Abstract
BACKGROUND:
Clinical investigations of shock in cardiac intensive care units (CICUs) have primarily focused on acute myocardial infarction (AMI) complicated by cardiogenic shock (AMICS). Few studies have evaluated the full spectrum of shock in contemporary CICUs.
METHODS AND RESULTS:
The Critical Care Cardiology Trials Network is a multicenter network of advanced CICUs in North America. Anytime between September 2017 and September 2018, each center (n=16) contributed a 2-month snap-shot of all consecutive medical admissions to the CICU. Data were submitted to the central coordinating center (TIMI Study Group, Boston, MA). Shock was defined as sustained systolic blood pressure <90 mm Hg with end-organ dysfunction ascribed to the hypotension. Shock type was classified by site investigators as cardiogenic, distributive, hypovolemic, or mixed. Among 3049 CICU admissions, 677 (22%) met clinical criteria for shock. Shock type was varied, with 66% assessed as cardiogenic shock (CS), 7% as distributive, 3% as hypovolemic, 20% as mixed, and 4% as unknown. Among patients with CS (n=450), 30% had AMICS, 18% had ischemic cardiomyopathy without AMI, 28% had nonischemic cardiomyopathy, and 17% had a cardiac cause other than primary myocardial dysfunction. Patients with mixed shock had cardiovascular comorbidities similar to patients with CS. The median CICU stay was 4.0 days (interquartile range [IQR], 2.5–8.1 days) for AMICS, 4.3 days (IQR, 2.1–8.5 days) for CS not related to AMI, and 5.8 days (IQR, 2.9–10.0 days) for mixed shock versus 1.9 days (IQR, 1.0–3.6) for patients without shock (P<0.01 for each). Median Sequential Organ Failure Assessment scores were higher in patients with mixed shock (10; IQR, 6–13) versus AMICS (8; IQR, 5–11) or CS without AMI (7; IQR, 5–11; each P<0.01). In-hospital mortality rates were 36% (95% CI, 28%–45%), 31% (95% CI, 26%–36%), and 39% (95% CI, 31%–48%) in AMICS, CS without AMI, and mixed shock, respectively.
CONCLUSIONS:
The epidemiology of shock in contemporary advanced CICUs is varied, and AMICS now represents less than one-third of all CS. Despite advanced therapies, mortality in CS and mixed shock remains high. Investigation of management strategies and new therapies to treat shock in the CICU should take this epidemiology into account.
Keywords: cardiogenic shock, epidemiology, hypotension, intensive care units, North America
Cardiogenic shock (CS), the most severe form of acute heart failure, is characterized by life-threatening end-organ hypoperfusion resulting from a low cardiac output state.1 CS occurs in ≈5% to 7% of patients presenting with acute myocardial infarction (AMI) and is more common in patients with ST-segment–elevation MI (STEMI) than non-STEMI.1 Although early revascularization of patients with AMI complicated by CS (AMICS) has improved survival,2 in-hospital mortality is reported to be high.3-7 A notable European registry conducted between 2010 and 2012 identified AMICS as accounting for ≈80% of CS cases8; however, this epidemiology has not been examined in a contemporary cohort in North America. Limited data suggest that clinical outcomes may be better in patients with CS not related to AMI, which is most commonly caused by severe chronic heart failure or severe valvular disease8; nevertheless, this condition is reported to be highly morbid and mortal as well.8,9 Given the poor outcomes and substantial variation in clinical practice patterns, models for developing dedicated cardiac shock centers and streamlining systems of care have been proposed.7,10 There is also a need for better clinical phenotyping of the heterogeneous causes and presentations of CS with the aim of tailoring therapies to improve outcomes.7,11
Most clinical investigations of shock in cardiac intensive care units (CICUs) have focused on AMICS, and few studies have evaluated the full spectrum of shock admitted to contemporary CICUs.8 Due to changes in preventive therapies and behaviors, and the shifting underlying pathophysiology of acute coronary syndromes,12 the incidence of STEMI has declined over the past 2 decades.13 Thus, it is uncertain whether the contemporary epidemiology of CS has evolved in parallel. Moreover, with a growing burden of noncoronary structural heart disease, related nonischemic causes of shock may be increasing.14 The objective of this analysis was to investigate the epidemiology and clinical management of shock in contemporary tertiary care CICUs in a well-characterized cohort from a large, multi-institutional clinical registry.
METHODS
Study Population
The Critical Care Cardiology Trials Network (CCCTN) is an investigator-initiated collaborative research network of American Heart Association Level 1 CICUs15 located in the United States and Canada. Scientific oversight of the CCCTN is conducted by its academic Executive and Steering Committees, and the data are coordinated by the TIMI Study Group (Boston, MA). For this analysis, between September 2017 and September 2018, each participating center (n=16) contributed a 2-month snap-shot of all consecutive medical admissions to the CICU. The CCCTN Registry protocol and waiver of informed consent were approved by the Institutional Review Board at Brigham and Women’s Hospital and at each of the participating centers. No personal identifying health information is collected in the Registry database. We encourage parties interested in collaboration and data sharing to contact the corresponding author directly for discussion.
Clinical data were collected both prospectively and retrospectively through comprehensive clinical review of each patient and entered into a central electronic case record form.16 Shock was defined by the presence of both sustained hemodynamic impairment (ie, systolic blood pressure <90 mm Hg or the need for inotropic or vasopressor support) and evidence of end-organ hypoperfusion (eg, altered mental status, oliguria, acute kidney injury, hepatic injury, metabolic acidosis, or elevated serum lactate [>2 mmol/L]) ascribed to the hypotension. The assessment was made by trained study staff and the site investigators based on the entirety of the medical record, including the managing clinician’s diagnoses. All patients from the 2-month snap-shot meeting clinical criteria for shock (either as a presenting diagnosis or with onset during the CICU admission) were included in this analysis.
