Abstract
Contemporary cardiac intensive care units (CICUs) provide care for an aging and increasingly complex patient population. The medical complexity of this population is partly driven by an increased proportion of patients with respiratory failure needing noninvasive or invasive positive pressure ventilation (PPV). PPV often plays an important role in the management of patients with cardiogenic pulmonary edema, cardiogenic shock, or cardiac arrest, and those undergoing mechanical circulatory support. Noninvasive PPV, when appropriately applied to selected patients, may reduce the need for invasive mechanical PPV and improve survival. Invasive PPV can be lifesaving, but has both favorable and unfavorable interactions with left and right ventricular physiology and carries a risk of complications that influence CICU mortality. Effective implementation of PPV requires an understanding of the underlying cardiac and pulmonary pathophysiology. Cardiologists who practice in the CICU should be proficient with the indications, appropriate selection, potential cardiopulmonary interactions, and complications of PPV.
Keywords: coronary intensive care unit, heart failure, mechanical ventilation, noninvasive ventilation, pulmonary edema, respiratory failure
The advanced cardiac intensive care unit (CICU) has evolved from a unit focused on arrhythmia monitoring and acute coronary interventions to a high-complexity environment caring for patients with cardiovascular diseases with multisystem organ dysfunction and severe noncardiac comorbidities (1,2). Previously common reasons for CICU admission, including acute myocardial infarction (MI), have diminished in their contribution, whereas noncardiac primary diagnoses, such as sepsis, acute renal failure, and respiratory failure, have increased (3). An analysis of Medicare data from 2003 to 2013 suggested that a substantial proportion of patients admitted to CICUs in the United States had noncardiac primary diagnoses, driven mainly by infections and respiratory diseases (4).
Within the changing CICU environment, it has become more important for cardiologists to be able to manage aspects of their patients’ general critical care, including noninvasive positive pressure ventilation (NI-PPV) and invasive mechanical positive pressure ventilation (IM-PPV) (3–5). Treatment with positive pressure ventilation (PPV) rose to nearly 15% of admissions in the Medicare population in 2013 (4,5). Cardiologists who take primary responsibility for patients in the CICU should have a sophisticated knowledge of evidence-based applications of NI- and IM-PPV and their cardiopulmonary interactions, and should be able to tailor ventilatory strategies to an individual patient’s underlying cardiovascular conditions. Moreover, any cardiologist who cares for patients in the CICU should have a working knowledge of these interventions. In the first half of this review, we address fundamental concepts of pulmonary mechanics and PPV; in the second half, we discuss specific clinical applications of these concepts in the CICU. In each section, we have highlighted key principles of broad relevance and identified those elements that are focused on the interests of the advanced provider. When possible, we have aimed to provide a practical framework or advice that may be useful for the clinician based on the experience of the writing group and on the available published data. Given the paucity of strong-quality studies specifically designed to assess PPV in the CICU, these practical points are not intended to be formal clinical practice guidelines.
BASIC CONCEPTS OF PULMONARY MECHANICS AND CARDIOPULMONARY PHYSIOLOGY
The cardiovascular and pulmonary systems work in a close relationship, and changes in one system often affect the other (6,7). Therefore, the basics of pulmonary mechanics are relevant to all cardiovascular providers who care for patients undergoing PPV. The key concepts are listed in Table 1.
TABLE 1.
Basic Pulmonary Physiology and Cardiopulmonary Interactions
| Ppleural Affects RV and LV Preload and Afterload |
|---|
| 1. Ppleural is determined by the balance of the tendency of alveolar units toward collapse (elastic recoil) versus of the thoracic wall to spring outwards and action of respiratory muscles |
| 2. Changes in Ppleural generally influence RV inflow and LV outflow, while changes in transpulmonary pressure (Palv-Ppleural) influence RV outflow and LV inflow. |
| 3. Negative Ppleural a) increases venous return and preload; b) decreases RV afterload; and c) increases LV afterload. |
| 4. Positive pressure ventilation increases Ppleural and a) decreases preload; b) increases RV afterload; and c) decreases LV afterload |
| 5. Large shifts in Ppleural (e.g., respiratory distress) can significantly increase LV afterload. |
| PEEP Affects RV and LV Hemodynamics |
| 1. Total PEEP is the sum of extrinsic PEEP (generated by the ventilator) and intrinsic or auto-PEEP (due to incomplete exhalation). |
| 2. Extrinsic PEEP is commonly used in the CICU for its beneficial effects on oxygenation, alveolar recruitment, airway patency, and preload. |
| 3. PEEP: a) increases pulmonary vascular resistance; b) decreases RV and LV preload; c) decreases LV afterload; and d) reduces LV compliance through interventricular dependence. |
| 4. The effect of PEEP on cardiac output varies with preload dependence and LV contractility and compliance. |
| Airway Pressure Influences Hemodynamics Via its Impact on Alveolar Pressure and Pleural Pressure |
| 1. Airway pressure is determined by the flow, airway resistance, tidal volume, compliance of the chest wall and lung parenchyma, and the total end-expiratory pressure. |
| 2. Positive pressure ventilation exerts its effects on cardiovascular hemodynamics principally through its impact on Palv and Ppleural. |
| 3. In poorly compliant lungs, changes in intrathoracic pressure will have more pronounced effects on hemodynamics. |
LV = left ventricular; Palv = alveolar pressure; Paw = airway pressure; PEEP = positive end-expiratory pressure; Ppleural = pleural pressure; RV = right ventricular.
DEFINITIONS AND BASICS OF PULMONARY MECHANICS.
Intrapleural pressure (Ppleural), also referred to as intrathoracic pressure, influences cardiovascular physiology. At rest, the functional residual capacity is determined by 2 opposing forces: the thoracic wall with a tendency to spring outwards, and the alveolar units with a tendency to collapse. In the resting state, Ppleural is slightly negative (Figure 1A). During spontaneous inspiration, contraction of the diaphragm and intercostal muscles renders Ppleural more negative, with resultant hemodynamic effects (Figure 1B). During respiratory distress, other auxiliary respiratory muscles (e.g., scalene, sternocleidomastoid) are recruited. Passive expiration occurs through alveoli and chest wall recoil, making Ppleural less negative (8). In patients with obstructive lung disease, exhalation might also require an active component, using abdominal muscles and intercostal muscles during forced expiration.
FIGURE 1. Effects of Pleural Pressure on Hemodynamics During Spontaneous Respiration.
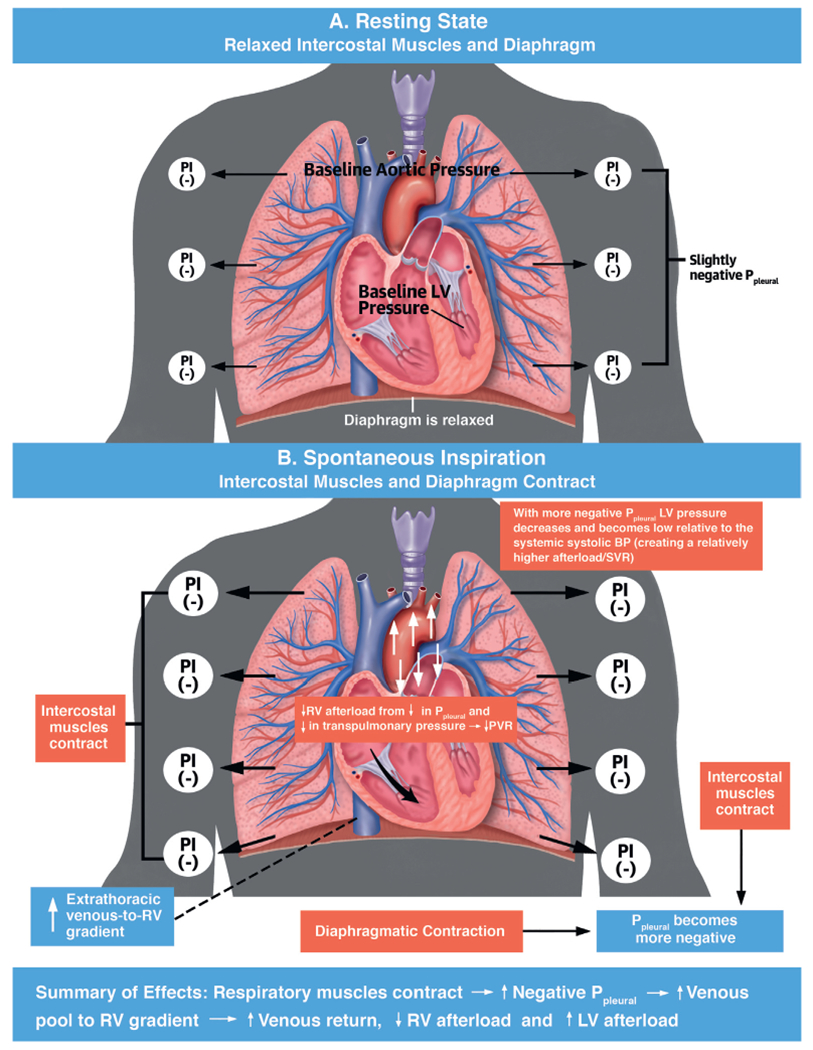
(A) In the resting state, Ppleural is slightly negative. (B) During spontaneous inspiration, Ppleural declines with diaphragmatic and intercostal muscle contraction. BP = blood pressure; LV = left ventricular; Ppleural = pleural pressure; PVR = pulmonary vascular resistance; RV = left ventricular; SVR = systemic vascular resistance.
Respiratory compliance and airway resistance are important components of the pulmonary mechanics of spontaneous breathing and PPV. Total compliance includes lung parenchymal and chest wall compliance. Lung compliance is defined as the change in volume over the change in pressure (ΔV/ΔP) in the alveoli. Chest wall compliance is influenced by extrapulmonary factors, including obesity, thoracic cage deformities, and intrabdominal pressure, as well as, rarely, medications (e.g., fentanyl-induced chest wall rigidity) (9). Plateau pressure (Pplat), which is relevant in patients undergoing PPV, refers to the alveolar pressure (Palv) at end-inspiration and is the maximal Palv in the respiratory cycle. In ventilated patients, Pplat is measured during an end-inspiratory pause (zero flow) and can be used to estimate total compliance (volume/[pplat - positive end-expiratory pressure]). Airway resistance is the change in pressure over flow as explained by Ohm’s law (resistance = ΔP/flow). Airway resistance is related to the diameter of the airways and can be influenced by bronchospasm, tracheal or upper airway pathology, mucus plugging, or airway remodeling. Increased airway resistance will also decrease dynamic lung compliance (Figure 2).
FIGURE 2. Pressure-Time Curve During Volume Control Ventilation.
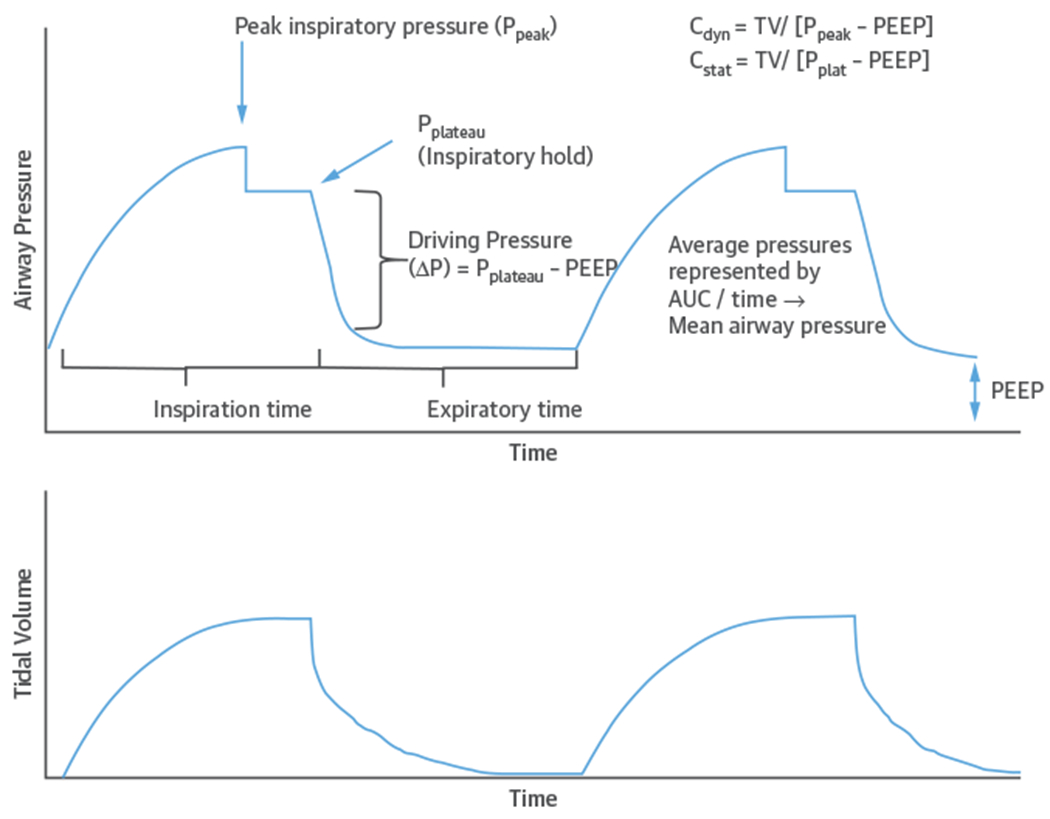
Relationship among airway pressure, flow, and time with static compliance (Cstat) and dynamic compliance (Cdyn). AUC = area under the curve; PEEP = positive end-expiratory pressure; Ppeak = peak pressure; Pplat = plateau pressure; Ppleural = pleural pressure; TV = tidal volume.
