Abstract
We report a rhodium-catalyzed asymmetric addition of aryl and alkenyl boronic acids to quinoxalinium salts that generates dihydroquinoxalines with high enantioselectivity. Functionalization of the reaction products, dihydroquinoxaline, allows the preparation of tetrahydroquinoxalines with various substitution patterns.
Introduction
Chiral tetrahydroquinoxalines (THQs) are common structural motifs in bioactive compounds (Figure 1).1 Despite their representation in important bioactive molecules, such as potent CETP and BET inhibitors, methods for their asymmetric synthesis are limited. Traditionally, synthetic access to chiral tetrahydroquinoxalines has been achieved through asymmetric hydrogenation of quinoxalines and various cyclization reactions (Scheme 1A).2 While these methods can produce THQs, the scope of asymmetric reductions of quinoxalines is limited, and when there are two substituents present, often syn-disubstituted THQs are obtained.3 Cyclization approaches to THQs require multistep syntheses to access starting materials, and often the desired stereogenic center is already present in one of the starting materials.
Figure 1.
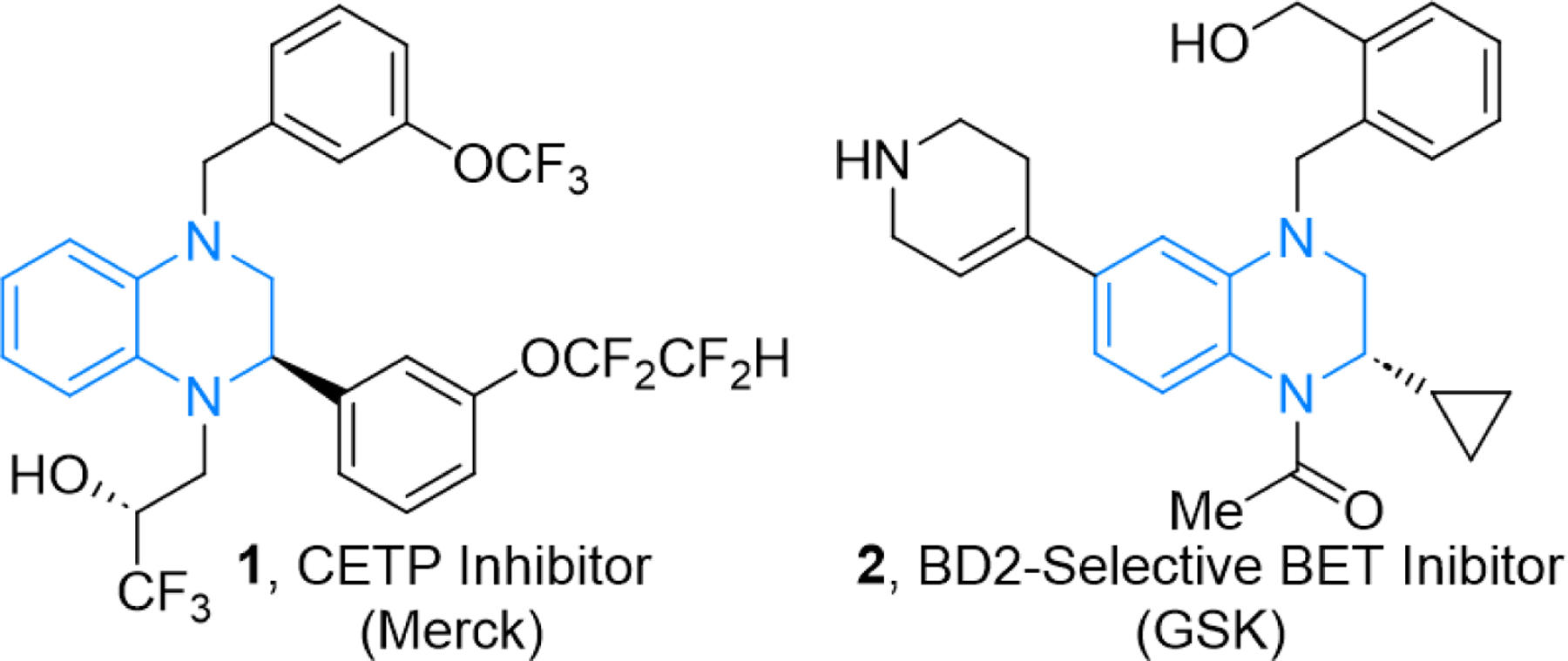
Examples of bioactive compounds with tetrahydroquinoxaline cores containing stereogenic centers.
Scheme 1.
