Abstract
Introduction
We aim to review the outcomes of shock wave lithotripsy (SWL), ureteroscopy, and percutaneous nephrolithotripsy (PCNL) for renal and ureteral stones in spinal cord neuropathy patients (SNP).
Material and methods
A literature search was performed on 8th March 2023 using PubMed, EMBASE, and Google Scholar with no date limit. Preclinical/animal studies, reviews, letters to the editor, case reports, and meeting abstracts were excluded. Only English papers were accepted.
Results
Thirty-five articles were accepted. Five studies focused on SWL, 17 on PCNL, and 6 on ureteroscopy. The remaining articles employed more than one procedure. Stone composition has shifted from struvite to the more common calcium phosphate. SWL showed a very poor stone-free rate (SFR) likely due to challenges in patient positioning, stone visualization, localization, and inability to pass fragments spontaneously. Flexible ureteroscopy and PCNL were associated with a high incidence of infectious complications, long hospital stays, high blood transfusion rate, and intensive care admissions. There were also cases of death. Both procedures were challenging due to genitourinary reconstruction, scoliosis and kyphosis, rib-cage deformity, lower limb contractures, and severe comorbidity which also affected anesthesia. SFR was lower than in non-neurological patients.
Conclusions
SWL, ureterolithotripsy, and PCNL should be considered challenging procedures in SNP due to positioning issues, an increased risk of intra and peri-operative morbidity, and even mortality. Computed tomography should be recommended to assess residual fragments as it becomes imperative to minimize a re-intervention in SNP who should be preferably treated in referral centers.
Keywords: kidney calculi, ureteral calculi, ureterolithotripsy, percutaneous nephrolithotripsy, extracorporeal shock wave lithotripsy, spinal neuropathy
INTRODUCTION
The incidence of nephrolithiasis has increased worldwide in the last twenty years with a prevalence ranging from 1–5% in Asia, 7–13% in North America, and 5–9% in Europe [1]. Spinal cord neuropathy patients [SNP) have a greater risk of nephrolithiasis due to multiple factors that increase the likelihood of developing urinary stones such as recurrent urinary tract infections, chronic indwelling/intermittent bladder catheterization, immobility with subsequent bone resorption and hypercalciuria, lower levels of urinary citrate, urinary stasis, and vesicoureteral reflux [2]. Despite improvements in the management of neurogenic bladder, 7% of spinal cord injury patients develop their first kidney stone within 10 years after injury with a peak of incidence in the first 6 months after trauma [3]. Yet, the recurrence rate is also high in these patients with a reported rate of 34% within 5 years of the first stone episode [4].
Current indications for the management of kidney stones in SNP are the same as in non-neurological patients. Depending on stone burden and location, extracorporeal shockwave lithotripsy (SWL) or flexible ureteroscopy are indicated in kidney stones up to 2 cm, whereas percutaneous nephrolithotripsy (PCNL) is preferred for larger stones [5]. An important consideration in SNP is the need for general anesthesia as spinal anesthesia is difficult when associated with a spinal deformity or for the risk of inadvertently introducing spinal cord infection [5]. A mid-stream urine specimen should always be sent for culture and preoperative infections must be treated [5]. However, mid-stream urine culture is a poor predictor of postoperative sepsis, and a pelvic urine culture or, even better, a stone culture should be collected to predict the actual pathogen in case of postoperative sepsis [6] considering that SNP are at high risk of postoperative infections.
The present study aimed to perform a scoping review on the outcomes of SWL, ureteroscopy, and PCNL for ureteral and renal stones in patients with spinal neuropathy.
MATERIAL AND METHODS
Literature search
A literature search was performed on 8th March 2023 using PubMed, EMBASE, and Google Scholar with no date limit. The following term and Boolean operators were used: (kidney stones OR renal stones OR ureteral stones) AND (neurogenic bladder OR paraplegia OR spinal cord injury) AND (shock wave lithotripsy OR SWL OR retrograde intrarenal surgery OR RIRS OR ureteroscopy OR percutaneous nephrolithotripsy OR percutaneous nephrolithotomy OR PCNL)
Selection criteria
The PICOS (Patient, Intervention, Comparison, Outcome, Study type) model was used to frame and answer the clinical question: P: patients with spinal cord neuropathy and kidney/ureteral stones; I: SWL; ureterolithotripsy; PCNL. C: none; O: complications and stone-free rate (SFR); stone composition. S: retrospective, prospective, and randomized.
Study Screening and Selection
Studies were accepted based on PICOS eligibility criteria. Preclinical and animal studies were excluded. Reviews, letters to the editor, case reports, and meeting abstracts were also excluded. Only English-language articles were accepted. Retrospective studies, prospective studies and prospective randomized studies were accepted.
All retrieved studies were screened by two independent authors through Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia). Discrepancies were solved by a third author through discussion. The full text of the screened papers was selected if found pertinent to the aim of this review.
RESULTS
Literature screening
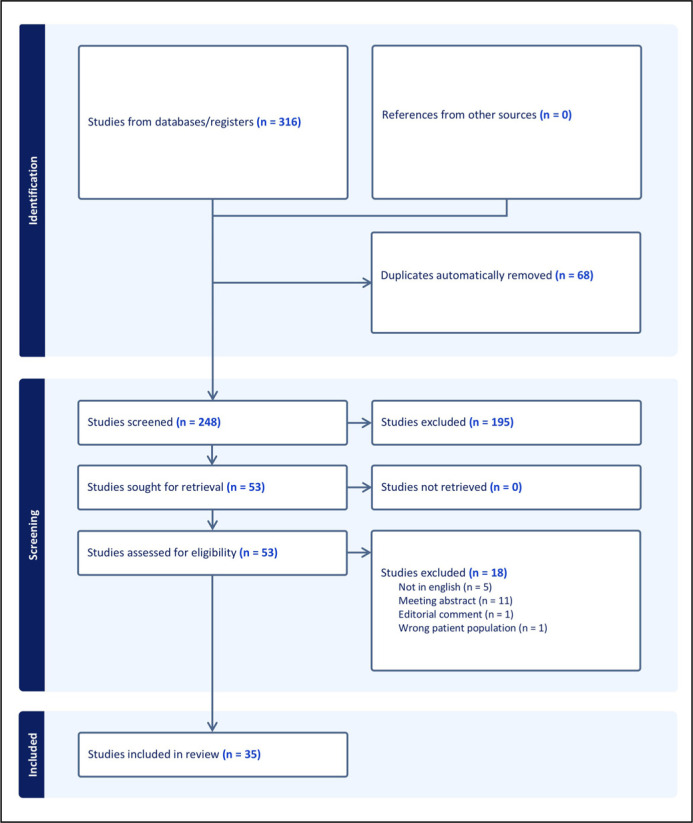
The literature search retrieved 316 papers. A total of 68 duplicates were automatically excluded. Then, 248 papers were screened against title and abstract and 195 papers were further excluded because they were irrelevant to the purpose of the present review. The remaining 53 full-text papers were screened for appropriateness and 18 papers were excluded. Finally, 35 papers were accepted and included [4, 7–40]. Figure 1 shows the flow diagram of the literature search.
Figure 1.
Flow diagram of the literature search.
Study characteristics
All studies were retrospective. There were 5 studies focusing on SWL alone [14, 15, 38–40], 17 on PCNL alone [10, 12, 13, 16, 19–22, 24, 25, 27–33] and 6 on ureteroscopy alone [8, 11, 17, 18, 26, 37]. The remaining studies concerned more than one procedure. Six studies compared SNP to non-neurological patients [23, 26, 27, 30–32]. There was one pediatric study [26]. Tables 1 and 2 show characteristics of the included studies.
Table 1.
