Abstract
Cyclooxygenase (COX) inhibitors have demonstrated efficacy in models of human cancer but the relevant mechanisms have not all been elucidated. Both Cox-dependent as well as Cox-independent mechanisms have been implicated. Using a syngeneic model of metastatic breast cancer, we have investigated the effect of Cox inhibitors on NK functions that are critical to the control of metastatic disease. NK recognition of target cells is governed by a balance of activating and inhibiting receptors that bind ligands including MHC class I. We now show that treatment of tumor cells with the nonselective COX-1/COX-2 inhibitor indomethacin or the selective COX-2 inhibitor celecoxib leads to decreased expression of the MHC class I molecules Ld and Kd . Downregulated class I expression is associated with concomitant increased sensitivity to NK cell-mediated lysis. Both COX inhibitors limit tumor metastasis and this therapeutic effect is dependent on NK but not T cell function. Antimetastatic activity is also lost in the absence of interferon- γ (IFN-γ). Both COX inhibitors also suppress local tumor growth of subcutaneously implanted mammary tumor cells in immune competent Balb/cByJ mice. This therapeutic activity is lost in the absence of either CD4+ or CD8+ T cells, but is not compromised by the loss of NK activity. Thus, the mechanism of tumor inhibition differs in the context of local versus metastatic disease. Taken together, these findings are consistent with a mechanism not previously described, whereby COX inhibitors may relieve MHC-mediated inhibition of NK cytotoxicity leading to recognition and lysis of metastatic tumor cells.
Keywords: Cyclooxygenase inhibitors, COX-2, NK recognition, Breast cancer immunity, Metastasis, MHC class I
Introduction
We have reported that nonselective (indomethacin) and selective (celecoxib, SC560) cyclooxygenase inhibitors limit growth and metastasis of highly aggressive murine mammary carcinomas [11]. Many mechanisms have been proposed to explain the therapeutic effects of cyclooxygenase inhibitors (coxibs) demonstrated against many tumor types. Evidence for direct inhibition of tumor cell proliferation, blockade of cell cycle progression, induction of apoptosis and indirect effects on tumor angiogenesis, have been reported [9, 16–18, 28]. Using a syngeneic murine model of metastatic breast cancer, we asked whether some of the beneficial effects of coxibs are attributable to modulation of tumor-protective immune mechanisms. The current report indicates that immune effector mechanisms contribute to therapeutic effects of coxibs, but the critical effector cell is context-dependent. We provide evidence for a novel mechanism by which coxibs modulate NK recognition leading to enhanced tumor cell lysis.
Materials and methods
Cell lines and tumors
Murine mammary tumor cell line 410.4 is maintained in DMEM supplemented with 10% FCS (Gemini BioProducts, Inc., Calabasas, CA, USA), 2 mM glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 1.5 g/L sodium bicarbonate and 0.1 mM nonessential amino acids. For growth studies in vivo, 5×105 viable cells were injected into the mammary fat pad of syngeneic immune competent Balb/cByJ and Balb/c IFN-γ-deficient (Jackson Laboratories, Bar Harbor, ME, USA), and immune compromised C.B-17/IcrCrl-Balb/SCID/BR and C.B-17/IcrCrl-Balb/SCID/Beige (Charles River Laboratories, Wilmington, MA, USA) female mice. For experimental metastasis assays, 3×105 tumor cells were injected into the lateral tail vein, and 21 days later, the mice were sacrificed and pulmonary metastases were quantified.
Cox inhibitor treatments
The selective COX-2 inhibitor, celecoxib, was a generous gift of Pharmacia (St. Louis, MO, USA). The dual COX inhibitor, indomethacin, was purchased from Sigma Chemical Co. (St. Louis). Both drugs were dissolved in a solution of methylcellulose (0.5%) and Tween 20 (0.025%) and administered by oral gavage twice/day to achieve a dose of 5/mg/kg/day (celecoxib) or 1 mg/kg/day (indomethacin). Control animals were gavaged daily with vehicle. To determine the direct effect of COX inhibitors on tumor cells, in the absence of host effects, 410.4 cells were cultured in the presence of indomethacin, celecoxib (1.0 μM) DMSO or ethanol for 48 h, washed, and 3×105 viable tumor cells were injected into the tail vein of untreated mice. No further drug treatments were carried out.
