Abstract
Human immunodeficiency virus type 1 (HIV-1) infection requires cell surface expression of CD4. Costimulation of CD8+/CD4− T lymphocytes by anti-CD3 and anti-CD28 antibodies or by allogeneic dendritic cells induced expression of CD4 and rendered these CD8 cells susceptible to HIV-1 infection. Naive CD45RA+ cells responded with greater expression of CD4 than did CD45RO+ cells. CD8+ lymphocytes derived from fetal or newborn sources exhibited a greater tendency to express CD4, consistent with their naive states. This mechanism of infection suggests HIV-induced perturbation of the CD8 arm of the immune response and could explain the generally rapid disease progression seen in HIV-infected children.
Human immunodeficiency virus type 1 (HIV-1) infection requires coordinated cell surface expression of CD4 as well as expression of one of several chemokine receptors, which function as coreceptors for HIV-1 entry (1, 4, 9, 12, 13, 16). The two accessory molecules most prevalently used by HIV-1 as coreceptors for infection are CXCR4 and CCR5 (10). In addition, the activation state of a cell affects many stages in the viral life cycle, including entry, reverse transcription, integration, and proviral expression (5, 6, 37, 42). Several studies have recently examined differential regulation of levels of CXCR4 and CCR5 expression on potential HIV target cells following cell cycle activation (2, 5, 6, 38, 40).
Optimal T-cell activation requires engagement of the T-cell receptor as well as engagement of costimulatory molecules such as CD28 (7, 20, 22). Our laboratory recently examined the effects of costimulation of primary peripheral blood lymphocytes (PBL) with anti-CD3 and anti-CD28 monoclonal antibodies (MAbs) on reverse transcription of HIV-1 (27). During these analyses, following stimulation, we noted a de novo appearance of CD4 on the surface of cells that were previously CD8 single positive.
Because of the critical role of CD4 in HIV infection, in this study, we further evaluated this phenomenon by analyzing enriched CD8+/CD4− lymphocytes from several human sources, including fetal thymus, fetal spleen, umbilical cord blood, and adult PBL. Stimulation either with mitogen (phytohemagglutinin [PHA]) or with anti-CD3 or anti-CD28 MAbs alone failed to promote expression of CD4 on any of these cells. In contrast, costimulation provided by the combination of anti-CD3 and anti-CD28 MAbs or by allogeneic dendritic cells during a mixed leukocyte reaction (MLR) resulted in expression of CD4 on a subpopulation of previously single-positive CD8 T cells. Depletion studies which enriched for either naive (CD45RA+) or memory (CD45RO+) T cells demonstrated that costimulation of the naive subset was primarily responsible for the de novo acquisition of CD4. Expression of CXCR4 and/or CCR5 coreceptors was also noted on these stimulated cells. Furthermore, we documented that CD8+/CD4− T cells stimulated in this manner became susceptible to HIV-1 infection.
This mechanism of infection, which allows HIV-1 entry into a cell type usually thought to be resistant to this virus, could have many pathogenic consequences, including increasing the reservoir of infected cells and perturbing the CD8 arm of the immune response. Heightened expression of CD4 by the naive CD8+/CD4− T cells that predominate in fetal and newborn samples suggests that this mechanism of infection may contribute to the more rapid disease progression seen in the infected pediatric population.
MATERIALS AND METHODS
Cell purification.
Fresh peripheral blood was obtained from healthy, HIV-seronegative donors. Spleen and thymus from fetuses ranging in gestational age from 20 to 24 weeks were obtained from the Anatomical Gift Foundation (Woodbine, Ga.), and umbilical cord blood was obtained from the UCLA Cord Blood Bank, as approved by the UCLA Human Subjects Protection Committee. Peripheral blood, fetal spleen, and cord blood mononuclear cells were isolated following Ficoll-Hypaque (Sigma, St. Louis, Mo.) separation. Cells were then passed through a nylon wool column to remove B cells and monocytes and further purified to remove macrophages by adherence to plastic for a minimum of 2 h. For purification of CD4−, CD4−/CD45RA−, or CD4−/CD45RO− populations, cells were incubated on ice with saturating amounts of MAbs either for CD4, CD4 and CD45RA, or CD4 and CD45RO (Becton Dickinson, San Jose, Calif.), respectively. Cells were extensively washed, resuspended in RPMI 1640 with l-glutamine (Bio-Whittaker, Walkersville, Md.), and subjected to panning in flasks coated with goat anti-mouse antibodies (Sigma) which depleted cells expressing either CD4, CD4 and CD45RA, or CD4 and CD45RO, respectively. Postdepletion purity was determined by flow cytometry.
Cell culture and cell activation.
