Abstract
We have demonstrated previously that the optimal method for inducing an antibody response against defined cancer antigens is covalent conjugation of the antigen to keyhole limpet hemocyanin (KLH) and use of the potent saponin adjuvant QS-21. Single molecules of glycolipids (tetrasaccharides, pentasaccharides, or hexasaccharides) and MUC1 peptides (containing between one and five MUC1 tandem repeats) conjugated to KLH have proven sufficient for antibody recognition and vaccine construction. However, cancer specificity of monoclonal antibodies against the monosaccharide Tn and disaccharide sTn comes largely from recognition of clusters (c) of these molecules on the cell surface. Tn consists of a monosaccharide (GalNAc) O-linked to serine or threonine on epithelial cancer mucins which are uniquely rich in serines and threonines. We test here several Tn constructs: Tn monosaccharide, Tn(c) prepared on a triple threonine backbone, and Tn prepared on a partially or fully glycosylated MUC1 backbone. We determine that Tn(c) is more effective than Tn, and conjugation to KLH is more effective than conjugation to BSA or polystyrene beads for inducing ELISA reactivity against Tn, and FACS reactivity against Tn-positive tumor cells. Surprisingly, MUC1 glycosylated with Tn at three or five sites per 20 amino acid MUC1 tandem repeat and conjugated to KLH, induced the strongest antibody response against Tn and tumor cells expressing Tn, and had the additional advantage of inducing antibodies against MUC1.
Keywords: Cancer vaccine, Conjugate vaccine, Glycosylated MUC1, MUC1, Tn antigen
Introduction
We have demonstrated previously that the optimal method for inducing an antibody response against a variety of cancer cell surface antigens is covalent conjugation of the antigen to keyhole limpet hemocyanin (KLH) and the use of a potent saponin adjuvant such as QS-21 (an immunological adjuvant consisting of a saponin fraction purified from the bark of the South American tree Quillaja saponaria Molina). Antigens studied in the past have been glycolipids such as GM2 [1, 2], GD3 [3, 4], fucosyl GM1 [5], globo H [6, 7] and Lewis y [8], and mucin epitopes such as MUC1 [9, 10] and sTn [11]. Single glycolipid and MUC1 molecules have been sufficient for optimal antibody recognition and vaccine construction. However, cancer specificity of monoclonal antibodies against the disaccharide sTn comes largely from recognition of clusters (c) of sTn molecules on the cell surface [11, 12]. Vaccination with sTn(c)-KLH plus a saponin adjuvant is the most effective approach for inducing antibodies against sTn-positive cancer cells [12].
Tn contains a monosaccharide (GalNAc) normally O-linked to serine or threonine on epithelial cancer mucins. Nakada et al. [13] have reported that the essential epitopic structure of Tn antigen recognized by mAb MLS 128 is a cluster composed of three or four consecutive GalNAc-serines/threonines. Cancer mucins may have repeating sequences of two serines or threonines as in the case of MUC1 tandem repeats, or four or five serines or threonines as in the case of MUC2 tandem repeats. These clusters can be mimicked by serine and/or threonine trimers with GalNAcα O-linked to each amino acid [14]. An alternative approach is to attach Tn to insoluble resin or polystyrene beads [15, 16]. Tn, Tn(c), or even Tn(c)-KLH can be adsorbed or chemically linked to such beads, and used for various purposes including vaccine production. We compare here the potency of these various approaches to Tn vaccination for inducing antibodies against Tn-positive cancer cells.
Materials and methods
Vaccines
Antigen synthesis
We prepared Tn and Tn(c) synthetically linked to a single threonine or a chain of three threonines (respectively), as described for sTn, each with a terminal-free sulfhydryl group [14, 17]. One and one half tandem repeats of MUC1 peptide (32aa) and MUC2 peptide (32aa) were synthesized on a peptide synthesizer with a terminal cysteine as previously described [10]. The peptides were enzymatically glycosylated using T2 and/or T4 N-acetylgalactosaminyltransferases (three or five sites per tandem repeat for MUC1 or fully glycosylated for MUC2) [10, 18, 19].The extent of glycosylation was confirmed by mass spectrophotometry.
