Abstract
DNA vaccination is an effective means of eliciting strong antibody responses to a number of viral antigens. However, DNA immunization alone has not generated persistent, high-titer antibody and neutralizing antibody responses to human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein (Env). We have previously reported that DNA-primed anti-Env antibody responses can be augmented by boosting with Env-expressing recombinant vaccinia viruses. We report here that recombinant Env protein provides a more effective boost of DNA-initiated antibody responses. In rabbits primed with Env-expressing plasmids, protein boosting increased titer, persistence, neutralizing activity, and avidity of anti-Env responses. While titers increased rapidly after boosting, avidity and neutralizing activity matured more slowly over a 6-month period following protein boosting. DNA priming and protein immunization with HIV-1 HXB-2 Env elicited neutralizing antibody for T cell line-adapted, but not primary isolate, viruses. The most effective neutralizing antibody responses were observed after priming with plasmids which expressed noninfectious virus-like particles. In contrast to immunizations with HIV-1 Env, DNA immunizations with the influenza virus hemagglutinin glycoprotein did not require a protein boost to achieve high-titer antibody with good avidity and persistence.
DNA immunization effectively elicits high-titer neutralizing antibody against influenza, measles, rabies, and herpesviruses but has been less successful in generating neutralizing antibody against human immunodeficiency virus type 1 (HIV-1) (reviewed by Robinson [53]). While single or singly boosted DNA immunizations often elicit strong and long-lasting neutralizing antibody responses comparable with those seen in virally infected and convalescent animals (52, 40, 66), multiple DNA immunizations are typically required to elicit even modest titers of HIV-1-neutralizing antibody (1, 4, 15–17, 33–36, 47, 51, 58, 63, 64). Furthermore, antibody responses elicited by DNA immunization (47, 51) or protein subunit immunization (21, 38) with Env are transient; titers rise and fall with successive immunizations.
Work with the simian immunodeficiency virus (SIV) system allows direct comparison of anti-Env antibody titers elicited by DNA immunization or viral infection. DNA immunization of macaques with SIV Env elicits neutralizing titers which are, at best, only 5 to 10% of those in SIV-infected macaques (53). If DNA immunization is to play a meaningful role in the development of the antibody component of a HIV-1 vaccine, we must identify ways of improving the titer and persistence of these neutralizing antibody responses.
Recently, attention has focused on the avidity, as well as the neutralizing titers, of antibody responses elicited by immunodeficiency virus infections and immunizations (8, 9, 18). Antibody responses induced by the envelope glycoprotein (Env) of the lentiviruses SIV (8, 9) and equine infectious anemia virus (24) mature slowly. Maturation, in this sense, is defined as development of significant avidity, high neutralizing titers, and some degree of cross-neutralizing activity. While antibody titers rise within several weeks of infection and neutralizing antibody specific for the autologous SIV peaks within several months, the avidity of polyclonal antisera increases more slowly, reaching maximal levels between 6 and 8 months after infection. Increased avidity is coincident with a broadening of protective neutralizing antibody responses to heterologous viruses (8, 9). Slow maturation of antibody and development of cross-neutralizing antibody, over 8 to 12 months, is also observed in HIV-infected patients (44). In contrast, the avidity of antibody responses to infection with nonlentiviruses, such as hepatitis C virus (65), varicella-zoster virus (28), and rubella virus (31), is fairly rapid; high-avidity responses are seen in a period of weeks to a few months after infection.
While affinity is an absolute thermodynamic measure of the strength of interaction determined at equilibrium, avidity can be defined as a more relative measure of the strength of interaction which is a function of antigenic valence and structure, antibody bivalence, the concentrations of antibody and antigen, and affinity. Affinity of polyclonal antisera cannot be determined. The relative avidity of polyclonal antisera can be estimated by using so-called avidity enzyme-linked immunosorbent assays (ELISAs) in which the ability of chaotropic agents (such as urea or sodium thiocyanate) to disrupt antigen-antibody interactions is determined (2, 7, 22, 37).
In this study, we examined the magnitude, persistence, avidity, and neutralizing activity of antibody elicited by priming rabbits with plasmids expressing various forms (and combination of forms) of HIV-1 Env and by boosting these responses with recombinant gp160. We compared these anti-Env antibody responses with antibody responses produced by DNA immunization of rabbits with a plasmid expressing influenza virus hemagglutinin type 1 (H1). While DNA immunization with influenza virus H1 elicited persistent high-titer antibody and neutralizing antibody, DNA immunization with HIV-1 Env did not. Furthermore, while the avidity of anti-H1 antibody was relatively strong, that of anti-Env antibody was weak. Protein boosting with rgp160 increased the titers, the persistence, and the avidity of anti-Env antibody. We conclude that DNA priming with HIV-1 Env is an effective means of priming anti-Env antibody responses but requires protein boosting to elicit high-titer and high-avidity antibody.
MATERIALS AND METHODS
Animals.
Female New Zealand White rabbits, 8 to 10 weeks old and 4 to 5 lb each, were purchased from Millbrook Farms (Amherst, Mass.) and housed in accordance with U.S. Department of Agriculture regulations. Rabbits were anesthetized with a 1:1 (vol/vol) mixture of ketamine-anased and bled by ear vein puncture.
Plasmids and protein expression.
Vaccine plasmids which express HXB-2 envelope glycoprotein gp120 (pJW4303/HXB-2gp120; abbreviated pHXB2gp120) and gp140 (pJW4303/HXB-2gp140; abbreviated pHXB2gp140) have been described by Mustafa et al. (47). Plasmids expressing gp160 (pCMVdHXB-2env; abbreviated pHXB2env) and defective virion constructs (pCMVHXB-2dpol; abbreviated pHXB2dpol) were modifications of those described by Lu et al. (34); in both cases, a downstream intron was removed from the rat preproinsulin polyadenylation sequence of the mammalian expression plasmid pBC/IL-2/CMV (34). A plasmid expressing A/PR/8/34 influenza virus hemagglutinin (pJW4303/H1 [pH1]) has been described by Robinson et al. (52). A plasmid which expresses human growth hormone (hGH), pWR61602, was provided by Joel Haynes, formerly of Geniva Inc. (Middleton, Wis.). Plasmids were grown in Escherichia coli HB101 and were purified with Qiagen (Santa Clarita, Calif.) anion-exchange resins. Lipofectamine (Life Technologies, Grand Island, N.Y.)-mediated transient transfection of Cos cells and an HIV-1 antigen-specific ELISA were used to quantitate expression of Env from plasmids (47). The hGH-expressing plasmid was included in each transfection as an internal control, and hGH expression was determined by using a commercial ELISA (Boehringer-Mannheim, Indianapolis, Ind.).
Indirect immunofluorescence assays were used to determine the localization of Env in transfected Cos cells. Cos monolayers were grown on coverslips prior to transfection and fixed with 4% paraformaldehyde or 100% methanol after transfection and prior to staining either with polyclonal rabbit anti-HXB-2 Env (elicited by DNA vaccination with various forms of HXB-2 Env) and goat anti-rabbit antibody conjugated with fluorescein isothiocyanate (Sigma, St. Louis, Mo.) or with mouse antihemagglutinin monoclonal antibodies (a kind gift of Walter Gerhard, Wistar Institute) and goat anti-mouse antibody conjugated with fluorescein isothiocyanate (Sigma). Fluorescence was observed with an Axioscope microscope (Carl Zeiss, Inc., Thornwood, N.Y.).
DNA priming.
Rabbits were primed by gene gun immunization. Gold beads, 0.95 μm in diameter (Geniva Inc.), were loaded with DNA at either 0.25 μg of DNA/mg of gold (Env-expressing plasmids) or 0.5 μg of DNA/mg of gold (H1-expressing plasmid). Each shot delivered 0.5 mg of gold and either 0.12 μg of Env-expressing DNA or 0.25 μg of H1-expressing DNA. Thirty-six shots, carrying a total of 4.5 μg of HIV Env-expressing plasmid or 9 μg of H1-expressing plasmid, were delivered to nonoverlapping areas of the shaved abdominal skin of anesthetized rabbits by using a helium-discharge Acell II gene gun (Geniva) at 375 to 450 lb/in2. Rabbits were primed three times at 1-month intervals.
rgp160 boosting.
