Abstract
The technique known as intravital microscopy (IVM), when used in conjunction with transgenic mice expressing fluorescent proteins in various cell populations, is a powerful tool with the potential to provide new insights into host-pathogen interactions in infectious disease pathogenesis in vivo. Yersinia pestis, the causative agent of plague, is typically deposited in a host’s skin during feeding of an infected flea. IVM has been used to characterize the innate immune response to Y. pestis in the skin and identify differences between the responses to needle-inoculated and flea-transmitted bacteria that would have been difficult, if not impossible, to detect by other means. Here we describe techniques used to image the neutrophil response to flea-transmitted Y. pestis in the dermis of live mice using conventional confocal microscopy.
Keywords: Intravital microscopy, Confocal microscopy, Skin, Yersinia pestis, Xenopsylla cheopis, Flea
1. Introduction
The disease plague, caused by the Gram-negative bacterium Yersinia pestis, can take three distinct forms, bubonic, pneumonic, and primary septicemic, with bubonic plague being the most common presentation in humans. Plague remains endemic in many countries around the world, where it is maintained in flea-rodent enzootic cycles with occasional outbreaks among humans after contact with infected animals or their fleas. Fleas become infected with Y. pestis by feeding on a bacteremic rodent and can transmit the bacteria to another host during subsequent feedings. Fleas deposit Y. pestis into the skin, where it adapts to conditions in the mammalian host and survives the host’s innate immune response. The bacteria eventually disseminate from the skin, via the lymphatics, to the regional draining lymph node. There, the pathogen replicates to high numbers, resulting in a large, swollen, painful lymph node called a bubo. There is a need for better understanding of host-Y. pestis interactions in the skin early after infection and how transmission of the bacteria by the natural route, the bite of an infected flea, might affect these interactions.
Numerous published studies describe the use of intravital microscopy to image host-pathogen interactions in the skin in a variety of animal models of infectious disease (reviewed in [1]). The bulk of these studies use multiphoton microscopy due to its ability to image tissues at depths of >100 μm. However, the skin of a mouse ear pinna is very thin, approximately 50–60 μm, allowing for imaging of fluorescently labeled cells and bacteria by conventional single-photon confocal microscopy. The main advantage of this approach is that conventional confocal microscopes are much more widely available than the more sophisticated and costly 2-photon systems. Additionally, even at research institutions that possess multiphoton microscopes, this equipment can be in high demand and getting access for the large blocks of time necessary for intravital experiments can be difficult. Thus, the ability to do this type of experiment with a conventional confocal microscope might allow more researchers to explore the use of IVM to study their model of interest.
A large number of transgenic mice have been created, many commercially available, that constitutively express fluorescent proteins in a variety of cell types relevant to the study of infections in the skin, including neutrophils [2], monocytes/macrophages [3, 4], dendritic cells [4, 5], Langerhans cells [5], and gamma-delta T-cells [6]. Lymphocytes can also be isolated, fluorescently labeled ex vivo, and adoptively transferred into animals to allow intravital imaging [7]. Additionally, fluorophore-conjugated antibodies or chemical labels can be injected into mice prior to IVM, permitting imaging of cell populations or tissues for which the use of transgenic mice is not possible or practical [8].
Here we describe the imaging of bacteria transmitted via the natural route of infection, the bite of an infected flea. These techniques permit the qualitative and quantitative assessment of neutrophil recruitment and bacteria-neutrophil interactions within the skin after Y. pestis infection [9, 10]. In addition to their utility in the evaluation of the innate cellular response to Y. pestis, these techniques may be adapted to the study of a variety of other pathogens transmitted by other arthropod vectors.
2. Materials
2.1. Reagents and Equipment
Brain-heart Infusion (BHI) broth.
Carbenicillin (100 mg/ml in 50% ethanol stock solution).
Blood agar plates.
Phosphate-buffered Saline (PBS), pH 7.2.
Sterile distilled water (ddH2O).
Coverslips (60 mm × 24 mm).
Coverslip forceps.
Membrane feeder device (Wade and Georgi [11]).
Heated waterbath.
Peristaltic pump and 2 lengths (approx. 1 meter each) of plastic tubing.
Flea storage capsules [11].
Custom plastic clip-on ear feeding device.
