Abstract
Background
Manual rotation is commonly performed to increase the chances of normal vaginal delivery and is perceived to be safe. Manual rotation has the potential to prevent operative delivery and caesarean section, and reduce obstetric and neonatal complications.
Objectives
To assess the effect of prophylactic manual rotation for women with malposition in labour on mode of delivery, and maternal and neonatal outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (31 October 2014), the Australian and New Zealand Clinical Trials Registry (ANZCTR), ClinicalTrials.gov, Current Controlled Trials and the WHO International Clinical Trials Registry Platform (ICTRP) (all searched 23 February 2014), previous reviews and, references of retrieved studies.
Selection criteria
Randomised, quasi‐randomised or cluster‐randomised clinical trials comparing prophylactic manual rotation in labour for fetal malposition versus expectant management, augmentation of labour or operative delivery. We defined prophylactic manual rotation as rotation performed without immediate assisted delivery.
Data collection and analysis
Two review authors independently assessed study eligibility and quality, and extracted data.
Main results
We included only one small pilot study (involving 30 women). The study, which we considered to be at low risk of bias, was conducted in a tertiary referral hospital in Australia, and involved women with cephalic, singleton pregnancies. The primary outcome was operative delivery (instrumental delivery or caesarean section).
In the manual rotation group, 13/15 women went on to have an instrumental delivery or caesarean section, whereas in the control group, 12/15 women had an operative delivery. The estimated risk ratio was 1.08 (95% confidence interval 0.79 to 1.49). There were no maternal or fetal mortalities in either group
There were no clear differences for any of the secondary maternal or neonatal outcomes reported (e.g. perineal trauma, analgesia use duration of labour).
In terms of adverse events, there were no reported cases of umbilical cord prolapse or cervical laceration and a single case of a non‐reassuring or pathological cardiotocograph during the procedure.
Authors' conclusions
Currently, there is insufficient evidence to determine the efficacy of prophylactic manual rotation early in the second stage of labour for prevention of operative delivery. One additional study is ongoing. Further appropriately designed trials are required to determine the efficacy of manual rotation.
Keywords: Adult; Female; Humans; Pregnancy; Labor Presentation; Analgesia, Obstetrical; Cesarean Section; Cesarean Section/statistics & numerical data; Extraction, Obstetrical; Extraction, Obstetrical/statistics & numerical data; Obstetric Labor Complications; Obstetric Labor Complications/therapy; Perineum; Perineum/injuries; Pilot Projects; Version, Fetal; Version, Fetal/methods
Plain language summary
Prophylactic manual rotation for fetal malposition to reduce operative delivery and reduce complications for mothers and babies
Background
Most babies are in a position where the baby is looking backwards (an anterior position for the back of the head) before and during delivery. When the baby is looking forwards (in the posterior position) or sideways (the transverse position), the baby's head has a wider profile in the birth canal so descent may be more difficult. The posterior and transverse positions are associated with longer labour, more painful labour, the need for epidural pain relief, higher rates of vaginal tears that sometimes includes the anus and rectum, bleeding after birth and infection in the uterus after the birth. It is also more common for the woman to have a forceps, vacuum or caesarean birth, in some instances in the late first or early second stage of labour, and usually when the mother's cervix is fully dilated.
Manual rotation may be performed to turn the baby's head to the anterior position. Manual rotation entails the use of the accoucheur's hand or fingers to rotate the baby's head. It may take two or three contractions to be performed and the position is commonly held for two contractions.
Study characteristics
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register and other databases for clinical trials comparing manual rotation with expectant management (waiting), speeding up of labour or operation. The women were at term (at least 37 weeks of pregnancy). The results are current to October 2014.
Key results
We found only one small pilot trial involving 30 women that assessed the feasibility of manual rotation versus routine care (no manual rotation). The study reported no clear difference for the review's primary outcomes of operative delivery (vacuum‐assisted delivery or forceps delivery (or both), or caesarean section) and no mother or baby deaths. In terms of adverse events, there were no reported cases of umbilical cord prolapse or cervical laceration and a single case of a non‐reassuring or pathological cardiotocograph during the procedure.
Further research is needed to assess the effect of manual rotation in second stage of labour to reduce operative delivery rates.
Quality of the evidence
We considered the study at low risk of bias but it did not have sufficient participants to detect important clinical benefits or harms of manual rotation to correct the baby's position in second stage of labour.
Background
Description of the condition
Fetal malposition in labour refers to any position other than occiput anterior (OA) in a fetus with a vertex presentation. Persistent occiput posterior (OP) position is the most common fetal malposition, with reported prevalence of 15% to 32% at the onset of labour (Cheng 2006a; Sherer 2002), and 5% to 8% at delivery (Lieberman 2005; Ponkey 2003). Occiput transverse (OT) position has a reported prevalence of 19% to 37% at the onset of labour and 3% to 16% at delivery (Akmal 2004; Souka 2003).
Obstetric associations with persistent OP position include more frequent induction and augmentation of labour, prolonged first and second stage of labour and pathological cardiotocograph (Cheng 2006a; Senecal 2005). There are increased rates of chorioamnionitis, postpartum haemorrhage, third‐ and fourth‐degree perineal tears, wound infection and endometritis (Benavides 2005; Souka 2003; Wu 2005). Less information is available regarding the potential complications of OT position (To 2000). However, there is evidence that this position is associated with increased use of oxytocin during labour and increased rates of third‐ and fourth‐degree perineal tears (Senecal 2005).
Adverse neonatal outcomes associated with the OP position include lower five‐minute Apgar scores, meconium‐stained liquor, cord blood acidaemia, birth trauma and admission to the neonatal intensive care unit (Cheng 2006b; Ponkey 2003). There is some evidence that the OT position is associated with lower five‐minute Apgar scores, birth trauma and admission to the neonatal intensive care unit (Senecal 2005).
OP position during labour is associated with higher rates of operative vaginal birth, failed attempts of vacuum‐assisted and forceps delivery, and caesarean section (De la Torre 2006; Ponkey 2003). While 5% of babies born vaginally are OP, this position has been reported present in 19% of babies born by emergency caesarean (Akmal 2004). Persistent OP position is more common in nulliparous women (Fitzpatrick 2001; Ponkey 2003), and only one‐third of nulliparous women with OP position achieve vaginal birth (Akmal 2004). Failure of rotation of the fetal occiput from the OT position or 'deep transverse arrest' is also associated with operative birth (Pearl 1993).
Operative delivery is associated with significant maternal morbidity. Over the last decades, caesarean delivery rates have been rising in many countries. There has been a 50% increase in the caesarean rate in Australia in the 10 years up to 2008 (Laws 2010), and in France, the rate increased from 11% in 1981 to 20% in 2003 (Deneux‐Tharaux 2006). Caesarean is a major contributing factor to maternal mortality and morbidity following childbirth in high‐income countries (Hall 1999; Minkoff 2003; Schuitemaker 1997), and low‐income countries (Lumbiganon 2010). Although overall risks are small, mortality associated with elective caesarean is higher than for vaginal birth (Cooper 2002; Hall 1999). The presence of a uterine scar puts future pregnancies at increased risk of complications, including ectopic pregnancy in the caesarean scar, placenta praevia and a morbidly adherent placenta leading to caesarean‐hysterectomy. There is an increased risk of uterine rupture in subsequent labours, which can lead to fetal or maternal death, or both (Dasche 2002; Gilliam 2002; Lyndon‐Rochelle 2001; Minkoff 2003). The complications of ventouse delivery include life‐threatening neonatal subgaleal haemorrhage, scalp lacerations, cephalohaematomas, retinal haemorrhages and neonatal jaundice (ACOG 2001).
Current trends in the management of OP and OT position in labour may be expectant, or involve manual rotation, instrumental delivery (with or without manual rotation) or caesarean section. Potential strategies for management of these positions include: active management of labour (O'Driscoll 1984); maternal positioning, such as hands and knees position in second stage of labour (Hunter 2007); expectant management; instrumental delivery (with or without manual rotation) or caesarean delivery; or prophylactic manual rotation alone. The evidence in relation to operative delivery for these alternative methods is inconclusive, unknown or increases operative delivery (Fitzpatrick 2001; Hunter 2007; Simkin 2010). Preliminary studies indicate prophylactic manual rotation may reduce operative delivery (ACOG 2001; Shaffer 2011; Simkin 2010).