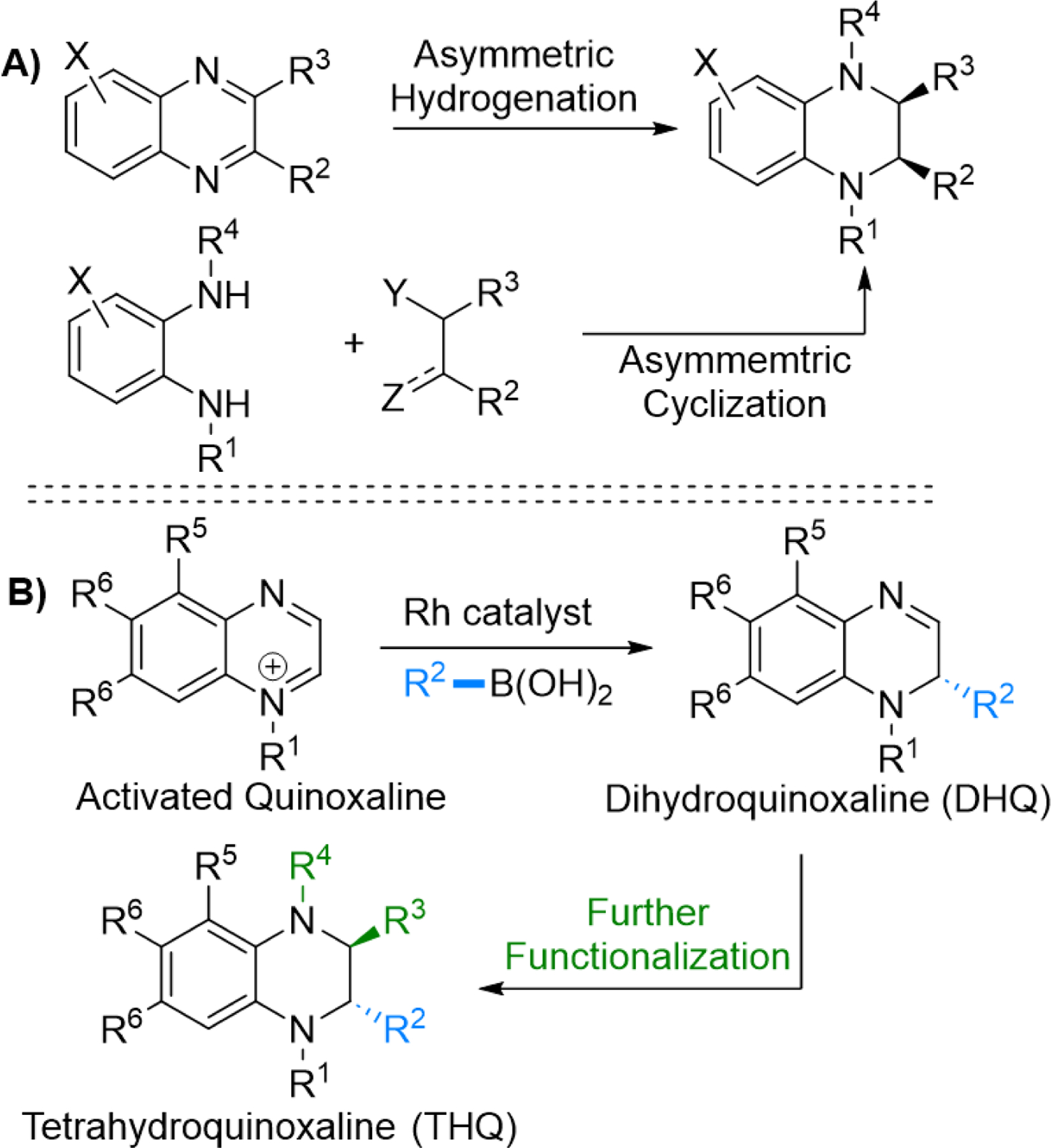
(A) Common Approaches to the Asymmetric Synthesis of Tetrahydroquinoxalines and (B) Our Approach to the Synthesis of Dihydro- and Tetrahydroquinoxalines
Inspired by asymmetric nucleophilic dearomatization of pyridines for the synthesis of piperidines, we envisioned an alternative approach to the asymmetric synthesis of substituted tetrahydroquinoxalines involving an enantioselective dearomatization of quinoxalinium salts to obtain dihydroquinoxalines (DHQs), which could then be converted into THQs (Scheme 1B).4 This is an attractive approach, as it utilizes readily available starting materials and allows for the stereoselective and lates-stage introduction of substituents on THQs. Specifically, herein, we report a rhodium-catalyzed dearomatization of quinoxalinium salts for the synthesis of chiral dihydroquinoxalines. In addition, we demonstrate that the C3=N4 imine bond of the DHQ reaction products can be functionalized through both reduction and addition reactions to produce substituted THQs with one and two stereocenters, respectively.
Results and Discussion
Our studies began using the reaction conditions we have developed for the dearomatization of pyridinium salts (Table 1).4g,i,x Thus, quinoxalinium salt 3 was reacted with PhB(OH)2 in the presence of the Rh(COD)2BF4/(S)-Binap combination as the catalyst, Na2CO3 as the base, and a 10:1 dioxane/H2O mixture as the solvent system. Under these conditions, dihydroquinoxaline 4a was obtained in 47% yield and 62% ee (Table 1, entry 1). Subsequent evaluation of Et2O, PhMe, and DME as solvents resulted in significantly lower yields of 4a (Table 1, entries 2−4, respectively). Diene ligands such as COD and L2−L4 instead of Binap gave DHQ 4a in low to moderate yield and in the case of L2−L4 moderate ee (Table 1, entries 5− 8, respectively). Commercial bis-phosphine ligands (R)-Segphos (L5) and (R)-(S)-Josiphos (L10) were evaluated, and while both ligands gave low yields, Josiphos (L10) gave 4a in 93% ee (Table 1, entries 9 and 10, respectively). (R,R)- QuinoxP* was identified as the best ligand for this transformation, affording 4a in 73% yield and 93% ee (Table 1, entry 11). Finally, increasing the catalyst loading to 6 mol % and increasing the reaction temperature to 80 °C afforded DHQ 4a in 80% yield and 94% ee (Table 1, entry 12). An increase or decrease in the dioxane:H2O ratio diminished the yields of 4a (entries 13−15). Without any catalyst, the desired dihydroquinoxaline 4a was obtained in only 8% yield (Table 1, entry 16).
Table 1.