Ketamine (13.9 mg/ml)/Xylazine (0.56 mg/ml) solution for injection.
Sytox Blue fluorescent DNA stain (ThermoFisher, 1:25 dilution of 1 mM stock solution in PBS).
Qtracker 655 vascular dye (ThermoFisher).
Microscope stage insert able to accommodate a 60 mm × 24 mm coverslip.
Medical adhesive tape.
Isoflurane.
Electric heating pad.
Image analysis software such as Imaris (BitPlane).
2.2. Instruments
An inverted confocal microscope with incubator chamber surrounding the entire microscope stage and lasers/detectors setup for imaging the desired fluorophores (blue [Sytox Blue], green [eGFP], red [mCherry], and far red [Qdot655 or Alex647]). We use a Zeiss LSR880 confocal microscope system.
Dissecting microscope with a chill table and external light source.
Isoflurane vaporizer anesthesia apparatus with induction chamber and small rodent breathing circuit and nose cone. We use the RC2 rodent circuit controller (VetEquip).
2.3. Organisms
Bacterial strains: Yersinia pestis strains constitutively expressing fluorescent protein suitable for confocal microscopy. We use the attenuated, BSL2 strain KIM6+ (pCD1 virulence plasmidnegative, pigmentation-positive) of Y. pestis transformed with the plasmid pMcherry [9].
-
Mice: This type of study requires an animal-use protocol describing the techniques to be used and approved by an institutional review board. The animal studies described here were performed in accordance with a protocol (#2012-040) approved by the Animal Care and Use Committee, Rocky Mountain Laboratories, NIAID, NIH. Transgenic mice expressing fluorescent protein in the cell population of interest are required. Here we use LysozymeM-eGFP transgenic mice expressing high levels of eGFP in neutrophils, originally described by Faust et al. [2].
Normal, inbred, or outbred mice to serve as a source of both heparinized mouse blood and fresh mouse skins for flea infections.
Fleas: The methods described here use the rat flea Xenopsylla cheopis. These fleas were reared in a breeding colony maintained at Rocky Mountain Laboratories. Detailed methods for the establishment and maintenance of flea colonies are described elsewhere [12].
3. Methods
3.1. Infection of Fleas with Y. pestis Expressing mCherry
Inoculate 4 ml of BHI broth containing 100 μg/ml carbenicillin with KIM6+ pMcherry from frozen stock.
Incubate overnight at 28 °C without aeration.
Use 1 ml from this overnight culture to inoculate 100 ml of fresh BHI broth containing 100 μg/ml carbenicillin.
Incubate overnight (approximately 18 h) at 37 °C without aeration.
Harvest bacteria by centrifugation, resuspend pellet in 1 ml BHI, and store on ice.
Prepare the artificial flea feeding device as described by Wade and Georgi [11], Hinnebusch et al. [13], and Bland et al. [12]. Briefly, affix a mouse hide to the glass feeding device with rubber bands and remove hair from the skin using an electric shaver. Attach hosing from 40 °C water bath, through a peristaltic pump to the feeding device. Attach the return hosing to the water bath. Turn on pump to bring the feeding device up to approximately 37 °C. Add 5 ml sterile heparinized mouse blood to the feeding device. Inoculate blood to approximately 1 × 109 KIM6+ pMcherry bacteria/ml by adding 1 ml bacterial suspension from step 5 (see Note 1). Collect approximately 300 X. cheopis fleas that have been starved for 4 days, chill fleas on ice, transfer fleas to plastic capsule, and affix to mouse skin side of the glass feeding device using tape. Allow fleas to feed for a minimum of 1 h. Collect all fleas in a 50 ml conical tube, chill on ice and use a dissecting microscope to select fleas that have fed, based on the presence of fresh blood in the digestive tract.
House the fed fleas in plastic flea capsules at 21 °C, 75% relative humidity. Provide the fleas with maintenance feedings on mice or artificial feeder every 3 to 4 days.
Assess flea blockage immediately after maintenance feeds. Collect fleas into plastic tube and place tube on ice until fleas are immobilized. Use a dissecting microscope with high quality optics placed over a chill table to identify fleas that have fresh, bright red blood in their esophagus, but not in their midgut, indicating that the proventricular valve has been “blocked” by a Y. pestis biofilm [12].
