Abstract
Background and Aims
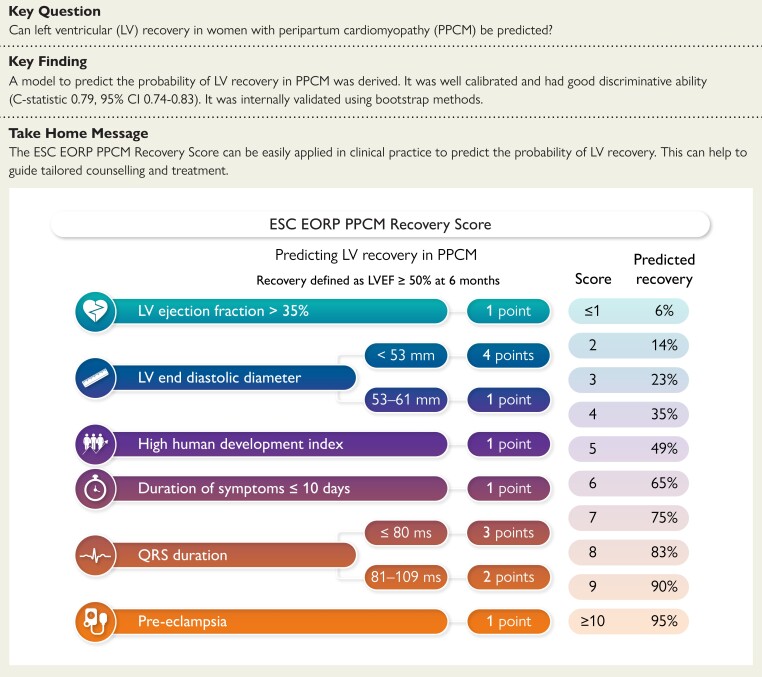
There are no established clinical tools to predict left ventricular (LV) recovery in women with peripartum cardiomyopathy (PPCM). Using data from women enrolled in the ESC EORP PPCM Registry, the aim was to derive a prognostic model to predict LV recovery at 6 months and develop the ‘ESC EORP PPCM Recovery Score’—a tool for clinicians to estimate the probability of LV recovery.
Methods
From 2012 to 2018, 752 women from 51 countries were enrolled. Eligibility included (i) a peripartum state, (ii) signs or symptoms of heart failure, (iii) LV ejection fraction (LVEF) ≤ 45%, and (iv) exclusion of alternative causes of heart failure. The model was derived using data from participants in the Registry and internally validated using bootstrap methods. The outcome was LV recovery (LVEF ≥50%) at six months. An integer score was created.
Results
Overall, 465 women had a 6-month echocardiogram. LV recovery occurred in 216 (46.5%). The final model included baseline LVEF, baseline LV end diastolic diameter, human development index (a summary measure of a country’s social and economic development), duration of symptoms, QRS duration and pre-eclampsia. The model was well-calibrated and had good discriminatory ability (C-statistic 0.79, 95% confidence interval [CI] 0.74–0.83). The model was internally validated (optimism-corrected C-statistic 0.78, 95% CI 0.73–0.82).
Conclusions
A model which accurately predicts LV recovery at 6 months in women with PPCM was derived. The corresponding ESC EORP PPCM Recovery Score can be easily applied in clinical practice to predict the probability of LV recovery for an individual in order to guide tailored counselling and treatment.
Keywords: Peripartum cardiomyopathy, Left ventricular recovery, Prediction model
Structured Graphical Abstract
Structured Graphical Abstract.
Graphical summary of the main study findings. Human development index (HDI) is a summary measure (between zero and one) of a country's social and economic development and can be found at: https://hdr.undp.org/data-center/human-development-index#/indicies/HDI. CI, confidence interval; EORP, EURObservational Research Programme; ESC, European Society of Cardiology; LV, left ventricular: LVEF, left ventricular ejection fraction; PPCM, peripartum cardiomyopathy
See the editorial comment for this article ‘Peripartum cardiomyopathy: the challenge of predicting cardiac function recovery', by C. Basic and M. Schaufelberger, https://doi.org/10.1093/eurheartj/ehae111.
Introduction
There are a number of established risk models and tools for predicting morbidity and mortality in people with chronic heart failure.1,2 They facilitate prediction of events within a specific population by utilizing characteristics to estimate the risk of an event occurring. Accurate quantification of risk for a particular individual, based on that individual’s own characteristics, allows a clinician to tailor counselling and treatment. There are currently no clinical tools that can be used to predict outcomes for women with peripartum cardiomyopathy (PPCM), a type of de novo heart failure which develops in approximately 1–100 in 1000 pregnancies.3,4 It is characterized by relatively frequent recovery of left ventricular (LV) function, when compared to dilated cardiomyopathy more generally.5,6 Although the definition of LV recovery used in studies varies, it is usually considered to be an improvement in LV ejection fraction (LVEF) to above a threshold of either 50% or 55%6–9. Estimating the propensity for LV recovery in PPCM is important and underpins a number of clinical decisions, such as recommending an implantable cardioverter defibrillator (ICD).
The European Society of Cardiology (ESC) EURObservational Research Programme (EORP) PPCM Registry is the largest prospective study of PPCM.7 Using patient-level data from women in the ESC EORP PPCM Registry, a prognostic model to predict LV recovery at 6 months was derived, internally validated and a ‘PPCM Recovery Score’ was generated—a tool for clinicians to estimate the probability of LV recovery at 6 months in this patient group.
Methods
Registry design
Registry design, patient selection and data collection have been published previously.7,10 Briefly, women who were diagnosed with PPCM within the preceding 6 months were prospectively enrolled into an observational registry. Inclusion criteria were: (i) a peripartum state, (ii) signs and/or symptoms of heart failure, (iii) a LVEF ≤45%, and (iv) the exclusion of alternative causes of heart failure. A total of 752 women from 51 countries were originally enrolled into the Registry (2012–2018). Core clinical data, such as data from the electrocardiogram and echocardiogram, were centrally validated by a data monitor who contacted the sites. LV recovery was defined as a LVEF ≥50% on the 6 month follow-up echocardiogram. Comorbidities, including diabetes, pregnancy-induced hypertension and pre-eclampsia, were clinician-recorded. Health expenditure (HE) is the share of gross domestic product within a country and was defined as low (<5%), medium (5%–8%) and high (>8%). Human development index (HDI) is a summary measure (between zero and one) of a country’s social and economic development, encompassing three domains—life expectancy, education, and standard of living. Human development index was defined as low (<0.550), medium (0.550–0.699), and high (≥0.700). Participating centres managed the approvals of national or regional ethics committees or institutional review boards according to local regulations. Locally appointed ethics committee approved the research protocol and informed consent was obtained from participants or a legally authorized representative.