Conjugation
Antigens were covalently attached to KLH (Sigma, St Louis, MO, USA) using m-malemidobenzoyl-N-hydroxysuccinimide ester (MBS) (Pierce, Rockford, IL, USA), which couples the free cysteine sulfhydryl on the antigen to amino groups on KLH [20], see Fig. 1. In brief, the conjugation procedure was as follows. MBS/dimethylformamide was mixed with KLH (Sigma, St Louis, MO, USA) and incubated at room temperature for 30 min. The unconjugated MBS was then eliminated by passage over a G25 Sephadex column. Maleimide-activated KLH was then added to the activated antigen and the mixture incubated at room temperature for 3 h. Free unreacted antigen was eliminated using a 30,000 molecular cutoff Centriprep filter. The epitope ratio (number of antigen molecules per KLH molecule) was determined using high-pH anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) assay for carbohydrate after acid hydrolysis [21] and Bio-Rad assay for proteins (as described in the instrument manual). Three batches of Tn(c)-KLH were prepared: batches A and B at the MSKCC, and batch C in Princeton. Tn(c) to KLH molecular epitope ratios were batch A, 201:1, batch B, 648:1; and batch C, not determined. The Tn to KLH ratio was 1,330:1. MUC1 to KLH ratios were MUC1, 560:1; MUC1 G3, 251:1; MUC1 G5, 173:1; MUC2, 977:1; and MUC2 G, 165:1.
Fig. 1.
Structure of Tn-TentaGel, Tn(c)-KLH, and MUC1G5-KLH conjugates.
Preparation of Tn and Tn(c) beads
TentaGel resin beads were purchased from Rapp Polymer (Tubingen, Germany) and either left unaltered, adsorbed, or chemically linked through an amide bond and on an MBS linker to Tn monosaccharide or Tn cluster molecules [15, 16] (see Fig. 1). Tn(c) beads were made by attaching three sugars to a tripeptide of Thr (one sugar on each alcohol), while Tn beads had single glycosylated amino acids. The Tn and Tn(c) molecules were either conjugated to KLH or BSA, or left unconjugated. All constructs were mixed with QS-21, which was obtained from Antigenics Pharmaceuticals (New York, NY, USA).
Immunization of mice
Pathogen-free C57BL/6 female mice age 6–10 weeks were obtained from Jackson Laboratory (Bar Harbor, ME, USA). Groups of five mice were immunized three times at 1-week intervals with 3 μg of Tn formulated in various ways plus 10 μg QS21 (with the exception of group 1-2, which was immunized once every 2 weeks). Vaccines were administered subcutaneously over the lower abdomen (with the exception of group 1-8, for which the administration was intraperitoneal). Three separate experiments were conducted.
Serological assays
Enzyme-linked immunosorbent assay (ELISA)
For ELISA, dOSM and Tn(c)-HSA were used as target antigens. Antigens were coated on ELISA plates at an antigen dose of 0.1–0.2 μg per well, and the assay was performed as previously described [11, 14]. Mouse sera were obtained from a bleed 10 days after the third immunization. Phosphatase-conjugated goat antimouse IgG or IgM antibody was added at a dilution of 1:200 (Southern Biotechnology, Birmingham, AL, USA). The plates were read at 405 nm, and antibody titer was determined with the highest serum dilution yielding absorbance of 0.10 or greater.
Flow cytometry
LSC human colon cancer cells expressing Tn, and MCF-7 human breast cancer cells expressing MUC1, were used (LSC cells were obtained from Dr. Steve Itzkowitz at Mount Sinai School of Medicine [22]; MCF7 cells were provided by the laboratory of Dr Neil Rosen at the Memorial Sloan-Kettering Cancer Center). Single-cell suspensions of 5×105 cells/tube were washed in PBS with 3% fetal calf serum and incubated with 25 μl of 1:20 diluted test sera for 30 min on ice. Twenty-five microliters of 1:25–diluted goat antimouse IgG or IgM antibody labeled with FITC was added. Percentage positive cells and mean fluorescence intensity (MFI) of stained cells were analyzed using a FACScan (Becton Dickinson, San Jose, CA, USA). Prevaccination and postvaccination sera were analyzed together and the pretreatment percentage positive cells set at 10% as background for postvaccination sera.
Statistical analysis
Results in serological assays for experimental groups were compared to controls or each other using the Mann–Whitney two-sample t test [23].
