Abstract
The C5a anaphylatoxin protein plays a central role in inflammation associated with complement activation. This protein is commonly regarded as one of the most potent inducers of the inflammatory response and a C5a peptide agonist was used as a molecular adjuvant. However, the full length C5a protein has not been tested as a potential tumor therapy. In this report, we describe the creation of a mini-gene construct that directs C5a expression to any cell of interest. Functional expression could be demonstrated in the murine mammary sarcoma, EMT6. When C5a expressing cells were injected into syngeneic mice, most C5a-expressing clones had significantly reduced tumor growth. Further characterization of a clone expressing low levels of C5a demonstrated that one-third of mice injected with this line had complete tumor regression. The mice whose tumors regressed were immune to subsequent challenge with unmodified EMT6 cells, suggesting that a component of the innate immune response can be used to augment adaptive immunity. Cellular analyses demonstrated that a significant difference in actual tumor cell number could be detected as early as day 10. A block in cell cycle progression was evident at all time points and high levels of apoptosis were observed early in the regression event. These data demonstrate that the complement protein C5a can play a significant protective role in tumor immunity.
Keywords: Rodent, Tumor immunity, Immunotherapy, C5a complement
Introduction
Cancerous cells arise via genetic changes that allow uncontrolled cell growth. Even though the products of these genetic changes (and other proteins from de novo gene expression that occurs in cancer) have the potential to be targets for the immune response, tumors often escape immune recognition [3]. In the past several years, a number of advances were made in the area of tumor immunotherapy (reviewed in [47]); however, most of the emphasis was placed on T lymphocyte recognition and killing of tumor cells [45]. Increasingly, there was evidence that a generalized inflammatory response may be significantly beneficial in tumor immunity [10]. For instance, high granulocytic infiltration of the tumor is a positive prognostic indicator for tumor regression in some cancers, suggesting that a factor which elicits generalized inflammation may be a beneficial therapeutic for the induction of tumor immunity [47]. In addition, a number of papers have shown that the innate immune response is sometimes critical for the generation of specific adaptive immunity [2, 14, 34, 57]. However, the role of generalized inflammation in tumor therapy is not entirely straightforward, as inflammation has also been found to be detrimental in some cases [13].
Complement component C5a is an important inflammatory mediator in the complement cascade [16]. It is formed when the fifth component of complement, C5, is cleaved by either the alternative or classical pathway C5 convertase, forming membrane associated C5b and the soluble 77 (rat and mouse) or 74 (human) amino acid C5a peptide. The specific receptor for C5a is CD88 (reviewed in [16, 56]), although a recently identified receptor, C5L2, also binds to C5a with unknown effector functions [6, 25, 42]. Messenger RNA analysis has shown that CD88 is expressed on a number of cell types; however, the functional consequences of this expression are often unknown [21, 38, 55]. The binding of C5a to its receptor on immune cells including macrophages and neutrophils elicits several functions depending upon the concentration in the local environment. In myeloid cells, C5a is a potent chemotactic and activation factor; while in endothelial cells, it induces changes in vascular permeability [16]. The attraction and facilitated entry of immune effector cells into an area via activated complement is key in the ability of C5a to induce an immune response.
C5a is known to induce the chemotaxis of several cell types including macrophages and granulocytes [55], mast cells [20], dendritic cells [28, 35, 36, 49] and B lymphocytes [29, 43]. The binding of C5a to CD88 induces morphological and phenotypic changes in the responding cell including actin cytoskeletal remodeling, cell spreading, adhesion and de-adhesion. Through mechanisms not yet fully understood, cells are able to sense the gradient of C5a along the axis of the cell and respond with directional movement toward the area of higher concentration [46]. When the C5a concentration is in the 100 nM range (i.e., when the cells migrate to the area of C5a production) binding of C5a to CD88 can induce degranulation, activation of NAPDH oxidase and changes in gene expression [56]. All of these mechanisms contribute to the role of C5a as one of the most potent inducers of an inflammatory response.
Complement proteins have been shown to be involved in a wide variety of autoimmune phenomena including systemic lupus erythematosus [1, 5], arthritis [23, 54], atherosclerosis [8, 40], autoimmune myocarditis [26] and neuroinflammation [11, 39]. In nearly every case, it has been hypothesized that complement plays a negative role during autoimmune attack. Inappropriate complement activation leading to the attraction and activation of immune effectors, as well as direct cell damage caused by terminal complement components may contribute to the autoimmune disease phenotype. It has been hypothesized that the goal of induction of an anti-cancer immune response is akin to induction of autoimmunity [44]; therefore, we have investigated the hypothesis that production of the complement anaphylatoxin in tumor cells can lead to the induction of antitumor immunity.
Since C5a is produced through the proteolytic cleavage of C5, it has been difficult to target expression to a specific cell and the role of C5a as a potential inflammatory factor for the control of tumor growth has not been investigated. Other inflammatory complement factors have cellular receptors that inactivate their function making their use in tumor immunity induction questionable; however, there are no known receptors or other proteins expressed in tumor cells that could modify the activity of C5a, although there are fluid-phase carboxypeptidases that cleave C5a to the des-Arg form [7]. In the present report, we demonstrate that functional C5a can be produced from a mini-gene construct utilizing a heterologous signal sequence. The expression of C5a in the poorly immunogenic mammary tumor model, EMT6, leads to decreased tumor growth kinetics and complete regression of tumors in one-third of mice injected with C5a-expressing cells. Furthermore, mice that have undergone tumor regression are completely immune to subsequent challenge with unmodified tumor cells. A block in cell cycle progression contributes to this phenotype, perhaps in concert with high levels of apoptosis. These data demonstrate a positive role for C5a in the induction of an antitumor response.
Materials and Methods
Cells and cellular assays
The EMT6.8 murine mammary tumor cell line (hereafter referred to as EMT6) [32] was obtained from Dr. J.G. Frelinger and was maintained in DMEM (Invitrogen, Carlsbad, California) with 7% fetal bovine serum (Invitrogen). The J774A.1 macrophage-like cell line (referred to as J774 hereafter) was purchased from American Type Culture Collection (Manassas, Virginia) and maintained in DMEM with 10% fetal bovine serum.
FACS analysis was done using chicken anti-mouse C5aR antibody (S.R. Barnum). Cells were washed in PBS, blocked in normal goat serum, and then stained with a 1:100 antibody dilution. After washing, cells were stained with biotinylated goat anti-chicken IgG (Vector Labs, Burlingame, CA), followed by streptavidin-Alexa Fluor 488 (Molecular Probes, Eugene, Oregon). Stained cells were analyzed on a FACScan (Becton-Dickinson, San Jose, California) using Summit software (Cytomation, Fort Collins, Colorado).
Constructs
The C5a fragment of C5 was cloned by reverse transcription/PCR as follows: Messenger RNA was isolated from the liver of a BALB/c mouse by the guanidinium/cesium chloride method [31]. The RNA pool was reverse transcribed using reverse transcriptase (Invitrogen) with the primer: 5′-CTA CCT TCC CAG TTG GAC AG-3′. Note that this primer modifies the 3′ end of the C5a sequence. The cleavage site for C5a contains the sequence: 5′-CTG GGA AGG ATC CAC-3′, which codes for the amino acids LGRIH, with the carboxy terminal end of the C5a peptide being LGR. The oligonucleotide above changes the 3′ end of the sequence to: 5′-CTG GGA AGG TAG-3′, with the terminal TAG now coding a stop codon. The cDNA pool was then amplified with Taq DNA polymerase (Invitrogen) using the following oligonucleotides: sense, 5′-CGG GAT CCG TTA ACC TGC ATC TCC TAA GGC-3′ and antisense, 5′-GCG GAT CCT ACC TTC CCA GTT GGA CAG-3′. These primers both contain BamHI cleavage sequences for cloning purposes. The antisense primer contains the aforementioned stop codon. The amino terminal amino acid in C5a is N, coded by 5′-AAC-3′. A 5′-GTT-3′ sequence was added to create 5′-GTTAAC-3′, creating an HpaI site (blunt cutter). Amplification was performed for five cycles with annealing at 55°C, then for thirty cycles with concurrent annealing/extension at 72°C. The product of 252 bp was purified, cut with BamHI and cloned into pBluescript SK- (Stratagene, La Jolla, CA). Lack of mutation and orientation were confirmed by sequencing.
The signal sequence used for the synthetic C5a gene was from the mouse IFN-γ gene. The gene was excised from the pHβ-mIFN-neo construct [33]. This DNA was used as a template for amplification of the signal sequence using the oligonucleotides: sense, 5′-CGG AAT TCT CTG AGA CAA TGA ACG CTA-3′; antisense, 5′-CGG AAT TCC TGC GCA GTA ACA GCC AGA AAC-3′. Both oligonucleotides create EcoRI sites for cloning. The last amino acid in the IFN-γ signal sequence is a cysteine coded by 5′-TGC-3′. The sequence GCA was added to create 5′-TGCGCA-3′, an FspI site. Amplification was performed as described for C5a. The 95 bp product was purified, cut with EcoRI and cloned into pBluescript SK-. Lack of mutation and orientation were confirmed by sequencing.
The interferon signal sequence (IFN-SS) construct was cut with FspI (creating a blunt site) and EcoRI and the 91 bp fragment was cloned into the C5a-pBluescript construct cut with EcoRI and HpaI (creating a blunt site). The junction between IFN-SS-C5a is predicted to be a precise cleavage site, resulting in only the native C5a sequence (Signal P web server [41]). This construct (IFN-SS-C5a-pBluescript) was cut with SalI and BamHI and the 314 bp fragment was cloned into pHβ [33] cut with the same enzymes.
