doi: 10.1056/NEJMoa1702079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tengyao S., Jones D.M., Homsi Y. Therapeutic effect of anti–IL-5 on eosinophilic myocarditis with large pericardial effusion. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2016-218992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bello F., Emmi G., Tamburini C., et al. Eosinophilic granulomatosis with polyangiitis-related myocarditis during mepolizumab therapy reveals a TH1/TH17-mediated vasculitic response. Clin Exp Rheumatol. 2022;40(4):863–864. doi: 10.55563/clinexprheumatol/envpc5. [DOI] [PubMed] [Google Scholar]