Abstract
The chimeric simian-human immunodeficiency virus SHIVKU-1, bearing the envelope of human immunodeficiency virus type 1 (HIV-1), causes fulminant infection with subtotal loss of CD4+ T cells followed by development of AIDS in intravaginally inoculated macaques and thus provides a highly relevant model of sexually transmitted disease caused by HIV-1 in human beings. Previous studies using this SHIV model had shown that the vpu and nef genes were important in pathogenesis of the infection, and so we deleted portions of these genes to create two vaccines, ΔvpuΔnefSHIV-4 (vaccine 1) and ΔvpuSHIVPPc (vaccine 2). Six adult macaques were immunized subcutaneously with vaccine 1, and six were immunized orally with vaccine 2. Both viruses caused infection in all inoculated animals, but whereas vaccine 1 virus caused only a nonproductive type of infection, vaccine 2 virus replicated productively but transiently for a 6- to 10-week period. Both groups were challenged 6 to 7 months later with pathogenic SHIVKU-1 by the intravaginal route. All four unvaccinated controls developed low CD4+ T-cell counts (<200/μl) and AIDS. The 12 vaccinated animals all became infected with SHIVKU-1, and two in group 1 developed a persistent productive infection followed by development of AIDS in one. The other 10 have maintained almost complete control over virus replication even though spliced viral RNA was detected in lymph nodes. This suppression of virus replication correlated with robust antiviral cell-mediated immune responses. This is the first demonstration of protection against virulent SHIV administered by the intravaginal route. This study supports the concept that sexually transmitted HIV disease can be prevented by parenteral or oral immunization.
Human immunodeficiency virus type 1 (HIV-1) is primarily a sexually transmitted virus which causes persistent systemic infection and loss of CD4+ T cells which culminates in loss of immunocompetence and development of AIDS. Although several effective anti-HIV drugs, notably viral protease inhibitors, are now available, their widespread use is hampered by their high cost and the need for multiple treatments daily for an indefinite period. Their use is thus not feasible in less developed countries, where the bulk of HIV infections occur. Under these conditions, development of a safe and effective vaccine is a priority. Moreover, such a vaccine should protect against sexual transmission of the virus. The inability of HIV-1 to infect animals other than chimpanzees has meant that macaque models using nonhuman primate lentiviruses have become the only practical alternative for testing proof-of-concept approaches to vaccines against HIV-1. The simian immunodeficiency virus SIVmac, which is closely related to HIV-2 and SIVsm, causes AIDS in macaques and has been extensively used in vaccine studies. Macaques vaccinated with attenuated strains of SIVmac239, produced by deleting auxiliary genes, including nef, resisted infection after challenge with virulent strains of SIVmac (1, 5, 21, 23, 24, 30). A major limitation of the SIVmac239 model of AIDS is that this virus is only distantly related to HIV-1 genetically, and although SIVmac causes AIDS in macaques, the biological properties of SIVmac vary greatly from those of HIV-1, especially with reference to neutralization and replication in macrophages (31). Since some of these differences are attributable to the envelopes of the viruses, chimeric simian-human immunodeficiency virus (SHIV), which contains the core of SIVmac and the envelope of HIV-1, was created in hopes of simulating the effects of HIV-1 in macaques more accurately than does SIVmac. Newly constructed SHIVs were infectious and induced neutralizing antibodies to HIV-1 but were not pathogenic, and thus vaccines could not be evaluated for efficacy in preventing disease. Our recent derivation of virulent SHIV which causes almost total loss of CD4+ T cells and AIDS in macaques 6 months after inoculation established a new and reproducible model which accurately reflected HIV-1 disease, although compressed into a short time frame (9, 11, 12). Moreover, the new virus is virulent after intravenous, oral, or intravaginal infusion (7, 9, 13). This model thus proved ideal for evaluating efficacy of vaccines against sexually transmitted HIV-1.
Our choice of live-virus vaccines was based on observations of genetic changes occurring in SHIV during the animal passages that yielded virulent SHIVKU-1. The original molecularly cloned SHIV-4 is avirulent and has a stop codon in vpu (16). SHIVKU-1 is extremely virulent and has an open vpu in addition to numerous mutations in the env and nef genes (25, 27). However, most of the changes in the latter two genes developed after vpu became functional (17). The genetic changes correlated with a newly acquired ability of the virus to replicate efficiently in macrophage cultures and at extremely high titers in the animals, with resultant subtotal loss of CD4+ T cells and development of AIDS. Assuming that the changes in the auxiliary genes vpu and nef were essential for development for virulence of the virus, we deleted portions of both genes from original SHIV-4 and used this virus as a vaccine (group 1). Oral inoculation of this virus into two newborn pig-tailed macaques failed to result in infection in the animals (19a). Therefore, in the vaccine trial described in this report, the animals were inoculated subcutaneously with this virus. Chimeric virus SHIVPPc was created by replacing the env and nef genes of SHIV-4 with the corresponding regions of virus isolated from macaque PPc, which died with AIDS (26). SHIVPPc is macrophagetropic (27). To test the attenuating potential of a vpu deletion alone, we created virus ΔvpuSHIVPPc and used this virus as a vaccine. This virus caused a transiently productive infection in two newborn macaques after oral inoculation. Therefore, in the vaccine experiment (group 2), this virus was inoculated orally. Four unvaccinated controls and the 12 vaccinated animals were challenged 6 to 7 months later by intravaginal infusion of 1 ml of undiluted SHIVKU-1 stock.
MATERIALS AND METHODS
Viruses.
We obtained a SHIV-4 DNA encoding the env, tat, rev, and vpu genes of HIV-1 HXB2c on a background of SIVmac239 (16) from Joseph Sodroski, Harvard University. Viral DNA was transfected into CEMx174 cells to produce a virus that was used to initiate passage in macaques. Virus from the fourth passage of SHIV-4, which was associated with AIDS and death of macaque PNb at 6 months, was amplified in culture of peripheral blood mononuclear cells (PBMC) from a healthy macaque (12). Supernatant fluid from this culture (SHIVKU-1) had a titer of 104 50% tissue culture infectious doses (TCID50)/ml in macaque PBMC and in C8166 cells. Aliquots of this SHIVKU-1 stock were stored in liquid nitrogen.
Construction of ΔvpuΔnef SHIV-4.
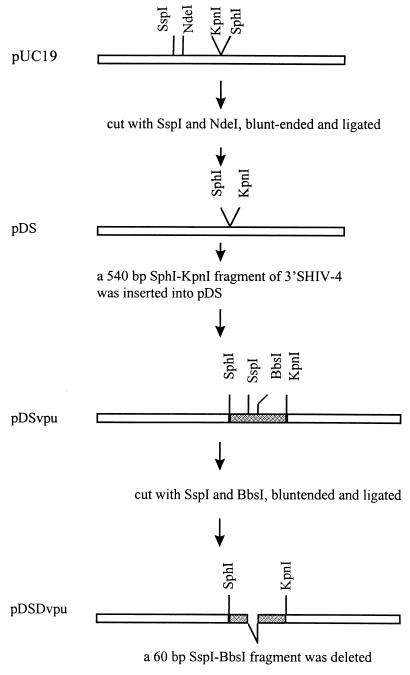
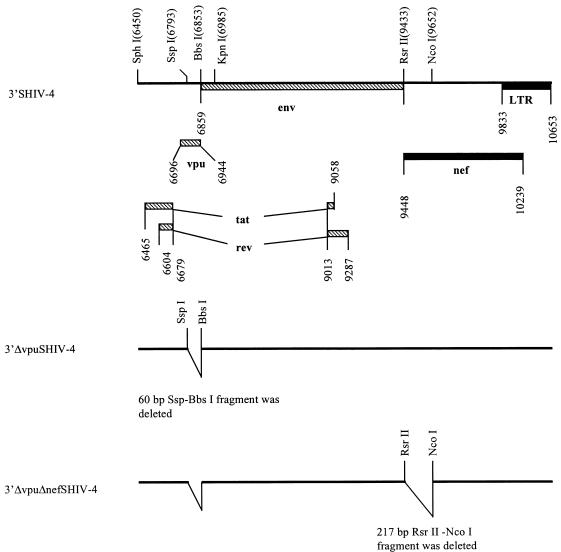
The original SHIV-4 DNA consisted of two plasmids with the 5′ and 3′ regions, respectively. All manipulations were performed with the p3′SHIV-4. In the first step, as shown in Fig. 1, plasmid pUC19 was digested with SspI and NdeI, blunt ended with the Klenow fragment of DNA polymerase, and ligated to generate pDS. The SphI-KpnI fragment of p3′SHIV-4 was subcloned into pDS to generate pDSvpu. Plasmid pDSvpu was digested with SspI and BbsI, blunt ended, and religated to delete a 60-bp SspI-BbsI fragment and generate pDSDvpu. Figure 2 shows the construction of ΔvpuΔnef SHIV-4. First, the SphI-KpnI fragment of 3′SHIV-4 was replaced with the corresponding fragment of pDSDvpu to yield p3′ΔvpuSHIV-4. Next, p3′ΔvpuSHIV-4 was digested with RsrII and NcoI, blunt ended, and ligated, resulting in the deletion of an RsrII-NcoI fragment (including 205 bp of the nef gene, encoding the first 69 amino acids of Nef). This plasmid was designated pΔvpuΔnefSHIV-4. Plasmids pΔvpuΔnefSHIV-4 and p5′SHIV-4 were digested with SphI and ligated with T4 DNA ligase, and the ligated DNA was used to transfect C8166 cells as described previously (27). Virus stocks were prepared, and aliquots were stored at −80°C. The virus stock had a titer of 104 TCID50/ml in C8166 cells.