Results
Comparison of Tn-KLH, Tn(c)-KLH, and Tn bead vaccines
In experiment 1, group 1-9 (Tn(c)-KLH-A) induced the highest IgG and IgM titers (see Table 1). The IgG antibody titers in 1-9 where significantly superior to those in all other groups except groups 1-1, 1-2, and 1-8. In experiment 2, group 2-11 (Tn(c)-KLH-B) produced the highest IgM and IgG antibody titers against dOSM. The IgM antibody titers in 2-11 were significantly superior to those of all other groups, and the IgG antibody titers were significantly superior to those of all other groups except group 2-4 (Tn(c)-KLH-C). There appears to be a variation between the preparations of Tn(c)-KLH. While even the batch A preparation producing the weaker titer is better than all the other Tn or Tn(c) vaccines, the batch B preparation is significantly superior. The B preparation had a higher Tn(c) to KLH ratio (648:1 vs 201:1 or lower), consistent with our previous experience that higher epitope ratios result in higher antibody titers. Consequently, in two different experiments, Tn(c)-KLH prepared at three different times and at two locations was the optimal immunogen for inducing antibodies against a natural source of Tn, dOSM.
Table 1.
Median ELISA and FACS results against natural sources of Tn after vaccination of groups of five mice with Tn or MUC1 vaccines. dOSM Desialylated ovine submaxillary mucin, a natural source of Tn; LSC a human colon cancer cell line that expresses Tn but not MUC1; Gly glycosylated with Tn at three or five sites per MUC1 20-aa tandem repeat or fully glycosylated MUC2 23-aa tandem repeat; MSKCC Memorial Sloan-Kettering Cancer Center
| Groupa | Vaccine | ELISA titer against dOSM | FACS on LSC cells % positive cells/mean fluorescence intensity |
||
|---|---|---|---|---|---|
| IgG | IgM | IgG | IgM | ||
| Experiment 1 | |||||
| gr 1-1 | Tn beads-KLH-adsorbed | 40 | 160 | 12/7 | 46/26 |
| gr 1-2 | Tn beads-KLH-adsorbed (14 day injection) | 40 | 320 | 10/6 | 47/23 |
| gr 1-3 | Tn beads | 0 | 80 | 9/6 | 19/22 |
| gr 1-4 | Acetylated-KLH-adsorbed | 0 | 40 | 10/6 | 13/21 |
| gr 1-5 | Acetylated beads-Tn-KLH-adsorbed | 0 | 0 | 11/7 | 21/27 |
| gr 1-6 | Acetylated beads-KLH-adsorbed | 0 | 40 | 9/6 | 20/23 |
| gr 1-7 | Tn-KLH | 0 | 40 | 11/8 | 17/27 |
| gr 1-8 | Tn-KLH(IP injection) | 20 | 80 | 11/8 | 16/23 |
| gr 1-9 | Tn(c)-KLH-MSKCC | 160 | 320 | 36/12 | 52/23 |
| Experiment 2 | |||||
