Abstract
A ganglioside fraction isolated from pooled intestines from newborn to 4-week-old piglets, which we previously partially characterized and showed to specifically inhibit the binding of porcine rotavirus (OSU strain) to host cells (M. D. Rolsma, H. B. Gelberg, and M. S. Kuhlenschmidt, J. Virol. 68:258–268, 1994), was further purified and found to contain two major monosialogangliosides. Each ganglioside was purified to apparent homogeneity, and their carbohydrate structure was examined by high-pH anion-exchange chromatography coupled with pulsed amperometric detection and fast atom bombardment mass spectroscopy. Both gangliosides possessed a sialyllactose oligosaccharide moiety characteristic of GM3 gangliosides. Compositional analyses indicated that each ganglioside was composed of sialic acid, galactose, glucose, and sphingosine in approximately a 1:1:1:1 molar ratio. Each ganglioside differed, however, in the type of sialic acid residue it contained. An N-glycolylneuraminic acid (NeuGc) moiety was found in the more polar porcine GM3, whereas the less polar GM3 species contained N-acetylneuraminic acid (NeuAc). Both NeuGcGM3 and NeuAcGM3 displayed dose-dependent inhibition of virus binding to host cells. NeuGcGM3 was approximately two to three times more effective than NeuAcGM3 in blocking virus binding. Inhibition of binding occurred with as little as 400 pmol of NeuGcGM3/50 ng of virus (∼2 × 107 virions) and 2 × 106 cells/ml. Fifty percent inhibition of binding was achieved with 0.64 and 1.5 μM NeuGcGM3 and NeuAcGM3, respectively. The free oligosaccharides 3′- and 6′-sialyllactose inhibited binding 50% at millimolar concentrations, which were nearly 1,000 times the concentration of intact gangliosides required for the same degree of inhibition. Direct binding of infectious, triple-layer rotavirus particles, but not noninfectious, double-layered rotavirus particles, to NeuGcGM3 and NeuAcGM3 was demonstrated by using a thin-layer chromatographic overlay assay. NeuGcGM3 and NeuAcGM3 inhibited virus infectivity of MA-104 cells by 50% at concentrations of 3.97 and 9.84 μM, respectively. NeuGcGM3 (700 nmol/g [dry weight] of intestine) was found to be the predominant enterocyte ganglioside (comprising 75% of the total lipid-bound sialic acid) in neonatal piglets, followed by NeuAcGM3 (200 nmol/g [dry weight] of intestine). NeuGcGM3 and NeuAcGM3 together comprised nearly 100% of the lipid-bound sialic acid in the neonatal intestine, but their quantities rapidly diminished during the first 5 weeks of life. These data support the hypothesis that porcine NeuGcGM3 and NeuAcGM3 are physiologically relevant receptors for porcine rotavirus (OSU strain). Further support for this hypothesis was obtained from virus binding studies using mutant or neuraminidase-treated cell lines. Lec-2 cells, a mutant clone of CHO cells characterized by a 90% reduction in sialyllation of its glycoconjugates, bound less than 5% of the virus compared to control cell binding. In contrast, Lec-1 cells, a mutant CHO clone characterized by a deficiency in glycosylation of N-linked oligosaccharides, still bound rotavirus. Furthermore, exogenous addition of NeuGcGM3 to the Lec-2 mutant cells restored their ability to bind rotavirus in amounts equivalent to that of their parent (CHO) cell line. In the virus-permissive MA-104 cell line, NeuGcGM3 was also able to partially restore rotavirus infectivity in neuraminidase-treated cells. These data suggest that gangliosides play a major role in recognition of host cells by porcine rotavirus (OSU strain).
Group A rotaviruses are a major cause of viral diarrhea in the young of most mammalian species. The significance of rotavirus infection in domestic animals, in terms of individual mortality and economic losses to producers, is well documented. Despite extensive efforts, effective control of rotavirus disease through vaccination programs has not been successful. Formulation of more-effective vaccines or new strategies for the control of rotaviral disease is needed.
The earliest and requisite step for productive viral infection is recognition and binding of the virus to villus-tip enterocytes. Interruption of virus binding to host cells by using natural receptor molecules or receptor mimics potentially provides an opportunity to intercede in the infectious cycle. Receptor therapy may be especially efficacious for enteric viral diseases since the intestinal tract, in contrast to most other tissue sites, is open to the environment. The increase in intestinal virus concentration due to production of new viral progeny by infected enterocytes is balanced by the loss of free virus via the feces and sloughing of infected enterocytes. Virions not able to bind to enterocytes as a consequence of binding soluble receptor analogues would be removed from the intestine and thus would no longer participate in the virus-host cell binding equilibrium. Thus, an open system such as the gut provides favorable conditions for reducing the virus load through receptor competition. It is likely, therefore, that soluble receptor mimics, when added to an appropriate steady-state concentration, will be able to reduce virus infectivity in diseased as well as naive animals.
The tissue- and cell type-specific tropisms displayed by rotaviruses are consistent with the hypothesis that a specific host cell surface receptor(s) mediates virus recognition (11). Earlier reports (2, 32, 39) provided strong evidence supporting the existence of specific rotavirus receptors. In fact, all of these studies implicated glycoconjugates as the putative receptors. We reported (21) the identification and partial purification of a ganglioside fraction, isolated from porcine intestine, that contained a small family of chromatographically separable monosialogangliosides capable of dose-dependent inhibition of rotavirus binding to host cells. Herein, we describe the further purification and carbohydrate structural characterization of the individual monosialogangliosides contained in this fraction as well as demonstrate their ability to block both rotavirus binding and infectivity of host cells in vitro. In addition, by measuring concentrations in intestinal tissue of each ganglioside as a function of piglet age and severity of natural rotavirus disease, as well as through the use of various glycosylation-deficient mutant or neuramidase-treated cell lines, we demonstrated that gangliosides are likely natural receptors that are exploited by porcine rotavirus (OSU strain) for host cell recognition in vivo.
(Part of this research was presented by M.S.K. at the First International Mt. Rushmore Conference on Mechanisms in the Pathogenesis of Enteric Diseases, Rapid City, S.D., 27 to 30 September 1995, and appeared in preliminary form as a part of those proceedings [14].)
MATERIALS AND METHODS
Materials.
Unless stated otherwise, all reagents and chemicals were obtained from Sigma Chemical Co. and were of the highest purity available. Ceramide glycanase (catalog no. L-1005), 3′-sialyllactose (catalog no. D211), and 6′-sialyllactose (catalog no. D212) were obtained from V-Labs Inc., Covington, La. Purified N-glycolyl-GM3 was a gift from Akemi Suzuki (The Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan). Purified N-acetyl-9-mono-O-acetylneuraminic acid and Vibrio cholerae neuraminidase were gifts from Eric Vimr (University of Illinois). Arthrobacter ureafaciens neuraminidase was obtained from Boehringer Mannheim Corp. All solvents were Burdick & Jackson high-performance liquid chromatography (HPLC) grade (Baxter Healthcare Corp.). Glass thin-layer chromatography (TLC) and high-performance TLC (HPTLC) plates were prepared with E. Merck Kieselgel 60 without a fluorescent indicator (VWR Scientific). Plastic-backed silica gel TLC plates (Macherey-Nagel) were obtained from Alltech (catalog no. 805013).
Cell culture.
MA-104 cells, derived from fetal rhesus monkey kidneys, were grown as previously described (21). Caco-2 cells were obtained from the American Type Culture Collection (ATCC) (HTB 37) and were grown in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS). Chinese hamster ovary cells (CHO-K1; ATCC CCL 61) were grown in Ham’s F-12 medium supplemented with 10% FBS. Lectin-resistant mutant cell lines Lec-1 and Lec-2 and the parental Pro-5 CHO cells from which they were derived (3, 29–31), all obtained from the ATCC (catalog no. crl 1735, crl 1736, and crl 1781, respectively), were grown in α-MEM supplemented with ribonucleosides, deoxyribonucleosides, and 10% FBS (Gibco; catalog no. 11900-024). For use in the virus binding assays, all cells were grown in their respective media to confluence in 150-cm2 flasks and harvested with an isotonic solution consisting of 0.05% trypsin in 0.53 mM EDTA (Gibco). All cells were washed two times and suspended in ice-cold MEM without serum to a density of 2 × 106 or 4 × 106 per ml, depending on the virus assay method.
Virus propagation, purification, and radiolabeling.
Group A porcine rotavirus strain OSU (P9[7],G5) was propagated in MA-104 cells, and triple-layered particles (TLP) and double-layered particles (DLP) were purified by CsCl centrifugation and radiolabeled with 125I as previously described (21). Viral protein was quantified colorimetrically (27) with human serum albumin as the standard (Micro BCA; Pierce Chemical Co.).
Virus binding assays.
Measurement of virus binding to MA-104 cells in the absence of added gangliosides was performed as previously described (21).
(i) Blocking assay 1.
Purified ganglioside pools were evaluated for their ability to block virus binding in the standard full-scale assay as described previously (21).
(ii) Blocking assay 2.
The blocking assay 1 method was scaled down to conserve the additives being tested. Various amounts (0.04 to 4 nmol) of a ganglioside pool or 0.04 to 0.8 μmol of 3′- or 6′-sialyllactose were dried in borosilicate glass tubes (6 mm by 50 mm). A 35-μl aliquot of virus buffer (50 mM Tris, 150 mM NaCl, 10 mM CaCl2, pH 7.5 [TNC]) was added, and the sample was mixed and sonicated. A 15-μl portion of diluted 125I-labeled porcine rotavirus (10 ng) was added, and the sample was gently mixed and incubated on ice for 30 min. MA-104 cells (2 × 105, in 50 μl) were added, and the tube was covered with Parafilm and inserted into a borosilicate tube (12 mm by 75 mm), which was then corked and rotated end over end as described previously for the full-scale binding assay (21). After 30 min of rotation, 80 μl of the cell suspension was removed and layered over a silicone-mineral oil mixture, and the cell-associated radioactivity was pelleted as in the full-scale assay (21). Controls (100% binding) were devoid of additive, and blanks were prepared from control tubes in which the cell-associated radioactivity was measured immediately after addition of the MA-104 cells.
Virus overlay assay.
Gangliosides (750 pmol) were applied to the origin of a plastic-backed silica gel TLC plate which had been prerun in chloroform-methanol (MeOH)-H2O (60:30:4.5, vol/vol/vol). The plate was developed in chloroform-MeOH-H2O (55:45:10), and after it was air dried, overlay of the 125I-labeled rotavirus was performed by a modification of a previous method (12). Briefly, the developed plate was treated with ice-cold TNC–1% bovine serum albumin (Sigma; catalog no. 7030) for 1 h at 4°C. The plate was then rinsed quickly in TNC buffer, placed in a glass dish with 7 ml of TNC containing 1.25 × 107 dpm (∼3 μg of viral protein) of 125I-labeled porcine rotavirus/100-cm2 plate, and gently rocked for 2.5 h at 4°C. The 125I-rotavirus suspension was removed by aspiration, and the plate was rinsed seven times in 400 ml of ice-cold filtered TNC. After air drying for 1 h at room temperature, the plate was wrapped in plastic, placed in a Cronex Lightning Plus cassette with intensifier screens, exposed to Kodak X-Omat film at −80°C for 4 h, and developed in an automatic developer.
Measurement of virus infectivity: focus-forming assay.
Confluent monolayers of MA-104 cells in 24-well plates were rinsed twice with phosphate-buffered saline (PBS). One milliliter of serum-free MEM was added to each well. The plates were incubated at 37°C in a 5% CO2 incubator for 3 h. The CsCl-purified TLP virus suspension was treated for 30 min at 37°C with crystallized trypsin (Sigma) at a final concentration of 10 μg/ml. The trypsinized virus suspension was diluted in serum-free MEM to a density of ∼1,000 focus-forming units (FFU)/75 μl. Identical 250-μl aliquots of the diluted virus suspension were treated with either enterocyte or brain gangliosides at a final concentration of 0 to 43.6 nmol/ml (measured as total sialic acid by a thiobarbituric acid-based HPLC method [20]) at room temperature (RT) for 15 min. Following the chilling of all reagents and 24-well plates on ice, the serum-free MEM was aspirated. Triplicate wells were inoculated with 75 μl of each virus-inhibitor dilution and incubated on ice with continuous rocker platform agitation for 15 min. Virus-inhibitor inoculates were aspirated from each well. The wells were rinsed with 1 ml of ice-cold serum-free MEM. Following aspiration of the rinse MEM and replacement with 1 ml of fresh serum-free MEM, the plates were warmed to 37°C and incubated in a 5% CO2 incubator for 16 to 18 h prior to quantification of infected cells by immunocytochemical detection of virus progeny.
