Significance
The class of membrane proteins known as secondary-active transporters mediate a wide range of critical cellular processes, including nutrient uptake, transmembrane signaling, and resistance to cytotoxic compounds, like human-made drugs. A detailed understanding of their molecular mechanisms is therefore of interest not only from a fundamental standpoint, but also because it will facilitate the design of inhibitors or stimulators that may be used as therapeutic agents. This study provides a conceptual mechanistic framework, grounded on statistical thermodynamics, that bridges the specific physiological function of these proteins and their molecular structure. While the study is focused on a particular subclass of transporters involved in cardiac physiology and cellular Ca2+ homeostasis, we envisage our conclusions will be broadly applicable.
Abstract
Secondary-active transporters catalyze the movement of myriad substances across all cellular membranes, typically against opposing concentration gradients, and without consuming any ATP. To do so, these proteins employ an intriguing structural mechanism evolved to be activated only upon recognition or release of the transported species. We examine this self-regulated mechanism using a homolog of the cardiac Na+/Ca2+ exchanger as a model system. Using advanced computer simulations, we map out the complete functional cycle of this transporter, including unknown conformations that we validate against existing experimental data. Calculated free-energy landscapes reveal why this transporter functions as an antiporter rather than a symporter, why it specifically exchanges Na+ and Ca2+, and why the stoichiometry of this exchange is exactly 3:1. We also rationalize why the protein does not exchange H+ for either Ca2+ or Na+, despite being able to bind H+ and its high similarity with H+/Ca2+ exchangers. Interestingly, the nature of this transporter is not explained by its primary structural states, known as inward- and outward-open conformations; instead, the defining factor is the feasibility of conformational intermediates between those states, wherein access pathways leading to the substrate binding sites become simultaneously occluded from both sides of the membrane. This analysis offers a physically coherent, broadly transferable route to understand the emergence of function from structure among secondary-active membrane transporters.
Compared with other classes of membrane proteins, secondary-active transporters are arguably the least understood from a mechanistic standpoint, despite their pervasive role in all aspects of human physiology, both in health and disease (1). Like passive-diffusion channels and ATP-driven transporters, these proteins mediate the uptake or efflux of substances across membranes, in a selective manner, and to do so they cycle through a series of distinct structural states. However, unlike channels and primary-active transporters, this conformational cycle is neither initiated nor terminated by extrinsic factors, but rather by the recognition or release of the transported substances themselves, which can be thus thought of as both substrates and agonists. Another distinct characteristic of this class of proteins is their inherent poly-specificity, i.e. their structures have evolved the ability to selectively recognize substrates of different types, either concurrently (symporters) or competitively (antiporters). This feature is key, as it enables these proteins to harness a preexisting transmembrane electrochemical gradient of one substrate, typically H+ or Na+ ions, to energize the accumulation or depletion of another, to a degree that would be unfeasible otherwise. Accordingly, cells employ this class of transporters for uptake of scarce nutrients and efflux of cytotoxic substances that penetrate the cell membrane, among other critical processes.
While much remains to be clarified about the interplay between secondary-active transporters and their substrates, the basic features of their conformational mechanism appear to have been delineated. Extensive biochemical and structural data demonstrate that these proteins undergo a spontaneous reversible transition between two major states, typically referred to as inward- and outward-facing (IF and OF) conformations; as a result, substrate binding sites in the protein interior become sequentially exposed to one or the other side of the membrane, but not both simultaneously (2–5). Meta-analyses of this class of transporters indicate this inherent conformational bistability owes to repeated topological units within their architectures, often inverted relative to the membrane plane (6); differences in the internal structure of these repeats naturally translate into asymmetric conformations for the transporter as a whole.
Despite these insights, fundamental questions about the nature of this “alternating-access” mechanism remain to be answered. For example, the factors that control the probability of the interconversion between OF and IF states differ from protein to protein and are not at all self-evident from analysis of structures alone. For symporters, this interconversion occurs only when all substrates are simultaneously bound to the protein, or when all binding sites are simultaneously empty. Antiporters, by contrast, do not undergo this transition when no substrates are bound; instead, antiporters require that a substrate be recognized, but only of one type at a time. Furthermore, for both symporters and antiporters it is increasingly apparent that the transported substrate is not necessarily the substance that binds with the most specificity or potency; indeed, known inhibitors very often occupy the same binding sites as the biological substrates, and yet they somehow cause the alternating-access mechanism to stall.
Evidently, the interconversion between OF and IF states is not abrupt; it entails a series of necessary intermediate conformations, whose nature and significance are also very much unclear. These intermediates have been rarely captured by conventional structural biology methods, indicating they are less energetically favorable than the OF and IF states, probably because they require the abovementioned structural repeats to adopt a similar arrangement, i.e. to form a quasi-symmetric conformation. At any rate, a logical expectation is that these intermediates will completely occlude access to the substrate binding sites in the protein interior, from either side of the membrane. The opposite scenario, i.e. simultaneous access to these sites from both sides, seems highly improbable as it would cause the dissipation of the electrochemical gradients that power active transport, as in a passive-diffusion membrane channel. An intriguing possibility, therefore, is that the mechanism by which substrates or inhibitors control the alternating-access transition somehow involves these occluded transient states that to date have been difficult to examine experimentally.
In this work we sought to obtain answers to these outstanding mechanistic questions, using as a model system a prokaryotic antiporter of Na+ and Ca2+, referred to as NCX_Mj (7–10). This transporter is a close homolog of NCX1, the human Na+/Ca2+ exchanger with a central role in the initiation and regulation of the heartbeat (11, 12). The primary function of NCX antiporters is to rapidly extrude cytosolic Ca2+, even when the concentration of free Ca2+ in the extracellular space exceeds that inside the cell. To minimize the backflow of Ca2+ into the cell in such conditions, this class of antiporters have coevolved the ability to independently recognize and transport Na+ ions, which are more abundant in the extracellular space than in the cytosol. Thus, in typical physiological conditions, the transporter captures cytosolic Ca2+ in the inward-facing state, delivers it extracellularly upon spontaneously transitioning to the outward-facing state, then loads Na+, and once again spontaneously transitions to the inward-facing state, where Na+ is released. (All these reactions are reversible, and some physiological conditions entail Ca2+ reuptake and Na+ efflux instead.)
As is the case for many other transporters, however, the causality behind these observed processes is not understood at the molecular level. For example, it is unknown why the conversion between outward and inward-facing states requires that either Na+ or Ca2+ be bound; other monovalent and divalent ions bind to the transporter, such as H+, Li+, Cd2+ or Mn2+ and yet they are not transported, but rather inhibit function (7, 8, 10, 13, 14). It is also unclear why the alternating-access mechanism stalls when the protein is unliganded—which, as mentioned, differentiates antiporters from symporters. But perhaps the most intriguing question is why the stoichiometry of the antiport reaction mediated by both NCX1 and NCX_Mj is exactly 3 Na+ to 1 Ca2+ (10). That is, among the variety of partial or mixed ion occupancy states that necessarily exist, at least transiently, it appears that only those with either 3 Na+ or 1 Ca+ bound permit the transporter to spontaneously alternate between OF and IF conformations. It is worth noting that the exchange stoichiometry is a key physiological quantity, as it determines the thermodynamic limit for uphill Ca2+ efflux, for a given Na+ electrochemical gradient; for example, a 10-fold Na+ concentration gradient (larger outside) would allow NCX to deplete the cytosolic concentration of Ca2+ down to a value 1,000 times smaller than that in the extracellular space. In other words, the exchange stoichiometry determines the intensity of the Ca2+ signals transduced by the transporter. To elucidate the molecular basis for such an important functional characteristic thus seems particularly worthwhile.
