Abstract
Purpose
Patients with multiple endocrine neoplasia type 1 (MEN1) are predisposed to develop duodenopancreatic neuroendocrine tumors (dpNETs), and metastatic dpNET is the primary cause of disease-related mortality. Presently, there is a paucity of prognostic factors that can reliably identify patients with MEN1-related dpNETS who are at high risk of distant metastasis. In the current study, we aimed to establish novel circulating molecular protein signatures associated with disease progression.
Experimental Design
Mass spectrometry-based proteomic profiling was conducted on plasmas procured through an international collaboration between MD Anderson Cancer Center, the National Institutes of Health, and the University Medical Center Utrecht from a cohort of 56 patients with MEN1 [14 with distant metastasis dpNETs (cases) and 42 with either indolent dpNETs or no dpNETs (controls)]. Findings were compared to proteomic profiles generated from serially collected plasmas from a mouse model of Men1-pancreatic neuroendocrine tumors (Men1fl/flPdx1-CreTg) and control mice (Men1fl/fl).
Results
A total of 187 proteins were found to be elevated in MEN1 patients with distant metastasis compared to controls, including 9 proteins previously associated with pancreatic cancer and other neuronal proteins. Analyses of mouse plasmas revealed 196 proteins enriched for transcriptional targets of oncogenic MYCN, YAP1, POU5F1, and SMAD that were associated with disease progression in Men1fl/flPdx1-CreTg mice. Cross-species intersection revealed 19 proteins positively associated with disease progression in both human patients and in Men1fl/flPdx1-CreTg mice.
Conclusions
Our integrated analyses identified novel circulating protein markers associated with disease progression in MEN1-related dpNET.
Keywords: biomarkers, prognosis, multiple endocrine neoplasia type 1, duodenopancreatic neuroendocrine tumors
Multiple endocrine neoplasia type 1 (MEN1) is an inherited autosomal dominant disease caused by germline loss-of-function mutations in the MEN1 gene that predisposes individuals to endocrine tumors (1). While MEN1 is a rare disease with an estimated prevalence of 1 in 20 000 to 1 in 40 000, 4 out of 5 patients with MEN1 will develop duodenopancreatic neuroendocrine tumors (dpNET) during their lifetime (2, 3). These MEN1-related dpNETs share common features, including the loss of MEN1 as driver event, foregut origin, and association with endocrine cell hyperplasia and micro-adenomas as precursor lesions. Notwithstanding, there is substantial clinical heterogeneity among dpNETs depending on, but not limited to, differences in primary site (duodenum vs pancreas) and functionality (nonfunctioning vs insulinomas, gastrinomas and more rare functioning tumors such as VIPomas). Moreover, even between dpNETs of the same primary site and functionality, disease progression varies. Metastatic dpNETs are the significant contributors to disease-related mortality in patients with MEN1 (2, 4). Yet, only a subset of MEN1 patients will develop distant metastases from a dpNET.
Currently available blood-based biomarkers such as chromogranin A, glucagon, or pancreatic polypeptide have limited prognostic value, and only elevated gastrin is reported to be adversely associated with survival (5–7). Other liquid biopsy tests, such as the NETest, a novel transcriptomic-based biomarker test for detection of neuroendocrine tumors (NETs) (8), have not been evaluated in the context of MEN1. The development of blood-based markers that can identified patients with MEN1-related dpNETs that are at high risk of distant metastasis remains an unmet need, which—if met—can lead to a better understanding of disease progression and potential targets of therapeutic intervention as well as better identify patients who would benefit from more intensive follow-up and early interception.
Genetically engineered mouse (GEM) models provide a powerful tool to study the initiation, progression, and maintenance of cancers and have a strong history in informing our understanding of MEN1 and the cancers driven by MEN1 loss. For example, germline heterozygous mice (Men1+/−) are predisposed to tumorigenesis and develop a tumor spectrum consistent with MEN1 patients, and these tumors undergo loss of heterozygosity (9, 10). Conditional alleles have subsequently been combined with tissue- and cell-type specific Cre drivers. In the pancreas, insulinomas are the major tumor type that arise when Men1 is lost from β-cells (11–13), α-cells (14, 15), or more generally from all epithelial cells of the pancreas (16). While RIP-CreTg is frequently used to target Men1 deletion from β-cells, mice also develop pituitary tumors (11). Using the Pdx1-CreTg line, which generally targets deletion in pancreatic progenitors during embryonic development, restricts tumor development to the pancreas (16, 17). The progressive transformation of normal islets to pancreatic neuroendocrine tumors (PanNETs) provides a strong experimental platform for understanding tumorigenesis when Men1 loss is the driving genetic lesion.
Previously, we reported a plasma acetylated polyamine metabolite signature that was associated with MEN1-dpNET disease progression (18). Specifically, we showed that a panel of 3 acetylated polyamines could distinguish patients with MEN1 with liver metastasis from a dpNET from those with an indolent, nonmetastatic dpNET as well as those without dpNETs. We further demonstrated that acetylated polyamines were elevated early and persisted through disease progression in a Men1-PanNET mouse model, Men1fl/flPdx1-CreTg (18).
In the current study, we expand upon our prior investigations by interrogating dynamic changes in the plasma proteome associated with MEN1-related dpNET progression. For proteomic profiling, we used 2 distinct yet complementary sources of biospecimens that converge on the loss of MEN1/Men1 as the initiating and driving genetic event: human plasmas from a unique cross-sectional cohort representing the disease progression in patients with MEN1 consisting of patients with liver metastases from a dpNET (cases, n = 14), patients with indolent dpNET(s) without metastases (controls-1, n = 28), and patients without visible dpNETs (controls-2, n = 14) and the Men1fl/flPdx1-CreTg mouse model of Men1-pNET and corresponding Men1fl/fl controls. Generated proteomic profiles for the human and mouse cohorts were analyzed separately and findings intersected to assess for concordant circulating protein signatures associated with MEN1-dpNET disease progression.
Materials and Methods
Human Subjects
Detailed information regarding human specimens is described in our prior publication. Briefly, biospecimens were collected through an international collaboration between MD Anderson Cancer Center (MDACC), the National Institutes of Health (NIH), and the University Medical Center Utrecht (UMCU). At each center, plasma and clinical data were collected under existing institutional review board-approved protocols after written informed consent or the approval for waiver of informed consent was obtained as applicable. Under institutional review board-approved MDACC protocol PA19-0498, these biospecimens and associated retrospectively collected clinical data were used for the present study, with a waiver of informed consent.
EDTA plasmas were obtained from 14 case subjects with MEN1 and liver metastases from a dpNET and 2 types of controls: patients with MEN1 and a nonmetastatic (distant or regional) indolent dpNET (n = 28; controls-1) and patients with MEN1 without a visible dpNET or other NET (n = 14; controls-2) (Table 1) (18).
Table 1.