Shock Classification
For all patients with shock, the type of shock was classified by the site investigator as cardiogenic, distributive, hypovolemic, or mixed (Methods in the Data Supplement). To support consistent definitions, all study staff underwent training by the central CCCTN team. In addition, all case entries were individually reviewed by the Coordinating Center via automated consistency checks and manual review, and data were amended as necessary by the site. The mixed category included patients for whom >1 type of shock was determined to contribute (eg, both CS and distributive shock). Patients assessed as having CS were further characterized based on (1) type of ventricular dysfunction (ie, left ventricular failure, right ventricular failure, biventricular failure, or other) and (2) underlying cause of CS (ie, ischemic, nonischemic, other cardiac, or unknown). Patients with CS due to an ischemic cause were further categorized according to whether the shock presentation was related to AMI or not. To be inclusive of all spontaneous AMIs, both STEMI and non-STEMI as diagnosed by the managing clinicians were included.
Data Analysis
Baseline patient characteristics, presenting features, and CICU resource utilization were summarized according to shock type. For these analyses, we separated patients in whom CS was precipitated by AMI versus not precipitated by AMI (ie, patients with nonischemic cardiomyopathies, ischemic cardiomyopathies without AMI, or other cardiac causes of shock). For categorical variables, the results are reported as counts and percentages, and for continuous variables, as medians and interquartile ranges (IQRs). In addition, both CICU and in-hospital mortality rates were calculated across shock types. Mortality rates in shock categories are also reported stratified by Sequential Organ Failure Assessment (SOFA) score (ie, a widely used clinical risk score for characterization of illness severity in patients with critical illness)17,18 ≥10 versus <10 based on externally established cutoffs17,18 and proximity to the median of our cohort. Absolute 95% CIs for mortality rates were calculated with a binomial method. In addition, we compared mortality rates adjusted for age, sex, and SOFA score using logistic regression with mortality as the outcome and shock categories as the independent variables. To account for potential misclassification as well as possible excess mortality associated with an initial cardiac arrest, we performed a sensitivity analysis restricted to patients who did not have a cardiac arrest before CICU admission.
Among patients with CS, we performed a subgroup analysis comparing patients who received mechanical circulatory support (MCS) versus those who did not. MCS included intraaortic balloon pump counterpulsation, Impella percutaneous ventricular assist systems, TandemHeart percutaneous ventricular assist systems, and venoarterial extracorporeal membrane oxygenation. In addition, to eliminate potential misclassification related to absence of invasive hemodynamic data, we performed a sensitivity analysis restricted to patients who underwent right heart catheterization or had an indwelling pulmonary artery catheter. For this analysis, we analyzed CS and mixed shock separately.
Statistical significance was assessed at a nominal α level of 0.05. All reported P values were 2-sided. All statistical computations were performed with SAS System V9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Shock Type
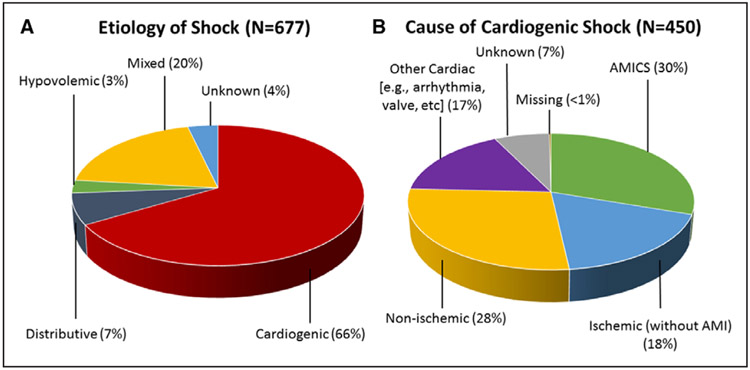
Among 3049 CICU admissions, 677 patients (22%) met clinical criteria for shock (Figure 1). Shock type was varied, with 66% assessed as CS, 7% as distributive shock, 3% as hypovolemic shock, 20% as mixed shock, and 4% as unknown. In patients with CS (n=450), 30% had an acute coronary syndrome as a precipitant (59% STEMI, 41% non-STEMI), 18% had an ischemic cardiomyopathy without AMI, 28% had a nonischemic cardiomyopathy, 17% had an identified cardiac cause that was not primarily related to myocardial dysfunction (eg, incessant ventricular tachycardia, severe valve disease, etc), 7% had an unknown cause, and <1% had a missing cause (Figure 1). Thus, overall, 20% of all shock in CCCTN CICUs was classified as AMICS. Forty-seven percent of CS patients were assessed as isolated left ventricular failure, 25% had biventricular failure, 9% had isolated right ventricular failure, and 19% had a cardiac cause that was not related to underlying myocardial dysfunction. Twenty-five percent of CS patients had cardiac arrest preceding their CICU admission. In a sensitivity analysis excluding patients with preceding cardiac arrest, the distribution of shock types was unchanged (68% CS [of which 32% was AMICS] and 19% mixed type). In patients with mixed shock (n=132), 17% had AMI as a contributor and 26% had initial cardiac arrest.
Figure 1. Shock type.
A, All patients meeting criteria for shock (n=677) were classified according to shock type. B, Patients with cardiogenic shock (n=450) were further divided according to the cause of cardiogenic shock. Patients with cardiogenic shock from an ischemic cause were categorized according to whether or not the shock presentation was precipitated by acute myocardial infarction (AMI). AMICS indicates acute myocardial infarction cardiogenic shock.