Following purification, cells were cultured in RPMI 1640 containing penicillin (100 U/ml), streptomycin (100 μg/ml) (Sigma), and 10% human AB serum (Gemini Bioproducts, Inc., Calabasas, Calif.). Cells were stimulated by culture in either PHA (1 μg/ml; Sigma), anti-CD3 MAb (1 μg/ml) immobilized on goat anti-mouse antibody-coated plates, or immobilized anti-CD3 MAb and soluble anti-CD28 MAb at a concentration of 1 μg/ml. Dendritic cells were prepared from fresh human PBL as described previously (23). MLRs were performed by the addition of 200,000 CD4−, CD4−/CD45RA−, or CD4−/CD45RO− cells to 10,000 allogeneic dendritic cells in a 96-well plate in a total volume of 200 μl of RPMI 1640 containing antibiotics, 10% human AB serum, and interleukin 12 (IL-12; 1 ng/ml; Pharmingen, San Diego, Calif.).
Flow cytometry.
Fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, biotin-, or allophycocyanin-conjugated MAbs specific for human CD3, CD4, CD8, CD45RA, and CD45RO and the activation markers CD25, CD69, and CD71 were obtained from Becton Dickinson. Biotinylated antibodies were stained in a second step with streptavidin conjugated with red 613 (Becton Dickinson). Conjugated MAbs specific for CXCR4 and CCR5 were obtained from Pharmingen. Four-color acquisition was performed with a FACStarPlus flow cytometer (Becton Dickinson). Immunophenotypic analysis was performed with the Cellquest program (Becton Dickinson). Live cells were gated by using forward-versus-side scatter dot plots. Conjugated mouse isotype antibodies were used as a negative control for gating of those cells staining negative for a cell surface marker. As a control for autofluorescence, unstimulated populations were acquired by the FACStarPlus, using instrument settings determined by unstimulated cells separately stained with either an FITC-, PE-, red 613-, or allophycocyanin-conjugated isotype control MAb. Stimulated populations were likewise acquired, using instrument settings from single-color-stained stimulated cells.
RT-PCR.
RNA was extracted from cells by using the RNeasy column extraction procedure (Qiagen, Chatsworth, Calif.) and DNase treated as described elsewhere (25). Reverse transcriptase PCR (RT-PCR) for CD4 mRNA was performed as previously described, using primers specific for CD4 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), with one of each set of primers being radiolabeled, to quantitate cellular RNA input (25). The sample was subsequently analyzed by 6% polyacrylamide gel electrophoresis (25) followed by radioanalytic image quantitation.
Virus stocks and infection.
HIV-1NL-thy has been previously described (21, 30); virus stocks were obtained by electroporation of CEM cells with plasmid containing full-length infectious DNA (30 μg), followed by coculture with uninfected CEM cells. Quantitation of p24gag in virally infected culture supernatants was performed by enzyme-linked immunosorbent assay (Coulter, Hialeah, Fla.). Determination of infectious units of virus per milliliter was performed by PCR on infected PBL as described previously (26). It was determined that 1 ng of p24 is approximately equivalent to 125 infectious units.
Infection of stimulated and unstimulated PBL was performed by the addition of HIV-1NL-thy virus stock with Polybrene (10 μg/ml) at a multiplicity of infection of approximately 0.5 for 1 h as described previously (27). For CD4 blocking experiments, 100 μg of CD4 immunoglobulin G per ml (19) was added to the virus stock immediately before infection and subsequently to cultures following infection.
RESULTS
Stimulation of CD4− PBL.
