Abstract
Background:
Surgeon performance has been investigated as a factor affecting patient outcomes after orthopaedic procedures to improve transparency between patients and providers.
Purpose/Hypothesis:
The purpose of this study was to identify whether surgeon performance influenced patient-reported outcomes (PROMs) 1 year after arthroscopic partial meniscectomy (APM). It was hypothesized that there would be no significant difference in PROMs between patients who underwent APM from various surgeons.
Study Design:
Case-control study; Level of evidence, 3.
Methods:
A prospective cohort of 794 patients who underwent APM between 2018 and 2019 were included in the analysis. A total of 34 surgeons from a large multicenter health care center were included. Three multivariable models were built to determine whether the surgeon—among demographic and meniscal pathology factors—was a significant variable for predicting the Knee injury and Osteoarthritis Outcome Score (KOOS)-Pain subscale, the Patient Acceptable Symptom State (PASS), and a 10-point improvement in the KOOS-Pain at 1 year after APM. Likelihood ratio (LR) tests were used to determine the significance of the surgeon variable in the models.
Results:
The 794 patients were identified from the multicenter hospital system. The baseline KOOS-Pain score was a significant predictor of outcome in the 1-year KOOS-Pain model (odds ratio [OR], 2.1 [95% CI, 1.77-2.48]; P < .001), the KOOS-Pain 10-point improvement model (OR, 0.57 [95% CI, 0.44-0.73), and the 1-year PASS model (OR, 1.42 [95% CI, 1.15-1.76]; P = .002) among articular cartilage pathology (bipolar medial cartilage) and patient-factor variables, including body mass index, Veterans RAND 12-Item Health Survey–Mental Component Score, and Area Deprivation Index. The individual surgeon significantly impacted outcomes in the 1-year KOOS-Pain mixed model in the LR test (P = .004).
Conclusion:
Patient factors and characteristics are better predictors for patient outcomes 1 year after APM than surgeon characteristics, specifically baseline KOOS-Pain, although an individual surgeon influenced the 1-Year KOOS-Pain mixed model in the LR test. This finding has key clinical implications; surgeons who wish to improve patient outcomes after APM should focus on improving patient selection rather than improving the surgical technique. Future research is needed to determine whether surgeon variability has an impact on longer-term patient outcomes.
Keywords: arthroscopic partial meniscectomy, patient-reported outcomes, predictive modeling, surgeon performance
Transparency within the medical community can be achieved to improve the relationship between patients and providers when patients can identify high-quality care. 6 When patients have access to information on the quality of their health care, they become more informed consumers. 12 Investigators have conducted research on surgeon performance as a method to identify transparency. Specifically, researchers have focused on the impact of surgeons on patient complications to identify why some patients undergoing elective procedures have avoidable problems. 19 Surgeon scorecards that display complication rates for individual surgeons have served as a useful methodologic tool to encourage surgeons to make quality improvements and help patients make informed selections for care. 5
Such reporting methods have been implemented to assess surgeon performance in bladder tumor resections, where improvements in resection quality were measured after scorecards were distributed with surgeon and institution detrusor sampling rates. 4 In colectomy cases, scorecards were distributed with items under 5 domains (survival, morbidity avoidance, anastomotic leak avoidance, surgical site infection bundle compliance, and utilization) to predict future surgeon performance. 6 In cardiac operations, surgeon characteristics 18 and experience 1 have been studied to identify associations with patient outcomes. Thus, scorecards with specialty-specific measurements and demographic characteristics have been utilized to measure surgeon performance for quality control and improved patient outcomes.
Specifically, surgeon performance has been closely analyzed in orthopaedic surgery to assess variability in patient outcomes. In knee and hip arthroplasties, surgeons utilized a scorecard to assess financial costs, resulting in a 20% decrease in total direct costs from surgery. 20 Sharing financial outcomes was deemed a clinically relevant method for promoting surgeon accountability and improved decision making. 20 Further, high-volume surgeons had decreased complications compared with low-volume surgeons in idiopathic scoliosis 14 and shoulder arthroscopy procedures. 17 Such findings can inform patients to make evidence-based decisions regarding their care. Arthroscopic partial meniscectomies (APMs) present a problem to patients because outcomes are variable.7,10,22 With over 400,000 APMs performed annually in the United States,8,21 information about surgeon performance on clinically relevant primary outcomes of pain relief and patient acceptable status should provide meaningful contributions to patients faced with the decision to undergo APM.
To the best of our knowledge, there is a gap in the literature that has investigated surgeon performance as a potential factor for impacting APM outcomes. To fill this gap, we analyzed patient outcomes from a cohort of 794 patients who underwent APM within a multicenter health enterprise, 3 with sites located across the United States, to determine whether there was a difference in outcome among surgeons. We hypothesized that there would be no significant difference in Knee injury and Osteoarthritis Outcome Score (KOOS)-Pain subscores and Patient Acceptable Symptom State (PASS) scores between surgeons.
Methods
Study Design and Population
The present study involved a prospective longitudinal cohort that originally included 1194 unique participants, with data collection captured from our institution’s Outcomes Measurement and Evaluation (OME) database. 13 This data collection system records patient-reported outcome measures (PROMs) in a consecutive series of patients using Research Electronic Data Capture software (RedCap; Vanderbilt University). 13 All included patients had undergone knee arthroscopy or open knee surgery at a large multicenter health care system between January 1, 2018, and December 31, 2019.
