Significance
IL-6 signaling has a pivotal role in the pathogenesis of pulmonary arterial hypertension (PAH). However, the target cells of IL-6 signaling in PAH have been elusive. Here, we revealed that SM22α-Cre mice show unexpected recombination in almost all hematopoietic cell lineages. Based on these findings, we demonstrated that IL-6/gp130 signaling in CD4+ T cells has a critical role in the pathogenesis of PAH. We next examined the effects of Il6 deletion on severe PAH models in rats and showed that IL-6 deficiency improves the pathophysiology in several rat PAH models. Furthermore, the additive effect of IL-6 deficiency with existing PAH therapies was also revealed. Taken together, IL-6/gp130 signaling in CD4+ cells is important in the pathogenesis of PAH.
Keywords: pulmonary arterial hypertension, interleukin-6, CD4+ T cell, inflammation, SM22α-Cre
Abstract
Pulmonary arterial hypertension (PAH) is characterized by stenosis and occlusions of small pulmonary arteries, leading to elevated pulmonary arterial pressure and right heart failure. Although accumulating evidence shows the importance of interleukin (IL)-6 in the pathogenesis of PAH, the target cells of IL-6 are poorly understood. Using mice harboring the floxed allele of gp130, a subunit of the IL-6 receptor, we found substantial Cre recombination in all hematopoietic cell lineages from the primitive hematopoietic stem cell level in SM22α-Cre mice. We also revealed that a CD4+ cell-specific gp130 deletion ameliorated the phenotype of hypoxia-induced pulmonary hypertension in mice. Disruption of IL-6 signaling via deletion of gp130 in CD4+ T cells inhibited phosphorylation of signal transducer and activator of transcription 3 (STAT3) and suppressed the hypoxia-induced increase in T helper 17 cells. To further examine the role of IL-6/gp130 signaling in more severe PH models, we developed Il6 knockout (KO) rats using the CRISPR/Cas9 system and showed that IL-6 deficiency could improve the pathophysiology in hypoxia-, monocrotaline-, and Sugen5416/hypoxia (SuHx)-induced rat PH models. Phosphorylation of STAT3 in CD4+ cells was also observed around the vascular lesions in the lungs of the SuHx rat model, but not in Il6 KO rats. Blockade of IL-6 signaling had an additive effect on conventional PAH therapeutics, such as endothelin receptor antagonist (macitentan) and soluble guanylyl cyclase stimulator (BAY41-2272). These findings suggest that IL-6/gp130 signaling in CD4+ cells plays a critical role in the pathogenesis of PAH.
Pulmonary arterial hypertension (PAH) is characterized by stenosis and occlusions of small pulmonary arteries, leading to elevated pulmonary arterial pressure and right heart failure. The importance of inflammation in the pathogenesis of PAH has been reported (1, 2). Interleukin (IL)-6 is a multifunctional proinflammatory cytokine linked to numerous autoimmune diseases (3, 4). The serum level of IL-6 increased in patients with idiopathic PAH, and the IL-6 level correlated with prognosis (5). In animal studies, it has been reported that transgenic mice overexpressing IL-6 exhibit manifestations of pulmonary hypertension (PH) pathology even under conditions of normoxia and that these manifestations are exacerbated by chronic hypoxia (6). Furthermore, it has been reported that the manifestations of hypoxia-induced pulmonary hypertension (HPH) were improved in IL-6-deficient mice (7).
We reported that inhibiting the IL-6 signal by MR16-1, an anti-mouse IL-6 receptor antibody, resulted in a significant improvement in the HPH phenotype (8). We also reported that the Pristane/Hypoxia model, a mouse PAH model that reflects the pathological features of connected tissue disease-associated PAH, is IL-6 dependent and that the phenotype of this model was ameliorated by MR16-1 (9). In addition, we found an association between the upstream molecules that regulate IL-6 expression and the pathogenesis of PAH. Regnase-1, encoded by the ZC3H12A gene, regulates the expression levels of various inflammatory cytokines, including IL-6, by degrading their mRNAs (10). We have recently reported that ZC3H12A expression in peripheral blood mononuclear cells was decreased in patients with PAH, and its expression is inversely correlated with disease severity (11). Furthermore, mice lacking Regnase-1 in alveolar macrophages spontaneously developed severe PAH without hypoxic exposure (11). These findings strongly indicate the significant role of Regnase-1 in the pathophysiology of PAH. However, the target cells of IL-6 are largely unknown. Two studies have analyzed the significance of IL-6 signaling in smooth muscle cells (SMCs) in the pathogenesis of PAH by deleting the IL-6-specific receptor alpha subunit (IL-6Rα), which triggers the IL-6 signaling in association with the IL-6 signal transduction (IL6ST, also called gp130), specifically in the smooth muscle lineage (12, 13). One study used SM22α-Cre, an SMC-specific Cre mouse model, and reported that IL-6 signaling in SMCs acts as an aggravating factor to promote vascular remodeling in PAH (13). The second study used SMMHC-CreERT2, another SMC-specific Cre mouse model, and concluded that IL-6 signaling in SMCs is protective against Schistosoma- and hypoxia-induced PH (12). This difference may be due to several factors: SM22α-Cre causes a defect in IL-6 signaling via IL-6Ra in SMCs from birth, whereas SMMHC-CreERT2 causes a defect in the IL-6Ra only after tamoxifen administration. The authors also discuss the possibility that the altitude of the experimental facility may have affected the results (12). However, differences in tissue specificity of Cre mice and Cre recombination in nonspecific organs also need to be noted. The SM22α-Cre (14) mouse is one of the SMC-specific Cre transgenic mice that is widely used in the field of vascular biology for vascular SMC-specific gene knockout and reporter purposes. However, a previous report has shown that unintentional Cre recombination also occurs in some myeloid lineage cells in SM22α-Cre mice (15). The mice have been used to study the pathogenesis of various diseases, including atherosclerosis (14), hypertension (16), aneurysms (17), and PH (13, 18).
Here, we investigated the tissue specificity of SM22α-Cre mice and found substantial Cre recombination in all hematopoietic cell lineages from the primitive hematopoietic stem cell level. Based on these results and the expression pattern of gp130, we proposed that IL-6/gp130 signaling in CD4+ T cells was important in the pathogenesis of PAH. To further examine the role of IL-6/gp130 signaling in more severe PH models, we developed Il6 knockout (KO) rats using the CRISPR/Cas9 system and showed that IL-6 deficiency could improve the pathophysiology in several rat PAH models including the Sugen5416/hypoxia model.
Results
Disruption of IL-6 Signaling via gp130 Deletion Using SM22α-Cre Resulted in an Improvement in the Pulmonary Hypertension (PH) Phenotype of an HPH Mouse Model.
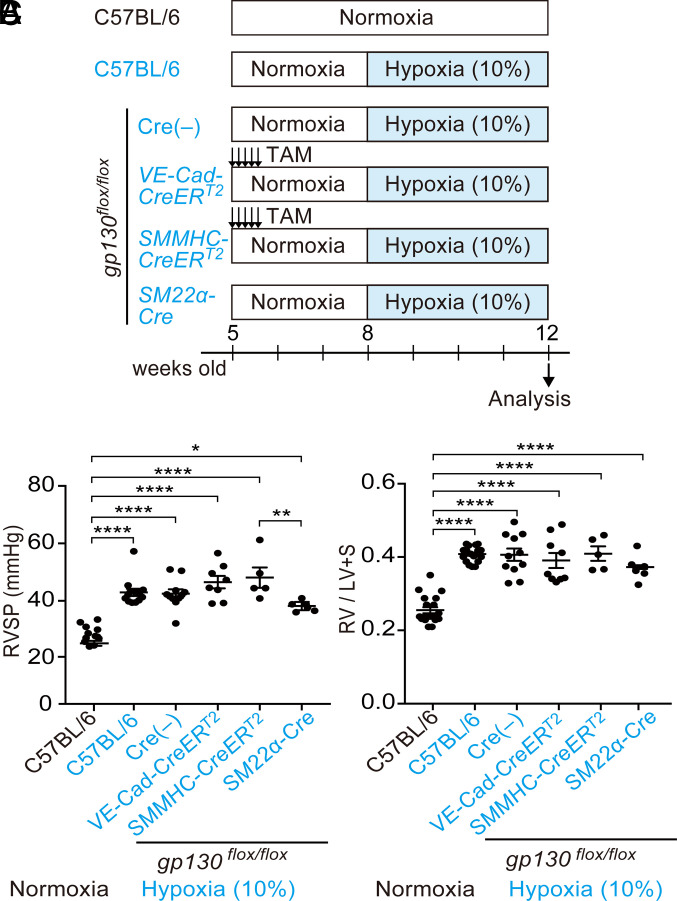
To clarify the effect of tissue-specific gp130 deficiency on the phenotypes of HPH, we crossed gp130flox (19) mice with three tissue-specific Cre mice, VE-Cad-CreERT2 (20) (for endothelial cells), SMMHC-CreERT2 (12, 21), and SM22α-Cre mice (13, 16) (for SMCs), and subjected these mice to 4 wk of hypoxia (Fig. 1A). Tamoxifen was administered intraperitoneally to induce Cre recombination in CreERT2 mice. In wild-type (WT) mice, 4 wk of hypoxia resulted in elevated right ventricular systolic pressure (RVSP) and an increase in Fulton’s index (Fig. 1 B and C). These elevations were also observed in mice with only the floxed gp130 allele and not Cre. Endothelial cell-specific gp130 deficiency induced by VE-Cad-CreERT2 mice did not improve the RVSP or Fulton’s index (Fig. 1 B and C). Two types of SMC-targeted Cre mice showed conflicting results. While deletion of gp130 using SMMHC-CreERT2 mice did not ameliorate the phenotype of HPH, deletion of gp130 using SM22α-Cre mice improved the RVSP (Fig. 1 B and C).