To clarify these rather fundamental questions, we have carried out a systematic single-molecule study of the structure and dynamics of NCX_Mj using advanced all-atom molecular-dynamics simulations; the trajectory time accumulated in this study, which exceeds 100 µs, illustrates the magnitude of the computational effort invested. Specifically, based on the only known structure of NCX_Mj, namely that of the OF state, we used enhanced-sampling simulation methodologies to map out the conformational free-energy landscape of the protein in a multidimensional space, and to examine how that landscape is reshaped by different ion occupancies including the apo state. These calculations enabled us to identify the structure of the IF conformation, as well as those of the most probable intermediates in the alternating-access mechanism, none of which had been known. Crucially, the calculated landscapes clearly explain why only the biological substrates catalyze this mechanism, but only for a unique stoichiometry. In summary, this study provides a conceptual mechanistic framework that bridges the specific physiological function of a paradigmatic human antiporter with its molecular structure and dynamics; we anticipate that this conceptualization will be readily transferable to other secondary-active transporters.
Results
Asymmetry in Topological Repeats Explains Why the Known Structure of NCX_Mj Is Outward-Facing.
All available evidence indicates that the known structures of NCX_Mj, obtained through X-ray diffraction, capture the architecture of the core ion-transporting domain of the proteins in the Ca2+-cation antiporter superfamily (TCDB database entry 2.A.19). These transporters are ubiquitous among eukaryotic and prokaryotic organisms, functioning primarily as either Na+/Ca2+ or H+/Ca2+ exchangers, in some instances modulated by other ions. This core domain contains a total of 10 transmembrane spans, all helical (Fig. 1). Closer examination reveals this assembly consists of two intertwined units of five helices each, whose transmembrane topology is inverted. The first repeat, TM1 to TM5, is fused to the second, TM6 to TM10, by a long, seemingly flexible linker. The amino-acid sequence identity between these two repeats is relatively low, however; in NCX_Mj, for example, it is only 32% (SI Appendix, Fig. S1). Not surprisingly, therefore, the two repeats tend not to adopt exactly the same structure. Specifically, while helices TM3-TM5 and TM8-TM10 are organized almost identically, the configurations of TM1-TM2 and TM6-TM7 relative to the rest of each repeat are very different (Fig. 1). This difference translates into a noticeably asymmetry in the structure of the complete assembly. This asymmetry is very consequential; it opens up a pathway from the exterior of the protein into the sites where Na+, Ca2+, and other ions bind, deep in the interior. In the known structures of NCX_Mj, this access pathway is on the extracellular side of the protein; hence these structures are believed to capture the outward-facing state.
Fig. 1.
Topological repeats within the architecture of NCX_Mj, the Na+/Ca2+ exchanger from M. jannaschii. (A) Crystal structure of NCX_Mj in the outward-facing state (7). The structure contains two inverted topological repeats of five helices each, namely TM1 to TM5 and TM6 to TM10, shown in orange and marine, respectively. The protein is bound to three Na+ ions, shown in green. (B and C) Overlays of the internal structures of the two repeats reveals they are in part similar, but clearly distinct. The angle formed between TM2a and TM2b differs from that observed between TM7a and TM7b; the relative position of TM2a and TM1 also differs from that between TM7a and TM6. Taken together, these two differences explain why the structure shown in (A) features an access pathway into the Na+ binding site from the extracellular side. (D) A hypothetical structural model wherein the repeats swap conformations (see SI Appendix for more details), with no further adjustment, shows an intracellular access pathway analogous to that in panel (A).
An interesting exercise that further illustrates the mechanistic significance of this structural asymmetry is to generate a model of the transporter wherein the two repeats swap conformations (15). That is, in this repeat-swapped model, TM1-TM5 are arranged as observed for TM6-TM10 in the experimental structure of NCX_Mj, while TM6-TM10 are arranged as TM1-TM5 (see SI Appendix for further details). Consistent with the logic outlined above, the resulting model features an access pathway into the ion binding sites on the intracellular side of the protein (Fig. 1). Needless to say, this simple model is unlikely to represent the actual structure of the inward-facing state, which to our knowledge has not been reported to date for this protein. However, it provides insights into the nature of the alternating-access mechanism, and a guide for a more rigorous characterization, as discussed in the following sections.
Calculated Free-Energy Landscapes Reveal Inward-Facing Structures Bound to Either Na+ or Ca2+.
To identify, or at least predict, the actual structure of the IF state of NCX_Mj, we employed advanced molecular dynamics (MD) simulations. Specifically, we used an adaptive enhanced-sampling methodology known as a bias-exchange Metadynamics (16, 17). This approach is in our experience more capable than alternative MD simulation methodologies, for two important reasons: First, it permits a wide exploration of conformational space, even when this space is defined in multiple dimensions; and second, it permits a straightforward derivation of the free-energy landscape underlying that multidimensional space (as well as the error of the calculations) (9, 18, 19). In other words, as the simulation gradually reveals unknown conformational states, it is possible to use the principles of statistical thermodynamics to evaluate whether or not they are likely to be mechanistically significant. The simulations presented in this study used as the only input the X-ray structures of NCX_Mj bound to either 3 Na+ ions or 1 Ca2+ ion (7–9) (SI Appendix, Fig. S2); however, selected features of the abovementioned repeat-swapped model (Fig. 1D) guided some aspects of the calculation design (see Methods and SI Appendix, Fig. S3 and Table S1 for further details). As is common, a hydrated phospholipid bilayer was constructed around the protein to mimic in vitro experimental conditions (10). The simulations represented NCX_Mj, its ligands, and environment in atomic detail (SI Appendix, Fig. S4A). The trajectory time accumulated in these simulations was 62 µs.
The free-energy landscapes resulting from these calculations are shown in Fig. 2A. To facilitate the presentation of our results, the landscapes are projected onto a two-dimensional space, wherein one coordinate quantifies the degree of opening of the extracellular access pathway to the ion binding sites, while the other does the same for the intracellular pathway. For both Na+ and Ca2+, these maps reveal two distinct free-energy minima, i.e. two most probable states, separated by a less favorable region. Reassuringly, one of these minima encompasses the experimental structures of the outward-facing state of NCX_Mj bound to either 3 Na+ ions or 1 Ca2+ ion (7, 9). (Note no aspect of the calculation design dictates this result.) In this state, there are numerous contacts between helices TM1-2 and TM7-8 on the intracellular side of the protein, which close off the ion binding sites to the interior, while few contacts exist between TM2-3 and TM6-7 on the opposite side, which translate into an open exterior pathway (Fig. 2B). In the second free-energy minimum, these contact patterns are reversed almost exactly, and so the exterior pathway closes while an analogous interior pathway opens (Fig. 2B); that is, the calculated landscapes reveal the inward-facing structures of NCX_Mj loaded with either 3 Na+ or 1 Ca2+, which as mentioned had not been previously determined.
Fig. 2.
Free-energy landscapes underlying the alternating-access mechanism of NCX_Mj. (A) Landscapes are shown for the transporter loaded with 3 Na+ ions (Left) or with 1 Ca2+ ion (Right). For clarity, the maps represent the conformational free energy as a function of the degree of opening or closing of intra- and extracellular access pathways into the ion binding site. To objectively quantify accessibility, we use the number of contacts between two sets of protein residues lining those pathways. On the extracellular side, these contacts are between TM6 or TM7 and TM2 or TM3; on the intracellular side, between TM1 or TM2 and TM7 or TM8. Note that these selections are topologically symmetric with respect to the membrane plane. The two free-energy minima featured in each map correspond to the outward and inward-facing states (OF and IF) of the transporter. Red circles mark the positions in these maps of the outward-facing crystal structures of NCX_Mj obtained at high Na+ (Left) or high Ca2+ (Right) concentrations (PDB entries 3V5U/5HXE and 5HXR, respectively), the repeat-swap model shown in Fig. 1D, and the recently determined cryo-EM structure of NCX1 (20). Contours are shown in intervals of 1 kcal/mol. Error estimates for each map are provided in SI Appendix, Fig. S4B. (B) Water density maps for each of the free-energy minima revealed in the maps in panel (A) are overlaid onto representative configurations. For clarity only water molecules within 12 Å of the ion binding sites are mapped. Note OF and IF states have opposing water accessibility patterns, though in all cases the binding sites are occluded from the solvent. (C) Close-up of the ion binding sites, highlighting the mode of ion coordination in each case. Note the binding site geometries for OF and IF states are nearly identical.
