Abstract
Background:
Acute limb ischemia (ALI) carries a 15–20% risk of combined death or amputation at 30 days and 50–60% at 1 year. Percutaneous mechanical thrombectomy (PT) is an emerging minimally invasive alternative to open thrombectomy (OT). However, ALI thrombectomy cases are omitted from most quality databases, limiting comparisons of limb and survival outcomes between PT and OT. Therefore, our aim was to compare in-hospital outcomes between PT and OT using the National Inpatient Sample (NIS).
Methods:
We analyzed survey-weighted NIS data (2015–2020) to include emergent admissions of aged adults (50+ years) with a primary diagnosis of lower extremity ALI undergoing index procedures within two days of hospitalization. We excluded hospitalizations with concurrent trauma or dissection diagnoses and index procedures using catheter-directed thrombolysis. Our primary outcome was composite in-hospital major amputation or death. Secondary outcomes included in-hospital major amputation, death, in-hospital reintervention (including angioplasty/stent, thrombolysis, PT, OT, or bypass), and extended length of stay (eLOS; defined as LOS >75th percentile). Adjusted odds ratios (aORs) with 95% confidence intervals (95%CI) were generated by multivariable logistic regression, adjusting for demographics, frailty (Risk Analysis Index), secondary diagnoses including atrial fibrillation and peripheral artery disease (PAD), hospital characteristics, and index procedure data including the anatomic thrombectomy level and fasciotomy. A priori subgroup analyses were performed using interaction terms.
Results:
We included 23,795 survey-weighted ALI hospitalizations (mean age 72.2 years, 50.4% female, 79.2% White, 22.3% frail) with 7,335 (30.8%) undergoing PT. Hospitalization characteristics for PT vs OT differed by atrial fibrillation (28.7% vs 36.5%, p<.0001), frequency of intervention at the femoropopliteal level (86.2% vs 88.8%, p=.009), and fasciotomy (4.8% vs. 6.9%, p=.006). In total, 2,530 (10.6%) underwent major amputation or died. Unadjusted (10.1% vs 10.9%, p=.43) and adjusted (aOR=0.96 [95%CI, 0.77–1.20], p=.74) risk did not differ between groups. PT was associated with increased odds of reintervention (aOR=2.10 [95%CI 1.72–2.56], p<.0001) when compared to OT, but this was not seen in the tibial subgroup (aOR=1.31 [95% CI, 0.86–2.01], p=.21, pinteraction<.0001). Further, 79.1% of PT hospitalizations undergoing reintervention were salvaged with endovascular therapy. Lastly, PT was associated with significantly decreased odds of eLOS (aOR=0.80 [95%CI 0.69–0.94], p=.005).
Conclusion:
PT was associated with comparable in-hospital limb salvage and mortality rates compared to OT. Despite an increased risk of reintervention, most PT reinterventions avoided open surgery and PT was associated with a decreased risk of eLOS. Thus, PT may be an appropriate alternative to OT in appropriately selected patients.
Keywords: acute limb ischemia, endovascular, percutaneous thrombectomy, suction thrombectomy, open thrombectomy
Table of Contents Summary
Limb salvage and mortality outcomes were equivalent in percutaneous vs. open thrombectomy in a retrospective study of 23,795 hospitalizations for acute limb ischemia undergoing revascularization within the National Inpatient Sample. The authors suggest percutaneous thrombectomy is a suitable alternative to open thrombectomy in properly selected patients.
Introduction
Acute limb ischemia (ALI) results from a sudden decrease in limb perfusion that threatens limb viability. Among adults in the United States (U.S.), ALI occurs in 1.5 per 10,000 person-years and despite significant advances in medical and surgical therapy, over half will ultimately require major amputation within one year1–3. Traditional treatment options include open thrombectomy (OT), surgical bypass, and catheter directed thrombolysis (CDT), with treatment selection based on both etiology and degree of ischemia as determined by Rutherford classification of ALI2,4,5. Typically, open approaches are preferred in patients with ALI secondary to embolic events or with compromised motor function, requiring rapid revascularization. Conversely, CDT is frequently favored among patients with acute thrombosis of pre-existing peripheral arterial disease (PAD) and/or prior interventions, as they often present with milder symptoms2,4,5.
Minimally invasive approaches, when appropriately selected, can be associated with lower physiologic stress when compared to open surgical interventions and may improve outcomes, especially in an aged and frail patient population6–8. Endovascular approaches can also be attractive in re-operative fields due to increased wound infection risk with open surgery9. However, the existing endovascular treatment options have limitations. Specifically, CDT can require 24–48 hours of thrombolytic infusion to clear thrombus and restore perfusion. Thus, CDT is not feasible in those with a high bleeding risk or Rutherford IIB ischemia, who have a dense motor deficit requiring prompt revascularization.
More recently, the addition of percutaneous thrombectomy (PT) to the armamentarium has broadened treatment options for ALI. Several devices are available with different mechanisms for thrombus clearance, including mechanical and suction thrombectomy, both alone and in combination with thrombolytics. PT offers several potential benefits. It allows for minimally invasive, single-session thrombus clearance, making it a viable option in Rutherford IIB ischemia. It also potentially obviates the need for thrombolytics, which carry an increased risk of bleeding, particularly amongst elderly patients4,10,11.
The study of ALI is excluded from commonly used quality improvement databases (OT is excluded from the Vascular Quality Initiative [VQI] and PT is excluded from the National Surgical Quality Improvement Program [NSQIP]). Thus, current literature on PT is limited to single-center retrospective studies comparing PT to CDT and studies comparing open revascularization to CDT only12–17. We therefore examined in-hospital outcomes between PT and OT using the National Inpatient Sample (NIS), hypothesizing that PT would be associated with improved combined amputation and death rates.
Methods
Our retrospective study using the NIS database was determined to be exempt from human subjects’ review by the University of Pittsburgh’s Human Research Protection Office (STUDY20110441). Data were presented in accordance with the NIS, Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality Use Agreements and Strengthening the Reporting of Observational Studies in Epidemiology guidelines18,19.
Data Source and Patient Cohort
We examined all hospitalizations for ALI in which patients underwent PT or OT in the U.S. using NIS (2015–2020) data. The NIS samples 20% of non-federal acute care hospital discharges in the United States from all payers, allowing for population-level, survey-weighted estimates of hospitalizations and outcomes20,21. NIS data includes demographics, up to 40 diagnosis codes using International Classification of Disease, 10th Edition, Clinical Modifications (ICD-10-CM), up to 25 procedure codes using International Classification of Disease, 10th Edition, Procedure Coding System (ICD-10-PCS), procedure day, in-hospital mortality and discharge disposition22.
In our primary cohort, we included non-elective hospitalizations with a primary ICD-10-CM diagnosis code of lower extremity ALI and underwent PT or OT within 2 days of admission as defined by ICD-10-PCS (Figure 1, Supplementary Table 1), mapped from previously described ICD-9-PCS3,23. Using procedure days from admission, we defined the index thrombectomy procedure as the one that occurred on the earliest hospital day. Those who underwent both PT and OT on the first procedural day were classified as PT and analyzed in an intention-to-treat fashion, assuming that both procedures were done within the same operation and the likelihood of PT failure converting to OT was higher than OT failure converting to PT. To minimize misclassification, we excluded hospitalizations with secondary diagnoses of trauma24 or dissection25 (Figure 1, Supplementary Table 1). We further excluded patients <50 years old to minimize the inclusion of outliers, such as patients with traumatic ischemia, within our cohort. We also eliminated hospitalizations with concomitant CDT, because its use is only a viable treatment among patients with limited ischemia and low bleeding risk.
