Abstract
Background
In malaria endemic regions of the Peruvian Amazon, rainfall together with river level and breeding site availability drive fluctuating vector mosquito abundance and human malaria cases, leading to temporal heterogeneity. The main variables influencing spatial transmission include location of communities, mosquito behaviour, land use/land cover, and human ecology/behaviour. The main objective was to evaluate seasonal and microgeographic biting behaviour of the malaria vector Nyssorhynchus (or Anopheles) darlingi in Amazonian Peru and to investigate effects of seasonality on malaria transmission.
Methods
We captured mosquitoes from 18:00 to 06:00 h using Human Landing Catch in two riverine (Lupuna, Santa Emilia) and two highway (El Triunfo, Nuevo Horizonte) communities indoors and outdoors from 8 houses per community, during the dry and rainy seasons from February 2016 to January 2017. We then estimated parity rate, daily survival and age of a portion of each collection of Ny. darlingi. All collected specimens of Ny. darlingi were tested for the presence of Plasmodium vivax or Plasmodium falciparum sporozoites using real-time PCR targeting the small subunit of the 18S rRNA.
Results
Abundance of Ny. darlingi varied across village, season, and biting behaviour (indoor vs outdoor), and was highly significant between rainy and dry seasons (p < 0.0001). Biting patterns differed, although not significantly, and persisted regardless of season, with peaks in highway communities at ~ 20:00 h in contrast to biting throughout the night (i.e., 18:00–06:00) in riverine communities. Of 3721 Ny. darlingi tested for Plasmodium, 23 (0.62%) were infected. We detected Plasmodium-infected Ny. darlingi in both community types and most (20/23) were captured outdoors during the rainy season; 17/23 before midnight. Seventeen Ny. darlingi were infected with P. vivax, and 6 with P. falciparum. No infected Ny. darlingi were captured during the dry season. Significantly higher rates of parity were detected in Ny. darlingi during the rainy season (average 64.69%) versus the dry season (average 36.91%) and by community, Lupuna, a riverine village, had the highest proportion of parous to nulliparous females during the rainy season.
Conclusions
These data add a seasonal dimension to malaria transmission in peri-Iquitos, providing more evidence that, at least locally, the greatest risk of malaria transmission is outdoors during the rainy season mainly before midnight, irrespective of whether the community was located adjacent to the highway or along the river.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-024-04940-z.
Keywords: Nyssorhynchus darlingi, Amazonian Peru, Malaria transmission, Vectors, Abundance, Seasonality
Background
Malaria remains a pressing global health issue despite numerous intervention and treatment efforts, i.e., following a decade-long decrease, the World Health Organization (WHO) reported a worldwide increase of 8 million cases between 2015 and 2017, with an annual death toll estimated at 450,000 persons [1–3]. Overall, in the Americas, Peru contributes annually about 15% of cases [3], and within Peru, Loreto Department in northeastern Amazonian Peru accounts for most (90%) of the country-wide cases [4].
Nyssorhynchus darlingi (also known as Anopheles darlingi ([5]) is the main vector of Plasmodium transmission in and around the capital of Loreto, Iquitos, since its initial detection there [6] at the beginning of a major malaria epidemic [7]. This mosquito species subsequently spread extensively, likely along river systems [8, 9] through many parts of Amazonian Peru. Transmission hotspots occur mainly in the extensive network of riverine villages and along the Iquitos-Nauta highway, linked frequently to occupation (logging, charcoal production, agriculture, fish farming) [10–12]. Understanding malaria transmission in such diverse community types could help elimination efforts and reduction of the overall malaria case burden in Peru.
Nyssorhynchus darlingi is a dominant malaria vector because of its anthropophily, behavioural plasticity [13, 14] and preference for secondary forest and forest fragment edges [15, 16]. Frequently collected biting outdoors in many Peruvian communities [17–19] in some villages in Colombia and Brazil it bites frequently indoors [20, 21]. In general malaria vector biting location (indoors, outdoors) and time, can be modified by the use of IRS (insecticide residual spray) and/or LLINs (long-lasting insecticide nets) [14, 22, 23]. Access to a human bloodmeal [18], and environmental factors such as local temperature and humidity, also influence vector biting behaviour. The average infectivity rate of Ny. darlingi in Peru ranges from 0.1 to 4% [17, 18, 24], but throughout the Amazon Basin elevated human biting rates (HBR) can contribute to higher-than-expected vectorial capacity [25, 26]. Feeding on humans is common but bloodmeal analyses have demonstrated that hosts can include chickens, dogs and cattle, depending on local availability and accessibility [18, 21]. Biting in Peru occurs mainly between dusk and midnight with reported unimodal and bimodal peaks [9, 14]. The Global Fund Malaria Project PAMAFRO programme [27] was introduced in the Loreto Region, Amazonian Peru, in 2005 for 5 years to reduce malaria transmission. The primary interventions were strengthening of malaria diagnosis and detection, improved malaria case-management, use of insecticide-treated nets (ITNs) and encouragement of community participation in environmental management [28, 29]. The main outcome, by 2010, was the reduction in annual incidence rates from 48.9 to 11.6/1000 [28]. Studies in Loreto prior to the 2005–2010 PAMAFRO initiative detected near-equal proportions of Ny. darlingi biting indoors and outdoors [24], but since the end of the PAMAFRO programme, a long-lasting insecticidal net (LLIN) distribution campaign by the Ministry of Health, and the Malaria Cero Programme (MCP) initiated in 2017 [30], several communities have shown increased outdoor-biting [17, 19]. However, in localities where insecticide pressure was relaxed, Ny. darlingi again began to bite indoors frequently [14]. This shift to increased indoor biting appears to have resulted from behavioural plasticity, and perhaps also aging of LLINs, as opposed to hypothesized genetic differences in Ny. darlingi populations in Loreto [9, 14].
The environment is a powerful driver of mosquito-borne disease prevalence [31, 32]. Throughout much of the Amazon, the greatest risk of malaria transmission is during the rainy season [15, 17, 33], or during the wet-dry transition period [34]. Across the Brazilian Amazon, the length of the rainy season, as well as a range of socioeconomic factors, contribute the most to malaria risk [35]. This finding is consistent with earlier observations that rainfall may predict vector abundance [34], although a study in French Guiana determined that relationships between Ny. darlingi densities, malaria incidence, rainfall and water level were quite variable, depending on local land-cover and availability of suitable breeding habitat [36]. In Amazonian Peru, rainfall leads to an estimated 10 m increase in river levels and contributes to the generation of larval habitats [15, 37]. Seasonal abundance makes studying vector ecology and behaviour in the dry season difficult as numbers of specimens collected are frequently very low; malaria incidence is also reduced [11].
Nyssorhynchus darlingi is highly adapted to anthropogenic landscapes. In Brazil, deforestation patches of ~ 5 km2 were significantly correlated with malaria prevalence [16], partly due to this species’ preference for forest fringe habitat [38, 39]. Along the Iquitos-Nauta highway in Peru, Vittor et al. [15] found a 278-fold increase in the Human Biting Rate (HBR) and greater numbers of larval habitats in sites with high deforestation. Despite higher forest coverage in riverine communities compared to those located along highways, Lainhart et al. [9] detected a 3.33-fold higher rate of Plasmodium transmission in riverine communities, in addition to higher rates of HBR, infection rates (IR), and entomological inoculation rates (EIR). Understanding habitat-specific influences on Ny. darlingi behaviour in anthropogenic landscapes can help predict areas of greatest local risk of Plasmodium transmission.
This study aimed to understand the environmental effects of community location and seasonality on mosquito abundance, biting behaviour, and entomological indices linked to malaria transmission of the primary malaria vector, Ny. darlingi, in Loreto Department south of Iquitos, Peru. Unlike previous temporal studies of Ny. darlingi abundance [9, 14, 17] we were able to include dry season data in addition to the more common rainy season findings from four communities.
