Key Points
Question
Is a self-administered acupressure (SAA) training program effective for relieving pain in patients with probable knee osteoarthritis (OA)?
Findings
In this randomized clinical trial of 314 middle-aged and older adults, the SAA group had a significantly greater improvement in the numerical rating scale pain score than the knee health education group over 12 weeks. A cost-effectiveness acceptability curve demonstrated a greater than 90% probability of SAA being cost-effective at a willingness to pay threshold of 1 GDP per capita.
Meaning
These findings suggest that the implementation of an SAA training program is an efficacious and cost-effective approach to relieve knee pain in middle-aged and older adults with probable knee OA.
This randomized clinical trial evaluates the effectiveness of self-administered acupressure on reducing knee osteoarthritis (OA) pain among middle-aged and older adults.
Abstract
Importance
The effects of self-administered acupressure (SAA) on knee osteoarthritis (OA) pain remain unclear.
Objective
To evaluate the effectiveness of SAA taught via a short training course on reducing knee OA pain in middle-aged and older adults.
Design, Setting, and Participants
This randomized clinical trial was conducted among community-dwelling individuals in Hong Kong who were aged 50 years or older with probable knee OA from September 2019 to May 2022.
Interventions
The intervention included 2 training sessions for SAA with a brief knee health education (KHE) session, in which participants practiced acupressure twice daily for 12 weeks. The control group (KHE only) received only education about maintaining knee health on the same schedule and duration.
Main Outcomes and Measures
The primary outcome was the numerical rating scale (NRS) pain score at 12 weeks. Other outcomes included Western Ontario and McMaster University Osteoarthritis Index, Short Form 6 Dimensions (SF-6D), Timed Up and Go, and Fast Gait Speed tests.
Results
A total of 314 participants (mean [SD] age, 62.7 [4.5] years; 246 [78.3%] female; mean [SD] knee pain duration, 7.3 [7.6] years) were randomized into intervention and KHE-only groups (each 157). At week 12, compared with the KHE-only group, the intervention group had a significantly greater reduction in NRS pain score (mean difference [MD], −0.54 points; 95% CI, −0.97 to −0.10 points; P = .02) and higher enhancement in SF-6D utility score (MD, 0.03 points; 95% CI, 0.003 to 0.01 points; P = .03) but did not have significant differences in other outcome measures. The cost-effectiveness acceptability curve demonstrated a greater than 90% probability that the intervention is cost-effective at a willingness to pay threshold of 1 GDP per capita.
Conclusions and Relevance
In this randomized clinical trial, SAA with a brief KHE program was efficacious and cost-effective in relieving knee pain and improving mobility in middle-aged and older adults with probable knee OA.
Trial Registration
ClinicalTrials.gov Identifier: NCT04191837
Introduction
Knee osteoarthritis (OA) is a debilitating condition that is common among people older than 50 years, and 23% of people aged 40 years or older are affected.1 Knee OA is associated with long-term health consequences, such as reduced physical fitness, low quality of life, and increased health care utilization.2,3,4 Conventional analgesic medications, such as nonsteroidal anti-inflammatory drugs and acetaminophen, are effective for the treatment of knee OA.5 However, the use of these agents is limited due to concerns regarding potential gastrointestinal adverse effects. Nonpharmacological treatments such as patient education, exercise, weight loss, and physical therapy produce substantial benefits,6 but the delayed therapeutic effects and requirement of significant changes in behavior discourage individuals from receiving such therapeutic measures. Given the limitations of the conventional treatments, complementary health approaches are commonly sought by patients with knee OA.7
Self-administered acupressure (SAA) has been used for different pain conditions,8,9,10 and it could be an effective treatment for knee OA pain.11 Acupressure stimulates the same acupoints as acupuncture with the use of fingers, hands, or elbows, based on traditional Chinese medicine (TCM) meridian theory. The analgesic mechanism of acupressure may primarily involve the suppression of peripheral inflammation through the regulation of inflammatory signal transduction12 and pain signal transduction pathway13 modulation of ion channels and inhibition of glial cell activation.14,15 Recently, clinical trials have attempted to examine the feasibility and effectiveness of SAA for the treatment of knee OA in postmenopausal women16 and older patients.17,18,19 These studies were limited by a nonrandomized design,19 no fidelity assessment,16 and high attrition rate.16 A randomized clinical trial (RCT) compared the effects of SAA with sham SAA and usual care in participants with knee OA aged 65 years or older,18 in which they found, compared with usual care, that the SAA and sham acupressure groups showed significantly greater improvements in the numerical rating scale (NRS) of pain, Western Ontario and McMaster University Osteoarthritis Index (WOMAC) pain, and function scores. However, the tested acupressure protocol was not empirically used for knee pain reduction in clinical practice20 but was borrowed from a previous trial targeting sleep disturbance and fatigue in cancer survivors.21
Therefore, the effectiveness of the traditionally used acupoints now being used in clinical practice for knee OA remains uncertain. To bridge this knowledge gap, we conducted this RCT to examine the short-term (4-week) and medium-term (8- and 12-week) effectiveness of SAA on traditionally used acupoints, along with health economic analyses, for relieving knee OA pain in middle-aged and older adults.
Methods
Setting and Design
This study was an assessor-blinded, 2-group RCT. All eligible participants were randomized to the SAA intervention group or the knee health education (KHE)–only comparison group at a 1:1 ratio. The methods and statistical analysis plan are described in Supplement 1. This study was reported following the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.22 The study protocol was approved by the Hong Kong Polytechnic University Human Subjects Ethics Subcommittee.