All meniscectomies included in the final cohort were performed arthroscopically without any additional open procedures, and patients undergoing concomitant procedures (eg, anterior cruciate ligament reconstruction) were excluded. Patients who underwent APM with chondroplasty were included in the final cohort. The final dataset for analysis included 794 participants diagnosed with a medial tear, lateral tear, or both medial and lateral tears and who completed PROMs at 1 year postoperatively (69% follow-up rate). All of the included patients pursued APM for treatment during the specified time window of 1 year. All surgeries were performed by 1 of 34 surgeons. A Strengthening the Reporting of Observational Studies in Epidemiology diagram showing the winnowing down of eligible participants after applying the inclusion and exclusion criteria is shown in Figure 1.
Figure 1.
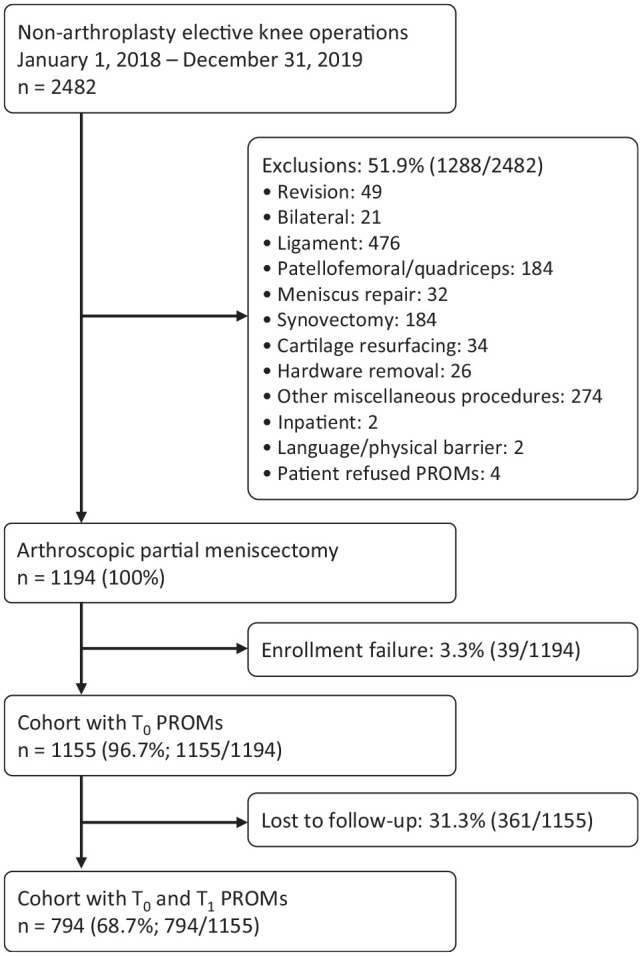
A STROBE diagram for the inclusion and exclusion criteria. PROMs, patient-reported outcome measures; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; T0, baseline; T1, 1 year postoperative.
Data Collection
At baseline, demographic variables were collected from all patients, including age, sex, race, body mass index (BMI), years of education, smoking status, and Area Deprivation Index (ADI). An ADI associates a geographic area’s socioeconomic conditions with its health outcomes and was established to better understand social determinants of health. 11 Further, all patients completed PROMs that included the Veterans RAND 12-Item Health Survey–Mental Component Score (VR12-MCS), the KOOS-Pain subscale, and the PASS at baseline. The VR12-MCS score is standardized using a T-score metric, with a mean of 50 (SD, 10). The KOOS-Pain is scored from 0 to 100, with higher scores representing less pain. The original question for the PASS was, “Do you consider the current state of your knee satisfactory?” Patients completed these same PROMs at 1 year postoperatively, at which time the 10-point improvement in the KOOS-Pain model was calculated for each participant.
The total number of cases per participating surgeon was collected.
Statistical Analyses
Continuous variables were summarized using medians and interquartile ranges (IQRs), and categorical variables were summarized using counts and percentages. Three multivariable mixed-effect models were used to investigate whether the surgeon—among other demographic and meniscal pathology factors—was a significant variable in predicting KOOS-Pain, a 10-point improvement in KOOS-Pain, and PASS scores at 1 year after APM. The mixed effect models included 2 parts—fixed effect and random effect. The fixed effects were patient age, sex, race, BMI, years of education, smoking status, baseline VR12-MCS score, medial cartilage, lateral cartilage, patellofemoral cartilage, tear type, effusion, synovitis, chondroplasty, and ADI. The random effect was the surgeon, where surgeon-specific random intercepts were estimated by the model. Ordinal response mixed models were used to model 1-year KOOS-Pain because of the skewed distribution and potential violation of the normality assumption of linear mixed models. Binary outcome mixed models were used to model KOOS 10-point improvement (yes or no) and PASS (yes or no). The significance of the surgeon variable was tested using likelihood ratio (LR) tests by halving the P values from the tests because the test of variance was 1-sided (variance cannot be negative). Variable importance plots were obtained for each outcome, ranking variables by the increase in the Akaike information criterion (AIC) upon its removal. An AIC increase of ≥2 indicated that the given variable contributed to a statistically better model. The ordinal response mixed models were built using the ordinal package in R software (R Core Team).
