Abstract
Context
Cardioprotective roles of endogenous estrogens may be particularly important in women with HIV, who have reduced estrogen exposure and elevated cardiovascular disease risk. The gut microbiome metabolically interacts with sex hormones, but little is known regarding possible impact on cardiovascular risk.
Objective
To analyze potential interplay of sex hormones and gut microbiome in cardiovascular risk.
Methods
Among 197 postmenopausal women in the Women's Interagency HIV Study, we measured 15 sex hormones in serum and assessed the gut microbiome in stool. Presence of carotid artery plaque was determined (B-mode ultrasound) in a subset (n = 134). We examined associations of (i) sex hormones and stool microbiome, (ii) sex hormones and plaque, and (iii) sex hormone–related stool microbiota and plaque, adjusting for potential confounders.
Results
Participant median age was 58 years and the majority were living with HIV (81%). Sex hormones (estrogens, androgens, and adrenal precursors) were associated with stool microbiome diversity and specific species, similarly in women with and without HIV. Estrogens were associated with higher diversity, higher abundance of species from Alistipes, Collinsella, Erysipelotrichia, and Clostridia, and higher abundance of microbial β-glucuronidase and aryl-sulfatase orthologs, which are involved in hormone metabolism. Several hormones were associated with lower odds of carotid artery plaque, including dihydrotestosterone, 3α-diol-17G, estradiol, and estrone. Exploratory mediation analysis suggested that estrone-related species, particularly from Collinsella, may mediate the protective association of estrone with plaque.
Conclusion
Serum sex hormones are significant predictors of stool microbiome diversity and composition. The gut microbiome may play a role in estrogen-related cardiovascular protection.
Keywords: microbiome, sex hormones, HIV, atherosclerosis
Cardiovascular disease (CVD) is the leading cause of death for women in the United States, accounting for 1 of 5 female deaths (1). CVD appears at higher rates in people living with HIV than the general population (2) and is a leading cause of non-AIDS death in HIV (3). However, the effect of HIV on CVD is more pronounced in women than men (4-6), which may hint at a hormonal driver of excess CVD risk in women with HIV. Women with HIV have lower ovarian reserve, testosterone, and estradiol than uninfected women (7-10) and also appear to have an earlier median age at menopause (11); these deficiencies have been associated with subclinical atherosclerosis (7, 12). Estradiol can inhibit HIV replication and is thought to maintain HIV latency (13, 14), further suggesting a role of female sex hormones in HIV-related disease. From a biological perspective, endogenous estrogens are cardioprotective (15, 16), and hormone therapy with estrogen is associated with favorable cardiometabolic endpoints among younger women (<60 years old) when initiated within 10 years of menopause (17, 18), including in women with HIV (19). Thus, hormonal perturbations in women with HIV, particularly for estrogens, may be important risk factors for CVD.
The gut microbiome, the community of microorganisms residing in the human gut, has bi-directional interactions with sex hormones that may also influence CVD risk. The sex hormone milieu appears to impact the gut microbiome, resulting in sexual dimorphism of the gut microbiome (20-22), while some gut microbiota (coined the “estrobolome”) may also be involved in regulating free circulating hormone levels (23). Specifically, sex hormones headed for fecal excretion due to hepatic conjugation with glucuronide or sulfate groups may be deconjugated by gut microbial enzymes such as β-glucuronidase and aryl-sulfatase, releasing the hormones for reabsorption into the enterohepatic circulation; through this “recycling” mechanism, estrogens and other hormones may again access the systemic circulation and some target tissues (23, 24). Despite compelling evidence for gut microbiome and sex hormone interactions, it remains unknown whether these interactions may influence CVD risk, either in the general population or in women with HIV who have sex hormone perturbations (7-10), gut dysbiosis (25-28), and elevated CVD risk (4-6).
Here, we present an analysis of serum endogenous sex hormones, the gut microbiome in stool, and subclinical atherosclerosis in postmenopausal women with or without HIV participating in the Women's Interagency HIV Study (WIHS) (Fig. 1A). Our objectives were to examine the relationship of serum sex hormones, measured by sensitive gas or liquid chromatography coupled to mass spectrometry methods, with the gut microbiome measured by shotgun metagenomic sequencing of stool samples, and with subclinical atherosclerosis assessed by B-mode carotid artery ultrasound. Additionally, we sought to examine whether sex hormone–related stool microbiota are associated with subclinical atherosclerosis and may partially mediate sex hormone effects. Our results provide the first insights into the potential interplay of sex hormones and the gut microbiome in CVD risk.
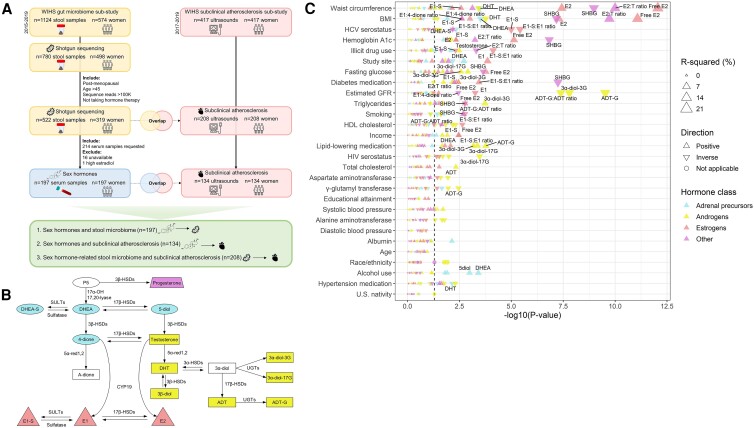
Figure 1.
Study design and sex hormone–related covariates in the Women's Interagency HIV study (WIHS). (A) Overview of sample size and study design. (B) Schematic representation of steroidogenesis (35), displaying in color the hormones measured. Abbreviations: 3α-diol-3G: androstane-3α,17β-diol 3-glucuronide; 3β-diol: 5α-androstane-3β,17β-diol; 4-dione: androstenedione; 5-diol: androst-5-enediol; ADT: androsterone; ADT-G: ADT-glucuronide; CYP: cytochrome P450; DHEA: dehydroepiandrosterone; DHEA-S: DHEA-sulfate; DHT: dihydrotestosterone; E1: estrone; E1-S: E1-sulfate; E2: estradiol; HSD: hydroxysteroid dehydrogenase; P5: pregnenolone; red: reductase; SULT: sulfotransferase; UGT: uridine 5′-diphospho-glucuronosyltransferase. (C) Univariate linear regressions were used to determine the associations of demographic, behavioral, and clinical characteristics (predictors) with inverse-normal transformed hormone outcomes. P values are from likelihood ratio tests comparing to a null model. Labeled hormone associations indicate an FDR-adjusted P value < 0.05.
Methods
Study Population
WIHS was a US multicenter cohort of women living with HIV and demographically similar women without HIV, now part of the MACS/WIHS Combined Cohort Study (MWCCS) (29). WIHS conducted semi-annual core visits that included interviews, standardized clinical measurements, physical exams, and laboratory tests (30). Institutional review boards at all sites approved the study, and participants provided written informed consent. In 2015, WIHS participants from the Bronx, Brooklyn, and Chicago sites began home collection of stool samples as part of a gut microbiome substudy, using study-provided kits with detailed instructions, described previously (31, 32). All WIHS participants at these sites were eligible to participate in the microbiome substudy. Stool samples collected until the end of 2018 were sent for shotgun sequencing. Among women participating in the gut microbiome substudy with available shotgun sequencing data, our goal was to select ∼200 serum samples for sex hormone measurement. Inclusion criteria for sex hormone measurement were: (i) classified sequence read depth >100 K; (ii) person-visit classified as postmenopausal, described briefly as being after an observed natural final menstrual period (defined previously (19)), after a bilateral ovariectomy, or uncertain status but age ≥54; (iii) >45 years of age at the person-visit, and at least 6 months after the final menstrual period (if observed); and (iv) not currently taking hormone therapy at the person-visit. From 319 women (522 person-visits) meeting these criteria, 214 person-visits from 214 unique women were randomly selected for serum sex hormone measurement. Sixteen serum samples were unavailable in the specimen repository, and one sample was excluded due to high estradiol (128 pg/mL; ie, likely premenopausal or taking exogenous estradiol), leaving 197 samples remaining for the sex hormone data analysis (Fig. 1A).
In parallel with the gut microbiome substudy, a substudy of carotid artery B-mode ultrasound was also ongoing among participants from the WIHS Bronx, Brooklyn, and Chicago sites. We identified 208 women with overlapping shotgun sequencing and carotid artery ultrasound data who met the 4 criteria listed above, which included 134 women with overlapping sex hormone and carotid artery ultrasound data (Fig. 1A).
Covariate Data
Data for analysis were taken from the WIHS core visit closest to the time of stool sample receipt in the lab. Covariates considered in our analysis are shown in Table 1. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI creatinine equation without race (33). Missing data for covariates were imputed based on the immediately prior study visit with available data.
Table 1.