Figure 1.
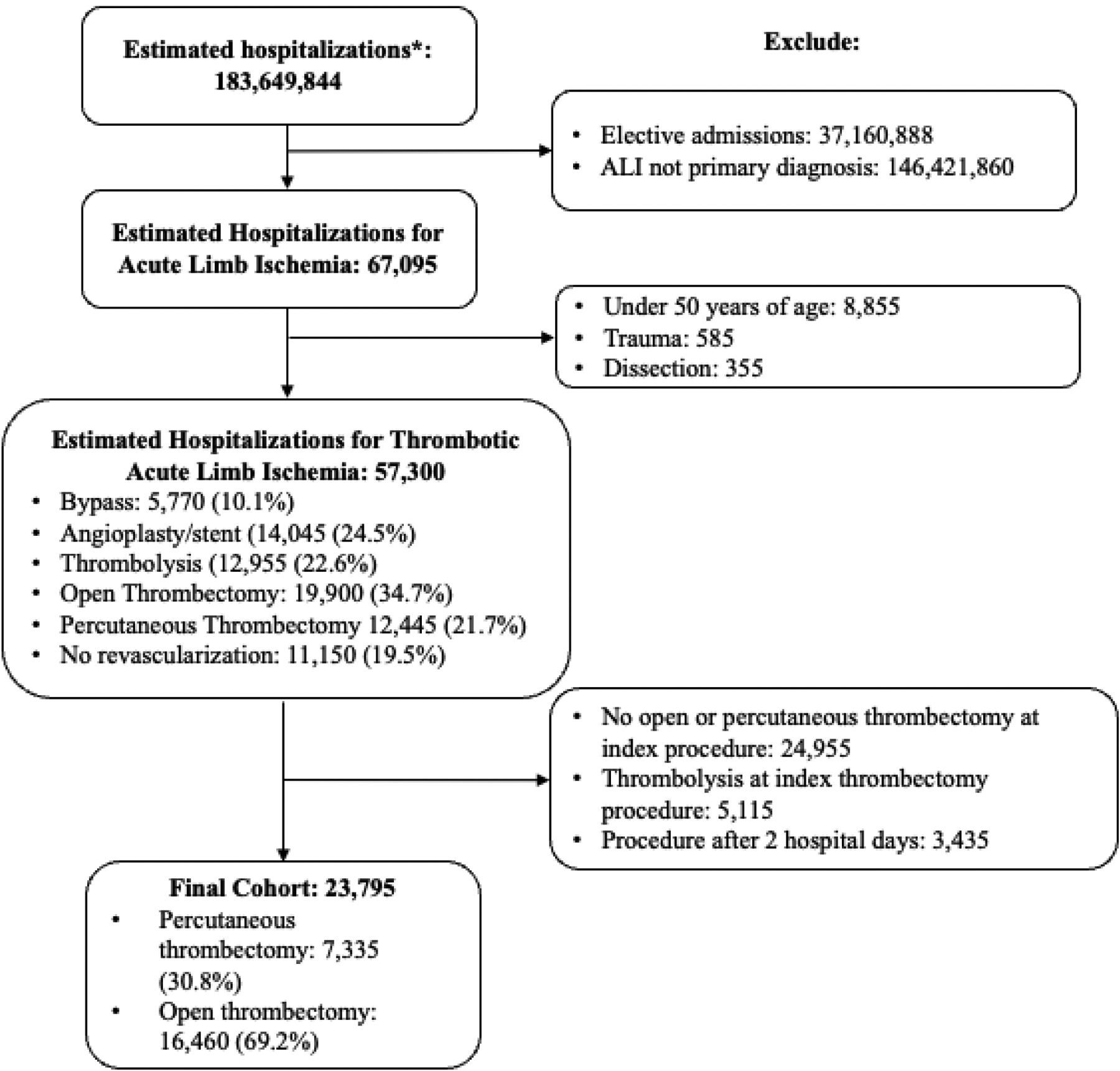
Inclusion and Exclusion Criteria
Patient demographic and admitting hospital information was available within the NIS at time of hospitalization. Hospital facility information includes geographic location, teaching status, and bed size. Comorbid conditions were defined using secondary ICD-10-CM codes in accordance with NIS guidance, using Elixhauser Comorbidity Index26 and Risk Analysis Index (RAI)27. Other covariates were taken from previously published codes3,28–30 (Supplementary Table 1). The RAI is a semi-continuous frailty score ranging from 0–81 that includes a variety of demographics and comorbidities (cancer, weight loss, renal failure, congestive heart failure, dyspnea, cognitive impairment, and functional status) that has been validated to predict postoperative complications and mortality in patients with vascular disease27. The RAI was used as a continuous variable in our initial multivariable model but was dichotomized into frail (RAI≥ 26) and not frail (RAI<26) for subgroup analyses27. Fasciotomy was used as a surrogate for ALI severity, as it’s independently associated with severity31. In-hospital amputation and reintervention procedures were also defined using ICD-10-PCS, recapitulated from the VQI32 (Supplementary Table 1).
Outcomes
The primary outcome was defined as the composite of in-hospital ipsilateral major (above-ankle) amputation and death. Secondary outcomes included in-hospital ipsilateral major amputation, death, in-hospital reintervention (including angioplasty/stent, thrombolysis, PT, OT, or bypass, regardless of laterality), and extended length of stay (eLOS; defined as LOS greater than 75th percentile within the entire thrombectomy cohort27).
Statistical Analysis
Survey-weighted data were reported in accordance with NIS guidelines. Continuous variables were presented as means with standard errors (SE) and categorical variables were presented as proportions and 95% confidence intervals (95%CI). Baseline characteristics were compared using Students t- and chi-squared testing.
Multivariable logistic regression generated adjusted odds ratios (aOR) for outcomes. A priori covariates included patient demographics (i.e. age, sex, race, insurance type, hospital type, and median income of the patients’ residential zip code), pertinent comorbid conditions (i.e. pre-existing PAD, atrial fibrillation, hypercoagulable states, and smoking status), ALI severity (using fasciotomy31), frailty (i.e. RAI), and treated anatomic level (i.e. aortoiliac, femoropopliteal, and tibial). Multilevel thrombectomy was defined as more than one anatomic level treated, as granular data were only present for procedures, not anatomic level of disease. A priori subgroup analyses (age over 70, sex, race, frailty, PAD, atrial fibrillation, diabetes, hypercoagulable state, smoker, multilevel treatment, distal anatomic level, and fasciotomy) were performed assessing the moderation of the treatment effect using interaction terms within multivariable modeling. Post-prediction rates were calculated from our multivariable model and used to determine adjusted outcome equivalence. Outcome equivalence was defined by a margin of 3% difference between treatment groups based off objective performance goals of catheter-based treatments in chronic limb threatening ischemia, with 90% confidence intervals (90%CI) as this is a two-sided test (CI=[1–2α]*100%)33,34.
Five sensitivity analyses were performed using alternative cohort and exposure definitions. First, we included an additional ICD-10-CM in our definition of ALI (I99.8, Other disorder of the circulatory system), given this non-specific code includes “ischemia of lower extremity” within its definition35. Second, we included patients who underwent concomitant thrombolysis during the index thrombectomy procedure. Third, hospitalizations with both PT and OT on the first procedural day were classified as OT instead of PT, as they were within the primary analysis. Fourth, we only included patients who underwent index thrombectomy within 1 day of hospitalization. Finally, we completed a sensitivity analysis including other clinically relevant patient (chronic antiplatelet/anticoagulation use, hypertension, prior angioplasty/stent, prior bypass), intraoperative (bilateral intervention), and postoperative factors (compartment syndrome), which were omitted from the primary a priori model in an effort to minimize both overfitting of the model and use of non-validated ICD-10 codes.
