Abstract
Normal cortical growth and the resulting folding patterns are crucial for normal brain function. Although cortical development is largely influenced by genetic factors, environmental factors in fetal life can modify the gene expression associated with brain development. As the placenta plays a vital role in shaping the fetal environment, affecting fetal growth through the exchange of oxygen and nutrients, placental oxygen transport might be one of the environmental factors that also affect early human cortical growth. In this study, we aimed to assess the placental oxygen transport during maternal hyperoxia and its impact on fetal brain development using MRI in identical twins to control for genetic and maternal factors. We enrolled 9 pregnant subjects with monochorionic diamniotic twins (30.03 ± 2.39 gestational weeks [mean ± SD]). We observed that the fetuses with slower placental oxygen delivery had reduced volumetric and surface growth of the cerebral cortex. Moreover, when the difference between placenta oxygen delivery increased between the twin pairs, sulcal folding patterns were more divergent. Thus, there is a significant relationship between placental oxygen transport and fetal brain cortical growth and folding in monochorionic twins.
Keywords: cortical folding, monochorionic twins, fetal brain, MRI, placental oxygen transport
Introduction
Folding of the cerebral cortex begins in utero, and it is a critical milestone of human brain evolution. Cerebral cortical growth and the resulting folding patterns are related to cognitive abilities and sensorimotor skills (Sun and Hevner 2014). Disrupted cortical folding is associated with a range of cognitive deficits and many developmental brain disorders (Molko et al. 2003; Nakamura et al. 2007; Cykowski et al. 2008; Kim et al. 2008; Shim et al. 2009; Barkovich et al. 2012; Bae et al. 2014). It is well known that both genetic and environmental factors impact cortical folding. However, most studies have explored the role of genetic factors under normal intrauterine conditions (Rakic 2004; O’Leary et al. 2007; Sun and Hevner 2014; de Juan et al. 2015; Llinares-Benadero and Borrell 2019), with little knowledge about the impact of an altered intrauterine environment on cortical development (Lo et al. 2018). Given that gene expression associated with cortical development can be modified by environmental factors in fetal life, it is likely that both the fetal genotype and intrauterine environment contribute to early cortical growth and folding.
The placenta serves as the critical interface between the environment and the fetus, which is responsible for the efficient transfer of nutrients—amino acids, glucose, and oxygen in particular—from the maternal uterine circulation to the fetus. Placental insufficiency causes fetal malnutrition, chronic fetal hypoxia, and an altered endocrine status, which can affect fetal brain development and results in alterations of neuronal connectivity and myelination, leading to long-term cognitive deficits (Tolsa et al. 2004; Rees et al. 2008; Miller et al. 2016; Malhotra et al. 2017). Human brain specimens who experienced a reduced cerebral oxygen supply during gestation showed impaired neurogenesis and reduced cortical surface growth in subregions of the frontal cortex, a site involved in high-order cognitive functions (Morton et al. 2017). Disrupted cortical neuronal connectivity and modified gene expression affected by intrauterine environmental factors may also result in atypical patterning of cortical functional areas, possibly causing disrupted cortical growth and folding patterns (Klyachko and Stevens 2003; Fischl et al. 2008; Tarui et al. 2018). However, there has been no detailed quantitative analysis assessing the effects of the fetal environment mediated by placental function on fetal brain cortical growth and folding. It is largely unknown how placental function influences early cortical growth and folding, which cortical regions are more influenced, and how cortical structures are altered. Examining these questions will be a first important step in identifying nongenetic, potentially modifiable, prenatal environmental factors that influence cortical growth and folding.
BOLD MRI with alternating maternal oxygenation has gained attention as a promising noninvasive method to monitor placental function in vivo (Sørensen et al. 2013; Sørensen, Peters, Simonsen, et al. 2013; Luo et al. 2017). In Luo et al.(Luo et al. 2017), a new measure, time to plateau (TTP), was defined as a relative regional quantification of placental oxygen transport by calculating the time required for the BOLD signal change due to maternal hyperoxia to reach a plateau. In the same study, the relationship between placental TTP and measures of fetal growth (e.g. brain volume and birth weight) were investigated. A significant correlation between the average placental TTP and whole-brain volume was observed, with larger placental TTP value associated with a smaller brain size. However, it is still unclear if the differences in placental oxygen transport altered the fetal cortical brain volume and folding.
In this study, we aimed to examine the relationship between placental oxygen transport using TTP and fetal brain development within monochorionic twins so that we could control for genetic and maternal factors. Global and lobar regional cortical surface growth and arrangement/patterning of primary sulci were measured to examine fetal cortical development, and these measures, as well as TTP, were compared between twin fetuses.
Materials and methods
Participants and MR image acquisition
All experimental protocols were approved by the Institutional Review Board (IRB) at the Boston Children’s Hospital. Informed consent was obtained from all subjects, and all methods were carried out in accordance with the guidelines and regulations of IRB. We enrolled 9 pregnant subjects with monochorionic diamniotic twins, whose gestational age ranged from 28 to 34 weeks with no apparent medical complication on fetuses (controls) and with the diagnosis of selective fetal growth restriction (sFGR; i.e. estimated fetal weight <10th percentile for 1 twin and weight discordance between twin pairs ≥25% based upon obstetrical ultrasound findings). Of the 9 subjects, 5 subjects were diagnosed with sFGR based upon their obstetrical ultrasound in the second trimester and no brain sparing effect was reported. We excluded subjects with twin-to-twin transfusion syndrome, fetal anomalies, maternal hypertension, and gestational or preexisting diabetes. The maternal age ranged from 28 to 43 with the median age of 33, and the maternal body mass index (BMI) ranged from 23 to 28 with the median BMI of 26.9 (Table 1).
Table 1.
