Abstract
Inflammatory bowel disease (IBD) is a complex chronic inflammatory intestinal disease. The development of de novo IBD after solid organ transplantation with immunosuppressive agents has been rarely reported. We present the case of a 65-year-old man with repeated colitis after heart transplantation (HTx) who was diagnosed with Crohn's disease (CD). The patient underwent HTx due to non-ischemic dilated cardiomyopathy. Six months after HTx, he developed serious diarrhea and a transient fever, which persisted for about 6 months. Valganciclovir or any antibiotic agents were not effective for his symptoms and longitudinal ulcers in colonoscopy aggravated during the course, so that we made a diagnosis of CD. We started 5-aminosalicylic acid and found improvement in his symptoms and colonoscopic findings. However, 7 months after improvement, CD worsened. We started ustekinumab by which his condition successfully went into remission again. While oral immunosuppressive drugs are thought to suppress autoimmune diseases in general, IBD should be included in the differential diagnoses for recurring enterocolitis after HTx. Poorly controlled CD can lead to serious and potentially fatal complications, but in this case, ustekinumab has been used safely and effectively for the treatment of CD.
Learning objective
Colitis is a common complication after heart transplantation (HTx). Although cytomegalovirus colitis or posttransplant lymphoproliferative disorder are observed commonly, de novo inflammatory bowel disease (IBD) should be considered when serious refractory colitis occurs. Not only 5-aminosalicylic acid but also ustekinumab, which is a monoclonal antibody to the p40 subunit of interleukin (IL)-12 and IL-23, may be a safe and effective treatment for de novo IBD after HTx.
Keywords: Heart transplantation, De novo inflammatory bowel disease, Ustekinumab
Introduction
Colitis after transplantation has been known to occur as a possible complication. Causes of colitis include cytomegalovirus (CMV), posttransplant lymphoproliferative disorder (PTLD), tuberculosis, and so forth. Development of inflammatory bowel disease (IBD) in patients on immunosuppressive therapy is said to be rare, but there is an estimated ten times higher risk of IBD after solid organ transplantation than that in the general population, and CMV mismatch (donor positive/recipient negative) was associated with a 4.5-fold higher risk of de novo IBD [1]. Here, we report the case of a 65-year-old man with CMV mismatch and repeated colitis after heart transplantation (HTx) who was finally diagnosed with de novo Crohn's disease (CD). Although he presented with severe CD at the diagnosis, he safely improved with 5-aminosalicylic acid (5-ASA) first, followed by ustekinumab.
Case report
A man with dilated cardiomyopathy received HTx at the age of 62 years. We started prednisolone (PSL), tacrolimus (TCR), and mycophenolate mofetil (MMF) as immunosuppressive therapy, and also valganciclovir (VGCV) as prophylactic therapy for CMV for 3 months because of CMV mismatch (donor positive/recipient negative). Considering neutropenia and prevention of CMV infection, we switched from MMF to everolimus (EVL) at 3 months. Although he had no episode suggestive of colitis before HTx (Fig. 1a), six months after HTx, he developed abdominal pain, severe diarrhea (7–8 motions/day), and a transient fever (37.8 °C) and was hospitalized for the first time with colitis. Laboratory test showed elevated C-reactive protein [CRP: 5.45 mg/dL (reference range: 0–0.3 mg/dL)]. The trends of TCR and EVL trough were controlled properly. CMV antigenemia was negative. No notable organisms were detected in stool culture and Clostridium difficile toxin was negative. Colonoscopy revealed small ulcers throughout the colon except the terminal ileum (Fig. 1b). Biopsy of the ulcers showed no evidence of CMV colitis, tuberculosis, or PTLD. Because both CMV antigenemia and immunohistochemistry staining are less sensitive, he was followed up with continued administration of VGCV as suspected CMV colitis and his symptoms improved gradually.