Cells were transfected with the C5a plasmid construct (see below) by calcium phosphate precipitation [30] and selected in Geneticin (Invitrogen). Limiting dilution was used to clone the cells from the polyclonal population. The clones were tested for functional C5a by chemotaxis assay and clones that directed J774 chemotaxis above background (media alone) were selected for further evaluation. Briefly, supernatants from the clonal cell populations were added to the lower well of a 96 well ChemoTx chemotaxis plate (5 μm pores; Neuroprobe, Gaithersburg, MD). J774 murine macrophage-like cells (1×105) were added to the upper part of the filter. Cells were allowed to migrate for three hours at 37°C. Cells that migrated to the lower well were quantitated using the CytoLite assay (Perkin-Elmer, Wellesley, MA). Assays were performed with triplicate samples and the mean ± the standard error is presented.
For measurement of in vitro cell growth, cells were plated at 1×105 cells per 10 cm plate. Cells were allowed to grow for 72 h at which time cells were harvested by trypsinization. At this time point, cells were approximately 50–70% confluent. Trypan-blue exclusion was used to quantitate the cell number. This assay was performed in triplicate and the experiment repeated three times with similar results.
Tumor studies
BALB/c mice were purchased from Jackson Labs (Bar Harbor, Maine). Cells in mid-log growth were harvested, washed thrice in PBS, and then resuspended at 2×105 cells per 50 μl. Mice were injected with 50 μl in the calf muscle of the hind limb. The mice were individually monitored for tumor growth. In each experiment, there were four to six mice per group. Graphs indicate the mean leg size ± the standard error. The primary tumor growth experiment for the C5a A1 clone was repeated four times and a representative experiment is shown. Composite data is shown as a Kaplan–Meier plot that represents data for four experiments. Mice were considered tumor-free if mean leg diameter was less than 5 mm.
Principles of laboratory animal care (NIH publication No. 85–23, revised 1985) were followed. All mouse experiments were approved by Institutional Animal Care and Use Committees of the University of North Carolina at Chapel Hill and The University of Iowa. Both institutions’ animal facilities are accredited by the American Association for the Accreditation of Laboratory Animal Care.
Tumor harvest
Mice were sacrificed on the day indicated. For each experiment, there were four mice per group and each experiment was repeated with a representative experiment shown. Tumors were carefully dissected from the leg of the mice while removing as much of the non-tumor tissue as possible. The tumors were weighed, then minced with scissors. The resulting tissue was treated with collagenase (Sigma, St. Louis, MO) for 75 min with rotation at 37°C. The cells were isolated by filtration through 70 μm mesh, followed by centrifugation. The erythrocytes were hypotonically lysed and the cells washed thrice in phosphate buffered saline. Cells were counted by trypan blue exclusion. For histological examination, tumors were harvested on the indicated days and dissected. Tissues were fixed in formalin and embedded in paraffin. Five μm sections were cut and then stained with hematoxylin and eosin.
Cell cycle and apoptosis analyses
Tumor cells (1×106) isolated above were incubated in ice cold 70% ethanol for 1 h, followed by two washes in PBS. Cells were resuspended in PBS containing 1 mg/ml RNAse A (Sigma) for 20 min on ice. The samples were transferred to a tube with propidium iodide (PI) to a final concentration of 67 mM. Cells were immediately analyzed on a FACScan (Becton-Dickinson) with the WinMDI software. Gates were set to address the number of cells with <2N DNA content (apoptotic), those with 2N DNA (resting to G1), and those with >2N DNA content (S, G2 and M phases). Data is presented as the mean ± the standard error. In all experiments in which statistical significance is presented, this was determined by use of Student’s t-Test, two-tailed distribution, homoscedastic.
Annexin V experiments were conducted by staining cells as recommended by the manufacturer (BD Biosciences). PI was used simultaneously to exclude dead cells (i.e., PI positive cells were excluded from the analyses). Cells were analyzed on a FACSCaliber (BD Biosciences) and dedicated software. All experiments with PI and Annexin V were repeated thrice with four mice per group.
Results
Construction of a mini-gene coding for secreted C5a
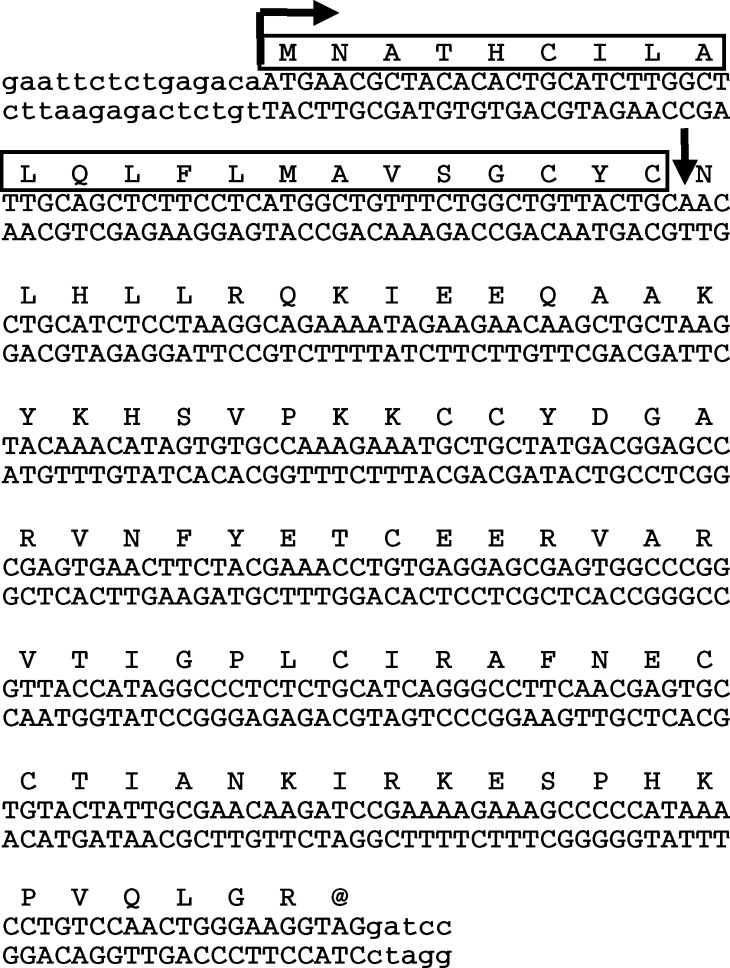
Complement proteins contain important inter- and intra-chain disulfide bonds and are posttranslationally processed, making them difficult to study in recombinant form. While there was a report on the production of C5a from synthetic constructs containing a novel initiating methionine in bacteria [4] and recombinant human C5a is readily available; there is no report in the literature of C5a expression using a heterologous signal sequence in eukaryotic cells. C5a is attractive as an immunotherapeutic since there are no known cellular proteins present on tumor cells that can specifically inactivate C5a function (although carboxypeptidases are active in the fluid phase [7]). To determine whether C5a expression in a tumor model system could alter tumor growth, we first developed a C5a mini-gene construct encoding the C5a sequence with a signal sequence for secretion. The C5a sequence was isolated by reverse-transcription/amplification and cloned. This product was fused to the signal sequence from the mouse IFN-γ gene. The resulting gene (IFN-SS-C5a, see Fig. 1) is predicted to be precisely cleaved by the signal protease (see vertical arrow in Fig. 1), leaving only the intact C5a coding portion for secretion [41]. This construct was cloned into the pHβ vector [33] with the C5a gene under the control of the β-actin promoter.
Fig. 1.