Patient and tumor characteristics for the discovery set at the time of sample collection
| Cases | Controls#1 | Controls#2 | |
|---|---|---|---|
| n | 14 | 28 | 14 |
| Sex, n (%) | |||
| Male | 6 (43) | 13 (46) | 7 (50) |
| Female | 8 (57) | 15 (54) | 7 (50) |
| Age (median, IQR) | 52.5 (41.8-60) | 39.5 (28.5-58) | 29.5 (22-38.5) |
| BMI (median, IQR)a | 26 (22.8-32.3) | 26 (23-36) | 23 (20.5-24.5) |
| Collection site, n (%) | |||
| MDACC | 5 (36) | 3 (11) | 1 (7) |
| NIH-NIDDK | 5 (36) | 0 (0) | 1 (7) |
| UMCU | 4 (29) | 25 (89) | 12 (86) |
| PanNET | 13b | 28 | — |
| Prior dpNET surgery, n (%) | 7 (50) | 1 (4) | — |
| Size largest PanNET resected | |||
| ≤20 mm | 2 | — | — |
| >20 mm | 3 | 1 | — |
| N/A (only duodenal/lymph node) | 2 | — | — |
| Size of largest PanNET, n (%) (in situ at sample collection) | 12 (4-29) | 11 (6-23) | — |
| <20 mm | 11 (79) | 26 (93) | — |
| ≥20 mm | 2 (14) | 2 (7) | — |
| Insulinoma, n (%)c | |||
| No | 13 (93) | 27 (96) | 14 (100) |
| Yes | 1 (7) | — | — |
| Suspected | — | 1 (4%) | — |
| Gastrinoma, n (%)d | |||
| No | 4 (29) | 26 (93) | 14 (100) |
| Yes | 7 (50) | — | — |
| Suspected | — | — | — |
| Unknown | 3 (21) | 2 (7) | — |
| Gastrin pg/mL (median, IQR)e | 351 (151-1448) | 75 (55-145) | 57.5 (48.8-80) |
| Other function in PanNETf | 1 (VIP) | 0 | — |
| Liver metastasis, origin, n (%) | |||
| PanNET | 10 (71) | — | — |
| Gastrinoma | 2 (14) | — | — |
| PanNET or gastrinoma | 2 (14) | — | — |
| Liver metastasis, n (%) | |||
| 1 | 6 (43) | — | — |
| 2 or 3 | 3 (21) | — | — |
| More than 3 | 5 (36) | — | — |
| Size largest liver metastasis in mm (median, IQR) | 11 (8.5-14) | — | — |
| Distant metastases outside liver, n (%) | 1 (7) | — | — |
| Systemic or liver-directed therapy, n (%) | — | ||
| None | 10 (71) | 28 (100%) | 14 (100%) |
| Previousg | 3 (21) | — | — |
| On active treatmenth | 1 (7) | — | — |
Abbreviations: BMI, body mass index; dpNET, duodenopancreatic neuroendocrine tumor; IQR, interquartile range; MDACC, MD Anderson Cancer Center; N/A, not applicable; NIH-NIDDK, National Institutes of Health-National Institute of Diabetes and Digestive and Kidney Diseases; PanNET, pancreatic neuroendocrine tumor; UMCU, University Medical Center Utrecht; VIP, vasoactive intestinal peptide.
a BMI data was not available for 10 control subjects.
b One case had total or partial pancreatectomy but presented with dpNET-related liver metastasis at the time of blood collection.
c Insulinoma was defined as a positive supervised fast or symptoms confirmed by low plasma glucose, inappropriate insulin levels, and resolution of symptoms with ingestion of calories, not in a supervised fast setting.
d A gastrinoma diagnosis was made when 1 of the following criteria were met: (1) gastrin >10 times the upper limit of normal (ULN) or (2) gastrin >2ULN twice consecutive in the absence of proton pump inhibitor use (no value <2ULN allowed in between) and not followed by 2 consecutive measurements <2ULN without surgery or start of systemic antitumor therapy or (3) gastrin is >5ULN twice consecutive in the presence of proton pump inhibitor use (no value <5ULN allowed in between) and not followed by 2 consecutive measurements <5ULN without surgery or start of systemic antitumor therapy or (4) positive secretin test or (5) there is dpNET or lymph nodes/liver metastases with positive immunohistochemistry for gastrin.
e Gastrin levels closest to sample collection within ± 12 months window (ULN 100 pg/mL). Gastrin levels were not available for 1 out of the 14 cases and 1 out of the 42 control patients.
f Other functioning tumors were defined as a clinical syndrome in conjunction with elevated hormone levels at least 2 times ULN.
g Three cases were previously treated; 1 was treated with neoadjuvant chemotherapy and somatostatin analogues 1.2 years prior to blood draw, 1 with Yttrium embolization of liver metastases 7 years prior to blood draw, and 1 with chemotherapy 12 years before sample collection and somatostatin analogue up until 9 years before sample collection. None of these 3 cases were on active treatment at the time of blood draw.
h One case was on active treatment with somatostatin analogues.
Patients were included for the present study if they had MEN1 according to any of the following: (1) a confirmed germline MEN1 mutation, (2) 1 of the 3 major manifestations (parathyroid, pituitary, dpNET) and a first-degree family member with a confirmed MEN1 mutation or if no genetic testing was performed, (3) 2 of the 3 major manifestations including a dpNET and a first-degree family member meeting the same criteria. Exclusion criteria were any active non-NET malignancy, an active thymus NET or active thymoma, rapidly progressive or metastatic lung or gastric NET, and poorly differentiated neuroendocrine carcinoma.
For cases, liver metastases were confirmed through histological examination or through consecutive positive imaging (magnetic resonance imaging, computed tomography, and/or somatostatin-receptor imaging). An expert panel [C.P. (all sites), G.V. (UMCU), D.H. (MDACC), J.B. (NIH)] reviewed all cases and in case of both pancreatic and duodenal primaries identified the most likely origin of the distant metastases considering all available biochemical, imaging, and histological information, showing that 71% of distant metastases were of pancreatic origin and 14% of duodenal origin; for 2 cases (14%) it could not be reliably determined if the duodenal or pancreatic primary was the origin (Table 1).
Criteria for controls-1 were a minimum of 3-year follow-up after diagnosis of the dpNET and imaging (magnetic resonance imaging, computed tomography, and/or somatostatin-receptor imaging) taken 1 or more years after blood draw demonstrating absence of distant or regional metastasis. Criteria for controls-2 were that patients had no diagnosis of other NETs (ie, lung, thymus, or gastric NET), had no prior diagnosis of dpNET, and were negative for dpNETs at the time of blood collection confirmed either by combined conventional and somatostatin-receptor imaging or through conventional imaging taken 6 or more months post blood draw.
Genetically Engineered Mouse Model of MEN1-PanNET
All mouse experiments were performed in compliance with the NIH guidelines for animal research and approved by the MDACC Institutional Animal Care and Use Committee. Detailed information regarding mouse experiments is provided in our previous publication (18).
Briefly, Men1fl/fl conditional knockout (Stock No. 005109) and Pdx1-CreTg (Stock No. 014647) mice were obtained from The Jackson Laboratory (11, 19). Men1fl/flPdx1-CreTg mice develop hyperplastic islets at 5 to 6 months, which progress to insulinomas at 10 to 12 months (16). Hypoglycemia caused by hyperinsulinemia as a result of functional PanNETs is the primary cause of morbidity in Men1fl/flPdx1-CreTg mice (16).
Mice were maintained on a mixed background (C57Bl/6, FVB, 129), Cre-negative littermates (Men1fl/fl) were used as functionally wild-type controls, and both male and female mice were used in our analyses. Blood was collected at 8- and 12-month time points through retro-orbital sampling under general anesthesia.
Proteomic Analyses
For analysis of the human and mouse plasma proteome, we used 6-plex and 10-plex Lys-tandem mass tag (TMT) channel quantification-based approaches, respectively. For human samples, each 6-plex Lys-TMT channel consisted of 1 case (patients with liver metastases from a dpNET), 2 controls-1 [patients with a nonmetastatic (distant or regional) indolent dpNET], 1 controls-2 (patients without any NET), a reference pooled quality control sample, and a batch-specific pooled quality control sample. For mouse samples, each 10-plex Lys-TMT channel consisted of serial samples collected at ages 8 and 12 months from 2 Men1fl/flPdx1-CreTg mice, 2 Men1fl/fl control mice, a reference pooled quality control sample, and a batch-specific pooled quality control sample.