To demonstrate surgeon variability based on the model results, we generated the predicted mean 1-year postoperative KOOS-Pain, predicted probability of achieving 10-point improvement in KOOS-Pain, and predicted probability of achieving PASS models at 1 year postoperatively based on 2 random patient profiles. The predicted mean or probabilities were calculated using the fixed-effect coefficient estimates, and per-surgeon intercepts estimated by the models. Missing values were singly imputed using multivariate imputation by chained equations, implemented in the mice package in R software (R Core Team).
Data management and analysis were performed in R software (Version 4.0; R Core Team). All tests are 2-sided, assuming an alpha level of .05.
Results
Study Population
The median age of the 794 patients who underwent APM was 56 years (IQR, 48-63 years), and 50.9% of the cohort patients were men. Also, 12.7% and 13.1% of the cohort patients had effusion and reactive synovitis on diagnostic arthroscopy, respectively. Most patients had a medial tear (86.3%), 32% of the patients had a lateral tear, and 18.3% had a tear in both compartments. The median KOOS-Pain at baseline was 47.2 (IQR, 38.9-58.3), and 81.7% of the cohort patients experienced a 10-point improvement in the KOOS-Pain 1 year after APM. One year after APM, 70.5% of the patients responded “yes” to the PASS. Among the 794 participants who were included in the analysis, 26 were missing race data, 20 were missing ADI, 3 were missing KOOS-Pain at 1 year, and 19 were missing PASS pain at 1 year. All other variables were complete. Table 1 displays the basic summary statistics to build the 3 models predicting PROMs 1 year after APM.
Table 1.
Summary Statistics of the Study Population (N = 794) a
| Variable | Value | Variable | Value |
|---|---|---|---|
| Age, y | 56 [48.0-63] | Lateral tear | |
| Sex | None | 540 (68) | |
| Male | 404 (50.9) | Root | 7 (0.88) |
| Female | 390 (49.1) | Bucket handle | 12 (1.51) |
| Race (n = 768) | Other | 235 (29.6) | |
| White | 677 (88.2) | Tear type | |
| Black | 68 (8.85) | Medial | 540 (68) |
| Other | 23 (2.99) | Lateral | 109 (13.7) |
| BMI, kg/m2 | 29.8 [26.1-34.2] | Both | 145 (18.3) |
| Education, y | 15 [12-16] | Effusion | |
| Smoking history | No | 693 (87.3) | |
| Never smoked | 483 (60.8) | Yes | 101 (12.7) |
| Quit ≥6 mo previous | 224 (28.2) | Reactive synovitis | |
| Quit <6 mo previous | 11 (1.39) | No | 690 (86.9) |
| Current smoker | 76 (9.57) | Yes | 104 (13.1) |
| T0 VR12-MCS | 55.1 [45.2-62.1] | Chondroplasty b | |
| Medial cartilage | None | 425 (53.5) | |
| Normal | 429 (54) | Grade 1 | 196 (24.7) |
| Unipolar | 243 (30.6) | Grade 2 | 138 (17.4) |
| Bipolar | 122 (15.4) | Grade 3 | 35 (4.41) |
| Lateral cartilage | ADI (n = 774) | 39 [22-60] | |
| Normal | 650 (81.9) | T0 KOOS-Pain | 47.2 [38.9-58.3] |
| Unipolar | 104 (13.1) | T1 KOOS-Pain (n = 791) | 80.6 [63.9-94.1] |
| Bipolar | 40 (5.04) | KOOS-Pain 10-point improvement (n = 791) | |
| Patellofemoral cartilage | No | 145 (18.3) | |
| Normal | 457 (57.6) | Yes | 646 (81.7) |
| Unipolar | 203 (25.6) | T1 PASS (n = 775) | |
| Bipolar | 134 (16.9) | No | 229 (29.5) |
| Medial tear | Yes | 546 (70.5) | |
| None | 109 (13.7) | ||
| Root | 30 (3.78) | ||
| Bucket handle | 20 (2.52) | ||
| Other | 635 (80) |
Data are presented as n (%) or mean [interquartile range]. ADI, Area Deprivation Index; BMI, body mass index; KOOS, Knee injury and Osteoarthritis Outcome Score; PASS, Patient Acceptable Symptom State; T0, baseline; T1, 1 year postoperative; VR12-MCS, Veterans RAND 12-Item Health Survey–Mental Component Score.
Modified Outerbridge grade.
The median case count of APMs per surgeon was 22 cases (range, 1-93 cases). The mean number of years in practice for all surgeons included in this model was 23, and 69% of all surgeons included in the model were sports fellowship trained. Also, 85% of the surgeons who completed >20 cases were fellowship trained.
