The creation of synthetic T cell states has captivated the field of cell-based therapies. Wang et al. describe how disruption of BCOR and ZC3H12A unleashes anti-tumor T cells with unprecedented lifespan and killer instinct. Are we witnessing the birth of immortal super-soldiers in medicine?
Abstract
The creation of synthetic T cell states has captivated the field of cell-based therapies. Wang et al. (https://doi.org/10.1084/jem.20232368) describe how disruption of BCOR and ZC3H12A unleashes anti-tumor T cells with unprecedented lifespan and killer instinct. Are we witnessing the birth of immortal super-soldiers in medicine?
Cellular immunotherapy is revolutionizing oncology by harnessing T cells’ unique ability to specifically target and potentially cure metastatic cancer, a feat not achievable with traditional treatments. T cells, whether naturally occurring tumor-infiltrating lymphocytes or genetically modified ones like chimeric antigen receptor T cells or those with enhanced T cell receptors (TCRs), can be engineered to have enhanced recognition of antigens, or to respond to antigens that natural T cells would typically ignore, which could involve novel receptors not found in nature. These innovative approaches have galvanized medical professionals, scientists, and industry leaders who are committed to unlocking the full potential of cell-based immunotherapies. Living T cells have proven they can eradicate even the most stubborn metastatic cells. However, challenges persist, as these therapies sometimes fail when T cells do not endure, often succumbing to exhaustion or senescence. This issue is being addressed by researchers like Wang et al. (2024), who are exploring methods to enhance T cell resilience and functionality, as discussed in this month’s Journal of Experimental Medicine (Wang et al., 2024).

Insights from Nicholas P. Restifo and Luca Gattinoni.
Evolution has shaped T cells to occasionally dampen their function in chronic viral infections to prevent autoimmunity and mitigate potential harm from an overly aggressive immune response. For example, the immune system’s complete elimination of a hepatitis virus could cause significant liver damage. Chronic activation can also drive T cells toward senescence and exhaustion, weakening the immune response to cancer. To address these challenges, researchers have developed checkpoint inhibitors and engineered T cells to create synthetic T cells that can reverse or bypass these evolutionary constraints with great success in some indications.
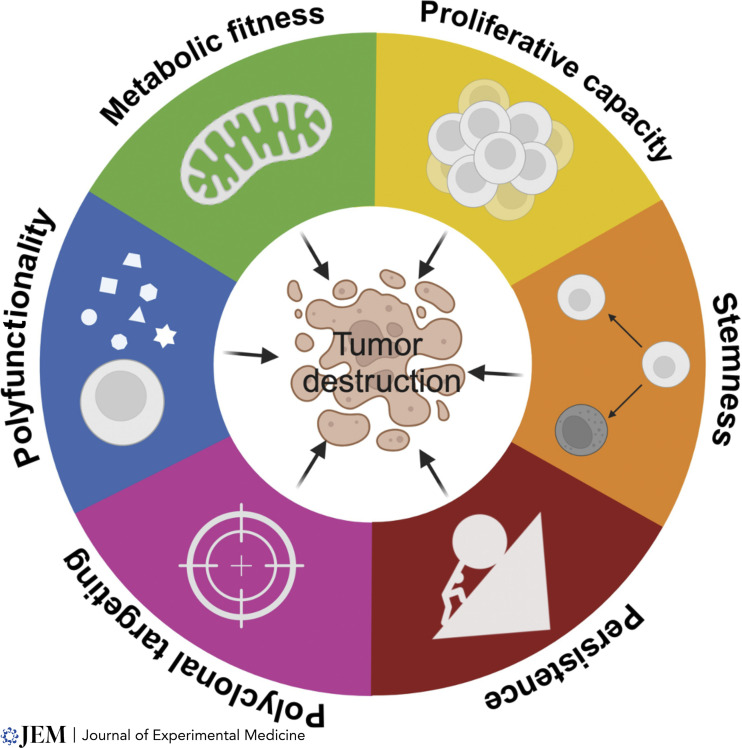
T cell immunotherapy has deepened our understanding of the qualities of cells that are highly effective at triggering tumor destruction (Fig. 1). T cell infusion products comprised of cells with stem-like qualities generally yield better patient responses compared to those enriched in mature T cells (Fraietta et al., 2018; Krishna et al., 2020). These results confirm observations made in wild-type mice and humanized mouse models showing that each T cell clonotype can behave like an adult stem cell system, capable of repeated cell divisions and giving rise to more specialized cells, while also renewing themselves (Gattinoni et al., 2009, 2011). These T cells resemble adult stem cells, capable of multiple divisions and evolving into specialized cells while self-renewing. T memory stem cells (TSCM) are vital for genetic integrity throughout life, akin to adult stem cells’ longevity (Beumer and Clevers, 2024). However, TSCM’s availability decreases with age (Farber et al., 2014). For therapy, they must transform into effector cells, which means sacrificing their multipotency and long-term survival for immediate anti-cancer activity.
