Abstract
Background
Children with congenital heart disease often undergo heart surgery at a young age. They are at risk for postoperative low cardiac output syndrome (LCOS) or death. Milrinone may be used to provide inotropic and vasodilatory support during the immediate postoperative period.
Objectives
This review examines the effectiveness of prophylactic postoperative use of milrinone to prevent LCOS or death in children having undergone surgery for congenital heart disease.
Search methods
Electronic and manual literature searches were performed to identify randomised controlled trials. We searched CENTRAL, MEDLINE, EMBASE and Web of Science in February 2014 and conducted a top‐up search in September 2014 as well as clinical trial registries and reference lists of published studies. We did not apply any language restrictions.
Selection criteria
Only randomised controlled trials were selected for analysis. We considered studies with newborn infants, infants, toddlers, and children up to 12 years of age.
Data collection and analysis
Two review authors independently extracted data according to a pre‐defined protocol. We obtained additional information from all study authors.
Main results
Three of the five included studies compared milrinone versus levosimendan, one study compared milrinone with placebo, and one compared milrinone verus dobutamine, with 101, 242, and 50 participants, respectively. Three trials were at low risk of bias while two were at higher risk of bias. The number and definitions of outcomes were non‐uniform as well. In one study comparing two doses of milrinone and placebo, there was some evidence in an overall comparison of milrinone versus placebo that milrinone lowered risk for LCOS (risk ratio (RR) 0.52, 95% confidence interval (CI) 0.28 to 0.96; 227 participants). The results from two small studies do not provide enough information to determine whether milrinone increases the risk of LCOS when compared to levosimendan (RR 1.22, 95% CI 0.32 to 4.65; 59 participants). Mortality rates in the studies were low, and there was insufficient evidence to draw conclusions on the effect of milrinone compared to placebo or levosimendan or dobutamine regarding mortality, the duration of intensive care stay, hospital stay, mechanical ventilation, or maximum inotrope score (where available). Numbers of patients requiring mechanical cardiac support were also low and did not allow a comparison between studies, and none of the participants of any study received a heart transplantation up to the end of the respective follow‐up period. Time to death within three months was not reported in any of the included studies. A number of adverse events was examined, but differences between the treatment groups could not be proven for hypotension, intraventricular haemorrhage, hypokalaemia, bronchospasm, elevated serum levels of liver enzymes, or a reduced left ventricular ejection fraction < 50% or reduced left ventricular fraction of shortening < 28%. Our analysis did not prove an increased risk of arrhythmias in patients treated prophylactically with milrinone compared with placebo (RR 3.59, 95% CI 0.83 to 15.42; 238 participants), a decreased risk of pleural effusions (RR 1.78, 95% CI 0.92 to 3.42; 231 participants), or a difference in risk of thrombocytopenia on milrinone compared with placebo (RR 0.86, 95% CI 0.39 to 1.88; 238 participants). Comparisons of milrinone with levosimendan or with dobutamine, respectively, did not clarify the risk of arrhythmia and were not possible for pleural effusions or thrombocytopenia.
Authors' conclusions
There is insufficient evidence of the effectiveness of prophylactic milrinone in preventing death or low cardiac output syndrome in children undergoing surgery for congenital heart disease, compared to placebo. So far, no differences have been shown between milrinone and other inodilators, such as levosimendan or dobutamine, in the immediate postoperative period, in reducing the risk of LCOS or death. The existing data on the prophylactic use of milrinone has to be viewed cautiously due to the small number of small trials and their risk of bias.
Keywords: Humans; Infant; Infant, Newborn; Cardiac Output, Low; Cardiac Output, Low/etiology; Cardiac Output, Low/mortality; Cardiac Output, Low/prevention & control; Cardiotonic Agents; Cardiotonic Agents/adverse effects; Cardiotonic Agents/therapeutic use; Dobutamine; Dobutamine/therapeutic use; Heart Defects, Congenital; Heart Defects, Congenital/mortality; Heart Defects, Congenital/surgery; Hydrazones; Hydrazones/therapeutic use; Milrinone; Milrinone/adverse effects; Milrinone/therapeutic use; Postoperative Complications; Postoperative Complications/mortality; Postoperative Complications/prevention & control; Pyridazines; Pyridazines/therapeutic use; Randomized Controlled Trials as Topic; Simendan; Syndrome; Time Factors
Plain language summary
Milrinone to prevent reduced heart function and death after heart surgery in children
Background: Children who are born with heart defects often undergo heart surgery at a young age. They are at risk for reduced heart function and death after surgery. Milrinone is a medication that may be used in this situation to make the heart stronger and make it easier for the heart to pump blood into the body.
Review question: We wanted to examine if the prophylactic use of milrinone prevents reduced heart function or death in babies and children from birth up to 12 years of age having had heart surgery. We planned to consider the number of children who died during the first 30 days after surgery as well as how many days they lived after surgery, followed up for three months. We searched a number of medical literature databases electronically which collect information about planned, ongoing, or finished studies, in order to find trials of this medication published by September 2014. Trials where children had received milrinone and another group of children had received another drug instead after heart surgery were considered. Data were collected by two review authors independently who had to use a pre‐prepared work sheet.
Study characteristics: We found five studies, and we asked the study authors for more information. Three studies compared milrinone versus levosimendan, one study compared milrinone versus placebo, and one compared milrinone versus dobutamine. The patients were given the study drugs for 24 to 48 hours and were watched for six to 78 days. A total of 393 participants were included.
Quality of evidence: Thus, the data are from a limited number of small trials and therefore must be viewed with caution. In addition, it was not always clear that the patient groups were formed and treated in a way that would make them completely comparable, that patients stayed in the trial for complete assessment, or that all study results were reported conscientiously.
Key results: In one study comparing two doses of milrinone and placebo, milrinone was better than placebo to prevent reduced heart function within 36 hours after surgery, but there was not enough information about long‐term heart function beyond the first postoperative days. It was not shown whether milrinone was better than placebo or than any of the other medications to prevent death, or whether the intensive care unit stay or hospital stay or time on mechanical ventilation was shorter if patients received milrinone. Similarly, when examining the studies regarding side effects of milrinone, we could not prove that milrinone caused more heart rhythm disturbances than dobutamine or placebo, or how it affected heart rhythm compared with levosimendan. We could not generate other useful information from comparing the trials regarding other harms which had been previously ascribed to milrinone, such as high heart rate, low blood pressure, bleeding into the brain's ventricular fluid, low potassium level in the blood, narrowing of the airways, low numbers of platelets in the blood, altered liver function tests, or low measurements of heart function by ultrasound. This was in part due to the different trial designs.
Background
With the technical and medical advances of the past few decades, approximately 85% of children with congenital heart disease now reach adulthood (Ermis 2011). Part of this improvement is due to the ability to operate on ever younger and smaller children (Warnes 2001). Those children suffering from the most severe forms of cardiac malformations need to undergo corrective or palliative surgery in their first year of life. For example, of the 12,495 procedures performed in 10,780 patients of all ages in Europe in 2009 that are registered in the European Association for Cardio‐Thoracic Surgery (EACTS) congenital database, 6717 procedures (53.8%) were done in 5777 (53.6%) neonates and infants (EACTS 2011). However, this comes at the cost of a high risk of morbidity and mortality in the postoperative period as evidenced by the fact that 77.6% of deaths in the 30 days following surgery involved neonates and infants (333 out of 429 cases) (EACTS 2011).
Description of the condition
One important condition associated with increased morbidity and mortality is low cardiac output syndrome (LCOS). LCOS is thought to be due to a combination of the underlying heart disease, myocardial ischaemia from aortic cross‐clamping, the residual effects of cardioplegia, and activation of inflammatory pathways from exposure of blood to foreign surfaces during cardiopulmonary bypass (Bailey 2004). It occurs in up to 25% of young children, even if there are no residual cardiac lesions after surgery (Bailey 2004) and typically occurs between six and 18 hours after surgery in a setting of elevated systemic and pulmonary vascular resistances, impaired myocardial function, and arrhythmias. LCOS is detected invasively or by signs of inadequate oxygen delivery to the organ systems, e.g. tachycardia, poor systemic perfusion, decreased urine output, elevated lactate, and reduced mixed venous oxygen saturation (Stocker 2006). If left untreated, LCOS can lead to cardiac arrest, the need for cardiopulmonary resuscitation or extracorporeal life support (Delmo Walter 2010), prolonged mechanical ventilation (Shi 2008), a prolonged intensive care stay and increased mortality (Baysal 2010). Therefore, prevention, early detection, and treatment of postoperative LCOS are paramount.
Cardiac output is regarded as low when the pumping capacity of the heart is insufficient to provide enough blood flow to satisfy the oxygen demand of the body tissues (Stocker 2006). In the adult intensive care setting, cardiac output can be measured directly by indicator dilution techniques like thermodilution (Lemson 2008), by Doppler echocardiography (Huntsman 1983), or by arterial pulse contour analysis (Kim 2006; Tibby 2002). A cardiac index of < 2.2 L/min/m2 is considered low (Hochman 1999; Rao 1996). In children, especially in neonates and infants, it is usually not feasible to employ these techniques due to device sizes, shunts, and other characteristics of cardiovascular physiology (Teng 2011), as well as poor correlation with tissue oxygen delivery (Bohn 2011). With lack of a clear definition, different authors describe various parameters, which are often used as a compound measure. Such a composite parameter for LCOS may consist of several of the following findings:
elevated blood lactate or rapid increase in blood lactate (Charpie 2000),
decreased central venous oxygen saturation (Stocker 2006),
increase in arterial to central venous oxygen saturation difference,
decreased urine output (Stocker 2006),
increased peripheral skin temperature to core body temperature difference,
echocardiographic Doppler‐derived low cardiac index
high inotrope requirement (Shore 2001).
The mainstays of treatment include catecholamines, calcium sensitisers, and phosphodiesterase inhibitors (usually milrinone) (Stocker 2006). A recent survey of hospitals in 31 European countries showed that treatment regimens are highly variable between centres, which was attributed to the lack of licensing and dosing guidance in many instances. It also showed that milrinone is being favoured in LCOS with elevated systemic vascular resistance and is also used in LCOS with elevated pulmonary vascular resistance (Vogt 2011a).
Description of the intervention
In the adult population, phosphodiesterase type III inhibitors have been used extensively for congestive heart failure in the past and in the postoperative management of patients undergoing coronary artery bypass grafting (Feneck 1992). The use of milrinone in adult acute heart failure has however diminished, supported by ADHERE registry data, which showed that patients treated with milrinone were among those with a higher mortality (Abraham 2005) compared to those receiving other medications. The drug did not play a large role in more recent surveys on heart failure treatment such as EuroHeart Failure Survey II (Nieminen 2006), ALARM‐HF (Follath 2011), and the Get With The Guidelines‐Heart Failure registry (Allen 2014). In contrast, children with acute heart failure from cardiomyopathy receive milrinone more commonly (Moffett 2015).
In children undergoing congenital heart surgery, milrinone is thought to decrease the incidence of low cardiac output syndrome without an increase in adverse reactions (Hoffman 2003). Adverse effects have been described in terms of arrhythmias (Fleming 2008), hypotension (Jeon 2006), tachycardia (Paradisis 2009), hypokalaemia, bronchospasm, headaches, thrombocytopenia (Ramamoorthy 1998), anaemia, and elevated serum levels of liver enzymes (Sanofi Aventis 2010). In neonates treated with milrinone, intraventricular haemorrhage has been observed as well (Bassler 2006). Milrinone as a prophylactic medication is administered intravenously, either as a bolus, or as a loading dose followed by continuous infusion, or by continuous infusion only. In clinical trials, doses have been chosen as follows: bolus doses given as 50 μg/kg of body weight (Bailey 1999). Typical loading doses ranged from 25 μg/kg intravenously (Hoffman 2003) to 250 μg/kg of body weight administered into the cardiopulmonary bypass priming volume (Zuppa 2006). Continuous infusion rates varied between 0.2 μg/kg/min (Zuppa 2006) and 0.75 μg/kg/min (Hoffman 2003). The medication is started immediately or within several hours after separation from cardiopulmonary bypass, when surgical correction or palliation of the heart defect is completed. Usual infusion periods for continuous administration were up to 36 hours (Hoffman 2003) or even several days. In clinical practice, milrinone is reported to be used either alone or in combination with other medications, at bolus doses of 50 to 300 μg/kg, followed by maintenance infusion rates of 0.2 to 1.5 μg/kg, with higher variability in combination regimens than with milrinone as a monotherapy (Vogt 2011a; Vogt 2011b).
How the intervention might work
Milrinone is a phosphodiesterase type III inhibitor, exerting its pharmacologic action by increasing the intracellular cAMP concentration, which in turn has inotropic and lusitropic effects relating to intracellular calcium handling in cardiac myocytes (el Allaf 1984). In the peripheral and pulmonary vasculature, phosphodiesterase type III inhibitors act as vasodilators, thereby decreasing systemic and pulmonary vascular resistance (Alousi 1986; Stocker 2007).
Why it is important to do this review
In the paediatric age group, a large percentage of drugs are used off‐label, that is in ways that are not formally approved, depending largely on information from adult trials. In cardiovascular medicine especially, there is a great discrepancy between the availability of trial information from many large adult studies and systematic reviews/meta‐analyses, and very little knowledge about drug effects in children (Pasquali 2008). This is even more striking as cardiac defects constitute the most common type of separate congenital organ malformations, affecting between 0.72% (Dilber 2010) and 1.08% of newborns (Lindinger 2010).
The most common indication for milrinone in the paediatric age group is in children with congenital heart defects undergoing palliative or corrective surgery, in order to prevent or treat low cardiac output syndrome after cardiopulmonary bypass. As of July 6, 2011, the European Medicines Agency has issued a Public Assessment Report (in accordance with article 45 of the Paediatric regulation) of milrinone, indicating the use of this substance for “the short‐term treatment (up to 35 hours) for severe congestive heart failure unresponsive to conventional maintenance therapy, and for the short‐term treatment (up to 35 hours) of paediatric patients with acute heart failure, including low output states following cardiac surgery” (Anonymous 2011). Prophylactic use has not been approved by the European Medicines Agency.
However, the EuLoCOS survey of hospitals in Europe showed that preventive drug administration in patients at risk for LCOS is common, and that milrinone is the most widely preferred drug, either alone or in combination with other drugs, in varying doses and modes of administration (Vogt 2011b). The frequent use of milrinone warrants further investigation into its effects in children undergoing surgery for congenital heart disease, especially if used prophylactically, in order to allow evidence‐based decisions.