Optimization of the Reaction Conditionsa
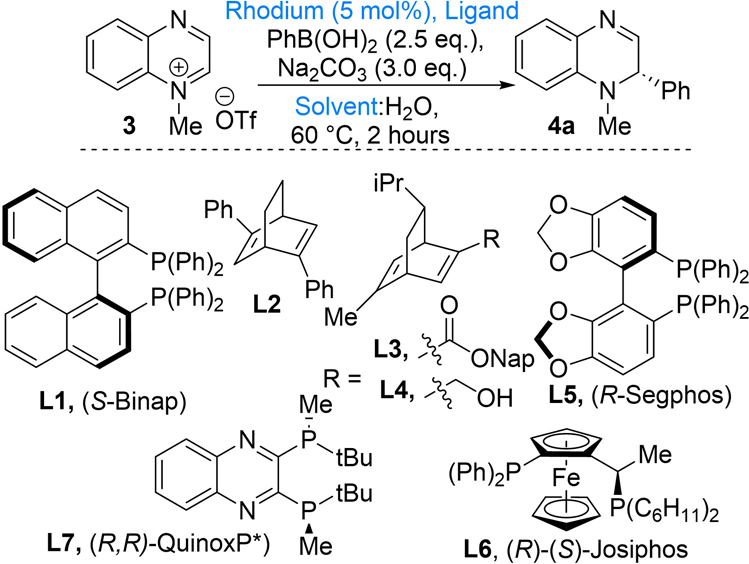
| |||
|---|---|---|---|
| entry | solvent/water | ligand (mol %) | yield (%)b (% eec) |
| 1 | dioxane (10:1) | L1 (5) | 47 (62) |
| 2 | Et2O (10:1) | L1 (5) | 12 (50) |
| 3 | PhMe (10:1) | L1 (5) | 9 (4) |
| 4 | DME (10:1) | L1 (5) | 18 (62) |
| 5 | dioxane (10:1) | none | 5 (ND) |
| 6d | dioxane (10:1) | L2 (10) | 17 (70) |
| 7d | dioxane (10:1) | L3 (20) | 39 (64) |
| 8d | dioxane (10:1) | L4 (20) | 57 (82) |
| 9 | dioxane (10:1) | L5 (5) | 27 (76) |
| 10 | dioxane (10:1) | L6 (5) | 20 (93) |
| 11 | dioxane (10:1) | L7 (5) | 73 (93) |
| 12e | dioxane (10:1) | L7 (7) | 80 (94) |
| 13 | dioxane (5:1) | L7 (5) | 68 (ND) |
| 14 | dioxane (20:1) | L7 (5) | 51 (ND) |
| 15 | dioxanef | L7 (5) | 53 (74) |
| 16g | dioxane (10:1 | none | 8 (ND) |
With the optimized reaction conditions in hand, we explored the scope of boronic acids that can be used in this reaction (Scheme 2). Electron-neutral and electron-rich aryl boronic acids afforded the corresponding dihydroquinoxalines in good to moderate yields and excellent %ee (Scheme 2, 4a−4e, 4m, and 4n, respectively). Electron-deficient boronic acid gave the corresponding DHQ 4k in diminished yields compared to those of electron-neutral and electron-rich aryl boronic acids, albeit with excellent %ee. When halogen-functionalized aryl boronic acids were used, DHQs were obtained in moderate to good yields depending on the nature and ring position of the halogens (Scheme 2, DHQs 4h−4j, 4t, and 4u). Thus, boronic acids with m-chlorophenyl, p-fluorophenyl, and p-chlorophenyl substituents gave DHQs 4h−4j, respectively, in good yields and excellent ee values, while o-fluorophenyl- and m-bromophenyl substituted DHQs (4t and 4u, respectively) were obtained in modest yields.
Scheme 2.
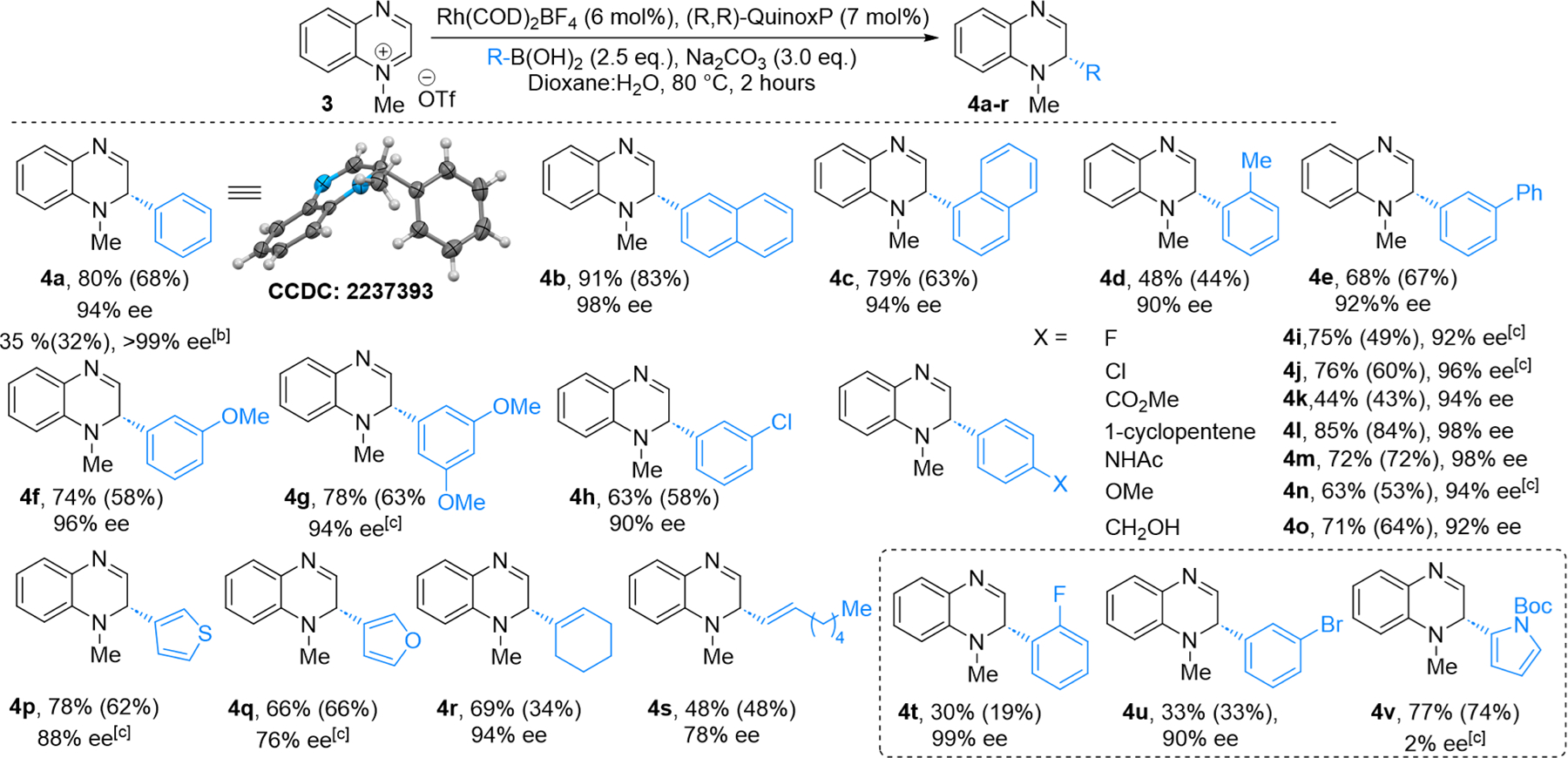
Scope of the Boronic Acids for Dearomatization of Quinoxalinium Salt 3a.
