Abstract
Context
Sexual dimorphism has direct consequences on the incidence and survival of cancer. Early and accurate diagnosis is crucial to improve prognosis.
Objective
This work aimed to characterized the influence of sex and adrenal asymmetry on the emergence of adrenal tumors.
Methods
We conducted a multicenter, observational study involving 8037 patients with adrenal tumors, including adrenocortical carcinoma (ACC), aldosterone-producing adenoma (APA), cortisol-secreting adrenocortical adenomas (CSAs), non-aldosterone-producing adrenal cortical adenoma (NAPACA), pheochromocytoma (PCC), and neuroblastoma (NB), and investigated tumor lateralization according to sex. Human adrenal tissues (n = 20) were analyzed with a multiomics approach that allows determination of gene expression, catecholamine, and steroid contents in a single sample. In addition, we performed a literature review of computed tomography and magnetic resonance imaging–based studies examining adrenal gland size.
Results
ACC (n = 1858); CSA (n = 68), NAPACA (n = 2174), and PCC (n = 1824) were more common in females than in males (female-to-male ratio: 1.1:1-3.8:1), whereas NBs (n = 2320) and APAs (n = 228) were less prevalent in females (0.8:1). ACC, APA, CSA, NAPACA, and NB occurred more frequently in the left than in the right adrenal (left-to-right ratio: 1.1:1-1.8:1), whereas PCC arose more often in the right than in the left adrenal (0.8:1). In both sexes, the left adrenal was larger than the right adrenal; females have smaller adrenals than males.
Conclusion
Adrenal asymmetry in both sexes may be related to the pathogenesis of adrenal tumors and should be considered during the diagnosis of these tumors.
Keywords: sexual dimorphism, adrenal asymmetry, tumor lateralization, cortical tumors, medullary tumors, bilateral disease, hypothalamic-pituitary-adrenal axis, Conn adenoma, Cushing syndrome
Epidemiological studies suggest that sexual dimorphism is an important factor for incidence and survival from cancer in non-reproductive organs, with males having a higher risk and worse prognosis than females (1, 2). Differences between the 2 biological sexes are due to various factors, including sex chromosome–related consequences, effects of gonadal hormones on the immune system and metabolism, and behavioral factors (Fig. 1) (2).
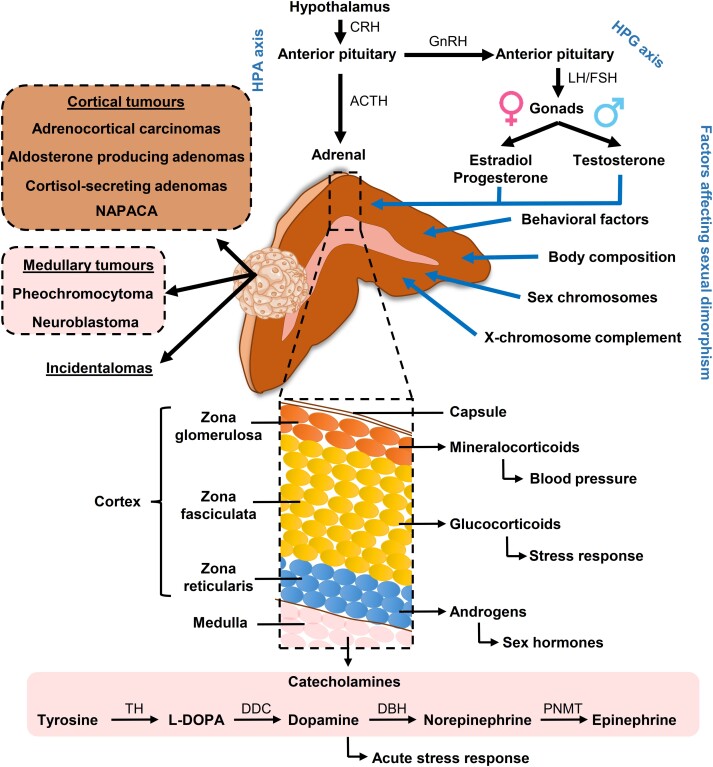
Figure 1.
Potential factors contributing to sexual dimorphism in adrenal in health and disease. The adrenal cortex is involved in the regulation of the stress response via the hypothalamic-pituitary-adrenal (HPA) axis, with a number of factors triggering the release of corticotropin-releasing hormone (CRH) from the hypothalamus. CRH afterward mediates the release of adrenocorticotropin (ACTH) from the pituitary and hence adrenocortical glucocorticoids. In response to acute stress, the chromaffin cells of the adrenal medulla secrete the catecholamines, norepinephrine and epinephrine, in the fight-or-flight response. The interaction between the HPA axis and hypothalamic-pituitary-gonadal (HPG) axis regulates production of the sex hormones testosterone, estradiol, and progesterone. Besides sex hormones, other factors may also contribute to a possible sexual dimorphism of the adrenal, with potential direct consequences on cortical and medullary tumors. DBH, dopamine beta-hydroxylase; DDC, dopa decarboxylase; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; NAPACA, non-aldosterone-producing cortical adrenal adenoma; PNMT, phenylethanolamine N-methyltransferase; TH, tyrosine hydroxylase.
There is increased evidence that adrenal glands are sexually dimorphic and asymmetric, potentially affecting the prevalence and progression of adrenal disease. The left and right adrenal differs markedly in their anatomy and vascularization; while the left adrenal is more crescent-shaped and extends toward the renal hilum, the right adrenal is pyramidal (3). As the main regulator of the endocrine stress response, the adrenal consists of 2 distinct endocrine tissues that are embedded under a common capsule: the outer cortex, responsible for the production of steroid hormones, and the inner medulla, the main source of epinephrine in the body (see Fig. 1) (3). The production of adrenal steroids takes place in 3 distinct zones of the adrenal cortex: zona glomerulosa (mineralocorticoids), zona fasciculata (glucocorticoids), and zona reticularis (androgens). Adrenal steroids play a central role in the homeostasis as part of the hypothalamic-pituitary-adrenal axis and the renin-angiotensin-aldosterone system. Moreover, there is a close interaction of the hypothalamic-pituitary-adrenal axis with the hypothalamic-pituitary-gonadal axis, which is responsible for the production of sex hormones.
More evidence is emerging that, unlike other neoplasms of non-reproductive organs, diseases of the adrenal cortex are more common in females than in males (4). In adrenocortical carcinoma (ACC; female-to-male ratio 1.5-2.5:1), female sex is associated with younger age at diagnosis and higher prevalence of functional tumors (4, 5). Adrenal incidentalomas are also predominantly detected in females, which was explained in the past by an increased frequency of diagnostic procedures in females (6). Cushing syndrome (CS) occurs with a female-to-male ratio of 3:1 (7). The adrenocorticotropin (ACTH)-independent form of endogenous CS accounts for about 20% of cases and is caused by cortisol-secreting adrenal adenomas (CSAs) (7). Pathogenic variants (PVs) in some genes also exhibit a sexual dimorphic occurrence in adrenal tumors; for example, PVs in KCNJ5 occur more frequently in females than in males in aldosterone-producing adenomas (APAs) (8). Nevertheless, studies that address these differences and draw conclusions for diagnosis and treatment of these tumors are limited. Moreover, possible differences between the left and right adrenal have to date not been considered. For primary aldosteronism (PA), knowledge of sexual dimorphism and morphological as well as functional differences between the right and left adrenal can be crucial for diagnosis and therapeutic success, since the treatment decision, surgical or pharmacological, is based on tumor lateralization (bilateral adrenal hyperplasia vs APA) diagnosed by steroid measurements in adrenal venous samples (AVS). Sex-specific differences in the adrenal medulla and a possible influence on tumorigenesis of medullary tumors, including pheochromocytomas (PCCs) and neuroblastomas (NBs), remain largely unknown.