Data analysis and figures were completed using Stata 17.0 (Stata Corp, College Station, TX) and PRISM 10 (GraphPad Software, La Jolla, CA). All graphs were created in Prism (GraphPad 9.0).
Results
Baseline characteristics
Among 183,649,844 estimated hospitalizations in the NIS (2015–2020), 67,095 (3.7%) had a primary diagnosis of ALI. Of those, 23,795 (35.5%) hospitalizations met procedure specific inclusion and exclusion criteria (mean age 72.2 [SE 0.2], 50.4% female, 79.2% White and 22.3% frail) and 7,335 (30.8%) underwent PT (Figure 1). Across index thrombectomy procedures, 35.8% were multilevel and 6.2% included fasciotomy. PT hospitalizations were more frequently located at urban non-teaching hospitals and in the Southern U.S., and less frequently located in the Northeast when compared to OT hospitalizations. PT hospitalizations were less likely to have diagnoses of atrial fibrillation, congestive heart failure, and chronic lung disease. PT procedures were less likely to include the femoropopliteal segment and fasciotomy (Table 1).
Table 1.
Baseline Characteristics by Index Thrombectomy Procedure
| Preoperative variables | Index Percutaneous Thrombectomy (n=7,335) | Index Open Thrombectomy (n=16,460) | p-value |
|---|---|---|---|
| Demographics | |||
| Age, years | 72.2(0.2) | 72.6(0.2) | .002 |
| Female sex | 49.1(46.5–51.6) | 50.9(49.3–52.6) | .23 |
| Race/ethnicity* | .97 | ||
| White | 79.5(77.2–81.6) | 79.1(77.6–80.6) | |
| Black | 10.5(9–12.3) | 10.8(9.8–12) | |
| Hispanic | 6.8(5.5–8.4) | 6.6(5.7–7.6) | |
| Asian or Pacific Islander | 1(0.6–1.7) | 1.2(0.9–1.7) | |
| American Indian or Alaska Native | 0.1(0–0.6) | 0.2(0.1–0.4) | |
| other | 2(1.4–2.9) | 2(1.6–2.6) | |
| Hospital Region | .0003 | ||
| Northeast | 14.8(12.9–17) | 20.2(18.4–22.2) | |
| Midwest | 24.6(22.1–27.3) | 24.1(22.2–26.2) | |
| South | 42.4(39.4–45.4) | 38(35.8–40.3) | |
| West | 18.2(16–20.7) | 17.6(16–19.4) | |
| Bed Size° | .40 | ||
| Small | 14.4(12.5–16.5) | 13.3(11.9–14.9) | |
| Medium | 27.5(24.9–30.2) | 26.4(24.5–28.4) | |
| Large | 58.1(55.2–61.1) | 60.3(58–62.5) | |
| Hospital Type | .001 | ||
| Rural | 3.1(2.3–41) | 3.3(2.6–4) | |
| Urban nonteaching | 19.8(17.5–22.2) | 15.2(13.8–16.8) | |
| Urban teaching | 77.2(74.6–79.5) | 81.5(79.8–83.1) | |
| Insurance type | .08 | ||
| Medicare | 67.8(65.3–70.1) | 71(69.4–72.6) | |
| Medicaid | 8.4(7.1–9.9) | 8.1(7.2–9.1) | |
| Private | 18.5(16.6–20.6) | 15.6(14.4–16.9) | |
| Self-pay | 2.4(1.7–3.3) | 2.8(2.3–3.5) | |
| Other** | 2.9(2.2–4) | 2.4(1.9–3) | |
| Income quartile⧺ | .66 | ||
| quartile 1 | 31.1(28.6–33.6) | 29.8(28.1–31.6) | |
| quartile 2 | 28.5(26.2–30.9) | 28(26.5–29.7) | |
| quartile 3 | 22.2(20.1–24.5) | 23.7(22.3–25.2) | |
| quartile 4 | 18.2(16.2–20.4) | 18.4(17–19.9) | |
| Comorbid conditions | |||
| Peripheral arterial disease | 70.8(68.4–73) | 68.6(66.9–70.2) | .13 |
| Prior peripheral vascular intervention | 6.4(5.3–7.9) | 4.8(4.2–5.6) | .021 |
| Lower extremity aneurysm | 4.0(3.1–5.2) | 2.7(2.3–3.4) | .024 |
| Atrial fibrillation | 28.7(26.4–31.1) | 36.5(34.9–38.2) | <0.0001 |
| Congestive heart failure | 25.8(23.6–28.1) | 29.6(28–31.1) | .007 |
| Hypertension | 79.1(76.9–81.1) | 80.3(78.8–81.6) | .35 |
| Smoker | 51.5(48.8–54.1) | 53.2(51.5–54.9) | .27 |
| Chronic lung disease | 23.7(21.6–26) | 28(26.5–29.6) | .002 |
| Renal failure | 21.4(19.3–23.6) | 21.1(19.8–22.6) | .84 |
| Diabetes | 30.8(28.5–33.3) | 29.5(28–31.1) | .38 |
| Hypercoagulable | 3.4(2.6–4.5) | 4.1(3.5–4.8) | .25 |
| Preoperative Anticoagulation | 22.6(20.5–24.7 | 23.9(22.4–25.4) | .32 |
| Cancer | 6.7(5.6–8.1) | 6.3(5.6–7.2) | .60 |
| Risk Analysis Index | 29.5(0.2) | 30.1(0.2) | .04 |
| Operative details | |||
| Multilevel | 34.8(32.3–37.3) | 36.3(34.6–38) | .32 |
| Bilateral Treated Levels | 13.0(11.3–14.8) | 14.1(13.0–15.5) | .27 |
| Aortoiliac | 27.1(24.8–29.4) | 25.5(24–27) | .25 |
| Femoropopliteal | 86.2(84.3–87.8) | 88.8(87.7–89.8) | .009 |
| Tibial | 23.2(21.1–25.5) | 25.8(24.3–27.3) | .07 |
| Fasciotomy | 4.8(3.8–6) | 6.9(6–7.8) | .006 |
| Compartment Syndrome | 3.9(3.01–5.0) | 5.5(4.8–6.4) | .02 |
| Weekend admission | 23.9(21.7–26.1) | 24.4(23–26) | .67 |
Amputation and Death
2,530 (10.6%) estimated ALI hospitalizations resulted in ipsilateral major amputation or death, with no difference between PT and OT on univariable (10.1% vs 10.9%, p=.43) or multivariable analysis (aOR=0.96 [95%CI, 0.77–1.20], p=.74). Post-prediction estimates of the composite primary outcome were equivalent for PT (10.2% [90%CI, 10.0–10.5%]) compared to OT (10.9% [90%CI, 10.7–11.1%]) based on a priori equivalence margins. Significant predictors for combined death and amputation include increasing frailty, pre-existing PAD, multilevel thrombectomy, and concurrent fasciotomy (Figure 2).
Figure 2.