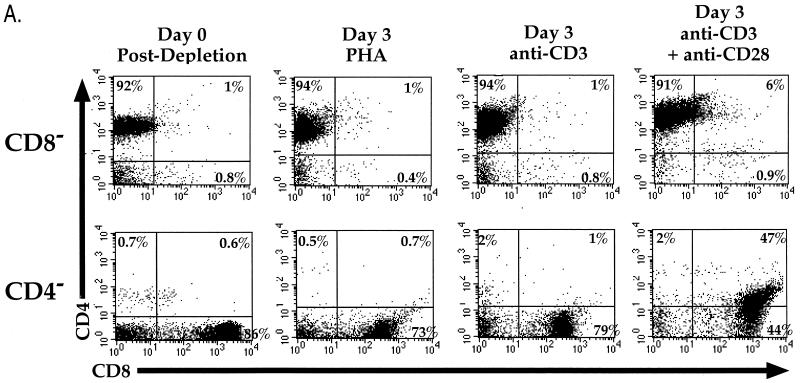
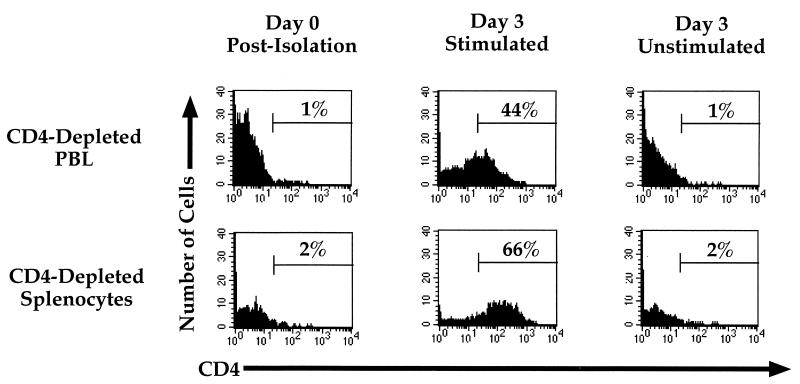
To determine the effects of different types of stimulation on the expression of surface markers on subsets of human PBL, CD4- or CD8-depleted cells from peripheral blood of adults were stimulated in the presence of either PHA, anti-CD3 MAb, or anti-CD28 MAb or costimulated with anti-CD3 and anti-CD28 MAbs. Three days later, the cells were analyzed for expression of CD4 and CD8 by flow cytometry. Costimulation resulted in de novo expression of CD4 (along with CD8) on cells that previously lacked CD4 expression. Stimulation by either PHA, anti-CD3 alone (Fig. 1A), or anti-CD28 alone (data not shown) did not result in this phenotype. Costimulation of cells lacking CD8 cell surface expression likewise resulted in de novo expression of CD8; however, expression of CD8 on these CD4+ cells was less frequent than the expression of CD4 on previously CD8+ cells. Costimulated cells also expressed greater levels of the activation markers CD25, CD69, and CD71, thus confirming their activated state (not shown).
FIG. 1.
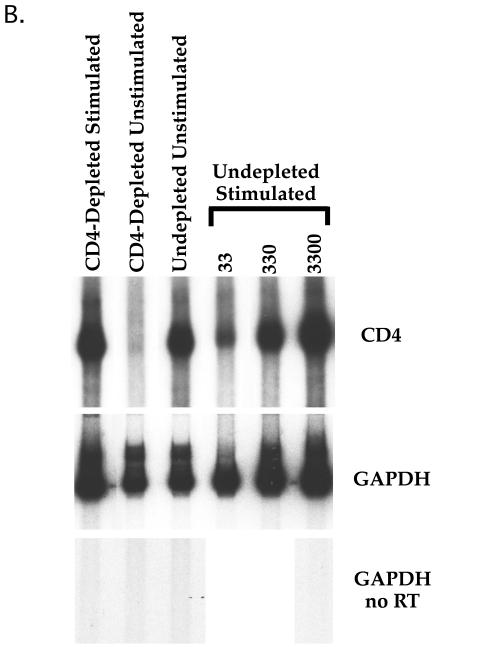
(A) Stimulation of CD8-depleted (top row) and CD4-depleted (bottom row) PBL. PBL were depleted by panning and stimulated with either PHA, anti-CD3 MAb, or anti-CD3 and anti-CD28 MAbs. Cells were analyzed by flow cytometry for expression of CD4 (red 613) and CD8 (allophycocyanin) following 3 days of stimulation. CD4+/CD8+ cells were not observed when cells were stimulated with anti-CD28 alone (data not shown). The percentage of cells in each quadrant is shown. (B) CD4 mRNA expression in costimulated and unstimulated CD4-depleted leukocytes. PBL were depleted of CD4+ cells by panning and stimulated with anti-CD3 and anti-CD28 MAbs or cultured unstimulated in parallel. Three days later, RNA was purified from the CD4-depleted and undepleted populations and subjected to RT-PCR for CD4 (top panel) and GAPDH (middle panel). Standards consisting of dilutions of undepleted stimulated PBL were amplified in parallel and are indicated by number of input cell equivalents. The primers for CD4 mRNA amplify a region containing an RNA splice site and therefore do not amplify potentially contaminating chromosomal DNA sequences. A “no RT” control was performed for GAPDH (bottom panel) to detect the presence of contaminating DNA sequences, of which there were none. The ratio of the level of CD4 mRNA signal to GAPDH signal was 22-fold higher in the costimulated CD8+ population than in the unstimulated CD8+ population.
Due to the importance of CD4 for HIV infection, we focused on characterizing the expression of CD4 on CD8 single-positive T lymphocytes. To determine whether expression of CD4 on the surface of the cell was the result of greater CD4 mRNA transcription, CD4 mRNA levels were assessed in purified CD4− cells following costimulation, using RT-PCR. Overall levels of CD4 mRNA (in relation to GAPDH expression) were approximately 20-fold higher in CD4− populations following costimulation than in unstimulated CD8+ cells (Fig. 1B). Therefore, costimulation by anti-CD3 and anti-CD28 MAbs resulted in increased transcription and protein expression of CD4 in cells that were previously CD4−, consistent with the increase in cell surface expression.