Interestingly, the calculated free-energy maps clearly show that conformations wherein the binding sites are simultaneously exposed to both sides of the membrane (i.e. few or no protein–protein contacts on both sides of the structure) are energetically forbidden, consistent with the alternating-access model of active transport. Instead, the conversion between OF and IF entails a series of states wherein the number of protein–protein contacts closing off the intracellular and extracellular pathways is gradually reduced, but not to the extent required to make the binding sites accessible from either side (Fig. 2 A and B). The nature of this conformational change will be discussed in more detail in the next sections. It is also worth noting that the detailed geometry of the ion binding sites is nearly identical when OF and IF states are compared, for both Na+ and Ca2+ (Fig. 2C). Consistent with these observations, conventional MD simulations initiated with configurations extracted from each of the free-energy minima revealed in the calculated landscapes showed minimal structural drift in the microsecond timescale; conversely, a conventional simulation of the repeat-swapped model shown above (Fig. 1D) showed a clear drift toward the minimum identified in the free-energy calculations (SI Appendix, Fig. S5).
Predicted IF State Is Consistent with HDX-MS Data and the Recently Reported Structure of NCX1.
To validate or refute our prediction for the IF state of NCX_Mj, we first turned to a series of biochemical and biophysical experiments previously reported by Khananshvili and coworkers (21–23). These experiments compared two forms of NCX_Mj: a WT-like construct, which structural studies had shown to strongly favor the OF state when solubilized in detergent (7, 9); and a functional mutant known as 5L6-8 (due to an elongation of the TM5-TM6 loop), which transport assays had indicated also populates the IF state (21). Consistent with those studies, Khananshvili and coworkers observed that cysteines engineered in positions G42 and G201, in the intracellular and extracellular sides of the protein, respectively, showed opposing reactivity patterns to TMRM maleimide probes introduced on either side of the membrane (23). As shown in Fig. 3B, these observations are in line with our prediction; G42 is maximally exposed to the solvent in the predicted IF state while G201 is buried within the protein, while the opposite is true for the OF state. Interestingly, the conformational intermediates show a reduced but equivalent accessibility for both positions, as might be expected for quasi-symmetric states.
Fig. 3.
Validation of the predicted IF structure of NCX_Mj against existing experimental data. (A) Graphical representation of published HDX-MS data (23) for two forms of NCX_Mj (WT and 5L6-8) with distinct propensities to adopt the OF of IF state (see text). Differences in measured deuteration levels for these two forms (after 15 s and for saturating [Na+] conditions) are indicated with a color scale, for the regions of the protein that were examined experimentally (TM2, TM7, and TM8). Data on overlapping protein fragments has been broken down into smaller regions by linear combination of the deuterated fractions. Residues G42 and G201, whose accessibility was probed through complementary labeling assays, are also highlighted. For reference, the figure also shows density isosurfaces for water molecules within 12 Å of the protein binding sites (gray mesh), based on our simulation data. (B) Solvent accessibility of G42 and G201 in either the OF or IF states identified in the free-energy maps in Fig. 2, as well as in the intermediate regions. This accessibility is quantified by the number of number water molecules found, on average, within 4.2 Å of either residue. (C) For both WT and 5L6-8, the degree of deuteration measured for a collection of protein fragments in TM2, TM7 and TM8 (after 15 s and for saturating [Na+]) is contrasted with deuteration levels calculated for an ensemble of OF and IF structures extracted from the free-energy basins in Fig. 2 (SI Appendix). Calculated and experimental data are compared when OF and IF are equally weighted, and for alternative weights that result in optimal agreement with measured data, for either WT or 5L6-8 (SI Appendix, Fig. S6). These population shifts are consistent with the known conformational propensities of these forms. (D) Comparison between the IF state of NCX_Mj identified in the free-energy landscapes shown in Fig. 2 with a recently reported cryo-EM structure of human NCX1 (20), coincidentally captured in the IF state. The RMS difference between the Cα traces is 1.9 Å.
Khananshvili and coworkers also compared the degree of hydrogen–deuterium exchange for WT and 5L6-8 NCX_Mj in various conditions and for multiple D2O exposure times (22, 23). The rate of this exchange is a complementary metric of solvent exposure (among other factors). The data obtained for saturating Na+ concentrations is particularly relatable to our simulation conditions, in that the binding sites within the transporter ought to be fully (and solely) occupied by Na+; likewise, only exposure times shorter than 15 s seem pertinent, as beyond that point local protein unfolding likely becomes increasingly dominant. To evaluate whether or not our simulation results are consistent with these data, we employed a variation of an analysis methodology we recently reported, named HDXer, based on the maximum-entropy principle (24) (see Methods and SI Appendix for further details). In a nutshell, this methodology considers an ensemble of protein conformations that includes two or more distinct populations (obtained through MD simulations or any other modeling method) and determines what population shifts would be required for that ensemble to optimally match an input HDX dataset. (Note that for this optimization to result in a good match or plausible population shifts, much of the underlying sample must be compatible with the experimental data.) Following this approach, we extracted an ensemble of OF and IF conformations from the free-energy minima identified in the calculations reported in Fig. 2A, populating each state equally. We then used the new version of HDXer to calculate the deuteration rates expected for the set of protein fragments examined experimentally, and to gradually reweight the initial populations of OF and IF states so as to obtain maximal agreement with experiment. As shown in Fig. 3C (and SI Appendix, Fig. S6), we found that plausible population shifts resulted in excellent agreement with the experimental data. Specifically, in order to reproduce the HDX dataset for the WT protein, the population of the OF state has to be upshifted, in line with the fact this form of the protein favors this particular state in experimental conditions. By contrast, reproducing the HDX dataset for the 5L6-8 mutant requires a depletion of the OF state and a larger population of the IF state, which is again in line with the experimental characterization of this mutant. In summary, while recognizing that HDX measurements offer limited structural resolution, we believe this objective analysis of the experimental data lends confidence to our prediction of the IF state of NCX_Mj.
As this manuscript was in its final stages of preparation, Jiang and coworkers reported the first known structure of NCX1, the human homolog of NCX_Mj found in cardiac muscle (20). As for other membrane proteins, complexation with an antibody fragment appears to have been crucial for this breakthrough and coincidentally, this approach captured the transporter in what appears to be the IF state. As demonstrated in Fig. 3D, our prediction for the IF of NCX_Mj is highly consistent with the NCX1 structure. Notwithstanding the low sequence identity between these proteins (~20%), it is evident from an overlay of the two structures that they represent the same functional state (RMS difference of 1.9 Å based on aligned Cα atoms). IF structures have also been reported for several H+/Ca2+ exchangers (25–27). Those structures are also comparable though logically not identical to that predicted here for NCX_Mj; for example, for CAX_Af (~20% sequence identity), the RMS difference between the structures is only 2.1 Å.
Alternating-Access Transition Entails a Distinct Structural Mechanism.