Antibody depletion
To deplete NK cells, mice were injected with rabbit asialoGM1 ganglioside antibody (20 μl, Wako Bioproducts, Richmond, VA, USA). CD4+ and CD8+ cells were depleted with rat hybridoma supernatants (500 μl ATCC 2.43 and ATCC GK1.5, respectively, ATCC, Rockville, MD, USA). Control animals were treated with normal rabbit serum (asialoGM1) or normal rat Ig (CD4, CD8 depletions). All antibody treatments were administered 1 day prior to and either 3 days (metastasis assays) or 7 days (mammary fat pad tumors) after tumor introduction. These protocols deplete approximately 50% of non-MHC-restricted tumor cell lysis; approximately 50% of CD4+ T cells and 90% of CD8+ cells spleen cells are depleted as indicated by flow cytometry.
Flow cytometry
Tumor cells were cultured in OPTIMEM medium for 48 h in the presence of COX inhibitors or appropriate vehicle for 48 h and stained for MHC class I as described previously [12]. Cells were reacted with antibody to H-2L d (clone 30-5-7) or H-2K d (clone SF1-1.1.1). Secondary antibody was fluorescein-conjugated goat-anti-mouse IgG (BD Biosciences Pharmingen, San Diego, CA, USA). Fluorescence analyzed by FACScan flow cytometer.
NK cytotoxicity assays
Assays were carried out as described in Ref. 12. Tumor target cells were labeled with [3 H] proline, washed and plated in 96-well flat-bottom microtiter plates in the presence of Cox inhibitors at the indicated concentrations. After a further 48 h, single cell suspensions of spleen effector cells from normal Balb/cByJ female mice (stimulated with 100 μg of poly-IC ip 24 h prior to sacrifice) were added at a 100:1 effector to target ratio. Lytic activity is linear using ratios of 1:10, 1:50 or 1:100. After a further 18 h incubation, nonadherent cells were aspirated, and radioactivity in the remaining adherent cells was determined. Cytotoxicity was determined as: % cytotoxicity = 1−(cpm remaining in experimental well/cpm remaining in medium control well)×100.
Statistical analysis
Degree of lysis, MHC class I expression, metastasis data and tumor size comparisons at individual time points by Student’s t test.
Results
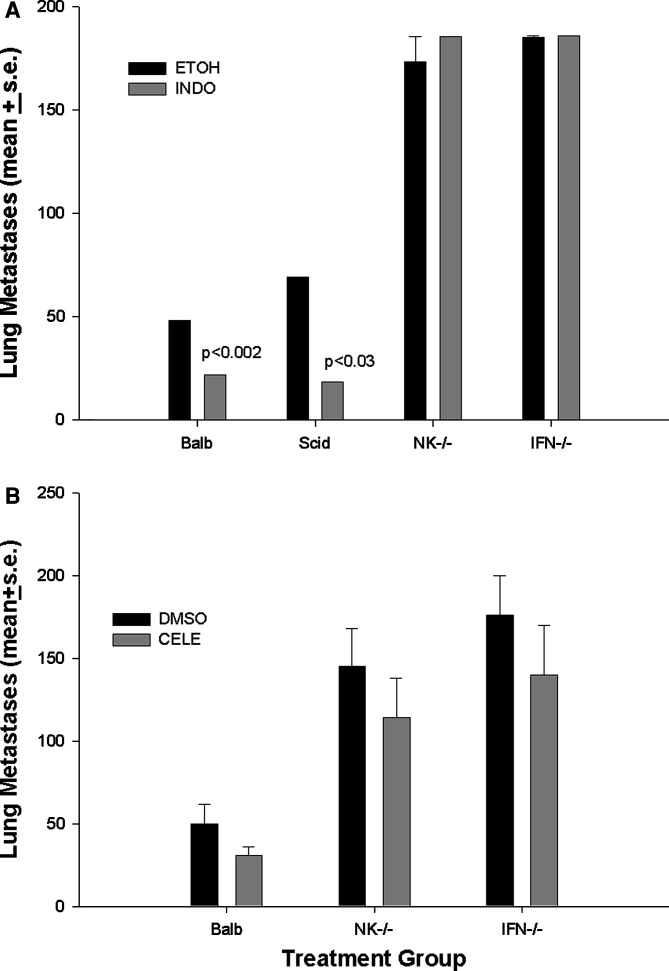
Therapeutic activity of COX inhibition has been examined in a number of rodent models [10,11, 17, 19, 22, 25, 27]. These studies have implicated several mechanisms of therapeutic action including tumor cell apoptosis, and inhibition of both proliferation and angiogenesis. Suppressive effects of the COX-2 product, PGE2, on immune effector cells are also well documented [3, 4]. Nevertheless, the precise mechanisms by which COX inhibitors limit tumor growth are not entirely elucidated. We determined the effect of COX inhibitors on tumor metastasis and examined the requirements for immune effector populations in control of