Characteristics of participants with sex hormone and stool microbiome data in the Women's Interagency HIV Study (WIHS)
| All (n = 197) | HIV + (n = 159) | HIV- (n = 38) | P-valuea | |
|---|---|---|---|---|
| Age, y, median [IQR] | 58.00 [54.00, 61.00] | 57.00 [54.00, 60.00] | 58.00 [55.25, 63.00] | .127 |
| Study site, n (%) | .004 | |||
| Bronx | 92 (46.7) | 68 (42.8) | 24 (63.2) | |
| Brooklyn | 61 (31.0) | 48 (30.2) | 13 (34.2) | |
| Chicago | 44 (22.3) | 43 (27.0) | 1 (2.6) | |
| Race/ethnicity, n (%) | .466 | |||
| Black non-Hispanic | 134 (68.0) | 105 (66.0) | 29 (76.3) | |
| White non-Hispanic or other | 19 (9.6) | 16 (10.1) | 3 (7.9) | |
| Hispanic | 44 (22.3) | 38 (23.9) | 6 (15.8) | |
| Not born in 50 U.S. states or DC, n (%) | 38 (19.3) | 32 (20.1) | 6 (15.8) | .704 |
| Annual income, n (%) | .696 | |||
| $12 000 or less | 120 (60.9) | 96 (60.4) | 24 (63.2) | |
| $12 001-$24 000 | 42 (21.3) | 33 (20.8) | 9 (23.7) | |
| $24 001 or more | 35 (17.8) | 30 (18.9) | 5 (13.2) | |
| Educational attainment, n (%) | .196 | |||
| Less than high school | 82 (41.6) | 63 (39.6) | 19 (50.0) | |
| Completed high school | 55 (27.9) | 43 (27.0) | 12 (31.6) | |
| Any college | 60 (30.5) | 53 (33.3) | 7 (18.4) | |
| Smoking status, n (%) | .075 | |||
| Never smoker | 37 (18.8) | 33 (20.8) | 4 (10.5) | |
| Current smoker | 69 (35.0) | 50 (31.4) | 19 (50.0) | |
| Former smoker | 91 (46.2) | 76 (47.8) | 15 (39.5) | |
| Alcohol use, n (%) | .289 | |||
| Abstainer | 112 (56.9) | 90 (56.6) | 22 (57.9) | |
| >0-7 drinks/wk | 70 (35.5) | 59 (37.1) | 11 (28.9) | |
| >7 drinks/wk | 15 (7.6) | 10 (6.3) | 5 (13.2) | |
| Illicit drug use, n (%) | 45 (22.8) | 33 (20.8) | 12 (31.6) | .225 |
| Hepatitis C virus serostatus, n (%) | 63 (32.0) | 51 (32.1) | 12 (31.6) | >.99 |
| Serum albumin, g/dL, median [IQR] | 4.20 [4.02, 4.40] | 4.20 [4.02, 4.40] | 4.21 [4.07, 4.40] | .762 |
| Estimated GFR, mL/min/1.73 m2, median [IQR] | 76.34 [60.08, 92.23] | 74.45 [59.96, 90.26] | 84.96 [63.36, 94.48] | .252 |
| Serum aspartate aminotransferase, IU/L, median [IQR] | 19.00 [16.00, 26.00] | 20.00 [16.00, 26.00] | 17.00 [15.00, 24.50] | .014 |
| Serum alanine aminotransferase, IU/L, median [IQR] | 17.00 [12.00, 22.00] | 17.00 [13.00, 22.50] | 14.00 [12.00, 18.75] | .051 |
| Serum γ-glutamyl transferase, U/L, median [IQR] | 26.00 [18.00, 42.00] | 26.00 [18.00, 38.00] | 30.00 [16.25, 47.75] | .441 |
| Waist circumference, cm, median [IQR] | 99.20 [89.00, 109.50] | 98.90 [90.05, 109.80] | 100.70 [87.05, 108.13] | .767 |
| BMI, kg/m2, median [IQR] | 29.10 [25.40, 35.10] | 29.10 [25.40, 35.05] | 29.45 [24.95, 35.20] | .955 |
| Fasting glucose, mg/dL, median [IQR] | 94.00 [87.00, 106.00] | 94.00 [86.00, 106.50] | 97.00 [90.00, 105.50] | .250 |
| Hemoglobin A1c, %, median [IQR] | 5.70 [5.30, 6.10] | 5.60 [5.30, 6.10] | 5.75 [5.50, 6.25] | .213 |
| Total cholesterol, mg/dL, median [IQR] | 187.00 [166.00, 221.00] | 188.00 [166.50, 222.00] | 182.00 [161.25, 215.25] | .753 |
| Triglycerides, mg/dL, median [IQR] | 112.00 [82.00, 158.00] | 112.00 [82.50, 149.50] | 115.50 [77.00, 186.25] | .555 |
| HDL cholesterol, mg/dL, median [IQR] | 58.00 [47.00, 70.00] | 58.00 [47.00, 71.50] | 57.50 [48.00, 69.00] | .924 |
| Systolic blood pressure, mmHg, median [IQR] | 130.00 [115.00, 144.00] | 128.00 [115.00, 143.00] | 136.50 [127.25, 149.25] | .024 |
| Diastolic blood pressure, mmHg, median [IQR] | 77.00 [71.00, 84.00] | 77.00 [71.50, 84.50] | 77.00 [70.25, 83.50] | .827 |
| Use of diabetes medication, n (%) | 44 (22.3) | 34 (21.4) | 10 (26.3) | .661 |
| Use of lipid-lowering medication, n (%) | 54 (27.4) | 39 (24.5) | 15 (39.5) | .098 |
| Use of hypertension medication, n (%) | 110 (55.8) | 84 (52.8) | 26 (68.4) | .119 |
| HIV viral load, copies/mL, median [IQR] | 1.00 [1.00, 20.00] | |||
| CD4+ cell count, cells/mm3, median [IQR] | 631.00 [463.50, 848.00] | |||
| Antiretroviral therapy, n (%) | 156 (98.1) | |||
| Antiretroviral therapy regimenb, n (%) | ||||
| None | 3 (1.9) | |||
| PI-based | 30 (18.9) | |||
| NNRTI-based | 40 (25.2) | |||
| NRTI-based | 22 (13.8) | |||
| Other | 64 (40.3) |
Abbreviations: BMI, body mass index; GFR, glomerular filtration rate; IQR, interquartile range.
a P value comparing HIV+ and HIV- groups from Wilcoxon rank-sum test for continuous variables and Chi-square test or Fisher exact test for categorical variables.
b Protease inhibitor (PI)-based: at least 1 PI and 1 nucleoside reverse transcriptase inhibitor (NRTI); nonnucleoside reverse transcriptase inhibitor (NNRTI)-based: at least 1 NNRTI and 1 NRTI; NRTI-based: 3 or more NRTIs; “Other” includes women on both PI and NNRTI, no NRTI, or < 3 NRTIs with no PI/NNRTI.
Sex Hormones
Sex steroid hormones and sex hormone binding globulin (SHBG) were measured in serum samples at the pharmacogenomics laboratory at Université Laval, using validated methods described previously (34, 35). The measured steroids, outlined in Fig. 1B, were dehydroepiandrosterone (DHEA), androst-5-enediol (5-diol), testosterone, dihydrotestosterone (DHT), androsterone (ADT), estrone (E1), estradiol (E2), androstenedione (4-dione), 5α-androstane-3β,17β-diol (3β-diol), progesterone, ADT-glucuronide (ADT-G), androstane-3α, 17β-diol 3-glucuronide (3α-diol-3G), 3α-diol-17G, DHEA-sulfate (DHEA-S), and estrone-sulfate (E1-S). Briefly, gas-chromatography (GC) coupled to mass spectrometry (MS) was used to quantify DHEA, 5-diol, 4-dione, testosterone, DHT, ADT, 3β-diol, E1, E2, and progesterone using 250 μL of serum, described previously in detail (34). Liquid chromatography (LC) tandem MS was used for conjugated steroids using 20 μL for sulfates (DHEA-S and E1-S) and 100 μL for glucuronides (ADT-G, 3α-diol-3G, and 3α-diol-17G) in 2 assays, described previously in detail (35). SHBG was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Diagnostics Biochem Canada, Inc, Ontario, Canada; RRID:AB_2940953), following the manufacturer’s instructions. Hormones with >50% below the lower limit of detection (3β-diol and progesterone) were excluded from our analysis; for other hormones, values below the lower limit of detection were imputed with half the lower limit of detection. Free testosterone and free estradiol were calculated using formulas that include the individual hormone, SHBG and a constant for albumin (43 g/L) (36, 37). We additionally considered ratios of estrone to androstenedione (E1:4-dione) and estradiol to testosterone (E2:testosterone), indicative of aromatase conversion of androgens to estrogens, as well as ratios reflective of conjugation with sulfate or glucuronide groups (E1-S:E1, DHEA-S:DHEA, and ADT-G:ADT). We refer to hormone features as any measured or derived hormone, SHBG, or hormone ratio.
Microbiome Measurement
Shallow shotgun sequencing was conducted in the Knight laboratory at the University of California San Diego (38), as previously described (39).
Briefly, DNA is extracted from fecal samples following the Earth Microbiome Project protocol (40). Input DNA is quantified in a 384-well plate using a PicoGreen fluorescence assay (ThermoFisher, Inc), and normalized to 1 ng using an Echo 550 acoustic liquid-handling robot (Labcyte, Inc). Enzyme mixes for fragmentation, end repair and A-tailing, ligation, and polymerase chain reaction (PCR) are added using a Mosquito HV micropipetting robot (TTP Labtech). Fragmentation is performed at 37 °C for 20 minutes, followed by end repair and A-tailing at 65 °C for 30 minutes. Sequencing adapters and barcode indices are added in 2 steps, following the iTru adapter protocol (41). Universal “stub” adapter molecules and ligase mix are first added to the end-repaired DNA using the Mosquito HV robot and ligation performed at 20 °C for 1 hour. Unligated adapters and adapter dimers are removed using AMPure XP magnetic beads and a BlueCat purification robot (BlueCat Bio). Next, individual i7 and i5 are added to the adapter-ligated samples using the Echo 550 robot. Then, eluted bead-washed ligated samples are added to PCR master mix and PCR-amplified for 15 cycles. The amplified and indexed libraries are purified again using magnetic beads and the BlueCat robot, resuspended in water, and transferred to a 384-well plate using the Mosquito HTS liquid-handling robot for library quantitation, sequencing, and storage. Samples are then normalized based on a PicoGreen fluorescence assay, for sequencing on Illumina NovaSeq.
Microbiome Bioinformatics Processing
FASTQ sequence reads were processed using the standard shotgun sequencing pipeline in Qiita. Briefly, per sample sequence adapters were removed via fastp, and sequence reads mapping to the human genome were filtered via minimap2. The sequence reads were then aligned against the WolR1 reference database of bacterial and archaeal genomes using Woltka with the Bowtie2 aligner (42), to generate an operational genomic unit (OGU) table, a gene table, and a KEGG (43) ortholog table. The sequence alignments were also classified at the species taxonomic rank, while functional profiles were obtained by collapsing the gene table into MetaCyc functional pathways; stratified output of functional pathway abundance per species was also obtained. Functional pathway abundance indicates the potential of the microbiome to carry out specific functions. Indices of α-diversity (observed species, Shannon diversity index) and β-diversity (Jensen-Shannon Divergence, generalized UniFrac) were calculated from the OGU table using “vegan,” “phyloseq,” and “GUniFrac” packages in R (44-46).