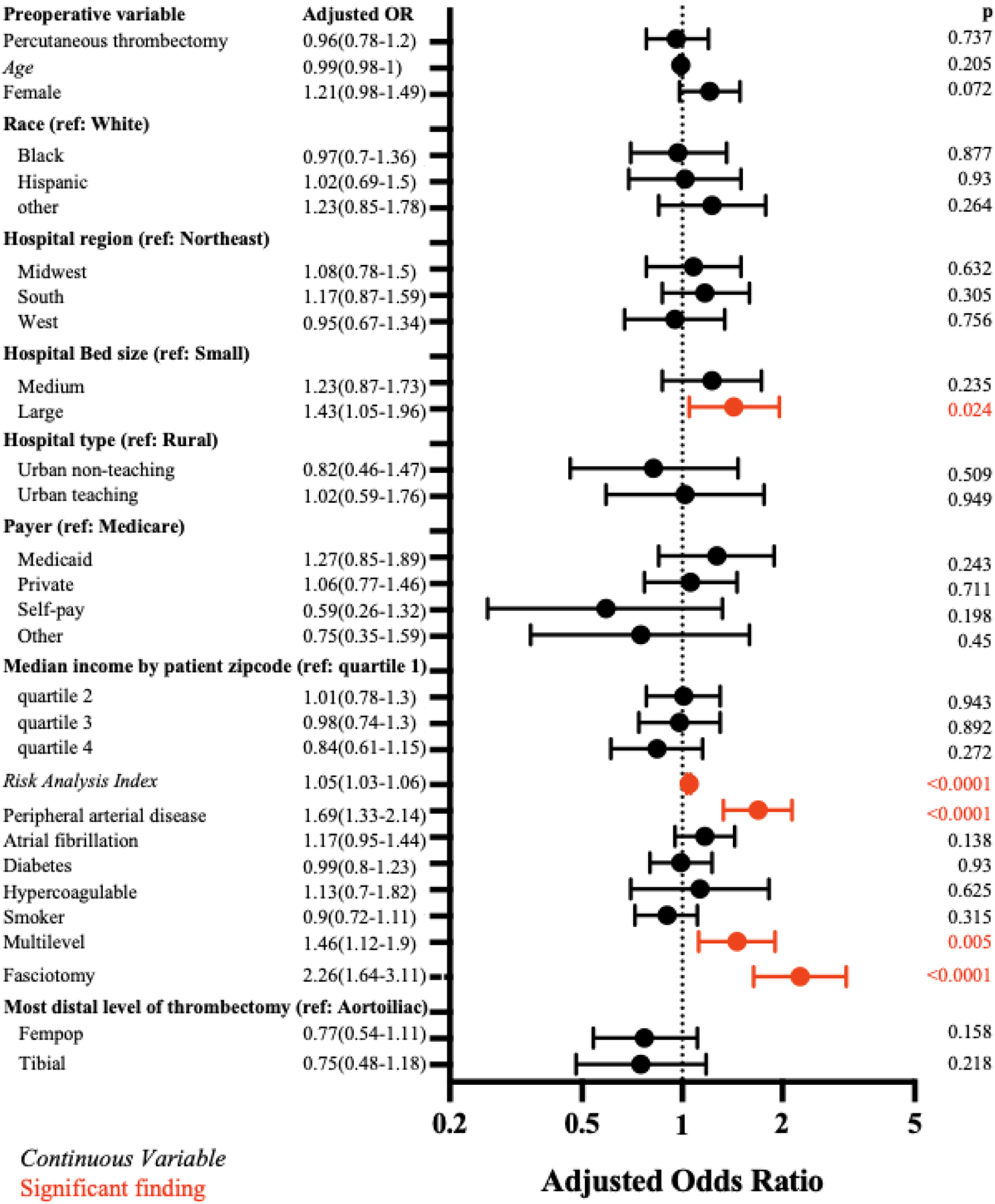
Multivariable Logistic Regression of Composite Amputation and Death
Major amputations occurred in 1,120 (4.7%) ALI hospitalizations with no difference between PT and OT on univariable (4.1% vs 5.0%, p=.28) or multivariable (aOR=0.82 [95%CI, 0.60–1.11]), p=.21) analysis. Older age was associated with a reduced risk of amputation. Procedural predictors for amputation included both femoropopliteal or tibial thrombectomy compared to aortoiliac thrombectomy as well as concurrent fasciotomy (Figure 3a)
Figure 3.
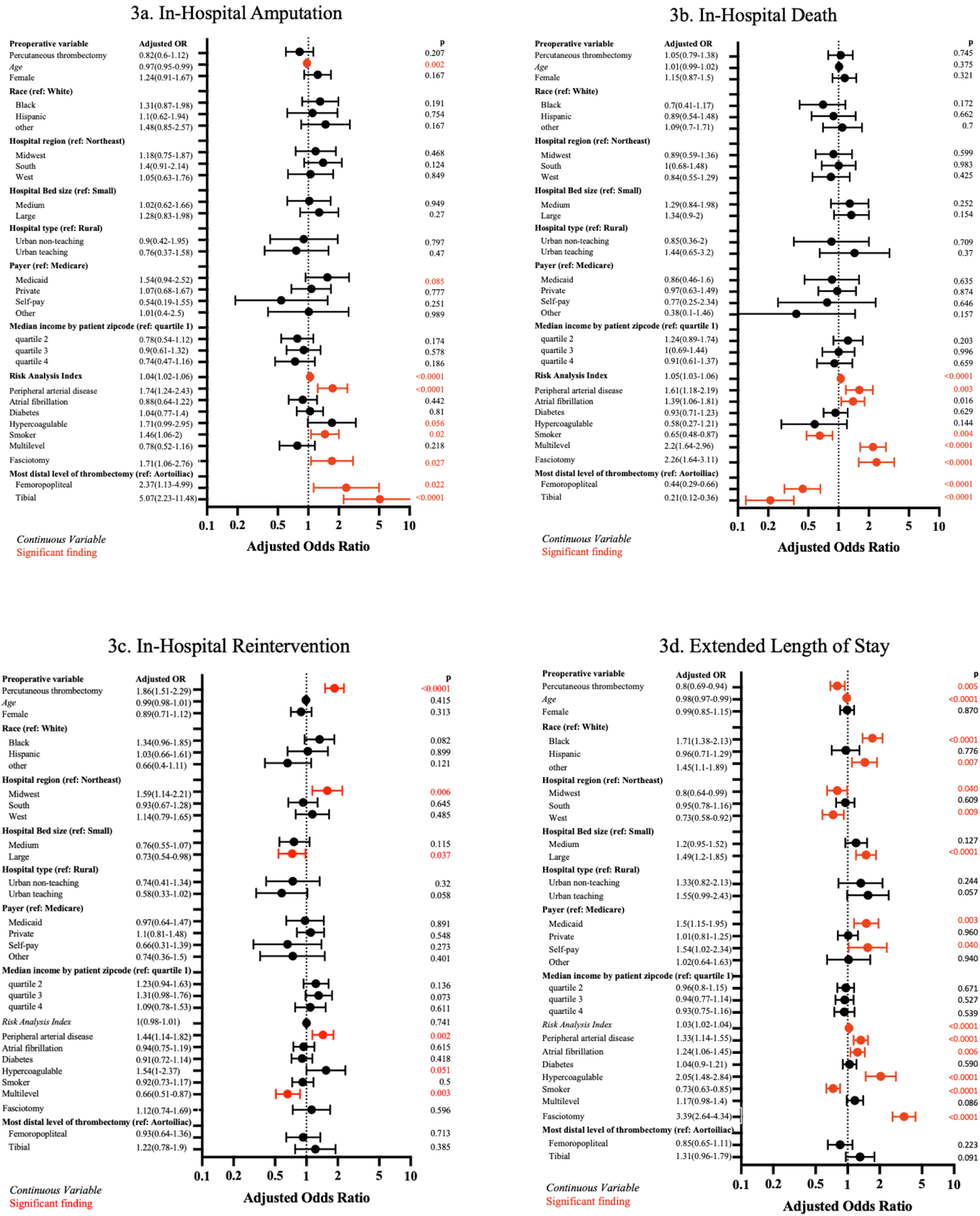
Multivariable Logistic Regression of Secondary Outcomes
Death occurred in 1,495 (6.3%) ALI hospitalizations with no difference between PT and OT for on univariable (6.2 vs 6.3%; p=.88) or multivariable (aOR=1.05 [95%CI, 0.79–1.38], p=.75) analysis. Predictors of death included multilevel thrombectomy and fasciotomy. Femoropopliteal or tibial thrombectomy (compared to aortoiliac thrombectomy) and smoking were protective (Figure 3b).