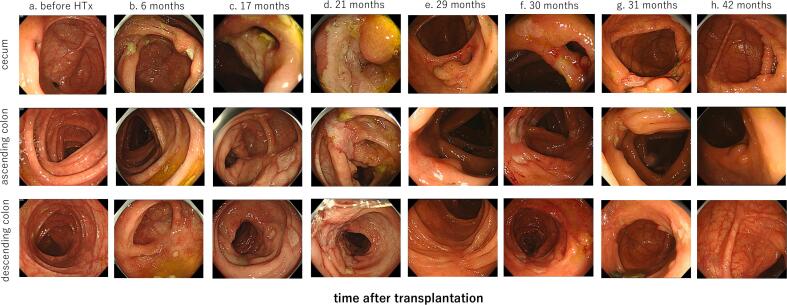
Fig. 1.
Colonoscopy findings of cecum, ascending colon, and descending colon.
(a) Before HTx, there was only nonspecific erythema at rectum, possibly due to the drug, and no malignant findings.
(b) Six months after HTx, first admission, small ulcers throughout the colon except the terminal ileum were detected.
(c) Seventeen months after HTx, second admission, small colonic ulcers gradually increased.
(d) Twenty-one months after HTx, third admission, colonic ulcers increased and fused together to form longitudinal ulcers.
(e) After administration of 5-ASA for treatment of de novo CD, colonic ulcers were almost healed.
(f) The small ulcers had reappeared, which suspected exacerbation of the de novo CD.
(g) Ulcers were generally improved after introduction of ustekinumab.
(h) Ulcers have maintained remission for >1 year after introduction of ustekinumab.
HTx, heart transplantation; 5-ASA, 5-aminosalicylic acid; CD, Crohn's disease.
Seventeen months after HTx, the same symptoms flared up and he was hospitalized for the second time. CMV antigenemia was negative. Colonoscopy showed that the small colonic ulcers had gradually increased, but biopsy or stool culture showed no obvious findings (Fig. 1c). Since there was no definite evidence of CMV colitis, we did not administer ganciclovir or foscarnet, and started fasting and antibiotics considering bacterial translocation from the ulcer lesions. Also, we returned from EVL to MMF considering side effects of delayed wound healing with EVL, and then his colitis was alleviated.
He became febrile 10 days after discharge and another 1 month later (21 months after HTx), his diarrhea flared up again and he was hospitalized for the third time. CMV antigenemia and C. difficile toxin results were still negative. No notable organisms were detected in stool culture. Colonoscopy showed that the colonic ulcers had increased and fused together to form longitudinal ulcers (Fig. 1d). Biopsy still showed no obvious findings. Capsule endoscopy revealed no small bowel lesions. From these findings, the possibility of de novo CD after HTx was considered as a diagnosis of exclusion, and 5-ASA and elemental diet were started. A few days after the treatment began, his symptoms and the inflammation improved. Colonoscopy also showed improvement in ulcerations (Fig. 1e). However, 5-ASA was discontinued due to drug-induced liver injury at 24 months after HTx, and 30 months after HTx, he was again hospitalized due to the exacerbation of the de novo CD (Fig. 1f). Because he had already taken TCR and MMF to prevent rejection, ustekinumab, a biological medicine, 90 mg/12-week interval was started. A few weeks later, his condition and colonoscopy findings generally improved in ulcerations (Fig. 1g), which proved that ustekinumab was effective for our patient. He responded well for >1 year (Fig. 1h) while using ustekinumab treatment without any specific adverse events (Fig. 2). Myocardial biopsy, which was performed 6 months after the initiation of ustekinumab, showed no rejection and cardiac function was well maintained.
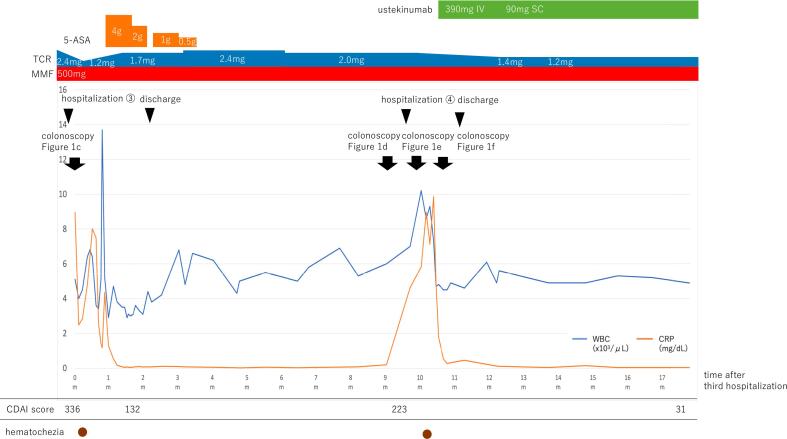
Fig. 2.