Six months after the final DNA inoculation, primed and naive rabbits were boosted with 100 μg of recombinant HIV-1 IIIb gp160 (rgp160) in incomplete Freund’s adjuvant (Sigma). rgp160 was produced by using the recombinant vaccinia virus, v11Kenv5 (30). rgp160 is in a presumed oligomeric form. Rabbits were anesthetized, and protein was injected both intradermally (six times, 50 μl each) and intramuscularly (twice, 100 μl each). A second rgp160 boost was given 6 months after the first. Sera were collected just prior to, and roughly 2, 4, 8, and 38 weeks after, each immunization.
Determination of HIV-1 Env-specific IgG titers.
An ELISA was used to determine anti-Env immunoglobulin G (IgG) titers of rabbit sera. Sera collected following DNA immunizations were assayed by using a mixture of the gp120 and gp140 forms of the BH8 Env produced by the recombinant vaccinia virus vCB-14 (12) as the solid-phase antigen. Sera collected after rgp160 boosting were assayed by using recombinant HIV-1 IIIb gp120 produced by using baculovirus (rgp120; Intracel, Seattle, Wash.) as the solid-phase antigen, to ensure that anti-vaccinia virus antibodies did not interfere with determination of anti-Env antibody titers in boosted rabbits. Details of the ELISA were described previously by Mustafa et al. (47) and Richmond et al. (51). Both solid-phase antigens gave identical anti-Env concentrations for preboost sera. All samples were run in duplicate at several serial dilutions. Titers of less than 0.1 μg of Env-specific IgG/ml of serum were not considered significant.
Determination of H1-specific antibody titers.
ELISA plates were coated with Triton X-100-lysed influenza virus A/PR/8/34, and rabbit sera were assayed as described by Boyle et al. (5) with the following modifications. Sera were assayed at threefold serial dilutions ranging from 1:500 to 1:8,900,000 for 60 min at 23°C. Bound antisera were detected with biotinylated goat anti-rabbit IgG and horseradish peroxidase-linked streptavidin (Vector Labs, Burlingame, Calif.) and 3,3′,5,5′-tetramethylbenzidine (Sigma). A standard curve was constructed by using threefold serial dilutions of blood from the fifth blood sample of rabbit R119, which contained approximately 84.5 μg of influenza virus-specific IgG/ml. This approximate concentration was determined as described by Mustafa et al. (47) by using lysed A/PR/8/34 influenza virus, rather than HIV-1 Env, as the solid-phase antigen. The optical density was measured at 450 nm, and titers were expressed as micrograms of A/PR/8/34-specific IgG/ml of serum.
NaSCN displacement ELISA.
Sodium thiocyanate (NaSCN) displacement ELISAs were performed by a modification of the methods of Charoenvit et al. (7) and Luxton and Thompson (37). ELISA plates were coated overnight with one of the following specific antigens: concanavalin A and vCB-14 at 0.1 μg/well, concanavalin A and rgp120 at 0.1 μg/well, rp24 at 0.1 μg/well, or Triton X-100-lysed A/PR/8/34. The plates were washed with phosphate-buffered saline (PBS)–0.1% Triton X-100 and blocked for 60 min at 23°C with whey buffer (PBS, 4% whey powder [Davisco, Le Sueur, Minn.], 0.05% Tween 20) containing 5% nonfat dry milk powder. Sera, serially diluted in whey buffer to equivalent initial concentrations of Env-specific IgG, were incubated in ELISA plates for 60 min at 23°C. The plates were washed three times with PBS–Triton X-100, once with PBS containing 0, 1, 2, 3, 4, or 5 M NaSCN for 15 to 20 min, and then six more times with PBS–Triton X-100. Bound antibody was detected as described for the H1-specific ELISA. A standard curve was constructed by using sera from rabbits immunized with pHXB2gp120 that were not subjected to NaSCN washing. All samples were assayed in duplicate over a range of dilutions, and results were expressed as the percentage of antibody bound in the absence of NaSCN.
HIV-1-neutralizing antibody assays.
Antibody-mediated neutralization of HIV-1 IIIb was measured by using the MT-2 cell killing assay described by Montefiori et al. (41) and Richmond et al. (51). Neutralization of HIV-1 primary isolates was assayed on peripheral blood mononuclear cells as previously described (43, 51). V3-loop specificity of neutralizing antibody was determined by preincubating serum samples with 20 μg of the V3-loop peptide (NNTRKSIRIQRGPGRAFVTIGKIG; amino acids 307 to 330 of IIIb Env) prior to performance of the MT-2 cell killing assay. Neutralizing titers are defined as that dilution of serum which resulted in 50% protection from virally induced cell killing in MT-2 cells or 90% reduction in p24 synthesis in peripheral blood mononuclear cells.
Influenza virus-neutralizing antibody assay.
Sterile 96-well tissue culture plates were seeded with 105 MDCK cells in modified Eagle’s medium (MEM) containing penicillin, streptomycin, l-glutamine, and 5% fetal calf serum and grown to confluence overnight at 37°C in 5% CO2. Serial threefold dilutions of rabbit serum, beginning at 1:50, were made in 100 μl of MEM. One hundred 50% tissue culture infective doses (TCID50), in 100 μl of MEM–4% bovine serum albumin–1 μg of TPCK (N-tosyl-l-phenylalanine chloromethyl ketone [Sigma]) per ml, were added to each serum sample. Virus-antiserum mixtures were incubated at 37°C, in 5% CO2, for 60 min. Confluent monolayers were washed twice with sterile PBS, overlaid with virus-antiserum mixtures (all conditions were assayed in duplicate wells, including control wells containing no antisera or neither antisera nor virus), and incubated for 72 h. Monolayers were visually scored for cytopathic effects, and neutralizing titer was defined as the highest antiserum dilution which prevented cytopathic effects.
RESULTS
Vaccine plasmids and expression in transiently transfected Cos cells.
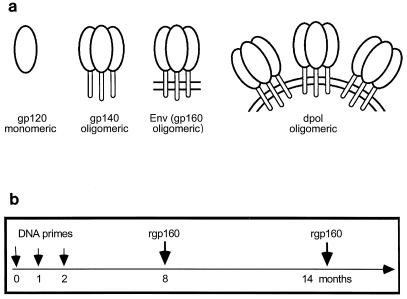
Vaccine plasmids expressed four forms of HIV-1 HXB-2 Env (Fig. 1a). The gp120 form represents a monomeric CD4-binding subunit of Env and terminates at amino acid 506 (numbered to include the signal sequence). In contrast, the gp140 form terminates at amino acid 675, includes the entire extracellular domain of Env, and forms an oligomer. Full-length Env (gp160) includes the transmembrane and cytoplasmic domains (Fig. 1a). The dpol plasmid encodes full-length Env and all HIV-1 HXB-2 proteins except Pol (HXB-2 is defective for vpr, vpu, and nef) and produces defective virus-like particles which bud from transfected cells (34). A fifth plasmid expressed the complete membrane-bound form of influenza virus H1 (52).
FIG. 1.
Study design. (a) Vaccine plasmids were constructed to express four forms of HIV-1 Env: gp120, gp140, gp160, and noninfectious, virus-like particles. gp120 is the nonreceptor-binding domain of Env. gp140 is the entire extracellular domain of Env and contains oligomerization sequences. (b) The immunization schedule used is shown.
Both the release of Env from cells and the localization of Env within cells were dependent on the form of Env. Nearly all of the HXB-2 gp120 was in culture medium, while only half of the gp140 and only 10% of the full-length Env were present in the medium (Table 1). About one-third of the HIV-1 antigens expressed from pHXB2dpol were secreted or shed into the medium (Table 1). In agreement with prior studies (11, 23), indirect immunofluorescence analyses revealed different localizations of the different forms of Env (data not shown). The cell-associated gp120 was located both in the cytosol and at the plasma membrane. In contrast, most gp140 was found in the perinuclear regions of the cell, while smaller amounts were seen in the cytosol and at the cell surface. Full-length Env and Env expressed by pHXB2dpol were observed only in the perinuclear regions of the cell. Like gp120, influenza virus H1 localized both in the cytosol and at the plasma membrane (data not shown).
TABLE 1.