Prophylactic manual rotation is performed by a minority of obstetricians and midwives in Australia and New Zealand yet is considered acceptable by the majority (Phipps 2012; Phipps 2014). However, obstetricians and midwives would perform a manual rotation if there was evidence it reduced the risk of operative delivery to 50% or less suggesting that demonstration of efficacy will translate into clinical practice (Phipps 2012; Phipps 2014).
Description of the intervention
Prophylactic manual rotation is defined as rotation performed without immediate assisted delivery. It is commonly performed in late first or early second stage of labour (Le Ray 2007; Shaffer 2011). Manual rotation entails the use of the accoucheur's hand or fingers to rotate the fetal head from the malpresenting (OP or OT) position to the usual OA position. It is most commonly performed at full dilatation after the malposition is diagnosed and is performed with an empty bladder and ruptured membranes. There appears to be variations in the practice of manual rotation (Cargill 2004). Two primary techniques have been described.
Constant pressure being exerted with the tips of the fingers against the lambdoid suture to rotate fetal head into the OA position.
The whole hand is introduced into the birth canal. The head is then rotated after positioning the fingers and thumb under the lateral posterior parietal bone and the anterior parietal bone.
For either technique, the occiput is rotated clockwise (using the left hand) or anticlockwise (using the right hand). Manual rotation may take two or three contractions to be performed and the position is commonly held for two contractions while the woman bears down to reduce the risk of the fetus reverting to the OP position (Cunningham 1997; Tarnier 1982).
How the intervention might work
The procedure of manual rotation in the management of OP position in labour could effectively reduce the rate of operative delivery by correcting the fetal malpresentation and allowing for normal descent and delivery of the fetus. Complications of this procedure appear to be uncommon. In settings where operative delivery is not readily available (low‐ and middle‐income countries), this intervention may reduce maternal and neonatal morbidity and mortality.
Why it is important to do this review
Manual rotation is commonly performed to increase the chances of normal vaginal delivery and is perceived to be safe. It is usually performed late in the second stage of labour, prior to an instrumental delivery. Manual rotation has the potential to prevent operative delivery and caesarean section, and reduce obstetric and neonatal complications.
Objectives
To assess the effect of prophylactic manual rotation for women with malposition in labour on mode of delivery, and maternal and neonatal outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included all published and non‐published randomised controlled trials (RCTs), quasi‐RCTs and cluster RCTs. We included studies with abstracts only.
Types of participants
Women at term planning a vaginal birth with a cephalic singleton fetal malposition in labour. Malpositions were cephalic presentations other than direct OA.
Types of interventions
Prophylactic manual rotation in labour for fetal malposition versus expectant management, augmentation of labour or operative delivery.
Prophylactic manual rotation was defined as rotation performed without immediate assisted delivery.
Types of outcome measures
We assessed all outcomes after treatment allocation.
Primary outcomes
Operative delivery (forceps or vacuum delivery or caesarean section).
Maternal mortality.
Perinatal mortality (stillbirth and neonatal death).
Secondary outcomes
Maternal
Caesarean section.
Forceps delivery.
Vacuum‐assisted delivery.
Third‐ or fourth‐degree perineal trauma.
Analgesia (nitrous oxide, opiate, regional).
Duration of the first and second stages of labour.
Blood loss (mL) measured or estimated at time of birth.
Primary postpartum haemorrhage of 500 mL or greater (clinically estimated or measured at the time of birth and within 24 hours of birth).
Secondary postpartum haemorrhage of 500 mL or greater (clinically estimated or measured after 24 hours and before six weeks after birth).
Maternal blood transfusion.
Postnatal infection.
Duration of hospital stay.
Postpartum re‐hospitalisation.
Other adverse events [not prespecified in protocol]
Longer‐term maternal outcomes
Negative experience of childbirth (as defined by the trial authors).
Postnatal depression (positive depression screen ‐ e.g. Edinburgh Postnatal Depression Scale (EPDS) greater than 12; or clinical diagnosis or treatment).
Breastfeeding failure (not exclusively breastfed; or not breastfeeding on discharge from hospital).
Relationship with baby (as defined by trial authors).
Perineal pain/dyspareunia.
Abdominal pain.
Backache reported six weeks postnatally.
Prolapse or urinary incontinence/faecal incontinence/fistulae.
Subsequent pregnancy complications.
Neonatal and infant outcomes
Non‐reassuring or pathological cardiotocograph during procedure [not prespecified in protocol]
Non‐reassuring or pathological cardiotocograph in first or second stage of labour.
Cord blood gas acidosis (e.g. pH less than 7.1 or base excess (BE) greater than ‐12 or lactate greater than 8 mmol/L).
Admission to neonatal intensive care unit.
Neonatal resuscitation (positive‐pressure ventilation, cardiac compression or drug therapy).
Mechanical ventilation (intermittent positive‐pressure ventilation (IPPV)/continuous positive airways pressure after resuscitation).
Neonatal jaundice treated with phototherapy.
Exchange transfusion.
Polycythaemia treated with partial volume exchange transfusion.
Neonatal stroke.
Intracranial bleed.
Fracture.
Scalp haematoma (e.g. cephalohaematoma or subgaleal haemorrhage).
Encephalopathy.
Neuropraxia.
Duration of stay at neonatal intensive care unit.
Duration of hospital stay.
Severe neurodevelopmental disability in infants (assessed at 12 months of age or older) defined as any one or combination of the following: non‐ambulant cerebral palsy, severe developmental delay assessed using validated tools, auditory and visual impairment.
Search methods for identification of studies
We based the following methods section of this review on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 October 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched the Australian and New Zealand Clinical Trials Registry (ANZCTR ), ClinicalTrials.gov, Current Controlled Trials and the WHO International Clinical Trials Registry Platform (ICTRP) (23 February 2014) using the search terms detailed in Appendix 1.
Searching other resources
We searched the reference list of reviews and other retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
This review included a single study.
Selection of studies
Two review authors (David Osborn and Bradley de Vries) independently assessed all the potential studies identified as a result of the search strategy for inclusion. We resolved any differences of opinion by discussion.
Data extraction and management
Two review authors extracted data from an unpublished draft of the included study (Graham 2014). We resolved discrepancies through discussion. We entered data into Review Manager (RevMan 2012) and checked them for accuracy.
When information regarding any of the above was unclear, we contacted the authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Sequence generation (checking for possible selection bias)
For each included study, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study, we described the method used to conceal the allocation sequence and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (e.g. open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study, we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low risk of bias for participants;
high risk of bias for participants;
unclear risk of bias for participants;
low risk of bias for personnel;
high risk of bias for personnel;
unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study, we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low risk of bias;
high risk of bias;
unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study and for each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total number of randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, we re‐included missing data in the analyses that we undertook.
We assessed methods as:
low risk of bias (e.g. less than 20% missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. 20% or more missing outcome data; numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study's pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study did not include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included study, we described any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there was risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to 1. to 6. above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses (see Sensitivity analysis).
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
For continuous data, we used the mean difference (MD) with 95% CI if outcomes were measured in the same way between trials. We used the standardised mean difference (SMD) with 95% CI to combine trials that measured the same outcome, but use different methods.
Unit of analysis issues
The unit of analysis was the individual woman and infant.
Cross‐over trials were not appropriate for addressing this review topic.
Cluster‐randomised trials
We did not identify any cluster‐randomised trial for inclusion. If, in future updates, we identify any cluster‐randomised trials, we will include them in the analyses, along with individually randomised trials. We will adjust their sample sizes or standard errors using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually randomised trials, we will synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Dealing with missing data
For the one included study, we noted levels of attrition.
In future updates, as more data become available, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analyses.