Subclinical Atherosclerosis
High-resolution B-mode ultrasound with automated computerized edge detection software assessed carotid artery arteriosclerosis in the right carotid arteries (47). Ultrasound images were read by the Atherosclerosis Research Unit at the University of Southern California. The primary outcome was presence/absence of carotid artery lesions (plaque), defined as focal intima-media thickness >1.5 mm in any imaged segment (common and internal carotid arteries and bifurcation). This definition of plaque is supported by the Mannheim carotid intima-media thickness and plaque consensus and the American Society of Echocardiography Carotid Intima-Media Thickness Task Force (48, 49), and has been used previously in the WIHS (47, 50).
Statistical Analysis
General principles
Sex hormone concentrations were inverse-normal transformed for all statistical models. Due to right skewness, γ-glutamyl transferase, fasting glucose, hemoglobin A1c, and triglycerides were log-transformed for analysis. Analyses were conducted among the entire study sample (non-stratified) and stratified by HIV serostatus. In regression models, cross-product terms were used to test effect modification by HIV serostatus (ie, HIV serostatus × hormone feature). The false discovery rate (FDR) was controlled at 5% for discovery of hormone feature–associated microbiome species and functional pathways; for other a priori outcomes (ie, α- and β- diversity, “estrobolome” orthologs, carotid artery plaque), unadjusted P values are presented in order to facilitate comparison among metabolically related and highly correlated hormone features. All analyses were conducted using R (version 4.2.2).
Sex hormone features and stool microbiome associations
(1) Selection of covariates. In statistical models relating sex hormone features to the stool microbiome, we determined a priori that analyses would adjust for age, race/ethnicity, study site, and HIV serostatus. Other covariates were chosen via univariate linear regressions for each covariate (predictors) with inverse-normal transformed hormone feature outcomes—covariates associated with at least one sex hormone feature (likelihood ratio test FDR-adjusted q < .05) in the non-stratified, HIV+, or HIV- groups were also included in all models (Fig. 1C; Supplementary Figure 1) (51). Thus, the final covariates for statistical adjustment in analyses relating sex hormone features to the stool microbiome were age, race/ethnicity, study site, HIV serostatus, income, smoking status, alcohol use, illicit drug use, hepatitis C virus (HCV) serostatus, eGFR, γ-glutamyl transferase, waist circumference, fasting glucose, hemoglobin A1c, total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, diabetes medication use, lipid-lowering medication use, and hypertension medication use. Since waist circumference and body mass index (BMI) are highly correlated, waist circumference was chosen rather than BMI due to its stronger association with sex hormones (Fig. 1C).
(2) Within-subject (α-) and between-subject (β-) diversity. Multivariable linear regression was used to examine the association of sex hormone features (predictors) with the number of observed species and the Shannon diversity index (outcomes), adjusting for covariates. Permutational multivariate analysis of variance (PERMANOVA) was used to assess the association of sex hormone features with overall microbiome composition, as measured by the Jensen-Shannon Divergence and generalized UniFrac distance, adjusting for covariates.
(3) Sensitivity analyses. For α- and β-diversity results, we conducted sensitivity analyses related to use of antibiotics and sex steroid-interacting HIV drugs. Antibiotic use was not assessed comprehensively in the cohort; however, 4 participants reported antibiotic use since their last study visit. In a sensitivity analysis we excluded these 4 participants. Certain HIV drugs have been implicated in interacting with sex steroids, including efavirenz, etravirine, nevirapine, cobicistat, and ritonavir as summarized in a recent review (52). 27, 7, 1, 42, and 32 women with HIV reported taking these drugs, respectively, since their last study visit. Some of the sex hormone features differed between women with HIV taking or not taking efavirenz, cobicistat, and ritonavir (etravirine and nevirapine did not have sufficient sample size for comparisons) (Supplementary Table 1) (51). In sensitivity analyses we adjusted for each of these drugs as covariates.
(4) Species and functional pathways. Microbial species and MetaCyc functional pathways were analyzed in 2 stages: first using the Analysis of Composition of Microbiomes (ANCOM2) method (53), followed by confirmatory multivariable linear regression, described previously (54). Briefly, ANCOM2 was used to detect species and pathways for which abundance was related to sex hormone features, adjusting for covariates. We controlled the FDR at 5%, and excluded species or pathways from testing if they were present in <20% of the participants. An ANCOM2 detection level ≥0.6 was considered significant—this level indicates that the ratios of the species or pathway to at least 60% of other species or pathways were detected to be significantly associated (FDR q < .05) with a hormone feature. To assess the direction and magnitude of the associations, we constructed multivariable linear regression models, with centered log ratio (clr)-transformed species/pathway abundance as outcomes, and sex hormone features as the main predictors, adjusting for covariates. Spearman correlations were used to examine associations among sex hormone–related species and functional pathways. We also used multivariable linear regression to examine the association of a priori KEGG orthologs for the “estrobolome” enzymes β-glucuronidase (K01195) and aryl-sulfatase (K01130) (clr-transformed outcomes) with sex hormone feature predictors, adjusting for covariates.
(5) Sex hormone–related microbiome scores. We developed sex hormone–related microbiome scores based on species associated with each hormone feature. First, clr-transformed abundance of species associated with a given hormone feature in ANCOM2 (detection level ≥0.6) were Z-score standardized to give equal weight to each species; then, those species positively related to the hormone feature were summed while species negatively related to the hormone feature were subtracted within each participant to derive the score. Spearman's correlations were used to examine the association of the derived microbiome scores with sex hormone features and gut “estrobolome” orthologs.
Sex hormone features, hormone-related stool microbiome, and subclinical atherosclerosis
(1) Sex hormone features and subclinical atherosclerosis. Multivariable logistic regression analyses were used to examine the associations of sex hormone features (predictors) with carotid artery plaque. Two levels of covariate adjustment were considered: Model 1 (base model) adjusted for age, race/ethnicity, study site, HIV serostatus (in non-stratified models only), income, smoking status, alcohol use, illicit drug use, HCV serostatus, eGFR, γ-glutamyl transferase, and waist circumference, while Model 2 (cardiometabolic model) additionally adjusted for fasting glucose, hemoglobin A1c, total cholesterol, triglycerides, HDL cholesterol, diabetes medication use, lipid-lowering medication use, and hypertension medication use.
(2) Sex hormone–related microbiome scores and subclinical atherosclerosis. Multivariable logistic regression was used to examine the associations of sex hormone–related microbiome scores (predictors) with carotid artery plaque. Two levels of covariate adjustment, base model and cardiometabolic model, as described above were used. For those hormone-related microbiome scores related to plaque, we further examined which species from the score were related to plaque, using clr-transformed species as predictors in logistic regression and adjusting for covariates.
(3) Exploratory mediation analysis. Bi-directional mediation analysis was conducted to explore whether stool microbiota may mediate effects of sex hormones on subclinical atherosclerosis, or alternatively whether sex hormones may mediate effects of stool microbiota on subclinical atherosclerosis. Candidates for mediation were selected if both the sex hormone feature and the respective sex hormone–related microbiome score were associated with carotid artery plaque at P < .10 in either the base or cardiometabolic model. The “mediation” package in R was used to test the average causal mediation effect (ACME) (55), using nonparametric bootstrap estimation with 5000 simulations.
Results
Participant Characteristics
Among 197 women with measured sex hormones, the median age was 58 (interquartile range, 54-61) and 81% were living with HIV. The majority were non-Hispanic Black (68%) and low income (82% with annual income <$24 K) (Table 1). Participants with and without HIV were similar on most characteristics, with the exception that women with HIV included more participants from the Chicago site and had lower systolic blood pressure (P < .05) (Table 1). Among women with HIV, 98% were on antiretroviral therapy and 77% were virally suppressed (ie, without detectable viral load).
Regarding sex hormone levels, women with HIV had lower DHT, 3α-diol-3G, 3α-diol-17G, ADT-G:ADT ratio, E2, and free E2 than women without HIV (Table 2), and these differences remained significant in multivariable linear regression models adjusting for covariates (Supplementary Table 2) (51). Most hormones were positively correlated with each other, while SHBG was inversely correlated with some hormones (eg, E2, 3α-diol-3G, and 3α-diol-17G) (Supplementary Figure 2) (51).
Table 2.