Expression of vaccine plasmids in transfected Cos cellsa
| Plasmid | Total amt of Env (ng) | % Env in supernatant | Amt of hGH in supernatant (ng) |
|---|---|---|---|
| pJW4303 | 0 | NAc | 316 |
| pCMV | 0 | NA | 502 |
| pHXB2gp120 | 12 | 92 | 242 |
| pHXB2gp140 | 15 | 49 | 302 |
| pHXB2env | 35 | 10 | 410 |
| pHXB2dpolb | 230 | 27 | NDd |
Tissue culture media and cellular lysates were collected at 24 h and subjected to HIV-1 antigen capture ELISA. An hGH-expressing plasmid (pWR61602) was included as an internal control. This experiment is representative of repeated assays.
Expression levels (in nanograms) of pHXB2dpol are high because the ELISA used detects total HIV-1 antigens rather than Env alone.
NA, not applicable.
ND, not done.
Temporal antibody responses following DNA priming with Env antigens.
To investigate the immunogenicity of Env-expressing plasmids, four groups of three rabbits were immunized with Env-expressing plasmids. The first group was primed with pHXB2env (the Env group), and the second was primed with pHXB2dpol (the dpol group). The third group was primed with a combination of pHXB2env, pHXB2gp120, and pHXB2gp140 (the Env++ group), and the fourth group was primed with pHXB2dpol, pHXB2gp120, and pHXB2gp140 (the dpol++ group). A fifth group of two rabbits was immunized with the plasmid expressing influenza virus H1 (the H1 group). All rabbits were primed three times at 1-month intervals (Fig. 1b).
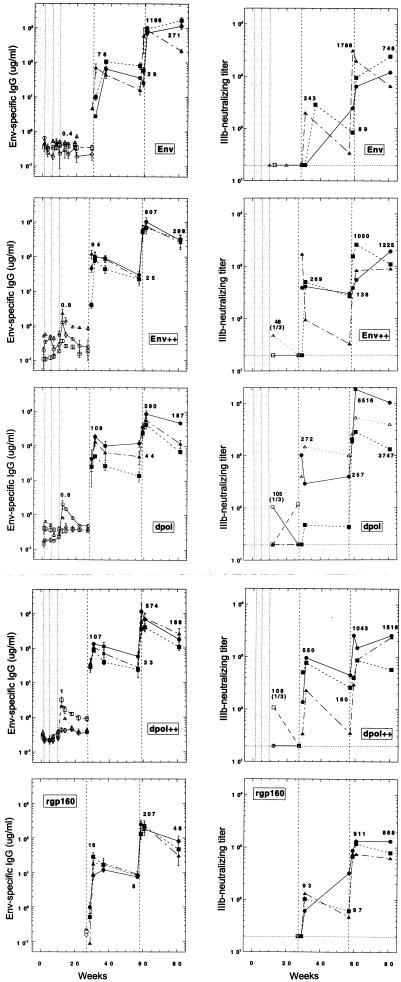
Consistent with previous studies (1, 4, 15–17, 33–36, 47, 51, 58, 63, 64), the induction of anti-Env antibody required multiple DNA immunizations (Fig. 2). One of three rabbits from the dpol group and two of three rabbits from the Env++ and dpol++ groups seroconverted after the third DNA immunization. None of the rabbits in the Env group seroconverted (Fig. 2). As noted in previous studies (47, 51), anti-Env titers were low (1 to 4 μg of Env-specific IgG/ml) (Table 2) and were not persistent (Fig. 2).
FIG. 2.
Temporal ELISA and IIIb-neutralizing antibody responses. Env indicates DNA priming with pHXB2env, while Env++ indicates priming with a combination of pHXB2env, pHXB2gp120, and pHXB2gp140. Similarly, dpol indicates DNA priming with pHXB2dpol and dpol++ indicates priming with a combination of pHXB2dpol, pHXB2gp120, and pHXB2gp140. Times of DNA immunizations are represented by thin vertical dotted lines, while times of protein immunizations are represented by heavier vertical dashed lines. Open symbols represent sera collected after DNA priming, while filled symbols represent sera collected after protein boosting. Individual rabbits are represented by different symbols (circles, squares, or triangles). ELISA values are arithmetic means ± standard deviations. Values on graphs are GMTs.
TABLE 2.
Peak ELISA and IIIb-neutralizing titersa
| Group | Rabbit | Anti-Env ELISA titers (μg of IgG/ml of serum)
|
IIIb-neutralizing titers
|
||||
|---|---|---|---|---|---|---|---|
| DNA | rgp160 × 1 | rgp160 × 2 | DNA | rgp160 × 1 | rgp160 × 2 | ||
| Env | R104 | 0.3 ± 0.1 | 65 ± 12 | 1,150 ± 202 | <20 | NDb | 1,198 |
| R105 | 0.4 ± 0.1 | 105 ± 14 | 1,693 ± 342 | <20 | 287 | 2,425 | |
| R106 | 0.5 ± 0.1 | 70 ± 28 | 879 ± 263 | <20 | 199 | 1,963 | |
| 0.4 | 78 | 1,196 | 243 | 1,786 | |||
| Env++ | R107 | 1 ± 0.2 | 101 ± 30 | 1,017 ± 331 | <20 | 411 | 546 |
| R108 | 2 ± 0.8 | 81 ± 14 | 678 ± 219 | <20 | 500 | 2,560 | |
| R109 | 4 ± 0.2 | 102 ± 34 | 762 ± 206 | 48 | 95 | 830 | |
| 0.9 | 94 | 807 | 269 | 1,050 | |||
| dpol | R110 | 2 ± 0.6 | 187 ± 29 | 864 ± 212 | 105 | 291 | 18,369 |
| R111 | 0.6 ± 0.1 | 50 ± 6 | 420 ± 100 | <20 | 47 | 2,829 | |
| R112 | 0.8 ± 0.1 | 133 ± 33 | 568 ± 137 | <20 | 1,487 | 5,318 | |
| 0.8 | 108 | 590 | 272 | 6,516 | |||
| dpol++ | R113 | 0.4 ± 0.1 | 131 | 674 ± 356 | <20 | 953 | 1,488 |
| R114 | 3 ± 0.6 | 87 ± 12 | 393 ± 102 | 108 | 765 | 861 | |
| R115 | 2 ± 0.1 | 107 ± 19 | 714 ± 296 | <20 | 228 | 886 | |
| 1 | 107 | 574 | 550 | 1,043 | |||
| rgp160 only | R126 | 8 ± 2 | 176 ± 59 | 61 | 869 | ||
| R127 | 28 ± 9 | 221 ± 58 | 102 | 1,174 | |||
| R128 | 18 ± 4 | 229 ± 85 | 131 | 741 | |||
| 16 | 207 | 93 | 911 | ||||
Peak DNA-elicited titers were determined 2 weeks after the third DNA priming (DNA), while boosted titers were determined 4 weeks after each protein boost (rgp160 × 1 and rgp160 × 2, first and second protein boosts with rgp160, respectively). All ELISAs were performed three times, and titers are expressed as arithmetic means ± standard deviations. IIIb-neutralizing titers (50% neutralization) of the same serum samples were determined on MT-2 cells by using a cell viability assay. Values in boldface type are GMTs. ELISA and neutralizing titers were determined either 2 or 4 weeks after the final DNA immunization or each protein immunization.
ND, this value was not determined.
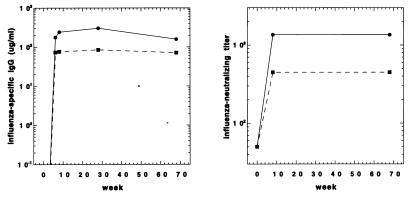
In contrast to immunization with HIV-1 Env, DNA immunization with influenza virus H1 elicited high titers of specific antibody that ranged from 100 to 300 μg of specific IgG per ml of serum (Fig. 3). Furthermore, these high titers were apparent after the second DNA immunization, and unlike anti-Env responses, anti-H1 antibody titers were persistent. Anti-H1 titers fell less than threefold over a 60-week period following the final DNA immunization (Fig. 3).
FIG. 3.
Temporal anti-H1 antibody and A/PR/8/34-neutralizing antibody responses. Antibody titers are expressed as micrograms of A/PR/8/34-specific IgG per milliliter of serum. A neutralizing antibody titer was defined as that dilution of serum which protected MDCK monolayers from infection with 100 TCID50 of influenza virus A/PR/8/34. Immunizations were at 0, 4, and 8 weeks.