FIG. 1.
Construction of recombinant pDSDvpu. Open bars, pUC19 sequences; shaded bars, HIV-1 sequences.
FIG. 2.
Genomic structures of 3′SHIV-4, 3′ΔvpuSHIV-4 and 3′ΔvpuΔnefSHIV-4. Striped bars, HIV-1 sequences; solid bars, SIVmac239 sequences. LTR, long terminal repeat.
Construction of ΔvpuSHIVPPc.
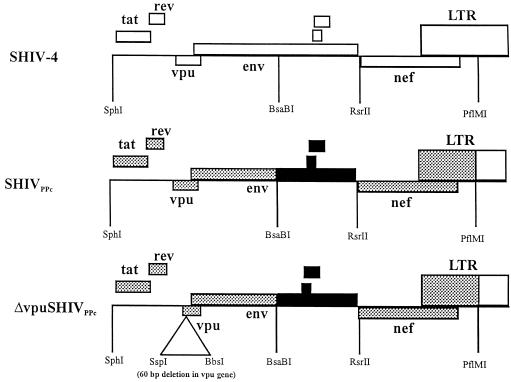
The construction of SHIVPPc has been described previously (26). Briefly, we used plasmid pSHIVPPc-3, which is a derivative of plasmid p3′SHIV-4 containing all of the changes found in the env and nef genes of SHIVPPc. The SphI/KpnI fragment from pDSDvpu, containing the 60-bp deletion in vpu, was subcloned into the SphI and KpnI sites of pSHIVPPc-3. The resulting plasmid was designated pΔvpuSHIVPPc (Fig. 3). Plasmids p5′SHIV-4 and pΔvpuSHIVPPc were digested with SphI and ligated with T4 DNA ligase, and the ligated DNA was used to transfect C8166 cells as described above.
FIG. 3.
Genomic structure of ΔvpuSHIVPPc. ░⃞, derived from PPc spleen; ■, derived from PPc lymph node. LTR, long terminal repeat.
Vaccination of macaques and challenge with SHIVKU-1.
Sixteen sexually mature (3- to 15-year-old) female pig-tailed macaques (Macaca nemestrina) were obtained from the Yerkes Primate Center, Atlanta, Ga. Six were inoculated subcutaneously, close to the inguinal and axillary lymph nodes, with 1.0 ml of ΔvpuΔnefSHIV-4 stock (vaccine 1), while two control animals (PFy and PLy) were mock inoculated with saline. Seven months (29 weeks) after vaccination, the six vaccinated and two control macaques were challenged with 1.0 ml of undiluted SHIVKU-1 stock which was inoculated intravaginally in nontraumatic fashion as described previously (9). This inoculum represents 30 50% animal infectious doses determined by titration of the virus in animals via the intravaginal route of inoculation (9). A second intravaginal challenge dose of SHIVKU-1 was given 5 weeks after the first challenge to ensure exposure to this virus. Six other macaques were vaccinated with 1.0 ml of ΔvpuSHIVPPc stock (vaccine 2) by the oral route, while two control animals (4267 and PDb) were mock inoculated with culture medium. Six months later, all eight macaques were challenged by intravaginal inoculation twice, 1 day apart, with 1.0 ml of undiluted SHIVKU-1 stock.
Cell cultures.
The human T-cell line C8166 was used as the indicator line to measure virus infectivity. Cells were cultured at a concentration of 106/ml in RPMI medium (RPMI 1640 supplemented with 10 mM HEPES buffer [pH 7.3], 50 μg of gentamicin per ml, 50 μM 2-mercaptoethanol, and 2 mM glutamine) with 10% fetal bovine serum (FBS).
Processing of samples.
Heparinized blood obtained from the femoral vein was centrifuged to separate plasma and buffy coats. Plasma was assayed for p27 by using a capture enzyme-linked immunosorbent assay kit (Coulter Laboratories, Hialeah, Fla.) and for infectivity in C8166 cells. PBMC were separated from buffy coats by centrifugation through a Ficoll-Paque (Pharmacia Biotech, Piscataway, N.J.) density gradient. Infectious cell frequency was measured by inoculation of serial 10-fold dilutions of PBMC, starting with 106 cells, into 24-well tissue culture plates containing 105 indicator C8166 T cells, which were observed for development of syncytial cytopathic effects during a 7-day period; then cells and supernatant fluid in 100 μl from each well were transferred to another plate, fresh indicator cells were added, and observation continued for a further 7 days (14). Results were expressed as the number of infectious cells per 106 PBMC. Mesenteric lymph nodes were obtained from vaccine group 1 at 19 weeks postchallenge, and axillary nodes were obtained from vaccine group 2 at 18 weeks postchallenge. Single-cell suspensions were prepared, and infectious cell frequency was assessed as described for PBMC. Other portions of the biopsy material were used for analysis of DNA. Inguinal lymph nodes were obtained later from animals in both groups (between weeks 54 and 60 for group 1 and weeks 31 and 37 for group 2) and snap-frozen in liquid nitrogen immediately upon removal. They were stored at −80°C until processed for RNA analysis.
Fluorescence-activated cell sorting analysis.
PBMC or lymph node cells were reacted with monoclonal antibody to CD4+ T or CD8+ T cells (Dako, Carpinteria, Calif.). After washing, the cells were stained with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (Dako), fixed in 1% buffered formalin, and analyzed on a fluorescence-activated cell counter (12, 14).
Neutralizing antibody assays.
We performed the test as described previously (10). Briefly, serial doubling dilutions of plasma in RPMI medium were prepared in quadruplicate in 96-well plates, 10 to 20 TCID50 of the virus was added to each well, plates were incubated 1 h at 37°C, and 104 indicator C8166 cells were added to each well. Plates were observed for cytopathic effect 7 days later, wells were scored individually, and the 50% neutralization endpoint was calculated by the Kärber method (15).
Detection of viral DNA in tissues.
Tissues from inoculated animals were first screened for the presence of the SIV region of SHIV by PCR using oligonucleotide primers specific for both SIV and SHIV. Total cellular genomic DNA was extracted from PBMC and/or lymph nodes of the animals and was used as a template in nested PCR to amplify SIV gag sequences which were common to both viruses. The gag-specific oligonucleotides and the conditions for amplification were exactly as described previously (14).
PCR techniques were also used to distinguish between the presence and absence of vaccine and/or challenge viruses in macaque tissues, using truncated or full-length vpu as markers of both viruses. For the first round of PCR amplification of vpu genes, we used oligonucleotide primers 5′-CCTAGACTAGAGCCCTGGAAGCATCC-3′ and GTACCTCTGTATCATATGCTTTAGCAT-3′ (antisense), which are complementary to nt 5845 to 5870 and 6393 to 6420 of the HIV-1 (HxB2) genome, respectively. One microgram of genomic DNA was used in the PCR mixture containing 4.0 mM MgCl2, 200 μM each of the four deoxynucleoside triphosphates, 100 pM each oligonucleotide primer, and 2.5 U of Taq polymerase (Perkin-Elmer Cetus, Norwalk, Conn.). The template was denatured at 95°C for 3 min, and PCR amplification performed with an automated DNA Thermal Cycler (Perkin-Elmer Cetus) for 35 cycles of denaturation at 92°C for 1 min, annealing at 55°C for 1 min, and primer extension at 72°C for 3 min. Amplification was completed by incubation of the PCR for 10 min at 72°C. One microliter of the resultant PCR product was used in a nested PCR using the reaction conditions described above. For the second round of amplification, we used oligonucleotide primers 5′-TTAGGCATCTCCTATGGCAGGAAGAAG-3′ (sense) and 5′-CACAAAATAGAGTGGTGGTTGCTTCCT-3′, which are complementary to nt 5956 to 5984 and 6386 to 6413 of the HIV-1 HxB2 genome, respectively. Following the second round of amplification, a 10-μl aliquot was removed and separated on a 1.5% agarose gel, and bands were visualized by staining with ethidium bromide. The result of this PCR was the amplification of a 397-bp fragment if the deleted vpu was present (i.e., the vaccine virus) or a 457-bp fragment if the intact vpu was present (i.e., the challenge virus).