Sex hormone levels among participants in the Women's Interagency HIV Study (WIHS)
| All (n = 197) | HIV+ (n = 159) | HIV− (n = 38) | P value | |
|---|---|---|---|---|
| Adrenal precursors | ||||
| Dehydroepiandrosterone (DHEA), ng/mL, median [IQR] | 0.95 [0.52, 1.55] | 0.95 [0.56, 1.50] | 0.94 [0.43, 1.72] | .977 |
| Androst-5-enediol (5-diol), pg/mL, median [IQR] | 172.29 [88.03, 278.31] | 169.60 [87.50, 266.83] | 229.65 [114.31, 329.54] | .093 |
| Androstenedione (4-dione), ng/mL, median [IQR] | 0.30 [0.22, 0.43] | 0.30 [0.21, 0.43] | 0.32 [0.25, 0.43] | .261 |
| DHEA-sulfate (DHEA-S), ug/mL, median [IQR] | 0.46 [0.22, 0.79] | 0.45 [0.23, 0.75] | 0.50 [0.15, 0.80] | .905 |
| DHEA-S:DHEA ratio, median [IQR] | 0.39 [0.27, 0.56] | 0.40 [0.27, 0.58] | 0.36 [0.27, 0.48] | .276 |
| Androgens | ||||
| Testosterone, ng/mL, median [IQR] | 0.15 [0.10, 0.24] | 0.15 [0.10, 0.23] | 0.18 [0.11, 0.33] | .134 |
| Dihydrotestosterone (DHT), pg/mL, median [IQR] | 20.99 [9.13, 35.76] | 19.34 [7.70, 34.12] | 26.53 [14.86, 44.10] | .040 |
| Androsterone (ADT), pg/mL, median [IQR] | 46.95 [12.50, 77.54] | 52.02 [12.50, 77.37] | 35.12 [12.50, 72.80] | .379 |
| Androsterone-glucuronide (ADT-G), ng/mL, median [IQR] | 9.62 [5.57, 17.40] | 8.82 [5.46, 16.95] | 12.10 [6.28, 19.40] | .165 |
| 5α-androstane-3α,17β-diol-3-glucuronide (3α-diol-3G), ng/mL, median [IQR] | 0.42 [0.13, 0.73] | 0.40 [0.13, 0.70] | 0.54 [0.29, 0.88] | .031 |
| 5α-androstane-3α,17β-diol-17-glucuronide (3α-diol-17G), ng/mL, median [IQR] | 0.35 [0.13, 0.68] | 0.32 [0.13, 0.59] | 0.59 [0.30, 0.90] | .002 |
| Free testosterone, nmol/L, median [IQR] | 0.01 [0.00, 0.01] | 0.01 [0.00, 0.01] | 0.01 [0.01, 0.01] | .068 |
| ADT-G:ADT ratio, median [IQR] | 0.15 [0.09, 0.30] | 0.14 [0.08, 0.30] | 0.21 [0.13, 0.54] | .026 |
| Estrogens | ||||
| Estrone (E1), pg/mL, median [IQR] | 13.85 [8.15, 20.51] | 13.37 [7.69, 20.24] | 16.93 [9.56, 21.48] | .126 |
| Estradiol (E2), pg/mL, median [IQR] | 3.50 [2.01, 5.85] | 3.31 [1.83, 5.06] | 4.97 [2.30, 7.56] | .023 |
| Estrone-sulfate (E1-S), ng/mL, median [IQR] | 0.13 [0.04, 0.26] | 0.11 [0.04, 0.24] | 0.16 [0.04, 0.28] | .363 |
| Free E2, nmol/L, median [IQR] | 0.15 [0.08, 0.30] | 0.15 [0.08, 0.25] | 0.24 [0.09, 0.44] | .022 |
| E1-S:E1 ratio, median [IQR] | 0.01 [0.00, 0.01] | 0.01 [0.00, 0.01] | 0.01 [0.00, 0.01] | .396 |
| Other | ||||
| Sex hormone binding globulin (SHBG), nmol/L, median [IQR] | 69.76 [46.31, 93.48] | 72.34 [48.23, 94.53] | 61.36 [41.41, 88.35] | .243 |
| Estradiol:Testosterone ratio, median [IQR] | 23.01 [13.04, 34.75] | 22.84 [13.13, 33.59] | 27.10 [13.36, 38.79] | .381 |
| Estrone:Androstenedione ratio, median [IQR] | 48.82 [30.05, 67.66] | 48.23 [29.37, 66.26] | 52.65 [31.92, 72.42] | .314 |
Abbreviation: IQR, interquartile range.
Sex Hormones and Stool Microbiome Overall Diversity and Composition
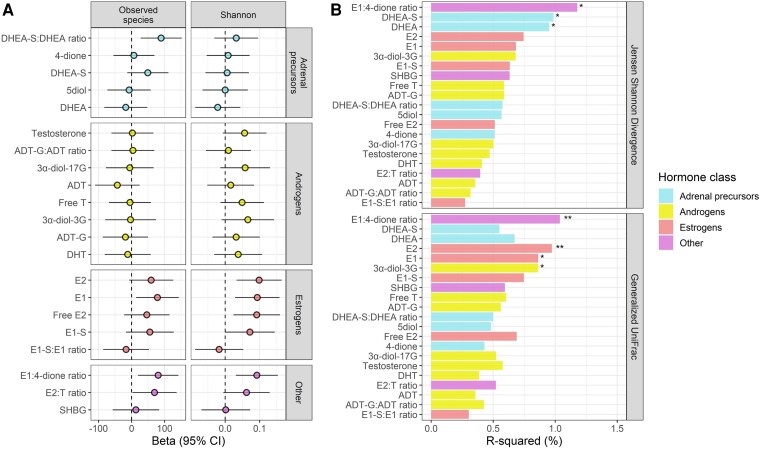
A number of sex hormone features were associated with higher stool microbiome within-person (α) diversity, indicated by the number of observed species and/or the Shannon diversity index, in multivariable linear regression models. These included the DHEA-S:DHEA ratio, E2, E1, free E2, E1-S, the E1:4-dione ratio, and the E2:testosterone ratio (Fig. 2A). These findings were consistent for women with and without HIV (Supplementary Table 3) (51). In regards to stool microbiome between-person (β) diversity, PERMANOVA revealed that the E1:4-dione ratio, DHEA, and DHEA-S were significantly associated with the Jensen-Shannon Divergence, while the E1:4-dione ratio, E2, E1, and 3α-diol-3G were significantly associated with the generalized UniFrac distance (Fig. 2B), and these findings were consistent for women with and without HIV (Supplementary Table 4) (51). Results for α- and β-diversity were unchanged when excluding 4 participants reporting antibiotic use since their last study visit (Supplementary Figure 3) (51), and when adjusting for use of HIV drugs known or suspected to interact with sex hormones (Supplementary Tables 3 and 4) (51).
Figure 2.
Sex hormone features and stool microbiome overall diversity and composition (n = 197). (A) Associations of hormones with number of observed species and the Shannon diversity index. Estimates are from multivariable linear regression with inverse-normal transformed hormone measures as predictors, adjusting for age, study site, race/ethnicity, income, smoking status, alcohol use, illicit drug use, HCV serostatus, estimated glomerular filtration rate, serum gamma-glutamyl transferase, waist circumference, fasting glucose, hemoglobin A1c, total cholesterol, triglycerides, HDL cholesterol, diabetes medication use, lipid-lowering medication use, hypertension medication use, and HIV serostatus. (B) Associations of hormones with the Jensen-Shannon Divergence and generalized UniFrac distance. Estimates are from permutational multivariate analysis of variance (PERMANOVA) with inverse-normal transformed hormone measures as predictors, adjusting for same covariates as in (a). *P < 0.05, **P < 0.01.
Sex Hormones and Stool Microbiome Species Abundance
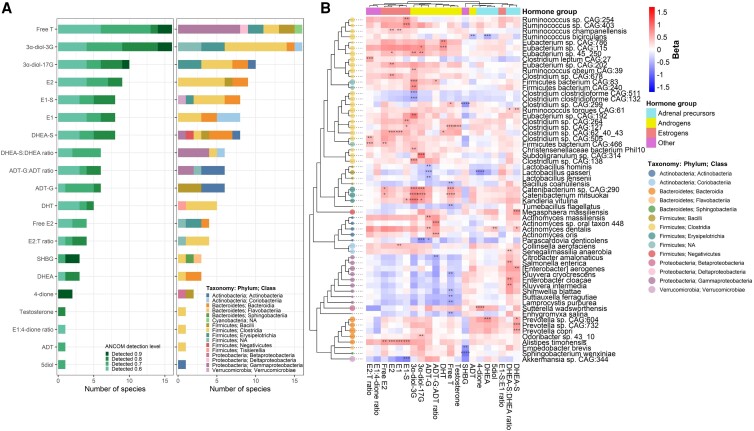
We tested the association of sex hormone features with 1723 species using ANCOM2. Free T and 3α-diol-3G were associated with the most species (16 species each), followed by 3α-diol-17G, E2, E1-S, E1, and DHEA-S (8-10 species each), while all other hormone features were associated with ≤ 6 species and the E1-S:E1 ratio was not associated with any species (Fig. 3A; Supplementary Table 5) (51). Though many significant associations were distinct to a specific hormone feature, directions of associations with species tended to be consistent within hormone groups (ie, estrogens, androgens, adrenal precursors) (Fig. 3B; Supplementary Table 5) (51). For example, estrogens were positively associated with species from Alistipes, Collinsella, Erysipelotrichia, and Clostridia; androgens were positively associated with species from Actinomyces, Erysipelotrichia, and Clostridia and inversely related to species from Bacilli and Gammaproteobacteria; and adrenal precursors were positively associated with species from Prevotella, Gammaproteobacteria, Actinomyces, and Megasphaera (Fig. 3B; Supplementary Table 5) (51). Most of the associations were of a consistent direction for women with and without HIV: out of 118 total hormone-species associations, only 18 had inconsistent direction between women with and without HIV, and only 2 had a statistically significant interaction with HIV serostatus (P-interaction <.05) (Supplementary Table 5; Supplementary Figure 4) (51). Among the sex hormone–related species, those within the same taxonomic class had strong positive correlations with each other, while the species from Gammaproteobacteria and Bacilli were inversely correlated with Clostridia species (Supplementary Figure 5) (51).
Figure 3.
Associations of sex hormone features with stool microbiome species (n = 197). (A) Analysis of Compositions of Microbiomes 2 (ANCOM2) was used to identify species associated with each inverse-normal transformed hormone feature, adjusting for age, study site, race/ethnicity, income, smoking status, alcohol use, illicit drug use, HCV serostatus, estimated glomerular filtration rate, serum gamma-glutamyl transferase, waist circumference, fasting glucose, hemoglobin A1c, total cholesterol, triglycerides, HDL cholesterol, diabetes medication use, lipid-lowering medication use, hypertension medication use, and HIV serostatus. Barplots show number of species selected with ANCOM detection level ≥ 0.6 per sex hormone feature, ANCOM detection level or taxonomic class. (B) For ANCOM-selected species, effect estimates shown in heatmap were obtained from multivariable linear regression of clr-transformed species (outcomes) on inverse-normal transformed hormone features (predictors), adjusting for above-mentioned covariates. Species are organized in a cladogram according to phylogenetic relatedness. Only species with ANCOM2 detection level ≥0.7 are shown due to space constraints (full list of detection level ≥ 0.6 in Supplementary Table 5) (51). Size of cladogram tips are based on species mean relative abundance. *ANCOM2 detection level = 0.6, **0.7, ***0.8, ****0.9.