Statistical methods
The main analysis included patients with complete data. Multiple imputations were used as a sensitivity analysis for model derivation. Patient characteristics were compared according to recovery status using Pearson’s chi-squared tests, two sample t-tests and Wilcoxon rank-sum test as appropriate. A total of 20 variables known or suspected to be prognostically significant in PPCM were included as candidate variables and are listed in the Appendix. Highly skewed data were log transformed. A logistic regression prediction model for LV recovery at 6 months was built using a backward selection process including all potential predictor variables, with a two-sided P-value of <.05 as the initial significance level. All selected variables were then examined for interactions, with a two-sided P-value threshold of <.05 considered to be statistically significant. The final predictor variables in the model were converted from continuous to categorical forms to allow clinical applicability of the model. Clinically relevant categories were determined by systematically assessing different cut-offs and identifying those resulting in a model with optimal discriminatory ability.
Model calibration was assessed by comparing predicted probability of LV recovery, estimated by the final model, with observed probability in deciles and the comparison was displayed graphically with a lowess smoother line using the ‘pmcalplot’ package in Stata. As a secondary measure of model calibration, a Hosmer–Lemeshow chi-squared goodness-of-fit test was performed. The discriminatory ability of the model was assessed using the C-statistic, which is equivalent to the area under the receiver operating characteristic curve; a value below 0.5 indicates a poor model, a value of 0.5 indicates that the model is no better at predicting the outcome than chance, and a value of 1 means that the model perfectly predicts individuals who will and will not experience the outcome. The model was internally validated using 1000 bootstrap samples. Bias-corrected 95% confidence intervals (CI) were obtained from bootstrap samples. The optimism-corrected C-statistic, which provides a measure of the extent to which the original model is too optimistic, or overfits the data, was calculated by generating the difference between the original C-statistic and the C-statistic obtained from each bootstrap sample, taking this difference from the original C-statistic and averaging this across the bootstrap samples. A PPCM recovery score was generated by converting the variable coefficients to corresponding integer points, by multiplying the coefficient by 1.75.
A number of sensitivity analyses were conducted:
Women who died were categorized as ‘unrecovered’ and a new model derived and performance assessed.
Model performance was assessed in a complete dataset using multiple imputation by chained equations according to Rubin’s rules.
Model-building was approached using alternative methods: forward selection (including all potential predictor variables, with a two-sided P-value of <.05 as the initial significance level) and stepwise selection (forward and backward, removing terms with P-value ≥.1 and adding those with a P-value of <.05).
Analyses were conducted using Stata SE v16.1 (StataCorp). The transparent reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) guidelines were followed.11
Results
Patient characteristics
In total, LVEF was available in 465 women at 6 months and LV recovery occurred in 216 (46.5%). The mean change in LVEF from baseline to 6 months was 15% (±13); 8% (±10) in women without recovery, and 24% (±12) in women with recovery.
Compared to unrecovered women, women with LV recovery were more often White, less often Black and Middle Eastern, and were more often from the highest HDI and HE categories (Table 1). They had a shorter median duration between symptom-onset and diagnosis, more frequently had pregnancy-induced hypertension and pre-eclampsia, had higher baseline systolic blood pressure and lower heart rate, and had a higher white blood cell count and serum sodium level. They also had a shorter QRS duration, higher baseline LVEF, smaller LV end diastolic and systolic diameter, smaller left atrial diameter, and less frequent right ventricular impairment and mitral regurgitation (moderate or worse). The extent of missing data is shown in Supplementary data online, Table S1. Characteristics are also presented by displaying proportions according to the characteristic, rather than recovery status, in Supplementary data online, Table S2.
