Abstract
INTRODUCTION
We set out to map evidence of disparities in Alzheimer's disease and Alzheimer's disease related dementias healthcare, including issues of access, quality, and outcomes for racial/ethnic minoritized persons living with dementia (PLWD) and family caregivers.
METHODS
We conducted a scoping review of the literature published from 2000 to 2022 in PubMed, PsycINFO, and CINAHL. The inclusion criteria were: (1) focused on PLWD and/or family caregivers, (2) examined disparities or differences in healthcare, (3) were conducted in the United States, (4) compared two or more racial/ethnic groups, and (5) reported quantitative or qualitative findings.
RESULTS
Key findings include accumulating evidence that minoritized populations are less likely to receive an accurate and timely diagnosis, be prescribed anti‐dementia medications, and use hospice care, and more likely to have a higher risk of hospitalization and receive more aggressive life‐sustaining treatment at the end‐of‐life.
DISCUSSION
Future studies need to examine underlying processes and develop interventions to reduce disparities while also being more broadly inclusive of diverse populations.
Keywords: dementia, dementia disparities, health equity, healthcare disparities, racial/ethnic disparities
1. INTRODUCTION
As the older adult population in the United States, including those living with dementia, becomes more ethnically and racially diverse, there is increased recognition of the importance of conducting research to identify and eliminate disparities in the care and outcomes for persons living with dementia (PLWD). 1 , 2 Reducing disparities in both Alzheimer's disease and Alzheimer's disease related dementias (AD/ADRD) disease burden and receipt of timely, high‐quality healthcare services is essential to advance health equity. 2 Despite substantial under‐representation of minoritized populations in AD/ADRD research, 3 there is now growing evidence of higher levels of AD/ADRD‐related risk factors in certain minoritized communities 4 , 5 , 6 as well as higher incidence 7 , 8 and prevalence rates 9 , 10 of AD/ADRD. The COVID‐19 pandemic further underscored the need for research on health and healthcare disparities for minoritized persons, especially those with dementia and their family caregivers. 11 , 12
An increasing number of studies have examined healthcare disparities for PLWD and family caregivers, from diagnosis to end‐of‐life care. 2 , 13 More than a decade ago, a meta‐analysis found evidence that Black and Hispanic American older adults with dementia access diagnostic services later in the course of their illness than non‐Hispanic White (NHW) older adults and were less likely to receive anti‐dementia medications. 14 Another review concluded that Hispanic and Black American older adults often receive delayed or inadequate healthcare services or were diagnosed in an emergency department (ED) or other non‐traditional setting. 15 Finally, a more recent review of access to health services among PLWD found that Black Americans were more likely be hospitalized. 16 Barriers to healthcare may stem from many sources, including inadequate health insurance, limited English language proficiency, transportation barriers, lack of specialty clinics in disadvantaged communities, 17 , 18 lack of diversity in the healthcare profession, and discrimination when seeking care.
As momentum builds to advance care and services for PLWD in healthcare systems, there is an urgent need to identify issues of access, quality, and outcomes for AD/ADRD in existing healthcare systems. 19 This study's goal was to map the evidence of disparities in AD/ADRD healthcare including issues of access, quality, and outcomes for racial/ethnic minoritized PLWD and their family caregivers, and to identify gaps in the field. This scoping review was undertaken because of the exponential growth of research in this area over the past decade and the need to both map and synthesize existing evidence to guide future systematic and/or meta‐analytic reviews and research. 20
2. METHODS
Our methodological approach for this scoping review was guided by Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines 21 for scoping reviews as well as the framework of Arksey and O'Malley. 22 To define the scope of our review, we focused on areas of healthcare that are included in the following definition of a healthcare disparity from the Kaiser Family Foundation to narrow the scope of our study: “A healthcare disparity typically refers to differences between groups in health insurance coverage, access to and use of care, and quality of care.” 23
2.1. Inclusion and exclusion criteria
Studies were included if they (1) focused on PLWD and/or their family caregivers, (2) examined disparities or differences in healthcare, (3) were conducted in the United States, (4) included the comparison of two or more racial/ethnic groups, (5) reported quantitative or qualitative findings, and (6) were published in a peer‐reviewed journal with full‐text available in English from January of 2000 to July of 2022. We excluded studies that used case‐study study designs as well as editorials, commentaries, and reviews.
We included a variety of settings, including hospitals, clinics, home‐based primary care, and long‐term care facilities, including healthcare delivered to the person with dementia and services or support delivered to a family member related to AD/ADRD.
RESEARCH IN CONTEXT
Systematic review: The scoping review was conducted to map evidence of disparities in healthcare including issues of access, quality, and outcomes for racial/ethnic minoritized persons living with dementia (PLWD) and their family caregivers and to identify gaps in the field.
Interpretation: Minoritized populations are less likely to receive an accurate and timely diagnosis, be prescribed with anti‐dementia medications, and use hospice care, and more likely to have a higher risk of hospitalization and receive more aggressive life‐sustaining treatment at the end of life.
Future directions: Future studies need to examine underlying processes and develop interventions to reduce disparities while also being more broadly inclusive of diverse populations.
2.2. Search strategy
This scoping review was conducted of the literature published from 2000 to 2022 in PubMed, PsycINFO, and CINAHL. To generate the search terms, we began with the MEDLINE/PubMed National Institute on Minority Health and Health Disparities Strategy 24 suggested search terms for health disparity populations, eliminated terms that were not relevant to this search, and added additional terms. Next, we added search terms for dementia due to AD and other related degenerative dementias (see the Appendix in supporting information for search terms).
A multi‐step process was used to identify records meeting inclusion criteria. First, duplicates were removed. Second, the remaining record abstracts were pulled and screened for eligibility by two independent reviewers. A random subset of 100 record abstracts was pulled and independently reviewed by the two study members, who achieved agreement at a level of kappa > 0.8, prior to screening the remaining abstracts. Full‐length articles were pulled for all abstracts that met eligibility criteria and were reviewed by the two independent study members to determine eligibility for inclusion. Discrepancies at the abstract or full‐length article review stage were resolved through team review and discussion until arriving at a consensus.
2.3. Data extraction and synthesis
Characteristics of full‐length articles meeting inclusion criteria were extracted and charted into tables. Extraction was done independently by two research staff who then compared their results and resolved any discrepancies in consultation with other authors. Data extraction elements included: study author and year of publication, stage of care and treatment (i.e., initial assessment, diagnosis, and referral; ongoing treatment and support; later stages of illness including end‐of‐life care), study design (i.e., cross‐sectional, longitudinal), racial/ethnic groups included, setting, primary study objective, whether racial/ethnic disparities or differences were a stated purpose of the study, how the sample was identified, and any reported differences in healthcare between racial/ethnic groups.
To synthesize the data, the lead author reviewed the findings from the studies in the chart and generated a list of healthcare categories that captured the range of findings from the studies. Multiple team members reviewed the categories, reviewed the chart data, and then the team developed a consensus about the categories related to healthcare that best described the findings/results. Findings from the studies were then categorized according to these healthcare categories. The abstracted results are the basis for the summaries in the next section. In abstracting and reporting the results in tables, we retained the racial/ethnic categories used by the study authors. To develop the final tables, we focused on reporting statistically significant (i.e., P < 0.05) results comparing two or more racial/ethnic categories from the studies related to each of the healthcare domains.
3. RESULTS
3.1. Search results
The search yielded 8550 articles and 6818 unique articles after removing duplicates. An additional 6729 articles were removed based on the review of record abstracts, leaving 89 articles. After review of full‐length articles for these 89 studies, 71 met full inclusion criteria (see Figure 1).
FIGURE 1.
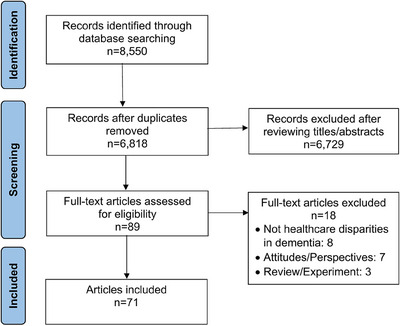
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow diagram of the study selection process.
3.2. Characteristics of studies
Of the 71 studies identified, all but one study reported one or more statistically significant differences between racial/ethnic groups. The most frequently addressed topic (see Figure 2) was medication treatment (38%), followed by end‐of‐life care (22.5%), health service use (18.3%), diagnosis (15.5%), family caregiving (7%), and long‐term care (5.6%). In terms of findings regarding representation of minoritized populations, 85.9% of studies reported findings for Black Americans, 57.7% for Hispanic Americans, 25.4% for Asian Americans/Pacific Islanders (AA/PI), 2.8% for American Indians/Alaska Natives (AI/AN), and 32.4% reported “other” or aggregate categories (e.g., other, non‐White, minority). For most studies, NHW was the reference for comparison. Overall, 18.3% (n = 13) of studies did not include racial/ethnic differences or disparities as a stated aim or focus but reported relevant findings in their results section. Studies varied widely in size and were primarily observational covering a range of services and settings, including community, outpatient clinics, EDs, hospitals, and long‐term care.
FIGURE 2.
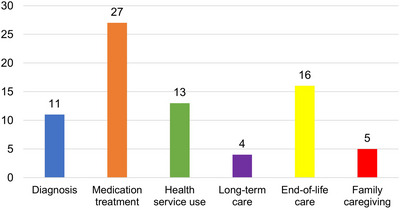
Number of articles for each healthcare domain (n = 71). Some studies report findings across multiple healthcare domains.
3.3. Synthesis of study results
We synthesized our results by grouping them into six broad topic areas reflecting different aspects of healthcare for PLWD: (1) diagnosis (timeliness, accuracy, initial referral), (2) medications (anti‐dementia medications, psychotropics, and medications for other health conditions), (3) healthcare service use and costs (excluding studies of healthcare services specific to the end of life), (4) long‐term care, (5) end‐of‐life care, and (6) family caregiving (see Figure 2). For each of these categories, Tables 1, 2, 3, 4, 5, 6 summarize statistically significant results (i.e., P < 0.05) for racial/ethnic comparisons with some of the studies reporting findings that crossed several topic areas.
TABLE 1.