Methods
Study sites
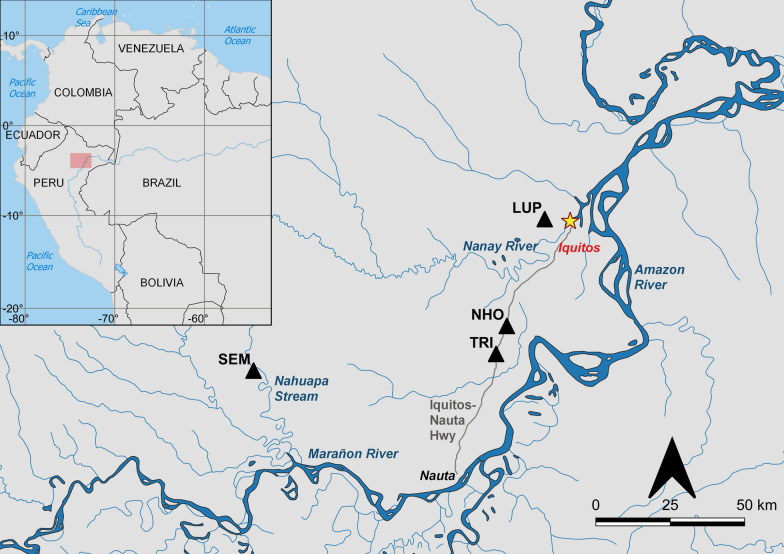
Adult mosquito collections were conducted in four communities in Loreto Department southwest of Iquitos (3.74°S, 73.25°W) that were each visited six times (except for El Triunfo that was visited seven times), in 2016–2017. Depending on locality, 3–4 collections were undertaken during the rainy season and 2–3 during the dry season (Table 1). Two communities studied are along rivers (Lupuna and Santa Emilia) and two along the Iquitos-Nauta highway (Nuevo Horizonte and El Triunfo) (Fig. 1). These sites have been described elsewhere: Lupuna (LUP) by Moreno et al. [17], Santa Emilia (SEM) by Prussing et al. [14], and Nuevo Horizonte (NHO) and El Triunfo (TRI) by Lainhart et al. [9]. As in Lainhart et al. [9], community habitat assignment was determined by proximity of the settlement to the nearest river: riverine localities were < 1 km and highway localities > 2 km from the nearest river.
Table 1.
Monthly abundance, HBR and EIR of Nyssorhynchus darlingi captured indoor and outdoor in Lupuna (LUP), Santa Emilia (SEM), El Triunfo (TRI), and Nuevo Horizonte (NHO), 2016–2017
| Indoor | Outdoor | ||||||
|---|---|---|---|---|---|---|---|
| Community | Mo-Yr | N | HBR(± SE) | EIR | N | HBR(± SE) | EIR |
| LUP | Feb-16 | 164 | 20.5(1) | 0.000 | 350 | 43.8(5.4) | 0.090 |
| Apr-16 | 154 | 19.2(3.3) | 0.000 | 328 | 41(9.1) | 0.088 | |
| Jul-16 | 95 | 11.9(0.5) | 0.000 | 192 | 24(4.9) | 0.000 | |
| Oct-16 | 34 | 4.2(1) | 0.000 | 57 | 7.1(0.9) | 0.000 | |
| Dec-16 | 27 | 3.8(0.5) | 0.000 | 43 | 5.4(0.7) | 0.000 | |
| Jan-17 | 172 | 21.5(1.5) | 0.000 | 273 | 34.1(3.4) | 0.000 | |
| Subtotal | 646 | 1243 | |||||
| SEM | Feb-16 | 152 | 19(1.2) | 0.041 | 317 | 39.7(5.1) | 0.427 |
| Apr-16 | 150 | 18.8(2.2) | 0.000 | 262 | 32.8(3) | 0.161 | |
| May-16 | 60 | 7.5(2) | 0.000 | 121 | 15.1(3) | 0.000 | |
| Sep-16 | 40 | 5(1.2) | 0.000 | 84 | 10.5(1.9) | 0.000 | |
| Nov-16 | 38 | 4.8(1.1) | 0.000 | 69 | 8.6(1.2) | 0.000 | |
| Jan-17 | 174 | 21.8(3) | N/A | 283 | 35.4(3.4) | N/A | |
| Subtotal | 614 | 1136 | |||||
| TRI | Jan-16 | 3 | 0.4(0.2) | 0.000 | 28 | 3.5(0.7) | 0.000 |
| Apr-16 | 7 | 0.9(0.4) | 0.000 | 18 | 2.3(1.1) | 0.000 | |
| Aug-16 | 8 | 1(0.3) | 0.000 | 17 | 2.1(0.4) | 0.000 | |
| Oct-16 | 5 | 0.6(0.1) | 0.000 | 13 | 1.6(0.3) | 0.000 | |
| Nov-16 | 3 | 0.4(0.1) | 0.000 | 12 | 1.5(0.4) | 0.000 | |
| Mar-17 | 6 | 1(0.6) | 0.000 | 21 | 3.5(1.3) | 0.000 | |
| Apr-17 | 3 | 0.8(0.8) | 0.000 | 11 | 2.8(2.3) | 0.000 | |
| Subtotal | 35 | 120 | |||||
| NHO | Jan-16 | 33 | 4.1(1) | 0.000 | 80 | 10(1.7) | 0.000 |
| Apr-16 | 35 | 4.4(1.4) | 0.000 | 71 | 8.9(1.1) | 0.000 | |
| Jul-16 | 29 | 3.6(0.9) | 0.000 | 32 | 4(0.6) | 0.000 | |
| Oct-16 | 16 | 2(0.5) | 0.000 | 23 | 2.9(0.4) | 0.000 | |
| Dec-16 | 18 | 2.3(0.6) | 0.000 | 24 | 3(0.2) | 0.000 | |
| Apr-17 | 49 | 6.1(1.1) | 0.085 | 95 | 11.9(1.2) | 0.909 | |
| Subtotal | 180 | 325 | |||||
| TOTAL | 1475 | 2824 | |||||
Mo-Yr: month-year of collection; N: number of Ny. darlingi captured; HBR: Human Biting Rate; HBR represents the average bites per person per night (b/p/n) calculated from the mean of collection days within each month/12 h per day per month (2 collectors inside and outside per night); EIR: Entomological Inoculation Rate. EIR not available for SEM January 2017. Italicized lines indicate rainy season collections
Fig. 1.
Map of communities (LUP: Lupuna, SEM: Santa Emilia, NHO: Nuevo Horizonte and TRI: El Triunfo) within Loreto Department, Peru, where Ny. darlingi were collected for this study. Red rectangle indicates the enlarged Loreto region of Peru. Made with Natural Earth data in QGIS v3
Mosquito sampling
We used the Human Landing Catch (HLC) method to collect adult mosquitoes indoors and outdoors (within five meters of the main house door) during four nights per collection event (a total of twenty-eight nights per community). We randomly chose eight houses and during each consecutive night, we captured mosquitoes from two of these houses. Every collection was conducted from 18:00 to 06:00 h, and collectors rotated every three hours to account for the effects of individual collector variation on attractiveness to mosquitoes. All mosquitoes captured were separated by hour, trap site location (indoor/outdoor), and by community, and subsequently identified using external morphology at our laboratory in Iquitos by trained personnel using standard keys [40–42]. Specimens were maintained on silica gel at 4 °C until DNA extraction.
Parity, daily survival rate, and life expectancy
To estimate female age composition of the mosquito population, a proportion (9% or more depending on total numbers captured, except for Jan-2016 in NHO where none were assessed for parity) of females collected were dissected to determine parity rates (PR), daily survival, and age estimation per community per season. Mosquito life expectancy (longevity) in days was calculated by Davidson’s method [43]: , where ℓ is the natural logarithm of the constant 2.71828 and P is the probability of a mosquito surviving one day (daily survival rate). P was calculated as: [44], where PR is the ratio between the number of parous mosquitoes to total number of females dissected, and gc the duration of the gonotrophic cycle (days). As in Moreno et al. [18], the gonotrophic cycle of 2.19 days was used for rainy season collections, and 2.43 for dry season collections [45].
Molecular detection of sporozoites in Ny. darlingi
Genomic DNA was extracted from each specimen of Ny. darlingi using Qiagen DNAeasy blood & tissue kits (Qiagen, Hilden, Germany), and DNA quantification conducted with a Qubit 2.0 Fluorometer (ThermoFisher Scientific, Waltham, MA). Detection of Plasmodium infection was conducted using real-time PCR targeting the small subunit of the 18S rRNA, with a triplex TaqMan assay (Life Technologies), as previously described [46]. RT-PCR was conducted on pools of DNA of up to five mosquitoes, in equal DNA concentration, for the presence of P. vivax and P. falciparum. Specimens from positive pools were tested individually to calculate the infection rate (IR).
Data analysis
The human biting rate (HBR) was evaluated as the average number of Ny. darlingi bites per collector per night. This was based on the total number of Ny. darlingi collected. As in Lainhart et al. [9], human biting time patterns were graphed, plotting the average proportion of Ny. darlingi collected per hour comparing riverine (LUP and SEM) and highway (NHO and TRI) communities; and then tested for significant differences with the nonparametric Kolmogorov–Smirnov (KS) test in GraphPad Prism version 9.2.0 (Graphpad Software, San Diego, CA). We calculated the Entomological Inoculation Rate (EIR) by multiplying the HBR by the proportion of Ny. darlingi that were determined to be Plasmodium-positive by RT-PCR. Sporozoite rates were calculated using the number of positive mosquitoes for Plasmodium divided by the total number of mosquitoes tested. To calculate the monthly EIR, we combined the numbers of P. vivax and P. falciparum and then multiplied the HBR by the proportion of infected specimens per month. Our rationale for combining these two Plasmodium species is that even though in the 1990s more Ny. darlingi were found to be infected by P. falciparum compared with P. vivax in Loreto [24], more recent studies have found the opposite [10, 14, 19, 47].