Participants
The participants were recruited from September 2019 to May 2022 through posters in the Hong Kong Polytechnic University, community centers, and social media. Written informed consent was obtained from all participants. Eligible participants aged 50 years or older were able to comprehend Chinese and fulfilled any 3 of the following criteria: (1) morning stiffness for 30 minutes or less; (2) crepitus on active joint motion; (3) bone tenderness; (4) bone enlargement; or (5) no palpable joint warmth. These criteria yielded 84% sensitivity and 89% specificity for OA knee diagnosis in those aged 50 years or older.23 Participants also had knee pain for at least 3 months24 and rated their pain levels as 3 or greater on the NRS. The exclusion criteria were (1) knee pain related to other conditions (eg, cancer, fracture, rheumatoid arthritis, and rheumatism) according to the red flags for further investigation or referral in the National Institute for Health and Care Excellence Guidelines for Osteoarthritis of the knee25; (2) history of acupressure, acupuncture, or steroid injection for knee pain in the past 6 months; (3) previous knee replacement surgery; (4) medical diagnoses or conditions that precluded individuals from active participation (eg, bleeding disorders, alcohol or drug abuse); (5) pregnancy; (6) a body mass index greater than 30 (body mass index is calculated as weight in kilograms divided by height in meters squared)26; (7) a score of less than 22 in Hong Kong Montreal Cognitive Assessment27; and (8) presence of skin lesions or infections at the treatment sites.
Intervention
The 2-hour training sessions for both groups were held over 2 consecutive weeks, 1 week apart (total of 4 hours; eFigure in Supplement 2). The SAA intervention protocol was developed based on TCM meridian theory by previous studies16,20 and modified by the experienced acupuncturists (W.-F. Y. and L.L.). The KHE protocol was developed based on the materials from the websites of Elderly Health Service, Department of Health, Hong Kong SAR28 and modified by a physiotherapist (T.C.C.).17,29 A brief version of KHE was included in the SAA course to provide a comprehensive coverage. The details of interventions are described in eTable 1 and 2 in Supplement 2. The SAA instructors were registered Chinese medicine practitioners with at least 5 years of clinical experience and trained by the experienced acupuncturists (W.-F.Y. and L.L.), while the KHE instructors were registered nurses and received training from a physiotherapist. The principal investigator (W.-F.Y.) randomly visited the classes to ensure the intervention delivery was in accordance with the protocol. The training was conducted in small groups of 4 to 7 participants. The participants’ acupressure performance, including the rhythm, force, technique, and acupoint location, were inspected by the instructor with a competency checklist in each session. Follow-up phone calls were made twice per week to remind the participants to practice and to answer any relevant queries during the first week.
Randomization and Masking
Randomization was conducted by an independent researcher by using block randomization with random block sizes of 4 to 6. The treatment allocation was enclosed in sealed, opaque, sequentially numbered envelopes. The envelopes were disclosed after the participants had completed all baseline assessments. Assessors were masked for group allocation.
Outcomes
Assessments were conducted at baseline and at weeks 4, 8, and 12. The primary outcome measure was pain severity NRS,30 which was an 11-point scale ranging from 0 (no pain) to 10 (greatest pain imaginable). Secondary outcomes included WOMAC, used to assess pain, stiffness and physical functional disability31,32; and the Short Form 6 Dimension (SF-6D), used to assess quality of life.33,34 Timed Up and Go (TUG) and Fast Gait Speed (FGS) tests were used to assess mobility, walking ability, balance, and fall risk.35,36
Intervention compliance throughout the 12-week period was assessed by providing a logbook to participants to record their daily acupressure practice (duration and frequency) in the SAA group or compliance to knee health instructions (whether they had followed the 6 instructions) in the KHE-only group. The acceptability of training content was assessed through a 10-point scale item and open-ended questions. Participants were also asked to report any adverse events (AEs) during their implementation of daily practice.
Cost-Effectiveness Analysis
Quality-adjusted life-years (QALYs) were determined from the SF-6D utility score using the standard area-under-the-curve approach.34 Costs of implementation were evaluated from the perspective of the health care system. Hong Kong’s health care system, a dual-track model, comprises a heavily government-subsidized public sector, providing 90% of inpatient services, and a private sector operating on a fee-for-service basis.37,38 The incremental cost-effectiveness ratio (ICER) was calculated as follows: different in costs divided by difference in effects, where difference in effects represents the difference in the QALYs (changes in SF-6D utility scores) between the SAA and KHE-only groups. To test the robustness and uncertainty of the ICER, we performed bootstrapping involving 5000 iterations, and the results were plotted in a cost-effectiveness plane. A cost-effectiveness acceptability (CEA) curve displaying the probability that the intervention was cost-effective for a range of willingness-to-pay threshold values was also plotted.
Statistical Analysis
The primary analysis was using intention-to-treat approach. The changes in the primary outcome (NRS score) and other secondary outcomes were compared using a linear mixed-effects model (LMM) with group-by–time point interaction (baseline to week 12). Missing data were handled by the LMM. Participants’ sociodemographic and clinical characteristics at baseline were examined for potential group differences by t test or χ2 test. Acceptability and compliance were presented using descriptive statistics. Clinical significance was examined by the proportion of participants who had a reduction of at least 2 points from baseline in the NRS using χ2 test.39 Significance level was set to .05 for all statistical analyses, and all tests were 2-tailed. Data analysis was conducted with SPSS version 28.0.1.0 (IBM Corp).
