Abstract
Pedigree analysis, clinical, gross, microscopic, ultrastructural, and lipidomic findings in four female superb bird-of-paradise (SBOP, Lophorina superba) siblings led to the diagnosis of a primary inherited glycerolipid storage disease, similar to neutral lipid storage disease with myopathy in humans. These birds were the offspring of a related breeding pair (inbreeding coefficient = 0.1797) and are the only known SBOPs to display this constellation of lesions. The birds ranged from 0.75 to 4.3 years of age at the time of death. Two birds were euthanized and one died naturally due to the disease, and one died of head trauma with no prior clinical signs. Macroscopic findings included hepatomegaly and pallor (4/4), cardiac and renal pallor (2/4), and coelomic effusion (1/4). Microscopic examination found marked tissue distortion due to cytoplasmic lipid vacuoles in hepatocytes (4/4), cardiomyocytes (4/4), renal tubular epithelial cells (4/4), parathyroid gland principal cells (2/2), exocrine pancreatic cells (3/3), and the glandular cells of the ventriculus and proventriculus (3/3). Ultrastructurally, the lipids were deposited in single to coalescing or fused droplets lined by an inconspicuous or discontinuous monolayer membrane. Lipidomic profiling found that the cytoplasmic lipid deposits were primarily composed of triacylglycerols. Future work, including sequencing of the SBOP genome and genotyping, will be required to definitively determine the underlying genetic mechanism of this disease.
Keywords: bird-of-paradise, lipid, lipidomics, Lophorina superba, pathology, storage disease
Lipid metabolism is a complex process involving tens of thousands of lipid molecular species and many pathways in which numerous errors can occur to disrupt homeostasis. Intracellular lipid accumulation is a common manifestation of multiple disease processes including genetic mutations; tissue damage; and diet, toxin, or endocrinopathy-related metabolic abnormalities.26 These diseases are typically divided into primary (genetic or idiopathic) and secondary (acquired) lipid accumulation disease. There is some overlap in these binary categories as there is often genetic predisposition to acquiring secondary lipid accumulation diseases.
Some secondary lipid accumulation diseases are common in avian species and are often associated with age, sex, reproductive status, and high-cholesterol diets, with variation in species susceptibility.2,5 The most common are lipid accumulation in the liver (hepatic lipidosis) and in the walls of great vessels (atherosclerosis).5 Other common secondary lipid-related lesions in birds include lipomas, xanthomas, endogenous lipid pneumonia, renal and splenic lipidosis, and corneal lipid deposition.5 Alternately, secondary sphingolipidosis, a lysosomal sphingolipid storage disease, is very rare and has only been reported twice in birds. Acquired sphinolipidosis was experimentally induced in mule ducks (Anas platyrhynchos domesticus and Cairina moschata domestica hybrid) fed fumonisins and presumptively diagnosed in captive Humboldt penguins (Spheniscus humboldti) treated with chloroquine for malaria.6,39
Primary lipid accumulation diseases are exceedingly rare in birds. Genetic lipid storage diseases in humans are well-described with known mutations and include four lysosomal storage diseases (the sphingolipidoses, ceroid lipofuscinosis, Niemann-Pick type C disease, and lysosomal-acid-lipase deficiency) and four cytoplasmic lipid droplet storage diseases, two of which are multisystemic (neutral lipid storage disease (NLSD) with myopathy and NLSD with ichthyosis), and two of which affect only muscle and liver (carnitine deficiency and multiple acyl-coenzyme A dehydrogenase deficiency).22,29,36,37 Of these, only ceroid lipofuscinosis and sphingolipidosis have been described in both avian and domestic species.9,34,41 Neuronal ceroid lipofuscinosis, characterized by an accumulation of lipid-based pigment in lysosomes, has been described in a lovebird (Agapornis roseicollis) and a mallard (Anas platyrhynchos) with neurological symptoms based on microscopic lesions and without isolation of a specific mutation.9,34 Gangliosidosis-2 (GM2) storage disease (Tay-Sachs disease), a sphingolipidosis, was diagnosed in an American flamingo (Phoenicopterus ruber) with genetic confirmation.40 A few other sphingolipidoses and Niemann-Pick type C disease have been described in mammalian domestic species.41
Lysosomal storage diseases with accumulation of non-lipids are also poorly described in avian species with many diagnoses relying solely on histopathologic comparisons to human cases. The majority of lysosomal storage diseases are due to mutations affecting lysosomal enzymes, with autosomal recessive inheritance, and are chronic, progressive, multisystemic diseases. One of the more thoroughly described primary lysosomal storage diseases in birds is mucopolysaccharidosis type IIIB (Sanfilippo syndrome type B) in farmed emu (Dromaius novaehollandiae) due to a deficiency in lysosomal alpha-N-acetylglucosaminidase, which causes mucopolysaccharide accumulation in neurons and neurologic signs.10 Mucopolysacchridosis type II (Hunter syndrome) was diagnosed in a kaka (Nestor meridionalis), an endangered New Zealand parrot, exhibiting seizures.16 Glycogenosis type II (Pompe disease) has been experimentally replicated in Japanese quail (Coturnix coturnix japonica) with acid maltase deficiency.20 An unspecified lysosomal storage disease was diagnosed in Costa’s hummingbirds (Calypte costae) based on the ultrastructural appearance of the lesions.15
Superb birds-of-paradise (Lophorina superba; SBOPs) are common throughout their range in the forests of Papua New Guinea, but are listed in appendix II of the Convention on International Trade in Endangered Species (CITES) to protect against over-exploitation.35,42 Small captive breeding populations have been maintained sporadically at zoological institutions over the last 55 years with increased success following new husbandry initiatives in the early 1990s.35,38 Female SBOPs become sexually mature at 3 to 5 years of age and the oldest recorded SBOP in captivity lived to be 30-years-old.35 The males are well-known for their elaborate mating dance.35 As with many frugivorous birds, such as mynahs and toucans, the most common acquired storage disease in SBOPs is hepatic iron accumulation, resulting in hemosiderosis and hemochromatosis, despite being fed low-iron diets.18
Materials and Methods
Birds
A breeding pair of SBOPs housed at the San Diego Zoo Wildlife Alliance (SDZWA) in San Diego, California, USA, sired 15 offspring between 2014 and 2019 (Fig. 1). Complete medical records were reviewed for all known ancestors and offspring of this breeding pair, as well as necropsy reports for all SBOPs housed at the SDZWA between 1990 and 2022. Of these 15 offspring, 12 died by the end of 2021 and were submitted for postmortem examination. Five of the deaths were embryonic or juvenile deaths, three were attributed to aspergillosis, and four are the focus of this study. Three offspring remained alive and were clinically normal at the time of publication. All of the offspring have been housed at the SDZWA for the entirety of their lives, except for case 2, which was transferred to another facility five months prior to death. Since 2003, all adult SBOPs at the SDZWA have been on an iron-restricted diet consisting primarily of pellet feed (Mazuri ZuLiFe Soft Bill Diet 5MI2, St. Louis, MO, USA) and mixed chopped fruit and greens, with mealworms added during the breeding season.
