Abstract
For the amphotropic murine leukemia virus (MuLV), a 208-amino-acid amino-terminal fragment of the surface unit (SU) of the envelope glycoprotein is sufficient to bind to its receptor, Pit2. Within this binding domain, two hypervariable regions, VRA and VRB, have been proposed to be important for receptor recognition. In order to specifically locate residues that are important for the interaction with Pit2, we generated a number of site-specific mutations in both VRA and VRB and analyzed the resulting envelope proteins when expressed on retroviral vectors. Concurrently, we substituted portions of the amphotropic SU with homologous regions from the polytropic MuLV envelope protein. The amphotropic SU was unaffected by most of the point mutations we introduced. In addition, the deletion of eight residues in a region of VRA that was previously suggested to be essential for Pit2 utilization only decreased titer on NIH 3T3 cells by 1 order of magnitude. Although the replacement of the amino-terminal two-thirds of VRA with the polytropic sequence abolished receptor binding, smaller nonoverlapping substitutions did not affect the function of the protein. We were not able to identify a single critical receptor contact point within VRA, and we suggest that the amphotropic receptor binding domain probably makes multiple contacts with the receptor and that the loss of some of these contacts can be tolerated.
The entry of murine leukemia virus (MuLV) into cells is initiated by an interaction between the envelope glycoprotein and the host cell receptor. The envelope glycoprotein is comprised of two polypeptides, the surface unit (SU) and the transmembrane protein (TM), which are processed from a polyprotein precursor by a host cell protease during transport to the cell surface and remain associated after cleavage (13). Five MuLV subgroups that bind to different cell surface receptors have been identified: ecotropic, amphotropic, polytropic, xenotropic, and 10A1 (29, 30, 34). Another murine retrovirus, Mus dunni endogenous virus, has recently been described, but it is as yet unclear whether this virus belongs in the MuLVs (6, 20).
Previous studies have demonstrated that specific receptor recognition by the different MuLV subgroups is a property of the amino-terminal domain of SU (2, 4, 5, 11, 22, 23, 25). This region contains several conserved cysteine residues that have been suggested to form hydrophilic, disulfide-linked loops (16), and such a structure has now been confirmed for the ecotropic MuLV SU (8). Within the amino-terminal region are two stretches of sequence, designated VRA and VRB, that vary among the different MuLV subgroups and are therefore likely to contain the residues involved in specific receptor interactions (4). The VRA regions can be further subdivided into three sections, comprised of an initial variable disulfide-linked loop(s) (residues 52 to 67), a more conserved interloop domain (residues 68 to 83), and a second variable disulfide-linked loop (residues 84 to 92), which has also been referred to as VRC (8). The VRB regions of all MuLVs contain two cysteine residues, which are predicted to form a single disulfide-linked loop (16). In addition, the amphotropic and 10A1 envelope proteins contain two more cysteine residues at the amino terminus of VRB that could form an additional small loop. Previous studies have localized residues involved in receptor interaction primarily within VRA (1, 10, 17–19, 24, 33, 37), although residues in VRB and sequences downstream of the amino-terminal domain may also influence receptor recognition (2, 10, 23).
The receptor for amphotropic MuLV has been identified as the phosphate symporter, Pit2 (14, 15, 21, 38–40). A 208-amino-acid amino-terminal fragment of amphotropic SU is capable of binding to Pit2. This domain is sufficient to bind to various cell lines susceptible to infection by amphotropic MuLV and can inhibit binding by viral particles bearing amphotropic envelope proteins (2). Within this domain, the Pit2 binding determinants have been suggested to reside in VRA. Retroviruses bearing amphotropic envelope proteins for which the VRB regions of either polytropic or xenotropic envelope proteins have been substituted retained the amphotropic interference pattern (4), and replacements of either the amino-terminal or carboxy-terminal half of VRB with a foreign linear epitope still allowed the infection of murine and human cells expressing Pit2 (3). However, these substitutions in VRB did have some effect on the host range, as they prevented infection of D17 cells in both cases (3, 4). In contrast, the replacement of residues 52 to 66 (residues 50 to 64 in Battini et al. [3]) in VRA with the same foreign linear epitope disrupted the ability of the envelope protein to interact with Pit2. Furthermore, studies analyzing the interactions of chimeric envelope proteins of amphotropic MuLV and feline leukemia virus type B with chimeric Pit1 and Pit2 receptors identified the VRA residues Tyr-62 and Val-63 (Tyr-60 and Val-61 in Tailor and Kabat [37]) as being critical for amphotropic recognition of Pit2. Taken together, these data suggest that residues in the first disulfide-linked loop of VRA are essential for Pit2 recognition.
We sought to determine whether less dramatic changes in the wild-type amphotropic SU would similarly affect Pit2 recognition. We generated a number of site-specific mutations in both VRA and VRB, including substitutions of residues Tyr-62 and Val-63. Concurrently, we replaced portions of the amphotropic SU with analogous regions from the polytropic SU in order to analyze larger regions of the amphotropic binding domain, which we predicted would cause minimal disruption of the overall structure of the region. Our analyses revealed that both VRA and VRB were refractory to multiple mutations and substitutions. Furthermore, the protein retained function despite a deletion within VRA that removed residues 56 to 62, although larger replacements of VRA residues 50 to 76 with polytropic sequence did abolish function. These data suggest that previously identified residues within VRA may not be as critical for Pit2 interaction within the context of the wild-type amphotropic envelope protein.
MATERIALS AND METHODS
Cell culture.
The 293T/17 cell line was obtained from the American Type Culture Collection (CRL 11268). 293/12 is a 293 cell line stably expressing the murine ecotropic MuLV receptor (28). All cell lines were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum (Hyclone, Logan, Utah) and 2 mM glutamine (Gibco BRL, Grand Island, N.Y.).
Plasmids and mutagenesis.
Plasmids pMo(4070A) and pMo(10A1) are infectious clones of Moloney MuLV (Mo-MuLV) containing 4070A and 10A1 env genes, respectively (23). Plasmid pRR66 is an infectious clone of Moloney mink cell focus-forming (Mo-MCF) virus (7). All three plasmids were kindly provided by A. Rein (National Cancer Institute-Frederick Cancer Research Facility). Plasmid pCgp is a cytomegalovirus-driven plasmid expressing Mo-MuLV Gag-Pol (45). Plasmid pCnBg is a retroviral vector expressing nuclear β-galactosidase and neomycin resistance genes (10). Plasmids pSCA (10), pSEC, and pSCP are 4070A, Mo-MuLV, and Mo-MCF virus env expression plasmids, respectively. The Mo-MuLV env was obtained from pCEE+ (17) as an EcoRI fragment, while the Mo-MCF virus env was obtained from pRR66 as an EagI-to-ClaI fragment. Plasmid E-T461P contains a single point mutation in the TM subunit of pCEE+ (45).
