Abstract
BHRF1, a component of the restricted early antigen complex of the Epstein-Barr virus lytic cycle, encodes a 17-kDa protein with both sequence and functional homology to the antiapoptotic Bcl-2 oncogene. Recent work has suggested that BHRF1 behaves like Bcl-2 in protecting cells from apoptosis induced by a range of stimuli. In this study, the effect of BHRF1 and Bcl-2 on the growth and differentiation of the SCC12F human epithelial cell line was examined. The levels of stable transfected BHRF1 expression achievable in SCC12F cells was consistently lower than that obtained with Bcl-2. While both BHRF1 and Bcl-2 inhibited epithelial differentiation, the effect of Bcl-2 was more pronounced, resulting in an almost complete blockade of differentiation in organotypic raft cultures. However, BHRF1-expressing SCC12F cells proliferated at a much higher rate than SCC12F cells expressing Bcl-2, and this effect was supported by cell cycle analysis which demonstrated that BHRF1, but not Bcl-2, promotes rapid transit through the cell cycle. These data highlight important differences between BHRF1 and Bcl-2 and suggest that BHRF1 may function to promote the survival and proliferation of lytically infected cells. The proliferative properties of BHRF1 described in this study, together with the demonstration that other oncogenic gamma herpesviruses encode Bcl-2 homologues, suggests that these proteins may serve to increase the susceptibility of virus-infected cells to oncogenic transformation, thereby contributing to the development of virus-associated tumors.
Epstein-Barr virus (EBV), a ubiquitous human herpesvirus with oncogenic potential, is predominantly associated with infection of two target tissues in vivo: B lymphocytes, in which the infection is largely nonproductive, and stratified squamous epithelium, in which virus replication occurs (39). Both of these cell types are susceptible to EBV-associated transformation resulting in tumors of B-cell origin (e.g., Burkitt’s lymphoma and immunoblastic lymphoma) or of epithelial cell origin (e.g., nasopharyngeal carcinoma and gastric adenocarcinomas) (25, 37, 41, 45). While the expression of EBV latent genes is strongly implicated in the development of these malignancies, it is also possible that viral proteins involved in EBV replication may influence the oncogenic process. In this respect BHRF1, an EBV lytic antigen with sequence homology to Bcl-2, is of particular interest (6). The BHRF1 gene encodes a 17-kDa putative transmembrane protein which is a component of the restricted early antigen complex expressed early during the EBV lytic cycle (35). Although not essential for viral replication or virus-mediated growth transformation in vitro (24, 26), the BHRF1 gene is highly conserved in all virus isolates (22) and, like Bcl-2, has a proven ability to act as a cell survival gene. Thus, BHRF1-expressing Burkitt’s lymphoma cell lines are more resistant to apoptosis induced by serum withdrawal (17), and rodent fibroblasts expressing BHRF1 display increased resistance to a variety of genotoxic drugs (43, 44). More recent work has demonstrated that BHRF1 can protect epithelial cells from apoptosis induced by tumor necrosis factor alpha, anti-Fas, activated monocytes, and serum deprivation (10, 21), lending support to the concept that this protein functions to enhance the survival of EBV-infected cells, particularly in response to host defense mechanisms in vivo. While BHRF1 is not consistently expressed in EBV-associated tumors, it is possible that expression of this protein at an early stage in the oncogenic process may influence the development of these malignancies (19). To date, the only known in vivo lesion where BHRF1 is abundantly expressed is oral “hairy” leukoplakia (HL), a benign lesion of oral tongue mucosa which represents a focus of chronic EBV replication (13, 14, 32, 51). Our previously published data demonstrating that BHRF1 can delay the terminal differentiation of epithelial cells through the prevention of apoptosis suggest that this protein may be responsible for HL pathology but may normally function to delay cell death during EBV replication so that full virus maturation can occur (8).
Recent studies demonstrate that both herpesvirus saimiri and human herpesvirus 8 encode functional Bcl-2 homologues (named ORF16 and KSbcl-2, respectively) (5, 31, 40), suggesting that these proteins provide a crucial antiapoptotic function during the gamma herpesvirus life cycle. Much of the previous work on the antiapoptotic properties of BHRF1, ORF16, and KSbcl-2 has been performed in heterologous systems with cell types and cytotoxic agents that may not be relevant to the in vivo function of these proteins. Thus, we have analyzed the effect of BHRF1 on epithelial cell growth and differentiation in comparison to Bcl-2 by using stable transfection of SCC12F cells, an immortalized but nontumorigenic epithelial cell line derived from a squamous cell carcinoma of facial epidermis (38). SCC12F retains several characteristics unique to normal epidermal keratinocytes, the most useful of which is its responsiveness to terminal differentiation signals (7, 34, 38). We report significant differences in the behavior of BHRF1 compared to Bcl-2 in this cellular environment, which may have important implications for our understanding of the normal role of viral Bcl-2 homologues in the biology of virus infection.
MATERIALS AND METHODS
Cell culture and isolation of stable BHRF1- and Bcl-2-expressing clones.
The SCC12F cell line was grown in a 3:1 mixture of Dulbecco’s modified Eagle’s medium and Ham’s F12 supplemented with 5% fetal calf serum (FCS) (Gibco BRL, Paisley, Scotland), 2 mM glutamine, hydrocortisone (0.4 μg/ml) (Glaxo, Greenford, United Kingdom) and the antibiotics penicillin (1,000 U/ml) and streptomycin (1 mg/ml) (Sigma Chemicals, Poole, United Kingdom). For routine culture, SCC12F cells were grown at clonal density (5 × 104 to 5 × 105 cells/9-cm petri dish) on an irradiated 3T3 fibroblast feeder cell layer (34). Stable clones of the SCC12F cell line expressing either BHRF1 or Bcl-2 were generated by electroporation with either pSG5 BHRF1 (8) or pCΔjBcl-2 (46) together with the pUC long terminal repeat neo at a ratio of 10:1 as previously described (8). Individual drug-resistant clones were isolated after a 3- to 4-week period with the aid of glass cloning cylinders (Sigma Chemicals). Stable drug-resistant clones were subsequently expanded for further analysis.
Analysis of BHRF1 and Bcl-2 expression in stable SCC12F cells.
The expression of BHRF1 in stable SCC12F cells was detected with [35S]methionine labelling and immunoprecipitation with the anti-BHRF1 monoclonal antibody (MAb) 5B11 (35) as previously described (8). Bcl-2 expression was detected by standard immunoblotting procedures with the Bcl-2-specific MAb, MAb 124 (Dako, Glostrup, Denmark) (16). The MAb was detected with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin (Ig) (Sigma Chemicals) and visualized by development with a chemiluminescence substrate (ECL; Amersham International) and subsequent exposure to X-ray film (Kodak, Rochester, N.Y.).