Summary of Significant Predictors
After controlling for all other variables, a higher baseline VR12-MCS was a significant predictor for better outcomes after surgery in the 1-year KOOS-Pain model (odds ratio [OR], 1.55 [95% CI, 1.27-1.9]; P < .001) and in the KOOS-Pain 10-point improvement model (OR, 1.5 [95% CI, 1.12-2.01]; P = .006). A higher baseline KOOS-Pain was a significant predictor of better outcomes after surgery in the 1-year KOOS-Pain model (OR, 2.1 [95% CI, 1.77-2.48]; P < .001), the KOOS-Pain 10-point improvement model (OR, 0.57 [95% CI, 0.44-0.73]; P < .001), and in the 1-year PASS model (OR, 1.42 [95% CI, 1.15-1.76]; P = .002) after controlling for all other variables (Table 2). Bipolar medial cartilage at baseline was a significant predictor for worse outcomes after surgery in the 1-year KOOS-Pain model (OR, 0.53 [95% CI, 0.35-0.81]; P = .004) and in the 1-year PASS model (OR, 0.51 [95% CI, 0.3-0.87]; P = .014) after controlling for all other variables (Table 2). A lower ADI at baseline was a significant predictor of better outcomes after surgery in the 1-year KOOS-Pain model (OR, 0.77 [95% CI, 0.62-0.95]; P = .014) and the KOOS-Pain 10-point improvement model (OR, 0.71 [95% CI, 0.52-0.99]; P = .042) after controlling for all other variables (Table 2). A higher BMI at baseline was a significant predictor of worse outcomes after surgery in the KOOS-Pain 10-point improvement model only (OR, 0.76 [95% CI, 0.6-0.96]; P = .022) after controlling for all other variables (Table 2).
Table 2.
Combined Results for the 3 Linear Multivariable Models a
| 1-Year KOOS-Pain (n = 791) | KOOS-Pain 10-Point Improvement (n = 791) | 1-Year PASS (n = 775) | ||||
|---|---|---|---|---|---|---|
| Factor | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Age, IQR increase, y | 1.11 (0.94-1.31) | .23 | 1.11 (0.86-1.42) | .42 | 1.13 (0.91-1.41) | .280 |
| Sex, female (vs male) | 0.81 (0.63-1.05) | .106 | 0.9 (0.61-1.34) | .604 | 0.98 (0.7-1.36) | .888 |
| Race | ||||||
| Black (vs White) | 0.88 (0.56-1.38) | .574 | 0.77 (0.41-1.46) | .428 | 0.79 (0.44-1.42) | .434 |
| Other (vs White) | 0.71 (0.35-1.43) | .336 | 1.01 (0.34-2.96) | .992 | 0.6 (0.24-1.49) | .274 |
| BMI, IQR increase, kg/m2 | 0.85 (0.72-1) | .052 | 0.76 (0.6-0.96) b | .022 | 0.84 (0.68-1.03) | .094 |
| Education, IQR increase, y | 1 (0.83-1.2) | .98 | 1.25 (0.93-1.68) | .14 | 0.84 (0.65-1.07) | .162 |
| Smoking | ||||||
| Quit ≥6 mo (vs never) | 1.06 (0.79-1.42) | .714 | 0.97 (0.62-1.53) | .906 | 0.88 (0.6-1.28) | .496 |
| Quit <6 mo (vs never) | 1.33 (0.44-3.98) | .614 | 3 (0.36-25.21) | .31 | 0.53 (0.14-1.97) | .342 |
| Current (vs never) | 0.66 (0.42-1.03) | .068 | 0.64 (0.35-1.19) | .16 | 0.79 (0.46-1.36) | .390 |
| T0 VR12-MCS, IQR increase | 1.55 (1.27-1.9) | <.001 | 1.5 (1.12-2.01) | .006 | 1.15 (0.89-1.48) | .288 |
| T0 KOOS-Pain, IQR increase | 2.1 (1.77-2.48) | <.001 | 0.57 (0.44-0.73) | <.001 | 1.42 (1.15-1.76) | .002 |
| Medial cartilage | ||||||
| Unipolar (vs normal) | 0.75 (0.55-1.03) | .076 | 0.99 (0.6-1.63) | .958 | 0.92 (0.61-1.39) | .682 |
| Bipolar (vs normal) | 0.53 (0.35-0.81) c | .004 | 0.58 (0.31-1.08) | .084 | 0.51 (0.3-0.87) | .014 |
| Lateral cartilage | ||||||
| Unipolar (vs normal) | 0.81 (0.54-1.23) | .32 | 0.73 (0.4-1.32) | .296 | 0.62 (0.37-1.03) | .068 |