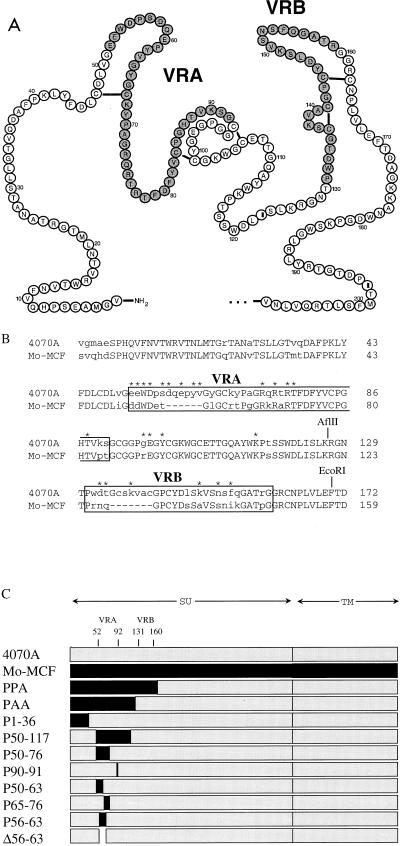
For site-directed mutagenesis, PCR splice overlap mutagenesis (12) was used to introduce mutations. Mutants are designated by the amino acid in the 4070A envelope protein followed by the residue number and the amino acid found in the mutant protein. The amino acid residues are numbered from the amino terminus of SU after the signal peptide is cleaved. The chimeric envelope plasmids pPPA and pPAA were constructed with conserved AflII and EcoRI restriction sites within amphotropic and polytropic SU (Fig. 1). All other chimeras were constructed by splice overlap PCR, and all PCR-derived fragments were completely sequenced to confirm their identity.
FIG. 1.
Comparison of the N-terminal amino acids of 4070A, Mo-MCF virus, and chimeric envelope proteins. (A) Schematic of the N-terminal 4070A SU, based on the disulfide linkages determined in the envelope protein of polytropic protein (16). Residues in VRA and VRB are shaded. (B) Amino acid alignment between 4070A and Mo-MCF virus envelope proteins. Conserved residues are shown in capital letters, variable residues are shown in lowercase, and gaps appear as dashes. The residues in 4070A that were mutated are identified with asterisks; the specific residues changes are listed in Table 1. VRA and VRB are boxed. (C) Schematic of the wild-type and chimeric 4070A and Mo-MCF virus envelope proteins. Chimeras are identified by the region of amino acids in the 4070A envelope protein that are replaced by residues from the homologous region of the Mo-MCF virus envelope protein (shown in black), except for PPA and PAA, which were generated by using conserved EcoRI and AflII sites, respectively.
Virus production and titer determination.
Retroviral vectors were produced by transient transfection of plasmids pCgp, pCnBg, and an env expression plasmid (5 μg each) into 293T/17 cells (5 × 105 cells in each 60-mm-diameter dish) by calcium phosphate precipitation, essentially as described previously (10, 35). Sixteen hours posttransfection, the precipitate was removed and replaced with 5 ml of medium containing 10 mM sodium butyrate (Sigma, St. Louis, Mo.) for 12 h. The cells were then incubated in 3 ml of fresh medium to allow production of retroviral vectors, which were harvested after a further 12-h incubation at 37 or 32°C, as specified.
Transductions were performed as described previously (10). Essentially, cells were exposed to 10-fold dilutions of retroviral vector supernatants for 24 h. The cells were then incubated for a further 48 h with fresh medium and then stained for β-galactosidase expression. Titers of viral stocks were expressed as β-galactosidase-expressing CFU per milliliter.
Interference assays.
Stocks of infectious viruses were produced by transfecting 15 μg of plasmids pMo(4070A), pMo(10A1), or pRR66 into 293T/17 cells (5 × 105 cells in each 60-mm-diameter dish) by calcium phosphate precipitation, as described previously (10). NIH 3T3 cells were infected with the resulting filtered supernatants, and the infections were monitored by reverse transcriptase (RT) assay (9) of the culture supernatants until chronically infected populations were obtained. The RT activities of the supernatants were calculated from a standard curve generated with serial dilutions of recombinant MuLV RT (Promega, Madison, Wis.). Typical chronically infected populations had supernatant RT activities within the range of 0.01 to 0.1 U/ml.
Interference assays were performed by titering retroviral vector supernatants on infected NIH 3T3 cells, produced as described above. A total of 3 × 104 chronically infected NIH 3T3 cells were seeded in the 30-mm-diameter wells of a six-well plate in 1 ml of medium and then transduced with retroviral vectors as described above for uninfected cells.
Binding assays.
Binding assays were performed on NIH 3T3 cells, essentially as described previously (42), except that the initial incubation of 2 × 105 NIH 3T3 cells with 1 ml of vector supernatant was performed at room temperature for 30 min.
Complementation assay.
Retroviral vectors were produced for complementation assays by transfecting equal amounts of two env expression plasmids (2.5 μg each) into 293T/17 cells (5 × 105 cells in each 60-mm-diameter dish), along with pCgp and pCnBg (5 μg each). Supernatants were harvested at 37°C as described above. The resulting retroviral vectors were titered on 293 and 293/12 cells, as described above for NIH 3T3 cells.
Western blot analysis of envelope proteins in retroviral vectors and cell lysates.
Western blotting was performed to detect specific viral proteins, essentially as described previously (45). To analyze proteins in retroviral vectors, the supernatants generated by transient transfection of 293T cells were pelleted for 30 min at 4°C through 20% sucrose at 16,000 × g. Cell lysates were prepared by lysing the transfected cells with lysis buffer (100 mM Tris-HCl [pH 7.4], 1% Triton X-100, 0.05% sodium dodecyl sulfate, 5 mg of sodium deoxycholate/ml, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride). The primary antibodies used were goat anti-Rauscher MuLV SU antiserum at 1:3,000 dilution (lot 79S656; Quality Biotech, Camden, N.J.), goat anti-Rauscher MuLV CA antiserum at 1:10,000 dilution (lot 78S221; Quality Biotech), and rat monoclonal antibody 42/114 against AKR MuLV TM at 1:2,000 dilution (27). The 42/114 hybridoma cell line was kindly provided by U. Hammerling (Memorial Sloan-Kettering Cancer Center, New York, N.Y.). The secondary antibodies used were horseradish peroxidase-conjugated rabbit anti-goat immunoglobulin G at 1:10,000 dilution and horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G at 1:10,000 dilution (Pierce, Rockford, Ill.).