Additional PPV parameters that influence cardiopulmonary interactions require definition. First, positive end-expiratory pressure (PEEP) is the pressure above atmospheric pressure maintained throughout the respiratory cycle to prevent alveolar collapse at end-expiration. Second, mean airway pressure is the arithmetic average pressure over the entire ventilatory cycle (Figure 2). Third, peak airway pressure reflects the pressure required to both overcome the airway resistance and generate the tidal volume (TV). When TV and compliance are constant (e.g., during volume control ventilation), peak airway pressure correlates with airway resistance. However, if lung compliance is decreased, peak pressures will increase. Measuring Pplat by performing an end-inspiratory hold can help distinguish between these contributors (Figure 2). Pplat will be higher in less compliant lungs. The fourth parameter is transpulmonary pressure, defined as the difference between Palv and pleural pressure (Palv – Ppleural), which influences hemodynamics in the left ventricle (LV) and right ventricle (RV). Because PPV increases both Palv and Ppleural, it can be difficult to accurately estimate transpulmonary pressures. For advanced providers, an esophageal balloon can be used to estimate Ppleural and to calculate transpulmonary pressure if needed (10).
PULMONARY MECHANICS OF PPV.
PPV creates a pressure gradient between the ventilator and the patient so that air moves in and out of the lungs as described by the equation of motion:
where Paw is the airway pressure, Pmuscle is the pressure generated by the patient, F is flow, R is resistance, V is volume, C is compliance, and PEEPT is total positive end-expiratory pressure (11). PEEPT is the total of extrinsic PEEP (generated by the ventilator) and intrinsic or auto-PEEP (due to incomplete exhalation). This equation reflects mathematically that peak pressure (Ppeak) in the system is the sum of the pressure at the end of expiration (PEEPT), the pressure created by distension of the alveoli during inspiration (V/C), and the pressure created by resistance in the airways (F ⋅ R). The pressure in the alveolus (Palv) is reflected by V/C + PEEPT.
Extrinsic PEEP is commonly used in the CICU due to beneficial effects on oxygenation (due to the linear relation between PEEP and partial pressure of oxygen), alveolar recruitment, airway patency, and preload (12,13). Low levels of PEEP (~5 cmH2O) are routinely used in intubated patients to maintain airway patency and avoid atelectasis. Although there is no established optimal PEEP, a range with higher levels of PEEP can be useful for some conditions, including heart failure (HF) and noncardiogenic pulmonary edema (13,14). PEEP is generally safe when properly used and monitored along with Ppeak, Pplat, and TV (13).
During PPV, exhalation is a passive process during which Palv becomes the driving pressure and progressively declines from its maximum (Pplat) down to the final end-expiratory pressure (PEEPT), determined by its extrinsic and intrinsic components defined in the previous text (Figure 2). During this phase, Palv > Paw, which is determined by the extrinsic PEEP set by the clinician. As an advanced concept, the amount of air trapped in the lungs at the end of exhalation depends on how quickly air exits the lungs, which in turn is determined by the expiratory time as influenced by resistance and compliance. Patients with high resistance (e.g., chronic obstructive pulmonary disease [COPD]) need longer exhalation time and are more prone to developing auto-PEEP, especially in those with high lung volumes (e.g., emphysema).
When considering the management of PPV in the cardiac patient, as described in the sections that follow, a fundamental concept is that the patient’s airway and lung characteristics (resistance and compliance) determine the relationship between physician-set parameters and resultant volume, pressures, and flow.
PPV AND LV PHYSIOLOGY.
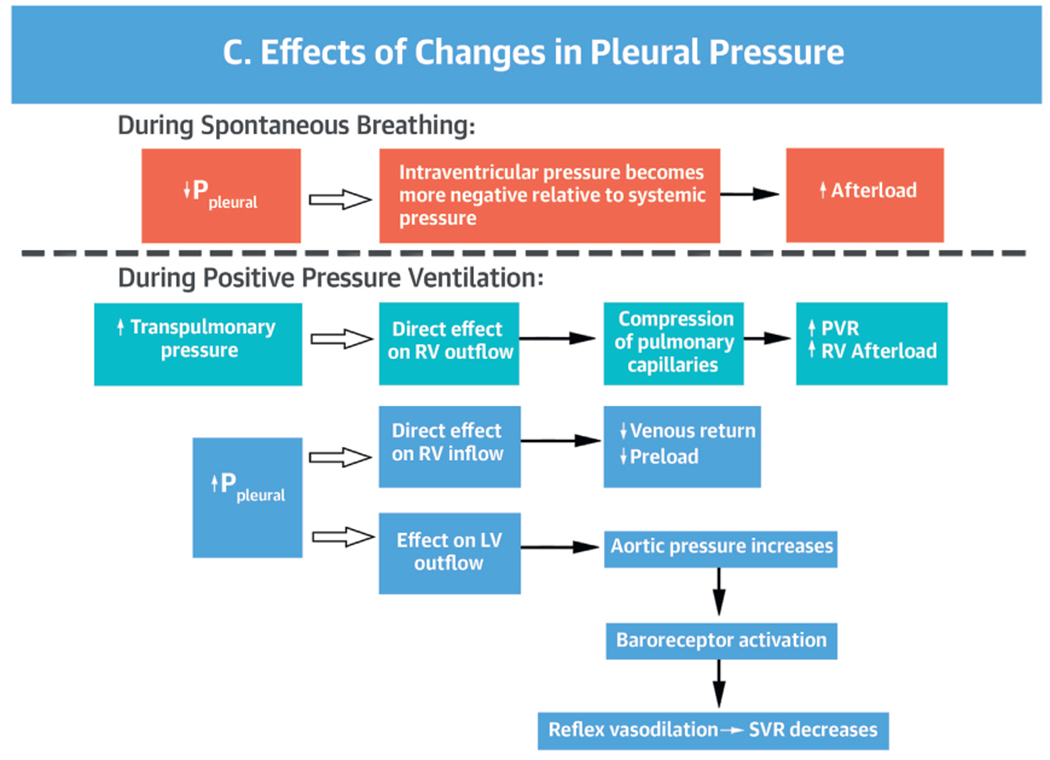
PPV exerts its effects on cardiovascular hemodynamics principally through its effect on Palv and Ppleural. Both Ppeak and end-expiratory pressure (determined by PEEP) influence hemodynamics (Figure 3). Transpulmonary pressure also affects the RV and LV. In general, changes in Ppleural influence inflow to the RV and outflow from the LV, whereas changes in transpulmonary pressure (Palv-Ppleural) influence outflow from the RV and inflow to the LV by affecting the pulmonary vasculature. Hence, when Ppleural drops, LV systolic pressure becomes more negative relative to systemic circulation increasing LV afterload (Figure 3C) (15).
FIGURE 3. Effects of Positive Pressure Ventilation on Hemodynamics.
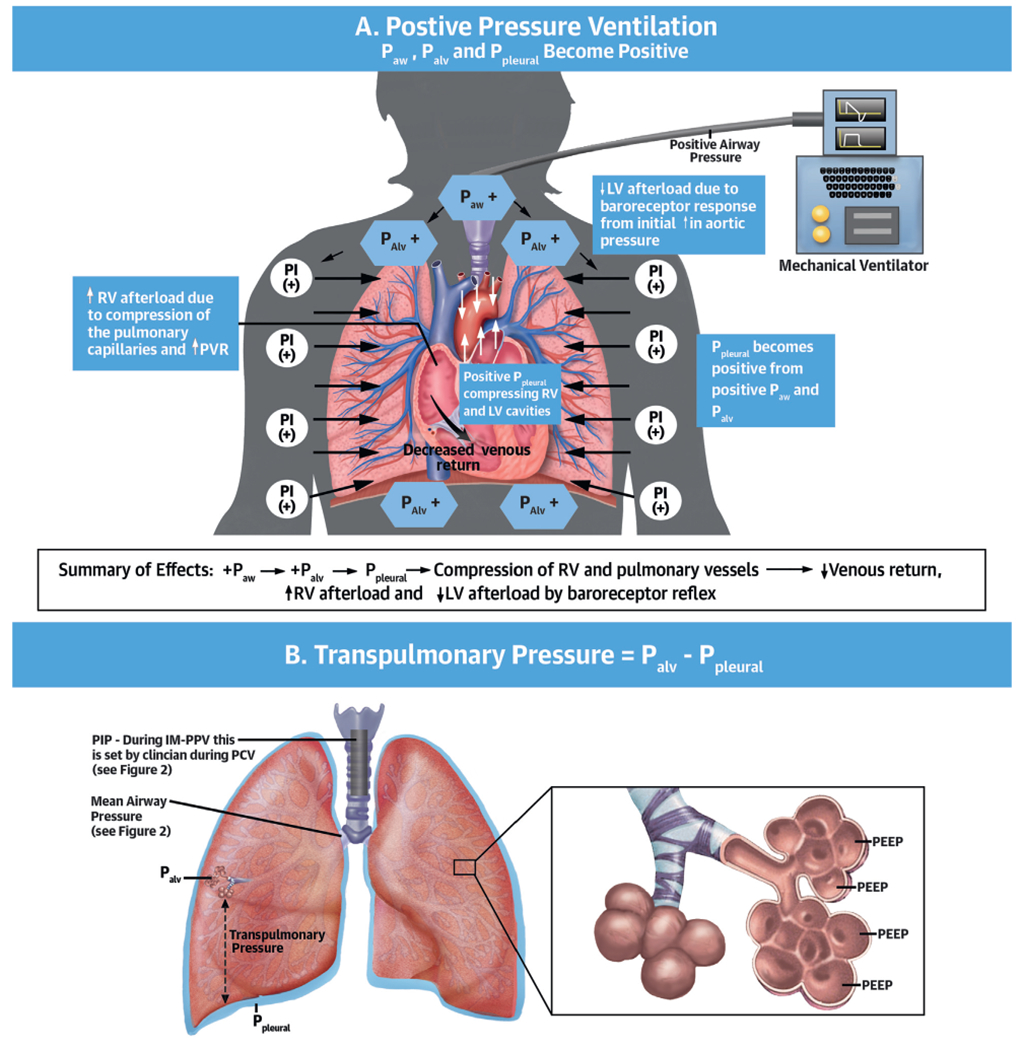
(A) Effects of positive pressure ventilation. (B) Schematic representation of transpulmonary pressures and mean airway pressure. (C) Effects of Ppleural and transpulmonary pressure. Palv = alveolar pressure; Paw = airway pressure; other abbreviations as in Figures 1 and 2.
Experimental and in vivo studies have demonstrated interactions between PPV and LV preload and afterload (Table 2) (6,7,16). During PPV, LV wall tension remains constant due to an equivalent increase in the Ppleural, aortic pressure, and LV pressure, generating a flow gradient between thorax and peripheral organs (intra-aortic balloon pump-like effect) (Online Figure 1). When Ppleural increases, aortic pressure initially goes up, triggering autoregulation by peripheral baroreceptors lowering systemic vascular resistance and LV afterload (17), improving cardiac output (CO) (Figure 3A) (14). Additionally, in patients with a dilated LV and high filling pressures in whom functional mitral regurgitation (MR) impairs CO, PEEP can improve ventricular diameter and MR (18,19). In addition, through ventricular interdependence, influences of PPV on the RV may ultimately affect LV performance. A pressure overloaded and dilated RV can displace the interventricular septum toward the LV, decreasing LV pre-load and stroke volume (Online Figure 2) (20,21). These effects of PEEP on LV and RV hemodynamics will determine the net influence on CO (Central Illustration).
TABLE 2.
Beneficial Effects of Positive Pressure Ventilation in Patients With Right and Left Ventricular Dysfunction
| Mechanism | Effect | |
|---|---|---|
| Direct effects from PEEP | • Decreases LV afterload • Decreases LV diameter, leading to decreased MR • Increases transmural pressure • Increases Palv at the end of expiration |
• LV unloading • Improved cardiac output • Improved cardiac output • Improved compliance (i.e., prevention of alveolar collapse) |
| Effects from or on gas exchange | • Reverses hypoxic vasoconstriction • Decreases in preload • Improves ventilation/perfusion matching |
• Lower RV afterload • Improved pulmonary congestion • Improved oxygenation |
| Effects from ventilatory support | • Improves work of breathing • Improves hypercarbia and acidosis |
• Improved tissue perfusion • Decreased myocardial consumption of oxygen • Improved RV afterload |
| Systemic effect | • Optimizes gas exchange, hence oxygenation and tissue perfusion | • Improved metabolic demand and peripheral perfusion |
FIO2 = fraction of inspired of oxygen; MR = mitral regurgitation; Palv = alveolar pressure; other abbreviations as in Table 1.
CENTRAL ILLUSTRATION. Potential Physiological Effects of Positive End-Expiratory Pressure on Ventricular Function and Cardiac Output.