| Bipolar (vs normal) | 0.82 (0.44-1.5) | .516 | 0.94 (0.37-2.37) | .896 | 1.3 (0.55-3.09) | .548 |
| Patellofemoral cartilage | ||||||
| Unipolar (vs normal) | 1.22 (0.89-1.68) | .224 | 1.12 (0.68-1.84) | .66 | 1.08 (0.71-1.63) | .728 |
| Bipolar (vs normal) | 1.36 (0.89-2.06) | .152 | 1.37 (0.71-2.62) | .344 | 1.51 (0.87-2.62) | .144 |
| Tear type | ||||||
| Lateral (vs medial) | 0.76 (0.52-1.13) | .182 | 0.72 (0.41-1.27) | .26 | 0.94 (0.56-1.57) | .808 |
| Both (vs medial) | 0.92 (0.65-1.31) | .656 | 0.97 (0.56-1.68) | .916 | 0.96 (0.6-1.52) | .854 |
| Effusion, yes (vs no) | 1.34 (0.91-1.96) | .136 | 1.52 (0.79-2.92) | .206 | 1.31 (0.79-2.17) | .304 |
| Synovitis, yes (vs no) | 0.89 (0.61-1.31) | .566 | 0.87 (0.48-1.55) | .63 | 1.36 (0.82-2.26) | .234 |
| Chondroplasty | ||||||
| Grade 1 (vs none) | 0.91 (0.67-1.24) | .556 | 0.99 (0.61-1.59) | .962 | 0.89 (0.6-1.32) | .548 |
| Grade 2 (vs none) | 0.82 (0.56-1.19) | .288 | 0.7 (0.4-1.22) | .208 | 0.78 (0.48-1.26) | .302 |
| Grade 3 (vs none) | 1.15 (0.59-2.26) | .682 | 2.09 (0.61-7.17) | .244 | 2.19 (0.81-5.89) | .120 |
| ADI, IQR increase | 0.77 (0.62-0.95) | .014 | 0.71 (0.52-0.99) | .042 | 0.96 (0.72-1.26) | .748 |
Bold values represent significant associations (P < .05). ADI, Area Deprivation Index; BMI, body mass index; IQR, interquartile range; KOOS, Knee injury and Osteoarthritis Outcome Score; OR, odds ratio; PASS, Patient Acceptable Symptom State; T0, baseline; VR12-MCS, Veterans RAND 12-Item Health Survey–Mental Component Score.
The OR for the BMI was 0.76 kg/m2 in the KOOS-Pain 10-point improvement model. Thus, the odds of getting a 10-point improvement in those with a third quartile BMI was 24% lower than those with a first quartile BMI after controlling for other variables in the model.
The OR for bipolar medial cartilage was 0.53 in the 1-year KOOS-Pain linear model. Thus, the odds of getting a 1-year KOOS-Pain of at least x in those with bipolar medial cartilage was 47% lower than those with normal medial cartilage after controlling for other variables in the model. The value of x is irrelevant since this is a proportional odds model.
Surgeon Variability and Relative Variable Importance
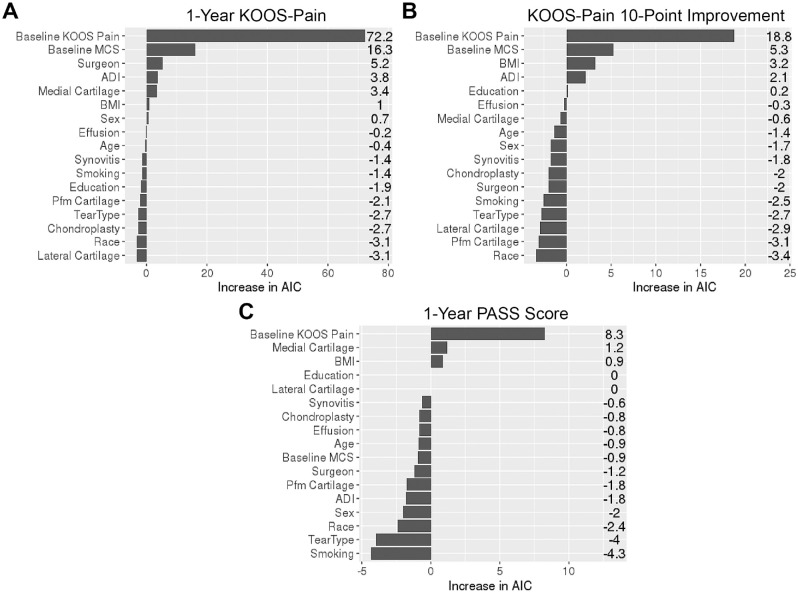
In the 1-year KOOS-Pain mixed model, surgeon term was significant for impacting PROMs at 1 year after APM in the LR test (P = .004). Surgeon term in the 1-year PASS mixed model was not significant for predicting outcomes at 1 year after APM (P = .185) and in the KOOS 10-point improvement mixed models (P = .5). Figure 2 displays the AIC relative importance plot of the 3 models. In the 1-year KOOS-Pain mixed model, baseline KOOS-Pain and baseline VR12-MCS contributed the most to the model, followed by the surgeon. In both the KOOS-Pain 10-point improvement and PASS mixed models, baseline KOOS-Pain was the most important predictor, and surgeons did not contribute as much as in the 1-year KOOS-Pain model.
Figure 2.
Variable importance ranked by AIC increase in the (A) 1-year KOOS-Pain model, (B) KOOS-Pain 10-point improvement model, and (C) 1-year PASS model. ADI, Area Deprivation Index; AIC, Akaike information criterion; BMI, body mass index; KOOS, Knee injury and Osteoarthritis Outcome Score; MCS, Veterans RAND 12-Item Health Survey–Mental Component Score; PASS, Patient Acceptable Symptom State; Pfm, patellofemoral.