In prespecified subgroup analyses, no interaction terms evaluating moderation reached significance for composite amputation and death, amputation alone, or death alone.
In-hospital Reintervention
In-hospital reintervention occurred in 2,480 (10.4%) ALI hospitalizations, with significantly more observed reinterventions in the PT group on univariable (15.7% vs 8.1%, p<.0001) and multivariable analysis (aOR=2.10 [95%CI, 1.72–2.56], p<.0001). Multilevel treatment was associated with decreased risk, while PAD was associated with increased risk (Figure 3c). In subgroup analysis, the association between PT and increased in-hospital reintervention was not present for those who underwent tibial (aOR=1.31 [95% CI, 0.86–2.01], p=.21) compared to femoropopliteal interventions (aOR=2.82 [95% CI, 2.21–3.60], p<.0001; pinteraction<.0001). Additionally, the association was lesser in magnitude for those who underwent multilevel (aOR=1.50 [95%CI 1.05–2.14] compared to single-level thrombectomy pinteraction=0.03; Supplementary Figure 1a).
In the PT group, 79.1% of hospitalizations requiring reintervention underwent endovascular salvage, 13.9% underwent open reintervention, and 7.0% underwent hybrid reintervention. Angioplasty/stent (53.5%) reinterventions were the most common, while 49.1% underwent repeat PT and 30.9% underwent CDT. In the OT group, only 17.7% of hospitalizations requiring reintervention underwent endovascular salvage, with the majority (60.5%) undergoing repeat open revascularization, and 21.8% undergoing both. The most common reintervention was repeated OT (72.9%) while 20.3% required a bypass (Table 2).
Table 2.
Reintervention Type by Index Thrombectomy Procedure
| Reintervention | Index Percutaenous Thrombectomy, (n=1150) | Index Open Thrombectomy (n=1330) |
|---|---|---|
| Bypass | 7.8(4.9–12.3) | 20.3(15.5–26.5) |
| Open Thrombectomy | 16.1(11.7–22.1) | 72.9(62.8–84.6) |
| Percutaneous Thrombectomy | 49.1(40.7–59.1) | 10.5(7.3–15.2) |
| Lysis | 30.9(24.4–38.9) | 12.0(8.4–17.1) |
| Angioplasty/stent | 53.5(45.0–63.4) | 31.2(24.9–39.1) |
Extended Length of Stay
eLOS occurred in 6,170 (25.9%) hospitalizations, with significantly less eLOS in PT compared to OT hospitalizations on univariable (22.6% vs 27.4%, p=.0005) and multivariable analysis (aOR=0.80 [95%CI, 0.69–0.94], p=.005). Black and other non-White races were associated with an increased risk of eLOS, as well as those with Medicaid or self-pay insurance. Greater frailty, pre-existing PAD, and hypercoagulable state were also associated with higher risk of eLOS (Figure 3d). The association between reduced eLOS and PT was moderated by race, with only white individuals having decreased eLOS with PT compared to black individuals (pinteraction=0.046; Supplementary Figure 1b).
Sensitivity Analysis
We performed five sensitivity analyses of our primary outcome, by altering the definition of ALI to include an additional ICD-CM-10 code, including concomitant CDT, reclassifying those who underwent both PT and OT as OT, limiting patients to those who underwent intervention within 1 day of hospitalization, and including additional covariates. The odds of amputation and death in the PT group remained similar and non-significant across sensitivity analyses (Table 3).
Table 3.
Sensitivity Analysis of Composite Amputation and Death
| Cohort | total n | PT n (%) | Adjusted Odds Ratio* |
|---|---|---|---|
| Primary cohort | 23,795 | 7,335 (30.8) | 0.98 (0.79–1.12), p=.89 |
| Sensitivity Analysis 1: including I998 ICD-CM-10** code | 25,235 | 7,960 (31.5) | 0.95 (0.77–1.17), p=64 |
| Sensitivity Analysis 2: including concomittant lysis | 28,310 | 10,860 (38.4) | 0.91 (0.75–1.11), p=35 |
| Sensitivity Analysis 3: concomittant PT and OT reclassified as OT | 23,795 | 6,515 (27.4) | 0.93 (0.74–1.18), p=0.57 |
| Sensitivity Analysis 4: OR within 1 day of hospitalization | 21,915 | 6,570 (30.0) | 0.95 (0.76–1.20), p=0.68 |
| Sensitivity Analysis 5: Additional covariates⧺ included | 23,795 | 7,335 (30.8) | 0.96 (0.78–1.20), p=0.75 |
Discussion
This present, large national study of 23,795 U.S. hospitalizations with a primary diagnosis of lower extremity ALI suggests that PT and OT are associated with equivalent in-hospital major amputation and mortality outcomes as measured by equivalence margins. However, our multivariable analysis of in-hospital outcomes suggests that these treatments are associated with differing secondary outcomes. PT was associated with an 110% increased relative risk of in-hospital major reintervention, although the majority of these reinterventions were endovascular. Additionally, this association was not present tibial interventions. We also demonstrated a 20% decreased relative risk of eLOS amongst PT hospitalizations. Our robust results were consistently observed across subgroups and in numerous sensitivity analyses.
Half of patients with ALI die or lose the affected limb within one year, reducing patient quality of life and functional independence1. Yet, the evaluation of ALI treatments is extremely limited with only 48 registered trials evaluating ALI36 on ClinicalTrials.gov in comparison to 334 evaluating chronic limb-threatening ischemia37 and 10,352 evaluating coronary artery disease38. The study of ALI is further impeded by the exclusion of thrombectomy procedures from important quality improvement datasets: OT is excluded from the VQI and PT is excluded from the NSQIP.
The prior data evaluating the association between ALI outcomes and open versus endovascular treatments are limited to three randomized controlled trials (STILE, TOPAS, and the Rochester trial), which compared open revascularization to CDT as opposed to PT39–41. Interestingly, two of three trials (TOPAS and Rochester) included patients with motor deficits. Two of the three trials (STILE and Rochester) reported decreased 30-day composite amputation and death rate with CDT, attributed to higher numbers of cardiopulmonary complications in the open surgical group39,40.
The evaluation of PT is limited to retrospective, single center studies or within larger studies comparing endovascular vs. open approaches. A retrospective analysis of endovascular (AngioJet™ [Boston Scientific®, MA] or CDT) vs. open surgical approaches showed similar major amputation risk but a higher 30-day mortality risk in the open revascularization group, attributed to the increased physiologic stress of open surgical interventions42. Another single-center, retrospective study comparing outcomes of Angiojet PT vs. CDT in patients with Rutherford I and II ALI showed PT had similar limb salvage outcomes with a mortality benefit, demonstrating a nearly 50% reduction in the adjusted risk of mortality12.