To test the hypothesis that the peak biting time of Ny. darlingi in riverine and highway communities differs, we used average proportions of Ny. darlingi collected per hour in the four communities and tested for significance with the KS statistical test [9].
Count data of all Ny. darlingi collected in 2016–2017 rainy and dry seasons, across all four collection sites, were analysed (Additional file 1) in RStudio version 1.2.5033 (R 4.0.2; R CORE TEAM). To accommodate the overdispersion in our dataset, negative binomial regression models, using forward and backward stepwise selection for variable selection, were conducted with the MASS package [48]. The glm.nb() function, was utilized with the following independent variables: season (dry/rainy), site, biting location (indoor/outdoor), 3-h time period (18:00–21:00, 21:00–24:00, 24:00–03:00, 03:00–06:00), and their interactions. A nonparametric Kruskal–Wallis analysis was also conducted on the count data for comparison to the negative binomial regression results.
Count data of the parous and nulliparous female Ny. darlingi in the rainy and dry seasons, across the four collection sites, were analysed (Additional file 2) in RStudio version 1.2.5033 (R 4.0.2; R CORE TEAM). We conducted negative binomial regression models as above but using the following independent variables: season (dry/rainy), site, and 6-h time period (18:00–24:00, 24:00–06:00), and their interactions. Some of the variable categories (site, indoor/outdoor, 3-h time period) were collapsed due to small sample sizes, e.g. of nulliparous females in TRI during the rainy season (see Table 2). The parity rates between community (LUP, SEM, TRI, NHO), season (rainy, dry) and community type (highway, riverine) were compared using the chi-square test with a statistical significance of P < 0.05 in GraphPad Prism version 9.5.1 for Windows, GraphPad Software (San Diego, California, USA).
Table 2.
Total number, percent and number of nulliparous, parous, and gravid female Nyssorhynchus darlingi collected from Lupuna (LUP), Santa Emilia (SEM), El Triunfo (TRI), and Nuevo Horizonte (NHO), 2016–2017, in addition to parity rate, daily survival rate, and life expectancy
| Community | Mo-Yr | Total Captured | % Total (N) | % Nulliparous (N) | % Parous (N) | % Gravid (N) | PR | Daily survival rate (P) | Age (days) |
|---|---|---|---|---|---|---|---|---|---|
| LUP | Feb-16 | 514 | 32.5 (167) | 2.4 (4) | 71.9 (120) | 25.7 (43) | 0.72 | 0.86 | 6.63 |
| Apr-16 | 482 | 41.1 (198) | 3.5 (7) | 28.3 (56) | 68.2 (135) | 0.28 | 0.56 | 1.73 | |
| Jul-16 | 287 | 46.3 (133) | 4.5 (6) | 13.5 (18) | 82 (109) | 0.14 | 0.44 | 1.22 | |
| Oct-16 | 91 | 97.8 (89) | 5.6 (5) | 23.6 (21) | 70.8 (63) | 0.24 | 0.55 | 1.68 | |
| Dec-16 | 70 | 98.6 (69) | 7.2 (5) | 23.2 (16) | 69.6 (48) | 0.23 | 0.55 | 1.66 | |
| Jan-17 | 445 | 38.4 (171) | 6.4 (11) | 60.2 (103) | 33.3 (57) | 0.60 | 0.79 | 4.32 | |
| Subtotal | 1889 | 43.8 (827) | 4.6 (38) | 40.4 (334) | 55 (455) | ||||
| SEM | Feb-16 | 469 | 9.4 (44) | 4.5 (2) | 70.5 (31) | 25 (11) | 0.70 | 0.85 | 6.25 |
| Apr-16 | 412 | 10.4 (43) | 11.6 (5) | 51.2 (22) | 37.2 (16) | 0.51 | 0.74 | 3.27 | |
| May-16 | 181 | 16 (29) | 17.2 (5) | 55.2 (16) | 27.6 (8) | 0.55 | 0.76 | 3.68 | |
| Sep-16 | 124 | 16.9 (21) | 19 (4) | 61.9 (13) | 19 (4) | 0.62 | 0.82 | 5.07 | |
| Nov-16 | 107 | 16.8 (18) | 16.7 (3) | 61.1 (11) | 22.2 (4) | 0.61 | 0.82 | 4.93 | |
| Jan-17 | 457 | 10.3 (47) | 12.8 (6) | 46.8 (22) | 40.4 (19) | 0.47 | 0.71 | 2.88 | |
| Subtotal | 1750 | 11.5 (202) | 12.4 (25) | 56.9 (115) | 30.7 (62) | ||||
| TRI | Jan-16 | 31 | 54.8 (17) | 0 (0) | 100 (17) | 0 (0) | 1.00 | 1.00 | N/A |
| Apr-16 | 25 | 80 (20) | 5 (1) | 60 (12) | 35 (7) | 0.60 | 0.79 | 4.29 | |
| Aug-16 | 25 | 100 (25) | 20 (5) | 36 (9) | 44 (11) | 0.36 | 0.66 | 2.38 | |
| Oct-16 | 18 | 100 (18) | 22.2 (4) | 38.9 (7) | 38.9 (7) | 0.39 | 0.68 | 2.57 | |
| Nov-16 | 15 | 100 (15) | 20 (3) | 40 (6) | 40 (6) | 0.40 | 0.69 | 2.65 | |
| Mar-17 | 27 | 33.3 (9) | 0 (0) | 77.8 (7) | 22.2 (2) | 0.78 | 0.89 | 8.71 | |
| Apr-17 | 14 | 50 (7) | 0 (0) | 100 (7) | 0 (0) | 1.00 | 1.00 | N/A | |
| Subtotal | 155 | 71.6 (111) | 11.7 (13) | 58.6 (65) | 29.7 (33) | ||||
| NHO | Jan-16 | 113 | 0 (0) | N/A (0) | N/A (0) | N/A (0) | N/A | N/A | N/A |
| Apr-16 | 106 | 53.8 (57) | 12.3 (7) | 59.6 (34) | 28.1 (16) | 0.60 | 0.79 | 4.24 | |
| Jul-16 | 61 | 100 (61) | 26.2 (16) | 37.7 (23) | 36.1 (22) | 0.38 | 0.67 | 2.49 | |
| Oct-16 | 39 | 100 (39) | 35.9 (14) | 30.8 (12) | 33.3 (13) | 0.31 | 0.62 | 2.06 | |
| Dec-16 | 42 | 100 (42) | 23.8 (10) | 38.1 (16) | 38.1 (16) | 0.38 | 0.67 | 2.52 | |
| Apr-17 | 144 | 44.4 (64) | 12.5 (8) | 59.4 (38) | 28.1 (18) | 0.59 | 0.79 | 4.20 | |
| Subtotal | 505 | 52.1 (263) | 20.9 (55) | 46.8 (123) | 32.3 (85) | ||||
| TOTAL | 4299 | 32.6 (1403) | 9.3 (131) | 45.4 (637) | 45.3 (635) |
*% Total: percent of females dissected for parous status of total captured
Results
Mosquito capture data and entomological indices
Overall, 4,330 Anophelinae were collected during this study, of which 4,299 were identified morphologically as Ny. darlingi. The rainy season accounted for 3,420 individual Ny. darlingi specimens (79.55%), and the dry season for 879 (20.45%). Using ITS2-PCR–RFLP as in Matson et al. [49], we identified one Nyssorhynchus benarrochi B individual from SEM. Thirty specimens that were provisionally morphologically identified as non-Ny. darlingi failed to amplify, could not be identified molecularly, and were excluded from all analyses.
