Abstract
Acute kidney injury (AKI) and cardiorenal syndrome (CRS) are increasingly prevalent in hospitalized patients with cardiovascular disease and remain associated with poor short- and long-term outcomes. There are no specific therapies to reduce mortality related to either AKI or CRS, apart from supportive care and volume status management. Acute renal replacement therapies (RRTs), including ultrafiltration, intermittent hemodialysis, and continuous RRT are used to manage complications of medically refractory AKI and CRS and may restore normal electrolyte, acid-base, and fluid balance before renal recovery. Patients who require acute RRT have a significant risk of mortality and long-term dialysis dependence, emphasizing the importance of appropriate patient selection. Despite the growing use of RRT in the cardiac intensive care unit, there are few resources for the cardiovascular specialist that integrate the epidemiology, diagnostic workup, and medical management of AKI and CRS with an overview of indications, multidisciplinary team management, and transition off of RRT.
Keywords: acute kidney injury, cardiorenal syndrome, dialysis, heart failure, hemofiltration, renal replacement therapy, ultrafiltration
The prevalence of acute kidney injury (AKI) and chronic kidney disease (CKD) in patients with acute cardiovascular disease is growing, highlighting an increasingly complex patient population at significant risk of adverse outcomes despite multispecialty provider input and optimal supportive care (1,2). Cardiorenal syndrome (CRS) represents a subset of AKI or CKD that develops in the context of worsening cardiovascular disease, recognizing that not all renal dysfunction developing in patients with cardiovascular disease is due to CRS (Central Illustration) (3,4).
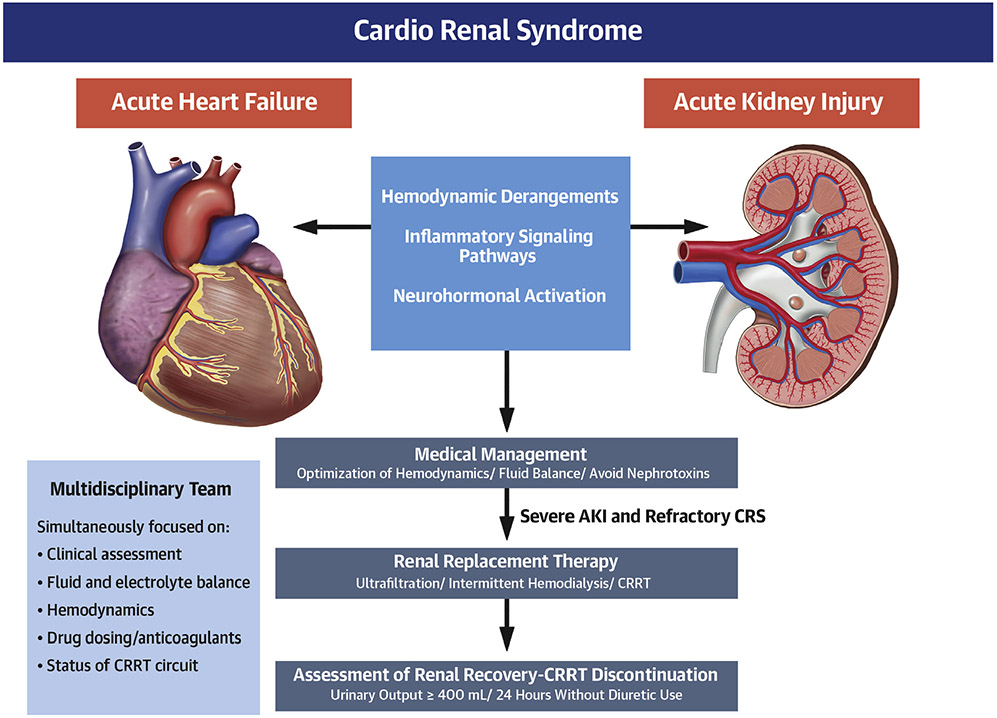
CENTRAL ILLUSTRATION. Pathophysiology, Medical Management, and Use of Renal Replacement Therapy in Severe Acute Kidney Injury and Refractory Cardiorenal Syndrome.
Cardiorenal syndrome (CRS) involves the interplay between hemodynamic, inflammatory, and neurohumoral abnormalities to produce worsening heart and kidney function. Management of patients with CRS involves multidisciplinary care, starting with medical management and avoidance of further acute kidney injury (AKI). For medically refractory CRS and severe AKI, renal replacement therapy, including continuous renal replacement therapy (CRRT), may be necessary.
PREVALENCE OF AKI IN CARDIAC INTENSIVE CARE UNIT PATIENTS
AKI occurs in approximately 1 in 4 patients hospitalized with cardiovascular disease, including up to 47% of patients with acute decompensated heart failure and 15% to 30% of patients with acute coronary syndrome (ACS) (3,5-7). In the cardiac intensive care unit (CICU), the reported prevalence of AKI is 25% to 50% (with up to one-third having severe AKI) and up to 38% having underlying CKD, emphasizing the substantial burden of CRS (1,2,8-11). These rates are comparable to general intensive care unit patients, in whom the incidence of AKI was 57% in a large, multinational study (12). AKI requiring dialysis (AKI-D) occurs in 20% of patients with AKI and in 1% to 3% of patients hospitalized with heart failure or ACS (5,13-15). AKI-D is more common in CICU patients (5% to 8%) and may exceed 13% in patients with cardiogenic shock (1,2,9,16,17).
The severity of underlying CKD is 1 of the strongest risk factors for AKI, reflecting reduced renal reserve and impaired ability of the kidneys to respond to stress (3,7,18). Older age, hypertension, diabetes mellitus, heart failure, and sepsis are also important risk factors for AKI, as are higher severity of illness, hypotension and/or shock, and the need for vasopressors (3,6,7,12,18). Larger infarct size and higher Killip Class are associated with a higher risk of AKI among patients with ACS (6,7). Furthermore, the use of iodinated radiocontrast material during cardiovascular interventional procedures is an important contributor to AKI risk, with contrast-associated AKI occurring in approximately 15% of patients with ACS who undergo percutaneous coronary intervention (19,20). Thus, common CICU admission diagnoses, comorbidities, and procedures represent significant risk factors for AKI (12).
OUTCOMES ASSOCIATED WITH AKI IN CICU PATIENTS.
AKI and CKD are consistently associated with higher short- and long-term mortality across populations of patients with acute cardiovascular disease and critical illness, including a persistent hazard for death and adverse cardiovascular events among hospital survivors (3,5,7,8,10-15,17,19,21-23). Elevated levels of creatinine, blood urea nitrogen, and cystatin C, which reflect a lower glomerular filtration rate, demonstrate a direct graded relationship with mortality in patients with acute cardiovascular disease, particularly among hospitalized patients with heart failure (3,23-25). Increases in serum creatinine during hospitalization for acute heart failure have frequently been associated with higher short-term mortality, although this mortality risk is dependent on other factors, such as the presence of residual congestion (3,4). The need for dialysis is a major risk factor for mortality among CICU patients and patients with AKI, and patients who develop AKI-D have poor short- and long-term outcomes, including high rates of mortality and dialysis dependence (11,16,26). In 1 study, patients admitted to the CICU for dialysis initiation had a mortality risk similar to patients admitted with cardiogenic shock or cardiac arrest (16). Mortality risk in patients with AKI depends on the severity of AKI, age, overall illness severity, presence and severity of other organ failures, and degree of renal function recovery. Low urine output, more severe volume overload, and greater net fluid accumulation are additional risk factors (12,22,27).
PATHOPHYSIOLOGY OF AKI AND CRS IN CARDIAC CRITICAL ILLNESS.
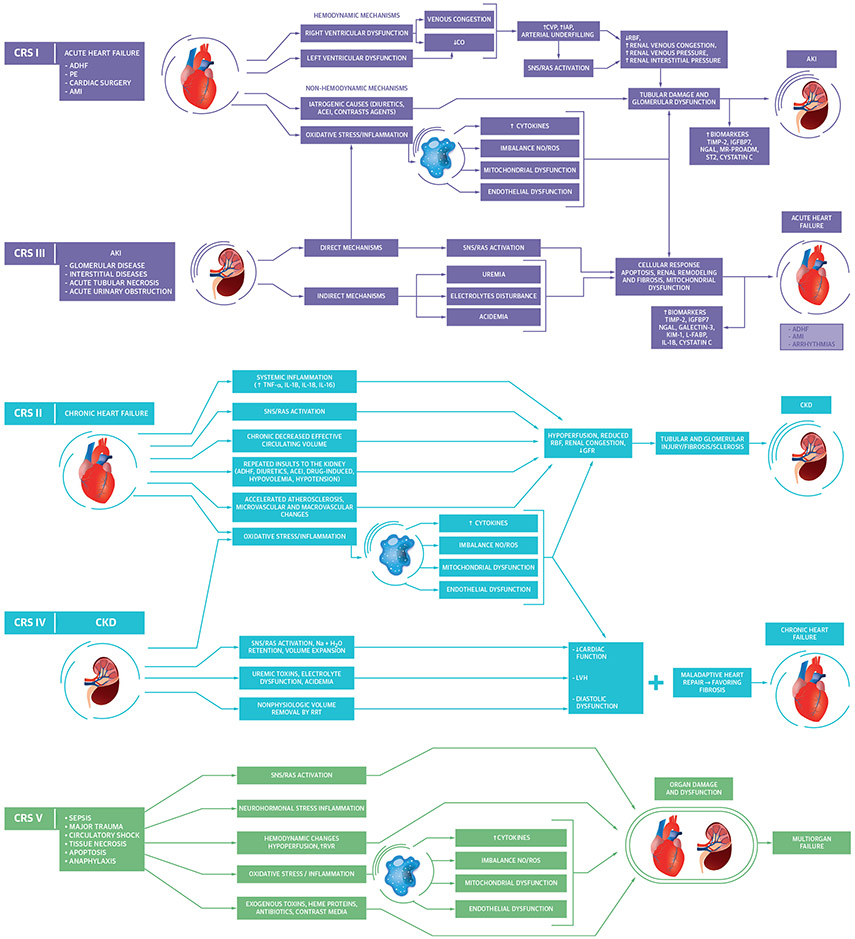
CRS was initially defined as worsening kidney function (i.e., AKI) that occurred during treatment for acute decompensated heart failure with persistent congestion and diuretic resistance; more recently, CRS has been envisioned as a spectrum of acute or chronic disorders of heart and kidney function characterized by mutual deterioration (4). CRS can be conceptually distinguished from other forms of AKI based on its intrinsic relationship with worsening cardiac function and often presents with a reversible decrease in kidney function without overt tubular injury (4). CRS has been sub-classified into 5 types based on chronicity and the primary organ dysfunction driving the syndrome (Figure 1) (4,28). Numerous inter-related pathophysiological mechanisms are involved in the reciprocal worsening of cardiac and renal functions seen in patients with CRS, including hemodynamic, neurohumoral, and inflammatory processes that potentially lead to reductions in glomerular filtration rate and tubular injury (Figure 1) (4,28).