Having validated our prediction for the IF state of NCX_Mj, we proceed to analyze in more detail the structural and energetic features of the mechanism of alternating access in NCX_Mj. An overlay of representative OF and IF structures underscores the significance of the structural asymmetry between the two topological repeats within the protein (Fig. 4 A and B); only the elements that distinguish these repeats in the experimental structure of the OF state, i.e. TM1, TM2, TM6, TM7, change upon transition to the IF state, while the rest of the protein remains largely unchanged. The resulting mechanism is one wherein TM1 to TM6 travels across the membrane midplane by about 1 nm, accompanied by more subtle changes in the intracellular and extracellular portions of TM2 and TM7; by contrast, the geometry of the ion binding sites and their position relative to the membrane are unaltered (Fig. 4 A and B). To our knowledge, this mechanism is unlike those previously described for other active transporters, though there are some commonalities with the so-called rocker-switch model (2–5).
Fig. 4.
Mechanism of alternating-access in NCX_Mj inferred from analysis of simulation data. (A and B) Comparison between the OF and IF states, using representative configurations of the free-energy minima in Fig. 2, for the Ca2+- and Na+-bound transporter, respectively. Note the displacement of the TM1 to TM6 unit across the membrane midplane, alongside localized changes in the intracellular and extracellular halves of TM2 (TM2ab) and TM7 (TM7ab), respectively. Close-ups of the ion binding sites are also shown, for the OF and IF states as well as for a doubly occluded intermediate (see below). (C and D) Change in free-energy along the minimum free-energy (most probable) multidimensional path connecting the OF and IF states, for the Ca2+- and Na+-bound transporter, respectively. The profiles reveal a metastable intermediate about halfway through the transition. These profiles were calculated in a four-dimensional space (Methods); additional dimensions did not significantly alter their features. (E and F) OF, intermediate and IF states are compared side by side, highlighting water molecules in proximity to the ion-binding sites, filling access pathways into the protein interior from either side of the membrane. Note the displacement of TM1-TM6 in panels (A and B) in fact entails two distinct movements in different directions, as indicated.
To adequately characterize this mechanism, however, we must go beyond this comparison and examine the intermediates of the transition. To do so, we reanalyzed our simulation data to identify the minimum free-energy pathway connecting the OF and IF states, i.e. the most probable trajectory followed by the transporter as it undergoes this conformational change (note this pathway is multidimensional and thus not discernible in the 2D projection used in Fig. 2; see Methods for further details). Key features of this structural mechanism are shown in Fig. 4 C–F (Movies S1 and S2, and SI Appendix, Figs. S7–S9). Starting from the OF state, the first stage of the transition entails displacement of TM1-TM6 toward the extracellular space and a change of TM2a from a sustained π-helical configuration to a more labile state (including α-helical, π-helical, and partially unstructured conformations); the change in TM2a requires disruption of a hydrogen-bond between G42 and A47 and displacement of F39 from a protected hydrophobic pocket. These changes permit water to begin to penetrate the structure from the intracellular side, while the access pathway on extracellular side begins to be depleted. However, water exposure of hydrophobic intracellular residues and disruption of several salt bridges on that side of the protein (R223-E28, R223-D21) lead to a sharp increase in free energy, peaking at around 5 kcal/mol. Soon thereafter, however, the protein settles down into a metastable state through a reconfiguration of sidechains and the surrounding solvent; at this point, TM1 and TM6 have moved toward the extracellular side by about ~8 Å, relative to the OF state (Fig. 4 E and F), and the ion binding sites are simultaneously occluded from both sides of the membrane. The next step entails crossing the highest free-energy barrier of the transition, which is ~7.5 kcal/mol when Ca+ is bound state and ~6.5 kcal/mol when 3 Na+ ions are carried instead. In this step, TM1 and TM6 continue to move, but this motion is now parallel to the membrane plane and requires reconfiguration of protein–protein and protein–lipid interactions involving bulky hydrophobic residues on both sides of the protein (e.g. F23 and F182). A second intermediate forms thereafter, and at this point TM1 and TM6 are essentially in their final position. However, complete progression toward the IF state requires a rearrangement of TM7a that mirrors the changes seen earlier in TM2a, but in reverse; i.e., TM7a becomes π-helical, as a hydrogen-bond between G201-A206 form and F202 becomes enclosed in a hydrophobic pocket. A substantial reduction in free energy is then achieved through dehydration of the extracellular access pathway and the formation of several salt bridges (K198-E257, K187-D197) on that side of the protein.
It is apparent from this analysis that the conformational change underlying the alternating mechanism of NCX_Mj would be very poorly described by a linear geometric interpolation between the OF and IF states. Instead, this mechanism entails a complex choreography of spontaneous progressive rearrangements, both in the protein and its local environment, which ultimately translate into larger-scale changes in tertiary structure. Interestingly, as the protein changes, we observe no significant effects on membrane shape or thickness (SI Appendix, Fig. S10), despite the motion of TM1 and TM6 across the lipid-bilayer midplane and the opening and closing of water channels near lipid–solvent interface; this observation is in line with the fact that the turnover rate of NCX_Mj is rather insensitive to the lipid composition of the membrane (28). In this regard, therefore, NCX_Mj is unlike other transporters that deform the membrane as a result of large domain motions perpendicularly to the bilayer plane (29), probably because the TM1-TM6 unit is comparatively much smaller.
As mentioned, the binding site region also retains the same structure throughout the transition (Fig. 4 A and B); we can thus infer that protein–substrate interactions, once fully engaged, are no longer a determining factor in the energetics of the transition between the OF and IF states. What other interactions might be more impactful? To examine this question, we recalculated the free-energy differences between the OF state and either the IF or the intermediate states, by resampling and reweighting our simulation data with modified versions of the original forcefield wherein the atomic charges of a selected number of residues are slightly scaled down (SI Appendix, Fig. S9). The rationale behind this analysis is that this modification of the energy function impacts both protein–protein and protein–solvent interactions, thus informing on the global electrostatic contribution of the selected residues. First, we considered a selection that includes all the polar residues near the ion-binding sites (those highlighted in Fig. 2C) as well as the ions themselves. Consistent with the inference made above, we found that scaling down the magnitude of these interactions had no significant effect on the free-energy difference between the OF and IF state, or that between the OF and intermediate states, relative to the original calculation with an intact forcefield. By contrast, perturbation of more peripheral polar interactions had a pronounced effect; relative to the OF state, the free energy of the intermediate is significantly diminished when salt bridges and other residues engaged in protein–protein or protein-water hydrogen-bonds are weakened. Interestingly the free-energy difference between the OF and IF states are also affected. These results underscore the mechanistic significance of these peripheral regions, often overlooked because they are less conserved.
Indeed, it is worth noting that, as is often observed for other transport families, NCX_Mj is markedly slower than its eukaryotic homologs, like cardiac NCX1 (30–32). The reasons are unclear, but we believe that the methodology deployed in this study, when applied to NCX1, will reveal the origins of this important difference. Distinct protein–protein and protein–solvent interaction patterns in peripheral regions of the protein are in our view the most likely explanation, rather than distinct protein-substrate interactions.
Functional Specificity Is Also Encoded in the Free-Energy Landscape of the Transporter.
Three characteristics summarize the functional specificity of a secondary-active transporter. First, what is its mode of transport? That is, does the protein function as an antiporter, a symporter, or a uniporter? Second, what are substrates that are translocated across the membrane, among all possible substances that might transiently bind to the protein? And third, what is the precise stoichiometry of the transport cycle? To anticipate or rationalize the answer to these questions on the basis of molecular structure is one of the central, largely unresolved problems in this field of research. For many transporters, these functional characteristics had been established through biochemical assays well before their molecular structure became known, and so the nature of this structure–function relationship, when examined post hoc, might appear straightforward. Even then, however, it can be argued that to convincingly deduce this relationship is not at all trivial; this difficulty is illustrated by many other cases wherein a transporter structure has been determined, typically that of an obscure prokaryotic homolog, before comprehensive biochemical assays exist. In those cases, to anticipate the functional specificity of the transporter is clearly not possible solely based on inspection of molecular structures. We reasoned, however, that analysis of conformational landscapes calculated for different occupancy states might provide answers to the questions outlined above, in the case of NCX_Mj.