Detection of viral RNA in lymph nodes.
Snap-frozen lymph nodes were homogenized in Trizol reagent (Gibco-BRL, Gaithersburg, Md.), by using an Omni-mixer homogenizer (Omni International, Waterbury, Conn.). Total RNA was isolated as described by the manufacturer, and the final RNA was dissolved in 50 μl of distilled H2O per 100 mg of original tissue. The quality of the RNAs was assessed by reverse transcriptase (RT)-mediated PCR (RT-PCR) for the cellular gene GAPDH mRNA as described previously (22), using the Titan One-Tube RT-PCR system (Boehringer Mannheim, Indianapolis, Ind.). The possibility of contaminating DNA in the RNA preparations was assessed by parallel reactions in which the RT activity in the reaction was first inactivated by 2 min at 99°C followed by 3 min at 95°C. If needed, RNase-free DNase (Gibco-BRL) was used to remove residual DNA from the samples, followed by extraction and precipitation. Sample RNAs were amplified by RT-PCR using primers for the SHIV pol gene (corresponding to the parent SIV sequence): SIVpolA (5′GAAAAGATGGAAAAGGATGG3′) and SIVpolB (5′TGGCTTCTAATGGCTTGC3′). One microgram of total RNA was used in the one-step reaction containing the manufacturer’s buffer and enzyme mix, appropriate primers, and 1.6 U of Prime RNase inhibitor (5 Prime→3 Prime, Inc., Boulder, Colo.). The reactions were performed with a Perkin-Elmer DNA Thermal Cycler 480 with the following thermal profile: 42°C for 30 min, 1 cycle; 94°C for 5 min, 1 cycle; 94°C for 30 s, 55°C for 30 s, and 68°C for 45 s, 10 cycles; 94°C for 30 s, 55°C for 30 s, 68°C for 45 s, with an additional 5-s extension/cycle, 25 cycles; 68°C for 6 min. Nine microliters of the product was loaded onto an agarose–Tris-borate-EDTA gel containing ethidium bromide, DNA was separated by electrophoresis, and the gel was photographed. When no visible product was detected, 1 μl of the initial reaction mixture was added to a nested PCR mixture containing primers SIVpolC (5′ACCAATCCATACAACACC3′) and SIVpolD (5′CTGCCCAATTTAATACTCC3′), 3 mM MgCl2, and 1.25 U of Taq enzyme (Sigma, St. Louis, Mo.), and a further 35 cycles were performed with the following thermal profile: 97°C for 1 min 45 s, 55°C for 2 min, and 72°C for 5 min, 1 cycle; 94°C for 30 s, 55°C for 30 s, 72°C for 45 s, with an additional 1-s extension/cycle, 33 cycles; 94°C for 30 s, 55°C for 30 s, and 72°C for 6 min, 1 cycle. The amplified SIVpolAB fragment is 869 bp, whereas the SIVpolCD nested fragment is 645 bp. For the detection of multiply spliced mRNAs, primers spanning the intron of the tat and rev genes were used (8, 20) without the additional restriction sites; the second-round primers are heminested, containing one of the outer primers plus a nested primer. The PCR products of the Msp1 AB primer pair range from 131 to 159 nt, and those for Msp1 CB primer pair range from 110 to 138 nt. Second-round reactions included 1 M betaine (Sigma).
Quantitation of plasma RNA viral load.
Plasma samples collected in acid citrate dextrose from animals in group 1 during weeks 52 and 58 and from group 2 during weeks 27 and 35 were analyzed by a real-time RT-PCR assay as previously described (28).
Lymphocyte proliferation assay.
PBMC from different macaques, collected 49 weeks after virus challenge, were cultured in triplicate at 105 cells/well in 96-well tissue culture plates in 200 μl of RPMI 1640 containing 10% FBS. Stock SHIVKU-1 (104.2 TCID50/ml) was UV irradiated for 30 minutes and heat treated at 56°C for 60 min before use as antigen; 20 μl of this material was added to each well. Three wells each containing unstimulated PBMC from different pig-tailed macaques served as negative controls. Cells were cultured for 6 days before the addition to each well of 1 μCi of [3H]thymidine (specific activity, 247.9 GBq/mmol; NEN, Boston, Mass.). Cultures were harvested onto glass fiber filter mats by using an automated plate harvester (Skatron, Sterling, Va.) 24 h after addition of [3H]thymidine, and [3H]thymidine incorporation was determined with a Microbeta liquid scintillation counter (Packard). Stimulation index (SI) was calculated as mean counts per minute in stimulated wells/mean counts per minute in control well, and an SI of greater than 1.5 was considered significant (3).
Development of CD4+ T-cell clones.
CD4+ cells from group 1 macaques PDj and PNa (week 50 postchallenge) were negatively selected from PBMC first by incubating PBMC with mouse anti-human CD8 monoclonal antibody and then with anti-mouse immunoglobulin G magnetic beads (Dynal, Lake Success, N.Y.). Cells enriched for CD4+ T cells were then inoculated with herpesvirus saimiri (American Type Culture Collection, Manassas, Va.) to generate immortalized CD4+ T-cell lines (2). CD4+ T-cell clones were derived by the limiting-dilution method, and the phenotype was confirmed by staining with mouse anti-human CD4+ antibody. These CD4+ T cell clones were inoculated with SHIVKU-1 at a multiplicity of infection of about 0.1 and used 7 days later as stimulators or targets.
Generation of bulk cytotoxic T-lymphocyte (CTL) population and chromium release assay.
PDj and PNa PBMC were collected 50 weeks after virus challenge and cocultured with UV-irradiated SHIVKU-1-infected autologous CD4+ T cells (effector/stimulator ratio of 10:1) in 24-well tissue culture plates in RPMI 1640 containing 10% FBS. They were restimulated on day 7 and used as effectors on day 14 in a chromium release assay.
Target cells (autologous CD4+ T-cell clone) were either infected with SHIVKU-1 or sham infected for 3 days and then used in chromium release assays. Cells were labeled with 100 μCi 51Cr (specific activity, 962 MBq/ml of sodium chromate; Amersham, Cleveland, Ohio) for 2 h and then washed thrice with Hanks balanced salt solution. Target cells (2,500/well) were dispensed in triplicate, and effector cells added in triplicate in effector/target (E/T) ratios of 80, 40, 20, 10, and 5 into each well of 96-well U-bottom plates. Chromium release was determined after 4 h of incubation at 37°C in a 5% CO2 incubator. Plates were spun at 1,200 rpm for 5 min, and 100 μl of supernatant from each well was counted in a gamma counter (Packard, Meriden, Conn.). Each experiment had three wells containing only targets and medium which provided the data for spontaneous release, while three wells containing targets and 0.5% sodium dodecyl sulfate served as wells for maximal release of chromium. The percent specific cytotoxicity was calculated as (test release − spontaneous release)/(maximum release − spontaneous release) × 100. Spontaneous lysis of relevant control target was always <25% of maximum release.
RESULTS
Uncoagulated blood samples were collected weekly for the first month, at 2-week intervals for the next month, and monthly thereafter from all inoculated animals. Fresh plasma was tested for infectivity, content of p27, viral RNA, and antiviral antibodies. PBMC were examined for virus content (infectious center assays) and for CD4+ and CD8+ T-cell markers and were used for cellular immunity studies.
Vaccine group 1.