FIGURE 1. Pathophysiological Interactions Between the Heart and Kidney in CRS.
Acute cardiorenal syndrome (CRS) (types 1 and 3) occurs when acutely worsening heart or kidney function, respectively, leads to worsening function of the other organ. Chronic CRS (types 2 and 4) occurs when chronic heart or kidney dysfunction, respectively, leads to worsening dysfunction of the other organ. Secondary CRS (type 5) occurs when a systemic process leads to simultaneous heart and kidney dysfunction (28,34). ACEI = angiotensin-converting enzyme inhibitor; ADHF = acute decompensated heart failure; AKI = acute kidney injury; AMI = acute myocardial infarction; CKD = chronic kidney disease; CO = cardiac output, CVP = central venous pressure; GFR = glomerular filtration rate; H2O = water; IAP = intra-abdominal pressure; IGFBP7 = insulin-like growth factor binding protein 7; IL = interleukin; KIM-1 = kidney injury molecule 1; L-FABP = liver-type fatty acid binding protein; LVH = left ventricular hypertrophy; MR-PROADM = mid regional pro adrenomedullin; Na = sodium; N-GAL = neutrophil gelatinase-associated lipocalin; NO/ROS = nitric oxide/reactive oxygen species; PE = pulmonary embolism; RBF = renal blood flow; RRT = renal replacement therapy; RVR = renal venous resistance; SNS/RAS = sympathetic nervous system/renin angiotensin system; ST2 = suppression tumorigenicity 2; TIMP-2 = tissue inhibitor of metalloproteinase 2; TNF = tumor necrosis factor.
Acute CRS is defined as an abrupt worsening of cardiac (CRS type 1) or kidney (CRS type 3) function that leads to AKI or acute heart failure, respectively. Right-sided heart failure often drives CRS type 1 by producing venous congestion, which reduces renal perfusion pressure and triggers intrarenal mechanisms that decrease renal function (3,29). AKI can trigger worsening heart failure and CRS type 3 via direct myocardial injury from oxidative stress, activation of the sympathetic nervous system and the renin-angiotensin-aldosterone system, and the harmful effects of fluid overload, uremia, electrolyte imbalance, and acidemia (28). Among other important mechanisms, the pro-inflammatory state of chronic heart failure can cause renal tubular injury, leading to fibrosis and CKD (CRS type 2) (28). CKD can likewise cause or intensify heart failure via volume and pressure overload and can aggravate cardiomyopathy due to uremic toxins and acidemia (CRS type 4) (28). CRS type 5 involves simultaneous acute or chronic cardiac and renal dysfunction secondary to a systemic disorder (e.g., sepsis). Chronic CRS (CRS types 2 and 4) often co-exists simultaneously with acute CRS (CRS types 1 and 3), reflecting acute chronic cardiac and renal dysfunction, making it difficult to distinguish CRS subtypes in patients presenting with combined heart and kidney failure (3,4).
DIAGNOSIS, WORKUP, AND STAGING OF AKI IN CICU PATIENTS.
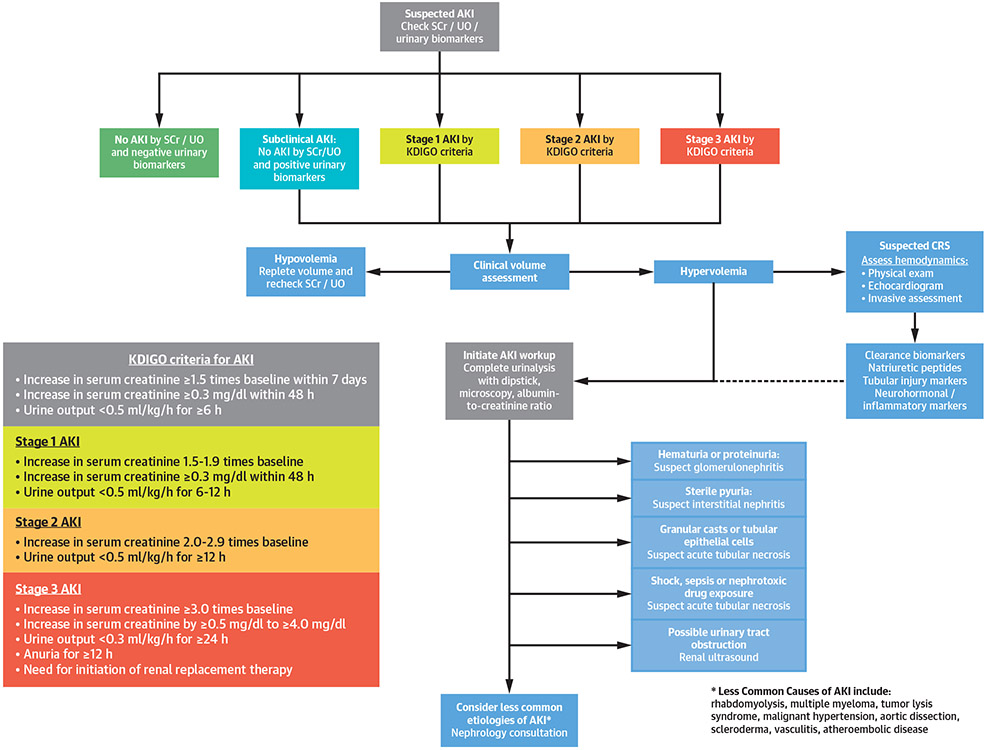
Standard criteria for AKI have been developed by the Kidney Disease: Improving Global Outcomes group, which classifies AKI into 3 progressive stages based on changes in serum creatinine and urinary output over hours to days (Figure 2) (30). Elevated levels of urinary tubular injury biomarkers have been validated to predict the development and progression of AKI, and these tests can improve prognostication when added to conventional functional indexes of AKI (31,32). A common sign of severe AKI is an acute reduction in urine output (unless masked by diuretic use), and oliguric AKI is more likely to progress and require dialysis. Any acute decrease in urine output in a CICU patient should be promptly evaluated to identify and treat reversible causes (33).
FIGURE 2. Diagnostic Approach to Patients With Suspected AKI.
AKI is defined as an acute reduction in urine output or increase in serum creatinine level and is divided into 3 Kidney Disease: Improving Global Outcomes (KDIGO) AKI stages of increasing severity (21,30). The diagnostic approach for patients with AKI includes assessment of volume status and focused testing to exclude potential etiologies, including biomarker testing (21,30,32,34). Natriuretic peptides include B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP). Urinary biomarkers include TIMP-2 and IGFBP-7. Clearance biomarkers include serum creatinine (SCr), blood urea nitrogen (BUN), and cystatin C. Tubular injury biomarkers include KIM-1, L-FABP, and NGAL. Neurohormonal and/or inflammatory biomarkers include growth differentiation factor (GDF)-15, ST-2, and cancer antigen 125 (CA-125). UO = urinary output; other abbreviations as in Figure 1.
For patients presenting with AKI or CRS, careful evaluation is needed to determine the etiology, including a detailed history and physical examination, volume status assessment, urinalysis, and urine microscopy, and a careful review of medication history to evaluate the use of renally eliminated and nephrotoxic drugs (Figure 2) (21). An essential step in the evaluation of patients with CRS is the assessment of filling pressures (congestion) and forward flow (perfusion) based on physical examination, echocardiography, and/or invasive hemodynamics (Figure 2) (3). Other causes of AKI, such as hypovolemia, nephrotoxins, or acute tubular necrosis due to hypotension and/or shock are not necessarily classified as CRS (3). A urinary albumin to creatinine ratio can be useful to evaluate for the presence and severity of CKD. A renal ultrasound can assess kidney size and echogenicity and exclude hydronephrosis as a cause of AKI.
Prognostic biomarkers in CRS include clearance biomarkers (which reflect glomerular filtration rate), tubular injury biomarkers, natriuretic peptides reflecting congestion, and biomarkers suggesting neurohumoral activation or inflammation (34). Natriuretic peptide levels can be elevated in all CRS subtypes, and a normal natriuretic peptide level suggests a cause of AKI other than CRS type 1. Increases in clearance biomarkers are characteristic of AKI, but reductions in glomerular filtration rate occur before biomarker changes, emphasizing the importance of oliguria as an early warning sign of AKI (32,33). Serum cystatin C is a clearance biomarker analogous to serum creatinine with higher sensitivity for AKI and additive prognostic value beyond serum creatinine levels (35,36).
MEDICAL MANAGEMENT OF AKI AND CRS BEFORE INITIATION OF RENAL REPLACEMENT THERAPY.
Management of AKI and CRS generally involve optimization of hemodynamics and fluid balance, and avoidance or discontinuation of potential nephrotoxins (Central Illustration). Common nephrotoxic medications include certain antimicrobial drugs (particularly aminoglycosides), nonsteroidal anti-inflammatory drugs, and iodinated radiocontrast. All renin-angiotensin-aldosterone system inhibitors can reduce the glomerular filtration rate and should be held during severe AKI (20,21). Because of the potentially beneficial effects of renin-angiotensin-aldosterone system inhibitors in patients with cardiovascular disease, continuing these medications initially may be reasonable during mild AKI and CRS, but caution is needed. Apart from preventative measures, there are no universally accepted therapies that consistently improve renal function recovery or clinical outcomes in patients with AKI or CRS (3,4,21). Cautious fluid administration guided by markers of fluid responsiveness can be considered (3). Excessive fluid administration should be avoided to prevent harmful volume overload (22,27).
Loop diuretics are useful for patients with CRS and AKI to prevent or treat fluid overload, and successful diuresis can potentially lead to improved renal function by relieving renal venous congestion (4,22). For most patients, intravenous bolus dosing or continuous infusion of loop diuretics at equivalent doses will produce similar diuresis, with a comparable safety profile and no demonstrated difference in clinical outcomes (37). In our clinical experience, the greater control of diuresis allowed by a continuous loop diuretic infusion may be advantageous for selected patients, such as those with hemodynamic instability who may not tolerate bolus dosing, as well as in patients in whom careful diuretic titration or high diuretic doses are required. Although loop diuretics can transiently reduce glomerular filtration rate, they are not considered directly nephrotoxic (3).
Although diuretics have not been demonstrated to reduce mortality or prevent the need for dialysis in patients with AKI, patients who respond to diuretics appear to have better outcomes (38). A loop diuretic challenge or furosemide stress test can be performed in patients with AKI by measuring the urine output after a dose of 1.0 to 1.5 mg/kg of intravenous furosemide (39-42). Patients who have a urine output <200 ml over the first 2 h are at increased risk of progressive AKI and potential need for renal replacement therapy (RRT); a 6-h urine output <600 ml is predictive of the need for RRT in patients with stage III AKI (39-42). Intravenous furosemide doses up to 200 to 240 mg can occasionally produce diuresis in patients with severe AKI who fail to respond to a standard furosemide challenge.