For human specimens, plasma volumes of 100 μL were processed using immuno-depletion affinity column Hu-14 10 × 100 mm (Agilent Technologies, Santa Clara, CA USA, catalog no. 5188-6559) to remove 14 high-abundance plasma proteins: albumin, IgG, IgA, transferrin, haptoglobin, fibrinogen, α1-antitrypsin, α1-acid glycoprotein, apolipoprotein AI, apolipoprotein AII, complement C3, transthyretin, IgM, and α2-macroglobulin. For mouse specimens, 50 μL of serially collected EDTA plasma was processed using an immuno-depletion affinity column Mu-3 6 × 50 mm (Agilent Technologies, Santa Clara, CA USA, catalog no. 5188-5217) to remove 3 high-abundance plasma proteins: albumin, IgG, and transferrin.
The respective flow-through fraction was then used for profiling the lower abundance free (non-Ig bound) plasma proteome. To prepare for proteomics analysis, samples were concentrated and reduced with Tris (2-chloroethyl) phosphate and alkylated by 2-chloro-N, N-diehtylcarbamidomethyl (diethylcarbamidomethyl). Next, the buffer was exchanged to triethylammonium bicarbonate and trypsin digested, 100 μg corresponding peptides from each sample was desalted by C18-CX Mono-spin column (GL Sciences, Torrance, CA, USA) and dried by SpeedVac (Thermo Fisher Scientific, Waltham, MA, USA).
Human samples were subsequently individually dissolved and labeled with 6 plex Lys-TMT channel whereas mouse samples were individually dissolved and labeled with 10 plex Lys-TMT channel (Thermo Fisher Scientific, catalog no. 90309). Respective human or mouse tandem mass tag-labeled samples were then combined and fractionated into 12 fractions with alkaline 0.1% triethylamine/acetonitrile reversed phase mode using a C18 Monospin Large column (GL Sciences, Torrance, CA, USA). The step elution was done by B concentration of 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 70%, 80%, and 100% using mobile phase A (0.1% triethylamine in water/acetonitrile 98/2) and Mobile phase B (0.1% triethylamine in water/acetonitrile 5/95), then the fractions were dried by the SpeedVac (Thermo Fisher Scientific). Dried samples were then reconstituted with acetonitrile/water/trifluoroacetic acid (2:98:0.1, v/v/v) and individually analyzed by Easy nanoLC 1000 system (Thermo Scientific) coupled to a Q-exactive mass spectrometer using a 15 cm column (75 μm ID, C18 3 μm, Column Technology Inc.) as a separation column, and a Symmetry C18 180 um ID × 20 mm trap column (Waters Inc., Milford, MA, USA) over a 120-minute gradient. Mass spectrometer parameters were spray voltage 3.0 kV, capillary temperature 275°C, full scan mass spectrometery of scan range 350 to 1800 m/z, resolution 70 000, AGC target 3e6, maximum It 50 msec, and data-dependent tandem mass spectrometry scan of resolution 17 500 in 6-plex and 30 000 for 10-plex in profile mode, AGC target 1e5, maximum IT 100 msec, and repeat count 10 in high-energy C-trap dissociation mode.
Acquired mass spectrometry data were processed using Proteome Discover 1.4 (Thermo Fisher Scientific). The tandem mass spectra from human samples were searched against Uniprot human database 2017 using Sequest HT whereas tandem mass spectra from mouse samples were searched against the Uniprot mouse database 2017 using Sequest HT. Modification parameters included: fixed modification of Cys alkylated with diethylcarbamidomethyl (+113.084064), Lys with multiplex TMT (+229.162932, N-terminal and Lys), and variable modification of methionine oxidation (+15.99491). The precursor mass tolerance of the parent and fragment mass were 10 ppm and 0.02 Da, respectively. Searched data was further processed with the Target Decoy PSM Validator function with a false-discovery rate of 0.05.
Ingenuity Pathway Analysis
Ingenuity pathway analysis (IPA) was conducted with proteins identified by mass spectrometry that were elevated in patients with distant metastatic MEN1-related dpNETs as well as in plasmas of Men1fl/flPdx1-CreTg mice compared to respective controls. Statistical significance of enriched pathways was determined by 2-sided Fisher's exact test.
Statistical Analyses
For human subjects, we report areas under the receiver operating characteristic curves (AUC); AUCs were generated using R statistical software (https://www.r-project.org/). The 95% confidence intervals for individual biomarker performance were based on the bootstrap procedure in which we resampled with replacement 2000 bootstrap samples.
A generalized linear-mixed effects model was used to determine statistical significance for association between plasma proteins and disease progression in a mouse model of Men1-pNET. Here, our 2 variables of interest were group (Men1fl/flPdx1-CreTg/Men1fl/fl) effects and time of blood draw; sex was considered as a random effect. Figures were generated in either GraphPad Prism v6 or R statistical software.
Results
Proteomic Profiling of Plasmas From Patients With Distant Metastatic MEN1-related dpNETs
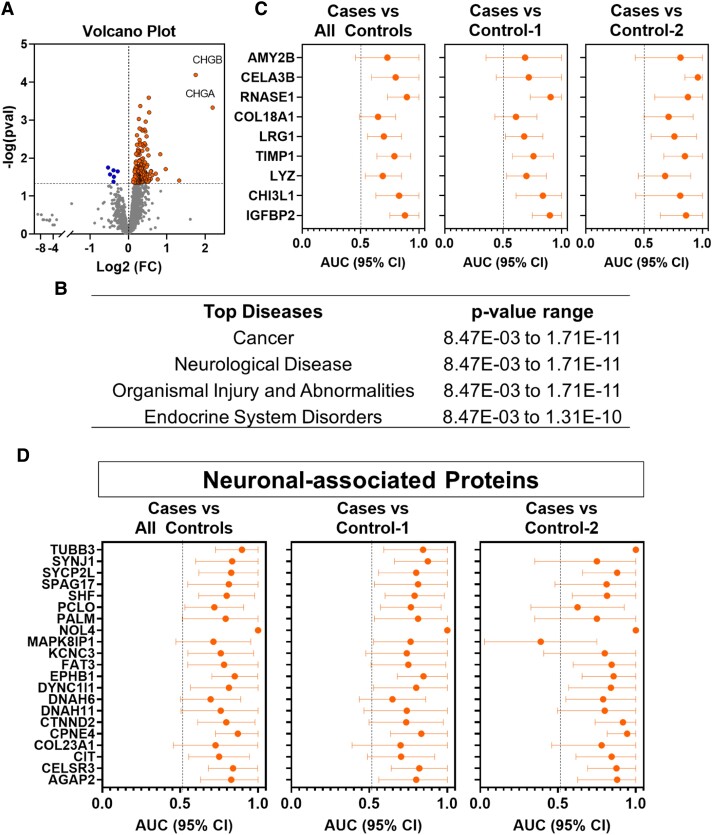
We performed mass spectrometry-based 6-plex TMT analyses on a set of plasmas from 14 MEN1 cases (patients with distant metastasis from a dpNET) and 42 MEN1 controls (28 patients with indolent dpNETs + 14 patients without dpNETs) (Table 1). A total of 3251 proteins were quantified in at least 25% of the samples. Of these, 194 were statistically significantly (raw P < .05) differential between case plasmas and respective controls, with 187 proteins being elevated in cases and 7 proteins being reduced (Fig. 1A; Supplementary Table S1) (20).
Figure 1.