Immunochemical detection of virus-infected cells (FFU assay).
The following steps were carried out at RT on a rocker plate unless otherwise noted.
The wells were rinsed twice with 1 ml of PBS. The monolayer in each well was fixed for 2 min with 500 μl of MeOH-glacial acetic acid (9:1). Rehydration of the monolayers was performed for 5 min with 500 μl of 70% ethanol per well, followed by a 5-min incubation with 500 μl of 50% ethanol. The 50% ethanol was replaced by 500 μl of wash buffer (WB; 125 mM Tris, 350 mM NaCl, 0.25% Triton X-100, pH 7.6). Cells were stained immediately or were held at 4°C until being stained. Endogenous peroxidase activity was quenched by treatment of each well for 10 min with 150 μl of 3% H2O2 diluted in WB. The wells were each rinsed with 500 μl of WB for 10 min. Nonspecific primary antibody binding was inhibited by addition of 150 μl of 5% normal goat serum (Vector Laboratories) in WB to each well and incubation at RT for 20 min. Following incubation, the normal goat serum was removed and, without rinsing, 150 μl of primary antibody (rabbit anti-human rotavirus; Dako; catalog no. B218), diluted 1:100 in WB, was added. The plates were incubated overnight at 4°C on a rocker platform. The wells were rinsed twice, for 10 min each, with 500 μl of WB. Monolayers were each incubated for 20 min with 150 μl of biotinylated goat anti-rabbit immunoglobulin G (Vector Elite Kit; Vector Laboratories) diluted 1:200 in WB with normal goat serum added to a dilution of 1:67. The wells were rinsed twice, for 10 min each, with 500 μl of WB. The monolayers were each incubated for 20 min with 150 μl of ABC reagent (Vector Elite Kit). The ABC reagent was made up 30 min prior to use and contained 1:50 dilutions of reagents A and B in WB. The wells were rinsed twice, for 10 min each, with 500 μl of WB. Seventy-five microliters of TruBlue peroxidase chromogen (Kirkegaard & Perry) was added to each well. Color development was observed with an inverted microscope, and development was stopped before nonspecific cell staining occurred (approximately 5 min). The wells were each rinsed in 500 μl of deionized water (dH2O) for 5 min. After aspiration of the rinse water, a 12-mm-diameter circular coverslip was mounted with an aqueous medium. The mounting medium consisted of 20 g of Airvol 205 (Air Products) dissolved, with heating (90°C), in 80 ml of Hanks’ balanced salt solution. After cooling, 40 ml (50.4 g) of glycerol was added. The mounting medium was stored tightly closed at RT.
The number of blue-stained (viral-antigen-positive) cells under the 12-mm-diameter coverslip was determined at a magnification of ×200 on an Olympus inverted microscope equipped with contrast enhancement and a mechanical stage. Counts in triplicate wells were averaged. Each inhibition experiment was repeated three times. Regression analysis of the inhibition curve for each experiment was performed to determine the 50% inhibition (I50) value. Individual values from each experiment were averaged. Statistical significance was tested by analysis of variance ANOVA.
Purification of porcine enterocyte monosialogangliosides.
A monosialoganglioside fraction, previously shown to specifically inhibit the binding of rotavirus to porcine enterocytes and MA-104 cells, was purified from colostrum-deprived, specific-pathogen-free, cross-bred newborn piglets by modifications of procedures previously described (21). Briefly, 30 g (wet weight) of intestinal mucosa was homogenized in 140 ml of water at 4°C by use of an Oscar mini-food processor. A polar ganglioside extract was prepared from the homogenate as previously described (34) and evaporated to dryness until further purified by Iatrobead (Iatron Chemical Products) chromatography, solvent partitioning, and HPLC.
(i) Iatrobead chromatography.
The polar ganglioside extract, dissolved in 16 ml of 4.5:1 chloroform-MeOH, was applied to an Iatrobead column (2.2 cm by 40 cm) equilibrated in 95:5 chloroform-MeOH. The column was eluted batchwise with three, 300-ml washes of 4.5:1 chloroform-MeOH followed by four, 250-ml washes of 1:1 chloroform-MeOH at a flow rate of 0.5 ml/min. The major monosialoganglioside fractions were found in the third wash of 4.5:1 chloroform-MeOH (67%) and in the first wash of 1:1 chloroform-MeOH (33%). These fractions were pooled and evaporated to dryness.
(ii) Solvent partitioning.
The dried ganglioside extract was suspended in 30 ml of di-isopropyl ether (DIPE)–1-butanol (6:4) and sonicated. Fifteen milliliters of 50 mM NaCl was added to the DIPE–1-butanol solution; after another sonication, the mixture was centrifuged (10 min, 750 × g) to aid partitioning. The upper, organic phase was carefully removed. The lower, aqueous phase was reextracted with another 30 ml of DIPE–1-butanol (6:4); this was followed by centrifugation as described above. After removal of the upper phase, the lower phase was dried by rotary evaporation. The ganglioside extract was suspended in 1.25 ml of dH2O, sonicated, and centrifuged for 5 min at 15,000 rpm (15,600 × g) in an Eppendorf model 5414 microcentrifuge to remove non-water-soluble material. The aqueous ganglioside solution was desalted on a Sephadex G-25 (coarse) column (10 mm by 200 mm) preequilibrated in dH2O. The column was eluted with dH2O. The effluent was monitored continuously at 206 nm in a Beckman model DU-50 spectrophotometer equipped with a flow cell. Fractions (2 min each) were collected with a Bio-Rad model 2110 fraction collector. Void-volume fractions containing gangliosides were pooled and dried by rotary evaporation.
(iii) HPLC purification.
The Iatrobead-purified, solvent-partitioned ganglioside fraction was dissolved in 10 ml of 65:35:3 chloroform-MeOH-H2O (solvent 1). One-milliliter aliquots were individually filtered through an Alltech syringe filter (catalog no. 2092), and the filter was rinsed with an additional 1 ml of solvent 1. The combined filtrates were evaporated, dissolved in 500 μl of solvent 1, and injected onto a semipreparative silica HPLC column (Alltech Econosil SI; 10-μm particles; 250 mm by 10 mm; catalog no. 6233) equilibrated in solvent 1. After 40 min of elution with solvent 1, at a flow rate of 2 ml/min, a linear gradient from 100% solvent 1 to 28% solvent 2 (chloroform-MeOH-H2O, 60:35:8) was begun. At 100 min, a second gradient, from 28% solvent 2 to 100% solvent 2, was begun, and it was terminated at 150 min. This second gradient was followed by a 10-min application of 100% solvent 2 to regenerate the column. The eluate stream from the column was split to send approximately 5% of the flow to a Varex model MKII evaporative light-scattering detector set at a drift tube temperature of 60.3°C. Samples from the collected fractions (3 min each) were spotted on silica TLC plates, and sialic acid-containing fractions were visualized by using a resorcinol reagent (13). The resorcinol-positive fractions were collected, pooled, and designated as pool 1 (80 to 100 min), pool 2 (101 to 130 min), and pool 3 (131 to 144 min). After reequilibration of the column, nine additional runs were performed, with the pools being collected and combined as described above. Following concentration by rotary evaporation, the pools were stored in chloroform-MeOH-H2O (60:30:4.5) at −20°C.
TLC.
Unless stated otherwise, samples were dissolved in chloroform–MeOH–0.25% CaCl2 in dH2O (55:45:10) and then applied in 0.5-cm-wide streaks to prechanneled silica gel 60 plates (E. Merck); this was followed by development in the above solvent. Ganglioside bands were visualized with resorcinol spray.
Sphingosine analysis.
The sphingosine contents of pool 1, pool 2, pool 3, and GM3 gangliosides were determined fluorimetrically as previously described (19), using d-erythrosphingosine (catalog no. 860025; Avanti Polar Lipids Inc., Alabaster, Ala.) as the standard.
Monosaccharide and oligosaccharide analyses. (i) Sialic acid analysis.
Determination of total sialic acid contents for use in the calculation of binding assay concentrations was done by a modification of a thiobarbituric acid-based HPLC method (20). Following acid hydrolysis and development of the chromagen, 100-μl aliquots were injected onto and chromatographed through a 4.6-mm by 250-mm C18 reversed-phase column (Alltech). The solvent (2× buffer stock-MeOH-water, 5:3:2) was run isocratically at a flow rate of 0.7 ml/min. Chromagen peaks were detected with a model LC-65T variable-wavelength UV detector (Perkin-Elmer), set at 549 nm, integrated with a model 3392A integrator (Hewlett-Packard). For individual determination of N-acetylneuraminic acid (NeuAc) and N-glycolylneuraminic acid (NeuGc) concentrations, dried portions of HPLC-Econosil pools 1, 2, and 3 and bovine GM3, or ceramide glycanase-released oligosaccharides (see below) from pools 1 and 3 and bovine GM1, were sonicated in 200 μl of 0.1 N HCl and heated for from 30 min to 2 h in an 80°C water bath (until maximum release of neuraminic acid was achieved). At the end of the heating time, the sample was chilled on ice and evaporated to dryness. The sample was dissolved in up to 300 μl of dH2O. Analyses for NeuAc and NeuGc were performed with a Dionex model DX-300 chromatography system equipped with a pulsed amperometric detector (detector settings: E1 = 0.05 mV, 420 ms (setting 4, range 2); E2 = 0.75 mV, 180 ms (setting 3, range 2); E3 = 0.15 mV, 360 ms (setting 6, range 2). Samples (200 to 1000 pmol) were injected onto a Carbopac PA-1 column run in 0.1 N NaOH–0.15 M sodium acetate, and the elution times were compared to those of NeuAc and NeuGc standards.
(ii) Neutral-sugar analysis.
Portions of HPLC-purified ganglioside pools or ceramide-released oligosaccharides were evaporated to dryness and hydrolyzed in 200 μl of 2 N trifluoroacetic acid at 100°C for from 2 to 6 h (until maximum release of sugar was achieved). In the case of intact gangliosides, maximum release of glucose was not obtained until 6 h of hydrolysis, whereas galactose was released at 2 h. The trifluoroacetic acid was removed by evaporation; the sample was rinsed once with H2O and, following evaporation, was dissolved in 300 μl of dH2O. Monosaccharide analyses was performed with a Dionex DX-300 chromatography system equipped with a pulsed amperometric detector (detector settings: E1 = 0.05 mV, 420 ms (setting 4, range 2); E2 = 0.75 mV, 180 ms (setting 3, range 2), E3 = 0.15 mV, 360 ms (setting 6, range 2) and a model 8880 autosampler (Spectrophysics). An aliquot of each sample (10 to 20 μl; 100 to 200 pmol) was injected onto an analytical Carbopac PA-1 column (4.6 mm by 250 mm) that had been equilibrated in 16 mM NaOH. After 20 min, the column was regenerated for 10 min with 200 mM NaOH; this was followed by a 25-min reequilibration in 16 mM NaOH prior to injection of the next sample. Data were collected and analyzed with the Dionex AI-450 chromatography workstation. Elution times of standard monosaccharides l-fucose, d-galactosamine, d-glucosamine, d-galactose, d-glucose, and d-mannose were compared with the elution times of the monosaccharides released by hydrolysis of ganglioside pools. Standard mixtures used for identification of monosaccharides were from Dionex. d-Galactose and d-glucose (Fluka Chemical Co.) were used as standards for the molar ratio determinations.
(iii) Oligosaccharide analysis.