The addition of a second diuretic, frequently a thiazide-type diuretic, to ongoing loop diuretic therapy can potentially overcome diuretic resistance and increase urine output (Figure 3) (43-46). Close monitoring of electrolytes is warranted when using combination diuretic therapy due to the elevated risk of electrolyte disturbances (43). Although tolvaptan and aldosterone antagonists can augment diuresis in selected patients with CRS, these agents have not improved kidney function or clinical outcomes in patients with acute heart failure (46-49). A randomized study in 60 loop diuretic-resistant patients hospitalized with heart failure demonstrated similar augmentation of urine output with the addition of oral metolazone, intravenous chlorothiazide, or oral tolvaptan (46). Small-volume hypertonic saline may also improve diuretic responsiveness and renal function in patients with CRS, potentially by amelioration of neurohormonal activation (50).
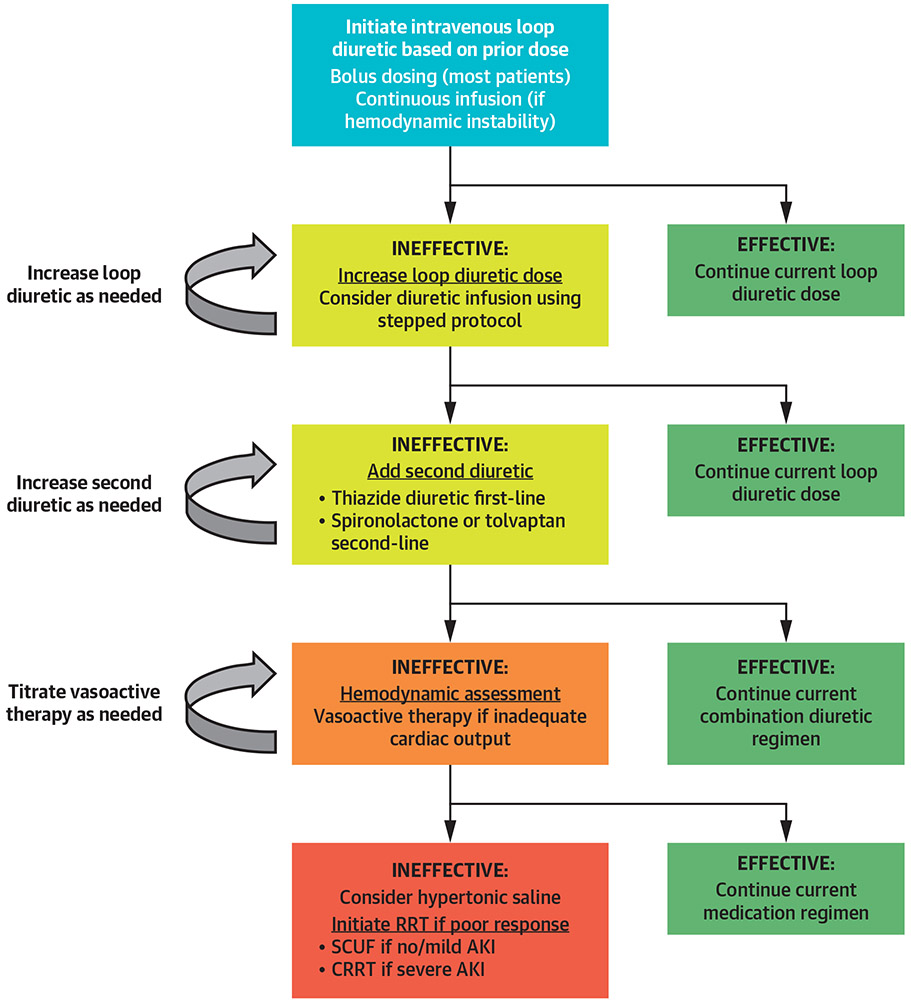
FIGURE 3. Volume Management in Patients With Acute Heart Failure and CRS.
Escalating doses of loop diuretics are the first-line of therapy for volume removal, with combination diuretic therapy used for patients who do not respond adequately to loop diuretics (3). A target urine output of 3 to 5 l/day has been used in studies of stepped-diuretic therapy (56,62). Hemodynamic assessment and vasoactive therapy can be helpful in selected patients with poor response to diuretics, with extracorporeal volume removal for patients in whom medical therapy fails. CRRT = continuous renal replacement therapy; RRT = renal replacement therapy; SCUF = slow continuous ultrafiltration; other abbreviations as in Figure 1.
The optimal systemic hemodynamic goals for the prevention and treatment of AKI and CRS are unknown, although avoidance of hypotension and low-output states is prudent (30). No specific vasoactive drug has been shown to prevent or treat AKI or CRS, including inotropes or vasodilators (3,4,21). Dopamine has cardiac inotropic and direct kidney effects that can enhance kidney function and diuresis in some patients, but dopamine has not improved clinical or renal outcomes in patients with AKI (51). Dopamine has not consistently improved kidney function, diuresis, or clinical outcomes in patients with CRS, although a possible beneficial effect of low-dose dopamine has been observed in patients with CRS with systolic heart failure (52-54). Inotropic therapy is most likely to be effective in patients with CRS with hypotension or objective evidence of reduced cardiac output, and empirical use of inotropes should be avoided due to their potential toxicity (44,45).
ULTRAFILTRATION IN REFRACTORY CRS.
Isolated ultrafiltration (aquapheresis) is a potential option for management of diuretic-resistant heart failure and refractory CRS (Central Illustration), although patients with severe kidney dysfunction (e.g., serum creatinine >2.5 mg/dl) may benefit from another RRT modality that provides clearance (44,45,55). No widely accepted standard criteria exist for initiating ultrafiltration in patients with heart failure, but it is reasonable to consider ultrafiltration for fluid-overloaded patients with severe diuretic resistance despite adequate kidney function (44,45,55). No studies have prospectively examined the optimal timing of ultrafiltration initiation in patients with heart failure and CRS, although ultrafiltration appears to be more beneficial if applied within 24 h of admission (55,56). The use of ultrafiltration may provoke less neurohormonal activation than diuretic therapy, which leads to greater sustainability of beneficial effects (57,58). Reversal of renal venous congestion using ultrafiltration can improve diuretic responsiveness, particularly in patients with significant right-sided heart failure (59,60).
Ultrafiltration is a safe alternative to diuretic therapy in patients with heart failure or CRS who are not medically refractory, and studies have generally shown greater decongestion and lower rates of subsequent rehospitalization with ultrafiltration, but no improvement in renal function or mortality (55,56,61,62). In the CARRESS-HF (Cardiorenal Rescue Study in Acute Decompensated Heart Failure), ultrafiltration was associated with a greater rise in serum creatinine without improved fluid removal or clinical outcomes in CRS patients compared with a stepped diuretic algorithm that involved escalating doses of furosemide infusion ± metolazone (Table 1) (62). For most patients with CRS, a stepped diuretic algorithm similar to that used in the CARRESS-HF and AVOID-HF (Aquapheresis versus Intravenous Diuretics and Hospitalization for Heart Failure) trials (Table 1) is a reasonable first-line strategy (56,62). Some patients will respond to diuretic doses greater than those used in CARRESS-HF, but the safety profile is uncertain, and transitioning to ultrafiltration is reasonable for patients who do not respond adequately to high-dose diuretic therapy (43-45).
TABLE 1.
| Current Diuretic Regimen |
Suggested Diuretic Regimen |
|||
|---|---|---|---|---|
| Step | Furosemide Dose | Thiazide | Furosemide Dose (IV) | Metolazone |
| 1 | ≤80 mg/day | +/− | 40 mg + 5 mg/h | 0 |
| 2 | 81–160 mg/day | +/− | 80 mg + 10 mg/h | 5 mg QD |
| 3 | 161–240 mg/day | +/− | 80 mg + 20 mg/h | 5 mg BID |
| 4* | >240 mg/day | +/− | 80 mg + 30 mg/h | 5 mg BID |
The starting diuretic dose is determined by the outpatient or current inpatient diuretic dose, and the patient is moved to a higher diuretic dose if urine output is <3 l/day on the current dose. All loop diuretic doses are given in furosemide equivalents, although an alternative loop diuretic could be used. *A vasodilator or inotrope can be added for patients who have urine output <3 l/day despite Step 4 diuretic dosing.
AVOID-HF = Aquapheresis versus Intravenous Diuretics and Hospitalization for Heart Failure; BID = twice daily; CARRESS-HF = Cardiorenal Rescue Study in Acute Decompensated Heart Failure; IV = intravenous; QD = every day.
USE OF RRT IN CRITICALLY ILL CARDIAC PATIENTS.
Traditionally, the decision to initiate urgent RRT in critically ill patients with AKI or CRS is based on the standard “AEIOU” indications: acidosis, electrolyte derangements, intoxications, volume overload, and uremia (Table 2) (63). The most common indication for continuous RRT (CRRT) initiation in CICU patients is medically refractory volume overload with hemodynamic instability (64). We support standard RRT initiation guidelines, although there may be some unique CICU-specific indications for initiating RRT (Table 2) (63,64).
TABLE 2.
Potential General and CICU-Specific CRRT Indications
| General Acute RRT Indications | Proposed CICU-Specific CRRT Indications |
|---|---|
| A: Severe metabolic acidosis (i.e., severe lactic acidosis with refractory shock and multiorgan failure) | Patients with severe cardiac and/or valvular dysfunction and borderline blood pressure with AKI and volume overload |
| E: Severe electrolyte disturbances, most commonly hyperkalemia | Cardiogenic shock or heart failure with pulmonary edema on mechanical ventilation and high FiO2 (>80% to 90%) despite diuretic therapy |
| I: Intoxication with dialyzable drugs or toxins | Pre-cardiac surgical volume removal to improve likelihood of chest closure and prevent post-operative right ventricular failure |
| O: Medically refractory volume overload | Refractory cardiorenal syndrome with progressive AKI (e.g., stage 2 to 3 AKI plus volume overload with inadequate diuretic response) |
| U: Severe azotemia or symptoms of uremia |
When initiating renal replacement therapy (RRT), the broader clinical context, presence of conditions that can be modified by RRT, and trends of laboratory tests should be considered instead of a single universal threshold (e.g., blood urea nitrogen or creatinine) (63).
AKI = acute kidney injury; CICU = cardiac intensive care unit; CRRT = continuous renal replacement therapy; FiO2 = fraction of inspired oxygen.