Store these blocked fleas in a plastic flea capsule overnight at 21 °C, 75% humidity. Use these blocked fleas for transmission experiments within 24 h of blockage diagnosis.
3.2. Feeding of Infected Fleas on Mouse Ears
Immobilize the blocked fleas by chilling on ice.
Place 1 to 10 fleas in custom plastic clip-on feeding chamber (Fig. 1a) (see Note 2). Store the chamber on ice until ready to feed fleas on a mouse ear.
Anesthetize the mouse to be used for intravital microscopy by subcutaneous injection (s.c.) of the ketamine/xylazine mixture (see Note 3).
To facilitate the later identification of fleabites on the mouse ear, inject the mouse intraperitoneally with 100 μl of Sytox Blue in PBS within ten to 20 min of flea feeding (see Note 4). Sytox Blue is a noncell permeant DNA stain that will stain the nuclei of cells at the bite site due to the dermal cell membrane damage caused by the flea mouthparts.
When the mouse is sufficiently anesthetized, place it on an electric heating pad on lowest setting to maintain body temperature (approximately 30 °C). Apply the clip-on feeding chamber to one of the mouse’s ears (Fig. 1b). As the tube warms to room temperature, the fleas will become active and attempt to feed. Allow fleas access to the mouse ear for at least 20 min, ideally closer to 40 min (see Note 5).
To terminate feeding, place entire mouse in a chamber containing ~2% isoflurane. Fleas will be anesthetized quickly upon isoflurane exposure. Remove clip-on chamber and collect fleas from ear surface.
The mouse can be returned to a temporary cage while the fleas are checked for feeding.
Examine the anesthetized fleas using a dissecting microscope to determine the number of fleas that fed. The presence of fresh, bright red blood in the esophagus indicates a feeding attempt.
Fig. 1.
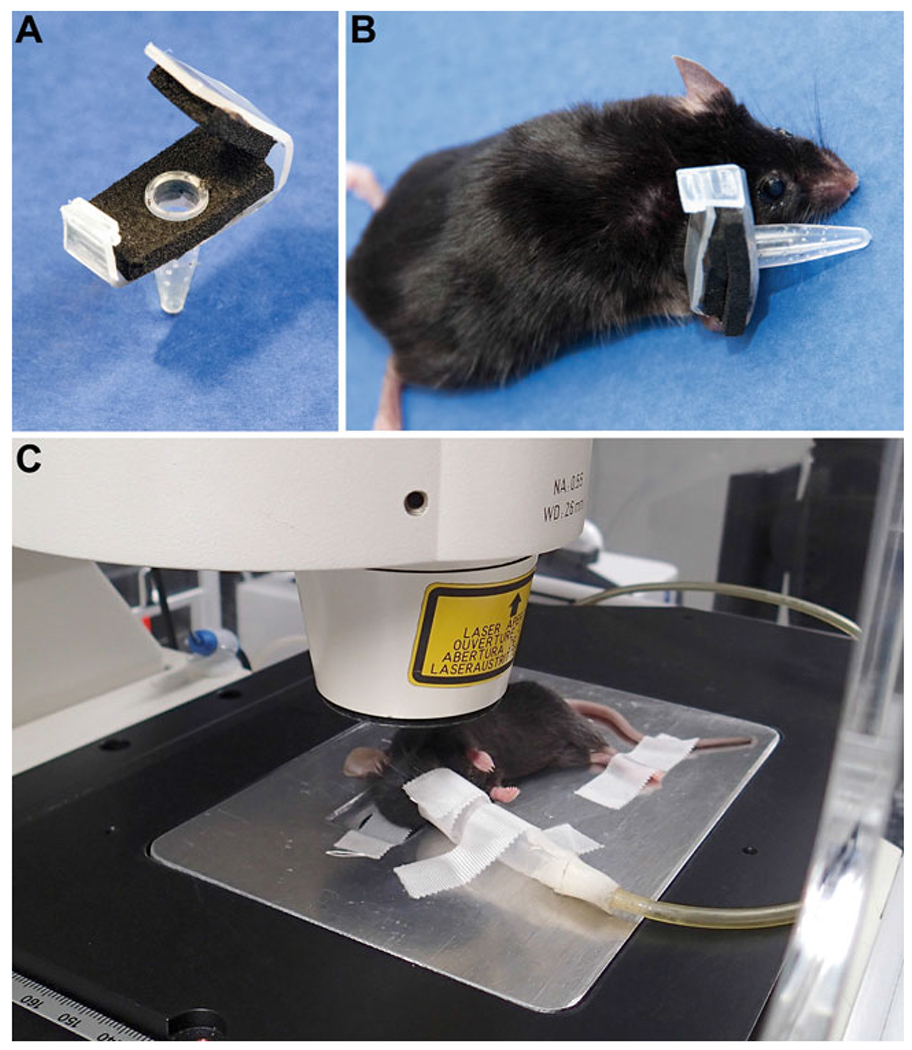
Clip-on device for feeding fleas on mouse ears and microscope stage insert set up. (a) The clip-on device is constructed using a 200 μl clear PCR tube, adhesive backed foam padding, and plastic cut from the lid of a pipette tip box. Several small holes are pierced in the tube using a 30-gauge needle to allow for air circulation. (b) Fleas are placed in the device before it is clipped onto the ventral surface of the ear of a ketamine/xylazine-anesthetized mouse. (c) After flea feeding, the mouse is switched to isoflurane anesthesia delivered via nosecone. The ear is gently pressed and spread out, ventral side down, onto a coverslip in the metal stage insert and secured in place with adhesive tape. The hind legs and nosecone tubing are secured to the stage insert with adhesive tape. The stage insert is placed within the incubated chamber and secured into the stage. Figure is reprinted from Shannon et al., PLOS Pathogens [9]