Figure 1. Hallmarks of anti-tumor efficacy in adoptively transferred T cells. This figure delineates six interrelated hallmarks that underpin the anti-tumor efficacy of adoptively transferred T cells. Collectively, these characteristics empower T cells with stem-like functions, allowing for the replenishment of cellular populations within the target tissues to combat tumor growth effectively. These features include (i) proliferative capacity, the ability of T cells to undergo multiple rounds of division, expanding their numbers within the tumor microenvironment; (ii) stemness, the capacity of transferred cells to undergo self-renewal, ensuring longevity and sustained presence in the host to maintain anti-tumor activity over time as well as the capacity to differentiate into effector cells; (iii) tumor recognition, which for solid tumors implies polyclonal attack to address tumor heterogeneity; (iv) polyfunctionality, the capacity of cells to produce the diverse cytokines and chemokines to trigger upregulation of antigen presentation on targets and to attract other immune cells to the site; (v) metabolic fitness whereby transferred T cells are utilizing oxidative phosphorylation and fatty acid oxidation but can acquire glycolytic metabolism upon activation in vivo. This integrative portrayal of T cell attributes emphasizes the multifaceted approach required to achieve a robust and lasting anti-tumor response. The hallmarks serve as a framework for the design and evaluation of adoptive T cell therapies, with the potential to guide future advancements in cancer immunotherapy.
Developing synthetic T cell states could be greatly enhanced by a thorough knowledge of natural T cell development. Epigenetics is critical in transitioning cells from a stem-like state to effector functionality, guiding CD8+ T cells through chromatin reorganization (Henning et al., 2018). This process, which involves DNA methylation and chemical modifications to histones, silences stemness-related genes while activating those essential for effector functions, enabling these cells to acquire the cytotoxic capabilities vital for attacking pathogens and cancer cells. The differentiation of T cells is characterized by specific gene regulatory networks that equip them with effector functions at the expense of their self-renewal and pluripotency. For example, genes like TCF7, FOXO1, and KLF2 maintain stemness, while BACH2 regulates the balance between stemness and effector differentiation by inhibiting AP-1–dependent gene activation, crucial during T cell activation (Gattinoni et al., 2012; Roychoudhuri et al., 2016).
As CD8+ T clonotypes evolve into cytotoxic lymphocytes (CTLs), they experience significant structural changes, including a 10-fold increase in size and the development of granules filled with cytotoxic molecules like granzymes and perforin. It is critical to consider the intrinsic toxicity of effector granules in developing synthetic T cell states because they are essential CTL to eliminate infected or cancerous cells. Effector molecules can increase the risk of self-damage due to potential leakage of these granules, reducing CTL lifespan (Weigelin and Friedl, 2022).
As stem-like T cells mature, they undergo a crucial metabolic transformation. Stem-like T cells relying on oxidative phosphorylation and fatty acid oxidation—metabolism suited for long-term survival. Effector differentiation involves a switch to glycolysis, an anabolic process similar to the Warburg effect in cancer cells, enhancing their rapid growth and division. This metabolic reprogramming involves upregulating glucose transporters and glycolytic enzymes, optimizing for immediate responses (Chapman and Chi, 2022; Kishton et al., 2017). Meanwhile, the mobility of these cells is characterized by specific trafficking behavior; stem-like T cells move through the body using receptors like CD62L and CCR7 to navigate to lymphoid tissues. Upon differentiation, effector T cells switch to receptors such as CD62E, adhesion molecules like LFA-1, and chemokine receptors like CXCR3, CCR5, CCR4, and CX3CR1, enabling them to migrate to areas of inflammation or infection (Farsakoglu et al., 2021).