Phenotypic analysis of CD8+/CD4− lymphocytes.
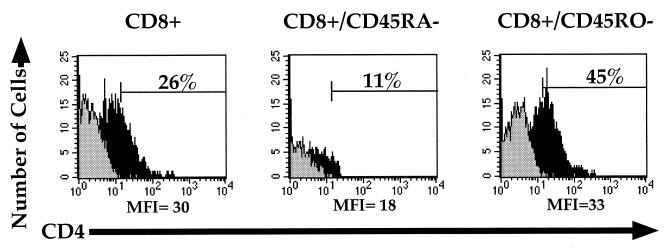
Adult CD8+/CD4− PBL are of predominantly two functional phenotypes: CD45RA+, which is thought to indicate cells that have not encountered sufficient stimulatory signals, and CD45RO+, the memory phenotype which is found on cells thought to have previously responded to antigenic stimulation (11, 32). Expression of CD45RA and CD45RO is mostly reciprocal on naive and memory T lymphocytes, respectively. However, there also exists a minor population of cells that express both isoforms; these cells are believed to more functionally resemble cells of the naive phenotype (3, 8, 34). To determine whether these phenotypes influence the ability of a CD4− cell to express CD4 following costimulation, CD4−/CD45RA− and CD4−/CD45RO− cells from adult PBL were separately purified by negative selection, subjected to costimulation, and examined for expression of CD4 (Fig. 2). Three days following costimulation, a greater percentage of cells expressing CD4 was observed in the previously naive CD8+/CD45RO− population than in the total CD8+ or CD8+/CD45RA− (memory/activated) population. The relative mean fluorescence intensity (MFI) of CD4 was also greater in the costimulated CD8+/CD45RO− population than in the CD8+/CD45RA− population. These levels of CD4 expression, however, are 5- to 10-fold lower than the level of CD4 expression seen on bona fide costimulated CD4+ cells cultured and examined in parallel (Fig. 1 and data not shown). CD8+/CD45RO− cells displayed a greater percentage of CD25, CD69, and CD71 than CD8+/CD45RA− cells following activation, confirming their differential susceptibility to costimulation (not shown).
FIG. 2.
Costimulation of CD45RA- or CD45RO-depleted, CD4-depleted PBL. PBL were depleted of either CD4, CD4 and CD45RA, or CD4 and CD45RO cells and stimulated with anti-CD3 and anti-CD28 MAbs. Three days following stimulation, cells were analyzed by flow cytometry for CD4 (red 613) and CD8 (allophycocyanin). CD8-expressing cells were gated, and CD4 expression was assessed. The gray histograms represent CD4 expression in unstimulated cells, and the black histograms represent CD4 expression in costimulated cells. Percentages of CD4+ cells in the unstimulated populations were all less than 0.2%. Percentages of costimulated CD4+ cells are given within the respective gates. The MFI of CD4 expression in the CD4+ gate is given below the respective histogram. The MFI of true CD4+ cells cultured in parallel was 197 (not shown).
Stimulation of CD8+/CD4− lymphocytes in an MLR.
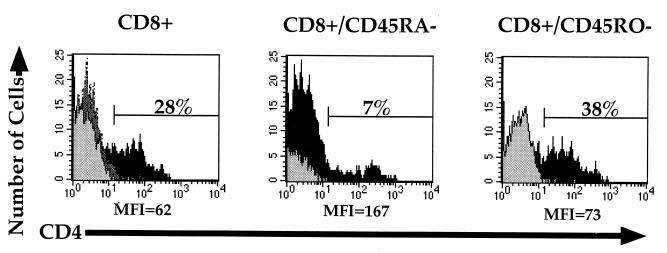
Lymphocytes are stimulated in vivo through contact with antigen in the context of an antigen-presenting cell (APC). Dendritic cells are professional APCs capable of stimulating T lymphocytes to a high degree of activation by presenting antigen on major histocompatibility complex (MHC) molecules while simultaneously expressing costimulatory ligands (36). To determine whether CD4 expression is induced on CD8+ lymphocytes in a setting more closely resembling in vivo cellular activation, MLRs with allogeneic dendritic cells were examined. Dendritic cells were derived from a CD14+ population of PBL treated with granulocyte-macrophage colony-stimulating factor and IL-4 (23). Purified allogeneic CD4−, CD4−/CD45RA−, or CD4−/CD45RO− PBL were then added to these cultures and later examined for expression of CD4 on CD8+ T cells. Seven days following coculture, CD4 expression was observed on CD8+ lymphocytes (Fig. 3). Similar to results seen with antibody costimulation, a greater percentage of CD8+/CD45RO− cells than of the CD8+/CD45RA− population responded by expressing CD4. Interestingly, in contrast to antibody costimulation, CD8+/CD45RO− cells responded with greater levels of CD4 expression on those few cells expressing CD4. The differences between these results and those observed with anti-CD3 and anti-CD28 costimulation may reflect the different methods of cellular activation or differences in the length of time in culture. The percentage of cells expressing activation markers CD71 and CD25 was also greater in the CD8+/CD45RO− population than in the CD8+/CD45RA− population (not shown), indicating greater overall cellular activation. Thus, costimulation during the process of antigen presentation also induces CD4 expression on purified CD8+/CD4− T lymphocytes, similar to that observed with antibody costimulation.