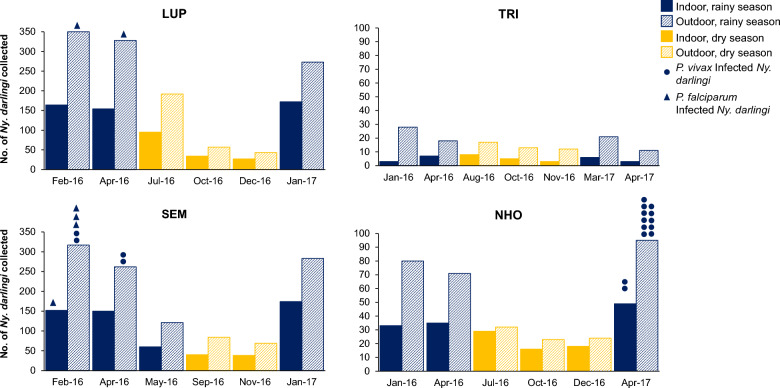
The HBR of Ny. darlingi ranged from 0.4 bites per night (b/p/n) indoors in TRI (highway) to 43.8 b/p/n outdoors in LUP (riverine) (Table 1) and was generally higher during the rainy season (Table 1). The final collection of Ny. darlingi in January 2017 from SEM was not analysed for Plasmodium for logistical reasons, thus a total of 3,721 Ny. darlingi were tested for Plasmodium. Of these, 23 (0.62%) were infected. In the localities where Plasmodium-infected Ny. darlingi were detected (Fig. 2), the EIR ranged from a high of 0.909 outdoors in NHO (highway) to a low of 0.041 indoors in SEM (riverine) (Table 1). No infected Ny. darlingi were collected during the dry season.
Fig. 2.
Abundance of Ny. darlingi collected indoors vs outdoors and rainy vs dry season in four collection sites in Loreto, Peru, 2016–2017. Collection times of infected specimens are indicated by a closed circle (P. vivax) or a closed triangle (P. falciparum)
Parity, daily survival rate, and life expectancy
We dissected 1,403/4,299 or 33% of the total number of Ny. darlingi from the four localities (Table 2). There were 637 parous, 635 gravid and 131 nulliparous Ny. darlingi detected (Table 2 and Additional file 2: Fig. S1). The PR in the rainy season ranged from 0.28 in LUP—1.00 in TRI whereas during the dry season it was 0.14 in LUP—0.62 in SEM. In the four localities, the average estimated mosquito age was somewhat higher during the rainy season (range 1.73–8.71 days) compared with the dry season (range 1.22–5.07 days), and the highest mosquito age was detected in TRI during the rainy season (8.71 days). There was a significant difference in the proportion of parous mosquitoes by site (χ2 = 8.04, df = 3, P = 0.0452) as well as season (χ2 = 8.621, df = 1, P = 0.0033), but not for community type (χ2 = 3.724, df = 1, P = 0.0536).
Seasonal, location (indoor/outdoor) and community effects
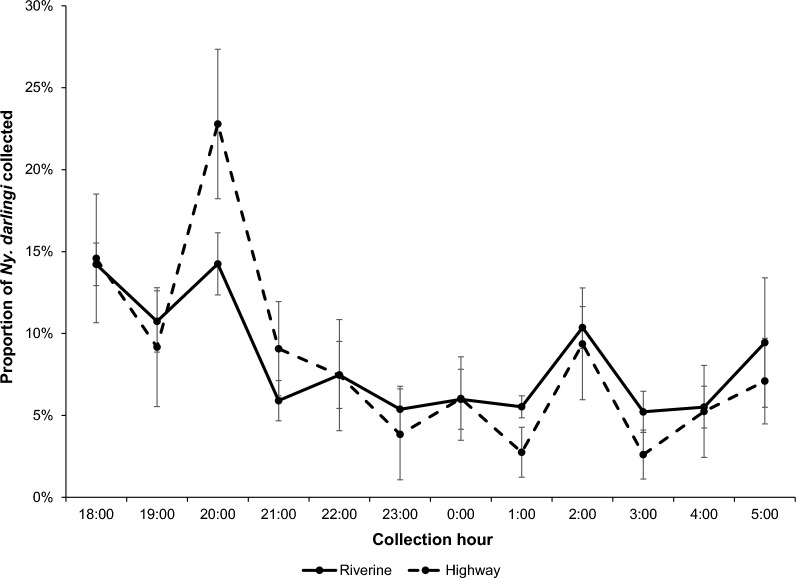
In general, more Ny. darlingi were captured during the rainy season compared with the dry season. Regardless of community or season, more Ny. darlingi were captured outdoors than indoors from all four sites; more were captured in riverine (LUP, SEM) than highway (TRI, NHO) communities (Table 1; Fig. 2). Regardless of location, season or community type, Ny. darlingi bit throughout the night, although there was a more pronounced early evening peak in the highway communities of TRI and NHO between 19:00 and 21:00 compared with LUP and SEM (Additional file 2: Figs. S2 and S3). There were no significant differences detected in biting patterns by the KS test (p = 0.8475; Fig. 3).
Fig. 3.
Average proportion of Ny. darlingi collected hourly by community type (LUP and SEM, riverine; NHO and TRI, highway). Error bars represent 95% confidence intervals
Results from negative binomial regression indicated significant differences in counts across sites, season, time period, and between indoor and outdoor captures (Table 3). Compared to the three other collection sites, significantly more Ny. darlingi were captured in LUP. In addition, more mosquitoes were collected during the rainy than dry season, outdoors versus indoors, and during the first time period (18:00–21:00) across all sites. Significant interactions (time period X indoor/outdoor, season X time period, season X site, and site X indoor/outdoor) suggest that these relationships are contextually dependent. A few of these interactions were significant only for TRI in comparison to LUP: season X site (indicating a lower rainy: dry season ratio in TRI than the other sites) and site X indoor/outdoor (indicating a higher outdoor: indoor ratio in TRI that the other sites). A Kruskal–Wallis analysis of the same Ny. darlingi count data supported the negative binomial regression findings (Supplemental Table 1).
Table 3.
Negative binomial regression model of Nyssorhynchus darlingi counts collected in four sites (Lupuna, Nuevo Horizonte, Santa Emilia, El Triunfo) during rainy and dry seasons
| Variable | β | eβ | SE | p value |
|---|---|---|---|---|
| Intercept | 1.71 | 5.60 | 0.11 | < 0.0001 |
| Site (ref = Lupuna) | ||||
| NHO | − 1.13 | 0.32 | 0.14 | < 0.0001 |
| SEM | − 0.27 | 0.76 | 0.13 | 0.0372 |
| TRI | − 2.49 | 0.08 | 0.22 | < 0.0001 |
| Season (ref = dry) | 1.17 | 3.23 | 0.11 | < 0.0001 |
| Time period (ref = 18:00—21:00) | ||||
| 21:00–24:00 | − 1.34 | 0.26 | 0.16 | < 0.0001 |
| 24:00–03:00 | − 0.66 | 0.52 | 0.14 | < 0.0001 |
| 03:00–06:00 | − 0.50 | 0.61 | 0.14 | < 0.0001 |
| Indoor/outdoor (ref = indoor) | 0.56 | 1.76 | 0.11 | < 0.0001 |
| Time period x indoor/outdoor | ||||
| 21:00–24:00 outdoor | 0.53 | 1.70 | 0.14 | < 0.0001 |
| 24:00–03:00 outdoor | 0.00 | 1.00 | 0.14 | 0.9973 |
| 03:00–06:00 outdoor | − 0.14 | 0.87 | 0.14 | 0.3190 |
| Season x time period | ||||
| Rainy × 21:00–24:00 | 0.34 | 1.41 | 0.15 | 0.0248 |
| Rainy × 24:00–03:00 | 0.02 | 1.02 | 0.14 | 0.8997 |
| Rainy × 03:00–06:00 | − 0.42 | 0.66 | 0.14 | 0.0037 |
| Season x site | ||||
| Rainy NHO | − 0.24 | 0.79 | 0.15 | 0.1049 |
| Rainy SEM | 0.05 | 1.05 | 0.13 | 0.7085 |
| Rainy TRI | − 0.74 | 0.48 | 0.20 | 0.0002 |
| Site x indoor/outdoor | ||||
| NHO outdoor | − 0.06 | 0.94 | 0.14 | 0.6710 |
| SEM outdoor | − 0.01 | 0.99 | 0.12 | 0.9037 |
| TRI outdoor | 0.60 | 1.83 | 0.22 | 0.0062 |
β: regression coefficient; eβ: exponentiated regression coefficient (z-value); SE: Standard error
Significance level p < 0.05 in bold
Malaria incidence data
During our study (2016–2017), the Annual Parasite Index (API), based on microscopic identification of blood smears, ranged from a low of 37.8 (TRI 2016) to a high of 504.1 in LUP (Table 4). In 2017, two of the communities registered a lower API compared with 2016: TRI, population 238, increased marginally from 37.8 to 54.6 and SEM, population 204, increased from 181.4 to 279.4. In the four communities during the time-frame of the present study (2016–2017) more malaria cases (~ 65%) were P. vivax (~ 65%) compared with P. falciparum (35%). These values are similar to national Peruvian averages for 2016–2017 [28, 50].
Table 4.