This review is an essential step to permit further relevant and ethical clinical trials in the paediatric population as needed, or to possibly prevent more children from being subjected to unnecessary trials, depending on its outcome.
Objectives
This review examines the effectiveness of prophylactic postoperative use of milrinone to prevent LCOS or death in children having undergone surgery for congenital heart disease.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials were considered.
Types of participants
Newborns, infants, toddlers, and children from birth to 12 years of age undergoing corrective or palliative heart surgery for congenital heart disease.
Types of interventions
Intervention: Prophylactic milrinone intravenous infusion alone or combined with other inotrope medications and/or vasopressin and/or calcium sensitisers and/or nitric oxide, started within six hours of surgery for congenital heart disease and irrespective of the administration protocol, provided that milrinone bolus doses, if any, are at least 25 μg/kg and continuous infusion rates are at least 0.2 μg/kg/min, and that milrinone is administered for a duration of at least four hours.
Comparative intervention: No milrinone infusion and a) or b):
(a) Placebo: Depending on the surgical intervention, children will rarely be weaned from cardiopulmonary bypass without inotropic medications. If any trials exist comparing milrinone with placebo, these will be included in future updates of this review.
(b) Other inotrope medications and/or vasopressin and/or calcium sensitisers and/or nitric oxide/nitroprusside alone. Inotropes (usually catecholamines) such as epinephrine, norepinephrine, dopamine, or dobutamine, may be used in combination with vasopressin and/or with calcium sensitisers such as levosimendan and/or combined with inhaled nitric oxide/ intravenous nitroprusside.
These combination regimens were regarded as eligible comparators, as long as they did not contain milrinone. Studies using medication regimens that contain other phosphodiesterase type III inhibitors (amrinone, inamrinone, enoximone, piroximone, pimobendane, imazodan, sulmazole, isomazole, flosequinan, indolidan, carbazeran, quazinone, adibendan, pelrinone, olprinone, siguazodan, cilostamide, cilostazol, zardaverine, alifedrine, lixazinone) were also excluded.
Types of outcome measures
Primary outcomes
Total mortality within 30 days
Time to death (censored after three months)
Low cardiac output syndrome as defined by authors of individual studies and typically based on two or more of the following:
blood lactate > 3 mmol/L (27 mg/dL) or increase in blood lactate of at least 2 mmol/L (18 mg/dL) from baseline,
central venous oxygen saturation < 50% in biventricular physiology without shunts,
increase in arterial to central venous oxygen saturation difference by at least 20% from baseline,
urine output < 1 mL/kg/hour,
peripheral skin temperature to core body temperature difference of > 7°C,
cardiac index as determined by Doppler echocardiography of < 2.2 L/min/m2
Secondary outcomes
Duration of intensive care stay, duration of hospital stay, duration of mechanical ventilation, inotrope score, number of patients requiring mechanical circulatory support (e.g. ECMO, pulsatile assist devices) or cardiac transplantation.
Safety outcomes
Number/proportion of adverse events. Adverse events include:
arrhythmias (number/proportion),
tachycardia (number/proportion of patients with heart rate above heart rate appropriate for age or body surface area),
hypotension (number/proportion of patients with blood pressures below blood pressure appropriate for age or body surface area),
intraventricular haemorrhage (number/proportion),
hypokalaemia (number/proportion),
bronchospasm (number/proportion),
thrombocytopenia (number/proportion of patients with platelet count < 50,000/mm3 or with a drop in platelet count of >100% from baseline prior to administration of milrinone),
elevated serum levels of liver enzymes (number/proportion of patients with serum enzymatic activities more than two‐fold the age‐appropriate normal values),
left ventricular ejection fraction < 50% or left ventricular fraction of shortening < 28% as assessed by biplane or M‐mode echocardiography.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2014, Issue 1 of 12) in The Cochrane Library, MEDLINE (OVID, 1946 to January Week 4 2014), EMBASE (OVID, 1980 to 2014 Week 04), and Web of Science (Thomson Reuters, 1970 to 31 January 2014) on 5 February 2014. No language restrictions were applied.
We performed an additional search in September 2014. The results of this search have been screened but have not yet been fully incorporated in this review (see Characteristics of studies awaiting classification).
Clinical trial registries were consulted for trials that were completed or nearing completion but had not been published yet. Specifically, the following registries were searched:
Clinicaltrials (www.clinicaltrials.gov), searched on 29 February 2012 (search term “milrinone”).
EU Clinical Trials Register, EU‐CTR Version: 1.1.2 (https://www.clinicaltrialsregister.eu/ctr‐search/search?query=milrinone), searched on 17 August 2012 (search term “milrinone”).
IFPMA (International Federation of Pharmaceutical Manufacturers & Associations) Clinical Trials Portal (http://clinicaltrials.ifpma.org/clinicaltrials/no_cache/de/suche/index.htm) searched on 17 August 2012 (search term “milrinone”).
metaRegister of current controlled trials (http://www.controlled‐trials.com/mrct/search.html), searched on 29 February 2012 (search terms “milrinone AND surgery”).
WHO International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/), searched on 29 February 2012 (search term “milrinone”).
Forschungsdatenbank Universität Zürich (http://www.research‐projects.uzh.ch/info/index.html), searched on 29 February 2012 (search term “milrinone”).
The Cochrane sensitivity‐maximising RCT filter (Lefebvre, 2011) has been used for MEDLINE and adaptations of it for the other databases except CENTRAL. The search strategies can be found in Appendix 1.
Searching other resources
We handsearched reference lists of published studies for further trials. Also, we scanned general reviews and overviews for relevant citations. We asked the manufacturer (Sanofi Aventis) to provide us with information about any additional trials. We contacted authors of published trials and expert colleagues from scientific medical societies (Association for European Paediatric Cardiology, American Academy of Pediatrics Section on Cardiology and Cardiac Surgery, American Heart Association Council on Cardiovascular Disease in the Young (Congenital Cardiac Defects Committee), and Japanese Society of Pediatric Cardiology and Cardiac Surgery) to find out about possible unpublished data. The German society for Pediatric Cardiology (Deutsche Gesellschaft für Pädiatrische Kardiologie) was not contacted separately, since BS is the 2014 president of the society.
Data collection and analysis
We carried out data analysis out using RevMan 5.2 software. Additionally, we performed some analyses using R package `meta' (R 2013; Schwarzer 2007).
Selection of studies
Two review authors (BB, BS) independently selected records for full‐text review. if there were any disagreements, we considered the full texts.. Again, the same two review authors examined the full texts for inclusion and arrived at the same selection of studies. We only selected randomised controlled trials for analysis. We documented study selection according to the PRISMA statement format (Moher 2009).
Data extraction and management
Two review authors (BB and BS) independently extracted data according to a pre‐defined protocol and using the same data extraction work sheet (Table 1). We settled any differences by discussion with the third author (GR). Whenever quality assessment of data revealed shortcomings of the publications identified, for example, lack of information of methods used for allocation concealment in randomised trials, we contacted trial authors and asked them to provide further information in order to obtain as complete a data set as possible concerning each individual study.
1. Data extraction form.
| Questions | Answers |
| AUTHORS | |
| PUBLICATION ID | |
| PUBLICATION SOURCE | |
| STUDY DESIGN | |
| DATES OF STUDY | |
| LOCATION and number of centres |
|
| SETTING | |
| SEQUENCE GENERATION | |
| ALLOCATION SEQUENCE CONCEALMENT | |
| BLINDING | |
| OTHER CONCERNS ABOUT BIAS | |
| PARTICIPANTS Number screened Number randomised Number completed Number of male/female participants Participant ages Inclusion criteria Exclusion criteria Co‐morbidity |
|
| INTERVENTIONS Total number of intervention groups Number of patients in each group Specific interventions: drugs Intervention details (bolus and infusion or infusion only, infusion rate, starting time of infusion after surgery, duration of infusion) Co‐interventions Follow‐up period |
|
| OUTCOMES Outcomes reported Subgroup analyses (infants/children, univentricular/biventricular) |
|
| RESULTS MORTALITY WITHIN 30 DAYS Sample size Number of missing participants Summary data for each intervention group Estimate of effect with confidence interval; P value |
|
| TIME TO DEATH (CENSORED AFTER 3 MONTHS) Sample size Number of missing participants Summary data for each intervention group Estimate of effect with confidence interval; P value |
|
| LCOS WITHIN 30 DAYS FROM SURGERY Sample size Number of missing participants LCOS definition (with diagnostic criteria) Summary data for each intervention group Estimate of effect with confidence interval; P value |
|
| DURATION OF ICU STAY Sample size Number of missing participants Summary data for each intervention group Estimate of effect with confidence interval; P value |
|
| DURATION OF HOSPITAL STAY Sample size Number of missing participants Summary data for each intervention group Estimate of effect with confidence interval; P value |
|
| DURATION OF MECHANICAL VENTILATION Sample size Number of missing participants Summary data for each intervention group Estimate of effect with confidence interval; P value |
|
| MAXIMUM INOTROPE SCORE Sample size Number of missing participants Summary data for each intervention group Estimate of effect with confidence interval; P value |
|
| NUMBER OF PATIENTS REQUIRING MECHANICAL CIRCULATORY SUPPORT WITHIN 30 DAYS FROM SURGERY Sample size Number of missing participants Summary data for each intervention group Estimate of effect with confidence interval; P value |
|
| NUMBER OF PATIENTS REQUIRING HEART TRANSPLANT WITHIN 30 DAYS FROM SURGERY Sample size Number of missing participants Summary data for each intervention group Estimate of effect with confidence interval; P value |
|
| NUMBER/PROPORTION OF ADVERSE EVENTS arrhythmias tachycardia hypotension intraventricular haemorrhage hypokalaemia bronchospasm thrombocytopenia elevated serum levels of liver enzymes left ventricular ejection fraction <50% or left ventricular fraction of shortening < 28% |
|
| FUNDING SOURCE | |
| INTENTION‐TO‐TREAT ANALYSIS | |
| KEY CONCLUSIONS OF THE STUDY AUTHORS | |
| MISCELLANEOUS COMMENTS FROM THE STUDY AUTHORS | |
| REFERENCES TO OTHER RELEVANT STUDIES | |
| CORRESPONDENCE REQUIRED? | Yes/No |
| MISCELLANEOUS COMMENTS FROM THE REVIEW AUTHORS | |
| RISK OF BIAS Random sequence generation Allocation concealment Blinding of participants and personnel Blinding of outcome assessment (Mortality) Blinding of outcome assessment (LCOS) Blinding of outcome assessment (Secondary outcomes) Blinding of outcome assessment (Safety outcomes) Incomplete outcome data (Mortality) Incomplete outcome data (LCOS) Incomplete outcome data (Secondary outcomes) Incomplete outcome data (Safety outcomes) Selective reporting Other sources of bias |
High risk/ Low risk/Unclear High risk/ Low risk/Unclear High risk/ Low risk/Unclear High risk/ Low risk/Unclear High risk/ Low risk/Unclear High risk/ Low risk/Unclear High risk/ Low risk/Unclear High risk/ Low risk/Unclear High risk/ Low risk/Unclear High risk/ Low risk/Unclear High risk/ Low risk/Unclear High risk/ Low risk/Unclear High risk/ Low risk/Unclear |
| CONSORT FLOW DIAGRAM |
ICU: intensive care unit LCOS: low cardiac output syndrome
Assessment of risk of bias in included studies
We used 'Risk of bias' tables to list possible concerns over the potential for bias of each individual study, evaluating sequence generation, allocation sequence concealment, blinding of participants, personnel, and outcome assessors, incomplete outcome data, selective outcome reporting, and other potential sources of bias, according to "The Cochrane Collaboration’s tool for assessing risk of bias" (Higgins 2011). This was performed independently by two review authors (BB, BS) and any disagreements were settled by discussion.
Measures of treatment effect
For total mortality within 30 days, the risk ratio (RR) with 95% confidence interval was used as an effect measure (as for other binary outcomes). Data were pooled using a random‐effects model.
For time to death, the effect measure was the log hazard ratio with its standard error. If the hazard ratio or its standard error were not directly reported, it was derived from other reported information if possible (Tierney 2007).
For evaluation of time to extubation (duration of mechanical ventilation), hazard ratios were calculated according to the Cox model.
For continuous variables (e.g. if mean and standard deviation of the duration of mechanical ventilation were available instead of hazard ratios), we used the mean difference (MD) with 95% confidence intervals.
We assessed binary outcomes (LCOS yes/no, 30‐day mortality) using RR as an effect measure.
Data were illustrated using forest plots.
Unit of analysis issues
The following outcomes were expected to be reported in repeated measurements of individual study participants at different time intervals following surgery: number of events of low cardiac output syndrome, number of patients requiring mechanical circulatory support, inotrope score.
Meta‐analysis of the effect of milrinone with the same comparator (placebo) was only useful within the study by Hoffman 2003, since the other studies used different comparison medications. This study used three treatment arms, of which two were assigned to different doses of milrinone (low‐dose group = LD and high‐dose group = HD). Therefore, we treated these as two separate studies for the purposes of the meta‐analysis, dividing the placebo population into two pro‐rata parts for comparison of each of the milrinone groups to one of the two parts of the placebo population, respectively.
For the numbers of LCOS and mechanical circulatory support, respectively, the incidence at any time within 30 days after surgery was considered relevant. For the inotrope score, the maximum value reported within 30 days after surgery was used.
Dealing with missing data
We contacted study authors and asked them to supply additional information in case of missing data. Some unpublished data were added as a result.
Assessment of heterogeneity
Heterogeneity between studies was planned to be assessed using Cochran’s Q and I2 according to Higgins and Thompson (Higgins 2002; Higgins 2011). Had we noted an I2 > 50%, we would have performed a subgroup analysis between patients with biventricular surgical repair of a congenital heart defect versus patients after univentricular palliation of a congenital heart defect.
Assessment of reporting biases
Wherever protocols of eligible studies were available, we compared the outcomes in the protocols and published reports. Additional information was obtained from all study authors. Funnel plots would have been applied to assess reporting bias if the number of studies had allowed this (Harbord 2006), and results would have been adjusted for in an additional sensitivity analysis (Rücker 2011; Schwarzer 2010).
Data synthesis
We pooled data using a random‐effects model. For evaluation of time to extubation, hazard ratios (Cox) would have been calculated, if data had been available. For continuous variables, the mean difference (MD) was used.