Figure 1.
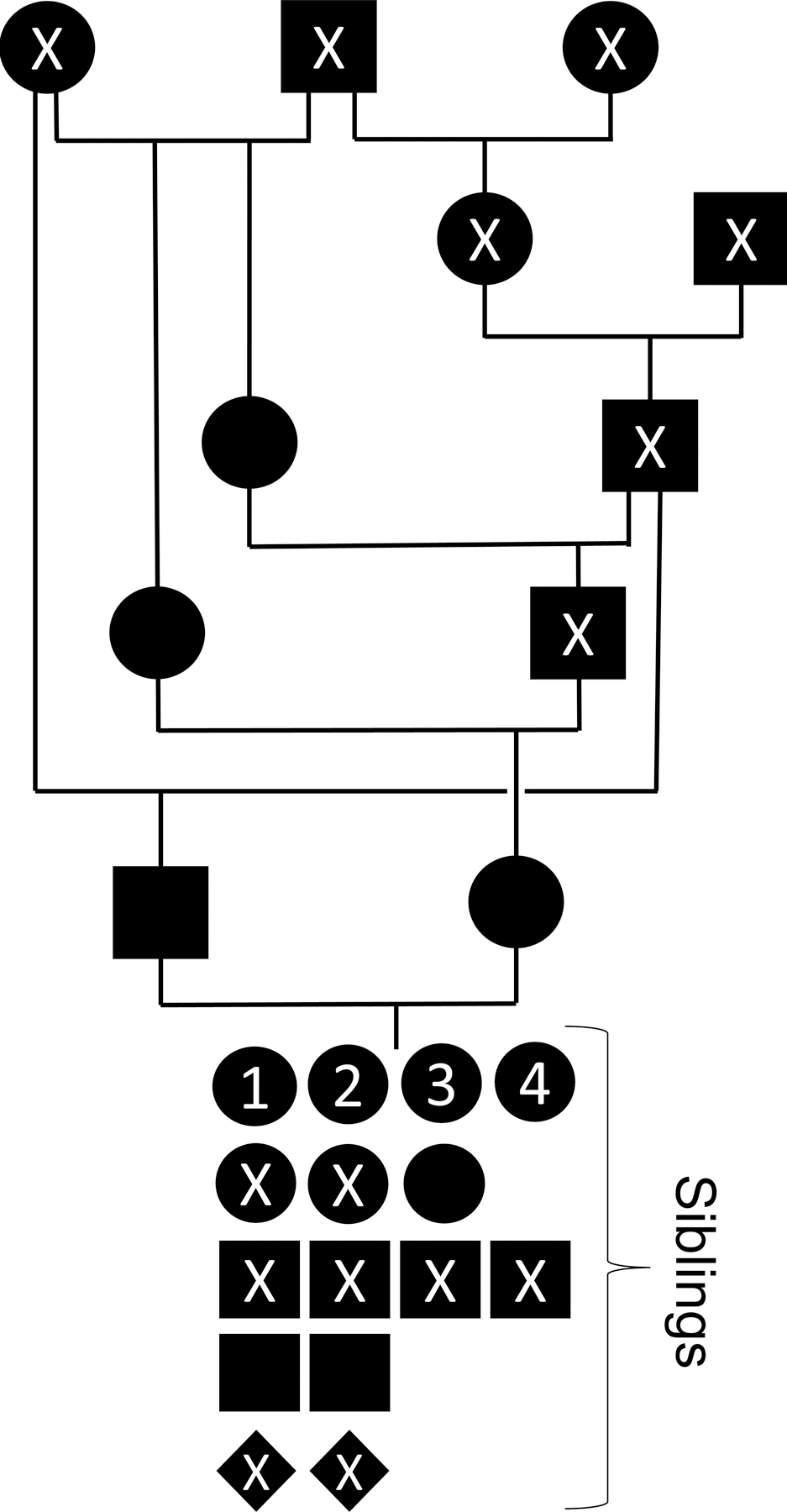
Pedigree of four superb bird-of-paradise siblings with a multisystemic lipid storage disease (labeled 1–4). Circles represent females, black squares represent males, and diamonds represent undetermined sex; X indicates unaffected, deceased birds. Birds with no parentage are founder birds collected from the wild.
SBOPs used as controls for lipidomic profiling in this study had no abnormal lipid accumulation or other evidence of storage disease histologically and were unrelated to each other and the affected birds. The first (control 1) was a 30-year-old female euthanized due to chronic degenerative joint disease. The second (control 2) was a 7.5-year-old male euthanized due to pulmonary aspergillosis. Both were in fair body condition and were fed a similar diet to the affected birds for at least 7 years prior to death. The authors confirm that no animals were sacrificed for the purpose of this study.
Inbreeding coefficients were calculated using PMx software21 and the SBOP studbook maintained at the SDZWA.43
Clinical Pathology, Necropsy, Histopathology, and Electron Microscopy
Complete blood counts were done manually. Blood chemistries were run on a HORIBA Pentra C400 (HORIBA Ltd., Japan). Results were compared to mean values from historical blood samples from a variety of bird-of-paradise species, when available.
Postmortem examination was performed within 24 hours of death. Representative samples of major organs were preserved in 10% neutral-buffered formalin, embedded in paraffin, sectioned at a 5 μm thickness, and stained with hematoxylin and eosin. For case 4, affected formalin-fixed paraffin-embedded tissues were additionally stained with Luxol-fast blue and periodic acid-Schiff (PAS) with and without diastase stains and frozen sections of liver and heart were cut at 8 μm thicknesses and stained with oil-red O stain.
In preparation for electron microscopy, one to two cubic millimeter formalin-fixed heart and liver samples were post-fixed in Karnovsky fixative and 1% osmium tetroxide 0.1 M cacodylate buffered solution. Using an automated processor, tissues were embedded in resin as described elsewhere.1 Briefly, samples were dehydrated using a 25–100% ethyl alcohol gradient and infiltrated with EMbed 812 resin (Electron Microscopy Sciences, Hatfeld, PA, USA). Resin tissue blocks were trimmed and sectioned on a Leica UC6 ultramicrotome (Leica Microsystems, Vienna, Austria). Contrasted thin sections (60–70 nm) were visualized using a JEOL 1400 transmission electron microscope (JEOL LTD, Tokyo, Japan). Images were obtained and analyzed using a OneView camera system Model 1095, 16 megapixels with the Gatan Microscope Suite (GMS 3.0; Gatan Inc, Pleasanton, CA, USA).