Sex Hormones and Stool Microbiome Functional Pathways
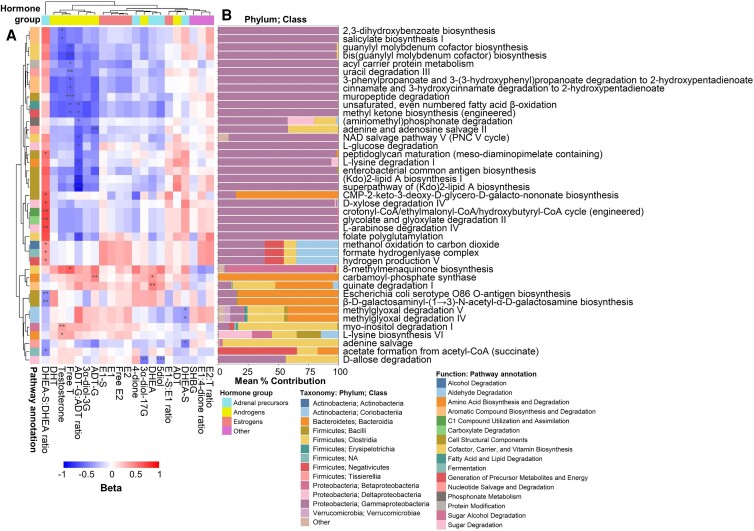
Among 812 pathways tested using ANCOM2, 41 pathways were associated with sex hormone features, mostly the DHEA-S:DHEA ratio, ADT-G:ADT ratio, and free testosterone, while estrogens were not associated with any pathways (Fig. 4A; Supplementary Table 6) (51). The DHEA-S:DHEA ratio was positively associated with pathways related to cell structure, hydrogen production, and sugar degradation, among others; free testosterone was inversely associated with pathways related to aromatic compound degradation and cofactor biosynthesis, among others; and the ADT-G:ADT ratio was inversely associated with pathways related to cell structure, cofactor/vitamin biosynthesis, amino acid and sugar degradation, among others (Fig. 4A; Supplementary Table 6; Supplementary Table 7) (51). Importantly, species from Gammaproteobacteria were the largest contributors to many of the pathways (Fig. 4B); thus, it is likely that the associations of DHEA-S:DHEA ratio, free testosterone, and ADT-G:ADT ratio with Gammaproteobacteria species were driving many of the observed functional pathway findings. Accordingly, functional pathways attributed to Gammaproteobacteria were highly correlated with each other (Supplementary Figure 6) (51) and with species from Gammaproteobacteria (Supplementary Figure 7) (51).
Figure 4.
Associations of sex hormone features with stool microbiome functional pathways (n = 197). (A) Analysis of Compositions of Microbiomes 2 (ANCOM2) was used to identify functional pathways associated with each inverse-normal transformed hormone feature, adjusting for age, study site, race/ethnicity, income, smoking status, alcohol use, illicit drug use, HCV serostatus, estimated glomerular filtration rate, serum gamma-glutamyl transferase, waist circumference, fasting glucose, hemoglobin A1c, total cholesterol, triglycerides, HDL cholesterol, diabetes medication use, lipid-lowering medication use, hypertension medication use, and HIV serostatus. Effect estimates shown in heatmap were obtained from multivariable linear regression of clr-transformed functional pathways (outcomes) on inverse-normal transformed hormone features (predictors), adjusting for above-mentioned covariates. All pathways with ANCOM2 detection level ≥ 0.6 are shown. Pathways are annotated by MetaCyc database ontology. *ANCOM2 detection level = 0.6, **0.7, ***0.8, ****0.9. (B) Mean percent contribution of taxonomic classes to the functional pathways. Stratified pathway by species output from Woltka was used to determine the abundance of each pathway per species, and percent contribution was determined per species for each pathway within each sample, then averaged across samples.
Sex Hormones and Gut “Estrobolome” Orthologs
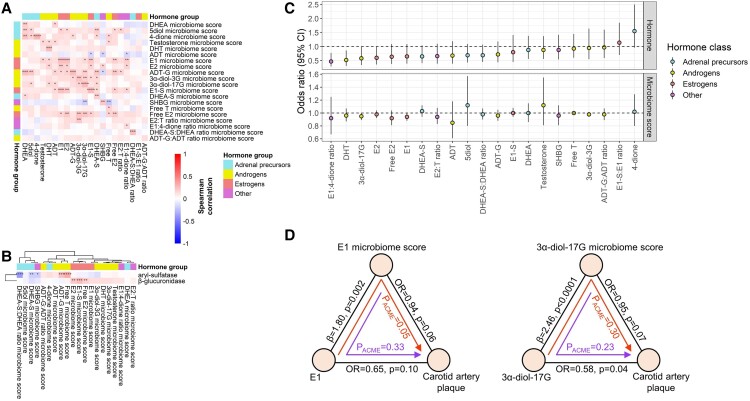
Free E2 had a significant positive association with aryl-sulfatase abundance (P = .04), while marginally significant positive associations were observed for E2 with aryl-sulfatase (P = .06), and for E1 and E1-S with β-glucuronidase (P = .10 and P = .06, respectively) (Supplementary Table 8) (51). Directions of these associations were consistent in women with and without HIV (Supplementary Table 8) (51). A microbiome score was derived for each hormone feature based on its associated species. Most of the scores were significantly positively correlated with their respective hormone feature (eg, E1 microbiome score correlated with E1, etc.) (Fig. 5A). We observed some correlations between the sex hormone–related microbiome scores and “estrobolome” orthologs; for example, aryl-sulfatase abundance was inversely correlated with the DHEA-S and DHEA-S:DHEA ratio microbiome scores and positively correlated with the free testosterone and ADT-G microbiome scores, while β-glucuronidase abundance was positively correlated with the E2, free E2, and E1-S microbiome scores (Fig. 5B). Alistipes timonensis and Alistipes sp. Zagget8, which were positively associated with serum estrogens, were strongly correlated with β-glucuronidase abundance (Spearman r = .35, P < .0001) (Supplementary Table 9) (51).
Figure 5.
Associations of sex hormone–related stool microbiome scores with “estrobolome” orthologs and subclinical atherosclerosis. (A) Spearman's correlations between sex hormone–related microbiome scores and sex hormone features (n = 197). (B) Spearman's correlations between sex hormone–related microbiome scores and microbial “estrobolome” orthologs, β-glucuronidase (K01195) and aryl-sulfatase (K01130) (n = 197). (C) Association of sex hormone features (n = 134) and sex hormone–related microbiome scores (n = 208) with carotid artery plaque, from multivariable logistic regression adjusting for age, study site, race/ethnicity, income, smoking status, alcohol use, illicit drug use, HCV serostatus, estimated glomerular filtration rate, serum gamma-glutamyl transferase, waist circumference, and HIV serostatus. (D) Exploratory bi-directional mediation analysis for sex hormone features/sex hormone–related microbiome scores with carotid artery plaque (n = 134). Betas and odds ratios (ORs) were from multivariable linear or logistic regression, respectively, adjusting for same covariates as in (C). P value for average causal mediation effect (ACME) estimated using “mediation” package in R. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Sex Hormones and Subclinical Atherosclerosis
A number of sex hormone features showed significant or marginally significant protective associations with carotid artery plaque in multivariable logistic regression models adjusted for all but cardiometabolic-related covariates, including the E1:4-dione ratio (P = .002), DHT (P = .01), 3α-diol-17G (P = .04), E2 (P = .05), free E2 (P = .10), E1 (P = .10), DHEA-S (P = .07), and DHEA-S:DHEA ratio (P = .10) (Fig. 5C; Supplementary Table 10) (51). Further adjustment for cardiometabolic risk factors did not materially attenuate the observed associations (Supplementary Table 10) (51). Most associations of sex hormone features with plaque appeared of consistent direction for women with and without HIV, with some exceptions (Supplementary Table 10) (51); however, the sample size in women without HIV was small (n = 29) and full covariate adjustment was precluded, thus making comparisons difficult.
Sex Hormone–Related Stool Microbiome and Subclinical Atherosclerosis
The E1 and 3α-diol-17G microbiome score were inversely associated with carotid artery plaque in multivariable logistic regression models (P = .06 and P = .07, respectively) (Fig. 5C; Supplementary Table 11) (51). Among the species comprising the microbiome scores, species from Collinsella (positively associated with E1) were related to lower odds of plaque, and Parascardovia denticolens (inversely associated with 3α-diol-17G) was related to higher odds of plaque (Supplementary Table 12) (51). These species did not differ in abundance by HIV serostatus or viral suppression (Supplementary Figure 8) (51). In an exploratory bi-directional mediation analysis, the E1 microbiome score appeared to mediate the protective association of E1 with carotid artery plaque (PACME = .05), while the other relationship tested (3α-diol-17G→microbiome score→plaque) did not reveal significant mediation effects (Fig. 5D).
Discussion
In this cross-sectional study of postmenopausal women with and without HIV, we found that serum sex hormones were significant predictors of stool microbiome diversity and composition independent of HIV status and demographic, behavioral, and clinical factors. In particular, higher levels of estrogens were associated with higher microbial diversity, while higher levels of certain estrogens, androgens, and adrenal precursors were related to altered overall microbiome composition and specific microbial species. Estrogens tended to be positively associated with microbial “estrobolome” orthologs, β-glucuronidase and aryl-sulfatase, while estrogen-related microbiome scores (derived from estrogen-related species) were positively correlated with β-glucuronidase, suggesting that postmenopausal systemic levels of estrogens are regulated in part by gut microbial deconjugation and “recycling” activity. Finally, a number of sex hormones including estrogens, androgens, and adrenal precursors tended to associate with lower odds of carotid artery plaque, with the association of E1 and plaque possibly mediated by the gut microbiome. Taken together, our results provide support for the existence of interactions between sex hormones and the gut microbiome, suggesting a possible role for the gut microbiome in estrogen-related cardiovascular protection.