Ceramide glycanase digestion of purified porcine intestinal gangliosides was performed by a modification of the method of Zhou et al. (41). Briefly, approximately 3 nmol of each ganglioside was sonicated in 200 μl of 50 mM sodium acetate (pH 5.0) containing 0.1 mg of sodium taurodeoxycholate and 0.38 mg of sodium cholate. After addition of 0.5 U of ceramide glycanase (catalog no. L-1005, V-Labs, Inc., Covington, La.), the sample was incubated at 37°C for 66 h. Removal of lipids was accomplished by passing the ceramide glycanase digestion mixture through an Alltech Extract-Clean C18 syringe (catalog no. 204900) equilibrated in H2O. The eluate (2 ml of H2O) was collected and concentrated by evaporation in a Savant Speed Vac apparatus. Approximately 1.0 nmol of this concentrate in water was analyzed by TLC for determination of completeness of digestion. About 1 nmol of each of the released oligosaccharide preparations from porcine intestinal ganglioside pools, the 3′- and 6′-sialyllactose standards, and released N-glycolyl-GM3 oligosaccharide was analyzed with the Dionex Dx-300 chromatography system. Released oligosaccharides from pool 1 or standard 3′- or 6′-sialyllactose oligosaccharides were injected onto a Dionex Carbopac PA1 column (4.6 mm by 250 mm) and run for 10 min in 150 mM NaOH (solvent 1). At 10 min, a linear gradient from 100% solvent 1 to 8% solvent 2 (150 mM NaOH–1 M sodium acetate) was applied and run for 55 min. From 65 to 70 min, solvent 2 was linearly increased to 50%. Released oligosaccharides from pool 3 or N-glycolyl-GM3 were chromatographed as described for pool 1 except the final gradient step started at 65 min and increased linearly from 8% solvent 2 to 100% solvent 2 in 20 min.
Analysis for presence of O-acetylated sialic acids.
Purified porcine intestinal ganglioside sialic acids were analyzed for the presence of O-acetyl esters by using hydrolysis conditions known to preserve O-acetyl groups (36). Briefly, dried gangliosides (50 to 70 nmol, as sphingosine) were pretreated with NH4OH (to remove putative O-acetyl groups) or sham treated (see below), and then 25 to 35 nmol of each ganglioside was mixed with 500 μl of 2 N acetic acid and heated at 80°C for 4 h. The acetic acid was removed by evaporation, and the hydrolysate was redissolved in a minimal volume of isopropanol-water (7:3) and then applied to a glass-backed silica HPTLC plate. The plate was developed in ethanol-butanol-pyridine-water-acetic acid (100:10:10:30:3) and then sprayed with resorcinol to detect sialic acids, and the relative amounts of each sialic acid were determined by quantitative densitometry. Base (NH4OH) treatment of purified gangliosides was performed according to a previously described method (6) used to detect O-acetylated sialic acids in ganglioside samples. Briefly, 200 μl of 1-propanol–2-propanol–concentrated NH4OH (35:35:30) was added to 50 to 70 nmol of dried ganglioside. After being mixed and sonicated, the samples were incubated for 3 h at RT. Solvent was removed by evaporation. Sham-treated samples were processed identically except that water was added in place of the concentrated NH4OH. Portions of the NH4OH- or sham-treated gangliosides were also analyzed for their ability to block virus binding by the use of blocking assay 1 as described above.
FAB-MS.
The molecular sizes of individual purified gangliosides were determined by negative-ion, low-resolution fast atom bombardment mass spectroscopy (FAB-MS) with a VG ZAB-SE ultra-high-resolution 8-kV mass spectrometer in the Mass Spectrometry Laboratory of the School of Chemical Sciences at the University of Illinois. Triethanolamine was used as the matrix. Approximately 10 μg of each ganglioside was evaporated to dryness and submitted for analysis at a resolution of 1,000 and a scan mass range of 100 to 2,000.
Reconstitution of mutant or neuraminidase-treated cells with NeuGcGM3.
Lec-2 cells (1.125 × 106 in 0.25 ml) were added to 12-mm by 75-mm borosilicate tubes containing 0.25 ml of either MEM alone or MEM containing 165 nmol of purified porcine intestinal NeuGcGM3. The tubes were capped and rotated end over end at 5 rpm and 37°C for 1 h. Cells were pelleted by centrifugation and washed two times in 500 μl of ice-cold MEM to remove remaining ganglioside. The cell pellets were each resuspended to 500 μl in ice-cold MEM. After 50 μl was removed for cell number determination, 10 μl (50 ng) of 125I-rotavirus (TLP) was added to the remaining treated or untreated Lec-2 cells or to freshly harvested CHO-K1 cells at the same number of cells per milliliter. Virus binding to these cells at 4°C was measured as previously described (21).
Confluent monolayers of MA-104 cells seeded in 24-well plates were washed and incubated for 3 h in serum-free MEM. Following serum-free medium exchange, cells were incubated with 100 μl of either fresh serum-free MEM or MEM containing 11.7 mU of V. cholerae neuraminidase and 3.3 mU of A. ureafaciens neuraminidase for 1 h at 37°C. Following three washes with MEM, the cell layers were either sham treated or treated for 1 h at 37°C with 100 μl of MEM containing 19 nmol of porcine intestinal NeuGcGM3. Following three washes with MEM, virus infectivity was measured by the focus-forming assay as described above.
Age-related changes in porcine intestinal ganglioside concentration.
Intestinal mucosa was isolated as previously described (21). Briefly, newborn (colostrum-deprived) and 1-, 2-, 3-, 4-, 5-, 6-, 8-, 12-, and 16-week-old pigs were obtained from the University of Illinois specific-pathogen-free swine herd and housed in confinement facilities until euthanized. The entire small intestine was removed from each piglet and thoroughly rinsed with ice-cold PBS (per liter, 8.0 g of NaCl, 0.2 g of KCl, 2.06 g of Na2HPO3 · 7H2O, 0.2 g of KH2PO4; pH 7.5). Segments of intestine were opened, and the mucosa was gently scraped with a microscope slide (1 in. by 3 in.). The mucosal scrapings were rinsed thoroughly by vortexing in an equal volume of PBS and then centrifuged in a Dupont Sorvall RT6000 centrifuge at 1,000 rpm (200 × g). Rinsing was repeated until the supernatant was clear. The rinsed mucosal pellet was stored at −70°C until further processing was performed. Rinsed intestinal mucosae from two pigs in each age group (1 g from each; 2 g total) were pooled in preweighed 50-ml polypropylene centrifuge tubes (Corning). The tissue was shell frozen and lyophilized. Following lyophilization, the tubes were reweighed to determine the dry weight of the mucosal sample.
Thirty milliliters of chloroform-methanol (1:1) was added to the dried intestinal tissue. Thorough probe sonication with a Heat Systems W-225R probe sonicator (Ultrasonics Inc.) was performed at 40% power for three 30-s intervals. The mixture was left overnight at 4°C. The extract was centrifuged at 4°C and 3,000 rpm (1,600 × g) for 10 min in a Sorvall RT-6000 centrifuge. The supernatant was saved. The pellet was reextracted with 30 ml of chloroform-methanol (1:1) by probe sonication and left overnight as before. Supernatants were pooled and rotary evaporated to 50% of their original volume (15 ml). The crude lipid extract was cooled to −20°C and left overnight. Insoluble material was removed by centrifugation, and the supernatant was vacuum filtered through a 0.20-μm-pore-size polytetrafluoroethylene filter and evaporated to dryness. Following solvent partitioning and desalting as described above, each ganglioside sample was suspended in 100 μl of chloroform-MeOH-H2O (60:30:4.5) in preparation for TLC. Five microliters of each enterocyte ganglioside preparation was spotted on a prechanneled silica gel 60 TLC plate (E. Merck). The plates were developed in a short-bed, continuous-development chamber (Regis), using chloroform–methanol–0.9% CaCl2 in water (55:45:10). Following development, the plates were sprayed with resorcinol to visualize gangliosides. Quantification of the relative concentrations of NeuGcGM3 and NeuAcGM3 was done by acid hydrolysis of each ganglioside fraction and analysis of released sialic acids by high-pH anion-exchange chromatography and pulsed-amperometric detection (HPAEC-PAD) as described above (see monosaccharide and oligosaccharide analyses). Ganglioside acid hydrolysis was performed as follows. A 10- to 25-μl aliquot (100 μl total) of each ganglioside sample (equivalent to 22 to 60 mg of dried intestinal mucosa) was placed in a screw-cap microcentrifuge tube and dried in a SpeedVac. Twenty microliters of tetrahydrofuran, 160 μl of dH2O, and 20 μl of 20 mM acetic acid were added sequentially, with sonication between the individual additions. Samples were incubated under N2 for 3 h at 80°C. Following incubation, the sample was dried in a SpeedVac. Addition of 75 μl of dH2O, sonication, and centrifugation for 5 min at 15,000 rpm (15,600 × g) in an Eppendorf model 5414 microcentrifuge to remove non-water-soluble material were performed to prepare the samples for sialic acid analysis by HPAEC-PAD as described above.
RESULTS
Purification of porcine enterocyte gangliosides.
Previous results (21) demonstrated that a ganglioside fraction isolated from porcine intestine displayed rotavirus receptor activity. The fraction also was shown to contain at least two chromatographically separable gangliosides. The two gangliosides displayed TLC and HPLC mobilities consistent with their designation as monosialogangliosides. Confirmation of their identity, however, required further purification and chemical characterization. A major hinderance in ganglioside purification is the removal of lipid-soluble, non-sialic acid contaminants. The addition of the DIPE–1-butanol–NaCl solvent partitioning purification step (see Materials and Methods) prior to preparative HPLC was effective in removing these contaminants. Especially reduced were the concentrations of non-sialic acid-containing lipids that stained yellow with resorcinol following TLC (data not shown) and which had consistently contaminated the ganglioside fraction during previous purifications. This modification resulted in a more pure monosialoganglioside preparation than had been previously achieved. The increased purity of the sample enabled the injection of larger amounts onto the preparative HPLC column without exceeding the column capacity.
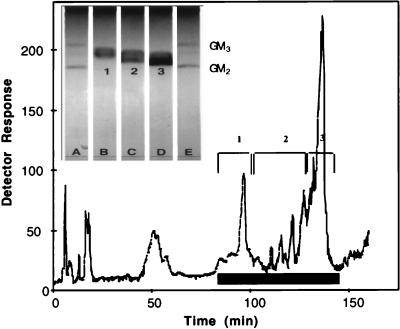
The DIPE–1-butanol–NaCl-extracted Iatrobead fraction was chromatographed on a silica semipreparative HPLC column. The column was monitored for both total solute, using evaporative laser light-scattering detection, and for sialic acid, using resorcinol reagent. A typical elution profile from this column is shown in Fig. 1. Several resorcinol-positive peaks with chromatographic mobilities between those of bovine brain GM3 and GM2 standards were identified. The less polar non-sialic acid components eluted earlier than the gangliosides. The resorcinol-positive peaks were combined into three separate pools as indicated in Fig. 1. Each of these pools was analyzed separately by TLC and shown to contain a single broad resorcinol-positive band. Although there were only minor differences in chromatographic resolution, each of the three bands displayed a unique mobility between those of bovine brain GM3 and GM2 standards. Consistent with the elution behavior seen on HPLC, pool 1 was the least polar and displayed a mobility close to bovine GM3, whereas pool 3 was the most polar and was nearest in mobility to bovine GM2. Pool 2 displayed a mobility intermediate between those of pools 1 and 3. Rechromatography of the individual pools on a preparative HPLC column showed that each pool eluted in the same fraction as originally seen upon chromatography of the crude mixture (data not shown).
FIG. 1.
The resorcinol-positive pool from the Iatrobead column (up to 5 μmol of sialic acid) was injected onto a semipreparative silica HPLC column as described in Materials and Methods. The response from the evaporative light-scattering detector is shown (versus elution time). Aliquots of fractions were spotted onto TLC plates and sprayed with resorcinol to locate sialic acid-containing fractions, which are designated by the solid black bar on the figure. Resorcinol-positive fractions combined to form pools 1, 2, and 3 are indicated by brackets. (Inset) Between 10 and 20 nmol of sialic acid equivalents of each ganglioside pool or approximately 3 nmol of standard GM3 or GM2 was applied to a channeled silica gel plate, developed as described in Materials and Methods, and stained with resorcinol to visualize ganglioside bands. Lanes A and E, GM3 and GM2; lanes B, C, and D, HPLC pools 1, 2, and 3, respectively.