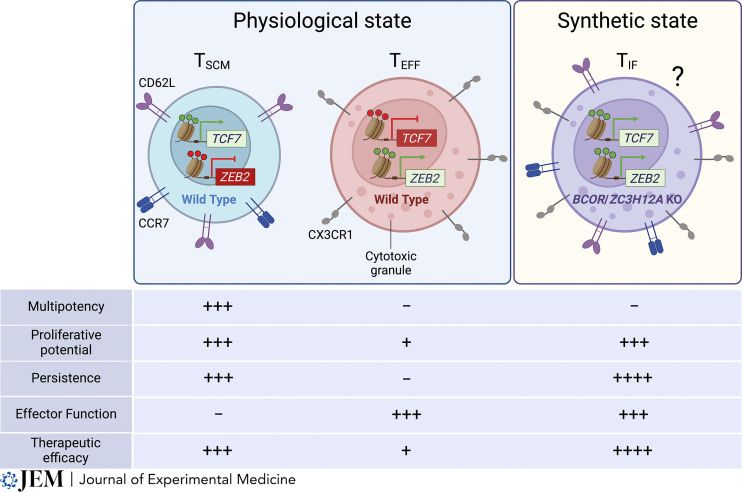
Wang et al. (2024) have developed a synthetic T cell state they call TIF (T cells with an immortal-like and functional state). TIF cells are the product of disrupting the BCOR and ZC3H12A genes, a result that is surprising because these genes are typically expressed at low levels in T cells and lack dynamic regulation. This approach is aimed at addressing the traditional trade-off in T cell therapies between longevity and potency, offering cells that not only persist longer but also retain robust anti-tumor capabilities. TIF cells demonstrate enhanced survival and can enter a reversible dormant state, like memory cells, providing long-term immunity. The synergy of eliminating BCOR following the knockout of ZC3H12A genes may augment T cell function, persistence, and anti-tumor activity through several mechanisms. ZC3H12A, also known as Regnase-1, has a role in the degradation of specific mRNA transcripts, including TCF7 (Zheng et al., 2021), thus regulating gene expression post-transcriptionally. It has a demonstrable impact on T cell function when used in combination with RC3H1 (Roquin-1) (Mai et al., 2023). BCOR (BCL6 corepressor) is known to be involved in gene repression and plays a role in the regulation of cell differentiation and proliferation. Without BCOR, and in combination with ZC3H12A deficiency, the authors show that genes that are usually repressed might become active, enhancing both stemness- and cytotoxicity-associated genes. Their knockouts could potentially remove brakes on the T cell stemness and cytotoxic programs, enhancing therapeutic efficacy in the mouse models used (Fig. 2).
Figure 2. Functional attributes of physiological and synthetic T cell states. This figure illustrates the distinct phenotypic and functional characteristics of three specialized T cell states. Plus signs indicate robustness of the designated function, minus signs indicate the absence of the function. Left: The first state, TSCM, is marked by the expression of the transcription factor TCF7 and the repression of the ZEB2. TSCM are identifiable by surface markers CD62L and CCR7, associated with their migratory capacity to lymphoid tissues. These cells are characterized by their ability to proliferate and persist without exerting effector functions, displaying high efficacy in adoptive transfer therapies. Center: The second state, T effector cells (TEFF), represents a terminally differentiated phenotype where TCF7 expression is silenced and ZEB2 is active. TEFF are distinguished by the surface expression of CX3CR1 and the production of cytotoxic granules, indicative of their effector functions. Despite their potent killing capacity, TEFF exhibit limited proliferation, persistence, and are less effective therapeutically upon adoptive transfer due to their short-lived nature. Right: The third hypothetical state described here by Wang et al. (2024) is a novel synthetic state designated as TIF, which purportedly combines the attributes of both TSCM and TEFF. This state is hypothesized to arise from the knockout (KO) of BCOR and ZC3H12A. According to Wang et al. (2024), TIF cells simultaneously maintain TCF7 and ZEB2 expression, bear stem-like and effector cell markers, and possess the ability to proliferate, persist, and execute effector functions, potentially offering a synergistic therapeutic advantage.