RESULTS
4070A envelope protein binding domain can tolerate single amino acid substitutions throughout variable regions A and B.
In order to identify one or more residues in the amphotropic envelope glycoprotein that may serve as contact points for Pit2, we generated a number of mutations in the amino-terminal binding domain of the 4070A envelope protein (Fig. 1B). For the most part, the residues targeted for site-directed mutagenesis were hydrophilic and/or charged residues in VRA and VRB which were expected to be surface exposed, and amino acids containing hydrophobic side chains were generally substituted for them. The resulting envelope protein mutants were incorporated into retroviral vector particles and assayed for their ability to transduce NIH 3T3 cells (Table 1).
TABLE 1.
Transduction ability of 4070A envelope protein mutants on NIH 3T3 cellsa
| Envelope proteinb | Relative titer of vectors (%) |
|---|---|
| None | <0.0001 |
| 4070A | 100c |
| VRA mutants | |
| E52V | 110 |
| E53V | 17 |
| W54R/K115E | 340 |
| D55V | 200 |
| S57A | 83 |
| D58E | 120 |
| E60V | 8.6 |
| Y62F | 160 |
| V63D | 120 |
| R73L | <0.0001 |
| R75L | 100 |
| R77L | 19 |
| T78V | 11 |
| T88R | 50 |
| Conserved region | |
| G97R/Y100H | 110 |
| E98K | 41 |
| VRB mutants | |
| D133V | 31 |
| T134V | 50 |
| K138E | 59 |
| K149E | 110 |
| N152V/F154L | 150 |
Mutants are grouped according to the regions identified by sequence alignment among mammalian type C retroviruses (4): VRA, amino acids 52 to 92; conserved region, amino acids 93 to 130; and VRB, amino acids 131 to 160.
Mutants are identified by the amino acid in the 4070A envelope protein, followed by the residue number and the amino acid found in the mutant protein.
Average of wild-type titers was 2.0 × 106 CFU/ml.
Except for the R73L mutant, all mutant envelope proteins allowed transduction of NIH 3T3 cells at levels greater than 10% of those achieved by the vectors bearing wild-type 4070A envelope protein, suggesting that the identities of mutated residues are not critical for the function of the protein. In contrast, the R73L mutation completely prevented transduction. Although residues Tyr-62 and Val-63 have been suggested previously to be essential for Pit2 recognition (37), the substitution of either of these residues individually had no apparent effect on the transduction of NIH 3T3 cells.
Residues 56 to 63 of VRA are not necessary for amphotropic receptor interaction.
Residues 52 to 66 (residues 50 to 64 in Battini et al. [3]) in the first disulfide loop of amphotropic VRA have been previously proposed as essential determinants of amphotropic receptor recognition, and Tyr-62 and Val-63 (Tyr-60 and Val-61 in Tailor and Kabat [37]) within this region have been suggested to be critical. Based on a sequence comparison between the amphotropic 4070A and the polytropic Mo-MCF virus envelope proteins in this region (Fig. 1B), we hypothesized that a possible amphotropic contact residue(s) would probably reside within amino acids 56 to 63. Therefore, we deleted these eight amino acids from the amphotropic envelope protein to produce mutant Δ56-63 (Fig. 1C). In addition, we replaced this region with the two amino acids (Glu and Thr) in the polytropic env sequence to give mutant P56-63 (Fig. 1). Retroviral vectors bearing the Δ56-63 mutant resulted in titers on NIH 3T3 cells that were still 8.5% of the wild-type titers, while vectors with the P56-63 mutant gave titers that were actually higher than that of the wild-type 4070A (Table 2). Both mutants were unable to transduce 4070A-infected NIH 3T3 cells (data not shown), indicating that the mutants utilized only the amphotropic receptor. Our results indicate that residues 56 to 63 are not critical for Pit2 utilization by the amphotropic envelope proteins on NIH 3T3 cells and contradict the findings of Tailor and Kabat (37).
TABLE 2.
Phenotypes of envelope protein chimeras
| Envelope proteina | Relative titer of vectors on NIH 3T3 cells (%) | Incorporationc | Tropismd |
|---|---|---|---|
| None | <0.0001 | N/Af | N/A |
| 4070A | 100e | + | Amphotropic |
| Mo-MCF | 18 | + | Polytropic |
| PPA | <0.0001 | + | N/A |
| PAA | <0.0001 | + | N/A |
| P1-36 | 100 | + | Amphotropic |
| P50-117 | <0.0001 | + | N/A |
| P50-76 | <0.0001 | + | N/A |
| P90-91 | 690 | + | Amphotropic |
| P50-63 | 27 | + | Amphotropic |
| P65-76 | 4.3 | + | Amphotropic |
| P56-63 | 390 | + | Amphotropic |
| Δ56-63b | 8.5 | + | Amphotropic |
Chimeras are identified by the amino acids in the 4070A envelope protein that are replaced by the homologous region of the Mo-MCF virus envelope protein, except for PPA and PAA, which were generated by using EcoRI and AflII sites, respectively.
Δ56-63 is the 4070A envelope protein with residues 56 to 63 deleted.
Incorporation of envelope proteins into viral vectors was determined by Western analysis of SU proteins. +, wild-type level of incorporation.
Tropism was determined by interference assays on NIH 3T3 cells chronically infected with Mo(4070A), Mo(10A1), or Mo-MCF virus.
Average of wild-type titer was 2.9 × 107 CFU/ml.
N/A, not applicable.
Substitution of the amphotropic receptor binding domain with polytropic sequences results in loss of function.
Since our initial analyses of amphotropic SU failed to identify residues that contributed to Pit2 binding, we decided to construct chimeras between the amphotropic (A) and polytropic (P) envelope proteins. Chimeras between the two envelope proteins, constructed by using one or both of the conserved AflII and EcoRI restriction sites, have been described previously, albeit with contradictory results (4, 23). The different isolates of polytropic envelope proteins utilized by the two groups were suggested as a possible reason for this discrepancy (23).
Accordingly, we obtained the Mo-MCF proviral clone that gave rise to functional chimeras (23) and reconstructed some of those chimeras in a nonreplicative vector system. A conserved EcoRI site was used to generate chimera PPA, while a conserved AflII site was employed for PAA (Fig. 1). These chimeras are equivalent to proteins MMA and MAA, respectively (4, 23). The chimeric envelope proteins were incorporated into retroviral vector particles and assayed for their ability to transduce NIH 3T3 cells.