Subject details.
| ID | GA | Sex | Maternal age | BMI | Diagnosis |
|---|---|---|---|---|---|
| S1 | 31w2d | F | 43 | 27.28 | control |
| S2 | 34w1d | M | 28 | 26.57 | sFGR |
| S3 | 28w1d | F | 32 | 23.2 | sFGR |
| S4 | 28w | F | 37 | 28 | sFGR |
| S5 | 33w | M | 33 | 27.98 | sFGR |
| S6 | 31w | F | 32 | 26.16 | sFGR |
| S7 | 32w5d | M | 34 | 27.49 | control |
| S8 | 27w5d | F | 31 | 24.1 | control |
| S9 | 27w4d | M | 34 | 21.41 | control |
We acquired MRI data on a 3 T Skyra scanner (Siemens Healthineers, Erlangen, Germany) using a combined 18-channel body and 12-channel spine-receive arrays, while the subject remained in left lateral position. Before BOLD imaging, half-Fourier acquisition single-shot turbo spin echo (HASTE) images (2 × 2 × 5 mm3) of the whole uterus were acquired for anatomical reference. BOLD imaging of the whole uterus was performed using single-shot gradient echo planar imaging sequence with field-of-view and matrix size adjusted to achieve in-plane resolution of 3 × 3 mm2, slice thickness of 3 mm, interleaved; TR = 5–8 s, TE = 32–38 ms, FA = 90°, and BW = 2.3 kHz/px. The number of time frames was adjusted so that the total acquisition time was 30 min. SAR did not exceed 2 W/kg at any time. The maternal oxygenation was adjusted to provide 10 min of room air (21% O2), followed by 10 min of 100% FiO2, and then 10 min of room air via a nonrebreathing facial mask.
BOLD MRI processing and TTP measurement
We used our previously demonstrated pipeline to correct signal nonuniformities and mitigate motion in the BOLD MRI time series (Turk et al. 2017). We first estimated the bias field accounting for signal nonuniformities using 3D-N4 bias correction implementation in Advanced Normalization Tools registration suite (Avants et al. 2009; Tustison et al. 2010). This bias field correction was then applied to each frame. Then, all motion correction steps were carried out in Elastix, open-source image processing software (Klein et al. 2010). For intravolume motion correction, we separated each volume into 2 subvolumes, even and odd slices, given the interleaved design, and then registered to the other using the group-wise registration approach (Guyader et al. 2015). For intervolume motion correction within the uterus, we selected a reference volume with the least sum of mean square error difference compared to the rest of the volumes in the series. Then, we employed a pairwise nonrigid body transformation between the reference volume and the other volumes following an initial 6 degrees of freedom rigid transformation. We manually delineated regions of interest for the placenta and the uterus in the reference frame using ITK-SNAP (Yushkevich et al. 2006). We smoothed the corrected BOLD MRI time series spatially with a Gaussian kernel with a width of 5 pixels to improve the signal to noise ratio. The BOLD signal was converted to normalized R2* (i.e. normalized 1/T2*) as the indicator of oxygen saturation level changes as follows:
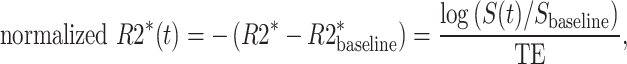 |
where t is time, S is the signal intensity, and TE is the echo time. Voxel-wise TTP in the placenta (i.e. the time required for the signal change due to maternal hyperoxia to reach a plateau) was estimated by fitting normalized R2* time series in placental voxels to a gamma variate function convolved with the oxygen paradigm, as described in Luo et al. (2017):
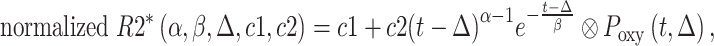 |
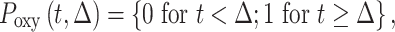 |
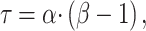 |
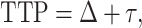 |
where α and β are gamma function parameters, Δ is the delay time of oxygen arrival, c1 is the baseline R2* signal, c2 is the amplitude of normalized R2* or change in R2* from baseline, t is time, and Poxy is a step function that describes the oxygen paradigm. Summation of Δ and the mode,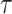 , of the gamma function across voxels give TTP (Fig. 1). To identify the placental regions belonging to each twin, initially placental cord insertions were detected by an experienced radiologist and then the regions were delineated by using the regional contrast differences, cotyledon structures around the cord insertions, and fetal positions according to the placenta. Average TTP values were then calculated by averaging the voxel-wise TTP estimates in placental regions for both twins and were categorized as lower and higher TTP per twin pairs for group comparisons.
, of the gamma function across voxels give TTP (Fig. 1). To identify the placental regions belonging to each twin, initially placental cord insertions were detected by an experienced radiologist and then the regions were delineated by using the regional contrast differences, cotyledon structures around the cord insertions, and fetal positions according to the placenta. Average TTP values were then calculated by averaging the voxel-wise TTP estimates in placental regions for both twins and were categorized as lower and higher TTP per twin pairs for group comparisons.
Fig. 1.
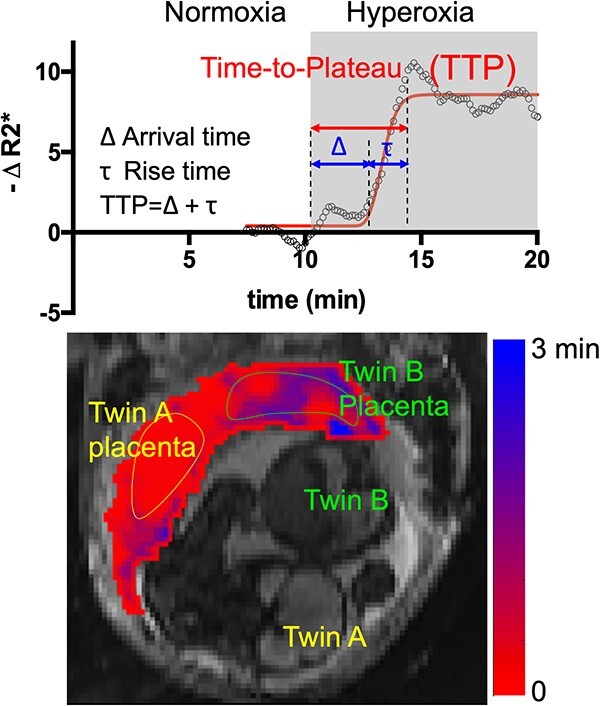
Voxel-wise dynamic BOLD MRI processing for TTP measurement and TTP map.