Characteristics of included studies
| Authors | Stone size | Staghorn stones: Partial/Full | Definition of stone-free status | Ancillary procedures for residual fragments | Stone-free rate (%) | Recurrence rate (%) | Intraoperative complications N°, (%) | Mean/median length of stay in neurological patients, days | Mean/median length of stay in non-neurological patients, days | Factors affecting complications | Complications in neurological patients | Complications in non-neurological patients |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alsinnawiet et al., 2013 | Mean 940 mm3 (range 360–1,200) | Not reported | Degree of stone clearance was determined by X-ray | SWL or second-look PCNL | 60% | 80% | Bleeding and Hypotension 1, (25%) | Not reported | / | Not reported | Fever (100%) | / |
| Baldea et al., 2017 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | LOS, mean (SD) 14.2 days (22.1) | LOS, mean (SD) 9.6 days (12.5) | Spinal cord injury | Pneumonia 96 (5.1%) Sepsis 143 (7.6%) Mortality 80 (4.2%) Myocardial infarction 21 (1.1%) |
Pneumonia 50 (2.7%) Sepsis 61 (3.2%) Mortality 59 (3.1%) Myocardial infarction 22 (1.2%) |
| Beraud et al., 2022 | Not reported | Not reported | Not reported | Not reported | Not reported | Neurological group 11.6% (12 months) 15.3% (18 months) 18.3% (24 months) |
Not reported | SM (3.0 ±5.6 days) SD (4.3 ±6.7 days) P (4.4 ±11.2 days) T (3.8 ±9.1 days) Overall (3.4 ±7.1) |
1.2 (± 2.9) | Not reported | UTI (<10 days following procedures) SM (15,34%), Spinal dysraphism (23.98%), Paraplegia (19.56%) Tetraplegia (20.70%) Neurological (17.70%) |
UTI (<10 days following procedures) (3.14%) |
| Chaudhry et al., 2017 | PCNL 2.6 cm (IQR 1.5–2.9) CLL 2.8 cm (IQR 2.2–4.3) |
Not reported | Not reported | PCNL | PCNL 60% CLL 71% |
Not reported | PCNL group Bleeding 1, (4.3%) | PCNL LOS 4.4 days (IQR 3.5–4.6) CLL LOS 1,4 days (IQR 1.2–1.9) |
/ | Cobb-S angle >60 degrees | SIRS in 4 patients after PCNL | / |
| Chen et al., 2002 | Not reported | Not reported | Not reported | Not reported | 83% | 34% (5 y) | Not reported | Not reported | / | Not reported | Not reported | / |
| Christman et al., 2013 | Median NGB 6 mm (4–8) Control group 7 mm (5–10) |
Not reported | No stones on renal/ bladder TC or US or URS | / | 63% NGB 86.6% Control |
45% NGB 14.5% Control |
/ | Not reported | Not reported | / | Bacteriuria 67% Pain with stone episode 24% |
Bacteriuria 16.4% Pain with stone episode 84.7% |
| Clifton et al., 2014 | Not reported | Not reported | No evidence of residual or recurrent nephrolithiasis on CT, urogram, nephrostogram and US | 13, PCN 7, URS 4, SWL |
47.4% | 19.0% | Not reported | Not reported | / | Hypercalciuria secondary to immobilization, chronic infection and indwelling catheters | / | / |
| Culkin et al., 1986 | Not reported | 7 (2/5) | Renal tomograms followed by nephrostogram | PCNL | 90.4% | 65.2% | RA (3.6%) Hydrothorax (3.6%), Perirenal abscess (7.2%) |
Not reported | Not reported | Atelectasis, Infected decubiti | Bacteriuria 100%, Indwelling Foley catheters 60.9%, Fever 64.3%, Dislodged nephrostomy 21.4%, Anemia 27.8% |
/ |
| Culkin et al., 1990 | Not reported | SCI 13 (8/5) Control Group 6 (4 /2) |
Renal tomograms followed by nephrostogram | PCNL | 88.6% SCI 98.5% Control 94% Overall |
Not reported | Perirenal abscesses, RA, pneumonia, nephrocolonic fistula, hydrothorax. 1 mortality for CID |
Not reported | Not reported | Respiratory problems, complex stone disease | Perirenal abscess (8.8%) Aspiration pneumonia (2.8%) Hydrothorax (2.8%; Nephrocolonic fistula 2.8%; Respiratory arrest 2.8% Hemorrhage (48.6%); fever (74.2%) Dislodged nephrostomy tube (17.1%) Retained stones (11.4%) |
Nephrodudenal fistula (1.4%) Hemorrhage (20%); Fever (12.3%) Dislodged nephrostomy tube (4.6%) Retained stones (1.5%) Ureteral edema (6.1%) |
| Eswara et al., 2013 | Median Same day PCNL 15 (range 7–40 mm) Delayed PCNL 18 (range 5–36 mm) |
Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Same-day PCNL: LOS 4 days (range 2–50) Delayed PCNL: LOS 3 days (range 2–14) |
/ | Urinary stasis, Recurrent UTI, Indwelling catheters, RVU |
/ | / |
| Ganesan et al., 2017 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | / | Not reported | Not reported | / |
| Gnessin et al., 2011 | Not reported | Not reported | Not reported | PCNL second look | Not reported | Not reported | Not reported | / | / | Infected urine, Vesicoureteral reflux, catheterization, neurogenic bladder | / | / |
| Irwin et al., 1991 | Mean stone burden SB 3.4 cm SCI 1.9 cm |
8 | Not reported | SWL | OPEN 71% PCNL 70% |
OPEN 0% PCNL 20% |
Not reported | OPEN: Mean LOS 22 days (8–60) PCNL: Mean LOS 7 days (5–14) |
/ | Stone burden Skeletal deformities |
Fever 16 (100%) Hemorrhage 1 (6.2%) pain 4 (25%) Wound infection 2 (12.5%) UTI 3 (18.85%) Pressure sores 2 (12.5%) |
/ |
| Knox et al., 2012 | Mean 3.31 cm | Not reported | No evidence of stone on imaging (RX, TC IVU) after procedure | 7, PCNL 4, SWL 1, URS |
Initial stone-free rate 60.6% Final stone free rate 69.7% |
Not reported | Not reported | Mean LOS 5.3 days 9 patients needed ICU-care and mean LOS was 13.9 days |
/ | Multiple access and increasing stone | Fever 12 (25.5%) Sepsis 8 (17%) Pyelonephritis 1 (2.1%) Acute respiratory distress syndrome 2 (4.2%) Bleeding/Transfusion 4 ((,4%) Arteriovenous fistula 1 (2.1%) Amputation extremity 1 (2.1%) Pressure wound (1 2.1%) Death 1 (2.1%) Acute renal failure 2 (4.2%) Hemothorax 1 (2.1%) |
/ |
| Lawrentschuk et al., 2005 | Mean stone area 480 (70–3500) mm2 | 24 | Stone fragments >2 mm | 2 monitored 2 SWL 2 URS 1 pyelolithotomy |
87% | Not reported | Not reported | Not reported | / | Not reported | Fever 58% Blood transfusion 12% Calyceal perforation 8% Pneumothorax 8% Urosepsis 4% |
/ |
| Lazare et al., 1988 | Mean stone burden was 2.9 cm (range 0.2 to 8.0 cm.) | 7 (2/5) | Free of stones or without any radiographic evidence of calcification overlying the collecting system | 2 nephrostomy 1 PCNL 1 cystolitholopaxy |
73% | Not reported | Not reported | Not reported | / | No Double J or nephrostomy insert before SWL | Sepsis 1 (3%) | / |
| Matlaga et al., 2006 | Not reported | Not reported | No evidence of calculi by CT imaging in first postoperative day | Not reported | 30% | Not reported | Not reported | Mean LOS 3.3 days (range 2 to 10) | / | Not reported | Fever 6, (18.75%) Pressure sore 1 (3.1%) |
/ |
| Mitchell et al., 2018 | Not reported | 43 | No evidence of calculi in postoperative imaging. (unenhanced CT, US, and/or intravenous urogram) | Not reported | 46% SB 82% non-SB |
23% SB 0% non-SB |