Results
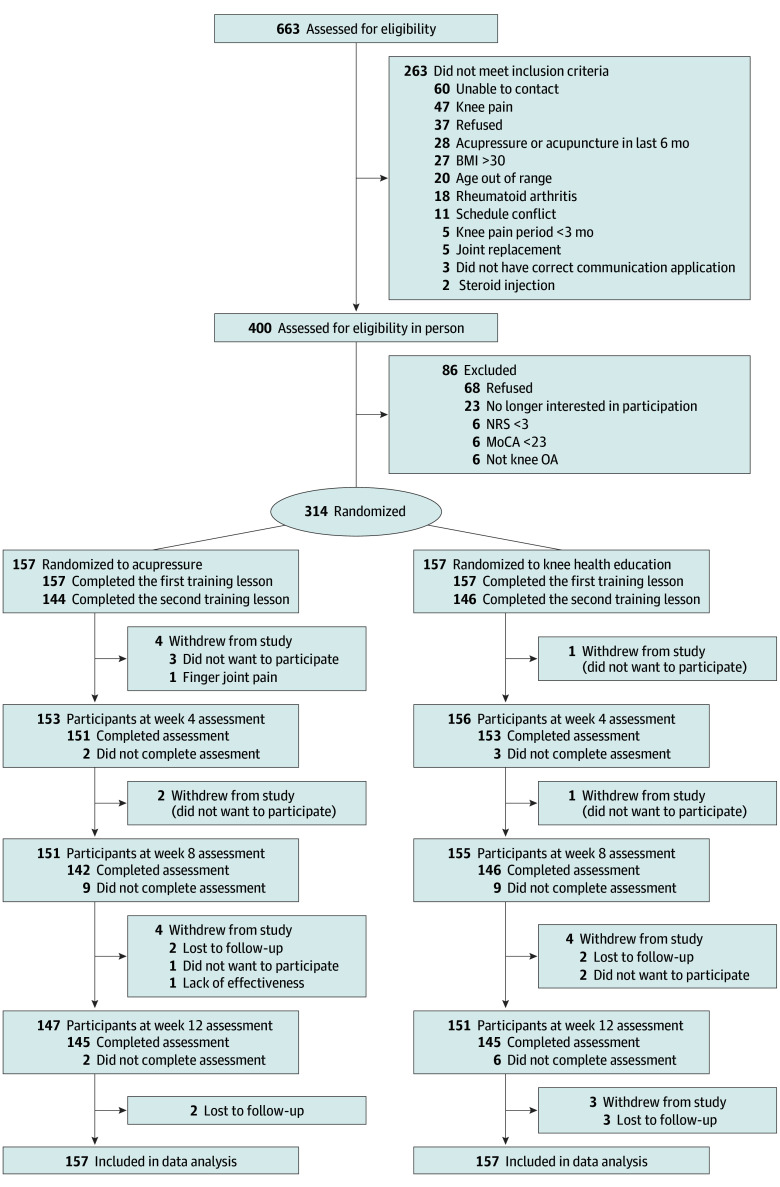
A total of 314 participants (246 female [78.3%]; mean [SD] age, 62.7 [4.5] years; mean [SD] knee pain duration, 7.3 [7.6] years) were randomized into either the SAA or KHE-only group (157 each) (Table 1). Overall, 12 participants in the SAA group (7.6%) and 9 participants in the KHE-only group (5.7%) withdrew, without significant difference (P = .50). The study flow diagram is shown in Figure 1.
Table 1. Baseline Characteristics of the Participants.
| Characteristic | Participants, No. (%) | ||
|---|---|---|---|
| All participants (N = 314) | Self-administered acupressure (n = 157) | Knee health education (n = 157) | |
| Age, mean (SD), y | 62.7 (4.54) | 62.6 (4.72) | 62.8 (4.36) |
| Sex | |||
| Male | 68 (21.7) | 34 (21.7) | 34 (21.7) |
| Female | 246 (78.3) | 123 (78.3) | 123 (78.3) |
| Education level | |||
| ≤Primary | 28 (8.9) | 14 (8.9) | 14 (8.9) |
| Junior high school | 60 (19.1) | 32 (20.4) | 28 (17.8) |
| Senior high school | 122 (38.9) | 63 (40.1) | 59 (37.6) |
| ≥Bachelor degree | 104 (33.1) | 48 (30.6) | 56 (35.7) |
| Marital statusa | |||
| Single | 40 (12.8) | 19 (12.2) | 21 (13.5) |
| Cohabitation | 14 (4.5) | 8 (5.1) | 6 (3.8) |
| Married | 222 (71.2) | 113 (72.4) | 109 (69.9) |
| Divorced | 19 (6.1) | 8 (5.1) | 11 (7.1) |
| Widowed | 17 (5.4) | 8 (5.1) | 9 (5.8) |
| Employment status | |||
| Professional or semiprofessional | 31 (9.9) | 18 (11.5) | 13 (8.3) |
| Skilled worker | 17 (5.4) | 9 (5.7) | 8 (5.1) |
| Nonskilled worker | 7 (2.2) | 2 (1.3) | 5 (3.2) |
| Retired | 169 (53.8) | 82 (52.2) | 87 (55.4) |
| Housewife | 69 (22) | 35 (22.3) | 34 (21.7) |
| Unemployed | 8 (2.5) | 2 (1.3) | 6 (3.8) |
| Others | 13 (4.1) | 9 (5.7) | 4 (2.5) |
| Body mass index, mean (SD)b | 23.5 (2.87) | 23.4 (2.91) | 23.5 (2.84) |
| Presence of health problemc | 220 (70.1) | 120 (76.4) | 100 (63.7) |
| Knee pain duration, mean (SD), y | 7.3 (7.58) | 7.5 (7.02) | 7.1 (8.13) |
| Current use of pain killers for knee pain | 43 (13.7) | 20 (12.7) | 23 (14.6) |
| Treatment received for knee osteoarthritis management | |||
| Western medicine | 140 (44.6) | 76 (48.4) | 64 (40.8) |
| Physiotherapy | 119 (37.9) | 56 (35.7) | 63 (40.1) |
| Chinese medicine (internal use)d | 42 (13.4) | 22 (14) | 20 (12.7) |
| Chinese medicine (external use)e | 55 (17.5) | 25 (15.9) | 30 (19.1) |
| Supplementsf | 218 (69.4) | 109 (69.4) | 109 (69.4) |
Two participants were missing data for marital status.
Body mass index was calculated as weight in kilograms divided by height in meters squared.