Fig. 1.
Gp130 deletion using SM22α-Cre showed a tendency to improve pulmonary hypertension in the HPH mouse model. (A) Experimental protocols and the groups are shown. Male 8-wk-old mice were exposed to a 10% hypoxic chamber for 4 wk. To induce CreERT2-mediated Cre recombination, 1 mg of tamoxifen was injected intraperitoneally on 5 consecutive days. (B) Right ventricular systolic pressure (RVSP) of each group is shown. (C) Right ventricle/left ventricle + septum (RV/LV+S) weight ratio (Fulton’s index) of each group is shown.
SM22α-Cre Mice Produced an Unexpected Cre Recombination in Hematopoietic Cells.
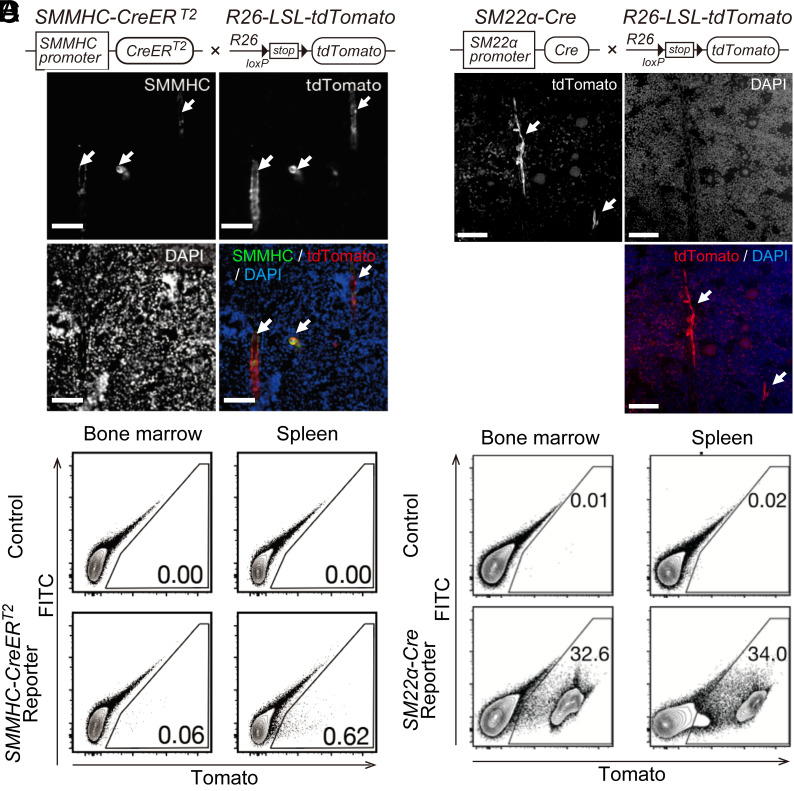
The discrepancy between the results obtained in the two SMC-targeted Cre mice was similar to that in previous reports (12, 13). One previous report showed that unintentional Cre recombination also occurs in some myeloid lineage cells in SM22α-Cre mice (15). We hypothesized that the discrepancy in the results of the two SMC-targeted Cre mice may be due to nonspecific Cre recombination in SM22α-Cre mice. To analyze Cre recombination in the hematopoietic lineage cells of SMMHC-CreERT2 and SM22α-Cre mice in detail, we generated reporter mice by crossing Rosa26-loxP-stop-loxP-tdTomato mice (22), which express tdTomato fluorescence by Cre recombination, with SMMHC-CreERT2 or SM22α-Cre mice (SI Appendix, Fig. S1 A and B). Using these reporter mice, we were able to visualize the Cre recombinant cells with tdTomato fluorescence.
In the bone marrow of SMMHC-CreERT2 reporter mice, Cre recombination (tdTomato-positive cells) was observed in the vascular SMCs surrounding the arteries in the bone marrow (Fig. 2A). Flow cytometry analysis of hematopoietic cells from the bone marrow and spleen of SMMHC-CreERT2 mice showed that Cre recombination did not occur in the hematopoietic cell lineage (Fig. 2B).
Fig. 2.
Unintended Cre recombination in all hematopoietic lineage cells in SM22α-Cre mice. (A) Representative image of immunofluorescence staining of the bone marrow from SMMHC-CreERT2 reporter mice. The arrows indicate smooth muscle cells around arteries in the bone marrow. SMMHC-CreERT2 mice were crossed with Rosa26-loxP-stop-loxP-tdTomato mice. (B) Flow cytometry analysis of hematopoietic tissues from SMMHC-CreERT2 reporter mice. FITC channels were used to remove cells that emit nonspecific autofluorescence. Gates indicate tdTomato-positive cells, and the percentages of cells in each gate are shown in each panel. (C) Representative image of immunofluorescence staining of the bone marrow from SM22α-Cre reporter mice. The arrows indicate smooth muscle cells around arteries in the bone marrow. SM22α-Cre mice were crossed with Rosa26-loxP-stop-loxP-tdTomato mice. (D) Flow cytometry analysis of hematopoietic tissues from SM22α-Cre reporter mice. FITC channels were used to remove cells that emit nonspecific autofluorescence. Gates indicate tdTomato-positive cells, and the percentages of cells in each gate are shown in each panel.
By contrast, SM22α-Cre reporter mice showed different results. As expected, Cre recombination occurred in the SMCs of the gastrointestinal tract and in the vascular SMCs of the aorta (SI Appendix, Fig. S1 C–E). Regarding the bone marrow, Cre recombination did occur in the SMCs surrounding the arteries in the bone marrow. However, tdTomato-positive cells, which experienced Cre recombination, were also widely found in hematopoietic cells around the bone marrow (Fig. 2C). Flow cytometric analysis also confirmed Cre recombination in CD45-positive hematopoietic cells of the bone marrow and spleen (Fig. 2D).
To examine the difference in Cre recombination depending on the hematopoietic cell lineage, the tdTomato positive rates of myeloid cells, T cells, and B cells in peripheral blood from SM22α-Cre reporter mice were analyzed by flow cytometry (SI Appendix, Fig. S2 A and B). Cre recombination was observed in all three lineages, but the ratio of Cre recombination was significantly higher in myeloid cells compared with lymphoid cells (SI Appendix, Fig. S2C). Regarding stem/progenitor cells, Cre recombination was observed at a low rate at the hematopoietic stem cell level, the most undifferentiated hematopoietic cell lineage (SI Appendix, Fig. S2 D and E). As the differentiation of blood cells progressed, an increase in the Cre recombination ratio was observed (SI Appendix, Fig. S2F). Cre recombination was also observed in the myeloid and lymphoid progenitor cells in the bone marrow (SI Appendix, Fig. S2 G–I). These results indicated that SM22α-Cre mice showed nonspecific Cre recombination in blood cells, suggesting that the improvement in HPH phenotype in gp130flox/flox; SM22α-Cre mice might be due to nonspecific gp130 deletion in blood cells.
SM22α-Cre Mice Had Partially Deleted gp130 in CD4+ Cells after Crossing with gp130 flox Mice.