| gr 2-1 | Tn(c) beads-KLH-adsorbed | 0 | 0 | 12/17 | 10/64 |
| gr 2-2 | Tn(c) beads-BSA-adsorbed | 20 | 80 | 18/23 | 20/63 |
| gr 2-3 | Tn(c) beads | 0 | 40 | 10/16 | 15/42 |
| gr 2-4 | Tn(c)-KLH | 160 | 0 | 77/103 | 52/90 |
| gr 2-5 | Tn(c)-BSA | 0 | 80 | 14/20 | 22/62 |
| gr 2-6 | Acetylated beads-Tn(c)-KLH-adsorbed | 40 | 40 | 11/17 | 21/49 |
| gr 2-7 | Tn-beads-BSA | 20 | 80 | 13/17 | 14/40 |
| gr 2-8 | Tn-BSA | 40 | 80 | 16/20 | 19/61 |
| gr 2-9 | Tn-KLH | 30 | 40 | 17/28 | 15/60 |
| gr 2-10 | Tn(c)-KLH(old)-MSKCC | 20 | 320 | 31/37 | 47/94 |
| gr 2-11 | Tn(c)-KLH(new)-MSKCC | 320 | 1,280 | 73/75 | 94/293 |
| Experiment 3—at the MSKCC | |||||
| gr 3-1 | MUC-1-1-KLH | 0 | 0 | 15/8 | 10/21 |
| gr 3-2 | MUC-1-1-Gly(3 site)-KLH | 3,200 | 20 | 49/13 | 24/39 |
| gr 3-3 | MUC-2-1-KLH | 0 | 0 | 17/7 | 15/28 |
| gr 3-4 | MUC-2-1-Gly-KLH | 0 | 20 | 40/21 | 14/26 |
| gr 3-5 | Tn(c)-KLH | 200 | 80 | 34/7 | 21/34 |
| gr 3-6 | Tn-KLH | 0 | 0 | 25/4 | 27/44 |
| gr 3-7 | MUC-1-3-KLH | 0 | 20 | 15/5 | 9/22 |
| gr 3-8 | MUC-1-3-Gly(5 site)-KLH | 1,600 | 20 | 29/10 | 12/27 |
| gr 3-9 | KLH | 0 | 0 | 15/8 | 12/23 |
aFive mice per group
The Tn(c) vaccine-induced antibodies also reacted strongly with Tn-positive tumor cells. LSC cells express Tn but express very little MUC1. In experiment 1, group 1-9 (Tn(c)-KLH-A) induced the highest IgM and IgG titers against LSC, with the IgG antibody titers statistically superior to those of all other groups. In experiment 2, group 2-11 (Tn(c)-KLH-B) had the highest IgM antibody titers against LSC, statistically significantly higher than all other groups. It also had statistically significantly higher IgG antibody titers against LSC than any other group with the exception of group 2-4 (Tn(c)-KLH-C). The individual FACS values for mice in the different groups, represented by median values in Table 1, were quite homogeneous, as demonstrated in Fig. 2 for the five mice in group 2-11 before and after immunization. These FACS results confirm the previous ELISA results for experiments 1 and 2; Tn(c)-KLH from various sources is the optimal immunogen of those tested in these two experiments for inducing relevant antibodies against Tn.
Fig. 2.
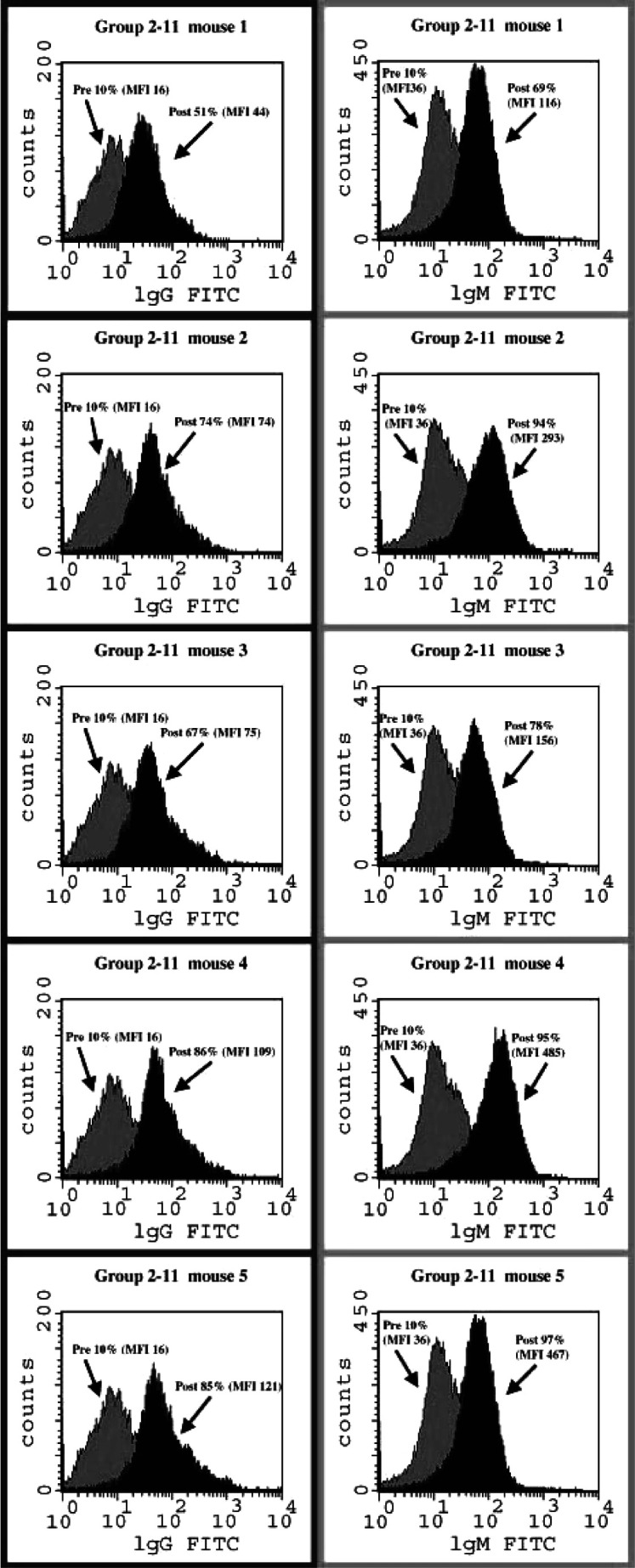
IgG and IgM FACS results before and after immunization with Tn(c)-KLH (MSKCC new) plus QS-21 for five mice in group 2-11. Data are percentage positive cells (MFI mean fluorescence intensity).