Demonstration of Surgeon Variability
Two patient profiles were derived from the 1-year KOOS-Pain model to understand how outcomes would be affected if a patient were to be operated by the surgeon with the highest ranking (surgeon 7) or lowest ranking (surgeon 4):
Patient 1: age, 48 years; BMI, 26.1 kg/m2; education, 16 years; never smoker; baseline KOOS-Pain, 58.33; baseline VR12-MCS, 67.1; normal medial, lateral, and patellofemoral cartilage; no effusion; no synovitis; chondroplasty, none; ADI, 22.
Patient 2: age, 63 years; BMI, 34.2 kg/m2; education, 12 years; current smoker; baseline KOOS-Pain, 38.89; baseline VR12-MCS, 45.2; bipolar medial, lateral, and patellofemoral cartilage; effusion present; synovitis present; chondroplasty, grade 3; ADI, 61.
Both patients were modeled to be White men with lateral tears. The profile of patient 1 had clinical and demographic characteristics shown to have better outcomes in the 1-year KOOS-Pain model, whereas patient 2 had characteristics shown to have worse outcomes in the 1-year KOOS-Pain model.
If surgeon 4 had performed surgery on patient 1, the 1-year mean KOOS-Pain would have been 80.7, compared with 87.3 if surgeon 7 had performed the surgery. The difference between 1-year postoperative KOOS-Pain levels for this patient was 6.6. With respect to patient 2, if surgeon 4 had performed the APM, the mean KOOS-Pain at 1 year after surgery would have been 53.8, compared with 64.6 if surgeon 7 had performed the procedure. The difference between 1-year postoperative KOOS-Pain levels for patient 2 was 10.8.
Discussion
In this study, we identified a 794-patient cohort who all underwent APM from a single hospital care system. In the 1-year KOOS-Pain model, baseline VR12-MCS (OR, 1.55 [95% CI, 1.27-1.9]; P < .001), baseline KOOS-Pain (OR, 2.1 [95% CI, 1.77-2.48]; P< .001), bipolar medial cartilage (OR, 0.53 [95% CI, 0.35-0.81]; P = .004), and ADI (OR, 0.77, 95% CI 0.62-0.95]; P = .014) were all significant predictors of outcome after controlling for all other variables. In the LR test, the surgeon significantly impacted outcomes 1 year after APM in the 1-year KOOS-Pain mixed model (P = .004). In the KOOS-Pain 10-point improvement model, BMI (OR, 0.76 [95% CI, 0.6-0.96]; P = .022), baseline VR12-MCS (OR, 1.5 [95% CI, 1.12-2.01]; P = .006), baseline KOOS-Pain (OR, 0.57 [95% CI, 0.44-0.73), and ADI (OR, 0.71 [95% CI, 0.52-0.99]; P = .042) were all significant predictors of outcome after controlling for all other variables. Only baseline KOOS-Pain (OR, 1.42 [95% CI, 1.15-1.76]; P = .002) and bipolar medial cartilage (OR, 0.51 [95% CI, 0.3-0.87]; P = .014) were significant predictors of outcome in the 1-year PASS model after controlling for all other variables. Baseline KOOS-Pain was a significant predictor of outcome in all 3 mixed effect models, suggesting that pain before APM is an important factor to consider when predicting how patients will respond 1 year after APM.
To our knowledge, this is the first study that has analyzed postoperative outcomes in patients after APM among various surgeons within a single health care system. Previous work has focused on patient characteristics to predict outcomes after APM. Current smoking patients at the time of APM had worse postoperative PROMs compared with nonsmokers. 9 Higher BMI, older age, less education, and lower VR12-MCS are all patient characteristics that have been shown to predict less improvement after APM. 2 However, none of these studies have identified surgeon performance as a potential factor affecting patient outcomes after APM. A similar analysis that has been completed for patients who had total hip arthroplasty found a significant association among surgeons and PROMs 1 year after total hip arthroplasty. 16 In our analysis, we only found a significant association in the LR test between the surgeon and outcomes in the 1-year KOOS-Pain mixed model (P = .004); however, this significant association was not seen in the KOOS-Pain 10-point improvement mixed model or the 1-year PASS mixed model.
The patient profiles derived from the 1-year KOOS-Pain model presented an interesting result. The mean KOOS-Pain score 1 year after APM for the patient with the most optimal clinical and demographic characteristics (patient 1) was 80.7 if operated on by the lowest-ranked surgeon (surgeon 4). The mean KOOS-Pain score 1 year after APM for the patient with the least optimal clinical and demographic characteristics (patient 2) was 64.6 if operated on by the highest-ranked surgeon (surgeon 7). This result suggested that the patients’ baseline characteristics were more predictive of their postoperative outcomes than surgeon variability. The difference between mean KOOS-Pain values 1 year after APM for patient 1 was 6.6. Ten points are regarded as the minimal clinically important difference on the KOOS-Pain scale. 15 Even when this patient was modeled to undergo APM by the lowest or highest ranked surgeon, the outcome difference was insignificant.
