Abstract
Pregabalin is the first-line treatment for neuropathic pain. Cases of cutaneous hypersensitivity reactions caused by pregabalin generally occur within 2 weeks of initiating medication. We report a rare case of a delayed cutaneous hypersensitivity reaction caused by pregabalin, which was confirmed by a drug provocation test. A 72-year-old man with severe herpes zoster neuralgia developed maculopapular drug eruption covering 80% to 90% of his total body surface area after 40 days of combined multidrug analgesia. A drug provocation test for pregabalin was positive. The time interval between initiating medication and the onset of the patient’s rash was the longest and he also had the largest area of skin affected compared with patients with a similar condition in previous related reports. Remaining vigilant for possible adverse cutaneous hypersensitivity reactions during treatment is important because of the long-term course of pregabalin treatment for neuropathic pain.
Keywords: Pregabalin, delayed cutaneous hypersensitivity reaction, maculopapular drug eruption, drug provocation test, case report, neuropathic pain, herpes zoster neuralgia
Introduction
Pregabalin is a commonly used antiepileptic medication and the first-line treatment for neuropathic pain. 1 This medication is a second-generation calcium channel modulator and is structurally similar to gabapentin. Pregabalin selectively binds to the α2-δ subunit of voltage-gated calcium channels and inhibits presynaptic calcium influx, which reduces the synaptic release of excitatory neurotransmitters, thereby exerting analgesic effects.2,3 Common adverse effects of pregabalin include somnolence, dizziness, peripheral nerve edema, and blurred vision. A hypersensitivity reaction is an uncommon side effect of pregabalin, occurring in 1/100 to 1/1000 patients. 4 Reported cases of cutaneous hypersensitivity reactions caused by pregabalin generally occur within 2 weeks of initiating medication.5–12
We report a rare case of a delayed cutaneous hypersensitivity reaction caused by pregabalin, which was confirmed by a drug provocation test. An elderly patient with severe herpes zoster (HZ) neuralgia developed maculopapular drug eruption covering 80% to 90% of the total body surface area after 40 days of combined multidrug analgesia. A drug provocation test for pregabalin was positive. The report conforms to the CARE guidelines. 13
Case report
A 72-year-old man was admitted to the Dermatology Department of the authors’ hospital with complaints of a 6-day history of right-sided facial and oral pain, accompanied by a facial rash for 4 days. The patient continued to report severe pain after undergoing a tooth extraction, describing the pain as “burning and tingling” that radiated to his upper lip and right eye. A dermatological examination showed scattered blisters in the second branch of the left trigeminal nerve. An ophthalmic examination revealed conjunctival congestion in the left eye with lid edema, but no evidence of herpetic keratitis or uveitis. He was diagnosed with HZ, and was prescribed acyclovir injection (0.5 g, every 8 hours), pregabalin capsules (75 mg, every 12 hours), compound ibuprofen and codeine phosphate tablets (0.2 g/12.5 mg, every 6 hours), compound oxycodone and acetaminophen tablets (5 mg/325 mg, every 6 hours), and acyclovir eye ointment (every 6 hours). The patient had no history of food or drug allergies, and no previous history of allergic diseases. He had a history of diabetes mellitus and his blood glucose concentrations were well controlled with gliquidone tablets (30 mg, every 8 hours). However, because the severe pain caused by HZ greatly affected his blood glucose concentrations, the hypoglycemic agent was changed to insulin glargine and insulin glulisine injections.