Comparison of vaccines containing Tn presented on MUC1, MUC2, or Tn(c) conjugates
Groups 3-2, 3-4, 3-5, and 3-8 were optimal for both IgM and IgG antibody titers against dOSM, with groups 3-2 (MUC1-G3-KLH) and 3-8 (MUC1-G5-KLH) having significantly higher IgG antibody responses than the other groups (Table 1). Using Tn(c)-HSA as target, group 3-8 had a significantly superior antibody response compared with all other groups except group 3-4 (Table 2). MUC1 Tn-glycosylated at three sites was surprisingly effective at inducing antibodies against dOSM, even more effective than Tn(c)-KLH.
Table 2.
Median ELISA titer against Tn(c), and FACS against MCF7 cell line (expressing MUC1), after vaccination against Tn or MUC1. MCF7 A human breast cancer cell line that expresses MUC1 but not Tn; Gly Glycosylated with Tn at three or five sites per MUC1 20-aa tandem repeat or at all 14 sites per fully glycosylated MUC2 23-aa tandem repeat
| Groupa | Vaccine | ELISA titer against Tn(c)-HSA | FACS on MCF7 cells % positive cells/mean fluorescence intensity |
||
|---|---|---|---|---|---|
| IgG | IgM | IgG | IgM | ||
| gr3-1 | MUC-1-1-KLH | 160 | 0 | 97/107 | 21/18 |
| gr3-2 | MUC-1-1-Gly(3 site)-KLH | 20,480 | 20 | 98/170 | 35/20 |
| gr3-3 | MUC-2-1-KLH | 5,120 | 20 | 15/6 | 18/13 |
| gr3-4 | MUC-2-1-Gly-KLH | 40,960 | 20 | 26/5 | 14/14 |
| gr3-5 | Tn(c)-KLH | 10,240 | 40 | 26/7 | 37/23 |
| gr3-6 | Tn-KLH | 0 | 0 | 14/6 | 21/24 |
| gr3-7 | MUC-1-3-KLH | 2,560 | 0 | 91/54 | 11/11 |
| gr3-8 | MUC1-3-Gly(5 site)-KLH | 40,960 | 40 | 99/258 | 17/13 |
| gr3-9 | KLH | 0 | 0 | 13/7 | 13/13 |
aFive mice per group
The highest IgG antibody response against LSC was seen in group 3-2 (MUC1-G3-KLH). The percentage positive cells and mean fluorescence intensity (MFI) in group 3-2 were significantly higher than in other groups except for groups 3-4, 3-5, and 3-8. MUC1 Tn-glycosylated at three sites was a surprisingly effective immunogen for induction of Tn antibodies.
Glycosylated MUC1-KLH conjugates also induced high-titer antibodies against MUC1. MCF7 cancer cells express MUC1 but little Tn. All MUC1-immunized groups produced high titer antibody responses against MCF7 but the highest responses were seen in groups 3-2 (MUC1-G3-KLH) and 3-8 (MUC1-G5-KLH), with group 3-8 showing responses which were significantly higher than those in any other group. As opposed to the predominantly IgM antibody response induced by Tn, antibodies induced by the MUC1 peptide vaccines were almost exclusively IgG.