Six macaques were inoculated subcutaneously with 1 ml of tissue culture fluid containing 104 TCID50 of ΔvpuΔnefSHIV-4, and heparinized blood was collected from all six according to the schedule outlined above to assess virus replication and host responses. Cocultivation of 106 PBMC of each animal with C8166 cells failed to yield infectious virus from any of the vaccinated animals at any time point (Table 1), even after depletion of CD8+ T cells. However, PCR analysis of PBMC from all six revealed SIVmac gag DNA sequences, showing that all six had become infected with the vaccine virus. Proof of infection was substantiated by immunoprecipitation analysis of plasma obtained 16 weeks after vaccination. This assay showed that all six had developed binding antibodies to SIVmac Gag and HIV-1 Env proteins (data not shown). By 19 weeks, all six had developed neutralizing antibodies at titers of 1:10 and 1:20 to SHIVKU-1. CD4+ T-cell counts remained normal in all of the six vaccinated macaques. Thus, although the vaccine virus caused a persistent infection, there was little evidence of productive replication of the virus.
TABLE 1.
Viral burden in macaques whose CD4+ T counts are shown in Table 2
| Wk | Infectious cells/106
PBMC
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control
|
Vaccine group
1
|
Vaccine group 2
|
||||||||||||||
| PFy | PLy | 4267 | PDb | 42105 | 42107 | PDj | PLk | PNa | PPm | 42106 | 7024 | 8124 | PEy | PWl | PWv | |
| −22 to −20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 10 | 10 | 10 | 10 | 10 |
| −19 to −17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 10 | 10 | 100 | 1,000 | 100 |
| −15 to −11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 100 | 0 |
| −9 to −3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 to 2 | 10,000 | NDa | 100 | 1,000 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | |
| 3 to 4 | 10 | 10 | 10,000 | 10,000 | 10 | 100 | 0 | 0 | 0 | 0 | 2 | 0 | 100 | 2 | 0 | 0 |
| 5 to 6 | 10 | 10 | 10 | 10 | 10 | 10 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 4 |
| 7 to 8 | 10 | 10 | 4 | 10 | 4 | 2 | 1 | 0 | 2 | 4 | 4 | 0 | 0 | 0 | 0 | 0 |
| 9 to 11 | 5 | 10 | 10 | 100 | 0 | 0 | 0 | 0 | ||||||||
| 12 to 13 | 10 | 10 | 10 | 10 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 14 to 17 | 10 | 10 | 1,000 | 10 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | |
| 18 to 20 | 10 | 2 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| 21 to 23 | 10 | 10 | 10 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ||
| 24 to 26 | 2 | 10 | 1 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 27 to 30 | 10 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 31 to 34 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 35 to 38 | 100 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 39 to 42 | 10 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 43 to 46 | 5 | 0 | 0 | 0 | 0 | 0 | ||||||||||
| 47 to 50 | 10 | 0 | 0 | 0 | 0 | 0 | ||||||||||
| 51 to 54 | 100 | 10 | 0 | 0 | 10 | 5 | ||||||||||
| 55 to 58 | 5 | 0 | 0 | 0 | 0 | |||||||||||
| 59 to 62 | 10 | 0 | 0 | 0 | 0 | |||||||||||
ND, not determined.
At 7 months following vaccination, the six vaccinated macaques and two unvaccinated controls (PFy and PLy) were inoculated intravaginally with 1 ml of undiluted SHIVKU-1 containing 104 TCID50. The inoculation was repeated 5 weeks later to ensure adequate exposure of the animals to the challenge virus. Following challenge, the two unvaccinated controls developed the same massive systemic infection typically caused by SHIVKU-1 in pig-tailed macaques (9, 11). These control animals developed infectious viremia of 103 to 104 TCID50/ml and plasma antigenemia with p27 levels of between 1,000 and 2,000 pg/ml during the first 3 weeks postchallenge. As shown in Table 1, this was accompanied by high numbers of circulating infectious PBMC. The productive infection was followed by rapid loss of more than 90% of CD4+ T cells within 3 weeks following exposure to the virus (Table 2). Macaque PLy developed AIDS approximately 12 weeks after challenge. The other animal (PFy) developed an acute infection similar to that in PLy but developed a more chronic disease course. It became progressively cachectic, and at 58 weeks, when it was euthanized in extremis, its viral RNA burden in plasma was 2.9 × 106 copies/ml (Table 3).
TABLE 2.
CD4+ counts in blood of macaques following intravaginal inoculation with SHIVKU-1
| Wk | No. of CD4+ cells/ml
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls
|
Vaccine group
1
|
Vaccine group 2
|
||||||||||||||
| PFy | PLy | 4267 | PDb | 42105 | 42107 | PDj | PLk | PNa | PPm | 42106 | 7024 | 8124 | PEy | PWl | PWv | |
| 0 | 866 | 1,729 | 467 | 1,394 | 1,249 | 1,152 | 1,507 | 630 | 1,217 | 1,197 | 1,056 | 1,636 | 676 | 1,149 | 1,053 | 1,137 |
| 1–2 | 106 | NDa | 9 | 290 | ND | ND | ND | ND | ND | ND | 974 | 1,355 | 666 | 1,721 | 909 | 1,104 |
| 3–4 | 0 | 26 | 1 | 51 | 538 | 449 | 1,728 | 592 | 792 | 1,254 | 1,151 | 1,033 | 734 | 1,531 | 995 | 1,304 |
| 5–6 | 0 | 1 | 1 | 99 | 884 | 584 | 1,446 | 548 | 974 | 670 | 940 | 968 | 523 | 1,588 | 1,008 | 1,235 |
| 7–8 | 0 | 22 | 0 | 127 | 554 | 415 | 1,174 | 389 | 1,439 | 1,066 | 972 | 1,372 | 616 | 1,557 | 1,008 | 1,092 |
| 9–11 | 0 | 0 | ND | ND | 505 | 360 | 1,158 | 472 | 1,341 | 748 | ND | ND | ND | ND | ND | ND |
| 12–13 | ND | 0 | 6 | 68 | 515 | 342 | 1,243 | 521 | 1,046 | 774 | 1,176 | 684 | 674 | 1,608 | 1,251 | 1,062 |
| 14–17 | 72 | 0 | 86 | 570 | 344 | 1,176 | 699 | 1,076 | 869 | 745 | 1,209 | 436 | 1,352 | 975 | 918 | |
| 18–20 | 55 | 0 | 531 | 98 | 1,479 | 764 | 1,617 | 961 | 236 | 430 | 113 | 812 | 616 | 573 | ||
| 21–23 | 0 | 54 | 696 | 226 | 1,216 | 743 | 915 | 513 | 575 | 825 | 86 | 694 | 324 | 646 | ||
| 24–26 | 160 | 0 | 514 | 139 | 1,601 | 722 | 960 | 460 | 857 | 1,466 | 442 | 1,088 | 886 | 1,331 | ||
| 27–30 | 141 | 163 | 283 | 1,527 | 592 | 706 | 508 | 255 | 359 | 121 | 561 | 319 | 544 | |||
| 31–34 | 9 | 65 | 818 | 1,434 | 693 | 1,071 | 544 | 255 | 916 | 72 | 689 | 624 | 290 | |||
| 35–38 | 15 | 145 | 515 | 566 | 522 | 392 | 480 | 255 | 779 | 156 | 291 | 724 | 702 | |||
| 39–42 | 22 | 5 | 569 | 749 | 439 | 1,056 | 501 | 386 | 858 | 195 | 1,421 | 734 | 900 | |||
| 43–46 | 0 | 573 | 497 | 272 | 182 | 69 | ||||||||||
| 47–50 | 68 | 656 | 528 | 271 | 511 | 277 | ||||||||||
| 51–54 | 0 | 639 | 1,003 | 763 | 668 | 446 | ||||||||||
| 55–58 | 0 | 280 | 516 | 169 | 258 | 214 | ||||||||||
| 59–62 | 833 | 947 | 693 | 895 | 534 | |||||||||||
ND, not determined.
TABLE 3.
Detection of viral load in plasma and viral RNA in lymph nodes of study animals
| Animal | Plasma viral RNA at indicated wk postinfection | Detection by lymph node RT-PCR
|
|||||
|---|---|---|---|---|---|---|---|
| GAPDH |
pol
|
Multiply
spliced
|
|||||
| Outer primer | Inner primer | Outer primer | Inner primer | ||||
| Vaccine group 1 | 52 | 58 | |||||
| PFya | 380,000 | 2,900,000 | ++ | +++ | +++ | + | ++ |
| PLya,b | NDc | ND | ND | ND | ND | ND | ND |
| 42105 | 5,200 | 70,000 | − | − | − | + | ++ |
| 42107b | ND | ND | ND | ND | ND | ND | ND |
| PDj | <600 | <600 | + | − | +++ | − | ++ |
| PLk | <600 | <600 | + | − | + | − | − |
| PNa | 6,100 | <300 | +++ | − | +++ | − | − |
| PPm | <600 | <300 | ++ | − | +++ | − | ++ |
| Vaccine group 2 | 27 | 35 | |||||
| PDba | 440,000 | 490,000 | ++ | + | +++ | + | ++ |
| 4267a,b | ND | ND | ND | ND | ND | ND | ND |
| 7024 | <600 | <300 | + | − | + | − | − |
| 8124 | <600 | <300 | + | − | + | − | + |
| 42106 | <600 | <300 | ++ | − | − | − | +++ |
| PEy | <600 | <300 | + | − | +++ | − | − |
| PWl | <600 | <300 | ++ | − | +++ | − | − |
| PWv | <600 | <300 | + | − | + | − | + |
Mock vaccinated.