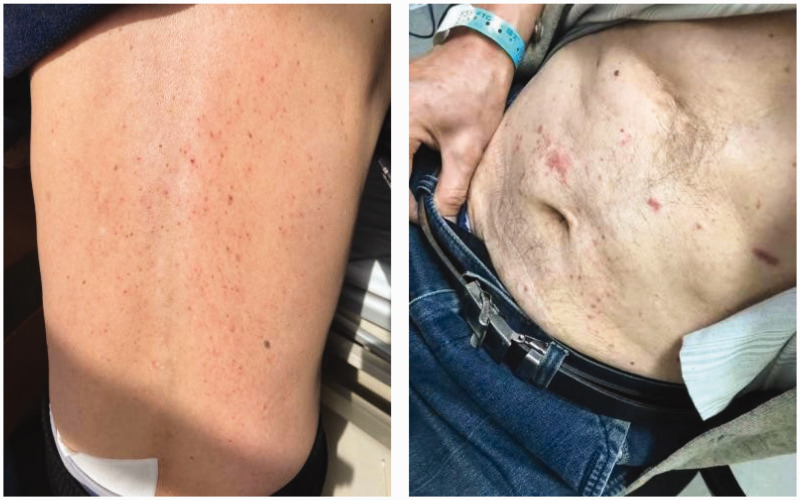
On day 17 after admission, the patient’s facial HZ was well controlled, although the pain did not greatly improve. Breakout pain occurred 7 to 10 times each day, with a pain score of 9 to 10 points on the visual analog scale. Each episode of pain persisted >10 minutes. The patient was transferred to the Department of Pain Management, and the analgesic regimen was adjusted to include a combination of pregabalin capsules (150 mg, every 12 hours), oxycodone hydrochloride prolonged-release tablets (10 mg, every 12 hours), morphine hydrochloride tablets (5 mg, as needed), and diclofenac sodium dual release enteric-coated capsules (75 mg, once daily). Despite these adjustments, the pain persisted with a visual analog scale score of 8 to 9 points while the rest pain was reduced. On day 23 after admission, he received infraorbital nerve blocks with 2.5 mL of 0.2% ropivacaine and diprospan (7 mg/mL), which resulted in temporary relief for several days. On day 39, the patient underwent subcutaneous peripheral nerve-field stimulation surgery, which targeted the maxillary branches of the trigeminal nerve. The patient’s pain was immediately relieved after the surgery, and the pain score was decreased to 2 to 3 points. On day 40 after admission, he developed a maculopapular rash scattered on his belly and back without pruritus or pain (Figure 1). He was treated with antihistamine ebastine tablets (10 mg, once daily). However, the maculopapular rash continued to progress and gradually spread to his trunk and limbs, affecting approximately 80% to 90% of his total body surface area (Figure 2). The maculopapular rash was symmetrically distributed and some rash fused without any blisters or bullae. The patient did not show any signs of systemic desquamation, Nikolsky’s sign, or skin necrosis. Regrettably, photographs of the rash were not captured without clothes. In addition, throughout the entire course of the patient’s rash, he did not report any other discomfort, such as gastrointestinal, respiratory, psychological, and cardiovascular symptoms, and no lymphadenopathy or mucosal lesions was observed. Furthermore, his vital signs, such as body temperature, pulse rate, respiration rate, and oxygen saturation, were normal. Laboratory tests showed that the patient's blood count (eosinophils, lymphocytes, monocytes, and platelets), liver function, kidney function, and complement levels were within the normal ranges. No reports have shown a correlation between known genetic polymorphisms and hypersensitivity reactions induced by these drugs. Therefore, genetic sequencing was not conducted.
Figure 1.
Photographs of a maculopapular rash on the 40th day after admission.
Figure 2.
Photographs of maculopapular rash on the 44th day after admission.
On day 44 of admission, all of the previously prescribed analgesic medications were discontinued. He was administered a diphenhydramine hydrochloride injection (20 mg, once daily), and the dose of methylprednisolone sodium succinate for injection was gradually increased from 40 to 80 mg each day. However, the rash was not effectively controlled. On day 47, a dermatologist prescribed recombinant human tumor necrosis factor receptor II: immunoglobulin (Ig) G Fc fusion protein (25 mg, twice weekly) to modulate inflammation and cellular immune responses, despite a lack of high-level evidence-based evidence. The patient consented to all of the above-mentioned treatment. After treatment with recombinant human tumor necrosis factor receptor II: IgG Fc fusion protein for 2 weeks, progression of the maculopapular rash was controlled. The patient continued to receive oral methylprednisolone and ebastine tablets after discharge.
Six months after discharge, the patient returned to the hospital for a follow-up because of slight postherpetic neuralgia after removal of the subcutaneous peripheral nerve-field stimulation device. A pain specialist prescribed pregabalin capsules. The patient developed a maculopapular rash on the trunk the same day after receiving 75 mg of pregabalin. Prior to this medical visit, he had no symptoms other than slight postherpetic neuralgia and had not been exposed to antihistamines, glucocorticoids, leukotriene antagonists, or novel immunosuppressive biologic agents such as anti-IgE monoclonal antibodies. Additionally, he had no history of smoking or alcohol consumption and did not smoke or drink before the drug provocation test. During the period from discharge to the occurrence of the second maculopapular rash, the patient had been consistently receiving the same medication for his underlying condition (diabetes), and the HZ had already healed. Unfortunately, images of the rash were not captured. Pregabalin was promptly discontinued and the rash rapidly dissipated after he was prescribed oral antihistamines. The patient was invited to undergo patch testing of pregabalin, but he declined because he did not want to be re-exposed to a drug that he considered potentially harmful. A timeline of the patient’s medications is shown in Figure 3. In a telephone follow-up 6 months later, he reported that he had not used any other medications except gliquidone tablets and had not experienced any other adverse reactions.