Lipidomic Profiling
Frozen liver from cases 1, 3, and 4; frozen heart from cases 1 and 4; and frozen liver from controls 1 and 2 were submitted to the LIPID MAPS Lipidomics Core facility at the University of California, San Diego, for lipidomic profiling using ultra high performance liquid chromatography coupled to mass spectrometry (MS) as previously described.13,14,33 Normal, frozen SBOP heart was not available for use as a control. For quantitative analysis, an Equisplash mix (Avanti) containing deuterated lipids was added to 100 μL heart or liver homogenate. Samples were extracted with butanol/methanol (3:1 = v/v), using a modified butanol/methanol method.24 The extracts were brought to dryness and reconstituted in isopropanol/dichloromethane/methanol (18:1:1, v/v). All lipids were measured according to the method described previously with slight modifications.14 Lipid separation was performed by a Vanquish Ultra High-Performance Liquid Chromatography (UHPLC, Thermo Fisher Scientific) interfaced with a Q Exactive mass spectrometer (Thermo Fisher Scientific). Individual lipid molecular species were analyzed using a data dependent acquisition using a Top N scan of 8 with a normalized collision energy of 30% in negative mode and a normalized collision energy of 25% in positive mode. All ions in the mass range of 200–1200 m/z were monitored. Lipid identification and quantification was done with Lipidomic Data Analyzer Software.13
The lipidomics data analyzed in this study are available as Supplemental Materials.
Results
Pedigree and Clinical Histories
The inbreeding coefficient for the affected birds was 0.1797, slightly less than the mean kinship of the captive SBOP population, which was 0.2779 as of May 2022. The most closely related shared ancestor is the paternal grandfather, who is also the maternal great grandfather (Fig. 1). The paternal grandmother of the affected birds was the most closely related founder animal, who was collected from the wild in 1987. This lipid storage disorder has not been identified in progenitor birds, other siblings, or other birds-of-paradise in captivity in North America.
All of the affected siblings were female, from different clutches, and ranged from 9 months to 4.3-years-old at the time of death (Table 1). Case 1 had marked coelomic distention and an increased respiratory rate two days prior to death. Bloodwork found decreased aspartate transaminase (AST; 290 units/L; normal mean 539 units/L). Alkaline phosphatase, creatinine kinase, cholesterol, glucose, and triglycerides were considered within normal limits. Lactate dehydrogenase quantification was not available. The venipuncture site was noted to bleed more and longer than expected, raising concerns of hepatic coagulopathy. On ultrasound evaluation, the coelomic distention was found to be due to a combination of hepatic enlargement and coelomic effusion. Ultrasound evaluation also revealed poor heart contractility and euthanasia was elected due to poor prognosis. Case 2 was found weak on exhibit and spent four days in hospital with a palpably enlarged liver, yellow mucosal membranes, and a high AST value (1581 units/L). This bird developed a “stargazing” behavior and was later found dead. Case 3 was clinically normal prior to being found dead on exhibit.
Table 1.
Case summaries of related superb birds-of-paradise with fatal lipid storage disease
| Case | Signalment (years) | History and cause of death | Tissues with lipid accumulation |
|---|---|---|---|
| 1 | 0.75, female | Euthanized due to heart failure secondary to cardiac lipidosis | Heart, kidney, liver, pancreas, proventriculus, ventriculusa |
| 2 | 3, female | Found dead after being in hospital with enlarged liver and jaundice secondary to hepatic lipidosis | Heart, kidney, liverb |
| 3 | 4.3, female | Found dead due to head trauma, previously clinically normal | Heart, kidney, liver, parathyroid gland, pancreas, proventriculus, ventriculus |
| 4 | 2.3, female | Euthanized after routine bloodwork showed elevated liver enzymes and biopsy showed massive hepatic lipidosis | Heart, kidney, liver, parathyroid gland, pancreas, proventriculus, ventriculus |
parathyroid glands not in histologic section for assessment;
parathyroid glands, pancreas, proventriculus, and ventriculus not described histologically
Case 4 was clinically normal when a preshipment workup revealed a mixed leukocytosis (31000 cells/μL) as well as elevated AST (1379 units/L) and creatine kinase (2294 units/L; normal mean 1307 units/L) levels. Other liver function indicators were unable to be measured due to small sample quantity. Due to concern for an infection given the bloodwork abnormalities, this bird was treated with a combination of antibiotics and antifungals and tested for aspergillosis, which was negative. When blood was retested 18 days later, the leukocytosis was slightly reduced (12000 cells/μL). At this time, AST was further increased (4060 units/L) and creatinine kinase was 2268 units/L. Ten days later, AST was reduced but still elevated (2160 units/L) and creatinine kinase was further increased (2388 units/L). In this sample, alkaline phosphatase (488 units/L), lactate dehydrogenase (3829 units/L), bile acids (94.5 μmol/L), and cholesterol (170 mg/dL) were also elevated. Glucose and triglyceride levels were considered within normal limits. Based on elevated liver values and the outcome of the other cases, hepatic biopsies were collected, revealing massive hepatic lipidosis. The first time the procedure was attempted, it was aborted due to excessive bleeding upon entry of the laparoscope, again raising concerns about hepatic coagulopathy. Given the lack of treatment options for a suspected hereditary storage disease and poor prognosis, euthanasia was elected.
Pathological Findings
At the postmortem examinations, all affected birds had enlarged (up to approximately four times normal size), pale tan, friable livers that floated in formalin (Fig. 2a). Cases 1 and 4 were noted to have pale tan hearts, which in case 1 was moderately dilated and in case 4 floated in formalin. Cases 1 and 4 also had diffusely pale tan kidneys. Case 1 had over 5 mL of coelomic fluid effusion and subcutaneous hemorrhage in the neck associated with a venipuncture site. Case 3 had hemorrhage in the air spaces of the skull due to head trauma. All birds were in adequate body condition with appropriate subcutaneous, pericardial, and mesenteric fat stores.
Figure 2.