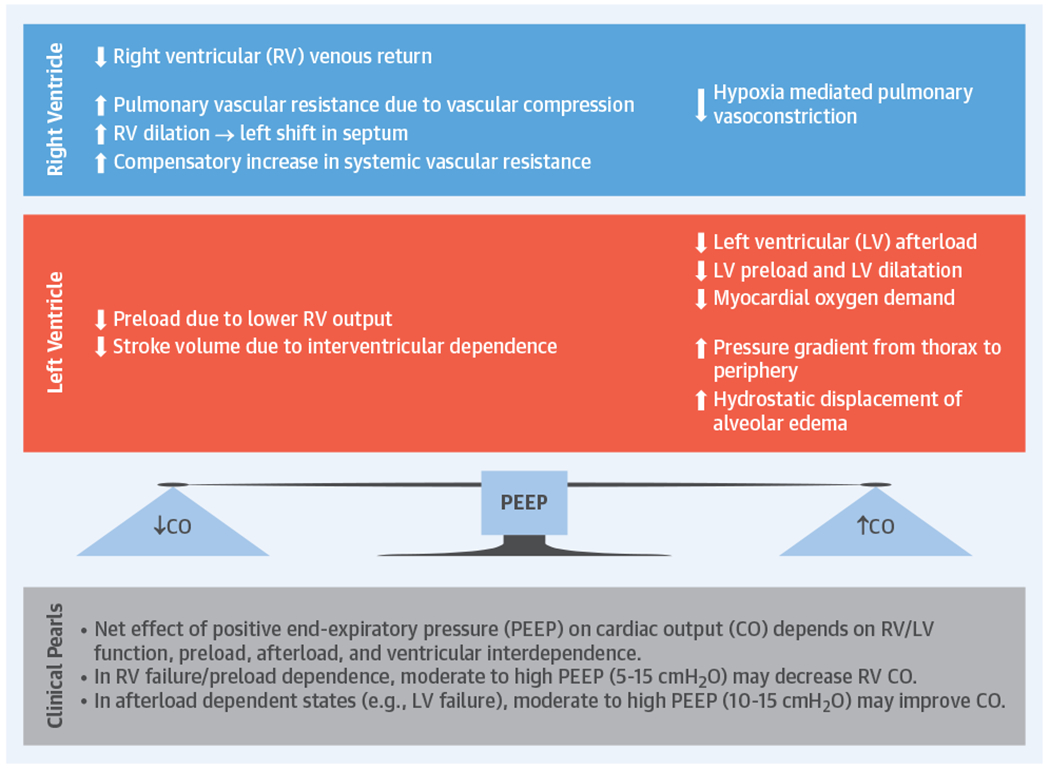
Positive end-expiratory pressure (PEEP) will have variable effects on cardiac output (CO) depending on left ventricular (LV) and right ventricular (RV) function, preload and filling pressures. Note that in patients with noncompliant lungs from causes other than cardiogenic pulmonary edema, the effect of PEEP on Ppleural and pre-load might not be as pronounced.
PPV AND RV PHYSIOLOGY.
PPV can have important effects on RV preload, afterload, and myocardial perfusion. During spontaneous breathing, the negative Ppleural created during inspiration augments venous return, preload, and RV filling (22). Because the pressure gradient between the venous circulation and the RV is normally only about 4 to 8 mm Hg (23), small changes in Ppleural can produce relatively large effects on venous return and CO (22,23). When Ppleural drops, the extrathoracic venous-to-RV gradient increases and preload increases.
In contrast, during PPV, the ventilator generates positive Paw, transmitted into Palv and Ppleural, leading to a positive transpulmonary pressure (Palv-Ppleural). Both PEEP and mean Paw have a direct effect on the loading conditions of the heart (Central Illustration). During invasive and noninvasive PPV, increased PEEP and Ppleural decrease venous return and thus RV preload (Figure 3) (15). At the same time, PPV increases RV afterload by increasing transpulmonary pressure causing microvascular collapse and increasing pulmonary vascular resistance (PVR). Similarly, the use of PEEP can increase RV afterload. Through its effects on lung volume, PEEP has a U-shaped effect on PVR. By relieving atelectasis, PEEP can open alveoli, enhance lung volume, and favorably tether blood vessels, decreasing PVR and improving blood flow (24). However, high PEEP can cause alveolar overdistension compressing extra-alveolar vessels, increasing PVR, and leading to a redirection of blood flow to poorly ventilated areas, which increases V/Q mismatch with consequent hypoxia and hypercarbia (Figure 4) (25).
FIGURE 4. Relationship Between Alveolar Volume/Pressure and Pulmonary Vascular Resistance.
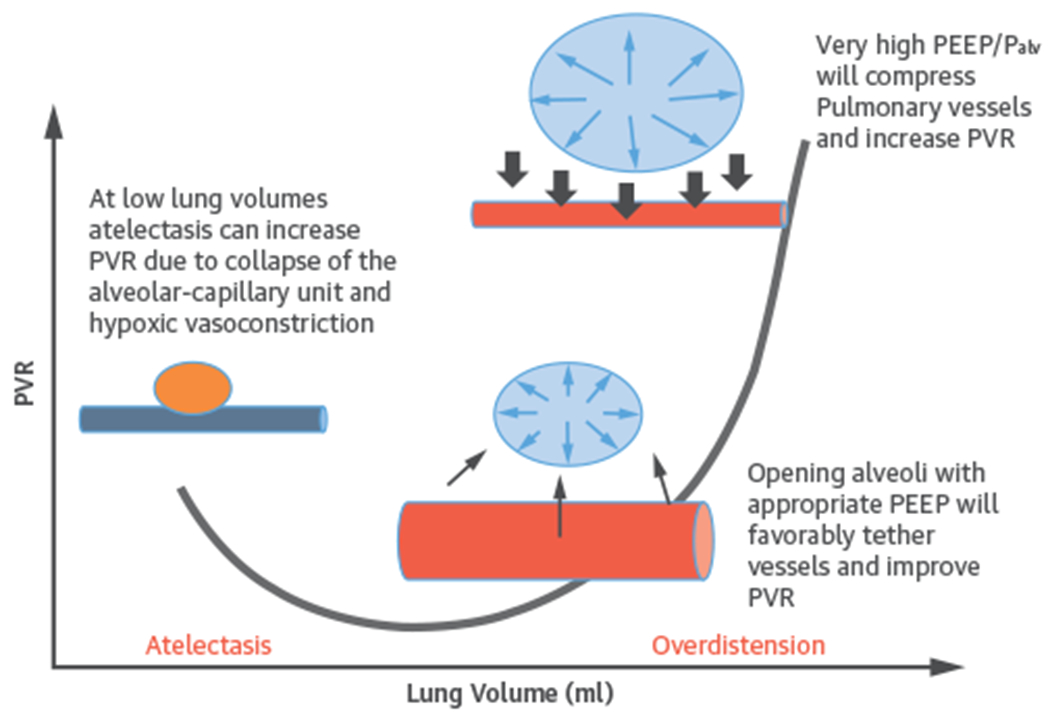
Adapted from West JB, Luks AM. Wes’s Respiratory Physiology: The Essentials. Philadelphia, PA: Wolters Kluwer Health Ed, 2015;92. PEEP = positive end-expiratory pressure; PVR = pulmonary vascular resistance. Blue vessel represents deoxygenated blood.
Changes in Ppleural reflecting changes in Palv from PEEP are highly dependent on alveolar compliance. For instance, in patients with cardiogenic pulmonary edema (CPE), lung compliance is decreased due to interstitial edema and fluid-filled alveoli. As PEEP decreases preload and concomitantly creates hydrostatic pressure that forces fluid out of the alveoli, compliance and gas exchange improve, and thus so does PVR. However, in patients with noncompliant lungs from other causes (e.g., acute respiratory distress syndrome [ARDS]), the effect of PEEP on Ppleural and preload is not as pronounced. In such patients, even small amounts of PEEP with small changes in Palv can increase RV afterload disproportionally by worsening PVR and impairing pulmonary blood flow (26). Patients with RV dysfunction are particularly sensitive to this adverse effect on PVR (Central Illustration).
Myocardial perfusion of the RV is dependent on Ppleural, RV pressure, and aortic pressure, and therefore is affected by PPV. During PPV in normotensive patients, aortic pressure remains higher than Ppleural and RV pressure, maintaining myocardial perfusion. However, in patients with low aortic pressure (e.g., shock), RV perfusion can be compromised by elevated Ppleural from high PEEP or high Paw (27). The limited myocardial mass of the RV wall makes it more sensitive to changes in afterload than to changes in preload (17), while the opposite is true for the LV (Online Figure 3). Therefore, taking each of these potential adverse effects into consideration, PEEP should be used with caution in patients with RV failure (17). Moreover, in patients with RV dysfunction secondary to obstructive lung disease (e.g., COPD), monitoring for and preventing auto-PEEP is particularly important to avoid precipitating hemodynamic instability and to prevent barotrauma (Online Figure 4) (28).
MODES AND APPLICATIONS OF OXYGENATION AND VENTILATORY SUPPORT IN THE CICU
HIGH-FLOW NASAL CANNULA.
Standard nasal cannulas are limited to a maximum flow of 6 l/min, and may not meet the demands of the severely hypoxemic patient. By comparison, high-flow nasal cannulas (HFNCs) deliver a heated and humidified flow of up to 60 l/min (29). By using flow rates higher than the inspiratory demand of the patient, HFNC can provide reliable inspired FiO2 (30). Along with improved oxygenation, with high flow rates, HFNC provides up to ~7 cmH2O of PEEP (29). Although 1 randomized trial has suggested lower mortality with HFNC compared with NI-PPV (31), other trials have indicated similar outcomes (32). There are no randomized controlled trials of HFNC specifically in patients with CPE (33).
NONINVASIVE PPV.
NI-PPV is an important modality for management of patients in the CICU. NI-PPV includes continuous positive airway pressure (CPAP) and bilevel positive airway pressure (BiPAP). Whereas CPAP provides continuous PPV, BiPAP provides separately titratable inspiratory positive airway pressure (IPAP) and expiratory positive airway pressure (EPAP) (which is functionally equivalent to PEEP in IM-PPV). BiPAP can reduce work of breathing, increase TV more than CPAP, and improve ventilation in patients with hypercarbia. Proper patient selection is integral to the successful use of NI-PPV (Table 3) (34).
TABLE 3.
Indications and Contraindications to Noninvasive Positive Pressure Ventilation
| Indications for NI-PPV | Contraindications to NI-PPV |
|---|---|
| • Cardiogenic pulmonary edema • Hypercapnic respiratory failure • COPD exacerbation • High-risk extubation • Immunocompromised patient with respiratory failure • Neuromuscular disease • Trauma (chest trauma) • Ventilatory support in the palliative patient • Post-operative hypoxic respiratory failure |
• Need for effective airway protection because of altered mental status, uncooperative patient, apnea, cardiac arrest, or seizures • Facial deformities because of pathology, recent surgery, or trauma • Hemodynamic instability • Aspiration risk (emesis, inability to control secretions) • Recent upper airway or gastrointestinal surgery |
COPD = chronic obstructive pulmonary disease.
There are several interfaces available for NI-PPV, including a total face mask (covering nose, mouth, and eyes), oronasal or full-face mask, nasal mask, and nasal pillows. Although all masks have advantages and disadvantages (Online Table 1), a mask covering both the nose and mouth is preferable to minimize leaks (35).
Initial NI-PPV settings in randomized controlled trials have varied. CPAP and EPAP are generally titrated to oxygenation, and IPAP (BiPAP only) is used to control ventilation (PaCO2). For CPAP, a typical starting pressure is 5 to 10 cmH2O, with titration by 2 cmH2O. If BiPAP is used, initial settings are often IPAP of 10 cmH2O and an EPAP of 5 cmH2O. Titration of the IPAP upward by 2 to 3 cmH2O can be made to improve hypercapnia, avoiding IPAP >20 cmH2O to prevent gastric insufflation and minimize aspiration risk (36). The FiO2 may start high and be titrated to a goal SaO2 (e.g., 94% to 98%) while avoiding SaO2 >98%. A trend in consensus toward avoiding hyperoxemia originates with observed associations between O2 therapy in nonhypoxemic patients and higher mortality (37), including some cardiovascular conditions, such as acute coronary syndromes, heart failure, stroke, and post-cardiac arrest (38,39).
If NI-PPV is going to be successful, the patient will generally show improvement, such as a decrease in respiratory rate, improved work of breathing, and improved gas exchange, in 1 to 2 h.
In well-selected patients, adverse effects are few and mostly related to the tolerability of the device/mask (40,41). More serious adverse effects can occur, including aspiration, mucus plugging, or hypotension (40). Online Table 2 describes factors associated with NI-PPV failure.
INVASIVE MECHANICAL PPV.
Indications for IM-PPV include refractory hypoxia or hypercarbia, unsustainable work-of-breathing, and need for airway protection in the setting of altered mental status, cardiorespiratory arrest, impending hemodynamic/respiratory collapse, active vomiting, or upper gastrointestinal bleeding. Modes of IM-PPV are preset algorithms that dictate the pattern of mandatory and spontaneous breaths delivered by the ventilator and translate into specific patient-ventilator interactions. The mode should be tailored to the clinical scenario to provide greatest benefit with the lowest risk of complications, while optimizing patient comfort and ease of liberation from IM-PPV (42).
More than 300 proprietary names represent about 50 modes. There are no compelling data to support the superiority of any specific mode for a given disease in adults. Although there is no consensus to classify IM-PPV modes, a simplified nomenclature has been proposed (42) based on three factors: The control variable (pressure, or volume/flow), the breath sequence (continuous mandatory ventilation, intermittent mandatory ventilation, or continuous spontaneous ventilation), and the targeting scheme (a specific ventilator pattern in response to patient-ventilator feedback) (Online Figure 5). Details of this taxonomy are explained elsewhere (42).
Management of ventilator settings.
When initiating and managing IM-PPV, 4 key elements (triggering, inspiratory control variable, cycling, and expiratory period PEEP) need to be considered in relation to each phase of a breath. These elements are included in Figure 5 and are explained in detail for the interested reader in Online Table 3. The control variable is selected by the clinician and describes how the ventilator regulates the amount of volume or pressure to deliver in each breath including pressure control ventilation or volume control ventilation. Another control type is volume-targeted ventilation, which is a dual-control mode with a set target TV, allowing the ventilator to automatically adjust the pressure level to achieve the targeted TV (e.g., “VC+” or “autoflow”).
FIGURE 5. Schematic Overview of Ventilator Modes and Settings.