Following that logic, we first tried to understand why NCX_Mj does not function as a Na+ or Ca2+ uniporter (or symporter), i.e., why translocation of one of these types of ions is necessarily coupled to the other. A uniporter mode, however, would require that the transporter undergo the alternating-access transition when neither Na+ or Ca2+ are bound (similarly for a symporter). To examine whether that is the case, we removed these ions from the OF and IF structures revealed by our free-energy calculations, and recalculated the free-energy landscape of the transporter in this apo state, using the same methodology used previously. As shown in Fig. 5A, this new landscape is radically different from that observed in the presence of 3 Na+ or 1 Ca2+. While the landscape still features an OF and an IF state, it is effectively impossible for the transporter to interconvert between those two states. Furthermore, the OF and IF states are distinctly unlike those observed when 3 Na+ or 1 Ca2+ are bound. In the absence of these ions, the binding site region is widely open to either the extracellular or intracellular solution (Fig. 5 B and C); indeed, comparison of the maps shown in Figs. 2 and 5 makes it clear that the occluded OF and IF states that are favored when 3 Na+ or 1 Ca2+ occupy the protein, and which precede the alternating-access transition, are energetically unattainable in this apo state, seemingly because the set of charged and polar sidechains that would be otherwise involved in ion coordination (e.g. E54 and E213) cannot reconfigure so as to eliminate unpaired groups, making dehydration of this region inviable. Hence, that NCX_Mj does not function as a uniporter ultimately owes to the unfeasibility to this occlusion process.
Fig. 5.
Free-energy landscapes dictate the functional specificity of NCX_Mj. (A) Free-energy landscapes analogous to those shown in Fig. 2, but for different ion-occupancy states, namely with no ions bound, with 2 H+ bound to E54 and E213, and with 2 Na+ bound only. Red circles mark the positions in these maps of the outward-facing crystal structures of NCX_Mj obtained at low pH and at low and high [Na+] (PDB entries 5HXH, 5HWY and 5HXE, respectively), and of the recently determined cryo-EM structure of NCX1 (20). The landscapes show the alternating-access transition is energetically unfeasible, and that the transporters are trapped either in OF or IF conformations that are distinctly more open to the solvent that those favored when either 3 Na+ or 1 Ca2+ ion are bound; Insets show an overlay of these different conformations, for both the OF (Left) and IF (Right) states. Note that we did not quantitate the precise magnitude of the free-energy barrier between the OF and IF states, as it appears to be in the order of tens of kcal/mol. The free-energy maps for OF and IF states are shown on the same scale starting at a zero value solely for conciseness. The actual value of the relative free-energy between OF and IF states is unknown; this quantity is however irrelevant, as these states are not in equilibrium with each other in these particular ion-occupancy configurations. Error estimates for each map are provided in SI Appendix, Fig. S4C. (B) Water density maps for each of the free-energy minima revealed in the maps in panel (A) are overlaid onto representative configurations. For clarity only water molecules within 12 Å of the ion binding sites are mapped. Note OF and IF states have opposing water accessibility patterns, and that in all cases the ion binding sites are readily exposed to the solvent, but only on one side of the membrane. (C) Close-up of the ion binding sites, highlighting the configuration of the ion coordination shell in each case.
As mentioned, the family of calcium/cation exchangers that includes all NCX proteins also includes a wide array of H+/Ca2+ exchangers, whose structures are highly similar (in the core transport domain). NCX_Mj, however, does not exchange Ca2+ for H+, nor does it exchange Na+ for H+ either (10). The reason is not that H+ do not bind to NCX_Mj; indeed, in conditions where the concentration of H+ is comparable to that of Na+ or Ca2+ (i.e., low pH), NCX_Mj binds H+, through protonation of E54 and E213, and becomes inhibited (8). A free-energy landscape calculated for this condition explains why this is the case. As noted for the apo state, the landscape demonstrates that occlusion of the binding sites in either the OF or the IF states is effectively unfeasible, and by extension, so is the alternating-access transition (Fig. 5A). That occlusion is so energetically unfavored despite the neutralization of E54 and E213 underscores that dehydration of the binding site region is unlikely without a well-defined interaction network wherein all polar groups are paired (Fig. 5 B and C); the net charge of the binding site is clearly of little or no consequence, and is thus not a good predictor of the viability of the alternating-access transition.
Last, we examined why the ion-exchange stoichiometry of NCX_Mj (and by extension its close homologs like cardiac NCX1) is precisely 3 Na+ for 1 Ca2+ (10), which as mentioned dictates the maximum concentration gradient against which the transporter will continue to drive Ca2+ efflux, for a given inward Na+ gradient (also known as sodium-motive-force). In particular, we examined why the nearest possibility, namely 2 Na+ for 1 Ca2+, is not viable (10). To do so, we removed one of the 3 Na+ ions from the OF and IF occluded structures (keeping in each case the two ions that are deeper into the binding site), and recalculated the free-energy landscape of the transporter. Like for the apo transporter and the H+-bound state, the landscape features clearly defined OF and IF states, but also demonstrates the interconversion between these states is effectively not possible (Fig. 5A). Interestingly, though, with 2 Na+ ions bound the binding site favors a configuration that is partially occluded, i.e. while the empty binding site remains exposed to the solvent, the self-organization of the other two sites occupied by Na+ appears to greatly facilitate their dehydration (Fig. 5 B and C). Nevertheless, it seems clear from our data that full occlusion, and therefore the initiation of the alternating-access transition, requires recognition of the third Na+ ion.
In summary, this analysis demonstrates that the functional specificity of a transporter can be in fact predicted or rationalized from structure alone, with limited a priori input from biochemical data. It is however important to recognize that purely visual or heuristic analyses of individual structures are insufficient to bridge the gap between structure and function, as are cursory simulation studies based on anecdotal data. By contrast, we posit that conformational free-energy landscapes, calculated with carefully designed advanced simulation methods, are a highly promising route.
Discussion and Conclusions
Overwhelming evidence indicates that the alternating-access model is the most plausible framework with which to rationalize secondary-active membrane transport at the molecular level. It is underappreciated, however, that this model rests on two seemingly paradoxical principles, which deserve further scrutiny. First, the interconversion between OF and IF conformations is spontaneous and stochastic, and perfectly reversible. Second, despite this inherent plasticity, this interconversion occurs with measurable likelihood only for two concrete substrate-occupancy states among all those that are possible, irrespective of whether these states are transient or long-lasting. Indeed, the nature of these two specific occupancy states defines whether the protein functions as a symporter, an antiporter, or a uniporter; it also defines how many and what type of substrates are actually translocated across the membrane, which in turns determines the maximum transport capacity of a given transporter population, and the energy source that sustains their biological activity.
It also underappreciated that while transmembrane voltages or electrochemical gradients can greatly influence the substrate turnover rate of a given active-transport reaction, no extrinsic forces are in fact required for a transporter to interconvert between the OF and IF states. Experiments wherein a population of transporters exchanges radioactive and nonradioactive substrates across the membrane in a zero-voltage, zero-gradient condition (i.e., a transport cycle lacking a net directionality) demonstrate this fact, for both symporters (33) and antiporters (10). Likewise, while binding-affinity differentials between the OF and IF states might have evolved in certain physiological settings to accelerate the transport rate, they do not drive the alternating-access transition, nor are they a requirement for active transport in general. Transporters for which the OF and IF conformations are perfectly identical, such as SMR antiporters (34), are perhaps the clearest example, but nearly identical binding affinities (actual, not apparent) in the OF and IF states have been reported for nonsymmetric transporters too (35).