Figure 3.
Timeline of the patient’s medications. EBS, ebastine (10 mg once a day); DHM, diphenhydramine (20 mg once a day); MPS, methylprednisolone (40–80 mg once a day); CIC, compound ibuprofen and codeine (0.2 g/12.5 mg every 6 hours); COA, compound oxycodone and acetaminophen (5 mg/325 mg every 6 hours); DLF, diclofenac sodium (75 mg daily); PGB1, pregabalin (75 mg every 12 hours); PGB2, pregabalin (150 mg every 12 hours); ACVE, acyclovir eye ointment (every 6 hours); MPE, morphine (5 mg as required); ACVI, acyclovir injection (0.5 g every 8 hours); OCD, oxycodone (10 mg every 12 hours); rhTNFR: Fc, recombinant human tumor necrosis factor receptor II: immunoglobulin G Fc fusion protein (25 mg twice weekly); GQD, gliquidone (30 mg every 8 hours); IGG, insulin glargine; IGL, insulin glulisine; sPNFS, subcutaneous peripheral nerve-field stimulation.
The causal relationship between maculopapular drug eruptions and each medication was assessed according to the Naranjo ADR Probability Scale. After excluding common causes of hypersensitivity reactions such as allergic diseases, viral infections, food, and other medications, we concluded that the drug provocation test results for pregabalin were positive. The Naranjo scores for pregabalin, NSAIDs, and opioid analgesics were 6, 4, and 4, respectively.
Discussion
We present a case of an elderly patient with severe HZ neuralgia who was treated with a combination of analgesics with different mechanisms of action, including pregabalin, non-steroidal anti-inflammatory drugs (NSAIDs), and opioid analgesics. After 40 days of treatment, he experienced an adverse cutaneous drug reaction (ACDR) that affected approximately 80% to 90% of his total body surface area. The patient’s ACDR was a type B adverse drug reaction, and more specifically, was a delayed-type drug hypersensitivity reaction.14,15 According to the severity of clinical symptoms, the anaphylaxis was classified as Grade I. 16
The ACDR in our patient was diagnosed as maculopapular drug eruption on the basis of clinical symptoms and laboratory investigations. The histopathological results were nonspecific. Therefore, a routine skin biopsy was not performed for the diagnosis of maculopapular drug eruptions. 17 Maculopapular drug eruption is one of the most common forms of delayed-type drug reactions, and it usually occurs 1 to 2 weeks after initiating medication. Maculopapular drug eruption may include type IV immunological reactions and other mechanisms. 18 Notably, simultaneous use of multiple drugs is more likely to cause drug eruptions, although the specific mechanisms remain unclear.19,20
In this patient, the relationship between maculopapular drug eruptions and each medication was assessed using the Naranjo ADR Probability Scale. The Naranjo scores for pregabalin, NSAIDs, and opioid analgesics indicated a probable association between pregabalin and the ACDR, and a possible association between NSAIDS or opioid analgesics and the ACDR.
To the best of our knowledge, eight cases of cutaneous hypersensitivity reactions caused by pregabalin have been reported.5–12 Three of these cases occurred within 1 week,5,6,12 three within 2 weeks,7,9,10 and one within 4 weeks after initiating medication, 8 and the time interval was not reported in one case. 11 In patients who are not previously sensitized, the onset of maculopapular drug eruption occurs within 4 to 14 days after the start of exposure, but rarely occurs at weeks to months. 21 In this patient, the latency period was 40 days, which may have been due to a difference in drug metabolism or polymorphisms in some unknown gene. Our case had the longest time interval between initiating pregabalin and the onset of the hypersensitivity reaction, and the largest skin area of involvement compared with other cases.5–12 These findings in our case were attributed to pregabalin and confirmed by a drug provocation test. Gabapentinoids are first-line drugs for treating HZ neuralgia.1,22 Pregabalin and gabapentin have similar chemical structures and are neither metabolized by the liver nor bound to plasma proteins. Therefore, unlike classical antiepileptic drugs, these drugs rarely cause hypersensitivity reactions through pharmacological interactions. Classical antiepileptic drugs may cause cross-hypersensitivity reactions.23,24 However, because of the different chemical structures of gabapentinoids and classic antiepileptic drugs, whether pregabalin and gabapentin have the potential to cause cross-hypersensitivity reactions remains unclear. Based on positive results of the pregabalin provocation test, which is the gold standard for the diagnosis of drug hypersensitivity reactions, patients should avoid using pregabalin in the future. The reason for this recommendation is because delayed hypersensitivity reactions may involve T-cell mediation, and re-exposure to pregabalin could potentially lead to more severe hypersensitivity reactions in a shorter period than the previous exposure. In our case, tricyclic antidepressants, such as amitriptyline, could be an alternative option for neuropathic pain. 22
If our patient’s hypersensitivity reaction was caused by an NSAID, based on clinical manifestations, this hypersensitivity reaction would be classified as a single NSAID-induced delayed hypersensitivity reaction. This reaction is non-cross-intolerant because its pathophysiological mechanism is unrelated to cyclooxygenase-1 inhibition.25,26 Therefore, when patients require NSAIDs, chemical structures different from those of ibuprofen and sodium diclofenac, such as etofenamate, celecoxib, and meloxicam, are preferred.