metastatic dissemination. In order to isolate effects of COX inhibitors on tumor cells, we pretreated 410.4 mammary tumor cells with COX inhibitors prior to intravenous administration of tumor cells into either immune competent Balb/cByJ mice, or immune incompetent severe combined immune deficient SCID mice, NK-depleted mice or mice lacking interferon-γ (IFN- γ). Pretreatment of 410.4 tumor cells with Indo (1 μM), prior to intravenous injection of tumor cells, resulted in a significant reduction in the number of experimental metastases (55% inhibition) (Fig. 1a). Indo was still effective at controlling metastases in SCID mice lacking mature T and B cell function. The ability of Indo to limit tumor metastases was, however, completely abrogated in mice lacking either NK function or IFN-γ. Likewise, treatment with celecoxib (1.0 μM) resulted in a 38% decrease in lung metastases (Fig. 1b). Like Indo, the therapeutic effect of celecoxib was compromised in either NK-depleted or IFN-γ-depleted mice. Thus, NK function is critical to the mechanism by which coxibs control metastatic dissemination.
Fig. 1.
a Tumor cells were cultured in Indo (1.0 μM) or ethyl alcohol for 48 h, washed and injected into the tail vein of syngeneic Balb/cByJ, Balb/Scid, or Balb/IFNγ -/- mice as well as Balb/cByJ mice treated with asialoGM1 antibody (NK-/-). b Tumor cells cultured with celecoxib (1.0 μM) or DMSO and injected into Balb/cByJ, Balb/IFNγ-/- or NK depleted mice. Twenty days later, the mice were sacrificed and surface lung tumor colonies were quantified. Mean ± SE of eight to ten mice per group. P values are for drug-treated cells versus vehicle-treated cells in the same host strain
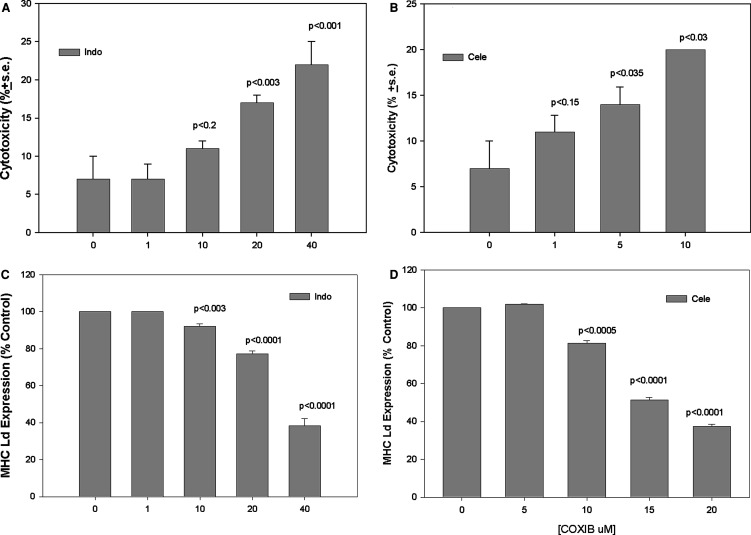
To examine potential mechanisms by which coxibs and NK interact, we determined the effect of coxibs on NK-mediated lysis of mammary tumor cells. Labeled 410.4 target cells were cultured in the presence of Indo or celecoxib for 48 h, spleen cells from normal mice were added to tumor targets and tumor cell lysis was analyzed. Figure 2a shows that vehicle-treated 410.4 cells are relatively resistant to non-MHC restricted cytotoxicity, however, the addition of either Indo (Fig. 2a) or celecoxib (Fig. 2b) resulted in statistically significant increased lytic activity in a dose-dependent manner. Coxibs induce apoptosis of many cells and the apparent increased NK-lytic sensitivity could have resulted from direct apoptosis of the tumor target cell. This is an unlikely mechanism, however, since these cells have been shown to be resistant to apoptosis-induction by coxibs [13]. Furthermore, lytic activity indicated in the figure is corrected for spontaneous killing in the absence of effector cells and thus reflects a true increase in lysis mediated by immune effector cells.