Sequence of the mouse IFN-SS-C5a construct. The mouse C5a sequence was cloned from mouse liver using reverse transcription-cDNA amplification. This gene was fused to the mouse IFN-γ signal sequence. The signal sequence is boxed and the junction between the signal sequence and the C5a sequence (and the point of predicted signal peptidase cleavage) is indicated by a vertical arrow. The translation initiation site is indicated by a horizontal arrow. The entire construct was confirmed by sequencing. These sequence data are available from EMBL/GenBank/DDBJ under accession number AF165982
Functional expression of C5a in the EMT6 mammary sarcoma cell line
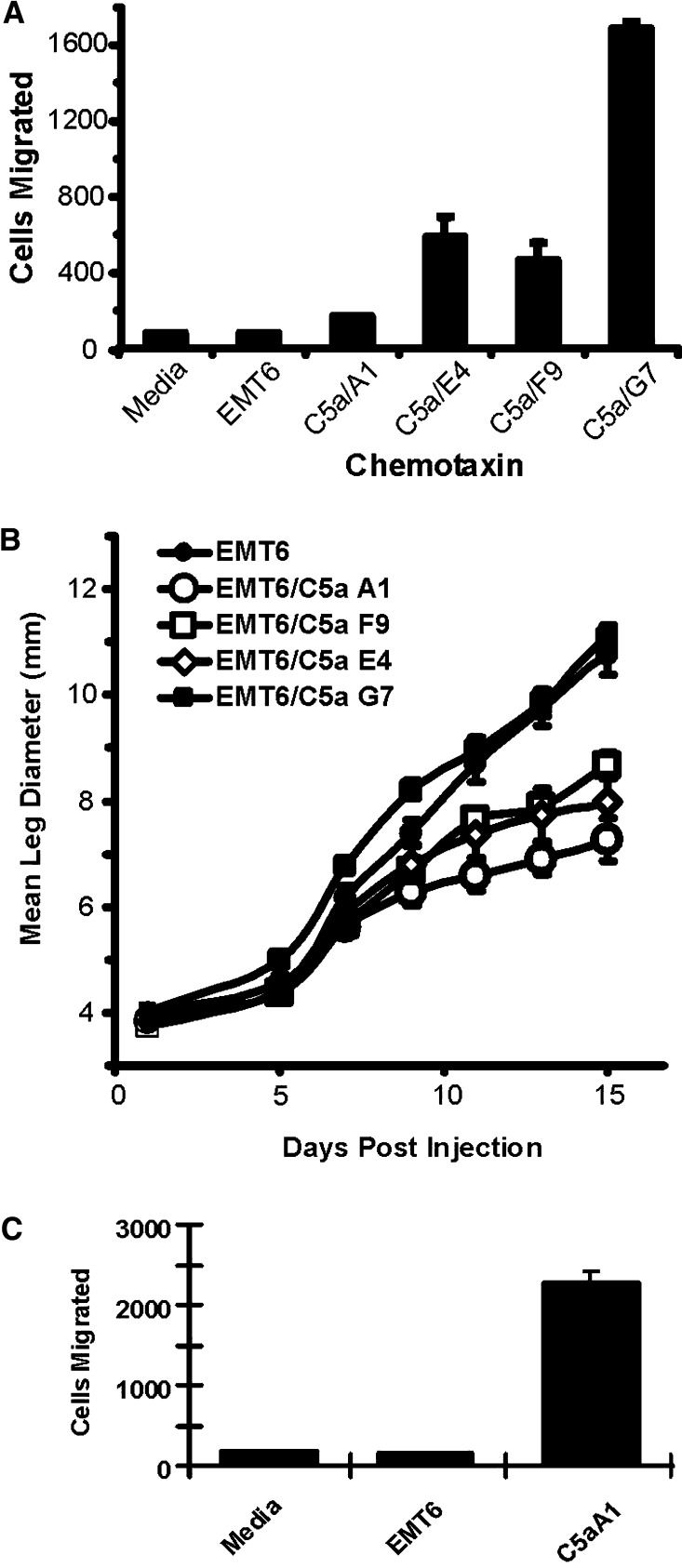
The murine tumor cell line, EMT6, is a poorly immunogenic BALB/c mammary sarcoma and it was used to determine whether C5a expression would alter the tumor growth properties in syngeneic mice. The IFN-SS-C5a construct was transfected into EMT6 cells and the cells were selected in Geneticin for expression of the construct. C5a was detected in the supernatants of the polyclonal population by J774 chemotactic assay (data not shown). Limiting dilution was used to clone cells from this pool and clones expressing C5a were identified by their ability to induce the chemotaxis of J774 cells. We selected a total of 33 clones, of which 17 (52%) secreted amounts of C5a that could induce J774 chemotaxis. EMT6 cells were also cloned from empty vector transfected cells and we never observed any chemotactic activity from vector transfected polyclonal cells, or individual clones (data not shown). Four C5a expressing clones were identified as inducing a wide range of chemotactic responses from J774 cells (see Fig. 2a). The difference in chemotactic activity for these clones ranged from P=0.014 for the F9 clone to P=2.3×10−7 for the G7 clone. All clones grew in vitro with kinetics indistinguishable from EMT6 cells for empty vector transfected cells (data not shown). These four clones were injected into syngeneic BALB/c mice and the growth properties of these tumors were measured. As shown in Fig. 2b, three clones grew with significantly delayed kinetics relative to untransfected EMT6 cells, while one clone, G7, had initially higher growth and tumor growth did not plateau as in the other C5a expressing clones. Interestingly, there was an inverse relationship between the level of C5a expression and tumor growth in vivo. The only clone in which mice had tumor regression was the A1 clone. We grew this clone in culture for an additional day in culture to highlight that it did indeed express significant quantities of C5a. As shown in Fig. 2c, the A1 clone did direct significant (P<0.005) (albeit low) levels of J774 chemotaxis, supporting our conclusion that this clone expressed C5a. These data disproved our original hypothesis that high C5a expression would lead to tumor regression. There are several possibilities that may account for high levels of C5a altering expected tumor survival. C5a may change growth kinetics in vivo through an autocrine pathway (although it does not change growth in vitro). Alternatively, C5a may induce other chemokines, cytokines or growth factors either in the cells themselves, or in parenchymal cells in the local microenvironment. However, our primary goal was to understand how low levels of C5a expression leads to tumor regression, thus based upon these results, the clone with the slowest growth rate in vivo (A1) was selected for further analyses.
Fig. 2.
Expression of C5a by transfected EMT6 cells and effect on tumor growth in vivo. a EMT6 cells were transfected with the C5a expression construct (see Fig. 1) and selected in geneticin. Limiting dilution was used to clone individual lines. Clones were plated in six well dishes and 3 days later, the supernatants harvested. Either control media, supernatants from untransfected EMT6 cells or clones transfected with the C5a construct were used as chemo-attractants in a 96-well J774 cell motility assay. The number of cells migrating to the lower chamber was measured and compared between samples. All selected clones had significantly more chemotactic activity than media alone or control EMT6 supernatant (P<0.05). b Tumor cells (2×105) from each clone were injected into syngenic BALB/c mice in the calf muscle and leg size was measured over time as a function tumor growth. The mean ± the SEM is presented for at least four mice per treatment group. c J774 cell migration in response to the A1 clone grown for 4 days to see the significance of the cell migration induced by A1 line supernatants. Difference in migration between media alone or EMT6 supernatants and supernatants from the A1 clone were significant at P<0.005. Mean ± SEM are presented
EMT6 cells express C5aR but expression of C5a does not alter logarithmic EMT6 cell growth in vitro
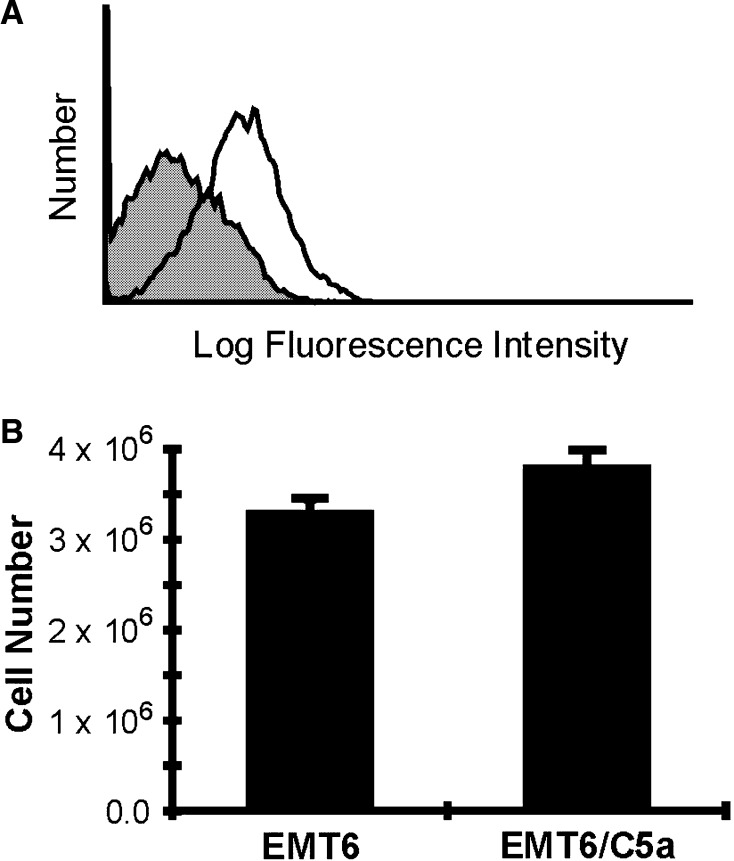
Our results with in vivo tumor growth suggested that C5a might alter cellular growth, so it is important to determine whether the receptor for C5a is expressed on these cells. As shown in Fig. 3a, the C5aR protein is indeed expressed on EMT6 cells, suggesting that C5a might modulate growth of the A1 clone. If the C5a expression changes the doubling time, then changes in vivo growth can be simply explained without the influence of the host immune response. In order to examine this question, we examined the doubling time of the A1 clone versus parental EMT6 cells. One hundred thousand EMT6 cells and EMT6 cells expressing C5a (EMT6/C5a) were plated and allowed to grow for 3 days. The total number of cells was then compared. As shown in Fig. 3b, the EMT6/C5a A1 clone grew slightly faster than did the EMT6 cells, although the difference did not reach statistical significance (P=0.08). These data indicate that if there was a slowing of growth in vivo, it could not be explained by simple changes in growth kinetics that manifest themselves in vitro.
Fig. 3.