Dead from AIDS at time of test.
ND, not determined.
The six vaccinated macaques all became infected with SHIVKU-1 after challenge, as indicated by PCR analysis of DNA obtained from lymph node biopsies. PCR analysis showed evidence of an open vpu gene, consistent with that of SHIVKU-1 (Fig. 4; Table 4). However, none of the six developed the highly productive type of infection seen in the two unvaccinated controls and as is typical of the infection caused by the SHIVKU-1. Examination of PBMC from PDj, PLk, PNa, and PPm showed that nearly all of the sequentially collected blood samples lacked infectious cells. Rarely, low numbers of infectious PBMC appeared, but these occurrences were transient. At 52 weeks, the viral RNA level in PPm, PDj, and PLk was less than 600 copies/ml (Table 3). One blood sample from PNa at week 52 showed infection in 10 cells per 106 PBMC, and this was accompanied by a plasma RNA level of approximately 6,000 copies/ml. Two weeks later, however, infected cells had again disappeared from blood samples, and at 58 weeks its viral RNA level in plasma was back to <300 copies/ml. Macaques 42105 and 42107 developed a more productive type of infection characterized by the persistent presence of small numbers of infectious cells in PBMC (Table 1), although neither animal developed infectious viremia or plasma antigenemia (data not shown). Both animals developed approximately 50% loss of CD4+ T cells by 3 weeks following virus challenge, and this cell count stabilized at this level in macaque 42105 (Table 2). However, in 42107, the cell count underwent a second period of decline at approximately 15 weeks, and the animal developed AIDS at 24 weeks. Macaque 42105 developed better control over virus replication, with no evidence of infectious cells in blood between weeks 26 and 50. However, infectious PBMC reappeared after this time period. At 52 weeks postchallenge, when the blood sample had 10 infectious cells per 106 PBMC, the corresponding plasma sample had approximately 5,000 copies/ml of viral RNA. At 58 weeks, the plasma had 70,000 copies/ml (Table 3). Thus, of the six animals that were vaccinated and challenged, all became infected with the challenge virus. One developed a productive infection and succumbed to AIDS, and another remains at risk for developing late-onset disease. Except for transient sporadic appearances of infectious cells in blood, the other four macaques developed total control over replication of the challenge virus and have resisted disease.
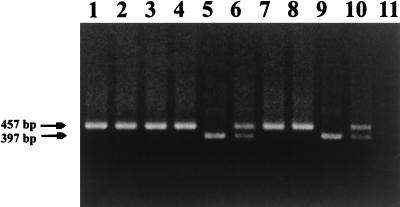
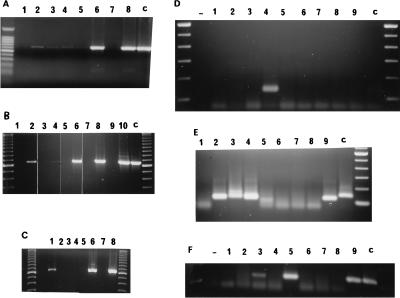
FIG. 4.
PCR detection of viral sequences from lymph node biopsies 19 weeks after challenge of vaccine group 1 with SHIVKU-1. Lymph node biopsies were performed; DNA was extracted and used in nested PCR with oligonucleotides that amplified the vpu gene of SHIVKU-1 as described in the text. Aliquots of the nested PCR were run on a 1.5% agarose gel and stained with ethidium bromide. Lanes 1 to 7, amplification of vpu sequences from lymph node DNA of macaques 42105, 42107, PLk, PPm, PDj, PNa, and PFy, respectively; lane 8, amplification of vpu sequences from plasmid p3′SHIV (positive control for full length vpu gene); lane 9, amplification of vpu sequences from plasmid pDSDvpu (positive control for truncated vpu gene); lane 10, amplification of vpu sequences from plasmids p3′SHIV and pDSDvpu; lane 11, amplification of vpu from uninfected PBMC DNA (negative control).
TABLE 4.
Infectious cell frequency and PCR analysis of mesenteric lymph nodes at week 19 postchallenge from vaccine group 1 and PFy and of axillary lymph nodes at week 18 postchallenge from PDj and vaccine group 2
| Animal | Infected cells/106 lymph node cells | PCR
|
|
|---|---|---|---|
| gag | vpua | ||
| Controls | |||
| PFy | 100 | + | +C |
| PDb | 10 | + | +C |
| Vaccine group 1 | |||
| 42105 | 5 | + | +C |
| 42107 | 1,000 | + | +C |
| PDj | 0 | − | +V |
| PLk | 0 | + | +C |
| PNa | 0 | + | +C,V |
| PPm | 10 | + | +C |
| Vaccine group 2 | |||
| 7024 | 0 | + | +V |
| 8124 | 0 | + | +C |
| PEy | 0 | + | +V |
| PWv | 0 | + | +C |
| 42106 | 0 | + | +C |
The vpu that was amplified is indicated as C (challenge virus [SHIVKU-1]) or V (vaccine virus). Both challenge and vaccine virus sequences were amplified from PNa.
Vaccine group 2.
Since the death of macaque 42107 and the low-grade persistent infection in macaque 42105 may have been the result of inadequate immunity resulting from poor replication of vaccine virus 1, we sought to use another vaccine virus that had the potential for more robust replication and also one that could be administered orally. As shown in previous reports, SHIV-4 had become virulent after sequential passage in macaques (12). Macaque PPc was the first animal to develop AIDS, and the disease course correlated with a number of factors, including high replication efficiency of the virus in CD4+ T cells and macrophages, infectious viremia, infection in high numbers of circulating PBMC in peripheral blood, and loss of CD4+ T cells (12). New genetic changes in the virus included an open, functional vpu gene and numerous consensus amino acid changes in the variable and constant regions of the env and nef genes (17, 25). Since vpu appeared to be important for pathogenicity, we deleted this gene but used the env and nef genes of the PPc virus in a new construct, termed ΔvpuSHIVPPc (Fig. 3). In contrast to SHIV-4, ΔvpuSHIVPPc is macrophagetropic as a consequence of mutations in the env genes of SHIVPPc. This property is probably important for the successful systemic infection that occurs following inoculation of this virus on mucosal surfaces.
Six macaques were inoculated orally with 104 TCID50 of vaccine 2 in 1 ml of tissue culture medium. The inoculum was deposited on both sides of the base of the tongue, aimed at the pharyngeal tonsils. As in tests on animals inoculated with ΔvpuΔnefSHIV-4, we bled the second vaccine group at indicated intervals and tested for evidence of virus replication. As shown in Table 1, all six animals developed a productive infection in PBMC by week 2 after inoculation. This was characterized by the appearance of infectious PBMC, although none of the vaccinated animals developed infectious viremia or plasma antigenemia. Infection in the PBMC lasted 6 to 10 weeks, after which productive replication was brought under control and virus could no longer be isolated from PBMC of the animals, even after depletion of CD8+ T cells. All six animals developed immunoprecipitating antibodies to the Gag of SIVmac and Env of HIV-1 (data not shown) and also neutralizing antibodies at titers of 1:10 to 1:80 to SHIVKU-1.