Table 1.
Characteristics in patients with and without left ventricular recovery
| All (n = 465) |
Unrecovered (n = 249) |
Recovered (n = 216) |
P-value | |
|---|---|---|---|---|
| Age, years | 31 ± 6 | 31 ± 6 | 31 ± 6 | .30 |
| Region | <.001 | |||
| Africa | 141 (30.3) | 88 (35.3) | 53 (24.5) | |
| Asia-Pacific | 75 (16.1) | 28 (11.2) | 47 (21.8) | |
| Europe | 171 (36.8) | 76 (30.5) | 95 (44.0) | |
| Middle East | 78 (16.8) | 57 (22.9) | 21 (9.7) | |
| Ethnicity | .002 | |||
| White | 149 (33.3) | 63 (26.2) | 86 (41.3) | |
| Black | 132 (29.5) | 84 (35.0) | 48 (23.1) | |
| Asian | 100 (22.3) | 54 (22.5) | 46 (22.1) | |
| Middle Eastern | 49 (10.9) | 32 (13.3) | 17 (8.2) | |
| Other | 18 (4.0) | 7 (2.9) | 11 (5.3) | |
| HDI category | <.001 | |||
| Low | 93 (20.1) | 62 (25.0) | 31 (14.5) | |
| Medium | 175 (37.9) | 103 (41.5) | 72 (33.6) | |
| High | 194 (42.0) | 83 (33.5) | 111 (51.9) | |
| Health expenditure | .038 | |||
| Low | 159 (34.5) | 98 (39.7) | 61 (28.5) | |
| Medium | 142 (30.8) | 72 (29.1) | 70 (32.7) | |
| High | 160 (34.7) | 77 (31.2) | 83 (38.8) | |
| Body mass index, kg/m2 | 26 ± 6 | 26 ± 6 | 26 ± 6 | .84 |
| Parity ≥2 | 221 (74.7) | 127 (75.1) | 94 (74.0) | .82 |
| Postpartum diagnosis | 396 (89.6) | 212 (89.8) | 184 (89.3) | .86 |
| Postpartum symptom-onset | 278 (68.1) | 151 (68.9) | 127 (67.2) | .70 |
| Duration of symptoms,days | 11 (3–34) | 17 (5–53) | 7 (1–22) | <.001 |
| Family history of heart failure or sudden death | 70 (15.2) | 34 (13.8) | 36 (16.8) | .37 |
| Diabetes | 15 (3.3) | 10 (4.0) | 5 (2.3) | .31 |
| Smoking (current/former) | 68 (15.3) | 28 (11.7) | 40 (19.4) | .024 |
| HIV Status | 19 (6.6) | 15 (9.3) | 4 (3.1) | .035 |
| Pregnancy-induced hypertension | 182 (39.9) | 83 (34.2) | 99 (46.5) | .007 |
| Pre-eclampsia | 119 (26.1) | 51 (21.0) | 68 (31.9) | .008 |
| Prior PPCM | 23 (7.7) | 15 (8.9) | 8 (6.2) | .40 |
| Systolic blood pressure, mmHg | 119 ± 23 | 116 ± 22 | 123 ± 24 | <.001 |
| Diastolic blood pressure, mmHg | 78 ± 16 | 77 ± 16 | 79 ± 16 | .20 |
| Heart rate, b.p.m. | 99 ± 21 | 101 ± 21 | 96 ± 22 | .016 |
| NYHA class | .23 | |||
| I | 39 (8.5) | 18 (7.3) | 21 (9.8) | |
| II | 122 (26.5) | 69 (28.0) | 53 (24.8) | |
| III | 159 (34.6) | 92 (37.4) | 67 (31.3) | |
| IV | 140 (30.4) | 67 (27.2) | 73 (34.1) | |
| S3 gallop | 198 (44.3) | 116 (48.7) | 82 (39.2) | .044 |
| Elevated jugular venous pressure (>6 cm) | 183 (41.3) | 106 (45.1) | 77 (37.0) | .084 |
| Peripheral oedema | 267 (57.5) | 147 (59.3) | 120 (55.6) | .42 |
| Pulmonary rales | 276 (60.7) | 147 (61.0) | 129 (60.3) | .88 |
| Serum creatinine, µmol/L | 70 (58–87) | 71 (59–88) | 67 (57–82) | .21 |
| Hemoglobin, g/L | 115 (105–129) | 114 (104–127) | 115 (105–130) | .47 |
| White blood cells, ×10^9/L | 9 (7–12) | 9 (6–11) | 10 (7–13) | .004 |
| Platelets, ×10^9/L | 266 (219–354) | 263 (221–344) | 268 (212–354) | .86 |
| Sodium, mmol/L | 138 (136–141) | 138 (135–140) | 139 (136–141) | .029 |
| Potassium, mmol/L | 4 (4–4) | 4 (4–4) | 4 (4–4) | .60 |
| QTc duration, ms | 461 ± 71 | 461 ± 76 | 461 ± 66 | .99 |
| QRS duration, ms | 89 ± 20 | 92 ± 22 | 84 ± 17 | <.001 |
| Left bundle branch block | 32 (7.1) | 25 (10.5) | 7 (3.3) | .003 |
| Atrial fibrillation/flutter | 7 (1.6) | 7 (2.9) | 0 (0.0) | .012 |
| LV ejection fraction, % | 31 ± 10 | 29 ± 9 | 34 ± 10 | <.001 |
| LV end diastolic diameter, mm | 59 ± 7 | 62 ± 7 | 56 ± 7 | <.001 |
| Indexed to body surface area, mm/m2 | 35 ± 5 | 36 ± 5 | 33 ± 5 | <.001 |
| LV end systolic diameter, mm | 49 ± 8 | 52 ± 7 | 46 ± 7 | <.001 |
| Indexed to body surface area, mm/m2 | 29 ± 5 | 31 ± 5 | 27 ± 5 | <.001 |
| Left atrial diameter, mm | 40 ± 7 | 41 ± 7 | 38 ± 7 | .002 |
| Right ventricular impairmenta | 153 (37.3) | 101 (45.7) | 52 (27.5) | <.001 |
| ≥ Moderate mitral regurgitation | 159 (43.8) | 95 (50.3) | 64 (36.8) | .010 |
Data are presented as n (%), mean ± standard deviation, or median (interquartile range).
HDI, human development index; HIV, human immunodeficiency virus; PPCM, peripartum cardiomyopathy; NYHA, New York Heart Association; LV, left ventricular.
aQualitatively assessed; function recorded by the investigator as normal, mildly impaired, or severely impaired.
Medical therapy
The proportions of women treated with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, a beta-blocker and bromocriptine were similar irrespective of whether or not LV recovery occurred. Women with LV recovery were less often on a mineralocorticoid receptor antagonist, diuretic, digoxin, and anticoagulant (see Supplementary data online, Table S3).