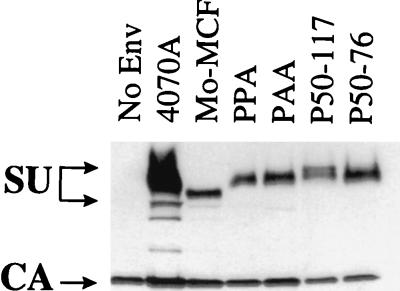
The vectors enveloped with PPA or PAA did not give detectable titer on NIH 3T3 cells (Table 2), consistent with the results published by Battini et al. (4). However, both PPA and PAA envelope proteins were incorporated into retroviral vector particles at reasonable levels (Fig. 2), suggesting that the block to transduction of NIH 3T3 cells was due to the lack of biological activity of the envelope chimeras.
FIG. 2.
Incorporation of chimeric envelope protein into retroviral vectors as determined by Western blotting. Specific viral proteins were detected with anti-SU and anti-CA antibodies. Lane No Env contains vectors produced without an env expression vector.
Extensive polytropic substitution of amphotropic VRA prevents Pit2 interaction.
In an effort to identify the minimal region(s) of polytropic sequence that was responsible for the inactivity of the PAA protein, smaller regions of the polytropic envelope protein were substituted into the homologous regions of the 4070A envelope protein (Fig. 1C). All chimeras were found to be incorporated into retroviral vectors at reasonable levels (Fig. 2 and data not shown). The smallest contiguous polytropic substitution that resulted in a loss of function was the substitution of amphotropic residues 50 through 76 (Table 2). Further subdivision of this VRA region by substituting either the first disulfide-linked loop domain (P50-63) alone or most of the interloop domain (P65-76) resulted in envelope proteins that were capable of transducing NIH 3T3 cells at near-wild-type levels (Table 2). Once we determined that P65-76 gave titer on NIH 3T3 cells, we expected the vectors bearing P65-76 to demonstrate a 10A1-like interference pattern and to be able to transduce 4070A-infected NIH 3T3 cells. We have previously demonstrated that changing residues Ala-71 and Gln-74 of amphotropic envelope protein to the 10A1 residues, Gly and Lys, respectively, resulted in a 10A1-like interference pattern (10), and the polytropic substitution from residues 65 to 76 results in the same changes at residues 71 and 74. However, P65-76 and all other chimeras capable of transducing NIH 3T3 cells demonstrated an amphotropic interference pattern when tested on the chronically infected cells (Table 2).
None of the vectors bearing the inactive chimeras (PPA, PAA, P50-76, and P50-117) were rescued by production at 32°C (data not shown). These vectors also failed to demonstrate measurable binding to NIH 3T3 cells compared to vectors bearing the 4070A envelope protein (data not shown). Thus, the primary defect of these inactive chimeras appears to be the lack of an interaction with Pit2.
Coexpression of P50-76 and Mo-MuLV mutant E-T461P leads to transduction.
Although our initial characterizations of mutant P50-76 and the other inactive chimeras appeared to indicate a binding-defective phenotype, other defects could also be present. To more fully analyze the defect in P50-76, we conducted a complementation assay. Previously, we have demonstrated that two classes of Mo-MuLV envelope protein that are individually defective in either the receptor binding or the postbinding (fusion) stages of entry can functionally complement each other in trans within a mixed hetero-oligomer (43). The coexpression of two such mutants in our vector production system resulted in vector particles that were then able to transduce NIH 3T3 cells. In addition, it has also been demonstrated that 10A1 and ecotropic envelope proteins can form functional hetero-oligomers (31). Therefore, we investigated whether P50-76 behaved as a purely binding-defective mutant in a complementation assay when coexpressed with a fusion-defective Mo-MuLV envelope protein mutant.
Mutant E-T461P contains a single point mutation in the amino terminus of the Mo-MuLV TM protein. It is fully competent for binding to the ecotropic receptor but is a fusion-defective mutant (45). We cotransfected plasmids expressing P50-76 and E-T461P into 293T/17 cells, together with pCgp and pCnBg. The resulting supernatants were titered on human 293 cells, which express functional Pit2, and also on 293/12 cells, which additionally express the murine ecotropic receptor (28). This approach determines whether any functional complementation is occurring through the use of amphotropic or ecotropic receptors. Although neither mutant gave titer on 293 or 293/12 cells alone, the coexpression of P50-76 with E-T461P resulted in retroviral vectors that were able to transduce 293/12 cells but not 293 cells (Table 3). Presumably, the transduction of 293/12 cells occurred through the use of the ecotropic receptor, with the receptor binding function provided by the wild-type SU domain of E-T461P and the P50-76 mutant providing the postbinding functions. These data indicate that the primary defect in protein P50-76 is its lack of ability to bind to Pit2.
TABLE 3.
Complementation for transduction by coexpression of envelope protein
| Envelope protein | Titer (CFU/ml)a of vectors on cell line:
|
|
|---|---|---|
| 293 | 293/12b | |
| None | <5 | <5 |
| 4070A | (5.8 ± 0.5) × 105 | (7.6 ± 0.8) × 105 |
| Mo-MuLV | <5 | (1.5 ± 0.9) × 106 |
| P50-76 | <5 | <5 |
| E-T461P | <5 | <5 |
| P50-76 + E-T461P | 11 ± 0.1 | (1.3 ± 0.5) × 103 |
Titers were averaged from at least three independent experiments and are expressed as the mean number of β-galactosidase-expressing colonies ± the standard error of the mean.
293/12 is a 293 cell line stably expressing the ecotropic MuLV receptor.
Mutant R73L exhibits temperature sensitivity due to aberrant processing.
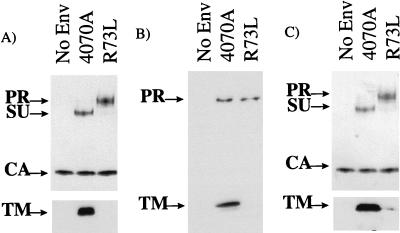
Retroviral vectors bearing the mutant R73L protein gave no titer on NIH 3T3 cells (Table 1). When this mutant protein was analyzed by Western blot analysis for its ability to be incorporated into retroviral vectors, a single band was detected with an anti-SU antiserum which migrated at a higher position than the wild-type SU (Fig. 3A). Probing the blot with an anti-TM antibody did not reveal any bands corresponding to the processed TM in vector particles (Fig. 3A). These results suggested that the higher-migrating band detected for R73L with the anti-SU antiserum was most likely the uncleaved precursor envelope protein, possibly due to a defect in the processing of the polyprotein precursor into SU and TM, as we have previously observed for certain mutants of Mo-MuLV SU (41).