SB 6, (23.5%) 2 anesthetic, 3 bleeding, 1 renal pelvic perforation; 2 difficult access non-SB 2 (4.9%) bleeding |
Median (IQR) hospital stay 7 days (6 to 8) | Median (IQR) hospital stay 4 days (3 to 5) | Mobilization was a problem for pressure sores; increased prevalence of matrix stone is a problem for sepsis; Risk of bleeding was much higher in SB for difficult percutaneous access. | Sepsis 38% Transfusion 11.8% | Sepsis 1.6%, Transfusion 1.6% |
| Morhardt et al., 2018 | Mean stone burden 15.7 mm ±11.2 | Not reported | CT, ultrasound, and plain radiography | Second and third look URS | 17% | Not reported | 0% | Mean LOS days ±SD (range) 3.1 ±1.9 (Range 1-8) | / | Primarily urinary tract infections | Primarily urinary tract infections 19 (15%), Sepsis requiring intensive care unit 2 (2%) |
/ |
| Nabbout et al., 2012 | Average 31.3 mm (exclude staghorn) |
8 (30.8%) | Complete absence of stones or presence of insignificant fragments (<2 mm) at CT scan one day after procedure | Second and third look PCNL | PCNL 53.8% 2nd PCNL 80.8% 3rd PCNL 88.5% |
Not reported | Bleeding 6 (28.6%) | Not reported | / | Limited pulmonary capacity and prolonged immobilization, making the anesthetic requirements more complicated | Urosepsis 3 (4.3%) Transfusion 6 (28.6%) Pneumothorax 1 (4.7%) Perforation 1 (4.7%) |
/ |
| Niedrach et al., 1991 | Average of the length of each stone 33.4 mm | 0 | No visible stone at plain-abdominal x-ray | Not reported | 44% | Not reported | Not reported | Average LOS 3.3 days (1–8) | / | Not reported | hypertension1, (9%) hypotension1 (9%) bradycardia 1 (9%) |
/ |
| Prattley et al., 2019 | Mean stone length, mm 26.7 ±14.5 [5–59] | 0 | Residual fragments were less than 2 mm at XRKUB, US or CTKUB | Second look URS | 47% | 42% | Not reported | Median LOS 1 days (range, 0–9) | / | Not reported | urinary infections 2 (10%) sepsis 3, (14%) lower respiratory tract infection 2, (10%) |
/ |
| Raj et al., 1999 | Mean aggregate stone diameter ±SD 22.3 mm ±18.0 (2–77) |
Not reported | Absence of stones on postoperative imaging (X-ray or CT) | Not reported | 55% | 73% | Not reported | Not reported | / | Not reported | Not reported | / |
| Robert et al., 1995 | Mean of maximum dimension 11 mm (range 5–35) | Not reported | Fragment <3 mm at plain abdomen radiograph | URS | 53% | 14% | Not reported | Not reported | / | Not reported | Few cases of gross hematuria which spontaneously regressed | / |
| Rubenstein et al., 2004 | Not reported | Not reported | Removal of the entire stone burden to visual completion with no fragments seen on follow-up imaging | Second PCNL | 96% | Not reported | Hydrothorax 1, (4.3%); Respiratory difficulty 1, (4.3%); Collecting system perforation 1, (4.3%) |
Average LOS 7.04 days (range 1 to 22) | / | Atelectasis poor thermoregulatory mechanisms, pyelolymphatic backflow of bacteria |
Fever 11, (47.8%) Hydrothorax 1, (4.3%) Hydrothorax 1, (4.3%) Retroperitoneal abscess 1, (4.3%) Ileus 1, (4.3%) |
/ |
| Sofimajidpour et al., 2016 | Mean of kidney stone size 35.7 ±6.1 mm (25 to 45 mm) | 8 0/8 |
KUB and kidney US first day after surgery, Non-contrast CT 6 months after surgery | Second PCNL | First PCNL, 53.1 % Second PCNL 78.1% |
Not reported | Not reported | Mean LOS 8.3 ±3.1 days | / | Not reported | Blood transfusion, 7 (24.1%) Visceral injury, 2 (6.8%) ICU stay, 7 (24.1%) Urosepsis, 6 (20.6%) Fever 11 (38%) |
/ |
| Spirnak et al., 1988 | Not reported | 1 0/1 |
Not reported | Not reported | 60% | Not reported | Hypertension | LOS ranged from 5 to 43 days (mean 17 days) | / | Not reported | Not reported | / |
| Stauffer et al., 2017 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Febrile UTI 9% | Febrile UTI 1.4% |
| Symons et al., 2006 | Stone size ranged from 10 to 60 mm; without staghorn the mean stone size was et al, 26 mm |
13 0/3 |
Not reported | PCNL SWL URS |
48.7% | Not reported | Not reported | Not reported | / | Difficult airways, abnormal anatomy increasing the risk of pneumothorax and a reduction in respiratory capacity | Death 2 (6.9%) major complications 5, (17.2%) minor complications 8 (27.5%) |
/ |
| Tepeler et al., 2015 | Mean stone size was 15.9 ±8.6 (6–40) mm | Not reported | Residual stone fragments <3 | 1 | 66.6 % | 5 | None | Mean LOS 2.0 ±2.4 (0–10) days | / | Not reported | Fever 6 (22.2 %) Hypotension 1 (3.7%) UTI 1 (3.7%) Urosepsis 1 (3.7%) Respiratory failure 2 (7.4%) |
/ |
| Torricelli et al., 2021 | Not reported | Not reported | Absence of any fragments on postoperative day 1 at CT scan. | Second look PCNL, FURS or SWL | 48.70% | Not reported | None | Mean LOS 5.8 ±4.7 days | Mean LOS 3.1 ±1.7 days | Infection, Hypotension Bleeding |
Clavien 1 = 1 (2.6%) Clavien 2 = 8 (20.5%) Clavien 3 = 0 (0%) Blood transfusion: 5.1% |
Clavien 1 = 0 (0%) Clavien 2 = 3 (3.9%) Clavien 3 = 3 (3.9%) Blood transfusion: 1.3% |
| Wahle et al., 1988 | Not reported | 22 | Not reported | 5 SWL 4 nephrostomy tube placement (1 steinstrasse requiring URS) |
25% 42% (had between 90 and 99% reduction in stone surface area). |
Not reported | None | Average of 4.2 days, (IQR 2–14) | / | Not reported | 1 nephrectomy five months after SWL. 1 patient with a proximal right ureteral stone underwent ureterolithotomy |
/ |
| Welk et al., 2013 | Not reported | Not reported | Not reported | Multiple PCNL | Not reported | Not reported | None | Median of LOS 5 days (IQR 3–8). | / | Not reported | The 30-day mortality rates after any stone-related procedure was low. Subsequent ICU admission was required in 12% of cases; none occurred after SWL. ACUTE KIDNEY INJURY with the need for dialysis was rare |
/ |
| Wolfe et al., 2013 | Stone size <1 cm: 32.8%; 1.0–2.0 cm: 35.8%; >2 cm: 31.3% |
Not reported | Removal of any stone larger than 4 mm | URS 31% SWL 16% PCNL 15% |
34.40% | 71.60% | Ureteral perforation (1.5%) | Not reported | / | Chronic obstructed pulmonary disease | Urosepsis (17.9% Respiratory failure (4.5%) Acute outlet obstruction (4.5%) |
/ |
| Wong et al., 2023 | Not reported | Not reported | Not reported | URS; SWL; PCNL | Not reported | Not reported | Not reported | Not reported | / | Not reported | Not reported | / |
CT – computed tomography; URS – ureteroscopy; SWL – extracorporeal shock wave lithotripsy; PCNL – percutaneous nephrolithotomy; LOS – length of stay; CLL – cystolitholapaxy; SIRS – systemic inflammatory response syndrome; MS – Multiple sclerosis; UTI – urinary tract infection; IQR – interquartile range; NGB – neurogenic bladder; ICU – intensive care unit; SD – standard deviation; FURS – ; KUB – kidney ureter bladder; US – ultrasound; IQR – interquartile range
Table 2.