Discussion
Of primary importance in the construction of vaccines against cancer is that the antigen in the vaccine replicates the antigen on the tumor. Concerning monosaccharides or disaccharides such as Tn and sTn, others report that epitopes recognized on cancer cells are clusters of these small molecules. Kurosaka et al. [24] demonstrated that sTn(c), but not sTn, was recognized by MLS102, a monoclonal antibody against sTn with cancer specificity. Subsequently, Nakada demonstrated monoclonal antibody MLS128 with cancer specificity reacted preferentially with clusters of three Tn residues. We have since confirmed that B72.3 monoclonal antibody against sTn with a striking cancer specificity, recognized sTn clusters [11], and have confirmed improved cancer specificity of antibodies raised in patients with vaccines containing sTn(c) as opposed to sTn monomers . Here we demonstrate in three separate experiments that among various Tn conjugates, Tn(c) is consistently a better form of Tn for induction of antibodies against two sources of naturally expressed Tn (dOSM and tumor cells).
Of great importance, as well, in the construction of vaccines is the method of presentation of the antigen to the immune system. The method and intensity of conjugation plays a major role in increasing the immunogenicity of an antigen. We have in the past described KLH as the optimal carrier [3]. Here again we demonstrate its clear superiority over BSA, and again demonstrate that simply mixing Tn(c) and KLH is significantly less effective than the Tn(c)-KLH conjugate.
Others describe different approaches to presentation of the antigen (with or without a carrier molecule). One such approach is the use of polystyrene beads. We attempted this approach with both Tn and Tn(c) using various means of preparation (adsorption, acetylation, linked or unlinked) and conjugation (with KLH, BSA, or unconjugated). The idea of this approach is to look at beads as a support for presenting carbohydrates. Here we test the efficacy of the beads as compared with our standard of using KLH as such a support. Specifically, we examine the possibility of mimicking the clustering of Tn with Tn-linked beads, as well as optimizing the presentation of Tn(c) with Tn(c)-linked beads. While the beads increased Tn immunogenicity slightly, the optimal approach was again Tn(c) linked to KLH with a high Tn(c) to KLH ratio.
Building on the results of the first two experiments which showed that Tn(c)-KLH was optimal, in experiment 3, Tn(c)-KLH was compared to vaccines containing Tn monomers O-linked to MUC1 or MUC2 in place of the three-threonine backbone of Tn(c). MUC1 is a glycosylated cell surface mucin expressed in a variety of epithelial cancers. We have previously demonstrated immunogenicity of MUC1 peptide-KLH in both mice and patients [10, 20]. Glycosylation of mucins such as MUC-1 and MUC-2 on tumor cells occurs predominantly with small sugars such as Tn and sTn. We have therefore created a series of MUC-1 and MUC-2 peptides with varying degrees of Tn-glycosylation as a means of reproducing this natural form. Surprisingly, we demonstrate here that this approach is highly effective in antibody production not only against MUC1, but also against Tn. In fact, MUC1 glycosylated at three sites per tandem repeat produced significantly higher antibody titers than even the Tn(c)-KLH conjugate, and also produced higher antibody titers against MUC1 than unglycosylated MUC1-KLH. Based on these studies we plan to test Tn-glycosylated MUC1-KLH in future clinical trials as immunogen for both Tn and MUC1.
Acknowledgements
This work is supported by NIH grants CA33049 and CA52477, and the Breast Cancer Research Fund. C.A.R. is supported by FCT/POCTI 363767/99.