Here, we retrospectively analyzed clinical data of 8472 patients with adrenal tumors, including ACC, APA, CSA, non-aldosterone-producing adrenal cortical adenoma (NAPACA), PCC and NB, to investigate possible sex-related differences in tumor lateralization.
Materials and Methods
Patients
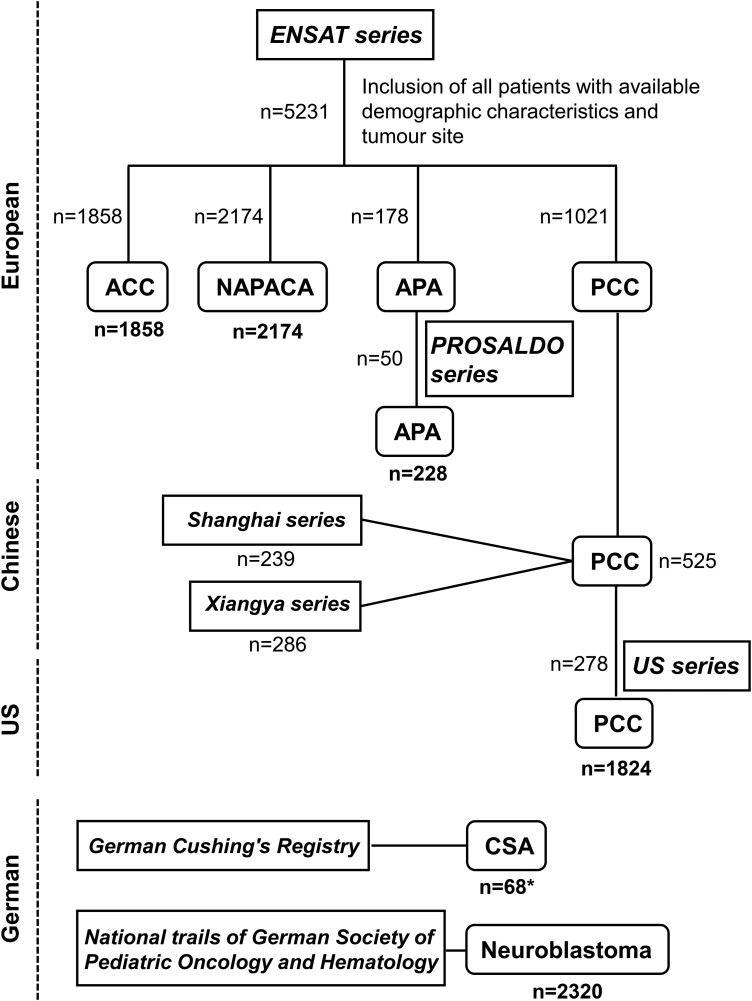
This retrospective cohort study included patients with confirmed ACC, APA, NAPACA, CSA, PCC, or NB investigated under multiple approved study protocols (Fig. 2). All patients were recruited through specialized centers and gave written informed consent before enrollment under respective study protocols. The local ethics committees approved all study protocols mentioned, which were used to collect the data presented in this study. Patients with confirmed ACC (n = 1858), APA (n = 178), PCC (n = 1021), or NAPACA (n = 2174) enrolled under the European Network for the Study of Adrenal Tumours (ENSAT) protocol were included in the present study. For the inclusion and characterization of NAPACA patients, further information is given in the supplementary material (9). The diagnosis of all tumors was pathologically confirmed, except in the case of NAPACAs, for which the final diagnosis may still be pending. The study was presented to all ENSAT members, and participation in the study was approved (additional information about all participating centers is provided in the supplementary material (9)). The last ENSAT data export (demographic characteristics, tumor site, tumor diameter, distant metastasis) was performed September 7, 2022. Patients with missing information or insufficient data quality were excluded from the study. In addition, patients with confirmed unilateral APA (n = 50) enrolled into the PROspective Study on the Diagnostic Value of Steroid Profiles in Primary ALDOsteronism (PROSALDO) study at 4 centers (Dresden, Munich, Würzburg, and Zurich) were included (total number of APA patients = 228). Patients with PCC (ntotal = 1824) were enrolled in addition to the ENSAT registry at 3 other centers: National Institutes of Health (n = 278; Bethesda, Maryland, USA), Xiangya Hospital (n = 286; China), and Zhongshan Hospital (n = 239; Shanghai, China).
Figure 2.
Flowchart patient inclusion from different international patient series. A total of 8472 patients with adrenal tumors were enrolled in the present study. Numbers indicated in bold are the final patient numbers included in the study. Square boxes contain the studies/registries from which patients were included. *The cohort of cortisol-secreting adrenocortical adenoma (CSA) patients includes 38 patients with clinically overt adrenal Cushing syndrome and 30 patients with mild autonomous cortisol secretion (MACS). ACC, adrenocortical carcinoma; APA, aldosterone-producing adenomas; NAPACA, non-aldosterone-producing cortical adrenal adenoma; NB, neuroblastoma; PCC, pheochromocytoma.
Patients diagnosed with clinically overt adrenal CS or mild autonomous cortisol secretion (n = 68) were recruited at the LMU Department of Internal Medicine IV, Munich, Germany (monocentric), as part of the German Cushing's Registry, a multicenter, longitudinal cohort study. Patients with unilateral and bilateral adrenal CS, including patients with bilateral micronodular or macronodular adrenal hyperplasia, were registered.
A total of 2320 NB patients registered in the national trials NB04, NB79, NB82, NB85, NB90, NB95, and NB97 of the German Society of Paediatric Oncology and Haematology were included in the study (10). NB patients were staged according to the International Neuroblastoma Staging System Committee (INSS) or Evans system (11).
Search Strategy
We searched in Google Scholar and PubMed to identify relevant articles published up to December 15, 2022, using combinations of the search items “adrenal,” “size,” “thickness,” “volume,” “magnetic resonance imaging (MRI),” “computed tomography (CT),” and “imaging.” The reference list of original articles, position statements, narrative and systematic reviews, and meta-analyses was screened for relevant publications. Only manuscripts published in English with sufficient information were considered.
Catecholamines and Steroid Measurements in Human Adrenal Specimens
Adrenal tissue specimens from operations of 20 patients with adrenal lesions at the University Hospital Dresden were examined. All patients were enrolled in ENSAT, PROSALDO, or the PROSpective PHEOchromocytoma study (PROSPHEO) and provided written informed consent. Tissue catecholamines and steroids were analyzed from the same tissue specimen as previously described (12). More details are given in the supplementary material (9).
Statistical Analysis
For statistical analyses, we used SigmaPlot 12.5 (Systat Software GmbH) or the JMP PRO 16 statistical software package (SAS Institute). Continuous data were tested for normal distribution using the Shapiro-Wilk test. The chi-square test on proportions was used to determine differences in the proportions between sexes or tumor lateralization. The Pearson chi-square test with contingency table was used to investigate whether the prevalence of metastatic disease was related to tumor lateralization. Mean values of continuous data were compared with the Wilcoxon test.