Type of patients and procedures, aim and outcomes of included studies
| Author | Number of patients | Type of spinal neuropathy and number of patients | Comparison Group | Procedure | Pre-stented patients or with a nephrostomy tube | Antibiotics prophylaxis | Aim of the study | Conclusion |
| Alsinnawiet et al., 2013 | 5 | SB, 5 | / | PCNLs | 20% (stent) | Broad-spectrum antibiotics | To present our experience in patients with SB and severe spinal abnormality undergoing PCNL for large stone burden. | PCNL in patients with SB is challenging but safe. Second-look PCNL and additional SWL/URS treatment may be required to completely clear stones. |
| Baldea et al., 2017 | 39868 | SCI, 1,918 | 37950 | PCNL | Not reported | Not reported | To evaluate outcomes of SCI patients undergoing PCNL in a large patient population and compared outcomes. | PCNL in SCI patients is associated with an increased complication rate and longer hospital stay. |
| Beraud et al., 2022 | 45,745,055 | MS, 116,730 SD 5,503 P 19,181 T 10,436 |
45,593,205 | SWL, rURS, fURS, PCNL, Open or Laparoscopic surgery | Not reported | Not reported | To compare the incidence and the safety outcomes associated with ASRP (active stone removal procedure) between neurological and non-neurological patients. | The results confirm and clarify the incidence and the safety outcomes associated with ASRP within the neurological population. |
| Chaudhry et al., 2017 | 23 | SB, 23 | / | 15, PCNL 17, CLL |
Not reported | Treatment based on pre-operative urine cultures | To determine whether stone-free rates after PCNL or CLL were associated with anatomical factors. Secondary outcomes included assessing whether SFR were associated with operative time, estimated blood loss and perioperative complications following PCNL and PC. | The SFR after a single procedure is modest. The severity of scoliosis and kyphosis may be predictive of a more challenging stone surgery. |
| Chen et al., 2002 | 77 | SCI, 77 | / | 60, Conservative 7, SWL 14, PCNa 4, SWL and PCN 1, Litholapaxy |
Not reported | Not reported | To document the recurrence rate of kidney stones in patients with SCI and to assess the potential contributing factors and long-term renal function outcome. | Despite advances in urologic treatment of persons with SCI, the recurrence rate of kidney stones is still substantial. The causality of stone formation is multifactorial. |
| Christman et al., 2013 | 147 | PNB, 20 | 127 | 147, URS | Not reported | Perioperative antibiotics against surgical related infection | To evaluate if URS in patients with neurogenic bladder would be associated with an increased risk of complications and a lower stone clearance rate than in patients without neurological impairment. | Patients with NGB have significantly increased morbidity related to URS with a predominance of infection related complications. |
| Clifton et al., 2014 | 95 | 13, CP 11, MS 15, SB 56, SCI |
/ | 40, PCNL 28, URS 26, SWL 1, Nephrectomy |
Not reported | Not reported | Identify changes in stone composition and surgical outcomes in patients with para and quadriplegia. | Para and quadriplegic patients with urolithiasis can be difficult to treat surgically with prolonged hospitalizations, low stone-free status, and often require additional procedures. |
| Culkin et al., 1986 | 23 | 18 quadriplegics 5 paraplegics |
/ | PCNL | Not reported | Perioperative antibiotic determined form urine culture. | To evaluate the success rate of stone removal and the incidence of operative complication. | PCNL is more effective and results in less morbidity than open lithotomy A repeat PCNL is safer and more effective in total stone excision than open lithotomy. |
| Culkin et al., 1990 | 100 | 27 quadriplegics 8 paraplegics |
65 | PCNL | Not reported | Systemic antibiotics by preoperative urine culture for 48 hours preoperatively and continued post-operatively until the patient was afebrile for 48 hours. | To evaluate the success of complete stone excision, operative morbidity and mortality were compared in two patient population: SCI patients and ambulatory patients. | SCI patients are at higher risk than the non-SCI patients for significant complications with PNL but still have a high overall success rate. |
| Eswara et al., 2013 | 246 | 16 MS 10 SB 4 quadriplegia 3 Paraplegia 2 CP |
/ | PCNL | 14% (stent) | Patients with positive urine cultures were treated with targeted antibiotics for 4–7 days prior to the surgery. The others received oral antibiotics for 4–7 days based on the most recent urine culture sensibility. In PCNL: intravenous levofloxacin or gentamicin at the time of percutaneous access |
To determine whether a delayed PCNL reduces the rate of bacteremia/ sepsis in patients with neuromuscular disorders. | Delayed PCNL results in lower rates of bacteremia and/or sepsis in patients with neuromuscular disorders. |
| Ganesan et al., 2017 | 587 | MS | / | PCNL, SWL, URS | Not reported | Not reported | To compare stone composition in patients with multiple sclerosis (MS) against patients without MS. | Patients with MS have a high incidence of calcium phosphate stones and struvite stones. |
| Gnessin et al., 2011 | 367 | 11 SCI 7 MMC 2 MS 2 CP 1 DM |
334 | PCNL | Not reported | One week of tailored antibiotic therapy before undergoing PCNL for at least 1 week before surgery. | To assess the composition of renal calculi and metabolic characteristics in a cohort of patients with MS who underwent PCNL. | Although patients with MS anomalies are traditionally thought to harbor infection-related calculi, most have calculi of metabolic etiology. |
| Irwin et al., 1991 | 16 | 11, SB 5, SCI |
/ | Open, PNL, SWL | Not reported | Parenteral antibiotics pre-operatively and after surgery. | To evaluate the management of renal stones in the spinal patient. | PCNL is the preferred initial treatment of renal stones in spinal patients leaving SWL to treat residual fragments. Open surgery will be reserved for patients in whom PCNL fails. |
| Knox et al., 2012 | 47 | 16, SB 26, SCI 2, MS 1, SLA 1, sacral agenesia 1, Triad syndrome |
/ | PCNL 30 Fr sheath 26 Fr rigid nephroscope |
Not reported | Antibiotics were instituted 1 week before procedure based on urine culture. | To evaluate predictors for increased length of stay, intensive care unit stay, stone-free rate and number of procedure between SCI and SB patients undergoing PCNL. | Increasing stone size and multiple access were predictors of adverse outcome. Location of access affected stone free status. No differences in outcome between SB and SCI patients. |
| Lawrentschuk et al., 2005 | 26 | 26 SCI | / | PCNL 28 Fr sheath | Not reported | Antibiotics 24 h before surgery until 48 h after procedure | To present our experience of PCNL for treating urolithiasis in patients with SCI using a single-stage dilatator for percutaneous access. | PCNL has high success rate and acceptable complication rate compared to SWL and remains a valid first line treatment opinion for kidney stone in patients with SCI. |
| Lazare et al., 1988 | 32 | 18, cervical SCI 9, thoracic, SCI 5, lumbar SCI |
/ | SWL | 66% (both stent and nephrostomy) | Antibiotics according to urine culture, discontinued 1 week after SWL | To evaluate the efficacy of SWL in the treatment of SCI patients with large stone burdens. | SWL is effective for the treatment of unbranched and partial staghorn calculi in the spinal cord injury. SWL alone is less effective for the treatment of full staghorn calculi. |
| Matlaga et al., 2006 | 32 | 14, SCI 18, MMC |
/ | PNL 30 Fr sheath Rigid and flex nephroscope |
Not reported | 2 weeks of tailored antibiotic therapy before undergoing PNL. | To defined the composition of renal calculi in a contemporary cohort of patients with neurogenic bladder who underwent PNL. | Many patients with NB due to SCI or MMC undergoing PNL will be found to have calculi that are metabolically derived rather than calculi secondary to chronic infection with urea-splitting organisms. |
| Mitchell et al., 2018 | 53 | 13, SB | 50 | PCNL | Not reported | Not reported | To evaluate peri- and postoperative outcome of PCNL in patients with SB undergoing PCNL with historically matched controls. | PCNL in patient with SB is associated with multiple parameters of poor outcome and should be counseled about increased perioperative risk and likelihood of recurrence |
| Morhardt et al., 2018 | 46 | 46, SCI (due to traumatic, vascular, or malignant mechanisms) |
/ | Ureteroscopy flexible and rigid | 16% (stent) | Positive urine cultures: 1 week preoperatively tailored therapy continued through surgery. Negative urine culture: standard perioperative prophylactic antibiotics. |
To evaluate the association of clinical factors on outcomes in patients with SCI undergoing ureteroscopy. | Ureteroscopy is a safe and effective method for treating kidney stones in patients with SCI. Patients with higher levels of SCI had lower SFRs and may warrant special consideration to limit complications. |
| Nabbout et al., 2012 | 21 | 14 SCI 7 SB |
/ | PCNL | Not reported | Treated with a culture specific oral antibiotic for at least 1 week before the procedure. Broad spectrum antibiotics were administered intravenously at admission | To evaluate the managing stones with PCNL in patients with spinal neuropathy. | PCNL in patients with spinal neuropathy had a stone clearance rate comparable with that of the general population. These patients, however, needed multiple PCNLs to be stone-free and had a higher incidence of complications (especially infectious). |
| Niedrach et al., 1991 | 11 | 5, congenital abnormalities 4, Trauma 2, MS |
/ | SWL | 27.3% (stent) 54.5% (nephrostomy tube) |
Treatment based on pre-operative urine cultures. Antibiotics including aminoglycosides, third-generations cephalosporins, for yeast infection amphotericin B. If patient remained afebrile for first postoperative night, the antibiotics were switched to oral dosing | To determine the effectiveness of SWL in patients with spinal cord impairment. | Fragmentation rate in patients with spinal cord dysfunction was excellent, the clearance of stones was poor and delayed. |
| Prattley et al., 2019 | 21 | 15, cervical SCI 6, thoracic SCI |
/ | URS (flexible and rigid) |