An association between hypersensitivity and opioid analgesics does not appear to be likely in our patient because there have been few reports of immune-mediated hypersensitivity reactions to opioid medications of types I, II, III, and IV. Many reactions are actually pseudo-allergic, which are provoked by direct cutaneous mast cell degranulation and subsequent histamine release, manifesting as urticaria, wheals, and itching.27,28 Owing to the infrequent occurrence of immune-mediated hypersensitivity reactions caused by opioid medications, there is a lack of reports addressing clinical and laboratory diagnostic methods, symptoms of different types of hypersensitivity reactions, cross-reactivity between natural, semi-synthetic, and fully synthetic opioids, and the appropriate selection of alternative drugs in cases in which sensitivity to ≥1 opioid is known.
Conclusion
We report a rare case of a delayed cutaneous hypersensitivity reaction caused by pregabalin, which was confirmed by a drug provocation test. This reaction occurred 40 days after initiating medication and involved 80% to 90% of the body surface area. Pregabalin treatment for neuropathic pain can be a long-term course. Therefore, remaining vigilant for possible adverse cutaneous hypersensitivity reactions, particularly when multiple medications are used in combination, is important.
Acknowledgement
The authors are grateful to the patient for providing consent to publish the details of his case.
Author contributions: XF and LL designed the study. WW and QJ collected the patient’s clinical information. YL analyzed the data. MZ and HY reviewed the literature. MZ and KD drafted the manuscript. XF, LL, and YL revised the manuscript. All authors read and approved the final manuscript.
The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Liang Liu https://orcid.org/0000-0003-3831-2352
Data availability statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Ethics statement
The study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University (approval number: 2022110 K). Written informed consent was obtained from the patient for publication of this case report. All of the patient’s details were de-identified.
References
- 1.Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol 2010; 17: 1113–e88. [DOI] [PubMed] [Google Scholar]
- 2.Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res 2007; 73: 137–150. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Taylor CP, Weber M, et al. Pregabalin is a potent and selective ligand for α(2)δ-1 and α(2)δ-2 calcium channel subunits. Eur J Pharmacol 2011; 667: 80–90. [DOI] [PubMed] [Google Scholar]
- 4.Electronic medicines compendium. Lyrica 75 mg hard capsules, summary of product characteristics. Updated February 28: https://www.medicines.org.uk/emc/product/10302/smpc/print. (2023, accessed 11 October 11 2023).