Health problem refers to any health problems other than osteoarthritis.
Chinese medicine (internal use) refers to prescribed or over-the-counter Chinese herbal medicine products intended for ingestion.
Chinese medicine (external use) refers to Chinese herbal medicine products intended for external application, as well as acupuncture, moxibustion, and cupping treatments.
Supplements refer to dietary supplements, including vitamins, minerals, and glucosamine.
Figure 1. Recruitment Flowchart.
BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); MoCA, Montreal Cognitive Assessment; NRS, numeric rating scale; OA, osteoarthritis.
Intervention Compliance and Acceptability
A total of 144 participants (91.7%) and 146 participants (93.0%) completed the 2 training sessions in the SAA group and KHE-only group, respectively. The mean (SD) ratings on degree of willingness to attend similar training courses in the SAA and KHE-only groups were 9.5 (0.9) and 9.3 (1.1), respectively. Of the 146 participants in the SAA group who had returned their acupressure log, 116 (79.5%) performed the acupressure at least 4 days per week during the 12-week period. The duration of self-practice at home was 16.5 minutes per day on average.
Effectiveness
Primary Outcome
The differences in the NRS score between the SAA and KHE-only groups across study time points were compared using the LMM (Table 2; eTable 3 in Supplement 2).40 Compared with the KHE-only group, the SAA group had a significantly greater reduction in NRS scores across all the assessment time points, with a between-group difference in changes from baseline to week 4 of −0.59 points (95% CI, −0.96 to −0.21 points; d = 0.35; P = .002); to week 8, −0.67 points (95% CI, −1.09 to −0.25 points; d = 0.35; P = .002), and to week 12, −0.54 points (95% CI, −0.97 to −0.10 points; d = 0.27; P = .02) (Table 2).
Table 2. Study Outcomes Across Study Time Points.
| Time | Mean (SE)a | Difference between groups in change from baseline (95% CI) | Effect size, db | P valuec | |
|---|---|---|---|---|---|
| SAA group (n = 157) | KHE-only group (n = 157) | ||||
| NRS pain | |||||
| Baseline | 5.14 (0.15) | 5.15 (0.15) | NA | NA | .006d |
| Week 4 | 3.74 (0.16) | 4.33 (0.15) | −0.59 (−0.96 to −0.21) | 0.35 | .002 |
| Week 8 | 3.43 (0.16) | 4.11 (0.16) | −0.67 (−1.09 to −0.25) | 0.35 | .002 |
| Week 12 | 3.04 (0.16) | 3.58 (0.16) | −0.54 (−0.97 to −0.10) | 0.27 | .02 |
| WOMAC-pain | |||||
| Baseline | 6.82 (0.27) | 7.59 (0.27) | NA | NA | .60d |
| Week 4 | 5.66 (0.27) | 6.64 (0.27) | −0.22 (0.81 to 0.37) | 0.08 | .46 |
| Week 8 | 5.07 (0.28) | 6.24 (0.27) | −0.41 (1.09 to 0.28) | 0.13 | .25 |
| Week 12 | 5.03 (0.28) | 5.86 (0.27) | −0.06 (0.78 to 0.65) | 0.02 | .86 |
| WOMAC-stiffness | |||||
| Baseline | 2.50 (0.13) | 2.99 (0.13) | NA | NA | .33d |
| Week 4 | 2.02 (0.13) | 2.65 (0.13) | −0.14 (−0.46 to 0.18) | 0.10 | .40 |
| Week 8 | 1.89 (0.13) | 2.30 (0.13) | 0.07 (−0.28 to 0.43) | 0.05 | .68 |
| Week 12 | 1.91 (0.13) | 2.22 (0.13) | 0.19 (−0.17 to 0.54) | 0.12 | .30 |
| WOMAC–physical function | |||||
| Baseline | 21.92 (0.94) | 25.38 (0.94) | NA | NA | .77d |
| Week 4 | 18.55 (0.96) | 21.50 (0.95) | 0.50 (−1.33 to 2.32) | 0.06 | .59 |
| Week 8 | 16.30 (0.97) | 20.07 (0.96) | −0.32 (−2.49 to 1.85) | 0.03 | .77 |
| Week 12 | 16.17 (0.97) | 19.25 (0.96) | 0.37 (−1.92 to 2.66) | 0.04 | .75 |
| TUG, s | |||||
| Baseline | 10.78 (0.15) | 10.99 (0.15) | NA | NA | .08d |
| Week 4 | 9.78 (0.16) | 10.36 (0.16) | −0.37 (−0.74 to 0.01) | 0.22 | .05 |
| Week 8 | 9.34 (0.17) | 10.02 (0.17) | −0.46 (−0.88 to −0.05) | 0.24 | .03 |
| Week 12 | 9.48 (0.17) | 9.76 (0.16) | −0.07 (−0.48 to 0.34) | 0.04 | .74 |
| Gait speed, s | |||||
| Baseline | 3.83 (0.07) | 3.81 (0.07) | NA | NA | .66d |
| Week 4 | 3.90 (0.07) | 3.80 (0.07) | 0.07 (−0.14 to 0.28) | 0.08 | .49 |
| Week 8 | 3.85 (0.08) | 3.84 (0.08) | −0.01 (−0.23 to 0.21) | 0.01 | .93 |
| Week 12 | 3.68 (0.08) | 3.72 (0.08) | −0.07 (−0.28 to 0.14) | 0.07 | .52 |
| SF-6D | |||||
| Baseline | 0.69 (0.01) | 0.69 (0.01) | NA | NA | .18d |
| Week 4 | 0.71 (0.01) | 0.70 (0.01) | 0.01 (−0.01 to 0.03) | 0.08 | .46 |
| Week 8 | 0.72 (0.01) | 0.70 (0.01) | 0.02 (−0.01 to 0.04) | 0.16 | .16 |
| Week 12 | 0.74 (0.01) | 0.71 (0.01) | 0.03 (0.003 to 0.01) | 0.25 | .03 |
Abbreviations: NA, not applicable; NRS, numerical rating scale; SF-6D, Short Form 6 Dimension; TUG, Timed Up and Go Test; WOMAC, Western Ontario and McMaster University Osteoarthritis Index.