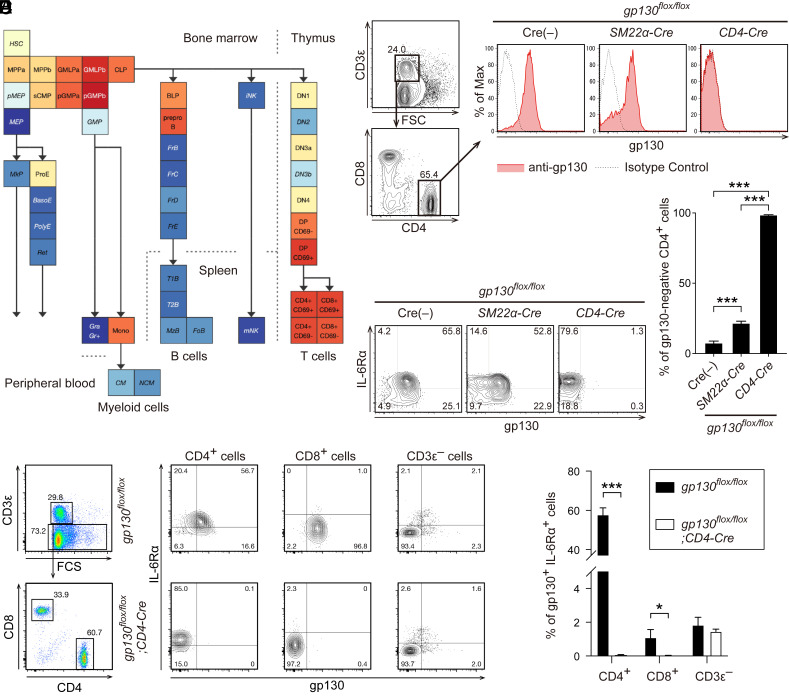
Using publicly available data to examine the expression of gp130 in differentiation stages of blood cells (23), we found that in mature blood cells, gp130 expression is particularly high in T cells (Fig. 3A). Indeed, gp130 was highly expressed on CD3ε+ CD4+ T cells in the spleen of control gp130flox/flox mice (Fig. 3B). Consistent with the reporter mice, gp130flox/flox; SM22α-Cre mice lacked gp130 expression in a subset of CD4+ T cells (Fig. 3 B and C). By contrast, in gp130flox/flox; CD4-Cre mice (24), gp130 expression in CD4+ T cells was almost completely absent, while IL-6Rα expression was retained (Fig. 3 B–D). In control gp130flox/flox mice, 60% of CD4+ T cells expressed both gp130 and IL-6Rα, whereas these double-positive cells were almost absent in gp130flox/flox; CD4-Cre mice (Fig. 3 E and F). CD8+ T cells showed a low abundance of IL-6Rα expression, even in control gp130flox/flox mice. The proportion of double-positive cells did not change in cells, other than CD3ε+ T cells, depending on the presence of CD4-Cre (Fig. 3 E and F).
Fig. 3.
Gp130 expression in CD4+ T cells was partially abolished in gp130flox/flox; SM22α-Cre mice. (A) Mouse gp130 (IL6ST) expression pattern in hematopoietic lineage cells was retrieved using the mouse hematopoiesis model on Gene Expression Commons (23). (B) Representative flow cytometry analyses of gp130 expression patterns on CD3ε+ CD4+ T cells from the spleen of Cre(−) control, gp130flox/flox; SM22α-Cre, and gp130flox/flox; CD4-Cre mice are shown. Solid red lines and dashed black lines represent gp130 and background levels, respectively. (C) The percentages of gp130-negative cells in CD3ε+ CD4+ cells from the spleen of each mouse are shown. (D) Representative flow cytometry analyses of gp130 and IL-6Rα expression patterns on CD3ε+ CD4+ T cells from the spleen of Cre(−) control, gp130flox/flox; SM22α-Cre, and gp130flox/flox; CD4-Cre mice are shown. (E) Representative flow cytometry analyses of gp130 and IL-6Rα expression patterns on CD3ε+ CD4+, CD3ε+ CD8+, and CD3ε− cells from the spleen of Cre(−) control, gp130flox/flox; SM22α-Cre, and gp130flox/flox; CD4-Cre mice are shown. (F) The percentages of gp130+ IL-6Rα+ cells in CD3ε+ CD4+ cells from the spleen of each mouse are shown.
To characterize CD4+ T cells as potential targets of SM22α-Cre, we sorted tdTomato-positive and tdTomato-negative CD4+ T cells derived from the spleens of SM22α-Cre reporter mice and performed RNA-seq analysis (SI Appendix, Fig. S3A). We found only three genes as a significant difference between the two groups (tdTomato+ up gene: 2 genes, tdTomato− up gene: 1 gene), indicating that there is little difference in gene expression between the tdTomato-positive and -negative CD4+ T cells (SI Appendix, Fig. S3B). Consistently, the expression levels of the genes related to IL-6-signaling including Il6st, Il6ra, Stat3, Jak1, Jak2, and Jak3 were almost comparable between the two groups (SI Appendix, Fig. S3C). We next analyzed the phosphorylation of STAT3 in the tdTomato-positive and -negative CD4+ T cells derived from the lungs of SM22α-Cre reporter mice exposed to hypoxia (SI Appendix, Fig. S3D). Hypoxia exposure caused stronger tyrosine-phosphorylation of STAT3 in the tdTomato-positive CD4+ T cells than in the tdTomato-negative CD4+ T cells (SI Appendix, Fig. S3 E and F). Based on these results, we hypothesized that improvement of the HPH phenotype in gp130flox/flox; SM22α-Cre mice was due to unintended gp130 loss in CD4+ T cells and that almost complete loss of gp130 expression in CD4+ T cells in gp130flox/flox; CD4-Cre mice would result in further improvement of the HPH phenotype.
Gp130 Deletion in CD4+ T Cells Ameliorated the PH Phenotype of HPH Mice.
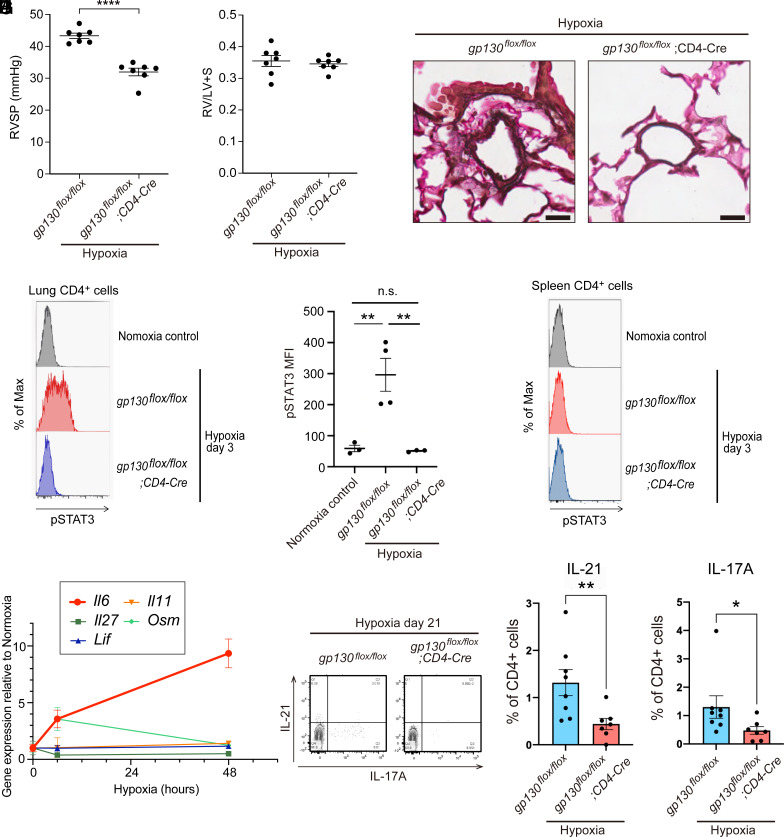
To determine the effect of gp130 deletion in CD4+ T cells on the pathogenesis of PAH, we subjected gp130flox/flox; CD4-Cre mice to 4 wk of hypoxia. Hemodynamic studies revealed that RVSP was significantly reduced in gp130flox/flox; CD4-Cre mice compared with control gp130flox/flox mice (Fig. 4A). Right ventricular hypertrophy was comparable in both groups (Fig. 4B). Histological analysis showed a decrease in medial wall thickness in gp130flox/flox; CD4-Cre mice compared with control gp130flox/flox mice (Fig. 4C). These results indicated that gp130-dependent signaling in CD4+ T cells plays a pivotal role in the pathogenesis of HPH.