Subgroup analysis and investigation of heterogeneity
With the small numbers of children studied in clinical trials, and owing to the fact that most surgical interventions for congenital heart disease are to date carried out in the first year of life, age‐based subgroup analysis would have been confined to two age groups: infants less than one year of age, and children and adolescents from one to 12 years of age. The infant group comprises children undergoing complicated palliative and corrective operations, necessitating long cardiopulmonary bypass times with concomitant long periods of cardioplegia, and with a high risk of morbidity and mortality. On the other hand, in the group of children ≥ 1 year old, there is a higher proportion of less complicated and lengthy procedures, which leads to an a priori lower morbidity and mortality risk.
Furthermore, two subgroups were pre‐defined based on cardiovascular physiology:
children with biventricular surgical repair of a congenital heart defect versus
children after univentricular palliation of a congenital heart defect.
Sensitivity analysis
We expected to find studies with different levels of risk of bias. For a sensitivity analysis, studies with high risk of bias would have been excluded.
Results
Description of studies
Results of the search
Electronic database searching yielded 1023 records by September 2014. From these, 349 records were removed as duplicates. A total of 656 records were excluded by screening, leaving 18 full‐text articles to be assessed, one of which was a partial duplicate (conference abstract and full report of the same study).
Registry searches yielded a total of 112 records on 29 February 2012 (Clinicaltrials.gov: 11, EU clinical trials register: seven, WHO International Clinical Trials Registry Platform: 11, metaRegister of current controlled trials: 23, International Federation of Pharmaceutical Manufacturers & Associations Clinical Trials Portal: 59, Forschungsdatenbank Universität Zürich: one), of which 101 were removed by screening, and of the remaining 11, nine were duplicates and two were excluded.
References from reviews yielded an additional 14 records, of which seven were duplicates and the remaining seven were removed by screening.
Expert consultation yielded no additional studies. The manufacturer did not answer our request about possible unpublished data.
From all sources, we assessed 20 full‐text articles for eligibility, and 12 of them were excluded. The remaining eight articles described six studies, five of which were included in the qualitative and quantitative synthesis and one remains unclassified so far (Costello 2014, Characteristics of studies awaiting classification).
See Figure 1 for the study flow diagram.
1.
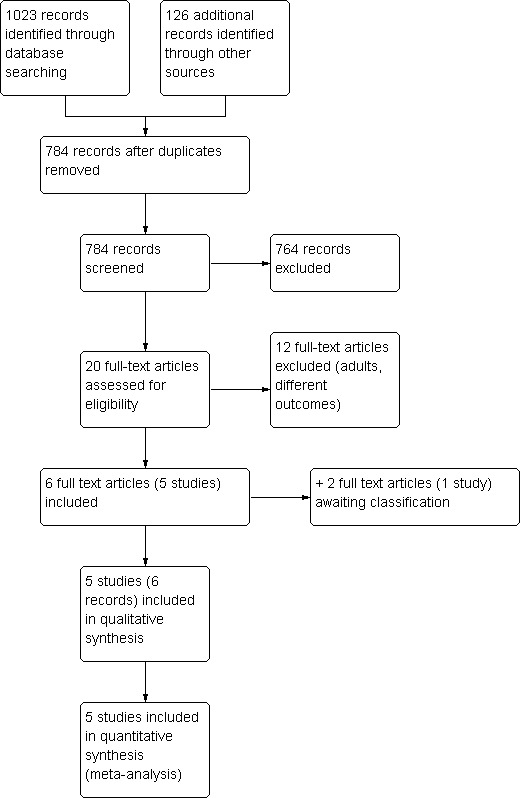
Study flow diagram
Included studies
Of the included studies (see also Characteristics of included studies below), one compared milrinone versus placebo (Hoffman 2003), three compared milrinone versus levosimendan (Lechner 2012; Momeni 2011; Pellicer 2013), and one study compared milrinone with dobutamine (Cavigelli 2013).
All studies were conducted in one or more tertiary care children's hospitals. All included neonates, and in addition, all except one study (Pellicer 2013) included infants beyond four weeks corrected gestational age, with one study (Lechner 2012) concentrating on patients one year old or younger. None examined subgroups of patients, neither by age nor by type of cardiac disease. Patients with single ventricle participated in one study only constituting four out of 50 participants) (Cavigelli 2013). The duration of study drug administration ranged from 24 to 48 hours, and follow‐up periods ranged from six to 78 days.
None of the studies evaluated time to death or adverse events as designated in our protocol. LCOS according to our definition was a defined outcome only in the Hoffman 2003 study. It could be reconstructed (unpublished data) by the authors of two of the levosimendan studies (Lechner 2012; Pellicer 2013), but not by the others, because not all of the parameters constituting this composite outcome were part of the endpoints of the other trials.
Only children who had undergone cardiopulmonary bypass were included.
Excluded studies
The studies excluded (see also Characteristics of excluded studies below) were not considered for review because of adult participants (e.g. Carmona 2010) or due to different outcomes, e.g. only pulmonary artery pressures and resistances reported (Cai 2008a).
Risk of bias in included studies
Three trials were considered at low risk of bias while two were considered at higher risk of bias. In cases of incomplete descriptions in the published articles, we sought additional information from the study authors, leading mostly to assessment as a low risk of bias (see 'Risk of bias' tables and Figure 2 and Figure 3).
2.
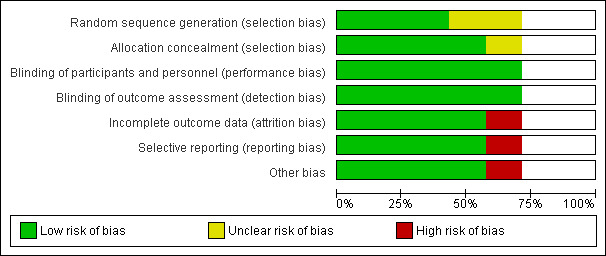
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
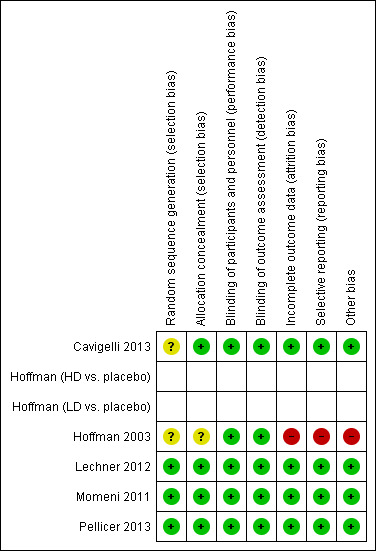
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Primary outcomes:
a) Total mortality within 30 days: Data available from the included trials are insufficient for additional comparisons. There was no 30‐day mortality in two trials (Lechner 2012 and Cavigelli 2013), no in‐hospital mortality among the patients who received the study drug postoperatively in one trial (Momeni 2011), three deaths during the 48‐hour study period (2/9 in the milrinone group and 1/11 in the comparison group) in one trial (Pellicer 2013), and no mortality during the 36‐hour study drug infusion period in the largest trial (Hoffman 2003), which also reports two later deaths (2/238 = 0.8% of patients), thought to be unrelated to the study drug but does not expressly report which study group these patients had been part of (the high‐dose group according to MHRA 2005).
For the comparison of mortality of patients treated with milrinone versus levosimendan (Analysis 1.1, mortality milrinone versus levosimendan), in two studies (Lechner 2012; Momeni 2011), no events were observed in any group. Therefore, these studies are omitted from the analysis, although they contribute more patients than the third study (Pellicer 2013), which contributed two events in the milrinone group and one event in the levosimendan group (risk ratio (RR) 2.44, 95% confidence interval (CI) 0.26 to 22.80). As a sensitivity analysis, we used a constant continuity correction of 0.5 for all groups of all studies (CCC) and also treatment arm continuity correction (TAC) (Sweeting 2004). The pooled results were (CCC) RR 1.61, (95% CI 0.35 to 7.38) and (TAC) RR1.58, (95% CI 0.35 to 7.18). These results indicate a risk ratio nearer to one than that of the primary analysis with more precision, that is, there is even less reason to assume a difference in mortality between milrinone and levosimendan than by using the primary analysis (see Analysis 1.1, Figure 4, and Figure 5).
1.1. Analysis.
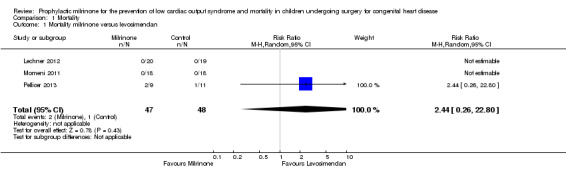
Comparison 1 Mortality, Outcome 1 Mortality milrinone versus levosimendan.
4.
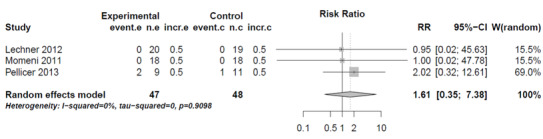
Forest plot of comparison: 1 Mortality, outcome: 1.1 Mortality milrinone versus levosimendan, Constant Continuity Correction.
event.e = event in experimental group; n.e. = non‐event in experimental group; incr.e. = increment experimental group.
event.c = event in control group; n.c. = non‐event in control group; incr.c. = increment control group.
5.
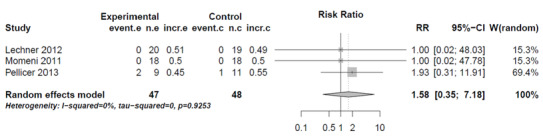
Forest plot of comparison: 1 Mortality, outcome: 1.1 Mortality milrinone versus levosimendan, Treatment Arm Continuity Correction.
event.e = event in experimental group; n.e. = non‐event in experimental group; incr.e. = increment experimental group.
event.c = event in control group; n.c. = non‐event in control group; incr.c. = increment control group.
b) Time to death (censored after three months): not reported in any of the included studies.
c) Low cardiac output syndrome: The study comparing milrinone to placebo (Hoffman 2003) used three treatment arms, of which two were assigned to different doses of milrinone (low‐dose group = LD and high‐dose group = HD). Therefore, we treated these as two separate studies for the purposes of the meta‐analysis, dividing the placebo population into two approximately equal parts for comparison of each milrinone group with one of the two halves of the placebo population, respectively. The incidence of LCOS in the first 36 hours postoperatively was lower in the milrinone group compared to placebo, (RR 0.52, 95% CI 0.28 to 0.96) (Analysis 2.1). Unfortunately, the study population in this trial (Hoffman 2003) showed a high attrition rate of over two thirds of the incidence of LCOS and missing data as to which groups the drop outs had belonged. Therefore, only data up to 36 hours follow‐up for LCOS could be used, which was the duration of postoperative study drug infusion. When considering high‐dose milrinone versus placebo separately, milrinone performed better than placebo for LCOS prevention within 36 hours after surgery, with a RR 0.35, (95% CI 0.15 to 0.86; 73 patients in the experimental and 37 in the pro‐rata placebo group). In contrast, the comparison of low‐dose milrinone versus placebo failed to show superiority of prophylactic milrinone in preventing LCOS (RR 0.67, 95% CI 0.33 to 1.37, with 79 patients in the experimental and 38 in the pro‐rata placebo group). However, there were no detectable subgroup differences, with a non‐significant heterogeneity (P = 0.27; I² = 19%) between the high‐dose milrinone versus placebo and the low‐dose milrinone versus placebo study components.
2.1. Analysis.
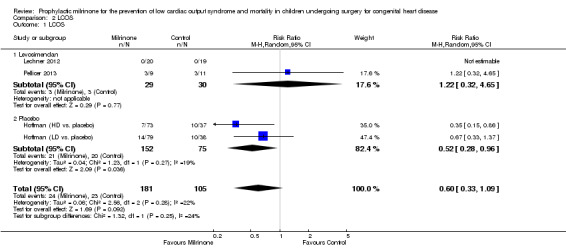
Comparison 2 LCOS, Outcome 1 LCOS.
When comparing milrinone to levosimendan, no statistically significant difference was found (RR 1.22, 95% CI 0.32 to 4.65, with 29 patients in the milrinone group and 30 patients in the levosimendan group) (Analysis 2.1). The study comparing milrinone to dobutamine (Cavigelli 2013) did not use LCOS as a defined outcome.
Secondary outcomes:
a) Duration of intensive care stay: Data are shown in Analysis 3.1. When using the numbers of the two trials comparing milrinone (38 patients) with levosimendan (37 patients), the resulting mean difference (MD) is ‐0.18 days with a 95% CI of ‐4.36 to 4.00 days. The other trial reporting on this outcome compared milrinone (24 patients) with dobutamine (26 patients) and did not show any differences either (MD 0.30 days, 95% CI ‐0.71 to 1.31 days).
3.1. Analysis.
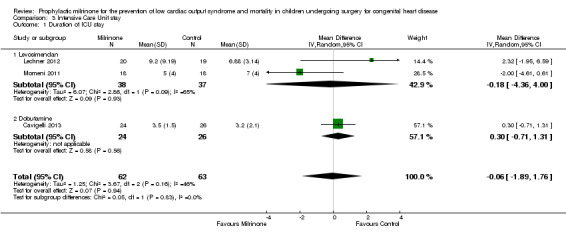
Comparison 3 Intensive Care Unit stay, Outcome 1 Duration of ICU stay.
b) Duration of hospital stay: Data are shown in Analysis 4.1. For the comparison of milrinone versus placebo (Hoffman 2003), a population of 209 patients is assumed after drop outs lost to follow‐up; however, only means are reported, not standard deviations, which therefore do not lend themselves to meta‐analysis. When taking into account milrinone versus levosimendan (38 versus 37 patients, respectively) and milrinone versus dobutamine (24 versus 26 patients), there is no significant difference in duration of hospital stay with either of these prophylactic medications (MD 0.72, 95% CI ‐2.75 to 4.19).
4.1. Analysis.
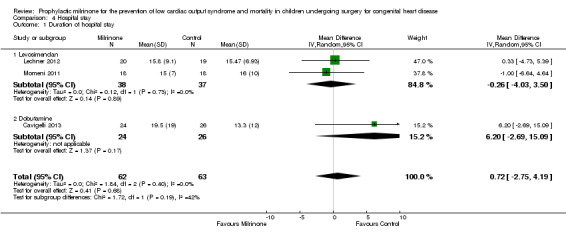
Comparison 4 Hospital stay, Outcome 1 Duration of hospital stay.
c) Duration of mechanical ventilation: Data are shown in Analysis 5.1. For the comparison of milrinone versus placebo (Hoffman 2003), the population of 209 patients is assumed after drop outs lost to follow‐up; however, only means are reported, not standard deviations, which therefore do not lend themselves to meta‐analysis. When taking into account milrinone versus levosimendan (38 versus 37 patients, respectively) and milrinone versus dobutamine (24 versus 26 patients), there is no significant difference in duration of mechanical ventilation with either of these prophylactic medications (MD 0.12, 95% CI ‐1.00 to 1.24).