Patient Characteristics
Baseline characteristics of patients in the largest shock categories—AMICS, CS not related to AMI, and mixed shock—are summarized in Table 1 (other shock categories are shown in Table I in the Data Supplement). Compared with patients in whom CS was not precipitated by AMI, AMICS patients had a significantly higher burden of atherosclerotic cardiovascular risk factors, including type 2 diabetes mellitus, hypertension, and active smoking (each P<0.01). By contrast, patients with CS not related to AMI had a significantly higher burden of preexisting heart failure (predominantly caused by reduced left ventricular ejection fraction), atrial and ventricular arrhythmias, pulmonary hypertension, and chronic kidney disease (each P<0.01). Patients with mixed shock had an overall burden of cardiovascular comorbidities similar to patients with AMICS and CS without AMI, including 45% with type 2 diabetes mellitus, 47% with coronary artery disease, and 55% with previous heart failure.
Table 1.
Baseline Characteristics of Patients With AMICS, CS Without AMI, and Mixed Shock
| AMICS, % (n) | CS Without AMI, % (n) |
Mixed, % (n) | P Value | |
|---|---|---|---|---|
| N=136 | N=314 | N=132 | ||
| Demographics | ||||
| Age, median (IQR), y | 68 (59–76) | 63 (53–73) | 65 (54–75) | 0.008 |
| Female sex | 33.1 (45) | 37.6 (118) | 37.1 (49) | 0.65 |
| White race | 76.5 (91) | 65.6 (181) | 66.7 (74) | 0.093 |
| Weight, median (IQR), kg | 83 (70–95) | 82 (69–97) | 82 (68–98) | 0.96 |
| BMI, median (IQR), kg/m2 | 28 (25–32) | 28 (24–32) | 28 (24–34) | 0.64 |
| Cardiovascular comorbidities | ||||
| Current smoker | 23.7 (32) | 13.8 (43) | 11.5 (15) | 0.002 |
| Diabetes mellitus | 50.0 (68) | 35.7 (112) | 44.7 (59) | 0.011 |
| Hypertension | 72.1 (98) | 53.5 (168) | 60.6 (80) | 0.001 |
| Coronary artery disease | 48.5 (66) | 40.1 (126) | 47.0 (62) | 0.18 |
| Cerebrovascular disease | 10.3 (14) | 8.3 (26) | 7.6 (10) | 0.70 |
| Peripheral artery disease | 13.2 (18) | 8.9 (28) | 11.4 (15) | 0.36 |
| Prior heart failure | 26.5 (36) | 62.1 (195) | 54.5 (72) | <0.001 |
| Historical LVEF* | ||||
| ≥50% | 8.3 (3) | 11.6 (22) | 19.7 (13) | 0.044 |
| 40–<50% | 13.9 (5) | 5.8 (11) | 4.5 (3) | |
| 30–<40% | 16.7 (6) | 10.0 (19) | 15.2 (10) | |
| 20–<30% | 38.9 (14) | 27.9 (53) | 31.8 (21) | |
| <20% | 22.2 (8) | 44.7 (85) | 28.8 (19) | |
| Prior heart transplant | 0.0 (0) | 1.3 (4) | 3.8 (5) | 0.036 |
| Atrial fibrillation | 10.3 (14) | 29.0 (91) | 27.3 (36) | <0.001 |
| Ventricular arrhythmia | 3.7 (5) | 12.1 (38) | 8.3 (11) | 0.017 |
| Severe valvular disease | 2.2 (3) | 15.6 (49) | 26.5 (35) | <0.001 |
| Pulmonary hypertension | 0.7 (1) | 9.9 (31) | 3.8 (5) | <0.001 |
| Congenital heart disease | 0.7 (1) | 1.6 (5) | 3.0 (4) | 0.34 |
| Noncardiovascular comorbidities | ||||
| Chronic kidney disease | 24.3 (33) | 35.4 (111) | 35.6 (47) | 0.053 |
| On dialysis† | 12.1 (4) | 15.3 (17) | 42.6 (20) | <0.001 |
| Significant pulmonary disease | 11.0 (15) | 18.8 (59) | 13.6 (18) | 0.086 |
| Significant liver disease | 2.9 (4) | 4.8 (15) | 6.8 (9) | 0.33 |
Categorical variables are shown as percentages with counts in parentheses. Continuous variables are shown as medians with interquartile ranges (IQRs). Differences between patients in each group were evaluated using Pearson χ2 test for categorical variables and Kruskal-Wallis test for continuous variables. *Among patients with prior heart failure. †Among patients with chronic kidney disease. AMI indicates acute myocardial infarction; AMICS, acute myocardial infarction with cardiogenic shock; BMI, body mass index; CS, cardiogenic shock; and LVEF, left ventricular ejection fraction.
Median SOFA scores were higher in patients with mixed shock (median, 10; IQR, 8–13) than in patients with AMICS (median, 8; IQR, 5–11) or CS without AMI (median, 7; IQR, 5–11; each P<0.01; Table 2). Median SOFA scores in all 3 shock categories were substantially higher than in CICU patients without shock (median, 2; IQR, 1–4, each P<0.01; Table II in the Data Supplement). Laboratory values demonstrated significant end-organ dysfunction across AMICS, CS without AMI, and mixed shock categories, as evidenced by lactic acidosis (range: 67%–81% with lactate >2.0 mmol/L), renal impairment (range: 72%–83% with estimated glomerular filtration rate <60 mL min−1 1.73 m−2), and elevated liver transaminases (range: 28%–48% with alanine aminotransferase or aspartate aminotransferase >3× the upper limit of normal). These biochemical derangements were most pronounced in patients with mixed shock (Table 2).
Table 2.