A meta-analysis that examined the timing of RRT initiation did not demonstrate any significant differences in mortality, renal recovery, or other outcomes in patients with severe AKI who were randomized to early or late RRT initiation (65). The AKIKI (Artificial Kidney Initiation in Kidney Injury) study randomized 620 critically ill patients with stage 3 AKI to immediate RRT initiation or deferred RRT initiation until patients developed severe hyperkalemia, metabolic acidosis, pulmonary edema, severe azotemia, or oliguria for >72 h (66). No difference in mortality was observed, and nearly one-half of the delayed RRT group did not require RRT (66). By contrast, the randomized ELAIN (Early versus Late Initiation of Renal Replacement Therapy in Critically Ill Patients With Acute Kidney Injury) study demonstrated lower mortality in 231 critically ill, predominantly post-operative patients with stage 2 AKI and elevated markers of tubular injury (plasma neutrophil gelatinase-associated lipocalin [NGAL] >150 ng/ml) who received immediate RRT initiation versus deferral of RRT initiation until the development of stage 3 AKI (67). The totality of evidence suggests that patients with AKI who do not have a specific indication for RRT initiation may safely be observed initially, even in the presence of oliguria (i.e., urine output <400 to 500 ml/day). The development of diuretic refractory symptomatic fluid overload should prompt consideration of RRT (63).
BASICS OF RRT.
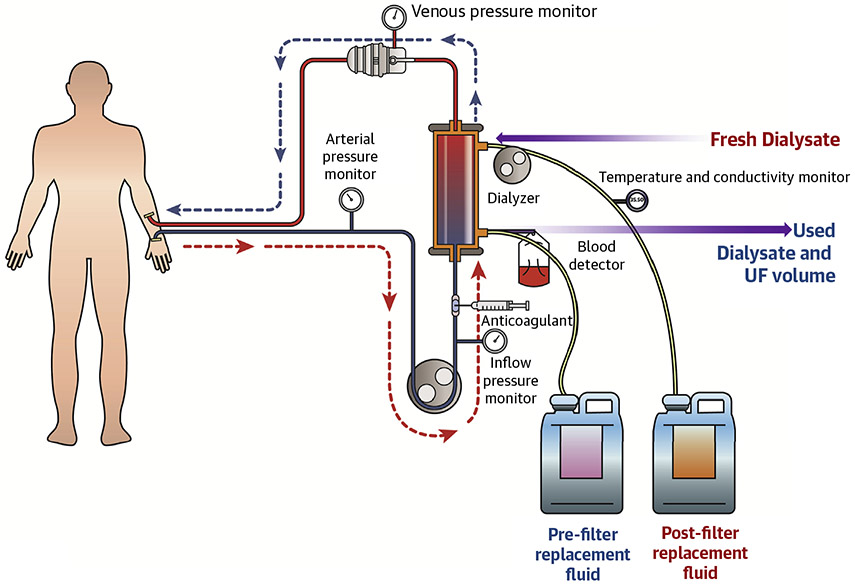
RRT removes metabolic wastes and excess water, balances electrolytes, and replenishes buffers in patients with severe AKI and patients with end-stage renal disease (30,63). In the United States, hemodialysis is used most commonly for acute RRT. The apparatus for performing RRT consists of a dialyzer with a semipermeable membrane, replacement fluid or dialysate (dialysis solution), sterile tubing for the transport of blood and dialysate, and a machine to power and monitor the procedure using sensors and alarms (Figure 4). The semipermeable dialyzer membrane allows the bidirectional exchange of solutes and fluid between a patient’s blood and dialysate without loss of large molecular weight substances or cells.
FIGURE 4. Components of a Dialysis Circuit.
The dialysis circuit is composed of a dialyzer with a semipermeable membrane, replacement fluid or dialysate (dialysis solution), sterile tubing for the transport of blood and dialysate, and a machine to power and monitor the procedure using sensors and alarms. A continuous venovenous hemofiltration (CVVH) circuit contains the same components, except that dialysate is not used. UF = ultrafiltration.
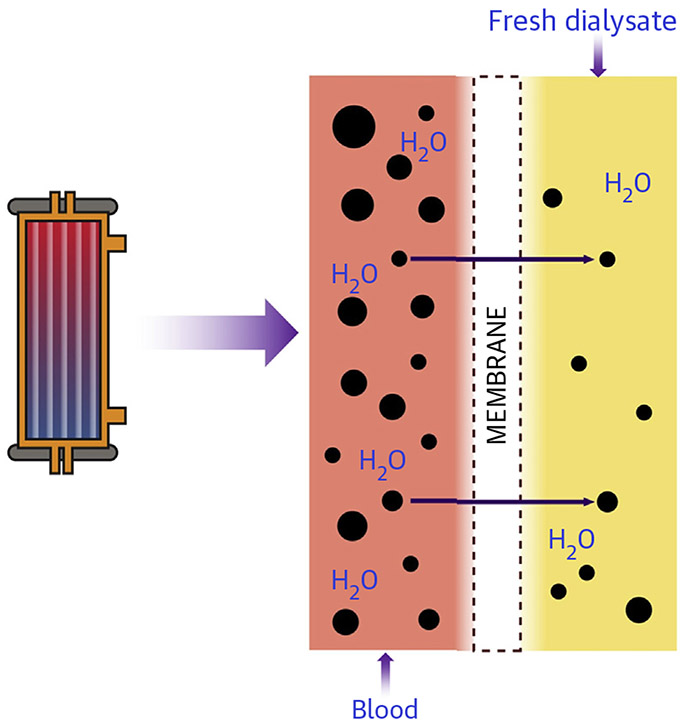
Metabolic clearance through the semipermeable membrane occurs primarily via diffusion (Figure 5) and convection (Figure 6), which can be used either alone or together during CRRT. Diffusive clearance is determined by the concentration gradient between the solute in the blood and dialysate, which is maximized during hemodialysis by countercurrent blood and dialysate flow. Convective clearance uses a hydrostatic pressure gradient to extract plasma water and small molecular weight solutes, leading to ultrafiltration of isotonic fluid. Fluid removed by convection during ultrafiltration increases intravascular plasma osmolality and oncotic pressure, which mitigates the potential hypotensive effects of rapid volume removal, whereas solute removal by diffusion during hemodialysis can result in decreased plasma osmolality and hypotension from fluid shifts (68).
FIGURE 5. Solute Clearance by Diffusion During Hemodialysis.
Blood and dialysate are separated by the semipermeable membrane, and solutes move across the membrane down a concentration gradient. With diffusive solute clearance, dialysate runs countercurrent to the blood flow to remove solutes.
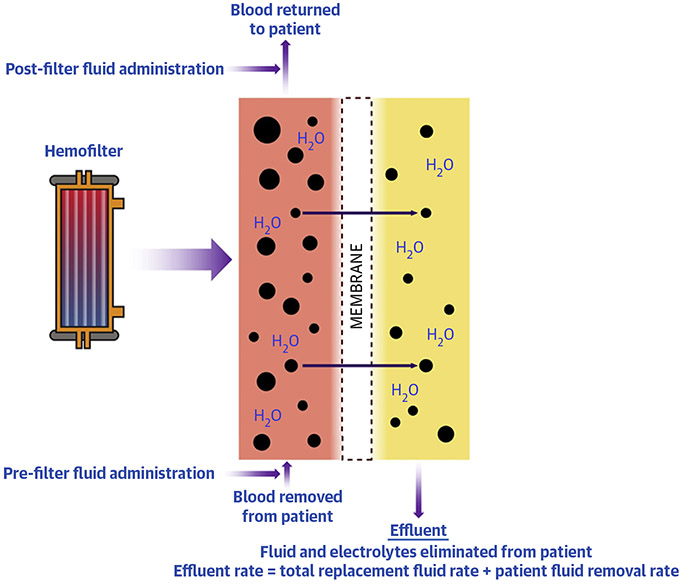
FIGURE 6. Function of the CVVH Filter and Solute Clearance by Convection.
Blood and ultrafiltrate are separated by the semipermeable membrane, and solutes move across the membrane down a hydrostatic pressure gradient. With convective solute clearance, solutes are removed from the blood by ultrafiltration and then balanced replacement fluid is administered. CVVH = continuous venovenous hemofiltration.
RRT MODALITIES.
Intermittent RRT modalities, such as intermittent hemodialysis (IHD), last 3 to 6 h/day, whereas CRRT ideally runs 24 h/day (Table 3). Standard modalities of CRRT include slow continuous ultrafiltration, continuous venovenous hemofiltration (CVVH), continuous venovenous hemodialysis (CVVHD), or continuous venovenous hemodiafiltration (CVVHDF). Aquapheresis is a volume removal procedure without the intent of solute removal by ultrafiltration that can be performed via a portable machine and peripheral venous access (55). Hybrid slow RRT modalities with intermediate or extended treatment durations, such as sustained low-efficiency dialysis, can be provided using standard IHD machines, with lower resource use, less need for anticoagulation, and less patient immobility compared with CRRT (69). The choice of RRT modality depends on the expertise of the treating physician, local availability, hemodynamic stability, vascular access, and indication (Table 3); data comparing different RRT modalities in patients with CRS are lacking (63,70).
TABLE 3.
Comparison Between CRRT Modalities (i.e., CVVH) and Intermittent RRT Modalities (i.e., IHD)
| Continuous RRT Modalities | Intermittent RRT Modalities | |
|---|---|---|
| Indication | Hemodynamic instability | Severe acid-base and electrolyte imbalances |
| Volume control | Intoxication | |
| Intracranial hypertension | Refractory filter clotting | |
| Advantages | Hemodynamic stability | (Relatively) inexpensive |
| Stable, effective, and predictable volume control | Flexible timing allows for mobility/transport | |
| Stable and predictable control of chemistry | Rapid correction of fluid overload | |
| Stable intracranial pressure | Rapid removal of dialyzable drugs | |
| Disease modification by cytokine removal | Minimizes anticoagulant exposure | |
| Potentially improved chances of renal recovery | Rapid correction of acidosis and electrolyte abnormality | |
| Disadvantages | Anticoagulation requirements | Intradialytic hypotension |
| Reduced patient mobility | Potential nephrotoxicity | |
| Higher potential for filter clotting | Risk of bowel and coronary ischemia | |
| Higher cost |
CVVH = continuous venovenous hemofiltration; IHD = intermittent hemodialysis; other abbreviations as in Table 1.
The choice of acute RRT modality (IHD vs. CRRT) does not appear to influence survival or subsequent renal recovery in patients with AKI (71). CRRT uses a lower blood flow rate than IHD, which allows better hemodynamic tolerance; aquapheresis can be performed using a blood flow rate as low as 40 ml/min (55). Because hemodynamic instability during RRT is influenced by the rate of fluid and solute removal, the major advantage of CRRT is slower and more controlled solute and fluid removal compared with IHD (Table 3) (63). The longer duration of CRRT compared with IHD allows greater daily fluid removal, making CRRT particularly useful in the presence of massive hypervolemia (63). CRRT is associated with improved cardiovascular stability, more consistent daily fluid removal, and better metabolic control compared with IHD (Table 3) (72,73). IHD is more effective for rapid fluid and solute removal and is preferred for patients with acute pulmonary edema, intoxications, or severe acid-base and electrolyte imbalances (e.g., hyperkalemia) (Table 3). Other potential advantages of IHD include improved patient mobility, a lower nursing workload, and lower costs (Table 3) (74-76). Hemodynamic effects, fluid removal, metabolic control, 30-day mortality, and RRT liberation are similar between CRRT and hybrid RRT modalities and between different CRRT modalities (77,78).