Fetal structural MRI processing and cortical surface reconstruction
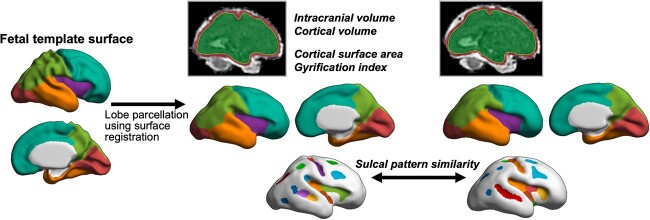
We used our pipeline for fetal MRI processing, which has been validated in several studies (Im et al. 2017; Ortinau et al. 2018 Oct 1; Tarui et al. 2018; Yun et al. 2020, 2021, 2022). Brain volume region (including ventricles) was extracted to exclude the maternal and nonbrain tissues from the raw fetal MRI using a 2D U-Net architecture (Ronneberger et al. 2015; Hong et al. 2021). The N4 bias field correction was performed to correct for intensity inhomogeneity (Tustison et al. 2010). We combined triplane data from multiple series to reconstruct motion-corrected volumes with isotropic super-resolution (0.75 mm) using slice-to-volume registration (Kuklisova-Murgasova et al. 2012). Among multiview images, low-quality images must be excluded for the best motion correction. We visually checked image quality and excluded the images with severe motion artifacts and then performed motion correction. We aligned the reconstructed volume images to fetal brain volume templates (Serag et al. 2012) using a linear registration tool (Jenkinson et al. 2002). The cortical plate was segmented using our deep learning method (Hong et al. 2020) and the mislabeled regions were manually edited. Then, we extracted the hemispheric inner cortical plate surface (boundary between cortical plate and subplate) as a triangular mesh. We geometrically smoothed the surfaces to reduce the noisy and small geometric features using Freesurfer (Fischl 2012).
Brain volume, cortical plate volume, surface area, and gyrification index
We measured brain volume and cortical plate volume, surface area, and 3D cortical gyrification index (GI) of the entire left and right cerebral hemispheres to estimate global cortical growth and folding (Fig. 2). Cortical plate volume was calculated from the segmentation volume and cortical surface area as the sum of the areas of all triangles making up the surface model. The GI is defined as the ratio of the whole cortical plate surface to their outer, convex hull surface area (Zilles et al. 1988). The inner volume of the cortical plate surface was isolated and constructed, and the 3D morphological closing operation was performed using a sphere of 15-mm diameter as the structural element to close the sulcal folding (Schaer et al. 2008; Im et al. 2017; Ortinau et al. 2018 Oct 1). The outer hull surface wrapping the cortical plate surface was created from the binary closed volume, and the 3D global GI of the cerebral hemispheres was calculated.
Fig. 2.
Fetal brain MRI processing and measures. Brain volume, cortical plate volume, cortical surface area, GI, and sulcal pattern similarity were measured from the brain and cortical plate regions and the reconstructed cortical surface model. Lobar regions were parcellated on individual cortical surfaces by alignment to the fetal brain template surface.
Lobe parcellation of cortical surface
For lobar regional surface area analysis, we manually parcellated and defined the left and right frontal, temporal, parietal, and occipital lobes on the template cortical surface of 31 gestational weeks reconstructed from the fetal brain templates (Serag et al. 2012). For lobar regional analysis, each individual fetal cortical surface and the template surface were aligned using 2D surface registration and cortical folding pattern matching that performs sphere-to-sphere warping (Robbins 2004; Lyttelton et al. 2007). Then, the lobar labels of the template were transformed and mapped onto the individual cortical surface (Fig. 2). The accuracy of surface registration in the fetal brain was validated in our previous study (Yun et al. 2019). We measured the cortical surface area for each lobar region.
Fetal body segmentation
We manually segmented the fetal body region in the reference BOLD MRI image after intravolume motion correction and the whole uterus HASTE image without motion correction using FreeView (Fischl 2012). Then, we counted the number of voxels and multiplied it by the voxel dimension for both images to estimate the fetal body volumes. For the subjects with the BOLD images with signal loss due to the susceptibility artifacts around the fetal region, we used the segmentation results from HASTE images.
Quantitative sulcal pattern similarity between twin pairs
We analyzed the global sulcal folding pattern using a sulcal pit-based graph structure (Im et al. 2011; Yun et al. 2019, 2020). Global patterning of the primary sulci was represented with a feature set of sulcal basins, including not only geometric and topological features (3D position, surface area, and mean sulcal depth of sulcal basin) but also the intersulcal relationships (intersulcal differences of position, area, and depth). Global sulcal pattern similarity can be quantitatively computed by comparing the sulcal feature set between different brain pairs using a spectral-based sulcal pattern matching and comparison technique, which ranges from 0 to 1 (Im et al. 2011, 2013, 2016, 2017; Ortinau et al. 2018, Oct 1; Tarui et al. 2018; Im and Grant 2019). After measuring the similarity with all features combined, we further calculated the similarity only using each individual feature by setting all weights of the other features to 0 to evaluate their relative impact on the composite sulcal pattern similarity. These methodological procedures are explained in more detail in our previous fetal MRI studies (Im et al. 2017; Ortinau et al. 2018, Oct 1; Tarui et al. 2018). We quantified the sulcal pattern similarities in the whole cortical area between twin pairs to quantify their difference in global cortical folding patterns according to different placental oxygen transports (Fig. 2).
Statistical analysis
To examine the relationship between placental oxygen transport and fetal cortical growth, we statistically compared the brain volume and hemisphere-specific cortical volume, surface area, and GI between fetal groups of low and high placental TTPs using a linear mixed-effects model. The group (higher vs. lower TTP relative to the other twin in the pair) was modeled as a fixed effect and the twin pairs was modeled as a random effect to account for within-pair correlation. For bilateral measures, the model also included fixed effects to assess and adjust for potential hemisphere effects (left–right differences) or group × hemisphere interaction (left–right variability in the effect of TTP). We employed iterative reweighting to detect and reduce the influence of extreme values (Rousseeuw and Leroy 1987). Since the change in the brain structure may be associated with the diagnosis of FGR, we analyzed the relationship between placental oxygen transport and metrics of fetal brain cortical growth using another mixed-effect model including and adjusting for the diagnosis of FGR. Fetal body volume may also be proportionally associated with the brain size and cortical volumetric and surface growth. We used an additional mixed-effect model including and adjusting for both the fetal body volume and the diagnosis of FGR to consider the effect of fetal body volume differences for the small fetuses without sFGR diagnosis.
We also assessed the association between sulcal pattern and placental transport function. The relationship between the sulcal pattern similarity and absolute TTP differences (i.e. absolute difference between average TTPs estimated in placental regions belonging to each twin pair) across all twin pairs was examined using correlation analysis, controlling for gestational age and the diagnosis of FGR. The correlations were tested with the 4 different feature sets (combined feature and position, depth, and area of sulcal basin) for the left and right hemispheres. P values < 0.05 were considered to be statistically significant. SPSS software version 28 (IBM, Armonk, NY, USA) and SAS version 9.4 (Cary, NC) were used for data analysis.