We will carry out analyses for all outcomes, as far as possible, on an intention‐to‐treat basis, that is, we will attempt to include all participants randomised to each group in the analyses, and analyse all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
In future updates of this review, we will assess statistical heterogeneity in each meta‐analysis using the Tau2, I2 and Chi2 statistics. We will regard heterogeneity as substantial if the I2 statistic is greater than 30% and either Tau2 is greater than zero, or there is a low P value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using Review Manager 5 (RevMan 2012). This review contained a single included study so it was not possible to combine data. In future updates, if we have sufficient data to perform meta‐analyses, we will use fixed‐effect meta‐analyses for combining data where it is reasonable to assume that studies were estimating the same underlying treatment effect (i.e. where trials were examining the same intervention, and the trials' population and we judged the methods sufficiently similar). If there is clinical heterogeneity sufficient to expect that the underlying treatment effect differs between trials, or if we detect substantial statistical heterogeneity, we will use random‐effects meta‐analyses to produce an overall summary if we consider a mean treatment effect across trials clinically meaningful. We will treat the random‐effects summary as the mean range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the mean treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, we will present the results as the mean treatment effect with its 95% CI, and the estimates of the Tau2 and I2 statistics.
Subgroup analysis and investigation of heterogeneity
We were unable to carry out planned subgroup analysis due to insufficient data. However, in future updates of this review, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and, if it is, use random‐effects analyses to produce it.
We will perform the following subgroup analyses.
OP versus OT position.
Nulliparous versus multiparous.
Epidural versus no epidural in labour.
Digital (fingers) versus whole‐hand rotation.
Term versus preterm.
Full dilatation versus less than full dilatation.
We will include the following outcomes in subgroup analysis.
Maternal mortality.
Perinatal mortality (stillbirth and neonatal deaths).
Severe neurodevelopmental disability in infants (assessed at 12 months of age or older) defined as any one or combination of the following: non‐ambulant cerebral palsy, severe developmental delay assessed using validated tools, and auditory and visual impairment.
Operative delivery (forceps or vacuum or caesarean delivery).
Caesarean section.
We will assess subgroup differences by interaction tests available within Review Manager 5 (RevMan 2012). We will report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I2 statistic.
Sensitivity analysis
In future updates, where sufficient data are available, we will explore methodological heterogeneity using sensitivity analyses. We will perform sensitivity analyses through excluding trials of lower quality, based on a lack of any of the following: allocation concealment, adequate randomisation, blinding of treatment and less than 10% loss to follow‐up. We will include the following outcomes in this sensitivity analysis.
Maternal mortality.
Perinatal mortality (stillbirth and neonatal deaths).
Severe neurodevelopmental disability in infants (assessed at 12 months of age or older) defined as any one or combination of the following: non‐ambulant cerebral palsy, severe developmental delay assessed using validated tools, and auditory and visual impairment.
Operative delivery (forceps or vacuum or caesarean delivery).
Caesarean section.
Results
Description of studies
Results of the search
See: Figure 1.
1.
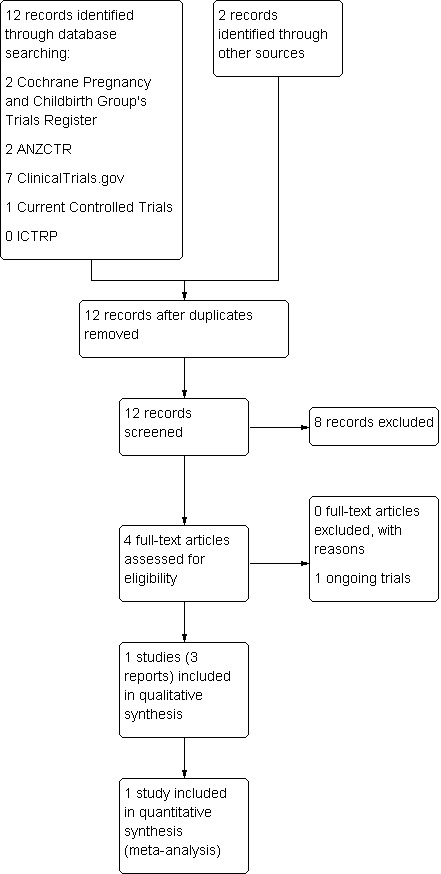
Study flow diagram.
The search retrieved one pilot study (Graham 2014, published in three reports) that met the inclusion criteria and one ongoing study (Phipps 2013 ‐ see Characteristics of ongoing studies).
Included studies
Types of participant
The included study enrolled 30 women with cephalic, singleton pregnancies from a tertiary referral hospital in Australia with 15 women in each arm of the trial (intervention and control) (Graham 2014).
Types of interventions
The study compared manual rotation with a sham (pretend) manual rotation (Graham 2014). Manual rotation used the digital technique, which involved the operator's fingers being placed along the lambdoid sutures and rotating the posterior fontanelle toward the pubic symphysis during contractions with maternal expulsive efforts over three contractions. The fetal occiput was then held in the OA position for a further one or two contractions. The procedure was reported as successful on ultrasound in nine of 15 (60%) women. The sham (pretend) manual rotation involved a vaginal examination over four or five contractions during which the attending midwife was asked not to observe.
Types of outcome measures
The primary outcomes of the study were operative delivery (defined as caesarean, forceps or vacuum delivery), and the feasibility of running a large trial (Graham 2014). Secondary outcomes were duration of the second stage of labour, the time between the intervention and delivery, the degree of perineal trauma, the incidence of postpartum haemorrhage (estimated blood loss greater than 500 mL), cervical laceration, cord prolapse, admission to the neonatal intensive care unit, perinatal mortality and serious neonatal morbidity. Neonatal outcomes included birthweight, sex of the infant, five‐minute Apgar score, cord umbilical artery lactate at delivery, neonatal trauma and admissions to the neonatal intensive care unit.
The study investigator used a repeat mobile transabdominal ultrasound, blinded to the treating clinicians, to confirm and record the final position of the fetal occiput. Digital rotation was considered to be successful if the fetal occiput was OA and within 45 degrees of the midline.
Excluded studies
There were no excluded studies.
Risk of bias in included studies
Overall, we assessed the one included study as having a low risk of bias (Graham 2014; see Characteristics of included studies table).
Allocation
The included study reported using computer‐generated random sized blocks of four, six or eight in opaque, sequentially numbered, sealed envelopes and was stratified by parity (nulliparous or multiparous) (Graham 2014). Investigators were blinded to randomisation group until after enrolment. We assessed the methods used as having a low risk of bias.
Blinding
The included study used a sham (pretend) manual rotation that involved a vaginal examination over four or five contractions during which the attending midwife was asked not to observe (Graham 2014). However, it was unclear how effective the blinding was to the midwives (author communication). We assessed the method as having an unclear risk of bias. Data collection was performed by a researcher who was blinded to the intervention allocation.
Incomplete outcome data
There were no losses to follow‐up in the included study (Graham 2014). We assessed this as a low risk of bias.
Selective reporting
The included study reported the prespecified outcomes (Graham 2014). This study appeared to be free of selective reporting (low risk of bias).
Other potential sources of bias
There were no statistically significant baseline imbalances in the included study (Graham 2014). There was a potentially clinically important difference in baseline incidence (pre‐randomisation) of meconium‐stained liquor between groups (manual rotation 40% versus sham 7%; P value = 0.08). We assessed the study as having an unclear risk of bias for this domain.
Effects of interventions
The results of this review were based on one study (involving 30 women), which compared manual rotation versus no manual rotation (Graham 2014).
Manual rotation versus no manual rotation
Primary outcomes
Operative delivery (forceps or vacuum delivery or caesarean section)
There was no difference in the rates of operative delivery between the manual rotation and the no manual rotation groups (RR 1.08; 95% CI 0.79 to 1.49) (Analysis 1.1).
1.1. Analysis.
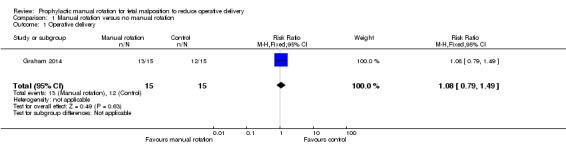
Comparison 1 Manual rotation versus no manual rotation, Outcome 1 Operative delivery.
Maternal mortality
For maternal mortality, there were no events in either the manual rotation or no manual rotation group (Analysis 1.2).
1.2. Analysis.
Comparison 1 Manual rotation versus no manual rotation, Outcome 2 Maternal mortality.