Based on these studies, we hypothesized that limb salvage rates would be similar between PT and OT with decreased in-hospital mortality in the PT group. However, we uncovered similar rates of amputation and mortality for PT and OT across all a priori subgroups and sensitivity analyses. Our findings may differ for several reasons. The aforementioned studies included bypass with OT in their analyses, and including higher surgical stress of bypass may have elevated the mortality rate above that of the endovascular group. Furthermore, in certain cases, a surgical embolectomy can be expeditious and minimally stressful, while a lengthy endovascular intervention can prolong exposure to anesthesia, stressing the importance of appropriate patient selection. Additionally, while our cohort was over 200 times larger than existing studies, the available NIS data cannot differentiate between Rutherford classes of ALI, necessitating use of fasciotomies as a surrogate for severity of ischemia. Lastly, NIS data is limited to in-hospital outcomes, rather than 30-day, mid or long-term outcomes. Our overall in-hospital amputation (4.7%) and mortality (6.3%) rates are comparable to previously reported rates of in-hospital events (6–9% amputation and 9–12% mortality), with outcome data from 1998 to 20091. However, morbidity remains high after hospitalization, with reported 1-year amputation rates ranging from 11–18% and 1-year mortality ranging from 26–42%1,43. Further study will be necessary to determine if the use of PT can decrease longer term mortality.
Several secondary outcomes and predictors of poor outcomes were consistent with existing literature. We demonstrated that PT had increased re-intervention but decreased eLOS, consistent with randomized controlled trials comparing open and endovascular treatments of chronic limb threatening ischemia44,45. We found that pre-existing PAD had increased amputation, mortality, reintervention, and eLOS, consistent with a retrospective review that showed ALI patients with thrombosed bypasses had 4-times the risk of 5-year amputation than those with ALI secondary to embolic disease43.
Counterintuitively, we found that younger patients were significantly more likely to undergo in-hospital amputation, consistent with a retrospective review comparing outcomes of patients over or above 50 years of age undergoing revascularization for ALI46. We believe this is likely due to the lower likelihood of underlying vascular disease and less resultant collateralization in younger patients, who thus may present with more dense ischemia and higher likelihood of underlying hypercoagulability, leading to worse outcomes47. One contradictory finding was that in our data, smoking was associated with a decreased risk of in-hospital death, as prior studies that show no association between ALI mortality and smoking1,43. We hypothesize this may be due to healthy user effect (i.e. those who have diagnosis codes for smoking are in cessation programs) or that smokers may represent a different population who is more likely to present with ALI at a younger, healthier state compared to those with other comorbidities such as diabetes or renal failure.
Our analysis suggests that PT is a viable alternative to OT, meeting the requirements for outcome equivalence as quantified by the non-inferiority margin33. Despite increased in-hospital reintervention rates for PT hospitalizations, almost 80% were able to avoid open salvage operations. Additionally, mortality and amputation rates were not affected, and eLOS frequency was decreased. This supports that this strategy may be attractive, particularly in poor surgical candidates who are frail, have contraindications to general anesthesia, or complex re-operative fields. While tibial disease appeared to be a strong predictor for amputation, we demonstrated no risk of reintervention with PT within the tibial subgroup. This may suggest that these patients had more complete revascularization, lessening the necessity of returning to the OR, tibial interventions with PT are more durable compared to OT, or these patients may have had a no reflow phenomenon such that reintervention was felt to be futile, leading to amputation. We also found that multilevel treatment was associated with increased mortality, likely due to both the greater ischemic burden and extent of the physiologically stressful operative intervention required. Therefore, the reduced risk of reintervention for this population may represent that many of these patients didn’t survive to undergo revascularization. ALI is extremely heterogeneous. Our work here, using administrative data to investigate outcomes in a national sample, must be complemented with a more granular dataset to further elucidate which patients may benefit most from this emerging technology. As our understanding of ALI expands to meet that of CTLI, we hope to develop evidence-based criteria to guide therapeutic interventions48,49.
Our study has several limitations. NIS data provide a large sample representative of the U.S., encompassing many sociodemographic communities and practice patterns, and remains the one of the only large databases that captures all ALI cases. However, it relies on ICD-10 coding for diagnoses and procedures, which cannot capture all clinical characteristics (e.g. Rutherford classification, reason for reintervention/fasciotomy, laterality, and device data), introducing potential selection bias. To combat these limitations, we used validated ICD coding strategies1,3,23,26,28,29,32 and excluded those who underwent CDT, with an age <50, and had concurrent trauma/dissection, capturing a patient cohort that was appropriate for either PT or OT based on severity and etiology. Furthermore, because Rutherford classification is not available within these data (Supplementary Table 1), we used intraoperative fasciotomy, occurring in 5–7% of hospitalizations, as a surrogate for ischemic severity. Although the reported rate of fasciotomy use ranges broadly (5–50% ALI revascularization) in the literature, representing variable practice patterns, our data mirror those reported in the literature46,50. Interestingly, Rutherford classification is also omitted from both the NSQIP and VQI, highlighting the omission of ALI specific data available in the literature. Additionally, outcomes may be misclassified as these data only include in-hospital events and do not account for planned reoperations or the competing risk of survival for those undergoing secondary procedures. Lastly, PT is a relatively recent treatment modality for ALI and the 2021 to 2023 NIS data were not yet released, omitting the most current uses and outcomes of PT from our analysis.
Conclusions
PT is an appropriate alternative to OT for the management of ALI with comparable in-hospital limb salvage and mortality rates. Despite the increased risk of in-hospital major reintervention associated with PT, most patients underwent endovascular reinterventions and the risk of eLOS remained lower than with OT. Further, our a priori subgroup analyses suggest that patients undergoing tibial intervention do not have an increased risk of reintervention with PT. However, more study working to identify which patients will benefit most from PT is necessary for appropriate, evidence-based application, particularly due to the higher rate of reintervention. Furthermore, the long-term outcomes of PT such as recurrent ALI and limb salvage could not be assessed and are important considerations that will need to be examined in future studies.
Supplementary Material
Article Highlights.
Type of Research:
Retrospective analysis of the prospectively collected National Inpatient Sample (NIS)
Key Findings:
In a cohort of 23,795 hospitalizations for acute limb ischemia undergoing thrombectomy, we found that 30.8% underwent percutaneous mechanical thrombectomy. Importantly, we found equivalent in-hospital limb-salvage and mortality between percutaneous and open thrombectomy (univariable 10.1% vs 10.9%, p=.43; multivariable aOR=0.96 [95%CI, 0.77–1.20], p=.74), which was consistent across subgroup analyses. However, despite having a higher likelihood of in-hospital reintervention (univariable 15.7% vs 8.1%, p<.0001; multivariable aOR=2.10 [95%CI, 1.72–2.56], p<.0001), 79.1% of the percutaneous group was able to avoid physiologically stressful open salvage operations. Furthermore, PT was not associated with increased reintervention for tibial interventions on subgroup analysis (aOR=1.31 [95% CI, 0.86–2.01], p=.21, pinteraction<.0001).
Take Home Message:
Percutaneous thrombectomy is an appropriate alternative to open thrombectomy given equivalent short-term limb salvage and mortality outcomes, but may potentially be most useful in patients with tibial disease or contraindications to open surgery.
Sources of Funding:
This research was supported by NIH T32HL098036 (Jarosinski). This funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting: Poster Presentation at the Eastern Vascular Society 37th Annual Meeting, Washington, D.C., September 7– 9, 2023.
Disclosures:
The authors report no disclosures or relevant financial interest related to the content of this manuscript.
