Abstract
A baculovirus-produced recombinant CEA (rCEA) protein comprising the extracellular region was used for vaccination of CRC patients with or without GM-CSF as an adjuvant cytokine. Ten patients with a significant proliferative T cell response against rCEA were selected for T cell epitope mapping. Fifteen-aa-long overlapping peptides covering the entire aa sequence of the external domain of CEA were used in a proliferation assay. In six of the patients a repeatable T cell response against at least one peptide was demonstrated. For the first time, nine functional HLA-DR epitopes of CEA were defined. Two of the peptides were recognized by more than one patient, i.e., two and three patients, respectively. Those 15-mer peptides that induced a proliferative T cell response fitted to the actual HLA-DR type (SYFPEITHI). The affinity of the native peptides for the T cell receptor was in the low to intermediate range (scores 6–19). The 15-mer peptides also contained 9-mer peptide sequences that could be predicted to bind to the actual HLA-ABC genotypes (SYFPEITHI/BIMAS). Blocking experiments using monoclonal antibodies indicated that the proliferative T cell response was both MHC class I and II restricted. The defined HLA-DR T cell epitopes were spread over the entire CEA molecule, but a higher frequency was noted towards the C-terminal. Peptides with a dual specificity may form a basis for production of subunit cancer vaccines, but modifications should be done to increase the T cell affinity, thereby optimizing the antitumoral effects of the vaccine.
Keywords: CEA, T cell epitopes, HLA-DR, Vaccination
Introduction
Tumor cells express a variety of tumor-associated antigens (TAA), most of which are normal cell counterparts [21]. Many TAAs have been shown to spontaneously induce a humoral and cellular immunity in patients, indicating that they can be recognized by the host immune system [17, 19]. Such antigens might be utilized for specific immunotherapy [24, 31]. Early clinical trials in colorectal carcinoma (CRC) patients have shown that passive as well as active specific immunotherapy might be of clinical benefit [23, 32].
Colorectal carcinoma cells exhibit the TAA CEA (carcinoembryonic antigen) [33]. CEA is a 180-kDa glycoprotein belonging to the family of cell adhesion molecules [1]. The antigen is expressed also in other gastrointestinal malignancies, as well as in lung and breast carcinoma [26]. CEA has been used as a target structure for active immunotherapy in humans [13, 16, 24].
With the aim to develop a therapeutic vaccination strategy, we used a recombinant CEA protein produced in baculovirus for immunization of CRC patients stages II and III in combination with the adjuvant cytokine GM-CSF. A strong humoral and cellular immune response against the protein molecule CEA was induced [24]. In the present study, we have further analyzed the cellular immune response by mapping HLA-DR-restricted T cell epitope regions in a genetically diverse population. Such information might be of value to further delineate a cellular response and to form a basis for improvement of the vaccination approach by producing subunit vaccines.
Materials and methods
Patients
Patients with surgically resected CRC, stages II and III, without remaining macroscopical tumors were immunized with recombinant human CEA produced in baculovirus (Protein Sciences Corp., Meriden, Conn.). Details of the study and the immunization procedure have been published previously [24]. rCEA in alum (see below) was administered s.c. at months 0, 0.5, 2, 4, 6, 9 and 12. Four dose levels of rCEA were used: 100 μg, 316 μg, 1,000 μg and 3,000 μg per immunization. Half of the patients received rCEA alone, while the other half also received GM-CSF (80 μg/d for 4 consecutive days) (Leucomax, Schering-Plough/Novartis, Kenilworth, N.J.) at each immunization time. GM-CSF was administered at the same site as rCEA. The majority (n=9) of the patients received GM-CSF.
Ten patients who developed an antigen-specific T-cell response were selected for the present study. Time from the first immunization to testing ranged from 9 to 31 months.
HLA tissue typing was done at the Tissue Typing Laboratory, Huddinge University Hospital, Stockholm.
Production of protein and peptide antigens
A recombinant human CEA protein covering the extracellular domain was produced in baculovirus-infected insect cells as previously described [24]. Briefly, the gene for CEA was cloned from human colon adenocarcinoma cells (LS174T) and engineered to contain all of the amino acids found in native CEA. CEA is glycosylated with N-linked glycans and has a molecular mass of 120 kD, which is smaller than the anticipated 180 kD of native CEA. The difference is probably due to the lack of complex carbohydrate structures in the CEA molecule. Recombinant CEA was purified under non-denaturing conditions and tested for purity (>99%), safety and sterility. Prior to immunization the purified CEA was formulated in situ with alum (0.5 mg/ml of aluminium ion as Al3PO4; Swedish Institute for Infectious Disease Control, Stockholm). For in vitro tests, CEA without alum was used.
Baculovirus and insect cell proteins may contaminate the purified CEA. Therefore, a baculovirus control protein (BCP) was prepared containing a mixture of insect cells and baculovirus proteins from cells infected with a control baculovirus expression vector as previously described [24].