Purified porcine intestinal gangliosides inhibit binding of rotavirus to host cells.
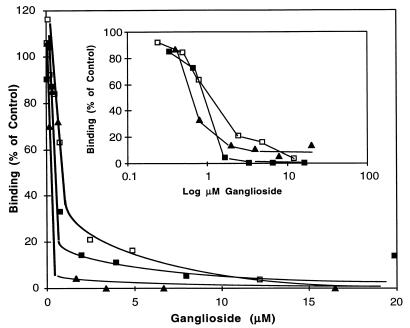
Our previous results (21) indicated that more than one enterocyte monosialoganglioside fraction might be recognized by porcine rotavirus. Therefore, we tested each of the three purified gangliosides for their ability to block the binding of 125I-labeled rotavirus to MA-104 cells. Each of the three purified gangliosides (Fig. 1) was tested over a range of concentration from 0.05 to 20 μM sialic acid. These data indicate that all three gangliosides were active inhibitors, displaying dose-dependent inhibition of rotavirus binding to MA-104 cells (Fig. 2). The concentration of each ganglioside required for 50% inhibition of binding ranged from 0.64 to 1.5 μM in this experiment. Pools 2 (I50 = 0.9 μM) and 3 (I50 = 0.64 μM) were always slightly more effective inhibitors than pool 1 (I50 = 1.5 μM), with pool 3 displaying two to three times more inhibitory activity than pool 1. Interestingly, bovine brain GM3 was at least fivefold less effective (I50 = 7.5 μM) than purified porcine intestinal GM3 (data not shown).
FIG. 2.
Various amounts of the ganglioside pools isolated by Econosil HPLC (Fig. 1) were tested for the ability to block the binding of 125I-labeled porcine rotavirus to MA-104 cells as described in Materials and Methods (blocking assay 1). Percentage of control binding (no blocking agent) is plotted versus the micromolar concentration of ganglioside in the final assay mixture of virus and cells. □, pool 1; ■, pool 2; ▴, pool 3. (Inset) Percentage of control virus binding is plotted versus the log of the micromolar concentration of added ganglioside in the final assay mixture of virus and cells.
Compositional analyses reveal a GM3-like structure for all three monosialoganglioside pools.
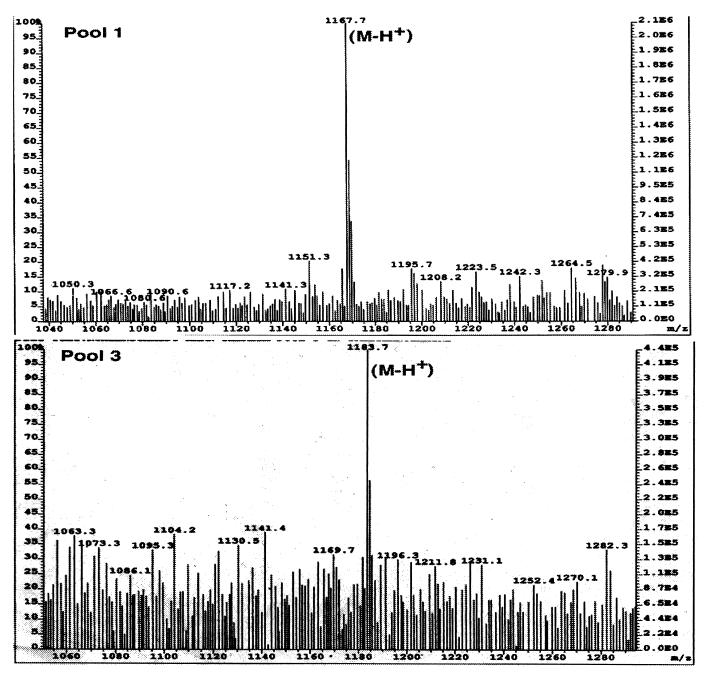
Although silica gel chromatography (both TLC and HPLC) demonstrated that the more polar ganglioside fraction (pool 3) migrated closer to GM2, monosaccharide and sphingosine analyses revealed that all three pools of purified enterocyte gangliosides contained approximately 1 mol of sialic acid, 1 mol of galactose, and 1 mol of glucose per mol of sphingosine (Table 1). No hexosamine (which would be expected for GM2) was detected. Thus, all three pools appeared to be GM3-type monosialogangliosides. In two of the purified monosialogangliosides, however, the type of sialic acid differed. Sialic acid released from pool 1 was identified as NeuAc, whereas pool 2 and 3 monosialogangliosides were both found to contain NeuGc. Confirmation of the presence of both NeuAc- and NeuGc-containing gangliosides in porcine intestine was obtained by MS analyses of the purified intact gangliosides (pools 1 and 3). Positive-ion FAB-MS revealed a single major peak corresponding to the molecular ion (M-H+) for both pool 1 and pool 3 (Fig. 3). One of the methyl group hydrogen atoms in the C-5 N-acetyl group of NeuAc is replaced by a hydroxyl group, resulting in a net 16-mass unit increase. The molecular mass of 1,183.7 for pool 3 was exactly 16 mass units greater than that observed for pool 1 (1,167.7). As predicted from monosaccharide compositional analyses, MS confirmed a 16-unit-greater molecular mass for the pool 3 ganglioside than for that of pool 1. These data are consistent with the designation of pool 3 as a GM3 ganglioside containing NeuGc (NeuGcGM3) and pool 1 as an NeuAc-containing GM3 ganglioside (NeuAcGM3). Although the mass spectrogram for pool 2 was heterogeneous, the 1,183.7-mass unit molecular ion was also observed, consistent with its monosaccharide analysis as well as its designation as NeuGcGM3 (data not shown). The presence of the more polar NeuGc explains the increased polarity of pools 2 and 3 compared to pool 1 that was seen during silica gel TLC and HPLC (Fig. 1).
TABLE 1.
Monosaccharide compositions of purified porcine intestinal gangliosidesa
| Sample | Amt. of monosaccharide or sphingosine (nmol)b
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fuc | GalNH2 | GlcNH2 | Gal | Glc | Man | NeuGc | NeuAc | Sph | |
| Pool 1 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 1.8 (0.95) | 2.0 (1.1) | 0.0 (0.0) | 0.0 (0.0) | 1.9 (1.0) | 1.9 (1.0) |
| Pool 2 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 5.9 (1.3) | 5.7 (1.2) | 0.0 (0.0) | 6.8 (1.4) | 0.0 (0.0) | 4.7 (1.0) |
| Pool 3 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 9.3 (0.85) | 9.1 (0.83) | 0.0 (0.0) | 11 (1.0) | 0.0 (0.0) | 11 (1.0) |
| GM3 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 3.1 (1.0) | 3.2 (1.1) | 0.0 (0.0) | 0.0 (0.0) | 2.5 (0.86) | 2.9 (1.0) |
Equivalent portions of each ganglioside pool were analyzed for sphingosine, monosaccharides, NeuAc, and NeuGc as described in Materials and Methods. Pools 1, 2, and 3 were purified porcine intestinal gangliosides as designated in Fig. 1 and the text. Commercial GM3 purified from bovine brain (Sigma) was used as a standard.
Values in parentheses are ratios of amounts of monosaccharides to amounts of sphingosine in the purified ganglioside pool or GM3 standard. Abbreviations: Fuc, fucose; GalNH2, galactosamine; GlcNH2, glucosamine; Gal, galactose; Glc, glucose; Man, mannose; Sph, sphingosine.
FIG. 3.
FAB-MS of purified monosialogangliosides. Low-resolution FAB-MS of purified porcine intestinal monosialogangliosides was performed as described in Materials and Methods. Pools 1 and 3 were as designated in Fig. 1.
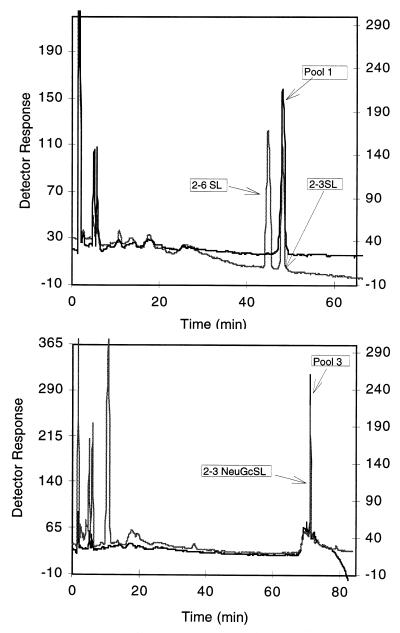
Oligosaccharides released from porcine enterocyte monosialogangliosides comigrate with authentic 3′-sialyllactose.
The purified monosialogangliosides (pools 1 to 3) were treated with ceramide glycanase to liberate the oligosaccharide from the ceramide portion of the molecule. The results of HPAEC-PAD analyses of the released oligosaccharides are shown in Fig. 4. Pool 1 comigrates with authentic α-2,3-linked N-acetylsialyllactose (top panel) but not with the corresponding α-2,6 standard. The oligosaccharide released from pool 3 is more polar than its pool 1 counterpart and comigrates with the α-2,3 oligosaccharide released from mouse liver NeuGcGM3 (bottom panel). Pool 2 shows a profile identical to that of pool 3 (data not shown). Carbohydrate compositional analyses of the oligosaccharides released from pools 1 and 3 indicated that the ratios of sialic acid, galactose, and glucose (Table 2) were equivalent (equimolar to those found in hydrolysates of the intact ganglioside (Table 1). It should be noted that no hexosamine was detected in any of the monosaccharide or oligosaccharide analyses. Analysis of bovine brain GM1 (used as a standard), however, revealed the expected ratio of 1 mol of galactosamine per mol of glucose (Table 2). These results further substantiate that the oligosaccharide from the pool 1 ganglioside is an α-2,3-linked N-acetylsialyllactose whereas the pool 2 and 3 oligosaccharides are N-glycolylsialyllactose molecules.
FIG. 4.
HPAEC-PAD chromatography of ceramide glycanase-released oligosaccharides from purified porcine monosialogangliosides. HPAEC-PAD chromatography of released oligosaccharides was done as described in Materials and Methods, and the profile was compared to the chromatographic mobilities of 3′-sialyllactose, 6′-sialyllactose, and the 3′-oligosaccharide released from mouse liver NeuGcGM3. Top: chromatography of oligosaccharide released from pool 1 (right ordinate) compared to that of 3′-sialyllactose and 6′-sialyllactose (left ordinate). Bottom: chromatography of oligosaccharide released from pool 3 (left ordinate) compared to that of oligosaccharide released from pure NeuGcGM3 (right ordinate).
TABLE 2.
Monosaccharide composition of oligosaccharides released from purified porcine intestinal gangliosidea
| Sample | Amt of monosaccharide released (nmol)b
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Fuc | GalNH2 | GlcNH2 | Gal | Glc | Man | NeuGc | NeuAc | |
| Pool 1 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 1.2 (0.86) | 1.4 (1.0) | 0.0 (0.0) | 0.0 (0.0) | 1.1 (0.79) |
| Pool 3 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 2.7 (0.82) | 3.3 (1.0) | 0.0 (0.0) | 5.0 (1.5) | 0.0 (0.0) |
| GM1 | 0.0 (0.0) | 0.30 (0.70) | 0.0 (0.0) | 0.71 (1.7) | 0.43 (1.0) | 0.0 (0.0) | 0.0 (0.0) | 0.46 (1.1) |
Pools 1 and 3 are porcine intestinal gangliosides purified by HPLC as described in the legend to Fig. 1. The oligosaccharides from each ganglioside pool or bovine brain GM1 standard (Sigma) were released from the ceramide moiety by the use of ceramide glycanase and analyzed for component monosaccharides as described in Materials and Methods.
Values in parentheses are ratios of amounts of monosaccharides to the amounts of glucose released from the purified ganglioside pool or GM1 standard.
O-Acetylated sialic acids are not critical as a recognition epitope for porcine rotavirus.