The OME database 13 was a key strength in this study that facilitated data collection across 1 entire health enterprise system. The large amount of patient data allowed us to carefully apply the inclusion and exclusion criteria. However, there are key limitations presented in this study. The follow-up was restricted to 1 year, and thus surgeon performance was not analyzed as a predictor for longer-term patient outcomes after APM. In addition, based on our data, it is difficult to conclude whether the type of meniscal injury—degenerative versus traumatic—has an impact on the surgeon’s performance and outcome. Surgeon volume and experience as factors for predicting patient outcomes after APM have not been studied, and analysis of a cohort with more surgeons could enable this analysis to be conducted.
Conclusion
In this study, we found that patient factors and characteristics are better predictors for patient outcomes 1 year after APM than surgeon characteristics, specifically baseline KOOS-Pain. This finding has key clinical implications for both patients and surgeons. Surgeons who wish to improve patient outcomes after APM should focus on improving patient selection rather than improving surgical techniques. Future research is needed to determine whether surgeon variability has an impact on longer-term patient outcomes.
Contributing Authors
The Cleveland Clinic Sports Health authors for this work include Morgan H. Jones, MD, MPH (Orthopaedic and Arthritis Center for Outcomes Research and Department of Orthopedic Surgery, Brigham and Women’s Hospital, Boston, Massachusetts); Julia R. Gottreich, BA (Orthopaedic and Arthritis Center for Outcomes Research, Brigham and Women’s Hospital, Boston, Massachusetts); Yuxuan Jin, MS (Department of Quantitative Health Sciences, Cleveland Clinic, Cleveland, Ohio); Michael W. Kattan, PhD (Department of Quantitative Health Sciences, Cleveland Clinic, Cleveland, Ohio); Kurt P. Spindler, MD (Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Florida, Weston, Florida); Lutul D. Farrow, MD (Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio); Salvatore J. Frangiamore, MD (Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio); Gregory J. Gilot, MD (Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Florida, Weston, Florida); Robert J. Hampton, DO (Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio); Brian M. Leo, MD (Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Florida, Weston, Florida); Robert J. Nickodem, MD (Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio); Richard D. Parker, MD (Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio); James T. Rosneck, MD (Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio); Paul M. Saluan, MD (Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio); Michael J. Scarcella, MD (Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio); Alfred Serna, MD (Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio); and Kim L. Stearns, MD (Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio).
Acknowledgments
The authors thank the additional Cleveland Clinic Orthopaedic Sports Medicine APM surgeons with insufficient case counts to be included as authors, as well as the research team, staff, and research personnel whose efforts related to regulatory requirements, data collection, data quality control, analyses, and manuscript preparation have made this consortium successful. Thanks to Elizabeth Sosic, MSL, for editorial management. The authors acknowledge Jack T. Andrish, MD; Wael K. Barsoum, MD; Alan W. Davis, MD; Joseph W. George, MD; Ryan C. Goodwin, MD; Michael W. Kolczun, MD; David H. Krahe, DO; Richard R. Masin, DO; Andrew J. Matko, DO; Brett W. McCoy, MD; John P. McLaughlin, DO; Anthony Miniaci, MD; Robert M. Molloy, MD; Trevor G. Murray, MD; Victor A. Nemeth, MD; Bradley A. Pierce, MD; Frank M. Sabo, MD; Joseph B. Scarcella, MD; Johnathan L. Schaffer, MD; Patrick E. Sziraky, MD; James S. Williams, MD (Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio); Aldo M. Riesgo, MD (Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Florida, Weston, Florida); Peter B. Imrey, PhD (Department of Quantitative Health Sciences, Cleveland Clinic, Cleveland, Ohio); and Greg Strnad, MS (Cleveland Clinic Foundation, Cleveland, Ohio).
Footnotes
Final revision submitted May 9, 2023; accepted May 19, 2023.
One or more of the authors has declared the following potential conflict of interest or source of funding: Funding was received from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (grants AR053684, R01 AR074131, AR053684, and AR075422). M.H.J. has received research support from Flexion Therapeutics and consulting fees from Biosplice and Regeneron. K.P.S. has received research support from DJO and Smith & Nephew; consulting fees from Flexion Therapeutics, National Football League, and NovoPedics; royalties from Oberd; and honoraria from NovoPedics. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Cleveland Clinic (ref No. 06-196).
Contributor Information
Morgan H. Jones, Orthopaedic and Arthritis Center for Outcomes Research and Department of Orthopedic Surgery, Brigham and Women’s Hospital, Boston, Massachusetts.
Julia R. Gottreich, Orthopaedic and Arthritis Center for Outcomes Research, Brigham and Women’s Hospital, Boston, Massachusetts.
Yuxuan Jin, Department of Quantitative Health Sciences, Cleveland Clinic, Cleveland, Ohio.
Michael W. Kattan, Department of Quantitative Health Sciences, Cleveland Clinic, Cleveland, Ohio.
Kurt P. Spindler, Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Florida, Weston, Florida.
Lutul D. Farrow, Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio.
Salvatore J. Frangiamore, Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio.
Gregory J. Gilot, Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Florida, Weston, Florida.
Robert J. Hampton, Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio.
Brian M. Leo, Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Florida, Weston, Florida.
Robert J. Nickodem, Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio.
Richard D. Parker, Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio.
James T. Rosneck, Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio.
Paul M. Saluan, Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio.
Michael J. Scarcella, Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio.
Alfred Serna, Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio.