Protein signatures associated with distant metastatic MEN1-related dpNETS. (A) Volcano plot showing differential circulating proteins in patients with metastatic MEN1-related dpNETs (Cases; n = 14) compared to MEN1 patients with indolent dpNETs and without distant or regional metastases (Control-1; n = 28) or MEN1 patients without dpNETs (Control-2; n = 14). Orange nodes represent those proteins that were statistically significantly (raw P < 0.05) elevated in cases compared to controls; blue nodes represented proteins that are statistically significantly decreased. Horizonal dashed line shows raw P-value threshold of 0.05. P-values were determined by 2-sided Student T-test. (B) Ingenuity pathway analysis of the 187 proteins elevated in case plasmas revealed cancer, neurological disease, organismal injury, and abnormalities and endocrine system disorders among top predicted diseases. (C) Dot plots showing AUCs (95% CI) of proteins with reported relevance to pancreatic cancer for distinguishing cases from respective controls. (D) Dot plot AUCs (95% CI) of neuronal-associated proteins for distinguishing cases from respective controls. Abbreviations: AUC, area under the receiver operating characteristic curve; CHG, chromogranin; CI, confidence interval; dpNET, duodenopancreatic neuroendocrine tumor.
IPA of the 187 proteins elevated in case plasmas revealed cancer, neurological disease, organismal injury and abnormalities, and endocrine system disorders among top predicted diseases (Fig. 2B and Supplementary Table S2) (20). Of the 187 elevated proteins, 32 (25.0%) were correlated (Spearman ρ coefficient >0.25 or < −0.25) with dpNET size and 122 (65.2%) correlated with extent of liver tumor burden (Supplementary Table S3) (20).
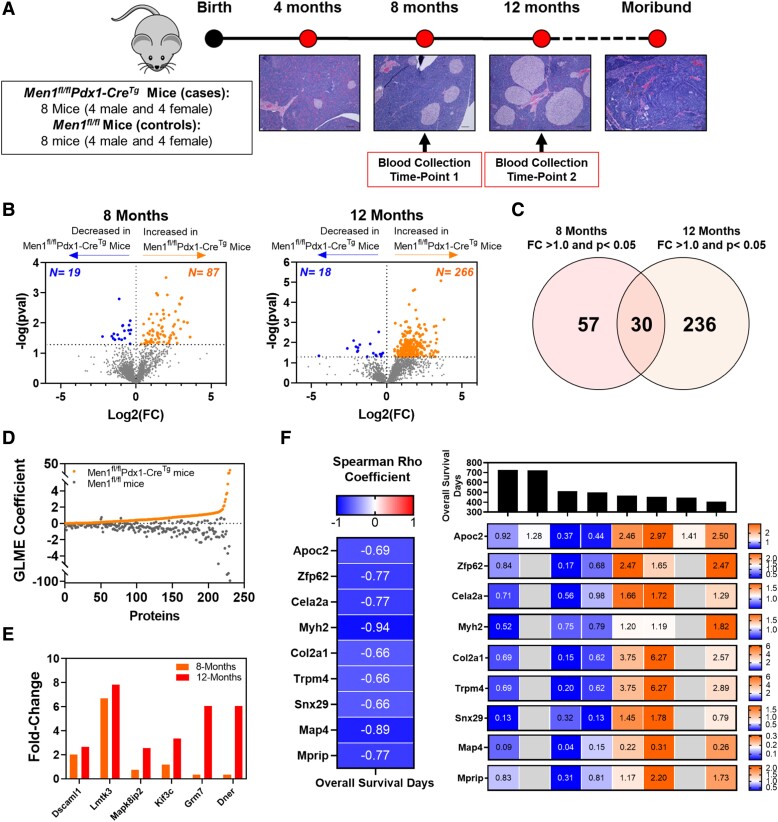
Figure 2.
Proteomic signatures associated with disease progression in a MEN1-PanNET mouse model. (A) Schematic of study design. Figure was adapted from a prior publication (18). (B) Volcano plots showing differential circulating proteins in Men1fl/flPdx1-CreTg compared to Men1fl/fl control mice at age 8 (left) and 12 months (right). Orange nodes represent those proteins that were statistically significantly (raw P < 0.05) elevated in Men1fl/fl Pdx1-CreTg mice; blue nodes represented proteins that are statistically significantly decreased. Horizonal dashed line shows raw P-value threshold of 0.05. P-values were determined by 2-sided Student T-test. (C) Venn diagram showing overlap between proteins that were statistically significantly elevated in Men1fl/flPdx1-CreTg mice at age 8 and 12 months. (D) Scatter plot showing coefficients from the generalized linear mixed effect models for proteins that were positively associated with disease progression in Men1fl/flPdx1-CreTg mice. (E) Bar plot showing fold change of proteins elevated in Men1fl/flPdx1-CreTg mice and that are associated with neuronal development and function. (F) Proteins that were statistically significantly [Log-rank (Mantel-Cox) test sided P < .05] with poor overall survival in Men1fl/flPdx1-CreTg mice at the 8-month sampling timepoint. For survival analyses, proteins were stratified into high or low based on median level. The left heatmap shows Spearman correlation coefficients for respective proteins and overall survival days. The right heatmap displays normalized circulating protein levels and overall survival times among individual Men1fl/flPdx1-CreTg mice. Grey boxes indicate that the protein was not quantified in the respective sample. Abbreviations: PanNET, pancreatic neuroendocrine tumor.
Among the proteins statistically significantly elevated in case plasmas were CHGA and CHGB, with reported relevance for detection of pancreatic neuroendocrine tumors (Fig. 1A) (21, 22), as well as several acute phase response proteins (12 out of 187) and proteins with reported relevance to hepatocellular carcinoma (21 out of 187) (Supplementary Tables S1 and S4) (20). Numerous proteins with relevance to pancreatic cancer (23–26) including IGFBP2, CHI3L1, LYZ, TIMP1, LRG1, and COL18A1 as well as pancreas-specific proteins with exocrine function (AMY2B, CELA3B, and RNASE1) were also found to be elevated (AUC ≥0.65) in case plasmas compared to respective controls (Fig. 1C and Supplementary Table S1) (20). Several neuronal proteins were also found to be elevated case plasmas, including AGAP2, CELSR3, CIT, COL23A1, CPNE4, CTNND2, DNAH6, DNAH11, DYNC1I1, EPHB1, FAT3, KCNC3, MAPK8IP1, NOL4, PALM, PCLO, SHF, SPAG17, SYCP2L, SYNJ1, and TUBB3. Individual performances (AUC) of neuronal proteins for distinguishing cases from controls ranged from 0.70 to 1.00. Subanalyses comparing cases relative to patients with MEN1 who had an indolent dpNET without liver metastases yielded comparable performance estimates (Fig. 1D and Supplementary Table S1) (20).
Circulating protein signatures associated with disease progression in a genetically engineered mouse model of MEN1-related pancreatic neuroendocrine tumors
To evaluate time-dependent changes in the plasma proteome associated with Men1-PanNET disease progression, we performed in-depth proteomic analyses on serially collected plasmas from 8 (1:1 female:male) Men1fl/flPdx1-CreTg mice and 8 (1:1 female:male) Men1fl/fl control mice. Serial blood samples were collected at 8 months (frequent islet hyperplasia) and 12 months (extensive hyperplasia and PanNET) of age (Fig. 2A) (18). Median survival among Men1fl/flPdx1-CreTg mice was 483 days (15.9 months) (Supplementary Table S5) (20).
Proteomic analyses of plasmas yielded 2379 proteins quantifiable in at least 25% of specimens (Supplementary Table S6) (20). Time-dependent analyses revealed 106 (87 increased/19 decreased) and 284 (266 increased/18 decreased) proteins to be statistically significantly (raw 2-sided Student T-test P < .05) differential between Men1fl/flPdx1-CreTg mice and Men1fl/fl control mice at ages 8 and 12 months, respectively. Thirty proteins were elevated (P < .05) at both time points (Fig. 2B and 2C; Supplementary Table S6) (20). IPA of the 106 and 284 differential proteins revealed cancer, endocrine system disorders, and neurological disease among the top predicted diseases (Supplementary Table S7) (20).