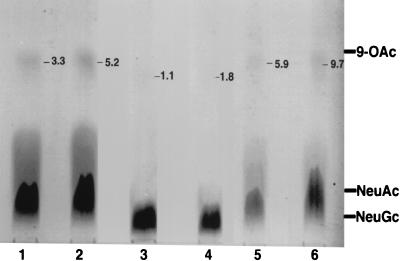
O-Acetylated sialic acids have been previously shown to modulate host cell recognition for viruses such as influenza virus (23). We examined for the presence of O-acetylated sialic acids in our purified porcine intestinal gangliosides and attempted to determine whether these substituents, if present, could function as critical epitopes for rotavirus host cell recognition. During our compositional analyses of the purified gangliosides, we used standard hydrolysis conditions (0.1 N HCl, 80°C, 1 h) to release the sialic acids. Some loss of putative O-acetyl groups may occur under these conditions (36) and certainly during HPAEC-PAD analyses, which are run in a strong base. Therefore, we performed additional analyses under acetic acid hydrolysis conditions, which have been documented to preserve O-acetyl groups (36), and using TLC in lieu of HPAEC-PAD. Figure 5 shows that even when samples were overloaded on the TLC plate in an attempt to detect minor sialic acid derivatives, greater than 90% of the sialic acid component in pool 1 and bovine brain GM3 (used as a standard) was NeuAc. A faint band representing a maximum of 10% of the total sialic acid seen in pool 1 migrated near the 9-O-NeuAc standard. Treatment of the pool 1 ganglioside prior to hydrolysis with NH4OH (to remove O-acetyl groups) reduced the intensity of this band to 5.9% of the total sialic acid but did not eliminate it. For the pool 3 ganglioside, it was even more difficult to detect any O-acetylated sialic acid. Although a trace band corresponding to approximately 1.8% of the total pool 3 sialic acid migrated near the 9-O-acetylsialic acid standard, it also was not eliminated by prior base treatment and was at the limit of detection of the densitometer. Interestingly, when the bovine brain GM3 used as a standard, which is not known to contain O-acetylated sialic acids, was processed identically to the purified porcine intestinal gangliosides, it also produced a minor component (5.2% of the total sialic acid) which migrated near the 9-O-acetylsialic acid standard and which also was reduced (to 3.3%), but not eliminated, by prior base treatment. When O-acetylated sialic acids isolated from bovine submaxillary mucins were treated with NH4OH under the same set of conditions as were used for the gangliosides and then analyzed by TLC, they were found to have been completely converted to nonacetylated sialic acid bands corresponding to NeuGc and NeuAc (data not shown). Because base treatment did not completely eliminate the minor bands migrating in the vicinity of the 9-O-acetylsialic acid standard seen in the pool 1 and pool 3 gangliosides, it is likely that these bands represent sialic acid lactones that formed in acetic acid rather than authentic O-acetylated sialic acids.
FIG. 5.
TLC of sialic acids released from purified porcine intestinal gangliosides and bovine brain GM3. Sialic acid was released from either sham-treated or NH4OH-treated pool 1, pool 3, or standard bovine brain GM3 with acetic acid, and the resultant sialic acid samples were applied to the origin of a glass-backed silica HPTLC plate, developed in ethanol-butanol-pyridine-water-acetic acid (100:10:10:30:3 [vol/vol/vol/vol/vol]), and sprayed with resorcinol to detect sialic acids as described in Materials and Methods. Lanes: 1, NH4OH-treated bovine brain GM3 (35 nmol); 2, sham-treated bovine brain GM3 (35 nmol); 3 and 4, NH4OH-treated (34 nmol) and sham-treated (34 nmol) pool 3, respectively; 5 and 6, NH4OH-treated (25 nmol) and sham-treated (25 nmol) pool 1, respectively. The migration positions of sialic acid standards, NeuGc, NeuAc, and N-5,9-di-O-NeuAc (9-OAc) are shown at the right of the figure. The number near the top right of each lane is the percentage of total sialic acid migrating near the 9-OAc standard, as determined by quantitative densitometry (see Materials and Methods).
To rule out the possibility that the small amount of putative O-acetylated sialic acids seen in pool 1 and pool 3 gangliosides was responsible for the virus-binding-inhibitory activity, pool 1, pool 3, and bovine brain GM3 gangliosides were treated with NH4OH to remove any O-acetyl groups and then examined for virus-binding-inhibitory activity. The results show no differences in the virus-binding-inhibitory activities of base-treated and sham-treated control samples. Binding-inhibitory activities of base-treated pool 1, pool 3, and bovine brain GM3 were 107, 100, and 91% of the sham-treated control values, respectively. If there are minor amounts of O-acetyl groups present in these gangliosides, it seems unlikely they are necessary as a critical virus binding epitope.
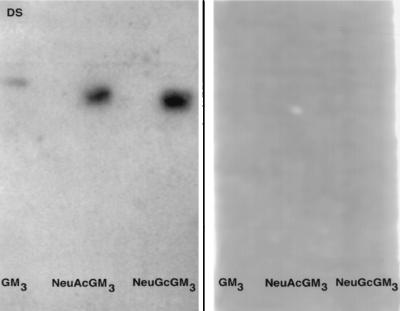
Porcine rotavirus binds directly to purified porcine intestinal GM3.
The results presented in Fig. 2 show that purified GM3 gangliosides (NeuGcGM3 and NeuAcGM3) from porcine enterocytes were active in blocking the binding of 125I-rotavirus to MA-104 cells. TLP also bind directly to the porcine intestinal gangliosides and standard bovine GM3, as determined in a virus overlay assay after chromatography on silica gel TLC plates (Fig. 6, left panel). As in the blocking assay (21), it is clear that virus TLP are required for the interaction with the ganglioside receptor, since DLP virus does not bind to the intact gangliosides (Fig. 6, right panel). This finding lends further support to the hypothesis that these gangliosides are functioning directly as receptors for rotavirus TLP rather than through some indirect inhibitory effect on either host cells or virus during the cell binding and infectivity assays.
FIG. 6.
Direct binding of 125I-rotavirus to purified porcine intestinal gangliosides immobilized on TLC plates. Standard bovine brain GM3 and purified porcine intestinal NeuAcGM3 and NeuGcGM3 were applied to each of two plastic-backed TLC plates, which were subsequently developed, overlayed with TLP (left panel) and DLP (right panel) 125I-rotavirus, and subjected to autoradiography as described in Materials and Methods.
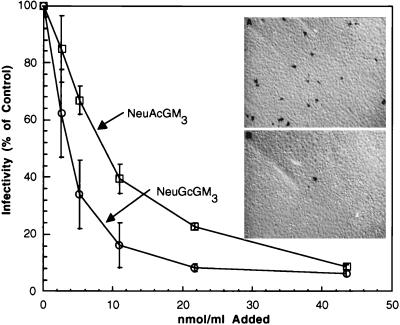
Porcine enterocyte NeuGcGM3 and NeuAcGM3 inhibit virus infectivity.
An immunocytochemical virus focus-forming assay was used to quantify the intracellular production of viral proteins in infected cells in the presence and absence of the purified enterocyte ganglioside NeuGcGM3. When increasing concentrations of enterocyte NeuGcGM3 and NeuAcGM3, over a range of 0 to 43.6 μM, were incubated with rotavirus TLP, a dose-dependent inhibition of virus infectivity with either ganglioside was observed. At the highest concentration tested (43.6 μM, as sialic acid), enterocyte NeuGcGM3 reduced virus progeny production by 90% (Fig. 7). There was an apparent difference in the doses of the two gangliosides required for inhibition of infectivity. Similar to the two- to three-fold-greater virus-binding-inhibitory power of the porcine NeuGcGM3 compared to that of the NeuAcGM3 (Fig. 2), the concentration of NeuGcGM3 required for 50% inhibition of infectivity was 3.97 μM and that of NeuAcGM3 was 9.84 μM. Bovine brain GM3 was a less effective inhibitor (data not shown), requiring a concentration of 15.5 μM for 50% inhibition, similar to the trend seen with bovine brain GM3 during virus binding inhibition assays. NeuGcGM3 appeared to reach a plateau of a maximal inhibition of 90% at a concentration of 21.3 μM. Doubling the NeuGcGM3 concentration did not significantly increase inhibition beyond 90%. NeuAcGM3 approached a similar degree of inhibition at the maximum concentration tested (43.6 μM).
FIG. 7.
Effect of NeuAcGM3 and NeuGcGM3 as competitive inhibitors of porcine rotavirus infection of MA-104 cells. Aliquots of CsCl-purified rotavirus TLP (1.3 × 104 FFU/ml) were incubated at RT for 15 min with 0 to 43.6 nmol of enterocyte NeuAcGM3, enterocyte NeuGcGM3, or bovine brain GM3 per ml. After cells and reagents were cooled to 4°C, triplicate wells in a 24-well plate were inoculated with 75 μl of the virus-ganglioside preparation as described in Materials and Methods. Following a 15-min incubation period, the virus inocula were aspirated and the monolayers were rinsed. The plates were warmed to 37°C and then incubated at that temperature for 16 to 18 h. Fixation of cell monolayers, immunocytochemical staining, and quantification of the FFU were performed as described in Materials and Methods. Symbols: ○, purified porcine NeuGcGM3; □, purified porcine NeuAcGM3. Inset: immunocytochemical detection of rotavirus in MA-104 cell monolayers infected in the presence or absence of NeuGcGM3. Aliquots of CsCl-purified rotavirus TLP (1.3 × 104 FFU/ml) were incubated at RT for 15 min with 0 (top panel) or 43.6 μM (bottom panel) NeuGcGM3 before inoculation of cell monolayers. Following incubation at 37°C for 16 to 18 h, monolayers were fixed and stained as described in Materials and Methods.
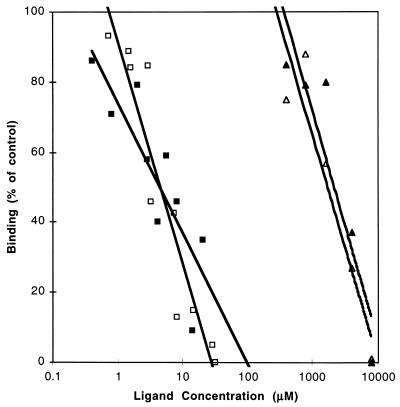
Sialyllactose blocks virus binding, but requires nearly a 1,000 times higher concentration than intact porcine GM3.
As indicated in our previous report (21), treatment of porcine enterocyte gangliosides with ceramide glycanase reduced their effectiveness in blocking the binding of 125I-rotavirus to MA-104 cells. These data suggested that the intact ganglioside is a more potent inhibitor of viral binding than are the free oligosaccharides. In the standard blocking assay, 50 μg (about 80 μM [final concentration]) of standard α2,3- or α2,6-sialyllactose failed to inhibit the binding of rotavirus to MA-104 cells; therefore, we scaled down the blocking assay to a final volume of 100 μl so that we could reduce the amount of sialyllactose needed to reach millimolar concentrations. Figure 8 shows that with this micro version of the standard blocking assay, approximately 5 μM porcine gangliosides are required to inhibit the binding of 125I-rotavirus by 50%. This I50 is in the same range as that seen in the standard blocking assay and validates the use of the microassay to compare the binding-inhibitory activities of individual oligosaccharides and intact gangliosides. In contrast to the data on intact gangliosides, 1.9 mM α2,6-sialyllactose and 2.7 mM α2,3-sialyllactose were required to inhibit rotavirus binding by 50%. Nearly 3 orders of magnitude more oligosaccharide than intact ganglioside is required to effect the same degree of inhibition of rotavirus binding.
FIG. 8.
Effect of introduction of various amounts of ganglioside pools or 3′- or 6′-sialyllactose oligosaccharides on rotavirus binding. Purified intestinal gangliosides (0.4 to 40 μM) and free oligosaccharides (0.4 to 8 mM) were tested for their ability to inhibit binding of 125I-labeled porcine rotavirus to MA-104 cells as described in Materials and Methods (blocking assay 2). The percentage of control binding of 125I-virus is shown versus the log of the micromolar concentration of blocking agent in the final cell suspension. Symbols: ■, NeuGcGM3; □, NeuAcGM3; ▵, 6′-sialyllactose; ▴, 3′-sialyllactose.