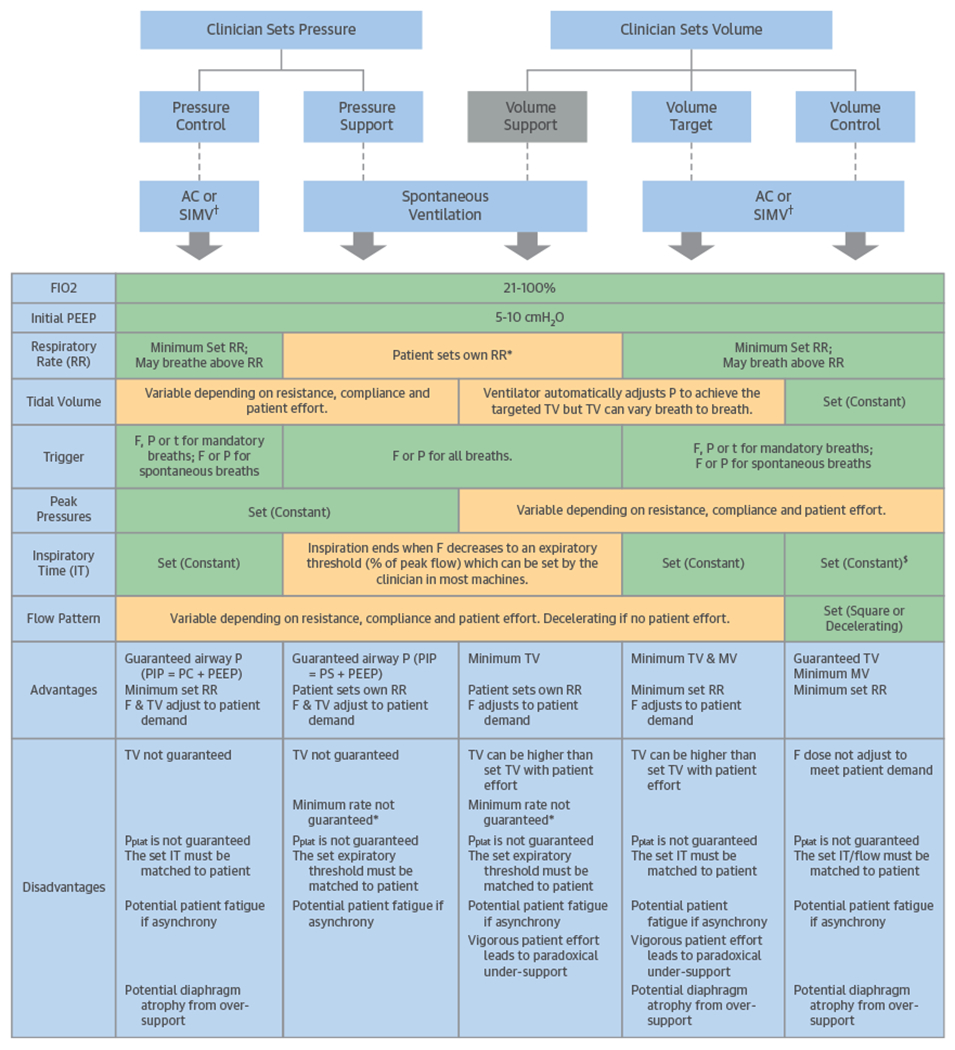
Each of the settings set by the clinician and the resulting ventilatory parameters are shown for the major modes of positive pressure ventilation. More advanced modes and breath types (proportional assist ventilation, neurally adjusted ventilatory assist, and airway pressure release ventilation) are not described. Green = variable set (constant) by the clinician; orange = variable changes according to patient-ventilator interaction. *Classically no back up. Alarm can be set if patien’s RR is < set limit. †For SIMV mode (SIMV PC, SIMV VC, or SIMV Volume Target), the descriptions apply only to set breaths and not spontaneous breaths. $For some machines, clinician sets TV and flow pattern, which determines IT. For other machines, IT is set directly. A/C = assist control; F = flow; FIO2 = fraction of inspired oxygen; IT = inspiratory time; MV = minute ventilation; P = pressure; PC = pressure control; PEEP = positive end expiratory pressure; PIP = peak inspiratory pressure; Pplat = plateau pressure; PS = pressure support; R = resistance; RR = respiratory rate; SIMV = synchronized intermittent mandatory ventilation; t = time; TV = tidal volume.
Currently, the most commonly used modes of IM-PPV can be classified by a simplified approach into pressure control, volume control, and pressure support (Tables 4 and 5). These modes can be sub-divided into assist control, synchronized intermittent mandatory ventilation (SIMV), and spontaneous ventilation (Figure 5). The distinguishing feature of assist control is that spontaneous patient effort results in delivery of an identical breath as the mandatory breath (same TV or driving pressure, same inspiratory time/flow pattern) (Figure 5). In contrast, during SIMV, these clinician-selected settings apply only to the mandatory breaths, while spontaneous breaths differ by being completely initiated and terminated by the patient effort with whatever volume, pressure, or flow the patient can exert without any ventilator assistance. SIMV is a form of intermittent mandatory ventilation. In the spontaneous ventilation mode, the clinician sets the FIO2, PEEP, and supporting volume or pressure, but not the respiratory rate, inspiratory time, or flow pattern, so the patient must initiate all breaths. An additional mode, airway pressure release ventilation (APRV), is a pressure control ventilation mode used in patients with refractory hypoxia. APRV has a prolonged inspiratory phase in an inverse ratio ventilation, where unrestricted spontaneous breathing can occur at any time (43).
TABLE 4.
Simplified Approach to Modes of Invasive Mechanical Positive Pressure Ventilation
| Volume Control Ventilation | Pressure Control Ventilation | Pressure Supported Ventilation |
|---|---|---|
| • The independent variable is volume and flow • The dependent variable is pressure |
• The independent variable is pressure • The dependent variables are volume and flow |
|
TABLE 5.
Advantages and Disadvantages of PS Mode
| Pressure Supported Ventilation | |
|---|---|
| Advantages | Disadvantages |
| 1. The patient can control the depth, length, and flow of each breath 2. Allows flexibility in ventilator support 3. Improves synchrony and diaphragmatic work |
• Excessive level of support can result in: 1. Respiratory alkalosis 2. Hyperinflation 3. Ineffective triggering 4. Apneic spells • Suboptimal support can result in: 1. Diaphragmatic fatigue 2. Respiratory acidosis |
PS = pressure support.
No ventilator mode is optimal for all cardiac patients. However, modes that allow full spontaneous breaths with no ventilator assistance (e.g., SIMV) can increase afterload and myocardial oxygen consumption when some of the spontaneous breaths are not fully supported, potentially leading to patient-ventilator dyssynchrony. Such modes might not be optimal for cardiac patients with cardiogenic shock or myocardial ischemia (44–46). Finding the appropriate level of support mitigates diaphragmatic fatigue and avoids diaphragmatic atrophy, both of which can increase duration of mechanical ventilation (47). Although there are no proven strategies to prevent diaphragmatic dysfunction, using minimally necessary supportive settings is reasonable.
After selecting a ventilator mode, the clinician is responsible for setting up the individual parameters (Figure 5, Online Table 4). FiO2 and PEEP have dominant effects on oxygenation and should be adjusted to achieve an acceptable oxygen saturation (SaO2), with early de-escalation of FiO2 to minimize the risk of oxygen toxicity. PEEP is typically set at 5 cmH2O and can be adjusted to the desired SaO2 while taking in consideration the effects of excessive PEEP (e.g., decreased preload, barotrauma) and those of inappropriately very-low PEEP (e.g., atelectrauma) as well as the effects on the RV and LV.
The product of TV and respiratory rate (minute ventilation) predominantly affects PCO2 and pH. TV should be set not to exceed 6 to 10 ml/kg (6 to 8 ml/kg of ideal body weight for ARDS), while keeping Pplat <30 cmH2O to minimize the risk of alveolar overdistension. Although low TV ventilation has been studied predominantly in ARDS, overdistension from high TV might also be harmful in cardiac patients according to 1 study, which showed that TV >9.3 ml/kg of ideal body weight was associated with adverse outcomes in the CICU (48). Inappropriately high TV may cause “volutrauma” from overdistension, but inappropriately low TV can cause atelectasis, patient-ventilator dyssynchrony, and suboptimal gas exchange.
The respiratory rate can be adjusted to achieve acceptable PCO2 and pH for the patient’s needs (e.g., permissive hypercapnia for refractory hypoxia during ARDS may be appropriate, whereas limiting acidosis and hypercapnia is beneficial in patients with RV dysfunction). It is important to underscore that data from studies in pulmonary syndromes may not be extrapolated to all cardiac patients, highlighting the need for research specifically done in CICU settings.
Each ventilator setting has both intended benefits and potential adverse consequences; therefore, to assess the patient’s response to IM-PPV and risk of complications, the clinician must monitor the hemodynamics, blood gases, TV, minute ventilation, Pplat, auto-PEEP, and patient-ventilator interactions. The clinician needs to be meticulous about avoiding auto-PEEP by allowing adequate exhalation time or by adjusting flow curves and inspiratory flow velocities. Auto-PEEP can lead to progressive air trapping and can compromise preload if not detected and resolved (Figure 6).
FIGURE 6. Effect of Auto-PEEP on Volume-Control Ventilation and Pressure-Control Ventilation.
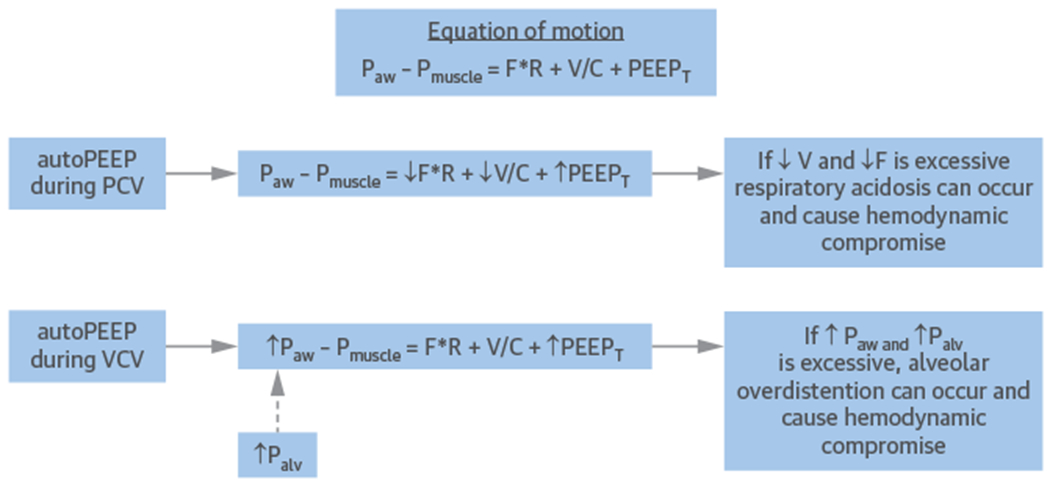
As auto-PEEP increases, a compensatory effect will occur on flow (F), volume (V), Paw, and Palv. If auto-PEEP is not addressed, hemodynamic compromise will ultimately occur by respiratory acidosis (during pressure control) or alveolar overdistension (during volume control). Abbreviations as in Figures 2 and 3.
APPLICATIONS OF PPV IN CARDIAC PATIENTS
NI-PPV IN CARDIOGENIC PULMONARY EDEMA.
Among critical cardiac conditions, CPE complicates 15% to 40% of HF admissions. Often, supplemental oxygen alone is insufficient for CPE, and more advanced support is necessary with NI-PPV. Through mechanisms already described in this review, NI-PPV increases intrathoracic pressure, reduces RV and LV preload and LV afterload (5,49), improves dyspnea, and augments oxygenation (41,50,51). Further, hospitals that use NI-PPV have lower intubation rates (52).
The 3CPO (Three Interventions in Cardiogenic Pulmonary Oedema) (41) was a multicenter, randomized, controlled trial that compared CPAP (n = 346) and BiPAP (n = 356) with standard oxygen therapy (n = 367) in patients with CPE. At 7 days, there was no difference in the primary outcome of mortality comparing NI-PPV with standard oxygen therapy (9.5% vs. 9.8%; p = 0.87). Furthermore, there was no difference in the combined endpoint of death or intubation rate between NI-PPV types. However, at 1 h, there were significant improvements in dyspnea, heart rate, acidosis, and hypercapnia with NI-PPV (41). This study was limited by low intubation rates and mortality, and considerable crossover between groups. In contrast, 2 subsequent meta-analyses reported reduced in-hospital mortality and intubations with NI-PPV in CPE (53,54), but most of the included trials were small, of lower quality, and with variable definitions and severity of CPE.
After a decision to use NI-PPV is made, it should be instituted as soon as possible (Figure 7) (55). Either CPAP or BiPAP is a reasonable first option for the patient with CPE. In the patient with acidosis, hypercapnia, or concomitant COPD, BiPAP is preferred as the initial modality. If NI-PPV is going to be successful, the patient will generally show improvement in respiratory and heart rate, work-of-breathing, and gas exchange in ~60 min (56). If the patient continues to deteriorate, intubation should immediately be considered.
FIGURE 7. Example Algorithm for Oxygen Therapy and NI-PPV in Patients With Pulmonary Edema.