FIG. 3.
Allogeneic dendritic cell stimulation of CD4− PBL populations. CD4-, CD4/CD45RA-, or CD4/CD45RO-depleted cells were placed in an MLR mixture with purified human allogeneic dendritic cells for 7 days and assessed for expression of CD3 (FITC), CD4 (red 613), and CD8 (allophycocyanin) by flow cytometry. CD4 expression was analyzed by gating on the CD3- and CD8-expressing populations to distinguish T cells from dendritic cells, which do not express CD3 (23). The light gray histograms represent CD4 expression on the unstimulated depleted PBL, the darker gray histogram represents CD4 expression in CD4-depleted PBL cultured for 7 days in IL-12 in the absence of dendritic cells, and the black histograms represent CD4 expression in cells cultured in the MLR. CD4 expression in the unstimulated and IL-12-cultured populations was less than 0.9%. Percentages of stimulated CD4+ cells are given within the respective gates. The MFI of CD4 expression in the CD4+ gate is given below the respective histogram.
Stimulation of fetal CD4− lymphocytes.
The fetal or newborn immune system is comprised of predominantly naive cells. Typically greater than 95% of fetal T lymphocytes express CD45RA (3, 8), whereas only 45 to 60% of circulating adult PBL express this marker (3, 34). To determine whether this difference influences the induction of expression of CD4 on CD8+ lymphocytes, purified CD4-depleted adult PBL and similarly purified fetal splenocytes were costimulated with anti-CD3 and anti-CD28 and cultured in parallel. Three days following costimulation, in three of three experiments using different cell donors, a greater percentage of CD8+ fetal splenocytes than of adult PBL expressed CD4 following costimulation (Fig. 4). Costimulation of CD4− lymphocytes derived from umbilical cord blood or from fetal thymus induced expression of CD4, similar to that seen on fetal splenocytes (data not shown). Thus, CD8+ lymphocytes derived from very young humans responded to costimulation by more readily expressing CD4 than did cells derived from adults. These differences are consistent with the relative distribution of CD45RA+ cells in each population.
FIG. 4.
CD4 expression on costimulated cells derived from the adult and the fetus. CD4-depleted adult PBL or fetal splenocytes were analyzed for CD4 (PE) on gated CD8-expressing cells immediately following purification and 3 days following costimulation with anti-CD3 and anti-CD28 MAbs. Percentages of cells within each gate are given. Data are representative of three experiments using different cell donors.
Expression of HIV coreceptors on costimulated CD8+ lymphocytes.
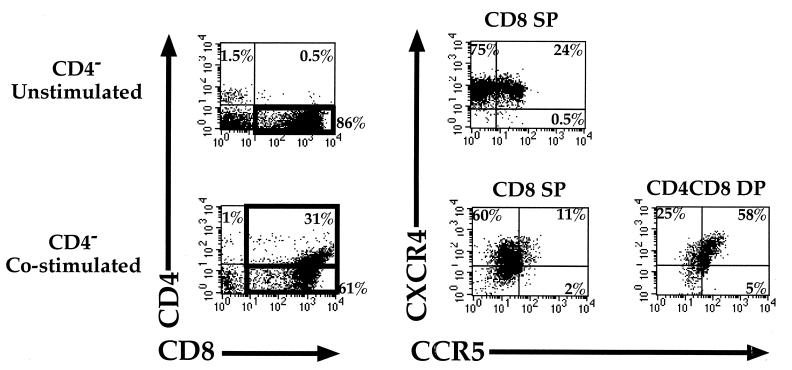
To determine whether CD8+ cells that have been stimulated to express CD4 could potentially be susceptible to HIV infection, cells were costimulated and analyzed for expression of the two major coreceptors for HIV-1, CXCR4 and CCR5 (Fig. 5). Greater overall levels of both coreceptors were seen on the costimulated CD8+ cells expressing CD4 than on unstimulated or activated CD8+/CD4− cells. In fact, the majority of lymphocytes in this newly CD4+ population expressed both coreceptors, suggesting that this population of cells might be susceptible to infection by most, if not all, HIV-1 strains.
FIG. 5.