Model derivation, performance and validation
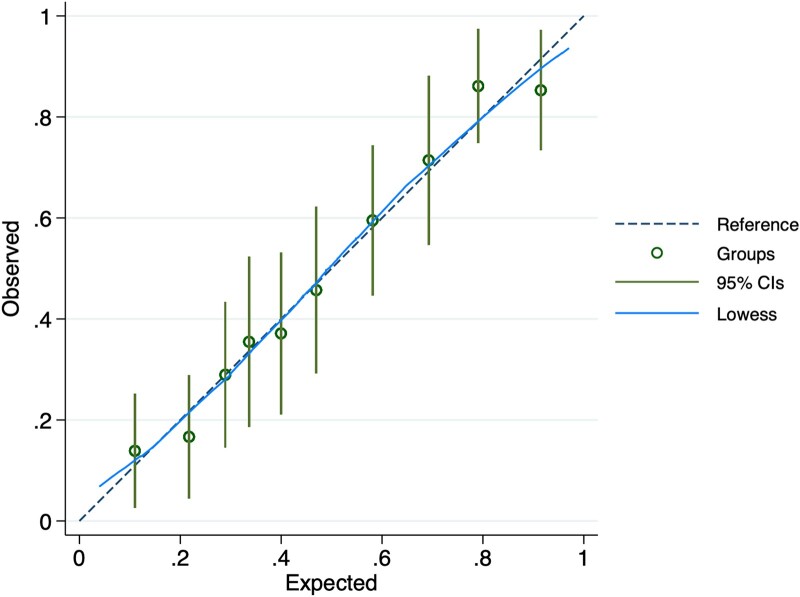
A total of 20 potential predictor variables were included in a backward selection process and are listed in the Appendix. The final multivariable predictors of LV recovery were the following: LVEF, LV end diastolic diameter, HDI, duration of symptoms, QRS duration, and pre-eclampsia (n = 351 in the final model) (Table 2). No significant interactions were identified. Clinically applicable categorical thresholds were determined as follows: LVEF >35% and ≤35%; LV end diastolic diameter <53 mm, 53–61 mm, and ≥62 mm (as per international categorizations12); duration of symptoms ≤10 days and >10 days; QRS duration ≤80 ms, 81–109 ms, and ≥110 ms (the model performed less well when bundle branch block vs. no bundle branch block, i.e. QRS durations <120 ms vs. ≥120 ms, was included instead). Predicted and observed probabilities of LV recovery were plotted according to decile of probability and are shown in Figure 1. Overall, the model was well-calibrated (Hosmer–Lemeshow chi2 goodness-of-fit test P = .87). The discriminative ability of the model was good, with a C-statistic of 0.79 (95% CI 0.74–0.83). The optimism-corrected C-statistic through internal validation by bootstrapping 1000 samples was 0.78 (95% CI 0.73–0.82).
Table 2.
Final multivariable prediction model for left ventricular recovery
| Odds ratio | Bias-corrected 95% CI |
Coefficient | |
|---|---|---|---|
| LV ejection fraction | |||
| ≤35% | 1.00 | ||
| >35% | 1.85 | 1.09–2.96 | 0.61 |
| LV end diastolic diameter | |||
| ≥62 mm | 1.00 | ||
| 53–61 mm | 1.69 | 0.94–2.88 | 0.52 |
| <53 mm | 8.43 | 3.34–19.18 | 2.13 |
| Human development index | |||
| Low | 1.00 | ||
| Medium | 0.97 | 0.49–1.92 | −0.03 |
| High | 2.27 | 1.01–4.95 | 0.82 |
| Duration of symptoms | |||
| >10 days | 1.00 | ||
| ≤10 days | 1.81 | 1.04–3.25 | 0.60 |
| QRS duration | |||
| ≥110 ms | 1.00 | ||
| 81–109 ms | 3.98 | 1.64–14.57 | 1.38 |
| ≤80 ms | 5.93 | 2.42–23.26 | 1.78 |
| Pre-eclampsia | |||
| No | 1.00 | ||
| Yes | 1.98 | 1.12–3.32 | 0.69 |
N = 351 in final model.
Figure 1.
Predicted and observed probability of left ventricular recovery according to decile of probability. The probabilities presented are those for each group of patients and the plot is a calibration plot of observed against expected probabilities. The dotted line represents perfectly matching predicted and observed probabilities, the green circles and extended lines represent the actual data point per decile with 95% confidence intervals and the blue line represents the lowess (locally weighted scatterplot smoothing) line, which is a smooth line through the scatterplot points
PPCM recovery score
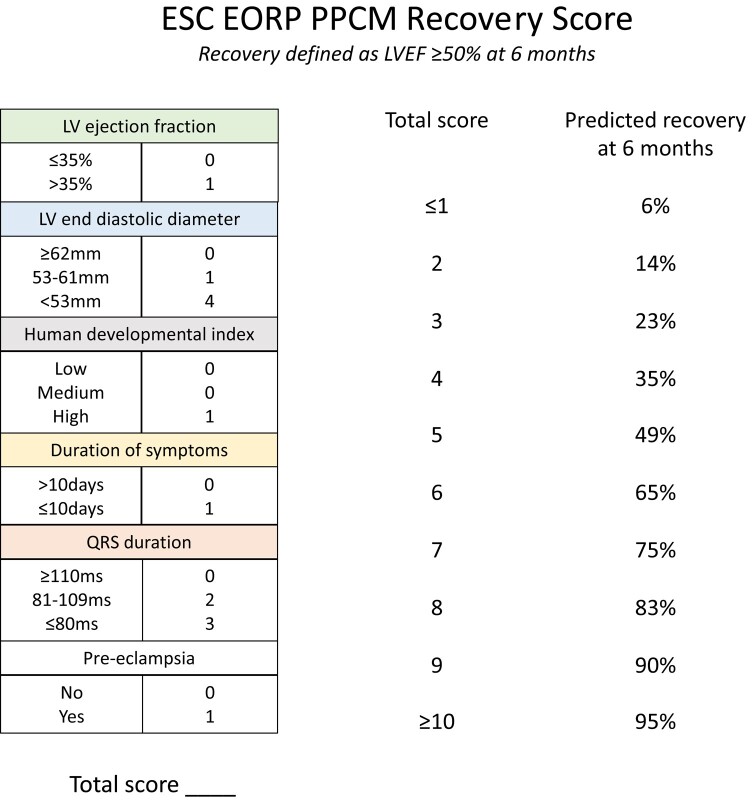
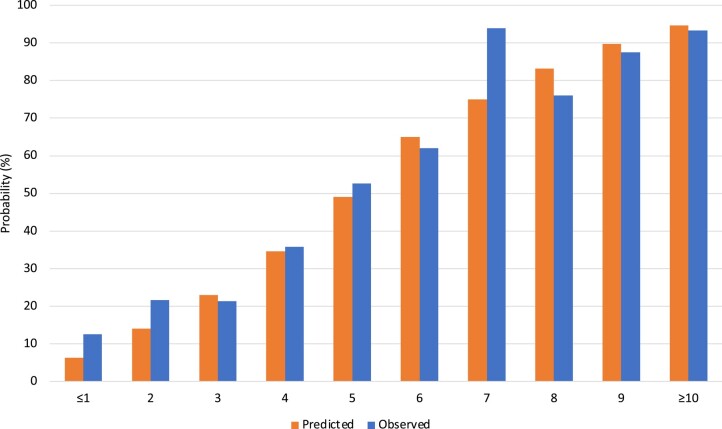
A score was generated to allow calculation of an individual’s predicted probability of LV recovery at 6 months (Figure 2). Variable coefficients were converted to integer points. The highest possible score was 11. Those with a score of 0 or 1 and of 10 or 11 were categorized as ≤1 or ≥10, respectively, due to small numbers with extremes of scores. The predicted probability of LV recovery was estimated for each integer score. The predicted and observed probabilities of LV recovery according to score are shown in Figure 3.