Generalized linear mixed effect models with sex as a variable were used to further prioritize circulating proteins associated with PanNET disease progression. These analyses yielded 196 proteins that were positively associated (Men1fl/flPdx1-CreTg generalized linear mixed effect coefficient >0; fold-change >1.2 at age 12 months) with PanNET disease progression (Fig. 2D). IPA of these 196 proteins revealed MYCN, YAP1, POU5F1, and SMAD3 to be among the top predicted transcriptional regulators, all of which have relevance to neuroendocrine tumor differentiation and progression (Supplementary Table S8) (20, 27, 28). Moreover, our analyses revealed several proteins involved in neuronal development and function, including Dner, DscamL1, Kif3c, Mapk8IP2, Lmtk3, and Grm7, to be associated with disease progression in Men1fl/flPdx1-CreTg mice (Fig. 2E).
Notably, at the 8-month sampling timepoint, 9 proteins (Apoc2, Col2a1, Cela2a, Trpm4, Snx29, Map4, Mprip, Myh2, and Zfp62) that were associated with disease progression were also prognostic for poor overall survival [Log-rank (Mantel-Cox) test 2-sided P < .05)] in Men1fl/flPdx1-CreTg mice (Fig. 2F).
Cross-species Intersection of Protein Signatures of MEN1-related dpNET Progression
Of the proteins quantified in mouse and human plasmas, 739 were overlapping between the 2 datasets. Of the overlapping proteins, 19 (NDC80, DEF8, SPAG17, ATM, IMMT, DNAH6, DSP, CIT, C3, HRG, F5, CD79A, BDP1, SERPINA11, PROS1, TARBP1, B2M, SERPIND1, and PROC) were associated with disease progression in Men1fl/fl Pdx1-CreTg mice and were concordantly elevated (AUC ≥ 0.65) in human MEN1 patients with liver metastasis from a dpNET. Three of the 19 proteins (NDC80, TARBP1, and SPAG17) had AUC estimates > 0.80 for distinguishing MEN1 patients with liver metastasis from respective controls (Table 2). Of relevance, several of these proteins (Ndc80, Def8, Spag17, Atm, Immt, Dnah6, Dsp, and Cit) were observed to be elevated (fold-change ≥1.2) as early as 8 months in Men1fl/flPdx1-CreTg mice when animals begin to develop hyperplastic islets and insulinomas (Table 2).
Table 2.
Cross-species intersection of plasma protein signatures of MEN1-related dpNET progression
| MEN1-PanNET micea | |||||
|---|---|---|---|---|---|
| 8 month | 12 month | Human MEN1 cohort | |||
| Protein | Description | Fold-changea | Fold-changea | AUCb | 95% CI |
| NDC80 | Kinetochore protein NDC80 | 3.58 | 4.30 | 0.84 | 0.64-1.00 |
| DEF8 | Differentially expressed in FDCP 8 homolog | 3.58 | 3.32 | 0.77 | 0.60-0.93 |
| SPAG17 | Sperm associated antigen 17 | 1.93 | 1.32 | 0.82 | 0.59-1.00 |
| ATM | ATM serine/threonine kinase | 1.54 | 1.99 | 0.66 | 0.50-0.82 |
| IMMT | Inner membrane mitochondrial protein | 1.36 | 2.95 | 0.77 | 0.57-0.96 |
| DNAH6 | Dynein axonemal heavy chain 6 | 1.32 | 1.55 | 0.69 | 0.50-0.88 |
| DSP | Desmoplakin | 1.31 | 1.57 | 0.77 | 0.49-1.00 |
| CIT | Citron Rho-interacting serine/threonine kinase | 1.22 | 6.16 | 0.75 | 0.56-0.94 |
| C3 | Complement C3 | 1.16 | 1.43 | 0.67 | 0.53-0.82 |
| HRG | Histidine rich glycoprotein | 1.15 | 1.24 | 0.66 | 0.49-0.82 |
| F5 | Coagulation factor V | 1.14 | 1.26 | 0.75 | 0.60-0.89 |
| CD79A | Cluster of differentiation CD79A | 1.08 | 3.22 | 0.69 | 0.44-0.95 |
| BDP1 | B double prime 1 | 1.07 | 2.86 | 0.68 | 0.53-0.84 |
| SERPINA11 | Serpin family A member 11 | 1.06 | 1.44 | 0.75 | 0.60-0.90 |
| PROS1 | Coagulation regulator protein S | 1.06 | 1.43 | 0.69 | 0.53-0.84 |
| TARBP1 | TAR (HIV-1) RNA binding protein 1 | 1.05 | 1.31 | 0.84 | 0.65-1.00 |
| B2M | B2 microglobulin | 1.02 | 1.25 | 0.68 | 0.52-0.84 |
| SERPIND1 | Serpin family D member 1 | 1.02 | 1.21 | 0.70 | 0.54-0.85 |
| PROC | Protein C | 1.00 | 1.23 | 0.68 | 0.53-0.84 |
Abbreviations: AUC, area under the receiver operating characteristics curve; CI, confidence interval; dpNET, duodenopancreatic neuroendocrine tumor.
a Fold change comparing plasma protein levels between cases (Men1fl/flPdx1-CreTg) and control (Men1fl/fl) mice.
b Predictive performance (AUC) for distinguishing cases from respective controls. Cases are defined as patients with MEN1 and liver metastases from a duodenopancreatic neuroendocrine tumor (dpNET). Controls are patients with MEN1 and a nonmetastatic (distant or regional) indolent dpNET or patients with MEN1 without a dpNET or other neuroendocrine tumor.
Discussion
In our prior investigations, we established the relevance of circulating acetylated polyamine metabolites for informing MEN1-dpNET-related disease progression (18). In the current study, we expanded upon the metabolomic findings and performed comprehensive proteomic analyses of plasmas from human MEN1 patients as well as a GEM model of Men1-PanNET (Men1fl/flPdx1-CreTg) to establish novel circulating molecular protein signatures associated with disease progression.
Many of the proteins we identified to be associated with MEN1-dpNET disease progression have previously established associations with cancer progression in other cancer types and have been reported to exert pro-tumorigenic activity in experimental models. For instance, among proteins elevated in plasmas of MEN1 patients with metastatic dpNET were IGFBP2, CHI3L1, LYZ, TIMP1, LRG1, and COL18A1, which have previously been reported to elevated in early-stage pancreatic ductal adenocarcinoma (PDAC) cases compared to healthy individuals or individuals presenting with benign conditions, including chronic pancreatitis and pancreatic cysts (23, 29). CHI3L1, TIMP1, and LRG1 have also been shown to be prognostic biomarkers for poor overall survival in various cancer types, including pancreatic cancer (30–34). Transcriptomic analyses of sporadic pancreatic neuroendocrine tumors showed mRNA levels of LYZ and LRG1 to be positively associated with tumor grade (35).
Similarly, in Men1-PanNET mice, disease associated protein signatures manifested in the blood reflect established oncogenic networks centered on MYCN, YAP1, POU5F1, and SMAD, which are key contributors to NET differentiation and progression (27, 28). For instance, MYCN overexpression induces neuroendocrine prostate cancer from epithelial precursors (36). Whole genome sequencing and transcriptomic analyses of metastatic PanNET tumors showed MYCN amplification and increased mRNA expression of MYCN target genes (37). Prior studies have also shown that MYC facilitates ductal-neuroendocrine lineage plasticity in pancreatic cancer; MYC ChIP-seq revealed MYC DNA binding peaks within 1000 bp of several genes that were found in neuroendocrine pancreatic cancer gene signatures compared to pancreatic adenocarcinoma (28). Notably, MYC is a transcriptional regulator of several polyamine metabolizing enzymes (18, 38). We have previously shown that plasma acetylated polyamines are associated with disease progression in MEN1-PanNET mice, thus providing a common oncogenic driver for revealed protein and metabolite signatures (18).