For immunostaining, cells were recovered by trypsinization and plated out onto Teflon-coated slides (Hendley-Essex, Loughton, United Kingdom) at 104 cells/well. After a brief rinse in phosphate-buffered saline (PBS), the slides were air dried and subjected to fixation in cold (−20°C) acetone for 5 min. BHRF1 and Bcl-2 expression was confirmed after immunostaining with the BHRF1- and Bcl-2-specific MAbs, 5B11 and MAb 124, which were used at 1:100 and 1:10 dilutions, respectively. After rehydration in PBS for 5 min, primary antibodies diluted in PBS plus 20% heat-inactivated normal goat serum (PBS-HINGS) were applied to the slides and incubated at 37°C for 60 min. The slides were then subjected to two 15-min washes in PBS followed by a further 60-min incubation with a 1/50 dilution of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Sigma Chemicals). After two further 15-min washes in PBS, the slides were mounted in DABCO solution consisting of the following: 90% glycerol, 10% PBS, and 2.5% (wt/vol) 1,4-diazabicyclo(2,2,2)octane, pH 8.6 (Sigma Chemicals) and examined with an Olympus UV-fluorescence microscope (560-nm excitation, 590-nm emission).
Collagen raft culture and immunostaining.
Vector control, BHRF1, and Bcl-2 clones of SCC12F were analyzed for their ability to terminally differentiate in the collagen raft system as previously described by Dawson et al. (8). Briefly, 2 × 105 trypsinized epithelial cells were seeded onto a collagen lattice (Collaborative Research) containing viable 3T3 fibroblasts (105 cells/ml) and grown until confluent. Thereafter, the lattice was carefully transferred to a stainless steel grid, and the epithelial culture was exposed at the air-liquid interface for a further 3 weeks. Under such conditions, SCC12F cells stratify and terminally differentiate (7). After the appropriate time, individual rafts were coated in Cryo-M-Bed (Bright Instrument Co. Ltd., Huntingdon, United Kingdom) and snap-frozen in liquid nitrogen. Frozen sections (6 μm) were cut from representative rafts, fixed in cold acetone, and stored at −70°C prior to use. After rehydration in PBS for 5 min, primary antibodies diluted in PBS-HINGS were applied to the sections, and the slides were incubated at 37°C for 60 min. The slides were then subjected to two 15-min washes in PBS followed by a further 60-min incubation with a 1/50 dilution of FITC-conjugated goat anti-mouse IgG (Sigma Chemicals), or in the case of the involucrin antiserum (Biogenesis, Poole, United Kingdom), a 1/50 dilution of FITC-conjugated goat anti-rabbit IgG (Sigma Chemicals). After a subsequent washing, slides were mounted in DABCO solution and examined with an Olympus UV-fluorescence microscope.
Suspension-induced terminal differentiation.
Exponentially growing cultures of SCC12F vector control, BHRF1, and Bcl-2 clones were removed from dishes by trypsinization. After counting, 105 cells were resuspended in 10 ml of serum-free medium made semisolid by the addition of 1.45% (wt/vol) Methocel (Sigma Chemicals) and plated out onto 9-cm bacteriological petri dishes (Bibby-Sterilin, Stone, United Kingdom) that had been pretreated with poly-HEMA (Aldrich, Poole, United Kingdom). After 24 to 48 h in suspension, cells were recovered by centrifugation and washed extensively in PBS, and cell spreads were made for immunostaining. Slides were fixed in 3.7% formaldehyde for 30 min followed by postfixation in ice-cold methanol for 5 min. Cell smears were immunostained as described above by using a polyclonal rabbit antiserum specific for involucrin (Biogenesis). For each time point, a minimum of 200 cells were scored, and the numbers of involucrin-positive cells were calculated as percentages of the total population.
Analysis of cell growth under conditions of reduced serum.
Exponentially growing cultures of SCC12F cells, vector controls, BHRF1, and Bcl-2 clones were rinsed free of irradiated 3T3 cells and collected as single-cell suspensions by trypsinization. Cells were plated out at a concentration of 5 × 103 cells/well in 96-well flat-bottomed plates (Nunc, Roskilde, Denmark). Twenty-four hours after seeding, the medium was aspirated and wells were refed with growth medium containing various concentrations of serum; thereafter, cells were cultured for an additional 5 days without further feeding. Cell viability and survival were assessed by the MTT assay (8, 30). Briefly, 20 μl of a 5-mg/ml stock of MTT (3-[4,4-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide [Sigma Chemicals]) in PBS was added to each well, and the cells were incubated with the substrate for 5 h. After the incubation, spent medium was aspirated from the wells, and the cells were solubilized by the addition of 200 μl of dimethyl sulfoxide (Fischer, Loughborough, United Kingdom). Absorbance at 550 nm was determined on a Becton Dickinson Multiscan. In this report, data are presented as the percentages of growth relative to those in standard growth medium which contained 5% FCS.
Analysis of cell growth kinetics.
Exponentially growing cultures of SCC12F cells, vector controls, BHRF1, and Bcl-2 clones were rinsed free of γ-irradiated 3T3 cells and collected as single-cell suspensions by trypsinization. Cells were plated out at a concentration of 104 cells/well in a six-well plate in duplicate (Nunc). Twenty-four hours after seeding, the medium was changed to growth medium containing either 10 or 1% serum; thereafter, cells were cultured for an additional 10 days with a medium change at day 4. Cell growth was assessed at 48-h intervals by counting viable cells with trypan blue exclusion after trypsinization.
Flow-cytometric analysis of cells for DNA cell cycle profiles.
Representative vector control and BHRF1- and Bcl-2-expressing clones were seeded onto 9-cm petri dishes (Nunc) at a density of 5 × 105 cells/dish in growth medium containing 5% FCS. Twenty-four hours after plating, the cell cultures were washed in two changes of PBS and refed with growth medium lacking serum. After incubation for 96 h in serum-free medium, cell cultures were refed with medium containing 5% FCS. Cells were harvested at this point (0 h), and at 12, 24, 48, 72, and 96 h after the readdition of serum. For cell cycle analysis, cells were recovered as single-cell suspensions, washed in two changes of PBS, and fixed in 5 ml of precooled 70% ethanol for 30 min at 4°C. After rehydration in PBS, the cells were collected by centrifugation (800 × g), and cells were processed for DNA analysis on a Coulter DNA prep workstation. Twenty microliters of propidium iodide was added (50 μg/ml), and the cells were analyzed by flow cytometry on an EPICS XL (Coulter) (488-nm excitation, 620-nm emission) gating out doublets and cell clumps. Cell cycle profiles were calculated with the Multicycle software package (version 2.53) (Phoenix Software, San Diego, Calif.).
RESULTS
Stable expression and subcellular localization of BHRF1 and Bcl-2 in SCC12F cells.