Presenting Features and ICU Resource Utilization
| AMICS, % (n) | CS Without AMI, % (n) |
Mixed, % (n) | P Value | |
|---|---|---|---|---|
| N=136 | N=314 | N=132 | ||
| Clinical features/illness severity | ||||
| SOFA score | 8 (5–11) | 7 (5–11) | 10 (6–13) | <0.001 |
| Worst laboratory values | ||||
| Lactate, median (IQR), mmol/L | 3.5 (2.2–7.6) | 2.9 (1.8–6.1) | 4.2 (2.4–8.1) | 0.005 |
| Lactate >2 mmol/L | 77.1 (84) | 66.7 (176) | 81.1 (103) | 0.006 |
| Arterial pH, median (IQR) | 7.32 (7.21–7.40) | 7.37 (7.21–7.43) | 7.29 (7.21–7.40) | 0.15 |
| Arterial pH <7.25 | 30.7 (31) | 31.3 (61) | 35.3 (36) | 0.73 |
| eGFR, median (IQR), mL min−1 1.73 m−2 | 42 (19–65) | 38 (20–54) | 31 (14–45) | 0.012 |
| eGFR <60 mL min−1 1.73 m−2 | 71.4 (85) | 79.8 (237) | 83.3 (105) | 0.061 |
| ALT, median (IQR), U/L | 81 (39–320) | 58 (26–292) | 73 (30–370) | 0.098 |
| AST, median (IQR), U/L | 190 (85–705) | 75 (32–363) | 112 (40–664) | <0.001 |
| Total bilirubin, median (IQR), mg/dL | 1.0 (0.6–1.6) | 1.2 (0.8–2.1) | 1.3 (0.7–2.7) | 0.003 |
| Platelets, median (IQR), K/μL | 150 (105–212) | 133 (94–195) | 116 (62–174) | <0.001 |
| INR, median (IQR) | 1.3 (1.1–1.7) | 1.5 (1.3–2.1) | 1.7 (1.3–2.8) | <0.001 |
| ICU resource utilization | ||||
| Days of ICU care, median (IQR) | 4.0 (2.5–8.1) | 4.3 (2.1–8.5) | 5.8 (2.9–10.0) | 0.068 |
| Mechanical ventilation | 60.3 (82) | 44.9 (141) | 65.9 (87) | <0.001 |
| Renal replacement therapy | 16.9 (23) | 13.7 (43) | 26.5 (35) | 0.005 |
| Invasive monitoring | 64.7 (88) | 70.7 (222) | 67.4 (89) | 0.43 |
| Central venous line | 46.3 (63) | 47.1 (148) | 54.5 (72) | 0.30 |
| Pulmonary artery catheter | 30.9 (42) | 38.2 (120) | 31.1 (41) | 0.19 |
| Arterial line | 55.1 (75) | 51.3 (161) | 59.8 (79) | 0.24 |
| Management of shock | ||||
| No. of vasoactive medications, median (IQR) | 1 (1–2) | 2 (1–2) | 2 (1–3) | 0.002 |
| Mechanical circulatory support | 61.0 (83) | 26.1 (82) | 22.7 (30) | <0.001 |
Categorical variables are shown as percentages with counts in parentheses. Continuous variables are shown as medians with interquartile ranges (IQRs). Differences between patients in each group were evaluated using Pearson χ2 test for categorical variables and Kruskal-Wallis test for continuous variables. For lactate, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, and INR, the worst values were the highest laboratory values; for arterial pH, estimated glomerular filtration rate (eGFR), and platelets, the worst values were the lowest laboratory values. AMI indicates acute myocardial infarction; AMICS, acute myocardial infarction with cardiogenic shock; AST, aspartate aminotransferase; CICU, cardiac intensive care unit; CS, cardiogenic shock; ICU, intensive care unit; INR, international normalized ratio; and SOFA, Sequential Organ Failure Assessment.
Intensive Care Unit Resource Utilization
The median CICU stay was 4.0 days (IQR, 2.5–8.1 days) for patients with AMICS, 4.3 days (IQR, 2.1–8.5 days) for patients with CS not related to AMI, and 5.8 days (IQR, 2.9–10.0 days) for patients with mixed shock (Table 2), compared with 1.9 days (IQR, 1.0–3.6) for CICU patients without shock (P<0.01 for each; Table II in the Data Supplement). Moreover, the median hospital stay was 8.3 days (IQR, 3.8–13.9) for patients with AMICS versus 11.9 days (IQR, 5.7–20.1) for patients with mixed shock (P<0.01). Patients with mixed shock had significantly higher rates of mechanical ventilation (66%) and renal replacement therapy (27%) than did patients with CS not related to AMI (45% and 14%, respectively; P<0.01 for both) and numerically higher rates than did patients with AMICS (60% and 17%, respectively; Table 2).
Approximately two-thirds of CS and mixed shock patients had some form of invasive monitoring, and approximately one-third of patients with CS and mixed shock had a pulmonary artery catheter. Nearly all patients with shock received vasoactive medications: AMICS (92%; median number of vasoactive medications = 1; IQR, 1–2), CS without AMI (93%; median number of vasoactive medications = 2; IQR, 1–2), and mixed shock (98%; median number of vasoactive medications = 2; IQR, 1–3). A substantially higher proportion of patients with AMICS received MCS (61%) compared with patients with CS not related to AMI (26%) or patients with mixed shock (23%; P<0.01 for each; Table 2).