Diagnosis (n = 11).
| Author, year | Study design |
Total sample (N), Racial/ethnic populations |
Results |
|---|---|---|---|
|
Chen et al., 27 2019 |
Longitudinal, Health, and Retirement Study linked to Medicare claims, 2000–2008 |
N = 10,450 Year 2000: White: n = 86.8% Black: n = 9.0% Hispanic: n = 4.1% |
|
|
Gianattasio et al., 28 2019 |
Longitudinal, Health and Retirement Study linked to fee‐for‐service Medicare claims, 2000–2010 |
N = 4647–5201 (varies by wave) Non‐Hispanic White: n = 91%–93% Non‐Hispanic Black: n = 7%–9% |
|
|
Amjad et al., 29 2018 |
Cross‐sectional, National Health, and Aging Trends Study linked to retrospective Medicare A and B claims data, 2011 |
N = 585 Undiagnosed: N = 229 Non‐Hispanic White: n = 63.7% Non‐Hispanic Black: n = 12.5% Hispanic or other: n = 21.4% |
|
|
Tsoy et al., 30 2021 |
Cross‐sectional, California Medicare fee‐for‐service data, 2013–2015 |
N = 10,472 Asian: n = 993 Black: n = 407 Hispanic: n = 1255 White: n = 7817 |
|
|
Lin et al., 31 2021 |
Longitudinal, Health and Retirement Study linked to Medicare and Medicaid claims, 2000–2014 |
N = 3966 Non‐Hispanic White: n = 2908 Non‐Hispanic Black: n = 687 Hispanic: n = 371 |
|
|
White et al., 32 2022 |
Longitudinal, Health and Retirement Study, 1995–2016 |
N with incident dementia = 4760 Non‐Hispanic White: n = 3039 Non‐Hispanic Black: n = 1031 Hispanic: n = 584 Other: n = 106 |
|
|
Kalkonde et al., 33 2009 |
Cross‐sectional, Veterans Affairs medical clinic chart data, 2004–2005 |
N = 410 Caucasian: n = 265 African American: n = 109 Hispanic: n = 34 Other ethnic groups: n = 2 |
|
|
Drabo et al., 34 2019 |
Longitudinal, Medicare claims data, 2008/2009 |
N = 226,604 White: n = 196,180 Black: n = 17,261 Hispanic: n = 9540 Asian: n = 3623 |
|
|
Chow et al., 35 2000 |
Longitudinal, Minimum Uniform Dataset from California Alzheimer's Disease Diagnostic and Treatment Centers, 1988, 1993, 1995 |
N = 9451 Asian (primarily Chinese): n = 4.2%, Filipino: n = 0.8%, Pacific Islander: n = 0.3% Caucasian: n = 75.9% |
|
|
Boustani et al., 36 2006 |
Cross‐sectional |
N = 434 White: n = 139 African American: n = 294 |
|
|
Hinton et al., 37 2004 |
Cross‐sectional, qualitative interview |
N = 39 Black: n = 10 Chinese: n = 14 Anglo Euro‐American: n = 15 |
|
TABLE 2.
Anti‐dementia, psychotropic, and other medication use (n = 26).
| Author, Year | Study design, data source if specified |
Total sample (N), Racial/ethnic populations |
Results |
|---|---|---|---|
|
Kalkonde et al., 33 2009 |
Cross‐sectional, Veterans Affairs medical clinic chart data, 2004–2005 |
N = 410 Caucasian: n = 265 African American: n = 109 Hispanic: n = 34 Other ethnic groups: n = 2 |
|
|
Lind et al., 38 2018 |
Longitudinal, Cost and Use Medicare Current Beneficiary Survey, 2003–2013 |
N = 4304 Non‐Hispanic White: n = 5586 Non‐Hispanic Black: n = 750 Hispanic: n = 530 Other: n = 359 (Note: racial/ethnic group n’s reported in person years) |
|
|
Mehta et al., 39 2005 |
Cross‐sectional, Minimum Uniform Dataset from Alzheimer's Disease Research Centers of California, 1999–2003 |
N = 2573 African American: n = 6% Asian: n = 7% Latino: n = 14% Other ethnicity: n = 2% White: n = 71% |
|
|
Giebel et al., 40 2020 |
Longitudinal, National Alzheimer's Coordinating Center, 2005–2019 |
N = 15,742 White: n = 82.4% Minority: n = 17.6% |
|
|
Lerner et al., 41 2008 |
Cross‐sectional |
N = 117 White: n = 94 African American: n = 23 |
|
|
McClendon et al., 42 2009 |
Cross‐sectional, Uniform Data Set from National Alzheimer's Coordinating Center, 2005–2007 |
Total N = 2512 N with Alzheimer's disease = 877 Hispanic/Latino: n = 7% Non‐Hispanic White: n = 80% Non‐Hispanic Black: n = 10% N with other dementia = 219 Hispanic/Latino: n = 4% Non‐Hispanic White: n = 86% Non‐Hispanic Black: n = 7% |
|
|
Barthold et al., 43 2020 |
Longitudinal, random 20% sample of Medicare beneficiaries, 2008–2016 |
N = 721,878 White: n = 600,358 Black: n = 57,412 Hispanic: n = 44,082 Asian: n = 20,026 |
|
|
Hernandez et al., 44 2010 |
Cross‐sectional, Uniform Data Set from National Alzheimer Coordinating Center, 2005–2007 |
N = 3049 White: n = 77.63% Black: n = 14.56% Non‐Black Hispanic: n = 7.81% |
|
|
Zuckerman et al., 45 2008 |
Longitudinal, Medicare Current Beneficiary Survey, 2001–2003 |
N = 1120 Non‐Hispanic White: n = 855 Non‐Hispanic Black: n = 131 Hispanic: n = 91 Other: n = 43 |
|
|
Poon et al., 46 2009 |
Longitudinal, the Veterans Health Administration: the Patient Treatment Files and the Pharmacy Benefit Management, 2000–2005 |
N = 56,561 White: n = 70.5% African American: n = 15.6% Hispanic: n = 6.6% Other races: n = 0.5% Unknown: n = 6.8% |
|
|
Sano et al., 47 2005 |
Cross‐sectional, baseline data of phase 4 study of patients with mild to moderate AD, 2001 |
N = 2114, Patient: N = 2105 White: n = 1807 Black: n = 143 Hispanic: n = 131 Asian n = 18 Other: n = 6 |
|
|
Zhu et al., 48 2022 |
Longitudinal, Uniform Data Set from National Alzheimer's Coordinating Center, 2005–2020 |
N = 3276 Non‐Hispanic White: n = 2454 Non‐Hispanic Black: n = 532 Hispanic: n = 290 |
|
|
Thorpe et al., 49 2016 |
Longitudinal, Medicare enrollment, Part A and B, medical claims, and Part D prescription data for a 10% national sample of Medicare fee‐for‐service beneficiaries, 2009–2010 |
N = 84,043 Non‐Hispanic White: n = 66,806 Non‐Hispanic Black: n = 9781 Hispanic: n = 7456 |
|
|
Gilligan et al., 50 2012 |
Cross‐sectional, Medicaid Analytic extract file from the Centers for Medicare and Medicaid Services, 2004 |
N = 158,974 White: n = 88,529 Black: n = 16,180 Hispanic: n = 21,483 Other: n = 10,649 Unknown: n = 22,133 |
|
|
Perryman et al., 51 2009 |
Longitudinal, Medicare Current Beneficiary Survey Cost and Use Files, 2000–2002 |
Y2000: N = 347, Y2001: N = 367, Y2002: N = 412 N by Year, 2000, 2001, 2002 Caucasian: n = 278, 291, 328 African American: n = 50, 52, 57 Hispanic: n = 9, 10, 12 |
|
|
Xiong et al., 52 2015 |
Cross‐sectional, National Alzheimer's Coordinating Center, 2008–2014 |
N = 8919 African American: n = 983 Hispanic: n = 849 Non‐Hispanic White: n = 7,087 |
|
|
Filshtein et al., 53 2016 |
Cross‐sectional, National Alzheimer's Coordinating Center, 2008–2014 |
N = 4741 Black: n = 401 Hispanic: n = 337 Non‐Hispanic White: n = 3389 |
|
|
Hsieh et al., 54 2021 |
Cross‐sectional, Medicare Current Beneficiary Survey, 2015–2017 |
N = 4,953,945 Non‐Hispanic White: n = 78.66% Non‐Hispanic Black: n = 9.67% Hispanic: n = 7.22% Other: n = 4.45% |
|
|
Grace et al., 55 2018 |
Cross‐sectional, baseline data of the Resources for Enhancing Alzheimer's Caregiver Health II |
N = 543 Non‐Hispanic White: n = 198 African American: n = 176 Hispanic/Latino: n = 169 |
|
|
Rivera‐Hernandez et al., 56 2022 |
Cross‐sectional, Residential History File, Medicare Beneficiary Summary File, Minimum Data Set, Certification and Survey Provider Enhanced Reports and the Long‐term Care: Facts on Care in the US, 2007 |
N = 1,005,781 White: n = 78% Black: n = 13% Hispanic: n = 6% Asian/Pacific Islander: n = 2% American Indian/Alaska Native: n = 0.4% |
|
|
Nili et al., 57 2020 |
Cross‐sectional, Medicare Current Beneficiary Survey and Medicare claims, 2006–2013 |
N = 2570 White: n = 1932 Latino: n = 209 Other race: n = 429 |
|
|
Thorpe et al., 58 2012 |
Cross‐sectional, baseline data of the Resources for Enhancing Alzheimer's Caregiver's Health |
N = 566 Non‐Hispanic White: n = 380 Non‐Hispanic Black: n = 96 Hispanic: n = 90 |
|
|
Schultz et al., 59 2017 |
Cross‐sectional, electronic health records, 2006–2010 |
N = 304 Caucasian: n = 101 Chinese: n = 26 Filipino: n = 21 Hawaiian: n = 28 Japanese: n = 92 Korean: n = 17 |
|
|
Poon et al., 60 2010 |
Cross‐sectional, medical chart review, 2003–2004 |
N = 304 Caucasian: n = 190 African American: n = 114 |
|
| Browning et al., 61 2022 | Cross‐sectional, Medicare data linked to the Area Health Resources Files, 2015–2017 |
N = 623,400 White: n = 75.75% Black: n = 10.40% Hispanic: n = 8.63% Asian: n = 3.63% Other: n = 1.58% |
|
|
Browning et al., 62 2022 |
Cross‐sectional, Medicare claims linked to the Master Beneficiary Summary File and Area Health Resource Files, 2013–2014 and 2016–2017 |
N = 381,485 White: n = 306,817 Black: n = 35,981 Hispanic: n = 22,532 Asian/Pacific Islander: n = 11,081 Other: n = 5074 |
|
TABLE 3.