In general, meta and para substituents were better tolerated than ortho substituents (e.g., 4e−4h vs 4d and 4t). It is worth noting that sterically hindered boronic acids with ortho substituents still gave moderate yields of the corresponding DHQs with excellent %ee (Scheme 2, 4d and 4t). N-Boc-pyrrole-2-boronic acid gave DHQ 4v in 74% yield but with only 2% ee.
The reaction tolerates a wide range of functional groups, including ether, ester, alkene, amide, primary alcohol, and heterocycle functionalities (Scheme 2, 4f, 4k−4m, and 4o−4q). We found that in addition to aryl boronic acids, alkenyl boronic acids could also be employed in this methodology. Cyclic alkene boronic acid gave the corresponding DHQ 4r in 69% yield and 94% ee. A linear alkene containing DHQ, 4s, was obtained in 48% yield and 78% ee. The latter result is particularly exciting as it opens the door for the synthesis of enantioenriched alkyl-functionalized tetrahydroquinoxalines after reduction of the C=C and C=N bonds (see Scheme 4, compound 7c). A preparative scale reaction (1 mmol) gave 4a in 76% isolated yield and 98% ee. Finally, under the standard reaction conditions, PhBpin as the nucleophile instead of PhB(OH)2 gave 4a in a lower yield but higher ee (for details, see the Supporting Information).
Scheme 4.
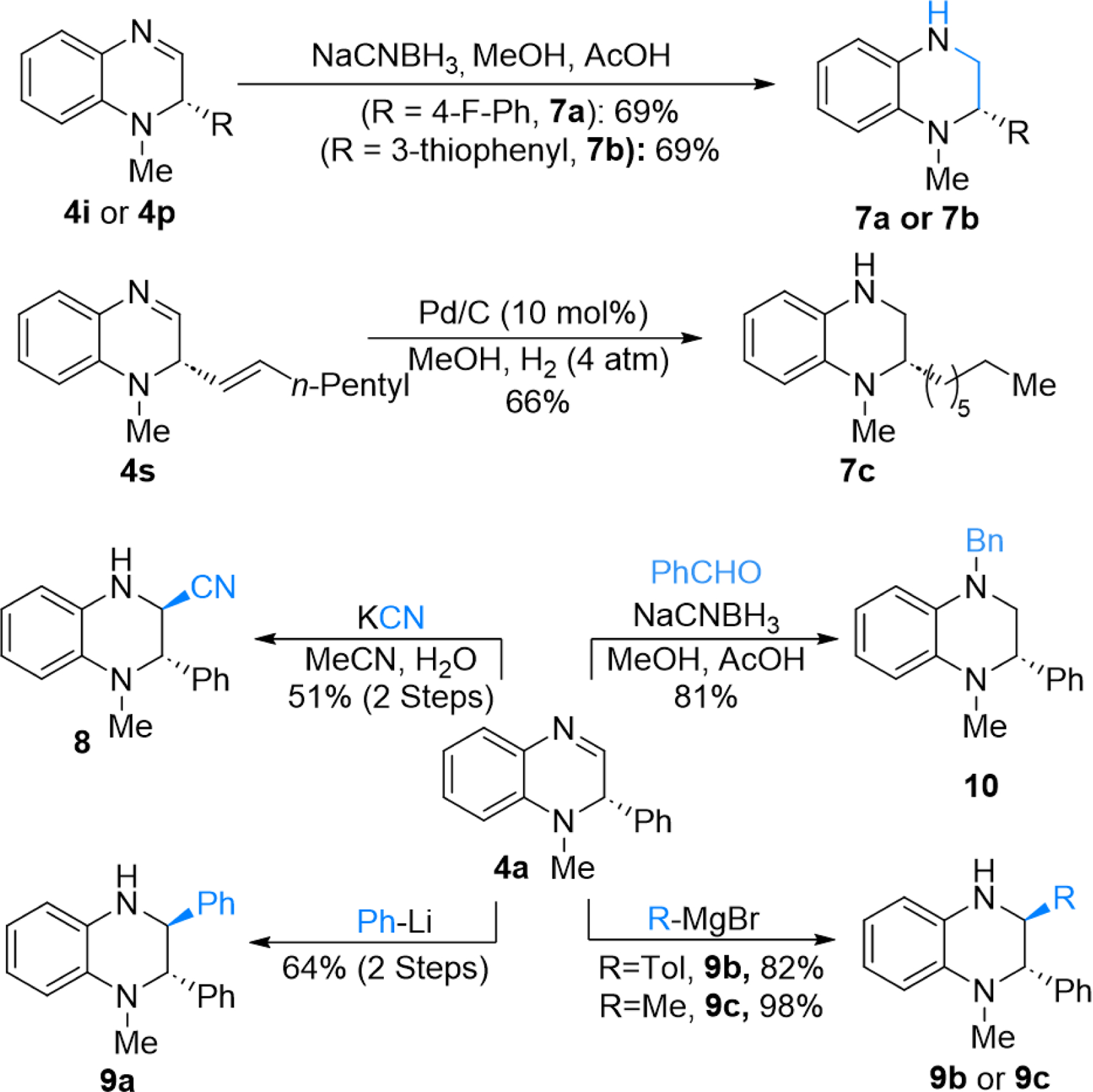
Synthesis of Tetrahydroquinoxaline Derivatives from Dihyroquinoxalines.