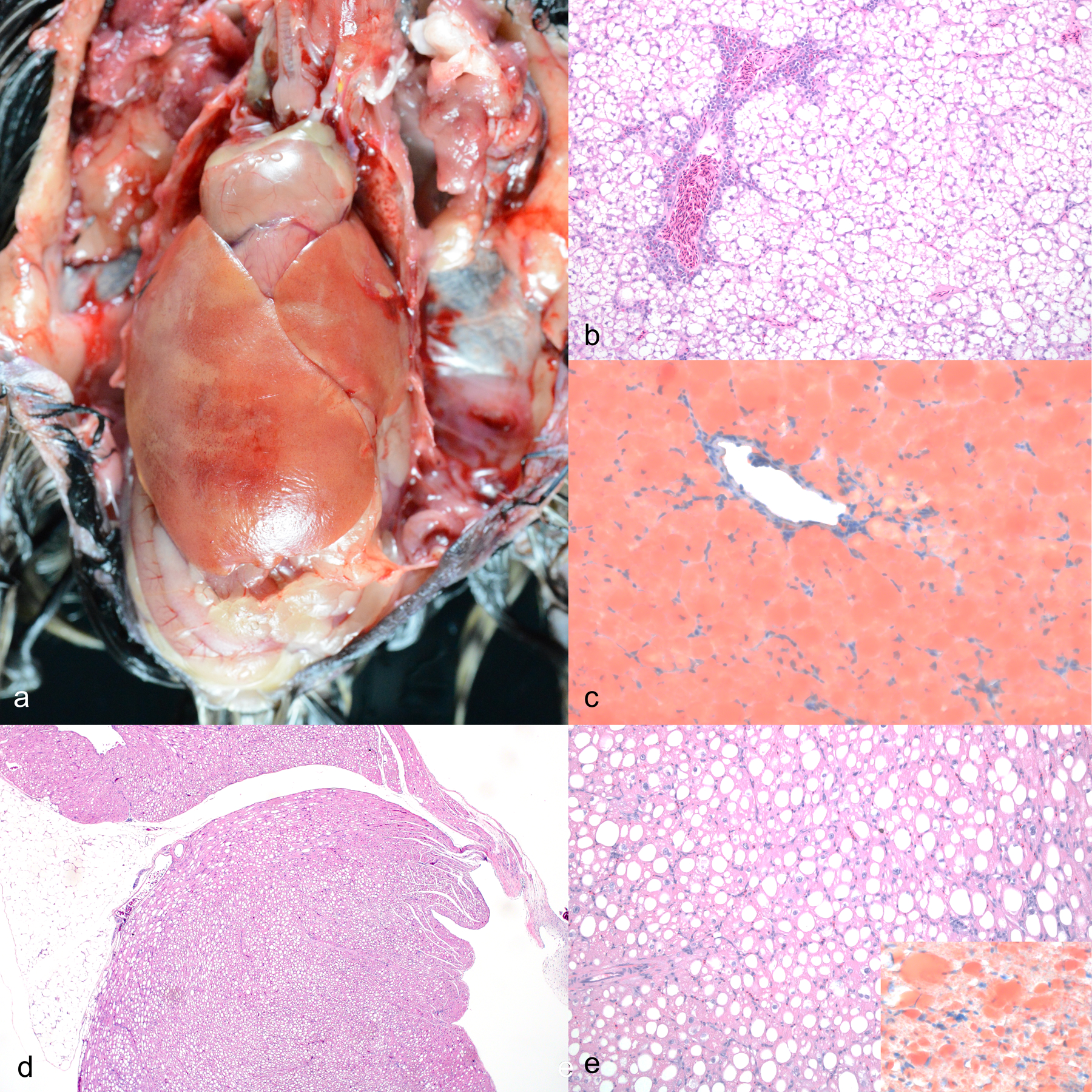
Gross and microscopic features of a multisystemic lipid storage disease in sibling superb birds-of-paradise. a) Coelomic cavity. The heart and liver are pale tan and the liver is massively enlarged with rounded edges. Case 4. b) Liver. Hepatocytes are diffusely expanded by round, clear, intracytoplasmic vacuoles consistent with lipid, which displace the nucleus. Central veins are surrounded by few hematopoietic cells. Case 4. Hematoxylin and eosin (HE). c) Liver. Hepatocellular cytoplasmic vacuoles diffusely stain red. Case 4. Oil red-O. d) Heart. Cardiomyocytes throughout the thickness of the atrium and ventricle are diffusely expanded by lipid vacuoles. Case 1. HE. e) Heart. Cardiomyocyte vacuoles disrupt the myofibers. Case 1. HE. Inset: Cardiomyocyte vacuoles stain red. Case 4. Oil red-O.
Microscopically in all cases, hepatocytes were diffusely enlarged, up to 4 times normal size, by numerous, variably sized, round, clear, intracytoplasmic vacuoles that displaced the nucleus peripherally (Fig. 2b). The vacuoles distended hepatocytes, compressed the surrounding sinusoids, and distorted overall hepatic architecture.The vacuoles in the liver stained red with oil red-O, confirming the presence of lipid (Figure 2c). The same vacuoles did not stain with Luxol fast blue or periodic acid-Schiff reaction. Similar vacuoles were present in cardiomyocytes throughout the thickness of the atrial and ventricular walls in all four cases (Figs. 2d–ee) and had similar staining properties as vacuoles in the liver (Fig. 2e, inset). Kidney in all four cases had diffuse cytoplasmic lipid vacuolation in the tubular epithelium. In cases 1, 3, and 4, the glandular epithelial cells of the ventriculus and proventriculus and the pancreatic islets were distended by lipid vacuoles. These tissues were not described in the histopathologic evaluation of case 2. In cases 3 and 4, the parathyroid gland principal cells were similarly affected. The parathyroid gland was not captured in section for case 1 and was not described for case 2. Incidental microscopic findings included pulmonary edema (case 1), intestinal protozoa (case 1), hepatic hemosiderosis (case 3), tracheitis (case 3), and thyroid gland colloid degeneration (cases 2, 3, and 4). Peripheral blood smears were unremarkable.
Ultrastructural examination of liver and heart from case 4 confirmed intracytoplasmic accumulation of lipid in droplets in hepatocytes and cardiomyocytes (Fig. 3). These lipid droplets varied in volume reaching up to 18.39 µm in diameter in the hepatocytes (Figs. 3a–b) and up to 26.34 µm in diameter in the cardiomyocytes (Fig. 3c). The lipid droplets were lined by an inconspicuous monolayer membrane, which was sometimes discontinuous (Figs. 3b, 3d, and 3e). Lipids droplets were single, coalescing, or fused (Figs. 3a and 3c). In hepatocytes, lipid droplets were in contact with mitochondria and rough endoplasmic reticulum and often surrounded by glycogen beta particles. Much of the soluble glycogen was artifactually lost due to suboptimal fixation leaving large empty spaces around lipid droplets. In cardiomyocytes, there was sarcoplasmic disruption due to the number and size of the lipid droplets, which separated and distorted the myofibrils, putting them in close contact with mitochondria (Fig. 3c). Cardiomyocyte mitochondria displayed several significant changes, including intramitochondrial accumulation of glycogen particles and lipid within vesicles, which displaced the mitochondrial cristae. These vesicles were lined by monolayered membranes. Affected mitochondria also had duplication and disarray of the cristae (Fig. 3d).
Figure 3.