Recombinant protein boosting.
In an effort to increase DNA-primed anti-Env antibody titers, all four Env-primed groups, as well as a group of naive rabbits, were boosted with rgp160 protein. rgp160 was chosen because it is likely to retain some native, oligomeric structure. Intradermal and intramuscular boosts of 100 μg of rgp160 in incomplete Freund’s adjuvant were given twice, at 6-month intervals (Fig. 1b).
The initial protein boost increased geometric mean titers (GMTs) of Env-specific antibody in the DNA-primed rabbits 100- to 200-fold to a GMT of ∼100 μg/ml (Fig. 2 and Table 2). Antibody titers increased rapidly in the Env++, dpol, and dpol++ groups, where titers were similar at 2 and 4 weeks postboost in most rabbits. In contrast, maximal antibody responses in the Env group did not occur until 4 weeks after the rgp160 boost. rgp160 immunization of naive rabbits elicited an anti-Env GMT of 16.0 μg/ml (Table 2). Maximal titers were not observed in naive rabbits until 4 weeks after immunization, consistent with induction of a primary B-cell response by rgp160 boosting. In both DNA-primed and naive rabbits, protein immunization produced a persistent antibody response that decreased by a factor of two to four over the next 6 months (Fig. 2).
A second protein boost was given 6 months after the first. This second boost increased antibody titers 5- to 15-fold. Antibody titers of DNA-primed and protein-boosted rabbits remained higher (GMT, 792 μg/ml) than those of animals which had not been primed with DNA (GMT, 207 μg/ml) (Table 2). The kinetics of the antibody response to the second boost were similar in all groups, and titers peaked within 2 weeks. Again, antibody responses were persistent, with titers decreasing only three- to fivefold over the next 6 months (Fig. 2).
Studies of antibody avidity using NaSCN displacement ELISAs.
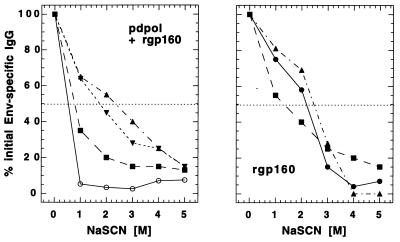
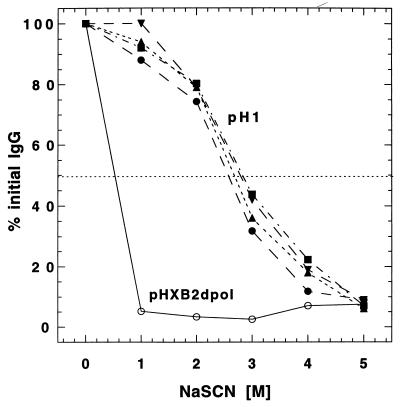
NaSCN displacement ELISAs demonstrated that the nature of the Env-specific antibody changed with both protein boosting and the passage of time (Fig. 4). Antibody elicited by pHXB2dpol priming of rabbit R110 was released at low concentrations of NaSCN. The effective concentration of NaSCN required to release 50% of antiserum from the ELISA plate (referred to as the ED50) was 0.8 M. Four weeks after the first rgp160 boost, the ED50 had increased to 1.0 M. However, 6 months later (just prior to the second protein boost), the ED50 had risen to 2.3 M. Four weeks after the second rgp160 boost, this value remained largely unchanged (2.0 M). In contrast, the ED50 of serum collected from a rabbit immunized with protein only (R126) was fairly high (2.2 M) by 4 weeks after the first immunization and was basically unchanged 4 weeks after the second protein boost (Fig. 4).
FIG. 4.
NaSCN displacement ELISAs of anti-Env antisera. Selected serum samples from a rabbit primed with pHXB2dpol and boosted with rgp160 (R110) or from a naive rabbit immunized with rgp160 (R126) were assayed by using an Env-specific NaSCN displacement ELISA. Sera were diluted to similar concentrations of Env-specific antibody prior to assay, and all sera were assayed at several dilutions. R110 serum samples were taken 2 weeks after DNA priming (open circles), 4 weeks after the first rgp160 boost (filled squares), 6 months after the first rgp160 boost (filled triangles), and 4 weeks after the second rgp160 boost (filled inverted triangles). R126 serum samples were taken 4 weeks (filled circles) and 6 months (filled squares) after the first immunization and 4 weeks after the second (filled triangles) rgp160 immunization.
In contrast to the low-avidity antibody elicited by DNA immunization with Env, a higher-avidity antibody was induced by DNA immunization with influenza virus H1 (Fig. 5). H1-specific antibody was released by higher levels of NaSCN (ED50, ranging from 2.4 to 3.0 M) than antibody elicited by Env-expressing plasmids (ED50, 0.8 M). Anti-H1 antibody avidity was high at the earliest time point tested and did not change with either time or number of DNA inoculations (Fig. 5).
FIG. 5.
NaSCN displacement ELISAs of anti-H1 antisera. Rabbit 119 was immunized three times with pH1. Selected serum samples were assayed by using an A/PR/8/34-specific NaSCN-displacement ELISA. Serum samples were collected 2 weeks after the second DNA immunization (filled circles), at the time of the third DNA immunization (filled squares), and 4 months (filled triangles) and 10 months (filled inverted triangles) after the third immunization. A serum sample collected after three DNA immunizations with pHXB2dpol (rabbit R110) (open circles) is included for comparison.
Temporal IIIb-neutralizing antibody responses.
The first rgp160 boost increased both the frequency and titer of IIIb-neutralizing antibodies. The kinetics of neutralizing antibody responses were similar to those of ELISA antibody responses (Fig. 2). Four weeks after the final DNA priming, serum from one of three rabbits in each of the Env++, dpol, and dpol++ groups exhibited low-titer IIIb-neutralizing antibody (1:48 to 1:108) (Table 2). Detectable neutralizing antibody (titer, 1:506 to 1:1,692) was present within 2 weeks after the first protein boost in those three rabbits. Within 4 weeks after the first protein boost, all rabbits, including the naive group, exhibited neutralizing antibody (Fig. 2). The geometric mean neutralizing titer of DNA-primed rabbits (titer, 333.5) was significantly greater than that of naive rabbits (titer, 93) (Table 2).
The second rgp160 boost further increased neutralizing titers in all rabbits within 2 weeks of this boost (Fig. 2). Again, the rabbit with the highest neutralizing titer (R110) had displayed DNA-primed neutralizing activity. By 4 weeks after the second protein boost, the IIIb-neutralizing titers of rabbits which had not been primed with DNA (naive) were approaching those of the DNA-primed rabbits (Table 2).
Protein boosts also increased the persistence of IIIb-neutralizing antibody (Fig. 2). Over the 6-month period between the first and second rgp160 boosts, geometric mean neutralizing titers decreased less than fourfold. Six months after the second rgp160 boost, geometric mean IIIb-neutralizing titers of the Env and Env++ groups had decreased twofold, while that of the naive animals had not changed. The mean neutralizing titers of animals in the dpol and dpol++ groups may have increased slightly (Fig. 2).
Quality of IIIb-neutralizing antibody.
Over the 6-month period following each protein boost, ELISA titers decreased more quickly than IIIb-neutralizing titers and neutralizing antibody/ELISA antibody ratios therefore rose (Table 3). For example, mean neutralizing antibody/ELISA antibody ratios of rabbits in the dpol group rose from 2.5 at four weeks after the first rgp160 boost to 5.8 at 6 months later. Similarly, neutralizing antibody/ELISA antibody ratios in this dpol group were higher 6 months after the second boost (ratio of 20) than immediately after the boost (ratio of 11). Throughout the experiment, the dpol group had the overall most favorable neutralizing antibody/ELISA antibody ratio, except for the period of time immediately following the first protein boost (Table 3). Interestingly, rabbits immunized with protein only also had quite favorable neutralizing antibody/ELISA antibody ratios. While determination of neutralizing antibody titers is neither simple nor absolutely quantitative, these data suggest that protein boosting increased the quality, as well as the titer, of neutralizing antibody.
TABLE 3.