Fig. 2.
a Tumor cells were cultured in the presence of Indo at the indicated concentrations for 48 h, drug was removed, after which spleen cells from poly IC-treated normal Balb/cByJ mice were added and degree of lysis determined 18 h later. Mean ± SE of triplicate determinations. b Lysis assays carried out as in a, with the addition of celecoxib at the indicated concentrations. c. Tumor cells were cultured in Optimem medium containing Indo at the indicated concentrations for 48 h, and stained for MHC class I Ld and analyzed by flow cytometry. Results expressed as percent change in mean fluorescence intensity. d Tumor cells cultured in medium containing celecoxib and analyzed by flow cytometry. Mean of triplicate determinations. All P values versus vehicle-treated cells
The immunosuppressive role of the cyclooxygenase product PGE2 on NK activities is documented [4, 15]. It is not surprising, therefore, that in the presence of Indo or celecoxib, increased NK lytic activity is observed. This data is consistent with a model in which tumor-PGE2 directly suppresses NK function. We considered the possibility that intrinsic properties of the tumor cells were also changed, rendering the cells more sensitive to NK-mediated killing. Activation of NK recognition and target cell lysis is mediated by loss of MHC class I expression [24]. We tested the hypothesis that coxibs might modulate expression of MHC class I molecules on tumor target cells. We cultured 410.4 tumor cells in the presence of increasing concentrations of Indo or celecoxib and examined MHC class I expression by flow cytometry. Figure 2c shows that treatment of 410.4 cells with Indo or celecoxib (2d) resulted in partial loss of expression of MHC class I at the Ld locus in a dose-dependent manner. Downregulation at the Kd locus was also observed. Treatment with celecoxib at 5,10, 15 or 20 μM reduced MHC Kd by 1, 4, 7 and 25%, respectively. Thus, celecoxib affected the expression of both loci, but the effect on Ld expression was more pronounced. To our knowledge, this is the first report that coxibs can modulate MHC class I expression.
To rule out nonspecific effects of coxibs on MHC expression, we determined if addition of exogenous PGE2 could reverse the coxib-mediated suppression of class I expression. Cells were treated with Indo (20.0 or 40.0 μM) and PGE2 (1.0 or 10 μM) was also added to some cultures. In the presence of Indo alone, MHC Kd was decreased in comparison to vehicle-treated control cells (Fig. 3). When PGE2 was added to Indo-treated cells, MHC expression was restored to vehicle control levels. Thus, exogenous PGE2 was able to reverse the Indo-mediated decrease in MHC class I expression. Similarly, downregulation of MHC expression by celecoxib was completely reversed when cells were also exposed to IFN-γ (100 units/ml) for 24 h (data not shown).
Fig. 3.
Tumor cells were cultured in vehicle (closed bar) or Indo at 20.0 or 40 μM (open bars) and some cells were also exposed to PGE2 (1.0 or 10.0 μM, hatched bars) for 48 h, stained for MHC class I Kd and analyzed by flow cytometry. All P values versus vehicle-treated cells
Immune effector mechanisms that control tumor behavior can differ depending on whether local tumor growth or metastatic dissemination is considered. Thus, NK cells are clearly important to coxib-mediated control of metastasis. We considered the possibility that different effector cells control local disease. To examine the potential contribution of immune function to the observed therapeutic effects of COX inhibitors on local tumor growth, we transplanted 5×105 of line 410.4 tumor cells to immunologically competent Balb/cByJ mice or to immunodeficient Balb/SCID or Balb/SCID/Beige female mice. Therapeutic efficacy of Indo and celecoxib was compared in these three strains of mice. Drugs or vehicle control were administered by oral gavage until day 28. Figure 4 shows that administration of either drug resulted in statistically significant inhibition of tumor size in immune-competent Balb/cByJ mice in comparison with vehicle-treated controls. Indo or celecoxib-mediated tumor growth inhibition was completely lost in animals lacking T and B lymphocyte effector functions (SCID) or mice with an additional loss of NK cell function (SCID/Beige).