Vascular access via an arteriovenous fistula is the preferred approach for maintenance IHD in patients with end-stage renal disease, but this approach is not effective for initiating acute IHD or CRRT. Venous access via a large-bore, high-flow, dual-lumen central venous dialysis catheter in the right internal jugular or femoral vein is necessary for acute CRRT or IHD. Catheter placement in the subclavian or brachial vein should be avoided to prevent subsequent venous stenosis (63). CRRT is preferred for management of AKI-D in patients receiving extracorporeal membrane oxygenator support, which is performed either using a dedicated catheter and separate circuit or via attaching a CRRT circuit to the venous limb of the extracorporeal membrane oxygenator circuit (79). Separate CRRT cannulation is the simplest setup to manage but requires additional large-caliber venous access.
EFFECTS OF CRRT ON DRUG PHARMACOKINETICS.
Depending on the mode, drug clearance during CRRT is determined by the ability of the drug to pass through the filter, pre-filter fluid administration (which reduces clearance), rate of blood flow, dialysate flow, and ultrafiltration rate (80-83). During CVVHDF, clearance occurs through a combination of convective and diffusive clearance, whereas drug clearance by ultrafiltration or aquapheresis is not clinically significant (80). Few published studies have characterized the effect of CRRT on the pharmacokinetics of common drugs used in the CICU (Supplemental Table 1).
For drugs with substantial renal excretion, dose reduction is necessary for patients with AKI, and dosing must be further adjusted when initiating or discontinuing RRT. Although dosing recommendations during IHD are available for many drugs, drug dosing recommendations for patients on CRRT are currently not included in the labeling for drugs approved by the Food and Drug Administration (84). Expert consultation is recommended to determine drug dosing specific to the CRRT modality being used. The clearance of drugs may be higher with prolonged CRRT than IHD, depending on the CRRT modality and dose. Close attention to appropriate drug dosing is necessary when CRRT is interrupted or discontinued for more than a brief period, or during transition onto or off of IHD. Patients who receive IHD require a diet that is restricted in fluid, sodium, potassium, and phosphorous, as well as modified in protein, whereas patients receiving CRRT may not need these electrolytes restricted and may require electrolyte and protein supplementation (30).
Cardiac medications such as intravenous inotropes, vasopressors, and vasodilators are not substantially cleared by either CRRT (Supplemental Table 1) or IHD. Milrinone accumulates during AKI, with a half-life of up to 20 h in patients on CVVH, leading to drug accumulation and toxicity even at low doses (85). Renally excreted antiarrhythmic drugs can accumulate to toxic levels in patients with AKI, and clearance by either CRRT or IHD is unreliable, so alternative drugs should be used in patients with AKI-D. The most commonly used drugs requiring altered dosing for patients with AKI-D are antimicrobial agents, which typically require higher and/or more frequent doses for patients on CRRT compared with that of IHD (82,83).
CRRT BEDSIDE MANAGEMENT AND TROUBLESHOOTING.
The CVVH prescription consists of 4 parts: blood flow rate, replacement fluid rate, replacement fluid composition, and net patient fluid removal (Supplemental Table 2). In CVVHD and CVVHDF, dialysate composition and dialysate flow rate are also prescribed. Blood flow rate is typically set at 150 to 250 ml/min in adults, because lower blood flow rates promote hemostasis and filter clotting; higher blood flow rates can lead to pressure alarms and CRRT interruption. CVVH removes a large volume of plasma ultrafiltrate via convection, exchanging it with a balanced replacement fluid mirroring the solute content of normal plasma (Figure 6). The dose of CVVH is the total effluent rate (roughly equivalent to the replacement fluid rate) and is typically set at 25 to 35 ml/kg/h; higher dose CRRT regimens have not been shown to improve outcomes in patients with AKI (86). Replacement fluid can be administered 100% pre-filter or split pre- and post-filter (Figure 6). Pre-filter fluid administration dilutes the blood in the filter, which reduces the risk of filter clotting at the expense of clearance, whereas post-filter fluid administration favors clearance at the expense of clotting risk. The bicarbonate concentration in the replacement fluid can be increased in patients with severe metabolic acidosis, but patients who receive high-bicarbonate replacement fluid are at increased risk of adverse outcomes (87). The net patient fluid removal (ultrafiltration) rate is adjusted to achieve fluid balance goals. Although higher ultrafiltration rates may be used temporarily for patients with severe volume overload, daily average net ultrafiltration rates >1.75 ml/kg/h may be associated with worse outcomes (88). High ultrafiltration rates can also dehydrate the filter and increase the risk of clotting (Supplemental Table 2).
Frequent monitoring of CRRT is necessary, and troubleshooting strategies for common CRRT circuit issues are outlined in Table 4. Monitoring transmembrane pressure helps to track filter patency, and a rising transmembrane pressure is a harbinger of filter clotting that may warrant electively returning the patient’s blood (before blood is wasted within a clotted filter) and replacing the circuit (Table 4). Frequent electrolyte monitoring every 6 to 12 h is necessary, including calcium, phosphorous, and magnesium. Phosphorous is cleared efficiently by CRRT, and patients may require phosphorous repletion during CRRT. Hypotension occurs in up to two-thirds of patients who are started on CRRT, many of whom are already in shock and require vasopressors (64,89). Hemodynamic intolerance to ultrafiltration in volume-overloaded patients with hypotension can be ameliorated in some patients by administration of hyperoncotic albumin (if hypoproteinemic), blood transfusion (if anemic), and/or lower extremity wrapping. CRRT can induce hypothermia and potentially mask fevers, warranting a high index of suspicion for infection.
TABLE 4.
Troubleshooting Common Alarms and Clinical Problems for Patients on CRRT
| Alarm/CRRT Issue | Evaluation | Resolution/Intervention |
|---|---|---|
| Negative pressure access alarms (high inlet pressure) | Evaluate patient for hypovolemia, intrabdominal hypertension, and respiratory distress | Remedy contributing patient factors (e.g., bolus fluids or add sedation) |
| Check catheter for kinking and clot formation | Reposition catheter | |
| Intra-catheter TPA administration | ||
| Reverse inlet and outlet positions | ||
| Place new catheter (longer if feasible) | ||
| Positive pressure access alarms (high outlet pressure) | Check catheter for kinking and clot formation | Adjust patient position |
| Reposition catheter | ||
| Intra-catheter TPA administration | ||
| Reverse inlet and outlet positions | ||
| Place new catheter (longer if feasible) | ||
| Transmembrane pressure alarms | Evaluate filter for evidence of clotting | Calculate and maintain filtration fraction <25% (e.g., increase pre-filter fluid or decrease net patient fluid removal) |
| Check adequacy of circuit anticoagulation | ||
| Add circuit anticoagulation | ||
| If clotting is imminent, return blood to patient | ||
| Refractory hyperkalemia | Assess patient for etiologies of elevated potassium: acidosis, hemolysis, tissue necrosis, drugs | Administer medical therapies including: insulin, bicarbonate, and laxatives |
| Attempt lower K replacement fluid administration | ||
| Increase prescription (replacement fluid rate) | ||
| Consider performing IHD | ||
| Refractory acidosis | Assess patient for etiologies of acidosis: hypoperfusion/lactic acidosis, ketosis, hyperchloremia, organic acids, toxic alcohols | Increase prescription (replacement fluid rate) |
| Increase replacement fluid bicarbonate concentration | ||
| Consider isotonic (150 mEq/l) bicarbonate infusion |
Clotting of the CRRT circuit can be prevented by adjusting the CRRT prescription or adding an anticoagulant to extend the life of the CRRT filter (Table 4). To perform CRRT without anticoagulation, high blood flow rates and pre-filter replacement fluid are used (Table 4). Heparin can be administered either systemically or regionally by infusing the heparin pre-filter (Supplemental Table 2). As an alternative to heparin, regional administration of citrate solution pre-filter prevents filter clotting by chelating calcium akin to banked blood. Although citrate is more effective for anticoagulation during CRRT and is associated with less bleeding than heparin, the use of citrate requires close monitoring of ionized calcium levels and additional central venous access for administration of a calcium infusion to maintain normal systemic ionized calcium levels (90,91). Accumulation of citrate can cause significant toxicity with worsening anion-gap metabolic acidosis and ionized hypocalcemia that triggers hypotension. Patients with liver failure and profound shock cannot efficiently clear citrate and may require lower citrate doses to avoid toxicity (92). An elevated ratio of total to ionized calcium >2.4 suggests citrate accumulation and an increased risk of death (93).
ASSESSMENT OF RENAL RECOVERY AND CRRT DISCONTINUATION.
Rates of CRRT discontinuation success vary widely (21% to 60%) among intensive care unit patients (94-96). In 1 study, 48% of CICU patients with AKI-D who survived hospitalization were ultimately transitioned to IHD (64). There are currently no widely accepted specific criteria to guide renal function recovery assessment for the discontinuation of CRRT. As an indicator of renal recovery in CRRT patients, urine output had a sensitivity of 66% and a specificity of 74% in a meta-analysis of observational studies that used different 24-h urine output thresholds (95-98). In a retrospective study of 1,006 intensive care unit patients who received CRRT, urine output was the strongest predictor of successful CRRT discontinuation (odds ratio: 1.08 per 100 ml/day increase; area under the curve: 0.81) (95). A cutpoint of 400 ml/day without the use of diuretics had a positive and negative predictive value of 80.9% and 76.5%, respectively. In a randomized study of 71 patients on CRRT, those who received a furosemide infusion after CRRT discontinuation had higher urine volume, with no differences in renal recovery or repeat need for CRRT (99). We suggest that urine output ≥400 ml over 24 h in the absence of diuretic administration is a reasonable predictor of renal recovery for patients on CRRT (Central Illustration); diuretics should be used primarily to enhance urine output after CRRT discontinuation. Although angiotensin-converting enzyme inhibitors and angiotensin receptor blockers should be held during AKI until recovery occurs, resuming these important drugs after AKI recovery appears to be associated with better long-term outcomes (100,101).
TRANSITIONS TO IHD.
The suitability for transitioning from CRRT to IHD should be assessed daily by the CICU and nephrology teams. There are no set criteria or timing standards for transitions from CRRT to IHD in either AKI or CRS, but potential considerations in CICU patients include the resolution of the initial indication for CRRT (Table 2), hemodynamic stability on minimal doses of vasoactive agents, and/or near-euvolemia with the anticipated ability to tolerate daily fluid removal goals with IHD. It is not known whether the criteria for transitioning from CRRT to IHD should differ for patients with end-stage renal disease versus patients with AKI-D or CRS, but caution should be taken in patients with AKI-D because recurrent hypotension associated with the transition to IHD could adversely affect renal recovery (Table 3). At least one-third of patients cannot be successfully liberated from CRRT, typically due to persistent hypotension that results in intolerance of IHD (64,95,102). Initiation of oral midodrine or droxidopa can allow weaning from intravenous vasopressors and prevent hypotension during IHD, but these alpha-agonists are potentially harmful to patients with cardiovascular disease (103).