5.1. Analysis.
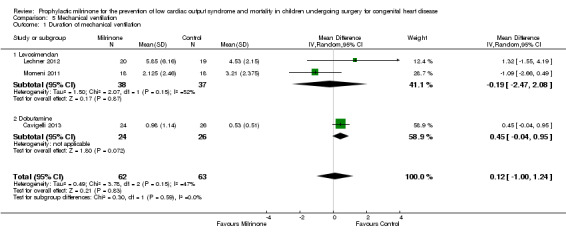
Comparison 5 Mechanical ventilation, Outcome 1 Duration of mechanical ventilation.
d) Inotrope score: Data are shown in Analysis 6.1. Data are available by patient groups from two studies comparing milrinone to levosimendan (Lechner 2012 and Pellicer 2013), with the maximum inotrope score reached in the milrinone groups after 24 hours (Lechner 2012) and after 12 hours (Pellicer 2013), respectively, versus after 18 hours in the levosimendan group (both studies, numbers taken from figure 4, page 547, Lechner 2012).
6.1. Analysis.
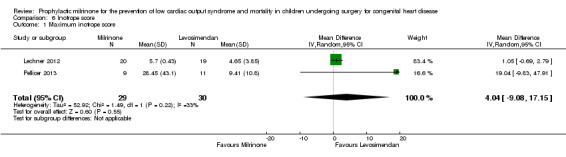
Comparison 6 Inotrope score, Outcome 1 Maximum inotrope score.
The original published report of another study (Hoffman 2003) did not report absolute or maximum inotrope scores, but claimed "no statistically significant differences among the 3 treatment groups with respect to [...] baseline inotropic support". Inotropic medication was increased or added in patients who developed LCOS. In an external publication detailing more data from this trial (MHRA 2005), initiation of pharmacological support was necessary in the intention‐to‐treat population's placebo group in 18 of 81 patients (22.2%), less frequently in the low‐dose milrinone group, that is in 14 of 80 patients (17.5%), and even less frequently in the high‐dose milrinone group, that is in five of 77 patients (6.5%). This is significantly higher for the placebo group than for the high‐dose milrinone group with a RR 0.29, 95% CI 0.10 to 0.80 and results in an overall RR of 0.51, 95% CI 0.19 to 1.37 for patients treated with any dose of prophylactic milrinone compared to patients on placebo. Escalation of existing pharmacological support was evenly distributed in the groups with six of 81 patients affected in the placebo group (7.4%), six of 80 patients in the low‐dose milrinone group (7.5%), and seven of 77 patients in the high‐dose milrinone group (9.1%).
e) Number of patients requiring mechanical circulatory support (e.g. ECMO, pulsatile assist devices) or cardiac transplantation: Mechanical circulatory support was rarely used. There was one out of 11 patients of the comparison group in one of the milrinone versus levosimendan trials (Pellicer 2013) and two out of 209 patients (population with complete follow‐up) in the milrinone versus placebo trial (Hoffman 2003) who needed extracorporeal membrane oxygenation, one in the high‐dose milrinone arm and one in the placebo arm (MHRA 2005). None of the participants of the other studies who had received the study drug for at least four hours needed mechanical circulatory support. No patient from any of the included studies received a heart transplantation during the study or during the respective follow‐up period.
Safety outcomes:
a) Arrhythmias (number/proportion): Two studies (Lechner 2012; Momeni 2011) had no incidences of arrhythmia. The largest study (Hoffman 2003) reported two patients (out of the assumed per protocol population of 227 patients) with arrhythmias but did not report which treatment groups they belonged to. Nodal arrhythmias were however reported to the UK regulatory agency (MHRA 2005) by the manufacturer at a frequency of two (2.5%) of 81 patients in the placebo group, six (7.5%) of 80 patients in the low‐dose milrinone group, and eight (10.4%) of 77 patients in the high‐dose milrinone group.
The remaining two studies report one of nine patients in the milrinone group and two of 11 patients in the levosimendan group with AV conduction abnormalities (RR 0.61, 95% CI 0.07 to 5.70) (Pellicer 2013), and seven of 24 patients (29%) in the milrinone group and four of 26 (15%) patients in the dobutamine group with arrhythmias (third degree atrio‐ventricular block (permanent or transient), second degree atrio‐ventricular block (Wenckebach type), junctional ectopic tachycardia, supraventricular tachycardia, atrial ectopic tachycardia) (RR 1.90, 95% CI 0.63 to 5.67) (Cavigelli 2013), respectively.
Meta‐analysis of the effect of milrinone with the same comparator (placebo) was performed within the study by Hoffman 2003, since the other studies used different comparison medications. This study used three treatment arms, of which two were assigned to different doses of milrinone (low‐dose group = LD and high‐dose group = HD). Therefore, we treated these as two separate studies for the purposes of the meta‐analysis, dividing the placebo population into two pro‐rata parts for comparison of each of the milrinone groups to one of the two parts of the placebo population, respectively. The incidence of arrhythmias was higher in the milrinone group compared to placebo, with the 95% confidence interval of the risk ratio still including 1, (RR 3.59, 95% CI 0.83 to 15.42.
When looking at all patients receiving milrinone versus different kinds of other interventions, the included studies did not prove an increased risk of arrhythmia in the milrinone groups (RR 1.99, 95% CI 0.88 to 4.50) (Analysis 7.1).
7.1. Analysis.
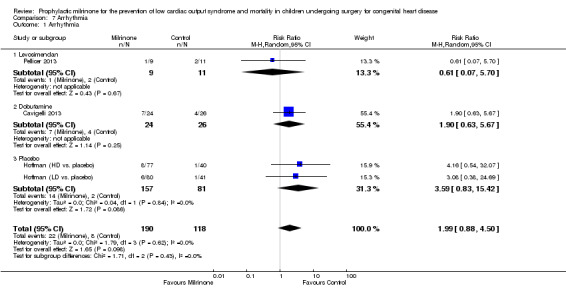
Comparison 7 Arrhythmia, Outcome 1 Arrhythmia.
b) Tachycardia (number/proportion of patients with heart rate above heart rate appropriate for age or body surface area): Tachycardia was part of the LCOS definition in one study (Hoffman 2003), which does not report cutoff values or patient numbers with tachycardia. Tachycardia occurred in 14 of 24 patients (58%) in the milrinone group versus 22 of 26 patients (85%) in the dobutamine group in one study (Cavigelli 2013). All other studies reported no incidence of tachycardia. A meta‐analysis for this outcome is therefore not possible.
c) Hypotension (number/proportion of patients with blood pressures below blood pressure appropriate for age or body surface area): Data from all studies are not comparable due to the different types of blood pressures recorded and manners of reporting. The largest trial (Hoffman 2003) reports hypotension (the definition of which is not mentioned) in one patient each in the placebo (1.2%) and low‐dose (1.3%) arms and in two patients (2.6%) in the high‐dose arm, which amounted to no differences between the groups. The manufacturer's report to the UK regulatory agency (MHRA 2005) mentions hypotension as an adverse event in 10 of 81 (12.3%) patients of the placebo group, in three of 80 (3.8%) patients in the low‐dose milrinone group, and in 10 (13%) of 77 patients in the high‐dose milrinone group.
One trial reports diastolic blood pressure means (Pellicer 2013), while there are systolic blood pressure data displayed in figures from one of the levosimendan trials (Lechner 2012): systolic blood pressure means with standard error of means markings (unclear which markings belong to which treatment arm) and diastolic blood pressure means with standard deviation markings in the dobutamine trial (Cavigelli 2013). Numerical data of mean arterial blood pressure means and standard deviations are available for one of the levosimendan trials (Momeni 2011), and of systolic arterial blood pressure means and standard deviations from the dobutamine trial (Cavigelli 2013).
However, our review definition of blood pressure values below those appropriate for patient age and body surface area at any point in time was not reported in any of the studies. A meta‐analysis of the effect of milrinone on the incidence of hypotension was not done due to the inconsistency of definition and reported values across studies.
d) Intraventricular haemorrhage (IVH) (number/proportion): This adverse event is not mentioned in the largest trial (Hoffman 2003) and in one of the levosimendan trials (Momeni 2011), and there were no cases of IVH in two trials (Cavigelli 2013; Lechner 2012). There were two of nine patients (22.2%) in the milrinone group with IVH of any grade and none of 11 patients (0%) in the comparison group in one of the levosimendan trials (Pellicer 2013), which however reports other new pathological cranial ultrasound findings in one of nine patients (11.1%) in the milrinone group and two of 11 patients (18.2%) in the comparison group. One other case of a pathological cranial ultrasound finding also occurred in one of 20 patients in the milrinone group and none out of 20 patients in the comparison group in one of the other levosimendan trials (Lechner 2012).
e) Hypokalaemia (number/proportion): No incidence of hypokalaemia was found in one trial (Pellicer 2013), whereas one other trial (Cavigelli 2013) reports hypokalaemia in 15 of 24 patients (62.5%) in the milrinone group and in 15 of 26 patients (57.7%) in the dobutamine group. All other trials did not assess this adverse reaction.
f) Bronchospasm (number/proportion): No incidences of bronchospasm are reported in any trial.
g) Thrombocytopenia (number/proportion): Using a definition of thrombocytopenia of a platelet count < 50,000/mm3, one trial (Hoffman 2003) found no difference in the incidence between the study groups at three different times of measurement. During the study drug infusion (first 36 hours), incidences were 7.4% in the placebo arm, 8.8% in the low‐dose milrinone arm, and 2.6% in the high‐dose milrinone arm, respectively. According to the numbers of the per protocol population, this equals roughly six of 75 patients in the placebo group, seven of 79 patients in the low‐dose milrinone group, and two of 73 patients in the high‐dose milrinone group, respectively. The manufacturer's information to the UK regulatory agency (MHRA 2005) reports nine out of 81 patients in the placebo group (11.1%), eight out of 80 patients in the low‐dose milrinone group (10%), and seven out of 77 patients in the high‐dose milrinone group (9.1%) who developed thrombocytopenia, respectively. Analysis 8.1 compares the treatment arms in this trial, with the placebo group split approximately equally between the high‐dose and the low‐dose arms of the study, thus treating them as separate studies with the same endpoint. The risk ratio of developing thrombocytopenia is 0.86 (95% CI 0.39 to 1.88) in the milrinone groups compared to placebo (Hoffman (HD vs. placebo); (Hoffman (LD vs. placebo), with a total of 157 participants in the milrinone groups and 81 participants in the placebo groups).
8.1. Analysis.

Comparison 8 Thrombocytopenia, Outcome 1 Thrombocytopenia.
No thrombocytopenia occurred in one of the levosimendan trials (Pellicer 2013), and a high incidence of thrombocytopenia was reported in the dobutamine trial (Cavigelli 2013), where 23 of 24 patients in the milrinone group (95.8%) and 23 of 26 patients in the comparison group (88.5%) had platelet counts below 80% of their individual baseline values. Thrombocytopenia was not assessed in the other trials.
h) Elevated serum levels of liver enzymes (number/proportion of patients with serum enzymatic activities more than two‐fold the age‐appropriate normal values): Serum levels of liver enzymes were only assessed in one trial (Pellicer 2013), which found no incidence.
i) Left ventricular ejection fraction < 50% or left ventricular fraction of shortening < 28%: While echocardiographic measurements are not reported in two trials (Hoffman 2003; Momeni 2011), one trial reports reduced echocardiographic left ventricular shortening fraction < 28% as one of two possible reasons (besides hypotension) for administration of at least one catecholamine on the decision of the senior consultant, which was the case in 14/20 patients from the milrinone group and 14/20 patients from the levosimendan group (Lechner 2012; page 545). LVFS values are shown in a figure (Lechner 2012; figure 4, page 547), with values at two hours postoperatively of mean/SEM 31%/1.5% (milrinone group) and 34%/2% (comparison group), at 24 hours postoperatively of mean/SEM 35%/1.5% (milrinone group) and 34%/2% (comparison group), and at 48 hours postoperatively of mean/SEM 35%/2% (milrinone group) and not shown for the comparison group. However, it is not possible to determine the numbers of patients with LVFS < 28% from these data. One other levosimendan trial (Pellicer 2013) reports the following numbers of patients with LVFS < 28%: At baseline: none in either group. At 24 hours: milrinone group three of nine patients (33.3%); comparison group three of 11 patients (27.3%). At 48 hours: milrinone group one of seven patients (14.3%); comparison group one of six patients (16.7%). The milrinone versus dobutamine trial (Cavigelli 2013) reports three time periods as well: a) two to six hours postoperatively: intervention five of 24 patients (20.8%) (LVFS values 16%; 27%, 13%, 25%, 25%); comparison 10 of 26 patients (38.5%) (LVFS values 26%; 13%; 25%; 23%; 24%; 9%; 26%; 25%; 24%; 16%). b) 24 to30 hours postoperatively: intervention six of 24 patients (25%); comparison five of 26 patients (19.2%). c) 48 to 54 hours postoperatively: intervention five of 24 patients (20.8%); comparison six of 26 patients (23.1%).
This leaves one trial comparing milrinone with levosimendan (Pellicer 2013) and one trial comparing milrinone with dobutamine (Cavigelli 2013), of which the incidences of LVFS < 28% could be compared at time points of at least two hours, at least 24 hours, and at least 48 hours postoperatively. Due to the different types of comparison interventions however, we refrained from doing this.
In addition to these adverse events we expected to analyse, the milrinone versus placebo trial (Hoffman 2003) generated some information about other possible side effects of milrinone (MHRA 2005). These were fever (placebo 14/81 (17.3%), low‐dose milrinone 12/80 (15%), high‐dose milrinone 10/77 (13%), resulting in no significant difference between the groups receiving milrinone and the placebo group, with a risk ratio of 0.81 (95% CI 0.44 to 1.50) and stridor (placebo 10/81 (12.3%), LD 10/80 (12.5%), HD 10/77 (13%), not meeting statistical significance either with a risk ratio of 1.03 (95% CI 0.51 to 2.10). In addition, incidences of pleural effusion were reported, with a risk ratio of 1.78 for the groups receiving milrinone as compared to placebo, where the 95% CI of the RR marginally includes 1 (RR 1.78, 95% CI 0.92 to 3.42; 231 participants). Thus, statistical significance is not formally established (Analysis 9.1) (Hoffman (HD vs. placebo); (Hoffman (LD vs. placebo).