FIG. 3.
(A) Incorporation of envelope proteins into retroviral vectors produced at 37°C. The upper blot was probed with anti-SU and anti-CA antibodies, and the lower blot was probed with anti-TM antibody. PR represents the precursor protein. (B) Envelope proteins in the lysates of transfected 293T cells produced at 37°C and detected with anti-TM antibody. (C) Incorporation of envelope protein into retroviral vectors produced at 32°C. The upper blot was probed with anti-SU and anti-CA antibodies, and the lower blot was probed with anti-TM antibody. Lanes No Env contain vectors produced without an env expression vector.
To verify our findings, we also analyzed the form of the R73L protein present in the lysates of transfected cells that reacted with an anti-TM antibody. While the precursor protein was detected for both the wild-type 4070A and R73L proteins, the processed TM protein was only detected for 4070A (Fig. 3B). Similarly, we also observed a lack of processing of the R73L protein into the mature SU subunit when the blot was probed with anti-SU antiserum (data not shown).
To determine whether the inability of the R73L protein to be processed properly was a result of protein misfolding, we analyzed whether any functional protein could be produced at a lower temperature. When produced at 32°C, retroviral vectors bearing the R73L protein were able to transduce NIH 3T3 cells with titers of 104, indicating a temperature-sensitive mutant (Table 4). In addition, Western blot analysis of the vectors now revealed the presence of a low level of processed TM protein (Fig. 3C). Although we were still not able to detect any processed SU in the vector particles (Fig. 3C), the amount of SU may have been at the limit of detection for this analysis. We have previously observed titer from MuLV mutants that gave rise to very small amounts of processed envelope proteins in vector particles (41). Thus, the low titer observed at 32°C for R73L probably arose from the small amount of correctly processed envelope protein. Therefore, it is unlikely that the defect of R73L produced at 37°C is due to a loss of interaction with Pit2, but rather is due to a structural defect that results in inefficient processing of the precursor protein into SU and TM.
TABLE 4.
Temperature sensitivity of R73L
| Envelope protein | Titer (CFU/ml)a of vectors on NIH 3T3 cells
|
|
|---|---|---|
| Vectors produced at 37°C | Vectors produced at 32°C | |
| None | <30 | <30 |
| 4070A | (2.9 ± 1.8) × 107 | (6.4 ± 3.0) × 106 |
| R73L | <30 | (1.0 ± 0.2) × 104 |
Titers were averaged from at least three independent experiments and are expressed as the mean number of β-galactosidase-expressing colonies ± the standard error of the mean.
Substitution of residue R102 in Mo-MuLV VRA also results in defective precursor processing.
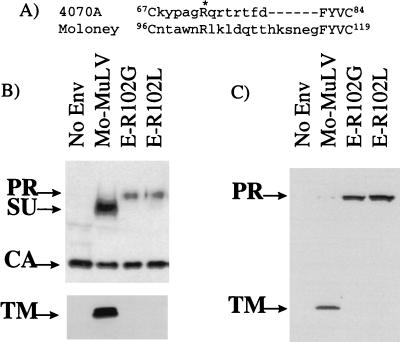
Sequence alignment of the interloop domain of the VRA regions revealed that Arg-73 of the amphotropic SU appears to be an invariant residue in all MuLV subgroups (4) (Fig. 4A). Therefore, we investigated whether mutation of the corresponding residue in Mo-MuLV SU, Arg-102, results in a phenotype similar to that of R73L. It has been reported previously that a Mo-MuLV virus bearing the double mutation of R102G and K104Q was noninfectious, and no interference with the Moloney murine sarcoma virus was observed in cells stably expressing this mutant protein, suggesting a defect in receptor binding (33). Since different substitutions of the same residue may result in different phenotypes, we generated two mutations of Mo-MuLV Arg-102. Mutant protein E-R102G corresponds to the substitution in the double mutation of Skov and Andersen (33), while the E-R102L mutant is analogous to the amphotropic mutation, R73L.
FIG. 4.
(A) Amino acid alignment of the VRA interloop domains of 4070A and Mo-MuLV envelope proteins. The conserved arginine residue is identified with an asterisk. Conserved residues are in capitals, and variable residues are in lowercase. (B) Incorporation of envelope proteins into retroviral vectors produced at 37°C. The upper blot was probed with anti-SU and anti-CA antibodies, and the lower blot was probed with anti-TM antibody. (C) Envelope proteins in the lysates of transfected 293T cells grown at 37°C, detected with anti-TM antibody. PR, precursor protein. Lanes No Env contain vectors produced without an env expression vector.
Retroviral vectors bearing either the E-R102G or E-R102L protein gave no titer on NIH 3T3 cells (data not shown). In contrast to the amphotropic R73L protein, production at 32°C did not rescue titer on NIH 3T3 cells for either ecotropic mutant. When the mutant proteins were analyzed for their ability to be incorporated into retroviral vector particles at 37°C, higher-migrating bands were detected with the anti-SU antiserum, suggesting the presence of uncleaved precursor protein (Fig. 4B). In addition, probing with the anti-TM antibody did not reveal any bands corresponding to TM for either of the two ecotropic mutants (Fig. 4B). When lysates of the transfected cells were probed with the anti-TM antibody, both mutants had higher levels of the precursor protein than of the wild-type Mo-MuLV protein, but processed TM protein was not detected (Fig. 3C). Western blot analysis of the R102G and R102L proteins present in vectors produced at 32°C were identical to those produced at 37°C (data not shown), implying that the lower temperature could not rescue the defect in the ecotropic mutants.