Estimated mean and standard error (SE) from linear mixed-effects model.
Effect size based on the mean change from baseline in the treatment group minus the mean change from baseline in the control group, divided by the pooled pretest standard deviation. Effect size was considered as small (0.2), medium (0.5), or large (0.8).40 A positive effect size denotes a better effect in the intervention group.
P value for interaction between groups and assessment time points in the linear mixed-effects model.
P value for interaction between groups and all assessment time points in the linear mixed-effects model.
Secondary Outcomes
WOMAC (pain, stiffness, and physical function) subscores decreased across the time points but without significant between-group difference. Compared with the KHE-only group, a significant better performance in TUG test was observed in the SAA group at week 8, with a between-group difference in changes from baseline of −0.46 seconds (95% CI, −0.88 to −0.05 seconds; d = 0.25; P = .03) but the differences were marginally insignificant at week 4 and not significant at week 12. The FGS test showed no significant changes across the time points. The SF-6D utility score improved in both groups across time points, and the SAA group showed a significantly higher enhancement than the KHE-only group at week 12 (mean difference, 0.03 points; 95% CI, 0.003 to 0.01 points; d = 0.24; P = .03).
Clinical Significance
A significantly higher proportion of participants in the SAA group showed a reduction of at least 2 points in NRS across study time points. At week 4, 70 SAA participants (44.6%) and 45 KHE-only participants (28.7%) had a clinically significant change in NRS (P = .003); at week 8, 79 SAA participants (50.3%) and 57 KHE-only participants (36.3%) (P = .01); and at week 12, 97 SAA participants (61.8%) and 77 KHE-only participants (49.0%) (P = .02).
AEs
Overall, 21 participants (13.4%) in the SAA group reported having AEs, including pain at the acupoints (5 participants), finger joint pain (5 participants), bruise at the acupoints (3 participants), worsening of knee pain (2 participants), worsening of crepitus (2 participants), worsening of stiffness (2 participants), thigh muscle pain (1 participant), and calf cramping (1 participants). All the reported AEs were mild and self-resolved, except for 1 participant who dropped out due to finger joint pain.
Cost-Effectiveness Analysis
The mean (SD) QALYs as measured by SF-6D utility scores from baseline to week 12 were 0.1651 (0.0212) in the SAA group, which was 0.0042 greater than that in the KHE-only group (mean [SD], 0.1609 [0.0235]), but the difference was not significant (P = .10). The mean (SD) cost of the SAA intervention per person in the SAA group was HK$698 (HK$152 [mean, US$89; SD, US$19]), which was significantly greater than that in the KHE-only group (mean [SD], HK$652 [HK$105] [mean, US$83; SD, US$13]; P = .002). The ICER for the SAA group relative to the KHE-only group was HK$10 874.
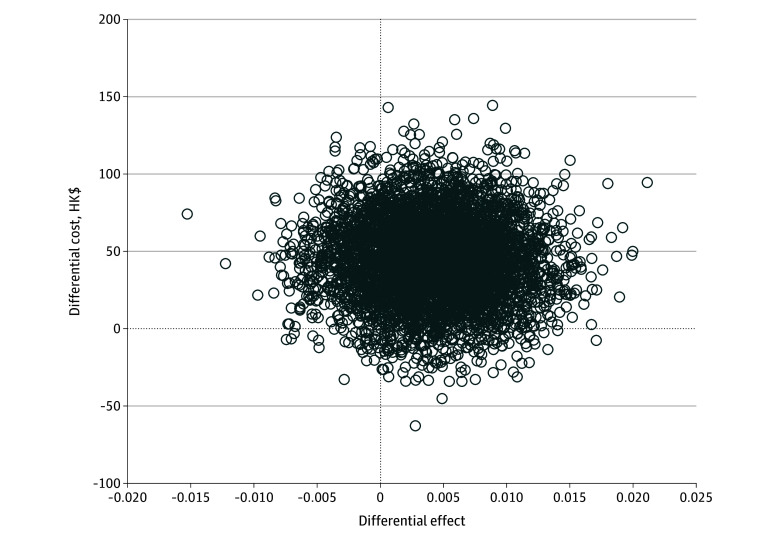
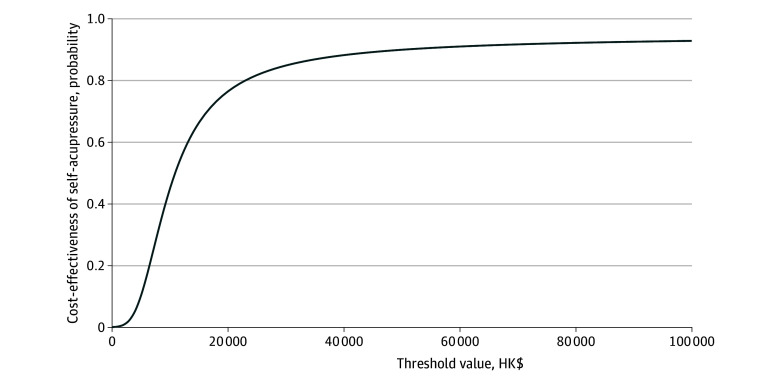
Sensitivity analysis on the ICER comparing SAA and KHE-only was conducted using 5000 bootstrapping iterations and plotted on the cost-effectiveness plane (Figure 2). Most boostrapped samples were located in the upper right quadrant, suggesting that SAA was associated with a larger improvement in QALY and higher costs than KHE alone. As illustrated by the parametric CEA curve, the probability of SAA being cost-effective reached 80% when the willingness-to-pay threshold was HK$23 000 (US$2948) (Figure 3). Figure 3 demonstrates an 80% probability of SAA being cost-effective at a willingness-to-pay threshold of HK$44 000 (US$5641), and greater than 90% of SAA being cost-effective at willingness-to-pay threshold of 1 GDP per capita (Figure 3).