In-Hospital Mortality by Shock Type
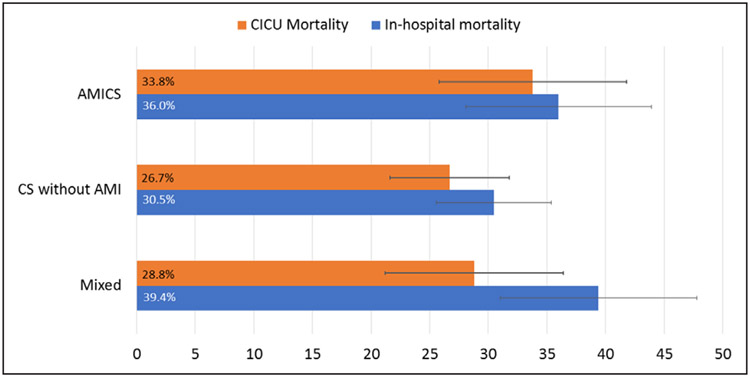
The in-hospital mortality rates were numerically highest in patients with mixed shock (39%; 95% CI, 31%–48%), followed by AMICS (36%; 95% CI, 28%–45%) and CS not related to AMI (31%; 95% CI, 26%–36%). When stratified by SOFA score as a metric of severity of illness, among patients with SOFA score ≥10, the in-hospital mortality rates were 65% (95% CI, 51%–78%), 61% (95% CI, 51%–70%), and 55% (95% CI, 43%–67%), for patients with AMICS, CS not related to AMI, and mixed shock, respectively. In patients with SOFA score <10, the rates were 18% (95% CI, 10%–28%), 16% (95% CI, 11%–22%), and 23% (95% CI, 14–35), respectively. In both unadjusted analysis and after adjusting for age, sex, and SOFA score, in-hospital mortality rates between mixed shock and CS categories were not statistically significant (Figure 2).
Figure 2. Mortality by shock type.
Cardiac intensive care unit (CICU) and in-hospital mortality rates are summarized for patients with acute myocardial infarction cardiogenic shock (AMICS), cardiogenic shock without acute myocardial infarction (AMI), and mixed shock. Absolute binomial 95% CIs are shown. In-hospital mortality rates between mixed shock vs AMICS and mixed shock vs cardiogenic shock (CS) without AMI were not statistically different when adjusted for age, sex, and severity of illness (P=0.50 and P=0.87, respectively).
In a sensitivity analysis excluding patients with initial cardiac arrest, the in-hospital mortality rates were 31% (95% CI, 22%–41%), 21% (95% CI, 16%–27%), and 37% (95% CI, 27%–47%) for patients with AMICS, CS not related to AMI, and mixed shock, respectively (Table III in the Data Supplement).
CS Patients Treated With MCS
In an analysis of all CS patients who were treated versus not treated with MCS, MCS patients had worse indices of illness severity, including higher SOFA scores, higher median lactate, and lower median arterial pH (P<0.01 for each; Table IV in the Data Supplement). Consistent with these findings, CS patients treated with MCS versus without MCS had higher in-hospital mortality rates (40%; 95% CI, 33%–48% versus 28%; 95% CI, 23%–33%), even after adjusting for differences in age, sex, and SOFA score (P<0.01; Table IVB in the Data Supplement).
Shock Patients With Invasive Hemodynamic Data
We performed sensitivity analyses excluding CS and mixed shock patients who did not undergo right heart catheterization or have an indwelling pulmonary artery catheter to eliminate potential misclassification related to not having invasive hemodynamic data. In these analyses, the median SOFA scores (CS: 7; IQR, 5–11 versus 8; IQR, 5–11; mixed shock: 9; IQR, 7–13 versus 10; IQR, 6–13) and other indicators of illness severity including degree of lactic acidosis, renal impairment, and liver injury were very similar to those from the overall cohort (Table V in the Data Supplement). Moreover, the in-hospital mortality rates were 30% (95% CI, 24%–37%) and 38% (95% CI, 25%–52%) in invasively confirmed patients with CS and mixed shock, respectively, consistent with the findings from the overall cohort (Table VB in the Data Supplement).
DISCUSSION
In this analysis of the contemporary epidemiology of shock within the CCCTN Registry, we found that shock type was varied, with one-fifth classified as AMICS, nearly one-half classified as CS not related to AMI, and one-fifth classified as mixed shock. Compared with patients with AMICS, patients with CS not related to AMI had a higher burden of preexisting heart failure, atrial and ventricular arrhythmias, pulmonary hypertension, and chronic kidney disease. Patients with mixed shock had the highest SOFA scores, driven by multisystem organ dysfunction, and highest intensive care unit (ICU) resource utilization rates. Despite the use of substantial ICU resources, more than one-third of patients with CS and mixed shock did not survive to hospital discharge. Although AMICS should remain a focal point for medical advances, CS not related to AMI and mixed shock are associated with high morbidity and mortality and warrant greater attention from clinical investigation.
Evolving Epidemiology of CS
Before the advent of medical and coronary revascularization therapies for treatment of AMI, in-hospital mortality rates from AMICS exceeded 80%.19 The paradigm for treating AMICS changed following the landmark SHOCK trial (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock?) in which early revascularization reduced mortality at 6 and 12 months post-AMICS.2 Indeed, in the decade following the widespread adoption of percutaneous coronary intervention, estimated mortality rates from AMICS significantly declined to ≈50%.3,4 Moreover, data from more contemporary cohorts suggest that outcomes after AMICS have continued to improve, with in-hospital mortality rates of 30% to 40%.5,6,20 This trend in AMICS is reinforced by our contemporary multicenter assessment with an in-hospital AMICS mortality rate of 36%. Although these temporal trends are encouraging, the fact that 1 in 3 patients with AMICS does not survive to hospital discharge indicates that there are still significant opportunities to improve outcomes.