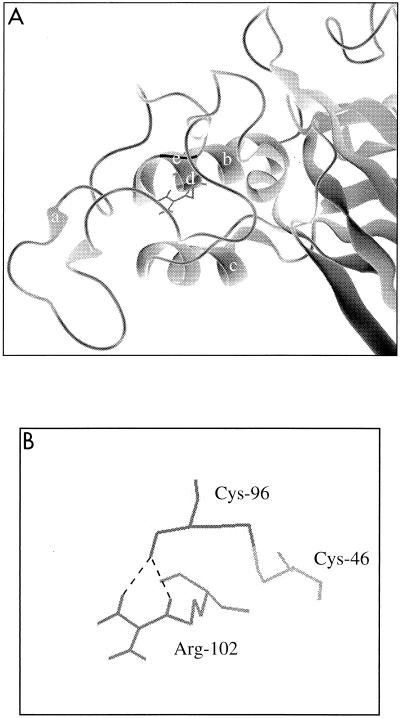
The results for the ecotropic Arg-102 substitutions were similar to the phenotype observed for the amphotropic R73L protein, suggesting that a similar structural defect is present that results in the inefficient processing of the precursor protein. Therefore, we analyzed the local environment of Arg-102 in the crystal structure of the ecotropic Friend MuLV binding domain (8). The structure revealed that the side chain of Arg-102 may be involved in a hydrogen bond with the backbone of Cys-96 (Fig. 5). Therefore, the substitution of Arg-102 could have serious structural consequence for the ecotropic protein, which could account for the lack of efficient processing of the R102 mutant proteins that we observed. In addition, the similar phenotypes observed for both the ecotropic and amphotropic substitutions suggest that this conserved arginine may play a similarly important role in envelope protein conformation for all of the MuLV subtypes.
FIG. 5.
(A) Ribbon representation of VRA and VRB of the ecotropic envelope proteins (8). The amino terminus of VRA contains an extended coil (a), while the interloop domain contains a helix (b). VRB also contains a helical region (c). Arg-102 (d) is shown with its side chain exposed. Cys-96 (e) is at the junction of the VRA amino-terminal loop and the interloop domain. (B) Potential hydrogen bond formation (dashed lines) between the side chain of Arg-102 and the carbonyl group of Cys-96 is shown relative to the disulfide bond between Cys-96 and Cys-46.
DISCUSSION
The critical receptor binding determinants of the amphotropic MuLV envelope protein are located in the amino-terminal 208 amino acids of SU (2) and have been suggested to be located, in particular, in the first disulfide loop domain of VRA (3, 37). Our analysis of this region failed to identify individual residues that were critical for receptor interaction, with the amphotropic envelope protein remaining functional despite several point mutations, polytropic sequence substitutions, and a deletion within this first disulfide-linked loop. However, the replacement of a more extensive region of VRA with polytropic sequence produced a protein that was unable to interact with the host cell receptor. Therefore, our findings suggest that multiple residues may be involved in the interaction between the envelope protein and its receptor.
Two previous studies have implicated the first disulfide-linked loop of VRA in receptor contact. In particular, residues Tyr-62 and Val-63 (Tyr-60 and Val-61 in Tailor and Kabat [37]) of amphotropic VRA have been previously suggested to be critical for receptor recognition. However, these observations were made in the context of chimeric envelope proteins of amphotropic MuLV and feline leukemia virus type B, titered on a chimeric Pit1-Pit2 receptor. When we mutated these two residues individually in the amphotropic envelope protein, there was no apparent effect on the transduction of NIH 3T3 cells. The deletion of both of these residues in Δ56-63 resulted in titers that were 8% of the level attained by vectors containing the wild-type amphotropic envelope protein. Although Δ56-63 did demonstrate a decrease in titer, the reinsertion of the two polytropic residues in this region (P56-63) restored titers to the wild-type level. Therefore, the decrease in titer for Δ56-63 probably reflected a compromise in the structural integrity of the region rather than a deletion of residues that are essential for receptor interaction. Taken together, our data clearly demonstrate that there is no requirement for Tyr-62 and Val-63 in the interaction of amphotropic envelope protein with murine Pit2.
A second study identified a 14-amino-acid segment between residues 52 and 66 (residues 50 to 64 in Battini et al. [3]) of the first disulfide loop of VRA as being an essential determinant of amphotropic receptor interaction. The replacement of this segment with a foreign linear peptide appeared to block interaction of the amphotropic envelope protein with its receptor. However, extensive mutagenesis within this first disulfide loop domain failed to identify any single residue as being essential for receptor interaction. In addition, the amphotropic protein was able to tolerate the loss of residues 56 to 63 and the replacement of residues 50 to 63 or 65 to 76 with the corresponding polytropic sequences, although the replacement of a larger segment, spanning residues 50 to 76, with the corresponding polytropic sequence (P50-76) did abolish receptor utilization.
Further analysis of P50-76 confirmed that it was indeed a binding-defective envelope protein. Although it was unable to bind to or transduce Pit2-expressing cells, it was competent to provide postbinding functions when coexpressed with a fusion mutant of the ecotropic MuLV envelope protein. As we have previously proposed (43, 44), it is likely that functional complementation occurred between P50-76 and the ecotropic fusion mutant as a result of the formation of hetero-oligomers. Our results indicate that envelope protein monomers from different subgroups of MuLV can oligomerize with each other, as has previously been demonstrated for the ecotropic and 10A1 MuLV proteins (31). These findings indicate that the primary domain(s) involved in oligomerization between MuLV envelope protein monomers is likely to be located within a conserved region(s) of the protein.
The binding-defective phenotype of the P50-76 mutant implicated both the first disulfide loop and the interloop domain of VRA as being essential for Pit2 interaction. The competency of both P50-63 and P65-76 argues against a single critical residue for Pit2 recognition being present in residues 50 to 76, at least among the variable residues between the amphotropic and polytropic envelope proteins, and instead points to a more complex receptor binding domain. It is possible that contact points exist in both the variable first disulfide loop and the more conserved interloop domain within VRA. Thus, although the loss of some of these contact residues could be tolerated individually, the combined loss of contact residues in P50-76 would reduce binding efficiency below a critical level. The involvement of multiple residues in receptor recognition has been suggested for other retrovirus envelope proteins, including the ecotropic MuLV envelope protein (1) and avian sarcoma and leukosis viruses (32).
The crystal structure of the amino terminus of the ecotropic SU reveals that the interloop domain forms a helix that may be important for the structure of the envelope protein (8). Various insertions in the interloop domain have been shown previously to result in impaired SU-TM cleavage, transport to the cell surface, and incorporation into virions (3, 26, 36), which are suggestive of structural defects. We observed a similarity in the phenotypes of both ecotropic and amphotropic mutants—a conserved arginine residue in this helical region—that was consistent with the region playing an important role in the overall structure of the protein. Therefore, at least this region of VRA in the amphotropic binding domain may be organized similarly to the ecotropic binding domain. The difference between the ecotropic and amphotropic arginine substitutions in terms of their ability to be rescued by production at 32°C may be due to the fact that VRA has a simpler composition in the amphotropic protein, allowing for partial correction at a lower temperature.