Figure 2.
Integer score for left ventricular recovery and predicted probability of left ventricular recovery for each score. Human development index (HDI) is a summary measure (between zero and one) of a country's social and economic development and can be found at: https://hdr.undp.org/data-center/human-development-index#/indicies/HDI
Figure 3.
Predicted and observed probabilities of left ventricular recovery according to score. The mean predicted probability of recovery for all patients within an integer score group is presented against the proportion of observed recovery within that group
Sensitivity analyses
As a sensitivity analysis, women who died were included and categorized as ‘unrecovered’. Patient characteristics are shown in Supplementary data online, Table S4. Backward selection using the same 20 candidate variables was performed. Results were similar, with the only difference being the removal of pre-eclampsia from the model (n = 384 in final model) (see Supplementary data online, Table S5). This model was well-calibrated (Hosmer–Lemeshow chi2 goodness-of-fit test P = .88) and demonstrated good discriminative ability (C-statistic 0.79, 95% CI 0.75–0.84).
In a further sensitivity analysis, model performance was assessed in a complete dataset using multiple imputation (n = 465 in final model with ×10 imputations). The mean C-statistic for the model across the imputed datasets was 0.79 (95% CI 0.75–0.84) (see Supplementary data online, Table S6).
In order to evaluate different methods of model-building, forward selection and stepwise selection (both forward and backward) were used. Using forward selection, the final variables included in the model were the same, with the exception of pre-eclampsia which was replaced with pregnancy-induced hypertension. The final variables included in the model were unchanged using stepwise selection.
Discussion
A prognostic model for LV recovery in women with PPCM in the ESC EORP PPCM Registry was derived, internally validated and an integer score generated (Structured Graphical Abstract). A small number of studies have investigated factors associated with LV recovery, but this is the first prospective study to systematically assess model calibration and discrimination, and to provide validation. Factors most strongly associated with LV recovery in this global registry were LVEF, LV end diastolic diameter, HDI, duration of symptoms, QRS duration and pre-eclampsia.
Higher LVEF and lower LV end diastolic diameter at baseline have previously been shown to be associated with more frequent LV recovery.6,8,9,13,14 In the IPAC (Investigations of Pregnancy-Associated Cardiomyopathy) study, which prospectively recruited 100 women with PPCM in North America, 86% of women who had recovered by 12 months (defined as LVEF >50%) had a baseline LVEF ≥30%.6 Moreover, LV recovery occurred in 91% of women in the IPAC cohort with a baseline LVEF ≥30% and LV end diastolic diameter <6 cm. Pre-eclampsia has also been shown to be associated with approximately two-times greater a likelihood of LV recovery, even after adjusting for baseline LVEF.15 The reasons for more frequent LV recovery in patients with pre-eclampsia may be related to the different pathophysiological mechanisms through which women with gestational hypertensive disorders develop heart failure, compared to women with a genetic cardiomyopathy for example. This may also, in part, explain why the New York Heart Association (NYHA) functional class was not independently predictive of LV recovery in this cohort, since it has previously been shown that women with PPCM with pre-eclampsia have more severe symptoms (i.e. higher NYHA class) than those without pre-eclampsia but have a higher rate of LV recovery despite this.15
A number of other prognostic factors were identified. First, a longer median time between symptom-onset and diagnosis predicted a lower likelihood of LV recovery. In the ESC EORP PPCM Registry, median delay to diagnosis was 10 days.7 Delay to diagnosis and later presentation of PPCM (e.g. >1 month postpartum) have been shown to be associated with a greater frequency of adverse cardiovascular events and lower rates of LV recovery.16,17 Conversely, the timing of diagnosis (pre- vs. postpartum) was not an independent predictor of LV recovery in the current study. Second, a greater QRS duration predicted a lower likelihood of LV recovery. QRS duration has been shown to be associated with a greater degree of LV dilatation.18 Bundle branch block could reflect a subgroup of people with conduction delay due to specific genetic variants, or with fibrosis affecting the conduction system. Genetic data and data on fibrosis on cardiac magnetic resonance imaging were not available in the ESC EORP PPCM Registry to confirm or refute these hypotheses. In PPCM, women without gestational hypertensive disorders have a higher prevalence of bundle branch block than those with gestational hypertensive disorders, and these women tend to have a phenotype more compatible with a persistent, dilated cardiomyopathy.15 Third, higher HDI predicted a greater likelihood of LV recovery. The ESC EORP PPCM Registry is the only global cohort of women with PPCM and the first to describe socioeconomic factors as determinants of outcomes. In an analysis of country-level (HDI, HE and GINI coefficient) and individual-level (educational attainment and income) factors, low HDI and HE were associated with less frequent recovery of LV function.19 HDI is a summary measure of average achievement in three aspects of human development—life expectancy, education, and standard of living.20 It is a more widely encompassing marker of disparity than factors such as ethnicity, education or income alone and, indeed, was a more robust predictor of LV recovery than ethnicity. Whether or not HDI also represents better access to heart failure treatments is uncertain, but there did not appear to be an association between treatment and LV recovery in the current study. In fact, fewer women with LV recovery than without were on a mineralocorticoid receptor antagonist at 6 months, which suggests that these women had less severe disease at presentation. Only a small number of patients in the ESC EORP PPCM Registry received bromocriptine, which was given a IIb recommendation in the ESC guidelines on cardiac disease in pregnancy in 2018, 6 years after the ESC EORP PPCM Registry began enrolling patients.21 The efficacy of bromocriptine with respect to LV recovery in women with PPCM is currently being studied in a prospective randomized controlled trial.22
Understanding the individualized chance of recovery is important to guide tailored counselling, risk stratification, more timely optimization of medical therapy, referral to specialist services, and to inform decisions about certain treatments such as an ICD.23 Early implantation of an ICD is generally not advised in women with PPCM because of a higher propensity for recovery relative to that seen in patients with other types of cardiomyopathy. In some places, wearable cardioverter defibrillators are used in the early phase.23,24 International guidelines suggest that ICD implantation should be considered in patients who have a LVEF <35% despite optimal medical therapy for at least 3 months.25 In patients with cardiomyopathy, ventricular arrhythmias are more likely to occur with impaired LVEF and this is also true in PPCM.26 Although the majority of women who recover will do so within the first 6 months, it has been shown that recovery can occur beyond this time in women with PPCM.27 Being able to predict more accurately who will go on to normalize LVEF may prevent unnecessary implantation of a primary prevention ICD or, conversely, identify those at greatest risk of persisting LV dysfunction who warrant closer follow-up, more timely optimization of therapies and early involvement of advanced heart failure teams. In the future, comprehensive phenotyping of women with LV dysfunction around the time of pregnancy is required to identify those with specific genetic variants or those with acquired cardiotoxic mechanisms that result in different rates of LV recovery.