Perinatal mortality (stillbirth and neonatal death)
For perinatal mortality, there were no events in either the manual rotation or no manual rotation group (Analysis 1.3).
1.3. Analysis.
Comparison 1 Manual rotation versus no manual rotation, Outcome 3 Perinatal mortality.
Maternal secondary outcomes
Caesarean section
There was no clear difference between the manual rotation and no manual rotation groups in terms of the numbers of women who had a caesarean section (RR 1.33; 95% CI 0.36 to 4.97) (Analysis 1.4).
1.4. Analysis.
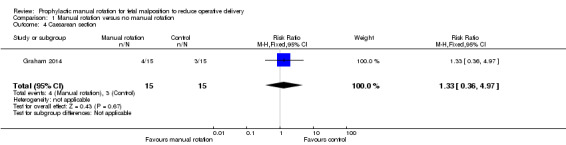
Comparison 1 Manual rotation versus no manual rotation, Outcome 4 Caesarean section.
Forceps delivery
There was no difference between the manual rotation and no manual rotation groups in the numbers of women who had forceps delivery (RR 0.75; 95% CI 0.20 to 2.79) (Analysis 1.5).
1.5. Analysis.
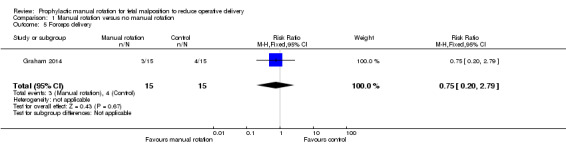
Comparison 1 Manual rotation versus no manual rotation, Outcome 5 Forceps delivery.
Vacuum‐assisted delivery
There was no difference between the manual rotation and no manual rotation groups in vacuum‐assisted deliveries (RR 1.20; 95% CI 0.47 to 3.09) (Analysis 1.6).
1.6. Analysis.
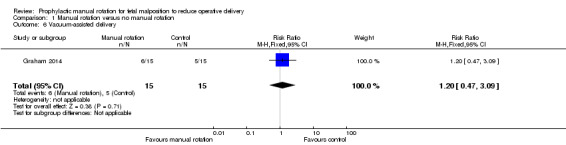
Comparison 1 Manual rotation versus no manual rotation, Outcome 6 Vacuum‐assisted delivery.
Third‐ or fourth‐degree perineal trauma
There was no difference between the manual rotation and no manual rotation groups in the incidence of third‐ or fourth‐degree perineal trauma (RR 0.20; 95% CI 0.01 to 3.85) (Analysis 1.7).
1.7. Analysis.
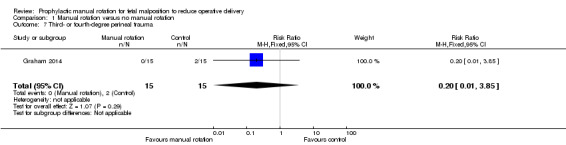
Comparison 1 Manual rotation versus no manual rotation, Outcome 7 Third‐ or fourth‐degree perineal trauma.
Analgesia in labour
Nitrous oxide analgesia in labour
There was no difference between the manual rotation and no manual rotation groups in the use of nitrous oxide analgesia (RR 0.73, 95% CI 0.41 to 1.28) (Analysis 1.8).
1.8. Analysis.
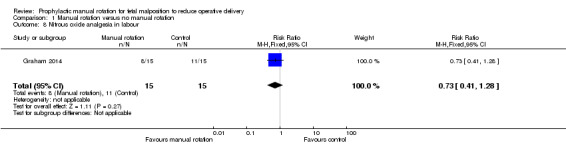
Comparison 1 Manual rotation versus no manual rotation, Outcome 8 Nitrous oxide analgesia in labour.
Opiate analgesia in labour
There was no significant difference between the manual rotation and no manual rotation groups in the use of opiate analgesia (RR 1.00; 95% CI 0.24 to 4.18) (Analysis 1.9).
1.9. Analysis.
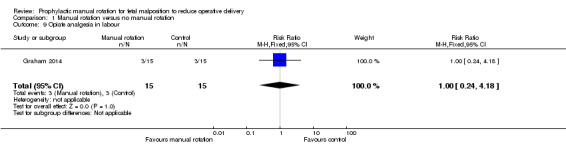
Comparison 1 Manual rotation versus no manual rotation, Outcome 9 Opiate analgesia in labour.
Epidural analgesia in labour
There was no difference between the manual rotation and no manual rotation groups in the use of epidural analgesia in labour (RR 1.17; 95% CI 0.88 to 1.55) (Analysis 1.10).
1.10. Analysis.
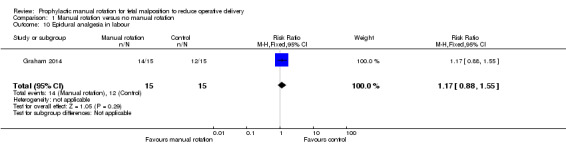
Comparison 1 Manual rotation versus no manual rotation, Outcome 10 Epidural analgesia in labour.
Duration of the first and second stage of labour
Duration of the first stage of labour
The included study did not report the duration of first stage of labour.
Duration of second stage of labour
There was no difference between the manual rotation and no manual rotation groups in the duration of second stage of labour (MD ‐5.70; 95% CI ‐58.34 to 46.94) (Analysis 1.11).
1.11. Analysis.
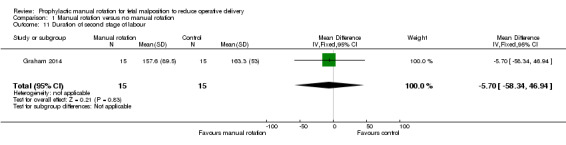
Comparison 1 Manual rotation versus no manual rotation, Outcome 11 Duration of second stage of labour.
Blood loss measured or estimated at time of birth
There was no difference between the manual rotation and no manual rotation groups in blood loss (MD 109.30 mL; 95% CI ‐78.51 to 297.11) (Analysis 1.12).
1.12. Analysis.
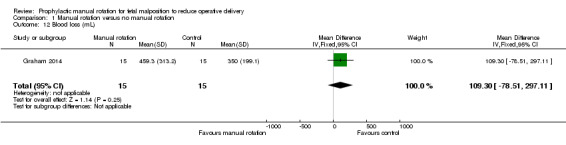
Comparison 1 Manual rotation versus no manual rotation, Outcome 12 Blood loss (mL).
Primary postpartum haemorrhage 500 mL or greater (clinically estimated or measured at the time of birth and within 24 hours of birth)
There was no difference between the manual rotation and no manual rotation groups in the incidence of primary postpartum haemorrhage of 500 mL or greater (RR 1.00; 95% CI 0.24 to 4.18) (Analysis 1.13).
1.13. Analysis.
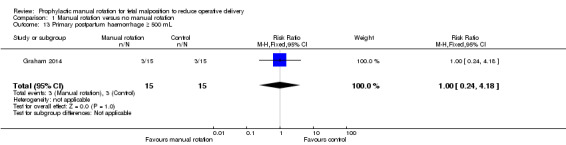
Comparison 1 Manual rotation versus no manual rotation, Outcome 13 Primary postpartum haemorrhage ≥ 500 mL.
Secondary postpartum haemorrhage 500 mL or greater (clinically estimated or measured after 24 hours and before six weeks)
The included study did not report secondary postpartum haemorrhage.
Maternal blood transfusion
There were no maternal blood transfusions (Analysis 1.14).
1.14. Analysis.
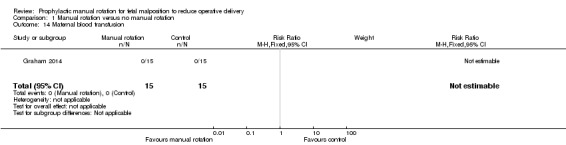
Comparison 1 Manual rotation versus no manual rotation, Outcome 14 Maternal blood transfusion.
Postnatal infection
There were no cases of postnatal infection (Analysis 1.15).
1.15. Analysis.
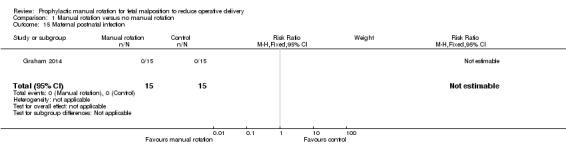
Comparison 1 Manual rotation versus no manual rotation, Outcome 15 Maternal postnatal infection.