Next, we investigated the dearomatization of quinoxaline derivatives to produce chiral DHQs (Scheme 3). 6,7- Disubstituted quinoxalinium salts gave the corresponding DHQs in moderate yield and excellent ee (Scheme 3, 6a−6c). DHQs substituted at position C5 also underwent dearomatization to give the corresponding dearomatization products in moderate yields and 96% ee (Scheme 3, 6d and 6e). It is worth noting that for the synthesis of 6e we used N-benzyl quinoxalinium salt as attempts to methylate the corresponding quinoxaline resulted in a mixture of quinoxalinium salts arising from methylation of N1 or N4. Next, the effect of quinoxalinium counterions was explored. Triflate and phosphorus hexafluoride counterions (Scheme 3, 6f) were well tolerated under our reaction conditions and gave DHQ 6f in moderate yield and 92% ee. A bromide counteranion gave DHQ 6f in only a trace amount. An attempt to form dihydroquinoxaline 6g containing a fully substituted stereocenter by dearomatization of the corresponding quinoxalinium salt resulted in the elimination product of the starting quinoxalinium salt (for details, see the Supporting Information).
Scheme 3.
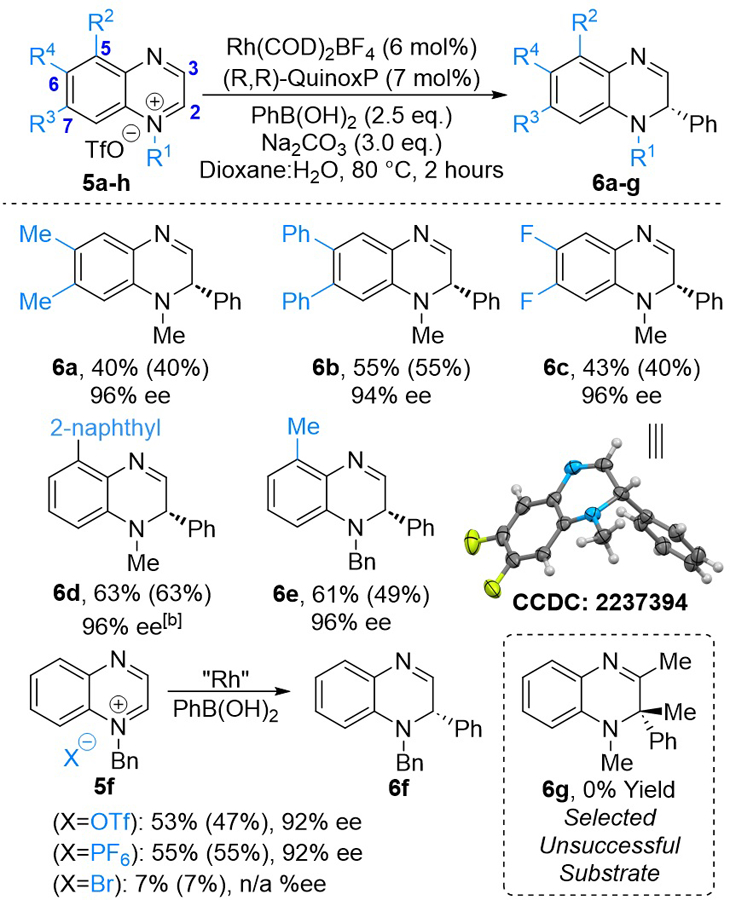
Scope of Substituted Quinoxalinium Saltsa
In addition, under the optimized reaction conditions, pyrazinium salts did not produce any of the corresponding functionalized dihydropyrazine product. Finally, we found that treatment of 3 with an additional portion of MeOTf did not provide the desired second alkylation at N4 for a potential double dearomatization.
To demonstrate the utility of DHQs, we explored their further functionalization to prepare tetrahydroquinoxalines (Scheme 4). Reduction of the C=N bond using NaCNBH3 provided the corresponding tetrahydroquinoxaline compounds 7a and 7b in good yields without significant erosion of the ee. Catalytic hydrogenation of 4s afforded enantioenriched tetrahydroquinoline 7c that contains an alkyl substituent at the stereogenic center. Reduction of 4a under the same catalytic hydrogenation conditions gave the reduced tetrahydroquinoxaline compound in yields lower than that of NaCNBH3 with erosion of the ee (for details, see page S43 of the Supporting Information). Catalytic hydrogenation of 4s afforded tetrahydroquinoxaline 7c that contains an alkyl substituent at the stereogenic center. Reaction of 4a under Streker-type reaction conditions afforded 8 in 51% yield. Aryl lithium, alkyl Grignard, and aryl Grignard reagents reacted with 4a and gave tetrahydroquinoxalines 9a−9c, respectively, with >20:1 dr and good yields. Furthermore, 4a was amenable to reductive amination conditions to give N,N-dialkylated THQ 10.