3.3. Preparation of a Mouse for Imaging by Intravital Confocal Microscopy
Once it has been confirmed that at least one blocked flea has fed on the mouse ear, anesthetize the mouse by placing it in the induction chamber of an Isoflurane vaporizer anesthesia apparatus set to administer 3–4% isoflurane in oxygen (see Note 6). When the mouse has reached a deep plane of anesthesia, it can be transferred to a breathing circuit with nosecone and the isoflurane can be reduced to 1.5–2% (see Note 7).
Attach a 60 mm × 24 mm coverslip to the microscope stage insert. Move the anesthetized mouse to the stage insert with the ear to be imaged facing down toward the coverslip. Secure the isoflurane tubing and nosecone to the stage insert with adhesive tape so that the nosecone remains over the mouse’s nose.
Using a cotton swab, thoroughly moisten the ventral surface of the ear to be imaged with sterile ddH2O and gently place the ear against the coverslip. Use a dry cotton swab to smooth the ear out and ensure as much of the ear as possible is in contact with the coverslip. Use a piece of medical adhesive tape over the ear to secure it to the coverslip. Immobilize the mouse’s legs to the stage insert using additional strips of tape (Fig. 1c).
Place the stage insert into the microscope stage. Set the incubated chamber enclosing the microscope to 30 °C (see Note 8).
3.4. Identifying and Imaging Flea Bite Sites in the Mouse Ear
Using a 10× or 20× objective and an epifluorescence filter set compatible with the 444/480 nm excitation/emission wavelengths of Sytox Blue, the ear can be visually scanned for areas of tissue damage indicative of a flea feeding (see Note 9). Nuclei of cells damaged by a flea’s mouthparts probing the skin will stain bright blue. The staining pattern will vary from a single stab of the mouthparts into the skin (as shown in Fig. 2), to what appears to be the result of multiple probes of the same area (not shown). If the mouse was also injected intravascularly (i.v.) with a vascular dye such as Qtracker655 prior to flea feeding, vascular leakage in close proximity to the Sytox Blue staining confirms the location is a flea bite site (Fig. 2).
Once a fleabite has been identified, use the software associated with the particular confocal microscope system to collect a single Z-stack containing the Sytox Blue, GFP, mCherry (see Note 10), and Far Red (if used) emission channels. Parameters such as laser voltage, gain, confocal pinhole size, and Z-step size will require optimization for the specific microscope and fluorophores used.
Once the various parameters have been optimized, use the software to collect a time series of Z-stacks at the desired interval and duration (see Note 11).