Duration of hospital stay
There was no difference between the manual rotation and no manual rotation groups in the length of maternal hospital stay (MD 0.84 days; 95% CI ‐0.04 to 1.72) (Analysis 1.16).
1.16. Analysis.
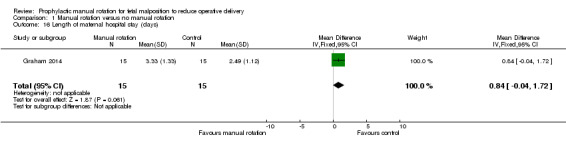
Comparison 1 Manual rotation versus no manual rotation, Outcome 16 Length of maternal hospital stay (days).
Postpartum re‐hospitalisation
The included study did not report postpartum re‐hospitalisation.
Longer‐term maternal outcomes
The included study did not report longer‐term maternal outcomes.
Neonatal and infant outcomes
Non‐reassuring or pathological cardiotocograph during procedure
There was no difference between the manual rotation and no manual rotation groups in non‐reassuring or pathological CTG (RR 3.00; 95% CI 0.13 to 68.26) (Analysis 1.17).
1.17. Analysis.
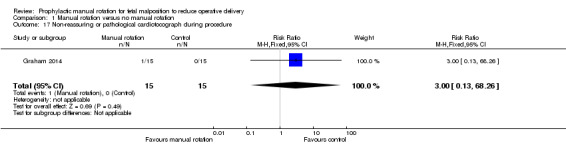
Comparison 1 Manual rotation versus no manual rotation, Outcome 17 Non‐reassuring or pathological cardiotocograph during procedure.
Non‐reassuring or pathological cardiotocograph in first or second stage of labour
There was no difference between the manual rotation and no manual rotation groups in non‐reassuring or pathological CTG at any time after allocation (RR 0.57; 95% CI 0.21 to 1.55) (Analysis 1.18).
1.18. Analysis.
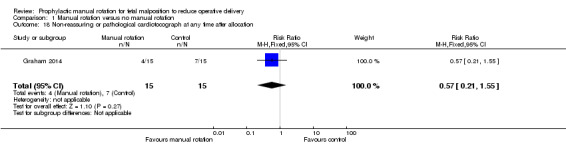
Comparison 1 Manual rotation versus no manual rotation, Outcome 18 Non‐reassuring or pathological cardiotocograph at any time after allocation.
Cord blood gas acidosis (e.g. pH less than 7.1 or base excess greater than ‐12 or lactate greater than 8 mmol/L)
In the Graham 2014 study, 12 participants in the manual rotation group and 11 participants in the no manual rotation group had three cord blood tests (lactate, pH and base excess). Of these, 2/12 had an abnormal result in the manual rotation group and 1/11 had an abnormal result in the no manual rotation group (abnormal result is defined as having a pH less than 7.1, BE less than ‐12 or lactate greater than 8 mmol/L) (RR 1.83; 95% CI 0.19 to 17.51) (Analysis 1.19).
1.19. Analysis.
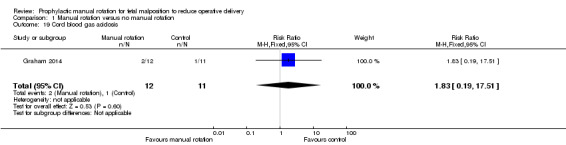
Comparison 1 Manual rotation versus no manual rotation, Outcome 19 Cord blood gas acidosis.
Admission to neonatal intensive care unit
There was no difference between the manual rotation and no manual rotation groups in the number of admissions to the neonatal intensive care unit (RR 2.00; 95% CI 0.61 to 6.55) (Analysis 1.20).
1.20. Analysis.
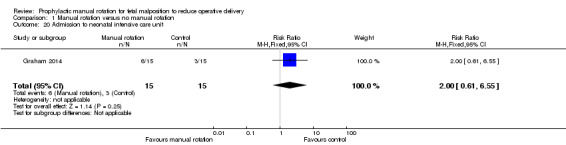
Comparison 1 Manual rotation versus no manual rotation, Outcome 20 Admission to neonatal intensive care unit.
Neonatal resuscitation (positive‐pressure ventilation, cardiac compression or drug therapy)
There was no difference between the manual rotation and no manual rotation groups in number of neonates who required resuscitation (RR 1.00; 95% CI 0.31 to 3.28) (Analysis 1.21).
1.21. Analysis.
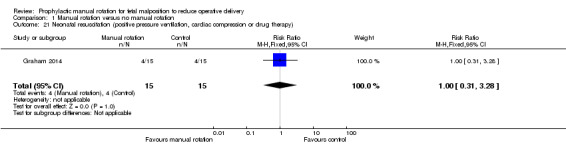
Comparison 1 Manual rotation versus no manual rotation, Outcome 21 Neonatal resuscitation (positive pressure ventilation, cardiac compression or drug therapy).
Mechanical ventilation (intermittent positive‐pressure ventilation/continuous positive airways pressure after resuscitation)
There was no clear difference between the manual rotation and no manual rotation groups in the number of neonates requiring ventilation (RR 3.00; 95% CI 0.35 to 25.68) (Analysis 1.22).
1.22. Analysis.

Comparison 1 Manual rotation versus no manual rotation, Outcome 22 Mechanical ventilation (intermittent positive‐pressure ventilation/continuous positive airways pressure after resuscitation).
Neonatal jaundice treated with phototherapy
There was no difference between the manual rotation and no manual rotation groups in the incidence of neonatal jaundice treated with phototherapy (RR 0.50; 95% CI 0.05 to 4.94) (Analysis 1.23).
1.23. Analysis.
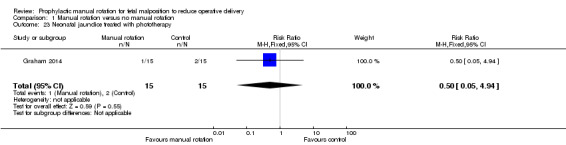
Comparison 1 Manual rotation versus no manual rotation, Outcome 23 Neonatal jaundice treated with phototherapy.
Exchange transfusion
No infant was reported receiving an exchange transfusion (Analysis 1.24).
1.24. Analysis.
Comparison 1 Manual rotation versus no manual rotation, Outcome 24 Neonatal exchange transfusion.
Polycythaemia treated with partial volume exchange transfusion
No infant was reported with polycythaemia treated with partial volume exchange transfusion (Analysis 1.25).
1.25. Analysis.
Comparison 1 Manual rotation versus no manual rotation, Outcome 25 Polycythaemia treated with partial volume exchange transfusion.
Neonatal stroke
No infant was reported as having had a stroke (Analysis 1.26).
1.26. Analysis.
Comparison 1 Manual rotation versus no manual rotation, Outcome 26 Neonatal stroke.
Intracranial bleed
No infant was reported as having had an intracranial bleed (Analysis 1.27).
1.27. Analysis.
Comparison 1 Manual rotation versus no manual rotation, Outcome 27 Neonatal intracranial bleed.
Fracture
No infant was reported as having had a fracture (Analysis 1.28).
1.28. Analysis.
Comparison 1 Manual rotation versus no manual rotation, Outcome 28 Neonatal fracture.
Scalp hematoma (e.g. cephalohaematoma or subgaleal haemorrhage)
One infant in the manual rotation group and no infants in the no manual rotation group had a subgaleal haemorrhage (RR 3.00; 95% CI 0.13 to 68.26) (Analysis 1.29).
1.29. Analysis.
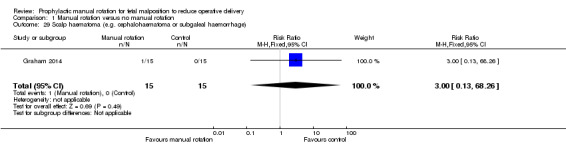
Comparison 1 Manual rotation versus no manual rotation, Outcome 29 Scalp haematoma (e.g. cephalohaematoma or subgaleal haemorrhage).
Encephalopathy
No infant was reported as having had an encephalopathy (Analysis 1.30).