Fig. 4.
Five×105 line 410.4 tumor cells were injected s.c. into either syngeneic Balb/cByJ, Balb/c/Scid or Balb/c/Scid/Beige mice on day zero, and animals were treated by oral gavage on a daily basis with either vehicle, indomethacin (Indo) or celecoxib (Cele) at a dosage of 1 mg/kg or 5 mg/kg/day, respectively. Tumor diameters were measured by caliper and plotted as mean ± SE of eight to ten mice per group. P values indicated as *, <0.05; **, < 0.01 comparing Indo, Balb versus Veh, Balb; Cele, Balb versus Veh, Balb
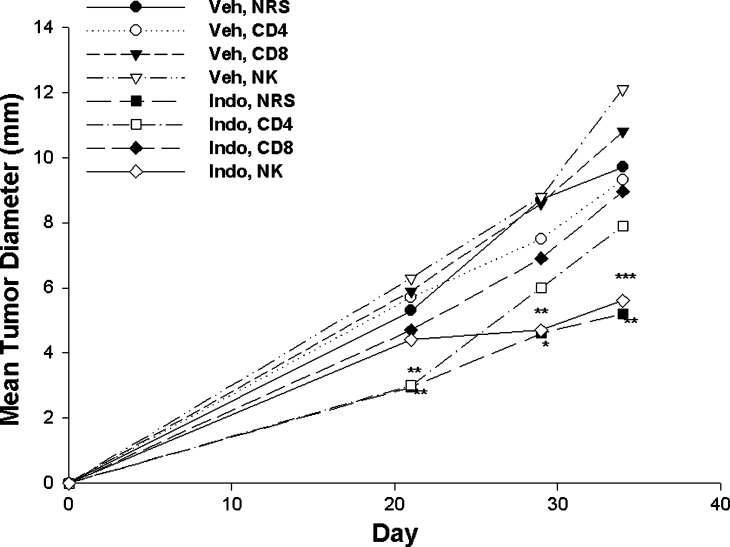
To further examine the role of T cell subsets and to more directly investigate the role of NK cells, the therapeutic activity of Indo was evaluated in Balb/cByJ mice depleted of CD4+ or CD8+ cells or NK cells (Fig. 5). Depletion of either CD4+ or CD8+ cells compromised the tumor growth inhibition mediated by Indo, whereas loss of NK activity had no apparent effect on therapeutic activity. In contrast to control of metastatic disease, these two experiments implicate a role for T lymphocytes in the mechanism by which Coxibs limit growth of locally growing tumors, but indicate that this therapeutic effect is not dependent on NK function.
Fig. 5.
Five×105 line 410.4 tumor cells were injected on day zero into syngeneic Balb/cByJ female mice treated with either normal rat or rabbit serum, anti-CD4 or anti-CD8 rat hybridoma supernatant or anti-asiaoloGM1 rabbit antibody on day −1 and day +7. Oral Indo or vehicle control administered as in Fig. 4. Tumor diameters were measured by caliper and plotted as mean ± SE of eight to ten mice per group. P values indicated as *, < 0.04; **, <0.006; ***, <0.001, comparing Indo, NRS to Veh, NRS; Indo, anti-asialoGM1 versus Veh, anti-asialoGM1
Discussion
High COX-2 expression is a common feature of many tumors including those of the breast [1, 21, 26]. It has been known for many years that increased COX activity, in the form of prostaglandin synthesis and, more recently, expression of the COX-2 protein, is associated with more aggressive disease and increased likelihood of metastatic disease [1, 21]. Furthermore, epidemiological evidence supports a protective role for nonsteroidal anti-inflammatory drugs in breast cancer incidence [6–8]. These findings have resulted in the initiation of a number of clinical trials in breast, as well as other cancers, to evaluate efficacy of COX-2 inhibitors.