Quality of neutralizing antibody with protein boosting and timea
| Group | Neutralizing antibody/ELISA antibody ratio
|
||||
|---|---|---|---|---|---|
| Peak DNA | rgp160 × 1
|
rgp160 × 2
|
|||
| <1 mo | 6 mo | <1 mo | 6 mo | ||
| Env | 3.1 | 3.1 | 1.5 | 2.8 | |
| Env++ | 12b | 2.9 | 5.5 | 1.3 | 4.2 |
| dpol | 52b | 2.5 | 5.8 | 11.0 | 20.0 |
| dpol++ | 36b | 5.1 | 4.8 | 1.8 | 8.9 |
| rgp160 only | NAc | 5.8 | 12.1 | 4.4 | 18.1 |
The quality of neutralizing antibody is assessed by calculation of the ratio of neutralizing antibody to ELISA (or binding) antibody. Increasing ratios are indicative of increasing effectiveness (or quality) of neutralizing antibody. Results are expressed as the neutralizing antibody/ELISA antibody ratios of geometric mean neutralizing and ELISA titers for each group (see Fig. 2). rgp160 × 1 and rgp160 × 2, first and second protein boosts with rgp160.
Values derived for the one rabbit which seroconverted in each group at this early time point.
NA, this group was not primed with DNA.
Breadth of neutralization.
To assess the breadth of neutralization elicited by the DNA prime-protein boost protocol, neutralizing assays were performed with two unrelated, T cell line-adapted (TCLA) viruses, HIV-1 MN and SF2 (Table 4). Sera collected four weeks after the second rgp160 boost exhibited moderate to strong neutralization of both MN and SF2. MN-neutralizing titers ranged from 1:149 to 1:3,598, while SF-neutralizing titers ranged from 1:37 to 1:269. Sera from all rabbits, including the rabbits immunized with protein only, exhibited cross-neutralizing activity (Table 4).
TABLE 4.
Neutralization of TCLA HIV-1 strains
| Group | Rabbit | Neutralizing antibody titera
|
IIIb % V3b | ||
|---|---|---|---|---|---|
| IIIb | MN | SF-2 | |||
| Env | R104 | 1,357 | 163 | 40 | NDc |
| R105 | 2,452 | 149 | 46 | ND | |
| R106 | 1,893 | 167 | 125 | ND | |
| Env++ | R107 | 2,635 | 1,885 | 93 | 0 |
| R108 | 3,327 | 274 | 225 | 87 | |
| R109 | 369 | 109 | 25 | 76 | |
| dpol | R110 | 4,223 | 240 | 138 | ND |
| R111 | 2,565 | 570 | 37 | ND | |
| R112 | 1,256 | 3,598 | 94 | ND | |
| dpol++ | R113 | 7,128 | 2,049 | 207 | 24 |
| R114 | 1,020 | 977 | 91 | 83 | |
| R115 | 696 | 357 | 101 | 77 | |
| rgp160 only | R126 | 658 | 161 | 173 | 88 |
| R127 | 629 | 1,353 | 269 | 100 | |
| R128 | 612 | 337 | 144 | 86 | |
Neutralizing titers for TCLA viruses were determined 4 weeks after the second rgp160 boost on MT-2 cells and were expressed as the dilution of serum required to prevent 50% of virally induced cell death observed in the absence of antiserum.
IIIb % V3, extent (percentage) to which IIIb neutralization was dependent on V3-loop-specific antibodies, as determined on MT-2 cells. Sera were preincubated with 20 μg of V3-loop peptide (NNTRKSIRIQRGPGRAFVTIGKIG, amino acids 307 to 330 of IIIb Env) per ml prior to the IIIb neutralization assay.
ND, not determined.
V3 loop dependence of IIIb-neutralizing antibody.
Nearly all IIIb-neutralizing antibody in sera of naive rabbits immunized with rgp160 only was V3-loop dependent (Table 4). However, the V3-loop specificity of neutralizing activity in DNA-primed and protein-boosted groups was much more variable. For example, within the Env++ group, the neutralizing activity of rabbit R107 was independent of V3-loop specificity, while that of rabbits R108 and R109 was nearly completely dependent on antibodies specific for the V3-loop. The V3-loop specificity in DNA-primed rabbits did not correlate with the ability to cross-neutralize other TCLA strains. Both sera with and without V3-loop-dependent IIIb-neutralizing antibody were able to cross-neutralize MN and SF-2 (Table 4).
Failure to neutralize primary isolates.
Primary isolate neutralization studies performed on PBMC did not detect primary isolate-neutralizing antibody in any rabbit sera. Sera were tested against two non-syncytium-inducing (P46471 and W97464) and two syncytium-inducing (V67970 and W179273) viruses (43). Some rabbit sera modestly inhibited production of p24, but none demonstrated the 90% inhibition viewed as a benchmark of neutralization (data not shown). However, p24 production by all four primary isolates was inhibited by greater than 90% by serum from an HIV-1-infected patient with an atypically high neutralizing titer, demonstrating that these primary isolates could be neutralized (data not shown).
DISCUSSION
DNA elicits low-titer, low-avidity, and transient IgG responses to Env.
This study demonstrates that immunization of rabbits with plasmids expressing HIV-1 Env or influenza virus H1 elicits very different humoral responses. Multiple DNA immunizations with Env were required for seroconversion, and antibody responses to Env were transient, low titer, and low avidity. In contrast, DNA immunization with H1 elicited a persistent, high-titer antibody response with relatively high avidity. Since both glycoproteins are expressed in the context of the same plasmid (pJW4303) and at generally similar levels (several nanograms) following gene gun delivery to skin (34; also unpublished observations), we do not believe that discordant titers can be attributed to differences in levels of expression.
We believe that the markedly different antibody responses elicited by DNA immunization with HIV-1 Env and influenza virus H1 reflect fundamental differences in these antigens and in their interaction with the immune system. While Env is not a classical T cell-independent (TI) antigen, it may exhibit some TI characteristics noted by Binley et al. (3). Env is a heavily glycosylated protein (32, 48) and may somewhat resemble TI bacterial polysaccharide antigens. This may affect the ability of Env to elicit T-cell help and may preclude development of germinal center reactions which are critical for antibody maturation and persistence (38, 59). The persistence of Env but not Gag antibody responses in HIV-1-positive patients with low CD4+ counts (i.e., low T-cell help) (3) supports the notion that antibody responses to Env, but not Gag, may be TI.
The antibody responses observed in this study, to what are essentially subunit immunizations, mirror antibody responses observed following infection with influenza and immunodeficiency viruses. Infection with influenza virus quickly elicits an effective, high-titer neutralizing antibody response (5). In contrast, antibody responses to infection with immunodeficiency virus (8, 9, 24, 44) mature more slowly. Development of significant avidity, neutralizing titer, and breadth of neutralizing activity lag behind the appearance of Env-binding antibodies. Thus, intrinsic antigenic differences between immunodeficiency virus Env and influenza virus H1 are apparent both in natural infection (8, 9, 49, 50) and in DNA immunization.
Protein boosting of DNA-primed responses.
Protein boosting effectively increased the titer, avidity, and persistence of anti-Env antibody responses (Fig. 2 and 4). In DNA-primed animals, the avidity of the anti-Env antibody increased fairly slowly after protein boosting and more slowly than that in rabbits immunized with protein alone (Fig. 4). This suggests that DNA priming may bias the antibody response towards recognition of complex, discontinuous epitopes that undergo slow affinity maturation (2). This would be consistent with epitope specificities detected in V3-loop inhibition of neutralization (Table 4). While neutralizing antibody elicited by protein alone was always specific for the linear V3-loop, the specificity of neutralizing antibody elicited by DNA priming and protein boosting appeared to be more complex. A recent study of chimpanzees by Girard et al. (18) has also found that the avidity of anti-Env antibodies, primed with recombinant canarypox vaccines, was increased by boosting with recombinant protein. Protein immunizations may effectively boost antibody responses by providing higher doses of antigen than either DNA immunization or inoculation with recombinant vaccinia viruses.
Forms of Env.