Once we observed that the substitution mutant P65-76 resulted in titer on NIH 3T3 cells that was similar to the wild-type amphotropic level, we investigated whether retroviral vectors bearing this protein demonstrated a 10A1-like interference pattern. We have previously demonstrated that changing the residues Ala-71 and Gln-74 of the amphotropic envelope protein to the 10A1 residues, Gly and Lys, respectively, resulted in a 10A1-like interference pattern, i.e., an ability to infect amphotropic MuLV-infected NIH 3T3 cells (10). The polytropic sequence between residues 65 and 76 contains the 10A1 residues at these two positions, but mutant P65-76 gave an amphotropic interference pattern. We have previously suggested that the 10A1-specific residues exert their effects indirectly on the binding domain rather than acting as direct contact points for the 10A1 receptor, Pit1 (10). In addition, this effect was proposed to be quite subtle, as amphotropic envelope proteins for which 10A1 residues were substituted retained their ability to interact with Pit2. Therefore, the additional residue changes contained within the polytropic substitution P65-76 may have prevented the conversion of the amphotropic binding domain to a structure that could additionally recognize Pit1. We are currently exploring this possibility.
Overall, our analysis suggests that multiple residues of the amphotropic binding domain participate in Pit2 recognition and that most or all of these residues may be located within the first disulfide loop and the interloop domain of VRA. In addition, our analysis of mutants of a conserved arginine residue in VRA of both the amphotropic and ecotropic envelope proteins suggests that despite the high variability in the VRA sequences, the overall organization of the region in different MuLV subtypes may be similar. One could speculate, therefore, that targeted in vitro evolution of the variable regions of the MuLV envelope proteins may generate envelope proteins with novel receptor specificity for the purpose of gene therapy while conserving important postbinding functions in the molecule.
ACKNOWLEDGMENTS
We thank Albert J. MacKrell, Nai-Wei Soong, Kin-Man Lai, and Jane Xia from the Gene Therapy Laboratories for their assistance and helpful comments.
This work was supported by GTI/Norvartis and by grant CA59318-04 from NIH.
REFERENCES
- 1.Bae Y, Kingsman S M, Kingsman A J. Functional dissection of the Moloney murine leukemia virus envelope protein gp70. J Virol. 1997;71:2092–2099. doi: 10.1128/jvi.71.3.2092-2099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battini J L, Danos O, Heard J M. Receptor-binding domain of murine leukemia virus envelope glycoproteins. J Virol. 1995;69:713–719. doi: 10.1128/jvi.69.2.713-719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battini J L, Danos O, Heard J M. Definition of a 14-amino-acid peptide essential for the interaction between the murine leukemia virus amphotropic envelope glycoprotein and its receptor. J Virol. 1998;72:428–435. doi: 10.1128/jvi.72.1.428-435.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battini J L, Heard J M, Danos O. Receptor choice determinants in the envelope glycoproteins of amphotropic, xenotropic, and polytropic murine leukemia viruses. J Virol. 1992;66:1468–1475. doi: 10.1128/jvi.66.3.1468-1475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battini J L, Rodrigues P, Muller R, Danos O, Heard J M. Receptor-binding properties of a purified fragment of the 4070A amphotropic murine leukemia virus envelope glycoprotein. J Virol. 1996;70:4387–4393. doi: 10.1128/jvi.70.7.4387-4393.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonham L, Wolgamot G, Miller A D. Molecular cloning of Mus dunni endogenous virus: an unusual retrovirus in a new murine viral interference group with a wide host range. J Virol. 1997;71:4663–4670. doi: 10.1128/jvi.71.6.4663-4670.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosselman R A, van Straaten F, Van Beveren C, Verma I M, Vogt M. Analysis of the env gene of a molecularly cloned and biologically active Moloney mink cell focus-forming proviral DNA. J Virol. 1982;44:19–31. doi: 10.1128/jvi.44.1.19-31.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fass D, Davey R A, Hamson C A, Kim P S, Cunningham J M, Berger J M. Structure of a murine leukemia virus receptor-binding glycoprotein at 2.0 angstrom resolution. Science. 1997;277:1662–1666. doi: 10.1126/science.277.5332.1662. [DOI] [PubMed] [Google Scholar]
- 9.Goff S, Traktman P, Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981;38:239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han J Y, Cannon P M, Lai K M, Zhao Y, Eiden M V, Anderson W F. Identification of envelope protein residues required for the expanded host range of 10A1 murine leukemia virus. J Virol. 1997;71:8103–8108. doi: 10.1128/jvi.71.11.8103-8108.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han L, Hofmann T, Chiang Y, Anderson W F. Chimeric envelope glycoproteins constructed between amphotropic and xenotropic murine leukemia retroviruses. Somatic Cell Mol Genet. 1995;21:205–214. doi: 10.1007/BF02254771. [DOI] [PubMed] [Google Scholar]
- 12.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 13.Hunter E, Swanstrom R. Retrovirus envelope glycoproteins. Curr Top Microbiol Immunol. 1990;157:187–253. doi: 10.1007/978-3-642-75218-6_7. [DOI] [PubMed] [Google Scholar]
- 14.Kavanaugh M P, Miller D G, Zhang W, Law W, Kozak S L, Kabat D, Miller A D. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci USA. 1994;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kavanaugh M P, Kabat D. Identification and characterization of a widely expressed phosphate transporter/retrovirus receptor family. Kidney Int. 1996;49:959–963. doi: 10.1038/ki.1996.135. [DOI] [PubMed] [Google Scholar]
- 16.Linder M, Wenzel V, Linder D, Stirm S. Structural elements in glycoprotein 70 from polytropic Friend mink cell focus-inducing virus and glycoprotein 71 from ecotropic Friend murine leukemia virus, as defined by disulfide-bonding pattern and limited proteolysis. J Virol. 1994;68:5133–5141. doi: 10.1128/jvi.68.8.5133-5141.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacKrell A J, Soong N W, Curtis C M, Anderson W F. Identification of a subdomain in the Moloney murine leukemia virus envelope protein involved in receptor binding. J Virol. 1996;70:1768–1774. doi: 10.1128/jvi.70.3.1768-1774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masuda M, Hanson C A, Alvord W G, Hoffman P M, Ruscetti S K, Masuda M. Effects of subtle changes in the SU protein of ecotropic murine leukemia virus on its brain capillary endothelial cell tropism and interference properties. Virology. 1996;215:142–151. doi: 10.1006/viro.1996.0017. [DOI] [PubMed] [Google Scholar]