C5aR expression on EMT6 cells and in vitro growth rates of C5a expressing clone A1 and control EMT6 mammary tumor cells. a EMT6 cells were treated with chicken anti-mouse C5aR (unfilled histogram) or without primary antibody (gray histogram). Cells were visualized with biotinylated anti-chicken antibody, followed by streptavidin-Alexa Fluor 488. b Cells were plated at 1×105 cells per 10 cm plate. Three days later, the cells were harvested and counted by microscopy using trypan-blue. On average, the EMT6/C5a A1 clone grew faster, but the difference does not reach statistical significance (P=0.08)
The C5a mini-gene product reduces tumor incidence and size
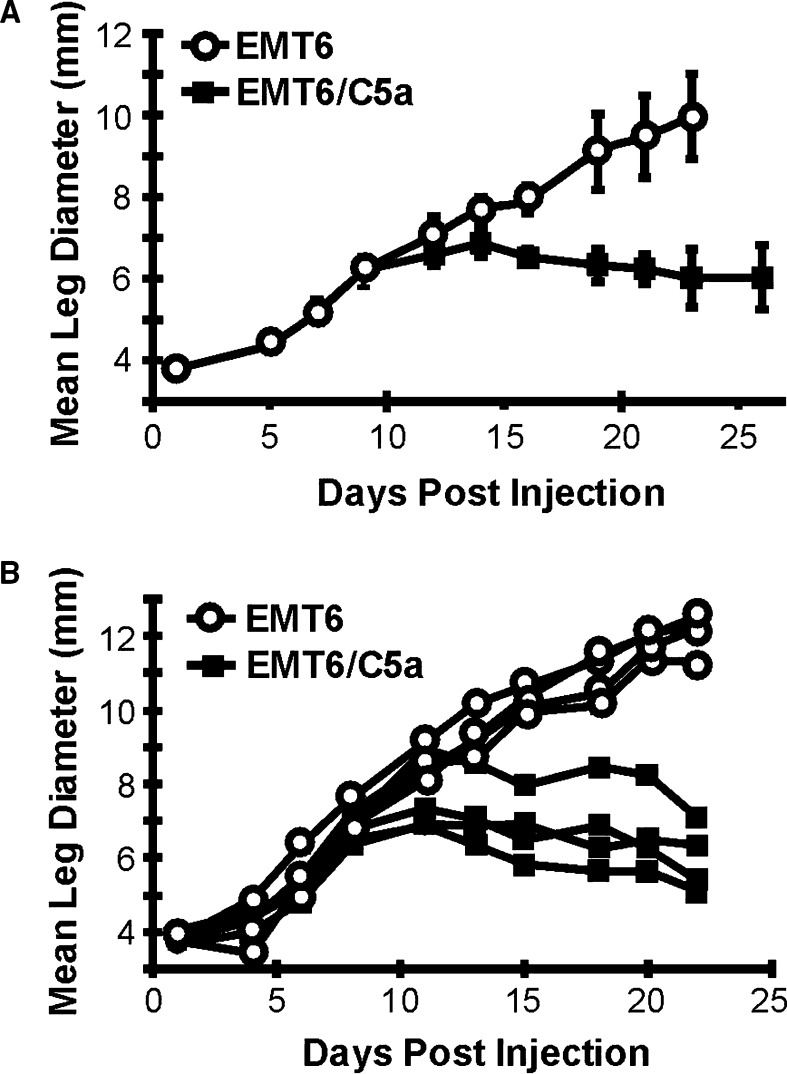
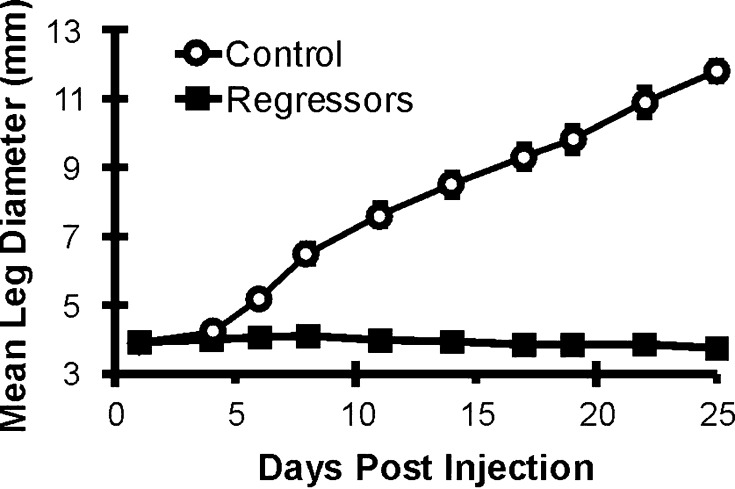
To further examine the effect that C5a expression may have on the growth of EMT6 tumors in vivo, cells were injected IM in the hind limb of syngeneic BALB/c mice and mice were individually monitored for tumor growth over a longer time period relative to that shown in Fig. 2b. As shown in Fig. 4a, initially the growth rates of control and the A1 C5a expressing clone were identical up to day 10. Beginning at day 12, the C5a tumors began to grow slower, and after 15 days of growth, there was an overall regression in tumor size. A different experiment is shown in Fig. 4b, with the measurements of individual mice shown, demonstrating the regressive phenotype. This regression was reproducible over four separate experiments. Thus, in the early phase of tumor growth around day 15, there is a significant difference in tumor size.
Fig. 4.
Tumor growth characteristics of mice injected with EMT6 and EMT6 C5a clone A1. Cells were injected into the calf muscle of BALB/c mice at day 0. After injection, mean leg diameter was monitored individually as a function of tumor size. Beginning at day 16, the difference in tumor size between mice injected with control and C5a expressing cells was statistically significant (P<0.05). a The data presented are a representative experiment that was repeated four times. Open circles, mice injected with control tumors; solid squares, mice injected with C5a-expressing tumors. b Measurements from individual mice in a representative experiment. Open circles, mice injected with control EMT6 tumors; solid squares, mice injected with EMT6 cells expressing C5a
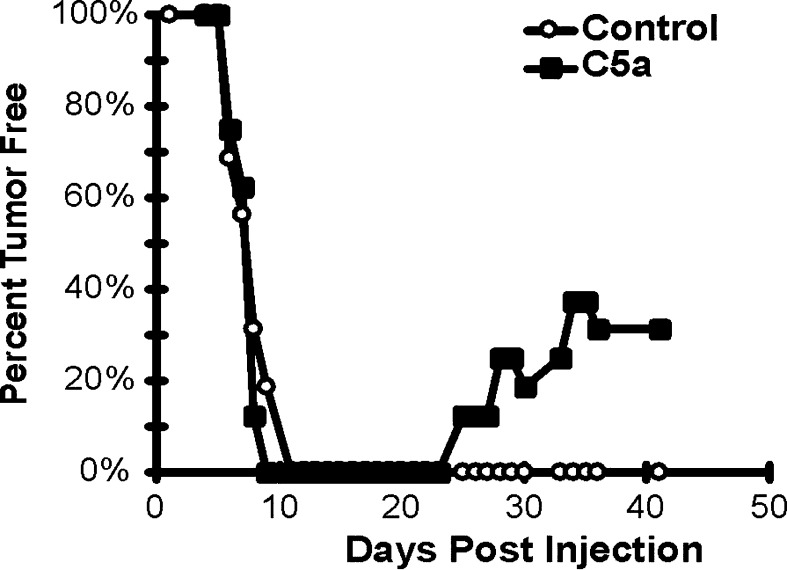
Another way to analyze the growth of tumors is to chart the percentage of mice that are tumor-free over a prolonged period. Figure 5 demonstrates that mice injected with control and C5a expressing tumors had similar onset of tumor formation with no mice being tumor-free by day 11 post inoculation. Starting at day 25, however, mice injected with C5a expressing cells became tumor-free. By day 37, ~31% of mice initially injected with clone A1 were tumor-free (a total of sixteen mice were analyzed in four experiments). It should also be noted that although in every experiment 50–75% of the mice injected with C5a expressing tumors eventually grew out those tumors, 80% of the growing C5a-expressing tumors were smaller than the smallest control tumor (data not shown). These data show that C5a expression in this mammary sarcoma model system can lead to the retardation of tumor growth; and more significantly, it leads to tumor rejection, even after tumor establishment.
Fig. 5.
Greater than 30% of mice injected with cells expressing C5a have complete regression of their tumors. Mice were injected as in Fig. 4. Mice were considered tumor bearing when the mean leg diameter was less than 5.0 mm and the number with tumor versus the total number injected is presented. The data are a composite of four separate experiments. Open circles, mice injected with control tumors; solid squares, mice injected with C5a-expressing clone A1 tumor cells
The C5a mini-gene induces immunity to unmodified tumors
If the specific immune response actually played a role in the rejection of C5a expressing tumors, then it is reasonable to hypothesize that mice that rejected the initial challenge with C5a expressing cells would be immune to challenge with unmodified EMT6 cells. Four mice that had rejected EMT6/C5a tumors from three separate experiments were subsequently challenged with unmodified EMT6 cells. As shown in Fig. 6, all mice were completely tumor-free throughout the course of the experiment. These data indicate that the rejection event mediated by C5a had induced an adaptive immune response.
Fig. 6.