Expression of HIV-1 coreceptors CXCR4 and CCR5 on costimulated adult PBL. CD4-depleted adult PBL were analyzed for CCR5 (FITC), CXCR4 (PE), CD8 (allophycocyanin), and CD4 (red 613) 3 days following costimulation with anti-CD3 and anti-CD28 MAbs. Coreceptor expression on the different CD8-expressing subsets was quantitated by gating. Gates are denoted by black boxes in the left column. Cells within the different gates expressing CCR5 and CXCR4 are represented on the right. The percentage of cells single positive (SP) or double positive (DP) for the indicated marker is given within each quadrant.
Susceptibility to HIV-1 infection.
To determine whether expression of CD4 following costimulation renders the CD8+ cell susceptible to infection by HIV-1, CD4-depleted adult PBL and fetal splenocytes were exposed in parallel to HIV-1NL-thy 3 days following stimulation. This CXCR4-tropic virus contains the murine thy1.2 gene inserted into the viral nef region, rendering productively infected cells detectable by flow cytometry specific for the murine protein (30). Thy1.2 expression was detected in CD8+ cells newly induced to express CD4 in both cultures (Fig. 6). The high levels of CD8 found on these virus-expressing cells confirm their origin. No Thy1.2 expression was detected in unstimulated cultures or in stimulated cultures pretreated with CD4 linked to immunoglobulin G, a reagent which has previously (19) been demonstrated to block infection (not shown). Thus, infection of these cells occurred through a CD4-mediated pathway. Although not directly tested here, the high levels of CCR5 on costimulated CD8+/CD4+ cells suggest that viruses tropic for this coreceptor should similarly infect these cells, and very recent studies by Yang et al. have demonstrated this to occur (41).
FIG. 6.
HIV-1 infection of costimulated, CD4-depleted adult PBL and fetal splenocytes. CD4-depleted adult PBL were costimulated by anti-CD3 and anti-CD28 MAbs for 3 days, assessed for CD4 (PE) on CD8-expressing cells (see Fig. 4), and infected with HIV-1NL-thy. Infected and mock-infected cells were cultured for 1 week following infection and analyzed for CD8 (FITC), CD4 (PE), and Thy1.2 (allophycocyanin) expression. (A) CD4 expression in unstimulated CD8+ cells following 10 days in culture. Thy1.2 expression in infected, unstimulated cells was not detectable (not shown). (B) Costimulated cells following 10 days in culture. (C) Phenotype of cells expressing Thy1.2, which was determined by gating on the cells that were positive for Thy1.2. Four percent of the total adult PBL and 5% of the total splenocyte population expressed Thy1.2. Percentages of CD4 and CD8 expression are given within the quadrants.
DISCUSSION
Our results indicate that costimulation of purified CD8+/CD4− lymphocytes induces CD4 expression. The levels of CD4 expression on these cells, however, are lower than those observed on bona fide CD4+ cells examined in parallel. The CD4 molecule functions as a coreceptor in antigen recognition during T-cell responses and thymic selection. CD4 binds to MHC class II and appears to provide stability to weak-affinity T-cell receptor–MHC interactions, and it is also involved in triggering the signal transduction cascade in antigen-specific responses (24). The biologic function of CD4 expression on CD8+ cells is not known. However, induction of expression of CD4 on the surface of a previously CD4− cell might allow a more efficient interaction with an APC. The ability of the naive population to express CD4 in greater amounts than memory cells may reflect the functional requirement of this population to receive additional signals from APCs to encourage differentiation into a memory phenotype.
Our results and those recently reported by two other groups (17, 41) further indicate that induction of CD4 expression on CD8+ cells renders these cells susceptible to HIV-1 infection. The lower levels of CD4 expression on these populations, however, suggest that these cells may be less susceptible to infection in vivo than are CD4 single-positive cells. Infection of CD8+ cells could have profound effects on altering cell number and function. Late in HIV disease progression, CD8+ cell numbers significantly decline in the peripheral blood (18, 29, 31). Whether this is due to indirect means or to virus-mediated killing is not known. However, recent clinical studies have demonstrated the presence of HIV-1 proviral DNA in CD8+ cells in the lungs of infected patients (35). Furthermore, it has been reported that late in disease progression, the major reservoir for HIV proviral sequences in the peripheral blood is the CD8+ lymphocyte (28). It is interesting to speculate that the high levels of CXCR4 present on activated CD4-bearing CD8+ cells might influence the acquisition of CXCR4-tropic virus strains seen late in disease. It is not known how frequently CD8+ cells express CD4 in vivo, although this phenomenon might occur most often in lymphoid tissues, where costimulation occurs. We have recently shown that infection of an immature CD8+/CD4+ thymocyte that undergoes further differentiation into a CD8+/CD4− cell results in CD8+ thymocytes that express HIV-1 (25). Thus, mechanisms such as infection of a CD4+ precursor or de novo acquisition of CD4 may explain the presence of proviral sequences in and loss of CD8+ cells during disease progression.