Fig. 4.
Gp130 deletion in CD4+ T cells ameliorated the PH phenotype of HPH mice. (A) Right ventricular systolic pressure of each group. (B) Right ventricle/left ventricle + septum (RV/LV+S) weight ratio (Fulton’s index) of each group. (C) Representative images of pulmonary arteries with a diameter ~50 µm stained with Elastica van Gieson. (Scale bar: 20 µm.) (D) Phosphorylation of STAT3 (pSTAT3) in CD4+ cells from the lungs of each mouse at hypoxia day 3 is shown. (E) Median fluorescence intensity (MFI) of pSTAT3 in CD4+ cells from the lungs of each mouse at hypoxia day 3 is shown. (F) Phosphorylation of STAT3 (pSTAT3) in CD4+ cells from the spleen of each mouse at hypoxia day 3 is shown. (G) mRNA expression levels of IL-6 family cytokines in the lungs of wild-type C57BL/6 mice after hypoxic exposure are shown. (H) Representative flow cytometry analysis of IL-17A and IL-21 expression in CD4+ cells in the lungs at hypoxia day 21. (I and J) Percentages of IL-21+ (I) and IL-17A+ (J) cells among the CD4+ cells at hypoxia day 21.
We then examined how the loss of gp130 in CD4+ T cells in the HPH model caused changes in downstream signaling. STAT3 is located downstream of gp130, and when IL-6 binds to the receptor, the JAK-STAT pathway is activated and STAT3 is phosphorylated. We previously reported that Il6 mRNA expression in the lungs peaks on day 2 of hypoxic exposure (8). Therefore, we analyzed the phosphorylation of STAT3 in lung CD4+ cells on day 3 of hypoxic exposure by flow cytometry. Hypoxic exposure caused STAT3 phosphorylation in CD4+ T cells, which was completely suppressed by gp130 deletion in these cells (Fig. 4 D and E). Interestingly, phosphorylation of STAT3 was not observed in CD4+ T cells in the spleen, suggesting that a gp130 ligand produced locally in the lungs was important (Fig. 4F). Because gp130 is a common subunit in IL-6 family cytokines, we examined the mRNA expression of IL-6 family cytokines in the lungs during the acute phase of hypoxic exposure. Family cytokines other than IL-6 showed little increase in expression following hypoxic exposure (Fig. 4G). These results suggested that IL-6 is the cytokine responsible for gp130 signaling in CD4+ T cells on hypoxic exposure. Th17 cells and IL-21-producing CD4+ T cells were increased in the lungs during the acute phase of hypoxic exposure, but deletion of gp130 in CD4+ T cells suppressed this increase (SI Appendix, Fig. S4 A–C). In the chronic phase of hypoxia, the increase in Th17 cells and IL-21-producing T cells was also suppressed by gp130 deletion in CD4+ T cells (Fig. 4 H–J).
Genetic Deletion of Il6 in Rats.
The pathological features observed in severe cases of human PAH do not develop in mouse PH models. To test whether IL-6/gp130 signaling is also important in severe PH models, we generated Il6 KO rats using the CRISPR-Cas9 system with a specific sgRNA-rIl6 and Cas9 mRNA, targeting the second exon of the Il6 gene. In one of the newborn rats, a deletion of 118 bp was found, which originated from an out-of-frame shift in the open reading frame that led to a premature stop codon and the generation of a completely different amino acid (aa) sequence (SI Appendix, Fig. S5A). To confirm IL-6 deficiency in the generated Il6 KO rats, we examined the plasma IL-6 levels after LPS administration. Four hours after intraperitoneal LPS administration, there was a marked increase in plasma IL-6 levels in WT rats, but this increase was abolished in Il6 KO rats (SI Appendix, Fig. S5B). We examined the tyrosine-phosphorylation of STAT3 in the lungs of each rat. Although tyrosine phosphorylation of STAT3 was strongly induced by LPS administration in the lungs of WT rats, it was attenuated in those of Il6 KO rats (SI Appendix, Fig. S5C). Thus, the lack of IL-6 signaling in the created Il6 KO rats was confirmed.
Il6 KO Rats Are Resistant to HPH.
We evaluated the effect of chronic hypoxia on Il6 KO rats (SI Appendix, Fig. S6A). WT rats exposed to hypoxia (10% O2) for 3 wk exhibited significantly increased RVSP, Fulton’s index, and medial wall thickness compared with rats exposed to normoxia. By contrast, the hypoxia-induced elevation of these parameters was inhibited significantly in Il6 KO rats (SI Appendix, Fig. S6 B and C). Similarly, remodeling of the medial vascular walls following hypoxia exposure in WT rats was also inhibited significantly in the lungs of Il6 KO rats (SI Appendix, Fig. S6 D and E). WT and Il6 KO rats showed no difference in physiological parameters under normoxia. We examined IL-6 mRNA levels in the lungs of WT rats after exposure to hypoxia using quantitative RT-PCR (qRT-PCR). Il6 mRNA levels peaked on day 7 after hypoxia exposure and did not return to basal levels even on day 21 (SI Appendix, Fig. S6F). We next examined tyrosine-phosphorylation of STAT3 in the lungs of WT and Il6 KO rats. Although tyrosine-phosphorylation of STAT3 was strongly induced by hypoxic exposure in the lungs of WT rats after 7 d hypoxic exposure, it was reduced in Il6 KO rats (SI Appendix, Fig. S6 G and H). These results suggested that Il6 KO rats are resistant to HPH, similar to the findings in Il6 KO mice (7).
Il6 KO Rats Are Resistant to MCT-Induced PH.
We evaluated the effect of MCT administration on Il6 KO rats (SI Appendix, Fig. S7A). Previous studies reported survival rates of around 30% in rats in the 35 d following MCT administration (25). In the present study, the survival rate in MCT-injected WT rats was 41% at 5 wk. Kaplan–Meier survival curves demonstrated that Il6 deficiency significantly improved the survival rate in MCT rats compared with WT rats (SI Appendix, Fig. S7B). The PH-associated changes in RVSP were significantly ameliorated in MCT-administered Il6 KO rats (SI Appendix, Fig. S7C). There was a trend for decreased right ventricular hypertrophy in Il6 KO rats compared with WT rats but this did not reach significance (SI Appendix, Fig. S7D). The vascular thickening and lumen closure observed in WT rats receiving MCT were mitigated in Il6 KO littermates (SI Appendix, Fig. S7 E and F). Similar to the lungs of WT rats during hypoxia, Il6 mRNA expression was also examined in the lungs of WT rats after MCT administration using qRT-PCR. Il6 mRNA levels continued to increase after MCT administration, rising approximately 20-fold after 4 wk (SI Appendix, Fig. S7G). We also examined the tyrosine-phosphorylation of STAT3 in MCT-treated rat lungs as well as in HPH model WT and Il6 KO rat lungs. Although tyrosine-phosphorylation of STAT3 was strongly induced by 5 wk after MCT administration in WT rat lungs, it was decreased in Il6 KO rat lungs (SI Appendix, Fig. S7 H and I). These results indicated that IL-6 plays an important role in PH pathogenesis in the MCT model as well as in the HPH model.