PROGNOSIS AND PREDICTORS OF OUTCOME AMONG CICU PATIENTS REQUIRING RRT.
Although the indication for CRRT influences the observed mortality, the provision of CRRT is associated with poor outcomes among CICU patients, with hospital mortality rates of at least 40% to 50% and 1-year mortality >70% (26,64,104). Among 199 CICU patients who received CRRT at a single center, 53% died in the hospital and 50% of hospital survivors required dialysis at discharge, which produced a dialysis-free hospital survival of 22% (64). Hospital survivors had 39% 1-year mortality, and overall 1-year survival was only 29% (64). In a multicenter study of 178 CICU patients who received dialysis (including CRRT and IHD), 42% died in the hospital (26). Among general intensive care unit patients who received CRRT in randomized clinical trials, the pooled hospital mortality was 58% (71). Short-term mortality was 30% in patients treated with ultrafiltration or CRRT for refractory CRS, but 1-year mortality was 95%; patients who responded to ultrafiltration without needing CRRT had better outcomes (105,106). Patients with acute coronary syndromes who required CRRT had a 41% hospital mortality rate, which contrasted with >60% mortality for patients with cardiogenic shock and AKI-D (14,17,26). Among patients who received CRRT, those with end-stage renal disease might have had lower short-term mortality than those with AKI-D (89). Predictors of higher hospital mortality in patients who receive CRRT include older age, hypotension (particularly early after CRRT initiation), greater vasopressor requirements, greater fluid overload, worse right ventricular function, higher overall illness severity, frailty, hyperphosphatemia, and worse oxygenation (64,89,104,106-110).
MULTIDISCIPLINARY TEAM-BASED CARE FOR RRT PATIENTS.
Acute RRT constitutes a resource-intensive intervention that requires specialized skills and a dynamic individualized approach to the patient’s changing clinical status in the context of the patient’s acute illness and underlying comorbidities (111). Appropriate delivery of CRRT requires simultaneous attention to the patient’s clinical status, fluid and electrolyte balance, hemodynamics, drug dosing, and anticoagulation (Central Illustration), as well as assessing the technical components of the CRRT circuit (Table 4). There is no single evidence-based approach to the staffing or care for patients undergoing CRRT, and each institution should determine the optimal composition of the multidisciplinary team as well as adequate nurse staffing ratios. We believe that multidisciplinary CICU teams caring for patients receiving CRRT should ideally include providers with expertise in critical care medicine, cardiovascular medicine, critical care nursing, pharmacy, nutrition, and nephrology. The American Society of Nephrology Acute Kidney Injury Advisory Group emphasizes the importance of nephrologists in supporting CRRT in collaboration with critical care physicians (112). Regular, structured, multidisciplinary evaluation of CRRT quality is essential (113). Nonrandomized studies suggest that having specialized CRRT nurses can free the general critical care nurse to focus on other aspects of patient care (114,115). A single-center retrospective observational study in South Korea that evaluated 1,104 patients who underwent RRT before and after the implementation of a specialized CRRT team, which consisted of a nephrologist and 2 nurses, found that time to CRRT initiation, downtimes, and in-hospital mortality rates decreased after implementation of the specialized CRRT team (114).
END-OF-LIFE ISSUES IN PATIENTS RECEIVING RRT.
End-of-life issues should be considered for any critically ill patient with severe or medically refractory CRS or AKI. Early consultation with a palliative medicine specialist can be beneficial for symptom control, patient and family emotional support, and assistance with goals of care discussions even when an aggressive care plan is desired. Prognosis and quality of life are often poor even among those patients who are successfully liberated from CRRT (64,105,106,116). Patients with cardiomyopathy or heart failure have a poor prognosis on maintenance IHD, and the high rates of post-discharge mortality, rehospitalization, and dialysis dependence among hospital survivors should be discussed with the patient and family members when considering initiation of acute RRT (117). Overall goals of care should be clarified before the initiation of RRT, allowing individualized decision-making regarding a patient’s quality of life and prospects for recovery. Although older patients with AKI-D with higher severity of illness and more nonrenal organ failures are at higher risk of death, there are no established criteria for declaring futility of RRT in medically refractory AKI and CRS (3).
The presence of end-stage heart failure is associated with poor outcomes, particularly in patients who are not candidates for advanced heart failure therapies. The presence of severe kidney dysfunction often makes patients ineligible for a left ventricular assist device or heart transplantation because of a high post-operative mortality. RRT may not be appropriate for these patients unless kidney recovery can be expected (118,119). Patients and families may be faced with choosing between continuing supportive care, including prolonged CRRT in the CICU without the certainty of recovery or deciding to forego artificial life–prolonging technologies and allowing natural death. Many patients who receive CRRT are intubated and unable to directly participate in goals of care discussions, so providers and families need to make these crucial decisions on their behalf (64). Ideally, decisions to forgo CRRT and allow natural death are made after determining the medical appropriateness of supportive therapies in the context of the goals, preferences, values, and anticipated prognosis of the patient. Although withdrawing any supportive therapy is emotionally challenging for patients and providers, discontinuing RRT is bioethically equivalent to never having started RRT, especially as a patient’s clinical trajectory and personal goals evolve.
The decision to forego or withdraw RRT for patients with unrecovered AKI-D or end-stage renal disease will result in death, with the time frame depending on the severity of the underlying illness and the degree of residual renal function. For patients who discontinue long-term maintenance IHD, death usually occurs within 7 days due to uremia and hyperkalemia, and hospice care is often appropriate (120). Death may occur sooner after withdrawal of CRRT for critically ill patients, particularly in the setting of discontinuing other supportive therapies (e.g., vasopressors or mechanical ventilation). A primary concern during withdrawal of life-sustaining measures is adequate control of end-of-life symptoms, most notably dyspnea due to volume overload. Family support and aggressive symptom management through pharmacotherapy is critical to proactively alleviate suffering at the end-of-life, allowing a peaceful death.
CONCLUSIONS
AKI and CRS are increasingly prevalent in patients with cardiovascular disease and remain associated with poor short- and long-term outcomes, with few established treatments available for clinicians. Patients with AKI-D and those requiring CRRT represent a growing subset of patients within the CICU environment who are at incrementally higher risk of post-discharge death and dialysis dependence. The successful provision of CRRT in the CICU environment requires an integrated multidisciplinary care team. There is a pressing need for future research to evaluate the optimal approach to the management of the growing population of cardiac patients with AKI-D and refractory CRS, including new therapies for AKI and CRS, and improved strategies for CRRT initiation, management, and transitions.
Supplementary Material
HIGHLIGHTS.
AKI is increasingly common in hospitalized patients with cardiac disease.
Initial medical therapy of acute CRS involves a stepped diuretic regimen.
The need for RRT in AKI and CRS is associated with poor outcomes.
Outcomes are similar for patients treated with different acute RRT strategies in the setting of AKI.
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institutes of Health Clinical Center. The opinions expressed in this article are the author’s own and do not reflect the view of the U.S. Food and Drug Administration, the Department of Health and Human Services, the National Institutes of Health, or the U.S. government. Dr. Kellum has received consulting and/or grant support from Baxter and NxStage. Dr. Kazory has received consulting fees and is on the medical Advisory Board of CHF Solutions, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC author instructions page.
ABBREVIATIONS AND ACRONYMS
- ACS
acute coronary syndrome
- AKI
acute kidney injury
- AKI-D
acute kidney injury requiring dialysis
- CICU
cardiac intensive care unit
- CKD
chronic kidney disease
- CRS
cardiorenal syndrome
- CRRT
continuous renal-replacement therapy
- CVVH
continuous venovenous hemofiltration
- CVVHD
continuous venovenous hemodialysis
- CVVHDF
continuous venovenous hemodiafiltration
- IHD
intermittent hemodialysis
- RRT
renal replacement therapy
Footnotes
APPENDIX For supplemental tables, please see the online version of this paper.