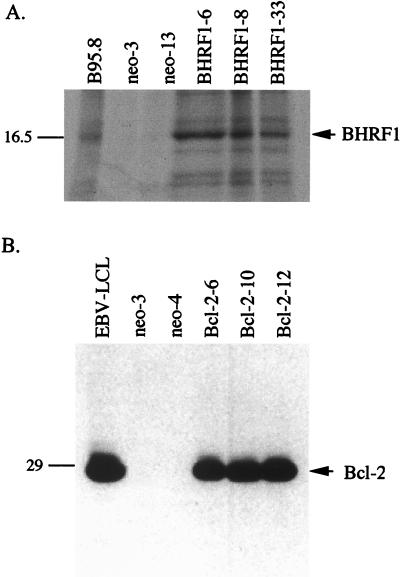
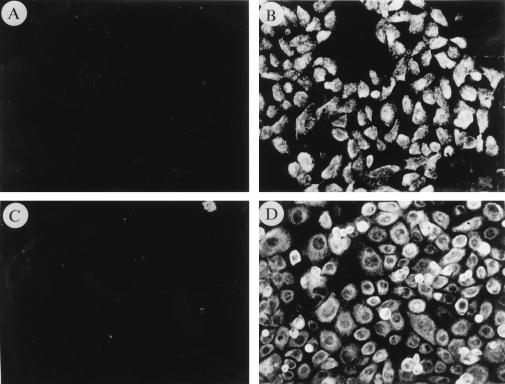
Expression of BHRF1 in clones of SCC12F cells was confirmed by immunoprecipitation with the 5B11 MAb (Fig. 1A). Clones 6, 8, and 33 all expressed detectable BHRF1 at levels that were comparable to that found in B95.8 cells, whereas two vector control clones (clones 1 and 3) were negative for expression. Attempts to detect BHRF1 in these cell lines by using immunoblotting with 5B11 were unsuccessful. Figure 1B shows expression of the 26-kDa Bcl-2 protein in stable clones of SCC12F by immunoblotting analysis with the Bcl-2-specific 124 MAb. Clones 6, 10, and 12 displayed levels of Bcl-2 expression that were comparable to that observed in the EBV-transformed lymphoblastoid cell line, X50-7. No expression was observed in the two SCC12F vector controls, clones 3 and 4. Immunostaining for BHRF1 and Bcl-2 in the stable clones revealed differences in the pattern of subcellular localization of these proteins (Fig. 2). Whereas both BHRF1 and Bcl-2 localized to the cytoplasm with intense granular staining (Fig. 2B and D), the most obvious difference related to the nuclear sparing of Bcl-2 (Fig. 2D) compared to BHRF1, which displayed diffuse reactivity over the nucleus (Fig. 2B). In both cases, immunostaining with either the BHRF1-specific or Bcl-2-specific MAb gave no staining on vector control clones (Fig. 2A and C).
FIG. 1.
Stable expression of BHRF1 and Bcl-2 in SCC12F cells. BHRF1 expression in SCC12F cells was determined by immunoprecipitation from [35S]methionine-labelled cells with the BHRF1-specific 5B11 MAb (A) whereas immunoblotting with the Bcl-2-specific 124 MAb was sufficient to detect stable Bcl-2 expression in transfected SCC12F cells (B). The molecular mass markers are indicated on the left (in kilodaltons) with the positions of BHRF1 and Bcl-2 shown on the right.
FIG. 2.
Distinct subcellular localization of BHRF1 and Bcl-2 in SCC12F cells. Representative clones of SCC12F expressing either BHRF1 or Bcl-2 were plated onto Teflon-coated microslides and allowed to grow for 48 h. After fixation in ice-cold acetone, the cell monolayers were immunostained with the 5B11 or 124 MAb specific for BHRF1 or Bcl-2, respectively. Unlike vector control cells (clone 3), which showed no specific reactivity with either the 5B11 (A) or 124 (C) MAbs, representative BHRF1- (B) (clone 6) and Bcl-2- (D) (clone 12) expressing clones displayed strong staining and revealed different patterns of subcellular localization.
Effects of BHRF1 and Bcl-2 on cell morphology and behavior in raft culture.
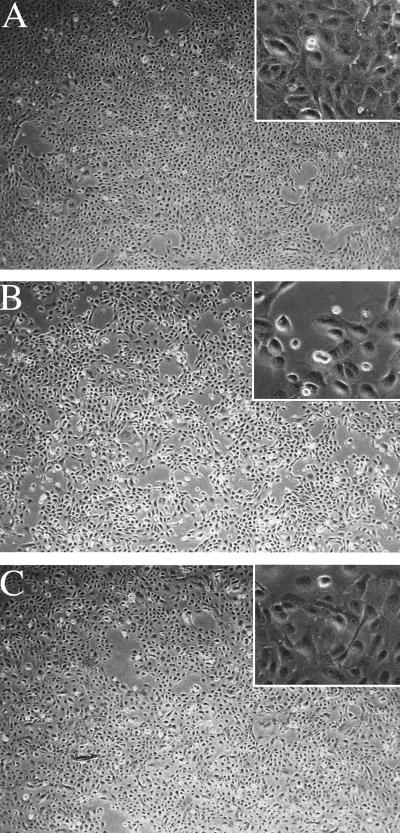
BHRF1 expression resulted in a marked alteration in the morphology of SCC12F cells in monolayer culture. BHRF1-expressing clones (i.e., clones 6, 8, and 33) displayed poorer intercellular contact (Fig. 3B) and failed to stratify to the same extent as representative vector control clones (Fig. 3A). Although clones expressing high levels of Bcl-2 (i.e., clones 6 and 10) did not stratify to the same degree as vector control clones, they still maintained a cuboidal morphology (Fig. 3B).
FIG. 3.
Stable expression of BHRF1 and Bcl-2 is associated with alterations in the morphology of SCC12F cells in monolayer culture. Representative clones of a SCC12F vector control (A) (clone 3), BHRF1 (B) (clone 6) or Bcl-2 (C) (clone 12) were cultured for 7 days in the absence of 3T3 feeder cells and photographed under phase contrast. Magnification, ×68; inset, ×272.