HIV-infected children display a more rapid disease progression than do adults (15, 39). Approximately one out of four perinatally infected children develops AIDS within the first year after birth, and the remainder typically develop AIDS within a mean of approximately 6 years, in contrast to the median 10-year period seen in adults (14, 15, 33). The abundance of naive cell types in the fetus and newborn and the greater ability of the CD8+/CD45RO− population to express CD4 following costimulation could influence infection of these cells and contribute to the increased rate of disease progression in children.
ACKNOWLEDGMENTS
We thank W. Aft and L. Duarte for manuscript preparation, and we thank I. S. Y. Chen and J. Ferbas for critical reading of the manuscript. We also thank C. Hunter, S. Laforge, and M. Mendenhall for technical assistance.
This work was supported by NIH grants AI36059, AI36554, and HL55205 (J.A.Z.) and grant 2CB-0160 from the Breast Cancer Research Program at the University of California (M.D.R.). J.A.Z. is an Elizabeth Glaser Scientist supported by the Pediatric AIDS Foundation.
REFERENCES
- 1.Alkhatib G, Broder C C, Berger E A. Cell-type-specific fusion cofactors determine human immunodeficiency virus type 1 tropism for T-cell lines versus primary macrophages. J Virol. 1996;70:5487–5494. doi: 10.1128/jvi.70.8.5487-5494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amara A, Gall S L, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier J L, Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1 alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amlot P L, Tahami F, Chinn D, Rawlings E. Activation antigen expression on human T cells. I. Analysis by two-colour flow cytometry of umbilical cord blood, adult blood and lymphoid tissue. Clin Exp Immunol. 1996;105:176–182. doi: 10.1046/j.1365-2249.1996.d01-722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berson J F, Long D, Doranz B J, Rucker J, Jirik F R, Doms R W. A seven-transmembrane domain receptor involved in fusion and entry of T-cell-tropic human immunodeficiency virus type 1 strains. J Virol. 1996;70:6288–6295. doi: 10.1128/jvi.70.9.6288-6295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll R G, Riley J L, Levine B L, Feng Y, Kaushal S, Ritchey D W, Bernstein O S, Brown C R, Berger E A, June C H, St. Louis D C. Differential regulation of HIV-1 fusion cofactor expression by CD28 costimulation of CD4+ T cells. Science. 1997;276:273–276. doi: 10.1126/science.276.5310.273. [DOI] [PubMed] [Google Scholar]
- 7.Chambers C A, Allison J P. Co-stimulation in T cell responses. Curr Opin Immunol. 1997;9:396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 8.Chheda S, Palkowetz K H, Rassin D K, Goldman A S. Deficient quantitative expression of CD45 isoforms on CD4+ and CD8+ T cell subpopulations and subsets of CD45RA(low)CD45RO(low) T cells in newborn blood. Biol Neonate. 1996;69:128–132. doi: 10.1159/000244287. [DOI] [PubMed] [Google Scholar]
- 9.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 10.Clapham P R, Weiss R A. Immunodeficiency viruses. Spoilt for choice of co-receptors. Nature. 1997;388:230–231. doi: 10.1038/40758. [DOI] [PubMed] [Google Scholar]
- 11.Clement L T. Isoforms of the CD45 common leukocyte antigen family: markers for human T-cell differentiation. J Clin Immunol. 1992;12:1–10. doi: 10.1007/BF00918266. [DOI] [PubMed] [Google Scholar]
- 12.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 13.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 14.The European Collaboratory Study. Children born to women with HIV-1 infection: natural history and risk of transmission. Lancet. 1991;337:253–260. [PubMed] [Google Scholar]
- 15.The European Collaboratory Study. Natural history of vertically acquired human immunodeficiency virus-1 infection. Pediatrics. 1994;94:815–819. [PubMed] [Google Scholar]
- 16.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 17.Flamand L, Crowley R W, Lusso P, Colombini-Hatch S, Margolis D M, Gallo R C. Activation of CD8+ T lymphocytes through the T cell receptor turns on CD4 gene expression: implications for HIV pathogenesis. Proc Natl Acad Sci USA. 1998;95:3111–3116. doi: 10.1073/pnas.95.6.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giorgi J V, Detels R. T-cell subset alterations in HIV-infected homosexual men: NIAID multicenter AIDS cohort study. Clin Immunol Immunopathol. 1989;52:10–18. doi: 10.1016/0090-1229(89)90188-8. [DOI] [PubMed] [Google Scholar]