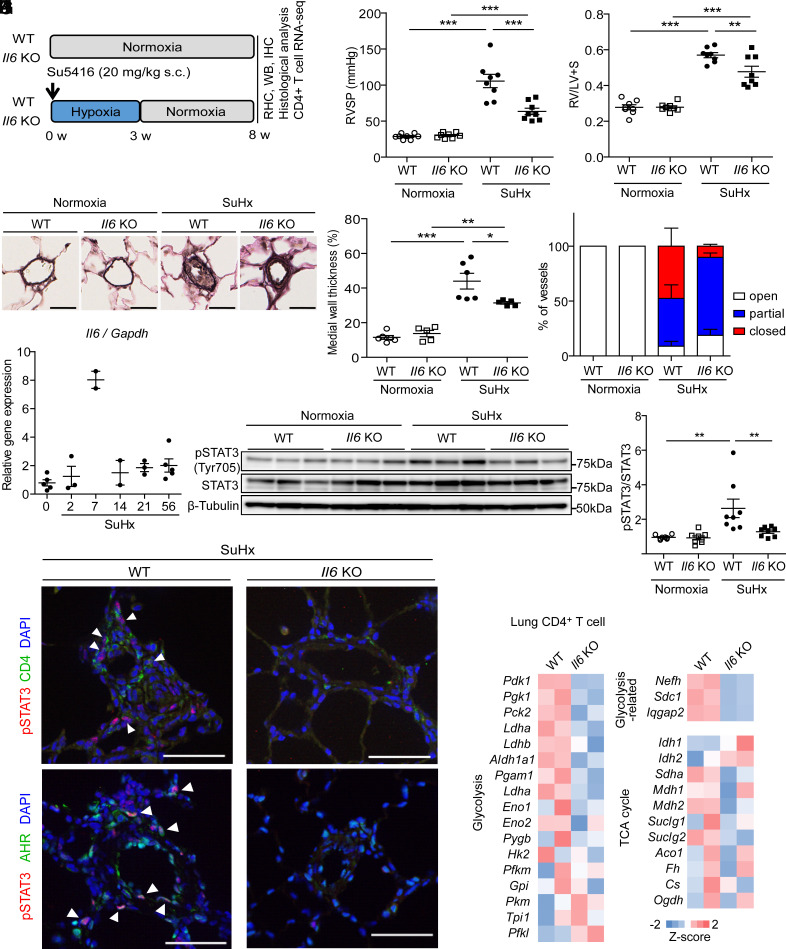
Il6 KO Rats Are Resistant to SuHx-Induced Severe PH.
We next examined the effect of IL-6 deficiency on the PH phenotypes in the SuHx rat PH model (Fig. 5A). As previously reported, WT rats showed a marked increase in RVSP to about 100 mmHg and marked right heart hypertrophy at 8 wk in the SuHx model (Fig. 5 B and C). In pulmonary vascular remodeling, not only medial thickening but also an increase in the number of occluded vessels due to intimal proliferation and plexiform-like lesions were observed (Fig. 5 D–F). In Il6 KO rats, there was a significant decrease in RVSP and right ventricular hypertrophy (Fig. 5 B and C). The medial wall thickness index of intra-acinar arterioles was considerably lower in Il6 KO rats than in WT rats (Fig. 5 D–F). Furthermore, a significant reduction in the percentage of occluded vessels in the pulmonary vessels was observed in Il6 KO rats. Similar to the lungs of WT rats during hypoxia, Il6 mRNA expression was increased after exposure to hypoxia and did not return to basal levels even 8 wk after Su5416 administration (Fig. 5G). We examined tyrosine-phosphorylation of STAT3 in the lungs of SuHx WT and Il6 KO rats as well as in the lungs of HPH and MCT rats and found that tyrosine phosphorylation of STAT3 was significantly induced in the lungs of WT rats at 8 wk in the SuHx model but was suppressed in Il6 KO rats (Fig. 5 H and I). These findings indicated that IL-6 also plays an essential role in the pathogenesis of severe PH.
Fig. 5.
Il6 KO rats are resistant to PH in the SuHx rat model. (A) Experimental protocol for examining the effect of Il6 deletion on SuHx rats. (B and C) Assessment of the effect of Il6 deletion on the PH phenotype of SuHx rats in terms of RVSP (B) and Fulton’s index (C) (n = 8 in each group). (D) Representative images of the vascular remodeling of distal acinar arterioles in lung sections subjected to EVG staining. (Scale bar: 20 μm.) (E) Medial wall thickness index of rats (normoxia WT: n = 6, normoxia Il6 KO: n = 5, SuHx WT: n = 6, SuHx Il6 KO: n = 5). (F) Pulmonary arterial occlusions were graded as open (no luminal occlusion; white), partial (<50% occlusion; blue), or closed (≥50% occlusion; red). Percentages of open, partial, and closed pulmonary arteries of outer diameter (OD) <100 μm in SuHx rats) (n = 4 in each group). (G) qRT-PCR analysis of Il6 mRNA expression in the lungs of WT rats after SuHx treatment. (H and I) Western blot analysis of STAT3 phosphorylation (Tyr705) and total STAT3 in lung homogenates from WT and Il6 KO rats 8 wk after normoxia or SuHx treatment (n = 8 in each group). (J) Representative immunofluorescence images of pulmonary arteries stained for pSTAT3 (red) and CD4 (green), and pSTAT3 (red) and Ahr (green) in the lung tissues of WT and Il6 KO rats 8 wk after SU5416 administration. Arrowheads indicate copositive cells. (K) Z-score of respiration-related genes in CD4+ cells. RHC: right heart catheterization, WB: western blotting, IHC: immunohistochemistry. Values are the means ± SEM. ***P < 0.001, **P < 0.01, and *P < 0.05.
IL-6 Induces Accumulation of STAT3-Activated CD4+ Cells around Pulmonary Vascular Lesions in SuHx Rats.
Next, we performed immunohistological studies to determine the cells involved in IL-6 signaling in the SuHx rat model of severe PH. We coimmunostained SuHx rat lungs harvested at 8 wk with antibodies against phospho-STAT3 (pSTAT3) with αSMA, vWF, CD68, or CD4 (Fig. 5J and SI Appendix, Figs. S8 and S9A). The concomitant expression of pSTAT3 and αSMA, vWF, or CD68 was not detected in the pulmonary arteries of naïve rats (SI Appendix, Fig. S8). In SuHx rats, we found increased perivascular pSTAT3-positive cells in WT rats, but they did not merge with SMA, vWF, or CD68 (SI Appendix, Fig. S8). Further, CD68+ and CD4+ cells were found to be increased in the perivascular lungs of WT rats in the SuHx model, but only some CD4+ cells merged with pSTAT3 (Fig. 5J). By contrast, in Il6 KO rats, few pSTAT3+ cells were observed around pulmonary vessels, and CD68+ cells were also reduced. In particular, CD4+ pSTAT3+ cells were hardly detected around the arteries. We also confirmed that the pSTAT3 and IL-21 copositive cells around the pulmonary vessels that resulted in remodeling in the SuHx model seen in WT rats were rarely seen in Il6 KO rats (SI Appendix, Fig. S8). We previously reported that activation of aryl hydrocarbon receptor (AHR), a nuclear receptor/transcription factor, induces upregulation of inflammatory signals and accumulation of CD4+ IL-21+ T Cells in vascular lesions in the advanced stage of SuHx rats (26). In this study, cells with nuclear copositivity of AHR and pSTAT3 were found to cluster around pulmonary vessels in SuHx rats (Fig. 5J and SI Appendix, Fig. S9B). These results suggested that IL-6 is involved in either proliferation or differentiation of helper T cells during the pathogenesis of PH and that the activation of AHR could trigger a positive feedback loop of Th17 cell differentiation by the IL-6/AHR signaling axis in PH.
IL-6 Induces Immunological Synapse Formation via a Th17 Differentiation Accompanied with Upregulation of Glycolysis in the Lungs of SuHx Rats.
To elucidate the mechanisms underlying the IL-6-dependent development of severe PH phenotypes, including intimal and plexiform-like lesions that are observed in the advanced stage, RNA-seq was performed using the 1- and 8-wk lungs of SuHx rats (SI Appendix, Fig. S10A). We identified 343 and 390 genes that were down-regulated in Il6 KO rats at 1 and 8 wk, respectively, and 81 of these down-regulated genes were commonly detected at both time points (SI Appendix, Fig. S10 B and C). Pathway analysis using these 81 IL-6-dependent genes revealed upregulation of genes involved in immune cell dynamics (i.e., phagosome, antigen processing and presentation, and cell adhesion molecules) (SI Appendix, Fig. S10D). GO enrichment analysis using these 81 IL-6-dependent genes revealed upregulation of genes involved in immune cell accumulation (i.e., immune response, neutrophil chemotaxis, lymphocyte chemotaxis, antigen processing and presentation of peptide antigen via MHC class 1, and immunological synapse formation), indicating that the interaction of antigen-presenting cells such as B cells, macrophages, and dendritic cells with helper T cells occurred in an IL-6-dependent manner in SuHx rat lungs (SI Appendix, Fig. S10E). Because STAT3 activation was observed primarily in CD4+ cells in the pulmonary perivasculature (Fig. 5J), we focused on CD4+ cell-specific genes (SI Appendix, Fig. S10F). Twenty-two IL-6 dependently down-regulated genes were detected in CD4+ cells (SI Appendix, Fig. S10G). Gene ontology (GO) enrichment analysis using the 22 genes down-regulated in CD4+ cells revealed downregulation of several inflammation-associated biological processes (i.e., response to lipopolysaccharide, response to bacterium, and defense response to bacterium) (SI Appendix, Fig. S10H). Furthermore, pathway analysis using these 22 IL-6-dependent genes revealed downregulation of genes involved in inflammatory cell signaling (i.e., IL-17 signaling pathway and cell adhesion molecules) (SI Appendix, Fig. S10I). Because Th17 cells are characterized by distinctive homeostasis involving the upregulation of glycolysis (27–29), we focused on the metabolic genes associated with respiratory functions. Numerous glycolysis-related gene expressions exhibited downregulation in Il6 KO rats, whereas the majority of TCA cycle gene expressions remain unaffected (Fig. 5K). These results suggest that immune cell activation, such as immunological synapse formation between T cells and APCs, is driven by IL-6 signaling-dependent glycolysis in T cells within the pulmonary lesions of severe PH models.