Abbreviations
- BSA
Bovine serum albumin
- dOSM
Desialylated ovine submaxillary mucin
- ELISA
Enzyme-linked immunosorbent assay
- FACS
Fluorescence-activated cell sorting
- HPAEC-PAD
High-pH anion-exchange chromatography with pulsed amperometric detection
- HSA
Human serum albumin
- KLH
Keyhole limpet hemocyanin
- MBS
m-Malemidobenzoyl-N-hydroxysuccinimide ester
- PBS
Phosphate buffer saline
- QS21
Quillaja saponaria Molina–based immunological adjuvant
References
- 1.Livingston PO, Wong GYC, Adluri S, Tao Y, Padavan M, Parente R, Hanlon C, Helling F, Ritter G, Oettgen HF, Old LJ. Improved survival in AJCC stage III melanoma patients with GM2 antibodies: a randomized trial of adjuvant vaccination with GM2 ganglioside. J Clin Oncol. 1994;12:1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 2.Chapman PB, Morrissey DM, Panageas KS, Hamilton WB, Zhan C, Destro AN, Williams L, Israel RJ, Livingston PO. Induction of antibodies against GM2 ganglioside by immunizing melanoma patients using GM2-KLH+QS21 vaccine: a dose-response study. Clin Cancer Res. 2000;6:874–879. [PubMed] [Google Scholar]
- 3.Helling F, Shang Y, Calves M, Oettgen HF, Livingston PO. Increased immunogenicity of GD3 conjugate vaccines: comparison of various carrier proteins and selection of GD3-KLH for further testing. Cancer Res. 1994;54:197–203. [Google Scholar]
- 4.Ragupathi G, Meyers M, Adluri S, Howard L, Musselli C, Livingston PO. Induction of antibodies against GD3 ganglioside in melanoma patients by vaccination with GD3-lactone-KLH conjugate plus immunological adjuvant QS-21. Int J Cancer. 2000;85:659–666. doi: 10.1002/(SICI)1097-0215(20000301)85:5<659::AID-IJC11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Dickler MN, Ragupathi G, Liu NX, Musselli C, Martino DJ, Miller VA, Kris MG, Brezicka FT, Livingston PO, Grant SC. Immunogenicity of the fucosyl-GM1-keyhole limpet hemocyanin (KLH) conjugate vaccine in patients with small cell lung cancer. Cancer Res. 1999;5:2773–2779. [PubMed] [Google Scholar]
- 6.Ragupathi G, Slovin S, Adluri S, Sames D, Kim IJ, Kim HM, Spassova M, Bornmann WG, Lloyd K, Scher HI, Livingston PO, Danishefsky SJ. A fully synthetic globo H carbohydrate vaccine induces a focused humoral response in prostate cancer patients: a proof of principle. Angewandte Chemie. 1999;38:563–566. doi: 10.1002/(SICI)1521-3773(19990215)38:4<563::AID-ANIE563>3.3.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 7.Gilewski T, Ragupathi G, Bhuta S, Williams LJ, Musselli C, Zhang XF, Bencsath KP, Panageas KS, Chin J, Norton L, Houghton AN, Livingston PO, et al. Immunization of metastatic breast cancer patients with a fully synthetic globo H conjugate: a phase I trial. Proc Natl Acad Sci U S A. 2001;98:3270–3275. doi: 10.1073/pnas.051626298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabbatini P, Kudryashov V, Ragupathi G, Danishefsky S, Livingston PO, Bornmann W, Spassova M, Spriggs D, Aghajanian C, Soignet S, Peyton M, O’Flaherty C, et al. Immunization of ovarian cancer patients with a synthetic LewisY–protein conjugate vaccine: clinical and serological results. Int J Cancer. 2000;87:79–85. doi: 10.1002/1097-0215(20000701)87:1<79::AID-IJC12>3.3.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 9.Adluri S, Gilewski T, Zhang S, Ramnath V, Ragupathi G, Livingston PO. Specificity analysis of sera from breast cancer patients vaccinated with Muc1-KLH plus QS-21. Br J Cancer. 1999;79:1806–1812. doi: 10.1038/sj.bjc.6690288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilewski T, Adluri S, Ragupathi G, Zhang S, Yao TJ, Panageas K, Moynahan M, Houghton A, Norton L, Livingston PO. Vaccination of high risk breast cancer patients with Mucin-1 keyhole limpet hemocyanin conjugate plus QS-21. Clin Cancer Res. 2000;6:1693–1701. [PubMed] [Google Scholar]