Number of malaria cases in the four sampled communities in Loreto, Peru (2016 and 2017)
| Community | Population | No. cases 2016 | API 2016 | No. cases 2017 | API 2017 |
|---|---|---|---|---|---|
| Lupuna | 365 | 184 | 504.1 | 163 | 446.6 |
| Santa Emilia | 204 | 37 | 181.4 | 57 | 279.4 |
| El Triunfo | 238 | 9 | 37.8 | 13 | 54.6 |
| N. Horizonte | 375 | 61 | 162.7 | 44 | 117.3 |
N Nuevo; Data from Ministry of Health, Iquitos, Peru; API: Annual Parasite Index calculated as number of confirmed malaria cases per 1,000 individuals
Discussion
Results are congruent with previous studies in Amazonian Peruvian of Ny. darlingi that found the highest risk of acquiring malaria to be outdoors before midnight during the rainy season. Our new data underscore the low risk of local malaria transmission during the dry season, due in part to a highly significant reduction in Ny. darlingi abundance together with significantly lower parity rates, compared with the rainy season. In agreement with previous studies in the peri-Iquitos region of Loreto Department in Amazonian Peru [10, 19], Ny. darlingi was the predominant anopheline species collected in the present study (99.3%). The HBR, IR and EIR were all within the range of other similar regional studies that have focused mainly on Ny. darlingi (Table 1).
In peri-Iquitos, Ny. benarrochi B appears to be relatively uncommon, as previous reports demonstrate [14, 19, 51, 52]. In some regions, notably Datem del Marañon province, it was [8] and remains [53, 54] highly abundant. It is a regional or secondary Plasmodium vector in southern Colombia, eastern Peru [55], eastern (Amazonian) Ecuador [53] and Datem del Marañon [54].
Despite high net distribution and cover and access (many residents in the present study use both tocuyos and LLINs as we have shown previously [17, 19], Plasmodium continues to be transmitted and the APIs, especially in the two riverine villages of LUP and SEM, were high in both 2016 and 2017 (Table 4). In similar villages in Mazan district, Loreto region, Peru, we demonstrated that during the early evening (17:00–20:00), between 20 and ~ 80% of the human population was protected under nets, leaving adequate hosts available for mosquitoes to obtain a blood meal [19]. The most likely explanation for transmission in the present study is that some of the residents were not using their bednets during the early evening hours and therefore not protected. We observed in this study as well as in [19], that residents indoors were occupied eating or watching television, not using their nets, or they were outdoors bathing in a nearby river or playing soccer. It has been suggested that interventions that are more in synch with the malaria endemic community lifestyles and can be incorporated into routine activities will have a better chance of protecting individuals [56].
Notably, the riverine village of LUP has persisted as a malaria transmission hot spot for several years [28]. In the present study, the highest Ny. darlingi HBR was reported in LUP and there were significantly more Ny. darlingi collected in this village compared with the other three communities (Tables 1 and 3). The possible explanations for the complex malaria transmission scenario in LUP are varied: (1) the high biting rate may be an adaptation to increase Plasmodium transmission as infectivity rates in Ny. darlingi are relatively low [25]; (2) our prior larval sampling in LUP revealed that a slow-moving stream traversing the village has been a productive and permanent breeding site for Ny. darlingi [37]; (3) high Plasmodium genetic diversity and gene flow among geographically relatively far-flung communities including LUP and SEM facilitates the movement of malaria parasites [57]; (4) recurrent seasonal flooding adds to the abundance and fluctuations of Ny. darlingi populations; and (5) there is transmission microheterogeneity within the village of LUP [58]. It is difficult to quantify the contribution of each of these factors but integrating multiple effective interventions such as regular distribution of LLINs and/or impregnated hammocks and hammock nets, (after testing for effectiveness in reduction of indoor and outdoor transmission) to every resident; detection and monthly treatment of larval breeding sites (as in [59]); and routine detection and treatment of both symptomatic and asymptomatic/sub-microscopic Plasmodium carriers [60] would undoubtedly reduce malaria transmission. A comparison of human blood samples tested for presence of Plasmodium spp. in Santa Emilia by microscopy versus PCR by Ramirez [61] underscore the importance of testing and treatment of both symptomatic and asymptomatic/sub-microscopic Plasmodium carriers to cut transmission. A multi-pronged programme (PAMAFRO) was successful in dramatically reducing malaria in Amazonian Peru from 2005 to 2010 [28]. The Plan Maria Cero in Loreto, implemented from 2017 to 2020, reduced malaria cases by 75% [62]. Subsequently, in 2022, the national malaria elimination plan was launched with the main objective of reducing malaria cases in Peru by 90% by 2030 [63].
The propensity of the primary regional vector Ny. darlingi to bite outdoors in the early evening, at least in many riverine communities in the Amazon [64, 65] when residents are outdoors eating, relaxing, or working, constitutes a major coverage gap [19]. One plausible vector control intervention for local communities with housing that frequently includes incomplete or fewer than four walls could be the use of eave ribbons impregnated with a mosquito repellent such as those that have been shown to be highly effective in several sub-Saharan malaria endemic countries [66–68]. Clearly, such interventions would need to be tested rigorously in the Latin American context.
We were able to collect adequate sample sizes during the dry season for analysis in this study (n = 879 (20.45%)) and detected significant seasonal differences in abundance (Table 3) and parity. The lack of dry season Ny. darlingi infected with Plasmodium supports earlier findings that the risk for transmission in this region is during the rainy season, although farther west, in Datem del Marañon province, even in the dry season there is a risk of acquiring malaria [54]. There are two probable reasons for this. First, during the low-transmission (dry) season, despite a substantial burden of sub-microscopic infections in Loreto [12, 61] and the fact that persons with submicroscopic malaria can infect anophelines [69], generally very few mosquitoes are infected at low gametocyte densities with standard membrane feeders and transmission is considered much less likely to occur [60, 70]. Nevertheless, a comparison of microscopy versus real time PCR results of Plasmodium-positive residents from the community of Santa Emilia from January–September 2016, demonstrated a very biologically significant difference i.e., 6.5% (92/1416) infectivity by microscopy and 24.34% (295/1212) by PCR. The data support the consideration of testing and treating asymptomatic inhabitants, particularly of malaria hotspots.
The second reason is that malaria transmission occurs not only in villages but also in temporary locations (for example, logging or mining camps) linked to seasonal occupation [10, 12]. We did not collect anopheline specimens from such localities to test for this study but the Parker et al. [10] study along the Mazan River during both rainy and dry seasons detected two of 967 Ny. darlingi specimens infected, one positive for P. falciparum and one for P. vivax, from occupation-related logging sites.
The peak biting patterns for Ny. darlingi reported here are similar to those in Lainhart et al. [9], in that they differ between riverine and highway communities, albeit not significantly. Thus, in the present study, there was no significant difference between seasonal patterns, and we reject our initial hypothesis of differences between village types (riverine and highway) and instead propose that these biting patterns result from the spatiotemporal availability of human (and possibly other animal and/or bird) hosts [9, 18, 71, 72].
Our parity data indicate that the majority of Ny. darlingi females seeking a human bloodmeal are parous, i.e., older, as previous studies have demonstrated in Amazonian Peru [18] and Brazil [45] and, therefore, they could potentially be infected with Plasmodium. However, only during the March collection in TRI were samples of Ny. darlingi old enough (range 7.24–9.13 days) to sustain the P. vivax sporogonic cycle, calculated with the Moshkovsky method in Barros et al. [45]). Parity rates were higher for Ny. darlingi during peak transmission (second half of the rainy season and beginning of the dry season) in LUP, NHO and TRI, although this does not hold for SEM (Table 2). Limitations of this study include the high variability in the range of mosquitoes that were dissected and analysed to estimate parity among sites and seasons, as well as in some cases, very low numbers of nulliparous mosquitoes, such that the vector age could not be calculated. Dissections can be inaccurately interpreted and training in the apparently simple technique is essential. Determination of age has long been a vexing issue in vector biology but a promising new surveillance method, based on deep learning of mid-infrared spectra of mosquito cuticle, was able to accurately and cost-effectively identify both species and age class among three closely related Africa vectors, Anopheles gambiae, Anopheles arabiensis and Anopheles coluzzii [73]. Hopefully, this method can soon be applied to Latin American and other regional malaria vectors and make a significant impact on malaria transmission reduction.
Conclusions
Malaria incidence in Peru is highest when the interactions between the various ecological and human factors are optimal for malaria transmission [28] and may be region specific. For example, in Roraima state, Brazil, peak malaria incidence and the highest parity rates occurred during the dry season [45], whereas in southern Venezuela, the peak malaria incidence occurred one month after peak biting rates by Ny. darlingi and Nyssorhynchus marajoara, and there was no correlation with rainfall [74]. In the present study, malaria transmission is optimal during the rainy season when there is an abundance of breeding sites, parous female Ny. darlingi, and available human hosts, especially outdoors during the early evening hours that coincide with the peak human biting rates of Ny. darlingi.