In this study, we show that the theory of conformational free-energy landscapes provides a means to rationalize these fundamental principles and empirical observations at the single-molecule level. Using the Na+/Ca2+ exchanger NCX_Mj as a model system (Fig. 6), we show that the OF and IF conformations are indeed the two most prominent states in the free-energy landscape of transporter, and show these states exist because each of two topological repeats within the protein architecture tend to adopt two distinct, alternative arrangements. The calculated landscapes also indicate that in most conditions the free-energy barrier between the OF and IF states is too high to be traversed in a timescale that is physiologically or experimentally relevant. For example, when no ligands are bound to the transporter or when the transporter is loaded with H+, the transporter is arrested in either the OF or IF state, but will not interconvert between those states. By contrast, recognition of either 1 Ca2+ ion or 3 Na+ ions radically reshapes the free-energy landscape of the protein; a series of conformational intermediates between the OF and IF states become energetically attainable, which facilitate a gradual interconversion between those states simply through thermal fluctuations of the molecular system.
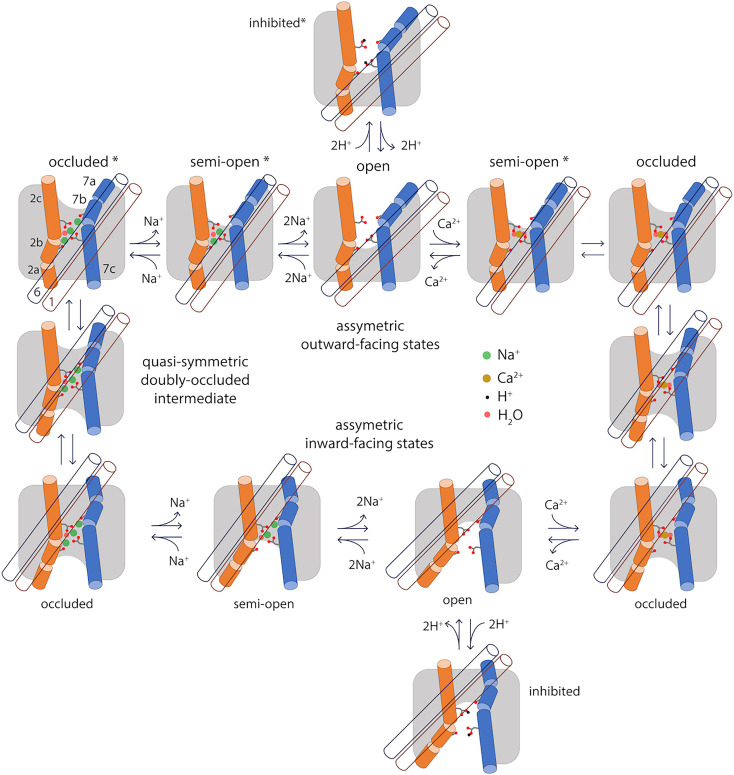
Fig. 6.
Specificity in the mechanism of alternating-access of the Na+/Ca2+ exchanger. This study demonstrates that the functionality of NCX_Mj, namely antiport of 3 Na+ and 1 Ca+, owes to the fact that the alternating-access transition is not viable for any other substrate-occupancy states, including the apo protein. The scheme highlights the essence of the conformational changes that the transporter undergoes when transitioning between the OF and IF states, namely a sliding motion of TM1 and TM6 (blue and brown empty cylinders, respectively) across the membrane mid-plane, and changes in secondary structure in TM2 and TM7 (orange and marine full cylinders, respectively). Also highlighted are the side chains of E54 (on TM2c) and E213 (on TM7c) and the carbonyl groups of A47 (on TM2b) and A206 (on TM7b), which coordinate Na+, Ca2+ and H+. The scheme shows only the states and connectivity deduced from the calculated free-energy landscapes in Figs. 2 and 5A; additional open or semiopen states with only 1 Na+ or 1 H+ bound, or a combination of Na+, Ca2+ and H+, are also conceivable. Asterisks mark the states that have been also determined by X-ray crystallography.
In this view, it becomes clear why the alternating-access mechanism is a spontaneous, reversible process that requires no extrinsic driving force, and yet one that is tightly controlled. In a hypothetical condition where free Ca2+ is similarly available across the membrane, but Na+ is much more abundant extracellularly, a population of NCX_Mj transporters will gradually deplete the cytosol from Ca2+, i.e., generate an outward electrochemical gradient of Ca2+ that ultimately balances that of Na+. However, this Na+ gradient does not cause or drive the protein toward one or other conformational state; it merely introduces a statistical bias or preferred directionality in the alternating-access cycle. That is, most (but not all) of the transitions from the OF to the IF state will occur with 3 Na+ ions bound, and many (but not all) of the transitions from the IF to the OF state will instead occur with 1 Ca2+ bound. Consistent with this notion, in the absence of Ca2+ NCX_Mj will not catalyze a net uptake of Na+, even under a strongly favoring electrochemical gradient; instead, the transporter will repeatedly interconvert between OF and IF states, but only with 3 Na+ ions bound, but not when empty, and therefore the Na+ concentrations at either side of the membrane will remain unchanged. Similarly, even in pH conditions such that H+ outcompete Na+, an inward gradient of H+ will not result in Ca2+ efflux despite the many structural similarities between Na+/Ca2+ and H+/Ca2+ exchangers, as the binding of H+ in fact precludes the transition between OF and IF states. All these functional characteristics are encoded in the free-energy landscape of the protein, or more precisely in the way this landscape is reshaped by one or other ligand, or the lack thereof.
But why does this landscape change? Our simulation data demonstrates that the viability of the alternating-access transition, for a certain binding-site occupancy state, hinges on the energetics of a relatively modest structural change, wherein the network of amino-acids that define the ion-binding sites, as well as the ions themselves, become completely occluded from hydration at either side of the membrane. This open-to-occluded transition, either in the OF or IF states, is only feasible when either 1 Ca2+ or 3 Na+ ions occupy these sites (alongside several structural water molecules), but not for partial Na+ occupancies, or when H+ replace Na+ or with no ions bound. It is likely that other divalent cations that bind to NCX_Mj with significant affinity but are not transported, such as Cd2+ and Mn2+, do not permit occlusion either, though other explanations are possible (7, 13, 36). At any rate, we posit that the feasibility of this open-to-occluded transition explains the functional specificity of a transporter at the molecular level. This is, in our view, a fundamental insight that is likely to be universally valid. Indeed, distinct conformational changes leading to binding-site occlusion appear to have been experimentally captured for other secondary-active transporters, such as Mhp1 (37) vSGLT (38, 39), LeuT (40–43), LacY (44, 45) and GltPh (46), though the actual mechanistic significance of each of these structures requires further verification, case by case. To do so, we believe it is imperative to examine these and other transporters through the lens of free-energy landscapes, even though this kind of calculation is technically very challenging and time consuming. A comprehensive analysis of a symporter would be of particular interest; calculated free-energy landscapes should show occlusion is viable only when the transporter is loaded with all of its substrates, or completely empty. For the latter state, we anticipate the data will show occlusion is feasible because the network of amino-acids involved in substrate recognition can adopt an alternate configuration that maximizes the number of protein–protein interactions, so as to counter the energetic cost of dehydration.