Results
To compare the cortical volume, surface area, GI, and lobar regional surface area between the low and high TTP groups, we used the diagnosis of FGR and fetal body volume to control for these effects in our statistical model. However, the whole fetal body was not covered in both BOLD and HASTE images in 1 twin pair (S5). Thus, we included 8 twin pairs in the group analyses. Fetuses with the higher TTP (mean ± SD = 1.423 ± 0.560 min) of the twin pair showed significantly decreased brain volume (P = 0.009), cortical volume (P = 0.0001), and cortical surface area (P < 0.0001) compared to paired fetus with the lower TTP (mean ± SD = 1.085 ± 0.568 min) in the unadjusted analysis. There was no significant difference in GI between the groups. When the diagnosis of FGR was adjusted, we still observed decreased brain volume (P = 0.049), cortical volume (P = 0.035), and cortical surface area (P < 0.0001) compared to paired fetus with the lower TTP, and when we adjusted for both the diagnosis and fetal body volume, a significant decrease in the cortical surface area was still observed in the higher TTP group (P = 0.002) (Table 2).
Table 2.
Comparison of brain volume and hemisphere-specific cortical volume, surface area, and GI between twins with higher and lower TTP with statistical findings.
| Measure | Mean (SD) | P, higher versus lower TTPa | |||
|---|---|---|---|---|---|
| Higher TTP | Lower TTP | Unadjusted | Adj FGR | Adj FGR and body volume | |
| Brain volume, mm3 | 263,043 (54,603) | 288,261 (68,322) | 0.009 | 0.049 | 0.57 |
| Cortical volume, mm3 | |||||
| Left | 21,990 (7,180) | 23,439 (8,019) | 0.0001 | 0.035 | 0.50 |
| Right | 21,998 (8,094) | 24,078 (8,219) | |||
| Surface area, mm2 | |||||
| Left | 10,474 (2,363) | 11,117 (2,607) | <0.0001 | <0.0001 | 0.002 |
| Right | 10,562 (2,500) | 11,101 (2,614) | |||
| Gyrification index | |||||
| Left | 1.139 (0.058) | 1.142 (0.059) | 0.63 | 0.14 | — |
| Right | 1.142 (0.062) | 1.139 (0.061) | |||
aMain-effect comparison in linear mixed model, including random effect for twin pairs. TTP × hemisphere interaction was nonsignificant in all cases.
In lobar regional analysis, cortical surface areas in the frontal (P < 0.0001), temporal (P = 0.016), and parietal (P = 0.013) lobes were significantly lower in the higher TTP than in the lower TTP group in the unadjusted model. In both adjusted models, we observed that cortical surface area in the frontal lobe was significantly lower in the higher TTP than in the lower TTP group (P = 0.0001 when adjusted for FGR and P = 0.011 when adjusted for FGR and fetal body volume) (Table 3). The TTP group × left and right hemisphere interaction was nonsignificant in every case (0.28 < P < 0.93).
Table 3.
Comparison of lobar regional surface area in left and right hemispheres between twins with higher and lower TTPs with statistical findings.
| Lobe | Mean surface, mm2 (SD) | P, higher versus lower TTPa | |||
|---|---|---|---|---|---|
| Higher TTP | Lower TTP | Unadjusted | Adj FGR | Adj FGR and body volume | |
| Frontal | |||||
| Left | 3,471 (729) | 3,822 (948) | <0.0001 | 0.0001 | 0.011 |
| Right | 3,530 (846) | 3,814 (986) | |||
| Temporal | |||||
| Left | 1,846 (455) | 1,923 (507) | 0.016 | 0.34 | 0.33 |
| Right | 1,794 (387) | 1,891 (442) | |||
| Parietal | |||||
| Left | 2,531 (588) | 2,674 (628) | 0.013 | 0.16 | 0.36 |
| Right | 2,567 (714) | 2,669 (641) | |||
| Occipital | |||||
| Left | 1,051 (346) | 1,093 (333) | 0.96 | 0.34 | 0.37 |
| Right | 1,137 (350) | 1,087 (383) | |||
aMain-effect comparison in linear mixed model, including random effect for twin pairs. TTP × hemisphere interaction was nonsignificant in all cases.
Absolute TTP differences between the twin pairs were correlated with sulcal pattern similarity values in all 9 twin pairs. A significant negative correlation was observed between the pattern similarity and TTP differences in the right hemisphere (correlation coefficient r = −0.754, P = 0.050) when all 3 sulcal features were combined (sulcal position, area, and depth). Among these features, the position pattern similarity of sulci was also negatively associated with the TTP difference (r = −0.762, P = 0.046) (Table 4), but the other 2 features were not.
Table 4.
Association between sulcal pattern similarity and TTP difference in 9 twin pairs adjusted for gestational age and the diagnosis of FGR in left and right hemispheres.
| Left | Right | |
|---|---|---|
| Combined features | −0.376 (0.406) | −0.754 (0.050a) |
| Position | −0.378 (0.403) | −0.762 (0.046a) |
| Depth | −0.013 (0.977) | −0.627 (0.132) |
| Area | −0.040 (0.933) | −0.345 (0.448) |
Data: Pearson’s correlation coefficient r (P value).
a P < 0.05.
Discussion
In monochorionic twins, the twin pairs are matched for genetic growth potential, gestational age, and maternal environment. As a result, differences in twin growth can be attributed primarily due to the differences in regional placental function. Oxygen is an important factor for the function. Most specifically, it is the most crucial element in the process of placental flux, and the failure of oxygen transport will likely deteriorate the overall nutrient transport. In other words, the efficiency of oxygen transport is monotonically correlated with the extent of placental insufficiency. This study demonstrates the significant relationship between placental oxygen transport and fetal brain cortical growth and folding in monochorionic twins in utero. We used TTP of the BOLD MRI time series acquired with maternal hyperoxia as a marker for placental oxygen transport and assessed its relationship with the fetal cortical volume, surface area, GI, and sulcal folding patterns. Higher TTP values are associated with slower placental oxygen transport. Using the unadjusted model and the model adjusted for the diagnosis of FGR, we demonstrated that (i) the fetal twin with the higher TTP showed reduced brain volume, cortical volume, and surface area growth and (ii) the fetal twin with the higher TTP showed reduced surface growth in the frontal lobe. When we used the model adjusted for both the diagnosis of FGR and fetal body volume, we still observed significantly reduced cortical surface area and reduced surface growth in the frontal lobe in the higher TTP group compared to the lower TTP group. This observation may indicate that cortical surface area and surface growth in the frontal lobe are most sensitive to the changes in placental oxygenation and that sFGR diagnosis might not be enough to identify a twin pair with a change in brain development. In sulcal pattern analysis, larger TTP differences were associated with a lower sulcal pattern similarity between twin pairs in the right hemisphere.