Previous studies have suggested that oligomeric forms of Env are superior antigens for raising neutralizing antibody (14, 39, 51, 57, 62) and that distinct sets of epitopes are exposed on oligomeric and monomeric forms of Env (6, 12, 57). All immunizations given in our study included one or more plasmids expressing an oligomeric form of Env (see Fig. 1). The most effective neutralizing antibody elicited by DNA priming and protein boosting was induced by priming with a plasmid that expressed noninfectious particles (dpol) (Table 3 and Fig. 2). It may be worthy of note that dpol presents Env as spikes exposed to the immune system on the surface of virus-like particles; the multivalent nature of these particles may enhance presentation to, and stimulation of, the humoral immune system. The addition of plasmids expressing secreted monomeric (gp120) and oligomeric (gp140) forms of Env increased antibody, but not neutralizing antibody, titers. These data support the finding of Moore and Sodroski (46) that many antibodies elicited by monomeric gp120 are specific for nonneutralizing epitopes of Env which are masked or sequestered in native, oligomeric Env complexes.
DNA priming with full-length Env alone elicited no antibody response but did provide some priming which may have been T-cell help. The majority of this membrane-bound form of Env was found in the endoplasmic reticulum and Golgi bodies of transfected Cos cells and may not have been available for stimulation of significant antibody titers. Intracellular localization may have resulted from endocytosis directed by a tyrosine-containing internalization motif in the cytoplasmic tail of Env; rapid internalization of Env occurs in the absence of Gag (11, 13, 55). In contrast, the more potent H1 and gp120 immunogens are found at the surface of Cos cells as well as in the cytoplasm. Recent manipulation of HIV-1 Env expressed by recombinant vaccinia virus demonstrates that alterations of transmembrane and cytoplasmic domains of Env increase its expression on the surface of vaccinia virions and dramatically increase humoral immunogenicity (29). Enhanced surface expression (or increased residence time at the plasma membrane) may make Env more accessible to antigen-presenting cells and antibody.
Neutralizing antibody.
Sera from all rabbits, either DNA primed and protein boosted or immunized with protein alone, exhibited high-titer neutralizing antibody with significant breadth for TCLA strains of HIV-1 (Table 4). Cross-neutralizing titers were somewhat higher for HIV-1 MN than for HIV-1 SF2 (data not shown). The good cross-neutralizing activity for TCLA strains raised by protein-only immunization may in part reflect the presumed oligomeric structure of the rgp160 used for boosts. Unfortunately, cross-neutralizing activity did not extend to the four primary isolates tested in this study. Previous studies have shown that priming with recombinant vaccinia virus followed by protein boosting elicited significant titers of TCLA neutralizing antibody (19, 20, 26, 27). A recent study by Letvin et al. (33) demonstrated that DNA immunization, followed by rgp160 boosting, elicited high titers of simian-human immunodeficiency virus HXB-2-neutralizing titers and protected macaques from challenge with this simian-human immunodeficiency virus) with a TCLA Env. None of these prime-boost schemes have elicited neutralizing antibody for primary isolates of HIV-1.
These results are consistent with other studies in which antibody elicited by TCLA Envs is able to neutralize other TCLA strains of HIV-1 (10, 19, 39, 42, 45, 56, 62) but not primary isolates (25, 39, 51), with the notable exception of an oligomeric gp160 study with rabbits (62). We have previously observed distinct patterns of neutralization in rabbit sera after DNA priming rabbits with primary isolate Env constructs and boosting them with recombinant vaccinia viruses which express a variety of primary isolate Envs (51).
Development of a DNA prime-protein boost protocol with primary isolate Envs which consistently elicits higher-titer, cross-reactive neutralizing antibody for primary isolates is our next goal. The observation that primary isolate-neutralizing antibody present in the HIV-1-positive patients is specific for complex, conformation-dependent epitopes (61) suggests that protein boosting reagents which maintain the neutralizing epitopes of primary isolate Envs will be critical.
ACKNOWLEDGMENTS
We are indebted to F. Vogel, N. Miller, and A. Schultz for discussion. We thank Helen Drake-Perrow for administrative assistance.
This research was supported in part by U.S. Public Health Service grants R01-AI-34241 (H. Robinson) and 5-T32 AI-07272 (J. Richmond), by a Howard Hughes Postdoctoral Research Fellowship for Physicians (S. Lu), and by contract NCI-6S-1649 (D. Montefiori).
REFERENCES
- 1.Barnett S W, Rajasekar S, Legg H, Doe B, Fuller D H, Haynes J R, Walker C M, Steimer K S. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine. 1997;15:869–873. doi: 10.1016/s0264-410x(96)00264-2. [DOI] [PubMed] [Google Scholar]
- 2.Binley J M, Arshad H, Fouts T R, Moore J P. An investigation of the high-avidity antibody response to glycoprotein 120 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1997;13:1007–1015. doi: 10.1089/aid.1997.13.1007. [DOI] [PubMed] [Google Scholar]
- 3.Binley J M, Klasse P J, Cao Y, Jones I, Markowitz M, Ho D D, Moore J P. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. J Virol. 1997;71:2799–2809. doi: 10.1128/jvi.71.4.2799-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyer J D, Ugen K E, Wang B, Agadjanyan M, Gilbert L, Bagarazzi M L, Chattergoon M, Frost P, Javadian A, Williams W V, Refaeli Y, Ciccarelli R B, McCallus D, Coney L, Weiner D B. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat Med. 1997;3:526–532. doi: 10.1038/nm0597-526. [DOI] [PubMed] [Google Scholar]
- 5.Boyle C M, Morin M, Webster R G, Robinson H L. Role of different lymphoid tissues in the initiation and maintenance of DNA-raised antibody responses to the influenza virus H1 glycoprotein. J Virol. 1996;70:9074–9078. doi: 10.1128/jvi.70.12.9074-9078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broder C C, Earl P L, Long D, Abdeon S T, Moss B, Doms R W. Antigenic implications of human immunodeficiency virus type 1 envelope quaternary structure oligomeric-specific and -sensitive monoclonal antibodies. Proc Natl Acad Sci USA. 1994;91:11699–11703. doi: 10.1073/pnas.91.24.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charoenvit, Y., M. Sedegah, L. F. Yuan, M. Gross, C. Cole, R. Bechara, M. F. Leef, F. A. Robey, G. H. Lowell, R. L. Beaudoin, and S. L. Hoffman. 1990. Active and passive immunization against Plasmodium yoelii sporozoites. Bull. W. H. O. 68(Suppl.):26–32. [PMC free article] [PubMed]
- 8.Clements J E, Montelaro R C, Zink M C, Amedee A M, Miller S, Trichel A M, Jagerski B, Hauer D, Martin L N, Bohm R P, Murphey-Corb M. Cross-protective immune responses induced in rhesus macaques by immunization with attenuated macrophage-tropic simian immunodeficiency virus. J Virol. 1995;69:2737–2744. doi: 10.1128/jvi.69.5.2737-2744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole K S, Rowles J L, Jagerski B A, Murphey-Corb M, Unangst T, Clements J E, Robinson J, Wyand M S, Derosiers R C, Montelaro R C. Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J Virol. 1997;71:5069–5079. doi: 10.1128/jvi.71.7.5069-5079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolin R. Human studies in the development of human immunodeficiency virus vaccines. J Infect Dis. 1995;172:1175–1183. doi: 10.1093/infdis/172.5.1175. [DOI] [PubMed] [Google Scholar]