Last, it is worth noting that our results confirm that the mechanism of alternating access in the superfamily of cation/Ca2+ transporters entails a structural change unlike that observed for other families. This mechanism involves a sliding motion of a two-helix unit across the lipid-bilayer midplane, namely TM1 and TM6, over a distance of about 1 nm. Hinge-like motions within helices TM2 and TM7 facilitate this sliding mechanism, while the remainder of the structure (TM3 to TM6, TM8 to TM10) is essentially unchanged (Fig. 6). Importantly, the configuration of the ion binding sites is also largely unchanged as the transporter switches between outward- and inward-occluded conformations; thus, no channel-like states are ever formed that could allow passive diffusion of the bound ions across the membrane. During the transition between OF and IF conformations, complementary sets of hydrophobic, hydrophilic, and lipid-mediated interactions alternatively form and disrupt at either side of the membrane (SI Appendix, Figs. S7–S9). Many of these residues have been highlighted by previous mutational analyses for their impact on protein function (T57, F202, G76, D197) or ion recognition (G42, G201, V205, G231, G235) (21, 47). Interestingly, many of these residues are also conserved when the two internal topological repeats are compared (SI Appendix, Fig. S1), further linking this architectural feature to the alternating-access mechanism. This gradual interconversion between comparable interaction patterns translates into a series of modest free-energy barriers, roughly consistent with measured turnover rate for comparable protein constructs (21). Mammalian orthologs such as cardiac NCX1 are over 1,000 faster than NCX_Mj (30–32), but based on our results, as well as other available data (28), it is unlikely that this acceleration stems from differences in the vicinity of the ion-binding sites; in our view, less conserved regions of the transmembrane domain are the most plausible explanation. Now that the molecular structure of cardiac NCX1 has become available, it will be possible to examine the functional coupling between regulatory and transport domains, through free-energy landscape calculations analogous to those reported here.
Methods
Molecular Dynamics Simulations.
Conventional and enhanced-sampling MD simulations were carried out using GROMACS2018 or GROMACS 4.5.5 with PLUMED (48–51), at constant temperature (298 K), pressure (1 bar) and periodic-boundary conditions. The simulation systems (52) comprise the protein (with or without ions bound) embedded in a POPC lipid bilayer (of 208 molecules), ~15,000 water molecules and Cl− counterions, for ~80,000 atoms in total (SI Appendix, Fig. S4A). The systems were set up using GRIFFIN (53). All ionizable residues in the protein were set to their default state at neutral pH, except D240, which is constitutively protonated (8, 9). To limit the number of possible protein conformations to be sampled and facilitate convergence of the free-energy calculations, we truncated the unstructured intracellular loop between residues A148 and N157, which is not required for substrate recognition or transport function (21). All simulations used the standard CHARMM36m (54) and CHARMM36 (55) force fields for protein and lipids respectively, except for the Lennard-Jones Rmin parameters for the interactions between carboxylate oxygens and Na+ or Ca2+, which had been previously optimized against experimental data (8, 9). All the enhanced-sampling simulations were carried out using the bias-exchange Metadynamics method (BE-META) (16). Further details are provided in SI Appendix.
Evaluation of Simulation Results by Comparison with Experimental HDX Data.
A methodology was developed to contrast the conformational ensembles obtained with our MD simulations with experimental measurements of hydrogen–deuterium exchange (HDX) carried out for WT NCX_Mj as well as the loop-elongation mutant 5L6-8 (52). This methodology builds upon a theoretical framework reported in a previous study (24) and is generally applicable to cases where multiple constructs and conformational states are evaluated; it explicitly accounts for the unknown degree of deuterium loss after quenching of the HDX reaction. Further details are provided in SI Appendix.
Supplementary Material
Appendix 01 (PDF)
Alternating-access mechanism of NCX_Mj bound to 3 Na+ ions, as predicted by enhanced-sampling all-atom MD simulations and minimum free-energy path calculations. Helices TM1 and TM6 are highlighted in purple, and water molecules in the proximity of the ion binding sites are shown as red spheres; the bound Na+ ions are shown in green. All other elements of the simulation system are omitted for clarity.
Alternating-access mechanism of NCX_Mj bound to 1 Ca2+ ions, as predicted by enhanced-sampling all-atom MD simulations and minimum free-energy path calculations. Helices TM1 and TM6 are highlighted in purple, and water molecules in the proximity of the ion binding sites are shown as red spheres; the bound Ca2+ ion is shown in cyan. All other elements of the simulation system are omitted for clarity.
Acknowledgments
We are thankful to Dr. Emel Ficici for constructing a repeat-swap model of NCX_Mj; to Prof. Daniel Khananshvili and coworkers for providing their published HDX-MS data; and to Dr. Lucy Forrest and Dr. Vanessa Leone for their feedback throughout this study and their comments on this manuscript. This research was funded by the Division of Intramural Research of the National Heart, Lung and Blood Institute (NHLBI), NIH (USA). Most of the computational resources were provided by the NIH high-performance computing facility Biowulf.
Author contributions
F.M. and J.D.F.-G. designed research; F.M. performed research; F.M. analyzed data; and F.M. and J.D.F.-G. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. J.C. is a guest editor invited by the Editorial Board.
Contributor Information
Fabrizio Marinelli, Email: fabrizio.marinelli@nih.gov.
José D. Faraldo-Gómez, Email: jose.faraldo@nih.gov.
Data, Materials, and Software Availability
A comprehensive package of input and output files is available at https://github.com/Faraldo-Gomez-Lab-at-NIH/Download (52). Some study data available (Simulation data amounts to tens of terabytes; this data will be shared with researchers upon request).
Supporting Information
References
- 1.Hediger M. A., Clemencon B., Burrier R. E., Bruford E. A., The ABCs of membrane transporters in health and disease (SLC series): Introduction. Mol. Aspects Med. 34, 95–107 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jardetzky O., Simple allosteric model for membrane pumps. Nature 211, 969–970 (1966). [DOI] [PubMed] [Google Scholar]
- 3.Forrest L. R., Kramer R., Ziegler C., The structural basis of secondary active transport mechanisms. Biochim. Biophys. Acta 1807, 167–188 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Drew D., Boudker O., Shared molecular mechanisms of membrane transporters. Annu. Rev. Biochem. 85, 543–572 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Beckstein O., Naughton F., General principles of secondary active transporter function. Biophys. Rev. 3, 011307 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forrest L. R., Structural symmetry in membrane proteins. Annu. Rev. Biophys. 44, 311–337 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao J., et al. , Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger. Science 335, 686–690 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Marinelli F., et al. , Sodium recognition by the Na+/Ca2+ exchanger in the outward-facing conformation. Proc. Natl. Acad. Sci. U.S.A. 111, E5354–5362 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao J., et al. , Mechanism of extracellular ion exchange and binding-site occlusion in a sodium/calcium exchanger. Nat. Struct. Mol. Biol. 23, 590–599 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shlosman I., Marinelli F., Faraldo-Gomez J. D., Mindell J. A., The prokaryotic Na(+)/Ca(2+) exchanger NCX_Mj transports Na(+) and Ca(2+) in a 3:1 stoichiometry J. Gen. Physiol. 150, 51–65 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaustein M. P., Lederer W. J., Sodium/calcium exchange: Its physiological implications. Physiol. Rev. 79, 763–854 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Kang T. M., Hilgemann D. W., Multiple transport modes of the cardiac Na+/Ca2+ exchanger Nature 427, 544–548 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Barthmes M., Liao J., Jiang Y., Bruggemann A., Wahl-Schott C., Electrophysiological characterization of the archaeal transporter NCX_Mj using solid supported membrane technology. J. Gen. Physiol. 