In our study, cross-species intersection of proteomic profiles revealed 19 proteins that were associated with disease progression in MEN1-PanNET mice and that were concordantly elevated in human cases. Notably, except for serpins, these proteins are not known to be secreted. We posit that the occurrence of these proteins in circulation may be attributed to tumor cell turnover, membrane shedding, or dissemination in cancer-associated microparticles, eg, exosomes (39, 40). Additionally, many of these proteins have previously been associated with poor prognosis in other cancers or functionally linked with tumor cell phenotypes, making them strong candidates for future evaluation. For example, NDC80, a key regulator of kinetochore assembly, is highly expressed in several cancer types, including PDAC (41–44). Knockdown of NDC80 in PDAC cells promoted G2/M phase arrest and induction of apoptosis (41).
Given the rare nature of this disease, procurement of specimens from MEN1 patients is challenging. Through a unique international collaboration between MDACC, the NIH, and the UMCU, we were able to procure of biospecimen with limited availability from well-annotated and characterized patient cohorts. This is the first study of its kind and represents a very large biospecimen cohort in the field of MEN1, despite being limited in sample size when compared with more common diseases and cancer types. Additionally, as most included cases were treatment naïve, this allowed us to identify markers of natural disease progression as these cases were compared to indolent dpNETs and patients without visible dpNETs. Given that these latter patients still have MEN1 and dpNETs in MEN1 are characterized by precursor lesions, it may well be possible that these patients already harbor small micro-adenomas not yet visible on imaging. However, this does not distract from the purpose of this subgroup, namely representing preclinical dpNET disease. Controls tend to be younger than cases, which is a limitation. This is inherent to the natural progression of the disease. Given the multifocality of dpNETs in MEN1, it can be challenging to identify the exact origin of distant metastases, and misclassification remains possible. Whether protein biomarkers will be high in patients with MEN1 who harbor NETs of non-duodenopancreatic origin remains to be determined. Moreover, whether other manifestations of the disease, such as primary hyperparathyroidism, pituitary adenomas, and adrenal adenomas, impact protein signatures remains to be determined. Men1fl/flPdx1-CreTg mice develop predominantly insulinomas whereas the human cohort consisted mainly of nonfunctioning PanNETs and gastrinomas. Despite this limitation, we believe this model has many advantages (including the ability to collect serial samples, and littermates are housed together in a controlled environment) and therefore has utility as a model to understand molecular changes and tumor progression in the context of Men1-loss being the driving genetic event. This is further supported by several preclinical studies using similar mouse models, including those evaluating somatostatin analogs (45, 46), VEGF inhibition (16), a hedgehog inhibitor (47), and epigenetic pathway inhibitors (48) as potential anticancer therapeutics. While some of the differences we detected in our proteomic profiling of mouse plasma may be attributed to specifically to insulinomas, our proteomic analysis revealed several candidate circulating protein markers associated with dpNET progression in Men1fl/flPdx1-CreTg mice as well as in human MEN1 patients, including 19 overlapping proteins that reflected oncogenic networks that promote PanNET development. These overlapping changes with the human cohort are notable and may provide insights pertinent to tumors driven by the loss of Men1 that are independent of the tumors being functional or nonfunctional.
In conclusion, through comprehensive and cross-species analysis, we have identified protein biomarker candidates that are associated with MEN1-dpNET disease progression. As in the case of our previous metabolomics profile analyses (18), given that patients in the present study had developed distant metastasis, prospective studies are needed to determine the extent to which plasma protein signatures are prognostic for future distant metastasis. Findings from the present study, together with our previously published work on acetylated polyamines, are a first step in the development of signatures that identify patients with MEN1 with more aggressive PanNETs. We envision biomarkers identified from our studies alone or together as part of a multianalyte panel, eg, proteins and acetylated polyamine metabolites, may provide a means for risk stratification in patients with MEN1-related dpNETs to inform clinical decision-making.
Acknowledgments
Supported by the generous philanthropic contributions to The University of Texas MD Anderson Cancer Center Moon Shots Program™, the McKee Early Career Investigator in Pancreatic Cancer Research, the Neuroendocrine Tumor Research Foundation, National Institutes of Health National Cancer Institute [(NIH/NCI) R21 CA252426-01], and the intramural research program of the NIH/National Institute of Diabetes and Digestive and Kidney Diseases and NIH/NCI. K.A.D. was partially supported by a Cancer Center Support Grant NCI Grant P30 CA016672, NIH grants UL1TR003167, 5R01GM122775, the prostate cancer SPORE P50 CA140388, CPRIT Grant RP160693. We additionally thank Nikita Williams for technical assistance.
Contributor Information
Johannes F Fahrmann, Department of Clinical Cancer Prevention, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
Amanda R Wasylishen, Department of Genetics, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA; Department of Cancer Biology, University of Cincinnati, Cincinnati, OH 45267, USA.
Carolina R C Pieterman, Department of Surgical Oncology, Section of Surgical Endocrinology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA; Department of Endocrine Oncology, University Medical Center Utrecht, Utrecht 3508 GA, the Netherlands.
Ehsan Irajizad, Department of Clinical Cancer Prevention, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA; Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
Jody Vykoukal, Department of Clinical Cancer Prevention, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
Ranran Wu, Department of Clinical Cancer Prevention, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
Jennifer B Dennison, Department of Clinical Cancer Prevention, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
Christine B Peterson, Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
Hua Zhao, Department of Epidemiology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA; Department of Family Medicine and Population Health, Virginia Commonwealth University, Richmond, VA 23284, USA.
Kim-Anh Do, Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
Daniel M Halperin, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
Sunita K Agarwal, Metabolic Diseases Branch, The National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA.
Jenny E Blau, Metabolic Diseases Branch, The National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA.
Smita Jha, Metabolic Diseases Branch, The National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA.
Jaydira Del Rivero, Developmental Therapeutics Branch, The National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA.
Naris Nilubol, Surgical Oncology Program, The National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA.
Mary F Walter, Core for Clinical Laboratory Services, The National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA.
James M Welch, Metabolic Diseases Branch, The National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA.
Lee S Weinstein, Metabolic Diseases Branch, The National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA.
Menno R Vriens, Department of Surgical Oncology and Endocrine Surgery, University Medical Center Utrecht, Utrecht 3584 CX, the Netherlands; Center for Neuroendocrine Tumors, ENETS Center of Excellence, Netherlands Cancer Institute Amsterdam, University Medical Center Utrecht, Utrect 1066 CX, the Netherlands.
Rachel S van Leeuwaarde, Center for Neuroendocrine Tumors, ENETS Center of Excellence, Netherlands Cancer Institute Amsterdam, University Medical Center Utrecht, Utrect 1066 CX, the Netherlands; Department of Cancer Biology, University of Cincinnati, Cincinnati, OH 45267, USA.
Mark J C van Treijen, Center for Neuroendocrine Tumors, ENETS Center of Excellence, Netherlands Cancer Institute Amsterdam, University Medical Center Utrecht, Utrect 1066 CX, the Netherlands; Department of Cancer Biology, University of Cincinnati, Cincinnati, OH 45267, USA.