- 11.Zhang S, Walberg LA, Ogata S, Itzkowitz SH, Koganty RR, Reddish M, Gandhi SS, Longenecker BM, Lloyd KO, Livingston PO. Immune sera and monoclonal antibodies define two configurations for the sialyl Tn tumor antigen. Cancer Res. 1995;55:3364–3368. [PubMed] [Google Scholar]
- 12.Dicker M, Gilewski T, Ragupathi G, Adluri R, Koganty RR, Longenecker M, Houghton AN, Norton L, Livingston PO. Vaccination of breast cancer patients (pts) with no evidence of disease (NED) with sialyl Tn cluster (sTa(c))-keyhole limpet hemocyanin (KLH) conjugate plus adjuvant QS-21: preliminary results. Proc ASCO. 1997;16:1572. [Google Scholar]
- 13.Nakada H, Inoue M, Numata Y, Tanaka N, Funakoshi J, Fukui S, Mellors A, Yamashina I. Epitopic structure of Tn glycophorin A for an anti-Tn antibody (MLS 128) Proc Natl Acad Sci U S A. 1993;90:2495–2499. doi: 10.1073/pnas.90.6.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ragupathi G, Howard L, Cappello S, Koganty RR, Qiu D, Longenecker BM, Reddish MA, Lloyd KO, Livingston PO. Vaccines prepared with sialyl-Tn and sialyl-Tn trimers using the 4-(4-maleimidomethyl) cyclohexane-1-carboxyl hydrazide linker group result in optimal antibody titers against ovine submaxillary mucin and sialyl-Tn-positive tumor cells. Cancer Immunol Immunother. 1999;48:1–8. doi: 10.1007/s002620050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang R, Yan L, Loebach J, Ge M, Uozumi Y, Sekanina K, Horan N, Gildersleeve J, Thompson C, Smith A, Biswas K, Still WC, et al. Parallel synthesis and screening of a solid phase carbohydrate library. Science. 1996;274:1520–1522. doi: 10.1126/science.274.5292.1520. [DOI] [PubMed] [Google Scholar]
- 16.Liang R, Loebach J, Horan N, Ge M, Thompson C, Yan L, Kahne D. Polyvalent binding to carbohydrates immobilized on an insoluble resin. Proc Natl Acad Sci U S A. 1997;94:10554–10559. doi: 10.1073/pnas.94.20.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ragupathi G, Koganty RR, Qui DS, Lloyd KO, Livingston PO. A novel and efficient method for synthetic carbohydrate conjugate vaccine preparation: synthesis of sialyl Tn KLH conjugate using a 4-(4-N-maleimidomethyl)cyclohexane-1-carboxyl hydrazide (MMCCH) linker arm. Glycoconjugate J. 1998;15:217–221. doi: 10.1023/A:1006936826730. [DOI] [PubMed] [Google Scholar]
- 18.White T, Bennett EP, Takio K, Sorensen T, Bonding N, Clausen H. Purification and cDNA cloning of a human UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase. J Biol Chem. 1995;270:24156–24165. doi: 10.1074/jbc.270.41.24156. [DOI] [PubMed] [Google Scholar]
- 19.Bennett EP, Hassan H, Mandel U, Mirgorodskaya EP, Burchell J, Taylor-Papadimitriou J, Hollingsworth MA, Merkx G, Vankessel AG, Eiberg H, Steffensen R, Clausen H. Cloning of a human UDP-N-acetyl-alpha-D-alactosaminepolypeptide N-acetylgalactosaminyltransferase that complements other GalNAc-transferases in complete O-glycosylation of the MUC1 tandem repeat. J Biol Chem. 1998;273:30472–30481. doi: 10.1074/jbc.273.46.30472. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Graeber LA, Helling F, Ragupathi G, Adluri S, Lloyd KO, Livingston PO. Augmenting the immunogenicity of synthetic MUC1 peptide vaccines in mice. Cancer Res. 1996;56:3315–3319. [PubMed] [Google Scholar]
- 21.Rohrer JS, Miller HI. Detecting O-linked oligosaccharides on glycoproteins. Anal Biochem. 2003;316:131–134. doi: 10.1016/S0003-2697(03)00041-1. [DOI] [PubMed] [Google Scholar]
- 22.Ogata S, Chen A, Itzkowitz SH. Use of model cell lines to study the biosynthesis and biological role of cancer-associated sialosyl-Tn antigen. Cancer Res. 1994;54:4036–4044. [PubMed] [Google Scholar]
- 23.Huntsberger DV, Leaverton PE (ed) (1970) In: Statistical inference in the biomedical sciences. Allyn Bacon, Boston, pp 138–140, 337–338
- 24.Kurosaka A, Kitagawa H, Fukui S, Numata Y, Nakada H, Fuakoshi I, Kawasaki T, Ogawa T, Iijima H, Yamashina I. A monoclonal antibody that recognizes a cluster of a disaccharide, NeuAcα2→6GalNAc, in mucin-type glycoproteins. J Biol Chem. 1988;263:8724–8726. [PubMed] [Google Scholar]