Prediction tools are also necessary to provide women with tailored counselling regarding a subsequent pregnancy. These risks are largely based on LVEF prior to the subsequent pregnancy, though all subsequent pregnancies carry a degree of risk.21,28 Discussions about subsequent pregnancies have been underpinned by data from small studies including patients with vastly different demographics. Applicability of these data on a wider scale is limited. Relapse is thought to occur in around 30% of subsequent pregnancies (approximately one in five when there has been recovery of cardiac function following the index pregnancy and just under half without recovery of cardiac function).29–31 All reported deaths associated with a subsequent pregnancy have occurred in women without recovery (equivalent to one in seven women without recovery). While this model concerns prediction of LV recovery after an index presentation of PPCM, and cannot be used to predict outcomes associated with a subsequent pregnancy, more reliable prediction of LV recovery at the time of initial presentation with PPCM will allow counselling with respect to future pregnancies earlier in the patient journey.
Strengths of the model are derivation in a large, global cohort, with inclusion of easily accessible clinical data. Applicability is not limited only to regions where more advanced and costly tests are routinely performed, such as cardiac magnetic resonance imaging or genetic or biomarker testing. The model was internally validated. The model was derived in a cohort receiving contemporary guideline-recommended heart failure therapies; in the ESC EORP PPCM Registry, 85% were prescribed angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, 81% were prescribed beta-blockers, and 45% were prescribed mineralocorticoid receptor antagonists.7 Heart failure therapies were not included at the model-building stage to avoid confounding by indication and to allow the tool to be used at the point of diagnosis, rather than after a period of up-titration of medication. A further strength is the generation of clinical useful score which can be quickly calculated to estimate probability of LV recovery.
Limitations of the study include the inability to include certain variables in model-building, either due to missing data or due to a low prevalence of the characteristic. Validation was done internally using accepted methods, but not externally, due to the lack of other large, prospective cohorts of women with PPCM with similar data capture (e.g. duration of symptoms). The score could not be validated in subgroups of patients with different phenotypes and, although many important variables were included in the model, calibration and precision could vary by phenotype. The model cannot be used to predict LV recovery following a subsequent pregnancy. Generating an integer score from 1–10 is less precise than using original coefficients, but produces a score which is simple and easy to use. Other limitations of the study are shared with many global registries, including lack of genotyping and more detailed cardiac imaging beyond echocardiography, lack of consecutive recruitment, and lack of biomarker measurement in all individuals; these are all difficult to achieve in a registry performed mostly in low GDP countries without payment to sites.
In summary, this model accurately predicts LV recovery at 6 months in women with PPCM. It relies on accessible, readily available data and includes a simple integer score which can be easily applied in clinical practice to predict the probability of LV recovery for an individual at the point of presentation. Better prediction of those who will and will not recover can guide information-giving and tailored counselling, referral to specialist services, more timely optimization of treatments when appropriate, and avoidance of unnecessary treatments when inappropriate.
Supplementary Material
Acknowledgements
The EORP Oversight Committee and the Registry Executive Committee of the EURObservational Research Programme (EORP). Data collection was conducted by the EORP department from the ESC by Rachid Mir Hassaine and Souad Mekhaldi as Clinical Project Managers, Emanuela Fiorucci as Project Officer, Marina Andarala as Data Manager. Overall activities were co-ordinated and supervised by Doctor Aldo P. Maggioni (EORP Scientific Coordinator).
Appendix
Variables included in model building
Age (years)
Body mass index (kg/m2)
Human development index (low, medium, high)
Health expenditure (low, medium, high)
Ethnicity
Pregnancy-induced hypertension
Pre-eclampsia
Family history of heart failure or sudden death
Symptom duration, log transformed (days)
Systolic blood pressure (mmHg)
Heart rate (b.p.m.)
QRS duration (ms)
QTc interval (ms)
Left ventricular ejection fraction (%)
Left ventricular end diastolic diameter (mm)
Right ventricular impairment
Left atrial diameter (mm)
New York Heart Association functional class
Bromocriptine
Timing of diagnosis (pre- vs. postpartum)
Contributor Information
Alice M Jackson, BHF Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, United Kingdom.
Sorel Goland, The Heart Institute, Kaplan Medical Center, Rehovot, Israel; Hadassah Medical School, Hebrew University, Jerusalem.
Hasan Ali Farhan, Iraqi Board for Medical Specializations, Scientific Council of Cardiology, Baghdad Heart Center, Medical City, Baghdad, Iraq.
Israa Fadhil Yaseen, Iraqi Board for Medical Specializations, Scientific Council of Cardiology, Baghdad Heart Center, Medical City, Baghdad, Iraq.