The results of our analysis also highlight another striking trend: in this contemporary cohort from tertiary care CICUs in North America, less than one-third of all CS cases were related to AMI. This evolution in epidemiology may be a consequence of several developments. First, although there are conflicting reports about whether the incidence of CS as a complication of AMI has been changing over time,4,5,21 the overall incidence of STEMI is unequivocally declining.13 Thus, the evolving epidemiology of acute coronary syndromes may be translating to a relative reduction in AMICS cases. Second, the prevalence of heart failure, including advanced (ie, stage C and D) heart failure, is increasing globally, in part because of improved treatment of patients with heart failure and AMI leading to longer survival from these diseases.13 In our cohort, nearly two-thirds of patients with CS not related to AMI had preexisting heart failure, and a substantial number had sequelae of advanced heart disease (eg, atrial and ventricular arrhythmias, pulmonary hypertension, and chronic kidney disease). It is likely that many of these patients developed CS due to progression of their underlying cardiomyopathy. These data from the CCCTN indicate that the emergence of CS without AMI, first noted in an inclusive study of CS (n=216) within the Global Research on Acute Conditions Team network in Europe,8 now carries an even larger contribution to contemporary shock care in North America.
To date, most clinical investigations of CS have focused on AMICS, in large part because the data are derived from clinical registries of patients with AMI. Consequently, there are limited data to guide clinical decision-making and additional research in patients with CS due to other causes. For example, patients with CS not related to AMI have generally been excluded from MCS trials, which may partly explain why a relatively smaller proportion of these patients were treated with MCS in our cohort (26% versus 61% in AMICS patients). Moreover, as patients with CS not related to AMI have distinct clinical features from patients with AMICS, extrapolating management approaches from one group to the other may be inappropriate. Given the changing clinical profile of CS patients in the CICU, future investigations of therapies and management approaches in patients with CS not related to AMI may be critical to improving outcomes from CS.
Mixed Shock
The results of our study also highlight another high-risk, understudied patient population, namely individuals with mixed shock. Although inconsistently defined, mixed shock has been described as vasodilatory CS, with a hemodynamic profile characterized by low cardiac output, elevated filling pressures, and inappropriately low systemic vascular resistance.7 Although the classic paradigm of CS predicts a compensatory increase in systemic vascular resistance to maintain mean arterial pressure in the setting of a low cardiac output state, ancillary analyses from the SHOCK trial challenged this model.22,23 Specifically, investigators noted that systemic vascular resistance varied widely in patients with AMICS but was typically near-normal, and thus inappropriately low, in the context of use of vasopressors.22,23 Mechanistic studies helped to explain this observation by establishing that inducible nitric oxide synthase expression and cytokine cascades triggered by myocardial necrosis can produce a systemic inflammatory response that leads to systemic vasodilation in some patients with AMICS.24,25 Even in the absence of myocardial ischemia and necrosis, major systemic insults including end-organ injury from shock (eg, ischemic hepatopathy) can produce a similar vasodilatory response. Other factors may contribute to distributive physiology in CS patients as well. For example, ICU patients frequently receive vasodilatory medications (eg, sedatives) and are at increased risk for infection, both because of factors related to critical illness (eg, increased risk of aspiration) and factors related to ICU care (eg, invasive monitoring). In other cases, mixed shock evolves in the opposite direction. For instance, distributive physiology can lead to coronary hypoperfusion and consequent myocardial depression or decompensation in patients with preexisting cardiomyopathy, which can produce the hemodynamic profile of mixed shock.
The results of our analysis highlight several important clinical features of mixed shock patients in contemporary CICUs. First, patients with mixed shock tended to have comorbidity profiles similar to patients with CS, including a high prevalence of baseline myocardial dysfunction and heart failure. Second, they tended to be the sickest patients among the shock categories, with the highest SOFA scores (median, 10; IQR, 8–13) and the highest rates of advanced ICU therapies including mechanical ventilation and renal replacement therapy. Finally, their outcomes were quite poor, with in-hospital mortality rates at least as high as patients with pure CS. Considering these observations, efforts to better phenotype these patients and develop evidence-based therapies specifically targeted at this population may be critical to reducing overall mortality from shock.
Strengths and Limitations
This analysis has several important strengths compared with other epidemiological investigations of shock. First, our study population was derived from a well-characterized clinical registry of all consecutive medical CICU patients, which allowed us to comprehensively profile the clinical characteristics of these shock patients with greater fidelity than is typical for large public administrative databases. Second, by virtue of the multicenter participation and consecutive enrollment, these data provide a broader view of shock patients treated in tertiary care medical centers than do studies focused on subsets of shock patients in disease-specific or device-specific registries. Finally, all patients in this analysis were treated for shock during a 1-year window from September 2017 to September 2018. Thus, these data offer a contemporary view of this population.
Several limitations of this analysis should be acknowledged as well. First, as a registry of routine practice, measurement of lactate was not required, nor was the timing mandated when measured. Therefore, even the worst reported lactates may have been obtained at a time when the patient was adequately supported hemodynamically rather than at the time of shock onset. Future study of the assessment of lactate in CICUs may provide insight into the timeliness of evaluation of this biomarker for emerging shock. Second, invasive hemodynamic assessment was not mandated and detailed hemodynamic data were not captured in the CCCTN registry, so we were unable to directly analyze the complete hemodynamic profiles of patients admitted to the CICU with shock. This limitation is especially relevant to patients with CS because some definitions of CS used in clinical trials (eg, the SHOCK trial) are based on invasive hemodynamic criteria. Mitigating this limitation, in a sensitivity analysis excluding patients without invasive hemodynamic data (52% of CS patients and 58% of mixed shock patients), the clinical characteristics, management patterns, and outcomes remained largely unchanged, suggesting that there was no meaningful misclassification bias in patients who did not have an invasive hemodynamic assessment. Third, although all case entries were individually reviewed by the Coordinating Center, the clinical characteristics and outcomes of shock patients included in this analysis were not formally adjudicated. Finally, as the patients in our cohort were treated in predominantly urban, tertiary care medical centers, these data may not reflect the clinical characteristics of patients treated in smaller community hospitals. Nonetheless, they are relevant to those centers aspiring to be shock referral centers.