9.1. Analysis.
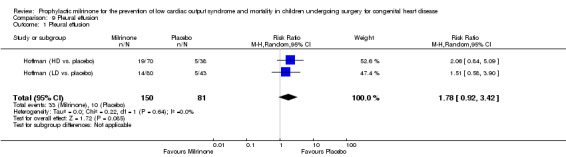
Comparison 9 Pleural effusion, Outcome 1 Pleural effusion.
No subgroups (age or physiology) were analysed within the patient population for the following reasons. Information about age or physiology was limited. In the studies using levosimendan as a comparative intervention, there were only very few patients older than one year. The number of patients with univentricular physiology was low (four out of 50 participants in Cavigelli 2013), or none were included for participation at all.
Discussion
Summary of main results
Our search for studies of prophylactic postoperative milrinone in children undergoing corrective surgery for congenital heart defects yielded records from five studies meeting the inclusion criteria. The five studies were heterogeneous in terms of population numbers, interventions (inodilators used), and which outcomes they reported. In addition, milrinone was used in different dosages, with only two studies reporting pharmacokinetic data of their study participants (Pellicer 2013 and Hoffman 2003: see Bailey 2004).
Our question of whether milrinone prevents low cardiac output syndrome (LCOS) and mortality in children undergoing heart surgery for congenital heart disease remains largely unanswered. While patients treated with milrinone showed a lower relative risk for LCOS than those treated with placebo, this could be shown only for the first 36 hours postoperatively and only in the high‐dose arm of the trial comparing milrinone with placebo (Hoffman 2003). There was no statistically significant LCOS risk reduction in patients treated prophylactically with milrinone versus levosimendan. If used prophylactically, milrinone resulted in an absolute risk reduction for developing LCOS within 36 hours of congenital cardiac surgery of 14.2% (high‐dose arm of the Hoffman 2003 study versus placebo arm), resulting in a number needed to treat of eight in order to prevent one case of LCOS within this time frame.
Mortality data were available from the three trials comparing milrinone and levosimendan, albeit with different durations of follow‐up, and no difference between both treatment groups was found. In studies of as small a size as the included studies, mortality differences are of course not to be expected.
Secondary outcomes such as duration of intensive care unit (ICU) stay, hospital stay, mechanical ventilation, or maximum inotrope score were also not statistically different between the milrinone and the comparison groups.
Safety outcomes or harms were reported so inconsistently that no comparisons between studies could be made. As far as arrhythmias are concerned, the prophylactic use of milrinone might increase patients' risk compared to placebo, according to Hoffman 2003, although the confidence interval was too wide to exclude no difference in arrhythmias between milrinone and placebo.
Overall completeness and applicability of evidence
There is insufficient evidence to judge the effect of milrinone on the studied population related to the primary outcomes of this review (30‐day mortality, time to death, or LCOS in the entire postoperative period) since follow‐up periods for mortality were variable between studies, and LCOS was not uniformly used as a defined outcome.
Another difficulty is encountered when comparing studies that use placebo as a comparison intervention with studies that compare different inodilator drugs, such as milrinone versus levosimendan or milrinone versus dobutamine. Not all of these comparison interventions can reasonably be combined to one "comparison group" for the purposes of a meta‐analysis.
Quality of the evidence
Unfortunately but inherently to the situation, patient numbers of all five studies were low. No treatment arm in any single trial comprised more than 80 patients.
Of note, while the earliest study (Hoffman 2003) compared milrinone with placebo, more recent pilot studies use milrinone as their comparison medication (which is now widely used in children after congenital heart surgery) in order to investigate a newer drug, levosimendan. The hypotheses of the respective studies that milrinone might prove better than placebo (except for short‐term reduction of LCOS), and possibly levosimendan better than milrinone, are not statistically supportable, according to our data. A robust conclusion regarding the objectives of the review can not be drawn based on the evidence available.
Risk of bias was low in most instances in the included studies, and if there were incomplete descriptions in the published articles, additional information from the study authors led mostly to assessment as low risk of bias in the respective area. Blinding of participants and personnel (performance bias) and blinding of outcome assessment (detection bias) were deemed satisfactory in all included studies. Risk of allocation concealment bias (selection bias) and random sequence generation bias (selection bias) were low in all studies except Hoffman 2003 and the latter in Cavigelli 2013. There was also concern about possible incomplete outcome data (attrition bias) and selective reporting (reporting bias) in the largest study (Hoffman 2003), as well as concern about other bias in terms of support of the study by a grant from the manufacturer (Sanofi Synthelabo) and co‐authorship of the study publication by manufacturer representatives (see 'Risk of bias' tables Figure 2 and Figure 3).
Potential biases in the review process
The identification of relevant studies comprised updated electronic literature searches 11 months, 24 months, and 31 months after the original search, which were successful in identifying additional relevant studies.
In order to obtain more information beyond which was reported in the literature and to complement incomplete outcome data, we contacted the authors of all included studies. All of them responded. However, some of the outcomes sought after for this review were not available, either due to the long time elapsed since the study had been conducted (Hoffman 2003), or to a smaller number of outcomes in the study protocols than in the protocol of this review (Cavigelli 2013; Lechner 2012; Momeni 2011; Pellicer 2013;).
Agreements and disagreements with other studies or reviews
We know of no other studies dealing with our review question that would not have been examined for inclusion or included in this review.
Authors' conclusions
Implications for practice.
There is insufficient evidence of the prophylactic effect of milrinone in preventing mortality or low cardiac output syndrome (LCOS) in children undergoing surgery for congenital heart disease. As much as prophylactic milrinone proved to provide a short‐term benefit in the reduction of LCOS in the immediate postoperative period compared to placebo, there might also be harm in terms of increased risk of arrhythmia, which was however not statistically significant. The existing data on the prophylactic use of milrinone has to be viewed cautiously due to the small number of small trials with different dosing regimens of milrinone and the risk of bias in some of these studies.
In the absence of a proven mortality benefit and in the absence of proven long‐term beneficial effects of prophylactic milrinone, clinicians must weigh the short‐term reduction of LCOS risk and concomitant reduction of additional pharmacological support against a possible increase in the risk of arrhythmias.
Implications for research.
There are a handful of randomised controlled trials addressing the prophylactic use of milrinone in children after congenital heart surgery. Participant numbers are limited, yielding results with low statistical significance. The number of outcomes reported is also variable.
Future studies should maintain the high methodological standards of randomised controlled trials, paying attention to the pharmacokinetic properties of milrinone in different age groups, and ideally include larger numbers of participants. This is especially the case when milrinone is not compared with placebo but instead to other inodilator substances. In order to allow comparisons, a standardised definition of LCOS, with criteria as objective as possible, should be adopted in future clinical trials. Even the use of end‐organ related outcomes, especially neuromonitoring, would make studies more relevant. We would also argue that follow‐up periods in future trials should be extended at least until hospital discharge or possibly longer, in order to detect meaningful long‐term outcomes in addition to mortality.
Acknowledgements
We thank Nicole Ackermann for her valuable help with design of the search strategy. We are very grateful to her and Joanne Abbott who conducted the electronic literature searches across databases. Also, we would like to thank the authors of the included studies very much, as all of them went back to the study data in order to answer as many of our questions as possible.
Appendices
Appendix 1. Search strategies
CENTRAL
#1 MeSH descriptor Milrinone, this term only #2 (milrinon*) #3 MeSH descriptor Phosphodiesterase 3 Inhibitors, this term only #4 (Phosphodiesterase 3 inhibitor*) #5 "win 47203" #6 (win‐47203) #7 (win47203) #8 (corotrop*) #9 (primacor) #10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9) #11 MeSH descriptor Cardiac Surgical Procedures explode all trees #12 (cardiac surg*) #13 ("heart surg*") #14 (cardiosurg*) #15 "thoracic surg*" #16 MeSH descriptor Thoracic Surgery, this term only #17 "cardiopulmonary bypass*" #18 MeSH descriptor Cardiopulmonary Bypass, this term only #19 MeSH descriptor Heart Defects, Congenital explode all trees #20 "congenital heart*" #21 "heart abnorm*" #22 "congenital card*" #23 (heart near/2 (repair* or replace* or operation* or procedure*)) #24 (cardiac near/2 (repair* or replace* or operation* or procedure*)) #25 ((heart or cardiac) next transplant*) #26 (cardiomyopath*) #27 (perioperative) #28 (postoperative) #29 (anesth*) #30 (anaesth*) #31 (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30) #32 (#10 AND #31)
MEDLINE
1. Milrinone/ 2. milrinon*.tw. 3. Phosphodiesterase 3 Inhibitors/ 4. Phosphodiesterase 3 inhibitor*.tw. 5. win 47203.tw. 6. win‐47203.tw. 7. win47203.tw. 8. corotrop*.tw. 9. primacor.tw. 10. or/1‐9 11. exp Cardiac Surgical Procedures/ 12. cardiac surg*.tw. 13. heart surg*.tw. 14. cardiosurg*.tw. 15. thoracic surg*.tw. 16. Thoracic Surgery/ 17. cardiopulmonary bypass*.tw. 18. Cardiopulmonary Bypass/ 19. exp Heart Defects, Congenital/ 20. congenital heart*.tw. 21. heart abnorm*.tw. 22. congenital card*.tw. 23. (heart adj2 (repair* or replace* or operation* or procedure*)).tw. 24. (cardiac adj2 (repair* or replace* or operation* or procedure*)).tw. 25. ((heart or cardiac) adj transplant*).tw. 26. cardiomyopath*.tw. 27. perioperative.tw. 28. postoperative.tw. 29. anesth*.tw 30. anaesth*.tw 31. or/11‐30 32. 10 and 31 33. randomized controlled trial.pt. 34. controlled clinical trial.pt. 35. randomized.ab. 36. placebo.ab. 37. drug therapy.fs. 38. randomly.ab. 39. trial.ab. 40. groups.ab. 41. 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 42. exp animals/ not humans.sh. 43. 41 not 42 44. 32 and 43
EMBASE
1 Milrinone/ 2 milrinon*.tw. 3 phosphodiesterase III Inhibitor/ 4 Phosphodiesterase 3 inhibitor*.tw. 5 win 47203.tw. 6 win‐47203.tw. 7 win47203.tw. 8 corotrop*.tw. 9 primacor.tw. 10 or/1‐9 11 exp Cardiac Surgical Procedures/ 12 cardiac surg*.tw. 13 heart surg*.tw. 14 cardiosurg*.tw. 15 thoracic surg*.tw. 16 Thoracic Surgery/ 17 cardiopulmonary bypass*.tw. 18 Cardiopulmonary Bypass/ 19 exp Heart Defects, Congenital/ 20 congenital heart*.tw. 21 heart abnorm*.tw. 22 congenital card*.tw. 23 (heart adj2 (repair* or replace* or operation* or procedure*)).tw. 24 (cardiac adj2 (repair* or replace* or operation* or procedure*)).tw. 25 ((heart or cardiac) adj transplant*).tw. 26 cardiomyopath*.tw. 27 perioperative.tw. 28 postoperative.tw. 29 anesth*.tw. 30 anaesth*.tw. 31 or/11‐30 32 10 and 31 33 random$.tw. 34 factorial$.tw. 35 crossover$.tw. 36 cross over$.tw. 37 cross‐over$.tw. 38 placebo$.tw. 39 (doubl$ adj blind$).tw. 40 (singl$ adj blind$).tw. 41 assign$.tw. 42 allocat$.tw. 43 volunteer$.tw. 44 crossover procedure/ 45 double blind procedure/ 46 randomized controlled trial/ 47 single blind procedure/ 48 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 49 (animal/ or nonhuman/) not human/ 50 48 not 49 51 32 and 50
Web of Science
#28 #27 AND #26 #27 Topic=(((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*))) #26 #25 AND #8 #25 #24 OR #23 OR #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 #24 Topic=(anaesth*) #23 Topic=(anesth*) #22 Topic=(postoperative) #21 Topic=(perioperative) #20 Topic=(cardiomyopath*) #19 Topic=(((heart or cardiac) near/1 transplant*)) #18 Topic=((cardiac near/2 (repair* or replace* or operation* or procedure*))) #17 Topic=((heart near/2 (repair* or replace* or operation* or procedure*))) #16 Topic=("congenital card*") #15 Topic=("heart abnorm*") #14 Topic=(" congenital heart*") #13 Topic=("cardiopulmonary bypass*") #12 Topic=("thoracic surg*") #11 Topic=(cardiosurg*) #10 Topic=("heart surg*") #9 Topic=("cardiac surg*") #8 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 #7 Topic=(primacor) #6 Topic=(corotrop*) #5 Topic=(win47203) #4 Topic=("win‐47203") #3 Topic=("win 47203") #2 Topic=("Phosphodiesterase 3 inhibitor*") #1 Topic=(milrinon*)
Data and analyses
Comparison 1. Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality milrinone versus levosimendan | 3 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.26, 22.80] |
Comparison 2. LCOS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 LCOS | 4 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.33, 1.09] |
| 1.1 Levosimendan | 2 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.32, 4.65] |
| 1.2 Placebo | 2 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.28, 0.96] |
Comparison 3. Intensive Care Unit stay.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of ICU stay | 3 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐1.89, 1.76] |
| 1.1 Levosimendan | 2 | 75 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐4.36, 4.00] |
| 1.2 Dobutamine | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.71, 1.31] |
Comparison 4. Hospital stay.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of hospital stay | 3 | 125 | Mean Difference (IV, Random, 95% CI) | 0.72 [‐2.75, 4.19] |
| 1.1 Levosimendan | 2 | 75 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐4.03, 3.50] |
| 1.2 Dobutamine | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 6.20 [‐2.69, 15.09] |
Comparison 5. Mechanical ventilation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of mechanical ventilation | 3 | 125 | Mean Difference (IV, Random, 95% CI) | 0.12 [1.00, 1.24] |
| 1.1 Levosimendan | 2 | 75 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐2.47, 2.08] |
| 1.2 Dobutamine | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐0.04, 0.95] |
Comparison 6. Inotrope score.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximum inotrope score | 2 | 59 | Mean Difference (IV, Random, 95% CI) | 4.04 [‐9.08, 17.15] |
Comparison 7. Arrhythmia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Arrhythmia | 4 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.88, 4.50] |
| 1.1 Levosimendan | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.07, 5.70] |
| 1.2 Dobutamine | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [0.63, 5.67] |
| 1.3 Placebo | 2 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 3.59 [0.83, 15.42] |
Comparison 8. Thrombocytopenia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Thrombocytopenia | 2 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.39, 1.88] |
Comparison 9. Pleural effusion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pleural effusion | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.92, 3.42] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cavigelli 2013.