Supplementary Information
Additional file 1: Count dataset. Nyssorhynchus darlingi by collection site, season (rainy/dry), biting location (indoor/outdoor), and time period for all four collection sites (LUP, NHO, SEM, TRI).
Additional file 2: Table S1. Kruskal-Wallis analysis on ranked abundance of Nyssorhynchus darlingi, in four collection sites (Lupuna, Nuevo Horizonte, Santa Emilia, El Triunfo), during rainy and dry seasons 2016-2017. Figure S1. Average parity rate for each collection site comparing: A Before vs. after midnight collections; B Indoor vs. outdoor collections; C Rainy vs. dry collections. Figure S2. Average proportion of Ny. darlingi collected hourly biting indoor vs. outdoor for each collection site. Confidence intervals not shown for clarity. Figure S3. Average proportion of Ny. darlingi collected hourly biting by season for each collection site. Confidence intervals not shown for clarity.
Acknowledgements
We thank all study participants and the leaders and residents of Lupuna, Santa Emilia, El Triunfo, and Nuevo Horizonte for agreeing to host this study in their communities and all field workers (David Arimuya, Abrahan Vilchez, Mario Florez, Delfin Mamerto, Gloria Rodriguez, Santiago Ruiz, Papa Segundo, Papa Wilson and Victor Aquituari) for their dedication during the seasonal field collections. We also thank all the people involved in the Amazon ICEMR project in Lima and Iquitos. We are grateful to Dirección Regional de Salud (DIRESA, Iquitos, Loreto) for collaboration and facilitating logistics in Loreto Department. This publication has been possible thanks to the authorization and permits (no. 0424-2012-AG-DGFFS-DGEFFS) from Dirección de Gestión Forestal y de Fauna Silvestre y la Dirección General Forestal y de Fauna Silvestre del Ministerio de Agricultura de la República del Perú.
Author contributions
CP, MM and JEC conceived and designed the study. CP, MM and MPS supervised the study. FA provided guidance on field site selection and malaria case data for each community. CP, MM and MPS conducted the study. SAB analysed the data with help from RL and MPS. SAB, RL and JEC wrote the manuscript. All authors read and approved the final manuscript.
Funding
This project was funded by NIH-NIAID (U19AI089681) to JMV and NIH-NIAID (R01AI110112) to JEC. The Biodefense and Emerging Infectious Disease training fellowship Grant T32AI05532901 provided partial support for CP.
Availability of data and materials
The data supporting the conclusions of this article are included within the article. The raw data used and/or analysed in this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Study protocols were approved by the Ethics Review Board of the Regional. Health Direction of Loreto (477-2016), Universidad Peruana Cayetano Heredia. in Lima (184-09-16), Asociación Benéfica PRISMA, Lima, Peru and the Human Subjects Protection Program of the University of California San Diego, La Jolla, CA, USA. The New York State Department of Health Institutional Review Board considers mosquito collecting to be a Risk Management issue. Collections were only conducted by trained professionals and standard precautions were taken by all collectors.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marta Moreno, Email: Marta.Moreno@lshtm.ac.uk.
Jan E. Conn, Email: jan.conn@health.ny.gov
References
- 1.World Health Organization . World Malaria Report 2016. Geneva: World Health Organization; 2016. p. 148. [Google Scholar]
- 2.World Health Organization . World Malaria Report 2017. Geneva: World Health Organization; 2017. p. 196. [Google Scholar]
- 3.World Health Organization . World Malaria Report 2018. Geneva: World Health Organization; 2018. p. 210. [Google Scholar]
- 4.World Health Organization . World malaria report 2020: 20 years of global progress and challenges. Geneva, Switzerland: World Health Organization; 2020. p. 247. [Google Scholar]
- 5.Foster PG, de Oliveira TMP, Bergo ES, Conn JE, Sant'Ana DC, Nagaki SS, et al. Phylogeny of Anophelinae using mitochondrial protein coding genes. R Soc Open Sci. 2017;4:170758. doi: 10.1098/rsos.170758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernández R, Carbajal F, Quintana J, Chauca H, Watts DM, de Presencia A. (N) darlingi (Diptera: Culicidae), en alrededores de la ciudad de Iquitos, Loreto-Peru. Boletín de la Soc Peruana de Enfermedades Infecciosas y Trop. 1996;5:10–20. [Google Scholar]
- 7.Aramburu Guarda J, Ramal Asayag C, Witzig R. Malaria reemergence in the Peruvian Amazon region. Emerg Infect Dis. 1999;5:209–215. doi: 10.3201/eid0502.990204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoeler GB, Flores-Mendoza C, Fernandez R, Davila JR, Zyzak M. Geographical distribution of Anopheles darlingi in the Amazon Basin region of Peru. J Am Mosq Control Assoc. 2003;19:286–296. [PubMed] [Google Scholar]
- 9.Lainhart W, Bickersmith S, Nadler K, Moreno M, Saavedra M, Chu VM, et al. Evidence for temporal population replacement and the signature of ecological adaptation in a major Neotropical malaria vector in Amazonian Peru. Malar J. 2015;14:375. doi: 10.1186/s12936-015-0863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker BS, Paredes Olortegui M, Penataro Yori P, Escobedo K, Florin D, Rengifo Pinedo S, et al. Hyperendemic malaria transmission in areas of occupation-related travel in the Peruvian Amazon. Malar J. 2013;12:178. doi: 10.1186/1475-2875-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosas-Aguirre A, Speybroeck N, Llanos-Cuentas A, Rosanas-Urgell A, Carrasco-Escobar G, Rodriguez H, et al. Hotspots of malaria transmission in the Peruvian Amazon: rapid assessment through a parasitological and serological survey. PLoS ONE. 2015;10:e0137458. doi: 10.1371/journal.pone.0137458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrasco-Escobar G, Gamboa D, Castro MC, Bangdiwala SI, Rodriguez H, Contreras-Mancilla J, et al. Micro-epidemiology and spatial heterogeneity of P. vivax parasitaemia in riverine communities of the Peruvian Amazon: a multilevel analysis. Sci Rep. 2017;7:8082. doi: 10.1038/s41598-017-07818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vezenegho SB, Adde A, Pommier de Santi V, Issaly J, Carinci R, Gaborit P, et al. High malaria transmission in a forested malaria focus in French Guiana: how can exophagic Anopheles darlingi thwart vector control and prevention measures? Mem Inst Oswaldo Cruz. 2016;111:561–569. doi: 10.1590/0074-02760160150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prussing C, Moreno M, Saavedra MP, Bickersmith SA, Gamboa D, Alava F, et al. Decreasing proportion of Anopheles darlingi biting outdoors between long-lasting insecticidal net distributions in peri-Iquitos. Amazonian Peru Malar J. 2018;17:86. doi: 10.1186/s12936-018-2234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Lozano WS, et al. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74:3–11. doi: 10.4269/ajtmh.2006.74.3. [DOI] [PubMed] [Google Scholar]