Sixty-four (numbered 4–67) 15-aa-long peptides (with 5-aa N- and C-terminal overlap) derived from the amino acid sequence of CEA were synthesized using the multiple peptide synthesized SyRo (Multisyn Tech, Bochum, Germany). Solid-phase peptide synthesis was carried out using the 9-fluorenyl-methoxycarbonyl (Fmoc) methodology [4]. The protocol and method described by Zsigo and Saneii [35] were followed with slight modifications.
Epitope prediction analysis
Twelve of the 64 15-mer peptides were shown to induce a proliferative response that could be reproduced at two different time points (see Results). Epitope prediction analyses were performed for these 12 15-mer peptides for fitting to the actual MHC class II molecules using the SYFPEITHI database (http://syfpeithi.bmi-heidelberg.com/). The score for binding capacity was recorded. The maximum score that can be obtained is 35. A peptide with a score <15 was defined as having a low affinity for the T cell receptor, a score of 15–25 as an intermediate affinity and a score of >25 as a high affinity [7]. The following class II molecules were included in the analyses: HLA-DRB1*0101, HLA-DRB1*0301, HLA-DRB1*0401 and HLA-DRB*0701.
The twelve 15-mer peptides were then examined for the expression of 9-aa peptide sequences, which could potentially bind to the actual MHC class I molecules, using the BIMAS database (http://bimas.dcrt.nih.gov/molbio/hla_bind) for epitope prediction. HLA-A1, HLA-A*0201, HLA-A*0205, HLA-A3, HLA-A24, HLA-B7, HLA-B40, HLA-B62, HLA-Cw*0301, HLA-Cw*0602 and HLA-Cw*0702 were analyzed. (MHC class II fitting can not be predicted in BIMAS.) A predicted half-life of 1 min is considered a threshold for binding [6]. Prediction analyses for MHC class I molecules were also done using SYFPEITHI. The following haplotypes were included in the analyses: HLA-A1, HLA-A*0201, HLA-A*0202, HLA-A*0203, HLA-A3, HLA-B*0702, HLA-B*1510. In SYFPEITHI HLA-C prediction analyses can not be done. A predicted score of ≥9 might be considered a threshold for binding as it has previously been shown for idiotypic immunoglobulin peptide sequences that a score of 9 corresponded to a half-life of 1 min in BIMAS [7].
Isolation of peripheral blood mononuclear cells (PBMC)
PBMC were obtained by centrifugation of heparinized venous blood on a Ficoll-Paque (Pharmacia, Uppsala, Sweden) gradient (density: 1.077 g/ml). The cells were washed three times in PBS [24].
Proliferation assay (DNA synthesis)
The method has been described in detail elsewhere [24]. PBMC (105 cells/well) were cultured in 96-well round-bottomed microtiter plates (Göteborgs Termometerfabrik, Stockholm, Sweden) at 37°C in humidified air with 5% CO2 for 6 days. Peptides were added at the concentrations of 1 μg/ml and 10 μg/ml. The proteins (rCEA and BCP) were added at concentrations of 0.01–1,000 μg/ml. Concanavalin A (ConA) (80 μg/ml) (Pharmacia Biotech, Uppsala, Sweden), phytohemagglutinin (PHA) (10 μg/ml) (Sigma-Aldrich Sweden AB, Stockholm), purified protein derivate of tuberculin (PPD) (2.5 μg/ml) (Statens Seruminstitut Köpenhamn, Denmark) and tetanus toxin (TT) (50 ng/ml) (SBL Vaccin, Stockholm) were used as positive controls. During the last 18 h of incubation, 1 μ Ci/well of 3H-thymidine (specific activity 5 Ci/mmol) (Amersham Life Sciences, UK) was added. Cells were harvested by an automatic cell harvester (Skatron, Lier, Norway). Radioactivity was measured in a liquid scintillation counter (Wallac, Åbo, Finland). The results are expressed as mean of triplicates. A stimulation index (SI) was calculated for each triplicate by dividing mean radioactivity (cpm) of stimulated cells by that of unstimulated cells.
To establish a cut-off level for a positive response, lymphocytes of healthy control donors (n=36) were stimulated with rCEA and BCP, respectively. Mean SI +2 SD for rCEA was 2.97. Based on these results an SI ≥3.0 for rCEA was considered a positive response (cut-off level) in rCEA vaccinated patients. The highest SI obtained by any of the tested concentrations is given [3]. Fifteen healthy control donors were also tested for a proliferative response against the 64 CEA derived peptides. Mean SI + 2 SD for all peptides of all control donors was 3.08. There was no significant difference between SI values of the different peptides (data not shown). An SI ≥3.1 was used as a cut-off level for a positive peptide response.
Major histocompatibility complex (MHC) blocking experiments
To study MHC restriction of the proliferative response mouse monoclonal antibodies (Mab) against human MHC class II molecules (HLA-DR) (IgG2B) (Immunotech, Marseilles, France) or class I molecules (HLA-ABC) (IgG2B) (Chemicon International Inc., Temecula, Calif.) were used. A non-relevant mouse Mab (IgG2B) was used as control (Immunotech). Antibodies (1 μg/ml) were added at the start of culture. Results are expressed as percent inhibition of cell proliferation according to the formula: inhibition (%)=(A−B)/A×100, where A is radioactivity (cpm) measured in cells stimulated with antigen alone and B is radioactivity (cpm) measured in cells incubated with both antigen and the monoclonal antibodies. The control mouse Mab induced an inhibition of 2.8±7.2% (mean ± SD) (n=9). Mean +3 SD was 24.4. More than 25% inhibition was considered a specific inhibition of the proliferative response.