Mice that have complete regression of their primary tumors are immune to subsequent challenge with unmodified EMT6 cells. Mice that had complete regression were rechallenged with control EMT6 tumor cells. Mice were monitored individually for tumor growth. The data represents four surviving mice from three experiments. There was a statistically significant difference between rechallenged and control mice beginning at day 6 (P<0.001). Both groups were injected with unmodified EMT6 cells. Open circles, control naïve mice; solid squares, mice that rejected C5a tumors
The C5a mini-gene greatly decreases tumor cellularity
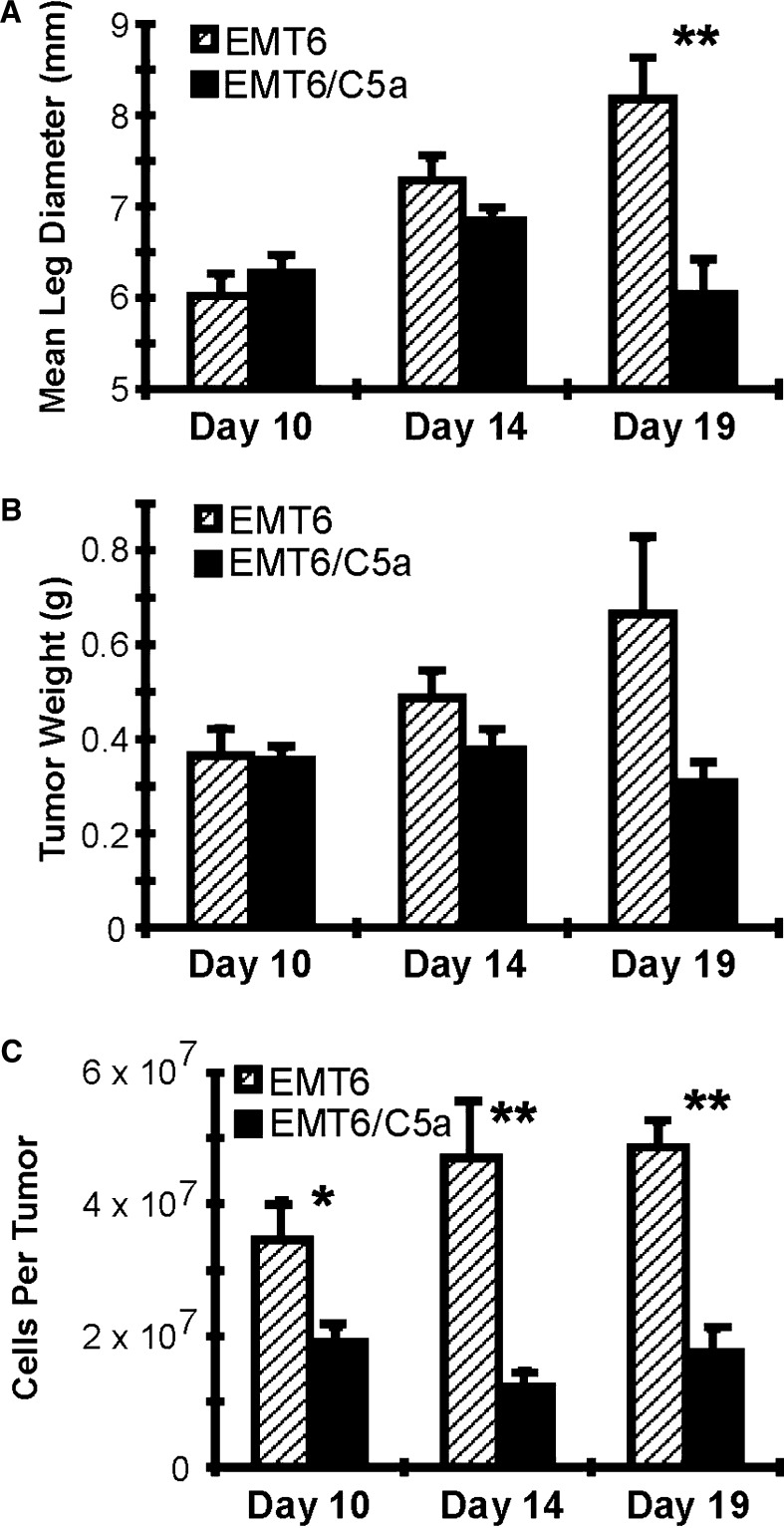
Kinetically, the long delay in tumor regression from the time of inoculation until the initiation of regression suggested an immune recognition event; however, the external measurement of leg diameter may not reflect all of the changes happening within the tumor itself. Figure 7a is the representation of mean leg diameter from mice in an experiment separate from those shown in Fig. 4 and serves to demonstrate the tumor size in relation to other measurements in this experiment. In this case, the difference between control and C5a expressing tumors was not statistically significant until day 19. Similarly, mean tumor weight was not significantly different until day 19 (Fig. 7b). Although the size and weight of the tumors was very similar at days 10 and 14, this did not necessarily indicate that the actual number of tumor cells was equal. To examine whether the tumor size correlated precisely with cellularity, viable cells derived from the dissected tumors were counted by trypan blue exclusion and the absolute number of cells per tumor was compared. As shown in Fig. 7c, even at day 10, the overall cellularity of the C5a expressing tumors is significantly less (P<0.05). At day 14, there is an even greater difference (P<0.005). At day 19, the cellularity of C5a expressing tumors was still significantly low. This change in cellularity suggests that the mechanism responsible for the tumor rejection is taking place at a much earlier stage than can be appreciated by grossly examining tumor size as an indicator of rejection, and underscores the importance of these different measurements to reveal a more complex biologic process during tumor rejection.
Fig. 7.
Mice injected with C5a expressing cells display reduced cellularity before they show overt external signs of decreased tumor size. Mice were injected as in Fig. 6. At the indicated days, mice were measured and sacrificed. a Mean leg diameter for each group. b After the mice were sacrificed, the tumors were carefully excised and weighed. The mean weight of each group is presented. c A single cell suspension was made by treating the tumors with collagenase. Cells were quantitated by microscopy, counting only cells that excluded trypan-blue. The mean number of cells per tumor for each group is presented. Symbols, * P<0.05; ** P<0.01. Hatched bars, control tumors; solid bars, C5a tumors
The C5a mini-gene product results in early apoptosis and fewer mitotic cells
Although data presented thus far clearly demonstrate a regressive phenotype in C5a expressing tumors, the mechanism responsible for these changes is unknown. The hypothesis behind using C5a as a tumor immunotherapeutic was that C5a expression from the tumor cells would lead to infiltration by predominantly myeloid cell types, resulting in an inflammatory reaction and tumor rejection.
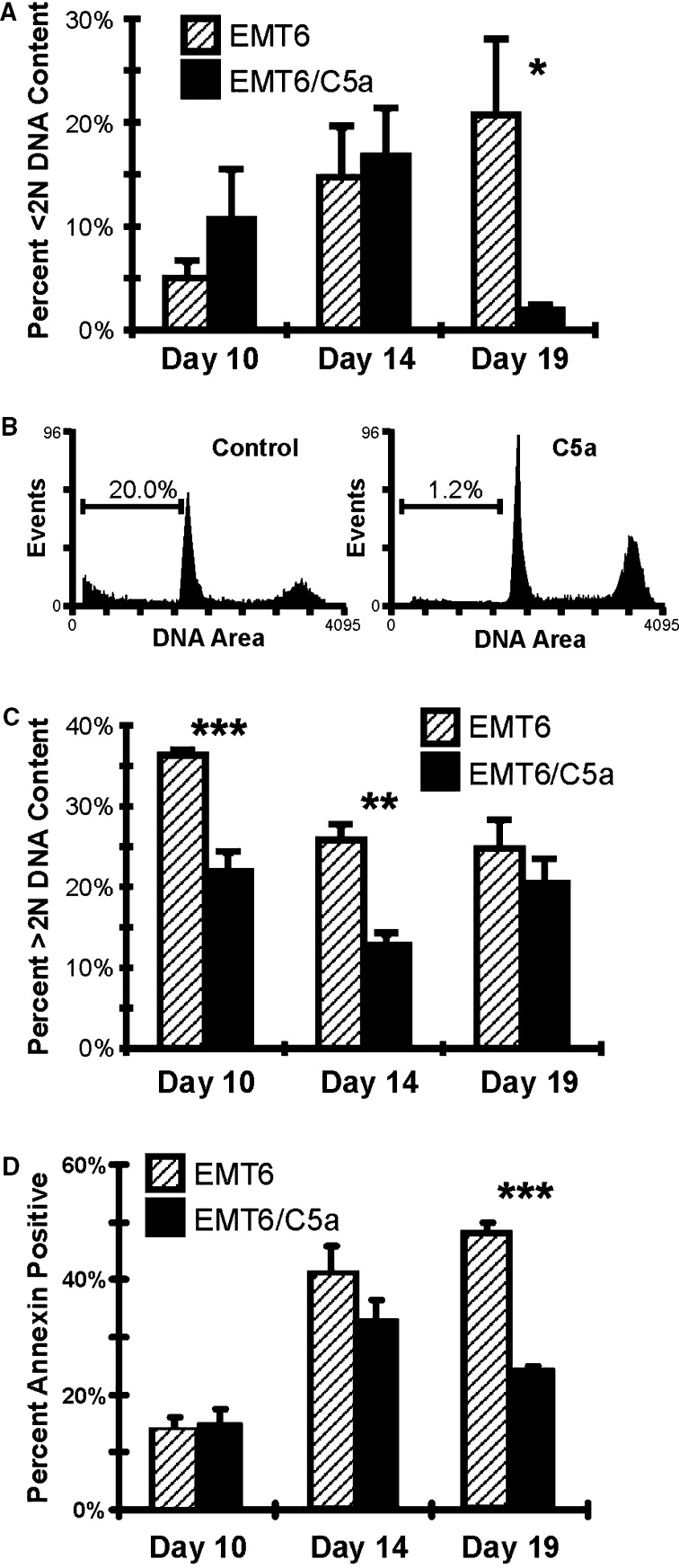
Possible cellular mechanisms for the decreased cell numbers in C5a expressing tumors include the induction of programmed cell death and/or changes in the cell cycle program. One downstream effect of the activation of the caspase cascade is the activation of cellular nucleases leading to degradation of chromosomal DNA [50]. When membrane blebs begin to form, various chromosome fragments are present and can be quantitated by showing a DNA content of <2N. Propidium iodide staining was employed to determine if cells with <2N DNA content could be detected. As shown in Fig. 8a, control tumor cells have a relatively high level of apoptosis, with 5% of the cells having <2N DNA content. C5a expressing tumors had nearly twice as many apoptotic cells as control tumors at day 10. At day 14, there was no difference between the two groups. But by day 19, there was a large difference in the number of cells with <2N DNA, with the C5a tumors having significantly fewer apoptotic blebs. A representative histogram for day 19 mice is presented in Fig. 8b. One explanation for the decreased number of <2N cells is that most of the tumor cells have already undergone cell death at this time point (see below) and that many of the cells are infiltrating cells, not tumor cells. Indeed, the overall architecture of the tumor is very different when control tumors are compared to C5a expressing cells (see Fig. 9). One caveat to this experiment is that, in some instances, necrotic cells may have <2N DNA content, suggesting that another method can be employed in the future to confirm apoptosis. In addition to DNA fragmentation, cells undergoing apoptosis also have a loss of membrane asymmetry. This can be measured using FITC labeled Annexin V, whose epitope is normally found only on the inner leaflet. Annexin V staining closely paralleled the results from cell cycle analysis, although there was no difference in apoptosis on day 10, there was a significant difference on day 19 (Fig. 8d). These data suggest that early apoptosis alone does not explain tumor regression.