- 5.Jaiswal S, Chakrabarti SS, Rai T, et al. Pregabalin Induced Maculopapular Eruption in an Elderly Male. Curr Drug Saf 2022; 17: 393–398. [DOI] [PubMed] [Google Scholar]
- 6.Khazaka M, Laverdière J, Bouchard A, et al. Identification of Possible Causative Agents in a Polymedicated Patient Presenting With Toxic Epidermal Necrolysis. J Pharm Pract 2021; 34: 970–974. [DOI] [PubMed] [Google Scholar]
- 7.Sahota S, Parry RG, Gentile G. Pregabalin-induced urticarial rash and neutropenia in a renal transplant recipient: a case report. BMC Nephrol 2019; 20: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gómez Torrijos E, Moreno Lozano L, Extrmera Ortega AM, et al. First case of skin allergy to pregabalin with positive patch test reaction. Contact Dermatitis 2019; 81: 78. [DOI] [PubMed] [Google Scholar]
- 9.Inoue A, Sawada Y, Ohmori S, et al. Maculopapular type drug eruption caused by pregabalin: A case and literature review. Allergol Int 2016; 65: 351–352. [DOI] [PubMed] [Google Scholar]
- 10.Bamanikar A, Dhobale S, Lokwani S. Pregabalin hypersensitivity in a patient treated for postherpetic neuralgia. Indian J Pharmacol 2013; 45: 522–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortega-Camarero MA, Avila R, Prados Castaño M, et al. Challenge-based pregabalin induced urticaria and angioedema. A case report. Allergol Immunopathol (Madr) 2012; 40: 323. [DOI] [PubMed] [Google Scholar]
- 12.Smith TL, Baldwin A, Cunningham LL, Jr, et al. Rash associated with pregabalin use. Ann Pharmacother 2008; 42: 1899–1902. [DOI] [PubMed] [Google Scholar]
- 13.Gagnier JJ, Kienle G, Altman DG, CARE Group et al. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 14. International drug monitoring: the role of national centres. Report of a WHO meeting. World Health Organ Tech Rep Ser 1972; 498: 1–25. [PubMed] [Google Scholar]
- 15.Davies DM, Ashton CH, Rao JG, et al. Comprehensive clinical drug information service: first year's experience. Br Med J 1977; 1: 89–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ring J, Behrendt H, De Weck A. History and classification of anaphylaxis. Chem Immunol Allergy 2010; 95: 1–11. [DOI] [PubMed] [Google Scholar]
- 17.Seitz CS, Rose C, Kerstan A, et al. Drug-induced exanthems: correlation of allergy testing with histologic diagnosis. J Am Acad Dermatol 2013; 69: 721–728. [DOI] [PubMed] [Google Scholar]
- 18.Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med 2003; 139: 683–693. [DOI] [PubMed] [Google Scholar]
- 19.Schaub N, Bircher AJ. Severe hypersensitivity syndrome to lamotrigine confirmed by lymphocyte stimulation in vitro. Allergy 2000; 55: 191–193. [DOI] [PubMed] [Google Scholar]
- 20.Pichler WJ, Daubner B, Kawabata T. Drug hypersensitivity: flare-up reactions, cross-reactivity and multiple drug hypersensitivity. J Dermatol 2011; 38: 216–221. [DOI] [PubMed] [Google Scholar]
- 21.Brockow K, Wurpts G, Trautmann A, et al. Guideline for allergological diagnosis of drug hypersensitivity reactions: S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) in cooperation with the German Dermatological Society (DDG), the Association of German Allergologists (ÄDA), the German Society for Pediatric Allergology (GPA), the German Contact Dermatitis Research Group (DKG), the German Society for Pneumology (DGP), the German Society of Otorhinolaryngology, Head and Neck Surgery, the Austrian Society of Allergology and Immunology (ÖGAI), the Austrian Society of Dermatology and Venereology (ÖGDV), the German Academy of Allergology and Environmental Medicine (DAAU), and the German Documentation Center for Severe Skin Reactions (dZh). Allergol Select 2023; 7: 122–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan T, Barry P, Reken S; Guideline Development Group et al. Pharmacological management of neuropathic pain in non-specialist settings: summary of NICE guidance. BMJ 2010; 340: c1079. [DOI] [PubMed] [Google Scholar]
- 23.Mani R, Monteleone C, Schalock PC, et al. Rashes and other hypersensitivity reactions associated with antiepileptic drugs: A review of current literature. Seizure 2019; 71: 270–278. [DOI] [PubMed] [Google Scholar]
- 24.Wang XQ, Lang SY, Shi XB, et al. Cross-reactivity of skin rashes with current antiepileptic drugs in Chinese population. Seizure 2010; 19: 562–566. [DOI] [PubMed] [Google Scholar]
- 25.Kowalski ML, Makowska JS, Blanca M, et al. Hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) - classification, diagnosis and management: review of the EAACI/ENDA(#) and GA2LEN/HANNA*. Allergy 2011; 66: 818–829. [DOI] [PubMed] [Google Scholar]
- 26.Laidlaw TM, Cahill KN. Current Knowledge and Management of Hypersensitivity to Aspirin and NSAIDs. J Allergy Clin Immunol Pract 2017; 5: 537–545. [DOI] [PubMed] [Google Scholar]
- 27.Baldo BA. Toxicities of opioid analgesics: respiratory depression, histamine release, hemodynamic changes, hypersensitivity, serotonin toxicity. Arch Toxicol 2021; 95: 2627–2642. [DOI] [PubMed] [Google Scholar]
- 28.Baldo BA. Opioid Analgesic Drugs: Misuse, Toxicity, and Hypersensitivity. J Allergy Clin Immunol Pract 2017; 5: 1607–1608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.