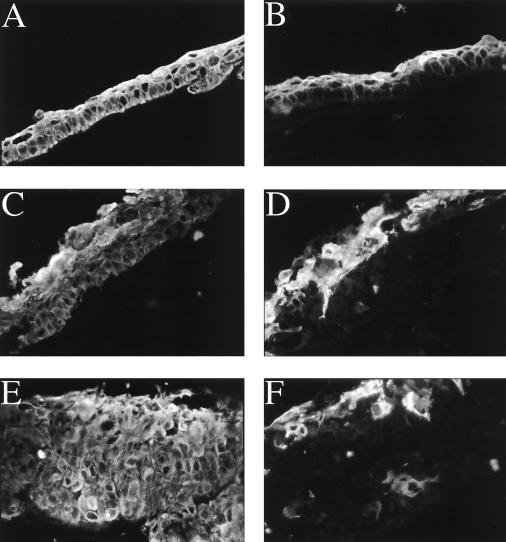
In organotypic raft culture, both the parental SCC12F cell line and vector control transfectants routinely gave rise to simple differentiating epithelial structures that generated a clearly defined basal cell layer and two to three layers of flatter differentiating cells as demonstrated by immunostaining with the AE-1 pan-keratin MAb (Fig. 4A). This contrasted with both the BHRF1- and Bcl-2-expressing SCC12F clones, which produced thicker and less well-organized raft structures compared to vector control rafts. Immunostaining of raft structures with the pan-keratin MAb, AE-1, clearly showed that both the BHRF1 and Bcl-2 clones produced overall thicker epithelial structures (Fig. 4C and E) compared to the vector controls (Fig. 4A). However, distinct differences were observed between BHRF1 and Bcl-2 raft structures. In general, structures formed by Bcl-2 clones were thicker than those formed by BHRF1 clones and tended to have weaker expression of differentiation-specific proteins compared to the BHRF1-expressing rafts. Thus, although the differentiation-associated involucrin antigen was clearly observed in differentiating suprabasal cell layers of both the SCC12F vector control (Fig. 4B) and BHRF1 rafts (Fig. 4D), expression of involucrin remained suprabasal but was relatively reduced and more diffuse in Bcl-2 raft structures (Fig. 4F). These results were confirmed by using MAbs against the differentiation-specific K1/10 keratins (reference 12 and data not shown). Similar results were obtained in at least three separate raft experiments with representative BHRF1 and Bcl-2 clones.
FIG. 4.
BHRF1 and Bcl-2 induce profound alterations in the growth of SCC12F cells in organotypic raft culture. Immunofluorescent staining of cross-sections of SCC12F transfectants grown on collagen rafts. Staining of sections of vector control transfectant clone neo 1 (A, B), BHRF1 clone 6 (C, D), and Bcl-2 clone 12 (E, F) with MAb AE1 (50) against type 1 keratins (A, C, E) or a polyclonal rabbit serum against involucrin (B, D, F). Magnification, ×150.
Bcl-2 and BHRF1 both delay commitment to terminal differentiation.
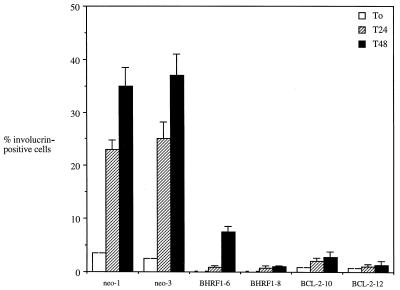
The aberrant differentiation programs displayed by BHRF1- and Bcl-2-positive clones in raft culture prompted us to investigate whether Bcl-2, like BHRF1, could influence the commitment of SCC12F cells to terminally differentiate. Figure 5 shows the results from three experiments where vector control, BHRF1, and Bcl-2 clones of SCC12F were induced to terminally differentiate in suspension culture. Expression of involucrin, a protein precursor of the cross-linked envelope, was used to identify terminally differentiated cells (1). In untreated starting populations, the number of involucrin-positive cells in all clones was relatively low (less than 5% of cells). However, after 24 h in suspension, up to 25% of cells in the vector control clones could be induced to express involucrin, these values increasing to between 35 to 38% after 48 h (Fig. 5). This level of induction contrasted with that achieved by both the two BHRF1 and Bcl-2 clones analyzed, where only 1 to 2% of cells could be induced to express involucrin after 24 h. Even after 48 h in suspension, less than 15% of the cells scored positive for involucrin expression.
FIG. 5.
BHRF1 and Bcl-2 suppress the terminal differentiation of SCC12F cells in suspension culture. SCC12F vector controls (neo-1 and neo-3), BHRF1- (clones 6 and 8), and Bcl-2 (clones 10 and 12)-expressing clones were induced to differentiate in suspension culture, and the number of terminally differentiated cells was assessed by staining for involucrin expression. The percentages of involucrin-positive cells were scored at the start of the experiment (time 0 [T0]), at 24 h (T24), and at 48 h (T48) after suspension culture. At least 200 cells were scored for each time point. Data are the means and standard errors of the means for three independent experiments.
Unlike BHRF1, Bcl-2 does not enhance cell survival in low serum.
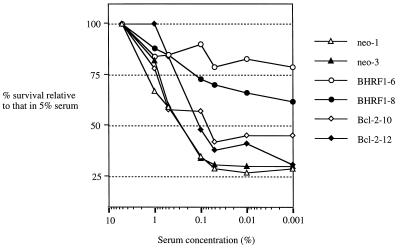
The ability of Bcl-2 and BHRF1 to enhance cell survival under conditions of growth factor withdrawal (serum deprivation) has been well documented for lymphoid cells (16, 17). In a previous study, we demonstrated that BHRF1 not only enhanced the survival of SCC12F cells under conditions of serum deprivation but also promoted cell growth (8). To investigate whether Bcl-2 could also impart increased cell survival and growth under low-serum conditions, we assessed the relative ability of BHRF1 and Bcl-2 to survive and grow under conditions of growth factor withdrawal (serum deprivation) by using the MTT assay as a measure of cell viability (30). The results of three independent experiments were pooled and are shown in Fig. 6. In these experiments, the growth and survival of SCC12F vector control transfectants and of BHRF1- and Bcl-2-expressing clones were assessed relative to those observed in medium containing 5% FCS over a 5-day period. As no loss of viability was observed over the experimental period by using trypan blue exclusion, any changes in MTT values relative to those obtained in 5% serum are a measure of cell survival and growth. Thus, reduction to 1% FCS for 5 days gave only a modest reduction in the survival of SCC12F vector controls and of the BHRF1 and Bcl-2 transfectants, but more dramatic differences became apparent in cells grown at lower concentrations of serum. Thus, the survival of vector control transfectants sloped off severely at concentrations of serum below 0.5%, while BHRF1 transfectants retained between 60 and 80% of their maximal survival potential even in 0.001% FCS (Fig. 6). However, both Bcl-2 transfectants achieved only 30 to 45% of their maximal survival potential in low serum.
FIG. 6.
BHRF1, but not Bcl-2, enhances the survival of SCC12F under conditions of serum deprivation. SCC12F vector control and BHRF1- and Bcl-2-expressing clones were assayed for their growth potential under conditions of serum withdrawal. In each case, 24 h after plating, individual clones were switched from 5% serum to medium supplemented with decreasing amounts of serum. Survival was assessed after 5 days by MTT assay. Data are the percentages of growth in medium supplemented with decreasing amounts of serum relative to that in 5% serum. Data are the means from three separate experiments. In each case, values deviated by no more than 10%.