Conclusions
The epidemiology of shock patients treated in contemporary CICUs is evolving. Whereas AMI leading to severe left ventricular dysfunction has previously dominated the causes of CS, AMICS now represents less than one-third of all CS cases represented in these tertiary CICUs. In addition, mixed shock patients, who have outcomes at least as poor as CS patients, constitute a significant proportion of shock patients treated in the CICU. Despite novel technologies for treating shock patients, mortality in CS and mixed shock remains high. In addition to ongoing research in AMICS, future investigations of therapies and management approaches in patients with CS not related to AMI and mixed shock will be critical to improving overall outcomes from shock treated in the CICU.
Supplementary Material
WHAT IS KNOWN
In past registries, the significant majority of shock in the cardiac intensive care unit was caused by acute myocardial infarction complicated by cardiogenic shock.
Few studies have evaluated the full spectrum of shock patients admitted to contemporary cardiac intensive care units.
WHAT THE STUDY ADDS
In the Cardiac Critical Care Trials Network Registry of advanced cardiac intensive care units in North America, more than two-thirds of all cardiogenic shock cases were related to causes other than acute myocardial infarction.
Patients with mixed shock, who comprised 20% of shock patients treated in the cardiac intensive care unit, had outcomes at least as poor as patients with cardiogenic shock.
By virtue of the multicenter participation and consecutive enrollment of patients in the Critical Care Cardiology Trials Network Registry, these data provide a broad view of shock patients treated in tertiary care medical centers.
Sources of Funding
Dr Berg is supported by a T32 postdoctoral training grant from the National Heart, Lung, and Blood Institute (T32 HL007604). Dr Solomon is supported by National Institutes of Health Clinical Center intramural research funds.
Footnotes
Disclosures
None.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCOUTCOMES.119.005618.
Contributor Information
David D. Berg, Levine Cardiac Intensive Care Unit, TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA.
Erin A. Bohula, Levine Cardiac Intensive Care Unit, TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA.
Sean van Diepen, Department of Critical Care and Division of Cardiology, Department of Medicine, University of Alberta, Edmonton, AB, Canada.
Jason N. Katz, Divisions of Cardiology and Pulmonary and Critical Care Medicine, University of North Carolina, Center for Heart and Vascular Care Chapel Hill.
Carlos L. Alviar, University of Florida, Gainesville.
Vivian M. Baird-Zars, Levine Cardiac Intensive Care Unit, TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA.
Christopher F. Barnett, Department of Cardiology, MedStar Washington Hospital Center, DC.
Gregory W. Barsness, Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN.
James A. Burke, Lehigh Valley Health Network, Allentown, PA.
Paul C. Cremer, Department of Cardiovascular Medicine, Cleveland Clinic Foundation, OH.
Jennifer Cruz, Section of Cardiology, Cooper University Hospital, Camden, NJ.
Lori B. Daniels, Sulpizio Cardiovascular Center, University of California San Diego, La Jolla.
Andrew P. DeFilippis, Division of Cardiovascular Medicine, Department of Medicine, University of Louisville, KY.
Affan Haleem, Lehigh Valley Health Network, Allentown, PA.
Steven M. Hollenberg, Section of Cardiology, Cooper University Hospital, Camden, NJ.
James M. Horowitz, New York University Langone Health.
Norma Keller, New York University Langone Health.
Michael C. Kontos, Virginia Commonwealth University, Richmond.
Patrick R. Lawler, Peter Munk Cardiac Centre, Toronto General Hospital, University of Toronto, ON, Canada.
Venu Menon, Department of Cardiovascular Medicine, Cleveland Clinic Foundation, OH.
Thomas S. Metkus, Division of Cardiology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD.
Jason Ng, New York University Langone Health.
Ryan Orgel, Divisions of Cardiology and Pulmonary and Critical Care Medicine, University of North Carolina, Center for Heart and Vascular Care Chapel Hill.
Christopher B. Overgaard, Peter Munk Cardiac Centre, Toronto General Hospital, University of Toronto, ON, Canada.
Jeong-Gun Park, Levine Cardiac Intensive Care Unit, TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA.
Nicholas Phreaner, Sulpizio Cardiovascular Center, University of California San Diego, La Jolla.
Robert O. Roswell, New York University Langone Health.
Steven P. Schulman, Division of Cardiology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD.
R. Jeffrey Snell, Rush University Medical Center, Chicago, IL.
Michael A. Solomon, Critical Care Medicine Department, National Institutes of Health Clinical Center and Cardiovascular Branch, National Heart, Lung, and Blood Institute, of the National Institutes of Health, Bethesda, MD.
Bradley Ternus, Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN.
Wayne Tymchak, Department of Critical Care and Division of Cardiology, Department of Medicine, University of Alberta, Edmonton, AB, Canada.
Fnu Vikram, Lehigh Valley Health Network, Allentown, PA.
David A. Morrow, Levine Cardiac Intensive Care Unit, TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA.