References
- 1.Baril DT, Ghosh K, Rosen AB. Trends in the incidence, treatment, and outcomes of acute lower extremity ischemia in the United States Medicare population. J Vasc Surg. 2014;60:669–677 e662. doi: 10.1016/j.jvs.2014.03.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Creager MA, Kaufman JA, Conte MS. Clinical practice. Acute limb ischemia. N Engl J Med. 2012;366:2198–2206. doi: 10.1056/NEJMcp1006054 [DOI] [PubMed] [Google Scholar]
- 3.Eliason JL, Wainess RM, Proctor MC, Dimick JB, Cowan JA, Jr., Upchurch GR, Jr., Stanley JC, Henke PK. A national and single institutional experience in the contemporary treatment of acute lower extremity ischemia. Ann Surg. 2003;238:382–389; discussion 389–390. doi: 10.1097/01.sla.0000086663.49670.d1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorck M, Earnshaw JJ, Acosta S, Bastos Goncalves F, Cochennec F, Debus ES, Hinchliffe R, Jongkind V, Koelemay MJW, Menyhei G, et al. Editor’s Choice - European Society for Vascular Surgery (ESVS) 2020 Clinical Practice Guidelines on the Management of Acute Limb Ischaemia. Eur J Vasc Endovasc Surg. 2020;59:173–218. doi: 10.1016/j.ejvs.2019.09.006 [DOI] [PubMed] [Google Scholar]
- 5.de Donato G, Pasqui E, Setacci F, Palasciano G, Nigi L, Fondelli C, Sterpetti A, Dotta F, Weber G, Setacci C. Acute on chronic limb ischemia: From surgical embolectomy and thrombolysis to endovascular options. Semin Vasc Surg. 2018;31:66–75. doi: 10.1053/j.semvascsurg.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 6.Reitz KM, Hall DE, Shinall MC Jr., Shireman PK, Silverstein JC. Using the Unified Medical Language System to Expand the Operative Stress Score - First Use Case. J Surg Res. 2021;268:552–561. doi: 10.1016/j.jss.2021.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shinall MC Jr., Arya S, Youk A, Varley P, Shah R, Massarweh NN, Shireman PK, Johanning JM, Brown AJ, Christie NA, et al. Association of Preoperative Patient Frailty and Operative Stress With Postoperative Mortality. JAMA Surg. 2020;155:e194620. doi: 10.1001/jamasurg.2019.4620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbertson EA, Bailey TR, Kraiss LW, Griffin CL, Smith BK, Sarfati M, Beckstrom J, Brooke BS. Long-Term Impact of Vascular Surgery Stress on Frail Older Patients. Ann Vasc Surg. 2021;70:9–19. doi: 10.1016/j.avsg.2020.06.048 [DOI] [PubMed] [Google Scholar]
- 9.Chang JK, Calligaro KD, Ryan S, Runyan D, Dougherty MJ, Stern JJ. Risk factors associated with infection of lower extremity revascularization: analysis of 365 procedures performed at a teaching hospital. Ann Vasc Surg. 2003;17:91–96. doi: 10.1007/s10016-001-0337-8 [DOI] [PubMed] [Google Scholar]
- 10.King EF A What Is the Best Treatment for Acute Limb Ischemia? Advances in Surgery. 2022;56:287–304. [DOI] [PubMed] [Google Scholar]
- 11.Smeds MR, Sandhu HK, Leake SS, Miller CC, Charlton-Ouw KM. Patterns in the Management of Acute Limb Ischemia: A VESS Survey. Ann Vasc Surg. 2017;38:164–171. doi: 10.1016/j.avsg.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 12.de Athayde Soares R, Matielo MF, Brochado Neto FC, Pereira de Carvalho BV, Sacilotto R. Analysis of the Safety and Efficacy of the Endovascular Treatment for Acute Limb Ischemia with Percutaneous Pharmacomechanical Thrombectomy Compared with Catheter-Directed Thrombolysis. Ann Vasc Surg. 2020;66:470–478. doi: 10.1016/j.avsg.2019.11.038 [DOI] [PubMed] [Google Scholar]
- 13.Katsargyris A, Ritter W, Pedraza M, Moehner B, Bruck M, Verhoeven EL. Percutaneous endovascular thrombosuction for acute lower limb ischemia: a 5-year single center experience. J Cardiovasc Surg (Torino). 2015;56:375–381. [PubMed] [Google Scholar]
- 14.Lopez R, Yamashita TS, Neisen M, Fleming M, Colglazier J, Oderich G, DeMartino R. Single-center experience with Indigo aspiration thrombectomy for acute lower limb ischemia. J Vasc Surg. 2020;72:226–232. doi: 10.1016/j.jvs.2019.10.079 [DOI] [PubMed] [Google Scholar]
- 15.Rossi M, Tipaldi MA, Tagliaferro FB, Pisano A, Ronconi E, Lucertini E, Daffina J, Caruso D, Laghi A, Laurino F. Aspiration Thrombectomy with the Indigo System for Acute Lower Limb Ischemia: Preliminary experience and analysis of parameters affecting the outcome. Ann Vasc Surg. 2021;76:426–435. doi: 10.1016/j.avsg.2021.04.016 [DOI] [PubMed] [Google Scholar]
- 16.Vorwerk D, Triebe S, Ziegler S, Ruppert V. Percutaneous Mechanical Thromboembolectomy in Acute Lower Limb Ischemia. Cardiovasc Intervent Radiol. 2019;42:178–185. doi: 10.1007/s00270-018-2129-3 [DOI] [PubMed] [Google Scholar]
- 17.Kronlage M, Printz I, Vogel B, Blessing E, Muller OJ, Katus HA, Erbel C. A comparative study on endovascular treatment of (sub)acute critical limb ischemia: mechanical thrombectomy vs thrombolysis. Drug Des Devel Ther. 2017;11:1233–1241. doi: 10.2147/DDDT.S131503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S, Chan PS, Krumholz HM. Adherence to Methodological Standards in Research Using the National Inpatient Sample. JAMA. 2017;318:2011–2018. doi: 10.1001/jama.2017.17653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghaferi AA, Schwartz TA, Pawlik TM. STROBE Reporting Guidelines for Observational Studies. JAMA Surg. 2021;156:577–578. doi: 10.1001/jamasurg.2021.0528 [DOI] [PubMed] [Google Scholar]
- 20.Healthcare Cost and Utilization Project AfHRaQ. Overview of the National (Nationwide) Inpatient Sample (NIS). https://hcup-us.ahrq.gov/nisoverview.jsp. 2022. Accessed 6/16/23.
- 21.Healthcare Cost and Utilization Project AfHRaQ. Producing National HCUP Estimates. https://hcup-us.ahrq.gov/tech_assist/nationalestimates/508_course/508course_2018.jsp#weights. 2018. Accessed 6/16/23.
- 22.Healthcare Cost and Utilization Project AfHRaQ. NIS Description of Data Elements. https://hcup-us.ahrq.gov/db/nation/nis/nisdde.jsp. 2022. Accessed 6/16/2023.