27% (stent) | Single dose gentamicin (3 mg/kg) at induction unless pre-operative urine cultures dictated otherwise. | To evaluate the experience at a regional SCI unit for ureteroscopy in upper tract stone disease in patients with SCI. | Ureteroscopy provides a useful treatment option for patients with SCI, but SFR are inferior to those in the general population. |
| Raj et al, 1999 | 20 | 20, Neural tube defects, (tumor, trauma and infarcts were excluded) |
/ | SWL; PCNL; URS; open | Not reported | Not reported | To identify the incidence of nephrolithiasis documented by radiography and elucidate risk factors that may have contributed to stone formation. | Risk factors for stone formation were analyzed and bacteriuria was invariably present. Vesicoureteral reflow, pelvicalicectasis, renal scarring and a thoracic level spinal defect were also associated with an increased risk of stone formation. |
| Robert et al., 1995 | 15 | 12 secondary to closed trauma 2 secondary to a bullet injury 1 iatrogenic |
/ | SWL | Not reported | Parenteral antibiotics immediately before SWL and for 1 week when preoperative urine cultures were positive. | To evaluate the efficacy of SWL in paraplegic and tetraplegic patients. | SWL is effective in spinal cord injury patients, but its particular usefulness and limitations in this patient population need to be kept in mind. Once fragmentation of the calculi is achieved, elimination from the urinary tract remains a problem. |
| Rubenstein et al, 2004 | 23 | 23, SB, SCI, exstrophy/epispadias, neonatal meningitis, stroke, and spine chondrosarcoma | / | PCNL | 100% (nephrostomy 24 hour prior to PCNL as routine care) | Culture-specific oral antimicrobial agents 48 hours before admission if they had a history of urinary tract infection or colonization and were admitted the night before the access procedure for administration of broad- spectrum intravenous antimicrobial agents | To reviewed our experience performing percutaneous nephrolithotomy (PNL) on patients with neurogenic bladder dysfunction with special attention paid to the risks of surgical complications and stone recurrence. | PNL in patients with neurogenic voiding dysfunction is safe and effective, with outcomes comparable to that of patients without such lesions. |
| Sofimajidpour et al, 2016 | 29 | 24, SCI 5, SB |
/ | PCNL | Not reported | Not reported | To investigate technical problems, complications and stone clearance rate in patients with spinal neuropathy undergoing PCNL. | Although patients with spinal cord injury have problems in terms of surgery and complications, percutaneous nephrolithotomy is an appropriate and safe treatment method for kidney stones. |
| Spirnak et al., 1988 | 5 | 5, Cervical SCI | / | SWL | Not reported | Intravenous antibiotics 48 h before the procedure based on culture and sensitivity results | To report results of SWL kidney stone treatment in traumatic quadriplegic patients. | SWL may be performed safely in quadriplegic patients without the added morbidity of a general or spinal anesthetic. |
| Stauffer et al., 2017 | 402 | 34, SCI | 368 | URS | 13.6% (nephrostomy tube) 40.1% (stent) |
In patients with prior infections treated with ureteral stenting or nephrostomy tube placement, a full 2-week course of antibiotic therapy was administered before ureteroscopy. In cases of a positive preoperative culture, targeted antibiotic treatment was initiated within a minimum of 3 days before the procedure. |
To characterize the rate of febrile UTI after ureteroscopy in patients with neurogenic bladder compared with those with physiologically normal bladders. | Although infectious complications in the neurogenic population are likely multifactorial, the reliance on catheterization and thus colonization appears to be a significant factor and extends to non-neurogenic patients. Bacterial colonization may be the significant underlying risk factor for febrile UTI after ureteroscopy. |
| Symons et al., 2006 | 29 | 9, SCI 10, SB 10, other causes |
/ | PCNL, SWL | 27.6% (nephrostomy tube) 17.2% (stent) |
Urine cultures were obtained in all patients preoperatively and appropriate antibiotic prophylaxis was used prior to the procedure. All patients received antibiotics postoperatively for 48 h | To assess the technical difficulties, associated complications and stone clearance rates in patients with spinal neuropathy undergoing percutaneous nephrolithotomy. | Technical difficulties and potential complications should be considered carefully before undertaking percutaneous nephrolithotomy in these patients. |
| Tepeler et al., 2015 | 19 | 6, quadriplegics 13, paraplegics |
/ | URS, RIRS | Not reported | Not reported | To evaluate the outcomes of fURS in patients with SCI. | fURS is an effective procedure for UTU stone in patients with spinal cord injury. |
| Torricelli et al., 2021 | 117 | 39, SCI | / | PCNL | Not reported | Patients with positive urine culture received antibiotic therapy for 1 week before surgery. Those with negative urine culture received third generation cephalosporin starting 24 h before surgery |
To assess the complication and SFR of PCNL in patients with SCI and to evaluate whether this population should be assigned a GSS of 4. | Patients with SCI should not be automatically assigned GSS 4. SFR is related to stone burden in these patients, but they have a higher complication rate and a longer hospital stay than non-neurological patients. |
| Wahle et al., 1988 | 31 | 8, quadriplegic 23, paraplegic |
/ | SWL | 12.1% (nephrostomy tube) | Not reported | To assess the efficacy and safety of SWL as a treatment modality for renal stones in paraplegic and quadriplegic patients. | In paralyzed patients, SWL plays an important role in the treatment of stones less than 3 cm. |
| Welk et al., 2013 | 5,121 | 66 | / | URS; ureteral stent/ percutaneous nephrostomy; SWL, PCNL |
Not reported | Not reported | To describe the incidence, management and outcomes of surgically treated kidney stones after SCI and to evaluate the impact of a past history of kidney stones on the occurrence of kidney stones. | During intermediate follow-up after SCI, surgically treated upper tract kidney stones occur in 1.3% of patients. URS is the most common treatment. A history of surgically managed kidney stones before SCI portends a higher risk of stones after SCI. |
| Wolfe et al., 2013 | 67 | SCI, 29 | / | URS | Not reported | Broad spectrum antibiotics for 48h post operatively | To review the outcomes and safety of URS for the treatment of urolithiasis in the SCI population. | URS in the SCI population is an effective treatment for ureteral or renal stones but may be associated with greater risks and reduced efficacy. |
| Wong et al., 2023 | 189 .739 | / | SWL, URS | Not reported | Not reported | To determine risk factors and time course for repeat procedures after URS or SWL procedure using a large employer-based claims database. | Patients with paralysis and neurogenic bladder had a significantly higher risk of repeat stone procedure. SWL was associated with higher risk of repeat procedure than URS. |
ABL – acute blood loss; AD – autonomic dysreflexia; ASRP – active stone removal procedure; CLL – cystolitholapaxy; CP – cerebral palsy; CT – computed tomography; fURS – flexible ureteroscopy; GA – general anesthesia; LOS – length of stay; MD – muscular dystrophy; MMC – myelomeningocele; MS – multiple sclerosis; NGB – neurogenic bladder; P – paraplegia; PCNL – percutaneous nephrolithotomy; PNB – pediatric neurogenic bladder; rURS – rigid ureteroscopy; SB – spina bifida; SCI – spinal cord injury; SD – spinal dysraphism; SFR – stone-free rates; SIRS – systemic inflammatory syndrome; SWL – extracorporeal shock wave lithotripsy; T – tetraplegia; UTI – urinary tract infections
DISCUSSION
Stone composition
In the past, most kidney stones in SNP were infection-related, namely struvite stones, with urea-splitting organisms such as Proteus mirabilis, Klebsiella species, and Pseudomonas aeruginosa as the main pathogens [2]. More recently, stone composition has shifted from struvite to the more common calcium phosphate as recent literature demonstrated. Clifton et al. [21] and Ganesan et al. [34] found calcium phosphate stones in 82.5% of paraplegic and quadriplegic patients and 42% of patients with multiple sclerosis, a rate much higher than in control groups (15%). This change may be attributed to the improvement of bladder management techniques, such as urological rehabilitation and the use of intermittent catheterization instead of chronic indwelling catheters which lead to a high percentage of infection stones [31]. Ileal-conduit diversion and intermittent catheterization are associated with less bacteriuria, especially from urea-splitting pathogens [36]. Even though struvite stones are still found in a great proportion of SNP, the most prominent crystal identified in this population is calcium phosphate. This can be explained by the increased level of serum calcium and phosphorus in the first several months following an injury during the immobilization period by the mechanism of resorptive bone disease which increase the risk of developing osteoporosis and a low bone mass [41]. The same mechanism contributes to increasing urinary pH (between 5.6 and 7) which is a well-known factor of active stone formation [31]. Compared with matched controls, SNP are less likely to have calcium oxalate monohydrate and dihydrate, calcium carbonate, uric acid, or cystine stones [21, 34].
Outcomes of shock wave lithotripsy
SWL was for many years the cornerstone treatment of kidney stones up to 2 cm in the largest diameter. Though remaining an option in current guidelines, the use of SWL has decreased both in the general population [42] and SNP as demonstrated by the presence of only 5 papers focusing on SWL, all dating back to the 80s and 90s. Wahle et al. treated 31 paraplegic and quadriplegic patients with a total of 54 treatments performed on 42 kidneys, with an average of 2,193 shocks per session [40]. Almost half of the patients required more than one session: 8 patients needed two treatments, 3 patients required three treatments and 3 more patients had four treatments. Postoperative fever >38.5°C occurred in 22% of the 54 sessions. Three months after SWL, SFR was 25.8% but 79% of the stones were reduced by more than 70%.