We have shown that, in a murine model that mimics metastatic human breast cancer, high COX-2 expression and enzymatic activity are associated with more tumorigenic and metastatic behavior [2, 14]. These tumors also express the COX-1 isoform, and we reported that selective COX-1 and COX-2 inhibitors, as well as a dual COX-1/COX-2 inhibitor, reduce tumor size and inhibit metastasis [11]. Other laboratories have also shown the protective effects of COX inhibitors both in preventing tumor induction and inhibiting growth of transplanted tumors [10, 17, 19, 22, 27]. Those studies have implicated several mechanisms underlying the therapeutic effects. Direct effects of COX inhibitors on tumor cells have been demonstrated including inhibition of proliferation [9] and induction of apoptosis [16]. Other studies support an indirect effect of COX inhibitors on tumor angiogenesis [17, 18, 22, 28].
We now demonstrate a role for immune effector cells in coxib-mediated tumor inhibition. Using both cell-depletion and genetic approaches, we show that control of local tumor growth depends on both CD4+ and CD8+ T cells, but not NK cells. Although NK cells appeared not to be important to the mechanism by which coxibs control local tumor growth, it was important to examine the role of NK cells in a model of metastatic disease. We had shown previously, and confirm here, that pretreatment of 410.4 tumor cells with sublethal concentrations of Indo, Celecoxib or NS398, prior to intravenous introduction into immune competent mice, reduced the number of lung tumor colonies by 50–89% [11]. The current results show that control of metastatic disease by COX inhibitors is dependent on both NK function and IFN-γ, but, in contrast to local disease, does not require functional T cells. Thus, coxibs are effective at controlling local tumors as well as metastatic disease, but the critical effector cell depends on the tumor site.
COX inhibitor-mediated control of tumor growth has been reported in other tumor models [10, 15, 17, 27]. Some of these studies were carried out using xenotransplants in immune-incompetent hosts. Those studies have identified apoptosis induction and inhibition of tumor angiogenesis as critical therapeutic mechanisms. The current studies identify additional immune-dependent mechanisms. The relative dependence on immunologic versus nonimmunologic mechanisms many vary for different model systems. Models in which tumor inhibition is achieved by other mechanisms typically employ higher doses of celecoxib (25 mg/kg) which may be necessary to reveal immune-independent mechanisms. In the current study, sub-optimal drug doses were used that do not result in complete tumor inhibition but may unmask previously unappreciated immune-dependent mechanisms. Furthermore, both Cox-dependent and Cox-independent effects of COX inhibitors have been described. It will be interesting to determine if COX-independent therapeutic effects are also independent of immune effector mechansisms.
Recently, the mechanisms by which NK cells recognize and kill tumor targets have been clarified [24]. Through a repertoire of receptors, NK cells receive both stimulatory and inhibitory signals from ligands expressed on the potential target cell. Inhibitory signals are mediated by binding of MHC molecules, expressed on the target cell, to cognate inhibitory receptors on the NK effector cells. The current data reports the novel finding that two COX inhibitors downregulate expression of MHC class I molecules (Ld and Kd) on tumor cells. The reversal of the MHC class I downregulation by exogenous PGE2 indicates that the effect of COX inhibitors on class I is COX-dependent. Decreased MHC class I expression is associated with increased NK-mediated lysis. These data suggest an additional mechanism whereby COX inhibitors can enhance NK-mediated lysis. By decreasing the expression of inhibitory signals mediated by MHC class I, NK cells are activated to lyse tumor cell targets. This study also shows that IFN-γ is critical to controlling metastasis by COX inhibitors. IFN-γ modulates many functions including NK activities. Consistent with our findings, a recent report shows that PGE2 suppresses IFN-γ synthesis by NK cells [29]. The finding that IFN- γ can upregulate MHC class I appears to conflict with the mechanism of NK activation proposed in this study. Although further studies will be required to resolve this issue, it is possible that the role of IFN-γ is context-dependent. Thus, IFN-γ may be required at the site of NK activation in peripheral sites, but does not affect MHC expression at the tumor site. Our studies and earlier work from other laboratories [4, 15] implicate NK cells as important targets of COX therapy. There is increasing evidence that dendritic cells are also important targets of PGE2-mediated immune suppression [5, 20, 23, 30]. Those findings have obvious implications for the induction of tumor-specific T cell-mediated immunologic responses. Thus, tumor-PGE2 has effects on both innate and adaptive anti-tumor immune responses.