The sex hormone and stool microbiome associations observed here confirm and expand upon prior knowledge of hormone-microbial relationships, which remains very sparse (23, 56). Several studies have previously observed positive associations of stool microbiome diversity with estrogen levels in urine (57, 58) or serum (59) from adult women. Further, studies have also identified that taxa from Clostridia (eg, Ruminococcus) are associated with higher estrogen levels (57, 58, 60), consistent with our findings. Most studies of androgens and the stool microbiome focused on men or women with polycystic ovary syndrome (PCOS) (56), a condition characterized by hyperandrogenism, though findings have been inconsistent regarding serum testosterone associations with gut microbiome diversity and taxa (59, 61-63). In a study of men and women with and without PCOS, serum testosterone was positively associated with stool microbiome diversity, and men and women with PCOS had higher abundance of Catenibacterium and Kandleria than women without PCOS (63); this is consistent with our results, where testosterone was marginally associated with higher stool microbiome diversity, and various androgens were positively associated with species from Catenibacterium and Kandleria. We found that free testosterone and androgen glucuronides (ie, 3α-diol-3G and 3α-diol-17G), rather than total testosterone, were more highly associated with stool microbiome species. Free testosterone represents the testosterone that is unbound and available for activation of the androgen receptor, while androgen glucuronides are a proxy for systemic androgen activity in tissues (64), so overall androgenic activity may be important in relation to the gut microbiome.
We hypothesized that metabolic interactions between the gut microbiota and sex hormones would account for some hormone-microbiome relationships, and our results support this notion. We observed marginally positive associations of serum estrogens, but not androgens, with abundance of the microbial aryl-sulfatase and β-glucuronidase orthologs, involved in deconjugation and “recycling” of hormones. Previous studies have found positive associations of fecal β-glucuronidase activity with urinary E1 (58), but not with serum testosterone (65), in line with our results. Further, when examining sex hormone–related microbiome scores and the “estrobolome” orthologs, we found positive correlations of the β-glucuronidase ortholog with estrogen-related scores, attributed largely to Alistipes species; positive correlations of the aryl-sulfatase ortholog with androgen-related scores; and an inverse correlation of aryl-sulfatase with the DHEA-S:DHEA ratio-related score. The relationship of Alistipes to β-glucuronidase is consistent with prior identification of β-glucuronidase genes in Alistipes species (66), while the inverse relationship of aryl-sulfatase and the DHEA-S:DHEA ratio score may indicate microbial sulfatase activity toward DHEA-S. Taken together, our results suggest that glucuronide deconjugation activity may underlie some estrogen-microbiome relationships, while sulfate deconjugation activity may underlie some androgen-microbiome relationships.
Though almost all of the measured sex hormones were associated with some stool microbiome species, only adrenal precursors and androgens, in particular free testosterone, the DHEA-S:DHEA ratio, and the ADT-G:ADT ratio, were associated with stool microbiome functional pathways. These functional pathways, encompassing a wide range of metabolic processes, including that of aromatic compounds, sugars, amino acids, fatty acids, vitamins, and cofactors, were largely attributed to Gammaproteobacteria. Thus, we speculate that the associations of free testosterone (inverse), the ADT-G:ADT ratio (inverse), and the DHEA-S:DHEA ratio (positive) with species from Gammaproteobacteria (mostly Enterobacteriaceae family), lead to functional shifts in the gut microbiome. Enterobacteriaceae are a family of gram-negative bacteria recognized for their pathogenicity in humans (67); this family was previously shown to be enriched in the stool microbiome of people with HIV, and associated with systemic immune activation and inflammation (68). Potential regulation of gut Enterobacteriaceae by adrenal precursors and androgens may be a protective mechanism in people with HIV, and warrants validation in additional studies.
We also found associations of sex hormones with subclinical atherosclerosis, which has not been previously studied in postmenopausal women with HIV, and therefore may have important implications irrespective of links with the gut microbiome. Endogenous estrogens are considered cardioprotective from a mechanistic standpoint; however, cohort studies examining serum estradiol and incidence of coronary heart disease in postmenopausal women from the general population have yielded conflicting results (69, 70). Discrepancies could relate to populations of different ages and the “timing hypothesis” which assumes that estrogens are protective only in younger postmenopausal women (71). Women with HIV are a unique population to study in regard to sex hormones and cardiovascular risk for several reasons. First, women with HIV may have sex hormone deficiencies compared to women without HIV—this has been observed previously for estradiol and testosterone (7, 8, 10), and is confirmed here in our study where women with HIV had lower levels of several estrogens and androgens than women without HIV. These sex hormone deficiencies could be due to direct actions by specific HIV drugs (52), or to the effects of HIV drugs on the liver where sex steroids are metabolized (liver enzymes were elevated in women with HIV compared to women without HIV, suggesting some liver damage). Second, while CVD risk is elevated in both men and women with HIV compared to their uninfected counterparts, the effect of HIV on CVD is more pronounced in women than men (4-6). Lastly, estrogens have been shown to inhibit HIV replication, maintain HIV latency (14), and downregulate the immune response to HIV (13), suggesting that estrogens can modify HIV-related disease risk. Taken together, estrogen deficiencies in women with HIV may contribute to excess CVD risk, either directly (ie, deficiency of cardioprotective estrogens) or indirectly through estrogen effects on HIV latency and response. Our results, in a younger postmenopausal population (median age 58), support protective associations of estrogens (and some androgens and adrenal precursors) with carotid artery plaque, a subclinical predictor of future clinical cardiovascular events in the general population (72) and in people with HIV (50, 73).
Prior studies have examined the stool microbiome in relation to CVD (74), and several microbiome-mediated mechanisms have been implicated in triggering or ameliorating cardiovascular dysfunction, including microbial synthesis of metabolites that are detrimental (eg, trimethylamine N-oxide, imidazole propionate) or beneficial (eg, short-chain fatty acids, indole propionate) to cardiovascular health, and microbial translocation leading to inflammatory sequelae (75). Previous research in women with HIV has linked a number of these metabolites with cardiovascular outcomes (76), including studies in the WIHS cohort (77-79). To our knowledge, this is the first study to explore potential sex hormone–related microbial mechanisms of CVD. Our study highlights that the gut microbiome may play a role in estrogen-related cardioprotection in postmenopausal women, specifically for the relationship of serum E1 with carotid artery plaque. E1 is the dominant form of estrogen after menopause, primarily present in its sulfated form (E1-S). Our results showed that E1-related species, particularly those from Collinsella, were associated with lower odds of carotid artery plaque. This finding is consistent with a prior report of lower gut Collinsella abundance in patients with coronary heart disease compared to healthy controls (80), but contrary to a small study where Collinsella was enriched in patients with atherosclerosis compared to healthy controls (81), as well as other studies linking Collinsella to coronary calcification among patients with coronary artery disease (82), and to cardiovascular complications in patients with diabetes (83). Collinsella do not harbor aryl-sulfatase (84) or β-glucuronidase (66) genes, and consistently, were not positively associated with either “estrobolome” ortholog in our study, indicating that the connection of E1, Collinsella, and subclinical atherosclerosis may be independent of microbial deconjugation activity. We previously reported lower abundance of gut Collinsella in women with HIV compared to women without HIV in the WIHS using 16S rRNA gene sequencing (25), but we did not observe that here, and other studies have indicated enriched Collinsella abundance in HIV (85, 86). Additional research is needed to determine whether associations of Collinsella with E1 and subclinical atherosclerosis are specific to this study population or generalizable to others.
Our study was strengthened by the use of shotgun metagenomic sequencing and measurement of a wide range of sex hormones, which can provide new insights into microbial species-level and functional relationships with multiple estrogens, androgens, and adrenal precursors, while most prior studies on sex hormones and the stool microbiome utilized 16S rRNA sequencing and measured few sex hormones. Moreover, we implemented stringent control for potentially confounding variables using extensive data from a well-characterized cohort. Our study was limited by a cross-sectional design, which precludes inferences of temporality and causality, and by low sample size for some analyses (ie, stratified analysis of women without HIV). Additionally, our results from a population of women with HIV and comparable women without HIV, may not be generalizable to other populations.
In conclusion, serum sex hormones were significantly associated with higher stool microbiome diversity and altered composition in postmenopausal women with and without HIV, with some associations likely attributed to metabolic interactions of the gut microbiome with hormones. Serum levels of estrogens and androgens were lower in women with HIV than women without HIV, and some were associated with lower odds of carotid artery plaque, which supports hormonal mechanisms of elevated cardiovascular risk in postmenopausal women with HIV. Finally, the protective association of E1 with plaque may involve the gut microbiome, though the mechanisms remain unclear. While it is difficult to disentangle temporal relationships in a cross-sectional study, our findings suggest that sex hormones are important in both gut microbiome composition and cardiovascular risk. Prospective studies, with and without hormone therapy intervention, should further examine the potential role of gut microbiome and sex hormones interactions in cardiovascular disease and other health outcomes. Looking forward, such research may reveal potential microbial intervention targets to support optimal sex hormone levels and/or reduce cardiovascular risk, in women with HIV and other populations as well.
Acknowledgments
B.A.P., D.B.H., Q.Q., and R.C.K. were supported by the National Heart, Lung, and Blood Institute (B.A.P. K01HL160146; R.C.K. R01HL148094; D.B.H. K01HL137557; R.C.K. and D.B.H. U01HL146204-04S1; Q.Q. and R.C.K. R01HL140976). Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS), now the MACS/WIHS Combined Cohort Study (MWCCS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos, David Hanna, and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D'Souza, Stephen Gange and Elizabeth Topper), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels and Matthew Mimiaga), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora and Michelle Floris-Moore), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR003098 (JHU ICTR), UL1-TR001881 (UCLA CTSI), P30-AI-050409 (Atlanta CFAR), P30-AI-073961 (Miami CFAR), P30-AI-050410 (UNC CFAR), P30-AI-027767 (UAB CFAR), P30-MH-116867 (Miami CHARM), UL1-TR001409 (DC CTSA), KL2-TR001432 (DC CTSA), and TL1-TR001431 (DC CTSA).