An example of the type of images of flea-transmitted Y. pestis obtained by this method is shown in Fig. 3. Blocked fleas were allowed to feed on the mouse ear and a flea bite site was identified by Sytox Blue staining. This particular flea bite deposited approximately hundreds of Y. pestis. Neutrophil recruitment over the first 1.5 h of imaging can be seen when compared to the 0 h time point (Fig. 3, lower panels).
Fig. 2.
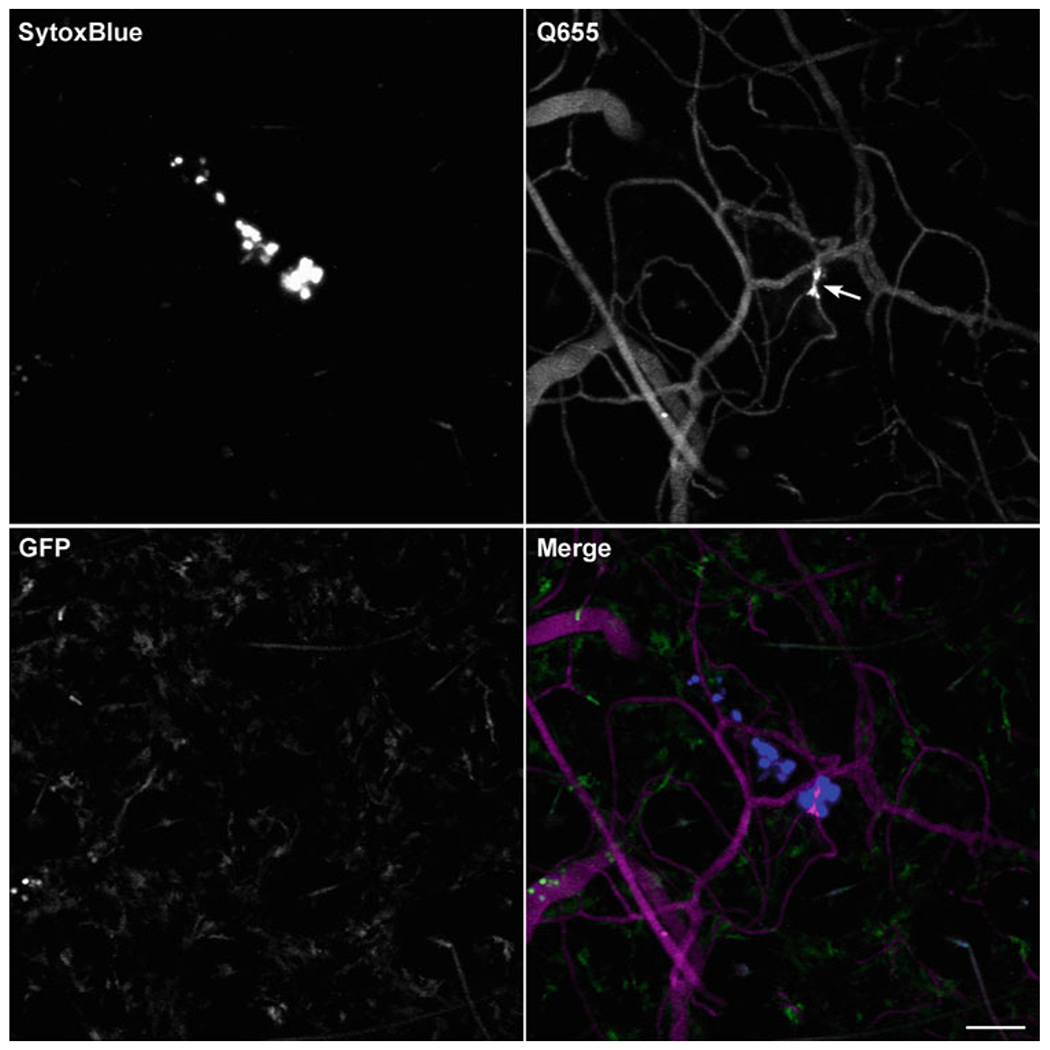
Use of the fluorescent DNA stain Sytox Blue to identify uninfected flea bite sites on mouse ears. Confocal images of the ear of a LysozymeM-eGFP mouse injected intraperitoneally (i.p.) with Sytox Blue and i.v. with Qtracker 655 vascular dye prior to being fed upon by uninfected flea. Imaris software was used to make maximum intensity projections of a Z-stack for each individual channel (blue, far red [shown as magenta in merge], and green) and a merged image. Nuclei of skin cells damaged by a flea’s mouthparts during feeding stain brightly with Sytox Blue. The arrow in the Q655 image indicates leakage of the dye from a blood vessel damaged during flea feeding. A few bright green neutrophils can be seen within a blood vessel at the left side of the image in the GFP channel. The GFP positive, but dim cells are likely tissue-resident macrophages. Scale bar equals 70 μm
Fig. 3.