The enhanced ability of BHRF1 to promote cell survival in low-serum conditions compared to Bcl-2 prompted us to examine the growth kinetics of the SCC12F-transfected clones in more detail. Figure 7 shows the results from two representative experiments in which the growth of SCC12F vector controls and of BHRF1 and Bcl-2 transfectants was assessed over a 10-day period by counting viable cells. Over this period, no differences were observed in total versus viable cell numbers, indicating that the observed effects were due to cell proliferation and not to differing rates of cell death. In 10% serum, both vector control clones achieved 20 population doublings over the 10-day period, whereas the two BHRF1 clones grew appreciably faster, achieving between 40 and 60 doublings (Fig. 7A). In marked contrast, the two Bcl-2 clones failed to proliferate to the same extent as the BHRF1 clones; clone 10 grew at a rate similar to that of the vector control clones, and clone 12 grew at a much slower rate. These differences were highlighted when experiments were performed in 1% serum (Fig. 7B). Under those conditions, the two vector controls achieved just over 10 population doublings over the 10-day period, whereas the two BHRF1 clones achieved 25 to 37 doublings. Both Bcl-2 clones grew at rates slower than that of either the vector control or BHRF1 clone.
FIG. 7.
BHRF1-expressing SCC12F clones display enhanced growth kinetics relative to Bcl-2-expressing clones. The growth kinetics of representative SCC12F vector control and BHRF1- and Bcl-2-expressing clones was analyzed over a 10-day period. Cell number was determined at two daily intervals by trypan blue exclusion to estimate viable cell number. Growth was assessed in 10% serum (A) and in 1% serum (B). Data are the means of triplicate counts and are representative of three separate experiments.
BHRF1 expression induces rapid transit through the cell cycle.
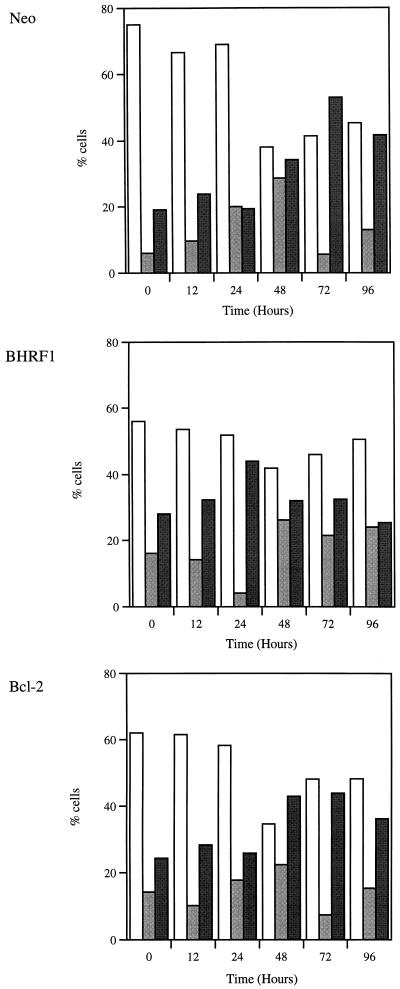
The above experiments indicate that BHRF1 promotes the proliferation of SCC12F cells. To examine this effect in more detail, SCC12F vector control cells and the BHRF1 and Bcl-2 transfectants were subjected to cell cycle analysis. Cultures were deprived of serum for 96 h, fed serum-containing medium, and sampled for analysis of the proportion of cells in each phase of the cell cycle. Although serum deprivation was unable to fully quiesce SCC12F cells, this treatment resulted in a significant reduction in cell proliferation, with around 75% of parental SCC12F cells or vector control cells in G1 and less than 10% in S phase (Fig. 8). Interestingly, a similar treatment of SCC12F cells expressing either BHRF1 or Bcl-2 always resulted in less of a growth-inhibitory effect. Of greater significance was the response of these cells after readdition of serum. Whereas both SCC12F vector controls and Bcl-2-expressing clones showed a gradual reentry into cycle with a peak in S phase at 48 h and progression through G2/M at 72 h, SCC12F cells expressing BHRF1 rapidly transited through the cell cycle such that by 24 h, the majority of cells had already progressed through S phase into G2/M (Fig. 8).
FIG. 8.
BHRF1, but not Bcl-2, induces rapid transit of SCC12F cells through the cell cycle. To determine the rate at which SCC12F cells expressing Bcl-2 or BHRF1 reentered the cell cycle after serum deprivation, the percentage of cells in each phase of the cell cycle was analyzed by flow cytometry at the depicted intervals after the readdition of serum. Data are presented in the form of histograms indicating the percentages of cells in each phase of the cell cycle at the indicated times and are representative of three similar analyses. Cell cycle phases are represented as follows: G1, open bars; S, light gray bars; G2/M, dark gray bars.
DISCUSSION
The ability of BHRF1 to function as a viral homologue of Bcl-2 is well established. Earlier work demonstrated that BHRF1 expression rendered EBV-negative B-cell lines more resistant to apoptosis induced upon serum withdrawal (17) and protected rodent fibroblasts from the apoptosis-inducing effects of DNA-damaging drugs and p53 (43). More recent studies have confirmed the antiapoptotic effects of BHRF1 in epithelial cells exposed to tumor necrosis factor alpha, anti-Fas antibody, cytotoxic monocytes, or serum deprivation (10, 21). While not essential in vitro for either EBV-induced B-cell transformation or EBV replication, the BHRF1 gene is present in all natural isolates of EBV so far examined, and its sequence and antiapoptotic function are highly conserved (22). Sequence homology has also been used to identify functional Bcl-2 homologues in both herpesvirus saimiri (ORF16) and human herpesvirus 8 (KSbcl-2) (5, 31, 40). The fact that gamma herpesviruses along with adenovirus (19K) and African swine fever virus (LMW5-HL) have pirated Bcl-2 homologues suggests that this antiapoptotic function is crucial to the virus life cycle, probably acting to prolong the survival of infected cells and thereby maximizing the production of progeny virus and/or facilitating the establishment of virus persistence (52).
While previous work has emphasized the similarities between BHRF1 and Bcl-2, the results of this study clearly highlight important differences in the function of these proteins in a human epithelial cell line. The first indication that the behavior of Bcl-2 may differ from that of BHRF1 in SCC12F cells came from an examination of the relative levels of stable expression of these proteins that were achievable in this cell background. While high-level Bcl-2 expression was readily obtained in stable SCC12F clones, this was not the case with BHRF1 even though the Bcl-2 and BHRF1 vectors drive transgene expression from the same simian virus 40 immediate-early promoter. This observation is consistent with previous studies where BHRF1 expression was detectable at only low levels in CHO cells or epithelial cells as determined by either immunoprecipitation (8, 43) or immunoblotting (10, 21). Interestingly, in agreement with our study, stable levels of transfected Bcl-2 in the BJAB cell line were shown to be much higher than those obtained with BHRF1 (10). Thus, even though the levels of achievable BHRF1 expression were low, BHRF1 was still able to induce phenotypic changes and impair epithelial differentiation. Perhaps even more impressive was the ability of these low levels of BHRF1 to promote cell proliferation relative to Bcl-2, and it may be that this growth-promoting property of BHRF1 is not consistent with the maintenance of high-level BHRF1 expression.