At 24 weeks following immunization, the six vaccinated macaques and two unvaccinated controls were inoculated intravaginally with SHIVKU-1, and the inoculation was repeated 24 h later. The two unvaccinated controls developed the typical massive infection caused by SHIVKU-1. Viremia, high numbers of infectious PBMC, and more than 90% loss of CD4+ T cells characterized the early phase of these infections (Tables 1 and 2). One was euthanized with AIDS at 12 weeks and the other, PDb, is still alive in a cachectic state. Biopsy and examination of axillary lymph nodes from five of the six vaccinated macaques at 18 weeks showed an open vpu gene in three (Table 4), demonstrating that the animals had become infected with SHIVKU-1. However, as for the four macaques in group 1, virus was recovered only rarely from PBMC of these vaccinated macaques at any time point. The animals have remained healthy and failed to develop virus-associated loss of CD4+ T cells during more than 40 weeks of examination. Considerable menstrual blood loss among these retired breeders, especially 8124, appeared to be associated with the wide fluctuations of CD4+ T-cell counts in individuals. Whatever the reason for loss of these cells, it was not associated with virus replication (Tables 1 and 4). Thus, although all six had become infected with virus crossing the vaginal mucosa and entering the regional lymph nodes, no systemic productive viral replication ensued in any of them. At 27 and 35 weeks postchallenge, plasma RNA levels in the six vaccinated macaques were less than 600 and 300 copies/ml, respectively, whereas the surviving unvaccinated challenge control animal had 490,000 copies/ml (Table 3).
Because nearly all of the animals receiving either vaccine had undetectable levels of virus in the plasma, we sought to determine the status of virus replication in lymph nodes. Studies of HIV-infected people have shown that replication-competent virus can be rescued from lymph nodes (4, 6, 29), and active virus replication can be demonstrated in lymph nodes with the use of primers for spliced mRNAs, even while the plasma has less than 400 copies of viral RNA/ml (8). Since lymph nodes of vaccinated macaques contained proviral DNA of the challenge virus, SHIVKU-1, we considered it important to determine whether the virus was inactive (trapped in follicular dendritic cells) or was replicating. Lymph node RNA was prepared from biopsies obtained from all surviving 11 of the 12 vaccinated macaques at weeks 54 and 60 (group 1) and weeks 31 and 37 (group 2). RT-PCR amplification of lymph node RNA from the five vaccinated macaques of group 1, using primers to the SIV pol gene, did not reveal detectable SHIV after 35 cycles despite obvious low-grade infection in PBMC of 42105 (Table 3). However, the RNA from the lymph node of 42105 was not of good quality, as shown by the lack of GAPDH signal. The one remaining unvaccinated challenged animal, PFy, showed a strong signal in the initial reaction with the outer primers. However, lymph nodes of four of the five vaccinated macaques in group 1 did show a specific signal upon nested set amplification of 34 further cycles (Fig. 5A, lanes 6 and 8; Fig. 5C, lanes 2 and 6). In group 2, again, only the unvaccinated animal showed a signal upon the initial RT-PCR (not shown), but five of the six vaccinated animals (the exception being 42106) showed a signal upon nested set amplification (Fig. 5A, lane 2; Fig. 5B, lanes 2, 4, 6, 8). These assays detected full-length mRNAs. Since these could have been viral RNA in virion particles trapped in the lymph nodes, we used a different set of primers spanning the introns of the tat and rev genes to detect multiply spliced viral mRNAs, indicative of replication. The two remaining unvaccinated challenge animals (PFy and PDb) and 1 of the 11 vaccinated macaques (macaque 42105 in group 1) had a detectable signal with these primers (Fig. 5D, lanes 4 and 2; data for PDb not shown). However, nested PCR showed that two other group 1 animals (PDj and PPm; Fig. 5E, lanes 3 and 5) and three group 2 animals (8124, 42106, and PWv; Fig. 5F, lanes 3, 5, and 9) had positive signals (summarized in Table 3). Thus, virus replication, albeit at an extremely low level, was still in progress in three of the five vaccinated macaques in group 1 and three of the six in group 2.
FIG. 5.
RT-PCR detection of full-length viral RNA and spliced mRNA in lymph nodes. Total RNA was extracted from snap-frozen inguinal lymph nodes from control and vaccinated animals. RT-PCRs were performed with 1 μg of total RNA, except as noted; nested or heminested PCRs were performed with 1 μl from the RT-PCR. (A to C) Nested PCR with SIVpolCD primers. (A) Macaque 8124, lanes 1 and 2; 42106, lanes 3 and 4; PNa, lanes 5 and 6; PPm, lanes 7 and 8; heated controls in the RT-PCR step, lanes 1, 3, 5, and 7. (B) PWv, lanes 1 and 2; 7024, lanes 3 and 4; PEy, lanes 5 and 6; PWl, lanes 7 and 8; PDb, lanes 9 and 10; heated controls, lanes 1, 3, 5, 7, and 9. (C) PLk, lanes 1 and 2; 42105 (5 μg), lanes 3 and 4; PDj, lanes 5 and 6; PFy, lanes 7 and 8; heated controls, lanes 1, 3, 5, and 7. (D to F) Multiply spliced primers. (D) RT-PCR with MsplAB primers. PLk, lane 1; 42015, lane 2; PDj, lane 3; PFy, lane 4; PWv, lane 5; 7024, lane 6; PEy, lane 7; PWl, lane 8; PDb, lane 9. (E) Heminested reactions with MsplBC primers. Lanes 1 to 9 are as described for panel D. (F) Heminested reactions with MsplBC primers. Macaque 8124, lanes 1 to 3 (lanes 1 and 2, 1 μg; lane 3, 5 μg); 42106, lanes 4 and 5; PNa, lanes 6 and 7; PPm, lanes 8 and 9; heated controls, lanes 1, 4, 6, and 8; reactions with no RNA added to original RT-PCR step, −. Lanes c in all panels represent control reactions with related KU2 RNA.
Since virus replication was in progress in the lymphoid tissues but had been kept in check in 10 of the 12 vaccinated macaques, we sought to determine whether the vaccinated macaques had developed antiviral cell-mediated immune (CMI) responses. We determined first whether the animals had acquired virus-specific T-helper (Th) cells by using assays for lymphocyte proliferation after exposure of PBMC to virus.
Four of the five macaques in group 1 and all six macaques in group 2 displayed moderate to excellent responses when stimulated in vitro with inactivated challenge virus (Table 5), suggesting that major histocompatibility complex class II-restricted antiviral Th cells may have been expanded in these vaccinated macaques. The single surviving unvaccinated challenge control animal, PDb, failed to prime any virus-specific Th cells, as evidenced by the inability of challenge virus to induce an in vitro Th cell expansion. Of the group 1 vaccinated macaques, only monkey PNa failed to display any significant proliferation of T cells in vitro. It is thus of interest that this animal, which had DNA of both vaccine and challenge viruses in its lymph node (Table 4) and had controlled virus replication completely, showed no significant proliferation of T cells. In contrast, macaque 42105, the only member of the group that had an ongoing productive infection, had the highest SI value. Primed relevant Th cells were detected in all of the vaccinated macaques in group 2. While SI values may not be markers of resistance to virus replication, they may contribute to the development of such resistance.
TABLE 5.
Lymphoproliferative responses to SHIVKU-1 in vaccinated animals
| Immunogen | Monkey | SI |
|---|---|---|
| Vaccine 1 | 42105 | 9.10 |
| PLk | 8.19 | |
| PPm | 7.87 | |
| PDj | 2.29 | |
| PNa | 1.31 | |
| Vaccine 2 | 42106 | 3.78 |
| 7024 | 7.17 | |
| 8124 | 12.39 | |
| PEy | 3.62 | |
| PWl | 2.78 | |
| PWv | 4.94 | |
| Virus control | PDb | 0.91 |
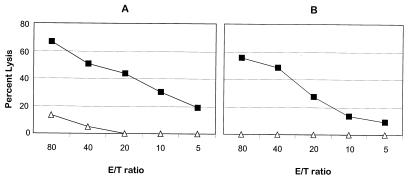
In addition to performing lymphoproliferation assays, we are also determining whether the resistant animals had developed antiviral CTL and report here on preliminary studies on two vaccinated macaques. We took advantage of the fact that not only did the disease-resistant, challenged vaccinated macaques have CD4+ T cells in peripheral blood that were uninfected with SHIVKU-1, but these cells were fully susceptible to infection with this virus in culture. We therefore immortalized CD4+ T cells by infection with herpesvirus saimiri and obtained clones which were then expanded, inoculated with SHIVKU-1, and used in autologous reactions as stimulators and targets. Two animals, PDj and PNa, from which we had derived our first T-cell clones, were selected for study. PBMC collected at 50 weeks postchallenge from these two animals were stimulated with SHIVKU-1-infected autologous CD4+ T cells at an effector/stimulator ratio of 10:1 and restimulated again in the same fashion on day 7. These bulk T-cell lines were used as effector cells for detecting CTL activity 2 weeks after the first stimulation (Fig. 6). Both cell populations displayed excellent cytotoxic activity while showing no (PNa) or very little (PDj) lysis against control targets (Fig. 6).