These findings add to the growing body of evidence that coxibs have therapeutic activity in models of human cancer. They expand our knowledge by showing that, in addition to direct effects of coxibs on tumor cell proliferation and apoptosis and indirect effects on tumor angiogenesis, coxibs also modulate protective antitumor immune effector functions leading to control of tumor growth and metastasis. Further elucidation of the relevant mechanisms will be important in the design of combination therapies employing immune-based and COX-targeted strategies.
Acknowledgements
Supported by the United States Department of Defense and the Department of Health and Human Services (to A.M.F.).
References
- 1.Bennett A, Berstock DA, Raja B, Stamford IF. Survival time after surgery is inversely related to the amounts of prostaglandins extracted from human breast cancers. Br J Pharmacol. 1970;66:451–55. [PMC free article] [PubMed] [Google Scholar]
- 2.Fulton AM, Heppner G. Relationship of prostaglandin E and natural killer sensitivity to metastatic potential in murine mammary adenocarcinomas. Cancer Res. 1985;45:4779–84. [PubMed] [Google Scholar]
- 3.Goodwin JS, Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983;3:295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- 4.Goto T, Herberman RB, Maluish A, Strong DM. Cyclic AMP as a mediator of prostaglandin E-induced suppression of human natural killer cell activity. J Immunol. 1983;130:1350–1357. [PubMed] [Google Scholar]
- 5.Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin E(2) enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol. 2002;168:2255–2263. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- 6.Harris RE, Chlebowski RT, Jackson RD, Frid DJ, Ascenseo JL, Anderson G, Loar A, Rodabough RJ, White E, McTiernan A. Breast cancer and nonsteroidal anti- inflammatory drugs: prospective results from the Women’s Health Initiative. Cancer Res. 2003;63:6096–6101. [PubMed] [Google Scholar]
- 7.Harris RE, Namboodiri KK, Farrar WB. Nonsteroidal antiinflammatory drugs and breast cancer. Epidemiology. 1996;7:203–205. doi: 10.1097/00001648-199603000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Harris RE, Namboodiri KK, Stellman SD, Wynder EL. Breast cancer and NSAID use: heterogeneity of effect in a case-control study. Prev Med. 1995;24:119–120. doi: 10.1006/pmed.1995.1022. [DOI] [PubMed] [Google Scholar]
- 9.Hung W-C, Chang H.-C, Pan M-R, Lee T-H, Chuang L-Y. Induction of p27 KIP1 as a mechanism underlying NS398-induced growth inhibition in human lung cancer cells. Mol Pharmacol. 2000;58:1398–1403. doi: 10.1124/mol.58.6.1398. [DOI] [PubMed] [Google Scholar]
- 10.Joarder FS, Abou-Issa H, Robertson FM, Parrett ML, Alshafie G, Harris RW. Growth arrest of DMBA-induced mammary carcinogenesis with ibuprofen treatment in female Sprague-Dawley rats. Oncol Rep. 1997;4:1271–1273. doi: 10.3892/or.4.6.1271. [DOI] [PubMed] [Google Scholar]
- 11.Kundu N, Fulton AM. Selective cyclooxygenase (COX)-1 or COX-2 inhibitors control metastatic disease in a murine model of breast cancer. Cancer Res. 2002;62:2343–2346. [PubMed] [Google Scholar]
- 12.Kundu N, Fulton AM. Interleukin-10 inhibits tumor metastasis, downregulates MHC class I and enhances NK lysis. Cell Immunol. 1997;180:55–61. doi: 10.1006/cimm.1997.1176. [DOI] [PubMed] [Google Scholar]
- 13.Kundu N, Smyth MJ, Samsel L, Fulton AM. Cyclooxygenase inhibitors block cell growth, increase ceramide and inhibit cell cycle. Breast Cancer Res Treat. 2002;76:57–64. doi: 10.1023/A:1020224503335. [DOI] [PubMed] [Google Scholar]