Our previous work demonstrated that BHRF1 can delay the terminal differentiation of epithelial cells supporting the apoptotic nature of this process. Within stratified squamous epithelium, the commitment of cells to differentiation appears to be initiated by loss of contact with extracellular matrix, and this event is coupled with the down-regulation of Bcl-2 expression and subsequent induction of apoptosis (11, 23, 29). Our observations indicate that Bcl-2 expression in SCC12F cells inhibits differentiation in both raft and suspension cultures. This supports previous studies in which Bcl-2 expression was shown to block terminal differentiation in both human and murine epithelial cell lines (11, 15, 27). The normal restricted expression of Bcl-2 to the basal epithelial compartment (18) is consistent with a role for Bcl-2 in regulating the commitment of epithelial cells to the terminal differentiation program. However, in the HL lesion, BHRF1 expression is observed throughout all suprabasal epithelial cells in which EBV replication occurs, suggesting that, unlike Bcl-2, BHRF1 serves to prevent apoptosis in cells already committed to the differentiation process. Interestingly, the BHRF1-expressing cells in HL are present within the expanded suprabasal layer responsible for the epithelial thickening (acanthosis) which typifies this lesion. These cells are postmitotic and may be able to tolerate higher levels of BHRF1 expression than proliferating cells. Thus, BHRF1 may normally function to delay cell death in nonproliferating cells during EBV replication so that full virus maturation can occur (8), whereas Bcl-2 acts at a much earlier stage to inhibit initial commitment to differentiation of proliferation-competent epithelial cells. This difference may explain the more pronounced inhibitory effect of Bcl-2 on epithelial differentiation compared to that of BHRF1 and may also underlie the contrasting effects of Bcl-2 and BHRF1 on epithelial cell growth. However, this conclusion must be qualified by the inability to achieve stable expression of equal protein levels of BHRF1 and Bcl-2 in SCC12F cells. Thus, it is possible that levels of BHRF1 expression equivalent to those of Bcl-2 could result in a more profound inhibition of differentiation. We are currently establishing inducible expression systems to address this issue.
Although the antiapoptotic properties of BHRF1 appear superficially similar to those of Bcl-2, we have identified additional differences which relate to the effects of these proteins on epithelial cell growth. Under normal growth conditions, we found that the BHRF1-expressing SCC12F clones proliferated at a rate higher than that of either the vector control or Bcl-2-expressing clones; these differences were particularly evident under conditions of serum deprivation. Our findings in epithelial cells are supported by previous work in lymphoid cells and fibroblasts demonstrating that Bcl-2 expression inhibits cell cycle progression (3, 28, 33, 36, 42, 48). This growth-inhibitory effect of Bcl-2 is clearly distinct from the growth-promoting properties of BHRF1. Previous studies have shown that deletion of a nonconserved region of Bcl-2 between the BH4 and BH3 conserved domains results in a gain-of-function mutant which is not only able to suppress apoptosis but also stimulates cell proliferation (47). This region of Bcl-2 (amino acids 30 to 80) has recently been implicated in the negative regulation of Bcl-2 function, possibly via phosphorylation (4). Another study demonstrated that mutation of a tyrosine residue (Y28) at the C-terminal end of the BH4 domain did not affect the antiapoptotic activity of Bcl-2 but reduced the ability of Bcl-2 to restrain the reentry of quiescent cells into the cell cycle (20). Thus, the BH4 and BH3 regions of Bcl-2 together with the intervening loop region appear to be important in constraining the growth-promoting properties of Bcl-2. Interestingly, BHRF1 and the other herpesvirus Bcl-2 proteins (ORF16 and KSbcl-2) are poorly conserved over this region compared to the other mammalian homologues of Bcl-2 (5). Importantly, the Y28 residue in the BH4 domain is not conserved in the herpesvirus Bcl-2 proteins, and the intervening loop (amino acids 30 to 80) is missing from these viral homologues. Thus, the ability of BHRF1 to confer a proliferative capacity on epithelial cells may result from the lack of these regulatory domains, and it is predicted that both ORF16 and KSbcl-2 will have similar properties.
The precise mechanism responsible for the functional differences between BHRF1 and Bcl-2 is likely to relate to the differential abilities of these proteins to interact with other cellular Bcl-2 homologues or with other nonhomologous proteins. Even the seemingly similar antiapoptotic effects of BHRF1 and Bcl-2 which result in the inhibition of epithelial differentiation are likely to be mediated by different interacting proteins. Thus, unlike Bcl-2, the antiapoptotic function of BHRF1 is not dependent on heterodimerization with Bax (2, 31, 44). In this regard, BHRF1 resembles KSbcl-2, which is able to suppress apoptosis even though it does not interact with either Bax or Bak (5). It would thus appear that the antiapoptotic function of both BHRF1 and KSbcl-2 does not require the inactivation of the death effector function of Bax, thereby relieving these viral Bcl-2 proteins from the negative regulation that controls the antiapoptotic cellular Bcl-2 family. Of the other cellular proteins known to interact with BHRF1, most if not all also interact with Bcl-2. Thus, both Bcl-2 and BHRF1 interact with Bik and with the Nip family of proteins (31, 44). Another cellular protein that interacts with BHRF1 is R-ras, a member of the ras superfamily that has previously been shown to interact with Bcl-2 (9, 44, 49). Interestingly, a mutation within the poorly conserved BH3 domain of BHRF1 (mutant 50-1) abrogates R-ras binding and confers a proliferative capacity on BHRF1 in BRK cells (44). This gain-of-function mutation suggests that the R-ras interaction with BHRF1 acts to restrain the proliferative activity of BHRF1. As we have observed enhanced growth induced by BHRF1 in SCC12F cells, it is possible that this effect results from a lack of R-ras expression in this cell line. If this is the case, then the BHRF1 50-1 mutant should confer no additional growth advantage to SCC12F cells relative to wild-type BHRF1. These possibilities are currently being examined.