Il6 Deficiency Suppresses Downregulation of BMPR2 Expression and Attenuates Apoptotic Signaling in the SuHx Model Lung.
We next examined the effects of IL-6 deficiency on bone morphogenetic protein receptor 2 (BMPR2) expression levels and apoptotic signaling, which have been shown to be associated with PH pathology. We found reduced levels of BMPR2 in the lung tissue protein lysate of SuHx WT rats at 8 wk (SI Appendix, Fig. S11A). The decrease in BMPR2 was suppressed in Il6 KO rats (SI Appendix, Fig. S11B). In addition, the levels of cleaved caspase-3 and p53 were increased in the lung tissue of SuHx WT rats at week 8, and these increases were suppressed in Il6 KO rats (SI Appendix, Fig. S11 C and D). Taken together, our data indicate that IL-6 deficiency suppresses both BMPR2 reduction and apoptotic responses in SuHx rat lungs.
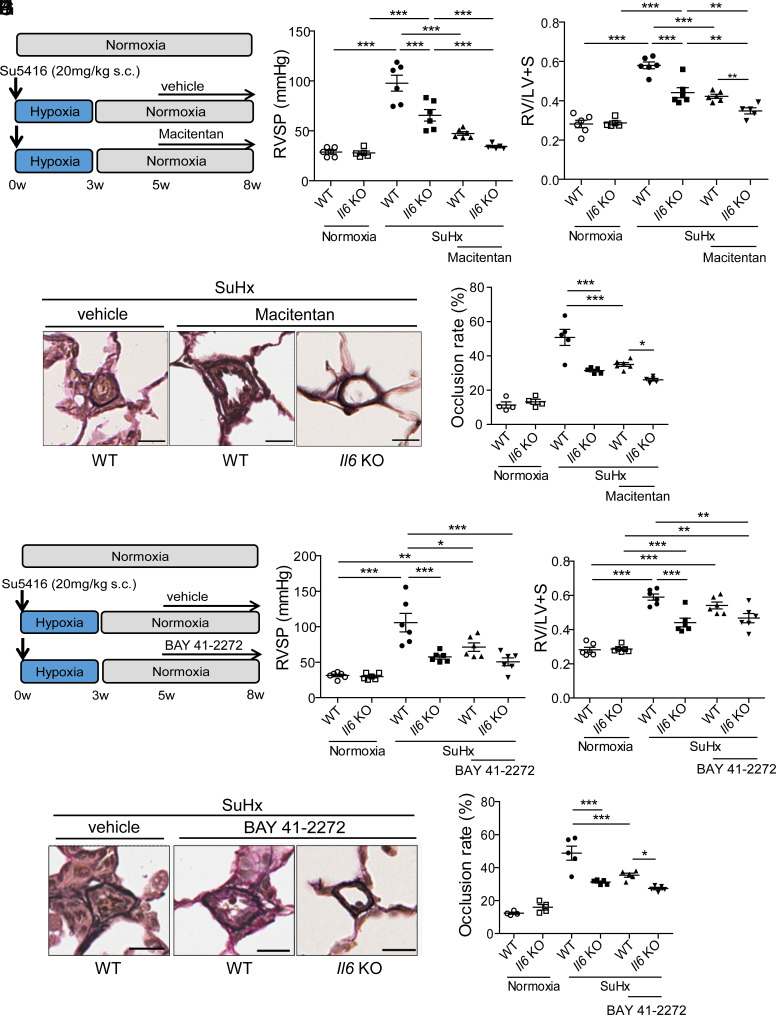
Il6 Deficiency and Standard Vasodilator Treatment Show an Additive Effect on the Pathogenesis of PH.
We examined whether IL-6 blockade has an additive effect on existing therapeutic agents, endothelin receptor antagonist (ERA), macitentan, and soluble guanylyl cyclase (sGC) stimulator, BAY41-2272. Rats were exposed to Su5416 and hypoxia (10%) for 3 wk before returning to room air for 2 wk to allow for the progression of pulmonary vascular remodeling. WT and Il6 KO rats were then randomly divided into three groups to receive a pulverized chow containing macitentan (30 mg/kg/d) or BAY41-2272 (10 mg/kg/d), or a control diet, from week 5 for 3 wk (Fig. 6 A and F). The treatment of WT SuHx rats with macitentan or BAY41-2272 resulted in comparable attenuation of the PH phenotype. RVSP and right ventricular hypertrophy were also significantly reduced in Il6 KO rats given a control diet. There were no significant effects on mean arterial pressure and heart rate indicating specific effects on pulmonary circulation. Interestingly, treatment of Il6 KO rats with macitentan or BAY41-2272 resulted in a further reduction of RVSP and right ventricular hypertrophy compared with the WT rats treated with the respective drugs (Fig. 6 B, C, G, and H). Histological analysis of the lungs suggested that the hemodynamic changes caused by ERA or sGC stimulator treatment with IL-6 deficiency were associated with a reduction in medial wall thickness and the percentage of occluded vessels (Fig. 6 D, E, I, and J). These results suggest that IL-6 inhibition and existing anti-PAH drugs can be expected to have an additive inhibitory effect on the pathogenesis of PAH.
Fig. 6.
Il6 deficiency and standard of care vasodilator therapy combination in rats with SuHx-induced PH. (A) Experimental protocol for disease initiation and the macitentan treatment time course. A macitentan-containing diet (30 mg/kg/d) or a control diet was fed to rats every day. (B and C) Assessment of the effect of Il6 deletion combined with macitentan treatment on the PH phenotype of SuHx rats in terms of RVSP (B) and Fulton’s index (C) (normoxia WT: n = 6, normoxia Il6 KO: n = 5, SuHx WT: n = 6, SuHx Il6 KO: n = 6, SuHx+macitentan WT: n = 6, SuHx+macitentan Il6 KO: n = 5). (D) Representative images of the vascular remodeling of distal acinar arterioles in lung sections subjected to EVG staining. (Scale bar: 20 μm.) (E) Vascular occlusion rate of rats (normoxia WT: n = 4, normoxia Il6 KO: n = 4, SuHx WT: n = 5, SuHx Il6 KO: n = 5, SuHx+macitentan WT: n = 5, SuHx+macitentan Il6 KO: n = 5). (F) Experimental protocol for disease initiation and the macitentan treatment time course. A BAY41-2272-containing diet (10 mg/kg/d) or a control diet was fed to rats every day. (G and H) Assessment of the effect of Il6 deletion combined with BAY41-2272 treatment on the PH phenotype of SuHx rats in terms of RVSP (G) and Fulton’s index (H) (n = 6 in each group). (I) Representative images of the vascular remodeling of distal acinar arterioles in lung sections subjected to EVG staining. (Scale bar: 20 μm.) (J) Vascular occlusion rate of rats (normoxia WT: n = 4, normoxia Il6 KO: n = 4, SuHx WT: n = 5, SuHx Il6 KO: n = 5, SuHx+BAY41-2272 WT: n = 5, SuHx+BAY41-2272 Il6 KO: n = 5). Values are the means ± SEM. ***P < 0.001, **P < 0.01, and *P < 0.05.
Discussion
In the present study, we used mouse and rat PH models to show that IL-6/gp130 signaling is important in the pathogenesis of PH. Phosphorylation of STAT3, a downstream molecule of IL-6/gp130 signaling, was observed in CD4+ T cells in the lungs of both mouse and rat PH models. We also showed that CD4+ T cells are an important target of IL-6 in the pathogenesis of PH in a tissue-specific gp130-deficient HPH mouse model. Deletion of gp130 in CD4+ T cells suppressed phosphorylation of STAT3 and Th17 subsequently increased following hypoxic exposure, contributing to amelioration of the PH phenotype.