REFERENCES
- 1.Jentzer JC, van Diepen S, Barsness GW, et al. Changes in comorbidities, diagnoses, therapies and outcomes in a contemporary cardiac intensive care unit population. Am Heart J 2019;215:12–9. [DOI] [PubMed] [Google Scholar]
- 2.Sinha SS, Sjoding MW, Sukul D, et al. Changes in primary noncardiac diagnoses over time among elderly cardiac intensive care unit patients in the United States. Circ Cardiovasc Qual Outcomes 2017;10:e003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jentzer JC, Chawla LS. A clinical approach to the acute cardiorenal syndrome. Crit Care Clin 2015;31:685–703. [DOI] [PubMed] [Google Scholar]
- 4.Rangaswami J, Bhalla V, Blair JEA, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation 2019;139:e840–78. [DOI] [PubMed] [Google Scholar]
- 5.Vandenberghe W, Gevaert S, Kellum JA, et al. Acute kidney injury in cardiorenal syndrome type 1 patients: a systematic review and meta-analysis. Cardiorenal Med 2016;6:116–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun YB, Liu BC, Zou Y, Pan JR, Tao Y, Yang M. Risk factors of acute kidney injury after acute myocardial infarction. Ren Fail 2016;38:1353–8. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Pei YY, Ma YH, et al. Risk factors for acute kidney injury in patients with acute myocardial infarction. Chin Med J (Engl) 2019;132:1660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holland EM, Moss TJ. Acute noncardiovascular illness in the cardiac intensive care unit. J Am Coll Cardiol 2017;69:1999–2007. [DOI] [PubMed] [Google Scholar]
- 9.Goldfarb M, van Diepen S, Liszkowski M, Jentzer JC, Pedraza I, Cercek B. Noncardiovascular disease and critical care delivery in a contemporary cardiac and medical intensive care unit. J Intensive Care Med 2019;34:537–43. [DOI] [PubMed] [Google Scholar]
- 10.Jentzer JC, Wiley B, Bennett C, et al. Early noncardiovascular organ failure and mortality in the cardiac intensive care unit. Clin Cardiol 2020;43:516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brueske B, Sidhu MS, Schulman-Marcus J, Kashani KB, Barsness GW, Jentzer JC. Hyperkalemia is associated with increased mortality among unselected cardiac intensive care unit patients. J Am Heart Assoc 2019;8:e011814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015;41:1411–23. [DOI] [PubMed] [Google Scholar]
- 13.Khera S, Kolte D, Aronow WS, et al. Trends in acute kidney injury and outcomes after early percutaneous coronary intervention in patients ≥75 years of age with acute myocardial infarction. Am J Cardiol 2013;112:1279–86. [DOI] [PubMed] [Google Scholar]
- 14.Marenzi G, Cosentino N, Marinetti A, et al. Renal replacement therapy in patients with acute myocardial infarction: rate of use, clinical predictors and relationship with in-hospital mortality. Int J Cardiol 2017;230:255–61. [DOI] [PubMed] [Google Scholar]
- 15.Correa A, Patel A, Chauhan K, et al. National Trends and outcomes in dialysis-requiring acute kidney injury in heart failure: 2002-2013. J Card Fail 2018;24:442–50. [DOI] [PubMed] [Google Scholar]
- 16.Bohula EA, Katz JN, van Diepen S, et al. Demographics, care patterns, and outcomes of patients admitted to cardiac intensive care units: The Critical Care Cardiology Trials Network Prospective North American Multicenter Registry of Cardiac Critical Illness. JAMA Cardiol 2019;4:928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauridsen MD, Gammelager H, Schmidt M, et al. Acute kidney injury treated with renal replacement therapy and 5-year mortality after myocardial infarction-related cardiogenic shock: a nationwide population-based cohort study. Crit Care 2015;19:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cartin-Ceba R, Kashiouris M, Plataki M, et al. Risk factors for development of acute kidney injury in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Res Pract 2012;2012:691013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, George KC, Luo R, et al. Contrast-induced acute kidney injury and adverse clinical outcomes risk in acute coronary syndrome patients undergoing percutaneous coronary intervention: a meta-analysis. BMC Nephrol 2018;19:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarese EP, Gurbel PA, Andreotti F, et al. Prevention of contrast-induced acute kidney injury in patients undergoing cardiovascular procedures-a systematic review and network meta-analysis. PLoS One 2017;12:e0168726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet 2019;394:1949–64. [DOI] [PubMed] [Google Scholar]
- 22.Teixeira C, Garzotto F, Piccinni P, et al. Fluid balance and urine volume are independent predictors of mortality in acute kidney injury. Crit Care 2013;17:R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy AK, Mc Gorrian C, Treacy C, et al. A comparison of traditional and novel definitions (RIFLE, AKIN, and KDIGO) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal Med 2013;3:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyle M, Wan SH, Murphree D, et al. Predictive value of the Get With The Guidelines heart failure risk score in unselected cardiac intensive care unit patients. J Am Heart Assoc 2020;9:e012439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jentzer JC, Anavekar NS, Bennett C, et al. Derivation and validation of a novel cardiac intensive care unit admission risk score for mortality. J Am Heart Assoc 2019;8:e013675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Diepen S, Tymchak W, Bohula EA, et al. Incidence, underlying conditions, and outcomes of patients receiving acute renal replacement therapies in tertiary cardiac intensive care units: an analysis from the Critical Care Cardiology Trials Network Registry. Am Heart J 2020;222:8–14. [DOI] [PubMed] [Google Scholar]
- 27.Wang N, Jiang L, Zhu B, Wen Y, Xi XM. Beijing Acute Kidney Injury Trial Workgroup. Fluid balance and mortality in critically ill patients with acute kidney injury: a multicenter prospective epidemiological study. Crit Care 2015;19:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCullough PA, Kellum JA, Haase M, et al. Pathophysiology of the cardiorenal syndromes: executive summary from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol 2013;182:82–98. [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Lee J, Johnson AE, Mark RG, Celi LA, Danziger J. Right ventricular function, peripheral edema, and acute kidney injury in critical illness. Kidney Int Rep 2017;2:1059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Summary of recommendation statements. Kidney Int Suppl (2011) 2012;2:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joannidis M, Forni LG, Haase M, et al. Use of cell cycle arrest biomarkers in conjunction with classical markers of acute kidney injury. Crit Care Med 2019;47:e820–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray PT, Mehta RL, Shaw A, et al. Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int 2014;85:513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin K, Murugan R, Sileanu FE, et al. Intensive monitoring of urine output is associated with increased detection of acute kidney injury and improved outcomes. Chest 2017;152:972–9. [DOI] [PubMed] [Google Scholar]
- 34.Forni LG, Chawla LS. Biomarkers in cardiorenal syndrome. Blood Purif 2014;37 Suppl 2:14–9. [DOI] [PubMed] [Google Scholar]
- 35.Feng Y, Zhang Y, Li G, Wang L. Relationship of cystatin-C change and the prevalence of death or dialysis need after acute kidney injury: a meta-analysis. Nephrology (Carlton) 2014;19:679–84. [DOI] [PubMed] [Google Scholar]
- 36.Yong Z, Pei X, Zhu B, Yuan H, Zhao W. Predictive value of serum cystatin C for acute kidney injury in adults: a meta-analysis of prospective cohort trials. Sci Rep 2017;7:41012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuriyama A, Urushidani S. Continuous versus intermittent administration of furosemide in acute decompensated heart failure: a systematic review and meta-analysis. Heart Fail Rev 2019;24:31–9. [DOI] [PubMed] [Google Scholar]
- 38.Bove T, Belletti A, Putzu A, et al. Intermittent furosemide administration in patients with or at risk for acute kidney injury: meta-analysis of randomized trials. PLoS One 2018;13:e0196088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chawla LS, Davison DL, Brasha-Mitchell E, et al. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care 2013;17:R207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lumlertgul N, Peerapornratana S, Trakarnvanich T, et al. Early versus standard initiation of renal replacement therapy in furosemide stress test non-responsive acute kidney injury patients (the FST trial). Crit Care 2018;22:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakhuja A, Bandak G, Barreto EF, et al. Role of loop diuretic challenge in stage 3 acute kidney injury. Mayo Clin Proc 2019;94:1509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rewa OG, Bagshaw SM, Wang X, et al. The furosemide stress test for prediction of worsening acute kidney injury in critically ill patients: a multicenter, prospective, observational study. J Crit Care 2019;52:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol 2010;56:1527–34. [DOI] [PubMed] [Google Scholar]
- 44.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 45.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 46.Cox ZL, Hung R, Lenihan DJ, Testani JM. Diuretic strategies for loop diuretic resistance in acute heart failure: The 3T trial. J Am Coll Cardiol HF 2020;8:157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greene SJ, Felker GM, Giczewska A, et al. Spironolactone in acute heart failure patients with renal dysfunction and risk factors for diuretic resistance: from the ATHENA-HF trial. Can J Cardiol 2019;35:1097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gheorghiade M, Konstam MA, Burnett JC Jr., et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST clinical status trials. JAMA 2007;297:1332–43. [DOI] [PubMed] [Google Scholar]
- 49.Konstam MA, Gheorghiade M, Burnett JC Jr., et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial. JAMA 2007;297:1319–31. [DOI] [PubMed] [Google Scholar]
- 50.Gandhi S, Mosleh W, Myers RB. Hypertonic saline with furosemide for the treatment of acute congestive heart failure: a systematic review and meta-analysis. Int J Cardiol 2014;173:139–45. [DOI] [PubMed] [Google Scholar]
- 51.Kellum JA, Decker J. Use of dopamine in acute renal failure: a meta-analysis. Crit Care Med 2001;29:1526–31. [DOI] [PubMed] [Google Scholar]
- 52.Triposkiadis FK, Butler J, Karayannis G, et al. Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: the Dopamine in Acute Decompensated Heart Failure II (DAD-HF II) trial. Int J Cardiol 2014;172:115–21. [DOI] [PubMed] [Google Scholar]
- 53.Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013;310:2533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wan SH, Stevens SR, Borlaug BA, et al. Differential response to low-dose dopamine or low-dose nesiritide in acute heart failure with reduced or preserved ejection fraction: results from the ROSE AHF Trial (Renal Optimization Strategies Evaluation in Acute Heart Failure). Circ Heart Fail 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costanzo MR, Ronco C, Abraham WT, et al. Extracorporeal ultrafiltration for fluid overload in heart failure: current status and prospects for further research. J Am Coll Cardiol 2017;69:2428–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Costanzo MR, Negoianu D, Jaski BE, et al. Aquapheresis versus intravenous diuretics and hospitalizations for heart failure. J Am Coll Cardiol HF 2016;4:95–105. [DOI] [PubMed] [Google Scholar]
- 57.Guazzi MD, Agostoni P, Perego B, et al. Apparent paradox of neurohumoral axis inhibition after body fluid volume depletion in patients with chronic congestive heart failure and water retention. Br Heart J 1994;72:534–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agostoni PG, Marenzi GC, Pepi M, et al. Isolated ultrafiltration in moderate congestive heart failure. J Am Coll Cardiol 1993;21:424–31. [DOI] [PubMed] [Google Scholar]
- 59.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 2009;53:582–8. [DOI] [PubMed] [Google Scholar]
- 60.Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009;53:589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwok CS, Wong CW, Rushton CA, et al. Ultrafiltration for acute decompensated cardiac failure: a systematic review and meta-analysis. Int J Cardiol 2017;228:122–8. [DOI] [PubMed] [Google Scholar]
- 62.Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Section 5: Dialysis interventions for treatment of AKI. Kidney Int Suppl (2011) 2012;2:89–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keleshian V, Kashani KB, Kompotiatis P, Barsness GW, Jentzer JC. Short, and long-term mortality among cardiac intensive care unit patients started on continuous renal replacement therapy. J Crit Care 2020;55:64–72. [DOI] [PubMed] [Google Scholar]
- 65.Yang XM, Tu GW, Zheng JL, et al. A comparison of early versus late initiation of renal replacement therapy for acute kidney injury in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. BMC Nephrol 2017;18:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gaudry S, Hajage D, Schortgen F, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 2016;375:122–33. [DOI] [PubMed] [Google Scholar]