- 19.Masuda M, Masuda M, Hanson C A, Hoffman P M, Ruscetti S K. Analysis of the unique hamster cell tropism of ecotropic murine leukemia virus PVC-211. J Virol. 1996;70:8534–8539. doi: 10.1128/jvi.70.12.8534-8539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller A D, Wolgamot G. Murine retroviruses use at least six different receptors for entry into Mus dunni cells. J Virol. 1997;71:4531–4535. doi: 10.1128/jvi.71.6.4531-4535.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller D G, Edwards R H, Miller A D. Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc Natl Acad Sci USA. 1994;91:78–82. doi: 10.1073/pnas.91.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan R A, Nussbaum O, Muenchau D D, Shu L, Couture L, Anderson W F. Analysis of the functional and host range-determining regions of the murine ecotropic and amphotropic retrovirus envelope proteins. J Virol. 1993;67:4712–4721. doi: 10.1128/jvi.67.8.4712-4721.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ott D, Rein A. Basis for receptor specificity of nonecotropic murine leukemia virus surface glycoprotein gp70su. J Virol. 1992;66:4632–4638. doi: 10.1128/jvi.66.8.4632-4638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park B H, Matuschke B, Lavi E, Gaulton G N. A point mutation in the env gene of a murine leukemia virus induces syncytium formation and neurologic disease. J Virol. 1994;68:7516–7524. doi: 10.1128/jvi.68.11.7516-7524.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peredo C, O’Reilly L, Gray K, Roth M J. Characterization of chimeras between the ecotropic and the amphotropic 4070A envelope proteins. J Virol. 1996;70:3142–3152. doi: 10.1128/jvi.70.5.3142-3152.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez L G, Hunter E. Mutations within the proteolytic cleavage site of the Rous sarcoma virus glycoprotein that block processing to gp85 and gp87. J Virol. 1987;61:1609–1614. doi: 10.1128/jvi.61.5.1609-1614.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinter A, Honnen W J, Tung J S, O’Donnell P V, Hammerling U. Structural domains of endogenous murine leukemia virus gp70s containing specific antigenic determinants defined by monoclonal antibodies. Virology. 1982;116:499–516. doi: 10.1016/0042-6822(82)90143-x. [DOI] [PubMed] [Google Scholar]
- 28.Ragheb J A, Yu H, Hofmann T, Anderson W F. The amphotropic and ecotropic murine leukemia virus envelope TM subunits are equivalent mediators of direct membrane fusion: implications for the role of the ecotropic envelope and receptor in syncytium formation and viral entry. J Virol. 1995;69:7205–7215. doi: 10.1128/jvi.69.11.7205-7215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rein A. Interference grouping of murine leukemia viruses: a distinct receptor for the MCF-recombinant viruses in mouse cells. Virology. 1982;120:251–257. doi: 10.1016/0042-6822(82)90024-1. [DOI] [PubMed] [Google Scholar]
- 30.Rein A, Schultz A. Different recombinant murine leukemia viruses use different cell surface receptors. Virology. 1984;136:144–152. doi: 10.1016/0042-6822(84)90255-1. [DOI] [PubMed] [Google Scholar]
- 31.Rein A, Yang C, Haynes J A, Mirro J, Compans R W. Evidence for cooperation between murine leukemia virus Env molecules in mixed oligomers. J Virol. 1998;72:3432–3435. doi: 10.1128/jvi.72.4.3432-3435.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rong L, Edinger A, Bates P. Role of basic residues in the subgroup-determining region of the subgroup A avian sarcoma and leukosis virus envelope in receptor binding and infection. J Virol. 1997;71:3458–3465. doi: 10.1128/jvi.71.5.3458-3465.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skov H, Andersen K B. Mutational analysis of Moloney murine leukemia virus surface protein gp70. J Gen Virol. 1993;74:707–714. doi: 10.1099/0022-1317-74-4-707. [DOI] [PubMed] [Google Scholar]
- 34.Sommerfelt M A, Weiss R A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990;176:58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- 35.Soneoka Y, Cannon P M, Ramsdale E E, Griffiths J C, Romano G, Kingsman S M, Kingsman A J. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szurek P F, Yuen P H, Ball J K, Wong P K Y. A Val-25-to-Ile substitution in the envelope precursor polyprotein, gPr80env, is responsible for the temperature sensitivity, inefficient processing of gPr80env, and neurovirulence of ts1, a mutant of Moloney murine leukemia virus TB. J Virol. 1990;64:467–475. doi: 10.1128/jvi.64.2.467-475.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tailor C S, Kabat D. Variable regions A and B in the envelope glycoproteins of feline leukemia virus subgroup B and amphotropic murine leukemia virus interact with discrete receptor domains. J Virol. 1997;71:9383–9391. doi: 10.1128/jvi.71.12.9383-9391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Zeijl M, Johann S V, Cross E, Cunningham J, Eddy R, Shows T B, O’Hara B. A human amphotropic retrovirus receptor is a second member of the gibbon ape leukemia virus receptor family. Proc Natl Acad Sci USA. 1994;91:1168–1172. doi: 10.1073/pnas.91.3.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson C A, Eiden M V, Anderson W B, Lehel C, Olah Z. The dual-function hamster receptor for amphotropic murine leukemia virus (MuLV), 10A1 MuLV, and gibbon ape leukemia virus is a phosphate symporter. J Virol. 1995;69:534–537. doi: 10.1128/jvi.69.1.534-537.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson C A, Farrell K B, Eiden M V. Properties of a unique form of the murine amphotropic leukemia virus receptor expressed on hamster cells. J Virol. 1994;68:7697–7703. doi: 10.1128/jvi.68.12.7697-7703.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu, B. W., P. M. Cannon, E. M. Gordon, F. L. Hall, and W. F. Anderson. Characterization of the proline-rich region of murine leukemia virus envelope protein. J. Virol. 72:5383–5391. [DOI] [PMC free article] [PubMed]
- 42.Yu H, Soong N, Anderson W F. Binding kinetics of ecotropic (Moloney) murine leukemia retrovirus with NIH 3T3 cells. J Virol. 1995;69:6557–6562. doi: 10.1128/jvi.69.10.6557-6562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Y, Lee S, Anderson W F. Functional interactions between monomers of the retroviral envelope protein complex. J Virol. 1997;71:6967–6972. doi: 10.1128/jvi.71.9.6967-6972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao, Y., L. Zhu, C. A. Benedict, D. Chen, W. F. Anderson, and P. M. Cannon. Functional domains in the retroviral transmembrane protein. J. Virol. 72:5392–5398. [DOI] [PMC free article] [PubMed]
- 45.Zhu N-L, Cannon P M, Chen D, Anderson W F. Mutational analysis of the fusion peptide of Moloney murine leukemia virus transmembrane protein p15E. J Virol. 1998;72:1632–1639. doi: 10.1128/jvi.72.2.1632-1639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]