Lazare et al. performed SWL in 41 renal units in 32 spinal cord injury male patients with a mean stone burden of 2.9 cm [15]. The authors found a good SFR of 78% after a single session but ancillary procedures, including insertion of nephrostomy tubes or double-J ureteral stents, were required before SWL in 66% of cases. Yet, they pointed out that partial staghorn stones required a staged fashion treatment (i.e. 3-4 sessions) with 2,400 shock waves per renal unit per session. Therefore, the authors argued that SWL was effective for the treatment of unbranched and partial staghorn stones only.
In another small series, Niedrach et al. performed SWL in 11 SNP with a total of 19 treatments in 13 renal units [14]. The average number of shock waves per renal unit was 2,350 with a mean power setting of 20 Kv. The main difficulty found by the authors was the shadow of gas and stool from the bowel which made it challenging to target the stone. This was demonstrated by the fact that 3 months after treatment no patient was stone-free and ancillary procedures post-lithotripsy were required in 10 renal units. Complications were mild and there were no symptoms of autonomic dysreflexia episodes.
Robert et al. performed 63 SWL sessions on 23 kidney/proximal ureteral stones in 15 spinal cord injury patients [39]. They demonstrated that SWL was safe with no episode of autonomic dysreflexia and a few cases of gross hematuria occurred which however ceased spontaneously. Auxiliary procedures were performed in two cases to remove ureteral stone fragments with a Dormia basket. SFR was 53%. Conversely, Spirnak et al. had 2 cases of significant intraoperative hypertension in traumatic quadriplegic patients with no anesthesia, despite no case of complete clinical syndrome of autonomic dysreflexia occurred [38]. Therefore, it is recommended that such patients should be carefully monitored throughout the treatment.
In summary, we found that SWL in this subset population has a very poor outcome likely due to challenges in patient positioning, stone visualization, localization, inability to pass fragments spontaneously, and need for anesthesia to prevent neuropathic events.
Outcome of ureteroscopy
Semirigid and flexible ureteroscopy for ureteral and kidney stones have shown a good safety profile and clearance rate in the general population. Ureteroscopy may be associated with more complications in SNP who are at higher risk of postoperative infectious complications. This was confirmed by Stauffer et al. who demonstrated that febrile urinary tract infections following ureteroscopy were significantly higher in patients with neurogenic bladder compared with control patients (9% vs 1.4%, p = 0.01) with higher rates in those dependent on bladder catheterization (12.5% vs 1.4%, p = 0.003) [18].
Christman et al. compared ureteroscopy outcomes for upper urinary tract stones of 20 pediatric patients with a neurogenic bladder with 127 controls [26]. The neurological group had 22 stone episodes, requiring a total of 45 ureteroscopy procedures, while the control group had 138 stones episodes with a total of 173 procedures required. Interestingly, non-neurogenic patients had a significantly higher percentage of pain associated with the stone episode (84.7%) than bladder-neurogenic children (24%). Conversely, the latter presented with a greater percentage of associated bacteriuria compared with controls (67% vs 16.4%, respectively). Surgical time was significantly longer in neurogenic patients. Similarly, complications were more common in neurological patients (25% vs 16.6%) than in controls, including one death. Specifically, infectious complications were again more frequent in the neurological population (23% vs 5.8%) with a lower SFR (63% vs 83.6%).
Ureteroscopy in SNP can be challenging not only for infectious complications but also for anatomical variation such as lower limb contractures, access via Mitrofanoff or suprapubic tracts, and increased comorbidity [37]. Prattley et al. performed flexible ureteroscopy for ureteral and kidney stones in 21 spinal cord injury patients for a total of 41 procedures [37]. A ureteral access sheath was used in 63% of cases. Seven patients required a repeat ureteroscopy as a multistage approach due to the level of stones. Postoperative outcomes were satisfactory with a median postoperative stay of one day (range, 0–9 days). Complications were acceptable with 3 cases of sepsis, 2 cases of lower respiratory tract infections, and one case of autonomic dysreflexia. Despite the use of baskets for active fragments removal, SFR was low at 47%. At a median follow-up of 46 months, stone recurrence occurred in 42% of patients. Ensuring a complete stone clearance, i.e. zero fragments, is essential in patients with a high level of spinal neuropathy since immobility makes the passage of residual stone fragments less likely. This partly explains the higher risk of stone recurrence in such patients.
Tepeler et al. had similar results in a series of 19 patients with upper ureteral and kidney stones [17]. There were 3 major complications (1 sepsis and 2 respiratory failure) that required admission to the intensive care unit. Single-stage SFR was 57.1% and after additional ureteroscopy sessions, 66.6% of the 21 renal units were finally stone-free.
The challenge of ureteroscopy in this population was also confirmed by Wolfe et al. in a cohort of 29 male patients who required an average of 2.3 ipsilateral ureteroscopies because stone clearance of any stone >4 mm after the first procedure was only 34.3% [8]. Interestingly, 45.5% of patients with residual fragments were secondary to technical or procedural limitations due to failure to identify or insert the ureteroscope through the ureteral orifice (45%) and inability to successfully access all of the stones (40%).
Some factors have been identified to be associated with worse SFR. Morhardt et al. showed that patients with no preservation of sensory or motor function in the sacral segments S4-S5 [OR 0.16, 95% CI 0.03–0.82) and an average of 2.2 procedures per patient were associated with lower odds of stone-free status (OR 0.83, 95% CI 0.03–0.32) [11].
Our review cautions urologists who perform flexible ureteroscopy in these patients that apart from infectious complications, they must be adequately prepared for genitourinary anatomical challenges, often superimposed with lower limb contractures, modifying access approaches through reconstructed lower tracts and, at times, even supported by percutaneous approach. All of the aforementioned factors increase the level of challenge and morbidity of a minimally invasive approach.
Outcomes of percutaneous nephrolithotomy
PCNL has become widely accepted as the preferred approach for managing large kidney stones, even in patients with anatomical anomalies of the kidney, severe obesity, spinal diseases, and prior renal surgery. However, most SNP present with immobilization, altered body habitus, severe scoliosis and kyphosis, rib-cage deformity, and often with reconstructed urinary tracts already from childhood. All the aforementioned factors could play a relevant role in performing PCNL. Prone PCNL could cause restrictive lung disease and ventilation difficulties and this problem is at least partly overcome by supine PCNL which should be preferred in such a patient [43]. Cautions must be paid in scoliotic patients who should be placed in an appropriate atraumatic position. Indeed, the short distance between the rib cage and the iliac crest, the curvature of the spine, the pelvic tilt, and the lower limbs contracture may all make positioning and access rather challenging due to a small window for percutaneous access in the prone position [19, 44]. An interesting study by Chaudhry et al. demonstrated the association between increasing anatomic complexity and lower SFR in patients with scoliosis and kyphosis [24]. Anatomic complexity was assessed using the Cobb angle which is the measurement between two lines drawn perpendicular to the superior and inferior vertebral endplates of the curved spine segment. The authors found that patients with worsening scoliosis (median Cobb angle of 43°) had a lower SFR compared with those having a lower median Cobb angle (24°, p = 0.058) [24]. Alsinnawi et al. [20] suggested paying particular attention to patient positioning in case of spina bifida to maintain a safe anesthetic, safeguard pressure points and maximize percutaneous access. The authors suggested using malleable supports, in particular below the curved part of the spine, and adhesive strapping to maintain position and facilitate exposure of percutaneous access. Despite these measures, they found that the range of movement of the nephroscope was inadequate, in particular, in accessing upper calyces [20]. The challenge of PCNL in SNP is additionally demonstrated by the low rate of single-stage stone-free status as demonstrated by several studies. Nabbout et al. performed PCNL in 26 renal units in 21 patients and found that SFR was low at only 53.8% after the first PCNL [25]. This result was in line with studies by Sofimajidpour et al. [12], Symons et al. [34], and Mitchell et al. [19] whereby the SFR of a single-stage procedure was 53.1%, 62%, and 50%, respectively. These studies found that multiple procedures were required to achieve complete stone-free status in several patients and this was mainly related to the presence of complete staghorn stones in up to 30% of their series.
In a series of 23 patients, Rubenstein et al. found that 6 patients required three or more procedures to clear their staghorn stones [10], highlighting that such patients should be counseled about the necessity of more than one PCNL session to achieve satisfactory stone clearance. This reveals that SNP commonly present with a significant stone burden which makes PCNL in this population more challenging. Also it is important to note that in most cases surgery can last more than two hours [12]. Therefore, urologists should be ready to face these situations with a variety of instruments and lithotripsy devices. The abnormal visceral anatomy that may result from skeletal deformities may be responsible not only for failure to access the kidney in PCNL but also in visceral injury and both such problems have been only partly attenuated by supine PCNL. Indeed, visceral injury [12, 32] pneumothorax, hemothorax, and hydrothorax [10, 16, 25, 28, 29, 32] are well-documented complications not only in historical series.