The authors gratefully acknowledge the contributions of the study participants and dedication of the staff at the MWCCS sites.
Abbreviations
- 3α-diol-3G
androstane-3α 17β-diol 3-glucuronide
- 3β-diol
5α-androstane-3β,17β-diol
- 4-dione
androstenedione
- 5-diol
androst-5-enediol
- ADT
androsterone
- ADT-G
androsterone-glucuronide
- ANCOM2
Analysis of Composition of Microbiomes
- BMI
body mass index
- clr
centered log ratio
- CVD
cardiovascular disease
- DHEA
dehydroepiandrosterone
- DHEA-S
dehydroepiandrosterone sulfate
- DHT
dihydrotestosterone
- E1
estrone
- E1-S
estrone sulfate
- E2
estradiol
- eGFR
estimated glomerular filtration rate
- FDR
false discovery rate
- HCV
hepatitis C virus
- HDL
high-density lipoprotein
- MWCCS
MACS/WIHS Combined Cohort Study
- PCR
polymerase chain reaction
- PERMANOVA
permutational multivariate analysis of variance
- SHBG
sex hormone binding globulin
- WIHS
Women’s Interagency HIV Study
Contributor Information
Brandilyn A Peters, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY 10461, USA.
David B Hanna, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY 10461, USA.
Yi Wang, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY 10461, USA.
Kathleen M Weber, Cook County Health/Hektoen Institute of Medicine, Chicago, IL 60608, USA.
Elizabeth Topper, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205, USA.
Allison A Appleton, Department of Epidemiology and Biostatistics, University at Albany School of Public Health, Rensselaer, NY 12144, USA.
Anjali Sharma, Department of Medicine, Albert Einstein College of Medicine, Bronx, NY 10461, USA.
Howard N Hodis, Departments of Medicine and Population and Public Health Sciences, Atherosclerosis Research Unit, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033, USA.
Nanette Santoro, Department of Obstetrics and Gynecology, University of Colorado School of Medicine, Aurora, CO 80045, USA.
Chantal Guillemette, Centre Hospitalier Universitaire (CHU) de Québec—Université Laval Research Center, Cancer research center (CRC) and Faculty of Pharmacy, Université Laval, Québec City, QC G1V 0A6, Canada.
Patrick Caron, Centre Hospitalier Universitaire (CHU) de Québec—Université Laval Research Center, Cancer research center (CRC) and Faculty of Pharmacy, Université Laval, Québec City, QC G1V 0A6, Canada.
Rob Knight, Departments of Pediatrics, Computer Science and Engineering, Bioengineering, and Center for Microbiome Innovation, University of California San Diego, La Jolla, CA 92093, USA.
Robert D Burk, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY 10461, USA; Departments of Microbiology and Immunology and Obstetrics & Gynecology and Women's Health, Albert Einstein College of Medicine, Bronx, NY 10461, USA.
Robert C Kaplan, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY 10461, USA; Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA.
Qibin Qi, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY 10461, USA.
Disclosures
A.S. reports grant support from Gilead Sciences (unrelated to the current work) and advisory board participation for Gilead Sciences. N.S. is a study investigator and consultant for Astellas, a member of the scientific advisory board for Amazon (Project Ember), MenoGeniX, and is a consultant for Ansh Labs and QUE Oncology. She is on the Board of Directors for the North American Menopause Society and is Past President of the Society for Reproductive Investigations. R. Knight is an advisor to DayTwo, an advisor to and equity holder of GenCirq and Cybele, and a co-founder, advisor, and equity holder of Micronoma and Biota. All other authors have nothing to disclose.
Data Availability Statement
The gut microbiome sequence data in this study are deposited in QIITA database (https://qiita.ucsd.edu/) with accession number 13223. Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS), now the MACS/WIHS Combined Cohort Study (MWCCS). Access to individual-level data from the MWCCS may be obtained upon review and approval of a MWCCS concept sheet. Links and instructions for online concept sheet submission are on the study website (https://statepi.jhsph.edu/mwccs/).
References
- 1. Centers for Disease Control and Prevention . Women and Heart Disease. Accessed September 6, 2023. https://www.cdc.gov/heartdisease/women.htm
- 2. Kaplan RC, Hanna DB, Kizer JR. Recent insights into Cardiovascular Disease (CVD) risk among HIV-infected adults. Curr HIV/AIDS Rep. 2016;13(1):44‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schwarcz SK, Vu A, Hsu LC, Hessol NA. Changes in causes of death among persons with AIDS: San Francisco, California, 1996–2011. AIDS Patient Care STDS. 2014;28(10):517‐523. [DOI] [PubMed] [Google Scholar]
- 4. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506‐2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanna DB, Ramaswamy C, Kaplan RC, et al. Sex- and poverty-specific patterns in cardiovascular disease mortality associated with human immunodeficiency virus, New York City, 2007–2017. Clin Infect Dis. 2020;71(3):491‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stone L, Looby SE, Zanni MV. Cardiovascular disease risk among women living with HIV in North America and Europe. Curr Opin HIV AIDS. 2017;12(6):585‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karim R, Mack WJ, Kono N, et al. Gonadotropin and sex steroid levels in HIV-infected premenopausal women and their association with subclinical atherosclerosis in HIV-infected and -uninfected women in the Women's Interagency HIV study (WIHS). J Clin Endocrinol Metab. 2013;98(4):E610‐E618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sinha-Hikim I, Arver S, Beall G, et al. The use of a sensitive equilibrium dialysis method for the measurement of free testosterone levels in healthy, cycling women and in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 1998;83(4):1312‐1318. [DOI] [PubMed] [Google Scholar]
- 9. Wessman M, Korsholm AS, Bentzen JG, et al. Anti-Müllerian hormone levels are reduced in women living with human immunodeficiency virus compared to control women: a case-control study from Copenhagen, Denmark. J Virus Eradication. 2018;4(2):123‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coburn SB, Dionne-Odom J, Alcaide ML, et al. The association between HIV Status, estradiol, and sex hormone binding globulin among premenopausal women in the Women's Interagency HIV study. J Womens Health (Larchmt). 2022;31(2):183‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schoenbaum EE, Hartel D, Lo Y, et al. HIV infection, drug use, and onset of natural menopause. Clin Infect Dis. 2005;41(10):1517‐1524. [DOI] [PubMed] [Google Scholar]
- 12. Looby SE, Fitch KV, Srinivasa S, et al. Reduced ovarian reserve relates to monocyte activation and subclinical coronary atherosclerotic plaque in women with HIV. AIDS. 2016;30(3):383‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Devadas K, Biswas S, Ragupathy V, Lee S, Dayton A, Hewlett I. Modulation of HIV replication in monocyte derived macrophages (MDM) by steroid hormones. PLoS One. 2018;13(1):e0191916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Das B, Dobrowolski C, Luttge B, et al. Estrogen receptor-1 is a key regulator of HIV-1 latency that imparts gender-specific restrictions on the latent reservoir. Proc Natl Acad Sci U S A. 2018;115(33):E7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. 2017;8(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clegg D, Hevener AL, Moreau KL, et al. Sex hormones and cardiometabolic health: role of estrogen and estrogen receptors. Endocrinology. 2017;158(5):1095‐1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El Khoudary SR, Aggarwal B, Beckie TM, et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation;142(25):e506‐e532. [DOI] [PubMed] [Google Scholar]
- 18. Hodis HN, Mack WJ, Henderson VW, et al. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peters BA, Hanna DB, Sharma A, et al. Menopausal hormone therapy and subclinical cardiovascular disease in women with and without human immunodeficiency virus. Clin Infect Dis. 2023;76(3):e661‐e670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yurkovetskiy L, Burrows M, Khan AA, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39(2):400‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Markle JG, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084‐1088. [DOI] [PubMed] [Google Scholar]
- 23. Peters BA, Santoro N, Kaplan RC, Qi Q. Spotlight on the gut microbiome in menopause: current insights. Int J Womens Health. 2022;14(2022):1059‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kwa M, Plottel CS, Blaser MJ, Adams S. The intestinal microbiome and estrogen receptor-positive female breast cancer. J Natl Cancer Inst. 2016;108(8):djw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Z, Usyk M, Sollecito CC, et al. Altered gut microbiota and host metabolite profiles in HIV-infected women. Clin Infect Dis. 2019;68(8):1274‐1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mutlu EA, Keshavarzian A, Losurdo J, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10(2):e1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tuddenham SA, Koay WLA, Zhao N, White JR, Ghanem KG, Sears CL. The impact of human immunodeficiency virus infection on gut microbiota alpha-diversity: an individual-level meta-analysis. Clin Infect Dis. 2020;70(4):615‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lozupone CA, Li M, Campbell TB, et al. Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe. 2013;14(3):329‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. D'Souza G, Bhondoekhan F, Benning L, et al. Characteristics of the MACS/WIHS combined cohort study: opportunities for research on aging with HIV in the longest US observational study of HIV. Am J Epidemiol. 2021;190(8):1457‐1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adimora AA, Ramirez C, Benning L, et al. Cohort profile: the Women's Interagency HIV study (WIHS). Int J Epidemiol. 2018;47(2):393‐394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Z, Zolnik CP, Qiu Y, et al. Comparison of fecal collection methods for microbiome and metabolomics studies. Front Cell Infect Microbiol. 2018;8(301):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moon JY, Zolnik CP, Wang Z, et al. Gut microbiota and plasma metabolites associated with diabetes in women with, or at high risk for, HIV infection. EBioMedicine. 2018;37(2018):392‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. 2022;79(2):268‐288.e1. [DOI] [PubMed] [Google Scholar]
- 34. Caron P, Turcotte V, Guillemette C. A chromatography/tandem mass spectrometry method for the simultaneous profiling of ten endogenous steroids, including progesterone, adrenal precursors, androgens and estrogens, using low serum volume. Steroids. 2015;104(2015):16‐24. [DOI] [PubMed] [Google Scholar]
- 35. Audet-Walsh E, Lépine J, Grégoire J, et al. Profiling of endogenous estrogens, their precursors, and metabolites in endometrial cancer patients: association with risk and relationship to clinical characteristics. J Clin Endocrinol Metab. 2011;96(2):E330‐E339. [DOI] [PubMed] [Google Scholar]
- 36. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666‐3672. [DOI] [PubMed] [Google Scholar]
- 37. Södergård R, Bäckström T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801‐810. [DOI] [PubMed] [Google Scholar]
- 38. Hillmann B, Al-Ghalith GA, Shields-Cutler RR, et al. Evaluating the information content of shallow shotgun metagenomics. mSystems. 2018;3(6):e00069‐e00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peters BA, Lin J, Qi Q, et al. Menopause is associated with an altered gut microbiome and estrobolome with implications for adverse cardiometabolic risk in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). mSystems. 2022;7(3):e0027322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thompson LR, Sanders JG, McDonald D, et al. A communal catalogue reveals Earth's Multiscale microbial diversity. Nature. 2017;551(7681):457‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Glenn TC, Nilsen RA, Kieran TJ, et al. Adapterama I: universal stubs and primers for 384 unique dual-indexed or 147,456 combinatorially-indexed illumina libraries (iTru & iNext). PeerJ. 2019;7:e7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Langmead B, Salzberg SL. Fast gapped-read alignment with bowtie 2. Nat Methods. 2012;9(4):357‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG As a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44(D1):D457‐D462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McMurdie PJ, Holmes S. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8(4):e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oksanen J, Blanchet FG, Kindt R, et al. Multivariate analysis of ecological communities in R: vegan tutorial. R package version 1.7. 2013.