Clinical course since the third hospitalization. Inflammation reaction reduced after 5-ASA treatment. Due to drug-induced liver injury, 5-ASA had to be discontinued and the colitis flared up. Ustekinumab improved colitis and did not induce rejection in grafted heart.
WBC, white blood cell; CRP, C-reactive protein; TCR, tacrolimus; MMF, mycophenolate mofetil; CDAI, Crohn's Disease Activity Index; 5-ASA, 5-aminosalicylic acid; IV, intravenous injection; SC, subcutaneous injection; m, month.
Discussion
We report an elderly heart transplant recipient who developed de novo CD after HTx and was treated successfully with ustekinumab. To the best of our knowledge, this is the first case treated with ustekinumab for de novo CD after HTx.
Colitis is one of the common complications after HTx. The incidence of de novo IBD after solid transplantation with immunosuppressive therapy was also reported to be higher than that in the general population (206 versus 20 per 100,000 cases annually) [1]. In the mechanism of developing CD, it is considered that the imbalance between pro-inflammatory and anti-inflammatory cytokines in the mucosa may lead to the development and potential perpetuation of mucosal inflammation in patients with IBD [2]. The use of immunomodulators, such as TCR, cyclosporine A, and MMF, causes a down-regulation of regulatory T-cells in the colonic mucosa. This effect may create a propensity to develop immune-mediated inflammations in the colon, as regulatory T-cells prevent activation of B and cytotoxic T-lymphocytes [3]. Therefore, it is currently known that post transplantation state is potentially responsible for giving rise to development of IBD [1], and this may also apply to recipients over 60 years of age, such as our patient.
Paradoxically, there is some evidence to support the use of TCR or MMF for treating active IBD and these medicines appear to be effective in treating IBD [4]. Also, various studies have confirmed that mammalian target of rapamycin (mTOR) is upregulated in active forms of IBD and inhibition of mTOR suppressed the gene expressions of several pro-inflammatory cytokines in IBD [5]. Immunosuppressive agents appear to be effective in treating IBD while these can induce colitis resembling IBD or lead to secondary IBD. Therefore, we think the relation between the change in immune balance by immunosuppressive agents and the development of IBD in post-transplant individuals need further investigation.
CD is a chronic inflammatory disease of the gastrointestinal tract with symptoms evolving in a relapsing and remitting manner, which leads to bowel damage and disability. Most patients present with an inflammatory phenotype at diagnosis, but some complications such as strictures, fistulas, or abscesses will develop in half of patients, often resulting in surgery [6].
In our case, cobblestone appearance in colonoscopy, anal lesions, and obvious pathological findings were absent. However, he developed a longitudinal ulcer in spite of several previous treatments, which suggested the possibility of de novo CD. Since he presented with severe CD [Crohn's Disease Activity Index (CDAI) = 336 points], even under the medications of TCR and EVL as maintenance of immunosuppressive therapy after HTx, we firstly administered 5-ASA for his CD. 5-ASA, which is thought to improve the imbalance of cytokines and is generally used for mild to moderate cases of CD, was effective in our case. However, it has been reported that some patients cannot tolerate 5-ASA [7]. Indeed, our case also had intolerance to 5-ASA, and he experienced a recurrence of CD. Ustekinumab is a monoclonal antibody to the p40 subunit of interleukin (IL)-12 and IL-23, which inhibits the differentiation of CD4-positive naive T cells into Th1 and the activation of Th17 differentiation, thereby reducing inflammation, and exerting therapeutic effects. It was approved for patients with moderate-to-severe CD who experienced treatment failure or intolerance with conventional therapies [8]. Ustekinumab leads to a lower incidence of antidrug antibodies than anti-tumor necrosis factor-α antibodies and has fewer side effects such as infection or malignancy [8]. Also, ustekinumab is reported to take effect more immediately than vedolizumab [9]. There were several reports about the effectiveness of ustekinumab for CD developed in liver transplant recipients [10]. However, there are no reports on the use of ustekinumab for the treatment of de novo CD in patients after HTx. In our case, ustekinumab improved CD status and has maintained remission for >1 year (CDAI 31 points) without any significant adverse effects. Also, it did not affect the maintenance of graft function post-HTx. In summary, we treated a case with repeated colitis which resembles CMV colitis and was diagnosed with CD. De novo IBD should be considered when serious refractory colitis occurs. Also, our case demonstrates that ustekinumab is safe and effective for de novo IBD after HTx.