Our study has provided a quantitative explanation for the previous human and nonhuman studies showing an association between FGR caused by placental insufficiency and cortical growth and folding (Sasaki et al. 2000; Tashima et al. 2001; Dubois et al. 2008; Egaña-Ugrinovic et al. 2013; Miller et al. 2016; McDougall et al. 2017; Lo et al. 2018) by analyzing the correlation between a metric associated with placental oxygen transport and metrics of fetal brain cortical growth. In our previous study with monochorionic twins, we demonstrated the association between the smaller brain volume and the higher TTP in twin pairs (Luo et al. 2017). Similarly, in the current study, we observed significantly decreased brain volume in the fetal twin with higher TTP compared to lower TTP in the unadjusted analysis. Even though the difference in cortical volume was not significant when we controlled for the fetal body volume and diagnosis of FGR, we observed significant change in the cortical surface area in higher TTP compared to lower TTP. Since the change in cortical surface area is affected by the cerebral cortical size as well as cortical gyrification, the reduced cortical surface area in higher TTP might be due to the combined effect of a decrease in cortical size and GI. Thus, the surface area might show a greater sensitivity in detecting significant group differences. Cortical surface size depends on the expansion of the neural progenitor pool. The decrease in cortical surface area and folding is related to a reduced number of cortical progenitor cells in the ventricular and subventricular zones and less production of neurons (Lui et al. 2011; Sun and Hevner 2014). We suggest that the low efficiency of oxygen transport to the brain during development may affect neural proliferation.
Earlier Doppler studies investigating cerebral blood flow in fetuses with FGR showed the redistribution of the blood flow with a relative decrease to the frontal areas in favor of basal ganglia (Hernandez-Andrade et al. 2012; Meher et al. 2015; Briana and Malamitsi-Puchner 2019). Decreases in blood flow to the cortex in growth-restricted fetuses could cause hypoxia and undernutrition, altering lobar development. Although, no change in the flow velocity in the cerebral arteries (i.e. change in the middle cerebral artery pulsatility index) was reported in the ultrasound reports of our study cohort, possible changes in fetal oxygenation due to the placental oxygenation might still explain the significantly reduced cortical surface growth in the frontal lobe in the fetuses with the lower TTP when we considered the fetal body volume and the diagnosis of FGR in the analysis model.
It is noteworthy that not only cortical areal growth but also the global patterns of arrangement, number, and size of sulcal folds are influenced by the placental oxygen transport. Previously, the sulcal pattern similarity in monozygotic co-twin pairs has been demonstrated (Lohmann et al. 1999; Im et al. 2011). Our graph-based sulcal pattern analysis found that the similarity of the sulcal graphs in twin pairs was significantly higher than in unrelated pairs, showing a genetic impact on sulcal patterning (Im et al. 2011). However, in this study, sulcal pattern similarity in twin pairs significantly decreased when the difference in TTP increased, demonstrating the importance of environmental factors in addition to the importance of genetic factors in fetal brain cortical development. Based on the protomap hypothesis, cortical neurons originated in the proliferative zones carry intrinsic programs for cortical functional arealization and migrate to their proper laminar and areal positions (Rakic 2009). Optimal cortical arealization and their white matter connectivity may result in the primary pattern of sulcal folds (Van Essen 1997; Klyachko and Stevens 2003; Rakic 2004; Fischl et al. 2008). Placental transport insufficiency may decrease neural proliferation in the ventricular and subventricular zones globally or regionally and may affect the protomap and cortical area patterning (Chenn and Walsh 2002; Rakic 2009). Placental transport may also alter neuronal connectivity patterns during fetal development (Tolsa et al. 2004; Rees et al. 2008; Miller et al. 2016; Malhotra et al. 2017). These factors may possibly cause alterations in cortical folding patterns and significant differences between twin pairs. In addition, fetal intrauterine environment influenced by placental oxygen and nutrient transport may modify the gene expression associated with brain development. Altered brain-related gene expression patterns may also directly affect the genetic protomap of cortical areas and change the sulcal folding patterns. We identified significant correlations of right-hemispheric sulcal pattern similarity with oxygen transport difference between twin pairs. Hemispheric asymmetries are a common feature in brain development (Sun and Hevner 2014) and the right hemisphere develops earlier than the left hemisphere (Geschwind and Galaburda 1985). In a previous study with neonates (Lin et al. 2013), more oxygen consumption and blood flow were reported in the right hemisphere compared to the left hemisphere, which may explain the importance of oxygen transport for the right hemisphere during the early development. As a result, changes in oxygen transport in the placenta may affect the sulcal pattern in the right hemisphere more dramatically.
The main limitation of our study is the small sample size. Although the results are statistically sound in that we were able to detect significant differences in cortical growth and folding, a larger prospective cohort is needed to confirm these findings. Previous studies on fetal brain development showed a direct correlation between fetal cerebral oxygenation and fetal brain volume (Sun et al. 2015; Masoller et al. 2016). In fetal growth-restricted fetuses, the fetus may redistribute its cardiac output to maximize the oxygen and nutrient supply to the brain (i.e. brain-sparing effect). Since no brain-sparing effect was reported in our subject cohort, the significant changes we observed in the brain for the lower TTP group, when we adjusted the analysis for fetal body volume and the diagnosis of FGR, might be associated with other factors related to placental oxygenation rather than the change in cerebral oxygenation. This observation requires further measurements and explorations.
In summary, we investigated the relationship between placental oxygen transport and fetal brain cortical growth and folding in monochorionic twins. The fetuses with the longer placental oxygen delivery timing showed reduced volumetric and surface growth of the cerebral cortex. In addition, when the difference between placenta oxygen delivery timing increased between the twin pairs, sulcal folding patterns were more divergent. Our MRI analysis identified placental nongenetic prenatal influences on human cortical development and provided critical information for understanding the developmental mechanisms of the human cerebral cortex.
Acknowledgments
Authors are grateful to the subjects who participated in this research.
Contributor Information
Esra Abaci Turk, Department of Pediatrics, Harvard Medical School, 300 Longwood Ave, Boston, MA 02115, United States; Division of Newborn Medicine, Boston Children’s Hospital, 300 Longwood Ave, Boston, MA 02115, United States; Fetal Neonatal Neuroimaging and Developmental Science Center, Boston Children’s Hospital, 401 Park Dr, Boston, MA 02115, United States.