Cells deficient in sialic acid do not bind porcine rotavirus.
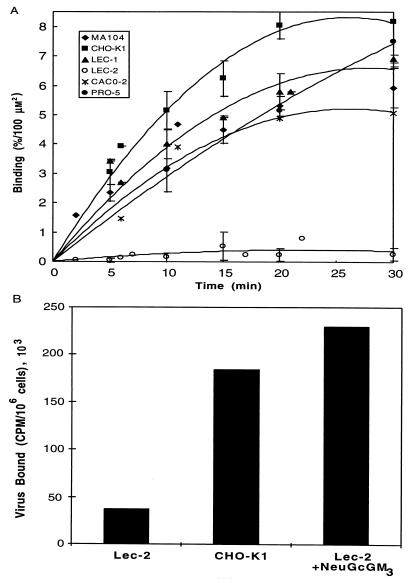
Further confirmation that porcine rotavirus requires sialic acid for host cell recognition was obtained by using Lec-2 cells (a mutant CHO cell line exhibiting a 90% reduction in sialylation of all glycoconjugates due to a drastically reduced rate of transport of CMP-sialic acids into the Golgi apparatus [3]) and Lec-1 cells (a mutant CHO cell line, deficient in GlcNAc-T1 glycosyltransferase, which results in blockage of the glycosylation and processing of N-linked glycoproteins at the Man5GlcNAc2Asn intermediate [30]). Both glycoproteins and glycolipids, of Lec-2 cells are deficient in sialic acid content, whereas Lec-1 cells are primarily deficient in sialic acid content on N-linked glycoproteins. Figure 9A shows the time course of binding of rotavirus TLP to a variety of control cell lines with no known alterations in sialic acid metabolism compared with the two sialic acid-deficient mutant cell lines (Lec-1 and Lec-2) and their parental cell line (Pro-5). Because the cell sizes varied, the surface areas of all cells examined in this experiment were determined from diameter measurements obtained with a Coulter counter. These values were then used to normalize the virus binding data. The cell surface areas ranged from 353 μm2 (Lec-2 cells) to 879 μm2 (Ma-104 cells). All control cell lines, including the Pro-5 parental cell line, from which the sialic acid-deficient mutants were derived, bound rotavirus within a range of 5 to 8% input virus particles bound per 100 μm2 of surface area. Lec-2 cells bound at a rate of 0.26%/100 μm2/30 min, while its parental Pro-5 cell line bound at a rate of 7.5%/100 μm2/30 min. Interestingly, Lec-1 cells, which are primarily deficient in the sialic acid content of N-linked glycoproteins, displayed essentially normal virus binding (6.9%/100 μm2/30 min) compared to any of the control cell lines. These data are consistent with our previous finding that neuraminidase treatment of cells prior to their use in the binding assay inhibits virus binding (21). Since Lec-1 cells are expected to be unaltered in ganglioside content, this result lends further support to the hypothesis that gangliosides, rather than sialoglycoproteins, are utilized in vivo as porcine rotavirus receptors.
FIG. 9.
(A) Comparison of binding of 125I-labeled porcine rotavirus to normal and glycosylation-defective cells. CHO-K1 cells, two lectin-resistant mutants (Lec-1 and Lec-2) and their parental Pro-5 cells, MA-104 cells, and Caco-2 cells were harvested from tissue culture, suspended to a density of 2 × 106 per ml, and tested for their ability to bind 125I-rotavirus as described in Materials and Methods. The percentage bound at each time point divided by the surface area of the respective cell type is plotted versus the length of incubation. Symbols: ○, Lec-2 cells; ×, Caco-2 cells; ⧫, MA104 cells; ▴, Lec-1 cells; ■, CHO cells; •, Pro-5 cells. (B) Reconstitution of rotavirus binding to sialic acid-defective cells by exogenous addition of NeuGcGM3. Lec-2 cells were preincubated with purified porcine intestinal NeuGcGM3, rinsed, and challenged with 125I-rotavirus TLP as described in Materials and Methods. Virus bound per 106 NeuGcGM3-treated, sham-treated, and or CHO-K1 cells is shown.
NeuGcGM3 can reconstitute rotavirus binding in a sialic acid-deficient cell line.
As described above, Lec-2 mutant cells deficient in cell surface sialoglycoconjugates were unable to support rotavirus binding. Since it has been previously shown that exogenously added glycosphingolipids functionally intercalate into cell surface membranes (16), we attempted to establish rotavirus binding in the mutant cells by adding exogenous NeuGcGM3. Preincubation of these cells with excess porcine intestinal NeuGcGM3 prior to challenge with rotavirus resulted in complete reconstitution of virus binding. The amount of virus binding in the ganglioside-reconstituted cells was equal to or greater than that of normal control cells (Fig. 9B). Thus, exogenously added NeuGcGM3 can completely correct the rotavirus binding-deficient phenotype of the Lec-2 mutant cells.
NeuGcGM3 can partially reconstitute rotavirus infectivity in sialic acid-depleted MA-104 cells.
Our previous work (21) as well as that of others (4) demonstrated that treatment of MA-104 cells with neuraminidase dramatically decreased rotavirus binding and that binding could be partially restored following incubation of the neuraminidase-treated cells with exogenous monosialogangliosides. Table 3 shows that, similar to the effect on rotavirus binding, rotavirus infectivity is markedly reduced (5 to 28% of control values) following neuraminidase treatment but can also be partially restored (15 to 47% of control values) by preincubation of the neuraminidase-treated cells with NeuGcGM3.
TABLE 3.
Reconstitution of rotavirus infectivity by NeuGcGM3a
| MA-104 cell treatment | Virus infectivity, FFU/well (mean ± SD)b
|
|
|---|---|---|
| Expt A | Expt B | |
| None | 615 ± 41 (100) | 934 ± 308 (100) |
| Nanase | 33 ± 10 (5) | 266 ± 127 (28) |
| Nanase + NeuGcGM3 | 90 ± 20 (15) | 438 ± 63 (47) |
MA-104 cells in quadruplicate wells of a 24-well plate were incubated with or without neuraminidase, washed, and further incubated with or without NeuGcGM3 as described in Materials and Methods. Following additional washing, the monolayers were incubated with 100 μl of purified porcine rotavirus TLP containing 1.7 (experiment A) or (experiment B) 17 ng of protein for 15 min. The plates were incubated and processed for measurement of FFU as described in the legend to Fig. 7 and in Materials and Methods.
Values in parentheses are percentages of control infectivity determined in the absence of neuraminidase treatment and added ganglioside.
Enterocyte ganglioside concentrations in neonatal pigs rapidly decline.
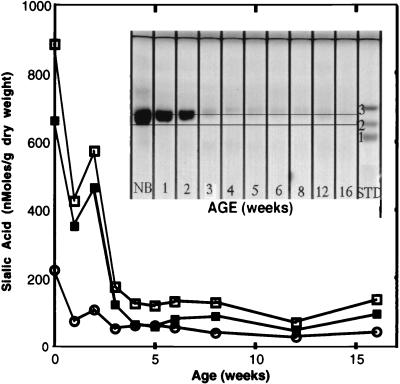
Porcine rotavirus diarrhea is a disease that has a distinct age-associated incidence; it is seen primarily in nursing piglets and during the immediate postweaning period (40). We investigated age-related changes in the concentrations of the enterocyte NeuGcGM3 and NeuAcGM3 ganglioside receptors isolated from the intestinal mucosa of pigs ranging in age from newborn to 16 weeks in order to determine if there is a correlation with the age-related incidence or severity of the natural disease.
Monosialylgangliosides migrating in the NeuAcGM3-NeuGcGM3 region predominate in the enterocyte extracts of all age groups and constitute nearly 100% of the sialic acid-positive bands present in these extracts. Based on this observation, the relative concentrations of NeuAcGM3 and NeuGcGM3 were determined. Since the NeuAcGM3 and NeuGcGM3 TLC bands overlap, TLC densitometry cannot be utilized for quantitation of the individual ganglioside concentrations. Therefore, the relative concentration of each ganglioside in each age group was determined by sialic acid hydrolysis and HPAEC-PAD as described in Materials and Methods. The thin-layer chromatograph shown in Fig. 10 clearly demonstrates that while enterocyte ganglioside concentrations in newborn piglets are high, a rapid and sharp decline in ganglioside concentration occurs over the following 3 to 4 weeks. By 4 to 5 weeks of age, the enterocyte monosialylganglioside bands are barely discernible. The total ganglioside concentration declines from approximately 900 nmol/g to 125 nmol/g over the first 5 weeks of life. NeuGcGM3 is the predominant ganglioside (sialic acid) present in all age groups and constitutes over 75% of the total ganglioside present in newborn pigs. Over a period of 5 weeks, concentrations of NeuGcGM3 decline nearly 10-fold, from approximately 700 nmol/g to 75 nmol/g. In comparison, NeuAcGM3 concentrations decline less than fourfold, from approximately 200 nmol/g to 50 nmol/g, over the same time course. The marked decline in total enterocyte ganglioside concentration is primarily due to a reduction in the NeuGcGM3 concentration.
FIG. 10.
Age-related changes in porcine intestinal NeuGcGM3 and NeuAcGM3. Gangliosides were extracted from intestinal mucosa with solvent, purified by DIPE–1-butanol–NaCl extraction, and analyzed for sialic acid composition as described in Materials and Methods. Enterocyte NeuGcGM3 and NeuAcGM3 ganglioside concentrations are expressed as nanomoles of ganglioside sialic acid per gram (dry weight) of intestinal mucosa. Symbols: ■, NeuGcGM3; ○, NeuAcGM3; □, total sialic acid. Inset: TLC of enterocyte gangliosides. Intestinal mucosa from piglets ranging in age from newborn (NB) to 16 weeks was extracted with DIPE–1-butanol–NaCl to obtain a total-ganglioside extract. Aliquots of the total-ganglioside extract (equivalent to ∼9.3 mg [dry weight] of tissue) were spotted on a pre-channeled TLC plate and developed as described in Materials and Methods. STD, standards GM1 (1), GM2 (2), and GM3 (3).
DISCUSSION
This study confirms and extends our previous findings that porcine enterocyte monosialogangliosides are able to specifically inhibit the binding of rotavirus to host cells and may therefore serve as cellular receptors for porcine rotavirus (OSU strain). In this study, the previously identified porcine enterocyte monsialogangliosides (GMX1 and GMX2) were further purified by HPLC into three ganglioside pools with mobilities between GM3 and GM2. TLC revealed that each pool contained a single, apparently homogeneous ganglioside species. Monosaccharide analysis of each ganglioside showed that there were equimolar amounts of sialic acid, galactose, and glucose per mole of sphingosine. No other sugars were detected. In particular, hexosamine, which would be present in GM2, was not found even though the hydrolysis conditions used were appropriate for its detection (8). Hydrolysis of commercial bovine-brain GM1 showed the expected ratios (1:2:1) of sialic acid, galactose, and galactosamine per mole of glucose. Thus, all three porcine enterocyte gangliosides contained a sialyllactose oligosaccharide and, accordingly, were designated as GM3 gangliosides. Further sialic acid analyses revealed that pool 1 differed qualitatively from pools 2 and 3; it contained only NeuAc, while pools 2 and 3 were found to have only NeuGc.
FAB-MS of the purified, intact gangliosides showed that the major parent ions consisted of 1,167.7 mass units for pool 1 and 1,183.7 mass units for pool 3. The 16-mass unit increase seen for pool 3 is exactly what would be expected if the only difference in these two gangliosides were the addition of a C-5 hydroxyl group in place of a hydrogen atom to form an N-glycolyl group in pool 3, compared to the N-acetyl group in pool 1. These data, along with the results of the monosaccharide analyses, demonstrate that the pool 1 ganglioside is NeuAcGM3 and the pools 2 and 3 gangliosides are NeuGcGM3.