Health service use (n = 11).
| Author, Year | Study design |
Total sample (N), Racial/ethnic populations |
Results |
|---|---|---|---|
|
Gilligan et al., 63 2013 |
Cross‐sectional, the Medicaid analytic extract file from Centers for Medicare and Medicaid Services, 2004 |
N = 158,974 California (N = 53,013): White: n = 25,935 Black: n = 4006 Hispanic: n = 7574 Other: n = 8503 Unknown: n = 6995 Florida (N = 41,292): White: n = 21,830 Black: n = 4542 Hispanic: n = 10,526 Other: n = 182 Unknown: n = 4212 New Jersey (N = 20,910): White: n = 13,458 Black: n = 2930 Hispanic: n = 1457 Other: n = 167 Unknown: n = 2898 New York (N = 43,759): White: n = 27,306 Black: n = 4702 Hispanic: n = 1926 Other: n = 1797 Unknown: n = 8028 |
|
|
Hermosura et al., 64 2020 |
Cross‐sectional, Agency for Healthcare Research and Quality, Health Care Cost and Utilization Project, Hawaii State Inpatient Databases, 2010–2014 |
N = 10,645 Non‐Hispanic White: n = 6926 Native Hawaiians and other Pacific Islanders (NHOPI): n = 3719 |
|
|
Ornstein et al., 65 2018 |
Longitudinal, Washington Heights‐Inwood Columbia Aging Project linked to Medicare claims, 1999–2010 |
Total N = 4604 Full sample at dementia diagnosis: N = 186 Non‐Hispanic White: n = 37 Non‐Hispanic Black: n = 47 Hispanic: n = 102 Decedent sample with dementia: N = 86 Non‐Hispanic White: n = 26 Non‐Hispanic Black: n = 23 Hispanic: n = 37 |
|
|
Gorges et al., 66 2019 |
Cross‐sectional, National Medicaid Analytic eXtract linked with Medicare claims, Master Beneficiary Summary Files for demographics and Medicare Provider Analysis and Review for hospitalizations, 2012 |
Total N = 1,659,645 elderly, dual‐eligible long‐term care users N with dementia = 51.7% Of total sample of elderly, dual‐eligible long‐term care users: Non‐Hispanic White: n = 57.3% Non‐Hispanic Black: n = 19% Hispanic: n = 14.8% Asian: n = 9% |
|
|
Husaini et al., 67 2003 |
Longitudinal, Tennessee Medicare claims, 1991–1993 |
Total N = 33,688 N with dementia = 1366 White: n = 1186 African American: n = 180 |
|
|
Husaini et al., 68 2015 |
Cross‐sectional, Tennessee Hospital Discharged database, 2008 |
Total N = 154,945 N with dementia = 5,556 White: n = 4847 Black: n = 709 |
|
|
Chen et al., 69 2021 |
Cross‐sectional, Healthcare Cost & Utilization Project with American Hospital Association Annual Survey, 2015 |
N = 14,135 White: n = 10,733 African American: n = 1651 Latinx: n = 1751 |
|
|
Pereira et al., 70 2022 |
Cross‐sectional, data collected using the Care Ecosystem Program, 2019–2020 |
N = 133 dyads White: n = 87 Black: n = 46 |
|
|
Miller et al., 71 2010 |
Longitudinal, Clinical Antipsychotic Trial of Intervention Effectiveness—AD at baseline and 3, 6, and 9 months |
N = 421 Non‐Hispanic White: n = 79% Black: n = 18%* Other: n = 3%* |
|
|
Miller et al., 72 2009 |
Longitudinal, Clinical Antipsychotic Trial of Intervention Effectiveness—AD at baseline and 3, 6, and 9 months |
N = 421 Non‐Hispanic White: n = 79% Black: n = 18%* Other: n = 3%* *pull information from another article (“Effectiveness of Atypical Antipsychotic Drugs in Patients with Alzheimer's Disease” by Schneider et al.) |
|
|
Park et al., 73 2020 |
Cross‐sectional, Medical Expenditure Panel Survey, 1996–2017 |
N without cognitive deficit = 57,057 N with cognitive deficits without AD/ADRD = 10,088 N with AD/ADRD = 3,420 White: n = 2028 Black: n = 693 Asian: n = 120 Latino: n = 579 |
|
Abbreviations: AD, Alzheimer's disease; ADRD, Alzheimer's disease and related dementias.
TABLE 4.
Long‐term care (n = 4).
| Author, Year | Study design |
Total sample (N), Racial/ethnic populations |
Results |
|---|---|---|---|
|
Rivera‐Hernandez et al., 56 2022 |
Cross‐sectional, Residential History File, Medicare Beneficiary Summary File, Minimum Data Set, Certification and Survey Provider Enhanced Reports and the Long‐term Care: Facts on Care in the US, 2007 |
N = 1,005,781 White: n = 78% Black: n = 13% Hispanic: n = 6% Asian/Pacific Islander: n = 2% American Indian/Alaska Native: n = 0.4% |
|
|
Rivera‐Hernandez et al., 74 2019 |
Cross‐sectional, Minimum Data Set, Master Benefit Summary File, Certification and Survey Provider Enhanced Reporting system, Long‐Term Care: Facts on Care in the US and Nursing Home Compare Five‐Star Ratings databases, 2014 |
Total N = 1,302,099 N with AD/ADRD = 268,181 White: n = 85.1% African American: n = 10.3% Hispanic: n = 4.6% |
|
|
Sengupta et al., 75 2012 |
Cross‐sectional, National Nursing Home Survey, 2004 |
N = 6332 White: n = 5624 Non‐White: n = 708 |
|
|
Resnick et al., 76 2022 |
Longitudinal, Evidence Integration Triangle for Behavioral and Psychological Symptoms of Dementia Implementation Study |
N = 553 White: n = 76% Black: n = 24% |
|
Abbreviations: AD, Alzheimer's disease; ADRD, Alzheimer's disease and related dementias; LTC, long‐term care.
TABLE 5.
End‐of‐life care (n = 16).
| Author, Year | Study design |
Total sample (N), Racial/ethnic populations |
Results |
|---|---|---|---|
|
Braun et al., 77 2005 |
Longitudinal, three national Veterans Affairs databases: patient treatment file, outpatient clinic, file and the Beneficiary Identification Record Locator Subsystem, 1990–2001 |
N = 413,627 White: n = 316,893 African American: n = 76,181 Hispanic: n = 14,007 Other: n = 2844 Unknown/missing: n = 3702 |
|
|
Owen et al., 78 2001 |
Cross‐sectional |
N = 63 White caregivers: n = 47 African American caregivers: n = 16 |
|
|
Meier et al., 79 2001 |
Longitudinal |
N = 99 Black: n = 39 White: n = 36 Hispanic: n = 22 Asian: n = 2 |
|
|
Mitchell et al., 80 2016 |
Longitudinal, Minimum Data Set, 2000–2015 |
N = 71,251 White: n = 85.6% Black: n = 9.5% |
|
|
Sharma et al., 81 2020 |
Longitudinal, Medicare Beneficiary Enrollment File, Minimum Data Set, and Medicare Claims, 2001–2014 |
N = 289,017 2001–2002 White: n = 38,450 Black: n = 10,712 2005–2006 White: n = 36,374 Black: n = 10,466 2009–2010 White: n = 23,696 Black: n = 6722 2013–2014 White: n = 20,277 Black: n = 6394 |
|
|
Shepard et al., 82 2021 |
Longitudinal, Texas Medicare beneficiaries from Medicare claims and Long‐term Care Minimum Data Set, 2011–2016 |
N = 20,582 Only reported for 2016: Non‐Hispanic White: w/ FT, n = 186 w/o FT, n = 2709 Non‐Hispanic Black: w/FT, n = 138 w/o FT, n = 408 Hispanic: w/ FT, n = 210 w/o FT, n = 769 Other: w/ FT, n = 42 w/o FT, n = 113 |
|
|
Henao et al., 83 2022 |
Cross‐sectional, Epic data from Novant Health, 2015–2018 |
N = 21,939 Black: n = 4138 White: n = 17,801 |
|
|
Kim et al., 84 2021 |
Cross‐sectional, National Hospital Discharge Survey, 2006–2010 |
Parkinson's disease and related disorders (PDRD): N = 2,862,778 White: n = 72% Black: n = 4.4% Asian: n = 0.9% Other minority group: n = 1.4% Not stated: n = 21.3% Non‐Parkinsonian dementia (NPD): N = 4,135,180 White: n = 67.2% Black: n = 9.5% Asian: n = 0.6%, Other minority group: n = 1.6% Not stated: n = 21.1% |
|
|
Luth et al., 85 2022 |
Cross‐sectional, administrative claims data for Medicare fee‐for‐service, 2016–2018 |
Total N = 463,590 N with dementia = 234,737: Non‐Hispanic White: n = 197,539 Non‐Hispanic Black: n = 19,874 Hispanic: n = 10,258 Asian/Pacific Islander: n = 4361 Other: n = 2705 |
|
|
Jia et al., 86 2022 |
Longitudinal, Medicare fee‐for‐service, 2000–2017 |
Medicare fee‐for‐service decedents with dementia: N = 2,170,759 No IMV: White: n = 13.3% Asian: n = 11.8% IMV: White: n = 4.0% Asian: n = 4.4% Medicare Advantage decedents with dementia: N = 282,037 No IMV: White: n = 18.5% Asian: n = 18.9% IMV: White: n = 5.2% Asian: n = 5.7% |
|
|
Austin et al., 87 2019 |
Cross‐sectional, National Sample of White and Black fee‐for‐service Medicare patients, 2013–2015 |
N = 389,922 Black: n = 61,708 White: n = 328,214 |
|
|
Temkin‐Greener et al., 88 2021 |
Cross‐sectional, Minimum Data Set, Medicare Beneficiary Summary Files, Medicare Provider Analysis and Review, Nursing Home Compare, 2014–2017 |
N = 665,033 White: n = 598,502 Black: n = 66,531 |
|
|
Lin et al., 89 2022 |
Cross‐sectional, Health and Retirement Study Linked Medicare & Medicaid data, 2000–2016 |
N = 5058 Non‐Hispanic Black: n = 809 Hispanic: n = 357 Non‐Hispanic White: n = 3892 |
|
|
Oud, 90 2017 |
Cross‐sectional, Texas Inpatient Public Use Data File, 2001–2010 |
N = 889,008 White: n = 590,806 Hispanic: n = 141,202 Black: n = 107,746 Other: n = 47,502 Missing: n = 2035 |
|
|
Luth et al., 91 2020 |
Cross‐sectional, Electronic Health Records from large not‐for‐profit hospice agency in New York City, 2013–2017 |
N = 2629 African American: n = 16% Hispanic: n = 20% Other race/ethnicity: n = 9% Non‐Hispanic White: n = 55% |
|
|
Luth et al., 92 2018 |
Cross‐sectional, National Health and Aging Trends Survey, 2011–2016 |
N = 1588 Non‐Hispanic White: n = 72% Non‐Hispanic Black: n = 23% Hispanic: n = 5% |
|
TABLE 6.