Kim L. Stearns, Department of Orthopaedic Surgery, Sports Medicine, Cleveland Clinic, Cleveland, Ohio; Investigation Performed at the Cleveland Clinic, Cleveland, Ohio, USA.
References
- 1. Anderson BR, Wallace AS, Hill KD, et al. Association of surgeon age and experience with congenital heart surgery outcomes. Circ Cardiovasc Qual Outcomes. 2017;10(7):e003533. doi: 10.1161/CIRCOUTCOMES.117.003533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cleveland Clinic Sports Health. Predictors of successful treatment 1 year after arthroscopic partial meniscectomy: data from the OME Cohort. JB JS Open Access. 2020;5(4):e19.00044. doi: 10.2106/JBJS.OA.19.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cleveland Clinic Sports Knee Group; Bessette MC, Westermann RW, et al. Predictors of pain and function before knee arthroscopy. Orthop J Sports Med. 2019;7(5):2325967119844265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Das A, Cohen JE, Ko OS, et al. Surgeon scorecards improve muscle sampling on transurethral resection of bladder tumor and recurrence outcomes in patients with nonmuscle invasive bladder cancer. J Urol. 2021;205(3):693-700. [DOI] [PubMed] [Google Scholar]
- 5. Friedberg MW, Pronovost PJ, Shahian DM, et al. A methodological critique of the ProPublica Surgeon Scorecard. RAND Health Q. 2016;5(4):1. [PMC free article] [PubMed] [Google Scholar]
- 6. Iyengar R, Mossner JM, Sekhri S, et al. A new composite measure for assessing surgical performance. Michigan J Med. 2019;4(1):77-89. [Google Scholar]
- 7. Katz JN, Wright J, Spindler KP, et al. Predictors and outcomes of crossover to surgery from physical therapy for meniscal tear and osteoarthritis: a randomized trial comparing physical therapy and surgery. J Bone Joint Surg Am. 2016;98(22):1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000. [DOI] [PubMed] [Google Scholar]
- 9. Kraus NR, Lowenstein NA, Garvey KD, Matzkin EG. Smoking negatively effects patient-reported outcomes following arthroscopic partial meniscectomy. Arthrosc Sports Med Rehabil. 2021;3(2):e323-e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liebensteiner MC, Nogler M, Giesinger JM, et al. Cartilage degeneration and not age influences the health-related quality of life outcome after partial meniscectomy. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):26-31. [DOI] [PubMed] [Google Scholar]
- 11. Maroko AR, Doan TM, Arno PS, et al. Integrating social determinants of health with treatment and prevention: a new tool to assess local area deprivation. Prev Chronic Dis. 2016;13:E128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mongan JJ, Ferris TG, Lee TH. Options for slowing the growth of health care costs. N Engl J Med. 2008;358(14):1509-1514. [DOI] [PubMed] [Google Scholar]
- 13. OME Cleveland Clinic Orthopaedics. Implementing a scientifically valid, cost-effective, and scalable data collection system at point of care: The Cleveland Clinic OME Cohort. J Bone Joint Surg Am. 2019;101(5):458-464. [DOI] [PubMed] [Google Scholar]
- 14. Perfetti D, Atlas AM, Galina J, et al. Surgeon volume affects short- and long-term surgical outcomes in idiopathic scoliosis. Spine Deform. 2020;8(3):455-461. [DOI] [PubMed] [Google Scholar]
- 15. Roos EM. 3 steps to improve reporting and interpretation of patient-reported outcome scores in orthopedic studies: using the KOOS as an example. Acta Orthop. 2018;89(1):1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sinclair ST, Klika AK, Jin Y, et al. The impact of surgeon variability on patient-reported outcomes in total hip arthroplasty. J Arthroplasty. 2022;37(7):S479-S487. [DOI] [PubMed] [Google Scholar]
- 17. Singh A, Yian EH, Dillon MT, et al. The effect of surgeon and hospital volume on shoulder arthroplasty perioperative quality metrics. J Shoulder Elbow Surg. 2014;23(8):1187-1194. [DOI] [PubMed] [Google Scholar]
- 18. Sun LY, Boet S, Chan V, et al. Impact of surgeon and anaesthesiologist sex on patient outcomes after cardiac surgery: a population-based study. BMJ Open. 2021;11(8):e051192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wei S, Pierce O, Allen M. Surgeon scorecard. Online tool. ProPublica. 2015. Accessed April 17, 2023. https://projects.propublica.org/surgeons/
- 20. Winegar AL, Jackson LW, Sambare TD, et al. A surgeon scorecard is associated with improved value in elective primary hip and knee arthroplasty. J Bone Joint Surg Am. 2019;101(2):152-159. [DOI] [PubMed] [Google Scholar]
- 21. Yazdi H, Moradi A, Sanaie A, Ghadi A. Does the hyperextension maneuver prevent knee extension loss after arthroscopic anterior cruciate ligament reconstruction? J Orthop Traumatol. 2016;17(4):327-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yim J-H, Seon J-K, Song E-K, et al. A comparative study of meniscectomy and nonoperative treatment for degenerative horizontal tears of the medial meniscus. Am J Sports Med. 2013;41(7):1565-1570. [DOI] [PubMed] [Google Scholar]