FAB-MS analysis of pool 2 also showed a major fragment at 1,183.7 mass units, although the spectrum was more heterogeneous than that obtained for either pool 1 or 3 (data not shown). This heterogeneity was not unexpected, since multiple small peaks were also seen during HPLC (Fig. 1) even though a single broad band was visualized by TLC. The difference in the mobilities of pool 2 and pool 3 NeuGcGM3 gangliosides on both HPLC and TLC and the multiple fragments seen during FAB-MS of the pool 2 ganglioside may represent fatty acid heterogeneity in the ceramide portion of the ganglioside (7, 24, 28) or different degrees of sialic acid substitution. Although pool 2 was chromatographically distinguishable from pool 3, virus-binding-inhibitory activity, its monosaccharide composition, and preliminary oligosaccharide structure were identical to those of pool 3. Therefore, pool 2 was considered to be a microheterogeneous form of pool 3 (NeuGcGM3) and was not further examined.
Although we have not completely ruled out the existence of O-acetylated sialic acids in the purified intestinal gangliosides, they are certainly not a major component of either NeuGcGM3 or NeuAcGM3. When pools 1 and 3 were hydrolyzed with acetic acid under conditions that preserve O-acetyl groups (36) and analyzed by HPTLC, greater than 90% of the released sialic acid appeared to be NeuAc in the case of pool 1 and NeuGc in the case of pool 3. Less than 10% of the sialic acid released from either of the ganglioside pools migrated on TLC in the vicinity of authentic 9-O-acetylneuraminic acid or with multi-O-acetylated sialic acids released from bovine submaxillary mucins. The minor resorcinol-positive bands that did migrate in the vicinity of O-acetylneuraminic acid standards appeared to be chromatographically identical to that seen with bovine brain GM3, which is not known to contain O-acetylated sialic acids. Furthermore, this material was still seen in pools 1 and 3 and in bovine brain GM3 following exposure to base under conditions that remove O-acetyl groups (6). It is known that NeuAc and NeuGc can form lactones in acetic acid (18). Because these putative O-acetyl sialic acid derivatives were present in only a minor concentration and did not disappear with base treatment of the intact ganglioside, it is likely that they represent corresponding N-acetyl- and N-glycolylneuraminyl lactones generated during exposure to acetic acid, rather than O-acetyl derivatives. More importantly, base treatment of pool 1 and 3 gangliosides showed no reduction in their ability to block the binding of rotavirus to MA-104 cells. Thus, it is unlikely that sialic acid O-acetyl groups are responsible for the in vitro virus-binding-inhibitory activity of the purified porcine enterocyte gangliosides. This apparent absence of an effect of sialic acid O acetylation on porcine OSU rotavirus binding is contrary to the striking effect of 9-O acetylation of 5-acetylneuraminic acid, which prevents binding of influenza A virus (10), or the requirement of O acetylation for attachment of influenza C virus (9). Furthermore, the simian SA-11 rotavirus stain appears to bind preferentially to O-acetylated sialic acids on O-linked glycoconjugates (38). In earlier work (39), the same SA-11 strain was found to recognize asialo-GM1 ganglioside. These seemingly contradictory results could be consistent with the hypothesis, as suggested by these investigators (38), that rotavirus binding and infectivity involve multiple receptors that differ in their preferential recognition by various rotavirus stains.
The structure of the NeuAcGM3 oligosaccharide after its removal from the ceramide backbone by ceramide glycanase was shown to be that of an α2,3-linked N-acetylsialyllactose. The oligosaccharides released from pool 2 and 3 gangliosides (NeuGcGM3) displayed increased polarity, as expected for N-glycolylsialyloligosaccharides, and cochromatographed with a polar oligosaccharide released from authentic NeuGcGM3 (33). This oligosaccharide corresponds to α2,3-linked N-glycolylsialyllactose.
Both porcine enterocyte GM3 gangliosides displayed dose-dependent inhibition of rotavirus binding to MA-104 cells. Additionally, using a cell-free, virus TLC-overlay assay, we also were able to demonstrate that rotavirus binds directly to immobilized enterocyte gangliosides. This result strongly suggests that the ganglioside-mediated inhibition of virus binding and infectivity (see below) observed during in vitro experiments is the result of a direct interaction between virions and exogenous ganglioside receptors rather than an indirect effect of the added gangliosides on the host cells or virus.
While we have shown that micromolar concentrations of intact enterocyte NeuAcGM3 and NeuGcGM3 inhibit rotavirus binding by 50%, millimolar concentrations of the free sialyloligosaccharides were required to inhibit virus binding by the same amount. Since the added gangliosides are in vesicles in aqueous solutions (28), this result lends support to the hypothesis that clustering or multivalency of sialic acid-binding epitopes is critical for rotavirus recognition (21, 39). A similar requirement for receptor multivalency has been shown in a variety of other systems. In a recent study by Simon et al. (26), millimolar concentrations of sialyllactose were necessary to inhibit the binding of Helicobacter pylori to cell monolayers by 50% as compared to micromolar concentrations of a multivalent albumin conjugate of 3′-sialyllactose (20 mol of oligosaccharide per mol of protein). On the basis of moles of 3′-sialyllactose, the multivalent form was approximately 1,000-fold more potent than the monovalent form.
Micromolar concentrations of the purified enterocyte gangliosides also effected dose-dependent inhibition of rotavirus infectivity in MA-104 cells. The porcine NeuGcGM3 showed a slightly (two to threefold) greater potency for blocking rotavirus binding or infection than did the N-acetyl derivative. Although we routinely observe this difference, it is a physiologically small effect compared to the influence of the N-glycolyl versus the N-acetyl group on the susceptibility of pigs to Escherichia coli K99 infection (15, 25, 35). E. coli K99 avidly binds to NeuGcGM3 but does not recognize NeuAcGM3. This result suggests that the sialic acid fine-structural requirement for rotavirus recognition is less stringent than that seen with the K99 pilus of E. coli. Curiously, the purified porcine intestinal NeuAcGM3 (pool 1), which our data indicate is identical in sugar composition and oligosaccharide structure to bovine brain N-acetyl GM3, is two to five times more effective at blocking rotavirus binding to and infectivity of MA-104 cells. Likewise, the purified porcine intestinal NeuAcGM3 immobilized on TLC plates is a more potent ligand than the corresponding bovine brain NeuAcGM3. These data suggest that there is a species- or tissue-specific variance in GM3 structure that is not reflected in sugar composition or sequence data. The ceramide structure of gangliosides often shows a wide variability and can be dependent on the tissue source (7, 24, 28). Furthermore, variations in ceramide structure are known to influence glycolipid function by modifying the relative conformation or mobility of the oligosaccharide moiety in the membrane (1, 17). Thus, it is plausible that the porcine intestinal NeuAcGM3 has a ceramide structure that presents the sialyllactose moiety in a more favorable conformation for rotavirus recognition than exists in the bovine brain NeuAcGM3. Although perhaps less likely, there may also be some direct contribution of the aglycon to rotavirus binding.
While evidence for the requirement for sialic acid-containing glycoconjugates for rotavirus binding accumulates, the relative participation of gangliosides and glycoproteins in virus recognition in vivo is not known. The availability of glycosylation-deficient mutant cell lines allowed us to specifically address the role of sialoglycoconjugates in rotavirus binding. We examined the binding of rotavirus to two glycosylation-defective clones of CHO cells. Lec-2 cells, which exhibit a 90% reduction in sialylation of both glycoproteins and glycolipids due to a drastically reduced rate of transport of CMP-sialic acids into the Golgi apparatus (3), exhibited less than 5% of the rotavirus binding achieved by parental and other control cells. However, Lec-1 cells, which are deficient only in N-linked glycosylation, bound rotavirus at the same rate and to the same extent as control cells. While these data do not unequivocally rule out a role for N-linked sialyloligosaccharides as porcine OSU rotavirus receptors, it is unlikely that they play a major role since a radical reduction in their cell surface concentration had no effect on rotavirus binding. These results also are in agreement with those of our earlier study demonstrating that a variety of serum glycoproteins are unable to inhibit binding of rotavirus to host cells (21). Furthermore, we have been able to completely reconstitute rotavirus binding in a sialic acid-deficient cell line (Lec-2 cells) by exogenous addition of porcine intestinal NeuGcGM3.
Although we were able to reconstitute virus binding in binding-deficient cells with exogenous NeuGcGM3, these data do not address the more interesting question of the relationship between binding to NeuGcGM3 and virus infectivity. In an effort to evaluate whether binding to cell surface NeuGcGM3 is required for, or at least functionally linked to, virus infectivity, we attempted to reconstitute virus infectivity in cells defective for virus binding. Interestingly, even though binding of virus to Lec-2 cells was completely restored following incubation with NeuGcGM3, we were not able to demonstrate reconstitution of infectivity as assayed by either plaque or focus-forming assays. However, when the virus-binding-competent parental Pro-5 and CHO-K1 cells were examined, they too were unable to be infected by the porcine OSU rotavirus strain under the conditions we use to measure MA-104 cell infectivity. Thus, it appears that these parental cells and their mutant cell clones are not permissive for OSU porcine rotavirus infection (we have also been unable to infect these cells with the human Wa rotavirus strain [data not shown]). This result is not particularly surprising since few tissue culture cell lines are permissive to rotavirus in the absence of adaptation by blind passage or culture in primary cells (11, 22, 37). This lack of rotavirus permissivity could be due to a lack of virus entry (because it is not restored by the presence of NeuGcGM3), or it is possible that viral entry occurs but cytoplasmic viral processing or assembly mechanisms are defective in these cells. We do not know whether virus entry occurred in the ganglioside-reconstituted Lec-2 cells; this clearly needs to be examined before conclusions about the role of NeuGcGM3 as a functionally relevant virus receptor in these cells can be made. Regardless, it was of interest to us to further explore the possibility that NeuGcGM3 might serve as a ubiquitous porcine OSU rotavirus-binding ligand. Accordingly, we examined whether NeuGcGM3 could reconstitute virus infectivity in MA-104 cells, which are permissive to the OSU rotavirus strain, that were made virus infectivity deficient by treatment with neuraminidase. Following preincubation of the MA-104 cells with neuraminidase, which reduced virus infectivity by 78 to 95%, the incorporation of NeuGcGM3 prior to virus challenge reconstituted from 15 to 47% of the infectivity, depending on the virus dose, compared to the non-neuramidase-treated control cells. The incomplete restoration of infectivity may suggest that proper intercalation of the ganglioside, rather than simple adherence to the cell surface, is required for viral entry and infectivity compared to virus binding. It is likely that virus infectivity is a dynamic process requiring multiple receptor interactions along the pathway from initial host cell recognition to virus entry. Therefore, the correct orientation of the ganglioside with other receptor molecules may be required for optimal infectivity of host cells. Regardless, these data suggest that, at least for the OSU strain of porcine rotavirus, NeuGcGM3 can serve as an in vivo-relevant virus receptor.
Porcine rotavirus diarrhea is a disease that has a distinct age-associated incidence; it is seen primarily in nursing piglets and during the immediate postweaning period (5, 11). To determine whether there is a correlation between the age-related incidence of natural disease and ganglioside receptor concentrations, this study investigated the age-related changes in the concentrations of NeuGcGM3 and NeuAcGM3 isolated from the intestinal mucosa of pigs ranging from newborn to 16 weeks of age. While the dramatic decline in NeuGcGM3, and especially NeuAcGM3, by 4 weeks of age appears to precede the decline in age-related incidence of spontaneous rotavirus disease in pigs (5), it is closely correlated with age-related severity of natural rotavirus disease. Newborn piglets are especially prone to rotavirus mortality, which is likely due in part to the number of infected enterocytes and longer time for crypt-to-enterocyte maturation compared to older or weanling animals. The relatively large amounts of NeuGcGM3 may well contribute to the increased enterocyte virus load, resultant cell loss, and death seen in newborn animals.
Not all cell lines that can bind rotavirus can support the entire process of virus binding, entry, and replication. Further study is required to fully delineate the complete mechanism of host cell infection. What is profoundly clear, however, is that porcine OSU rotavirus has an affinity for NeuAcGM3 and NeuGcGM3 and that we may be able to exploit this activity for treatment of rotavirus disease. Our current work involves further characterization of the role of the porcine GM3 rotavirus receptor in viral entry into the host cell. We are also actively pursuing the design of active derivatives of NeuGcGM3 that when presented in an appropriate manner—on a molecule incorporated into piglet creep feed, for example—might prove to be useful agents for the receptor-therapeutic prevention or control of rotavirus disease.