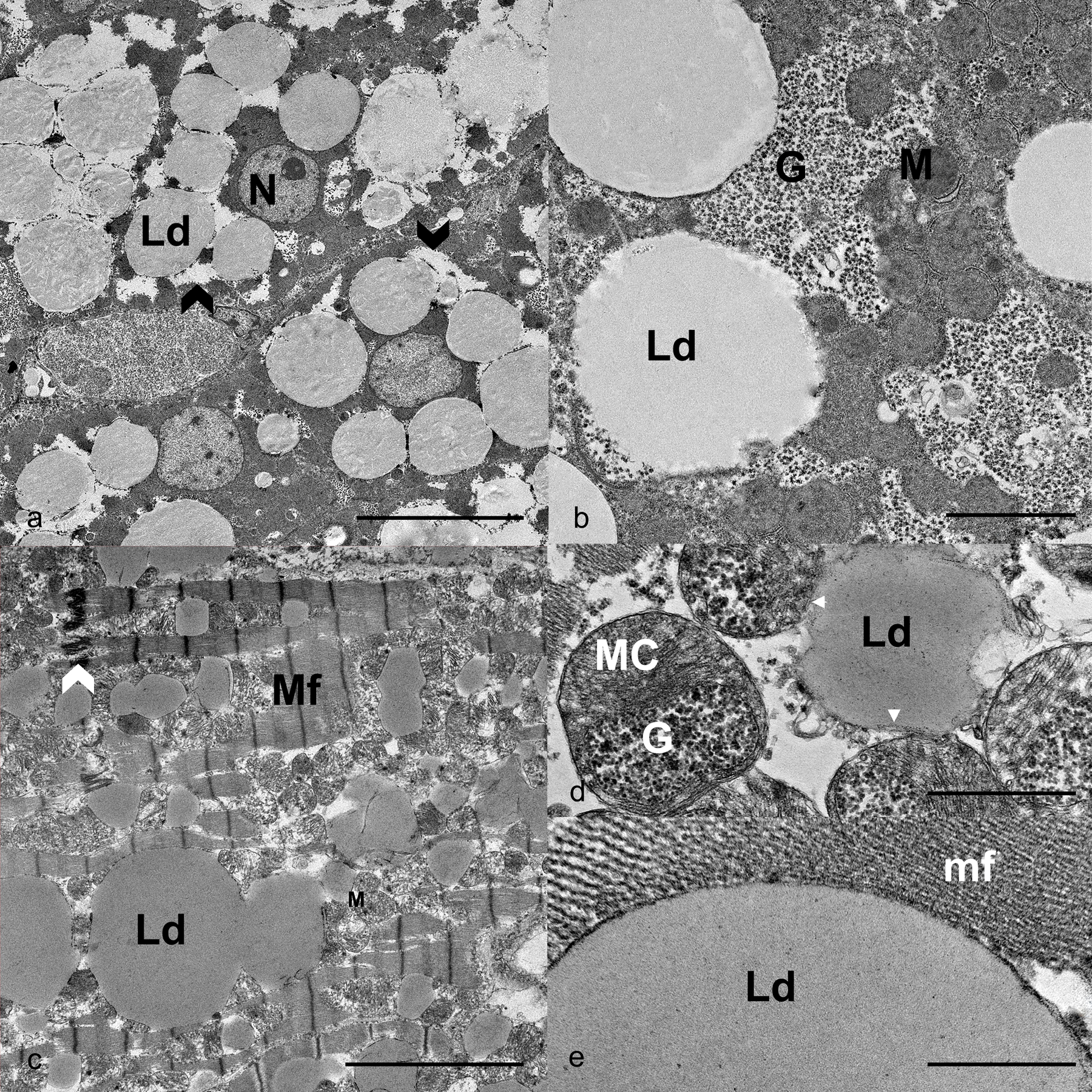
Ultrastructural features of a multisystemic lipid storage disease in sibling superb birds-of-paradise. a) Hepatocytes. Individual and coalescing, up to 18 nm diameter, lipid droplets (Ld) expand the cytoplasm and displace organelles, including the nucleus (N). Clear spaces around lipid droplets are occupied by soluble glycogen (black chevron). Scale bar is 10 μm. b) Hepatocytes. Lipids droplets (Ld) are in contact with beta-glycogen particles (G) and mitochondria (M). Scale bar is 2 μm. Case 4. c) Cardiomyocytes. Sarcoplasmic accumulation of individual and coalescing or fused lipid droplets (Ld). Myofibrils (Mf) are disarrayed or disrupted, and mitochondria (M) are displaced by accumulation of large lipid droplets. Intercalated disc = white chevron. Scale bar is 5 μm. d) Cardiomyocyte. A lipid droplet with inconspicuous monolayer membrane (white arrowheads) is in contact with mitochondria, one of which is distended by beta glycogen particles (G). Mitochondrion cristae are numerous and displaced (MC). Scale bar is 1 μm. e) Cardiomyocyte. A lipid droplet (Ld) displaying a monolayered membrane in contact with the myofilaments (mf) comprising a myofibril. Scale bar is 0.5 μm. Case 4.
Lipidomic Profiling
Lipidomic profiling was performed by liquid chromatography MS/MS and identified 1185 analytes, of which 923 were MS2 confirmed analytes present in at least four of the seven samples (Supplemental Dataset 1). The most striking difference between the control and affected birds was in the glycerolipids, which made up 31.6 – 47.2% of total measured lipid mass in the affected bird livers and 16.9 – 19.3% of the total measured lipid mass in control livers based on normalized intensities (Fig. 4). On average, triacylglycerols (TAGs) comprised 24.5% and diacylgycerols (DAGs) 13.0% of the total measured lipid mass in affected livers, which is an over twofold increase from the levels in the control livers (11.9% TAGs and 6.2% DAG). Affected heart contained similar levels of glycerolipids (24.3% TAG and 12.9% DAG). Of the 923 MS2 confirmed analytes, there were 59 analytes found in affected livers and hearts that were not found in control livers (35 TAGs, 16 phospholipids, 4 DAGs, three acylcarnitines, one ceramide). Of the 100 analytes with the largest measurable mass difference between affected and control liver samples, 99 were TAGs and one was a DAG. The range of these differences was enormous, with the individual lipid molecular species being 10 – 776 times more abundant in the affected livers compared with the controls. The analyte with the largest average increase in quantity between affected and control liver samples was TAG 54:4_30.96 | 18:0_18:1_18:3. Of the 100 analytes with the largest difference between affected heart and control liver samples, 89 were TAGs, nine were phospholipids, and two were DAGs, with a range of 9 – 473 times higher by mass of individual lipid metabolites in affected hearts. The analyte with the largest average increase in quantity between affected heart and control livers was TAG 52:6_29.57 | 16:1_18:2_18:3. This was also the analyte with the second largest difference in the liver, with an increase of 661 times, compared to control liver. The analyte with the largest difference in the liver (TAG 54:4_30.96 | 18:0_18:1_18:3) was present in six times higher quantities in the affected hearts than in the control livers. Seven analytes were found in control livers that were not present in affected livers; three of which were TAGs, three were phospholipids, and one DAG. Cholesterol esters comprised 9.6% of the lipidome of affected livers, 0.2% of the affected hearts, and 7.2% of the control livers. Ninety-one analytes were found in control livers that were not found in affected hearts.
Figure 4.