Wang et al. (2024) interpretations of data regarding T cell longevity face challenges. Advances in single-cell RNA sequencing provide a window into the nuanced states of these cells. However, what appears to be the longevity of TIF cells may actually be clonal succession—where some clones outlive and outgrow others, eventually succumbing to senescence or exhaustion, only to be replaced by new clonal populations. This cyclic dominance among clonotypes necessitates careful data interpretation to avoid conflating the collective behavior of a population with that of individual T cells. Such complexity in clonal dynamics requires tracking of individual cell trajectories and at the very least measurement of clonal succession through TCR sequencing. The use of multiplicative models, like those used by Soerens et al. (2023), yield overestimations of T cell growth (i.e., 30,000 earth masses). A more refined approach, involving additive mathematics, would yield a much smaller number, and would better account for clonal succession, offering a more realistic representation of T cell population expansion with finite life spans for individual clones. Thus, Wang’s claims for creating “immortal” T cells will require deeper analyses.
Moving forward, synthetic T cells may involve supraphysiological properties, not easily understood in the context of normal T cell ontogeny. Investigators may aim to create cells that have enhanced proliferation beyond normal limits, designed to surpass the counter regulation of division observed in physiological T cell responses. Ideally, these synthetic states would have resistance to exhaustion and indefinite persistence. But such idealized cells of the future might not carry effector molecules such as granzymes and perforin because of the potential for leakiness of these profoundly toxic molecules. Synthetic T cells could be designed with inducible effector functions and on-demand control systems for activation and deactivation, representing a paradigm shift in cellular engineering beyond natural T cell development.
Thus, the age of synthetic forms of T cell states is upon us, necessitated by deficiencies in the natural physiology of T cells. Although the subject of the ontogeny of TIF and other synthetic states is not yet explored, it may be possible to reprogram cells that have already entered senescent or terminally exhausted states. In this context, T cell “rejuvenation” may more specifically imply “re-functionalization” through a synthetic state, rather than a return of dysfunctional T cells to naturally occurring stem-like cells. Armed with increasingly powerful methods of genome editing, investigators moving forward into the clinic must be mindful of unintended consequences, such as autoimmunity or unregulated cell growth. A fulsome understanding of the risks and benefits of using synthetic T cell states represents seemingly infinite potential, akin to unlocking a new dimension of immunotherapy where T cells are tailored to be architects of immune resilience, revolutionizing our approach to the ravages of aging and to chronic human diseases.
Acknowledgments
Figures created with https://BioRender.com.
References
- Beumer, J., and Clevers H.. 2024. Cell Stem Cell. 10.1016/j.stem.2023.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, N.M., and Chi H.. 2022. Immunity. 10.1016/j.immuni.2021.12.012 [DOI] [Google Scholar]
- Farber, D.L., et al. 2014. Nat. Rev. Immunol. 10.1038/nri3567 [DOI] [Google Scholar]
- Farsakoglu, Y., et al. 2021. Cold Spring Harb. Perspect. Biol. 10.1101/cshperspect.a038075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraietta, J.A., et al. 2018. Nat. Med. 10.1038/s41591-018-0010-1 [DOI] [Google Scholar]
- Gattinoni, L., et al. 2012. Nat. Rev. Cancer. 10.1038/nrc3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni, L., et al. 2011. Nat. Med. 10.1038/nm.2446 [DOI] [Google Scholar]
- Gattinoni, L., et al. 2009. Nat. Med. 10.1038/nm.1982 [DOI] [Google Scholar]
- Henning, A.N., et al. 2018. Nat. Rev. Immunol. 10.1038/nri.2017.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishton, R.J., et al. 2017. Cell Metab. 10.1016/j.cmet.2017.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna, S., et al. 2020. Science. 10.1126/science.abb9847 [DOI] [Google Scholar]
- Mai, D., et al. 2023. Proc. Natl. Acad. Sci. USA. 10.1073/pnas.2218632120 [DOI] [Google Scholar]
- Roychoudhuri, R., et al. 2016. Nat. Immunol. 10.1038/ni.3441 [DOI] [Google Scholar]
- Soerens, A.G., et al. 2023. Nature. 10.1038/s41586-022-05626-9 [DOI] [Google Scholar]
- Wang, L., et al. 2024. J. Exp. Med. 10.1084/jem.20232368 [DOI] [Google Scholar]
- Weigelin, B., and Friedl P.. 2022. Trends Cancer. 10.1016/j.trecan.2022.07.007 [DOI] [PubMed] [Google Scholar]
- Zheng, W., et al. 2021. Blood. 10.1182/blood.2020009309 [DOI] [Google Scholar]