Hyuk Jin Yun, Department of Pediatrics, Harvard Medical School, 300 Longwood Ave, Boston, MA 02115, United States; Division of Newborn Medicine, Boston Children’s Hospital, 300 Longwood Ave, Boston, MA 02115, United States; Fetal Neonatal Neuroimaging and Developmental Science Center, Boston Children’s Hospital, 401 Park Dr, Boston, MA 02115, United States.
Henry A Feldman, Department of Pediatrics, Harvard Medical School, 300 Longwood Ave, Boston, MA 02115, United States; Division of Newborn Medicine, Boston Children’s Hospital, 300 Longwood Ave, Boston, MA 02115, United States; Institutional Centers for Clinical and Translational Research, Boston Children’s Hospital, 300 Longwood Ave, Boston, MA 02115, United States.
Joo Young Lee, Department of Pediatrics, Hanyang University College of Medicine, 222, Wangsimni-ro, Seongdong-gu, Seoul, 04763, South Korea.
Hyun Ju Lee, Department of Pediatrics, Hanyang University College of Medicine, 222, Wangsimni-ro, Seongdong-gu, Seoul, 04763, South Korea.
Carolina Bibbo, Department of Obstetrics and Gynecology, Brigham and Women's Hospital, 75 Francis St, Boston, MA 02115, United States.
Cindy Zhou, Division of Newborn Medicine, Boston Children’s Hospital, 300 Longwood Ave, Boston, MA 02115, United States; Fetal Neonatal Neuroimaging and Developmental Science Center, Boston Children’s Hospital, 401 Park Dr, Boston, MA 02115, United States.
Rubii Tamen, Division of Newborn Medicine, Boston Children’s Hospital, 300 Longwood Ave, Boston, MA 02115, United States; Fetal Neonatal Neuroimaging and Developmental Science Center, Boston Children’s Hospital, 401 Park Dr, Boston, MA 02115, United States.
Patricia Ellen Grant, Department of Pediatrics, Harvard Medical School, 300 Longwood Ave, Boston, MA 02115, United States; Division of Newborn Medicine, Boston Children’s Hospital, 300 Longwood Ave, Boston, MA 02115, United States; Fetal Neonatal Neuroimaging and Developmental Science Center, Boston Children’s Hospital, 401 Park Dr, Boston, MA 02115, United States; Department of Radiology, Boston Children’s Hospital, 300 Longwood Ave, Boston, MA 02115, United States.
Kiho Im, Department of Pediatrics, Harvard Medical School, 300 Longwood Ave, Boston, MA 02115, United States; Division of Newborn Medicine, Boston Children’s Hospital, 300 Longwood Ave, Boston, MA 02115, United States; Fetal Neonatal Neuroimaging and Developmental Science Center, Boston Children’s Hospital, 401 Park Dr, Boston, MA 02115, United States.
Authors’ contributions
Esra Abaci Turk (Data curation, Formal analysis, Methodology, Software, Writing—original draft), Hyuk Jin Yun (Software, Writing—review & editing), Henry A. Feldman (Formal analysis, Writing—review & editing), Joo Young Lee (Formal analysis, Writing—review & editing), Hyun Ju Lee (Supervision, Writing—review & editing), Carolina Bibbo (Resources, Writing—review & editing), Cindy Zhou (Data curation, Resources, Writing—review & editing), Rubii Tamen (Data curation, Resources, Writing—review & editing), P. Ellen Grant (Funding acquisition, Methodology, Resources, Supervision, Writing—review & editing), and Kiho Im (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft).
Funding
This work was supported by National Institute of Child Health and Human Development at the National Institutes of Health (R01-EB032708, R01-HD100009 to PEG, R21-HD094130 to KI); National Institute of Neurological Disorders and Stroke at the National Institutes of Health (R01-NS114087 to KI).
Conflict of interest statement
None declared.
Data availability
Data generated or analyzed during the study will be available upon request and shared after signing a data sharing/usage agreement required by the corresponding author’s institution.
References
- Avants BB, Tustison N, Song G. Advanced normalization tools (ANTS). Insight J. 2009:2:1–35. [Google Scholar]
- Bae B-I, Tietjen I, Atabay KD, Evrony GD, Johnson MB, Asare E, Wang PP, Murayama AY, Im K, Lisgo SN, et al. Evolutionarily dynamic alternative splicing of GPR56 regulates regional cerebral cortical patterning. Science. 2014:343(6172):764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. 2012:135(5):1348–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briana DD, Malamitsi-Puchner A. Twins and neurodevelopmental outcomes: the effect of IVF, fetal growth restriction, and preterm birth. J Matern Fetal Neonatal Med. 2019:32(13):2256–2261. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002:297(5580):365–369. [DOI] [PubMed] [Google Scholar]
- Cykowski MD, Kochunov PV, Ingham RJ, Ingham JC, Mangin J-F, Rivière D, Lancaster JL, Fox PT. Perisylvian sulcal morphology and cerebral asymmetry patterns in adults who stutter. Cereb Cortex. 2008:18(3):571–583. [DOI] [PubMed] [Google Scholar]
- de Juan RC, Bruder C, Tomasello U, Sanz-Anquela JM, Borrell V. Discrete domains of gene expression in germinal layers distinguish the development of gyrencephaly. EMBO J. 2015:34(14):1859–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Benders M, Borradori-Tolsa C, Cachia A, Lazeyras F, Ha-Vinh Leuchter R, Sizonenko SV, Warfield SK, Mangin JF, Hüppi PS. Primary cortical folding in the human newborn: an early marker of later functional development. Brain. 2008:131(Pt 8):2028–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egaña-Ugrinovic G, Sanz-Cortes M, Figueras F, Bargalló N, Gratacós E. Differences in cortical development assessed by fetal MRI in late-onset intrauterine growth restriction. Am J Obstet Gynecol. 2013:209(2):126.e1–126.e8. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage. 2012:62(2):774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Rajendran N, Busa E, Augustinack J, Hinds O, Yeo BTT, Mohlberg H, Amunts K, Zilles K. Cortical folding patterns and predicting cytoarchitecture. Cereb Cortex. 2008:18(8):1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: III. A hypothesis and a program for research. Arch Neurol. 1985:42(7):634–654. [DOI] [PubMed] [Google Scholar]