Results
Lateralization and Sex Discrepancies in Adrenal Tumors
To investigate whether sex and tumor lateralization influence the prevalence of cortical and medullary adrenal tumors, we retrospectively analyzed clinical data of 8472 patients with adrenal tumors collected under comprehensive international protocols (see Fig. 2).
Cortical Tumors
Adrenocortical carcinomas
In our series of 1858 patients with ACC (mean age at diagnosis: 53.1 years; Table 1), the female-to-male ratio was 1.7:1 (P < .0001). Patients aged 18 years or younger had a further increased ratio (2.3:1). Women were significantly younger than men at diagnosis (age 52.4 vs 54.2 years; P = .0476). ACC occurred more often in the left than the right adrenal (1027 vs 825; P < .0001). Furthermore, the left adrenal was associated with larger tumor size (11.5 vs 10.5 cm; P < .0001). ACC lateralization had no association with age at diagnosis. The total rate of distant metastasis was 28.7% in this series. Left-sided ACCs were not only larger but showed an increased metastatic rate compared to right-sided ACCs (30.7% vs 26.3%; P = .0155).
Table 1.
Lateralization and sex discrepancies of adrenal tumors
| Cortical tumors | Medullary tumors | |||||
|---|---|---|---|---|---|---|
| ACC | APA | CSA | NAPACA | PCC | NB | |
| Total No. of patients | 1858 | 228 | 68 | 2174 | 1824 | 2320a |
| Sex | ||||||
| Female, n (%) | 1159 (62.4) | 100 (43.9) | 54 (79.4) | 1376 (63.3) | 951 (52.1) | 1028 (44.3) |
| Male, n | 699 (P < .0001b) | 128 (P = .0368b) | 14 (P < .0001b) | 798 (P < .0001b) | 873 (P = .0357b) | 1291 (P < .0001b) |
| Age at diagnosis | ||||||
| Mean ± SD, yc | 53.1 ± 16.4 | 52.9 ± 12.4 | 53.2 ± 15.7 | 62.2 ± 12.4 | 50.5 ± 16.4 | 803.6 ± 10018.1 d |
| Median (range), yc | 53.9 (1-93) | 52.9 (23-80) | 56.5 (15-79) | 63.3 (1-96) | 62.0 (5-99) | 470 (0-9207) d |
| Sex differences—dependent on age | ||||||
| % females ≤18 y at diagnosis (n) | 70.0 (28/40) | NA | 100 (2/2) | 75.0 (3/4) | 38.5 (20/52) | NA |
| % females >18 y at diagnosis (n) | 62.3 (1129/1812) | 78.8 (52/66) | 63.3 (1373/2170) | 52.9 (931/1761) | ||
| Age at diagnosis—dependent on sex | ||||||
| Mean ± SD, females, yc | 52.4 ± 17.0 | 48.7 ± 12.5 | 51.6 ± 16.2 | 61.6 ± 12.4 | 51.3 ± 16.2 | 803.2 ± 1034.8 d |
| Median (range) females, yc | 53.5 (191) | 48.0 (2380) | 55 (1579) | 62.7 (194) | 51 (699) | 466.5 (09207) d |
| Mean ± SD, males, yc | 54.2 ± 15.3 (P = .0476d) | 56.2 ± 11.3 (P < .0001d) | 58.9 ± 11.9 (P = .1410) | 63.2 ± 12.2 (P = .0016d) | 49.7 ± 16.5 (P = .0585d) | 804.90 ± 1005.3 d (P = .8997d) |
| Median (range) males, yc | 54.5 (3-93) | 55.6 (32-80) | 57.5 (25-76) | 64.3 (10-96) | 50 (5-98) | 477 (0-531) d |
| Tumor location, n | 1858 | 228 | 68 | 2174 | 1824 | 2320 |
| Left, n (%) | 1027 (55.3) | 140 (61.4) | 28 (41.2) | 1024 (47.1) | 756 (41.4) | 1183 (51.0) |
| Females, left, n (%) | 613 (59.7) | 62 (44.3) | 21 (75.0) | 654 (63.9) | 394 (52.1) | 524 (44.3) |
| Right, n (%) | 825 (44.4; P < .0001b) | 88 (38.6; P = .0005b) | 16 (23.5; P = .0481b) | 720 (33.1; P < .0001b) | 944 (51.8; P < .0001b) | 1067 (46.0; P = .0144b) |
| Females, right, n (%) | 544 (65.9) | 38 (43.2) | 13 (81.3) | 437 (60.7) | 490 (51.9) | 459 (43.0) |
| Bilateral, n (%) | 6 (0.3) | 0 (0) | 24 (35.3) | 430 (19.8) | 124 (6.8) | 70 (3.0) |
| Age at diagnosis—dependent on tumor location | ||||||
| Left: mean ± SD, age, yc | ||||||
| Left: median age (range), yc | 53.1 ± 19.9 | 52.4 ± 12.3 | 51.6 ± 17.3 | 61.9 ± 12.7 | 51.2 ± 16.3 | 839.3 ± 1028.3 d |
| Right: mean ± SD, age, yc | 54.4 (1-93) | 52.3 (26-77) | 56.5 (15-76) | 63.3 (1-96) | 51 (6-93) | 513 d (0-9207) |
| Right: median age (range), yc | 53.0 ± 16.9 (P = .9976d) | 53.7 ± 12.5 (P = .4903d) | 53.9 ± 15.3 (P = .9611d) | 61.8 ± 12.9 (P = .9440d) | 51.2 ± 16.0 (P = .9193d) | 797.0 ± 1009.3 d (P = .0227d) |
| 53.6 (1-93) | 53.7 (23-80) | 54 (27-79) | 63.2 (14-94) | 51 (5-93) | 470 d (0-7531) | |
| Size unilateral tumors, n | 1765 | 195 | 21 | 2072 | 785 | NR |
| Mean ± SD, size, cm | 11.0 ± 5.0 | 1.7 ± 1.0 | 3.6 ± 1.5 | 3.0± | 4.6 ± 3.0 | |
| Median size (range), cm | 10.0 (1-32) | 1.5 (0.5-10.2) | 3.5 (0.1-6.5) | 2.5 (0.17-28) | 4 (0.2-32) | |
| Mean ± SD, size left mass, cm | 11.5 ± 5.2 | 1.6 ± 1.1 | 3.7 ± 0.9 | 2.9± | 4.58 ± 2.8 | |
| Mean ± SD, size right mass, cm | 10.5 ± 4.7 (P < .0001d) | 1.8 ± 0.9 (P = .2266d) | 3.8 ± 1.4 | 3.2 ± (P < .0001d) | 4.6 ± 3.2 (P = .7095d) | |
| Distant metastasis | ||||||
| Total, n (metastasis rate %) | 527/1834 (28.7) | NR | NR | NR | 117/1754 (6.7) | NR |
| Females, n (metastasis rate %) | 324/1143 (28.3) | 59/905 (6.5) | ||||
| Males, n (metastasis rate %) | 203/691 (29.4) | 58/849 (6.8) | ||||
| Left, n (metastasis rate %) | 311/1014 (30.7) | 55/729 (7.5) | ||||
| Right, n (metastasis rate %) | 216/820 (26.3) (P = .0155e) | 56/909 (6.1) (P = .2681e) | ||||
Abbreviations: ACC, characteristics of patients with adrenocortical carcinomas; APA, aldosterone-producing adenomas; CSA, cortisol-secreting adrenocortical adenoma; NAPACA, non-aldosterone-producing adrenal cortical adenomas; PCC, pheochromocytomas; NA, not applicable; NB, neuroblastomas; NR, not recorded.
a One patient with unknown sex included.
b Chi-square test on proportions vs females; or left adrenal location.
c If not indicated otherwise.
d Wilcoxon test vs females; or vs left adrenal location.
e Pearson chi-square test with contingency table for left vs right and metastatic vs non-metastatic.