Family caregiving (n = 5).
| Author, year | Study design | Total sample (N), racial/ethnic populations | Results |
|---|---|---|---|
|
Chow et al., 35 2000 |
Longitudinal, Minimum Uniform Dataset from California Alzheimer's Disease Diagnostic and Treatment Centers, 1988, 1993, 1995 |
N = 9451 Asian: n = 4.2% Filipino: n = 0.8% Pacific Islander: n = 0.3% Caucasian: n = 75.9% |
|
|
Badana et al., 93 2019 |
Cross‐sectional, National Alliance for Caregiving and the American Association of Retired Persons’ Caregiving in the U.S. survey, 2015 |
Total N = 887 N with dementia = 218 White: n = 173 African American: n = 45 |
|
|
Parker et al., 94 2020 |
Cross‐sectional, National Health and Aging Trends Study and National Study of Caregiving, 2015 |
N = 4,590,650 White: n = 75.4% Black: n = 24.6% |
|
|
Sleath et al., 95 2005 |
Cross‐sectional, National Longitudinal Caregiver, 1997 |
N = 2032 African American: n = 316 White: n = 1716 |
|
|
Parker et al., 96 2019 |
Cross‐sectional, data from two randomized controlled trials: Advancing Caregiver Training (ACT; n = 272) and Care of Persons with Dementia in Their Environments (COPE; n = 237) |
N = 509 Black: n = 135 White: n = 362 |
|
To further synthesize and integrate the results, we mapped key results onto an adapted and simplified version of the dementia care trajectory, 25 , 26 beginning with initial assessment, diagnosis, and referral at the early stages of the clinical disease process and awareness of symptoms (i.e., diagnosis), followed by ongoing treatment and support as disease and disability progresses (i.e., medications, health service use and costs), and finally to the later stages of the disease (i.e., long‐term care, end‐of‐life care). The family caregiving topic cuts across the three stages. Figure 3 summarizes our synthesis of results onto the adapted model, which we refer to as the dementia healthcare trajectory.
FIGURE 3.
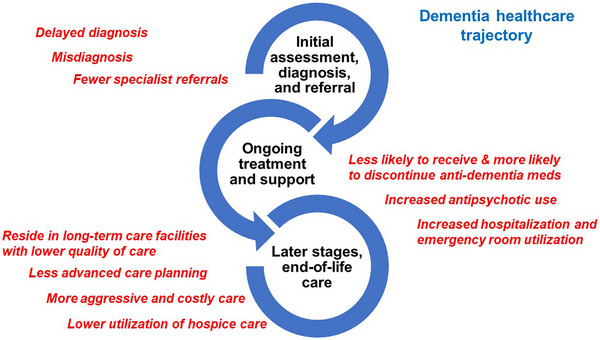
Evidence of racial/ethnic disparities in Alzheimer's disease and Alzheimer's disease related dementias healthcare.
3.3.1. Diagnosis
The healthcare trajectory begins when awareness of signs and symptoms of AD/ADRD, including cognitive changes and associated functional decline, trigger care seeking on the part of the PLWD and family members and/or are observed by healthcare providers. Diagnosis is a critical aspect of healthcare during this early stage. Eleven studies examined diagnosis, including accuracy, timeliness, quality of care, and initial referral (see Table 1). All 11 studies reported one or more statistically significant differences, primarily in the direction of NHW receiving more optimal care and with several studies reporting differences among minoritized populations. Six studies examined timeliness and accuracy of diagnosis and all found evidence of disparities (i.e., higher rates of misdiagnosis and/or delayed diagnosis) in minoritized populations compared to NHW. 27 , 28 , 29 , 30 , 31 , 32 The study by Lin et al., 31 for example, estimated that the delay in time to diagnosis was 11% longer for Black Americans and 40% longer for Hispanic Americans compared to NHW. Five studies examined other aspects of diagnostic assessment and post‐diagnostic care, with most finding evidence of less optimal care for one or more minoritized populations compared to NHW. 30 , 33 , 34 , 35 , 36 For example, one study found that Hispanic and Asian American older adults were less likely to have a return visit within a year to a dementia specialist and more likely to lack follow‐up compared to NHW and Black American patients, 34 while another study found that NHW patients were more likely to be referred for neuropsychological testing compared to Black and Hispanic American patients. 33 Asian American older adults were less likely to receive recommended diagnostic work‐up for cognitive impairment compared to NHW older adults. 30 NHW patients who were < 80 years old were more likely to refuse a work‐up for dementia compared to Black American patients. 36 A study conducted at state‐funded AD diagnostic centers in California found that compared to NHW, Asian American older adults were less likely to seek a second opinion or receive referrals from a physician or community support groups for dementia diagnosis. In that same study, Asian Americans were less likely to receive referrals for home health services yet more likely to be referred for financial help, case management, day centers, and state‐funded caregiver resource centers. 35 Finally, one qualitative study found evidence of racial/ethnic differences in patterns of help seeking leading to diagnosis. 37
3.3.2. Anti‐dementia, psychotropic, and other medication use
After diagnosis, ongoing treatment and support of the PLWD and family caregivers becomes a major focus of healthcare. Early in the clinical treatment process, this may include prescription of anti‐dementia medications as well as the use of psychotropic medication for more severe behavioral changes. Out of 27 studies, 26 reported racial/ethnic differences in medication use among PLWD, including anti‐dementia medications, psychotropics, as well as medications for other medical conditions (see Table 2). Studies were conducted in a range of settings including several conducted in long‐term care. Among the 15 studies examining rates of anti‐dementia medication (i.e., cholinesterase inhibitors and/or memantine) use, 12 reported higher rates of prescription or receipt of these medications in NHW compared to minoritized populations 33 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 while 3 found higher rates of anti‐dementia medication use in Hispanic 49 , 50 , 51 and Black 51 Americans compared to NHW. Higher discontinuation rates of anti‐dementia medications were also reported for Hispanic and Black American older adults compared to NHW individuals. 49
Six studies reported on use of psychotropic medications across racial/ethnic groups. 52 , 53 , 54 , 55 , 56 , 57 Three of these studies found higher use of anti‐psychotic medication in Hispanic Americans compared to NHW 52 , 53 , 54 adults while one study reported higher use in NHW compared to Hispanic Americans. 55 One study reported lower use of anti‐psychotic medication in Black Americans and AA/PI compared to NHW, Hispanic Americans, and AI/AN groups 56 and another study found higher use of anxiolytics in Black Americans compared to NHW individuals. 55 Anti‐depressant use was also higher in NHW compared to other racial/ethnic groups. 56 Finally, one study found higher rates of anti‐psychotic medication (i.e., “low value care”) in Hispanic Americans and NHW compared to the “other race” category. 57
Two studies reported on potentially inappropriate medication use—“drugs that should generally be avoided because they are ineffective or pose an unnecessarily high risk for older adults, and drugs that are appropriate to use in older persons only at certain doses, frequencies, or duration of therapies.” 58 One study reported higher rates of potentially inappropriate medication use in Hispanic Americans compared to NHW persons 58 and another found higher rates of concurrent use of medications with anti‐cholinergic properties and anti‐dementia medications in NHW and Native Hawaiians compared to Asian Americans. 59 Finally, several studies reported racial/ethnic differences in patterns of use of drugs for hypertension and other medical conditions in PLWD. 46 , 60 , 61 Among the studies of medication for other medical conditions, a Medicare Comprehensive Medication Review was found to reduce disparities in statin non‐adherence for Hispanic American PLWD. 61 In a related study, Hispanic and Asian Americans were more likely to be similarly enrolled in Medication Therapy Management (MTM) programs compared to NHW, and Black Americans were less likely to be enrolled in MTM than NHW. 62 ,
3.3.3. Healthcare service use (excluding end‐of‐life care)
Another set of studies examined aspects of healthcare service use (i.e., inpatient, outpatient, ED) after the point of diagnosis and addressed use, costs, and quality of care (see Table 3). These 13 studies, 11 of which reported racial/ethnic differences, varied substantially, with some examining use and costs from the earliest stages of dementia to the end of life, while others had a more discrete focus. Eleven studies reported on hospitalizations, including the likelihood of admission or readmission, length of stay, and costs. 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 In all but two of these studies, Black American PLWD were found to have increased admission rates, longer lengths of stay, and higher costs. 63 , 65 , 66 , 67 , 68 , 69 This included two studies examining potentially preventable hospitalization, 66 , 69 with one study showing higher rates of preventable hospitalization in both Black and Hispanic Americans compared to NHW. 69 In the two remaining studies, NHW had higher rates of non‐emergency hospitalizations compared to Black Americans; 70 in a study comparing NHW to Native Hawaiians and other Pacific Islanders (NHOPI), the former had long inpatient lengths of stay while the latter had higher rates of readmission. 64 Four studies examined use and/or costs of outpatient healthcare or community‐based supports, with all finding higher rates or costs for NHW compared to “other” races 66 , 71 , 72 or Asian Americans. 73 ED use was examined separately in two studies, with one finding higher ED and ambulance use in Black Americans compared to NHW 70 and another finding higher ED costs for NHW compared to Asian Americans. 73 Finally, two studies examined different aspects of total expenditures or costs without finding a clear pattern. 66 , 73 Relatively few studies reported findings separately for minoritized populations other than Black Americans, with a number of studies referring to minoritized populations in aggregate.