Conclusions
In summary, we have developed the first asymmetric Rh-catalyzed dearomatization of quinoxalinium salts for the synthesis of chiral dihydroquinoxalines. The reaction tolerates the addition of electron-rich, electron-neutral, and electron-poor aryl and alkenyl boronic acids with varying functional groups, including amides, free alcohols, and heterocycles. Subsequent functionalization of DHQ derivatives enables the enantioselective synthesis of tetrahydroquinolines containing one or two stereogenic centers.
Supplementary Material
References
- 1.(a) Anand R; Colandrea VJ; Reiter M; Vachal P; Zwicker A; Wilson JE; Zhang F; Zhao K Benzopiperazine Derivatives as Cetp Inhibitors. WO 2013/028382 A1, 2013. [Google Scholar]; (b) Wilson JE; Kurukulasuriya R; Reibarkh M; Reiter M; Zwicker A; Zhao K; Zhang F; Anand R; Colandrea VJ; Cumiskey A-M; Crespo A; Duffy RA; Murphy BA; Mitra K; Johns DG; Duffy JL; Vachal P Discovery of Novel Indoline Cholesterol Ester Transfer Protein Inhibitors (Cetp) through a Structure-Guided Approach. ACS Med. Chem. Lett. 2016, 7, 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Law RP; Atkinson SJ; Bamborough P; Chung C.-w.; Demont EH; Gordon LJ; Lindon M; Prinjha RK; Watson AJB; Hirst DJ. Discovery of Tetrahydroquinoxalines as Bromodomain and Extra-Terminal Domain (Bet) Inhibitors with Selectivity for the Second Bromodomain. J. Med. Chem. 2018, 61, 4317–4334. [DOI] [PubMed] [Google Scholar]; (d) Kuhl A; Kolkhof P; Telan L; Peters J-G; Lustig K; Kast R; Münter K; Stasch J-P; Tinel H. Tetrahydroquinoxalines and Their Use as M2 Acetylchloine Receptor Agonists. WO 2005/028451 A1, 2005. [Google Scholar]; (e) Bissantz C; Dehmlow H; Erickson S; Kim K; Martin R; Sander U; Pietrancio-Cole S; Richter H; Ulmer C 4-Phenoxy-Nicotinamide or 4-PhenoxyPyridmidine-5-Carboxamide Compounds. US 2011/0178089, 2011. [Google Scholar]; (f) Torisu K; Kobayashi K; Iwahashi M; Nakai Y; Onoda T; Nagase T; Sugimoto I; Okada Y; Matsumoto R; Nanbu F; Ohuchida S; Nakai H; Toda M Discovery of a New Class of Potent, Selective, and Orally Active Prostaglandin D2 Receptor Antagonists. Bioorgan. Med. Chem. 2004, 12, 5361–5378. [DOI] [PubMed] [Google Scholar]; (g) Eary CT; Jones ZS; Groneberg RD; Burgess LE; Mareska DA; Drew MD; Blake JF; Laird ER; Balachari D; O’Sullivan M; Allen A; Marsh V Tetrazole and Ester Substituted Tetrahydoquinoxalines as Potent Cholesteryl Ester Transfer Protein Inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 2608–2613. [DOI] [PubMed] [Google Scholar]; (h) Duan H; Ning M; Chen X; Zou Q; Zhang L; Feng Y; Zhang L; Leng Y; Shen J Design, Synthesis, and Antidiabetic Activity of 4-Phenoxynicotinamide and 4-Phenoxypyrimidine-5-Carboxamide Derivatives as Potent and Orally Efficacious Tgr5 Agonists. J. Med. Chem. 2012, 55, 10475–10489. [DOI] [PubMed] [Google Scholar]; (i) Millan DS; Kayser-Bricker KJ; Martin MW; Talbot AC; Schiller SER; Herbertz T; Williams GL; Luke GP; Hubbs S; Alvarez Morales MA; Cardillo D; Troccolo P; Mendes RL; McKinnon C Design and Optimization of Benzopiperazines as Potent Inhibitors of Bet Bromodomains. ACS Med. Chem. Lett. 2017, 8, 847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Hu J; Tian C-Q; Damaneh MS; Li Y; Cao D; Lv K; Yu T; Meng T; Chen D; Wang X; Chen L; Li J; Song S-S; Huan X-J; Qin L; Shen J; Wang Y-Q; Miao Z-H; Xiong B Structure-Based Discovery and Development of a Series of Potent and Selective Bromodomain and Extra-Terminal Protein Inhibitors. J. Med. Chem. 2019, 62, 8642–8663. [DOI] [PubMed] [Google Scholar]
- 2.(a) Zhou YG Asymmetric Hydrogenation of Heteroaromatic Compounds. Acc. Chem. Res. 2007, 40, 1357–1366. [DOI] [PubMed] [Google Scholar]; (b) Wang DS; Chen QA; Lu SM; Zhou YG Asymmetric Hydrogenation of Heteroarenes and Arenes. Chem. Rev. 2012, 112, 2557–2590. [DOI] [PubMed] [Google Scholar]; (c) Cartigny D; Berhal F; Nagano T; Phansavath P; Ayad T; Genêt, J-P; Ohshima T; Mashima K; Ratovelomanana-Vidal V. General Asymmetric Hydrogenation of 2-Alkyl- and 2-Aryl-Substituted Quinoxaline Derivatives Catalyzed by Iridium-Difluorphos: Unusual Halide Effect and Synthetic Application. J. Org. Chem. 2012, 77, 4544–4556. [DOI] [PubMed] [Google Scholar]; (d) Fleischer S; Zhou S; Werkmeister S; Junge K; Beller M Cooperative