Figure 2. Cost-Effectiveness Plane for Quality-Adjusted Life-Years After Self-Administered Acupressure or Knee Health Education for Knee Osteoarthritis.
To convert Hong Kong dollars to US dollars, multiply by 0.13.
Figure 3. Cost-Effectiveness Acceptability Curve of Self-Administered Acupressure for Knee Osteoarthritis by Varying the Willingness-to-Pay Threshold Values.
To convert Hong Kong dollars to US dollars, multiply by 0.13.
Discussion
The results of this large-scale RCT showed that the SAA training program embedded with a brief KHE program outperformed the full KHE program alone in relieving general knee pain in middle- and older-aged adults throughout the 12 weeks. In addition, participants in the SAA group performed significantly better in the TUG test and had a better quality of life in the medium term than those in the KHE-only group. Notably, participants had high acceptability and compliance with the SAA training program. However, the examined SAA training program did not show significantly higher QALYs than the KHE-only group. Even so, our cost-effectiveness analysis supported the SAA program as a cost-effective intervention.
This study, to our knowledge, was the largest RCT that examined the effectiveness of an SAA training program for probable knee OA along with health economic analyses. Our study found a small effect size for the SAA training program in relieving knee pain by NRS compared with KHE alone. Despite decreasing WOMAC scores in both groups, no significant between-group difference was found. These findings aligned with the RCT by Li et al,18 which reported significantly greater improvements in NRS scores in both SAA (d = 0.42) and sham SAA (d = 0.29) groups compared with usual care. However, their report of significantly greater reduction in WOMAC pain score (d = 0.53) and WOMAC function (d = 0.64) contradicted our results. This discrepancy may be attributed to our use of KHE alone as a comparison group. In our study, KHE-only participants also experienced a decrease in NRS and WOMAC scores over time. Consequently, the between-group differences were smaller than those in the study by Li et al,18 which used usual care for comparison.
The KHE-only group also showed an improvement in knee pain and other outcome measures, which narrowed the between-group differences. A previous systematic review on the effectiveness of education programs for knee OA reported inconsistent results,41 whereas a recent meta-analysis of group-based education for KOA demonstrated additional benefits only when delivered in combination with exercise.42 Therefore, more research is still needed to confirm the findings. Another issue is that the content, development, and delivery of the education programs in previous trials were usually unclear and not thorough,43 which may hinder other researchers from replicating the protocol or translation into clinical practice. Future reporting guidelines should be developed for reporting educational interventions.
Given that acupressure training involves much contact time between the instructor and participants, the improvement in participants’ pain, if any, might be attributed to nonspecific effects from the participant-practitioner interaction. Care as usual and waiting list control used in previous studies were not able to control for such nonspecific effects and even led to nocebo effects due to not being treated.44 Sham control design has been proposed in SAA studies.45 However, the use of sham control in acupuncture-related trials remains controversial, as the intervention characteristics, such as palpation of acupoints and general physical pressure stimulation, may also contribute to the nonspecific effects of the treatment, which can be considered part of the therapeutic elements.46,47 These characteristics may not be as clearly discerned as nonspecific effects. Therefore, the present study adopted KHE for the same length as the SAA intervention to control the contact hours between the instructor and participants.
The safety data showed that, consistent with previous findings, SAA is a reliable, well-tolerated nonpharmacological intervention.48,49 The most frequently reported AE was finger joint pain, likely due to prolonged or improper pressing the acupoints, which was also found in other studies of SAA.16,17,50 Other probable intervention-related AEs are mild and self-resolving but can be prevented by proper acupressure training. To enhance the safety of SAA, future studies should promote the use acupressure rods to prevent finger overuse and overexerting pressure on acupoints.
Limitations
The present RCT had several limitations. First, the absence of a sham control group and longer-term follow-up limited the ability to discern the specific effects and long-term effects of SAA. However, we adopted a pragmatic approach using KHE alone as a comparison, which allows us to compare the effects of the SAA training program with a clinically used intervention. Second, the absence of objective measures to evaluate the swelling or range of motion of the knee might weaken the validity of the study outcomes, but we had adopted TUG and FGS tests to assess functional mobility. Third, we cannot rule out the potential for social desirability bias, which could result in participants overreporting the benefits. However, given that some outcome measures did not demonstrate significant improvements, the impact of such bias, if present, is likely to be minimal. Furthermore, individuals in the KHE-only group may access external information regarding acupressure and conduct the intervention by themselves, thereby potentially underestimating the treatment effect size of the SAA group.
Conclusions
In this RCT, we found that a short SAA training program, accompanied with a brief KHE session, could effectively alleviate knee pain and improve mobility in middle- and older-aged adults with probable knee OA. It was noteworthy that participants showed high acceptability and compliance with the SAA training program. Our cost-effectiveness analysis indicated that the SAA was a cost-effective intervention.