3.3.4. Long‐term care
As functional impairment and caregiving needs intensify during the middle and later stages of dementia, PLWD may transition from the community to long‐term care (LTC) settings and memory care units. Four studies reported racial/ethnic differences in the characteristics of their LTC facilities (see Table 4). NHW individuals with AD/ADRD were more likely to be residents in LTC facilities with AD special care units compared to minoritized populations. 56 , 74 , 75 Black and Hispanic American older adults were more likely to be admitted to for‐profit facilities 74 and facilities that scored lower on quality indicators including rates of hospital readmission. 56 , 74 However, one study examined a variety of quality‐of‐care indicators and found mixed results. 76
3.3.5. End‐of‐life care
In the later stages of dementia, treatment shifts toward end‐of‐life (EOL) and palliative care. Sixteen studies reported on EOL care for PLWD including on life‐prolonging procedures, advanced directives, hospitalizations, and hospice care (see Table 5). Ten studies examined the use of more intensive and life‐sustaining procedures, such as feeding tubes and mechanical ventilation in PLWD, with all finding higher rates of use of more intensive life‐sustaining procedures in Black American 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 PLWD and several studies showing similar results for other minoritized populations 82 , 85 , 86 compared to NHW. In terms of specific life‐prolonging procedures, compared to NHW adults, Black Americans had higher rates of feeding tube placement. 77 , 79 , 80 , 81 , 82 , 83 , 84 One study found higher rates of feeding tube placement in Hispanic Americans compared to NHW. 82 Asian Americans were less likely to receive gastrostomy tube placement than NHW 84 ; however, Asian Americans were more likely to receive invasive mechanical ventilation than NHW. 86 One study found that PLWD from racial/ethnic minoritized groups received more intensive EOL care (e.g., resuscitation, mechanical ventilation, intubation, feeding tube insertion, or new dialysis) than NHW. 85 Finally, NHW caregivers were also more likely to make life‐sustaining treatment decisions prior to a relative's death than Black American caregivers. 78
The remaining studies examined a variety of other aspects of EOL care. Five studies examined rates of hospitalization, including intensive care unit use, at the EOL, with higher rates reported for Black Americans, 81 , 87 , 88 , 89 Hispanic Americans, 89 and Asian Americans 86 compared to NHW. Consistent with these findings, inpatient hospital expenditures at the EOL were higher for Black and Hispanic Americans compared to NHW. 89 Four studies examined hospice care use, with all reporting higher use in NHW compared to minoritized populations. 86 , 87 , 89 , 90 Potentially burdensome and disruptive discharges (“live discharge”) from hospice, on the other hand, were more common in Black and Hispanic American patients compared to NHW patients. 91 Proxies for Black American decedents were less likely to report poor EOL care compared to NHW. 92 Advanced care planning was more frequent in NHW compared to Black and Hispanic Americans. 89
3.3.6. Family caregiving
Family caregivers often play a very important role in helping PLWD navigate the healthcare system and communicate with formal care providers. Support of family members in their role as caregivers is an important aspect of clinical care and can include psychoeducation, referral to community programs and supports, skills for non‐pharmacological management of behavioral changes, and assistance with respite care and legal issues. Five studies reported significant ethnic/racial differences in healthcare and services for the family caregivers of PLWD (see Table 6). Black American caregivers reported greater service use compared to NHW caregivers 93 while in another study Black American caregivers were less likely to use respite services compared to NHW caregivers. 94 In one study, NHW caregivers were more than twice as likely as Black American caregivers to be taking anti‐depressants or anti‐anxiety medications. 95 Another study found that Asian American caregivers were more likely to be referred for a variety of support services compared to NHW caregivers. 35 Finally, Black American caregivers were more likely than NHW caregivers to miss physician appointments. 96
4. DISCUSSION
This scoping review of healthcare disparities for PLWD and their family caregivers provides an important update to previous reviews and demonstrates substantial, accumulated evidence for healthcare disparities in several areas, emerging evidence of disparities in several additional areas, as well as important remaining gaps in knowledge. Our scoping review found substantial evidence for healthcare disparities for PLWD in diagnosis, treatment with anti‐dementia medications, hospitalization rates, and quality of care at the EOL. The evidence of disparities is strongest for Black Americans and to a lesser extent Hispanic Americans compared to NHW, with a striking lack of reported findings and gaps in evidence for AA/PI and AI/AN groups.
There was substantial evidence of disparities in the accuracy and timeliness of dementia diagnosis. Timely and accurate diagnosis of dementia is recognized as critical and central to good‐quality care. 13 , 97 Delays in assessment and diagnosis in turn lead to delays in initiation or continuation of goals of care discussions, evaluating healthcare decisions, identifying healthcare surrogates, completing and registering advanced healthcare directives, and engaging in financial and estate planning. In addition to these consequences of diagnostic inaccuracy and delays, PLWD and their families may experience distress due to uncertainties regarding the nature of cognitive and behavioral changes. The evidence of delays and misdiagnosis was strongest for Black and Hispanic Americans compared to NHW and is further supported by a recently published large epidemiological study. 98 However, surprisingly few studies have focused on AA/PI and AI/AN groups. In addition, less is known about the underlying processes (e.g., individual level factors, structural racism) that may contribute to disparities in diagnosis as well as strategies to reduce these disparities.
There now appears to be abundant evidence that minoritized populations are less likely to use or be prescribed anti‐dementia medications. While much of this evidence has focused on comparisons of Black and Hispanic Americans to NHW, there is also accumulating evidence that this pattern extends to other minoritized populations as well. Current guidelines recommend a trial of an anti‐dementia medication for most types of dementia, yet an overwhelming majority of studies included in this review found that anti‐dementia medication use was lower in racial/ethnic populations. Few studies shed light on underlying factors responsible for these differences, but one study found higher rates of discontinuation of these medications in minoritized populations. 49 This area is ripe for a deeper investigation to identify individual (i.e., PLWD, family caregivers, providers, and clinical communication) and systemic factors contributing to these prescribing patterns. Understanding the underlying factors responsible for disparities in anti‐dementia medication prescribing is particularly urgent as new, disease‐modifying treatments become available. 99
The most striking finding in the category of service use is substantial evidence that Black American PLWD have a higher likelihood of hospitalization as well as longer duration and costs compared to NHW and in several studies with other minoritized populations. Hospitalizations can be very difficult and distressing for PLWD because their cognitive impairment makes adjusting to the medical setting more difficult and hospitalization places them at higher risk of delirium. Much more research is needed to elucidate underlying processes resulting in more frequent and longer duration hospitalizations for Black American PLWD compared to NHW. One hypothesis is that Black Americans have less access to, or poorer quality of, outpatient services compared to their NHW counterparts, an interpretation supported by the higher rates of NHW use of outpatient services and higher costs compared to Black Americans as reported by several studies in this review. We also identified emerging evidence for additional gaps and disparities in care, including increased use of emergency services for minoritized populations, a finding that was also highlighted in our review of EOL studies.
The findings from our review highlight less optimal EOL care for minoritized populations, including more aggressive life‐sustaining interventions, higher hospitalization rates, and lower use of hospice and advance directives. Our findings are broadly consistent with recent reviews of end‐of‐life disparities in nursing homes 100 and among patients with cancer 101 not specific to PLWD, as well as a recent large study of Medicare beneficiaries with dementia. 85 These reviews highlight the possible role of multiple factors, including the cultural competence of staff, socioeconomic inequality, and systemic racism, as well as individual‐level preferences and values.
Our scoping review has identified important gaps in the field, the most striking of which is the lack of studies on AA/PI and AI/AN populations. Fewer than one third of studies reported findings separately for AA/PI and < 3% for AI/AN populations. An additional issue is that when studies did include minoritized populations other than Black Americans, the numbers were often relatively small and underpowered to detect meaningful differences. Perhaps in part because of low numbers, almost one third of studies used an aggregate category for minoritized populations. Finally, and related to the points above, few studies provided explicit criteria for use of racial/ethnic categories and/or were reliant on administrative datasets that are limited with respect to categorization of minoritized populations.
Our review also identified additional emerging areas and gaps. For example, there is almost no evidence on access to non‐pharmacological interventions for behavioral symptoms, and family caregiver supports. In addition, relatively few studies were designed with an explicit focus on racial/ethnic disparities in healthcare and may have important methodological limitations (e.g., underpowered for certain racial/ethnic populations). Finally, very few studies have looked further to examine the underlying processes (e.g., patient/family caregiver preferences, physician implicit bias or racism) that may account for the racial/ethnic differences identified in this scoping review.
The abundant evidence of disparities, summarized in Figure 3, has important implications for clinical care. Knowledge of existing disparities is the first step in addressing these disparities at the individual, clinical, and health systems levels, through monitoring at the local level, educating and training clinicians to reduce bias and improve communication, enhancing the cultural and linguistic competence of clinical care, and developing targeted initiatives to empower PLWD and family caregivers as they navigate the healthcare system. This knowledge can also form the basis for policy change at the health system level to address systemic racism and promote health equity. 102 , 103 , 104
5. LIMITATIONS
One important limitation of this review is the possibility that studies, particularly those without a specific focus on racial/ethnic disparities, with null findings may have been missed because our inclusion criteria required that racial/ethnic comparisons were mentioned in the title/abstract. It should also be stressed that while this scoping review identified a range of healthcare disparities and differences, the underlying factors responsible for these differences is unclear and may be driven by a variety of factors at the individual, interpersonal, and systemic levels, including systemic racism and social determinants of health. Additional limitations are artifacts with the goals of a scoping review, including lack of assessment of the quality of studies to weigh the relative importance of study findings. Finally, it is important to emphasize that the focus of this paper was on racial/ethnic populations and did not include consideration of other disparity populations, such as PLWD who identify as LGBTQ, or geography including residing in rural versus urban or suburban areas.
Three identified gaps and recommendations for future research are:
1. Study processes and interventions where substantial evidence exists. We recommend that in the areas where substantial evidence of disparities exists, research accelerates by moving beyond description to an examination of underlying processes and more importantly to interventions that reduce disparities. This scoping review has identified some priority areas to accelerate research toward impactful reduction of disparities, including diagnosis, prescription of anti‐dementia medications, prevention of hospitalization, and improvement in the EOL quality of care. Our scoping review may be useful in determining the strength of the evidence for specific minoritized populations. For example, our evidence in the areas above is ample for Black Americans and to some extent for Hispanic Americans but less strong for other groups. Research on understanding the mechanism of disparities is critical to advance science using appropriate frameworks, such as the National Institute on Aging Health Disparities Research Framework 105 as well as other frameworks and approaches that specifically address factors that drive healthcare disparities. 106 , 107 , 108 , 109 In particular, there is a need to examine the role of social determinants of health as drivers of racial and ethnic disparities in healthcare. 110 Finally, there is also an urgent need for studies that examine the impact of policies and interventions on disparities reduction.
2. Include a wider range of minoritized populations in sufficient numbers. Many studies included in this review limited their analyses to a comparison of NHW to “other” populations or to Black Americans or Hispanic Americans. Future studies should strive to include a broader range of minoritized populations, particularly AA/PI and AI/AN groups. In our review, many of the studies that included results identified differences between minoritized populations, highlighting the importance of including diverse populations in sufficient numbers for meaningful analysis and avoiding, where possible, the practice of aggregating minoritized populations. Aggregating groups masks importance differences among minoritized populations.