Consent statement
Informed consent was obtained from the patient for the publication of the case.
Funding statement
None.
Declaration of competing interest
EA belongs to the Department, endowed by NIPRO-Corp, Terumo-Corp., Senko Medical-Instrument-Mfg., Century-Medical, Inc., ONO-pharmaceutical-Co., Ltd. Medtronic-JAPAN Co., Ltd., Nippon-Shinyaku Co., Ltd., Mochida Pharmaceutical Co., Boehringer Ingelheim Pharmaceuticals Inc., Abiomed-Inc, AQuA-Inc, Fukuda-Denshi Co., Ltd., and Sun-Medical-Technology-Research Corp. EA received research fund from Bristol-Myers Squibb Co. The other authors declare that there is no conflict of interest regarding the publication of this article.
Acknowledgment
None.
Contributor Information
Chie Bujo, Email: bujoc-int@h.u-tokyo.ac.jp.
Eisuke Amiya, Email: amiyae-tky@umin.ac.jp.
References
- 1.Ghouri Y.A., Tahan V., Shen B. Secondary causes of inflammatory bowel diseases. World J Gastroenterol. 2020;26:3998–4017. doi: 10.3748/wjg.v26.i28.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neurath M.F. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 3.Nepal S., Navaneethan U., Bennett A.E., Shen B. De novo inflammatory bowel disease and its mimics after organ transplantation. Inflamm Bowel Dis. 2013;19(7):1518–1527. doi: 10.1097/MIB.0b013e3182813365. [DOI] [PubMed] [Google Scholar]
- 4.Allen P.B., Peyrin-Biroulet L. Immunomodulator for the treatment of Crohn’s disease in adults: optimal use and prospects for future drug treatments. Expert Rev Clin Immunol. 2016;12:741–749. doi: 10.1586/1744666X.2016.1154789. [DOI] [PubMed] [Google Scholar]
- 5.Lashgari N.A., Roudsari N.M., Momtaz S., Ghanaatian N., Kohansal P., Farzaei M.H., et al. Targeting mammalian target of rapamycin: prospects for the treatment of inflammatory bowel diseases. Curr Med Chem. 2021;28:1605–1624. doi: 10.2174/0929867327666200504081503. [DOI] [PubMed] [Google Scholar]
- 6.Torres J., Mehandru S., Colombel J.F., Peyrin-Biroulet L. Crohn’s disease. Lancet. 2017;389:1741–1755. doi: 10.1016/S0140-6736(16)31711-1. [DOI] [PubMed] [Google Scholar]
- 7.Hiraoka S., Fujiwara A., Toyokawa T., Higashi R., Moritou Y., Takagi S., et al. Multicenter survey on mesalamine intolerance in patients with ulcerative colitis. J Gastroenterol Hepatol. 2021;36:137–143. doi: 10.1111/jgh.15138. [DOI] [PubMed] [Google Scholar]
- 8.Bots S.J., Parker C.E., Brandse J.F., Löwenberg M., Feagan B.G., Sandborn W.J., et al. Anti-drug antibody formation against biologic agents in inflamatory bowel disease: a systematic review and meta-analysis. BioDrugs. 2021;35:715–733. doi: 10.1007/s40259-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer F., Wil-Verhoeven D., Prati C., Wendling D., Verhoeven F. Corrigendum to safety of biologic treatments in solid organ transplant recipients: a systematic review. Semin Arthritis Rheum. 2021;51:1263–1273. doi: 10.1016/j.semarthrit.2021.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Shibuya T., Nomura O., Nagahara A. Safety and efficacy of ustekinumab for ulcerative colitis in a liver transplant patient. Inflamm Bowel Dis. 2021;27 doi: 10.1093/ibd/izab169. e150-e51. [DOI] [PubMed] [Google Scholar]