Aldosterone-producing adenomas
Patients with confirmed unilateral APA (n = 228) included in this analysis had a mean age of 52.9 years (see Table 1). APA patients were more often male (56%, P = .0368) and older at diagnosis than women (mean age 56.2 vs 48.7 years; P < .0001). APAs occurred more often in the left adrenal (61.4%; P = .0005) with comparable tumor size and similar age at diagnosis.
Cortisol-secreting adrenocortical adenomas
Patients with clinically overt adrenal CS (overt CS) or mild autonomous cortisol secretion (MACS) caused by unilateral CSAs (n = 44) or micronodular or macronodular hyperplasia (n = 24) were predominantly female (n = 68; mean age: 53.2 years; 54 women vs 14 men; P < .0001; see Table 1). CSAs showed a higher prevalence in the left compared to the right adrenal (28 vs 16; P = .0481). Age at diagnosis and tumor size were comparable in left- and right-sided CSAs and showed no differences between sexes. Differences between women and men were primarily due to differences in patients with covert CS (Supplementary Table S1 (9)), as patients with overt CS showed a higher female proportion compared to MACS patients (89.5% vs 66.7%; P = .0332, female-to-male ratio: 8.5:1 vs 2:1) and women were significantly younger at diagnosis (45.4 vs 62.3 years; P = .0002; see Supplementary Table S1 (9)).
Non-aldosterone-producing adrenal cortical adenoma
Our series includes 2174 patients with NAPACA, who had a mean age of 62.2 years (see Table 1). Women predominated in the NAPACA series (P < .0001) and were significantly younger at diagnosis than men (61.6 vs 63.2 years; P = .0016). NAPACAs were more often diagnosed in the left than the right adrenal (47.1% vs 33.1%; P < .0001). In the present series, right-sided NAPACAs were larger than left-sided (3.2 vs 2.9 cm; P < .0001), while tumor size was comparable for bilateral disease (n = 430, 19.8%; mean: right: 2.7 cm, left: 2.6 cm).
Medullary Tumors
Pheochromocytoma
In the present series, which comprised 1824 patients with PCC from 3 different cohorts (European, Chinese, and US cohort), we found a female predominance (951 females vs 873 males; P = .0357), with a slight tendency of males being younger than females at diagnosis (age 49.7 vs 51.3 years; P = .0585; see Table 1). Female predominance was mainly due to patients from the European cohort, while sex differences were not observed in the Chinese and US cohorts (see Supplementary Fig. S1 (9)). In contrast to cortical tumors, PCCs occurred more frequently in the right adrenal (51.8% vs 41.4%; P < .0001) and 6.8% of patients had bilateral disease (see Table 1 and Supplementary Fig. S1 (9)). Unilateral PCCs presented with comparable tumor size independent of tumor lateralization (see Table 1), and bilateral PCCs showed no differences in tumor size (Supplementary Fig. S2 (9)). In the present series, 6.7% of PCC patients had distant metastases, with no differences in the ratio between females and males (see Table 1). PCCs of the left adrenal tended to be more frequently associated with metastatic disease than those of the right adrenal (7.5% vs 6.1%; P = .2681).
Neuroblastoma
The present series includes 2320 pediatric patients with adrenal NB (mean age: 803.6 days; see Table 1). We observed a male predominance (55.7% boys; P < .0001), but sex did not affect age of diagnosis. In contrast to PCC, NB occurred more frequently in the left than the right adrenal (51.0% vs 46.0%; P = .0144). Bilateral disease was present in 3% of cases. Patients diagnosed with left-sided NB were older compared to those with right-sided NB (839.3 days vs 766.4 days; P = .0227).
Malignant spread of NB often seems to be triggered by MYCN amplification or/and PVs in ALK and ATRX (13). In our series, 28.8% of NBs exhibited MYCN amplification, with no differences detected between female and male patients (22.5% vs 23.2%; Supplementary Fig. S3A (9)). NB with MYCN amplification occurred more frequently in the left than in the right adrenal (32.4% vs 26.0%; P < .005; Supplementary Fig. S3B (9)). We further investigated whether sex or tumor lateralization had an effect on tumor stage. Sex had no influence on tumor stage (Supplementary Fig. S3C (9)). Left-sided NB presented significantly more often with distant metastasis, for example, in the lymph nodes, bones, and liver (stage 4) than right-sided tumors (56.7% vs 41.4%; P < .001; Supplementary Fig. S3D (9)).
Larger adrenals in males—sex independent larger left adrenal in humans
CT- and MRI-based studies demonstrated that the left adrenal is larger than the right one regardless of race and sex (Table 2 and Supplementary Table S2) (14-23). In addition, it is known that males present with larger adrenals than females and that the total adrenal volume is related to body size, which may explain the smaller adrenals in females compared to males (14). Adrenals of males further present with a lower density than those of females, with a difference on average of 8 Hounsfield unit (HU) (14). This observation was confirmed by Chen and colleagues (24), who additionally showed that this difference in HU is independent of age, although adrenal volume changes depend on age. Despite the smaller size and higher density in CT scans of female compared with male adrenals, adrenal medulla and cortex functionality based on measurements of catecholamine and steroid content was similar between the 2 sexes (n = 20; Supplementary Fig. S4-S6 (9)). The medulla of left adrenals showed a slight increase in catecholamine content compared to the right adrenal; however, statistical significance was reached only for epinephrine (P = .037).
Table 2.