1.30. Analysis.
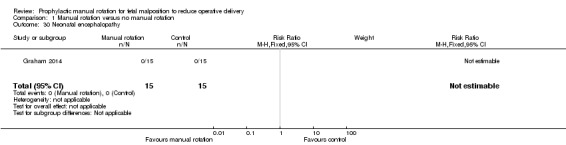
Comparison 1 Manual rotation versus no manual rotation, Outcome 30 Neonatal encephalopathy.
Neuropraxia
No infant was reported as having had neuropraxia (Analysis 1.31).
1.31. Analysis.
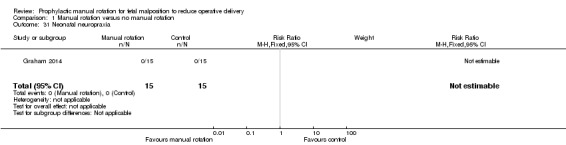
Comparison 1 Manual rotation versus no manual rotation, Outcome 31 Neonatal neuropraxia.
Duration stay at neonatal intensive care unit
The included study did not report duration of stay at neonatal intensive care unit.
Duration of hospital stay
The included study did not report the duration of hospital stay.
Severe neurodevelopmental disability in infants (assessed at 12 months of age or older)
The included study did not report severe neurodevelopmental disability in infants.
Other adverse events
There were no reported cases of umbilical cord prolapse or cervical laceration and a single case of a non‐reassuring or pathological cardiotocograph during the procedure.
Subgroup analyses
The study has insufficient power to perform any of the pre‐specified subgroup analyses (Graham 2014).
Sensitivity analysis
We planned to perform a sensitivity analysis based on the following: allocation concealment, inadequate randomisation, blinding of treatment and less than 10% loss to follow‐up. The study reported adequate randomisation, allocation procedures and no losses (Graham 2014). Blinding of the interventions to participants and clinical staff was reported, although it is unclear how effective the blinding to the midwives was (author communication).
Discussion
Summary of main results
We identified one small pilot study (involving 30 women) in our review. The study enrolled women from a tertiary referral hospital in Australia, with cephalic, singleton pregnancies (Graham 2014). The procedure was reported as successful on ultrasound in 60% of women. No difference was reported for our primary outcomes of operative delivery rate or for any maternal mortality or perinatal mortality. No clear difference was reported for any of the review's secondary outcomes. In terms of adverse events, there were no reported cases of umbilical cord prolapse or cervical laceration and a single case of a non‐reassuring or pathological cardiotocograph during the procedure. One additional study is ongoing (Phipps 2013).
Overall completeness and applicability of evidence
The study is substantially underpowered to detect important clinical benefits and harms of manual rotation for malposition in second stage of labour (Graham 2014). The study suggested that manual rotation is feasible in the context of a clinical trial.
Quality of the evidence
We assessed the included study as being of low risk of bias (Graham 2014).
Potential biases in the review process
We performed an extensive search for published and unpublished literature including searches of trial registries for ongoing studies. Two review authors independently assessed eligibility, study quality and extracted data. We reached agreement through consensus. One of the two review authors was an author on the study included in the review and one review author was independent.
Agreements and disagreements with other studies or reviews
There are no other known published studies or reviews.
Authors' conclusions
Implications for practice.
There was insufficient evidence to determine the efficacy of prophylactic manual rotation early in the second stage of labour for prevention of operative delivery. Currently, there is inadequate evidence to guide clinical practice.
Implications for research.
Appropriately designed, adequately powered randomised controlled trials are required to determine the efficacy and safety of manual rotation for reducing operative delivery and improving maternal and neonatal outcomes in both high‐income and low‐to middle‐income settings. Outcomes reported in future studies should include maternal and perinatal mortality, which are still common in low‐ and middle‐income countries, as well as operative delivery, caesarean delivery, and maternal and neonatal morbidity. Longer‐term outcomes including infant and childhood neurodevelopmental outcomes and maternal pelvic floor outcomes should also be assessed.
Acknowledgements
Frances Kellie and Denise Atherton for administrative assistance.
As part of the pre‐publication editorial process, this review was commented on by two peers (an editor and referee who was external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's statistical adviser.
Appendices
Appendix 1. Search terms
ICTRP Advanced search screen
occipito OR occipito‐posterior OR occiput OR malposition OR position (Title)
labour OR labor OR birth OR childbirth OR delivery OR pregnancy (Condition)
rotate OR rotation OR rotating OR manual (Intervention)
ClinicalTrials.gov – advanced search screen
occipito OR occipito‐posterior OR occiput OR malposition OR position (Search terms)
labour OR labor OR birth OR childbirth OR delivery OR pregnancy (Condition)
rotate OR rotation OR rotating OR manual OR turn OR turning (Intervention)
Current Controlled Trials
fetal AND (rotat* OR malposition OR position)
(labour OR labor) AND (occipito OR occiput OR occipito‐posterior OR malposition)
Australian New Zealand Clinical Trials Registry
fetal position
labour position
labor position
fetal malposition
occiput position
occipito position
(For Current Controlled Trials and Australian New Zealand Clinical Trials Registry, each line was searched separately)
Data and analyses
Comparison 1. Manual rotation versus no manual rotation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Operative delivery | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.79, 1.49] |
| 2 Maternal mortality | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Perinatal mortality | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Caesarean section | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.36, 4.97] |
| 5 Forceps delivery | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.20, 2.79] |
| 6 Vacuum‐assisted delivery | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.47, 3.09] |
| 7 Third‐ or fourth‐degree perineal trauma | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.85] |
| 8 Nitrous oxide analgesia in labour | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.41, 1.28] |
| 9 Opiate analgesia in labour | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.24, 4.18] |
| 10 Epidural analgesia in labour | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.88, 1.55] |
| 11 Duration of second stage of labour | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐5.70 [‐58.34, 46.94] |
| 12 Blood loss (mL) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 109.30 [‐78.51, 297.11] |
| 13 Primary postpartum haemorrhage ≥ 500 mL | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.24, 4.18] |
| 14 Maternal blood transfusion | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Maternal postnatal infection | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Length of maternal hospital stay (days) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.84 [‐0.04, 1.72] |
| 17 Non‐reassuring or pathological cardiotocograph during procedure | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] |
| 18 Non‐reassuring or pathological cardiotocograph at any time after allocation | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.21, 1.55] |
| 19 Cord blood gas acidosis | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.19, 17.51] |
| 20 Admission to neonatal intensive care unit | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.61, 6.55] |
| 21 Neonatal resuscitation (positive pressure ventilation, cardiac compression or drug therapy) | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.31, 3.28] |
| 22 Mechanical ventilation (intermittent positive‐pressure ventilation/continuous positive airways pressure after resuscitation) | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.35, 25.68] |
| 23 Neonatal jaundice treated with phototherapy | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 4.94] |
| 24 Neonatal exchange transfusion | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Polycythaemia treated with partial volume exchange transfusion | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Neonatal stroke | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Neonatal intracranial bleed | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Neonatal fracture | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Scalp haematoma (e.g. cephalohaematoma or subgaleal haemorrhage) | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] |
| 30 Neonatal encephalopathy | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Neonatal neuropraxia | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Graham 2014.