Fig. 8.
Cell cycle analysis of tumor cells isolated from control and C5a expressing tumors demonstrates increased apoptosis and a block in cell cycle progression at early time points. At the indicated times, freshly isolated cells from control and C5a-expressing tumors were stained with propidium iodide (Panels A, B and C) or annexin V (Panel D) and the percentage of cells with different DNA content was calculated. a Cells with <2N DNA content, indicative of apoptotic cells. b Representative histograms showing the DNA content of cells from tumors harvested at 19 days (control tumor cells, left side; cells from EMT6/C5aA1 injected mice, right side). Numbers indicate the cells with, 2N DNA content. c Cells with >2N DNA content, indicative of cells that are proceeding through the cell cycle. d Percent annexin positive cells from tumors harvested at the indicated days. Cells were gated to be propidium iodide negative, annexin positive. Symbols, * P<0.05; ** P<0.01; *** P<0.001, Students t-Test. Hatched bars, control tumors; solid bars, C5a tumors. All experiments were repeated three times with four mice in each group
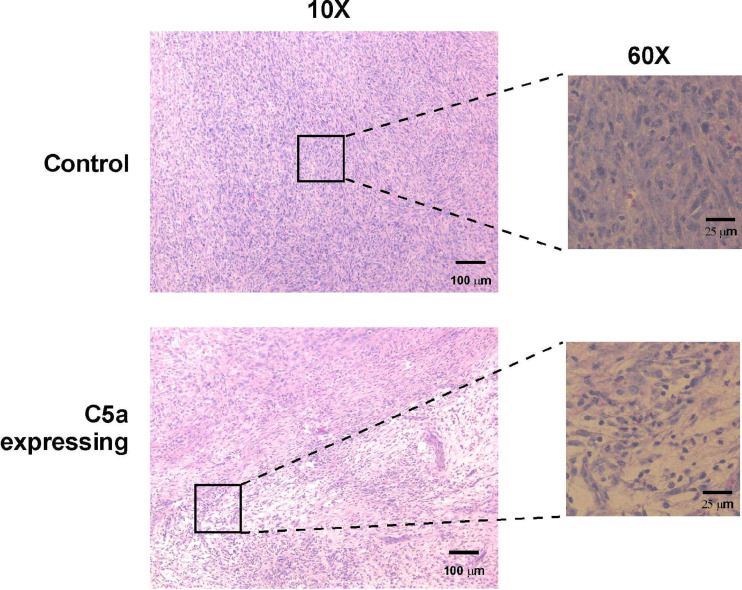
Fig. 9.
Histological analyses of EMT6 tumors expressing C5a demonstrate increased cellular infiltration and large areas of decreased cellularity. Tumors were harvested on day 15 and dissected. Tissues were fixed in formalin and embedded in paraffin. Five μm sections were cut and then stained with hematoxylin and eosin. Sections are shown at 10× and 60× objective magnification
In addition to giving information on the number of cells with <2N DNA content, propidium iodide staining also allows the detection of the number of cells within the tumor that are proceeding through the cell cycle. This can be determined by examining the number of cells that have >2N DNA content; that is, those that are in the S-G2-M portion of the cycle. As shown in Fig. 8c, at day 10, there are ~40% fewer C5a expressing cells in the G2 phase of the cell cycle compared with control tumors, while at day 14, the difference is ~50%. By day 19, the percentages are nearly identical. These data indicate that in addition to the induction of programmed cell death, there also appears to be a block in the ability of cells to proceed with DNA synthesis. This suggests that there are multiple (but possibly related) mechanisms by which C5a expression in the EMT6 cell line induces delayed tumor growth and rejection.
If the decreased levels of apoptosis and increased number of cells in the cell cycle at day 19 reflected an increase in infiltrating cells with a concomitant decrease in the absolute number of tumor cells present within the tumor mass, then this should be able to be visualized by histological analysis. As shown in Fig. 9, at day 15, control tumors are composed almost entirely of well-defined tumor cells with few infiltrating cells or necrotic regions. In contrast, at day 15, C5a expressing tumors had areas of massive infiltrates (Fig. 9) along with areas that had apparent decreased cellularity. These data demonstrate that the most likely explanation for the lack of apoptosis in day 19 tumors (see Fig. 8a) is that the cells undergoing apoptosis (i.e., tumor cells) are lost by this time point and the infiltrating cells are becoming the predominant cell population present in the tumor mass. Surprisingly, FACS analyses did not show large differences in most cell types. Control and C5a-expressing tumors had equal numbers of B cells, T cells and dendritic cells (all very low). C5a-expressing tumors had slightly higher numbers of macrophages (CD11B + cells, 12.7% in control tumors, versus 17.8% in C5a expressing tumors) and granulocytes (Gr-1 positive cells, 15.7% versus 18.2%). These data suggest that the increase in macrophages and granulocytes may be due to C5a-mediated chemotaxis, although it may also be possible that the increased numbers of dead and dying cells may be attracting these cell types.
Discussion
There were few published reports examining C5a expression targeted to an individual cell. This is likely due to the fact that C5a is part of the large C5 protein whose structure and function are highly dependent upon specific secondary structure involving disulfide bonds and posttranslational processing. C5 is not functional as a recombinant protein expressed in bacteria due to these constraints. Murine studies done with C5 and C5a in the past have largely been performed with human proteins since it is relatively easy to obtain large amounts of these proteins from serum; however, the number of mice required to obtain working quantities of these factors often precludes the use of species specific reagents in this most common model system. This is the first report to demonstrate functional C5a expression from a recombinant construct with a heterologous signal sequence in eukaryotic cells. The ability to express C5a in this novel form in vivo is a positive step in the determination of the functionality of this protein in a number of experimental systems. For instance, in systems where complement activation and specifically C5a is expected to play a role, such as joint inflammation in arthritis, C5a expression could be placed under control of a tissue-specific promoter to examine its contribution to these phenomena.
In this report, we have expressed C5a in the murine mammary sarcoma EMT6 and have shown that this induced tumor regression in >30% of mice injected with a lethal dose of tumor cells. When using external measurements to determine tumor regression, it appears that differences between control versus C5a expressing tumors do not appear until days 14–18; however, the mean cell number per tumor is actually much smaller at day 10, indicating that gross external measurements are not indicative of internal processes. Mechanistic analyses indicate that there are both high levels of apoptosis early during regression as well as a block in the ability of cells expressing C5a to progress through the cell cycle.
When external measurements of tumor size are used to examine tumor growth, it would appear that the immune response to the tumor cells is not able to initially control the rapid growth. It is not until day 14–18 post-injection that there is a significant difference in mean tumor size. This would seem to be consistent with a T cell-mediated event whereby the lag time required to mount an immune response is due to the time in which there is expansion of the naïve T lymphocyte population into effector cells. When the tumors themselves are examined, however, it is readily evident that even by day 10, there are already processes underway that are controlling tumor growth including apoptosis and a block in cell cycle progression. Our data are consistent with both innate immune responses, perhaps due to macrophages and granulocytes and autocrine type responses mediated through interaction of C5a with the cell’s surface receptor.
One important issue in the in vivo experiments is the presence of carboxypeptidases. These enzymes (especially carboxypeptidase N) are responsible for cleaving the C-terminal arginine, which drastically decreases C5a function [7]. In our in vitro cultures, the only carboxypeptidases would come from fetal bovine serum, while in vivo, these enzymes would be abundant. This suggests that activity in vitro might not directly correlate with in vivo activity. This may be important in our proposed autocrine response because upon secretion, the C5a protein would not be exposed for long to extracellular carboxypeptidases before binding to the surface receptor, retaining full functionality.
The cellular populations that are responsible for the delayed tumor growth and regression are as yet unknown. Although histological analysis has demonstrated an increase in tumor infiltrates, the cell type responsible for tumor regression has not been identified. Significant numbers of T or B lymphocytes are not found in EMT6 tumors (data not shown). These data suggest that the normal infiltrating cells (macrophages and/or granulocytes) are activated upon contact with the high levels of C5a present in the tumor, leading to the direct (i.e., phagocytosis) or indirect (i.e., cytokine secretion or reactive oxygen production) killing of the tumor cells. It is important to note, however, that although the cells infiltrating the tumor would suggest an innate immune response, there is also a component of the acquired immune response since mice that reject their primary inoculation are immune to subsequent challenge with normal tumor cells (see Fig. 6). This may suggest that dendritic cells may also be involved in the post-rejection immunity. Although we were unable to detect significant number of DC in C5a-expressing EMT6 tumors (data not shown), DC are typically present in very small numbers and it may be that few cells are actually required to confer protective immunity upon secondary challenge.