Adrenal asymmetry and effect of sex on the adrenal dimension
| Publication | n | Sex | Mean age, y | Disease | Volume, cm3 | Pa | Total volume, cm3 | Cumulative thickness, mm | Pa | County | Modality (n) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | ||||||||||
| Chen et al (2023) (24) | 1043 | 489 F | 50 (18-77)b | Normal | 2.2 ± 0.6 | 2.6 ± 0.8 | — | 2.4 ± 0.7 | — | — | — | China | CT (1043) |
| 554 M | 2.7 ± 0.8 | 3.2 ± 0.9 | — | 3.0 ± 0.9 | — | — | — | ||||||
| Askani et al (2022) (23) | 229 | 117 M + 112 F | 54.2 ± 8.8 | Normal | 4.6 ± 1.9c | 5.4 ± 2.3c | <.001 | 5.0 ± 1 .9c | — | — | — | Germany | MRI |
| Zhang et al (2022) (22) | 1272 | 664 F | Normal | 2.8 (2.3, 3.3)d | 3.4 (2.9, 4.0)d | — | — | — | China | CT | |||
| 608 M | 3.3 (2.7, 3.9)d | 4.0 (3.3, 4.6)d | — | — | — | — | — | ||||||
| Gurun et al (2021) (21) | 115 | 56 M + 59 F | 49.5 ± 17.7 | Normal | 3.5 ± 1.3 | 4.8 ± 1.7 | <.001 | 4.13 ± 1.37 | — | — | — | Turkey | MDCT |
| Aggarwal et al (2019) (18) | 1250 | 665 M + 585 F | 38.0 ± 7.2 | Normal | — | — | — | 14.2 ± 0.5 | 13.4 ± 0.4 | <.001 | Asian-Indian | CT | |
| John et al (2018) (17) | 586 | 201 F | Normal | — | — | — | 13.8 ± 3.15 | 16.6 ± 3.1 | <.001 | Asian-Indian | CT | ||
| 385 M | — | — | — | 16.6 ± 3.60 | 19.4 ± 19.4 | <.001 | |||||||
| Akin et al (2017) (16) | 420 | 200 F | 61.2 ± 8.0 | Normal | 3.4 ± 1.2 | 3.4 ± 1.1 | .013 | 13.7 ± 4.0 | 14.0 ± 4.1 | .230 | Turkey | CT | |
| 220 M | 63.4 ± 8.0 | 3.0 ± 1.0 | 3.5 ± 1.3 | .026 | 13.9 ± 3.8 | 14.5 ± 4.4 | .063 | ||||||
| Serifoglu et al (2016) (20) | 62 | 31 M + 21 F | 57.5 | Normal | 1.8 (0.7-4.2b) | 2.2 (1.0-5.1b) | — | 4.0 (1.9-7.5b) | — | — | — | Turkey | CT |
| Carsin-Vu et al (2016) (15) | 154 | 89 F | 57 ± 17 | Normal, diabetic, alcoholic, micronodular patients | 3.8 ± 1.3 | 4.5 ± 1.6 | <.001 | 11.9 ± 2.8 | 13.0 ± 3.4 | .001 | France | MDCT | |
| 65 M | |||||||||||||
| Schneller et al (2014) (14) | 105 | 36 F | — | Normal | 3.0 ± 1.0 | 4.2 ± 1.7 | <.001 | 15.8 ± 3.1 | 19.0 ± 3.4 | <.001 | German | MDCT (94)/PET-CT (11) | |
| 69 M | — | 4.0 ± 1.2 | 5.2 ± 1.6 | <.001 | |||||||||
| Nemeroff et al (1992) (19) | 11 | 4 F | 39.8 ± 9.5 | Normal | — | — | — | 4.7 ± 1.4 | — | — | — | USA | CT |
| 7 M | 35.1 ± 10.0 | — | — | — | 4.3 ± 1.3 | — | — | — | |||||
| 38 | 16 F | 50.9 ± 16.1 | Depressed | — | — | — | 5.7 ± 2.5 | — | — | — | |||
| 20 M | 50.4 ± 16.7 | — | — | — | 8.1 ± 2.9 | — | — | — | |||||
Abbreviations: CT, computed tomography; F, female; M, male; MDCT, multidetector computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
a Volume in mL.
b Range.
c Median (interquartile range).
d t-test comparing left and right adrenal gland.
Discussion
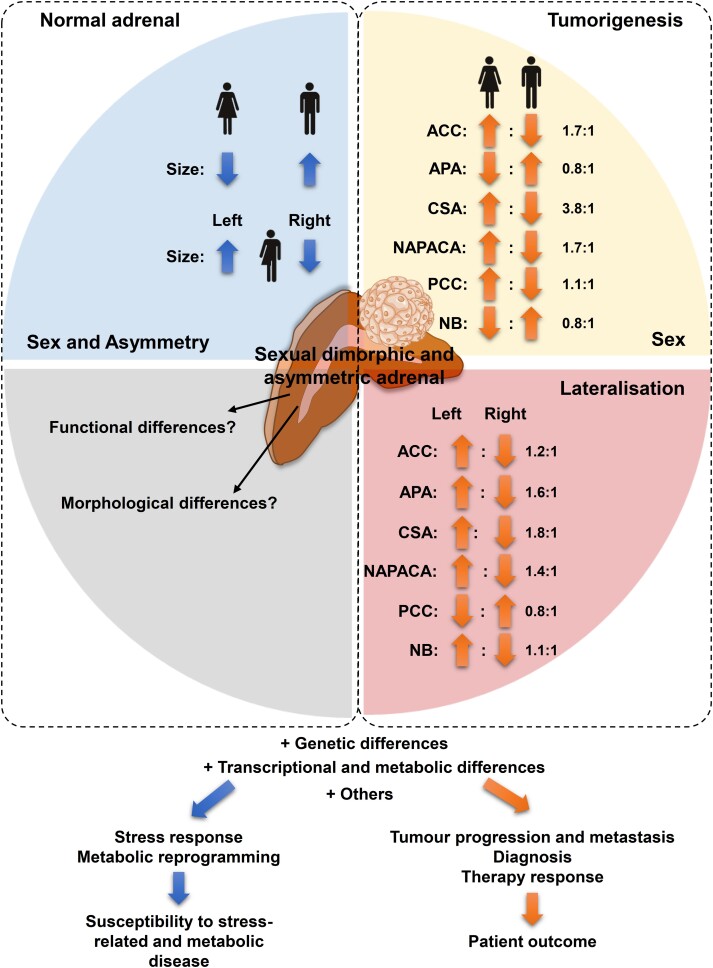
This retrospective registry study revealed that sex-specific adrenal asymmetry in humans is not restricted to differences in adrenal size, but has direct associations with side-dependent detection of adrenal tumors (Fig. 3). ACCs, CSAs, NAPACAs, and PCCs were more prevalent in females than males (female-to-male ratio: 1.1:1-3.8:1), whereas APAs and NBs were more common in males. Adrenal tumors occurred more frequently in the larger left adrenal (left-to-right ratio: 1.1:1-1.8:1), with the exception of PCCs (0.8:1). Our data provide evidence that sex-dependent adrenal asymmetry has potential consequences for the occurrence of adrenal tumors, and should be considered during the diagnosis of these tumors.
Figure 3.
The sexual dimorphic and asymmetric adrenal in health and disease. Sex-dependent differences in adrenal size and adrenal asymmetry have a direct implication in adrenal tumorigenesis and progression. Genetic, transcriptional, and metabolic differences between the 2 sexes further contribute to disparities in diagnosis, course, and response to specific therapies. An improved understanding of these mechanisms will thus contribute to a decisive improvement in the course and the outcome of adrenal diseases. Furthermore, the adrenal is a central relay of the stress response. Sex-related differences may therefore also be associated with an increased susceptibility to stress-related and metabolic diseases, which may contribute to adrenal tumorigenesis. Arrows indicate increases or decreases in dependence of sex or adrenal/tumor localization. The numbers reflect the ratio between females/males or left/right tumor occurrence. ACC, adrenocortical carcinoma; APA, aldosterone-producing adenomas; CSA, cortisol-secreting adrenocortical adenoma; NAPACA, non-aldosterone-producing cortical adrenal adenoma; NB, neuroblastoma; PCC, pheochromocytoma.
Various factors and diseases, such as sex, body mass index, and depression (19, 25), influence adrenal size. Patients with PA, regardless of subtype, have higher adrenal volumes than healthy individuals, suggesting a general dysregulation of adrenal growth in these patients (26). We identified a higher prevalence of APAs in males than females. There is increasing evidence of sex- and race-related differences in the prevalence of APA-driver PVs (8), which may contribute to a higher prevalence in males. The younger age of females at APA diagnosis in the present series is consistent with a Japanese cohort that included 2122 patients with PA and showed that females were significantly younger at diagnosis of unilateral disease (27). In addition to the fact that female patients tend to consult a physician earlier and undergo more frequently diagnostic procedures than males (6), the occurrence of somatic PVs in KCNJ5 is often associated with female sex, younger age, and unilateral disease (28), which may contribute to the differences found. We further observed a higher prevalence of left-sided APAs, which is consistent with findings from a CT- and AVS-based study (29). The left-vs-right adrenal volume ratio was even proposed as a screening index to identify unilateral PA (30). PA subtyping is currently achieved by AVS; however, the informative value is somewhat limited. A better understanding of the adrenal asymmetry and consequences on lateralization of these tumors is necessary to make the best possible therapy decision.