| Methods | RCT | |
| Participants | n = 50 Mean age: intervention = 0.7 years; control = 1.7 years |
|
| Interventions | Intervention group: 24 = continuous infusion of milrinone 0.75 µg/kg/min for 36 hours after cardiopulmonary bypass (started at half the dose for the first 4 hours postoperatively). Control group: 26 = continuous infusion of dobutamine 6 µg/kg/min for 36 hours after cardiopulmonary bypass (started at half the dose for the first 4 hours postoperatively). | |
| Outcomes |
Mortality within 30 days: none. Time to death (censored after 3 months): not reported. LCOS within 30 days from surgery yes/no: not reported. Duration of ICU stay: Median (range): intervention = 3 (2‐7) days; control = 2 (2‐10) days. Mean +/‐ standard deviation: intervention 3.5 +/‐ 1.5 days; control 3.2 +/‐ 2.1; P = 0.55. Duration of hospital stay: Median (range): intervention = 14 (8‐78) days; control = 10 (7‐69) days. Mean +/‐ standard deviation: intervention 19.5 +/‐ 19 days; control 13.3 +/‐ 12.0 days; P = 0.18. Duration of mechanical ventilation: Median (range): intervention = 8 (1‐78) hours; control = 7 (0‐50) hours. Mean +/‐ standard deviation: intervention 23.6 +/‐ 27.4 hours; control 12.6 +/‐ 12.2 hours; P = 0.069. Max. inotrope score: not reported. Number of patients requiring MCS or HTX within 30 days from surgery: none. Number/proportion of adverse events: ‐ Arrhythmias (third degree AV block (permanent or transient), second degree AV block II (Wenckebach), JET, SVT, AET): intervention n = 7 (29%); control n = 4 (15%) ‐ Tachycardia: not reported ‐ Hypotension: not reported ‐ LVEF < 28%: a) 2‐6 hours postoperatively: intervention n = 5 of 24 patients (20.8%) (LVFS values 16%; 27%, 13%, 25%, 25%); comparison n = 10 of 26 patients (38.5%) (LVFS values 26%; 13%; 25%; 23%; 24%; 9%; 26%; 25%; 24%; 16%). b) 24‐30 hours postoperatively: intervention n = 6 of 24 (25%) patients; comparison n = 5 of 26 patients (19.2%). c) 48‐54 hours postoperatively: intervention n = 5 of 24 patients (20.8%); comparison n = 6 of 26 patients (23.1%). ‐ Thrombocytopenia: intervention 23/24; comparison 23/26. ‐ Elevated liver enzymes: not reported |
|
| Notes | Intention‐to‐treat: yes, no drop outs. Comparison of milrinone with dopamine. Follow‐up until hospital discharge (6.9 to 78 days). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation by independent pharmacy, algorithm not known. |
| Allocation concealment (selection bias) | Low risk | Sequence generation by independent pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study drug prepared by independent pharmacy, same infusion rates. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Envelopes with allocation code kept closed until the end of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Hoffman (HD vs. placebo).
| Methods | Hoffman 2003 study, using only high‐dose milrinone treatment arm and half of the placebo population. Sample size calculation described in study methods to compare each dose with placebo. | |
| Participants | see Hoffman 2003 table below. | |
| Interventions | ||
| Outcomes | ||
| Notes | ||
Hoffman (LD vs. placebo).
| Methods | Hoffman 2003 study, using only low‐dose milrinone treatment arm and half of the placebo population. Sample size calculation described in study methods to compare each dose with placebo. | |
| Participants | see Hoffman 2003 table below. | |
| Interventions | ||
| Outcomes | ||
| Notes | ||
Hoffman 2003.
| Methods | Randomised, double‐blind, placebo‐controlled study with three parallel treatment groups. | |
| Participants | 242 paediatric patients undergoing cardiac surgery. Age: All gestational age 36 weeks or more up to 6 years of age: 8.3 (+/‐ 14.8) months in the placebo group, 5.9 (+/‐ 10.2) months in the low‐dose (LD) milrinone group, 8.6 (+/‐ 16.5) months in the high‐dose (HD) milrinone group. Male = 48% of placebo (36/75), 60.8% of LD milrinone (48/75), 53.4% of HD milrinone group (39/75). Female = 52% of placebo (39/75), 39.2% of LD milrinone (31/75), 46.6% of HD milrinone group (34/75). Of 242 randomised patients, 238 received the study drug. 11 were excluded "due to major protocol violations". Of the remaining 227 "per‐protocol population" (placebo n = 75; LD n = 79; HD n = 73), 13 received open‐label milrinone after 36 hours and were not included in further analyses (figure 3, p. 999), and 5 were lost to follow‐up, leaving 209 patients (placebo n = 71; LD n = 74; HD n = 64). Two patients (unknown group) died on the 5th and the 13th postoperative day, respectively, leaving 207 patients for 30‐day follow‐up. |
|
| Interventions |
Milrinone low‐dose: 79 = Bolus of 25 mcg/kg over 1 hour, followed by infusion of 0.25 μg/kg/min for (23‐)35 hours, starting within 90 minutes after arrival to the ICU from the operating room. Milrinone high‐dose: 73 = Bolus of 75 mcg/kg over 1 hour, followed by infusion of 0.75 μg/kg/min for (23‐)35 hours, starting within 90 minutes after arrival to the ICU from the operating room. Placebo: 75. |
|
| Outcomes |
Mortality within 30 days: Not reported beyond hospital discharge. Mortality within 36 hours (during study drug infusion): none in any group. Two deaths after 36 hours ("deemed to be unrelated to study drug"), study group unknown. Time to death (censored after 3 months): not reported. LCOS within 30 days from surgery yes/no: a) Numbers given for 227 (per protocol population) within the first 36 hours = during study drug infusion: HD Milrinone (n = 73) n = 7 (9.6%); LD Milrinone (n = 79) n = 14 (17.7%); Placebo (n = 75) n = 20 (26.7%). Relative risk reduction: HD Milrinone vs. Placebo 55% (P = 0.023) in all treated patients vs. 64% (P = 0.007) in the per‐protocol population of 227 patients. LD Milrinone vs. Placebo 34% (P = 0.183). b) Numbers given for 209 patients for the entire follow‐up period (missing participants: 4 received no study medication, 11 with "major protocol violations", 13 participants who received open‐label milrinone after the study drug infusion, and 5 participants who were lost to follow‐up), as measured from figure 3, p. 999: HD Milrinone (n = 64) n = 10 (15.6%); LD Milrinone (n = 74) n = 16 (21.6%); Placebo (n = 71) n = 21 (29.6%). Duration of ICU stay: not reported. Duration of hospital stay: Geometric mean of duration of hospital stay: Placebo (n= 71) 10.2 days, LD Milr (n= 74) 8.6 d, HD Milr (n= 64) 9.3 days. (Numbers assuming population of n = 209 with complete follow‐up.) Duration of mechanical ventilation: Geometric mean duration of mechanical ventilation: Placebo (n = 71) 1.6 days, LD Milr (n = 74) 1.7 days, HD Milr (n = 64) 1.7 days. (Numbers assuming population of n = 209 with complete follow‐up.) Max. inotrope score: not reported, "no statistically significant differences among the 3 treatment groups with respect to [...] baseline inotropic support". Number of patients requiring MCS or HTX within 30 days from surgery: 2 patients received ECMO for LCOS (treatment group unknown), heart transplantation not reported. Number/proportion of adverse events: ‐ Arrhythmia: Ventricular arrhythmia (n = 1), SVT (n = 1), groups unknown; "no differences between the groups" ‐ Tachycardia: "HR was significantly higher (mean, 10 beats per minute) in the treatment arms at 1, 12, and 24 hours compared with placebo”. No patient numbers reported using a cutoff heart rate value. ‐ Hypotension: “In both milrinone treatment arms, systolic and diastolic blood pressures decreased between 5% and 9% immediately after the bolus and were not significantly different from placebo by 12h into the study infusion.” [Circulation 2003, p.999]. Hypotension occurred in 1 patient each in the placebo (1.2%) and LD (1.3%) arms and in 2 patients (2.6%) in the HD arm. No differences between the groups. ‐ LVEF < 28%: not reported ‐ Thrombocytopenia: not reported (“no statistical difference in platelet count over time (baseline, 36 hours, 72 hours, and discharge) by treatment arm”, “no difference in the incidence of thrombocytopenia (platelet count < 50 000) during the study infusion”) ‐ Elevated liver enzymes: "no differences between the groups" According to extended information about this trial by MHRA 2005: "Adverse events were reported in 79% of placebo treated patients, 78.8% of low dose milrinone patients and 71% of high dose milrinone patients. Those occurring at a frequency of >10% in any group are as follows:" (absolute numbers in the treatment groups calculated according to percentages, mostly adding up to the intention‐to‐treat population of n = 238) ‐ Fever: Placebo 14/81 (17.3%), LD 12/80 (15%), HD 10/77 (13%). ‐ Cardiac failure: Placebo 21/81 (25.9%), LD 18/80 (22.5%), HD 12/77 (15.6%). ‐ Hypotension: Placebo 10/81 (12.3%), LD 3/80 (3.8%), HD 10/77 (13%). ‐ Nodal arrhythmia: Placebo 2/81 (2.5%), LD 6/80 (7.5%), HD 8/77 (10.4%). ‐ Thrombocytopenia: Placebo 9/81 (11.1%), LD 8/80 (10%), HD 7/77 (9.1%). ‐ Pleural effusion: Placebo 10/81 (12.3%), LD 14/80 (17.5%), HD 19/70 (27.4%). ‐ Stridor: Placebo 10/81 (12.3%), LD 10/80 (12.5%), HD 10/77 (13%). |
|
| Notes | Cutoffs for outcome variables (tachycardia, oliguria, cold extremities) were determined by the principal investigator (not reported in detail), the composite endpoint was later reviewed by an adjudication committee. Comparison of milrinone with placebo. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Performed by commercial CRO, no additional information available. |
| Allocation concealment (selection bias) | Unclear risk | Performed by commercial CRO, no additional information available. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Same infusion rate for all study drugs. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded clinical end point committee. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop out of a total of 33 participants at different stages (treatment groups not always known), calculation of outcomes using different populations. |
| Selective reporting (reporting bias) | High risk | Outcomes are only partially reported as absolute numbers or percentages. The review authors contacted the study authors and obtained additional information. However, due to the long time elapsed since the study had been conducted, many outcomes could not be comprehensively reconstructed. |
| Other bias | High risk | Study supported by a grant from the manufacturer (Sanofi Synthelabo) and reports co‐authored by manufacturer representatives. |
Lechner 2012.
| Methods | RCT (double‐blind) | |
| Participants | n = 40 (22 M, 18 F) Age: milrinone 63.9 (+/‐ 81.3) days, non‐milrinone 78.4 (+/‐ 80.5) days |
|
| Interventions | Milrinone group: n = 20: infusion at 0.5 μg/kg/min for 24 hours, starting at the time of weaning from CPB. Non‐milrinone group: n = 19 (n = 20 were randomised, but one did not receive the study drug due to immediate reoperation): levosimendan infusion at 0.1 μg/kg/min for 24 hours, starting at the time of weaning from CPB. |
|
| Outcomes |
Mortality within 30 days: none. Time to death (censored after 3 months): not reported. LCOS within 30 days from surgery yes/no: no LCOS in either group until hospital discharge. Duration of ICU stay: Non‐milrinone group: mean (SD) 6.88 (+/‐ 3.14); Milrinone group: mean (SD) 9.2 (+/‐ 9.19) days Duration of hospital stay: Non‐milrinone group: mean (SD) 15.47 (+/‐ 6.93); Mirinon: mean (SD) 15.8 (+/‐ 9.1) days Duration of mechanical ventilation: Non‐milrinone group: 19 = median 4; interquartile range 3‐6 days; mean (SD) 4.53 (+/‐ 2.15) days; milrinone group: 20 = median 4 days, interquartile range 2‐8 days; mean (SD) 5.85 (+/‐ 6.16) days. For mean/interquartile range: P 0.95 (2‐sided), Mann‐Whitney‐U test. Inotrope score at 24 hours: Mean and SD: Non‐milrinone group: 19 = 4.26 (+/‐ 0.46); milrinone group: 20 = 5.7 (+/‐ 0.43). Number of patients requiring MCS or HTX within 30 days from surgery: none Number/proportion of adverse events: ‐ Tachycardia: none ‐ Hypotension: Only figure available, not absolute numbers, for the non‐milrinone group (n = 19) and the milrinone group (n = 20). Mean values measurable from figure, but SEM markings not group‐specific. ‐ Intraventricular haemorrhage: none. Thrombosis (partial) of the sagittal sinus in 1 patient from the milrinone group. |
|
| Notes | Intention‐to‐treat: yes, plus per protocol analysis. 3 drop outs not included in per‐protocol‐analysis (1 did not receive non‐milrinone study drug due to immediate reoperation). Comparison of milrinone with levosimendan. Conclusions of the study authors: No difference due to low sample size, speculation that non‐milrinone study drug might have proven better otherwise. Follow‐up until hospital discharge. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Preparation and labelling of drugs by the local pharmacy on pharmacy premises. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Same infusion rates for both drugs, opaque black syringes and catheters. Record indicating the type of study drug was kept in a closed envelope in the patient’s chart to allow unblinding in case of emergency (never used throughout the study). All patients, parents, medical, and nursing staff members remained unaware of the study group assignments throughout the whole study period. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data were collected continuously before unblinding at the end of the study, statistical analysis by an external statistician. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors were contacted and asked for missing values. |
| Selective reporting (reporting bias) | Low risk | Study authors were contacted and asked for missing values. |
| Other bias | Low risk | |
Momeni 2011.