- 11.Earl P L, Koenig S, Moss B. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J Virol. 1991;65:31–41. doi: 10.1128/jvi.65.1.31-41.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earl P L, Broder C C, Long D, Lee S A, Peterson J, Chakrabarti S, Doms R W, Moss B. Native oligomeric human immunodeficiency virus type 1 envelope glycoprotein elicits diverse monoclonal antibody responses. J Virol. 1994;68:3015–3026. doi: 10.1128/jvi.68.5.3015-3026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan M A, Carruth L M, Rowell J F, Yu X, Siliciano R F. Human immunodeficiency virus type 1 envelope protein endocytosis mediated by a highly conserved intrinsic internalization signal in the cytoplasmic domain of gp41 is suppressed in the presence of the Pr55gag precursor protein. J Virol. 1996;70:6547–6556. doi: 10.1128/jvi.70.10.6547-6556.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fouts T R, Binley J M, Trkola A, Robinson J E, Moore J P. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J Virol. 1997;71:2779–2785. doi: 10.1128/jvi.71.4.2779-2785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuller D H, Haynes J R. A qualitative progression in HIV type 1 glycoprotein 120-specific cytotoxic cellular and humoral immune responses in mice receiving a DNA-based glycoprotein 120 vaccine. AIDS Res Hum Retroviruses. 1994;10:1433–1441. doi: 10.1089/aid.1994.10.1433. [DOI] [PubMed] [Google Scholar]
- 16.Fuller D H, Murphey-Corb M, Clements J, Barnett S, Haynes J R. Induction of immunodeficiency virus-specific immune responses in rhesus monkeys following gene gun-mediated DNA vaccination. J Med Primatol. 1996;25:236–241. doi: 10.1111/j.1600-0684.1996.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 17.Fuller D H, Corb M M, Barnett S, Steimer K, Haynes J R. Enhancement of immunodeficiency virus-specific immune responses in DNA-immunized rhesus macaques. Vaccine. 1997;15:924–925. doi: 10.1016/s0264-410x(96)00271-x. [DOI] [PubMed] [Google Scholar]
- 18.Girard M, van der Ryst E, Barré-Sinoussi F, Nara P, Tartaglia J, Paoletti E, Blondeau C, Jennings M, Verrier F, Meignier B, Fultz P N. Challenge of chimpanzees immunized with a recombinant canarypox-HIV-1 virus. Virology. 1997;232:98–104. doi: 10.1006/viro.1997.8560. [DOI] [PubMed] [Google Scholar]
- 19.Graham B S, Matthews T J, Belshe R B, Clements M L, Dolin R, Wright P F, Gorse G J, Schwartz D H, Keefer M C, Bolognesi D P, Corey L, Stablein D M, Esterlitz J R, Hu S-L, Smith G E. Augmentation of human immunodeficiency virus type 1 and neutralizing antibody by priming with gp160 recombinant vaccinia and boosting with rgp160 in vaccinia-naive adults. J Infect Dis. 1993;167:533–537. doi: 10.1093/infdis/167.3.533. [DOI] [PubMed] [Google Scholar]
- 20.Graham B S, Matthews T J, Belshe R B, Clements M L, Dolin R, Corey L, Gorse G J, Schwartz D H, Keefer M C, McElrath J, Bolognesi D P, Stablein D M, Esterlitz J R, Hu S L, Smith G, Wright P F. Determinants of antibody response after rgp160 boosting in vaccinia-naive volunteers primed with gp160 recombinant vaccinia. J Infect Dis. 1994;170:782–786. doi: 10.1093/infdis/170.4.782. [DOI] [PubMed] [Google Scholar]
- 21.Graham B S, Wright P F. Candidate AIDS vaccines. N Engl J Med. 1995;333:1331–1339. doi: 10.1056/NEJM199511163332007. [DOI] [PubMed] [Google Scholar]
- 22.Gray B M, Shaw D R. Artifacts with the thiocyanate elution method for estimating relative antibody avidity. J Immunol Methods. 1993;157:269–271. doi: 10.1016/0022-1759(93)90096-p. [DOI] [PubMed] [Google Scholar]
- 23.Hallenberger S, Tucker S P, Owens R J, Bernstein H B, Compans R W. Secretion of a truncated form of the human immunodeficiency virus type 1 envelope glycoprotein. Virology. 1993;193:510–514. doi: 10.1006/viro.1993.1156. [DOI] [PubMed] [Google Scholar]
- 24.Hammond S A, Cook S J, Lichtenstein D L, Issel C J, Montelaro R C. Maturation of the cellular and humoral immune responses to persistent infection in horses by equine infectious anemia virus is a complex and lengthy process. J Virol. 1997;71:3840–3852. doi: 10.1128/jvi.71.5.3840-3852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanson C V. Measuring vaccine-induced HIV neutralization: report of a workshop. AIDS Res Hum Retroviruses. 1994;10:645–648. doi: 10.1089/aid.1994.10.645. [DOI] [PubMed] [Google Scholar]
- 26.Hu S-L, Klaniecki J, Dykers T, Sridhar P, Travis B M. Neutralizing antibodies against HIV-1 BRU and SF2 isolates generated in mice immunized with recombinant vaccinia virus expressing HIV-1 envelope glycoproteins and boosted with gp160. AIDS Res Hum Retroviruses. 1991;7:615–620. doi: 10.1089/aid.1991.7.615. [DOI] [PubMed] [Google Scholar]
- 27.Hu S-L, Abrams K, Barber G N, Moran P, Zarling J M, Langlois A J, Kuller L, Morton W R, Benveniste R E. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science. 1992;255:456–459. doi: 10.1126/science.1531159. [DOI] [PubMed] [Google Scholar]
- 28.Kangro H O, Manzoor S, Harper D R. Antibody avidity following varicella-zoster virus infections. J Med Virol. 1991;33:100–105. doi: 10.1002/jmv.1890330207. [DOI] [PubMed] [Google Scholar]
- 29.Katz E, Moss B. Immunogenicity of recombinant vaccinia viruses that display the HIV type 1 envelope glycoprotein on the surface of infectious virions. AIDS Res Hum Retroviruses. 1997;13:1497–1500. doi: 10.1089/aid.1997.13.1497. [DOI] [PubMed] [Google Scholar]
- 30.Klaniecki J, Dykers T, Travis B, Schmitt R, Wain M, Watson A, Sridhar P, McClure J, Morein B, Ulrich J T, Hu S-L. Cross-neutralizing antibodies in rabbits immunized with HIV-1 gp160 purified from simian cells infected with a recombinant vaccinia virus. AIDS Res Hum Retroviruses. 1991;7:791–798. doi: 10.1089/aid.1991.7.791. [DOI] [PubMed] [Google Scholar]
- 31.Leinikki P O, Shekarchi I, Dorsett P, Sever J L. Enzyme-linked immunosorbent assay determination of specific rubella antibody levels in micrograms of immunoglobulin G per milliliter of serum in clinical samples. J Clin Microbiol. 1978;8:419–423. doi: 10.1128/jcm.8.4.419-423.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonard C K, Spellman M W, Ruddle L, Harris R J, Thomas J N, Gregory T J. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990;265:10373–10382. [PubMed] [Google Scholar]
- 33.Letvin N L, Montefiori D C, Yasutomi Y, Perry H C, Davies M E, Lekutis C, Alroy M, Freed D C, Lord C I, Handt L K, Liu M A, Shiver J W. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc Natl Acad Sci USA. 1997;94:9378–9383. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu S, Santoro J C, Fuller D H, Haynes J E, Robinson H L. Use of DNAs expressing HIV-1 env and noninfectious HIV-1 particles to raise antibody responses in mice. Virology. 1995;209:147–154. doi: 10.1006/viro.1995.1238. [DOI] [PubMed] [Google Scholar]
- 35.Lu S, Arthos J, Montefiori D C, Yasutomi Y, Manson K, Mustafa F, Johnson E, Santoro J C, Wissink J, Mullins J I, Haynes J R, Letvin N L, Wyand M, Robinson H L. Simian immunodeficiency virus DNA vaccine trial in macaques. J Virol. 1996;70:3973–3991. doi: 10.1128/jvi.70.6.3978-3991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu S, Wyatt R, Richmond J F L, Mustafa F, Wang S, Weng J, Montefiori D C, Sodroski J, Robinson H L. Immunogenicity of DNA vaccines expressing human immunodeficiency virus type 1 envelope glycoprotein with and without deletions in the V1/2 and V3 regions. AIDS Res Hum Retroviruses. 1998;14:151–155. doi: 10.1089/aid.1998.14.151. [DOI] [PubMed] [Google Scholar]
- 37.Luxton R W, Thompson E J. Affinity distributions of antigen-specific IgG in patients with multiple sclerosis and in patients with viral encephalitis. J Immunol Methods. 1990;131:277–282. doi: 10.1016/0022-1759(90)90199-6. [DOI] [PubMed] [Google Scholar]