3. Advance research in promising and understudied areas. Our understanding of healthcare disparities for minoritized populations is, in many respects, at a very early stage, with many areas of clinical and public health importance receiving little or no attention. As an example, there is wide recognition that non‐pharmacological management of dementia‐related behavioral problems is often the preferred method of treatment. However, none of the studies we identified examined access to these approaches, either through delivery in healthcare settings or referral to community resources. Surprisingly, there were also relatively few studies that examined the use of psychotropics or inappropriate medications in PLWD, an area of significant public health importance given the increased risk of mortality associated with anti‐psychotics. We found early evidence suggesting disparities in the use of potentially inappropriate medications in the PLWD, another area of high public health importance. There should be efforts to engage minoritized PLWD and their caregivers who have lived experiences of dealing with healthcare systems and important insights to inform the research agenda. Examination of the role of place is another important area for research to identify community/neighborhood factors that drive inequities in access and quality of care. 111 Additional studies of the impact of changes in healthcare policies and models of payment impact healthcare quality and access for PLWD are needed, including programs such as Medicare Advantage, Medicaid Home and Community‐Based Services, and Care Coordination.
6. CONCLUSIONS
Given the strength of accumulated evidence, there is an urgent need to address disparities in healthcare for minoritized populations. Minoritized populations such as Black Americans face a “double disparity” in AD/ADRD, with both a higher burden of clinical disease compared to NHW and a lower quality of healthcare for those who live with dementia. This brings the equity issues that must be addressed into sharp focus. We need to design studies that simultaneously examine interventions, disparities reduction, and mechanisms to accelerate the pace and progress of research. Researchers need to design studies that incorporate equity consideration from the start, with particular attention to inclusion of diverse populations. Disparities research focused on a disease or health condition moves through different phases, beginning with the demonstration of differences, then understanding underlying mechanisms to explain differences, and then developing and implementing interventions to eliminate or reduce disparities in care. 112 While the field of healthcare disparities for PLWD and family caregivers is clearly in its early stages, this scoping review has identified areas where there is substantial converging evidence of disparities as well as gaps in research that may help the field move forward to generate a more robust body of evidence and move toward understanding the underlying processes, designing interventions that address these mechanisms, and ultimately reducing healthcare disparities in AD/ADRD assessment and treatment.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare. Author disclosures are available in the supporting information.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
This work was supported by the National Institute on Aging (NIA) of the National Institutes of Health under Award Number U54AG063546, which funds the NIA IMbedded Pragmatic Alzheimer's Disease and AD‐Related Dementias Clinical Trials Collaboratory (NIA IMPACT Collaboratory). The sponsor did not have a role in the design, methods, data collection, analysis, and preparation of the paper. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Hinton L, Tran D, Peak K, Meyer OL, Quiñones AR. Mapping racial and ethnic healthcare disparities for persons living with dementia: A scoping review. Alzheimer's Dement. 2024;20:3000–3020. 10.1002/alz.13612
Contributor Information
Ladson Hinton, Email: lwhinton@ucdavis.edu.
Ana R. Quiñones, Email: quinones@ohsu.edu.
REFERENCES
- 1. Babulal GM, Quiroz YT, Albensi BC, et al. Perspectives on ethnic and racial disparities in Alzheimer's disease and related dementias: update and areas of immediate need. Alzheimers Dement. 2019;15(2):292‐312. doi: 10.1016/j.jalz.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aranda MP, Kremer IN, Hinton L, et al. Impact of dementia: health disparities, population trends, care interventions, and economic costs. J Am Geriatr Soc. 2021;69(7):1774‐1783. doi: 10.1111/jgs.17345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilmore‐Bykovskyi AL, Jin Y, Gleason C, et al. Recruitment and retention of underrepresented populations in Alzheimer's disease research: a systematic review. Alzheimers Dement (N Y). 2019;5:751‐770. doi: 10.1016/j.trci.2019.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413‐446. doi: 10.1016/s0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee M, Whitsel E, Avery C, et al. Variation in population attributable fraction of dementia associated with potentially modifiable risk factors by race and ethnicity in the US. JAMA Netw Open. 2022;5(7):e2219672. doi: 10.1001/jamanetworkopen.2022.19672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peterson RL, George KM, Gilsanz P, et al. Racial/ethnic disparities in young adulthood and midlife cardiovascular risk factors and late‐life cognitive domains: the Kaiser healthy aging and diverse life experiences (KHANDLE) study. Alzheimer Dis Assoc Disord. 2021;35(2):99‐105. doi: 10.1097/wad.0000000000000436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kornblith E, Bahorik A, Boscardin WJ, Xia F, Barnes DE, Yaffe K. Association of race and ethnicity with incidence of dementia among older adults. JAMA. 2022;327(15):1488‐1495. doi: 10.1001/jama.2022.3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216‐224. doi: 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer's disease and related dementias in the United States (2015‐2060) in adults aged ≥65 years. Alzheimers Dement. 2019;15(1):17‐24. doi: 10.1016/j.jalz.2018.06.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manly JJ, Jones RN, Langa KM, et al. Estimating the prevalence of dementia and mild cognitive impairment in the US: the 2016 health and retirement study harmonized cognitive assessment protocol project. JAMA Neurol. 2022;79(12):1242‐1249. doi: 10.1001/jamaneurol.2022.3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moon HE, Rote SM, Sears J, Schepens Niemiec SL. Racial differences in the dementia caregiving experience during the COVID‐19 pandemic: findings From the National Health and Aging Trends Study (NHATS). J Gerontol B Psychol Sci Soc Sci. 2022;77(12):e203‐e215. doi: 10.1093/geronb/gbac098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Q, Davis PB, Gurney ME, Xu R. COVID‐19 and dementia: analyses of risk, disparity, and outcomes from electronic health records in the US. Alzheimers Dement. 2021;17(8):1297‐1306. doi: 10.1002/alz.12296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawas CH, Corrada MM, Whitmer RA. Diversity and disparities in dementia diagnosis and care: a challenge for all of Us. JAMA Neurol. 2021;78(6):650‐652. doi: 10.1001/jamaneurol.2021.0285 [DOI] [PubMed] [Google Scholar]
- 14. Cooper C, Tandy AR, Balamurali TB, Livingston G. A systematic review and meta‐analysis of ethnic differences in use of dementia treatment, care, and research. Am J Geriatr Psychiatry. 2010;18(3):193‐203. doi: 10.1097/JGP.0b013e3181bf9caf [DOI] [PubMed] [Google Scholar]
- 15. Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis Assoc Disord. 2011;25(3):187‐195. doi: 10.1097/WAD.0b013e318211c6c9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Co M, Couch E, Gao Q, Mac‐Ginty S, Das‐Munshi J, Prina M. Access to health services in older minority ethnic groups with dementia: a systematic review. J Am Geriatr Soc. 2021;69(3):822‐834. doi: 10.1111/jgs.16929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dilworth‐Anderson P, Hendrie HC, Manly JJ, Khachaturian AS, Fazio S. Diagnosis and assessment of Alzheimer's disease in diverse populations. Alzheimers Dement. 2008;4(4):305‐309. doi: 10.1016/j.jalz.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 18. Alzheimer's Association . Special Report—Race, Ethnicity and Alzheimer's in America. Alzheimer's Association. 2021. [Google Scholar]
- 19. Quiñones AR, Mitchell SL, Jackson JD, et al. Achieving health equity in embedded pragmatic trials for people living with dementia and their family caregivers. J Am Geriatr Soc. 2020;68(Suppl 2):S8‐S13. doi: 10.1111/jgs.16614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. doi: 10.1186/s12874-018-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467‐473. doi: 10.7326/m18-0850 [DOI] [PubMed] [Google Scholar]
- 22. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19‐32. doi: 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 23. Orgera K, Artiga S. Disparities in Health and Health Care: Five Key Questions and Answers. Kaiser Family Foundation. https://www.kff.org/racial‐equity‐and‐health‐policy/issue‐brief/disparities‐in‐health‐and‐health‐care‐5‐key‐question‐and‐answers/ [Google Scholar]
- 24. MEDLINE®/PubMed® Health Disparities and Minority Health Search Strategy. https://www.nlm.nih.gov/services/queries/health_disparities_details.html
- 25. Gitlin LN, Schulz R. Family caregiving of older adults. In: Prohaska TR, Anderson LA, Binstock RH, eds. Public Health for an Aging Society. Johns Hopkins University Press; 2012:181‐204. [Google Scholar]
- 26. NASEM (National Academies of Sciences, Engineering, and Medicine) . Families Caring for an Aging America. The National Academies Press; 2016. [PubMed] [Google Scholar]
- 27. Chen Y, Tysinger B, Crimmins E, Zissimopoulos JM. Analysis of dementia in the US population using Medicare claims: insights from linked survey and administrative claims data. Alzheimers Dement (N Y). 2019;5:197‐207. doi: 10.1016/j.trci.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gianattasio KZ, Prather C, Glymour MM, Ciarleglio A, Power MC. Racial disparities and temporal trends in dementia misdiagnosis risk in the United States. Alzheimers Dement (N Y). 2019;5:891‐898. doi: 10.1016/j.trci.2019.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Amjad H, Roth DL, Sheehan OC, Lyketsos CG, Wolff JL, Samus QM. Underdiagnosis of dementia: an observational study of patterns in diagnosis and awareness in US older adults. J Gen Intern Med. 2018;33(7):1131‐1138. doi: 10.1007/s11606-018-4377-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsoy E, Kiekhofer RE, Guterman EL, et al. Assessment of racial/ethnic disparities in timeliness and comprehensiveness of dementia diagnosis in California. JAMA Neurol. 2021;78(6):657‐665. doi: 10.1001/jamaneurol.2021.0399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin PJ, Daly AT, Olchanski N, et al. Dementia diagnosis disparities by race and ethnicity. Med Care. 2021;59(8):679‐686. doi: 10.1097/mlr.0000000000001577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. White L, Ingraham B, Larson E, Fishman P, Park S, Coe NB. Observational study of patient characteristics associated with a timely diagnosis of dementia and mild cognitive impairment without dementia. J Gen Intern Med. 2022;37(12):2957‐2965. doi: 10.1007/s11606-021-07169-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kalkonde YV, Pinto‐Patarroyo GP, Goldman T, et al. Ethnic disparities in the treatment of dementia in veterans. Dement Geriatr Cogn Disord. 2009;28(2):145‐152. doi: 10.1159/000235577 [DOI] [PubMed] [Google Scholar]