Compared to non-neurological patients, SNP demonstrated significantly longer hospital stays, infectious complications, and blood transfusion rates. Baldea et al. performed a one-to-one matching based on age, race, gender, presence of major comorbidities, and preoperative urinary infections, and compared 1885 spinal cord injury patients with the same number of non-neurological patients [27]. The former had a significantly longer length of stay (mean 14.2 ±22.1 vs. 9.6 ±12.5 days, p <0.001). The authors also found that spinal cord injury status independently increases patient’s adjusted odds of both minor and major complications and mortality.
In another comparative study, Torricelli et al. demonstrated that surgical time was significantly longer in spinal cord injury patients (119.41 ±45.58 minutes) compared to controls (141.00 ±45.23, p = 0.018) [30]. Again, the former had a significantly longer postoperative stay (mean 5.8 ±4.7 vs. 3.1 ±1.7 days, p = 0.002). In a historical series back in the 1990s, Culkin et al. compared 35 spinal cord injury patients with 65 ambulatory patients; blood transfusion rate was 48.6% in the former as opposed to 20% in the latter [32]. Despite improved instruments and surgical techniques, more recent series still found a higher rate of transfusion in SNP [19, 30].
Undoubtedly, infections are among the most serious complication following PCNL in SNP. Pneumonia has been reported in many series with a rate ranging from 3% [21, 29, 32, 33] to 5.1% [27]. Spine deformity and spinal neuropathy have also a detrimental effect on lung function due to impairment of respiratory muscles, ineffective cough reduced vital capacity, and reduction in chest wall compliance [45] which could convert into prolonged intubation and acute respiratory distress syndrome [29]. Postoperative fever/urinary tract infections were commonly reported ranging from 10.2% [30] to 25.5% [29], 34% [13], and up to 58% [28]. Perirenal abscess formation was also reported, despite being not common [10, 16, 32]. Sepsis rate with intensive care admission was reported to range from 4% [28], 7.6% [27], 14.3% [25], 17% [29] up to 26% [22]. The risk of postoperative sepsis was found to be twofold higher in SNP compared with non-neurological patients even when controlling for the presence of preoperative urinary infections [27]. The presence of multiple risk factors for infections, such as indwelling catheters, neurogenic bladder, vesicoureteral reflux, and struvite stones, predisposes this population to postoperative sepsis. Delayed PCNL could be an option to reduce the postoperative rate of sepsis. Eswara et al. evaluated 35 patients with neuromuscular disorders and assessed the difference in postoperative bacteremia/sepsis between those who had or had not placed a percutaneous nephrostomy tube at least 24 hours before PCNL [22]. The rate of PCNL bacteremia/sepsis was 14 % but patients undergoing same-day surgery had a 26% rate of bacteremia/sepsis compared to the group who had a preoperative nephrostomy tube which has no cases of it. There were also reported several cases of death [29, 33] with a mortality rate of up to 4.2% [27].
From our review, we infer that PCNL in SNP must be approached with experience and caution. Apart from positioning and anesthesia challenges, these patients are at higher risk of intraoperative complications related to bleeding, organ injuries, need for multiple interventions, poorer SFR and, despite the best precautions, postoperative sepsis and even mortality are a reality. In the bargain for rendering them stone-free, especially if associated with larger stone burdens and infectious stones, a technically challenging surgery can become a life-threatening procedure and this needs to be clearly discussed due to a reverberating impact on the psychosocial profile of patients and caregivers alike.
Summary points
Our review provides a very succinct yet clear message to urologists who manage patients with spinal anomalies/deformities with or without associated neuropathy. The three main challenges include:
Anatomical challenges: they may include physical or bony or limb alterations as well as genitourinary reconstructions.
Metabolic anomalies: there is an increased risk of stone formation and recurrence with an equal propensity for non-infectious and infectious stones, especially when patients have neurogenic bladders complicated by autonomic dysreflexia when the spinal cord injury is associated with paralysis.
Interventional challenges: whether from simple positioning issues or the need for multiple general anesthesia for repeated interventions, there is an increased risk of intra and peri-operative morbidity and even mortality. Urologists should emphasize there is no simple intervention. Any procedure, including SWL, ureteroscopy and PCNL, is far more challenging in this subset of patients and should therefore be preferably carried out in referral centers.
SFR assessment: we feel that a low-dose computed tomography scan should be recommended to declare that patient is stone-free as in this subset of patients it becomes imperative to minimize a re-intervention.
Study limitations
We were unable to provide any recommendation from a technical or technological standpoint as rightfully for these patients one treatment does not fit all and hence only a tailored approach may be the best option [46].
CONCLUSIONS
SWL in SNP has a very poor outcome probably due to challenges in patient positioning, stone visualization and localization, and a high rate of residual fragments. Challenges in ureteroscopy and PCNL are linked to frequent difficulties in reaching the stone due to anatomical anomalies, a significantly increased risk of infectious complications, and the need for repeat interventions under general anesthesia. Also these potential problems need to be clearly addressed during counseling. We feel that endoscopic combined intrarenal surgery maybe be a possible best approach for personalized care in these patients and perhaps we need more studies to see if now is the “prime time” for this modality in managing urolithiasis in these patients [47].
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Sorokin I, Mamoulakis C, Miyazawa K, Rodgers A, Talati J, Lotan Y. Epidemiology of stone disease across the world. World J Urol. 2017; 35: 1301-1320. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey S, McIlhenny C. Evidence-based management of upper tract urolithiasis in the spinal cord-injured patient. Spinal Cord. 2011. Sep; 49: 948-954. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, DeVivo MJ, Roseman JM. Current trend and risk factors for kidney stones in persons with spinal cord injury: a longitudinal study. Spinal Cord. 2000; 38: 346-353. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, DeVivo MJ, Stover SL, Lloyd LK. Recurrent kidney stone: A 25-year follow-up study in persons with spinal cord injury. Urology. 2002; 60: 228-232. [DOI] [PubMed] [Google Scholar]
- 5.Geraghty RM, Davis NF, Tzelves L, et al. Best Practice in Interventional Management of Urolithiasis: An Update from the European Association of Urology Guidelines Panel for Urolithiasis 2022. Vol. 9, European urology focus. Netherlands; 2023. p. 199-208. [DOI] [PubMed] [Google Scholar]
- 6.Castellani D, Teoh JY-C, Pavia MP, et al. Assessing the Optimal Urine Culture for Predicting Systemic Inflammatory Response Syndrome After Percutaneous Nephrolithotomy and Retrograde Intrarenal Surgery: Results from a Systematic Review and Meta-Analysis. J Endourol. 2022; 36: 158-168. [DOI] [PubMed] [Google Scholar]
- 7.Wong DG, Monda S, Vetter J, et al. Time Course and Risk Factors for Repeat Procedures After Ureteroscopy or Shockwave Lithotripsy. Urology. 2022; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfe T, Klausner AP, Goetz LL, King AB, Hudson T, Gater DR. Ureteroscopy with laser lithotripsy for urolithiasis in the spinal cord injury population. Spinal Cord. 2013; 51: 156-160. [DOI] [PubMed] [Google Scholar]