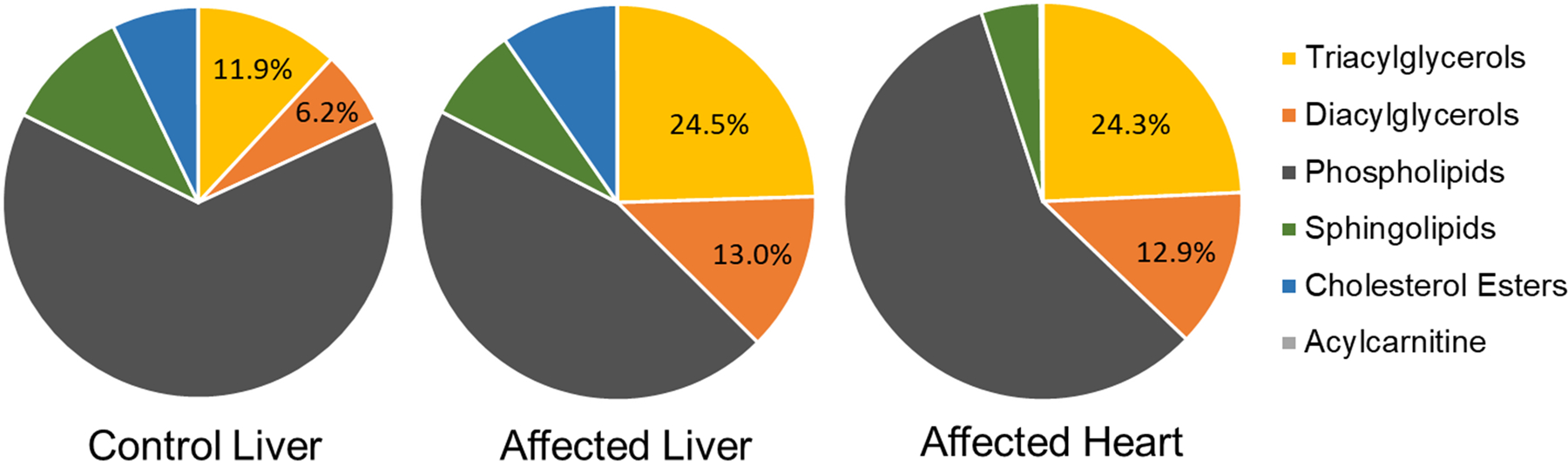
Charts showing proportions of the major categories of lipids comprising the lipidome of tissues from control superb birds-of paradise (control liver, n=2) and siblings affected by a multisystemic lipid storage disease (affected liver, n=3; affected heart, n=2). Acylcarnitines were present at levels of < 0.01% in all tissues.
Discussion
Based on the pedigree, clinical, gross, microscopic, ultrastructural, and lipidomic findings in these four female SBOP siblings, a primary inherited glycerolipid storage disease was diagnosed. Etiologies other than an underlying genetic disease were considered and discarded. The range of affected cell types (hepatocytes, cardiomyocytes, renal tubular epithelial cells, parathyroid gland principal cells, exocrine pancreatic cells, and glandular cells of the ventriculus and proventriculus), lack of lesions typical of secondary lipid-associated metabolic syndromes in birds, and identical diets fed to affected and unaffected SBOPs indicate that this is not an acquired lipid metabolism disease.5 These birds were the progeny of a related breeding pair, resulting in an inbreeding coefficient of 0.1797, which is between the kinship levels of breeding half-siblings (0.125) and full-siblings (0.25). This level of inbreeding was considered acceptable given the relatively small captive SBOP breeding population, which has a mean kinship of 0.2779. The overall level of genetic diversity in free-ranging SBOP populations is unknown. The proportion of affected progeny to unaffected (4:15, presuming those that died as embryos were unaffected) is similar to what would be expected for a disease with simple autosomal recessive inheritance (1:4), as is seen in most primary storage disorders.41 All of the affected birds were either juveniles or young adults just reaching sexual maturity, further supporting the hypothesis that this is a genetic disease.
Despite marked lipid accumulation in key organs such as the liver and heart, two of the four affected birds showed no external or behavioral clinical signs. Replacement of these tissues by lipid appears to have been progressive and had minimal impact on organ function until the end-stages of the disease. The disease progressed at different rates in each bird, with the youngest bird (0.75-years-old) displaying the most severe clinical signs of heart and liver failure and the oldest (4.3-years-old) displaying no clinical signs up until being found dead due to head trauma. Both of these birds had similar degrees of lipid accumulation in tissues at the time of death. In birds where antemortem blood work was done, there was evidence that liver function was impaired, with AST levels showing the largest changes and alkaline phosphatase, lactate dehydrogenase, bile acid, and cholesterol elevations in some cases. In two cases, there was suspicion of coagulopathy secondary to hepatopathy based on prolonged bleeding times observed clinically. Only one case developed grossly appreciable icterus and in only one case was cardiac insufficiency detected antemortem. It is surprising that signs of cardiac malfunction were not seen in the other birds, given the extreme lipid accumulation in the cardiomyocytes. Two cases displayed elevated circulating creatinine kinase levels, which was most likely secondary to handling. A less probable cause of elevated creatinine kinase is that there were underlying skeletal muscle metabolism abnormalities, similar to many human lipid storage myopathies, that were not appreciable histologically in these birds.22
Negative staining of the vacuoles with periodic acid-Schiff reaction with and without diastase and Luxol fast blue stain helped rule out glycogenoses and ceroid lipofuscin lysosomal storage diseases, while positive staining with oil red-O confirmed the presence of lipids. The ultrastructural appearance of the lipid vacuoles enabled the exclusion of some of the more common lysosomal lipid storage diseases. Both sphingolipidoses and Niemann-Pick type C lipid accumulations display characteristic lamellar bodies, which were absent in case 4. Ultrastructurally in this case, the lipid aggregates were homogenous deposits of lipid lined by discontinuous monolayer membranes, consistent with lipid droplets. Lipid droplets are organelles found in many cell types used for storage of neutral lipids, primarily TAGs, and are integral to lipid homeostasis and protection from oxidative stress.28,31 Unlike other organelles, including lysosomes, lipid droplets have a phospholipid monolayer rather than a bilayer as the contained lipids are hydrophobic.31 Thus, the presence of a monolayer membrane around the lipid deposits, instead of a bilayer membrane, rules out lysosomal storage diseases.
Ultrastructural examination found mitochondrial changes in the heart suggestive of metabolic stress or dysregulation.17,23,25 These changes included pleomorphism, replication, and disorganization of cristae and accumulation of glycogen and lipid. It has been suggested that glycogen within the mitochondria is due to rupture of the outer membrane, allowing beta-glycogen particles to leak and accumulate within the intermembrane space.17 Presumably, these changes are secondary to the primary accumulation of lipid droplets.