FIG. 6.
SHIVKU-1-specific CTL activity in macaques immunized with vaccine 1 and challenged with SHIVKU-1 at various E/T ratios. PBMC from macaques PDj (A) and PNa (B) collected 50 weeks postchallenge were stimulated in vitro with SHIVKU-1-infected, UV-irradiated autologous CD4+ T cells and used as effectors in a chromium release assay on day 14. Target cells used in the experiment were autologous CD4+ T cells either infected with SHIVKU-1 (■) or sham infected (▵) and labeled with 51Cr.
DISCUSSION
Six sexually mature macaques inoculated orally on a single occasion with ΔvpuSHIVPPc (group 2) developed an uneventful, transiently productive infection that conferred protection against AIDS caused by intravaginally inoculated, highly virulent SHIVKU-1 (χ2 test, P = 0.005). All four of the unvaccinated control macaques inoculated intravaginally with this virus developed highly productive infection, subtotal loss of CD4+ T cells, and AIDS, confirming our identical, previously reported finding (9) on development of disease in six of six animals that became infected after nontraumatic intravaginal infusion of this virus. The first vaccine virus, ΔvpuΔnefSHIV-4, was incapable of causing infection after mucosal inoculation (and therefore was injected subcutaneously) and replicated poorly in all six macaques, but nevertheless induced binding and neutralizing antibodies to the virus, proving infection. Four of these six vaccinated macaques resisted productive infection and disease following intravaginal challenge with SHIVKU-1 (χ2 test, P = 0.102). Of the two animals that developed productive infection, one developed AIDS and another remains at risk for developing AIDS. Given this prognosis, only 2 of the 12 vaccinated macaques, compared to four of four of the control animals, developed disease (χ2 test, P = 0.003). Mechanisms of vaccine-induced protection among the 10 resistant vaccinated macaques are still being evaluated, but the purpose of this report is to illustrate proof of concept that the sexually transmitted disease caused by HIV-1 in human beings could probably be prevented by prophylactic immunization.
Both vaccine viruses caused persistent infection irrespective of whether they replicated productively during the first few weeks following inoculation. Six months following immunization, even though infectious virus could no longer be isolated from PBMC from any of the 12 vaccinated macaques, vaccine virus DNA was still detectable. All 12 animals had developed binding and very low neutralizing antibody titers to SHIVKU-1 in plasma by the time of virus challenge (approximately 6 months following vaccination). As shown, all 12 became infected with the challenge virus, since its DNA was clearly detectable in PBMC or mesenteric lymph node tissue a few months following challenge. Two of the six vaccinated macaques in group 1 developed a persistent productive infection in PBMC and one, 42107, succumbed to AIDS approximately 6 months following challenge. The other macaque, 42105, had a phase of low-grade productive infection for 30 weeks followed by a 20-week period when it had no circulating infectious PBMC in blood. However, from week 50 onward, infectious PBMC, accompanied by relatively low concentrations of viral RNA (5,000 to 70,000, versus 4 × 105 to 3 × 106 in controls) have reappeared in blood. At 60 weeks postchallenge, this animal still has large numbers of uninfected CD4+ T cells (which are highly susceptible to infection), in contrast to control animals, nearly all of whose CD4+ T cells became infected and eliminated during the first 3 weeks of infection. Nevertheless, the resurgence of virus in macaque 42105 suggests that this animal may be at risk for developing late-onset AIDS.
The other 10 vaccinated macaques, 4 in group 1 and all six in orally immunized group 2, developed a unique type of control over replication of SHIVKU-1. Rarely and transiently, small numbers of infectious cells have appeared in blood, but at nearly all time points, replication-competent virus could not be isolated from PBMC or lymph nodes (sampled two to three times during this period of observations) by any procedure. Attempts have included three successive cocultivations of CD8-depleted PBMC with mitogen-activated normal PBMC or T-cell lines. Nevertheless, both viral DNA and viral RNA have persisted in lymph node tissue. Further, the detection of spliced viral RNA in lymph nodes of a few animals in both groups suggested that an extremely low level of replication of SHIVKU-1 has probably been in progress continuously in all of the vaccinated macaques. This type of replication of this virus had never been observed before. In our earlier study on pathogenesis of infection following intravaginal inoculation of SHIVKU-1 (9), we had shown that viral DNA became detectable by day 2 after inoculation in mesenteric lymph nodes and that explosive replication of the agent occurred during the following week. In this study, although the intravaginal route of inoculation was shown to be about 30-fold less sensitive than the intravenous route, all animals that became infected after intravaginal inoculation nevertheless developed disease. Extrapolating these data to our vaccinated macaques, since all had developed infection in lymph nodes, we surmised that systemic rather than mucosal factors were responsible for curtailment of virus replication.
It is doubtful that neutralizing antibodies had a significant role in curtailment of virus replication in the vaccinated macaques. In an earlier report (7), we showed that passively administered immune serum was effective only when given before parenteral inoculation of the virus. This serum had no therapeutic effect when given 2 h following virus inoculation. Worse still, unlike the success at preventing infection after parenteral inoculation of the virus, we were unable to protect macaques from the dire effects of infection by infusing the serum 24 h before mucosal inoculation of the agent (13). Immune serum therefore had no apparent effect on an already established infection, a well-known phenomenon in lentivirus infections, nor apparently could the previously administered serum prevent infection across mucosal surfaces. Restriction of virus replication in our 10 vaccinated macaques must therefore have been mediated by factors other than neutralizing antibodies.
Studies on CMI responses of the vaccinated macaques have only recently begun. That this type of immunity is important came from an earlier finding that macaques that had “recovered” from infection with avirulent SIVmac were protected from effects of infection with SHIVKU-1 (24). Since these two viruses have distinctly different envelopes, the protection was probably mediated by CMI responses to viral core proteins shared by the two viruses. In the present study, our findings that CD4+ T cells in the vaccinated macaques at week 50 in group 1 and in week 30 in group 2 proliferated when exposed to antigens of SHIVKU-1 showed that the animals had developed anti-SHIV CMI responses that have persisted along with the infection. The further preliminary finding that 2 of the 10 vaccinated macaques which have controlled virus replication have developed CTL to the virus has strengthened the premise that CMI responses are responsible for control and curtailment of replication of the virulent virus. Possibly, a dynamic interaction between virus replication and CTL curtailment responses is continuously at play. A recent study of CTL responses in HIV-infected humans showed an inverse correlation between the levels of CTL and plasma viral RNA loads (19), implicating a role for CTL control of viral replication and delay of disease progression. We hope that more complete studies on CMI responses among our vaccinated macaques, currently in progress, will shed new light on the mechanisms of curtailment of virus replication in immune animals. However, although mechanisms of protection against SHIVKU-1 are still not understood fully, the present study clearly lends support to the concept that sexually transmitted HIV-1 disease can be averted by immunization resulting from a single exposure to a live attenuated vaccine.
ACKNOWLEDGMENTS
This study was supported by grants AI-38492, AI-40372, RR-06753, DK-49516, and NS-32203 from the National Institutes of Health and by BioStratum Inc.
We thank Wu Zhuge for performing immunoprecipitation studies, Sampa Mukherjee and Manisha Sahni for technical assistance, and Erin McDonough for help in preparation of the manuscript.