A recent clinical study examining the effects of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in patients with group 1 PAH reported that treatment with tocilizumab was feasible but demonstrated no significant effect on hemodynamics (30). Because IL-6 is a multifaceted cytokine, it may have opposite effects on PAH depending on the cell type. Indeed, despite our study showing that blocking IL-6 signaling specifically in CD4+ T cells improved the HPH phenotype in mice, another group reported that disrupting IL-6 signaling in SMCs using SMMHC-CreERT2 mice worsened the phenotype (12). The balance of protective or promotive effects of IL-6 signaling on PH phenotypes by cell type may differ among species or PH models. Cell type-specific inhibition of IL-6 signaling, or inhibition of downstream molecules of IL-6 signaling in CD4+ T cells, may be alternative strategies.
A previous study using SM22α-Cre mice to analyze the effects of tissue-specific IL-6α deficiency reported that smooth muscle is a target for IL-6 in the pathogenesis of PH (13). However, previous reports and our results show that some degree of nonspecific Cre recombination also occurs in hematopoietic cells in SM22α-Cre mice (15), and the results should be interpreted with caution. Although a previous report showed that SM22α-Cre mice showed Cre recombination in only myeloid lineage cells, not in lymphoid lineage cells (15), our data showed that Cre recombination occurred to some extent even in the most undifferentiated hematopoietic stem cell stage, and as differentiation progressed, cells with Cre recombination accumulated. Our study showed that CD4+ cells from the spleen did not show substantial difference in gene expression dependent on the SM22α-Cre-dependent recombination. On the other hand, hypoxia exposure to mice induced significant tyrosine-phosphorylation of STAT3 more strongly in the CD4+ cells positive for SM22α-Cre-dependent recombination than in those negative for SM22α-Cre-dependent recombination. These data suggest that attenuation of the HPH phenotypes observed in the previous study and in our study using SM22α-Cre mice might be attributed to more preferential attenuation of IL-6-dependent signaling in the CD4+ cells (13).
To compensate for the inability to induce severe lung lesions in mouse PH models, we generated Il6 KO rats using the CRISPR/Cas9 system and examined the effects of IL-6 deficiency on conventional animal models of PH, such as the chronic hypoxia-induced PH model, the MCT-induced PH model, and the recently developed SuHx PH rat model, which exhibits plexiform-like lesions similar to those seen in patients with severe PAH. We found that in these three PH models, Il6 KO rats showed a significant improvement in PH pathology. In addition, RNA-seq analysis revealed IL-6-dependent immunological synapse formation in SuHx model lungs. Furthermore, inflammatory cells such as macrophages and helper T cells that congregate around pulmonary blood vessels were rarely observed in Il6 KO rats. In addition, the decreased expression levels of BMPR2 and increased apoptotic signaling associated with PH pathology observed in the lungs of WT rats were suppressed in the lungs of Il6 KO rats. Furthermore, the additive effect of IL-6 deficiency with existing PAH therapies was also revealed. These results suggest that the IL-6 signaling pathway may be a promising therapeutic target in the treatment of mild to severe PH.
Inflammatory processes are thought to be prominent in various types of human PAH and experimental PH and are recognized as major pathogenic components of pulmonary vascular remodeling. Our group and others have shown that IL-6 plays an important role in PH pathogenesis in a murine HPH model. The response to hypoxia varies among animal species, and in particular, it has been reported that the mesangial thickening of pulmonary vessels in the rat HPH model is more severe than that in mice (31). In the present study, we found that IL-6 plays an important role in the pathogenesis of PH in HPH in rats as well as in mice. We have previously shown that Il6 mRNA expression in the lungs of HPH mice peaks on day 2 after exposure to hypoxia and returns to basal levels on day 7. Further, we have shown that IL-6 mRNA expression in the lungs of the rat HPH model peaks on day 7 of hypoxic challenge and does not recover to baseline even after 21 d. These differences in IL-6 kinetics may contribute to the differences between mice and rats in the formation of pulmonary vascular remodeling in the HPH model.
Next, we examined the effects of IL-6 deficiency in another classic PH model, the MCT rat model. We observed a significant improvement in mortality and a significant decrease in RVSP in Il6 KO rats after MCT administration. This and other previous studies have shown that Il6 mRNA increases in a time-dependent manner in the lungs of MCT models (32). Furthermore, STAT3 phosphorylation in the lungs occurs in WT rats. Tamura et al. showed that administration of IL-6R/sIL6R antagonists significantly improved PH pathology in the MCT model (13). These results indicate that IL-6 is also important for the development of PH in the MCT model.
Furthermore, we investigated the effect of IL-6 deficiency in the SuHx model, which exhibits pulmonary vascular lesions similar to those of severe PAH in humans, and found that the PAH pathology in the SuHx model was markedly suppressed in Il6 KO rats. In the SuHx model, the localization of phosphorylated STAT3 in the lungs was examined by immunohistological staining to clarify the target cells of IL-6. The results showed that there was little colocalization of phosphorylated STAT3 in remodeled pulmonary vascular smooth muscle, as previously reported (13), and that it merged into a subset of CD4+ cells. IL-6, in cooperation with TGF-β, is reported to be critical for the differentiation of Th17 cells from naive CD4+ T cells (33–35). We previously showed that Th17 cells (CD4+ IL-21+ IL-17+ T cells) accumulated in the lung vascular lesions of HPH mice (8). In addition, we recently reported that activation of AHR plays an essential role in the pathogenesis of PH in the SuHx rat model (26). IL-6-dependent expression of AHR is crucial for the differentiation of Th17 cells, and AHR agonists promote Th17 cell differentiation induced by a combination of IL-6 and TGF-β (36, 37). In the present study, IL-21+ cells around pulmonary vessels, which resulted in remodeling in the SuHx model in WT rats, were rarely seen in Il6 KO rats. mRNA levels of genes involved in glycolysis, which is enhanced during differentiation of Th17 cells (27–29), were up-regulated in an IL-6-dependent manner in CD4+ cells of the SuHx lungs. Taken together, these results suggest that activation of AHR triggers a positive feedback loop of Th17 cell differentiation via the IL-6/AHR signaling axis in PAH. It is necessary to mention the limitation of the present study. Although we showed STAT3 activation in the CD4+ cells around the occlusive pulmonary arteries of the SuHx rats in immunohistochemistry and IL-6-dependent Th17 cell differentiation through RNA-seq analysis, we could not provide direct evidence for the necessity for CD4+ cells in rat severe PAH models due to lack of Cre-loxP system in rats. Creation of the rat Cre-loxP system will make it possible to demonstrate the indispensability of CD4+ cells downstream of IL-6-signaling in rat PAH models.
IL-6 has been shown to suppress BMPR2, one of the major disease-related genes in hereditary PAH, through overexpression of miR17/92 (38). Consistent with this finding, the decrease in BMPR2 expression in the lungs of the SuHx model was significantly suppressed in Il6 KO rats. Although the underlying mechanisms remain unclear, dysfunctional BMPR2 signaling is known to be involved in sustained inflammation and impaired resolution. Endothelial cells from PAH patients with BMPR2 mutations exhibit enhanced proliferation, altered glucose metabolism, reduced monolayer integrity, and increased susceptibility to apoptosis (39, 40). Consistent with this finding, apoptotic signaling was enhanced in the lungs of WT rats of the SuHx model but was significantly suppressed in the lungs of Il6 KO rats with normalized BMPR2 expression.
In conclusion, our findings indicate that gp130/IL-6 signaling in CD4+ T cells plays an important role in the pathogenesis of PH and suggest that IL-6 blockade concurrent with existing treatments may be effective in patients with PAH.