The differences identified in this study between BHRF1 and Bcl-2 are likely to reflect the different functional roles of these proteins in vivo. Thus, while Bcl-2 and its closely related antiapoptotic family members have evolved to suppress apoptosis and ensure that damaged cells do not reenter the cell cycle prematurely, BHRF1 and the other viral Bcl-2 proteins have evolved to protect virus-infected cells from host defense mechanisms and to prolong the life span of lytically infected cells to ensure the efficient replication and dissemination of infectious virus. That this function of BHRF1 is crucial to the EBV life cycle is evidenced by our previous work demonstrating that this protein is highly conserved at both the sequence and functional levels among a range of different EBV isolates (22). The physiological rationale for the ability of BHRF1 to initiate or drive cell proliferation is more difficult to explain. Clearly, this effect is likely to promote the survival of lytically-infected cells under conditions of growth factor deprivation but could also increase the susceptibility of infected cells to oncogenic transformation. In this context, the fact that other oncogenic gamma herpesviruses encode Bcl-2 homologues is intriguing. In future studies, it will be interesting to investigate the potential proliferative effects of ORF16 and of KSbcl-2 and to further explore the structural and functional basis for the differences of these viral homologues with their cellular counterparts.
ACKNOWLEDGMENTS
This work was supported by the Cancer Research Campaign, London, United Kingdom.
We thank Sue Williams for assistance with the photographic work.
REFERENCES
- 1.Adams J C, Watt F M. Fibronectin inhibits the terminal differentiation of human keratinocytes. Nature. 1989;340:307–309. doi: 10.1038/340307a0. [DOI] [PubMed] [Google Scholar]
- 2.Boise L H, Gonzalezgarcia M, Postema C E, Ding L Y, Lindsten T, Turka L A, Mao X H, Nunez G, Thompson C B. Bcl-X, a Bcl-2 related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 3.Borner C. Diminished cell-proliferation associated with the death-protective activity of Bcl-2. J Biol Chem. 1996;271:12695–12698. doi: 10.1074/jbc.271.22.12695. [DOI] [PubMed] [Google Scholar]
- 4.Chang B S, Minn A J, Muchmore S W, Fesik S W, Thompson C B. Identification of a novel regulation domain in Bcl-XL and Bcl-2. EMBO J. 1997;16:968–977. doi: 10.1093/emboj/16.5.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng E H-Y, Nicholas J, Bellows D S, Hayward G S, Guo H-G, Reitz M S, Hardwick J M. A Bcl-2 homolog encoded by Kaposi’s sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci USA. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleary M L, Smith S D, Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14-18) translocation. Cell. 1986;47:19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- 7.Dawson C W, Rickinson A B, Young L S. Epstein-Barr-virus latent membrane-protein inhibits human epithelial-cell differentiation. Nature. 1990;344:777–780. doi: 10.1038/344777a0. [DOI] [PubMed] [Google Scholar]
- 8.Dawson C W, Eliopoulos A G, Dawson J, Young L S. BHRF1, a viral homologue of the Bcl-2 oncogene, disturbs epithelial cell differentiation. Oncogene. 1995;9:69–77. [PubMed] [Google Scholar]
- 9.Fernandez-Sarabia M J, Bischoff J R. Bcl-2 associates with the ras-related protein R-Ras p23. Nature. 1993;366:274–275. doi: 10.1038/366274a0. [DOI] [PubMed] [Google Scholar]
- 10.Foghsgaard L, Jäättelä M. The ability of BHRF1 to inhibit apoptosis is dependent on stimulus and cell type. J Virol. 1997;71:7509–7517. doi: 10.1128/jvi.71.10.7509-7517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frisch S M, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gigi-Leitner O, Geiger B, Levy R, Czernobilsky B. Cytokeratin expression in squamous metaplasia of the uterine cervix. Differentiation. 1986;31:191–205. doi: 10.1111/j.1432-0436.1986.tb00400.x. [DOI] [PubMed] [Google Scholar]
- 13.Gilligan K, Rajadurai P, Resnick L, Raab-Traub N. Epstein-Barr virus small nuclear RNAs are not expressed in permissively infected cells in AIDS-associated leukoplasia. Proc Natl Acad Sci USA. 1990;87:8790–8794. doi: 10.1073/pnas.87.22.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenspan J S, Greenspan D, Lennette E T, Abrams D I, Canant M A, Petersen V, Freese U K. Replication of Epstein-Barr virus within the epithelial cells of oral hairy leukoplakia, an AIDS-associated lesion. N Engl J Med. 1985;313:1564–1571. doi: 10.1056/NEJM198512193132502. [DOI] [PubMed] [Google Scholar]
- 15.Harada H, Mitsuyasu T, Seta Y, Maruoka Y, Toyoshima K, Yasumoto S. Overexpression of bcl-2 protein inhibits terminal differentiation of oral keratinocytes in vitro. J Oral Pathol Med. 1998;27:11–17. doi: 10.1111/j.1600-0714.1998.tb02084.x. [DOI] [PubMed] [Google Scholar]
- 16.Henderson S, Rowe M, Gregory C, Croom-Carter D, Wang F, Longnecker R, Kieff E, Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 17.Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci USA. 1993;90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hockenbery D M, Zutter M, Hickey W, Nahm M, Korsmeyer S J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci USA. 1991;88:6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horner D, Lewis L, Farrell P J. Novel hypotheses for the roles of EBNA-1 and BHRF1 in EBV-related cancers. Intervirology. 1995;38:195–205. doi: 10.1159/000150433. [DOI] [PubMed] [Google Scholar]
- 20.Huang D C S, O’Reilly L A, Strasser A, Cory S. The anti-apoptosis function of Bcl-2 can be genetically separated from its inhibitory effect on cell cycle entry. EMBO J. 1997;16:4628–4638. doi: 10.1093/emboj/16.15.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawanishi M. Epstein-Barr virus BHRF1 protein protects intestine 407 epithelial cells from apoptosis induced by tumor necrosis factor alpha and anti-Fas antibody. J Virol. 1997;71:3319–3322. doi: 10.1128/jvi.71.4.3319-3322.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khanim F, Dawson C, Meseda C A, Dawson J, Mackett M, Young L S. BHRF1, a viral homologue of the Bcl-2 oncogene, is conserved at both the sequence and functional level in different Epstein-Barr virus isolates. J Gen Virol. 1997;78:2987–2999. doi: 10.1099/0022-1317-78-11-2987. [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi K, Tsutsumi K, Ohta Y, Yasumoto S. Time correlation of commitment to calcium-induced and terminal differentiation in human ectocervical keratinocytes in suspension cultures. Cell Growth Differ. 1997;8:571–579. [PubMed] [Google Scholar]