- 23.Columbo JA, Kang R, Trooboff SW, Jahn KS, Martinez CJ, Moore KO, Austin AM, Morden NE, Brooks CG, Skinner JS, et al. Validating Publicly Available Crosswalks for Translating ICD-9 to ICD-10 Diagnosis Codes for Cardiovascular Outcomes Research. Circ Cardiovasc Qual Outcomes. 2018;11:e004782. doi: 10.1161/CIRCOUTCOMES.118.004782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran Z, Zhang W, Verma A, Cook A, Kim D, Burruss S, Ramezani R, Benharash P. The derivation of an International Classification of Diseases, Tenth Revision-based trauma-related mortality model using machine learning. J Trauma Acute Care Surg. 2022;92:561–566. doi: 10.1097/TA.0000000000003416 [DOI] [PubMed] [Google Scholar]
- 25.Treffalls JA, Sylvester CB, Parikh U, Zea-Vera R, Ryan CT, Zhang Q, Rosengart TK, Wall MJ, Coselli JS, Chatterjee S, et al. Nationwide database analysis of one-year readmission rates after open surgical or thoracic endovascular repair of Stanford Type B aortic dissection. JTCVS Open. 2022;11:1–13. doi: 10.1016/j.xjon.2022.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Healthcare Cost and Utilization Project AfHRaQ. Elixhauser Comorbidity Software Refined for ICD-10-CM. https://hcup-us.ahrq.gov/toolssoftware/comorbidityicd10/comorbidity_icd10.jsp. 2022. Accessed 6/6/2023.
- 27.Rothenberg KA, George EL, Trickey AW, Barreto NB, Johnson TM 2nd, Hall DE, Johanning JM, Arya S. Assessment of the Risk Analysis Index for Prediction of Mortality, Major Complications, and Length of Stay in Patients who Underwent Vascular Surgery. Ann Vasc Surg. 2020;66:442–453. doi: 10.1016/j.avsg.2020.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Rein N, Heide-Jorgensen U, Lijfering WM, Dekkers OM, Sorensen HT, Cannegieter SC. Major Bleeding Rates in Atrial Fibrillation Patients on Single, Dual, or Triple Antithrombotic Therapy. Circulation. 2019;139:775–786. doi: 10.1161/CIRCULATIONAHA.118.036248 [DOI] [PubMed] [Google Scholar]
- 29.Delate T, Hsiao W, Kim B, Witt DM, Meyer MR, Go AS, Fang MC. Assessment of algorithms to identify patients with thrombophilia following venous thromboembolism. Thromb Res. 2016;137:97–102. doi: 10.1016/j.thromres.2015.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desai RJ, Solomon DH, Shadick N, Iannaccone C, Kim SC. Identification of smoking using Medicare data--a validation study of claims-based algorithms. Pharmacoepidemiol Drug Saf. 2016;25:472–475. doi: 10.1002/pds.3953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karonen E, Wrede A, Acosta S. Risk Factors for Fasciotomy After Revascularization for Acute Lower Limb Ischaemia. Front Surg. 2021;8:662744. doi: 10.3389/fsurg.2021.662744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanken ZJ, Anderson PB, Bessen SY, Rode JB, Columbo JA, Trooboff SW, Moore KO, Goodney PP. Translating coding lists in administrative claims-based research for cardiovascular procedures. J Vasc Surg. 2020;72:286–292. doi: 10.1016/j.jvs.2019.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26:192–196. doi: 10.1007/s11606-010-1513-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conte MS, Geraghty PJ, Bradbury AW, Hevelone ND, Lipsitz SR, Moneta GL, Nehler MR, Powell RJ, Sidawy AN. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. J Vasc Surg. 2009;50:1462–1473 e1461–1463. doi: 10.1016/j.jvs.2009.09.044 [DOI] [PubMed] [Google Scholar]
- 35.2023. ICD-10-CM DIagnosis Code I99.8 https://www.icd10data.com/ICD10CM/Codes/I00-I99/I95-I99/I99-/I99.8. Accessed 07/06/2023.
- 36.Medicine NLo. Search Results: acute limb ischemia https://clinicaltrials.gov/search?cond=acute%20limb%20ischemia. Accessed 7/19/23.
- 37.Medicine NLo. Search Results: chronic limb threatening ischemia. https://clinicaltrials.gov/search?cond=chronic%20limb%20threatening%20ischemia. Accessed 7/19/23.
- 38.Medicine NLo. Search Results: coronary artery disease. https://clinicaltrials.gov/search?cond=coronary%20artery%20disease. Accessed 7/19/23.
- 39.Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann Surg. 1994;220:251–266; discussion 266–258. doi: 10.1097/00000658-199409000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouriel K, Shortell CK, DeWeese JA, Green RM, Francis CW, Azodo MV, Gutierrez OH, Manzione JV, Cox C, Marder VJ. A comparison of thrombolytic therapy with operative revascularization in the initial treatment of acute peripheral arterial ischemia. J Vasc Surg. 1994;19:1021–1030. doi: 10.1016/s0741-5214(94)70214-4 [DOI] [PubMed] [Google Scholar]
- 41.Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med. 1998;338:1105–1111. doi: 10.1056/NEJM199804163381603 [DOI] [PubMed] [Google Scholar]
- 42.Taha AG, Byrne RM, Avgerinos ED, Marone LK, Makaroun MS, Chaer RA. Comparative effectiveness of endovascular versus surgical revascularization for acute lower extremity ischemia. J Vasc Surg. 2015;61:147–154. doi: 10.1016/j.jvs.2014.06.109 [DOI] [PubMed] [Google Scholar]
- 43.Genovese EA, Chaer RA, Taha AG, Marone LK, Avgerinos E, Makaroun MS, Baril DT. Risk Factors for Long-Term Mortality and Amputation after Open and Endovascular Treatment of Acute Limb Ischemia. Ann Vasc Surg. 2016;30:82–92. doi: 10.1016/j.avsg.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, Fowkes FG, Gillepsie I, Ruckley CV, Raab G, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5 [DOI] [PubMed] [Google Scholar]
- 45.Farber A, Menard MT, Conte MS, Kaufman JA, Powell RJ, Choudhry NK, Hamza TH, Assmann SF, Creager MA, Cziraky MJ, et al. Surgery or Endovascular Therapy for Chronic Limb-Threatening Ischemia. N Engl J Med. 2022;387:2305–2316. doi: 10.1056/NEJMoa2207899 [DOI] [PubMed] [Google Scholar]
- 46.Andraska EA, Phillips AR, Reitz KM, Asaadi S, Ho J, McDonald MM, Madigan M, Liang N, Eslami M, Sridharan N. Young patients without prior vascular disease are at increased risk of limb loss and reintervention after acute limb ischemia. J Vasc Surg. 2022;76:1354–1363 e1351. doi: 10.1016/j.jvs.2022.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torrealba JI, Osman M, Kelso R. Hypercoagulability predicts worse outcomes in young patients undergoing lower extremity revascularization. J Vasc Surg. 2019;70:175–180. doi: 10.1016/j.jvs.2018.09.062 [DOI] [PubMed] [Google Scholar]
- 48.Beropoulis E, Stavroulakis K, Schwindt A, Stachmann A, Torsello G, Bisdas T. Validation of the Wound, Ischemia, foot Infection (WIfI) classification system in nondiabetic patients treated by endovascular means for critical limb ischemia. J Vasc Surg. 2016;64:95–103. doi: 10.1016/j.jvs.2016.01.040 [DOI] [PubMed] [Google Scholar]
- 49.Shirasu T, Takagi H, Gregg A, Kuno T, Yasuhara J, Kent KC, Clouse WD. Predictability of the Global Limb Anatomic Staging System (GLASS) for Technical and Limb Related Outcomes: A Systematic Review and Meta-Analysis. Eur J Vasc Endovasc Surg. 2022;64:32–40. doi: 10.1016/j.ejvs.2022.03.044 [DOI] [PubMed] [Google Scholar]
- 50.Lin JH, Humphries MD, Hasegawa J, Saroya J, Mell MW. Outcomes After Selective Fasciotomy for Revascularization of Nontraumatic Acute Lower Limb Ischemia. Vasc Endovascular Surg. 2022;56:18–23. doi: 10.1177/15385744211045493 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