In a cross-sectional CT- and MRI-based study, left-sided adrenal adenomas were detected in 65%, right-sided adenomas in 21%, and bilateral adenomas in 14% of patients (31). In case of bilateral disease, left-sided adenomas were significantly larger than right-sided ones in 61% of patients (31). In addition to a potential pathophysiological basis for these differences, possible bias in the detection of left-sided adrenal adenomas is conceivable. However, perhaps more important, time from tumor onset until detection is longer for adenomas on the right compared to the left. This may have 2 important implications: (1) detection of right-sided adrenal adenomas may be delayed until a later stage of progressive growth; and (2) underappreciation of right-sided adrenal adenomas may lead to considerable inaccuracy in the detection of bilateral disease, which is of particular importance in PA and adrenal CS, in which treatment decisions depend on reliable information about lateralization (31). To address the potential imaging bias due to difficulties in the detection of right-sided tumors, we took the size of the tumor into consideration (Supplementary Table S3 (9)). Based on the assumption that tumors 4 cm or larger are equally detectable in both adrenals, the analysis in APA, CSA, and NAPACA revealed that the difference in tumor lateralization exists only for tumors smaller than 4 cm. However, in APA and CSA the number of patients with tumors 4 cm or larger is rather limited. ACCs 4 cm or larger occur significantly more frequently in the left than in the right adrenal, whereas no significant differences were found in the smaller than 4 cm group. This suggests, at least for ACCs, that differences in tumor lateralization are not exclusively due to imaging bias. Nonetheless, heterogeneity in the interpretation of cross-sectional images by different radiologists as well as the usage of different imaging modalities and the time of imaging (different resolution) could affect the results.
Our data show that the larger left adrenal is more prone to develop functional and non-functional adrenal tumors, except for PCCs, in a sex-independent manner. In addition, we investigated whether age, sex, and/or tumor type could be used as a predictor for adrenal tumor lateralization (Supplementary section 9, Supplementary Tables S4-S5 (9)). While age and sex had no effect on lateralization, tumor type had a significant effect on whether the tumor was more likely to occur in the right or left adrenal. PCCs were associated with a significantly higher probability of occurring in the right adrenal than ACCs, NAPACAs, or APAs. Apart from a possible bias in recognition, differences in the size of adrenals, which may be due to morphological, functional, or molecular differences, may account for these differences. Based on the steroid and catecholamine tissue content of the adrenal cortex and medulla, we could not determine any sex-related differences in the functionality of the tissue, but possible differences in the ratio of cortex to medulla could not be taken into account. However, the medullary tissue of the left female adrenal had higher contents of epinephrine and total catecholamines than the right one, which should be further investigated in additional studies.
Tumors of the adrenal cortex originate from different cells located in different zones of the adrenal cortex. In mice, the adult adrenal cortex undergoes extensive cell renewal, with profound sex differences (32, 33). Tissue turnover is higher in female than male mice and can be abolished in females by androgen treatment (32). This may contribute to an increased susceptibility of females to develop ACCs. In addition, sexual dimorphic activation of innate antitumor immunity, especially phagocytic macrophages triggered by testosterone, may further contribute to sex-dependent progression of ACCs in mice (34). Our findings confirmed observations of a smaller study that identified 51 ACCs (56%) in the left adrenal, while 40 (44%) ACCs occurred in the right adrenal (35). Moreover, more left-sided tumors were diagnosed in males than females (72% vs 47%) (35), which is in contrast to our large series, in which 59.7% of the left-sided ACCs were diagnosed in females.
The increased ratio of females to males described for endogenous CS (3:1) (7) was even higher when only adrenal CS was considered in the present series (3.8:1). If we consider only the patients with overt CS, the ratio is 8.5:1, which is significantly higher compared with patients with MACS (2:1). Chronic and excessive secretion of cortisol is associated with impaired quality of life through the occurrence of sometimes severe and even fatal comorbidities, including metabolic syndrome, musculoskeletal disorders, neuropsychiatric disorders, impaired reproductive and sexual function, and dermatological manifestations, with sometimes profound differences depending on sex (36). A recent retrospective cohort study including incidentaloma patients with (a) non-functional adenoma, (b) possible autonomous cortisol secretion, and (c) autonomous cortisol secretion revealed that autonomous cortisol secretion is associated with increased all-cause mortality, in particular in women younger than 65 years (37). This underscores the need for greater consideration of sex in the diagnosis and treatment of adrenal disease, especially with respect to systemic effects of adrenal hormone excess.
NB, like PCCs, originates from neural crest–derived cells. Clinical course of the disease ranges from spontaneous tumor regression to fatal malignant progression (38, 39). The occurrence of NB before puberty reduces the influence of sex hormones on the evolution of these tumors and might explain the higher prevalence in males, while PCCs are more common in females. In addition, fetal to postnatal alterations in the function of chromaffin and cortical cells in an age- and sex-dependent manner may further contribute to these differences (3, 40). Here, the interactions between the medulla and cortex are of particular importance, as glucocorticoid action is directly linked to maturation of chromaffin cells through induction of PNMT (phenylethanolamine N-methyltransferase) expression (3).
Our PCC series showed no differences with respect to metastasis rates between the sexes or in relation to tumor location. This is in contrast to another study that included besides PCC also extra-adrenal paragangliomas (PGLs) and head/neck PGL and found that metastases were more common in males than females (41). Jugulotympanic head/neck PGLs are more common in females than males and are associated in only 24% of cases with loss of succinate dehydrogenase (SDH) compared to 55% in males (42). PCCs and PGLs with PVs in genes that lead to activation of hypoxia signaling pathways, and in particular PVs in SDHB, are more frequently associated with metastatic disease (43). A lower rate of SDH loss in females with head/neck PGL may contribute to an apparent higher prevalence of metastases in males, underlining the importance of considering the effect of PVs on sex differences in this context.
This study has several limitations. Although we were able to keep the selection bias low by including comprehensive international register data, the study is highly dependent on the quality of data input at each center. This is particularly relevant where tumor sizes were available. In case of NB and CSA, only patients enrolled in Germany were included; in case of CSA, only monocentric. Even though the data quality of the CSA patients is high, the number of patients is limited, which restricts the validity. Despite the inclusion of a second study protocol, the APA cohort was also comparatively small. Genetic and race disparities were not taken into account, but may have an influence on sex- and lateralization-related differences, as exemplified by PCCs. The NAPACA cohort also includes non-adrenocortical lesions such as metastases, myelolipomas, and ganglioneuromas, and adrenal tumors whose diagnosis is not yet complete. Thus, it cannot be excluded that the NAPACA cohort also includes patients with MACS or other hormone-producing tumors with incomplete diagnosis. Although this cohort allows no conclusions about a specific tumor entity, it may allow general relationships to be made regarding the sex-dependent lateralization of adrenal neoplasms. A general selection bias in the detection of functional adrenal tumors may also be related to epidemiological differences, such as hypertension and obesity, and pathophysiological differences, such as the presence of amenorrhea and hirsutism, which may cause higher imaging rates in females than in males. This is consistent with the predominance of incidentalomas in females, which have often been attributed to more diagnostic procedures being performed in females compared to males (6). Information about the imaging modality used in each case was also not available, which limits the assessment of potential imaging bias. Due to their retrospective nature, the data collected do not allow any mechanistic conclusions to be drawn. Further studies that include, for example, genetic information in relation to sex, are urgently needed to shed light on the sexual dimorphism and lateralization of adrenal tumors and to draw conclusions for optimizing diagnosis and treatment of these multifaceted tumors.