To characterize the accumulated lipid, we used lipidomics. The field of lipidomics uses MS and liquid chromatography to comprehensively identify and quantify all of the lipid species in cells (the “lipidome”), creating large datasets that can be analyzed to better understand basic physiology as well as numerous metabolic disorders.12,33 The use of lipidomics in human medicine has rapidly increased over the last 20 years, providing insights into complex diseases such as diabetes, obesity, cardiovascular disease, and Alzheimer’s.12,27 This type of bioinformatics has rarely been applied to veterinary medical research, and has only very recently been applied to avian species.3,4 Lipidomic profiling of SBOP livers and hearts identified over a thousand analytes. Although our sample size was limited due to both the rarity of the disease and available SBOP tissues, precluding statistical analysis, there were clear differences between the lipidomes of affected and control tissues. The comparison of affected heart to control liver is imperfect, given the differences in metabolic activity, but was necessary due to a lack of normal frozen heart to use as a control. Despite the comparison of different tissues, similar changes were seen in the affected liver and heart, with over twice the amount of TAGs and DAGs in the affected hearts and livers as in the control liver. Some TAG compounds showed massive increases in the affected tissues, with TAG 54:4_30.96 | 18:0_18:1_18:3 increased 776 times in affected livers and TAG 52:6_29.57 | 16:1_18:2_18:3 increased 473 times in the affected hearts. The majority of the lipids with the largest increases in the affected liver and heart were TAGs, as were the majority of compounds found in affected tissues that were not present in the control livers. These findings indicate that the disease process in these SBOPs impacted glycerolipid metabolism overall and affected TAG metabolism more than DAG. We also found that the cholesterol esters were similar or lower in affected tissues than the control livers, which removed lysosomal-acid-lipase deficiency-like disease from the list of differentials. Lysosomal-acid-lipase deficiency, also known as Wolman’s Disease if complete or cholesterol ester storage disease if incomplete, is an autosomal recessive disease in humans caused by a mutation in the LIPA gene leading to massive storage of triglycerides and cholesterol esters in many tissues.7
Taken as a whole, these findings are suggestive of a disease process analogous to NLSD in humans. There are two forms of this disease, NLSD with myopathy and NLSD with ichthyosis (Chanarin-Dorfman syndrome).22,32 These rare diseases are due to autosomal recessive mutations in the patatin-like phospholipase domain-containing 2 (PNPLA2) gene in NLSD with myopathy and ABHD5/CGI58 gene in NLSD with ichthyosis.22 These mutations lead to reduction of adipose triglyceride lipase (ATGL) activity, which is necessary for the first step of TAG catabolism, causing TAG accumulation in cytoplasmic lipid droplets in a wide range of tissues including skeletal muscle, liver, intestine, and leukocytes.28 In NLSD wth myopathy, cardiac muscle is frequently affected, and in NLSD with ichthyosis there is lipid deposition in the skin causing nonbullous congenital ichthyosiform erythroderma and there is no lipid deposition in the heart.22 Both forms of this disease in humans primarily affect the skeletal muscle, even very early in the disease process, often presenting as progressive muscular dystrophy in adulthood28
ATGL has been identified in a range of avian species with variable tissue expression.30 In parrots, ATGL is expressed primarily in pectoral and limb skeletal muscle, cardiac muscle, abdominal fat, and subcutaneous fat.30 In quail, ATGL expression was found in skeletal muscle, but levels were much lower in the heart.30 Ducks express it primarily in subcutaneous and abdominal fat, but also in heart, spleen, and pectoral and limb skeletal muscles.30 This indicates that not only is ATGL very likely present in SBOPs, it is probably expressed at different levels in different tissues. Variable tissue expression of ATGL may explain why lipid accumulation occurred in a different subset of tissues than seen in NLSD in humans. TAGs and lipid droplets play integral roles in both hepatocyte and cardiomyocyte metabolism, so accumulation in these tissues is not surprising.8,31 What is less clear is why skeletal muscle was not affected by lipid accumulation in these SBOPs, despite its reliance on TAGs, while an unexpected combination of cells with secretory functions were affected. Mice have been used to model ATGL deficiency analogous to NLSD, causing lipid accumulation in virtually all tissues, but most obviously in the heart, liver, kidney, and testes.11 Despite the lipid accumulation in numerous tissues, the only clinically significant effect in the mice was decreased cardiac function leading to failure, similar to case 1.11 In order to fully characterize this disease, sequencing of the SBOP genome followed by genetic testing would be required to identify the specific mutation causing the accumulation of lipids in these birds.
Despite the fact that a hereditary storage disease such as this poses a low risk to genetically diverse, healthy, free-ranging populations, the inherent restrictions of captive breeding programs mean that these diseases pose a potential problem and highlights the risks of small breeding populations. Although this breeding pair had an acceptable inbreeding coefficient given the level of kinship in the breeding program overall, the recognition of a deleterious genetic disease in the offspring has prompted their separation.
Supplementary Material
Acknowledgements
We thank the SDZWA Disease Investigations pathologists and technicians for their assistance in preparing these cases, as well as everyone involved in caring for these birds. We are grateful to Asako Chaille and Jessica Thule at the SDZWA for assistance with pedigree analysis and SBOP studbook maintenance respectively. Thanks also to Kristy Harmon at the Leukocyte Antigen Biology Lab at UC Davis for preparing frozen sections and Sergio Ayala at University of California, Davis for oil-red O staining. We thank Christina Heard at the California Animal Health & Food Safety laboratory electron microscopy unit for the outstanding preparation. We thank Felix Hubert from the University of California, San Diego LIPID MAPS Lipidomics core for help with the lipidomics analysis.
Funding
Lipidomics work was supported by NIGMS MIRA grant R35 GM139641, which is a renewal of RO1 GM20501-44 (EAD).
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Christina M. McKenzie, San Diego Zoo Wildlife Alliance, San Diego, CA
Matt Marinkovich, San Diego Zoo Wildlife Alliance, San Diego, CA.
Aníbal G. Armién, California Animal Health & Food Safety Laboratory System, School of Veterinary Medicine, University of California, Davis, Davis, CA
Judy St. Leger, Department of Population Medicine and Diagnostic Sciences, Cornell University, Ithaca, NY
Aaron M. Armando, Department of Pharmacology, School of Medicine, University of California San Diego, San Diego, CA
Edward A. Dennis, Department of Pharmacology, School of Medicine, University of California San Diego, San Diego, CA
Oswald Quehenberger, Department of Pharmacology, School of Medicine, University of California San Diego, San Diego, CA.
Alison Righton, San Diego Zoo Wildlife Alliance, San Diego, CA.