| Methods | RCT | |
| Participants | n = 40 (+ one randomised but did not receive the study drug (randomised to milrinone) due to a modification in the surgical plan). Ages: 0‐5 years allowed, but only 2 children in each group in the 1‐year‐and‐older age range. |
|
| Interventions |
Milrinone group: 18 (9M/9F) = Infusion of milrinone at 0.4 μg/kg/min for up to 48 hours, starting at the onset of CPB. Non‐milrinone group: 18 (6M/12F) = Infusion of levosimendan at 0.05 μg/kg/min for up to 48 hours, starting at the onset of CPB. Infusion rate ”was allowed to be doubled if haemodynamic and/or echocardiographic signs of poor ventricular function” were present, which applied to 9 patients in each group by the end of the operation. “The study drug could be stopped earlier in case of favourable clinical and haemodynamic parameters.” |
|
| Outcomes |
Primary endpoint = lactate levels 4 hours postoperatively. Mortality within 30 days: 2 drop outs in each group intraoperatively, no in‐hospital mortality in the 36 patients who completed the study Time to death (censored after 3 months): not reported, some patients left the country. LCOS within 30 days from surgery yes/no: not reported as a defined end point (items collected: HR, mean arterial blood pressure, serum lactate (until T8: 2 patients in each group, that is 4 patients in total, had a lactate level of more than 3 mmol/L. Until T9, no patient with a lactate level of more than 3 mmol/L), central venous oxygen saturation, arterial to venous oxygen saturation difference (from this, patients in whom an atrial shunt was created were excluded, see page 423), urine output (given only as an average over 48 hours). Duration of ICU stay: Non‐milrinone group: median 7 days, range 2‐15 days, non‐normal distribution with mean 7 and SD 4 days. Milrinone group: median 3 days, range 2‐20 days, non‐normal distribution with mean 5 and SD 4 days. For median/range P = 0.17 (2‐tailed). Duration of hospital stay: Non‐milrinone group: median 13 days, range 7‐42 days, non‐normal distribution with mean 16 and SD 10 days. Milrinone group: median 13 days, range 8‐32 days, non‐normal distribution with mean 15 and SD 7 days. For median/range P = 0.96 (2‐tailed). Duration of mechanical ventilation: Non‐milrinone group: median 77 hours, range 2‐167 hours, non‐normal distribution with mean 77 hours and SD 57 hours. Milrinone group: median 34 hours, range 3‐237 hours, non‐normal distribution with mean 51 and SD 59 hours. For median/range P = 0.11 (2‐tailed). Inotrope score: not reported. Number of patients requiring MCS or HTX within 30 days from surgery: 2 patients (1 in each group) required MCS. No HTX. Number/proportion of adverse events: no arrhythmias. |
|
| Notes | Intention‐to‐treat: for the 36 patients who received the study drug and were alive and not on MCS after weaning from CPB. Duration of ICU stay, of hospital stay, and of mechanical ventilation: analysis of 36 patients (18 per group) each, including 3 patients from the milrinone group (including 1 who did not receive milrinone for at least 4 hours in the ICU) and 2 patients from the levosimendan group who did not complete 48 hours of study drug infusion. Comparison of milrinone with levosimendan. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Concealment of the allocation code “in an envelope that was opened by the study nurse who was also in charge of preparing the medication” but who did not participate in patient care and who did not analyse the data. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Same infusion rates for both drugs (1 mL/hour), syringes and tubing system covered with aluminium foil to ensure the blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Separate personnel for study drug preparation and blinded outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and exclusions are reported: 1 drop out in each group requiring ECMO at the end of cardiopulmonary bypass, 1 drop out in each group who "died intraoperatively because of unexpected anatomic cardiac lesions and severe myocardial dysfunction”. No re‐inclusions by the review authors in analysis. |
| Selective reporting (reporting bias) | Low risk | Study authors were contacted and asked for missing values. |
| Other bias | Low risk | Departmental funding. |
Pellicer 2013.
| Methods | RCT | |
| Participants | n = 20 (11M/9F) Age 6‐34 days |
|
| Interventions | Milrinone: 9 = Infusion starting at 0.5 μg/kg/min for the duration of surgery, starting when the central lines were placed (dose 1), increase to 0.75 μg/kg/min at the time of admission to the ICU (dose 2) for 2 hours, increase to 1 μg/kg/min 2 hours after ICU admission. Infusion of milrinone for 48 hours, followed by unblinding and open‐label infusion if needed. Non‐milrinone: 11 = Levosimendan infusion at 0.1 μg/kg/min for the duration of surgery, starting when the central lines were placed (dose 1), increase to 0.15 μg/kg/min at the time of admission to the ICU (dose 2) for 2 hours, increase to 0.2 μg/kg/min 2h after ICU admission. Infusion of levosimendan for 48 hours. |
|
| Outcomes |
Mortality within 30 days: during first 6 days, 1 death in non‐milrinone group and 2 deaths in milrinone group (leaving 7 alive patients in milrinone and 10 alive patients in non‐milrinone group). Time to death (censored after 3 months): not reported LCOS within 30 days from surgery yes/no: not defined as a composite outcome (separate items: Central‐peripheral temperature gradient during the first 24 hours: milrinone 4.7 °C (SD 0.24), comparison ‐0.42 °C (SD 0.32), P = 0.021. Lactate (mmol/L): (figure only)). Duration of ICU stay: not reported. Duration of hospital stay: not reported. Duration of mechanical ventilation: not reported. Inotrope score: (figure only). Number of patients requiring MCS or HTX within 30 days from surgery: 1 MCS in the non‐milrinone group. Number/proportion of adverse events: ‐ Arrhythmias: surgery‐related third degree AV block requiring pacemaker in 1 patient in each group, 1 patient from the comparison group with AV asynchrony at 48 hours post surgery. ‐ Tachycardia: none ‐ Hypotension: diastolic blood pressure reported ‐ LVFS < 28%: at baseline, at 24 hours, and at 48 hours (and also available for later, after un‐blinding): At baseline: none in either group. At 24 hours: milrinone group (n = 9) 33.3%, comparison group (n = 11) 27.3%. At 48 hours: milrinone group (n = 7) 14.3%, comparison group (n = 6) 16.7%. |
|
| Notes | Intention‐to‐treat: yes. Comparison of milrinone with levosimendan. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list. The randomisation was stratified by type of congenital heart defect and risk adjustment using the congenital heart surgery method (Jenkins KJ et al. J Thorac Cardiovasc Surg 2002;123:110‐8), with 3 strata (20% of patients in low‐risk, 60% in moderate‐low risk, 20% in moderate‐high risk). |
| Allocation concealment (selection bias) | Low risk | “A study nurse who was not involved in the clinical care of the infants prepared the study medication and was the custodian of the allocation code.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Same infusion rates for both drugs, opaque black syringes and catheters. Drugs were prepared by a study nurse who was not involved with the care of the patients. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for the first 48 hours while blinded according to the study protocol (including the 48 hours measurements). Then high risk while continued as open study, follow‐up until 6 days post surgery. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors were contacted and asked for additional data. |
| Selective reporting (reporting bias) | Low risk | Study authors were contacted and asked for additional data. |
| Other bias | Low risk | The manufacturer of the non‐milrinone intervention (comparison intervention, levosimendan, Orion Pharma Spanish Division, Esmoo, Finland) supported the pharmacokinetic part of the study financially. Not deemed an important source of bias because this financial support was restricted to pharmacokinetics instead of the RCT itself. |
AET: atrial ectopic tachycardia, AV: atrio‐ventricular CPB: cardiopulmonary bypass CRO: contract research organisation ECMO: extracorporeal membrane oxygenation HD: high dose HTX: heart transplantation ICU: intensive care unit JET: junctional ectopic tachycardia LCOS: low cardiac output syndrome LD: low dose LVEF: left ventricular ejection fraction LVFS: left ventricular fractional shortening MCS: mechanical circulatory support RCT: randomised controlled trial SD: standard deviation SEM: standard error of the mean SVT: supraventricular tachycardia
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bailey 2004 | Pharmacokinetics of an included study (Hoffman 2003) without additional information regarding LCOS outcome. |
| Cai 2008a | Non‐prophylactic use of milrinone. Milrinone used for different indication. |
| Cai 2008b | Non‐prophylactic use of milrinone. Milrinone used for different indication. |
| Carmona 2010 | Adults only. |
| Doolan 1997 | Adults only. |
| Farah 2013 | Different outcomes: pulmonary artery pressures. |
| Khazin 2004 | Different outcomes: pulmonary artery pressures and blood gases. |
| Maslow 2004 | Adults only. |
| Oztekin 2007 | Adults only. |
| Ricci 2012 | Use of milrinone in both intervention (levosimendan) and placebo groups. |
| Thul 1999 | Non‐prophylactic use of milrinone. |
| Zuppa 2006 | Different outcomes: pharmacokinetics. |
LCOS: low cardiac output syndrome
Characteristics of studies awaiting assessment [ordered by study ID]
Costello 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | Not known |
Differences between protocol and review
More specified definition of intervention (addition in upper case characters): "Prophylactic milrinone intravenous infusion alone or combined with other inotrope medications and/or vasopressin and/or calcium sensitisers and/or nitric oxide, started within 6 hours of surgery for congenital heart disease and irrespective of the administration protocol, provided that milrinone bolus doses, IF ANY, are at least 25 μg/kg and continuous infusion rates are at least 0.2 μg/kg/min, and that milrinone is administered for a duration of at least 4 hours."
Contributions of authors
BEUB, BS, and GR: conceived and designed the review.
BEUB and BS: extracted and interpreted data.
GR: provided methodological input, analysed data.
BEUB: wrote the protocol and the review.
BS: revised the review critically for important intellectual content.
All authors read and approved the final version of the review.
Sources of support
Internal sources
Department of Congenital Heart Disease and Paediatric Cardiology, University Hospital Freiburg, Germany.
External sources
No sources of support supplied
Declarations of interest
Barbara E.U. Burkhardt: Joined the institution where one of the trials had been conducted (Cavigelli 2013) seven months after identifying the study for this review in a clinical trial database. The study had already been finished by then, and this author was not involved in its conduct. Eight months later, it was published as an abstract, again without this author's involvement.
Brigitte Stiller: none known.
Gerta Rücker: none known.
New
References
References to studies included in this review
Cavigelli 2013 {published and unpublished data}
- Cavigelli‐Brunner A, Hug MI, Dave H, Cannizzaro V, Baenziger O, Buerki C, et al. Therapy of low cardiac output syndrome after cardiac surgery in infants and children: a double blind randomized study comparing dobutamine and milrinone. Cardiology in the Young. May 2013; Vol. 23, Supplement S1:S34; O11‐3.
Hoffman (HD vs. placebo) {published and unpublished data}
- Hoffman TM, Wernovsky G, Atz AM, Bailey JM, Akbary A, Kocsis JF, et al. Prophylactic intravenous use of milrinone after cardiac operation in pediatrics (PRIMACORP) study. American Heart Journal 2002;143(1):15‐21. [DOI] [PubMed] [Google Scholar]
- Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation 2003;107(7):996‐1002. [DOI] [PubMed] [Google Scholar]
Hoffman (LD vs. placebo) {published and unpublished data}
- Hoffman TM, Wernovsky G, Atz AM, Bailey JM, Akbary A, Kocsis JF, et al. Prophylactic intravenous use of milrinone after cardiac operation in pediatrics (PRIMACORP) study. American Heart Journal 2002;143(1):15‐21. [DOI] [PubMed] [Google Scholar]
- Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation 2003;107(7):996‐1002. [DOI] [PubMed] [Google Scholar]
Hoffman 2003 {published and unpublished data}
- Hoffman TM, Wernovsky G, Atz AM, Bailey JM, Akbary A, Kocsis JF, et al. Prophylactic intravenous use of milrinone after cardiac operation in pediatrics (PRIMACORP) study.. American Heart Journal 2002;143(1):15‐21. [DOI] [PubMed] [Google Scholar]
- Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation 2003;107(7):996‐1002. [DOI] [PubMed] [Google Scholar]
Lechner 2012 {published and unpublished data}
- Lechner E, Hofer A, Leitner‐Peneder G, Freynschlag R, Mair R, Weinzettel R, et al. Levosimendan versus milrinone in neonates and infants after corrective open‐heart surgery: a pilot study. Pediatric Critical Care Medicine 2012;13(5):542‐8. [DOI] [PubMed] [Google Scholar]
Momeni 2011 {published data only}
- Momeni M, Rubay J, Matta A, Rennotte MT, Veyckemans F, Poncelet AJ, et al. Levosimendan in congenital cardiac surgery: a randomized, double‐blind clinical trial. Journal of Cardiothoracic and Vascular Anesthesia 2011;25(3):419‐24. [DOI] [PubMed] [Google Scholar]
Pellicer 2013 {published data only}
- Pellicer A, Riera J, Lopez‐Ortego P, Bravo MC, Madero R, Perez‐Rodriguez J, et al. Phase 1 study of two inodilators in neonates undergoing cardiovascular surgery. Pediatric Research 2013;73(1):95‐103. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bailey 2004 {published data only}
- Bailey JM, Hoffman TM, Wessel DL, Nelson DP, Atz AM, Chang AC, et al. A population pharmacokinetic analysis of milrinone in pediatric patients after cardiac surgery. Journal of Pharmacokinetics and Pharmacodynamics 2004;31(1):43‐59. [DOI] [PubMed] [Google Scholar]
Cai 2008a {published data only}
- Cai J, Su Z, Shi Z, Zhou Y, Xu Z, Xu Z, et al. Nitric oxide and milrinone: combined effect on pulmonary circulation after Fontan‐type procedure: a prospective, randomized study. Annals of Thoracic Surgery 2008;86(3):882‐8. [DOI] [PubMed] [Google Scholar]
Cai 2008b {published data only}
- Cai J, Su Z, Shi Z, Zhou Y, Xu Z, Liu J, et al. Nitric oxide in conjunction with milrinone better stabilized pulmonary hemodynamics after Fontan procedure. Artificial Organs 2008;32(11):864‐9. [DOI] [PubMed] [Google Scholar]
Carmona 2010 {published data only}
- Carmona MJ, Martins LM, Vane MF, Longo BA, Paredes LS, Malbouisson LM. Comparison of the effects of dobutamine and milrinone on hemodynamic parameters and oxygen supply in patients undergoing cardiac surgery with low cardiac output after anesthetic induction. Revista Brasileira de Anestesiologia 2010;60(3):237‐46. [DOI] [PubMed] [Google Scholar]
Doolan 1997 {published data only}
- Doolan LA, Jones EF, Kalman J, Buxton BF, Tonkin AM. A placebo‐controlled trial verifying the efficacy of milrinone in weaning high‐risk patients from cardiopulmonary bypass. Journal of Cardiothoracic and Vascular Anesthesia 1997;11(1):37‐41. [DOI] [PubMed] [Google Scholar]
Farah 2013 {published data only}
- Farah P, Ahmad‐Ali A, Hanane G, Abbas E. Additive effect of phosphodiesterase inhibitors in control of pulmonary hypertension after congenital cardiac surgery in children. Iranian Journal of Pediatrics 2013;23(1):19‐26. [PMC free article] [PubMed] [Google Scholar]
Khazin 2004 {published data only}
- Khazin V, Kaufman Y, Zabeeda D, Medalion B, Sasson L, Schachner A, et al. Milrinone and nitric oxide: combined effect on pulmonary artery pressures after cardiopulmonary bypass in children. Journal of Cardiothoracic and Vascular Anesthesia 2004;18(2):156‐9. [DOI] [PubMed] [Google Scholar]
Maslow 2004 {published data only}
- Maslow AD, Regan MM, Schwartz C, Bert A, Singh A. Inotropes improve right heart function in patients undergoing aortic valve replacement for aortic stenosis. Anesthesia and Analgesia 2004;98(4):891‐902. [DOI] [PubMed] [Google Scholar]
Oztekin 2007 {published data only}
- Oztekin I, Yazici S, Oztekin DS, Goksel O, Issever H, Canik S. Effects of low‐dose milrinone on weaning from cardiopulmonary bypass and after in patients with mitral stenosis and pulmonary hypertension. Yakugaku Zasshi 2007;127(2):375‐83. [DOI] [PubMed] [Google Scholar]
Ricci 2012 {published data only}
- Ricci Z, Garisto C, Favia I, Vitale V, Chiara L, Cogo PE. Levosimendan infusion in newborns after corrective surgery for congenital heart disease: randomized controlled trial. Intensive Care Medicine 2012;38(7):1198‐204. [DOI] [PubMed] [Google Scholar]
Thul 1999 {published data only}
- Thul J, Hundt F, Will J, Schindler F, Akintürk H, Valeske K, et al. Enoximon vs. milrinon by myocardial dysfunction after cardio‐surgical operations in infants. Zeitschrift fur Kardiologie 1999;88(9):758. [CN‐00340079] [Google Scholar]
Zuppa 2006 {published data only}
- Zuppa AF, Nicolson SC, Adamson PC, Wernovsky G, Mondick JT, Burnham N, et al. Population pharmacokinetics of milrinone in neonates with hypoplastic left heart syndrome undergoing stage I reconstruction. Anesthesia and Analgesia 2006;102(4):1062‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Costello 2014 {published data only}
- Costello JM, Dunbar‐Masterson C, Allan CK, Gauvreau K, Newburger JW, McGowan FX Jr, et al. Impact of empiric nesiritide or milrinone infusion on early postoperative recovery after Fontan surgery: a randomized, double‐blind, placebo‐controlled trial. Circulation Heart Failure 2014;7(4):596‐604. [DOI] [PubMed] [Google Scholar]
- Costello JM, Masterson CD, Allan CK, Gauvreau K, Newburger JW, McGowan FX, et al. Impact of empiric nesiritide or milrinone infusion on early postoperative recovery following fontan surgery: A randomized, double‐blind, placebo‐controlled clinical trial. Circulation. 2013; Vol. 128(22 suppl):A12099. [DOI] [PubMed]
Additional references
Abraham 2005
- Abraham WT, Adams KF, Fonarow GC, Costanzo MR, Berkowitz RL, LeJemtel TH, et al. In‐hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). Journal of the American College of Cardiology 2005;46(1):57‐64. [DOI] [PubMed] [Google Scholar]
Allen 2014
- Allen LA, Fonarow GC, Grau‐Sepulveda MV, Hernandez AF, Peterson PN, Partovian C, et al. Hospital variation in intravenous inotrope use for patients hospitalized with heart failure: insights from Get With The Guidelines. Circulation Heart Failure 2014;7(2):251‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alousi 1986
- Alousi AA, Johnson DC. Pharmacology of the bipyridines: amrinone and milrinone. Circulation 1986;73(3 Pt 2):III10‐24. [PubMed] [Google Scholar]
Anonymous 2011
- Anonymous. Heads of Medicines Agencies EU worksharing procedure for assessment of paediatric data for milrinone. http://www.hma.eu/fileadmin/dateien/Human_Medicines/CMD_h_/Paediatric_Regulation/Assessment_Reports/Article_45_work‐sharing/Milrinone‐_2011_06_Art.45PaedPdAR.pdf 06/07/2011.