- 16.Chaves LSM, Conn JE, López RVM, Sallum MAM. Abundance of impacted forest patches less than 5 km2 is a key driver of the incidence of malaria in Amazonian Brazil. Sci Rep. 2018;8:7077. doi: 10.1038/s41598-018-25344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno M, Saavedra MP, Bickersmith SA, Lainhart W, Tong C, Alava F, et al. Implications for changes in Anopheles darlingi biting behaviour in three communities in the peri-Iquitos region of Amazonian Peru. Malar J. 2015;14:290. doi: 10.1186/s12936-015-0804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreno M, Saavedra MP, Bickersmith SA, Prussing C, Michalski A, Tong Rios C, et al. Intensive trapping of blood-fed Anopheles darlingi in Amazonian Peru reveals unexpectedly high proportions of avian blood-meals. PLoS Negl Trop Dis. 2017;11:e0005337. doi: 10.1371/journal.pntd.0005337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saavedra MP, Conn JE, Alava F, Carrasco-Escobar G, Prussing C, Bickersmith SA, et al. Higher risk of malaria transmission outdoors than indoors by Nyssorhynchus darlingi in riverine communities in the Peruvian Amazon. Parasit Vectors. 2019;12:374. doi: 10.1186/s13071-019-3619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Almeida NCV, Louzada J, Neves M, Carvalho TM, Castro-Alves J, Silva-do-Nascimento TF, et al. Larval habitats, species composition and distribution of malaria vectors in regions with autochthonous and imported malaria in Roraima state. Brazil Malar J. 2022;21:13. doi: 10.1186/s12936-021-04033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piedrahita S, Álvarez N, Naranjo-Díaz N, Bickersmith S, Conn J, Correa M. Anopheles blood meal sources and entomological indicators related to Plasmodium transmission in malaria endemic areas of Colombia. Acta Trop. 2022;233:106567. doi: 10.1016/j.actatropica.2022.106567. [DOI] [PubMed] [Google Scholar]
- 22.Musiime AK, Smith DL, Kilama M, Rek J, Arinaitwe E, Nankabirwa JI, et al. Impact of vector control interventions on malaria transmission intensity, outdoor vector biting rates and Anopheles mosquito species composition in Tororo. Uganda Malar J. 2019;18:445. doi: 10.1186/s12936-019-3076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bugoro H, Iro'ofa C, Mackenzie DO, Apairamo A, Hevalao W, Corcoran S, et al. Changes in vector species composition and current vector biology and behaviour will favour malaria elimination in Santa Isabel Province. Solomon Islands Malar J. 2011;10:287. doi: 10.1186/1475-2875-10-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reinbold-Wasson DD, Sardelis MR, Jones JW, Watts DM, Fernandez R, Carbajal F, et al. Determinants of Anopheles seasonal distribution patterns across a forest to periurban gradient near Iquitos. Peru Am J Trop Med Hyg. 2012;86:459–463. doi: 10.4269/ajtmh.2012.11-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sallum MAM, Conn JE, Bergo ES, Laporta GZ, Chaves LSM, Bickersmith SA, et al. Vector competence, vectorial capacity of Nyssorhynchus darlingi and the basic reproduction number of Plasmodium vivax in agricultural settlements in the Amazonian Region of Brazil. Malar J. 2019;18:117. doi: 10.1186/s12936-019-2753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmerman RH, Galardo AKR, Lounibos LP, Galardo C, Bahar AK, van Santen E. Vectorial capacities for malaria in eastern Amazonian Brazil depend on village, vector species, season, and parasite species. Malar J. 2022;21:237. doi: 10.1186/s12936-022-04255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosas-Aguirre A, Guzman-Guzman M, Moreno-Gutierrez D, Rodriguez-Ferrucci H, Vargas-Pacherrez D, Acuna-Gonzalez Y. Long-lasting insecticide - treated bednet ownership, retention and usage one year after their distribution in Loreto, Peru. Rev Peru Med Exp Salud Publica. 2011;28:228–236. doi: 10.1590/S1726-46342011000200009. [DOI] [PubMed] [Google Scholar]
- 28.Soto-Calle V, Rosas-Aguirre A, Llanos-Cuentas A, Abatih E, DeDeken R, Rodriguez H, et al. Spatio-temporal analysis of malaria incidence in the Peruvian Amazon Region between 2002 and 2013. Sci Rep. 2017;7:40350. doi: 10.1038/srep40350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janko MM, Recalde-Coronel GC, Damasceno CP, Salmón-Mulanovich G, Barbieri AF, Lescano AG, et al. The impact of sustained malaria control in the Loreto region of Peru: a retrospective, observational, spatially-varying interrupted time series analysis of the PAMAFRO program. Lancet Reg Health Am. 2023;20:100477. doi: 10.1016/j.lana.2023.100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez-Castro EE, Cahuana GM, García-Ríos CJ, Guerra-Duarte C, Chauca P, Tapia-Limonchi R, et al. Health and economic burden due to malaria in Peru over 30 years (1990–2019): findings from the global burden of diseases study 2019. Lancet Reg Health Am. 2022;15:100347. doi: 10.1016/j.lana.2022.100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norris DE. Mosquito-borne diseases as a consequence of land use change. EcoHealth. 2004;1:19–24. doi: 10.1007/s10393-004-0008-7. [DOI] [Google Scholar]
- 32.Shocket MS, Anderson CB, Caldwell JM, Childs ML, Couper LI, Han S, et al. Environmental drivers of vector-borne diseases. In: Drake JM, Bonsall M, Strand M, et al., editors. Population biology of vector-borne diseases. Oxford: Oxford University Press; 2020. [Google Scholar]
- 33.da Silva-Vasconcelos A, Kato MY, Mourao EN, de Souza RT, Lacerda RN, Sibajev A, et al. Biting indices, host-seeking activity and natural infection rates of anopheline species in Boa Vista, Roraima, Brazil from 1996 to 1998. Mem Inst Oswaldo Cruz. 2002;97:151–161. doi: 10.1590/S0074-02762002000200002. [DOI] [PubMed] [Google Scholar]
- 34.Galardo AK, Zimmerman RH, Lounibos LP, Young LJ, Galardo CD, Arruda M, et al. Seasonal abundance of anopheline mosquitoes and their association with rainfall and malaria along the Matapi River, Amapa, Brazil. Med Vet Entomol. 2009;23:335–349. doi: 10.1111/j.1365-2915.2009.00839.x. [DOI] [PubMed] [Google Scholar]
- 35.Canelas T, Castillo-Salgado C, Baquero OS, Ribeiro H. Environmental and socioeconomic analysis of malaria transmission in the Brazilian Amazon, 2010–2015. Rev Saude Publica. 2019;53:49. doi: 10.11606/S1518-8787.2019053000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girod R, Roux E, Berger F, Stefani A, Gaborit P, Carinci R, et al. Unravelling the relationships between Anopheles darlingi (Diptera: Culicidae) densities, environmental factors and malaria incidence: understanding the variable patterns of malarial transmission in French Guiana (South America) Ann Trop Med Parasitol. 2011;105:107–122. doi: 10.1179/136485911X12899838683322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prussing C, Saavedra MP, Bickersmith SA, Alava F, Guzmán M, Manrique E, et al. Malaria vector species in Amazonian Peru co-occur in larval habitats but have distinct larval microbial communities. PLoS Negl Trop Dis. 2019;13:e0007412. doi: 10.1371/journal.pntd.0007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Barros FS, Honorio NA. Deforestation and malaria on the Amazon frontier: larval clustering of Anopheles darlingi (Diptera: Culicidae) determines focal distribution of malaria. Am J Trop Med Hyg. 2015;93:939–953. doi: 10.4269/ajtmh.15-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaves LSM, Bergo ES, Conn JE, Laporta GZ, Prist PR, Sallum MAM. Anthropogenic landscape decreases mosquito biodiversity and drives malaria vector proliferation in the Amazon rainforest. PLoS ONE. 2021;16:e0245087. doi: 10.1371/journal.pone.0245087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Consoli RA, Lourenco-de-Oliveira R. 1994. Principais mosquitos de importância sanitária no Brasil. Fundação Oswaldo Cruz. Brazil: Editora Fiocruz.