- 67.Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA 2016;315:2190–9. [DOI] [PubMed] [Google Scholar]
- 68.Henrich WL, Woodard TD, Blachley JD, Gomez-Sanchez C, Pettinger W, Cronin RE. Role of osmolality in blood pressure stability after dialysis and ultrafiltration. Kidney Int 1980;18:480–8. [DOI] [PubMed] [Google Scholar]
- 69.Kumar VA, Craig M, Depner TA, Yeun JY. Extended daily dialysis: a new approach to renal replacement for acute renal failure in the intensive care unit. Am J Kidney Dis 2000;36:294–300. [DOI] [PubMed] [Google Scholar]
- 70.Palevsky PM. Dialysis modality and dosing strategy in acute renal failure. Semin Dial 2006;19:165–70. [DOI] [PubMed] [Google Scholar]
- 71.Nash DM, Przech S, Wald R, O’Reilly D. Systematic review and meta-analysis of renal replacement therapy modalities for acute kidney injury in the intensive care unit. J Crit Care 2017;41:138–44. [DOI] [PubMed] [Google Scholar]
- 72.John S, Griesbach D, Baumgartel M, Weihprecht H, Schmieder RE, Geiger H. Effects of continuous haemofiltration vs intermittent haemodialysis on systemic haemodynamics and splanchnic regional perfusion in septic shock patients: a prospective, randomized clinical trial. Nephrol Dial Transplant 2001;16:320–7. [DOI] [PubMed] [Google Scholar]
- 73.Bellomo R, Farmer M, Parkin G, Wright C, Boyce N. Severe acute renal failure: a comparison of acute continuous hemodiafiltration and conventional dialytic therapy. Nephron 1995;71:59–64. [DOI] [PubMed] [Google Scholar]
- 74.Rauf AA, Long KH, Gajic O, Anderson SS, Swaminathan L, Albright RC. Intermittent hemodialysis versus continuous renal replacement therapy for acute renal failure in the intensive care unit: an observational outcomes analysis. J Intensive Care Med 2008;23:195–203. [DOI] [PubMed] [Google Scholar]
- 75.Brownback CA, Fletcher P, Pierce LN, Klaus S. Early mobility activities during continuous renal replacement therapy. Am J Crit Care 2014;23:348–51. quiz 352. [DOI] [PubMed] [Google Scholar]
- 76.Toonstra AL, Zanni JM, Sperati CJ, et al. Feasibility and safety of physical therapy during continuous renal replacement therapy in the intensive care unit. Ann Am Thorac Soc 2016;13:699–704. [DOI] [PubMed] [Google Scholar]
- 77.Kitchlu A, Adhikari N, Burns KE, et al. Outcomes of sustained low efficiency dialysis versus continuous renal replacement therapy in critically ill adults with acute kidney injury: a cohort study. BMC Nephrol 2015;16:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kielstein JT, Kretschmer U, Ernst T, et al. Efficacy and cardiovascular tolerability of extended dialysis in critically ill patients: a randomized controlled study. Am J Kidney Dis 2004;43:342–9. [DOI] [PubMed] [Google Scholar]
- 79.Chen H, Yu RG, Yin NN, Zhou JX. Combination of extracorporeal membrane oxygenation and continuous renal replacement therapy in critically ill patients: a systematic review. Crit Care 2014;18:675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schetz M, Ferdinande P, Van den Berghe G, Verwaest C, Lauwers P. Pharmacokinetics of continuous renal replacement therapy. Intensive Care Med 1995;21:612–20. [DOI] [PubMed] [Google Scholar]
- 81.Schetz M Drug dosing in continuous renal replacement therapy: general rules. Curr Opin Crit Care 2007;13:645–51. [DOI] [PubMed] [Google Scholar]
- 82.Moriyama B, Henning SA, Neuhauser MM, Danner RL, Walsh TJ. Continuous-infusion beta-lactam antibiotics during continuous venovenous hemofiltration for the treatment of resistant gram-negative bacteria. Ann Pharmacother 2009;43:1324–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pistolesi V, Morabito S, Di Mario F, Regolisti G, Cantarelli C, Fiaccadori E. A guide to understanding antimicrobial drug dosing in critically ill patients on renal replacement therapy. Antimicrob Agents Chemother 2019;63:e00583–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mueller BA, Smoyer WE. Challenges in developing evidence-based drug dosing guidelines for adults and children receiving renal replacement therapy. Clin Pharmacol Ther 2009;86:479–82. [DOI] [PubMed] [Google Scholar]
- 85.Taniguchi T, Shibata K, Saito S, Matsumoto H, Okeie K. Pharmacokinetics of milrinone in patients with congestive heart failure during continuous venovenous hemofiltration. Intensive Care Med 2000;26:1089–93. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y, Gallagher M, Li Q, et al. Renal replacement therapy intensity for acute kidney injury and recovery to dialysis independence: a systematic review and individual patient data meta-analysis. Nephrol Dial Transplant 2018;33:1017–24. [DOI] [PubMed] [Google Scholar]
- 87.Kashani K, Thongprayoon C, Cheungpasitporn W, Iacovella GM, Akhoundi A, Albright RC Jr. Association between mortality and replacement solution bicarbonate concentration in continuous renal replacement therapy: a propensity-matched cohort study. PLoS One 2017;12:e0185064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murugan R, Kerti SJ, Chang CH, et al. Association of net ultrafiltration rate with mortality among critically ill adults with acute kidney injury receiving continuous venovenous hemodiafiltration: a secondary analysis of the Randomized Evaluation of Normal vs Augmented Level (RENAL) of Renal Replacement Therapy Trial. JAMA Netw Open 2019;2:e195418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shawwa K, Kompotiatis P, Jentzer JC, et al. Hypotension within one-hour from starting CRRT is associated with in-hospital mortality. J Crit Care 2019;54:7–13. [DOI] [PubMed] [Google Scholar]
- 90.Stucker F, Ponte B, Tataw J, et al. Efficacy and safety of citrate-based anticoagulation compared to heparin in patients with acute kidney injury requiring continuous renal replacement therapy: a randomized controlled trial. Crit Care 2015;19:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gattas DJ, Rajbhandari D, Bradford C, Buhr H, Lo S, Bellomo R. A randomized controlled trial of regional citrate versus regional heparin anticoagulation for continuous renal replacement therapy in critically ill adults. Crit Care Med 2015;43:1622–9. [DOI] [PubMed] [Google Scholar]
- 92.Slowinski T, Morgera S, Joannidis M, et al. Safety and efficacy of regional citrate anticoagulation in continuous venovenous hemodialysis in the presence of liver failure: the Liver Citrate Anticoagulation Threshold (L-CAT) observational study. Crit Care 2015;19:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Link A, Klingele M, Speer T, et al. Total-to-ionized calcium ratio predicts mortality in continuous renal replacement therapy with citrate anticoagulation in critically ill patients. Crit Care 2012;16:R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jeon J, Kim DH, Baeg SI, et al. Association between diuretics and successful discontinuation of continuous renal replacement therapy in critically ill patients with acute kidney injury. Crit Care 2018;22:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Uchino S, Bellomo R, Morimatsu H, et al. Discontinuation of continuous renal replacement therapy: a post hoc analysis of a prospective multicenter observational study. Crit Care Med 2009;37:2576–82. [DOI] [PubMed] [Google Scholar]
- 96.Wu VC, Ko WJ, Chang HW, et al. Risk factors of early redialysis after weaning from postoperative acute renal replacement therapy. Intensive Care Med 2008;34:101–8. [DOI] [PubMed] [Google Scholar]
- 97.Mendu ML, Ciociolo GR Jr., McLaughlin SR, et al. A decision-making algorithm for initiation and discontinuation of RRT in severe AKI. Clin J Am Soc Nephrol 2017;12:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Katulka RJ, Al Saadon A, Sebastianski M, et al. Determining the optimal time for liberation from renal replacement therapy in critically ill patients: a systematic review and meta-analysis (DOnE RRT). Crit Care 2020;24:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van der Voort PH, Boerma EC, Koopmans M, et al. Furosemide does not improve renal recovery after hemofiltration for acute renal failure in critically ill patients: a double blind randomized controlled trial. Crit Care Med 2009;37:533–8. [DOI] [PubMed] [Google Scholar]
- 100.Gayat E, Hollinger A, Cariou A, et al. Impact of angiotensin-converting enzyme inhibitors or receptor blockers on post-ICU discharge outcome in patients with acute kidney injury. Intensive Care Med 2018;44:598–605. [DOI] [PubMed] [Google Scholar]
- 101.Brar S, Ye F, James MT, et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with outcomes after acute kidney injury. JAMA Intern Med 2018;178:1681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stads S, Kant KM, de Jong MFC, et al. Predictors of short-term successful discontinuation of continuous renal replacement therapy: results from a prospective multicentre study. BMC Nephrol 2019;20:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Poveromo LB, Michalets EL, Sutherland SE. Midodrine for the weaning of vasopressor infusions. J Clin Pharm Ther 2016;41:260–5. [DOI] [PubMed] [Google Scholar]
- 104.Li J, Sheng X, Cheng D, et al. Is the mean platelet volume a predictive marker of a high in-hospital mortality of acute cardiorenal syndrome patients receiving continuous renal replacement therapy? Medicine (Baltimore) 2018;97:e11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Patarroyo M, Wehbe E, Hanna M, et al. Cardiorenal outcomes after slow continuous ultrafiltration therapy in refractory patients with advanced decompensated heart failure. J Am Coll Cardiol 2012;60:1906–12. [DOI] [PubMed] [Google Scholar]
- 106.Wehbe E, Patarroyo M, Taliercio JJ, et al. Renal failure requiring dialysis complicating slow continuous ultrafiltration in acute heart failure: importance of systolic perfusion pressure. J Card Fail 2015;21:108–15. [DOI] [PubMed] [Google Scholar]
- 107.Jung SY, Kwon J, Park S, et al. Phosphate is a potential biomarker of disease severity and predicts adverse outcomes in acute kidney injury patients undergoing continuous renal replacement therapy. PLoS One 2018;13:e0191290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kao CC, Yang JY, Chen L, et al. Factors associated with poor outcomes of continuous renal replacement therapy. PLoS One 2017;12:e0177759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kee YK, Kim D, Kim SJ, et al. Factors associated with early mortality in critically ill patients following the initiation of continuous renal replacement therapy. J Clin Med 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Woodward CW, Lambert J, Ortiz-Soriano V, et al. Fluid overload associates with major adverse kidney events in critically ill patients with acute kidney injury requiring continuous renal replacement therapy. Crit Care Med 2019;47:e753–60. [DOI] [PubMed] [Google Scholar]
- 111.Kellum JA, Ronco C. The 17th Acute Disease Quality Initiative International Consensus Conference: introducing precision renal replacement therapy. Blood Purif 2016;42:221–3. [DOI] [PubMed] [Google Scholar]
- 112.Askenazi DJ, Heung M, Connor MJ Jr., et al. Optimal role of the nephrologist in the intensive care unit. Blood Purif 2017;43:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rewa OG, Villeneuve PM, Lachance P, et al. Quality indicators of continuous renal replacement therapy (CRRT) care in critically ill patients: a systematic review. Intensive Care Med 2017;43:750–63. [DOI] [PubMed] [Google Scholar]
- 114.Rhee H, Jang GS, Han M, et al. The role of the specialized team in the operation of continuous renal replacement therapy: a single-center experience. BMC Nephrol 2017;18:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oh HJ, Lee MJ, Kim CH, et al. The benefit of specialized team approaches in patients with acute kidney injury undergoing continuous renal replacement therapy: propensity score matched analysis. Crit Care 2014;18:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schiffl H, Lang SM, Fischer R. Long-term outcomes of survivors of ICU acute kidney injury requiring renal replacement therapy: a 10-year prospective cohort study. Clin Kidney J 2012;5:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abdo AS. Diagnosis and management of heart failure in long-term dialysis patients. Curr Heart Fail Rep 2017;14:404–9. [DOI] [PubMed] [Google Scholar]
- 118.Hendawy A, Pouteil-Noble C, Villar E, Boissonnat P, Sebbag L. Chronic renal failure and end-stage renal disease are associated with a high rate of mortality after heart transplantation. Transplant Proc 2005;37:1352–4. [DOI] [PubMed] [Google Scholar]
- 119.Bansal N, Hailpern SM, Katz R, et al. Outcomes associated with left ventricular assist devices among recipients with and without end-stage renal disease. JAMA Intern Med 2018;178:204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen JC, Thorsteinsdottir B, Vaughan LE, et al. End of life, withdrawal, and palliative care utilization among patients receiving maintenance hemodialysis therapy. Clin J Am Soc Nephrol 2018;13:1172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.