ACKNOWLEDGMENTS
M.D.R. and T.B.K. contributed equally to this work.
This work was supported in part by grants from the U.S. Department of Agriculture NRICGP (ILLU-44-6310 and ILLU-44-6225), the Illinois Department of Agriculture (ILLU-70-0203), and the North Central Region USDA-CSRS Regional Research Funds. The ZAB-SE mass spectrometer used at the University of Illinois School of Chemical Sciences Mass Spectrometry Laboratory for these analyses was purchased in part with funds from grants from the Division of Research Resources, National Institutes of Health (RR01575), The National Science Foundation (PCM8121494), and The National Institute of General Medical Sciences (GM27029).
We thank William Hanafin for expert technical assistance.
REFERENCES
- 1.Arab S, Lingwood C A. Influence of phospholipid chain length on verotoxin/globotriaosyl ceramide binding in model membranes: comparison of a supported bilayer film and liposomes. Glycoconj J. 1996;13:159–166. doi: 10.1007/BF00731490. [DOI] [PubMed] [Google Scholar]
- 2.Bass D M, Mackow E R, Greenberg H B. Identification and partial characterization of a rhesus rotavirus binding glycoprotein on murine enterocytes. Virology. 1991;183:602–610. doi: 10.1016/0042-6822(91)90989-o. [DOI] [PubMed] [Google Scholar]
- 3.Deutscher S L, Nuwayhid N, Stanley P, Briles E I B, Hirschberg C B. Translocation across Golgi vesicle membranes: a CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell. 1984;39:295–299. doi: 10.1016/0092-8674(84)90007-2. [DOI] [PubMed] [Google Scholar]
- 4.Fukudome K, Yoshie O, Konno T. Comparison of human, simian, and bovine rotaviruses for requirement of sialic acid in hemagglutination and cell adsorption. Virology. 1989;172:196–205. doi: 10.1016/0042-6822(89)90121-9. [DOI] [PubMed] [Google Scholar]
- 5.Gelberg H B. Studies on the age-resistance of swine to group A rotavirus infection. Vet Pathol. 1992;29:161–168. doi: 10.1177/030098589202900209. [DOI] [PubMed] [Google Scholar]
- 6.Ghidoni R, Sonnino S, Tettamanti G, Baumann N, Reuter G, Schauer R. Isolation and characterization of a trisialoganglioside from mouse brain containing 9-O-acetyl-N-acetylneuraminic acid. J Biol Chem. 1980;255:6990–6695. [PubMed] [Google Scholar]
- 7.Hakomori S, Nudelman E, Levery S B, Kannagi R. Novel fucolipids accumulating in human adenocarcinoma. J Biol Chem. 1984;259:4672–4680. [PubMed] [Google Scholar]
- 8.Hardy M R, Townsend R R. High pH anion-exchange chromatography of glycoprotein derived carbohydrate. Methods Enzymol. 1994;230:208–225. doi: 10.1016/0076-6879(94)30014-3. [DOI] [PubMed] [Google Scholar]
- 9.Herrler G, Reuter G, Rott R, Klenk H D, Schauer R. N-Acetyl-9-O-acetylneuraminic acid, the receptor determinant for influenza C virus, is a differentiation marker on chicken erythrocytes. Biol Chem Hoppe-Seyler. 1987;368:451–454. doi: 10.1515/bchm3.1987.368.1.451. [DOI] [PubMed] [Google Scholar]
- 10.Higa H H, Rogers G N, Paulson J C. Influenza virus hemagglutinin differentiates between receptor determinants bearing N-acetyl-, N-glycollyl and N,O-diacetylneuraminic acid. Virology. 1985;144:279–282. doi: 10.1016/0042-6822(85)90325-3. [DOI] [PubMed] [Google Scholar]
- 11.Kapikian A Z, Chanock R M. Rotaviruses. In: Fields B N, Knipe D M, Chanock R M, Hirsch M S, Melnick J L, Monath T P, Roizman B, editors. Virology. 2nd ed. New York, N.Y: Raven Press; 1990. pp. 1353–1404. [Google Scholar]
- 12.Karlsson K A, Stromberg N. Overlay and solid phase analysis of glycolipid receptors for bacteria and viruses. Methods Enzymol. 1987;138:220–232. doi: 10.1016/0076-6879(87)38019-x. [DOI] [PubMed] [Google Scholar]
- 13.Kates M. Techniques of lipidology. In: Burdon R H, van Knippenberg P H, editors. Laboratory techniques in biochemistry and molecular biology. Vol. 3. New York, N.Y: Elsevier; 1986. pp. 186–278. [Google Scholar]
- 14.Kuhlenschmidt M S, Rolsma M D, Kuhlenschmidt T B, Gelberg H B. Characterization of a porcine enterocyte receptor for group A rotavirus. Adv Exp Med Biol. 1997;412:135–143. doi: 10.1007/978-1-4899-1828-4_21. [DOI] [PubMed] [Google Scholar]
- 15.Kyogashima M, Ginsburg V, Krivan H C. Escherichia coli K99 binds to N-glycolylsialoparagloboside and N-glycolyl-GM3 found in piglet small intestine. Arch Biochem Biophys. 1989;270:391–397. doi: 10.1016/0003-9861(89)90042-8. [DOI] [PubMed] [Google Scholar]
- 16.Laine R A, Hakomori S. Incorporation of exogenous glycosphingolipids in plasma membranes of cultured hamster cells and concurrent change of growth behavior. Biochem Biophys Res Commun. 1973;54:1039–1045. doi: 10.1016/0006-291x(73)90798-5. [DOI] [PubMed] [Google Scholar]
- 17.Lingwood C A. Aglycone modulation of glycolipid receptor function. Glycoconj J. 1996;13:495–503. doi: 10.1007/BF00731435. [DOI] [PubMed] [Google Scholar]
- 18.Manzi A E, Dell A, Azadi T, Varki A. Studies of naturally occurring modifications of sialic acid by fast atom bombardment mass spectrometry. Analysis of positional isomers by periodate cleavage. J Biol Chem. 1990;265:8094–8107. [PubMed] [Google Scholar]
- 19.Naoi M, Lee Y C, Roseman S. Rapid and sensitive determination of sphingosine bases and sphingolipid with fluorescamine. Anal Biochem. 1974;58:571–577. doi: 10.1016/0003-2697(74)90226-7. [DOI] [PubMed] [Google Scholar]
- 20.Powell L D, Hart G W. Quantitation of picomole levels of N-acetyl- and N-glycolylneuraminic acids by HPLC-adaptation of the thiobarbituric acid assay. Anal Biochem. 1986;157:179–185. doi: 10.1016/0003-2697(86)90211-3. [DOI] [PubMed] [Google Scholar]
- 21.Rolsma M D, Gelberg H B, Kuhlenschmidt M S. Assay for evaluation of rotavirus-cell interactions: identification of an enterocyte ganglioside fraction that mediates porcine group A porcine rotavirus recognition. J Virol. 1994;68:258–268. doi: 10.1128/jvi.68.1.258-268.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saif L J, Rosen B I, Kang S-Y, Miller K L. Cell culture propagation of rotaviruses. J Tissue Culture Methods. 1988;11:147–156. doi: 10.1007/BF01404267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schauer R. Biosynthesis and function of N- and O-substituted sialic acids. Glycobiology. 1991;1:449–452. doi: 10.1093/glycob/1.5.449. [DOI] [PubMed] [Google Scholar]
- 24.Schnaar R L. Isolation of glycosphingolipids. Methods Enzymol. 1994;230:348–370. doi: 10.1016/0076-6879(94)30024-0. [DOI] [PubMed] [Google Scholar]
- 25.Seignole D, Mouricout M, Duval-Iflah Y, Quintard B, Julien R. Adhesion of K99 fimbriated Escherichia coli to pig intestinal epithelium: correlation of adhesive and non-adhesive phenotypes with the sialoglycolipid content. J Gen Microbiol. 1991;137:1591–1601. doi: 10.1099/00221287-137-7-1591. [DOI] [PubMed] [Google Scholar]
- 26.Simon P M, Goode P L, Mobasseri A, Zopf D. Inhibition of Helicobacter pylori binding to gastrointestinal epithelial cells by sialic acid-containing oligosaccharides. Infect Immun. 1997;65:750–757. doi: 10.1128/iai.65.2.750-757.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 28.Sonnino S, Cantu L, Corti M, Acquotti D, Venerando B. Aggregative properties of gangliosides in solution. Chem Phys Lipids. 1994;71:21–45. doi: 10.1016/0009-3084(94)02304-2. [DOI] [PubMed] [Google Scholar]
- 29.Stanley P, Caillibot V, Siminovitch L. Selection and characterization of eight phenotypically distinct lines of lectin-resistant Chinese hamster ovary cells. Cell. 1975;6:121–128. doi: 10.1016/0092-8674(75)90002-1. [DOI] [PubMed] [Google Scholar]
- 30.Stanley P, Narasimhan S, Siminovitch L, Schachter H. Chinese hamster ovary cells selected for resistance to the cytotoxicity of phytohemagglutinin are deficient in a UDP-N-acetylglucosamine-glycoprotein N-acetylglucosaminyltransferase activity. Proc Natl Acad Sci USA. 1975;72:3323–3327. doi: 10.1073/pnas.72.9.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanley P, Siminovitch L. Complementation between mutants of CHO cells resistant to a variety of plant lectins. Somatic Cell Genet. 1977;3:391–405. doi: 10.1007/BF01542968. [DOI] [PubMed] [Google Scholar]
- 32.Superti F, Donelli G. Gangliosides as binding sites in SA-11 rotavirus infection of LLC-MK2 cells. J Gen Virol. 1991;72:2467–2474. doi: 10.1099/0022-1317-72-10-2467. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki M, Nakamura K, Yasuhiro H, Suzuki A, Yamakawa T. Mouse liver gangliosides. Carbohydr Res. 1986;151:213–223. doi: 10.1016/s0008-6215(00)90342-2. [DOI] [PubMed] [Google Scholar]
- 34.Svennerholm L, Fredman P. A procedure for the quantitative isolation of brain gangliosides. Biochim Biophys Acta. 1980;617:97–109. doi: 10.1016/0005-2760(80)90227-1. [DOI] [PubMed] [Google Scholar]
- 35.Teneberg S, Willemsen P, de Graaf F K, Karlsson K A. Receptor-active glycolipids of epithelial cell of the small intestine of young and adult pigs in relation to susceptibility to infection with Escherichia coli K99. FEBS Lett. 1990;263:10–14. doi: 10.1016/0014-5793(90)80693-d. [DOI] [PubMed] [Google Scholar]
- 36.Varki A, Diaz S. The release and purification of sialic acids from glycoconjugates: methods to minimize the loss and migration of O-acetyl groups. Anal Biochem. 1984;137:236–247. doi: 10.1016/0003-2697(84)90377-4. [DOI] [PubMed] [Google Scholar]
- 37.Ward R L, Knowlton D R, Pierce M J. Efficiency of human rotavirus propagation in cell culture. J Clin Microbiol. 1984;19:748–753. doi: 10.1128/jcm.19.6.748-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willoughby R E. Rotaviruses preferentially bind O-linked sialylglycoconjugates and sialomucins. Glycobiology. 1993;3:437–445. doi: 10.1093/glycob/3.5.437. [DOI] [PubMed] [Google Scholar]
- 39.Willoughby R E, Yolken R H, Schnaar R L. Rotaviruses specifically bind to the neutral glycosphingolipid asialo-GM1. J Virol. 1990;64:4830–4835. doi: 10.1128/jvi.64.10.4830-4835.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woode G N. Porcine rotavirus infection. In: Lehman A D, Straw B, Glock R D, Mengeling W L, Penny R H C, School E, editors. Diseases of swine. 6th ed. Ames: Iowa State University Press; 1986. pp. 368–382. [Google Scholar]
- 41.Zhou B, Li S-C, Laine R A, Huang R T C, Li Y-T. Isolation and characterization of ceramide glycanase from the leech, Macrobdella decora. J Biol Chem. 1989;264:12272–12277. [PubMed] [Google Scholar]