Iron−Brønsted Acid Catalysis: Enantioselective Hydrogenation of Quinoxalines and 2 H-1,4-Benzoxazines. Chem. - Eur. J. 2013, 19, 4997–5003. [DOI] [PubMed] [Google Scholar]; (e) He YM; Song FT; Fan QH Advances in Transition Metal-Catalyzed Asymmetric Hydrogenation of Heteroaromatic Compounds. Top. Curr. Chem. 2013, 343, 145–190. [DOI] [PubMed] [Google Scholar]; (f) Zhang Z; Du H A Highly Cis-Selective and Enantioselective Metal-Free Hydrogenation of 2,3-Disubstituted Quinoxalines. Angew. Chem., Int. Ed. Engl. 2015, 54, 623–626. [DOI] [PubMed] [Google Scholar]; (g) Chen ZP; Zhou YG Asymmetric Hydrogenation of Heteroarenes with Multiple Heteroatoms. Synthesis 2016, 48, 1769–1781. [Google Scholar]; (h) Kim AN; Stoltz BM Recent Advances in Homogeneous Catalysts for the Asymmetric Hydrogenation of Heteroarenes. ACS Catal. 2020, 10, 13834–13851. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Das TK; Ghosh A; Balanna K; Behera P; Gonnade RG; Marelli UK; Das AK; Biju AT N-Heterocyclic CarbeneCatalyzed Umpolung of Imines for the Enantioselective Synthesis of Dihydroquinoxalines. ACS Catal. 2019, 9, 4065–4071. [Google Scholar]; (j) Huang J; Li G.-x.; Yang G.-f.; Fu D.-q.; Nie X.-k.; Cui X; Zhao J.-z.; Tang Z. Catalytic Asymmetric Synthesis of N-Substituted Tetrahydroquinoxalines Via Regioselective Heyns Rearrangement and Stereoselective Transfer Hydrogenation in One Pot. Chem. Sci. 2021, 12, 4789–4793. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Qian C; Tang WJ A Versatile Synthesis of Vinyl-Substituted Heterocycles Via Regio-and Enantioselective Pd-Catalyzed Tandem Allylic Substitution. Org. Lett. 2020, 22, 4483–4488. [DOI] [PubMed] [Google Scholar]; (l) Ghorai MK; Sahoo AK; Kumar S Synthetic Route to Chiral Tetrahydroquinoxalines Via Ring-Opening of Activated Aziridines. Org. Lett. 2011, 13, 5972–5975. [DOI] [PubMed] [Google Scholar]
- 3.Luo Z; Li Z; Zhao H; Yang J; Xu L; Lei M; Fan Q; Walsh PJ Borane-Catalyzed Tandem Cyclization/Hydrosilylation Towards Enantio- and Diastereoselective Construction of Trans-2,3-Disubstituted-1,2,3,4-Tetrahydroquinoxalines. Angew. Chem., Int. Ed. 2023, 62, No. e202305449. [DOI] [PubMed] [Google Scholar]
- 4.(a) Jia J; Hu FD; Xia Y Transition-Metal-Catalyzed Nucleophilic Dearomatization of Electron-Deficient Heteroarenes. Synthesis 2022, 54, 92–110. [Google Scholar]; (b) Kratena N; Marinic B; Donohoe TJ Recent Advances in the Dearomative Functionalisation of Heteroarenes. Chem. Sci. 2022, 13, 14213–14225. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ding QP; Zhou XL; Fan RH Recent Advances in Dearomatization of Heteroaromatic Compounds. Org. Biomol. Chem. 2014, 12, 4807− 4815. [DOI] [PubMed] [Google Scholar]; (d) Comins DL; Higuchi K; Young DW Dihydropyridine Preparation and Application in the Synthesis of Pyridine Derivatives. Adv. Heterocycl. Chem. 2013, 110, 175–235. [Google Scholar]; (e) Bull JA; Mousseau JJ; Pelletier G; Charette AB Synthesis of Pyridine and Dihydropyridine Derivatives by Regio- and Stereoselective Addition to N-Activated Pyridines. Chem. Rev. 2012, 112, 2642–2713. [DOI] [PubMed] [Google Scholar]; (f) Ahamed M; Todd MH Catalytic Asymmetric Additions of Carbon-Centered Nucleophiles to Nitrogen-Containing Aromatic Heterocycles. Eur. J.Org. Chem. 2010, 5935–5942. [Google Scholar]; (g) Robinson DJ; Ortiz KG; O’Hare NP; Karimov RR Dearomatization of Heteroarenium Salts with Arbpin Reagents. Application to the Total Synthesis of a Nuphar Alkaloid. Org. Lett. 2022, 24, 3445–3449. [DOI] [PubMed] [Google Scholar]; (h) Nallagonda R; Karimov RR Copper-Catalyzed Regio- and Diastereoselective Additions of Boron-Stabilized Carbanions to Heteroarenium Salts: Synthesis of Azaheterocycles Containing Contiguous Stereocenters. ACS Catal. 2021, 11, 248–254. [Google Scholar]; (i) Robinson DJ; Spurlin SP; Gorden JD; Karimov RR Enantioselective Synthesis of Dihydropyridines Containing Quaternary Stereocenters through Dearomatization of Pyridinium Salts. ACS Catal. 