147, 485–496 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giladi M., et al. , Exploring the Li(+) transporting mutant of NCX_Mj for assigning ion binding sites of mitochondrial NCLX. Cell Calcium 107, 102651 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forrest L. R., et al. , Mechanism for alternating access in neurotransmitter transporters. Proc. Natl. Acad. Sci. U.S.A. 105, 10338–10343 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piana S., Laio A., A bias-exchange approach to protein folding. J. Phys. Chem. B. 111, 4553–4559 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Marinelli F., Faraldo-Gomez J. D., Force-correction analysis method for derivation of multidimensional free-energy landscapes from adaptively biased replica simulations. J. Chem. Theory Comput. 17, 6775–6788 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Marinelli F., et al. , Evidence for an allosteric mechanism of substrate release from membrane-transporter accessory binding proteins. Proc. Natl. Acad. Sci. U.S.A. 108, E1285–E1292 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corbi-Verge C., et al. , Two-state dynamics of the SH3-SH2 tandem of Abl kinase and the allosteric role of the N-cap. Proc. Natl. Acad. Sci. U.S.A. 110, E3372–E3380 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue J., et al. , Structural mechanisms of the human cardiac sodium-calcium exchanger NCX1. Nat. Commun. 14, 6181 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almagor L., et al. , Functional asymmetry of bidirectional Ca2+-movements in an archaeal sodium-calcium exchanger (NCX_Mj). Cell Calcium 56, 276–284 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Giladi M., et al. , Asymmetric preorganization of inverted pair residues in the sodium-calcium exchanger. Sci. Rep. 6, 20753 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giladi M., et al. , Dynamic distinctions in the Na(+)/Ca(2+) exchanger adopting the inward- and outward-facing conformational states. J. Biol. Chem. 292, 12311–12323 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradshaw R. T., Marinelli F., Faraldo-Gomez J. D., Forrest L. R., Interpretation of HDX data by maximum-entropy reweighting of simulated structural ensembles. Biophys. J. 118, 1649–1664 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishizawa T., et al. , Structural basis for the counter-transport mechanism of a H+/Ca2+ exchanger. Science 341, 168–172 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Wu M., et al. , Crystal structure of Ca2+/H+ antiporter protein YfkE reveals the mechanisms of Ca2+ efflux and its pH regulation. Proc. Natl. Acad. Sci. U.S.A. 110, 11367–11372 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waight A. B., et al. , Structural basis for alternating access of a eukaryotic calcium/proton exchanger. Nature 499, 107–110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Refaeli B., Hiller R., Khananshvili D., Characteristic attributes limiting the transport rates in NCX orthologs. Biochim. Biophys. Acta Biomembr. 1864, 183792 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Zhou W., et al. , Large-scale state-dependent membrane remodeling by a transporter protein. Elife 8, e50576 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niggli E., Lederer W. J., Molecular operations of the sodium-calcium exchanger revealed by conformation currents. Nature 349, 621–624 (1991). [DOI] [PubMed] [Google Scholar]
- 31.Hilgemann D. W., Nicoll D. A., Philipson K. D., Charge movement during Na+ translocation by native and cloned cardiac Na+/Ca2+ exchanger. Nature 352, 715–718 (1991). [DOI] [PubMed] [Google Scholar]
- 32.Baazov D., Wang X., Khananshvili D., Time-resolved monitoring of electrogenic Na+-Ca2+ exchange in the isolated cardiac sarcolemma vesicles by using a rapid-response fluorescent probe. Biochemistry 38, 1435–1445 (1999). [DOI] [PubMed] [Google Scholar]
- 33.Kaback H. R., A chemiosmotic mechanism of symport. Proc. Natl. Acad. Sci. U.S.A. 112, 1259–1264 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burata O. E., Yeh T. J., Macdonald C. B., Stockbridge R. B., Still rocking in the structural era: A molecular overview of the small multidrug resistance (SMR) transporter family. J. Biol. Chem. 298, 102482 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reyes N., Oh S., Boudker O., Binding thermodynamics of a glutamate transporter homolog. Nat. Struct. Mol. Biol. 20, 634–640 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trosper T. L., Philipson K. D., Effects of divalent and trivalent cations on Na+-Ca2+ exchange in cardiac sarcolemmal vesicles Biochim. Biophys. Acta 731, 63–68 (1983). [DOI] [PubMed] [Google Scholar]
- 37.Weyand S., et al. , Structure and molecular mechanism of a nucleobase-cation-symport-1 family transporter. Science 322, 709–713 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faham S., et al. , The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science 321, 810–814 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe A., et al. , The mechanism of sodium and substrate release from the binding pocket of vSGLT. Nature 468, 988–991 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamashita A., Singh S. K., Kawate T., Jin Y., Gouaux E., Crystal structure of a bacterial homologue of Na+/Cl–dependent neurotransmitter transporters. Nature 437, 215–223 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Krishnamurthy H., Gouaux E., X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature 481, 469–474 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malinauskaite L., et al. , A conserved leucine occupies the empty substrate site of LeuT in the Na(+)-free return state. Nat. Commun. 7, 11673 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gotfryd K., et al. , X-ray structure of LeuT in an inward-facing occluded conformation reveals mechanism of substrate release. Nat. Commun. 11, 1005 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar H., et al. , Structure of sugar-bound LacY. Proc. Natl. Acad. Sci. U.S.A. 111, 1784–1788 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang X., et al. , Crystal structure of a LacY-nanobody complex in a periplasmic-open conformation. Proc. Natl. Acad. Sci. U.S.A. 113, 12420–12425 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verdon G., Oh S., Serio R. N., Boudker O., Coupled ion binding and structural transitions along the transport cycle of glutamate transporters. Elife 3, e02283 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Dijk L., et al. , Key residues controlling bidirectional ion movements in Na(+)/Ca(2+) exchanger. Cell Calcium 76, 10–22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hess B., Kutzner C., van der Spoel D., Lindahl E., GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Bonomi M., et al. , PLUMED: A portable plugin for free-energy calculations with molecular dynamics. Comput. Phys. Commun. 180, 1961–1972 (2009). [Google Scholar]
- 50.Bonomi M., et al. , Promoting transparency and reproducibility in enhanced molecular simulations. Nat. Methods 16, 670–673 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Abraham M., et al. , GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015). [Google Scholar]
- 52.Marinelli F., Faraldo-Gómez J. D., Data for Conformational free-energy landscapes of a Na+/Ca2+ exchanger explain its alternating-access mechanism and functional specificity. Github. https://github.com/Faraldo-Gomez-Lab-at-NIH/Download. Deposited 15 March 2024. [DOI] [PMC free article] [PubMed]
- 53.Staritzbichler R., Anselmi C., Forrest L. R., Faraldo-Gomez J. D., GRIFFIN: A versatile methodology for optimization of protein-lipid interfaces for membrane protein simulations. J. Chem. Theory Comput. 7, 1167–1176 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang J., et al. , CHARMM36m: An improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klauda J. B., et al. , Update of the CHARMM all-atom additive force field for lipids: Validation on six lipid types. J. Phys. Chem. B 114, 7830–7843 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Alternating-access mechanism of NCX_Mj bound to 3 Na+ ions, as predicted by enhanced-sampling all-atom MD simulations and minimum free-energy path calculations. Helices TM1 and TM6 are highlighted in purple, and water molecules in the proximity of the ion binding sites are shown as red spheres; the bound Na+ ions are shown in green. All other elements of the simulation system are omitted for clarity.
Alternating-access mechanism of NCX_Mj bound to 1 Ca2+ ions, as predicted by enhanced-sampling all-atom MD simulations and minimum free-energy path calculations. Helices TM1 and TM6 are highlighted in purple, and water molecules in the proximity of the ion binding sites are shown as red spheres; the bound Ca2+ ion is shown in cyan. All other elements of the simulation system are omitted for clarity.
Data Availability Statement
A comprehensive package of input and output files is available at https://github.com/Faraldo-Gomez-Lab-at-NIH/Download (52). Some study data available (Simulation data amounts to tens of terabytes; this data will be shared with researchers upon request).