- 24.Lee M A, Yates J L. BHRF1 of Epstein-Barr virus, which is homologous to human proto-oncogene bcl2, is not essential for transformation of B cells or for virus replication in vitro. J Virol. 1992;66:1899–1906. doi: 10.1128/jvi.66.4.1899-1906.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magrath I, Jain V, Bhatia K. Epstein-Barr virus and Burkitt’s lymphoma. Semin Cancer Biol. 1992;3:285–295. [PubMed] [Google Scholar]
- 26.Marchini A, Tomkinson B, Cohen J I, Kieff E. BHRF1, the Epstein-Barr virus gene with homology to Bcl2, is dispensible for B-lymphocyte transformation and virus replication. J Virol. 1991;65:5991–6000. doi: 10.1128/jvi.65.11.5991-6000.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marthinuss J, Lawrence L, Seiberg M. Apoptosis in pam212, an epidermal keratinocyte cell-line: a possible role for bcl-2 in epidermal differentiation. Cell Growth Differ. 1995;6:239–250. [PubMed] [Google Scholar]
- 28.Mazel S, Burtrum D, Petrie H T. Regulation of cell-division cycle progression by bcl-2 expression: a potential mechanism for inhibition of programmed cell-death. J Exp Med. 1996;183:2219–2226. doi: 10.1084/jem.183.5.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCall C A, Cohen J J. Programmed cell-death in terminally differentiating keratinocytes: role of endogenous endonuclease. J Investig Dermatol. 1991;97:111–114. doi: 10.1111/1523-1747.ep12478519. [DOI] [PubMed] [Google Scholar]
- 30.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 31.Nava V E, Cheng E H-Y, Veliuona M, Zou S, Clem R J, Mayer M L, Hardwick J M. Herpesvirus saimiri encodes a functional homolog of the human bcl-2 oncogene. J Virol. 1997;71:4118–4122. doi: 10.1128/jvi.71.5.4118-4122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niedobitek G, Young L S, Lau R, Brooks L, Greenspan D, Greenspan J S, Rickinson A B. Epstein-Barr virus infection in oral hairy leukoplakia: virus replication in the absence of a detectable latent phase. J Gen Virol. 1991;72:3035–3046. doi: 10.1099/0022-1317-72-12-3035. [DOI] [PubMed] [Google Scholar]
- 33.O’Reilly L A, Huang D C S, Strasser A. The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry. EMBO J. 1996;15:6979–6990. [PMC free article] [PubMed] [Google Scholar]
- 34.Parkinson E K, Grabham P, Emmerson A. A sub-population of cultured human keratinocytes which is resistant to the induction of terminal differentiation-related changes by phorbol, 12-myristate, 13-acetate: evidence for an increase in the resistant population following transformation. Carcinogenesis. 1983;4:857–861. doi: 10.1093/carcin/4.7.857. [DOI] [PubMed] [Google Scholar]
- 35.Pearson G R, Luka J, Petti L, Sample J, Birkenbach M, Braun D, Kieff E. Identification of an Epstein-Barr virus early gene encoding a second component of the restricted early antigen complex. Virology. 1987;160:151–161. doi: 10.1016/0042-6822(87)90055-9. [DOI] [PubMed] [Google Scholar]
- 36.Pietenpol J A, Papadopoulos N, Markowitz S, Willson J K V, Kinzler K W, Vogelstein B. Paradoxical inhibition of solid tumor-cell growth by bcl2. Cancer Res. 1994;54:3714–3717. [PubMed] [Google Scholar]
- 37.Raab-Traub N. Epstein-Barr virus and nasopharyngeal carcinoma. Semin Cancer Biol. 1992;3:297–307. [PubMed] [Google Scholar]
- 38.Rheinwald J G, Beckett M A. Tumourigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981;41:1657–1663. [PubMed] [Google Scholar]
- 39.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2397–2446. [Google Scholar]
- 40.Sarid R, Sato T, Bohenzky R A, Russo J J, Chang Y. Kaposi’s sarcoma-associated herpesvirus encodes a functional Bcl-2 homologue. Nat Med. 1997;3:293–298. doi: 10.1038/nm0397-293. [DOI] [PubMed] [Google Scholar]
- 41.Shibata D, Weiss L M. Epstein-Barr virus-associated gastric adenocarcinoma. Am J Pathol. 1992;140:769–774. [PMC free article] [PubMed] [Google Scholar]
- 42.Subramanian T, Boyd J M, Chinnadurai G. Functional substitution identifies a cell survival promoting domain common to adenovirus E1B-19 kDa and Bcl-2 proteins. Oncogene. 1995;11:2403–2409. [PubMed] [Google Scholar]
- 43.Tarodi B, Subramanian T, Chinnadurai G. Epstein-Barr virus BHRF1 protein protects against cell death induced by DNA-damaging agents and heterologous viral infection. Virology. 1994;201:404–407. doi: 10.1006/viro.1994.1309. [DOI] [PubMed] [Google Scholar]
- 44.Theodorakis P, D’Sa-Eipper C, Subramanian T, Chinnadurai G. Unmasking of a proliferation-restraining activity of the anti-apoptosis protein EBV BHRF1. Oncogene. 1996;12:1707–1713. [PubMed] [Google Scholar]
- 45.Thomas J A, Allday M, Crawford D H. Epstein-Barr virus-associated lymphoproliferative disorders in immunocompromised disorders. Adv Cancer Res. 1991;57:329–380. doi: 10.1016/s0065-230x(08)61003-9. [DOI] [PubMed] [Google Scholar]
- 46.Tsujimoto Y. Overexpression of the human Bcl-2 gene product results in growth enhancement of Epstein-Barr virus-immortalized B cells. Proc Natl Acad Sci USA. 1989;86:1958–1962. doi: 10.1073/pnas.86.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uhlmann E J, D’Sa-Eipper C, Subramanian T, Wagner A J, Hay N, Chinnadurai G. Deletion of a nonconserved region of bcl-2 confers a novel gain of function-suppression of apoptosis with concomitant cell-proliferation. Cancer Res. 1996;56:2506. [PubMed] [Google Scholar]
- 48.Vairo G, Innes K M, Adams J M. Bcl-2 has a cell-cycle inhibitory function separable from its enhancement of cell-survival. Oncogene. 1996;13:1511–1519. [PubMed] [Google Scholar]
- 49.Wang H G, Millan J A, Cox A D, Der C J, Rapp U R, Beck T, Zha H B, Reed J C. R-Ras promotes apoptosis caused by growth-factor deprivation via a Bcl-2 suppressible mechanism. J Cell Biol. 1995;129:1103–1114. doi: 10.1083/jcb.129.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodcock-Mitchell J, Eichner R, Nelson W G, Sun T T. Immunolocalization of keratin polypeptides in human-epidermis using monoclonal-antibodies. J Cell Biol. 1982;95:580–588. doi: 10.1083/jcb.95.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young L S, Lau R, Rowe M, Niedobitek G, Packham G, Shanaham F, Rowe D T, Greenspan D, Greenspan J S, Rickinson A B, Farrell P J. Differentiation-associated expression of the Epstein-Barr virus BZLF1 transactivator protein in oral hairy leukoplakia. J Virol. 1991;65:2868–2874. doi: 10.1128/jvi.65.6.2868-2874.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young L S, Dawson C W, Eliopoulos A G. Viruses and apoptosis. Br Med Bull. 1997;53:509–521. doi: 10.1093/oxfordjournals.bmb.a011627. [DOI] [PubMed] [Google Scholar]