Bailey 1999
- Bailey JM, Miller BE, Lu W, Tosone SR, Kanter KR, Tam VK. The pharmacokinetics of milrinone in pediatric patients after cardiac surgery. Anesthesiology 1999;90(4):1012‐8. [DOI] [PubMed] [Google Scholar]
Bassler 2006
- Bassler D, Choong K, McNamara P, Kirpalani H. Neonatal persistent pulmonary hypertension treated with milrinone: four case reports. Biology of the Neonate 2006;89(1):1‐5. [DOI] [PubMed] [Google Scholar]
Baysal 2010
- Baysal A, Saşmazel A, Yıldırım Aİ, Koçak T, Sunar H, Zeybek R. The effects of thyroid hormones and interleukin‐8 levels on prognosis after congenital heart surgery [Doğuştan kalp cerrahisinde tiroit fonksiyonları ve interlökin‐8 değerlerinin prognoz üzerine etkisi.]. Turk Kardiyoloji Dernegi Arsivi 2010;38(8):537‐43. [PubMed] [Google Scholar]
Bohn 2011
- Bohn D. Objective assessment of cardiac output in infants after cardiac surgery. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual 2011;14(1):19‐23. [DOI] [PubMed] [Google Scholar]
Charpie 2000
- Charpie JR, Dekeon MK, Goldberg CS, Mosca RS, Bove EL, Kulik TJ. Serial blood lactate measurements predict early outcome after neonatal repair or palliation for complex congenital heart disease. Journal of Thoracic and Cardiovascular Surgery 2000;120(1):73‐80. [DOI] [PubMed] [Google Scholar]
Delmo Walter 2010
- Delmo Walter EM, Alexi‐Meskishvili V, Huebler M, Loforte A, Stiller B, Weng Y, et al. Extracorporeal membrane oxygenation for intraoperative cardiac support in children with congenital heart disease. Interactive Cardiovascular and Thoracic Surgery 2010;10(5):753‐8. [DOI] [PubMed] [Google Scholar]
Dilber 2010
- Dilber D, Malcić I. Spectrum of congenital heart defects in Croatia. European Journal of Pediatrics 2010 May;169(5):543‐50. [DOI] [PubMed] [Google Scholar]
EACTS 2011
- EACTS Congenital Database [European Association for Cardio‐Thoracic Surgery Congenital Database]. http://www.eactscongenitaldb.org 31.08.2011.
el Allaf 1984
- Allaf D, D'Orio V, Carlier J. The new inotropic phosphodiesterase inhibitors. Archives of Physiology and Biochemistry 1984;92(4):S69‐79. [DOI] [PubMed] [Google Scholar]
Ermis 2011
- Ermis PR, Morales DL. The adult Fontan patient: update for 2011. Methodist Debakey Cardiovascular Journal 2011;7(2):3‐8. [DOI] [PubMed] [Google Scholar]
Feneck 1992
- Feneck RO. Intravenous milrinone following cardiac surgery: I. Effects of bolus infusion followed by variable dose maintenance infusion. The European Milrinone Multicentre Trial Group. Journal of Cardiothoracic and Vascular Anesthesia 1992;6(5):554‐62. [DOI] [PubMed] [Google Scholar]
Fleming 2008
- Fleming GA, Murray KT, Yu C, Byrne JG, Greelish JP, Petracek MR, et al. Milrinone use is associated with postoperative atrial fibrillation after cardiac surgery. Circulation 2008;118(16):1619‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Follath 2011
- Follath F, Yilmaz MB, Delgado JF, Parissis JT, Porcher R, Gayat E, et al. Clinical presentation, management and outcomes in the Acute Heart Failure Global Survey of Standard Treatment (ALARM‐HF). Intensive Care Medicine 2011;37(4):619‐26. [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hochman 1999
- Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. New England Journal of Medicine 1999;341(9):625‐34. [DOI] [PubMed] [Google Scholar]
Huntsman 1983
- Huntsman LL, Stewart DK, Barnes SR, Franklin SB, Colocousis JS, Hessel EA. Noninvasive Doppler determination of cardiac output in man. Clinical validation. Circulation 1983;67(3):593‐602. [DOI] [PubMed] [Google Scholar]
Jeon 2006
- Jeon Y, Ryu JH, Lim YJ, Kim CS, Bahk JH, Yoon SZ, et al. Comparative hemodynamic effects of vasopressin and norepinephrine after milrinone‐induced hypotension in off‐pump coronary artery bypass surgical patients. European Journal of Cardiothoracic Surgery 2006;29(6):952‐6. [DOI] [PubMed] [Google Scholar]
Kim 2006
- Kim J, Dreyer J, Chang A. Arterial pulse wave analysis: an accurate means of determining cardiac output in children. Pediatric Critical Care Medicine 2006;7:532–5. [DOI] [PubMed] [Google Scholar]
Lefebvre, 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lemson 2008
- Lemson J, Boode WP, Hopman JC, Singh SK, Hoeven JG. Validation of transpulmonary thermodilution cardiac output measurement in a pediatric animal model. Pediatric Critical Care Medicine 2008;9(3):313‐9. [DOI] [PubMed] [Google Scholar]
Lindinger 2010
- Lindinger A, Schwedler G, Hense HW. Prevalence of congenital heart defects in newborns in Germany: Results of the first registration year of the PAN Study (July 2006 to June 2007) [Prävalenz angeborener Herzfehler bei Neugeborenen in Deutschland: Ergebnisse des ersten Geburtsjahrganges (Juli 2006 bis Juni 2007)]. Klinische Pädiatrie 2010;222(5):321‐6. [DOI] [PubMed] [Google Scholar]
MHRA 2005
- Medicines and Healthcare Products Regulatory Agency (UK), Paediatric Working Party ‐ Committee on Safety of Medicines. Review of paediatric data ‐ Primacor Injection. http://www.mhra.gov.uk/home/groups/pl‐p/documents/websiteresources/con2030490.pdf 2005, Apr 27; Vol. Accessed 2014, Sep 11.
Moffett 2015
- Moffett BS, Price JF. National prescribing trends for heart failure medications in children. Congenital Heart Disease 2015;10(1):78‐85. [DOI: 10.1111/chd.12183] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nieminen 2006
- Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. European Heart Journal 2006;27(22):2725‐36. [DOI] [PubMed] [Google Scholar]
Paradisis 2009
- Paradisis M, Evans N, Kluckow M, Osborn D. Randomized trial of milrinone versus placebo for prevention of low systemic blood flow in very preterm infants. Journal of Pediatrics 2009;154(2):189‐95. [DOI] [PubMed] [Google Scholar]
Pasquali 2008
- Pasquali SK, Hall M, Slonim AD, Jenkins KJ, Marino BS, Cohen MS, et al. Off‐label use of cardiovascular medications in children hospitalized with congenital and acquired heart disease. Circulation: Cardiovascular Quality and Outcomes 2008;1(2):74‐83. [DOI] [PubMed] [Google Scholar]
R 2013 [Computer program]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna 2013. Vienna: R Foundation, 2013.
Ramamoorthy 1998
- Ramamoorthy C, Anderson GD, Williams GD, Lynn AM. Pharmacokinetics and side effects of milrinone in infants and children after open heart surgery. Anesthesia and Analgesia 1998;86(2):283‐9. [DOI] [PubMed] [Google Scholar]
Rao 1996
- Rao V, Ivanov J, Weisel RD, Ikonomidis JS, Christakis GT, David TE. Predictors of low cardiac output syndrome after coronary artery bypass. Journal of Thoracic and Cardiovascular Surgery 1996;112(1):38‐51. [DOI] [PubMed] [Google Scholar]
Rücker 2011
- Rücker G, Schwarzer G, Carpenter JR, Binder H, Schumacher M. Treatment‐effect estimates adjusted for small‐study effects via a limit meta‐analysis. Biostatistics 2011;12(1):122‐42. [DOI] [PubMed] [Google Scholar]
Sanofi Aventis 2010
- Sanofi Aventis. Drug reference Corotrop® (Milrinone) [Fachinformation Corotrop®]. Rote Liste® Service GmbH • Mainzer Landstr. 55 • 60329 Frankfurt/Main August 2010.
Schwarzer 2007
- Schwarzer G. meta: An R package for Meta‐Analysis. R News 2007;7(3):40‐5. [Google Scholar]
Schwarzer 2010
- Schwarzer G, Rücker G. Statistical methods for detecting and adjusting for publication bias. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen (ZEFQ) 2010;104(4):306‐13. [DOI] [PubMed] [Google Scholar]
Shi 2008
- Shi S, Zhao Z, Liu X, Shu Q, Tan L, Lin R, et al. Perioperative risk factors for prolonged mechanical ventilation following cardiac surgery in neonates and young infants. Chest 2008;134(4):768‐74. [DOI] [PubMed] [Google Scholar]
Shore 2001
- Shore S, Nelson DP, Pearl JM, Manning PB, Wong H, Shanley TP, et al. Usefulness of corticosteroid therapy in decreasing epinephrine requirements in critically ill infants with congenital heart disease. American Journal of Cardiology 2001;88(5):591‐4. [DOI] [PubMed] [Google Scholar]
Stocker 2006
- Stocker CF. Recent developments in the perioperative management of the paediatric cardiac patient. Current Opinion in Anaesthesiology 2006;19(4):375‐81. [DOI] [PubMed] [Google Scholar]
Stocker 2007
- Stocker CF, Shekerdemian LS, Nørgaard MA, Brizard CP, Mynard JP, Horton SB, et al. Mechanisms of a reduced cardiac output and the effects of milrinone and levosimendan in a model of infant cardiopulmonary bypass. Critical Care Medicine 2007;35(1):252‐9. [DOI] [PubMed] [Google Scholar]
Sweeting 2004
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23(9):1351‐75. [DOI] [PubMed] [Google Scholar]
Teng 2011
- Teng S, Kaufman J, Pan Z, Czaja A, Shockley H, Cruz E. Continuous arterial pressure waveform monitoring in pediatric cardiac transplant, cardiomyopathy and pulmonary hypertension patients. Intensive Care Medicine 2011;37(8):1297‐301. [DOI] [PubMed] [Google Scholar]
Tibby 2002
- Tibby SM, Murdoch IA. Measurement of cardiac output and tissue perfusion. Current Opinion in Pediatrics 2002;14:303–9. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vogt 2011a
- Vogt W, Läer S. Treatment for paediatric low cardiac output syndrome: results from the European EuLoCOS‐Paed survey. Archives of Disease in Childhood 2011;96(12):1180‐6. [DOI] [PubMed] [Google Scholar]
Vogt 2011b
- Vogt W, Läer S. Prevention for pediatric low cardiac output syndrome: results from the European survey EuLoCOS‐Paed. Paediatric Anaesthesia 2011;21(12):1176‐84. [DOI] [PubMed] [Google Scholar]
Warnes 2001
- Warnes CA, Liberthson R, Danielson GK, Dore A, Harris L, Hoffman JI, et al. Task force 1: the changing profile of congenital heart disease in adult life. Journal of the American College of Cardiology 2001;37(5):1170‐5. [DOI] [PubMed] [Google Scholar]