- Guyader J-M, Bernardin L, Douglas NHM, Poot DHJ, Niessen WJ, Klein S. Influence of image registration on apparent diffusion coefficient images computed from free-breathing diffusion MR images of the abdomen. J Magn Reson Imaging. 2015:42(2):315–330. [DOI] [PubMed] [Google Scholar]
- Hernandez-Andrade E, Serralde JAB, Cruz-Martinez R. Can anomalies of fetal brain circulation be useful in the management of growth restricted fetuses? Prenat Diagn. 2012:32(2):103–112. [DOI] [PubMed] [Google Scholar]
- Hong J, Yun HJ, Park G, Kim S, Laurentys CT, Siqueira LC, Tarui T, Rollins CK, Ortinau CM, Grant PE, et al. Fetal cortical plate segmentation using fully convolutional networks with multiple plane aggregation. Front Neurosci. 2020:14:591683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Yun HJ, Park G, Kim S, Ou Y, Vasung L, Rollins CK, Ortinau CM, Takeoka E, Akiyama S, et al. Optimal method for fetal brain age prediction using multiplanar slices from structural magnetic resonance imaging. Front Neurosci. 2021:15:714252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Grant PE. Sulcal pits and patterns in developing human brains. NeuroImage. 2019:185:881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Pienaar R, Lee J-M, Seong J-K, Choi YY, Lee KH, Grant PE. Quantitative comparison and analysis of sulcal patterns using sulcal graph matching: a twin study. NeuroImage. 2011:57(3):1077–1086. [DOI] [PubMed] [Google Scholar]
- Im K, Pienaar R, Paldino MJ, Gaab N, Galaburda AM, Grant PE. Quantification and discrimination of abnormal sulcal patterns in polymicrogyria. Cereb Cortex. 2013:23(12):3007–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Raschle NM, Smith SA, Ellen Grant P, Gaab N. Atypical sulcal pattern in children with developmental dyslexia and at-risk kindergarteners. Cereb Cortex. 2016:26(3):1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Guimaraes A, Kim Y, Cottrill E, Gagoski B, Rollins C, Ortinau C, Yang E, Grant PE. Quantitative folding pattern analysis of early primary sulci in human fetuses with brain abnormalities. AJNR Am J Neuroradiol. 2017:38(7):1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002:17(2):825–841. [DOI] [PubMed] [Google Scholar]
- Kim H, Bernasconi N, Bernhardt B, Colliot O, Bernasconi A. Basal temporal sulcal morphology in healthy controls and patients with temporal lobe epilepsy. Neurology. 2008:70(22 Pt 2):2159–2165. [DOI] [PubMed] [Google Scholar]
- Klein S, Staring M, Murphy K, Viergever MA, Pluim JPW. Elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2010:29(1):196–205. [DOI] [PubMed] [Google Scholar]
- Klyachko VA, Stevens CF. Connectivity optimization and the positioning of cortical areas. Proc Natl Acad Sci U S A. 2003:100(13):7937–7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklisova-Murgasova M, Quaghebeur G, Rutherford MA, Hajnal JV, Schnabel JA. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Med Image Anal. 2012:16(8):1550–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P-Y, Roche-Labarbe N, Dehaes M, Fenoglio A, Grant PE, Franceschini MA. Regional and hemispheric asymmetries of cerebral hemodynamic and oxygen metabolism in newborns. Cereb Cortex. 2013:23(2):339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinares-Benadero C, Borrell V. Deconstructing cortical folding: genetic, cellular and mechanical determinants. Nat Rev Neurosci. 2019:20(3):161–176. [DOI] [PubMed] [Google Scholar]
- Lo JO, Roberts VHJ, Schabel MC, Wang X, Morgan TK, Liu Z, Studholme C, Kroenke CD, Frias AE. Novel detection of placental insufficiency by magnetic resonance imaging in the nonhuman primate. Reprod Sci. 2018:25(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann G, von Cramon DY, Steinmetz H. Sulcal variability of twins. Cereb Cortex. 1999:9(7):754–763. [DOI] [PubMed] [Google Scholar]
- Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011:146(1):18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Abaci Turk E, Bibbo C, Gagoski B, Roberts DJ, Vangel M, Tempany-Afdhal CM, Barnewolt C, Estroff J, Palanisamy A, et al. In vivo quantification of placental insufficiency by BOLD MRI: a human study. Sci Rep. 2017:7(1):3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. NeuroImage. 2007:34(4):1535–1544. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Ditchfield M, Fahey MC, Castillo-Melendez M, Allison BJ, Polglase GR, Wallace EM, Hodges R, Jenkin G, Miller SL. Detection and assessment of brain injury in the growth-restricted fetus and neonate. Pediatr Res. 2017:82(2):184–193. [DOI] [PubMed] [Google Scholar]
- Masoller N, Sanz-Cortés M, Crispi F, Gómez O, Bennasar M, Egaña-Ugrinovic G, Bargalló N, Martínez JM, Gratacós E. Severity of fetal brain abnormalities in congenital heart disease in relation to the main expected pattern of in utero brain blood supply. Fetal Diagn Ther. 2016:39(4):269–278. [DOI] [PubMed] [Google Scholar]
- McDougall ARA, Wiradjaja V, Azhan A, Li A, Hale N, Wlodek ME, Hooper SB, Wallace MJ, Tolcos M. Intrauterine growth restriction alters the postnatal development of the rat cerebellum. Dev Neurosci. 2017:39(1–4):215–227. [DOI] [PubMed] [Google Scholar]
- Meher S, Hernandez-Andrade E, Basheer SN, Lees C. Impact of cerebral redistribution on neurodevelopmental outcome in small-for-gestational-age or growth-restricted babies: a systematic review. Ultrasound Obstet Gynecol. 2015:46(4):398–404. [DOI] [PubMed] [Google Scholar]
- Miller SL, Huppi PS, Mallard C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J Physiol. 2016:594(4):807–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molko N, Cachia A, Rivière D, Mangin JF, Bruandet M, Le Bihan D, Cohen L, Dehaene S. Functional and structural alterations of the intraparietal sulcus in a developmental dyscalculia of genetic origin. Neuron. 2003:40(4):847–858. [DOI] [PubMed] [Google Scholar]
- Morton PD, Korotcova L, Lewis BK, Bhuvanendran S, Ramachandra SD, Zurakowski D, Zhang J, Mori S, Frank JA, Jonas RA, et al. Abnormal neurogenesis and cortical growth in congenital heart disease. Sci Transl Med. 2017:9(374):eaah7029. 10.1126/scitranslmed.aah7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, McCarley RW, Levitt JJ, Hsu L, Kawashima T, Niznikiewicz M, Shenton ME. Altered orbitofrontal sulcogyral pattern in schizophrenia. Brain. 2007:130(Pt 3):693–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DDM, Chou S-J, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007:56(2):252–269. [DOI] [PubMed] [Google Scholar]