Gerlof D Valk, Center for Neuroendocrine Tumors, ENETS Center of Excellence, Netherlands Cancer Institute Amsterdam, University Medical Center Utrecht, Utrect 1066 CX, the Netherlands; Department of Cancer Biology, University of Cincinnati, Cincinnati, OH 45267, USA.
Nancy D Perrier, Department of Surgical Oncology, Section of Surgical Endocrinology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
Samir M Hanash, Department of Clinical Cancer Prevention, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
Hiroyuki Katayama, Department of Clinical Cancer Prevention, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
Author Contributions
Conceptualization, J.F.F., A.W., C.R.P., and H.K.; methodology, J.F.F., A.W., and H.K.; validation, J.F.F., A.W., C.R.P., and H.K.; formal analysis, J.F.F., E.I., and H.K.; investigation, J.F.F., A.W., C.R.P., and H.K.; resources, A.W., C.R.P., C.B.P., H.Z., D.H., S.A., J.B., S.J., J.R., N.N., M.W., J.W., L.W., M.R., R.L., M.T., G.V., N.P., and S.H.; data curation, J.F.F., and H.K.; writing—original draft preparation, J.F.F., and H.K.; writing—review and editing, A.W., C.R.P., E.I., J.V., R.W., J.B.D., C.B.P., H.Z., K.A.D., D.H., S.A., J.B., S.J., J.R., N.N., M.W., J.W., L.W., M.R., R.L., M.T., G.V., N.P., and S.H.; visualization, J.F.F., E.I., and H.K.; supervision, N.P, and S.H.; funding acquisition, S.H. All authors have read and agreed to the published version of the manuscript.
Disclosures
D.M.H. received research funding from AAA, Lexicon, Incyte, Genentech, and Tarveda. He also had consulting roles with AAA, Lexicon, Ipsen, and Curium. J.B. is a full-time employee at AstraZeneca.
Data Availability
Relevant data supporting the findings of this study are available within the article and supplementary information or are available from the authors on reasonable request.
References
- 1. Chandrasekharappa SC, Guru SC, Manickam P, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276(5311):404‐407. doi: 10.1126/science.276.5311.404 [DOI] [PubMed] [Google Scholar]
- 2. de Laat JM, van der Luijt RB, Pieterman CR, et al. MEN1 redefined, a clinical comparison of mutation-positive and mutation-negative patients. BMC Med. 2016;14(1):182. doi: 10.1186/s12916-016-0708-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Triponez F, Dosseh D, Goudet P, et al. Epidemiology data on 108 MEN 1 patients from the GTE with isolated nonfunctioning tumors of the pancreas. Ann Surg. 2006;243(2):265‐272. doi: 10.1097/01.sla.0000197715.96762.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goudet P, Murat A, Binquet C, et al. Risk factors and causes of death in MEN1 disease. A GTE (Groupe d'Etude des Tumeurs Endocrines) cohort study among 758 patients. World J Surg. 2010;34(2):249‐255. doi: 10.1007/s00268-009-0290-1 [DOI] [PubMed] [Google Scholar]
- 5. van Beek DJ, Nell S, Verkooijen HM, et al. Prognosis after surgery for multiple endocrine neoplasia type 1-related pancreatic neuroendocrine tumors: functionality matters. Surgery. 2021;169(4):963‐973. doi: 10.1016/j.surg.2020.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Beek DJ, Nell S, Pieterman CRC, et al. Prognostic factors and survival in MEN1 patients with gastrinomas: results from the DutchMEN Study Group (DMSG). J Surg Oncol. 2019;120(6):966‐975. doi: 10.1002/jso.25667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gibril F, Venzon DJ, Ojeaburu JV, Bashir S, Jensen RT. Prospective study of the natural history of gastrinoma in patients with MEN1: definition of an aggressive and a nonaggressive form. J Clin Endocrinol Metab. 2001;86(11):5282‐5293. doi: 10.1210/jcem.86.11.8011 [DOI] [PubMed] [Google Scholar]
- 8. Puliani G, Di Vito V, Feola T, et al. NETest: a systematic review focusing on the prognostic and predictive role. Neuroendocrinology. 2022;112(6):523‐536. doi: 10.1159/000518873 [DOI] [PubMed] [Google Scholar]
- 9. Crabtree JS, Scacheri PC, Ward JM, et al. A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. Proc Natl Acad Sci U S A. 2001;98(3):1118‐1123. doi: 10.1073/pnas.98.3.1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bertolino P, Tong W-M, Galendo D, Wang Z-Q, Zhang C-X. Heterozygous Men1 mutant mice develop a range of endocrine tumors mimicking multiple endocrine neoplasia type 1. Mol Endocrinol. 2003;17(9):1880‐1892. doi: 10.1210/me.2003-0154 [DOI] [PubMed] [Google Scholar]
- 11. Crabtree JS, Scacheri PC, Ward JM, et al. Of mice and MEN1: insulinomas in a conditional mouse knockout. Mol Cell Biol. 2003;23(17):6075‐6085. doi: 10.1128/MCB.23.17.6075-6085.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bertolino P, Tong W-M, Herrera PL, Casse H, Zhang CX, Wang Z-Q. Pancreatic beta-cell-specific ablation of the multiple endocrine neoplasia type 1 (MEN1) gene causes full penetrance of insulinoma development in mice. Cancer Res. 2003;63(16):4836‐4841. [PubMed] [Google Scholar]
- 13. Biondi CA, Gartside MG, Waring P, et al. Conditional inactivation of the MEN1 gene leads to pancreatic and pituitary tumorigenesis but does not affect normal development of these tissues. Mol Cell Biol. 2004;24(8):3125‐3131. doi: 10.1128/MCB.24.8.3125-3131.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu J, Herrera PL, Carreira C, et al. α Cell-specific Men1 ablation triggers the transdifferentiation of glucagon-expressing cells and insulinoma development. Gastroenterology. 2010;138(5):1954‐1965. doi: 10.1053/j.gastro.2010.01.046 [DOI] [PubMed] [Google Scholar]
- 15. Shen H-C, Ylaya K, Pechhold K, et al. Multiple endocrine neoplasia type 1 deletion in pancreatic alpha-cells leads to development of insulinomas in mice. Endocrinology. 2010;151(8):4024‐4030. doi: 10.1210/en.2009-1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shen HC, He M, Powell A, et al. Recapitulation of pancreatic neuroendocrine tumors in human multiple endocrine neoplasia type I syndrome via Pdx1-directed inactivation of Men1. Cancer Res. 2009;69(5):1858‐1866. doi: 10.1158/0008-5472.CAN-08-3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wasylishen AR, Sun C, Moyer SM, et al. Daxx maintains endogenous retroviral silencing and restricts cellular plasticity in vivo. Sci Adv. 2020;6(32):eaba8415. doi: 10.1126/sciadv.aba8415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fahrmann JF, Wasylishen AR, Pietevbvrman CRC, et al. A blood-based polyamine signature associated with MEN1 duodenopancreatic neuroendocrine tumor progression. J Clin Endocrinol Metab. 2021;106(12):e4969‐e4980. doi: 10.1210/clinem/dgab554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4(6):437‐450. doi: 10.1016/s1535-6108(03)00309-x [DOI] [PubMed] [Google Scholar]
- 20. Fahrmann JF, Wasylishen AR, Pieterman, CRC, et al. Supplemental data for “Blood-based proteomic signatures associated with Men1-related duodenopancreatic neuroendocrine tumor progression.” Figshare. Uploaded June 9, 2023. https://figshare.com/articles/dataset/Blood-based_Proteomic_Signatures_Associated_With_MEN1-related_Duodenopancreatic_Neuroendocrine_Tumor_Progression/23458136 [DOI] [PMC free article] [PubMed]