We have demonstrated in a comprehensive cohort study that sex-specific adrenal asymmetry in humans is not limited to differences in adrenal size, but is also directly associated with the side-dependent detection of adrenal tumors. Thus, our data provide evidence that the sex-dependent asymmetry of the adrenals has potential implications for the occurrence of adrenal tumors, which should be given greater consideration in the diagnosis and treatment of these tumors in the future.
Acknowledgment
The authors would like to thank Susan Richter, PhD, (University Hospital Carl Gustav Carus) for scientific discussion of the data and Matthias Kuhn (Institute of Medical Informatics and Biometry, Medical Faculty Carl Gustav Carus) for assistance with the statistical analysis.
Abbreviations
- ACC
adrenocortical carcinoma
- ACTH
adrenocorticotropin
- APA
aldosterone-producing adenoma
- AVS
adrenal venous sample
- CS
Cushing syndrome
- CSA
cortisol-secreting adrenocortical adenoma
- CT
computed tomography
- ENSAT
European Network for the Study of Adrenal Tumours
- HU
Hounsfield unit
- MACS
mild autonomous cortisol secretion
- MDCT
multidetector computed tomography
- MRI
magnetic resonance imaging
- NAPACA
non-aldosterone-producing adrenal cortical adenoma
- NB
neuroblastoma
- PA
primary aldosteronism
- PCC
pheochromocytoma
- PET
positron emission tomography
- PGL
paraganglioma
- PNMT
phenylethanolamine N-methyltransferase
- PROSALDO
PROspective Study on the Diagnostic Value of Steroid Profiles in Primary ALDOsteronism
- PV
pathogenic variant
- SDH
succinate dehydrogenase
Contributor Information
Nicole Bechmann, Institute of Clinical Chemistry and Laboratory Medicine, University Hospital Carl Gustav Carus, Medical Faculty Carl Gustav Carus, Technische Universität Dresden, 01307 Dresden, Germany.
Mats Leif Moskopp, Department of Neurosurgery, Vivantes Friedrichshain Hospital, Charité Academic Teaching Hospital, 10249 Berlin, Germany.
Georgiana Constantinescu, Department of Internal Medicine III, University Hospital Carl Gustav Carus, Technische Universität Dresden, 01307 Dresden, Germany.
Anthony Stell, School of Computing and Information Systems, University of Melbourne, 3052 Melbourne, Australia.
Angela Ernst, Institute of Medical Statistics and Computational Biology, Faculty of Medicine, University of Cologne, 50931 Cologne, Germany.
Frank Berthold, Children's Hospital, University of Cologne, 50735 Cologne, Germany.
Frank Westermann, Hopp Children's Cancer Center Heidelberg (KiTZ), 69120 Heidelberg, Germany; Division of Neuroblastoma Genomics, German Cancer Research Center (DKFZ), 69120 Heidelberg, Germany.
Jingjing Jiang, Department of Endocrinology and Metabolism, Zhongshan Hospital, 200031 Shanghai, China.
Longfei Lui, Department of Urology, Xiangya Hospital, Central South University, 410017 Changsha, China.
Elisabeth Nowak, Department of Medicine IV, University Hospital, Ludwig-Maximilians-Universität Munich, 80539 Munich, Germany.
Stephanie Zopp, Department of Medicine IV, University Hospital, Ludwig-Maximilians-Universität Munich, 80539 Munich, Germany.
Karel Pacak, Section on Medical Neuroendocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Rockville, MD 20892, USA.
Mirko Peitzsch, Institute of Clinical Chemistry and Laboratory Medicine, University Hospital Carl Gustav Carus, Medical Faculty Carl Gustav Carus, Technische Universität Dresden, 01307 Dresden, Germany.
Andreas Schedl, Université Côte d’Azur, Inserm, CNRS, Institut de Biologie Valrose, 06108 Nice, France.
Martin Reincke, Department of Medicine IV, University Hospital, Ludwig-Maximilians-Universität Munich, 80539 Munich, Germany.
Felix Beuschlein, Department of Medicine IV, University Hospital, Ludwig-Maximilians-Universität Munich, 80539 Munich, Germany; Department of Endocrinology, Diabetology and Clinical Nutrition, University Hospital Zurich (USZ) and University of Zurich (UZH), 8091 Zurich, Switzerland; Institute of Neuropathology, University of Zurich, 8091 Zurich, Switzerland.
Stefan R Bornstein, Department of Internal Medicine III, University Hospital Carl Gustav Carus, Technische Universität Dresden, 01307 Dresden, Germany.
Martin Fassnacht, Division of Endocrinology and Diabetes, Department of Internal Medicine I, University Hospital of Würzburg, University of Würzburg, 97080 Würzburg, Germany.
Graeme Eisenhofer, Department of Internal Medicine III, University Hospital Carl Gustav Carus, Technische Universität Dresden, 01307 Dresden, Germany.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) within the CRC/Transregio 205/1, project No. 314061271—TRR205 “The Adrenal: Central Relay in Health and Disease”.
Disclosures
The authors have nothing to disclose.
Data Availability
Sharing of unidentified participant data will be considered on request made to the corresponding author. After discussion of the request by the corresponding author with the principal investigators, a decision will be made whether data sharing is appropriate.