Trial Protocol
eFigure. Schedule of Self-Administered Acupressure Training Course and Knee Health Education Course
eTable 1. Acupoints Used in the Treatment Protocol
eTable 2. Self-Administered Acupressure Protocol
eTable 3. Study Outcomes Across Study Time Points
Data Sharing Statement
References
- 1.Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. 2020;29-30:100587. doi: 10.1016/j.eclinm.2020.100587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60(2):91-97. doi: 10.1136/ard.60.2.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma L. Osteoarthritis of the knee. N Engl J Med. 2021;384(1):51-59. doi: 10.1056/NEJMcp1903768 [DOI] [PubMed] [Google Scholar]
- 4.Vokó Z, Pitter JG. The effect of social distance measures on COVID-19 epidemics in Europe: an interrupted time series analysis. Geroscience. 2020;42(4):1075-1082. doi: 10.1007/s11357-020-00205-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duong V, Oo WM, Ding C, Culvenor AG, Hunter DJ. Evaluation and treatment of knee pain: a review. JAMA. 2023;330(16):1568-1580. doi: 10.1001/jama.2023.19675 [DOI] [PubMed] [Google Scholar]
- 6.McKenzie S, Torkington A. Osteoarthritis—management options in general practice. Aust Fam Physician. 2010;39(9):622-625. [PubMed] [Google Scholar]
- 7.Yang S, Dubé CE, Eaton CB, McAlindon TE, Lapane KL. Longitudinal use of complementary and alternative medicine among older adults with radiographic knee osteoarthritis. Clin Ther. 2013;35(11):1690-1702. doi: 10.1016/j.clinthera.2013.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng HL, Yeung WF, Wong HF, Lo HT, Molassiotis A. Self-acupressure for symptom management in cancer patients: a systematic review. J Pain Symptom Manage. 2023;66(1):e109-e128. doi: 10.1016/j.jpainsymman.2023.03.002 [DOI] [PubMed] [Google Scholar]
- 9.Selçuk AK, Yanikkerem E. Effect of acupressure on primary dysmenorrhea: review of experimental studies. J Acupunct Meridian Stud. 2021;14(2):33-49. doi: 10.51507/j.jams.2021.14.2.33 [DOI] [PubMed] [Google Scholar]
- 10.Song HJ, Seo HJ, Lee H, Son H, Choi SM, Lee S. Effect of self-acupressure for symptom management: a systematic review. Complement Ther Med. 2015;23(1):68-78. doi: 10.1016/j.ctim.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 11.Mahanani S, Kertia N, Madyaningrum E, Lismidiati W. Acupressure for pain of osteoarthritis: a systematic review. J Nurse Pract. 2023;7(1):191-208. doi: 10.30994/jnp.v7i1.341 [DOI] [Google Scholar]
- 12.Wang Q, Lin J, Yang P, et al. Effect of massage on the TLR4 signalling pathway in rats with neuropathic pain. Pain Res Manag. 2020;2020:8309745. doi: 10.1155/2020/8309745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao C, Ren J, Huang R, et al. Transcriptome profiling of microRNAs reveals potential mechanisms of manual therapy alleviating neuropathic pain through microRNA-547-3p-mediated Map4k4/NF-κb signaling pathway. J Neuroinflammation. 2022;19(1):211. doi: 10.1186/s12974-022-02568-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z, Wang H, Yu T, et al. A review on the mechanism of tuina promoting the recovery of peripheral nerve injury. Evid Based Complement Alternat Med. 2021;2021:6652099. doi: 10.1155/2021/6652099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu ZF, Wang HR, Yu TY, Zhang YQ, Jiao Y, Wang XY. Tuina for peripherally-induced neuropathic pain: a review of analgesic mechanism. Front Neurosci. 2022;16:1096734. doi: 10.3389/fnins.2022.1096734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Shen CL, Peck K, et al. Training self-administered acupressure exercise among postmenopausal women with osteoarthritic knee pain: a feasibility study and lessons learned. Evid Based Complement Alternat Med. 2012;2012:570431. doi: 10.1155/2012/570431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung DST, Yeung WF, Suen LK, et al. Self-administered acupressure for knee osteoarthritis in middle-aged and older adults: a pilot randomized controlled trial. Acupunct Med. 2020;38(2):75-85. doi: 10.1177/0964528419883269 [DOI] [PubMed] [Google Scholar]
- 18.Li LW, Harris RE, Tsodikov A, Struble L, Murphy SL. Self-acupressure for older adults with symptomatic knee osteoarthritis: A randomized controlled trial. Arthritis Care Res (Hoboken). 2018;70(2):221-229. doi: 10.1002/acr.23262 [DOI] [PubMed] [Google Scholar]
- 19.Sorour AS, Ayoub AS, Abd El Aziz EM. Effectiveness of acupressure versus isometric exercise on pain, stiffness, and physical function in knee osteoarthritis female patients. J Adv Res. 2014;5(2):193-200. doi: 10.1016/j.jare.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou PW, Fu PK, Hsu HC, Hsieh CL. Traditional Chinese medicine in patients with osteoarthritis of the knee. J Tradit Complement Med. 2015;5(4):182-196. doi: 10.1016/j.jtcme.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zick SM, Alrawi S, Merel G, et al. Relaxation acupressure reduces persistent cancer-related fatigue. Evid Based Complement Alternat Med. 2011;2011:142913. doi: 10.1155/2011/142913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD; CONSORT PRO Group . Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309(8):814-822. doi: 10.1001/jama.2013.879 [DOI] [PubMed] [Google Scholar]
- 23.Altman R, Asch E, Bloch D, et al. ; Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association . Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29(8):1039-1049. doi: 10.1002/art.1780290816 [DOI] [PubMed] [Google Scholar]
- 24.Macpherson H. Pragmatic clinical trials. Complement Ther Med. 2004;12(2-3):136-140. doi: 10.1016/j.ctim.2004.07.043 [DOI] [PubMed] [Google Scholar]
- 25.National Clinical Guideline Center . Osteoarthritis: Care and Management in Adults. National Institute for Health and Care Excellence; 2014. [PubMed] [Google Scholar]