- 9.Welk B, Shariff S, Ordon M, Catharine Craven B, Herschorn S, Garg AX. The surgical management of upper tract stone disease among spinal cord-injured patients. Spinal Cord. 2013; 51: 457-460. [DOI] [PubMed] [Google Scholar]
- 10.Rubenstein JN, Gonzalez CM, Blunt LW, Clemens JQ, Nadler RB. Safety and efficacy of percutaneous nephrolithotomy in patients with neurogenic bladder dysfunction. Urology. 2004; 63: 636-640. [DOI] [PubMed] [Google Scholar]
- 11.Morhardt DR, Hadj-Moussa M, Chang H, et al. Outcomes of Ureteroscopic Stone Treatment in Patients With Spinal Cord Injury. Urology. 2018. Jun; 116:41-6. [DOI] [PubMed] [Google Scholar]
- 12.Sofimajidpour H, Kolahghoci P, Gharibi F. Outcome of Percutaneous Nephrolithotomy in Patients with Spinal Cord Neuropathy. Urol J. 2016; 13: 2672-6. [PubMed] [Google Scholar]
- 13.Matlaga BR, Kim SC, Watkins SL, Kuo RL, Munch LC, Lingeman JE. Changing composition of renal calculi in patients with neurogenic bladder. J Urol. 2006; 175: 1716-1719. [DOI] [PubMed] [Google Scholar]
- 14.Niedrach WL, Davis RS, Tonetti FW, Cockett AT. Extracorporeal shock-wave lithotripsy in patients with spinal cord dysfunction. Urology. 1991; 38: 152-156. [DOI] [PubMed] [Google Scholar]
- 15.Lazare JN, Saltzman B, Sotolongo J. Extracorporeal shock wave lithotripsy treatment of spinal cord injury patients. J Urol. 1988; 140: 266-269. [DOI] [PubMed] [Google Scholar]
- 16.Culkin DJ, Wheeler JSJ, Nemchausky BA, Fruin RC, Canning JR. Percutaneous nephrolithotomy in the spinal cord injury population. J Urol. 1986; 136: 1181-1813. [DOI] [PubMed] [Google Scholar]
- 17.Tepeler A, Sninsky BC, Nakada SY. Flexible ureteroscopic laser lithotripsy for upper urinary tract stone disease in patients with spinal cord injury. Urolithiasis. 2015; 43: 501-505. [DOI] [PubMed] [Google Scholar]
- 18.Stauffer C, Snyder E, Ngo T, Elliott C. Is neurogenic bladder a risk factor for febrile urinary tract infection after ureteroscopy and if so, why? J Urol. 2017; 197: e1260. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell S, Gurung PMS, Choong S, et al. Percutaneous Nephrolithotomy and Spina Bifida: Complex Major Stone Surgery? J Endourol. 2018; 32: 205-212. [DOI] [PubMed] [Google Scholar]
- 20.Alsinnawi M, Torreggiani WC, Flynn R, McDermott TED, Grainger R, Thornhill JA. Percutaneous nephrolithotomy in adult patients with spina bifida, severe spinal deformity and large renal stones. Ir J Med Sci. 2013; 182: 357-361. [DOI] [PubMed] [Google Scholar]
- 21.Clifton MM, Gettman MT, Patterson DE, Rangel L, Krambeck AE. The change in upper tract urolithiasis composition, surgical treatments and outcomes of para and quadriplegic patients over time. Urolithiasis. 2014; 42: 415-419. [DOI] [PubMed] [Google Scholar]
- 22.Eswara JR, Lee H, Dretler SP, Sacco D. The effect of delayed percutaneous nephrolithotomy on the risk of bacteremia and sepsis in patients with neuromuscular disorders. World J Urol. 2013; 31: 1611-1615. [DOI] [PubMed] [Google Scholar]
- 23.Beraud F, Clément G, Lenne X, Biardeau X. Incidence and safety outcomes associated with active stone removal procedures (ASRP): a comparison between neurological and non-neurological patients using the French National Health Data Base. World J Urol. 2022; 40: 1821-1827. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhry R, Theisen KM, Stephany HA, Schneck FX, Cannon GM, Ost MC. Percutaneous Stone Surgery in Spina Bifida Patients--Are Stone-Free Rates Worth the Risk? J Endourol. 2017; 31: S81-86. [DOI] [PubMed] [Google Scholar]
- 25.Nabbout P, Slobodov G, Mellis AM, Culkin DJ. Percutaneous nephrolithotomy in spinal cord neuropathy patients: a single institution experience. J Endourol. 2012; 26: 1610-1613. [DOI] [PubMed] [Google Scholar]
- 26.Christman MS, Kalmus A, Casale P. Morbidity and efficacy of ureteroscopic stone treatment in patients with neurogenic bladder. J Urol. 2013; 190: 1479-1483. [DOI] [PubMed] [Google Scholar]
- 27.Baldea KG, Blackwell RH, Vedachalam S, et al. Outcomes of percutaneous nephrolithotomy in spinal cord injury patients as compared to a matched cohort. Urolithiasis. 2017; 45: 501-506. [DOI] [PubMed] [Google Scholar]
- 28.Lawrentschuk N, Pan D, Grills R, et al. Outcome from percutaneous nephrolithotomy in patients with spinal cord injury, using a single-stage dilator for access. BJU Int. 2005; 96: 379-384. [DOI] [PubMed] [Google Scholar]
- 29.Knox ML, Cantor AM, Bryant JE, Burns JR. Predictive factors for percutaneous nephrolithotomy outcomes in neurogenic bladder population. J Endourol. 2012; 26:823-827. [DOI] [PubMed] [Google Scholar]
- 30.Torricelli FCM, Vicentini FC, Zanetti L, et al. Percutaneous nephrolithotomy in patients with spinal cord injury: should all these patients be automatically assigned a Guy’s stone score of 4? World J Urol. 2021; 39: 2129-2134. [DOI] [PubMed] [Google Scholar]
- 31.Gnessin E, Mandeville JA, Handa SE, Lingeman JE. Changing composition of renal calculi in patients with musculoskeletal anomalies. J Endourol. 2011; 25: 1519-1523. [DOI] [PubMed] [Google Scholar]
- 32.Culkin DJ, Wheeler JS, Nemchausky BA, Fruin RC, Canning JR. Percutaneous nephrolithotomy: spinal cord injury vs. ambulatory patients. J Am Paraplegia Soc. 1990; 13: 4-6. [DOI] [PubMed] [Google Scholar]
- 33.Symons S, Biyani CS, Bhargava S, et al. Challenge of percutaneous nephrolithotomy in patients with spinal neuropathy. Int J Urol. 2006; 13: 874-879. [DOI] [PubMed] [Google Scholar]
- 34.Ganesan V, Chen WM, Jain R, De S, Monga M. Multiple sclerosis and nephrolithiasis: a matched-case comparative study. BJU Int. 2017; 119: 919-925. [DOI] [PubMed] [Google Scholar]
- 35.Irwin PP, Evans C, Chawla JC, Matthews PN. Stone surgery in the spinal patient. Paraplegia. 1991; 29:161-166. [Google Scholar]
- 36.Raj G V, Bennett RT, Preminger GM, King LR, Wiener JS. The incidence of nephrolithiasis in patients with spinal neural tube defects. J Urol. 1999; 162: 1238-1242. [DOI] [PubMed] [Google Scholar]
- 37.Prattley S, Oliver R, New F, Davies M, Brewin J. Ureteroscopy in patients with spinal cord injury: outcomes from a spinal injury unit and a review of literature. Transl Androl Urol. 2019; 8: S352-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spirnak JP, Bodner D, Udayashankar S, Resnick MI. Extracorporeal shock wave lithotripsy in traumatic quadriplegic patients: can it be safely performed without anesthesia? J Urol. 1988; 139: 18-19. [DOI] [PubMed] [Google Scholar]
- 39.Robert M, Bennani A, Ohanna F, Guiter J, Avérous M, Grasset D. The management of upper urinary tract calculi by piezoelectric extracorporeal shock wave lithotripsy in spinal cord injury patients. Paraplegia. 1995; 33: 132-135. [DOI] [PubMed] [Google Scholar]
- 40.Wahle S, Kramolowsky E, Loening S. Extracorporeal shock wave lithotripsy in paraplegic and quadriplegic patients. J Am Paraplegia Soc. 1988; 11: 6-9. [DOI] [PubMed] [Google Scholar]
- 41.Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord. 2001; 39: 208-214. [DOI] [PubMed] [Google Scholar]
- 42.Monga M, Murphy M, Paranjpe R, Cutone B, Eisner B. Prevalence of Stone Disease and Procedure Trends in the United States. Urology. 2023; In press. [DOI] [PubMed] [Google Scholar]
- 43.Mak DK-C, Smith Y, Buchholz N, El-Husseiny T. What is better in percutaneous nephrolithotomy - Prone or supine? A systematic review. Arab J Urol. 2016; 14: 101-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kara C, Resorlu B, Ozyuvali E, Unsal A. Is percutaneous nephrolithotomy suitable for patients with scoliosis: single-center experience. Urology. 2011; 78: 37-42. [DOI] [PubMed] [Google Scholar]
- 45.Benditt JO. Pathophysiology of Neuromuscular Respiratory Diseases. Clin Chest Med. 2018; 39: 297-308. [DOI] [PubMed] [Google Scholar]
- 46.Lim EJ, Osther PJ, Valdivia Uría JG, et al. Personalized stone approach: can endoscopic combined intrarenal surgery pave the way to tailored management of urolithiasis? Minerva Urol Nephrol. 2021; 73: 428-430. [DOI] [PubMed] [Google Scholar]
- 47.Gauhar V, Castellani D, Cracco CM, et al. Is endoscopic combined intrarenal surgery ready for primetime in endourology? Outcomes from a systematic review and meta-analysis. Cent Eur J Urol. 2022; 75: 171-181. [DOI] [PMC free article] [PubMed] [Google Scholar]