Materials and Methods
In mice, hypoxia-induced pulmonary hypertension model was evaluated in gp130flox/flox (control) mice (19)and gp130 conditional KO mice using the tissue-specific Cre mouse such as VE-Cad-CreERT2 mice (20), SMMHC-CreERT2 mice (12, 21), SM22α-Cre mice (13, 16) and CD4-Cre mice (24), on a C57BL/6 background. In rats, hypoxia-induced, monocrotaline-induced, and SuHx-induced pulmonary hypertension models were examined using WT and Il6 KO rats generated using the CRISPR-Cas9 system in an SD background. All experiments were carried out under the guidelines of the Animal Ethics Committee of the National Cerebral and Cardiovascular Center Research Institute and were also approved by the Institutional Review Board of the National Cerebral and Cardiovascular Center.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank Yuko Iwai and Nao Araki for secretarial assistance; Manami Nishimura, Saori Mizushima, and Mika Ejiri for technical assistance; and Manami Sone for technical assistance with histological analyses. We also thank Dr. Werner Müller (University of Manchester) for providing gp130flox mice. We also thank Pharma Foods International Co., Ltd. (Kyoto, Japan) for the creation of the Il6 KO rats. We thank Janssen Pharmaceutical K.K., Japan, and Bayer AG for providing macitentan and BAY41-2272, respectively. This work was supported in part by JSPS KAKENHI (Grant Numbers 19K17622 and 21K08070 to T. Ishibashi; 19K08506 and 22K08144 to T. Inagaki; 22H04379 to T.C.-K.; 23K07568 to M.O.; and 16H05298 to Y.N.); by JST, PRESTO Grant Number JPMJPR13M5, Japan, to Y.N.; by the Intramural Research Fund for Cardiovascular Diseases of the National Cerebral and Cardiovascular Center (29-6-5 and 30-6-4 to T. Ishibashi and 30-2-3 to T. Inagaki); by The Cell Science Research Foundation to T. Inagaki; by research grant in pulmonary hypertension from Nippon Shinyaku Ltd. to R.A.; and by the Takeda Science Foundation, SENSHIN Medical Research Foundation, Daiichi Sankyo Foundation of Life Science, Smoking Research Foundation, the Uehara Memorial Foundation, Janssen Pharmaceutical K.K. Contracted Research Grant, Chugai Pharmaceutical Co., Ltd. Contracted Research Grant, and Kishimoto Foundation to Y.N. Y.N. granted from Bayer Yakuhin, Ltd., outside the submitted work.
Author contributions
T. Ishibashi, T. Inagaki, T.K., and Y.N. designed research; T. Ishibashi, T. Inagaki, A.Y., K.O.-O., R.A., T.M., Y. Kotani, X.D., T.C.-K., N.M., and M.S. performed research; T. Ishibashi, T. Inagaki, M.O., A.Y., K.O.-O., T.C.-K., N.M., K.H., Y. Kubota, T.K., and Y.N. analyzed data; and T. Ishibashi, T. Inagaki, and Y.N. wrote the paper.
Competing interests
Y.N. reports consulting fees from Chugai Pharmaceutical Co., Ltd. outside the submitted work; consulting fees from Janssen Pharmaceutical K.K. outside the submitted work.
Footnotes
Reviewers: T.W.M., University of Toronto; and R.M., Yale University.
Contributor Information
Tadamitsu Kishimoto, Email: kishimoto@ifrec.osaka-u.ac.jp.
Yoshikazu Nakaoka, Email: ynakaoka@ncvc.go.jp.
Data, Materials, and Software Availability
RNA-seq data have been deposited at GEO (https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE255994 (41). All other data are included in the manuscript and/or SI Appendix.
Supporting Information
References
- 1.Dorfmuller P., Perros F., Balabanian K., Humbert M., Inflammation in pulmonary arterial hypertension. Eur. Respir. J. 22, 358–363 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Schermuly R. T., Ghofrani H. A., Wilkins M. R., Grimminger F., Mechanisms of disease: Pulmonary arterial hypertension. Nat. Rev. Cardiol. 8, 443–455 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choy E. H., et al. , Translating IL-6 biology into effective treatments. Nat. Rev. Rheumatol. 16, 335–345 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kishimoto T., Interleukin-6: Discovery of a pleiotropic cytokine. Arthritis Res. Ther. 8, S2 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soon E., et al. , Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 122, 920–927 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Steiner M. K., et al. , Interleukin-6 overexpression induces pulmonary hypertension. Circ. Res. 104, 236–244, 228p following 244 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savale L., et al. , Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir. Res. 10, 6 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto-Kataoka T., et al. , Interleukin-6/interleukin-21 signaling axis is critical in the pathogenesis of pulmonary arterial hypertension. Proc. Natl. Acad. Sci. U.S.A. 112, E2677–E2686 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mori H., et al. , Pristane/hypoxia (PriHx) mouse as a novel model of pulmonary hypertension reflecting inflammation and fibrosis. Circ. J. 84, 1163–1172 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Matsushita K., et al. , Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature 458, 1185–1190 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Yaku A., et al. , Regnase-1 prevents pulmonary arterial hypertension through mRNA degradation of interleukin-6 and platelet-derived growth factor in alveolar macrophages. Circulation 146, 1006–1022 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Mickael C., et al. , IL-6Ra in smooth muscle cells protects against schistosoma- and hypoxia-induced pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 61, 123–126 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura Y., et al. , Ectopic upregulation of membrane-bound IL6R drives vascular remodeling in pulmonary arterial hypertension. J. Clin. Invest. 128, 1956–1970 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boucher P., Gotthardt M., Li W. P., Anderson R. G., Herz J., LRP: Role in vascular wall integrity and protection from atherosclerosis. Science 300, 329–332 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Shen Z., et al. , Smooth muscle protein 22 alpha-Cre is expressed in myeloid cells in mice. Biochem. Biophys. Res. Commun. 422, 639–642 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holtwick R., et al. , Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc. Natl. Acad. Sci. U.S.A. 99, 7142–7147 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang J., et al. , Angiotensin-converting enzyme-induced activation of local angiotensin signaling is required for ascending aortic aneurysms in fibulin-4-deficient mice. Sci. Transl. Med. 5, 183ra158, 111–181 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansmann G., et al. , An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J. Clin. Invest. 118, 1846–1857 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Betz U. A., et al. , Postnatally induced inactivation of gp130 in mice results in neurological, cardiac, hematopoietic, immunological, hepatic, and pulmonary defects. J. Exp. Med. 188, 1955–1965 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okabe K., et al. , Neurons limit angiogenesis by titrating VEGF in retina. Cell 159, 584–596 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Wirth A., et al. , G12–G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat. Med. 14, 64–68 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Madisen L., et al. , A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seita J., et al. , Gene expression commons: An open platform for absolute gene expression profiling. PLoS One 7, e40321 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee P. P., et al. , A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15, 763–774 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Itoh T., et al. , C-type natriuretic peptide ameliorates monocrotaline-induced pulmonary hypertension in rats. Am. J. Respir. Crit. Care Med. 170, 1204–1211 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Masaki T., et al. , Aryl hydrocarbon receptor is essential for the pathogenesis of pulmonary arterial hypertension. Proc. Natl. Acad. Sci. U.S.A. 118, e2023899118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerriets V. A., et al. , Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Invest. 125, 194–207 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi L. Z., et al. , HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208, 1367–1376 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Z., et al. , The PRAK-NRF2 axis promotes the differentiation of Th17 cells by mediating the redox homeostasis and glycolysis. Proc. Natl. Acad. Sci. U.S.A. 120, e2212613120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toshner M., et al. , Mendelian randomisation and experimental medicine approaches to interleukin-6 as a drug target in pulmonary arterial hypertension. Eur. Respir. J. 59, 2002463 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoshikawa Y., et al. , Hypoxia induces different genes in the lungs of rats compared with mice. Physiol. Genomics 12, 209–219 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Tang C., et al. , Characteristics of inflammation process in monocrotaline-induced pulmonary arterial hypertension in rats. Biomed. Pharmacother. 133, 111081 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Bettelli E., et al. , Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Mangan P. R., et al. , Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441, 231–234 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Veldhoen M., Hocking R. J., Atkins C. J., Locksley R. M., Stockinger B., TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Kimura A., Naka T., Nohara K., Fujii-Kuriyama Y., Kishimoto T., Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc. Natl. Acad. Sci. U.S.A. 105, 9721–9726 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakahama T., et al. , Aryl hydrocarbon receptor-mediated induction of the microRNA-132/212 cluster promotes interleukin-17-producing T-helper cell differentiation. Proc. Natl. Acad. Sci. U.S.A. 110, 11964–11969 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brock M., et al. , Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ. Res. 104, 1184–1191 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Diebold I., et al. , BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab. 21, 596–608 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long L., et al. , Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat. Med. 21, 777–785 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishibashi T., et al. , Data from “IL-6/gp130 signaling in CD4+ T cells drives the pathogenesis of pulmonary hypertension”. Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE255994. Deposited 16 February 2024. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
RNA-seq data have been deposited at GEO (https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE255994 (41). All other data are included in the manuscript and/or SI Appendix.