- 34. Drabo EF, Barthold D, Joyce G, Ferido P, Chang Chui H, Zissimopoulos J. Longitudinal analysis of dementia diagnosis and specialty care among racially diverse Medicare beneficiaries. Alzheimers Dement. 2019;15(11):1402‐1411. doi: 10.1016/j.jalz.2019.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chow TW, Ross L, Fox P, Cummings JL, Lin KM. Utilization of Alzheimer's disease community resources by Asian‐Americans in California. Int J Geriatr Psychiatry. 2000;15(9):838‐847. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boustani M, Perkins AJ, Fox C, et al. Who refuses the diagnostic assessment for dementia in primary care Int J Geriatr Psychiatry. 2006;21(6):556‐563. doi: 10.1002/gps.1524 [DOI] [PubMed] [Google Scholar]
- 37. Hinton L, Franz C, Friend J. Pathways to dementia diagnosis: evidence for cross‐ethnic differences. Alzheimer Dis Assoc Disord. 2004;18(3):134‐144. doi: 10.1097/01.wad.0000127444.23312.ff [DOI] [PubMed] [Google Scholar]
- 38. Lind KE, Hildreth K, Lindrooth R, Morrato E, Crane LA, Perraillon MC. Effect of Medicare part D on ethnoracial disparities in antidementia medication use. J Am Geriatr Soc. 2018;66(9):1760‐1767. doi: 10.1111/jgs.15494 [DOI] [PubMed] [Google Scholar]
- 39. Mehta KM, Yin M, Resendez C, Yaffe K. Ethnic differences in acetylcholinesterase inhibitor use for Alzheimer disease. Neurology. 2005;65(1):159‐162. doi: 10.1212/01.wnl.0000167545.38161.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Giebel C, Cations M, Draper B, Komuravelli A. Ethnic disparities in the uptake of anti‐dementia medication in young and late‐onset dementia. Int Psychogeriatr. 2023;35(7):381‐390. doi: 10.1017/s1041610220000794 [DOI] [PubMed] [Google Scholar]
- 41. Lerner AJ, McClendon MJ, Sami SA, Ogrocki PK, Adams KB, Smyth KA. Factors affecting usage patterns of memantine in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22(2):137‐143. doi: 10.1097/WAD.0b013e31815ccd68 [DOI] [PubMed] [Google Scholar]
- 42. McClendon MJ, Hernandez S, Smyth KA, Lerner AJ. Memantine and acetylcholinesterase inhibitor treatment in cases of CDR 0.5 or questionable impairment. J Alzheimers Dis. 2009;16(3):577‐583. doi: 10.3233/jad-2009-0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barthold D, Joyce G, Ferido P, et al. Pharmaceutical treatment for Alzheimer's disease and related dementias: utilization and disparities. J Alzheimers Dis. 2020;76(2):579‐589. doi: 10.3233/jad-200133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hernandez S, McClendon MJ, Zhou XH, Sachs M, Lerner AJ. Pharmacological treatment of Alzheimer's disease: effect of race and demographic variables. J Alzheimers Dis. 2010;19(2):665‐672. doi: 10.3233/jad-2010-1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zuckerman IH, Ryder PT, Simoni‐Wastila L, et al. Racial and ethnic disparities in the treatment of dementia among Medicare beneficiaries. J Gerontol B Psychol Sci Soc Sci. 2008;63(5):S328‐S333. doi: 10.1093/geronb/63.5.s328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Poon I, Lal LS, Ford ME, Braun UK. Racial/ethnic disparities in medication use among veterans with hypertension and dementia: a national cohort study. Ann Pharmacother. 2009;43(2):185‐193. doi: 10.1345/aph.1L368 [DOI] [PubMed] [Google Scholar]
- 47. Sano M, Amatniek J, Feely M, et al. Undertreatment of patients with Alzheimer's disease in an elderly United States population. Alzheimers Dement. 2005;1(2):136‐144. doi: 10.1016/j.jalz.2005.09.011 [DOI] [PubMed] [Google Scholar]
- 48. Zhu CW, Neugroschl J, Barnes LL, Sano M. Racial/ethnic disparities in initiation and persistent use of anti‐dementia medications. Alzheimers Dement. 2022;18(12):2582‐2592. doi: 10.1002/alz.12623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thorpe CT, Fowler NR, Harrigan K, et al. Racial and ethnic differences in initiation and discontinuation of antidementia drugs by Medicare beneficiaries. J Am Geriatr Soc. 2016;64(9):1806‐1814. doi: 10.1111/jgs.14403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gilligan AM, Malone DC, Warholak TL, Armstrong EP. Racial and ethnic disparities in Alzheimer's disease pharmacotherapy exposure: an analysis across four state Medicaid populations. Am J Geriatr Pharmacother. 2012;10(5):303‐312. doi: 10.1016/j.amjopharm.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 51. Perryman M, Lewis M, Rivers PA. Treatment disparities in medication prescribing for Alzheimer's: disease among ethnic groups. J Health Care Finance. 2009;35(4):64‐73. [PubMed] [Google Scholar]
- 52. Xiong GL, Filshtein T, Beckett LA, Hinton L. Antipsychotic use in a diverse population with dementia: a retrospective review of the National Alzheimer's Coordinating Center Database. J Neuropsychiatry Clin Neurosci. 2015;27(4):326‐332. doi: 10.1176/appi.neuropsych.15010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Filshtein T, Beckett LA, Godwin H, Hinton L, Xiong GL. Incident antipsychotic use in a diverse population with dementia. J Am Geriatr Soc. 2016;64(9):e44‐e46. doi: 10.1111/jgs.14209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hsieh S, Yuan J, Lu ZK, Li M. Deprescribing antipsychotics based on real‐world evidence to inform clinical practice: safety considerations in managing older adults with dementia. Front Pharmacol. 2021;12:706750. doi: 10.3389/fphar.2021.706750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Grace EL, Allen RS, Ivey K, Knapp SM, Burgio LD. Racial and ethnic differences in psychotropic medication use among community‐dwelling persons with dementia in the United States. Aging Ment Health. 2018;22(4):458‐467. doi: 10.1080/13607863.2017.1286451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rivera‐Hernandez M, Kumar A, Roy I, Fashaw‐Walters S, Baldwin JA. Quality of care and outcomes among a diverse group of long‐term care residents with Alzheimer's disease and related dementias. J Aging Health. 2022;34(2):283‐296. doi: 10.1177/08982643211043319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nili M, Shen C, Sambamoorthi U. Low‐value care: antipsychotic medication use among community‐dwelling Medicare beneficiaries with Alzheimer's disease and related dementias and without severe mental illness. Aging Ment Health. 2020;24(3):504‐510. doi: 10.1080/13607863.2018.1544211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Thorpe JM, Thorpe CT, Kennelty KA, Gellad WF, Schulz R. The impact of family caregivers on potentially inappropriate medication use in noninstitutionalized older adults with dementia. Am J Geriatr Pharmacother. 2012;10(4):230‐241. doi: 10.1016/j.amjopharm.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schultz BR, Takeshita J, Goebert D, et al. Simultaneous usage of dementia medications and anticholinergics among Asians and Pacific Islanders. Psychogeriatrics. 2017;17(6):423‐429. doi: 10.1111/psyg.12267 [DOI] [PubMed] [Google Scholar]
- 60. Poon I, Lal LS, Ford ME, Braun UK. Racial/ethnic differences in blood pressure control and medication utilization in a cohort of older veterans with dementia. Am J Ther. 2010;17(1):34‐41. doi: 10.1097/MJT.0b013e318197eaa3 [DOI] [PubMed] [Google Scholar]
- 61. Browning JA, Tsang CCS, Dong X, et al. Effects of Medicare comprehensive medication review on racial/ethnic disparities in nonadherence to statin medications among patients with Alzheimer's disease: an observational analysis. BMC Health Serv Res. 2022;22(1):159. doi: 10.1186/s12913-022-07483-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Browning JA, Tsang CCS, Zeng R, et al. Racial/ethnic disparities in the enrollment of medication therapy management programs among Medicare beneficiaries with Alzheimer's disease and related dementias. Curr Med Res Opin. 2022;38(10):1715‐1725. doi: 10.1080/03007995.2022.2103962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gilligan AM, Malone DC, Warholak TL, Armstrong EP. Health disparities in cost of care in patients with Alzheimer's disease: an analysis across 4 state Medicaid populations. Am J Alzheimers Dis Other Demen. 2013;28(1):84‐92. doi: 10.1177/1533317512467679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hermosura AH, Noonan CJ, Fyfe‐Johnson AL, Seto TB, Kaholokula JK, MacLehose RF. Hospital disparities between native Hawaiian and other Pacific Islanders and non‐Hispanic Whites with Alzheimer's disease and related dementias. J Aging Health. 2020;32(10):1579‐1590. doi: 10.1177/0898264320945177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ornstein KA, Zhu CW, Bollens‐Lund E, et al. Medicare expenditures and health care utilization in a multiethnic community‐based population with dementia from incidence to death. Alzheimer Dis Assoc Disord. 2018;32(4):320‐325. doi: 10.1097/wad.0000000000000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gorges RJ, Sanghavi P, Konetzka RT. A national examination of long‐term care setting, outcomes, and disparities among elderly dual eligibles. Health Aff (Millwood). 2019;38(7):1110‐1118. doi: 10.1377/hlthaff.2018.05409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Husaini BA, Sherkat DE, Moonis M, Levine R, Holzer C, Cain VA. Racial differences in the diagnosis of dementia and in its effects on the use and costs of health care services. Psychiatr Serv. 2003;54(1):92‐96. doi: 10.1176/appi.ps.54.1.92 [DOI] [PubMed] [Google Scholar]
- 68. Husaini B, Gudlavalleti AS, Cain V, Levine R, Moonis M. Risk factors and hospitalization costs of dementia patients: examining race and gender variations. Indian J Community Med. 2015;40(4):258‐263. doi: 10.4103/0970-0218.164396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen J, Benjenk I, Barath D, Anderson AC. Disparities in preventable hospitalization among patients with Alzheimer diseases. Am J Prev Med. 2021;60(5):595‐604. doi: 10.1016/j.amepre.2020.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pereira C, LaRoche A, Arredondo B, et al. Evaluating racial disparities in healthcare system utilization and caregiver burden among older adults with dementia. Clin Neuropsychol. 2022;36(2):353‐366. doi: 10.1080/13854046.2021.1951844 [DOI] [PubMed] [Google Scholar]