- 21. Pulvirenti A, Rao D, McIntyre CA, et al. Limited role of chromogranin A as clinical biomarker for pancreatic neuroendocrine tumors. HPB (Oxford). 2019;21(5):612‐618. doi: 10.1016/j.hpb.2018.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Granberg D, Stridsberg M, Seensalu R, et al. Plasma chromogranin A in patients with multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 1999;84(8):2712‐2717. doi: 10.1210/jcem.84.8.5938 [DOI] [PubMed] [Google Scholar]
- 23. Capello M, Bantis LE, Scelo G, et al. Sequential validation of blood-based protein biomarker candidates for early-stage pancreatic cancer. J Natl Cancer Inst. 2017;109(4):djw266. doi: 10.1093/jnci/djw266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamura K, Yu J, Hata T, et al. Mutations in the pancreatic secretory enzymes CPA1 and CPB1 are associated with pancreatic cancer. Proc Natl Acad Sci U S A. 2018;115(18):4767‐4772. doi: 10.1073/pnas.1720588115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peracaula R, Cleary KR, Lorenzo J, de Llorens R, Frazier ML. Human pancreatic ribonuclease 1: expression and distribution in pancreatic adenocarcinoma. Cancer. 2000;89(6):1252‐1258. [PubMed] [Google Scholar]
- 26. Hayakawa T, Kondo T, Shibata T, et al. Sensitive serum markers for detecting pancreatic cancer. Cancer. 1988;61(9):1827‐1831. doi: [DOI] [PubMed] [Google Scholar]
- 27. Nelakurti DD, Pappula AL, Rajasekaran S, Miles WO, Petreaca RC. Comprehensive analysis of MEN1 mutations and their role in cancer. Cancers (Basel). 2020;12(9):2616. doi: 10.3390/cancers12092616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Farrell AS, Joly MM, Allen-Petersen BL, et al. MYC regulates ductal-neuroendocrine lineage plasticity in pancreatic ductal adenocarcinoma associated with poor outcome and chemoresistance. Nat Commun. 2017;8(1):1728. doi: 10.1038/s41467-017-01967-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen IM, Johansen AZ, Dehlendorff C, et al. Prognostic value of combined detection of serum IL6, YKL-40, and C-reactive protein in patients with unresectable pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2020;29(1):176‐184. doi: 10.1158/1055-9965.EPI-19-0672 [DOI] [PubMed] [Google Scholar]
- 30. Hermann CD, Schoeps B, Eckfeld C, et al. TIMP1 expression underlies sex disparity in liver metastasis and survival in pancreatic cancer. J Exp Med. 2021;218(11):e20210911. doi: 10.1084/jem.20210911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Y-S, Han L, Yang C, Liu Y-J, Zhang X-M. Prognostic value of LRG1 in breast cancer: a retrospective study. Oncol Res Treat. 2021;44(1-2):36‐42. doi: 10.1159/000510945 [DOI] [PubMed] [Google Scholar]
- 32. Wang C-H, Li M, Liu L-L, et al. LRG1 expression indicates unfavorable clinical outcome in hepatocellular carcinoma. Oncotarget. 2015;6(39):42118‐42129. doi: 10.18632/oncotarget.5967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang J, Sheng Z, Yang W, Cai Y. Elevated serum concentration of chitinase 3-like 1 is an independent prognostic biomarker for poor survival in lung cancer patients. Cell Physiol Biochem. 2016;38(2):461‐468. doi: 10.1159/000438643 [DOI] [PubMed] [Google Scholar]
- 34. Chen H-T, Zheng J-M, Zhang Y-Z, et al. Overexpression of YKL-40 predicts poor prognosis in patients undergoing curative resection of pancreatic cancer. Pancreas. 2017;46(3):323‐334. doi: 10.1097/MPA.0000000000000751 [DOI] [PubMed] [Google Scholar]
- 35. Simbolo M, Bilotta M, Mafficini A, et al. Gene expression profiling of pancreas neuroendocrine tumors with different Ki67-based grades. Cancers (Basel). 2021;13(9):2054. doi: 10.3390/cancers13092054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dardenne E, Beltran H, Benelli M, et al. N-Myc induces an EZH2-mediated transcriptional program driving neuroendocrine prostate cancer. Cancer Cell. 2016;30(4):563‐577. doi: 10.1016/j.ccell.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wong H-L, Yang KC, Shen Y, et al. Molecular characterization of metastatic pancreatic neuroendocrine tumors (PNETs) using whole-genome and transcriptome sequencing. Cold Spring Harb Mol Case Stud. 2018;4(1):a002329. doi: 10.1101/mcs.a002329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fahrmann JF, Vykoukal J, Fleury A, et al. Association between plasma diacetylspermine and tumor spermine synthase with outcome in triple-negative breast cancer. J Natl Cancer Inst. 2020;112(6):607‐616. doi: 10.1093/jnci/djz182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vykoukal J, Sun N, Aguilar-Bonavides C, et al. Plasma-derived extracellular vesicle proteins as a source of biomarkers for lung adenocarcinoma. Oncotarget. 2017;8(56):95466‐95480. doi: 10.18632/oncotarget.20748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fahrmann JF, Mao X, Irajizad E, et al. Plasma-derived extracellular vesicles convey protein signatures that reflect pathophysiology in lung and pancreatic adenocarcinomas. Cancers (Basel). 2020;12(5):1147. doi: 10.3390/cancers12051147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meng Q-C, Wang H-C, Song Z-L, et al. Overexpression of NDC80 is correlated with prognosis of pancreatic cancer and regulates cell proliferation. Am J Cancer Res. 2015;5(5):1730‐1740. [PMC free article] [PubMed] [Google Scholar]
- 42. Zeng L, Fan X, Wang X, et al. Involvement of NEK2 and its interaction with NDC80 and CEP250 in hepatocellular carcinoma. BMC Med Genomics. 2020;13(1):158. doi: 10.1186/s12920-020-00812-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xing XK, Wu HY, Chen HL, Feng HG. NDC80 Promotes proliferation and metastasis of colon cancer cells. Genet Mol Res. 2016;15(2). doi: 10.4238/gmr.15028312 [DOI] [PubMed] [Google Scholar]
- 44. Sun Z-Y, Wang W, Gao H, Chen Q-F. Potential therapeutic targets of the nuclear division cycle 80 (NDC80) complexes genes in lung adenocarcinoma. J Cancer. 2020;11(10):2921‐2934. doi: 10.7150/jca.41834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Walls GV, Stevenson M, Soukup BS, et al. Pasireotide therapy of multiple endocrine neoplasia type 1-associated neuroendocrine tumors in female mice deleted for an men1 allele improves survival and reduces tumor progression. Endocrinology. 2016;157(5):1789‐1798. doi: 10.1210/en.2015-1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Quinn TJ, Yuan Z, Adem A, et al. Pasireotide (SOM230) is effective for the treatment of pancreatic neuroendocrine tumors (PNETs) in a multiple endocrine neoplasia type 1 (MEN1) conditional knockout mouse model. Surgery. 2012;152(6):1068‐1077. doi: 10.1016/j.surg.2012.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gurung B, Feng Z, Iwamoto DV, et al. Menin epigenetically represses hedgehog signaling in MEN1 tumor syndrome. Cancer Res. 2013;73(8):2650‐2658. doi: 10.1158/0008-5472.CAN-12-3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lines KE, Stevenson M, Filippakopoulos P, et al. Epigenetic pathway inhibitors represent potential drugs for treating pancreatic and bronchial neuroendocrine tumors. Oncogenesis. 2017;6(5):e332. doi: 10.1038/oncsis.2017.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Relevant data supporting the findings of this study are available within the article and supplementary information or are available from the authors on reasonable request.