| Methods | Randomised blinded, parallel arm controlled trial. | |
| Participants |
Setting and population: 30 women from a tertiary referral hospital in Australia, with cephalic, singleton pregnancies. 15 women were randomised to each arm (intervention and control). Inclusion criteria: women who had completed 37 weeks of gestation with a cephalic singleton pregnancy, planned a vaginal delivery, had a cervix at full dilatation with an OP position of the fetal head confirmed by a mobile transabdominal ultrasound scan, aged > 16 years old and had given written informed consent. A vaginal examination at full dilatation was performed. A bedside mobile ultrasound scan was performed to confirm fetal head position by the labour ward registrar or consultant. If the position was OP, a study investigator performed either the manual rotation or sham procedure. Fetal OP position was established by obtaining a transverse view of the fetal orbits. OP was defined as fetal occiput within 45 degrees of the midline. At the first urge to push or 1 hour after full dilatation (whichever came first), the study investigator repeated the bedside ultrasound and if the OP position persisted, randomised the participant to either the control or intervention arm of the study. Exclusion criteria: clinical suspicion of cephalo‐pelvic disproportion, a history of previous uterine surgery (caesarean section, open myomectomy), a brow or face presentation, a pathologic CTG according to the RCOG guidelines plus either abnormal baseline or reduced variability for more than 90 minutes, suspected fetal compromise, an anatomical fetal abnormality, suspected or known chorioamnionitis, any condition requiring immediate delivery, any condition requiring an elective caesarean section, an intrapartum haemorrhage > 50 mL, a temperature more than 38.4 °C in the first stage of labour or a suspected fetal bleeding diathesis. |
|
| Interventions |
Intervention: manual rotation using the digital technique, with the operator's fingers placed along the lambdoid sutures and rotating the posterior fontanelle towards the pubic symphysis during contractions with maternal expulsive efforts over 3 contractions. The fetal occiput was then held in the OA position for a further 1 or 2 contractions. Control: sham manual rotation that involved a vaginal examination over 4 or 5 contractions during which the attending midwife was asked not to observe. |
|
| Outcomes |
Primary outcomes: operative delivery (defined as caesarean, forceps or vacuum delivery), and the feasibility of running a large trial. Other outcomes: duration of the second stage of labour, the time between intervention and delivery, degree of perineal trauma, incidence of postpartum haemorrhage (estimated blood loss > 500 mL), cervical laceration, cord prolapse, admission to the NICU, perinatal mortality and serious neonatal morbidity. Neonatal outcomes: birthweight, sex of the infant, 5‐minute Apgar score, cord umbilical artery lactate at delivery, neonatal trauma and admissions to the NICU Study investigator used a repeat mobile transabdominal ultrasound, blinded to the treating clinicians, to confirm and record the final position of the fetal occiput. Digital rotation was considered to be successful if the fetal occiput was OA and within 45 degrees of the midline. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using computer‐generated random sized blocks with variable block sizes of 4, 6 or 8. |
| Allocation concealment (selection bias) | Low risk | Women were consented, enrolled and then randomised. Treatment allocations were placed into opaque, sequentially numbered, sealed envelopes stratified by parity (nulliparous or multiparous). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Women in the control group underwent a sham manual rotation that involved a vaginal examination over 4 or 5 contractions during which the attending midwife was asked not to observe. However, it is unclear how effective the blinding was (author communication). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data collection performed blind to intervention allocation by independent researcher. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses reported. |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes. |
| Other bias | Unclear risk | No statistically significant baseline imbalances. Clinical baseline difference in thick meconium‐stained liquor (manual rotation 40% vs. sham 7%; P value = 0.08). |
CTG: cardiotocograph; NICU: neonatal intensive care unit; OA: occiput anterior; OP: occiput posterior; RCOG: Royal College of Gynaecologists.
Characteristics of ongoing studies [ordered by study ID]
Phipps 2013.
| Trial name or title | Does Manual Rotation of the Occiput Posterior Fetus during the Second Stage of Labour Increase the Likelihood of Vaginal Birth? |
| Methods | Double‐blind randomised controlled trial ‐ pilot study |
| Participants | Women with a singleton term pregnancy with a cephalic presentation and in OP position (on ultrasound) at full dilatation |
| Interventions | Eligible women were randomised to receive either a real or a 'sham' manual rotation by an independent investigator, such that both the woman and the labour ward care providers were blinded to the randomisation result. The procedure was performed either 1 hour after full dilatation was diagnosed or with onset of maternal urge to push, whichever occurred first |
| Outcomes | Primary outcomes: operative delivery (caesarean section, forceps‐assisted vaginal delivery or vacuum extraction) |
| Starting date | December 2010 and December 2011 |
| Contact information | Ms Hala Phipps, Research Midwife, RPA Women and Babies, Royal Prince Alfred Hospital, Camperdown, NSW, Australia Email: hala.phipps@sswahs.nsw.gov.au |
| Notes |
OP: occiput posterior.
Differences between protocol and review
We have updated the methods text for the following sections in accordance with the Cochrane Pregnancy and Childbirth Group's standard methods text.
Assessment of reporting biases.
Subgroup analysis and investigation of heterogeneity.
We have added the following secondary outcomes that were not prespecified on our published protocol (Phipps 2011).
Maternal: Other adverse events.
Neonatal and infant: Non‐reassuring or pathological cardiotocograph during procedure.
Contributions of authors
Hala Phipps, Bradley de Vries, Jon Hyett and David Osborn wrote the protocol and review.
Hala Phipps, Bradley de Vries and Professor David Osborn assessed eligibility, study quality and extracted data.
Hala Phips is the guarantor for the review.
Sources of support
Internal sources
No sources of support supplied
External sources
Australasian Satellite of the Cochrane Neonatal Review Group, Australia.
Australasian Cochrane Centre, Australia.
Declarations of interest
David A Osborn: none known.
Dr Bradley de Vries, Hala Phipps and Clinical Professor Jon Hyett were involved in the designing and conducting of the one study (pilot randomised controlled trial) included in this review (Graham 2014). Brad de Vries, Hala Phipps and Jon Hyett have an NHMRC (National Health and Medical Research Council) grant and are currently performing a randomised controlled trial to assess the efficacy of manual rotation for malposition in the second stage of labour for reducing operative delivery. This randomised controlled trial is potentially eligible for inclusion in this review. All decisions relating to the inclusion of this trial, as well as assessment of risk of bias and data extraction, will include a member of the review team (David Osborn) who is not directly involved in the trials.
New
References
References to studies included in this review
Graham 2014 {published data only}
- Graham K, Phipps H, Hyett JA, Ludlow JP, Mackie A, Marren A, et al. Persistent Occiput‐Posterior: OUTcomes following digital rotation: a pilot randomised controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2014;54(3):268‐74. [DOI] [PubMed] [Google Scholar]
- Graham K, Phipps H, Hyett JA, Ludlow JP, Mackie A, Marren A, et al. Persistent occipito‐posterior position: outcomes following digital rotation. A pilot study. Ultrasound in Obstetrics & Gynecology 2012;40(Suppl 1):241. [Google Scholar]
- Phipps H. Persistent occipito‐posterior: outcomes following digital rotation. The "POPOUT" Study, 2011. Australian New Zealand Clinical Trials Registry (http://www.anzctr.org.au/trial_view.aspx?ACTRN=12609000833268) (accessed 31 August 2011) 2011.
References to ongoing studies
Phipps 2013 {published data only}
- Phipps H. Persistent Occiput Transverse: OUTcomes following manual rotation. Australian New Zealand Clinical Trials Registry (accessed 10 July 2013) 2013.