- 38.MacLennan I C M. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 39.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M L, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S the National Institute of Allergy and Infectious Disease AIDS Vaccine Evaluation Group. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 40.Michel M-L, Davis H L, Schleef M, Mancini M, Tiollais P, Whalen R G. DNA-mediated immunization to the hepatitis B surface antigen in mice: aspects of the humoral response mimic hepatitis B viral infection in humans. Proc Natl Acad Sci USA. 1995;92:5307–5311. doi: 10.1073/pnas.92.12.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montefiori D C, Robinson W E, Jr, Schuffman S S, Mitchell W M. Evaluation of antiviral drugs and neutralizing antibodies to human immunodeficiency virus by a rapid and sensitive microtiter infection assay. J Clin Microbiol. 1988;26:231–237. doi: 10.1128/jcm.26.2.231-235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montefiori D C, Graham B S, Zhou J, Zhou J, Bucco R A, Schwartz D H, Cavacini L A, Posner M R the NIH-NIAID AIDS Vaccine Clinical Trials Network. V3-specific neutralizing antibodies in sera from HIV-1 gp160-immunized volunteers block virus fusion and act synergistically with human monoclonal antibody to the conformation-dependent CD4 binding site of gp120. J Clin Investig. 1993;92:840–847. doi: 10.1172/JCI116658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montefiori D C, Pantaleo G, Fink L M, Zhou J T, Zhou J Y, Bilska M, Miralles G D, Fauci A S. Neutralizing and infection-enhancing antibody responses to human immunodeficiency virus type 1 in long-term nonprogressors. J Infect Dis. 1996;173:60–67. doi: 10.1093/infdis/173.1.60. [DOI] [PubMed] [Google Scholar]
- 44.Moog C, Fleury H J A, Pellegrin I, Kirn A, Aubertin A M. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J Virol. 1997;71:3734–3741. doi: 10.1128/jvi.71.5.3734-3741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore J P, Ho D D. HIV-1 neutralization: the consequences of viral adaptation to growth on transformed T cells. AIDS 1995. 1995;9:S117–S136. [PubMed] [Google Scholar]
- 46.Moore J P, Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70:1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mustafa F, Richmond J F L, Fernandez-Larsson R, Lu S, Fredricksson R, Fenyö E M, O’Connell M, Johnson E, Weng J, Santoro J C, Robinson H L. HIV-1 Env glycoproteins from two series of primary isolates: replication phenotype and immunogenicity. Virology. 1997;229:269–278. doi: 10.1006/viro.1997.8445. [DOI] [PubMed] [Google Scholar]
- 48.Myers G, Wain-Hobson S, Henderson L E, Korber B, Jeang K-T, Pavlakis G N. Human retroviruses and AIDS 1994. Los Alamos, N. Mex: Los Alamos Theoretical Biology and Biophysics Group, Los Alamos National Laboratory; 1994. [Google Scholar]
- 49.Pincus S H, Messer K G, Schwartz D H, Lewis G K, Graham B S, Blatter W A, Fisher G. Differences in the antibody response to human immunodeficiency virus-1 envelope glycoprotein (gp160) in infected laboratory workers and vaccinees. J Clin Investig. 1993;91:1987–1996. doi: 10.1172/JCI116420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pincus S H, Messer K G, Cole R, Ireland R, VanCott T C, Pinter A, Schwartz D H, Graham B S, Gorse G J. Vaccine-specific antibody responses induced by HIV-1 envelope subunit vaccines. J Immunol. 1997;158:3511–3520. [PubMed] [Google Scholar]
- 51.Richmond J F L, Mustafa F, Lu S, Santoro J C, Weng J, O’Connell M, Fenyö E M, Hurwitz J L, Montefiori D C, Robinson H L. Screening HIV-1 glycoproteins for the ability to elicit neutralizing antibody using DNA immunization and recombinant vaccinia virus boosting. Virology. 1997;230:265–274. doi: 10.1006/viro.1997.8478. [DOI] [PubMed] [Google Scholar]
- 52.Robinson H L, Feltquate D M, Morin M J. DNA vaccines: a new approach to immunization. In: Brown F, Chancock R, Ginsberg H, Norrby E, editors. Vaccines 95. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 69–75. [Google Scholar]
- 53.Robinson, H. L. 1997. DNA vaccines for immunodeficiency viruses. AIDS 1997 11(Suppl. A):S109–S119. [PubMed]
- 54.Robinson J E, Holton D, Lui J, McMurdo H, Murciano A, Gohd R. A novel enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to HIV-1 envelope glycoproteins based on immobilization of viral glycoproteins in microtiter wells coated with concanavalin A. J Immunol Methods. 1990;132:63–71. doi: 10.1016/0022-1759(90)90399-g. [DOI] [PubMed] [Google Scholar]
- 55.Rowell J F, Stanhope P E, Siliciano R F. Endocytosis of endogenously synthesized HIV-1 envelope protein. J Immunol. 1995;155:473–488. [PubMed] [Google Scholar]
- 56.Salmon-Céron D, Excler J L, Sicard D, Blanche P, Finkielstzjen L, Gluckman J C, Autran B, Matthews T J, Meignier B, Kieny M P, Valentin C, Gonnet P, Diaz I, Salomon H, Pialoux G, Gonzalez-Canali G, Plotkin S the Agis Group and L’Agence Nationale de Recherche sur le SIDA. Safety and immunogenicity of a recombinant HIV type 1 glycoprotein 160 boosted by a V3 synthetic peptide in HIV-negative volunteers. AIDS Res Hum Retroviruses. 1995;11:1479–1486. doi: 10.1089/aid.1995.11.1479. [DOI] [PubMed] [Google Scholar]
- 57.Sattentau Q J, Moore J P. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J Exp Med. 1995;182:185–196. doi: 10.1084/jem.182.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shiver J W, Davies M-E, Yasutomi Y, Perry H C, Freed D C, Letvin N L, Liu M A. Anti-HIV Env immunities elicited by nucleic acid vaccines. Vaccine. 1997;15:884–887. doi: 10.1016/s0264-410x(96)00251-4. [DOI] [PubMed] [Google Scholar]
- 59.Tew J G, DiLosa R M, Burton G F, Kosco M H, Kupp L I, Masuda A, Szakal A K. Germinal centers and antibody production in bone marrow. Immunol Rev. 1992;126:99–112. doi: 10.1111/j.1600-065x.1992.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 60.VanCott T C, Bethke F R, Burke D S, Redfield R, Birx D L. Lack of induction of antibodies specific for conserved, discontinuous epitopes of HIV-1 envelope glycoprotein by candidate AIDS vaccines. J Immunol. 1995;155:4100–4110. [PubMed] [Google Scholar]
- 61.VanCott T C, Polonis V R, Loomis L D, Micheal N L, Nara P L, Birx D L. Differential role of V3-specific antibodies in neutralization assays involving primary and laboratory-adapted isolates of HIV type 1. AIDS Res Hum Retroviruses. 1995;11:1379–1391. doi: 10.1089/aid.1995.11.1379. [DOI] [PubMed] [Google Scholar]
- 62.VanCott T C, Mascola J R, Kaminski R W, Kalyanaraman V, Hallberg P L, Burnett P R, Ulrich J T, Rechtman D J, Birx D L. Antibodies with specificity to native gp120 and neutralization activity against primary human immunodeficiency virus type 1 isolates elicited by immunization with oligomeric gp160. J Virol. 1997;71:4319–4330. doi: 10.1128/jvi.71.6.4319-4330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang B, Ugen K E, Srikantan V, Agadjanyan M G, Dang K, Refaeli Y, Sato A, Boyer J, Williams W V, Weiner D B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1993;90:4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang B, Boyer J, Srikantan V, Coney L, Carrano R, Phan C, Merva M, Dang K, Agadjanyan M, Gilbert L, Ugen K E, Williams W V, Weiner D B. DNA inoculation induces neutralizing immune responses against human immunodeficiency virus type 1 in mice and nonhuman primates. DNA Cell Biol. 1993;12:799–805. doi: 10.1089/dna.1993.12.799. [DOI] [PubMed] [Google Scholar]
- 65.Ward K N, Dhaliwal W, Ashworth K L, Clutterbuck E J, Teo C G. Measurement of antibody avidity for hepatitis C virus distinguishes primary antibody responses from passively acquired antibody. J Med Virol. 1994;43:367–372. doi: 10.1002/jmv.1890430409. [DOI] [PubMed] [Google Scholar]
- 66.Yang K, Mustafa F, Valsamakis A, Santoro J C, Griffen D E, Robinson H L. Early studies on DNA-based immunizations for measles virus. Vaccine. 1997;15:888–891. doi: 10.1016/s0264-410x(96)00261-7. [DOI] [PubMed] [Google Scholar]