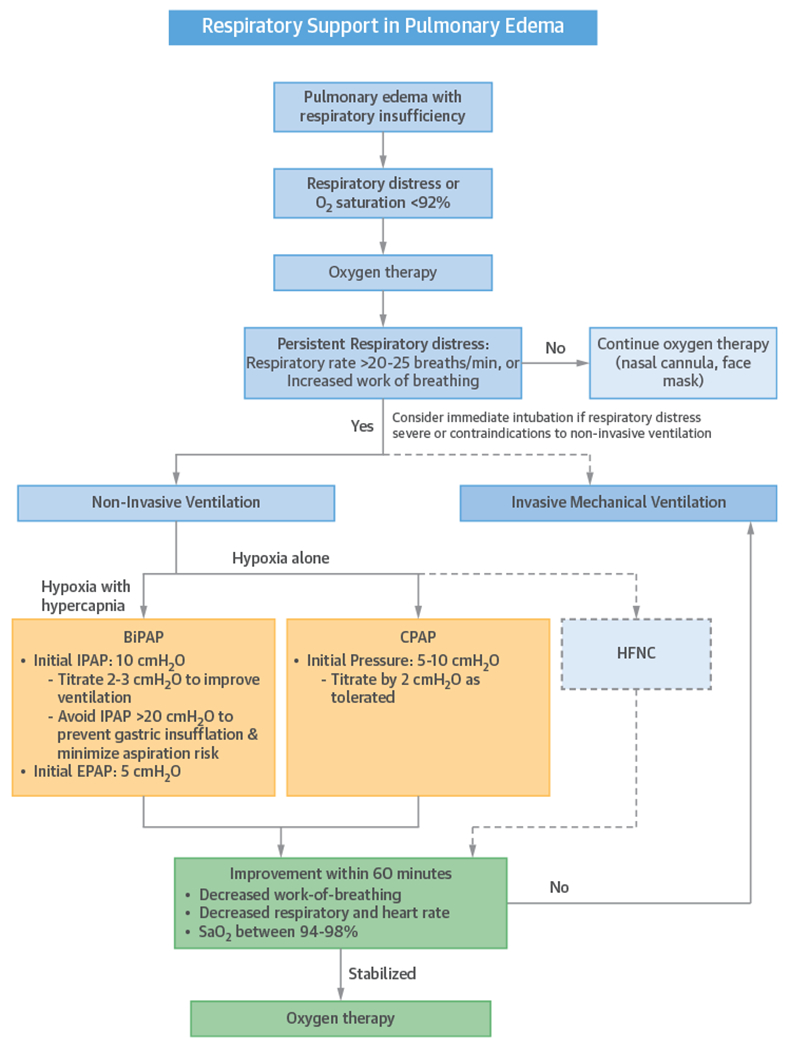
BiPAP = bilevel positive airway pressure; CPAP = continuous positive airway pressure; EPAP = expiratory positive airway pressure; HFNC = high-flow nasal cannula; IPAP = inspiratory positive airway pressure.
IM-PPV IN THE PATIENT WITH LEFT HEART FAILURE AND CARDIOGENIC SHOCK.
Up to 80% of patients presenting with cardiogenic shock (57–59) develop respiratory failure. As described already in this review (see the “Pulmonary Mechanics of PPV” section), IM-PPV with PEEP has favorable effects on hemodynamics in patients with HF (Table 2). These effects include decreasing LV afterload by lowering transthoracic and transpulmonary pressure (60); improving congestion and unloading the LV by decreasing preload (61); optimizing LV and RV myocardial perfusion and relieving ischemia by providing better oxygenation, reversing hypoxic pulmonary vasoconstriction, and decreasing work-of-breathing; and improving LV geometry and related functional MR (18,19,62). Through these effects on preload and afterload, PEEP improves alveolar and interstitial edema. An example algorithm for implementation of IM-PPV in patients with heart failure or shock is shown in Figure 8.
FIGURE 8. Example Algorithm for IM-PPV in Patients With Heart Failure or Cardiogenic Shock.
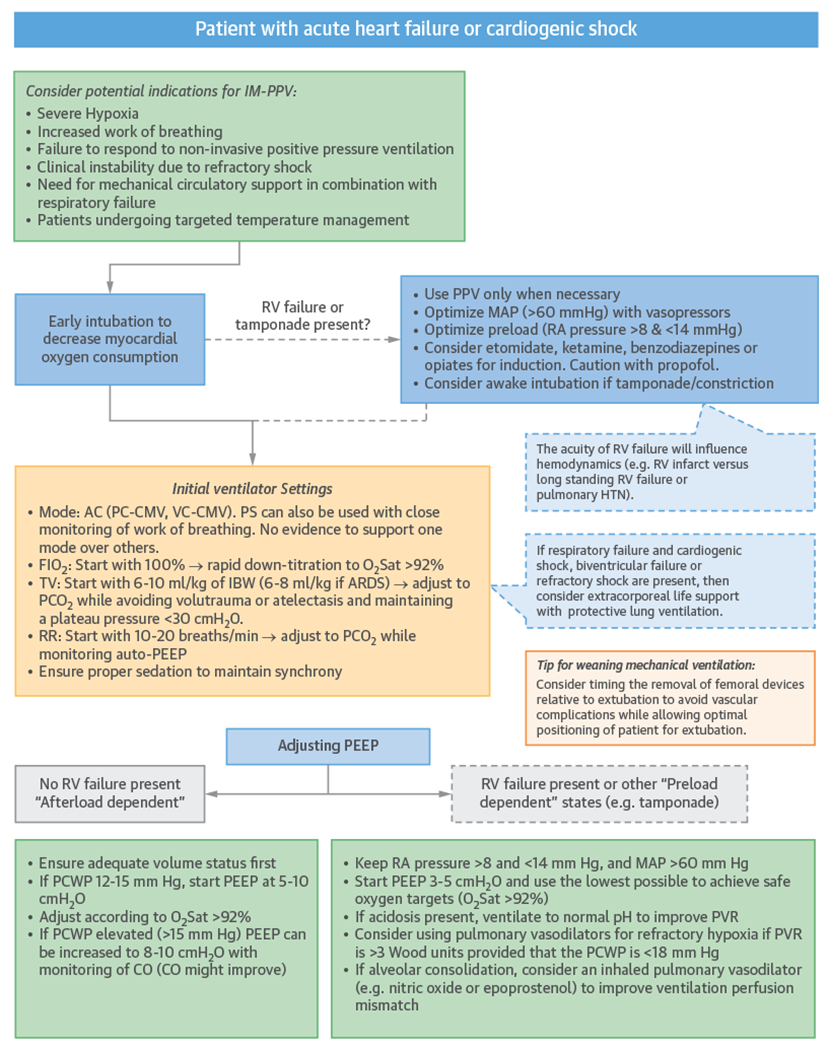
ARDS = acute respiratory distress syndrome; CO = cardiac output; IBW = ideal body weight; IM-PPV = invasive mechanical positive pressure ventilation; MAP = mean arterial pressure; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RA = right atrium; other abbreviations as in Figure 5.
Despite these benefits, there are concerns about potential detrimental effects of PEEP in patients with LV dysfunction, resulting in decreased CO due to decreased RV preload and increased afterload (16). However, in vivo studies have not supported this concern, especially when RV function and volume status are normal (63,64). Therefore, in practice, it appears relevant to differentiate physiological effects of PEEP in normal versus failing ventricles. The normal LV is preload dependent and more sensitive to a reduction in venous return with PEEP. In contrast, in patients with a dilated and hypokinetic LV who are afterload-dependent, PEEP will decrease preload and congestion and will promote forward flow (65). In patients with elevated pulmonary capillary wedge pressure (PCWP), CO improves as PEEP is increased (from 0 to 8 cmH2O) (66); however, in patients with normal or low pulmonary capillary wedge pressure, PEEP may lower CO (61,67). Consequently, in patients with pure cardiogenic shock with high filling pressures and high systemic vascular resistance, PEEP can provide benefit if the RV is not compromised and the patient is not hypovolemic (Figure 8).
IM-PPV IN THE PATIENT WITH RV FAILURE.
The management of respiratory failure with IM-PPV in patients with RV failure is particularly challenging (27). IM-PPV should be avoided when possible or used cautiously in these patients because of the risk of adverse hemodynamic effects (68,69). Simultaneous loss of sympathetic tone with sedation, and alveolar de-recruitment due to hypopnea from loss of respiratory drive can contribute to a precipitous development of hypotension for which the clinician must be prepared. When endotracheal intubation is necessary, preload and mean arterial pressure should be optimized before sedating the patient for intubation. After volume optimization, a vasopressor and, if necessary, a pulmonary vasodilator should be used to maintain mean arterial pressure above the mean pulmonary artery pressure (ideally above 60 mm Hg). In addition, it is preferred to consult a cardiac anesthesiologist for intubation if time permits. Preferred anesthetic agents are those with less negative effects on inotropy and vascular tone, such as ketamine or etomidate (Table 6). However, ketamine should be avoided in patients with poor sympathetic compensation as its negative inotropic properties can still result in a hypotensive response. Once intubated, ongoing attention to maintain adequate preload is crucial (20,27).
TABLE 6.
Cardiovascular Effects of Key Agents Used in the Management of PPV in the CICU
| Drug Class/Common Agents | Drug Dosage | Pharmacological Class | Most Frequent Adverse Cardiac Effects | Comments |
|---|---|---|---|---|
| Sedation/induction agents | All agents can worsen myocardial dysfunction. | |||
| Propofol | Bolus: 0.25-2.00 mg/kg IV Infusion: 5-50 μg/kg/min IV |
General anesthetic | Hypotension, bradycardia | Hypertension and tachycardia are also possible. Caution if LVEF <50%. Monitor for propofol-related infusion syndrome, a rare but important side effect. Negative inotropic effect, especially when infused as a bolus. |
| Etomidate | 0.3-0.4 mg/kg IV | General anesthetic | Hypotension because of adrenal suppression | Single dose inhibits adrenal steroid production for 6-24 h; hence, need to closely monitor fluid balance in patients with heart failure. May require stress steroids. Concerns for adrenal insufficiency have been raised, but it has not been demonstrated to increase mortality in septic patients. Useful for intubation in RV failure. Consider an alternative agent in patients with sepsis (e.g., ketamine, methohexital). |
| Dexmedetomidine | Loading: 1 mcg/kg over 10 min IV Infusion: 0.2-0.7 μg/kg/h IV* for 24 h |
α2 adrenergic agonist, sedative | Hypotension, bradycardia | Hypotension due to cardiovascular depression and hypertension due to peripheral vasoconstriction (α2B receptor-mediated) have been described during bolus dosing. Hypertension and tachycardia may improve with dose reduction. Caution if pre-existing heart block or bradycardia. |
| Methohexital | Loading: IV: 0.75-00 mg/kg; can re-dose 0.5 mg/kg every 2 to 5 min as needed | General anesthetic (barbiturate derivative) | Hypotension, tachycardia | Hypotension due to cardiovascular depression and tachycardia. |
| Ketamine | Bolus: 0.1-0.5 mg/kg IV Infusion: 5-20 μg/kg/min IV |
General anesthetic | Hypertension, tachycardia | Hypotension may also occur. Reactions may require additional sedation with other agents; use with caution in CAD and catecholamine depletion, such as in states of prolonged illness/hospitalization. Although superiority over other agents has not been demonstrated, ketamine may cause less hypotension by increasing systemic vascular resistance, so careful patient selection and monitoring in patients with LV dysfunction and hypertension is warranted. Useful for intubation in RV failure in patients without suspicion for catecholamine depletion. |
|
| ||||
| Neuromuscular blockade agents | Ensure adequate sedation and analgesia if using NMB; monitor NMB through stimulation of peripheral nerves (79). | |||
| Succinylcholine | Bolus: 1-2 mg/kg IV | Depolarizing neuromuscular blockade | Bradycardia (most often in children); hypotension, hypertension, tachycardia; Arrhythmias because of hyperkalemia |
Avoid if severe electrolyte abnormalities including hyperkalemia, muscle disorders, plasma pseudo cholinesterase disorders. |
| Rocuronium | Bolus: 0.6-1.2 mg/kg IV Infusion: 4-16 μg/kg/min IV | Nondepolarizing neuromuscular blockade | Infrequent side effects (<1%); Anaphylaxis, hypersensitivity reactions; Hypertension, tachycardia | Use with caution in conditions that potentiate or antagonize NMB. Maintenance bolus dosing used in operating room. |
| Vecuronium | Bolus: 0.08-0.10 mg/kg IV Infusion: 1 μg/kg/min IV |
Nondepolarizing neuromuscular blockade | Infrequent side effects (<1%); Hypersensitivity, bradycardia, hypotension; Cross-reactivity with other NMBs |
Use with caution in conditions that potentiate or antagonize NMB. Maintenance bolus dosing used in operating room. |
| Cisatracurium | Bolus: 0.15-0.20 mg/kg IV Infusion: 0.5-10.0 μg/kg/min IV |
Nondepolarizing neuromuscular blockade | Infrequent side effects (<1%); Hypersensitivity, bradycardia, hypotension; Cross-reactivity with other NMB |
Use with caution in conditions that potentiate or antagonize NMB. Maintenance bolus dosing used in operating room. May be preferred in ARDS. |
|
| ||||
| Reversal agents | ||||
| Sugammadex | Bolus: 2-16 mg/kg IV depending on level of block | Selective relaxant binding agent | Anaphylaxis is major side effect, Hypotension, hypertension, bradycardia, tachycardia, QT prolongation | Reverses neuromuscular blockade only for rocuronium and vecuronium, not nonsteroidal neuromuscular blocking agents such as succinylcholine or benzylisoquinolinium compounds. |
| Naloxone | 0.4-2.0 mg IV, can be repeated at 2-3 min intervals to maximum dose 10 mg | Opioid antagonist | Flushing, hypertension, hypotension, arrhythmias | For reversal in chronic use cases, reduce dose and/or dilute in 10 ml 0.9% NaCl. Infuse slowly, to avoid acute opioid withdrawal and catecholamine release that can be very concerning in cardiac patients. |
|
| ||||
| Analgesics | ||||
| Fentanyl | Bolus: 25-75 mg q1-2 h IV Infusion: 50-100 mcg/h IV |
Anilidopiperidine opioid; general anesthetic | Hypotension, bradycardia | Many other reported adverse reactions. Fentanyl-induced chest wall rigidity (rare complication). Other dose ranges depend on diagnosis and route of administration. |
| Hydromorphone | Bolus: 0.2-0.6 mg q1-2 h IV Infusion: 0.5-3.0 mg/h IV |
Opioid | Hypotension, bradycardia Hypertension, tachycardia | Dose varies depending on route of delivery. |
| Morphine | Bolus: 2-5 mg q4 h IV for Infusion: 2-30 mg/h IV |
Opioid | Bradycardia, tachycardia, hypotension; histamine release | Dose varies depending on route of delivery. Has a venodilatory effect so it should be used with caution in preload-dependent states (e.g., RV failure). |
| Methadone | 2.5-10.0 mg q8-12 h IV or 10-40 mg q6-12 h by mouth | Opioid | Hypotension, arrhythmias, QT prolongation | Monitor QT interval; discontinue if significant QT prolongation. Dose varies depending on route of delivery and patient. Half-life variable and very long. |
| Ketorolac | Bolus: 30 mg IV, then 15-30 mg q6 h up to 5 days | NSAID | Edema, hypertension, hyperkalemia | Adjust dose for weight <50 kg, age ≥65 yrs and renal impairment. Contraindicated in setting of coronary artery bypass surgery. |
|
| ||||
| Anxiolytic agents | ||||
| Midazolam | Bolus: 0.01-0.05 mg/kg IV Infusion: 0.02-0.10 mg/kg/h IV† |
Anticonvulsant, benzodiazepine | Hypotension, especially in children | |
| Lorazepam | Bolus: 0.02-0.04 mg/kg (max 2 mg per dose) IV Infusion: 0.01-0.10 mg/kg/h IV† |
Anticonvulsant, benzodiazepine | Hypotension | Monitor for propylene glycol toxicity, especially if doses above 0.1 mg/kg/h and/or renal failure. |
|
| ||||
| Antipsychotic/hypnotic agents | ||||
| Quetiapine | No intravenous dosing. For ICU delirium: 50 mg by mouth twice daily; may increase to maximum dose 200 mg twice daily‡ |
Atypical antipsychotic, 2nd generation | Hypotension, hypertension, tachycardia | Monitor QT interval, discontinue if significant QT prolongation. |
| Haloperidol | Bolus: 0.5-10.0 mg q2-4 h IV Infusion: 0.5-2.0 mg/h IV |
Typical antipsychotic, 1st generation | Hypotension, hypertension, arrhythmias | Monitor QT interval, discontinue if significant QT prolongation. |
Higher infusion rates up to 1.5 mcg/kg/h are reported and infusions have extended to several days.