References
- 1.Armién AG, Wolf TM, Mor SK, et al. Molecular and biological characterization of a cervidpoxvirus isolated from moose with necrotizing dermatitis. Vet Pathol 2020;57(2):296–310. [DOI] [PubMed] [Google Scholar]
- 2.Beaufrère H, Ammersbach M, Reavill DR, et al. Prevalence of and risk factors associated with atherosclerosis in psittacine birds. J Am Vet Med Assoc 2013;242(12):1696–1704. [DOI] [PubMed] [Google Scholar]
- 3.Beaufrère H, Gardhouse S, Ammersbach M. Lipoprotein characterization in Quaker parrots (Myiopsitta monachus) using gel‐permeation high‐performance liquid chromatography. Vet Clin Pathol 2020;49(3):417–427. [DOI] [PubMed] [Google Scholar]
- 4.Beaufrère H, Gardhouse SM, Wood RD, Stark KD. The plasma lipidome of the Quaker parrot (Myiopsitta monachus). Clugston RD, ed. PLOS ONE. 2020;15(12):e0240449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaufrère H, Reavill D, Heatley J, Susta L. Lipid-related lesions in Quaker parrots (Myiopsitta monachus). Vet Pathol 2019;56(2):282–288. [DOI] [PubMed] [Google Scholar]
- 6.Benlasher E, Geng X, Nguyen NTX, et al. Comparative effects of fumonisins on sphingolipid metabolism and toxicity in ducks and turkeys. Avian Dis 2012;56(1):120–127. [DOI] [PubMed] [Google Scholar]
- 7.Boldrini R, Devito R, Biselli R, Filocamo M, Bosman C. Wolman disease and cholesteryl ester storage disease diagnosed by histological and ultrastructural examination of intestinal and liver biopsy. Pathol - Res Pract 2004;200(3):231–240. [DOI] [PubMed] [Google Scholar]
- 8.Drosatos K, Schulze PC. Cardiac lipotoxicity: Molecular pathways and therapeutic implications. Curr Heart Fail Rep 2013;10(2):109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans EE, Jones MP, Crews AJ, Newkirk K. Neuronal ceroid lipofuscinosis in a mallard duck (Anas platyrhynchos). J Avian Med Surg 2012;26(1):22–28. [DOI] [PubMed] [Google Scholar]
- 10.Genger SC, Mizukami K, Martin MP, Jr JRA, Barnes HJ, Giger U. Mucopolysaccharidosis IIIB (Sanfilippo syndrome B) in a commercial emu (Dromaius novaehollandiae) flock. Avian Pathol 2018;47(1):1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haemmerle G, Lass A, Zimmerman R, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312(5774):734–737. [DOI] [PubMed] [Google Scholar]
- 12.Han X Lipidomics for studying metabolism. Nat Rev Endocrinol 2016;12(11):668–679. [DOI] [PubMed] [Google Scholar]
- 13.Hartler J, Armando AM, Trötzmüller M, Dennis EA, Köfeler HC, Quehenberger O. Automated annotation of sphingolipids including accurate identification of hydroxylation sites using MSn data. Anal Chem 2020;92(20):14054–14062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartler J, Triebl A, Ziegl A, et al. Deciphering lipid structures based on platform-independent decision rules. Nat Methods. 2017;14(12):1171–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Proudfoot JS, Garner MM, Prieur D, Brown R. Lysosomal Storage Disease in Costa’s Hummingbirds (Calypte costae). IAAAM 2000. [Google Scholar]
- 16.Jolly RD, Hunter SA, Alley MR, et al. Mucopolysaccharidosis II (MPSII) in a free-living Kaka (Nestormeridionalis) in New Zealand. J Wildl Dis 2021;57(4):884–890. [DOI] [PubMed] [Google Scholar]
- 17.Jones M, Ferrans VJ. Intramitochondrial glycogen in hypertrophied infundibular muscle of patients with congenital heart diseases. Am J Pathol 1973;70(1):69–88. [PMC free article] [PubMed] [Google Scholar]
- 18.Klasing KC, Dierenfeld ES, Koutsos EA. Avian iron storage disease: Variations on a common theme? J Zoo Wildl Med 2012;43(3s):S27–S34. [DOI] [PubMed] [Google Scholar]
- 19.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. [DOI] [PubMed] [Google Scholar]
- 20.Kunita R, Nakabayashi O, Wu J-Y, et al. Molecular cloning of acid α-glucosidase cDNA of Japanese quail (Coturnix coturnix japonica) and the lack of its mRNA in acid maltase deficient quails. Biochim Biophys Acta BBA - Mol Basis Dis 1998;1362(2–3):269–278. [DOI] [PubMed] [Google Scholar]
- 21.Ballou JD, Lacy RC, Pollak JP. PMx: Software for demographic and genetic analysis and management of pedigreed populations (Version 1.6.5.20220325). Chicago Zoological Society, Brookfield, Illinois, USA. 2022. http://www.scti.tools [Google Scholar]
- 22.Liang W-C, Nishino I Lipid Storage Myopathy. Curr Neurol Neurosci Rep 2011;11(1):97–103. [DOI] [PubMed] [Google Scholar]
- 23.Liao Y, Tham DKL, Liang F-X, et al. Mitochondrial lipid droplet formation as a detoxification mechanism to sequester and degrade excessive urothelial membranes. Mostov KE, ed. Mol Biol Cell. 2019;30(24):2969–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Löfgren L, Forsberg G-B, Ståhlman M. The BUME method: A new rapid and simple chloroform-free method for total lipid extraction of animal tissue. Sci Rep 2016;6(1):27688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luciani A, Denley MCS, Govers LP, Sorrentino V, Froese DS. Mitochondrial disease, mitophagy, and cellular distress in methylmalonic acidemia. Cell Mol Life Sci 2021;78(21–22):6851–6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lydic TA, Goo Y-H. Lipidomics unveils the complexity of the lipidome in metabolic diseases. Clin Transl Med 2018;7(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meikle TG, Huynh K, Giles C, Meikle PJ. Clinical lipidomics: Realizing the potential of lipid profiling. J Lipid Res 2021;62:100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Missaglia S, Coleman R, Mordente A, Tavian D. Neutral lipid storage diseases as cellular model to study lipid droplet function. Cells. 2019;8(2):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mole SE, Cotman SL. Genetics of the neuronal ceroid lipofuscinoses (Batten disease). Biochim Biophys Acta BBA - Mol Basis Dis. 2015;1852(10):2237–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nie Q, Hu Y, Xie L, Zhang C, Shen X, Zhang X. Identification and characterization of adipose triglyceride lipase (ATGL) gene in birds. Mol Biol Rep. 2010;37(7):3487–3493. [DOI] [PubMed] [Google Scholar]
- 31.Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. 2019;20(3):137–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pennisi EM, Arca M, Bertini E, et al. Neutral lipid storage diseases: Clinical/genetic features and natural history in a large cohort of Italian patients. Orphanet J Rare Dis. 2017;12(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quehenberger O, Armando AM, Brown AH, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma1 [S]. J Lipid Res. 2010;51(11):3299–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reece R, MacWhirter P. Neuronal ceroid lipofuscinosis in a lovebird. Vet Rec. 1988;122(8):187–187. [DOI] [PubMed] [Google Scholar]
- 35.Rimlinger D, Theule J, Bass K. Breeding history and husbandry of the Superb Bird‐of‐paradise (Lophorina superba). Zoo Biol. 2021;40(5):485–490. [DOI] [PubMed] [Google Scholar]
- 36.Schulze H, Sandhoff K. Lysosomal lipid storage diseases. Cold Spring Harb Perspect Biol. 2011;3(6):a004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schweiger M, Lass A, Zimmermann R, Eichmann TO, Zechner R. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am J Physiol-Endocrinol Metab. 2009;297(2):E289–E296. [DOI] [PubMed] [Google Scholar]
- 38.Timmis WH. Breeding the superb bird of paradise: at Chester Zoo. Int Zoo Yearb. 1970;10(1):102–104. [Google Scholar]
- 39.Wunschmann. Neuronal storage disease in a group of captive Humboldt penguins (Spheniscus humboldti). Vet Pathol. 2006;43:1029–1033. [DOI] [PubMed] [Google Scholar]
- 40.Zeng BJ, Torres PA, Viner TC, et al. Spontaneous appearance of Tay–Sachs disease in an animal model. Mol Genet Metab. 2008;95(1–2):59–65. [DOI] [PubMed] [Google Scholar]
- 41.Zachary JF, editor. Pathologic basis of veterinary disease Elsevier; 2017. [Google Scholar]
- 42.Lophorina superba. CITES. Retrieved from https://cites.org/eng/taxonomy/term/2730.
- 43.ZIMS for Studbooks for Association of Zoos & Aquariums/Lophorina superba. (Theule J, April/18/2023). Species360 Zoological Information Management System. Retrieved from http://zims.Species360.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