REFERENCES
- 1.Almond N, Kent K, Cranage M, Rud E, Clarke B, Stott E J. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet. 1995;345:1342–1344. doi: 10.1016/s0140-6736(95)92540-6. [DOI] [PubMed] [Google Scholar]
- 2.Beisinger B, Muller-Fleckenstein I, Simmer B, Lang G, Wittman S, Platzer E, Desrosiers R C, Fleckenstein B. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc Natl Acad Sci USA. 1992;89:3116–3119. doi: 10.1073/pnas.89.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buge S L, Richardson R, Alipanah S, Markham P, Cheng S, Kalyan N, Miller C J, Lubeck M, Udem S, Eldridge J, Robert-Guroff M. An adenovirus-simian immunodeficiency virus envvaccine elicits humoral, cellular, and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J Virol. 1997;71:8531–8541. doi: 10.1128/jvi.71.11.8531-8541.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chun T W, Stuyver L, Mizell S B, Ehler L A, Mican J A M, Baseler M, Lloyd A L, Nowak M A, Fauci A S. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 6.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 7.Foresman L, Jia F, Li Z, Wang C, Stephens E B, Sahni M, Narayan O, Joag S V. Neutralizing antibodies administered before but not after virulent SHIV prevent infection in macaques. AIDS Res Hum Retroviruses. 1998;14:1035–1043. doi: 10.1089/aid.1998.14.1035. [DOI] [PubMed] [Google Scholar]
- 8.Gunthard H F, Wong J K, Ignacio C C, Guatelli J C, Riggs N L, Havlir D V, Richman D D. Human immunodeficiency virus replication and genotypic resistance in blood and lymph nodes after a year of potent antiretroviral therapy. J Virol. 1998;72:2422–2428. doi: 10.1128/jvi.72.3.2422-2428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joag S V, Adany I, Li Z, Foresman L, Pinson D M, Wang C, Stephens E B, Raghavan R, Narayan O. Animal model of mucosally transmitted human immunodeficiency virus type 1 disease: intravaginal and oral deposition of simian/human immunodeficiency virus in macaques results in systemic infection, elimination of CD4+T cells, and AIDS. J Virol. 1997;71:4016–4023. doi: 10.1128/jvi.71.5.4016-4023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joag S V, Anderson M G, Clements J E, McEntee M F, Sharma D P, Adams R J, Narayan O. Antigenic variation of molecularly cloned SIVmac239 during persistent infection in a rhesus macaque. Virology. 1993;195:406–412. doi: 10.1006/viro.1993.1390. [DOI] [PubMed] [Google Scholar]
- 11.Joag S V, Li Z, Foresman L, Pinson D, Raghavan R, Zhuge W, Adany I, Wang C, Jia F, Sheffer D, Ranchalis J, Watson A, Narayan O. Characterization of the pathogenic KU-SHIV model of acquired immunodeficiency syndrome in macaques. AIDS Res Hum Retroviruses. 1997;13:635–645. doi: 10.1089/aid.1997.13.635. [DOI] [PubMed] [Google Scholar]
- 12.Joag S V, Li Z, Foresman L, Stephens E B, Zhao L J, Adany I, Pinson D M, McClure H M, Narayan O. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+T cells and AIDS in pigtailed macaques. J Virol. 1996;70:3189–3197. doi: 10.1128/jvi.70.5.3189-3197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joag, S. V., Z. Li, C. Wang, L. Foresman, F. Jia, E. B. Stephens, and O. Narayan. Passively administered neutralizing serum which protected macaques against infection with parenterally-inoculated pathogenic SHIV failed to protect against mucosally-inoculated virus. Submitted for publication. [DOI] [PubMed]
- 14.Joag S V, Stephens E B, Adams R J, Foresman L, Narayan O. Pathogenesis of SIVmacinfection in Chinese and Indian rhesus macaques: effects of splenectomy on virus burden. Virology. 1994;200:436–446. doi: 10.1006/viro.1994.1207. [DOI] [PubMed] [Google Scholar]
- 15.Lennette E H. General principles underlying laboratory diagnosis of viral and rickettsial infections. In: Lennette E H, Schmidt N J, editors. Diagnostic procedures for viral and rickettsial infections. New York, N.Y: American Public Health Association; 1969. pp. 1–65. [Google Scholar]
- 16.Li J, Lord C I, Haseltine W, Letvin N L, Sodroski J. Infection of cynomolgus monkeys with a chimeric HIV-1/SIVmacX virus that expresses the HIV-1 envelope glycoproteins. J Acquired Immune Defic Syndr. 1992;5:639–646. [PubMed] [Google Scholar]
- 17.McCormick-Davis C, Zhao L, Mukherjee S, Leung K, Sheffer D, Joag S V, Narayan O, Stephens E B. Chronology of genetic changes in the vpu, env and nefgenes of chimeric simian-human immunodeficiency virus (strain HXB2) during acquisition of virulence for pig-tailed macaques. Virology. 1998;248:275–283. doi: 10.1006/viro.1998.9300. [DOI] [PubMed] [Google Scholar]
- 18.Merkenschlager M, Buck D, Beverley P C, Sattentau Q J. Functional epitope analysis of the human CD4 molecule. The MHC class II-dependent activation of resting T cells is inhibited by monoclonal antibodies to CD4 regardless whether or not they recognize epitopes involved in the binding of MHC class II or HIV gp120. J Immunol. 1990;145:2839–2845. [PubMed] [Google Scholar]
- 19.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath M J. Cytotoxic-T-cell responses, viral load and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 19a.Narayan, O., and H. M. McClure. Unpublished data.
- 20.Saksela K, Muchmore E, Girard M, Fultz P, Baltimore D. High viral load in lymph nodes and latent human immunodeficiency virus (HIV) in peripheral blood cells of HIV-1-infected chimpanzees. J Virol. 1993;67:7423–7427. doi: 10.1128/jvi.67.12.7423-7427.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharpe S A, Whatmore A M, Hall G A, Cranage M P. Macaques infected with attenuated simian immunodeficiency virus resist superinfection with virulence-revertant virus. J Gen Virol. 1997;78:1923–1927. doi: 10.1099/0022-1317-78-8-1923. [DOI] [PubMed] [Google Scholar]
- 22.Smith M S, Bloomer C, Horvat R, Goldstein E, Casparian J M, Chandran B. Detection of human herpesvirus 8 DNA in Kaposi’s sarcoma lesions and peripheral blood of human immunodeficiency virus-positive patients and correlation with serologic measurements. J Infect Dis. 1997;176:84–93. doi: 10.1086/514043. [DOI] [PubMed] [Google Scholar]
- 23.Stahl Hennig C, Dittmer T, Nisslein T, Petry H, Jurkiewicz E, Fuchs D, Wachter H, Matz-Rensing K, Kuhn E M, Kaup F J, Rud E W, Hunsmann G. Rapid development of vaccine protection in macaques by live-attenuated simian immunodeficiency virus. J Gen Virol. 1996;77:2969–2981. doi: 10.1099/0022-1317-77-12-2969. [DOI] [PubMed] [Google Scholar]
- 24.Stephens E B, Joag S V, Atkinson B, Sahni M, Li Z, Foresman L, Adany I, Narayan O. Infected macaques that controlled replication of SIVmac or nonpathogenic SHIV developed sterilizing resistance against pathogenic SHIVKU-1. Virology. 1997;234:328–339. doi: 10.1006/viro.1997.8662. [DOI] [PubMed] [Google Scholar]
- 25.Stephens E B, Joag S V, Sheffer D, Liu Z Q, Zhao L, Mukherjee S, Foresman L, Adany I, Li Z, Pinson D M, Narayan O. Initial characterization of viral sequences from a SHIV-inoculated pigtailed macaque that developed AIDS. J Med Primatol. 1996;25:175–185. doi: 10.1111/j.1600-0684.1996.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 26.Stephens E B, Mukherjee S, Liu Z, Sheffer D, Lamb-Wharton R, Leung K, Joag S V, Li Z, Foresman L, Adany I, Narayan O. Simian-human immunodeficiency virus (SHIV) containing the nef/long terminal repeat region of the highly virulent SIVsmmPBj14 causes PBj-like activation of cultured resting peripheral blood mononuclear cells, but the chimera showed no increase in virulence. J Virol. 1998;72:5207–5214. doi: 10.1128/jvi.72.6.5207-5214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephens E B, Mukherjee S, Sahni M, Zhuge W, Raghavan R, Singh D K, Leung K, Atkinson B, Li Z, Joag S V, Liu Z Q, Narayan O. A cell-free stock of simian-human immunodeficiency virus that causes AIDS in pig-tailed macaques has a limited number of amino acid substitutions in both SIVmacand HIV-1 regions of the genome and has altered cytotropism. Virology. 1997;231:313–321. doi: 10.1006/viro.1997.8534. [DOI] [PubMed] [Google Scholar]
- 28.Suryanarayana K, Wiltrout T A, Vasquez G M, Hirsch V M, Lifson J D. Plasma SIV RNA viral load by real time quantification of product generation in RT PCR. AIDS Res Hum Retroviruses. 1998;14:183–189. doi: 10.1089/aid.1998.14.183. [DOI] [PubMed] [Google Scholar]
- 29.Wong J K, Hezareh M, Gunthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 30.Wyand M S, Manson K H, Garcia Moll M, Montefiori D, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhuge W, Jia F, Stephens E B, Li Z, Wang C, Joag S V, Narayan O. Failure of SIVmac to be neutralized in macrophge cultures is unique to SIVmacand not observed with neutralization of SHIV or HIV-1. AIDS Res Hum Retroviruses. 1998;14:1045–1051. doi: 10.1089/aid.1998.14.1045. [DOI] [PubMed] [Google Scholar]