Dose for ICU sedation; may vary in other circumstances.
Dosage varies based on condition being treated.
ARDS = acute respiratory distress syndrome; CAD = coronary artery disease; ICU = intensive care unit; IM = intramuscular; IV = intravenous; LVEF = left ventricular ejection fraction; NMB = neuromuscular blockers; NSAID = nonsteroid anti-inflammatory drugs; RV = right ventricle; SC = subcutaneously.
During IM-PPV in patients with RV failure, low and high tidal volumes (atelectasis and overdistention) (Figure 4), high Pplat, hypoxia and hypercarbia (70), as well as high PEEP can all increase RV afterload. In RV failure, ventilator strategies should aim to achieve alveolar recruitment, while limiting overdistension. Other more specific goals of IM-PPV in this patient population include improving oxygenation, avoiding hypercarbia, and in general using low to moderate tidal volumes (e.g., ~8 ml/kg of ideal body weight), while maintaining Pplat <30 cmH2O and minimizing PEEP (initial settings of 3 to 5 cmH2O if the patient has cardiogenic shock [64] and <12 cmH2O if the patient is not in shock [71,72]). As acidosis increases PVR, maintaining the PaCO2 <60 mmHg will improve PVR, even if the etiology of acidosis is metabolic (73) (Figure 8). As an advanced topic, serial evaluation of the pressure/volume curves and stress index (the ratio of recruitment/hyperinflation of a lung at different pressure/time points) in the ventilator can assist the clinician to achieve these goals and tailor the IM-PPV strategy to the patient (74,75).
Advanced approaches to IM-PPV may be considered for some patients with RV failure, including APRV, high frequency oscillatory ventilation (HFOV), and prone ventilation (see the “Management of Refractory Hypoxemia” section). While the use of prone ventilation can improve PVR, RV hemodynamics, and oxygenation (76), the evidence supporting this approach, as well as APRV and HFOV, is limited to preclinical studies and small series (17,73,76,77).
OTHER PRELOAD DEPENDENT STATES: TAMPONADE, CONSTRICTION.
Among patients with tamponade, IM-PPV should, in general, be reserved for patients who become unstable, lose their ability to protect their airway, or are having surgical interventions. Because of the preload dependence and compensatory tachycardia present in patients with tamponade and constriction, PPV and associated induction and sedation can precipitate hemodynamic collapse. Therefore, NI-PPV may be preferable, limiting PEEP. Moreover, optimization of preload is important. In patients for whom intubation is the only option, an awake-intubation technique may be considered with vasopressors ready and with capacity to perform emergent pericardiocentesis or pericardial window if cardiac collapse occurs. Very low PEEP should be used (0 to 3 cmH2O) to avoid compromising venous return, until the pericardial fluid has been evacuated (78).
IM-PPV DURING EXTRACORPOREAL LIFE SUPPORT.
Cardiogenic shock is one of the fastest growing indications for ECMO in adults (79). Veno-arterial ECMO provides both biventricular circulatory and respiratory support (80). After removal of venous blood via the drainage cannula, deoxygenated blood passes through the oxygenator before returning to the arterial system (81).
Gas exchange through ECMO allows the clinician to select “rest settings” to minimize ventilator-associated lung injury (VALI). Although wide variation in practice exists and clinical guidelines are limited (82), most experts advocate for low tidal volumes (4 to 6 ml/kg of ideal body weight), low respiratory rates (5 to 10 breaths/min), allowance of spontaneous breaths if possible, PEEP ≤10 cmH2O, Pplat <25 cmH2O, and the lowest tolerable FiO2 (81,83). Many centers use pressure-control ventilation based on the protocol from the CESAR (Efficacy and Economic Assessment of Conventional Ventilatory Support Versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure) trial (82,84). However, others advocate for volume-controlled ventilation (82). Ventilation strategies in patients receiving ECMO remain an important area for future research.
CARDIAC EFFECTS OF SEDATIVES, NEUROMUSCULAR BLOCKERS, ANALGESICS, AND ANXIOLYTICS
Practicing cardiologists should be aware of the multiple relevant cardiovascular effects of many of the pharmacotherapies necessary for general critical care in the CICU, as well as the central role of pharmacological management of pain and delirium in effective IM-PPV and successful extubation. Observational data suggest that participation of a critical care pharmacist in ICU rounds can reduce adverse drug events, prescribing errors, and enhance therapeutic choices (85,86). Many drugs, including opiates, dexmedetomidine, and propofol, can cause hypotension and bradycardia through different mechanisms (Table 6). Guidelines for management of pain, agitation, delirium, and neuromuscular blockade are available (87,88).
MANAGEMENT OF REFRACTORY HYPOXEMIA
When patients do not respond to the ventilation strategies already described, available rescue therapies include recruitment maneuvers, advanced ventilation strategies, neuromuscular blockade to improve ventilation synchrony, prone positioning, vasodilators, and ECMO. These strategies have been studied predominantly in ARDS; nevertheless, they are of increasing relevance to the broad spectrum of patients cared for in some advanced CICUs.
Dependent lung regions are perfused but may have inadequate aeration. Lung recruitment maneuvers are based on transiently increasing PEEP to higher than normal to improve oxygenation and reduce VALI. Although early clinical trials and a meta-analysis indicated no harm from recruitment maneuvers, a recent clinical trial suggested that lung recruitment maneuvers and progressive PEEP titration may be associated with harm (13,89–91).
Survival data for other ventilatory modes such as HFOV or APRV are lacking. HFOV improves oxygenation, but does not improve survival and was associated with harm in one trial (92–94).
In a clinical trial of 340 subjects, neuromuscular blockade for 48 h prevented patient-ventilator dyssynchrony and lung injury, and reduced 90-day mortality (95). Although delirium and critical illness neuromyopathy are concerns, short-term use of neuromuscular blockade has not been associated with increased muscle weakness (95). When neuromuscular blockade is prescribed, sufficient sedation and analgesia are essential to avoid awareness (87).
Prone positioning has been advocated in severe hypoxemia to improve outcomes by lung recruitment and reduced ventilation-perfusion mismatch. Early clinical trials did not demonstrate benefit from a short duration of proning, but in a subsequent trial, proning for at least 16 h daily reduced mortality (96–98). Proning appears to be more effective if initiated within 3 days of respiratory failure and to be less useful after 7 days (99).
Vasodilators, such as inhaled nitric oxide and inhaled epoprostenol, have been used in severe hypoxemia to improve ventilation-perfusion mismatch and to reduce pulmonary hypertension with transient improvements in oxygenation, but no reduction in mortality. Inhaled pulmonary vasodilators can be utilized as rescue therapy for pulmonary hypertension associated with severe hypoxemia as a bridge to other therapies (100–102). However, pulmonary vasodilators may precipitate pulmonary edema in patients with poor LV performance and high filling pressures, due to the sudden increase in pulmonary blood flow. Nitric oxide can be associated with development of renal dysfunction (103) and methemoglobinemia (104).
MAJOR COMPLICATIONS OF IM-PPV
Along with avoidance of the deleterious hemodynamic effects of IM-PPV described in the previous text, the CICU clinician should be aware of other important complications of IM-PPV and strategies to prevent them. VALI and ventilator-associated pneumonia (VAP) are frequent and potentially preventable complications that are associated with significant morbidity and mortality.
Although more common in patients with ARDS, VALI has been estimated to occur in >25% of ventilated patients without ARDS. The most clinically recognized form of VALI is barotrauma (pneumothorax and pneumomediastinum), but it also includes volutrauma, atelectrauma, biotrauma (inflammatory response), and oxygen toxicity (105). Unlike in ARDS, there is not a defined TV goal for critically ill ventilated patients without lung injury (106). Several studies, including meta-analyses, have suggested that lower TV is associated with a decreased incidence of pulmonary infection, ARDS, and death (106,107). However, these studies are limited by lack of a standard of care control arm. In addition to tolerating hypercapnia and lower tidal volumes, avoidance of alveolar overdistension should be attempted with Pplat <30 cmH2O. Minimizing driving pressure (“delta P”: Pplat - PEEP) (Figure 2) may be more relevant than low TV to improve outcomes in ARDS (108).
However, ventilation at low lung volumes can lead to the continuous recruitment and derecruitment of alveolar units resulting in shear stress and atelectrauma (105). Clinically, atelectrauma can be difficult to detect and often requires assessment of pressure-volume loops with heavy sedation or neuromuscular blockade (109). Prevention of atelectrauma is accomplished by PEEP, and in certain clinical scenarios (e.g., perioperative care), by periodic recruitment maneuvers, while its role in other conditions such as ARDS is less clear. Placement of an esophageal balloon to more accurately measure transpulmonary pressure and lung compliance may be useful (110).
VAP is associated with a considerable increase in patient morbidity, mortality, and cost (111). Although evidence is limited, the incidence of VAP in the CICU appears to be as high or higher than other ICUs (112). Simple cost-effective measures shown to reduce VAP include elevation of the head of the bed >30°, targeted sedation, daily spontaneous awakening trials with or without spontaneous breathing trials (SBT) to minimize ventilator days, proper maintenance of ventilator circuits, and early mobilization (113,114). Incorporating these strategies with oral chlorhexidine has also reduced VAP (115). Subglottic secretion drainage may also reduce ICU length of stay and duration of IM-PPV, and increase time to first episode of VAP (116).
PRINCIPLES OF WEANING FROM IM-PPV
The most effective intervention to reduce complications is early liberation from IM-PPV. With increasing IM-PPV duration, complications, mortality, and cost increase (117). However, patients who fail extubation after tolerating SBT show an increased risk of complications and mortality compared with similar patients who were successfully extubated (118).
IM-PPV and associated analgesics and sedatives often reduce blood pressure, heart rate, myocardial work, and oxygen consumption. Liberation from IM-PPV removes these effects and, therefore, can precipitate adverse consequences in cardiac patients, including “weaning-induced pulmonary edema” (119). Professional guidelines for liberation emphasize SBT with inspiratory pressure augmentation, minimized sedation, early mobilization, and extubation of high-risk patients to NI-PPV or HFNC (120,121).
Assessment of readiness for extubation includes determining if the precipitating cause of respiratory failure has been sufficiently mitigated, electrical or hemodynamic stability is present, and the patient can cooperate. Second, an SBT can be attempted with pressure support (e.g., 5 cmH2O) to overcome endotracheal tube resistance, and is generally preferred over T-piece or CPAP alone. Although a successful SBT with T-piece or CPAP alone might predict extubation success, this strategy can increase myocardial oxygen consumption and may be best avoided in patients with compromised ventricular function, ischemic heart disease, or in scenarios where afterload increases are detrimental (e.g., severe valvular regurgitation) (44,122). Predictors of successful extubation, such as a rapid shallow breathing index (respiratory rate/TV in liters) ≤105, a negative inspiratory force <−30 cmH2O, a strong cough, or the presence of a cuff leak, are all imperfect but can supplement the general clinical assessment (123).
Failure to liberate from IM-PPV should trigger suspicion for weaning-induced pulmonary edema (124). Consideration should be given to persistent volume overload, myocardial ischemia, diastolic dysfunction, or mitral regurgitation. Preload, inotropy, and afterload optimization can be successful in managing this problem. Finally, it is important to consider timing of extubation relative to removal of mechanical support, particularly for femoral access, so the head of the bead can be elevated during the immediate post-extubation period to facilitate pulmonary mechanics, improve mobilization of secretions, and avoid aspiration events.