Hawani Sasmaya Prameswari, Department of Cardiology and Vascular Medicine, Hasan Sadikin General Hospital, Bandung, Indonesia.
Michael Böhm, Klinik für Innere Medizin III, Kardiologie, Angiologie und Internistische Intensivmedizin, Universitätsklinikum des Saarlandes, Saarland University, Homburg, Germany.
Pardeep S Jhund, BHF Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, United Kingdom.
Aldo P Maggioni, ANMCO Research Center, Heart Care Foundation, Firenze, Italy.
Peter van der Meer, Department of Cardiology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Karen Sliwa, Faculty of Health Sciences, Department of Medicine and Cardiology University of Cape Town, Cape Heart Institute, Cape Town, South Africa.
Johann Bauersachs, Department of Cardiology and Angiology, Medical School Hannover, Hannover, Germany.
Mark C Petrie, BHF Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, United Kingdom.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
A.M.J., S.G., I.F.Y., H.S.P., K.S., J.B., and M.C.P.: nothing to declare in relation to this manuscript. M.B.: supported by the Deutsche Forschungsgemeinschaft (German Research Foundation; TTR 219, project number 322900939) and reports personal fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Medtronic, Novartis, Servier, and Vifor during the conduct of the study. P.S.J.: reports speakers’ fees from AstraZeneca, Novartis, Alkem Metabolics, ProAdWise Communications, Sun Pharmaceuticals, and Intas Pharma; advisory board fees from AstraZeneca, Boehringer Ingelheim, and Novartis; research funding from AstraZeneca, Boehringer Ingelheim, Analog Devices Inc. P.S.J.’s employer, the University of Glasgow, has been remunerated for clinical trial work from AstraZeneca, Bayer AG, Novartis, and Novo Nordisk. Director, Global Clinical Trial Partners (GCTP). APM: received personal fees from Bayer, AstraZeneca, and Novartis for the participation in study committees, outside the present work. P.v.d.M.: the UMCG which employs P.v.d.M. received consultancy fees and/or grants from Novartis, Pharmacosmos, Vifor Pharma, AstraZeneca, Pfizer, Pharma Nord, BridgeBio, Novo Nordisk, and Ionis.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Funding
Since the start of EORP, the following companies have supported the whole research programme: Abbott Vascular Int. (2011–21), Amgen Cardiovascular (2009–18), AstraZeneca (2014–21), Bayer AG (2009–18), Boehringer Ingelheim (2009–19), Boston Scientific (2009–12), The Bristol Myers Squibb and Pfizer Alliance (2011–19), Daiichi Sankyo Europe GmbH (2011–20), The Alliance Daiichi Sankyo Europe GmbH and Eli Lilly and Company (2014–17), Edwards (2016–19), Gedeon Richter Plc. (2014–16), Menarini Int. Op. (2009–12), MSD-Merck & Co. (2011–14), Novartis Pharma AG (2014–20), ResMed (2014–16), Sanofi (2009–11), SERVIER (2009–21), and Vifor (2019–22). A.M.J. was supported by a British Heart Foundation Clinical Research Training Fellowship (FS/18/14/33330), M.C.P. by a British Heart Foundation Centre of Excellence Research Grant (grant number 18/6/34217), P.v.d.M. by the European Research Council (ERC CoG 101045236, DISSECT-HF), and K.S. by Servier: Institut La Conference Hippocrate.
Ethical Approval
Participating centres managed the approvals of national or regional ethics committees or institutional review boards according to local regulations. Locally appointed ethics committee approved the research protocol and informed consent was obtained from participants or a legally authorized representative.
Pre-registered Clinical Trial Number
None supplied.
References
- 1. Alba AC, Agoritsas T, Jankowski M, Courvoisier D, Walter SD, Guyatt GH, et al. Risk prediction models for mortality in ambulatory patients with heart failure: a systematic review. Circ Heart Fail 2013;6:881–9. 10.1161/CIRCHEARTFAILURE.112.000043 [DOI] [PubMed] [Google Scholar]
- 2. Simpson J, Jhund PS, Lund LH, Padmanabhan S, Claggett BL, Shen L, et al. Prognostic models derived in PARADIGM-HF and validated in ATMOSPHERE and the Swedish heart failure registry to predict mortality and morbidity in chronic heart failure. JAMA Cardiol 2020;5:432–41. 10.1001/jamacardio.2019.5850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Isogai T, Kamiya CA. Worldwide incidence of peripartum cardiomyopathy and overall maternal mortality. Int Heart J 2019;60:503–11. 10.1536/ihj.18-729 [DOI] [PubMed] [Google Scholar]
- 4. Sliwa K, Bauersachs J, Arany Z, Spracklen TF, Hilfiker-Kleiner D. Peripartum cardiomyopathy: from genetics to management. Eur Heart J 2021;42:3094–102. 10.1093/eurheartj/ehab458 [DOI] [PubMed] [Google Scholar]
- 5. Aimo A, Gaggin HK, Barison A, Emdin M, Januzzi JL Jr. Imaging, biomarker, and clinical predictors of cardiac remodeling in heart failure with reduced ejection fraction. JACC Heart Fail 2019;7:782–94. 10.1016/j.jchf.2019.06.004 [DOI] [PubMed] [Google Scholar]
- 6. McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, Ewald G, et al. Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol 2015;66:905–14. 10.1016/j.jacc.2015.06.1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sliwa K, Petrie MC, van der Meer P, Mebazaa A, Hilfiker-Kleiner D, Jackson AM, et al. Clinical presentation, management, and 6-month outcomes in women with peripartum cardiomyopathy: an ESC EORP registry. Eur Heart J 2020;41:3787–97. 10.1093/eurheartj/ehaa455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haghikia A, Podewski E, Libhaber E, Labidi S, Fischer D, Roentgen P, et al. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol 2013;108:366. 10.1007/s00395-013-0366-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goland S, Bitar F, Modi K, Safirstein J, Ro A, Mirocha J, et al. Evaluation of the clinical relevance of baseline left ventricular ejection fraction as a predictor of recovery or persistence of severe dysfunction in women in the United States with peripartum cardiomyopathy. J Card Fail 2011;17:426–30. 10.1016/j.cardfail.2011.01.007 [DOI] [PubMed] [Google Scholar]
- 10. Sliwa K, Hilfiker-Kleiner D, Mebazaa A, Petrie MC, Maggioni AP, Regitz-Zagrosek V, et al. EURObservational Research Programme: a worldwide registry on peripartum cardiomyopathy (PPCM) in conjunction with the Heart Failure Association of the European Society of Cardiology Working Group on PPCM. Eur J Heart Fail 2014;16:583–91. 10.1002/ejhf.68 [DOI] [PubMed] [Google Scholar]
- 11. TRIPOD Checklist. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis. [Internet]. https://www.tripod-statement.org/wp-content/uploads/2020/01/Tripod-Checlist-Prediction-Model-Development.pdf (30 March 2023, date last accessed).