- Ortinau CM, Rollins CK, Gholipour A, Yun HJ, Marshall M, Gagoski B, Afacan O, Friedman K, Tworetzky W, Warfield SK, et al. Early-emerging sulcal patterns are atypical in fetuses with congenital heart disease. Cereb Cortex. 2018:29(8):3605–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Genetic control of cortical convolutions. Science. 2004:303(5666):1983–1984. [DOI] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009:10(10):724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees S, Harding R, Walker D. An adverse intrauterine environment: implications for injury and altered development of the brain. Int J Dev Neurosci. 2008:26(1):3–11. [DOI] [PubMed] [Google Scholar]
- Robbins S. Tuning and comparing spatial normalization methods. Med Image Anal. 2004:8(3):311–323. [DOI] [PubMed] [Google Scholar]
- Ronneberger O, Fischer P, Brox T. U-net: convolutional networks for biomedical image segmentation. In: München TU, Hornegger J, Wells WM, Frangi AF. (eds), Medical image computing and computer-assisted intervention – MICCAI. Switzerland: Springer International Publishing; 2015. pp. 234–241. [Google Scholar]
- Rousseeuw PJ, Leroy AM. Robust regression and outlier detection. New York (NY): John Wiley& Sons; 1987. [Google Scholar]
- Sasaki J, Fukami E, Mimura S, Hayakawa M, Kitoh J, Watanabe K. Abnormal cerebral neuronal migration in a rat model of intrauterine growth retardation induced by synthetic thromboxane A2. Early Hum Dev. 2000:58(2):91–99. [DOI] [PubMed] [Google Scholar]
- Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran J-P. A surface-based approach to quantify local cortical gyrification. IEEE Trans Med Imaging. 2008:27(2):161–170. [DOI] [PubMed] [Google Scholar]
- Serag A, Aljabar P, Ball G, Counsell SJ, Boardman JP, Rutherford MA, Edwards AD, Hajnal JV, Rueckert D. Construction of a consistent high-definition spatio-temporal atlas of the developing brain using adaptive kernel regression. NeuroImage. 2012:59(3):2255–2265. [DOI] [PubMed] [Google Scholar]
- Shim G, Jung WH, Choi J-S, Jung MH, Jang JH, Park J-Y, Choi C-H, Kang D-H, Kwon JS. Reduced cortical folding of the anterior cingulate cortex in obsessive-compulsive disorder. J Psychiatry Neurosci. 2009:34(6):443–449. [PMC free article] [PubMed] [Google Scholar]
- Sørensen A, Peters D, Fründ E, Lingman G, Christiansen O, Uldbjerg N. Changes in human placental oxygenation during maternal hyperoxia estimated by blood oxygen level-dependent magnetic resonance imaging (BOLD MRI). Ultrasound Obstet Gynecol. 2013:42(3):310–314. [DOI] [PubMed] [Google Scholar]
- Sørensen A, Peters D, Simonsen C, Pedersen M, Stausbøl-Grøn B, Christiansen OB, Lingman G, Uldbjerg N. Changes in human fetal oxygenation during maternal hyperoxia as estimated by BOLD MRI. Prenat Diagn. 2013:33(2):141–145. [DOI] [PubMed] [Google Scholar]
- Sun T, Hevner RF. Growth and folding of the mammalian cerebral cortex: from molecules to malformations. Nat Rev Neurosci. 2014:15(4):217–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Macgowan CK, Sled JG, Yoo S-J, Manlhiot C, Porayette P, Grosse-Wortmann L, Jaeggi E, McCrindle BW, Kingdom J, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. 2015:131(15):1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarui T, Madan N, Farhat N, Kitano R, Ceren Tanritanir A, Graham G, Gagoski B, Craig A, Rollins CK, Ortinau C, et al. Disorganized patterns of sulcal position in fetal brains with agenesis of corpus callosum. Cereb Cortex. 2018:28(9):3192–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashima L, Nakata M, Anno K, Sugino N, Kato H. Prenatal influence of ischemia-hypoxia-induced intrauterine growth retardation on brain development and behavioral activity in rats. Neonatology. 2001:80(1):81–87. [DOI] [PubMed] [Google Scholar]
- Tolsa CB, Zimine S, Warfield SK, Freschi M, Sancho Rossignol A, Lazeyras F, Hanquinet S, Pfizenmaier M, Huppi PS. Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr Res. 2004:56(1):132–138. [DOI] [PubMed] [Google Scholar]
- Turk EA, Luo J, Gagoski B, Pascau J, Bibbo C, Robinson JN, Grant PE, Adalsteinsson E, Golland P, Malpica N. Spatiotemporal alignment of in utero BOLD-MRI series. J Magn Reson Imaging. 2017:46(2):403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010:29(6):1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997:385(6614):313–318. [DOI] [PubMed] [Google Scholar]
- Yun HJ, Chung AW, Vasung L, Yang E, Tarui T, Rollins CK, Ortinau CM, Grant PE, Im K. Automatic labeling of cortical sulci for the human fetal brain based on spatio-temporal information of gyrification. NeuroImage. 2019:188:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun HJ, Vasung L, Tarui T, Rollins CK, Ortinau CM, Grant PE, Im K. Temporal patterns of emergence and spatial distribution of sulcal pits during fetal life. Cereb Cortex. 2020:30(7):4257–4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun HJ, Perez JDR, Sosa P, Valdés JA, Madan N, Kitano R, Akiyama S, Skotko BG, Feldman HA, Bianchi DW, et al. Regional alterations in cortical sulcal depth in living fetuses with down syndrome. Cereb Cortex. 2021:31(2):757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun HJ, Lee HJ, Lee JY, Tarui T, Rollins CK, Ortinau CM, Feldman HA, Grant PE, Im K. Quantification of sulcal emergence timing and its variability in early fetal life: hemispheric asymmetry and sex difference. NeuroImage. 2022:263:119629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. 2006:31(3):1116–1128. [DOI] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anat Embryol. 1988:179(2):173–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated or analyzed during the study will be available upon request and shared after signing a data sharing/usage agreement required by the corresponding author’s institution.