Among patients passing SBTs but with a high risk of extubation failure, NI-PPV and HFNC offer options to improve success in the post-extubation period. Indicators of high risk of failure are common in the CICU population: age >65 years, morbid obesity (body mass index ≥35 kg/m2), heart failure, and a history of other cardiac comorbidities (125,126). The American College of Chest Physicians and the American Thoracic Society guidelines give post-extubation NI-PPV a “strong recommendation” in high-risk patients (125). If this strategy is taken, NI-PPV should be applied immediately following extubation. Applying NI-PPV after development of post-extubation respiratory failure may be detrimental by delaying reintubation (127).
CONCLUSIONS
Cardiologists who practice in the CICU should be familiar with the effects of PPV on cardiopulmonary physiology so that ventilator management can be tailored to optimize hemodynamics, oxygenation, and ventilation (Table 7). As critical care cardiology continues to evolve, dedicated ventilation management studies in the CICU are of heightened importance to achieve this goal.
TABLE 7.
Key Points
| • Positive pressure ventilation decreases RV preload, increases RV afterload, and decreases LV afterload. |
| • High-flow oxygen can avoid the need for positive pressure ventilation in some patients. |
| • BiPAP is preferred over CPAP when there is a need to reduce work of breathing and/or improve ventilation in patients with hypercarbia. |
| • PEEP is useful for oxygenation and to avoid or treat atelectasis, but can also be useful in unloading a failing LV. |
| • Auto-PEEP should be watched for in patients with increased airway resistance, hyperinflation, or ineffective triggering, and can lead to hemodynamic instability or barotrauma. |
| • Positive pressure should be avoided if possible, or used cautiously, in conditions dependent on adequate RV filling, such as acute pulmonary hypertension, RV failure. and tamponade. |
BiPAP = bilevel positive airway pressure; CPAP = continuous positive airway pressure; other abbreviations as in Table 1.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful for the support of the Heart Failure and Cardiac Transplant Council, Sarah Sears, and Alicia McClarin of the American College of Cardiology.
This work was supported in part by the National Institutes of Health Clinical Center. Dr. Soble is a cofounder of Ascend, a health care IT company. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CICU
cardiac intensive care unit
- IM-PPV
invasive mechanical positive pressure ventilation
- LV
left ventricle
- NI-PPV
noninvasive positive pressure ventilation
- PEEP
positive end-expiratory pressure
- Ppleural
pleural pressure
- PPV
positive pressure ventilation
- PVR
pulmonary vascular resistance
- RV
right ventricle
Footnotes
The views expressed in this paper by the American College of Cardiology’s (ACC’s) Critical Care Cardiology Working Group does not necessarily reflect the views of the Journal of the American College of Cardiology or the ACC.
APPENDIX For supplemental figures and tables, please see the online version of this paper.
REFERENCES
- 1.Morrow DA, Fang JC, Fintel DJ, et al. Evolution of critical care cardiology: transformation of the cardiovascular intensive care unit and the emerging need for new medical staffing and training models: a scientific statement from the American Heart Association. Circulation 2012;126:1408–28. [DOI] [PubMed] [Google Scholar]
- 2.Katz JN, Shah BR, Volz EM, et al. Evolution of the coronary care unit: clinical characteristics and temporal trends in healthcare delivery and outcomes. Crit Care Med 2010;38:375–81. [DOI] [PubMed] [Google Scholar]
- 3.Morrow DA. Trends in cardiac critical care: reshaping the cardiac intensive care unit. Circ Cardiovasc Qual Outcomes 2017;10:e004010. [DOI] [PubMed] [Google Scholar]
- 4.Sinha SS, Sjoding MW, Sukul D, et al. Changes in primary noncardiac diagnoses over time among elderly cardiac intensive care unit patients in the United States. Circ Cardiovasc Qual Outcomes 2017;10:e003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metkus TS, Albaeni A, Chandra-Strobos N, Eid SM. Incidence and prognostic impact of respiratory support in patients with ST-segment elevation myocardial infarction. Am J Cardiol 2017;119:171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feihl F, Broccard AF. Interactions between respiration and systemic hemodynamics. Part I: basic concepts. Intensive Care Med 2009;35:45–54. [DOI] [PubMed] [Google Scholar]
- 7.Feihl F, Broccard AF. Interactions between respiration and systemic hemodynamics. Part II: practical implications in critical care. Intensive Care Med 2009;35:198–205. [DOI] [PubMed] [Google Scholar]
- 8.Truwitt JD. Lung mechanics. In: Dantzer DR, MacIntyre N, Bakow ED, editors. Comprehensive Respiratory Care. Philadelphia: WB Saunders Co, 1995:18–31. [Google Scholar]
- 9.Coruh B, Tonelli MR, Park DR. Fentanyl-induced chest wall rigidity. Chest 2013;143:1145–6. [DOI] [PubMed] [Google Scholar]
- 10.Grinnan DC, Truwit JD. Clinical review: respiratory mechanics in spontaneous and assisted ventilation. Crit Care 2005;9:472–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodarte JR, Rehder K. Dynamics of respiration. In: Macklem PT, Mead J, editors. American Physiological Society Handbook of Physiology. Bethesda, MD: American Physiological Society, 1986:131–44. [Google Scholar]
- 12.Esteban A, Anzueto A, Frutos F, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA 2002;287:345–55. [DOI] [PubMed] [Google Scholar]
- 13.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus Lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327–36. [DOI] [PubMed] [Google Scholar]
- 14.Pang D, Keenan SP, Cook DJ, Sibbald WJ. The effect of positive pressure airway support on mortality and the need for intubation in cardiogenic pulmonary edema: a systematic review. Chest 1998;114:1185–92. [DOI] [PubMed] [Google Scholar]
- 15.Magder SA, Lichtenstein S, Adelman AG. Effect of negative pleural pressure on left ventricular hemodynamics. Am J Cardiol 1983;52:588–93. [DOI] [PubMed] [Google Scholar]
- 16.Rankin JS, Olsen CO, Arentzen CE, et al. The effects of airway pressure on cardiac function in intact dogs and man. Circulation 1982;66:108–20. [DOI] [PubMed] [Google Scholar]
- 17.Cheifetz IM. Cardiorespiratory interactions: the relationship between mechanical ventilation and hemodynamics. Respir Care 2014;59:1937–45. [DOI] [PubMed] [Google Scholar]
- 18.Patzelt J, Zhang Y, Seizer P, et al. Effects of mechanical ventilation on heart geometry and mitral valve leaflet coaptation during percutaneous edge-to-edge mitral valve repair. J Am Coll Cardiol Intv 2016;9:151–9. [DOI] [PubMed] [Google Scholar]
- 19.Tkacova R, Liu PP, Naughton MT, Bradley TD. Effect of continuous positive airway pressure on mitral regurgitant fraction and atrial natriuretic peptide in patients with heart failure. J Am Coll Cardiol 1997;30:739–45. [DOI] [PubMed] [Google Scholar]
- 20.Cassidy SS, Ramanathan M. Dimensional analysis of the left ventricle during PEEP: relative septal and lateral wall displacements. Am J Physiol 1984;246:H792–805. [DOI] [PubMed] [Google Scholar]
- 21.Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation 2008;117:1717–31. [DOI] [PubMed] [Google Scholar]
- 22.Robotham JL, Lixfeld W, Holland L, et al. The effects of positive end-expiratory pressure on right and left ventricular performance. Am Rev Respir Dis 1980;121:677–83. [DOI] [PubMed] [Google Scholar]
- 23.Nanas S, Magder S. Adaptations of the peripheral circulation to PEEP. Am Rev Respir Dis 1992;146:688–93. [DOI] [PubMed] [Google Scholar]
- 24.Price LC, Wort SJ, Finney SJ, Marino PS, Brett SJ. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care 2010;14:R169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersson J, Ax M, Frey J, Sanchez-Crespo A, Lindahl SG, Mure M. Positive end-expiratory pressure redistributes regional blood flow and ventilation differently in supine and prone humans. Anesthesiology 2010;113:1361–9. [DOI] [PubMed] [Google Scholar]
- 26.Tomashefski JF Jr. , Davies P, Boggis C, Greene R, Zapol WM, Reid LM. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol 1983;112:112–26. [PMC free article] [PubMed] [Google Scholar]
- 27.Green EM, Givertz MM. Management of acute right ventricular failure in the intensive care unit. Curr Heart Fail Rep 2012;9:228–35. [DOI] [PubMed] [Google Scholar]
- 28.Marini JJ. Dynamic hyperinflation and autopositive end-expiratory pressure: lessons learned over 30 years. Am J Respir Crit Care Med 2011;184:756–62. [DOI] [PubMed] [Google Scholar]
- 29.Hernandez G, Roca O, Colinas L. High-flow nasal cannula support therapy: new insights and improving performance. Crit Care 2017;21:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JH, Rehder KJ, Williford L, Cheifetz IM, Turner DA. Use of high flow nasal cannula in critically ill infants, children, and adults: a critical review of the literature. Intensive Care Med 2013;39:247–57. [DOI] [PubMed] [Google Scholar]
- 31.Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015;372:2185–96. [DOI] [PubMed] [Google Scholar]
- 32.Stephan F, Barrucand B, Petit P, et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA 2015;313:2331–9. [DOI] [PubMed] [Google Scholar]
- 33.Spoletini G, Alotaibi M, Blasi F, Hill NS. Heated humidified high-flow nasal oxygen in adults: mechanisms of action and clinical implications. Chest 2015;148:253–61. [DOI] [PubMed] [Google Scholar]
- 34.Organized Jointly by the American Thoracic Society, the European Respiratory Society, the European Society of Intensive Care Medicine, and the Société de Réanimation de Langue Française, and approved by the ATS Board of Directors, December 2000. International consensus conferences in intensive care medicine: noninvasive positive pressure ventilation in acute Respiratory failure. Am J Respir Crit Care Med 2001;163:283–91.11208659 [Google Scholar]
- 35.Girault C, Briel A, Benichou J, et al. Interface strategy during noninvasive positive pressure ventilation for hypercapnic acute respiratory failure. Crit Care Med 2009;37:124–31. [DOI] [PubMed] [Google Scholar]
- 36.Hess D, Kacmarek RM. Essentials of Mechanical Ventilation. Third edition. New York: McGraw Hill Education, Medical Publishing Division, 2014. [Google Scholar]
- 37.Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: The Oxygen-ICU Randomized Clinical Trial. JAMA 2016;316:1583–9. [DOI] [PubMed] [Google Scholar]
- 38.Stub D, Smith K, Bernard S, et al. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation 2015;131:2143–50. [DOI] [PubMed] [Google Scholar]
- 39.Roberts BW, Kilgannon JH, Hunter BR, et al. Association between early hyperoxia exposure after resuscitation from cardiac arrest and neurological disability: prospective multicenter protocol-directed cohort study. Circulation 2018;137:2114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gay PC. Complications of noninvasive ventilation in acute care. Respir Care 2009;54:246–57. [PubMed] [Google Scholar]
- 41.Gray A, Goodacre S, Newby DE, et al. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med 2008;359:142–51. [DOI] [PubMed] [Google Scholar]
- 42.Chatburn RL, El-Khatib M, Mireles-Cabodevila E. A taxonomy for mechanical ventilation: 10 fundamental maxims. Respir Care 2014;59:1747–63. [DOI] [PubMed] [Google Scholar]
- 43.Porhomayon J, El-Solh AA, Nader ND. Applications of airway pressure release ventilation. Lung 2010;188:87–96. [DOI] [PubMed] [Google Scholar]
- 44.Scharf SM, Bianco JA, Tow DE, Brown R. The effects of large negative intrathoracic pressure on left ventricular function in patients with coronary artery disease. Circulation 1981;63:871–5. [DOI] [PubMed] [Google Scholar]
- 45.Corredor C, Jaggar SI. Ventilator management in the cardiac intensive care unit. Cardiol Clin 2013;31:619–36, ix. [DOI] [PubMed] [Google Scholar]
- 46.Dursun A, Okumus N, Erol S, Bayrak T, Zenciroglu A. Effect of ventilation support on oxidative stress and ischemia-modified albumin in neonates. Am J Perinatol 2016;33:136–42. [DOI] [PubMed] [Google Scholar]
- 47.Goligher EC, Dres M, Fan E, et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med 2018;197:204–13. [DOI] [PubMed] [Google Scholar]
- 48.Shorofsky M, Jayaraman D, Lellouche F, Husa R, Lipes J. Mechanical ventilation with high tidal volume and associated mortality in the cardiac intensive care unit. Acute Card Care 2014;16:9–14. [DOI] [PubMed] [Google Scholar]
- 49.Chadda K, Annane D, Hart N, Gajdos P, Raphael JC, Lofaso F. Cardiac and respiratory effects of continuous positive airway pressure and noninvasive ventilation in acute cardiac pulmonary edema. Crit Care Med 2002;30:2457–61. [DOI] [PubMed] [Google Scholar]
- 50.Masip J, Betbese AJ, Paez J, et al. Non-invasive pressure support ventilation versus conventional oxygen therapy in acute cardiogenic pulmonary oedema: a randomised trial. Lancet 2000;356:2126–32. [DOI] [PubMed] [Google Scholar]
- 51.Park M, Sangean MC, Volpe Mde S, et al. Randomized, prospective trial of oxygen, continuous positive airway pressure, and bilevel positive airway pressure by face mask in acute cardiogenic pulmonary edema. Crit Care Med 2004;32:2407–15. [DOI] [PubMed] [Google Scholar]
- 52.Kulkarni VT, Kim N, Dai Y, et al. Hospital variation in noninvasive positive pressure ventilation for acute decompensated heart failure. Circ Heart Fail 2014;7:427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vital FM, Ladeira MT, Atallah AN. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev 2013:CD005351. [DOI] [PubMed] [Google Scholar]