- 19.Hays E F, Uittenbogaart C H, Brewer J C, Vollger L W, Zack J A. In vitro studies of HIV-1 expression in thymocytes from infants and children. AIDS. 1992;6:265–272. doi: 10.1097/00002030-199203000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Janeway C A, Jr, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–285. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 21.Jowett J B M, Planelles V, Poon B, Shah N P, Chen M-L, Chen I S Y. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.June C H, Ledbetter J A, Linsley P S, Thompson C B. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990;11:211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- 23.Kiertscher S M, Roth M D. Human CD14+ leukocytes acquire the phenotype and function of antigen-presenting dendritic cells when cultured in GM-CSF and IL-4. J Leukoc Biol. 1996;59:208–218. doi: 10.1002/jlb.59.2.208. [DOI] [PubMed] [Google Scholar]
- 24.Killeen N, Littman D R. The regulation and function of the CD4 coreceptor during T lymphocyte development. Curr Top Microbiol Immunol. 1996;205:89–106. doi: 10.1007/978-3-642-79798-9_5. [DOI] [PubMed] [Google Scholar]
- 25.Kitchen S G, Uittenbogaart C H, Zack J A. Mechanism of human immunodeficiency virus type 1 localization in CD4-negative thymocytes: differentiation from a CD4-positive precursor allows productive infection. J Virol. 1997;71:5713–5722. doi: 10.1128/jvi.71.8.5713-5722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitchen S G, Zack J A. CXCR4 expression during lymphopoiesis: implications for human immunodeficiency virus type 1 infection of the thymus. J Virol. 1997;71:6928–6934. doi: 10.1128/jvi.71.9.6928-6934.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korin Y, Zack J A. Progression to the G1 phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J Virol. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livingstone W J, Moore, M. M, Innes D, Bell J E, Simmonds P. Frequent infection of peripheral blood CD8-positive T-lymphocytes with HIV-1. Edinburgh Heterosexual Transmission Study Group. Lancet. 1996;348:649–654. doi: 10.1016/s0140-6736(96)02091-0. [DOI] [PubMed] [Google Scholar]
- 29.Margolick J B, Munoz A, Donnenberg A D, Park L P, Galai N, Giorgi J V, O’Gorman M R, Ferbas J. Failure of T-cell homeostasis preceding AIDS in HIV-1 infection. The Multicenter AIDS Cohort Study. Nat Med. 1995;1:674–680. doi: 10.1038/nm0795-674. [DOI] [PubMed] [Google Scholar]
- 30.Planelles V, Haislip A, Withers-Ward E S, Stewart S A, Xie Y, Shah N P, Chen I S Y. A new reporter system for detection of viral infection. Gene Ther. 1995;2:369–376. [PubMed] [Google Scholar]
- 31.Roederer M. Getting to the HAART of T cell dynamics. Nat Med. 1998;4:145–146. doi: 10.1038/nm0298-145. [DOI] [PubMed] [Google Scholar]
- 32.Roth M D. Interleukin 2 induces the expression of CD45RO and the memory phenotype by CD45RA+ peripheral blood lymphocytes. J Exp Med. 1994;179:857–864. doi: 10.1084/jem.179.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scarlatti G. Paediatric HIV infection. Lancet. 1996;348:863–868. doi: 10.1016/S0140-6736(95)11030-5. [DOI] [PubMed] [Google Scholar]
- 34.Schiavon V, Roth P, Bolton W E, Farcet J P, Bensussan A, Boumsell L. Lymphocyte subsets in normal individuals: analysis by four color immunofluorescence and flow cytometry on whole blood. Tissue Antigens. 1996;48:312–318. doi: 10.1111/j.1399-0039.1996.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 35.Semenzato G, Agostini C, Ometto L, Zambello R, Trentin L, Chieco-Bianchi L, De Rossi A. CD8+ T lymphocytes in the lung of acquired immunodeficiency syndrome patients harbor human immunodeficiency virus type 1. Blood. 1995;85:2308–2314. [PubMed] [Google Scholar]
- 36.Steinman R M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 37.Stevenson M, Stanwick T L, Dempsey M P, Lamonica C A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Unutmaz D, Littman D R. Expression pattern of HIV-1 coreceptors on T cells: implications for viral transmission and lymphocyte homing. Proc Natl Acad Sci USA. 1997;94:1615–1618. doi: 10.1073/pnas.94.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiznia A A, Lambert G, Pavlakis S. Pediatric HIV infection. Med Clin North Am. 1996;80:1309–1336. doi: 10.1016/s0025-7125(05)70492-2. [DOI] [PubMed] [Google Scholar]
- 40.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, MacKay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang L P, Riley J L, Carroll R G, June C H, Hoxie J, Patterson B K, Ohshima Y, Hodes R J, Delespesse G. Productive infection of neonatal CD8+ T lymphocytes by HIV-1. J Exp Med. 1998;187:1139–1144. doi: 10.1084/jem.187.7.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S Y. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]