- 41.Faran ME, Linthicum KJ. A handbook of the Amazonian species of Anopheles (Nyssorhynchus) (Diptera: Culicidae) Mosq Syst. 1981;13:1–81. [Google Scholar]
- 42.Forattini OP. Entomologia Medica. São Paulo, Brazil: Faculdade de Higiene e Sáude Publica; 1962. [Google Scholar]
- 43.Davidson G. Estimation of the survival-rate of anopheline mosquitoes in nature. Nature. 1954;174:792–793. doi: 10.1038/174792a0. [DOI] [PubMed] [Google Scholar]
- 44.Service MW. Community participation in vector-borne disease control. Ann Trop Med Parasitol. 1993;87:223–234. doi: 10.1080/00034983.1993.11812760. [DOI] [PubMed] [Google Scholar]
- 45.de Barros FS, Honorio NA, Arruda ME. Survivorship of Anopheles darlingi (Diptera: Culicidae) in relation with malaria incidence in the Brazilian Amazon. PLoS ONE. 2011;6:e22388. doi: 10.1371/journal.pone.0022388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bickersmith SA, Lainhart W, Moreno M, Chu VM, Vinetz JM, Conn JE. A sensitive, specific and reproducible real-time PCR method for detection of Plasmodium vivax and P. falciparum infection in field-collected anophelines. Mem Inst Oswaldo Cruz. 2015;110:573–576. doi: 10.1590/0074-02760150031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pizzitutti F, Pan W, Barbieri A, Miranda JJ, Feingold B, Guedes GR, et al. A validated agent-based model to study the spatial and temporal heterogeneities of malaria incidence in the rainforest environment. Malar J. 2015;14:1–19. doi: 10.1186/s12936-015-1030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venables WN, Ripley BD. Modern applied statistics with S. 4. New York: Springer; 2002. [Google Scholar]
- 49.Matson R, Rios CT, Chavez CB, Gilman RH, Florin D, Sifuentes VL, et al. Improved molecular technique for the differentiation of neotropical anopheline species. Am J Trop Med Hyg. 2008;78:492–498. doi: 10.4269/ajtmh.2008.78.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosas-Aguirre A, Gamboa D, Manrique P, Conn JE, Moreno M, Lescano AG, et al. Epidemiology of Plasmodium vivax malaria in Peru. Am J Trop Med Hyg. 2016;95:133–144. doi: 10.4269/ajtmh.16-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conn JE, Moreno M, Saavedra M, Bickersmith SA, Knoll E, Fernandez R, et al. Molecular taxonomy of Anopheles (Nyssorhynchus) benarrochi (Diptera: Culicidae) and malaria epidemiology in southern Amazonian Peru. Am J Trop Med Hyg. 2013;88:319–324. doi: 10.4269/ajtmh.2012.12-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prussing C, Emerson KJ, Bickersmith SA, Sallum MAM, Conn JE. Minimal genetic differentiation of the malaria vector Nyssorhynchus darlingi associated with forest cover level in Amazonian Brazil. PLoS ONE. 2019;14:e0225005. doi: 10.1371/journal.pone.0225005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morales Viteri D, Herrera-Varela M, Albuja M, Quiroga C, Diaz G, Del Aguila Morante C, et al. New records of Anopheles benarrochi B (Diptera: Culicidae) in malaria hotspots in the Amazon regions of Ecuador and Peru. J Med Entomol. 2021 doi: 10.1093/jme/tjaa293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conn JE, Bickersmith SA, Saavedra MP, Morales JA, Alava F, Diaz Rodriguez GA, et al. Natural infection of Nyssorhynchus darlingi and Nyssorhynchus benarrochi B with Plasmodium during the dry season in the understudied low-transmission setting of Datem del Marañon Province, Amazonian Peru. Am J Trop Med Hyg. 2023 doi: 10.4269/ajtmh.23-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flores-Mendoza C, Fernandez R, Escobedo-Vargas KS, Vela-Perez Q, Schoeler GB. Natural Plasmodium infections in Anopheles darlingi and Anopheles benarrochi (Diptera: Culicidae) from eastern Peru. J Med Entomol. 2004;41:489–494. doi: 10.1603/0022-2585-41.3.489. [DOI] [PubMed] [Google Scholar]
- 56.Monroe A, Moore S, Okumu F, Kiware S, Lobo NF, Koenker H, et al. Methods and indicators for measuring patterns of human exposure to malaria vectors. Malar J. 2020;19:207. doi: 10.1186/s12936-020-03271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manrique P, Miranda-Alban J, Alarcon-Baldeon J, Ramirez R, Carrasco-Escobar G, Herrera H, et al. Microsatellite analysis reveals connectivity among geographically distant transmission zones of Plasmodium vivax in the Peruvian Amazon: a critical barrier to regional malaria elimination. PLoS Negl Trop Dis. 2019;13:e0007876. doi: 10.1371/journal.pntd.0007876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosas-Aguirre A, Guzman-Guzman M, Chuquiyauri R, Moreno M, Manrique P, Ramirez R, et al. Temporal and micro-spatial heterogeneity in transmission dynamics of co-endemic Plasmodium vivax and Plasmodium falciparum in two rural cohort populations in the Peruvian Amazon. J Infect Dis. 2020;jiaa26:1–12. doi: 10.1093/infdis/jiaa526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fontoura PS, Silva MF, da Costa AS, Ribeiro FS, Ferreira MS, Ladeia-Andrade S, et al. Monthly biological larviciding associated with a tenfold decrease in larval density in fish farming ponds and reduced community-wide malaria incidence in northwestern Brazil. Parasit Vectors. 2021;14:445. doi: 10.1186/s13071-021-04964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferreira MU, Corder RM, Johansen IC, Kattenberg JH, Moreno M, Rosas-Aguirre A, et al. Relative contribution of low-density and asymptomatic infections to Plasmodium vivax transmission in the Amazon: pooled analysis of individual participant data from population-based cross-sectional surveys. Lancet Reg Health Am. 2022;9:100169. doi: 10.1016/j.lana.2021.100169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramirez RS. Micro-epidemiologia molecular des las infecciones que contribuyen a mantener la transmission de Plasmodium vivax en comunidades en vias de eliminacion de la region Loreto. San Martín de Porres: Universidad Peruana Cayetano Heredia Facultad de Ciencias y Filosofia; 2024. [Google Scholar]
- 62.MINSA. Ministerio de Salud del Perú: Boletín epidemiológico del Perú SE 31-2022. vol. 31. Lima, Perú: Centro Nacional de Epidemiología, Prevención y Control de Enfermedades; 2022.
- 63.Ministerio de Salud. Documento technico: plan hacia la malaria en el Peru 2022-2030. 2022:60
- 64.Iyer M, Skelton J, de Wildt G, Meza G. A qualitative study on the use of long-lasting insecticidal nets (LLINs) for the prevention of malaria in the Peruvian Amazon. Malar J. 2019;18:301. doi: 10.1186/s12936-019-2937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prado CC, Alvarado-Cabrera LA, Camargo-Ayala PA, Garzón-Ospina D, Camargo M, Soto-De León SC, et al. Behavior and abundance of Anopheles darlingi in communities living in the Colombian Amazon riverside. PLoS ONE. 2019;14:e0213335. doi: 10.1371/journal.pone.0213335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mmbando AS, Ngowo H, Limwagu A, Kilalangongono M, Kifungo K, Okumu FO. Eave ribbons treated with the spatial repellent, transfluthrin, can effectively protect against indoor-biting and outdoor-biting malaria mosquitoes. Malar J. 2018;17:368. doi: 10.1186/s12936-018-2520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaindoa EW, Mmbando AS, Shirima R, Hape EE, Okumu FO. Insecticide-treated eave ribbons for malaria vector control in low-income communities. Malar J. 2021;20:415. doi: 10.1186/s12936-021-03945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Masalu JP, Finda M, Killeen GF, Ngowo HS, Pinda PG, Okumu FO. Creating mosquito-free outdoor spaces using transfluthrin-treated chairs and ribbons. Malar J. 2020;19:109. doi: 10.1186/s12936-020-03180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alves FP, Gil LH, Marrelli MT, Ribolla PE, Camargo EP, Da Silva LH. Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. J Med Entomol. 2005;42:777–779. doi: 10.1603/0022-2585(2005)042[0777:ACOPSA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 70.Lin JT, Saunders DL, Meshnick SR. The role of submicroscopic parasitemia in malaria transmission: what is the evidence? Trends Parasitol. 2014;30:183–190. doi: 10.1016/j.pt.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fouque F, Gaborit P, Carinci R, Issaly J, Girod R. Annual variations in the number of malaria cases related to two different patterns of Anopheles darlingi transmission potential in the Maroni area of French Guiana. Malar J. 2010;9:80. doi: 10.1186/1475-2875-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oliveira TMP, Laporta GZ, Bergo ES, Chaves LSM, Antunes JLF, Bickersmith SA, et al. Vector role and human biting activity of Anophelinae mosquitoes in different landscapes in the Brazilian Amazon. Parasit Vectors. 2021;14:236. doi: 10.1186/s13071-021-04725-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siria DJ, Sanou R, Mitton J, Mwanga EP, Niang A, Sare I, et al. Rapid age-grading and species identification of natural mosquitoes for malaria surveillance. Nat Commun. 2022;13:1501. doi: 10.1038/s41467-022-28980-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moreno JE, Rubio-Palis Y, Paez E, Perez E, Sanchez V. Abundance, biting behaviour and parous rate of anopheline mosquito species in relation to malaria incidence in gold-mining areas of southern Venezuela. Med Vet Entomol. 2007;21:339–349. doi: 10.1111/j.1365-2915.2007.00704.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Count dataset. Nyssorhynchus darlingi by collection site, season (rainy/dry), biting location (indoor/outdoor), and time period for all four collection sites (LUP, NHO, SEM, TRI).
Additional file 2: Table S1. Kruskal-Wallis analysis on ranked abundance of Nyssorhynchus darlingi, in four collection sites (Lupuna, Nuevo Horizonte, Santa Emilia, El Triunfo), during rainy and dry seasons 2016-2017. Figure S1. Average parity rate for each collection site comparing: A Before vs. after midnight collections; B Indoor vs. outdoor collections; C Rainy vs. dry collections. Figure S2. Average proportion of Ny. darlingi collected hourly biting indoor vs. outdoor for each collection site. Confidence intervals not shown for clarity. Figure S3. Average proportion of Ny. darlingi collected hourly biting by season for each collection site. Confidence intervals not shown for clarity.
Data Availability Statement
The data supporting the conclusions of this article are included within the article. The raw data used and/or analysed in this study are available from the corresponding author upon reasonable request.