2020, 10, 51–55. [Google Scholar]; (j) Yedoyan J; Wurzer N; Klimczak U; Ertl T; Reiser O Regio- and Stereoselective Synthesis of Functionalized Dihydropyridines, Pyridines, and 2h-Pyrans: Heck Coupling of Monocyclopropanated Heterocycles. Angew. Chem., Int. Ed. Engl. 2019, 58, 3594–3598. [DOI] [PubMed] [Google Scholar]; (k) Gribble MW; Guo S; Buchwald SL Asymmetric Cu-Catalyzed 1,4-Dearomatization of Pyridines and Pyridazines without Preactivation of the Heterocycle or Nucleophile. J. Am. Chem. Soc. 2018, 140, 5057–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Bertuzzi G; Sinisi A; Pecorari D; Caruana L; Mazzanti A; Bernardi L; Fochi M Nucleophilic Dearomatization of Pyridines under Enamine Catalysis: Regio-, Diastereo-, and Enantioselective Addition of Aldehydes to Activated N-Alkylpyridinium Salts. Org. Lett. 2017, 19, 834–837. [DOI] [PubMed] [Google Scholar]; (m) Bertuzzi G; Sinisi A; Caruana L; Mazzanti A; Fochi M; Bernardi L Catalytic Enantioselective Addition of Indoles to Activated N-Benzylpyridinium Salts: Nucleophilic Dearomatization of Pyridines with Unusual C-4 Regioselectivity. ACS Catal. 2016, 6, 6473–6477. [Google Scholar]; (n) Lutz JP; Chau ST; Doyle AG Nickel-Catalyzed Enantioselective Arylation of Pyridine. Chem. Sci. 2016, 7, 4105–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Wang Y; Liu YL; Zhang DD; Wei H; Shi M; Wang FJ Enantioselective Rhodium-Catalyzed Dearomative Arylation or Alkenylation of Quinolinium Salts. Angew. Chem., Int. Ed. Engl. 2016, 55, 3776–3780. [DOI] [PubMed] [Google Scholar]; (p) Zhang M; Sun WS; Zhu GM; Bao GJ; Zhang BZ; Hong L; Li M; Wang R Enantioselective Dearomative Arylation of Isoquinolines. ACS Catal. 2016, 6, 5290–5294. [Google Scholar]; (q) Fischer T; Bamberger J; Mancheno OG Asymmetric Nucleophilic Dearomatization of Diazarenes by Anion-Binding Catalysis. Org. Biomol. Chem. 2016, 14, 5794–5802. [DOI] [PubMed] [Google Scholar]; (r) García Mancheno O; Asmus S; Zurro M; Fischer T Highly Enantioselective Nucleophilic Dearomatization of Pyridines by Anion-Binding Catalysis. Angew. Chem., Int. Ed. 2015, 54, 8823–8827. [DOI] [PubMed] [Google Scholar]; (s) Chau ST; Lutz JP; Wu K; Doyle AG Nickel-Catalyzed Enantioselective Arylation of Pyridinium Ions: Harnessing an Iminium Ion Activation Mode. Angew. Chem., Int. Ed. Engl. 2013, 52, 9153–9156. [DOI] [PMC free article] [PubMed] [Google Scholar]; (t) Nadeau C; Aly S; Belyk K Rhodium-Catalyzed Enantioselective Addition of Boronic Acids to N-Benzylnicotinate Salts. J. Am. Chem. Soc. 2011, 133, 2878–2880. [DOI] [PubMed] [Google Scholar]; (u) Fernandez-Ibanez MA; Macia B; Pizzuti MG; Minnaard AJ; Feringa BL Catalytic Enantioselective Addition of Dialkylzinc Reagents to N-Acylpyridinium Salts. Angew. Chem., Int. Ed. Engl. 2009, 48, 9339–9341. [DOI] [PubMed] [Google Scholar]; (v) Black DA; Beveridge RE; Arndtsen BA Copper-Catalyzed Coupling of Pyridines and Quinolines with Alkynes: A One-Step, Asymmetric Route to Functionalized Heterocycles. J. Org. Chem. 2008, 73, 1906–1910. [DOI] [PubMed] [Google Scholar]; (w) Sun ZK; Yu SY; Ding ZD; Ma DW Enantioselective Addition of Activated Terminal Alkynes to 1-Acylpyridinium Salts Catalyzed by CuBis(Oxazoline) Complexes. J. Am. Chem. Soc. 2007, 129, 9300–9301. [DOI] [PubMed] [Google Scholar]; (x) Ortiz KG; Dotson JJ; Robinson DJ; Sigman MS; Karimov RR Catalyst-Controlled Enantioselective and Regiodivergent Addition of Aryl Boron Nucleophiles to N-Alkyl Nicotinate Salts. J. Am. Chem. Soc. 2023, 145, 11781–11788. [DOI] [PMC free article] [PubMed] [Google Scholar]; (y) Mishra S; Karabiyikoglu S; Fletcher SP Catalytic Enantioselective Synthesis of 3-Piperidines from Arylboronic Acids and Pyridine. J. Am. Chem. Soc. 2023, 145, 14221–14226. [DOI] [PMC free article] [PubMed] [Google Scholar]; (z) Gressies S; Susse L; Casselman T; Stoltz BM Tandem Dearomatization/Enantioselective Allylic Alkylation of Pyridines. J. Am. Chem. Soc. 2023, 145, 11907–11913. [DOI] [PMC free article] [PubMed] [Google Scholar]; (aa) Liu Z; Shi ZJ; Liu L; Zhang M; Zhang MC; Guo HY; Wang XC Asymmetric C3-Allylation of Pyridines. J. Am. Chem. Soc. 2023, 145, 11789–11797. [DOI] [PubMed] [Google Scholar]; (ab) Harawa V; Thorpe TW; Marshall JR; Sangster JJ; Gilio AK; Pirvu L; Heath RS; Angelastro A; Finnigan JD; Charnock SJ; Nafie JW; Grogan G; Whitehead RC; Turner NJ Synthesis of Stereoenriched Piperidines Via ChemoEnzymatic Dearomatization of Activated Pyridines. J. Am. Chem. Soc. 2022, 144, 21088–21095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