Imaging neutrophils at a flea bite site containing flea-transmitted Y. pestis. Confocal images of the ear of a LysozymeM-eGFP mouse injected i.p. with Sytox Blue prior to being fed upon by fleas infected (blocked) with Y. pestis expressing mCherry. Imaris software was used to make maximum intensity projections of a Z-stack for the Sytox Blue and mCherry channels (upper panels). Merged images of the blue, red and green (GFP) channels show the 0 h and 1.5 h time points (lower panels). This flea bite site was identified by the Sytox Blue staining. The dashed ellipse in the red channel indicates an area where Y. pestis were deposited in the skin (note that hair and hair follicles autofluoresce in the red channel). Only a few GFP-bright neutrophils can be seen in the green channel at 0 h; large numbers of neutrophils were recruited to this bite site by the 1.5 h time point. Scale bar equals 70 μm
3.5. Image Processing and Analysis
The image files can be opened within software platforms like Imaris, where the results can be converted to maximum intensity projections or three dimensional still images, montages or movie files suitable for presentation and publication (Fig. 3).
The Imaris software can also quantify parameters like bacteria-neutrophil colocalization/interaction, or neutrophil recruitment, migration speed, and directionality as described in the developer’s instructions [9] (see Note 12).
Acknowledgements
We thank Clayton Jarrett, Chris Bosio, Dave Bland, Dustin Van Hofwegen, Adelaide Miarinjara, and Ashley Schwarzer for their careful review of the manuscript, David Dorward and Vinod Nair for assistance with confocal microscopy, and Anita Mora for assistance with graphic arts. This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
4 Notes
Following the procedure as described in steps 5 and 6 typically results in bacterial concentrations in the infectious blood meal ~1 × 109/ml. A small aliquot of blood should be set aside, diluted, and plated on blood agar plates to determine actual concentration of colony-forming units in the blood meal.
We construct this clip-on feeding device by gluing together a 200 μl clear PCR tube, adhesive-backed foam padding, and some plastic cut from the lid of a pipette tip box (Fig. 1a). Alternatively, we have had success cutting the tip off of a 10 ml disposable syringe and gluing a fine mesh fabric, with openings large enough for fleas to feed through, across the opening [14]. The mesh end of this syringe feeder can be pressed against the ear of the anesthetized mouse allowing the fleas to feed.
Ketamine/xylazine mixture is used for anesthesia because fleas are highly sensitive to isoflurane, and use of isoflurane delivered to the mouse by nosecone can result in inadvertent anesthesia of the fleas placed on the ear. Inject 100 μl/20 g mouse weight of ketamine/xylazine mixture s.c.
When first establishing this method, i.v. injection of a vascular dye like Qtracker 655 prior to flea feeding can help confirm that the Sytox Blue is staining flea bite sites. Qtracker 655 will allow visualization of the vasculature in the far red fluorescence channel and small areas of blood vessel damage can be identified by leakage of the dye. Flea feeding will result in a small amount of vascular leakage at the bite site as can be seen in Fig. 2.
This method typically results in a plane of anesthesia deep enough to permit flea feeding for a minimum of 40 min. Blocked fleas are unable to ingest a blood meal and will become weak and dehydrated, and thus can be more reluctant to feed than uninfected fleas. Allowing 4 to 5 blocked fleas access to the mouse ear for 40 min may be required to ensure at least 1 flea will attempt to feed.
For initial experiments, it may be better to use uninfected fleas to verify that flea bites can be identified using the Sytox Blue method.
Periodically monitor respiration rate/effort and pedal reflex to evaluate depth of anesthesia according to IACUC guidelines.
If the microscope does not have an incubated chamber, a heated stage insert may be adapted for maintaining mouse body temperature. If neither is available, the heating pad used during flea feeding can be placed over the mouse on the stage.