REFERENCES
- 1.Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008; 117:686–697. doi: 10.1161/CIRCULATIONAHA.106.613596 [DOI] [PubMed] [Google Scholar]
- 2.Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, LeJemtel TH. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901 [DOI] [PubMed] [Google Scholar]
- 3.Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation. 2009;119:1211–1219. doi: 10.1161/CIRCULATIONAHA.108.814947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aissaoui N, Puymirat E, Tabone X, Charbonnier B, Schiele F, Lefèvre T, Durand E, Blanchard D, Simon T, Cambou JP, Danchin N. Improved outcome of cardiogenic shock at the acute stage of myocardial infarction: a report from the USIK 1995, USIC 2000, and FAST-MI French nationwide registries. Eur Heart J. 2012;33:2535–2543. doi: 10.1093/eurheartj/ehs264 [DOI] [PubMed] [Google Scholar]
- 5.Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S, Jain D, Gotsis W, Ahmed A, Frishman WH, Fonarow GC. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3:e000590. doi: 10.1161/JAHA.113.000590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg RJ, Makam RC, Yarzebski J, McManus DD, Lessard D, Gore JM. Decade-long trends (2001-2011) in the incidence and hospital death rates associated with the in-hospital development of cardiogenic shock after acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2016;9:117–125. doi: 10.1161/CIRCOUTCOMES.115.002359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIR.0000000000000525 [DOI] [PubMed] [Google Scholar]
- 8.Harjola VP, Lassus J, Sionis A, Køber L, Tarvasmäki T, Spinar J, Parissis J, Banaszewski M, Silva-Cardoso J, Carubelli V, Di Somma S, Tolppanen H, Zeymer U, Thiele H, Nieminen MS, Mebazaa A; CardShock Study Investigators; GREAT network. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. 2015;17:501–509. doi: 10.1002/ejhf.260 [DOI] [PubMed] [Google Scholar]
- 9.Berg DD, Sukul D, O’Brien M, Scirica BM, Sobieszczyk PS, Olenchock BA, Bohula EA, Morrow DA. Outcomes in patients undergoing percutaneous ventricular assist device implantation for cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2016;5:108–116. doi: 10.1177/2048872615584079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rab T, Ratanapo S, Kern KB, Basir MB, McDaniel M, Meraj P, King SB 3rd, O’Neill W. Cardiac shock care centers: JACC review topic of the week. J Am Coll Cardiol. 2018;72:1972–1980. doi: 10.1016/j.jacc.2018.07.074 [DOI] [PubMed] [Google Scholar]
- 11.van Diepen S. Norepinephrine as a first-line inopressor in cardiogenic shock: oversimplification or best practice? J Am Coll Cardiol. 2018;72:183–186. doi: 10.1016/j.jacc.2018.04.052 [DOI] [PubMed] [Google Scholar]
- 12.Libby P, Pasterkamp G. Requiem for the ‘vulnerable plaque’. Eur Heart J. 2015;36:2984–2987. doi: 10.1093/eurheartj/ehv349 [DOI] [PubMed] [Google Scholar]
- 13.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong TY, Liao YB, Zhao ZG, Xu YN, Wei X, Zuo ZL, Li YJ, Cao JY, Tang H, Jilaihawi H, Feng Y, Chen M. Causes of death following transcatheter aortic valve replacement: a systematic review and meta-analysis. J Am Heart Assoc. 2015;4:e002096. doi: 10.1161/JAHA.115.002096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrow DA, Fang JC, Fintel DJ, Granger CB, Katz JN, Kushner FG, Kuvin JT, Lopez-Sendon J, McAreavey D, Nallamothu B, Page RL 2nd, Parrillo JE, Peterson PN, Winkelman C; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Council on Quality of Care and Outcomes Research. Evolution of critical care cardiology: transformation of the cardiovascular intensive care unit and the emerging need for new medical staffing and training models: a scientific statement from the American Heart Association. Circulation. 2012;126:1408–1428. doi: 10.1161/CIR.0b013e31826890b0 [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. [DOI] [PubMed] [Google Scholar]
- 18.Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. [DOI] [PubMed] [Google Scholar]
- 19.Killip T 3rd, Kimball JT Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967;20:457–464. [DOI] [PubMed] [Google Scholar]
- 20.Wayangankar SA, Bangalore S, McCoy LA, Jneid H, Latif F, Karrowni W, Charitakis K, Feldman DN, Dakik HA, Mauri L, Peterson ED, Messenger J, Roe M, Mukherjee D, Klein A. Temporal trends and outcomes of patients undergoing percutaneous coronary interventions for cardiogenic shock in the setting of acute myocardial infarction: a report from the CathPCI Registry. JACC Cardiovasc Interv. 2016;9:341–351. doi: 10.1016/j.jcin.2015.10.039 [DOI] [PubMed] [Google Scholar]
- 21.Jeger RV, Radovanovic D, Hunziker PR, Pfisterer ME, Stauffer JC, Erne P, Urban P; AMIS Plus Registry Investigators. Ten-year trends in the incidence and treatment of cardiogenic shock. Ann Intern Med. 2008;149:618–626. doi: 10.7326/0003-4819-149-9-200811040-00005 [DOI] [PubMed] [Google Scholar]
- 22.Menon V, Slater JN, White HD, Sleeper LA, Cocke T, Hochman JS. Acute myocardial infarction complicated by systemic hypoperfusion without hypotension: report of the SHOCK trial registry. Am J Med. 2000;108:374–380. [DOI] [PubMed] [Google Scholar]
- 23.Hochman JS. Cardiogenic shock complicating acute myocardial infarction: expanding the paradigm. Circulation. 2003;107:2998–3002. doi: 10.1161/01.CIR.0000075927.67673.F2 [DOI] [PubMed] [Google Scholar]
- 24.Wildhirt SM, Dudek RR, Suzuki H, Bing RJ. Involvement of inducible nitric oxide synthase in the inflammatory process of myocardial infarction. Int J Cardiol. 1995;50:253–261. [DOI] [PubMed] [Google Scholar]
- 25.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.