- 46. Chen J, Bittinger K, Charlson ES, et al. Associating microbiome composition with environmental covariates using generalized UniFrac distances. Bioinformatics. 2012;28(16):2106‐2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hanna DB, Post WS, Deal JA, et al. HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin Infect Dis. 2015;61(4):640‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media thickness task force endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21(2):93‐111. [DOI] [PubMed] [Google Scholar]
- 49. Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). an update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European stroke conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34(4):290‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hanna DB, Moon JY, Haberlen SA, et al. Carotid artery atherosclerosis is associated with mortality in HIV-positive women and men. AIDS. 2018;32(16):2393‐2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peters BA. Data from: Supplementary Data for “Sex hormones, the stool microbiome, and subclinical atherosclerosis in women with and without HIV”. 2023. Deposited V1. doi: 10.17632/26d7rwk8yx.1, https://data.mendeley.com/datasets/26d7rwk8yx/1. [DOI] [PMC free article] [PubMed]
- 52. Cirrincione LR, Senneker T, Scarsi KK, Tseng A. Drug interactions with gender-affirming hormone therapy: focus on antiretrovirals and direct acting antivirals. Expert Opin Drug Metab Toxicol. 2020;16(7):565‐581. [DOI] [PubMed] [Google Scholar]
- 53. Mandal S, Van Treuren W, White RA, Eggesbo M, Knight R, Peddada SD. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb Ecol Health Dis. 2015;26(1):27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Peters BA, Qi Q, Usyk M, et al. Association of the gut microbiome with kidney function and damage in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Gut microbes. 2023;15(1):2186685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kosuke I, Luke K, Teppei Y. Identification, inference and sensitivity analysis for causal mediation effects. Stat Sci. 2010;25(1):51‐71. [Google Scholar]
- 56. d'Afflitto M, Upadhyaya A, Green A, Peiris M. Association between sex hormone levels and gut microbiota composition and diversity-a systematic review. J Clin Gastroenterol. 2022;56(5):384‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fuhrman BJ, Feigelson HS, Flores R, et al. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J Clin Endocrinol Metab. 2014;99(12):4632‐4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Flores R, Shi J, Fuhrman B, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012;10(1):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shin J-H, Park Y-H, Sim M, Kim S-A, Joung H, Shin D-M. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res Microbiol. 2019;170(4-5):192‐201. [DOI] [PubMed] [Google Scholar]
- 60. Wu J, Zhuo Y, Liu Y, Chen Y, Ning Y, Yao J. Association between premature ovarian insufficiency and gut microbiota. BMC Pregnancy Childbirth. 2021;21(1):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mammadova G, Ozkul C, Yilmaz Isikhan S, Acikgoz A, Yildiz BO. Characterization of gut microbiota in polycystic ovary syndrome: findings from a lean population. Eur J Clin Invest. 2021;51(4):e13417. [DOI] [PubMed] [Google Scholar]
- 62. Torres PJ, Siakowska M, Banaszewska B, et al. Gut microbial diversity in women with polycystic ovary syndrome correlates with hyperandrogenism. J Clin Endocrinol Metab. 2018;103(4):1502‐1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Insenser M, Murri M, Del Campo R, Martínez-García M, Fernández-Durán E, Escobar-Morreale HF. Gut microbiota and the polycystic ovary syndrome: influence of sex, sex hormones, and obesity. J Clin Endocrinol Metab. 2018;103(7):2552‐2562. [DOI] [PubMed] [Google Scholar]
- 64. Labrie F, Bélanger A, Bélanger P, et al. Androgen glucuronides, instead of testosterone, as the new markers of androgenic activity in women. J Steroid Biochem Mol Biol. 2006;99(4-5):182‐188. [DOI] [PubMed] [Google Scholar]
- 65. Łagowska K, Malinowska AM, Kapczuk K, Mikołajczyk-Stecyna J, Chmurzyńska A, Schmidt M. β-glucuronidase activity is associated with carbohydrate metabolism but not with androgen status in overweight and obese women with polycystic ovary syndrome. Nutrition. 2022;97(2022):111606. [DOI] [PubMed] [Google Scholar]
- 66. Sui Y, Wu J, Chen J. The role of gut microbial β-glucuronidase in estrogen reactivation and breast cancer. Front Cell Dev Biol. 2021;9(2021):631552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Janda JM, Abbott Sharon L. The changing face of the family Enterobacteriaceae (order: “enterobacterales”): new members, taxonomic issues, geographic expansion, and new diseases and disease syndromes. Clin Microbiol Rev. 2021;34(2):e00174‐e00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Williams B, Landay A, Presti RM. Microbiome alterations in HIV infection a review. Cell Microbiol. 2016;18(5):645‐651. [DOI] [PubMed] [Google Scholar]
- 69. Wang H, Li Y, Wang X, Bu J, Yan G, Lou D. Endogenous sex hormone levels and coronary heart disease risk in postmenopausal women: A meta-analysis of prospective studies. Eur J Preventive Cardiol. 2017;24(6):600‐611. [DOI] [PubMed] [Google Scholar]
- 70. Zhao D, Guallar E, Ouyang P, et al. Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Cardiol. 2018;71(22):2555‐2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Scarabin PY. Endogenous sex hormones and cardiovascular disease in postmenopausal women: new but conflicting data. Ann Transl Med. 2018;6(23):448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7(10):1025‐1038. [DOI] [PubMed] [Google Scholar]
- 73. Janjua SA, Staziaki PV, Szilveszter B, et al. Presence, characteristics, and prognostic associations of carotid plaque among people living with HIV. Circ Cardiovasc Imaging. 2017;10(10):e005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Trøseid M, Andersen GØ, Broch K, Hov JR. The gut microbiome in coronary artery disease and heart failure: current knowledge and future directions. eBioMedicine. 2020;52(2020):102649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res. 2020;127(4):553‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Peters BA, Burk RD, Kaplan RC, Qi Q. The gut microbiome, microbial metabolites, and cardiovascular disease in people living with HIV. Curr HIV/AIDS Rep. 2023;20(2):86‐99. [DOI] [PubMed] [Google Scholar]
- 77. Shan Z, Clish CB, Hua S, et al. Gut microbial-related choline metabolite trimethylamine-N-oxide is associated with progression of carotid artery atherosclerosis in HIV infection. J Infect Dis. 2018;218(9):1474‐1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Luo K, Wang Z, Peters BA, et al. Tryptophan metabolism, gut microbiota, and carotid artery plaque in women with and without HIV infection. AIDS. 2023. doi: 10.1097/QAD.0000000000003596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang Z, Peters BA, Bryant M, et al. Gut microbiota, circulating inflammatory markers and metabolites, and carotid artery atherosclerosis in HIV infection. Microbiome. 2023;11(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang Y, Xu J, Wang X, Ren X, Liu Y. Changes of intestinal bacterial microbiota in coronary heart disease complicated with nonalcoholic fatty liver disease. BMC Genomics. 2019;20(1):862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Karlsson FH, Fåk F, Nookaew I, et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. 2012;3(1):1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nakajima A, Mitomo S, Yuki H, et al. Gut microbiota and coronary plaque characteristics. J Am Heart Assoc. 2022;11(17):e026036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Siegel EG, Lorenzo Bermejo J, Flade I, Hasslacher C. Cardiovascular complications and composition of the intestinal microbiome in patients with type 2 diabetes. Int J Diabetes Clin Res. 2018;5(3):1-7. [Google Scholar]
- 84. Ervin SM, Simpson JB, Gibbs ME, et al. Structural insights into endobiotic reactivation by human gut microbiome-encoded sulfatases. Biochemistry. 2020;59(40):3939‐3950. [DOI] [PubMed] [Google Scholar]
- 85. Rocafort M, Noguera-Julian M, Rivera J, et al. Evolution of the gut microbiome following acute HIV-1 infection. Microbiome. 2019;7(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ishizaka A, Koga M, Mizutani T, et al. Unique gut microbiome in HIV patients on antiretroviral therapy (ART) suggests association with chronic inflammation. Microbiol Spectrum. 2021;9(1):e00708‐e00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The gut microbiome sequence data in this study are deposited in QIITA database (https://qiita.ucsd.edu/) with accession number 13223. Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS), now the MACS/WIHS Combined Cohort Study (MWCCS). Access to individual-level data from the MWCCS may be obtained upon review and approval of a MWCCS concept sheet. Links and instructions for online concept sheet submission are on the study website (https://statepi.jhsph.edu/mwccs/).