- 71. Miller EA, Rosenheck RA, Schneider LS. Assessing the relationship between health utilities, quality of life, and health care costs in Alzheimer's disease: the CATIE‐AD study. Curr Alzheimer Res. 2010;7(4):348‐357. doi: 10.2174/156720510791162386 [DOI] [PubMed] [Google Scholar]
- 72. Miller EA, Schneider LS, Rosenheck RA. Assessing the relationship between health utilities, quality of life, and health services use in Alzheimer's disease. Int J Geriatr Psychiatry. 2009;24(1):96‐105. doi: 10.1002/gps.2160 [DOI] [PubMed] [Google Scholar]
- 73. Park S, Chen J. Racial and ethnic patterns and differences in health care expenditures among Medicare beneficiaries with and without cognitive deficits or Alzheimer's disease and related dementias. BMC Geriatr. 2020;20(1):482. doi: 10.1186/s12877-020-01888-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rivera‐Hernandez M, Kumar A, Epstein‐Lubow G, Thomas KS. Disparities in nursing home use and quality among African American, Hispanic, and White Medicare residents with Alzheimer's disease and related dementias. J Aging Health. 2019;31(7):1259‐1277. doi: 10.1177/0898264318767778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sengupta M, Decker SL, Harris‐Kojetin L, Jones A. Racial differences in dementia care among nursing home residents. J Aging Health. 2012;24(4):711‐731. doi: 10.1177/0898264311432311 [DOI] [PubMed] [Google Scholar]
- 76. Resnick B, Van Haitsma K, Kolanowski A, et al. Racial disparities in care interactions and clinical outcomes in Black versus White nursing home residents with dementia. J Nurs Care Qual. 2022;37(3):282‐288. doi: 10.1097/ncq.0000000000000606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Braun UK, Rabeneck L, McCullough LB, et al. Decreasing use of percutaneous endoscopic gastrostomy tube feeding for veterans with dementia‐racial differences remain. J Am Geriatr Soc. 2005;53(2):242‐248. doi: 10.1111/j.1532-5415.2005.53109.x [DOI] [PubMed] [Google Scholar]
- 78. Owen JE, Goode KT, Haley WE. End of life care and reactions to death in African‐American and white family caregivers of relatives with Alzheimer's disease. Omega (Westport). 2001;43(4):349‐361. doi: 10.2190/yh2b-8vve-la5a-02r2 [DOI] [PubMed] [Google Scholar]
- 79. Meier DE, Ahronheim JC, Morris J, Baskin‐Lyons S, Morrison RS. High short‐term mortality in hospitalized patients with advanced dementia: lack of benefit of tube feeding. Arch Intern Med. 2001;161(4):594‐599. doi: 10.1001/archinte.161.4.594 [DOI] [PubMed] [Google Scholar]
- 80. Mitchell SL, Mor V, Gozalo PL, Servadio JL, Teno JM. Tube feeding in US nursing home residents with advanced dementia, 2000‐2014. JAMA. 2016;316(7):769‐770. doi: 10.1001/jama.2016.9374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sharma RK, Kim H, Gozalo PL, Sullivan DR, Bunker J, Teno JM. The Black and White of invasive mechanical ventilation in advanced dementia. J Am Geriatr Soc. 2020;68(9):2106‐2111. doi: 10.1111/jgs.16635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shepard V, Chou LN, Kuo YF, Raji M. Characteristics associated with feeding tube placement: retrospective cohort study of Texas nursing home residents with advanced dementia. J Am Med Dir Assoc. 2021;22(7):1471‐1476. doi: 10.1016/j.jamda.2020.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Henao D, Gregory C, Walters G, Stinson C, Dixon Y. Race and prevalence of percutaneous endoscopic gastrostomy tubes in patients with advanced dementia. Palliat Support Care. 2023;224‐229. doi: 10.1017/s1478951521002042 [DOI] [PubMed] [Google Scholar]
- 84. Kim DS, Kunicki ZJ, Philips OW, et al. Racial and geographic disparities with gastrostomy tube placement in dementia and parkinsonian disorders. Parkinsonism Relat Disord. 2021;91:28‐31. doi: 10.1016/j.parkreldis.2021.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Luth EA, Manful A, Prigerson HG, et al. Associations between dementia diagnosis and end‐of‐life care utilization. J Am Geriatr Soc. 2022;70(10):2871‐2883. doi: 10.1111/jgs.17952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jia Z, Leiter RE, Sanders JJ, et al. Asian American Medicare beneficiaries disproportionately receive invasive mechanical ventilation when hospitalized at the end‐of‐life. J Gen Intern Med. 2022;37(4):737‐744. doi: 10.1007/s11606-021-06794-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Austin AM, Carmichael DQ, Bynum JPW, Skinner JS. Measuring racial segregation in health system networks using the dissimilarity index. Soc Sci Med. 2019;240:112570. doi: 10.1016/j.socscimed.2019.112570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Temkin‐Greener H, Yan D, Wang S, Cai S. Racial disparity in end‐of‐life hospitalizations among nursing home residents with dementia. J Am Geriatr Soc. 2021;69(7):1877‐1886. doi: 10.1111/jgs.17117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lin PJ, Zhu Y, Olchanski N, et al. Racial and ethnic differences in hospice use and hospitalizations at end‐of‐life among Medicare beneficiaries with dementia. JAMA Netw Open. 2022;5(6):e2216260. doi: 10.1001/jamanetworkopen.2022.16260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Oud L. Predictors of transition to hospice care among hospitalized older adults with a diagnosis of dementia in Texas: a population‐based study. J Clin Med Res. 2017;9(1):23‐29. doi: 10.14740/jocmr2783w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Luth EA, Russell DJ, Brody AA, et al. Race, ethnicity, and other risks for live discharge among hospice patients with dementia. J Am Geriatr Soc. 2020;68(3):551‐558. doi: 10.1111/jgs.16242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Luth EA, Prigerson HG. Associations between race and dementia status and the quality of end‐of‐life care. J Palliat Med. 2018;21(7):970‐977. doi: 10.1089/jpm.2017.0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Badana ANS, Marino V, Haley WE. Racial differences in caregiving: variation by relationship type and dementia care status. J Aging Health. 2019;31(6):925‐946. doi: 10.1177/0898264317743611 [DOI] [PubMed] [Google Scholar]
- 94. Parker LJ, Fabius CD. Racial differences in respite use among Black and White caregivers for people living with dementia. J Aging Health. 2020;32(10):1667‐1675. doi: 10.1177/0898264320951379 [DOI] [PubMed] [Google Scholar]
- 95. Sleath B, Thorpe J, Landerman LR, Doyle M, Clipp E. African‐American and White caregivers of older adults with dementia: differences in depressive symptomatology and psychotropic drug use. J Am Geriatr Soc. 2005;53(3):397‐404. doi: 10.1111/j.1532-5415.2005.53155.x [DOI] [PubMed] [Google Scholar]
- 96. Parker LJ, Gaugler JE, Samus Q, Gitlin LN. Adult day service use decreases likelihood of a missed physician's appointment among dementia caregivers. J Am Geriatr Soc. 2019;67(7):1467‐1471. doi: 10.1111/jgs.15995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Galvin JE, Sadowsky CH. Practical guidelines for the recognition and diagnosis of dementia. J Am Board Fam Med. 2012;25(3):367‐382. doi: 10.3122/jabfm.2012.03.100181 [DOI] [PubMed] [Google Scholar]
- 98. Davis MA, Lee KA, Harris M, et al. Time to dementia diagnosis by race: a retrospective cohort study. J Am Geriatr Soc. 2022;70(11):3250‐3259. doi: 10.1111/jgs.18078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER‐ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512‐527. doi: 10.1001/jama.2023.13239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Estrada LV, Agarwal M, Stone PW. Racial/ethnic disparities in nursing home end‐of‐life care: a systematic review. J Am Med Dir Assoc. 2021;22(2):279‐290. doi: 10.1016/j.jamda.2020.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. LoPresti MA, Dement F, Gold HT. End‐of‐life care for people with cancer from ethnic minority groups: a systematic review. Am J Hosp Palliat Care. 2016;33(3):291‐305. doi: 10.1177/1049909114565658 [DOI] [PubMed] [Google Scholar]
- 102. Balls‐Berry JJE, Babulal GM. Health disparities in dementia. Continuum (Minneap Minn). 2022;28(3):872‐884. doi: 10.1212/con.0000000000001088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. King RK, Green AR, Tan‐McGrory A, Donahue EJ, Kimbrough‐Sugick J, Betancourt JR. A plan for action: key perspectives from the racial/ethnic disparities strategy forum. Milbank Q. 2008;86(2):241‐272. doi: 10.1111/j.1468-0009.2008.00521.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chin MH, Clarke AR, Nocon RS, et al. A roadmap and best practices for organizations to reduce racial and ethnic disparities in health care. J Gen Intern Med. 2012;27(8):992‐1000. doi: 10.1007/s11606-012-2082-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Alvidrez J, Castille D, Laude‐Sharp M, Rosario A, Tabor D. The national institute on minority health and health disparities research framework. Am J Public Health. 2019;109(S1):S16‐S20. doi: 10.2105/ajph.2018.304883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care . In: Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; 2003. [PubMed] [Google Scholar]
- 107. Bourgois P, Holmes SM, Sue K, Quesada J. Structural vulnerability: operationalizing the concept to address health disparities in clinical care. Acad Med. 2017;92(3):299‐307. doi: 10.1097/acm.0000000000001294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Melino K. Structural competency in health care. Nurs Clin North Am. 2022;57(3):433‐441. doi: 10.1016/j.cnur.2022.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Quiñones AR, Talavera GA, Castañeda SF, Saha S. Interventions that reach into communities–promising directions for reducing racial and ethnic disparities in healthcare. J Racial Ethn Health Disparities. 2015;2(3):336‐340. doi: 10.1007/s40615-014-0078-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Butkus R, Rapp K, Cooney TG, Engel LS. Envisioning a better U.S. health care system for all: reducing barriers to care and addressing social determinants of health. Ann Intern Med. 2020;172(2):S50‐S59. doi: 10.7326/m19-2410 [DOI] [PubMed] [Google Scholar]
- 111. Fashaw‐Walters SA, Rahman M, Gee G, Mor V, White M, Thomas KS. Out of reach: inequities in the use of high‐quality home health agencies. Health Affairs. 2022;41(2):247‐255. doi: 10.1377/hlthaff.2021.01408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kilbourne AM, Switzer G, Hyman K, Crowley‐Matoka M, Fine MJ. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96(12):2113‐2121. doi: 10.2105/ajph.2005.077628 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