References
- 1. Clocchiatti A, Cora E, Zhang Y, et al. Sexual dimorphism in cancer. Nat Rev Cancer. 2016;16(5):330‐339. [DOI] [PubMed] [Google Scholar]
- 2. Haupt S, Caramia F, Klein SL, et al. Sex disparities matter in cancer development and therapy. Nat Rev Cancer. 2021;21(6):393‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bechmann N, Berger I, Bornstein SR, et al. Adrenal medulla development and medullary-cortical interactions. Mol Cell Endocrinol. 2021;528:111258. [DOI] [PubMed] [Google Scholar]
- 4. Lyraki R, Schedl A. The sexually dimorphic adrenal cortex: implications for adrenal disease. Int J Mol Sci. 2021;22(9):4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scollo C, Russo M, Trovato MA, et al. Prognostic factors for adrenocortical carcinoma outcomes. Front Endocrinol. 2016;7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barzon L, Sonino N, Fallo F, et al. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol. 2003;149(4):273‐285. [DOI] [PubMed] [Google Scholar]
- 7. Lacroix A, Feelders RA, Stratakis CA, et al. Cushing's syndrome. Lancet. 2015;386(9996):913‐927. [DOI] [PubMed] [Google Scholar]
- 8. Nanba K, Rainey WE. Genetics in endocrinology: impact of race and sex on genetic causes of aldosterone-producing adenomas. Eur J Endocrinol. 2021;185(1):R1‐R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bechmann N, Moskopp ML, Constantinescu G, et al. Supplementary material for “The asymmetric adrenals: Sexual dimorphism of adrenal tumours”. Deposited 1 September 2023. 10.5281/zenodo.8307990 [DOI]
- 10. Berthold F, Spix C, Kaatsch P, et al. Incidence, survival, and treatment of localized and metastatic neuroblastoma in Germany 1979–2015. Pediatr Drugs. 2017;19(6):577‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sokol E, Desai AV. The evolution of risk classification for neuroblastoma. Children. 2019;6(2):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bechmann N, Watts D, Steenblock C, et al. Adrenal hormone interactions and metabolism: a single sample multi-omics approach. Horm Metab Res. 2021;53(05):326‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pugh TJ, Morozova O, Attiyeh EF, et al. The genetic landscape of high-risk neuroblastoma. Nat Genet. 2013;45(3):279‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schneller J, Reiser M, Beuschlein F, et al. Linear and volumetric evaluation of the adrenal gland—MDCT-based measurements of the adrenals. Acad Radiol. 2014;21(11):1465‐1474. [DOI] [PubMed] [Google Scholar]
- 15. Carsin-Vu A, Oubaya N, Mulé S, et al. MDCT Linear and volumetric analysis of adrenal glands: normative data and multiparametric assessment. Eur Radiol. 2016;26(8):2494‐2501. [DOI] [PubMed] [Google Scholar]
- 16. Akin D, Tugrul Yilmaz M, Ozbek O, et al. Morphometric analysis of suprarenal glands (adrenal glands) with multislice computerized tomography. Int J Morphol. 2017;35(1):120‐127. [Google Scholar]
- 17. John R, Putta T, Simon B, et al. Normal adrenal gland thickness on computerized tomography in an Asian Indian adult population. Indian J Radiol Imaging. 2018;28(04):465‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aggarwal N, Bhoil R, Sharma S, et al. Computed tomography measurements of normal adrenal glands in Indian population. J Anat Soc India. 2019;68(1):23. [Google Scholar]
- 19. Nemeroff CB, Krishnan KRR, Reed D, et al. Adrenal gland enlargement in major depression: a computed tomographic study. Arch Gen Psychiatry. 1992;49(5):384‐387. [DOI] [PubMed] [Google Scholar]
- 20. Serifoglu I, Oz II, Bilici M. The adrenal gland volume measurements in manifestation of the metabolic status in type-2 diabetes mellitus patients. Int J Endocrinol. 2016;2016:7195849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gurun E, Kaya M, Gurun KH. Evaluation of normal adrenal gland volume and morphometry and relationship with waist circumference in an adult population using multidetector computed tomography. Sisli Etfal Hastan Tip Bul. 2021;55(3):333‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang W, Wang J, Shao M, et al. The performance of left/right adrenal volume ratio and volume difference in predicting unilateral primary aldosteronism. J Endocrinol Invest. 2023;46(4):687‐698. [DOI] [PubMed] [Google Scholar]
- 23. Askani E, Rospleszcz S, Lorbeer R, et al. Association of MRI-based adrenal gland volume and impaired glucose metabolism in a population-based cohort study. Diabetes Metab Res Rev. 2022;38(5):e3528. [DOI] [PubMed] [Google Scholar]
- 24. Chen Y, Yang J, Zhang Y, et al. Age-related morphometrics of normal adrenal glands based on deep learning-aided segmentation. Heliyon. 2023;9(6):e16810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kessing LV, Willer IS, Knorr U. Volume of the adrenal and pituitary glands in depression. Psychoneuroendocrinology. 2011;36(1):19‐27. [DOI] [PubMed] [Google Scholar]
- 26. Degenhart C, Schneller J, Osswald A, et al. Volumetric and densitometric evaluation of the adrenal glands in patients with primary aldosteronism. Clin Endocrinol. 2017;86(3):325‐331. [DOI] [PubMed] [Google Scholar]
- 27. Akasaka H, Yamamoto K, Rakugi H, et al. Sex difference in the association between subtype distribution and age at diagnosis in patients with primary aldosteronism. Hypertension. 2019;74(2):368‐374. [DOI] [PubMed] [Google Scholar]
- 28. Lenzini L, Rossitto G, Maiolino G, et al. A meta-analysis of somatic KCNJ5 K+ channel mutations in 1636 patients with an aldosterone-producing adenoma. J Clin Endocrinol Metab. 2015;100(8):E1089‐E1095. [DOI] [PubMed] [Google Scholar]
- 29. Wada N, Shibayama Y, Yoneda T, et al. Lateralizing asymmetry of adrenal imaging and adrenal vein sampling in patients with primary aldosteronism. J Endocr Soc. 2019;3(7):1393‐1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li S, Sun H, Ma L, et al. Left-versus-right-adrenal-volume ratio as a screening index before adrenal venous sampling to identify unilateral primary aldosteronism patients. J Hypertens. 2020;38(2):347‐353. [DOI] [PubMed] [Google Scholar]
- 31. Hao M, Lopez D, Luque-Fernandez MA, et al. The lateralizing asymmetry of adrenal adenomas. J Endocr Soc. 2018;2(4):374‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grabek A, Dolfi B, Klein B, et al. The adult adrenal cortex undergoes rapid tissue renewal in a sex-specific manner. Cell Stem Cell. 2019;25(2):290‐6.e2. [DOI] [PubMed] [Google Scholar]
- 33. Dumontet T, Sahut-Barnola I, Septier A, et al. PKA Signaling drives reticularis differentiation and sexually dimorphic adrenal cortex renewal. JCI Insight. 2018;3(2):e98394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilmouth JJ, Olabe J, Garcia-Garcia D, et al. Sexually dimorphic activation of innate antitumor immunity prevents adrenocortical carcinoma development. Sci Adv. 2022;8(41):eadd0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng S, Cherniack AD, Dewal N, et al. Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer cell. 2016;29(5):723‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pivonello R, Isidori AM, De Martino MC, et al. Complications of Cushing's Syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4(7):611‐629. [DOI] [PubMed] [Google Scholar]
- 37. Deutschbein T, Reimondo G, Di Dalmazi G, et al. Age-dependent and sex-dependent disparity in mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: an international, retrospective, cohort study. Lancet Diabetes Endocrinol. 2022;10(7):499‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ackermann S, Cartolano M, Hero B, et al. A mechanistic classification of clinical phenotypes in neuroblastoma. Science. 2018;362(6419):1165‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kastriti ME, Kameneva P, Adameyko I. Stem cells, evolutionary aspects and pathology of the adrenal medulla: A new developmental paradigm. Mol Cell Endocrinol. 2020;518:110998. [DOI] [PubMed] [Google Scholar]
- 40. Kempná P, Flück CE. Adrenal gland development and defects. Best Pract Res Clin Endocrinol Metab. 2008;22(1):77‐93. [DOI] [PubMed] [Google Scholar]
- 41. Pamporaki C, Prodanov T, Meuter L, et al. Determinants of disease-specific survival in patients with and without metastatic pheochromocytoma and paraganglioma. Eur J Cancer. 2022;169:32‐41. [DOI] [PubMed] [Google Scholar]
- 42. Richter S, Qiu B, Ghering M, et al. Head/neck paragangliomas: focus on tumor location, mutational status and plasma methoxytyramine. Endocr Relat Cancer. 2022;29(4):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bechmann N, Moskopp ML, Ullrich M, et al. HIF2α Supports pro-metastatic behavior in pheochromocytomas/paragangliomas. Endocr Relat Cancer. 2020;27(11):625‐640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sharing of unidentified participant data will be considered on request made to the corresponding author. After discussion of the request by the corresponding author with the principal investigators, a decision will be made whether data sharing is appropriate.