Sytox Blue fluorescence can be visualized with filter sets used for DNA stains like DAPI or Hoescht; however, these filters typically are suboptimal for the peak emission (480 nm) of Sytox Blue. Use of a filter set specifically suited for this dye will improve results.
Small hairs on the surface of the mouse ear as well as hair follicles will autofluoresce in multiple channels, but predominantly in the red channel, as is evident in Fig. 3.
The interval between scans will be determined by the cell type being studied and the types of post-acquisition analyses to be applied to the images. Scan intervals between one and two minutes are typically sufficient to track neutrophil accumulation and interaction with bacteria. Shorter intervals may be required to trace the movement of individual cells over time.
Tutorials available as part of these software packages are highly informative and cover quantitative image analyses beyond the scope of what can be discussed here.
References
- 1.Jain R, Weninger W (2013) Shedding light on cutaneous innate immune responses: the intravital microscopy approach. Immunol Cell Biol 91(4):263–270. 10.1038/icb.2012.76 [DOI] [PubMed] [Google Scholar]
- 2.Faust N, Varas F, Kelly LM, Heck S, Graf T (2000) Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood 96(2):719–726 [PubMed] [Google Scholar]
- 3.Burnett SH, Kershen EJ, Zhang J, Zeng L, Straley SC, Kaplan AM, Cohen DA (2004) Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukoc Biol 75(4):612–623. 10.1189/jlb.0903442 [DOI] [PubMed] [Google Scholar]
- 4.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR (2000) Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20(11):4106–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC (2004) Visualizing dendritic cell networks in vivo. Nat Immunol 5(12):1243–1250. 10.1038/ni1139 [DOI] [PubMed] [Google Scholar]
- 6.Unutmaz D, Xiang W, Sunshine MJ, Campbell J, Butcher E, Littman DR (2000) The primate lentiviral receptor Bonzo/STRL33 is coordinately regulated with CCR5 and its expression pattern is conserved between human and mouse. J Immunol 165(6):3284–3292 [DOI] [PubMed] [Google Scholar]
- 7.Benson RA, Brewer JM, Garside P (2017) Visualizing and tracking T cell motility in vivo. Methods Mol Biol 1591:27–41. 10.1007/978-1-4939-6931-9_3 [DOI] [PubMed] [Google Scholar]
- 8.McArdle S, Mikulski Z, Ley K(2016) Live cell imaging to understand monocyte, macrophage, and dendritic cell function in atherosclerosis. J Exp Med 213(7):1117–1131. 10.1084/jem.20151885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shannon JG, Bosio CF, Hinnebusch BJ (2015) Dermal neutrophil, macrophage and dendritic cell responses to Yersinia pestis transmitted by fleas. PLoS Pathog 11(3):e1004734. 10.1371/journal.ppat.1004734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shannon JG, Hasenkrug AM, Dorward DW, Nair V, Carmody AB, Hinnebusch BJ (2013) Yersinia pestis subverts the dermal neutrophil response in a mouse model of bubonic plague. MBio 4(5):e00170–e00113. 10.1128/mBio.00170-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wade SE, Georgi JR (1988) Survival and reproduction of artificially fed cat fleas, Ctenocephalides felis Bouché (Siphonaptera: Pulicidae). J Med Entomol 25:186–190 [DOI] [PubMed] [Google Scholar]
- 12.Bland DM, Brown LD, Jarrett CO, Hinnebusch BJ, Macaluso KR (2017) Methods in flea research. Biodefense and emerging infections research resources repository. https://www.beiresources.org/Catalog/VectorResources.aspx
- 13.Hinnebusch BJ, Perry RD, Schwan TG (1996) Role of the Yersinia pestis hemin storage (hms) locus in the transmission of plague by fleas. Science 273(5273):367–370 [DOI] [PubMed] [Google Scholar]
- 14.Bosio CF, Viall AK, Jarrett CO, Gardner D, Rood MP, Hinnebusch BJ (2014) Evaluation of the murine immune response to Xenopsylla cheopis flea saliva and its effect on transmission of Yersinia pestis. PLoS Negl Trop Dis 8(9):e3196. 10.1371/journal.pntd.0003196 [DOI] [PMC free article] [PubMed] [Google Scholar]