- 12. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 13. Karaye KM, Sa’idu H, Balarabe SA, Ishaq NA, Adamu UG, Mohammed IY, et al. Clinical features and outcomes of peripartum cardiomyopathy in Nigeria. J Am Coll Cardiol 2020;76:2352–64. 10.1016/j.jacc.2020.09.540 [DOI] [PubMed] [Google Scholar]
- 14. Ersbøll AS, Johansen M, Damm P, Rasmussen S, Vejlstrup NG, Gustafsson F. Peripartum cardiomyopathy in Denmark: a retrospective, population-based study of incidence, management and outcome. Eur J Heart Fail 2017;19:1712–20. 10.1002/ejhf.882 [DOI] [PubMed] [Google Scholar]
- 15. Jackson AM, Petrie MC, Frogoudaki A, Laroche C, Gustafsson F, Ibrahim B, et al. Hypertensive disorders in women with peripartum cardiomyopathy: insights from the ESC EORP PPCM registry. Eur J Heart Fail 2021;23:2058–69. 10.1002/ejhf.2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goland S, Modi K, Bitar F, Janmohamed M, Mirocha JM, Czer LSC, et al. Clinical profile and predictors of complications in peripartum cardiomyopathy. J Card Fail 2009;15:645–50. 10.1016/j.cardfail.2009.03.008 [DOI] [PubMed] [Google Scholar]
- 17. Lewey J, Levine LD, Elovitz MA, Irizarry OC, Arany Z. Importance of early diagnosis in peripartum cardiomyopathy. Hypertension 2019;75:91–7. 10.1161/HYPERTENSIONAHA.119.13291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mbakwem AC, Bauersachs J, Viljoen C, Hoevelmann J, van der Meer P, Petrie MC, et al. Electrocardiographic features and their echocardiographic correlates in peripartum cardiomyopathy: results from the ESC EORP PPCM registry. ESC Heart Fail 2021;8:879–89. 10.1002/ehf2.13172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sliwa K, van der Meer P, Viljoen C, Jackson AM, Petrie MC, Mebazaa A, et al. Socio-economic factors determine maternal and neonatal outcomes in women with peripartum cardiomyopathy: a study of the ESC EORP PPCM registry. Int J Cardiol 2023;17:131596. doi: 10.1016/j.ijcard.2023.131596. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 20. Human Development Index Human Development Reports. [Internet]. https://hdr.undp.org/data-center/human-development-index#/indicies/HDI (8 March 2023, date last accessed).
- 21. Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018 Sep 7;39:3165–241. 10.1093/eurheartj/ehy340 [DOI] [PubMed] [Google Scholar]
- 22. ClinicalTrials.gov. Impact of bromocriptine on clinical outcomes for peripartum cardiomyopathy. [Internet]. https://classic.clinicaltrials.gov/ct2/show/NCT05180773 (8 September 2023, date last accessed).
- 23. Bauersachs J, König T, van der Meer P, Petrie MC, Hilfiker-Kleiner D, Mbakwem A, et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail 2019;21:827–43. 10.1002/ejhf.1493 [DOI] [PubMed] [Google Scholar]
- 24. Duncker D, Westenfeld R, Konrad T, Pfeffer T, Correia de Freitas CA, Pfister R, et al. Risk for life-threatening arrhythmia in newly diagnosed peripartum cardiomyopathy with low ejection fraction: a German multi-centre analysis. Clin Res Cardiol 2017;106:582–9. 10.1007/s00392-017-1090-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;2021:3599–726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 26. Hoevelmann J, Sliwa K, Briton O, Ntsekhe M, Chin A, Viljoen C. Effectiveness of implantable loop recorder and Holter electrocardiographic monitoring for the detection of arrhythmias in patients with peripartum cardiomyopathy. Clin Res Cardiol 2023;112:379–91. 10.1007/s00392-022-02101-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Biteker M, İlhan E, Biteker G, Duman D, Bozkurt B. Delayed recovery in peripartum cardiomyopathy: an indication for long-term follow-up and sustained therapy. Eur J Heart Fail 2012;14:895–901. 10.1093/eurjhf/hfs070 [DOI] [PubMed] [Google Scholar]
- 28. Sliwa K, Petrie MC, Hilfiker-Kleiner D, Mebazaa A, Jackson A, Johnson MR, et al. Long-term prognosis, subsequent pregnancy, contraception and overall management of peripartum cardiomyopathy: practical guidance paper from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail 2018;20:951–62. 10.1002/ejhf.1178 [DOI] [PubMed] [Google Scholar]
- 29. Elkayam U, Tummala PP, Rao K, Akhter MW, Karaalp IS, Wani OR, et al. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med 2001;344:1567–71. 10.1056/NEJM200105243442101 [DOI] [PubMed] [Google Scholar]
- 30. Fett JD, Fristoe KL, Welsh SN. Risk of heart failure relapse in subsequent pregnancy among peripartum cardiomyopathy mothers. Int J Gynaecol Obstet 2010;109:34–6. 10.1016/j.ijgo.2009.10.011 [DOI] [PubMed] [Google Scholar]
- 31. Codsi E, Rose CH, Blauwet LA. Subsequent pregnancy outcomes in patients with peripartum cardiomyopathy. Obstet Gynecol 2018;131:322–7. 10.1097/AOG.0000000000002439 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.