- 26.Anuurad E, Shiwaku K, Nogi A, et al. The new BMI criteria for Asians by the regional office for the Western Pacific region of WHO are suitable for screening of overweight to prevent metabolic syndrome in elder Japanese workers. J Occup Health. 2003;45(6):335-343. doi: 10.1539/joh.45.335 [DOI] [PubMed] [Google Scholar]
- 27.Wong A, Xiong YY, Kwan PWL, et al. The validity, reliability and clinical utility of the Hong Kong Montreal Cognitive Assessment (HK-MoCA) in patients with cerebral small vessel disease. Dement Geriatr Cogn Disord. 2009;28(1):81-87. doi: 10.1159/000232589 [DOI] [PubMed] [Google Scholar]
- 28.Elderly Health Service. Osteoarthritis of knee. Department of Health, Hong Kong SAR Government . Accessed May 29, 2023. https://www.elderly.gov.hk/english/health_information/bones_and_joints/osteoarthritisknee.html
- 29.Cheung T, Ho YS, Yuen CS, et al. Electromoxibustion for knee osteoarthritis in older adults: a pilot randomized controlled trial. Complement Ther Clin Pract. 2020;41:101254. doi: 10.1016/j.ctcp.2020.101254 [DOI] [PubMed] [Google Scholar]
- 30.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63(suppl 11):S240-S252. doi: 10.1002/acr.20543 [DOI] [PubMed] [Google Scholar]
- 31.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833-1840. [PubMed] [Google Scholar]
- 32.Symonds T, Hughes B, Liao S, Ang Q, Bellamy N. Validation of the Chinese Western Ontario and McMaster Universities Osteoarthritis Index in patients from mainland China with osteoarthritis of the knee. Arthritis Care Res (Hoboken). 2015;67(11):1553-1560. doi: 10.1002/acr.22631 [DOI] [PubMed] [Google Scholar]
- 33.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271-292. doi: 10.1016/S0167-6296(01)00130-8 [DOI] [PubMed] [Google Scholar]
- 34.Lam CL, Brazier J, McGhee SM. Valuation of the SF-6D health states is feasible, acceptable, reliable, and valid in a Chinese population. Value Health. 2008;11(2):295-303. doi: 10.1111/j.1524-4733.2007.00233.x [DOI] [PubMed] [Google Scholar]
- 35.Fransen M, Crosbie J, Edmonds J. Reliability of gait measurements in people with osteoarthritis of the knee. Phys Ther. 1997;77(9):944-953. doi: 10.1093/ptj/77.9.944 [DOI] [PubMed] [Google Scholar]
- 36.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142-148. doi: 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 37.Leung GM, Bacon-Shone J. Hong Kong’s Health System: Reflections, Perspectives and Visions. Hong Kong University Press; 2006. [Google Scholar]
- 38.Government of Hong Kong SAR. Overview of the health care system in Hong Kong. Accessed March 6, 2024. https://www.gov.hk/en/residents/health/hosp/overview.htm.
- 39.Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole MR. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158. doi: 10.1016/S0304-3959(01)00349-9 [DOI] [PubMed] [Google Scholar]
- 40.Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Lawrence Erlbaum Associates, Publishers; 1988. [Google Scholar]
- 41.Uritani D, Koda H, Sugita S. Effects of self-management education programmes on self-efficacy for osteoarthritis of the knee: a systematic review of randomised controlled trials. BMC Musculoskelet Disord. 2021;22(1):515. doi: 10.1186/s12891-021-04399-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamada K, Tamura H, Hirohama K, et al. The effectiveness of group education in people over 50 years old with knee pain: a systematic review and meta-analysis of randomized control trials. Musculoskelet Sci Pract. 2022;62:102627. doi: 10.1016/j.msksp.2022.102627 [DOI] [PubMed] [Google Scholar]
- 43.Goff AJ, de Oliveira Silva D, Ezzat AM, et al. Knee osteoarthritis education interventions in published trials are typically unclear, not comprehensive enough, and lack robust development: ancillary analysis of a systematic review. J Orthop Sports Phys Ther. 2022;52(5):276-286. doi: 10.2519/jospt.2022.10771 [DOI] [PubMed] [Google Scholar]
- 44.Paterson C, Dieppe P. Characteristic and incidental (placebo) effects in complex interventions such as acupuncture. BMJ. 2005;330(7501):1202-1205. doi: 10.1136/bmj.330.7501.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan JY, Suen LK, Wang T, Molassiotis A. Sham acupressure controls used in randomized controlled trials: a systematic review and critique. PLoS One. 2015;10(7):e0132989. doi: 10.1371/journal.pone.0132989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birch S, Lee MS, Kim TH, Alraek T. Historical perspectives on using sham acupuncture in acupuncture clinical trials. Integr Med Res. 2022;11(1):100725-100725. doi: 10.1016/j.imr.2021.100725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen H, Ning Z, Lam WL, et al. Types of control in acupuncture clinical trials might affect the conclusion of the trials: A review of acupuncture on pain management. J Acupunct Meridian Stud. 2016;9(5):227-233. doi: 10.1016/j.jams.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 48.Ang L, Song E, Lee H, Lee M. Acupressure for managing osteoarthritis: a systematic review and meta-analysis. Appl Sci-Basel. Published online April 21, 2022. doi: 10.3390/app11104457 [DOI] [Google Scholar]
- 49.Yeung WF, Chung KF, Poon MMK, et al. Acupressure, reflexology, and auricular acupressure for insomnia: a systematic review of randomized controlled trials. Sleep Med. 2012;13(8):971-984. doi: 10.1016/j.sleep.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 50.Li LW, Harris RE, Murphy SL, Tsodikov A, Struble L. Feasibility of a randomized controlled trial of self-administered acupressure for symptom management in older adults with knee osteoarthritis. J Altern Complement Med. 2016;22(5):396-403. doi: 10.1089/acm.2015.0231 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure. Schedule of Self-Administered Acupressure Training Course and Knee Health Education Course
eTable 1. Acupoints Used in the Treatment Protocol
eTable 2. Self-Administered Acupressure Protocol
eTable 3. Study Outcomes Across Study Time Points
Data Sharing Statement