Additional references
ACOG 2001
- American College of Obstetrics and Gynecology. Operative vaginal delivery. Clinical management guidelines for obstetrician‐gynecologists. International Journal of Gynecology and Obstetrics 2001;74(1):69‐76. [DOI] [PubMed] [Google Scholar]
Akmal 2004
- Akmal S, Kametas N, Tsoi E, Howard R, Nicolaides KH. Ultrasonographic occiput position in early labour in the prediction of caesarean section. BJOG: an international journal of obstetrics and gynaecology 2004;111:532‐6. [DOI] [PubMed] [Google Scholar]
Benavides 2005
- Benavides L, Wu JM, Hundley AF, Invester TS, Viscose AG. The impact of occiput posterior fetal head position on the risk of anal sphincter injury in forceps‐assisted vaginal deliveries. American Journal of Obstetrics and Gynecology 2005;192:1702‐6. [DOI] [PubMed] [Google Scholar]
Cargill 2004
- Cargill YM, MacKinnon CJ, Arsenault M‐Y, Bartellas E, Daniels S, Gleason T. Guidelines for operative vaginal birth. Journal of Obstetrics & Gynaecology Canada 2004;26(4):747‐61. [DOI] [PubMed] [Google Scholar]
Cheng 2006a
- Cheng Y, Shaffer B, Caughey A. The association between persistent occiput posterior position and neonatal outcomes. Obstetrics & Gynecology 2006;107(4):837‐44. [DOI] [PubMed] [Google Scholar]
Cheng 2006b
- Cheng YW, Shaffer BL, Caughey AB. Associated factors and outcomes of persistent occiput posterior position: a retrospective cohort study from 1976 to 2001. Journal of Maternal‐Fetal and Neonatal Medicine 2006;19:563‐8. [DOI] [PubMed] [Google Scholar]
Cooper 2002
- Cooper GM, Lewis G, Neilson J. Confidential enquiries into maternal deaths, 1997‐1999. British Journal of Anaesthesia 2002;89(3):369‐72. [PubMed] [Google Scholar]
Cunningham 1997
- Cunningham FG, MacDonald PC, Gant NF, Levenno KJ, Gistrap III LC, Hankins GD, et al. Williams Obstetrics. 20th Edition. Stamford, Connecticut: Appleton and Lange, 1997:448‐50. [Google Scholar]
Dasche 2002
- Dashe JS, McIntire DD, Ramus RM, Santos‐Ramos R, Twickler DM. Persistence of placenta previa according to gestational age at ultrasound. Obstetrics & Gynecology 2002;99:692‐7. [DOI] [PubMed] [Google Scholar]
De la Torre 2006
- Torre L, Conzales‐Quintero VH, Mayor‐Lynn K, Smarkusky L, Hoffman MC, Saab A, et al. Significance of accidental extensions in the lower uterine segment during caesarean delivery. American Journal of Obstetrics and Gynecology 2006;194:e4‐e6. [DOI] [PubMed] [Google Scholar]
Deneux‐Tharaux 2006
- Deneux‐Tharaux C, Carmona E, Bouvier‐Colle MH, Bréart G. Postpartum maternal mortality and caesarean delivery. Obstetrics & Gynecology 2006;108(3 Pt 1):541‐8. [DOI] [PubMed] [Google Scholar]
Fitzpatrick 2001
- Fitzpatrick M, McQuillan K, O'Herlihy C. Influence of persistent occiput posterior position on delivery outcome. Obstetrics & Gynecology 2001;98:1027‐31. [DOI] [PubMed] [Google Scholar]
Gilliam 2002
- Gilliam M, Rosenberg D, Davis F. The likelihood of placenta previa with greater number of cesarean deliveries and higher parity. Obstetrics & Gynecology 2002;99:976‐80. [DOI] [PubMed] [Google Scholar]
Hall 1999
- Hall M, Bewley S. Maternal mortality and mode of delivery. Lancet 1999;354:776. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hunter 2007
- Hunter S, Hofmeyr GJ, Kulier R. Hands and knees posture in late pregnancy or labour for fetal malposition (lateral or posterior). Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD001063.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laws 2010
- Laws PJ, Li Z, Sullivan EA. Australia's Mothers and Babies 2008. Perinatal Statistics Series no. 24. Cat. no. PER 50. Canberra: Australian Institute of Health and Welfare, 2010. [Google Scholar]
Le Ray 2007
- Ray C, Serres P, Schmitz T, Cabrol D, Goffinet F. Manual rotation in occiput posterior or transverse positions. Obstetrics & Gynecology 2007;110(4):873‐9. [DOI] [PubMed] [Google Scholar]
Lieberman 2005
- Lieberman E, Davidson K, Lee‐Parritz A, Shearer E. Changes in fetal position during labor and their association with epidural analgesia. Obstetrics & Gynecology 2005;105:974‐82. [DOI] [PubMed] [Google Scholar]
Lumbiganon 2010
- Lumbiganon P, Laopaiboon M, Gulmezoglu AM, Souza JP, Taneepanichskul S, Ruyan P, et al. Method of delivery and pregnancy outcomes in Asia: the WHO global survey on maternal and perinatal health 2007‐08. Lancet 2010;375:490‐9. [DOI] [PubMed] [Google Scholar]
Lyndon‐Rochelle 2001
- Lyndon‐Rochelle M, Holt VL, Easterling TR, Martin DP. Risk of uterine rupture during labor among women with a prior cesarean delivery. New England Journal of Medicine 2001;345:3‐8. [DOI] [PubMed] [Google Scholar]
Minkoff 2003
- Minkoff H, Chervenak FA. Elective primary cesarean delivery. New England Journal of Medicine 2003;348:946‐50. [DOI] [PubMed] [Google Scholar]
O'Driscoll 1984
- O'Driscoll K, M Foley, MacDonald D. Active management of labor as an alternative to cesarean section for dystocia. Obstetrics & Gynecology 1984;63(4):485‐90. [PubMed] [Google Scholar]
Pearl 1993
- Pearl ML, Roberts JM, Laros RK, Hurd WW. Vaginal delivery from the persistent occiput posterior position. Influence on maternal and neonatal morbidity. Journal of Reproductive Medicine 1993;38:955‐61. [PubMed] [Google Scholar]
Phipps 2011
- Phipps H, Vries B, Hyett J, Osborn DA. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD009298] [DOI] [PMC free article] [PubMed] [Google Scholar]
Phipps 2012
- Phipps H, Vries B, Lee PN, Hyett J. A Survey of obstetric practices in the management of occipito‐posterior position in labour and delivery among obstetricians in Australia and New Zealand. Australian and New Zealand Journal of Obstetrics and Gynaecology 2012;52:450‐54. [DOI] [PubMed] [Google Scholar]
Phipps 2014
- Phipps H, Vries B, Jagadish U, Hyett J. Management of occiput posterior position in the second stage of labour: a survey of midwifery practice in Australia. Birth 2014;41(1):64‐69. [DOI] [PubMed] [Google Scholar]
Ponkey 2003
- Ponkey SE, Cohen AP, Heffner LJ, Lieberman E. Persistent fetal occiput posterior position: obstetric outcomes. Obstetrics & Gynecology 2003;101:915‐20. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schuitemaker 1997
- Schuitemaker N, Van Roosmalenj, Dekker G, Dongen P, Geijn H, Gravenhorst JB. Maternal mortality after cesarean section in the Netherlands. Acta Obstetricia et Gynecologica Scandinavica 1997;76:332‐4. [DOI] [PubMed] [Google Scholar]
Senecal 2005
- Senecal J, Xiong X, Fraser WD. Effect of fetal position on second‐stage duration and labor outcome. Obstetrics & Gynecology 2005;105:763‐72. [DOI] [PubMed] [Google Scholar]
Shaffer 2011
- Shaffer BL, Cheng YW, Vargas JE, Caughey AB. Manual rotation to reduce caesarean delivery in occiput posterior or transverse position. Journal of Maternal‐Fetal and Neonatal Medicine 2011;24(1):65‐72. [DOI] [PubMed] [Google Scholar]
Sherer 2002
- Sherer D, Miodovnik M, Bradley K, Langer O. Intrapartum fetal head position 1: comparison between transvaginal digital examination and transabdominal ultrasound assessment during the active stage of labor. Ultrasound in Obstetrics and Gynecology 2002;19:258‐63. [DOI] [PubMed] [Google Scholar]
Simkin 2010
- Simkin, P. The fetal occiput posterior position: state of the science and a new perspective. Birth 2010;37(1):61‐71. [DOI] [PubMed] [Google Scholar]
Souka 2003
- Souka AP, Haritos T, Basayiannis K, Noikokyri N, Antsaklis A. Intrapartum ultrasound for the examination of the fetal head position in normal and obstructed labor. Journal of Maternal‐Fetal and Neonatal Medicine 2003;13:59‐63. [DOI] [PubMed] [Google Scholar]
Tarnier 1982
- Tarnier S, Chantreuil G. In: Lauwereyus H editor(s). Traitê de L'art des Accouchements (Tome 2). 2nd Edition. Paris: Figure, 1982. [Google Scholar]
To 2000
- To WWK, Li ICF. Occipital posterior and occipital transverse positions: reappraisal of the obstetric risks. Australian and New Zealand Journal of Obstetrics and Gynaecology 2000;40(3):275‐9. [DOI] [PubMed] [Google Scholar]
Wu 2005
- Wu JM, Williams KS, Hundley AF, Connolly A, Visco AG. Occiput posterior fetal head position increases the risk of anal sphincter injury in vacuum‐assisted deliveries. American Journal of Obstetrics and Gynecology 2005;193:525‐8. [DOI] [PubMed] [Google Scholar]