- 14.Kundu N, Yang Q, Dorsey R, Fulton AM. Increased cyclooxygenase-2 (Cox-2) expression and activity in a murine model of metastatic breast cancer. Int J Cancer. 2001;93:681–686. doi: 10.1002/ijc.1397. [DOI] [PubMed] [Google Scholar]
- 15.Lala PK, Parhar RS, Singh P. Indomethacin therapy abrogates the prostaglandin-mediated suppression of natural killer activity in tumor-bearing mice and prevents tumor metastasis. Cell Immunol. 1986;99:108–118. doi: 10.1016/0008-8749(86)90220-0. [DOI] [PubMed] [Google Scholar]
- 16.Liu X-H, Yao S, Kirschenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58:4245–4249. [PubMed] [Google Scholar]
- 17.Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner M, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 18.Narko K, Ristimaki A, MacPhee M, Smith E, Haudenschild CC, Hla T. Tumorigenic transformation of immortalized ECV endothelial cells by cyclooxygenase-1 overexpression. J Biol Chem. 1997;272:21455–21460. doi: 10.1074/jbc.272.34.21455. [DOI] [PubMed] [Google Scholar]
- 19.Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. Suppression of intestinal polyposis in APC delta716 knockout mice by inhibition of cyclooxygenase (COX-2) Cell. 1996;87:803–809. doi: 10.1016/S0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 20.Pockaj BA, Basu GD, Pathangey LB, Gray RJ, Hernandez JL, Gendler S, Mukherjee P. Reduced T-cell and dendritic cell function is related to cyclooxygenase-2 overexpression and prostaglandin E2 secretion in patients with breast cancer. Ann Surg Oncol. 2004;11:328–339. doi: 10.1245/ASO.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 21.Ristimaki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, Joensuu H, Isola J. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635. [PubMed] [Google Scholar]
- 22.Rozic JG, Chakraborty C, Lala PK. Cyclooxygenase inhibitors retard murine mammary tumor progression by reducing tumor cell migration, invasiveness and angiogenesis. Int J Cancer. 2001;93:497–506. doi: 10.1002/ijc.1376. [DOI] [PubMed] [Google Scholar]
- 23.Sharma S, Stolina M, Yang SC, Baratelli F, Lin JF, Atianzar K, Luo J, Zhu L, Lin Y, Huang M, Dohadwala M, Batra RK, Dubinett SM. Tumor cyclooxygenase-2-dependent suppression of dendritic cell function. Clin Cancer Res. 2003;9:961–968. [PubMed] [Google Scholar]
- 24.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural killer cell surveillance and therapy of cancer. Nat Rev (Cancer) 2002;2:850–61. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 25.Stolina M, Sharma S, Lin Y, Dohadwala M, Gardner B, Luo J, Zhu L, Kronenberg M, Miller PW, Portanova J, Lee JC, Dubinett SM. Specific inhibition of cyclooxygenase-2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 26.Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (part I) J Natl Cancer Inst. 1998;90:1529–36. doi: 10.1093/jnci/90.20.1529. [DOI] [PubMed] [Google Scholar]
- 27.Thompson HJ, Jiang C, Lu J, Mehta RG, Piazza GA, Paranka NS, Pamukcu R, Ahnen DJ. Sulfone metabolite of sulindac inhibits mammary carcinogenesis. Cancer Res. 1997;57:267–271. [PubMed] [Google Scholar]
- 28.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/S0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 29.Walker W, Rotondo D. Prostaglandin E2 is a potent regulator of interleukin-12 and interleukin-18-induced natural killer cell interferon-γ synthesis. Immunology. 2004;111:298–305. doi: 10.1111/j.1365-2567.2004.01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L, Yamagata N, Yadav R, Brandon S, Courtney RL, Morrow JD, Shyr Y, Boothby M, Joyce S, Carbone DP, Breyer RM. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Invest. 2003;111:727–735. doi: 10.1172/JCI200316492. [DOI] [PMC free article] [PubMed] [Google Scholar]