The relationship between complement and tumor immunity is not clear. There were several reports demonstrating resistance of tumor cells to complement mediated lysis through enhanced expression of complement control proteins [9, 17, 24, 53]. In other cases, the susceptibility of tumors to complement was demonstrated. For instance, one report demonstrated direct complement-mediated destruction of micrometastases and small solid tumors from breast carcinoma and ovarian teratocarcinoma cell lines [18]. Along the same lines, the significance of complement activation during monoclonal antibody treatment in B-cell lymphoma was demonstrated as a fundamental mechanism in Rituximab therapeutic activity in vivo [12, 19]. In addition, a new pathway of complement action against tumors was described. It was shown that mannose binding protein (MBP) recognizes and binds specifically to oligosaccharide residues expressed on the surfaces of human colorectal carcinoma [37]. It was shown that C5a causes apoptosis in neuroblastoma cells. Expression of a C5aR-like molecule was demonstrated in the TGW neuroblastoma cell line and NC5aR (neuronal C5a receptor)-associated signal transduction system was linked to an apoptotic pathway in TGW neuroblastoma cells [15]. Finally, recent data from Ross et al. [22] have suggested that C5a may be involved in granulocytes recruitment and tumor killing in β-1,3-glucans treated mice. Collectively, these data demonstrate that there is a role for complement in tumor immunity.
The use of C5a as a direct therapeutic compound in cancer treatment is also suggested by our research. Indeed, C5a was utilized to induce tumor immunity in another report via a very different mechanism than the one described in our studies. A minimally active C5a peptide was fused to a tumor-specific peptide [51, 52]. The C5a portion bound to and was internalized by antigen presenting cells and presented in the context of MHC class II, which lead to the induction of an immune response to the C5a-bound peptide. Although this report presented data distinct from our study, it lends additional support for harnessing the complement system for induction of specific immune responses.
A difficulty with trying to utilize complement as a way to induce tumor immunity is that nearly all cells have receptors that control homologous lysis [27]. The method used in this report is not subject to control by these proteins since there is no known receptor expressed on tumor cells that inactivates C5a function. Although we have demonstrated that C5a enhances apoptosis and decreases cell cycle progression, the precise mechanism for these effects is not clear. One likely possibility is that C5a induces the migration of myeloid cells into the tumor and inflammatory mediators contribute to tumor cell death. Alternatively, C5a may be acting directly on EMT6 cells through an autocrine mechanism. Indeed, there are a number of non-myeloid cells that express the C5a receptor [21, 48, 55], and the function of the receptor on these cell types has not been examined. The results of the present study suggest that further investigation into the function of C5a in tumor immunology is warranted.
Acknowledgments
The authors would like to thank the following persons: Dr. Jenny P.-Y. Ting, in whose laboratory these experiments were initiated. Her help and guidance were invaluable for the completion of these studies; Dr. J. G. Frelinger for the gift of EMT6 tumor cells, the pHβ construct and the mouse IFN-γ gene; Dr. T. Collins for his help in setting up the apoptosis assays and Ting laboratory members for their helpful discussions; Dr. Scott Barnum for the gift of C5aR antibody. This work was supported by an American Cancer Society Seed Grant (IRG - IN122V) administered through the Holden Comprehensive Cancer Center of The University of Iowa.
References
- 1.Abe K, Miyazaki M, Koji T, Furusu A, Nakamura-Kurashige T, Nishino T, Ozono Y, Harada T, Sakai H, Kohno S. Enhanced expression of complement C5a receptor mRNA in human diseased kidney assessed by in situ hybridization. Kidney Int. 2001;60:137–146. doi: 10.1046/j.1523-1755.2001.00780.x. [DOI] [PubMed] [Google Scholar]
- 2.Ahearn JM, Fischer MB, Croix D, Goerg S, Ma M, Xia J, Zhou X, Howard RG, Rothstein TL, Carroll MC. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4:251–262. doi: 10.1016/S1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- 3.Antonia SJ, Extermann M, Flavell RA. Immunologic nonresponsiveness to tumors. Crit Rev Oncog. 1998;9:35–41. doi: 10.1615/critrevoncog.v9.i1.30. [DOI] [PubMed] [Google Scholar]
- 4.Bautsch W, Emde M, Kretzschmar T, Kohl J, Suckau D, Bitter-Suermann D. Human C5a anaphylatoxin: gene cloning and expression in Escherichia coli. Immunobiology. 1992;185:41–52. doi: 10.1016/S0171-2985(11)80316-5. [DOI] [PubMed] [Google Scholar]
- 5.Belmont HM, Hopkins P, Edelson HS, Kaplan HB, Ludewig R, Weissmann G, Abramson S. Complement activation during systemic lupus erythematosus. C3a and C5a anaphylatoxins circulate during exacerbations of disease. Arthritis Rheum. 1986;29:1085–1089. doi: 10.1002/art.1780290905. [DOI] [PubMed] [Google Scholar]
- 6.Cain SA, Monk PN. The orphan receptor C5L2 has high affinity binding sites for complement fragments C5a and C5a des Arg74. J Biol Chem. 2001;31:31. doi: 10.1074/jbc.C100714200. [DOI] [PubMed] [Google Scholar]
- 7.Campbell WD, Lazoura E, Okada N, Okada H. Inactivation of C3a and C5a octapeptides by carboxypeptidase R and carboxypeptidase N. Microbiol Immunol. 2002;46:131–134. doi: 10.1111/j.1348-0421.2002.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 8.Chakraborti T, Mandal A, Mandal M, Das S, Chakraborti S. Complement activation in heart diseases. Role of oxidants. Cell Signal. 2000;12:607–617. doi: 10.1016/S0898-6568(00)00111-X. [DOI] [PubMed] [Google Scholar]
- 9.Chen S, Caragine T, Cheung NK, Tomlinson S. Surface antigen expression and complement susceptibility of differentiated neuroblastoma clones. Am J Pathol. 2000;156:1085–1091. doi: 10.1016/S0002-9440(10)64976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colombo MP, Forni G. Immunotherapy. I: Cytokine gene transfer strategies. Cancer Metastasis Rev. 1997;16:421–432. doi: 10.1023/A:1005980418533. [DOI] [PubMed] [Google Scholar]
- 11.Davoust N, Nataf S, Reiman R, Holers MV, Campbell IL, Barnum SR. Central nervous system-targeted expression of the complement inhibitor sCrry prevents experimental allergic encephalomyelitis. J Immunol. 1999;163:6551–6556. [PubMed] [Google Scholar]
- 12.Di Gaetano N, Cittera E, Nota R, Vecchi A, Grieco V, Scanziani E, Botto M, Introna M, Golay J. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2003;171:1581–1587. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- 13.Ernst P. Review article: the role of inflammation in the pathogenesis of gastric cancer. Aliment Pharmacol Ther. 1999;13(Suppl 1):8–18. doi: 10.1046/j.1365-2036.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 14.Fang Y, Xu C, Fu YX, Holers VM, Molina H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J Immunol. 1998;160:5273–5279. [PubMed] [Google Scholar]
- 15.Farkas I, Baranyi L, Liposits ZS, Yamamoto T, Okada H. Complement C5a anaphylatoxin fragment causes apoptosis in TGW neuroblastoma cells. Neuroscience. 1998;86:903–11. doi: 10.1016/S0306-4522(98)00108-0. [DOI] [PubMed] [Google Scholar]
- 16.Gerard C, Gerard NP. C5A anaphylatoxin and its seven transmembrane-segment receptor. Annu Rev Immunol. 1994;12:775–808775808. doi: 10.1146/annurev.iy.12.040194.004015. [DOI] [PubMed] [Google Scholar]
- 17.Gorter A, Meri S. Immune evasion of tumor cells using membrane-bound complement regulatory proteins. Immunol Today. 1999;20:576–582. doi: 10.1016/S0167-5699(99)01537-6. [DOI] [PubMed] [Google Scholar]
- 18.Hakulinen J, Meri S. Complement-mediated killing of microtumors in vitro. Am J Pathol. 1998;153:845–855. doi: 10.1016/S0002-9440(10)65626-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harjunpaa A, Junnikkala S, Meri S. Rituximab (anti-CD20) therapy of B-cell lymphomas: direct complement killing is superior to cellular effector mechanisms. Scand J Immunol. 2000;51:634–641. doi: 10.1046/j.1365-3083.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann K, Henz BM, Kruger-Krasagakes S, Kohl J, Burger R, Guhl S, Haase I, Lippert U, Zuberbier T. C3a and C5a stimulate chemotaxis of human mast cells. Blood. 1997;89:2863–2870. [PubMed] [Google Scholar]
- 21.Haviland DL, McCoy RL, Whitehead WT, Akama H, Molmenti EP, Brown A, Haviland JC, Parks WC, Perlmutter DH, Wetsel RA. Cellular expression of the C5a anaphylatoxin receptor (C5aR): demonstration of C5aR on nonmyeloid cells of the liver and lung. J Immunol. 1995;154:1861–1869. [PubMed] [Google Scholar]