- 54.Weng CL, Zhao YT, Liu QH, et al. Meta-analysis: Noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med 2010;152:590–600. [DOI] [PubMed] [Google Scholar]
- 55.Mebazaa A, Yilmaz MB, Levy P, et al. Recommendations on pre-hospital and early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine–short version. Eur Heart J 2015;36:1958–66. [DOI] [PubMed] [Google Scholar]
- 56.Anton A, Guell R, Gomez J, et al. Predicting the result of noninvasive ventilation in severe acute exacerbations of patients with chronic airflow limitation. Chest 2000;117:828–33. [DOI] [PubMed] [Google Scholar]
- 57.Ariza Sole A, Salazar-Mendiguchia J, Lorente-Tordera V, et al. Invasive mechanical ventilation in acute coronary syndromes in the era of percutaneous coronary intervention. Eur Heart J Acute Cardiovasc Care 2013;2:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287–96. [DOI] [PubMed] [Google Scholar]
- 59.Puymirat E, Fagon JY, Aegerter P, et al. Cardiogenic shock in intensive care units: evolution of prevalence, patient profile, management and outcomes, 1997-2012. Eur J Heart Fail 2017;19:192–200. [DOI] [PubMed] [Google Scholar]
- 60.Fessler HE, Brower RG, Wise RA, Permutt S. Mechanism of reduced LV afterload by systolic and diastolic positive pleural pressure. J Appl Physiol (1985) 1988;65:1244–50. [DOI] [PubMed] [Google Scholar]
- 61.Grace MP, Greenbaum DM. Cardiac performance in response to PEEP in patients with cardiac dysfunction. Crit Care Med 1982;10:358–60. [DOI] [PubMed] [Google Scholar]
- 62.Bellone A, Barbieri A, Ricci C, et al. Acute effects of non-invasive ventilatory support on functional mitral regurgitation in patients with exacerbation of congestive heart failure. Intensive Care Med 2002;28:1348–50. [DOI] [PubMed] [Google Scholar]
- 63.Calvin JE, Driedger AA, Sibbald WJ. Positive end-expiratory pressure (PEEP) does not depress left ventricular function in patients with pulmonary edema. Am Rev Respir Dis 1981;124:121–8. [DOI] [PubMed] [Google Scholar]
- 64.Wiesen J, Ornstein M, Tonelli AR, Menon V, Ashton RW. State of the evidence: mechanical ventilation with PEEP in patients with cardiogenic shock. Heart 2013;99:1812–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peters J. Mechanical ventilation with PEEP–a unique therapy for failing hearts. Intensive Care Med 1999;25:778–80. [DOI] [PubMed] [Google Scholar]
- 66.Kontoyannis DA, Nanas JN, Kontoyannis SA, Stamatelopoulos SF, Moulopoulos SD. Mechanical ventilation in conjunction with the intra-aortic balloon pump improves the outcome of patients in profound cardiogenic shock. Intensive Care Med 1999;25:835–8. [DOI] [PubMed] [Google Scholar]
- 67.Mathru M, Rao TL, El-Etr AA, Pifarre R. Hemodynamic response to changes in ventilatory patterns in patients with normal and poor left ventricular reserve. Crit Care Med 1982;10:423–6. [DOI] [PubMed] [Google Scholar]
- 68.Hoeper MM, Granton J. Intensive care unit management of patients with severe pulmonary hypertension and right heart failure. Am J Respir Crit Care Med 2011;184:1114–24. [DOI] [PubMed] [Google Scholar]
- 69.Aqel RA, Aljaroudi W, Hage FG, Tallaj J, Rayburn B, Nanda NC. Left ventricular collapse secondary to pericardial effusion treated with pericardicentesis and percutaneous pericardiotomy in severe pulmonary hypertension. Echocardiography 2008;25:658–61. [DOI] [PubMed] [Google Scholar]
- 70.Balanos GM, Talbot NP, Dorrington KL, Robbins PA. Human pulmonary vascular response to 4 h of hypercapnia and hypocapnia measured using Doppler echocardiography. J Appl Physiol (1985) 2003;94:1543–51. [DOI] [PubMed] [Google Scholar]
- 71.Jardin F, Vieillard-Baron A. Right ventricular function and positive pressure ventilation in clinical practice: from hemodynamic subsets to respirator settings. Intensive Care Med 2003;29:1426–34. [DOI] [PubMed] [Google Scholar]
- 72.Paternot A, Repesse X, Vieillard-Baron A. Rationale and description of right ventricle-protective ventilation in ARDS. Respir Care 2016;61:1391–6. [DOI] [PubMed] [Google Scholar]
- 73.Malik AB, Kidd BS. The independent effects of changes in H + and CO 2 concentrations on pulmonary hemodynamics of intact dogs. Can J Physiol Pharmacol 1973;51:139–47. [DOI] [PubMed] [Google Scholar]
- 74.Grasso S, Terragni P, Mascia L, et al. Airway pressure-time curve profile (stress index) detects tidal recruitment/hyperinflation in experimental acute lung injury. Crit Care Med 2004;32:1018–27. [DOI] [PubMed] [Google Scholar]
- 75.Henderson WR, Chen L, Amato MBP, Brochard LJ. Fifty years of research in ARDS. Respiratory mechanics in acute respiratory distress syndrome. Am J Respir Crit Care Med 2017;196:822–33. [DOI] [PubMed] [Google Scholar]
- 76.Vieillard-Baron A, Charron C, Caille V, Belliard G, Page B, Jardin F. Prone positioning unloads the right ventricle in severe ARDS. Chest 2007;132:1440–6. [DOI] [PubMed] [Google Scholar]
- 77.Harjola VP, Mebazaa A, Celutkiene J, et al. Contemporary management of acute right ventricular failure: a statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur J Heart Fail 2016;18:226–41. [DOI] [PubMed] [Google Scholar]
- 78.Ho AM, Graham CA, Ng CS, et al. Timing of tracheal intubation in traumatic cardiac tamponade: a word of caution. Resuscitation 2009;80:272–4. [DOI] [PubMed] [Google Scholar]
- 79.Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J 2017;63:60–7. [DOI] [PubMed] [Google Scholar]
- 80.Miller PE, Solomon MA, McAreavey D. Advanced percutaneous mechanical circulatory support devices for cardiogenic shock. Crit Care Med 2017;45:1922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Extracorporeal Life Support Organization. ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support. Ann Arbor, MI: Extracorporeal Life Support Organization, 2017. [Google Scholar]
- 82.Marhong JD, Telesnicki T, Munshi L, Del Sorbo L, Detsky M, Fan E. Mechanical ventilation during extracorporeal membrane oxygenation. An international survey. Ann Am Thorac Soc 2014;11:956–61. [DOI] [PubMed] [Google Scholar]
- 83.Schmidt M, Pellegrino V, Combes A, Scheinkestel C, Cooper DJ, Hodgson C. Mechanical ventilation during extracorporeal membrane oxygenation. Crit Care 2014;18:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351–63. [DOI] [PubMed] [Google Scholar]
- 85.Preslaski CR, Lat I, MacLaren R, Poston J. Pharmacist contributions as members of the multidisciplinary ICU team. Chest 2013;144:1687–95. [DOI] [PubMed] [Google Scholar]
- 86.Shulman R, McKenzie CA, Landa J, et al. Pharmacis’s review and outcomes: Treatment-enhancing contributions tallied, evaluated, and documented (PROTECTED-UK). J Crit Care 2015;30:808–13. [DOI] [PubMed] [Google Scholar]
- 87.deBacker J, Hart N, Fan E. Neuromuscular blockade in the 21st century management of the critically ill patient. Chest 2017;151:697–706. [DOI] [PubMed] [Google Scholar]
- 88.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263–306. [DOI] [PubMed] [Google Scholar]
- 89.Cavalcanti AB, Suzumura ÉA, Laranjeira LN, et al. for the Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial Investigators. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA 2017;318:1335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:637–45. [DOI] [PubMed] [Google Scholar]
- 91.Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 2010;303:865–73. [DOI] [PubMed] [Google Scholar]
- 92.Bollen CW, van Well GT, Sherry T, et al. High frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial [ISRCTN24242669]. Crit Care 2005;9:R430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Derdak S, Mehta S, Stewart TE, et al. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med 2002;166:801–8. [DOI] [PubMed] [Google Scholar]
- 94.Sud S, Sud M, Friedrich JO, et al. High frequency oscillation in patients with acute lung injury and acute respiratory distress syndrome (ARDS): systematic review and meta-analysis. BMJ 2010;340:c2327. [DOI] [PubMed] [Google Scholar]
- 95.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107–16. [DOI] [PubMed] [Google Scholar]
- 96.Taccone P, Pesenti A, Latini R, et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA 2009;302:1977–84. [DOI] [PubMed] [Google Scholar]
- 97.Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010;36:585–99. [DOI] [PubMed] [Google Scholar]
- 98.Guerin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159–68. [DOI] [PubMed] [Google Scholar]
- 99.Pelosi P, Brazzi L, Gattinoni L. Prone position in acute respiratory distress syndrome. Eur Respir J 2002;20:1017–28. [DOI] [PubMed] [Google Scholar]
- 100.Taylor RW, Zimmerman JL, Dellinger RP, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA 2004;291:1603–9. [DOI] [PubMed] [Google Scholar]
- 101.Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ 2007;334:779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lundin S, Mang H, Smithies M, Stenqvist O, Frostell C, for the European Study Group of Inhaled Nitric Oxide. Inhalation of nitric oxide in acute lung injury: results of a European multicentre study. Intensive Care Med 1999;25:911–9. [DOI] [PubMed] [Google Scholar]
- 103.Gries A, Herr A, Kirsch S, et al. Inhaled nitric oxide inhibits platelet-leukocyte interactions in patients with acute respiratory distress syndrome. Crit Care Med 2003;31:1697–704. [DOI] [PubMed] [Google Scholar]
- 104.Gebistorf F, Karam O, Wetterslev J, Afshari A. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst Rev 2016:CD002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013;369:2126–36. [DOI] [PubMed] [Google Scholar]
- 106.Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA 2012;308:1651–9. [DOI] [PubMed] [Google Scholar]
- 107.Neto AS, Simonis FD, Barbas CS, et al. Lung-protective ventilation with low tidal volumes and the occurrence of pulmonary complications in patients without acute respiratory distress syndrome: a systematic review and individual patient data analysis. Crit Care Med 2015;43:2155–63. [DOI] [PubMed] [Google Scholar]
- 108.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 2017;377:562–72. [DOI] [PubMed] [Google Scholar]
- 109.Decailliot F, Demoule A, Maggiore SM, Jonson B, Duvaldestin P, Brochard L. Pressure-volume curves with and without muscle paralysis in acute respiratory distress syndrome. Intensive Care Med 2006;32:1322–8. [DOI] [PubMed] [Google Scholar]
- 110.Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 2008;359:2095–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med 2005;33:2184–93. [DOI] [PubMed] [Google Scholar]
- 112.Ensminger SA, Wright RS, Baddour LM, Afessa B. Suspected ventilator-associated pneumonia in cardiac patients admitted to the coronary care unit. Mayo Clin Proc 2006;81:32–5. [DOI] [PubMed] [Google Scholar]
- 113.Alexiou VG, Ierodiakonou V, Dimopoulos G, Falagas ME. Impact of patient position on the incidence of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. J Crit Care 2009;24:515–22. [DOI] [PubMed] [Google Scholar]
- 114.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008;371:126–34. [DOI] [PubMed] [Google Scholar]
- 115.Morris AC, Hay AW, Swann DG, et al. Reducing ventilator-associated pneumonia in intensive care: impact of implementing a care bundle. Crit Care Med 2011;39:2218–24. [DOI] [PubMed] [Google Scholar]
- 116.Muscedere J, Rewa O, McKechnie K, Jiang X, Laporta D, Heyland DK. Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care Med 2011;39:1985–91. [DOI] [PubMed] [Google Scholar]
- 117.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med 2005;33:1266–71. [DOI] [PubMed] [Google Scholar]
- 118.Seymour CW, Martinez A, Christie JD, Fuchs BD. The outcome of extubation failure in a community hospital intensive care unit: a cohort study. Crit Care 2004;8:R322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu J, Shen F, Teboul JL, et al. Cardiac dysfunction induced by weaning from mechanical ventilation: incidence, risk factors, and effects of fluid removal. Crit Care 2016;20:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Girard TD, Alhazzani W, Kress JP, et al. An official American Thoracic Society/American College of Chest Physicians clinical practice guideline: liberation from mechanical ventilation in critically ill adults. rehabilitation protocols, ventilator liberation protocols, and cuff leak tests. Am J Respir Crit Care Med 2017;195:120–33. [DOI] [PubMed] [Google Scholar]
- 121.Hernandez G, Vaquero C, Colinas L, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and post-extubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA 2016;316:1565–74. [DOI] [PubMed] [Google Scholar]
- 122.Tobin MJ. Extubation and the myth of “minimal ventilator settings.” Am J Respir Crit Care Med 2012;185:349–50. [DOI] [PubMed] [Google Scholar]
- 123.McConville JF, Kress JP. Weaning patients from the ventilator. N Engl J Med 2012;367:2233–9. [DOI] [PubMed] [Google Scholar]
- 124.Ruiz-Bailen M, Cobo-Molinos J, Castillo-Rivera A, et al. Stress echocardiography in patients who experienced mechanical ventilation weaning failure. J Crit Care 2017;39:66–71. [DOI] [PubMed] [Google Scholar]
- 125.Ouellette DR, Patel S, Girard TD, et al. Liberation from mechanical ventilation in critically ill adults: an official American College of Chest Physicians/American Thoracic Society clinical practice guideline: inspiratory pressure augmentation during spontaneous breathing trials, protocols minimizing sedation, and noninvasive ventilation immediately after extubation. Chest 2017;151:166–80. [DOI] [PubMed] [Google Scholar]
- 126.Thille AW, Harrois A, Schortgen F, Brun-Buisson C, Brochard L. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med 2011;39:2612–8. [DOI] [PubMed] [Google Scholar]
- 127.Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med 2004;350:2452–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