- 22.Hong F, Yan J, Baran JT, Allendorf DJ, Hansen RD, Ostroff GR, Xing PX, Cheung NK, Ross GD. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J Immunol. 2004;173:797–806. doi: 10.4049/jimmunol.173.2.797. [DOI] [PubMed] [Google Scholar]
- 23.Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, Takahashi K, Holers VM, Walport M, Gerard C, Ezekowitz A, Carroll MC, Brenner M, Weissleder R, Verbeek JS, Duchatelle V, Degott C, Benoist C, Mathis D. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–168. doi: 10.1016/S1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 24.Jurianz K, Ziegler S, Garcia-Schuler H, Kraus S, Bohana-Kashtan O, Fishelson Z, Kirschfink M. Complement resistance of tumor cells: basal and induced mechanisms. Mol Immunol. 1999;36:929–939. doi: 10.1016/S0161-5890(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 25.Kalant D, Cain SA, Maslowska M, Sniderman AD, Cianflone K, Monk PN. The chemoattractant receptor-like protein C5L2 binds the C3a des-Arg77/acylation-stimulating protein. J Biol Chem. 2003;278:11123–11129. doi: 10.1074/jbc.M206169200. [DOI] [PubMed] [Google Scholar]
- 26.Kaya Z, Afanasyeva M, Wang Y, Dohmen KM, Schlichting J, Tretter T, Fairweather D, Holers VM, Rose NR. Contribution of the innate immune system to autoimmune myocarditis: a role for complement. Nat Immunol. 2001;2:739–745. doi: 10.1038/90686. [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita T. Biology of complement: the overture. Immunol Today. 1991;12:291–295. doi: 10.1016/0167-5699(91)90001-A. [DOI] [PubMed] [Google Scholar]
- 28.Kirchhoff K, Weinmann O, Zwirner J, Begemann G, Gotze O, Kapp A, Werfel T. Detection of anaphylatoxin receptors on CD83+ dendritic cells derived from human skin. Immunology. 2001;103:210–217. doi: 10.1046/j.1365-2567.2001.01197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kupp LI, Kosco MH, Schenkein HA, Tew JG. Chemotaxis of germinal center B cells in response to C5a. Eur J Immunol. 1991;21:2697–2701. doi: 10.1002/eji.1830211108. [DOI] [PubMed] [Google Scholar]
- 30.Martin BK, Chin KC, Olsen JC, Skinner CA, Dey A, Ozato K, Ting JP. Induction of MHC class I expression by the MHC class II transactivator CIITA. Immunity. 1997;6:591–600. doi: 10.1016/S1074-7613(00)80347-7. [DOI] [PubMed] [Google Scholar]
- 31.Martin BK, Weis JH. Functional identification of transcription control sequences of the mouse Crry gene. J Immunol. 1993;151:857–869. [PubMed] [Google Scholar]
- 32.McAdam AJ, Felcher A, Woods ML, Pulaski BA, Hutter EK, Frelinger JG, Lord EM. Transfection of transforming growth factor-beta producing tumor EMT6 with interleukin-2 elicits tumor rejection and tumor reactive cytotoxic T-lymphocytes. J Immunother Emphasis Tumor Immunol. 1994;15:155–164. doi: 10.1097/00002371-199404000-00001. [DOI] [PubMed] [Google Scholar]
- 33.McAdam AJ, Pulaski BA, Harkins SS, Hutter EK, Lord EM, Frelinger JG. Synergistic effects of co-expression of the TH1 cytokines IL-2 and IFN- gamma on generation of murine tumor-reactive cytotoxic cells. Int J Cancer. 1995;61:628–634. doi: 10.1002/ijc.2910610508. [DOI] [PubMed] [Google Scholar]
- 34.Molina H, Holers VM, Li B, Fung Y, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, Chaplin DD. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci USA. 1996;93:3357–3361. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morelli A, Larregina A, Chuluyan I, Kolkowski E, Fainboim L. Expression and modulation of C5a receptor (CD88) on skin dendritic cells. Chemotactic effect of C5a on skin migratory dendritic cells. Immunology. 1996;89:126–34. doi: 10.1046/j.1365-2567.1996.d01-701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mrowietz U, Koch WA, Zhu K, Wiedow O, Bartels J, Christophers E, Schroder JM. Psoriasis scales contain C5a as the predominant chemotaxin for monocyte-derived dendritic cells. Exp Dermatol. 2001;10:238–245. doi: 10.1034/j.1600-0625.2001.100403.x. [DOI] [PubMed] [Google Scholar]
- 37.Muto S, Sakuma K, Taniguchi A, Matsumoto K. Human mannose-binding lectin preferentially binds to human colon adenocarcinoma cell lines expressing high amount of Lewis A and Lewis B antigens. Biol Pharm Bull. 1999;22:347–352. doi: 10.1248/bpb.22.347. [DOI] [PubMed] [Google Scholar]
- 38.Nataf S, Davoust N, Ames RS, Barnum SR. Human T cells express the C5a receptor and are chemoattracted to C5a. J Immunol. 1999;162:4018–4023. [PubMed] [Google Scholar]
- 39.Nataf S, Stahel PF, Davoust N, Barnum SR. Complement anaphylatoxin receptors on neurons: new tricks for old receptors? Trends Neurosci. 1999;22:397–402. doi: 10.1016/S0166-2236(98)01390-3. [DOI] [PubMed] [Google Scholar]
- 40.Niculescu F, Rus H. Complement activation and atherosclerosis. Mol Immunol. 1999;36:949–955. doi: 10.1016/S0161-5890(99)00117-0. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. A neural network method for identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Int J Neural Syst. 1997;8:581–599. doi: 10.1142/S0129065797000537. [DOI] [PubMed] [Google Scholar]
- 42.Okinaga S, Slattery D, Humbles A, Zsengeller Z, Morteau O, Kinrade MB, Brodbeck RM, Krause JE, Choe HR, Gerard NP, Gerard C. C5L2, a nonsignaling C5A binding protein. Biochemistry. 2003;42:9406–9415. doi: 10.1021/bi034489v. [DOI] [PubMed] [Google Scholar]
- 43.Ottonello L, Corcione A, Tortolina G, Airoldi I, Albesiano E, Favre A, D’Agostino R, Malavasi F, Pistoia V, Dallegri F. rC5a directs the in vitro migration of human memory and naive tonsillar B lymphocytes: implications for B cell trafficking in secondary lymphoid tissues. J Immunol. 1999;162:6510–6517. [PubMed] [Google Scholar]
- 44.Pardoll DM. Inducing autoimmune disease to treat cancer. Proc Natl Acad Sci USA. 1999;96:5340–5342. doi: 10.1073/pnas.96.10.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–594. doi: 10.1016/S0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 46.Parent CA, Devreotes PN. A cell’s sense of direction. Science. 1999;284:765–770. doi: 10.1126/science.284.5415.765. [DOI] [PubMed] [Google Scholar]
- 47.Sogn JA. Tumor immunology: the glass is half full. Immunity. 1998;9:757–763. doi: 10.1016/S1074-7613(00)80641-X. [DOI] [PubMed] [Google Scholar]
- 48.Soruri A, Kim S, Kiafard Z, Zwirner J. Characterization of C5aR expression on murine myeloid and lymphoid cells by the use of a novel monoclonal antibody. Immunol Lett. 2003;88:47–52. doi: 10.1016/S0165-2478(03)00052-X. [DOI] [PubMed] [Google Scholar]
- 49.Sozzani S, Sallusto F, Luini W, Zhou D, Piemonti L, Allavena P, Van Damme J, Valitutti S, Lanzavecchia A, Mantovani A. Migration of dendritic cells in response to formyl peptides, C5a, and a distinct set of chemokines. J Immunol. 1995;155:3292–3295. [PubMed] [Google Scholar]
- 50.Stroh C, Schulze-Osthoff K. Death by a thousand cuts: an ever increasing list of caspase substrates. Cell Death Differ. 1998;5:997–1000. doi: 10.1038/sj.cdd.4400451. [DOI] [PubMed] [Google Scholar]
- 51.Tempero RM, Hollingsworth MA, Burdick MD, Finch AM, Taylor SM, Vogen SM, Morgan EL, Sanderson SD. Molecular adjuvant effects of a conformationally biased agonist of human C5a anaphylatoxin. J Immunol. 1997;158:1377–1382. [PubMed] [Google Scholar]
- 52.Ulrich JT, Cieplak W, Paczkowski NJ, Taylor SM, Sanderson SD. Induction of an antigen-specific CTL response by a conformationally biased agonist of human C5a anaphylatoxin as a molecular adjuvant. J Immunol. 2000;164:5492–5498. doi: 10.4049/jimmunol.164.10.5492. [DOI] [PubMed] [Google Scholar]
- 53.Varsano S, Rashkovsky L, Shapiro H, Ophir D, Mark-Bentankur T. Human lung cancer cell lines express cell membrane complement inhibitory proteins and are extremely resistant to complement-mediated lysis; a comparison with normal human respiratory epithelium in vitro, and an insight into mechanism(s) of resistance. Clin Exp Immunol. 1998;113:173–182. doi: 10.1046/j.1365-2249.1998.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Rollins SA, Madri JA, Matis LA. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. Proc Natl Acad Sci USA. 1995;92:8955–8959. doi: 10.1073/pnas.92.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wetsel RA. Expression of the complement C5a anaphylatoxin receptor (C5aR) on non- myeloid cells. Immunol Lett. 1995;44:183–187. doi: 10.1016/0165-2478(94)00212-A. [DOI] [PubMed] [Google Scholar]
- 56.Wetsel RA. Structure, function and cellular expression of complement anaphylatoxin receptors. Curr Opin Immunol. 1995;7:48–53. doi: 10.1016/0952-7915(95)80028-X. [DOI] [PubMed] [Google Scholar]
- 57.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Recognition of gram-positive bacterial cell wall components by the innate immune system occurs via toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]