Results
Ten patients were considered for the present study. None of them had a proliferative T cell response against rCEA before vaccination (data not shown). At the time the patients entered the present study, they had received six to seven vaccinations. SI value against rCEA was 68.4±101.5 (mean ± SD; range: 3.8–364; n=10).
The ten patients were first tested against all the 64 CEA derived peptides. Patients that had a positive response (SI ≥3.1) against any peptide were tested a second time using blood cells drawn at another time point than the first sample. At the first testing time all ten patients had a positive response against at least one peptide. A repeatable proliferative T cell response against at least one peptide at the second testing time could be noticed in six of the ten patients. Only those six patients were considered for further analysis.
The individual SI values against the protein antigen rCEA in these six patients are shown in Table 1. Nine different 15-mer peptides were found to induce a repeatable proliferative T cell response. In five of the six patients the 15-aa-long peptides fitted to the HLA-DR molecules of the respective patients. The scores varied between 6 and 19 (SYFPEITHI). In patient no. 3, five of six 15-mer peptides which induced a proliferative T cell response were found to fit to the actual MHC class II molecule (HLA-DR7). One peptide (pp 40) that induced a T cell response could not be found to fit to the actual class II molecule.
Table 1.
Amino-acid sequence of 15-mer CEA-derived HLA-DR fitting peptides, the T cell receptor affinity (score) and proliferative activity as well as predicted class I binding sequences within the 15-mer sequence and their T cell receptor affinity
| Patient | Peptide sequence | HLA type of the patient | Affinity for the T cell receptor | Stimulation index (SI) | |||
|---|---|---|---|---|---|---|---|
| BIMAS | SYFPETHI | Peptide | CEA | ||||
| (T 1/2) | (Score) | (Score) | |||||
| MHC I* | MHC I* | MHC II | |||||
| No. 2 | TYLWWVNSQSLPVSP (pp 54) | DR1 | 19 | 4.5 | 21.1 | ||
| TYLWWVNSQ | CW7 | 0.600 | – | – | |||
| No. 3 | YECGIQNELSVDHSD (pp 40) | DR7 | – | 4.5 | 12.8 | ||
| QNELSVDHS | A1 | 0.225 | 11 | – | |||
| PKPSISSNNSKPVED (pp 51) | DR7 | 12 | 44.8 | ||||
| SSNNSKPVE | A1 | – | 7 | – | |||
| PSISSNNSK | A1 | 0.03 | – | – | |||
| TYLWWVNGQSLPVSP (pp 54) | DR7 | 10 | 17.4 | ||||
| WVNSQSLPV | A1 | – | 8 | – | |||
| LWWVNSQSL | B37 | 1 | – | – | |||
| YGPDTPIISPPDSSY (pp 60) | DR7 | 14 | 26.7 | ||||
| IISPPDSSY | A1 | 5 | 20 | – | |||
| GIPQQHTQVLFIAKI (pp 64) | DR7 | 16 | 55.8 | ||||
| HTQVLFIAK | A1 | – | 13 | – | |||
| QQHTQVLFI | B37 | 5 | – | – | |||
| GTYACFVSNLATGRN (pp 66) | DR7 | 6 | 21.8 | ||||
| GTYACFVSN | A1 | – | 9 | – | |||
| TYACFVSNL | B37 | 1 | – | – | |||
| No. 6 | TTVTTITVYAEPPKP (pp 32) | DR6/9 | – | 4.0 | 153 | ||
| TVTTITVYA | A2 | 2.39 | 11 | – | |||
| No. 7 | LNLSCHSASNPSPQY (pp 62) | DR4 | 12 | 3.6 | 29.8 | ||
| NLSCHSASN | A2 | 0.075 | 14 | – | |||
| No. 16 | GYVIGTQQATPGPAY (pp 9) | DR3 | 10 | 11.1 | 42 | ||
| QQATPGPAY | B62 | 80,000 | – | – | |||
| QQATPGPAY | A1 | – | 17 | – | |||
| TYLWWVNSQSLPVSP (pp 54) | DR3 | 8 | 7.7 | ||||
| YLWWVNSQS | B62 | 0.8 | – | – | |||
| YLWWVNSQS | A3 | – | 17 | – | |||
| No. 21 | GIPQQHTQVLFIAKI (pp 64) | DR4 | 6 | 3.9 | 49.5 | ||
| IPQQHTQVL | B7 | 80,000 | – | – | |||
| GIPQQHTQV | A2 | – | 19 | – | |||
*The highest predicted value is indicated; – not available in the database
Two patients (nos. 3 and 16) had a proliferative T cell response against more than one peptide, six and two peptides respectively (Table 1). Two of the peptides, pp 54 and 64, were recognized by three patients (nos. 2, 3 and 16) and two patients (nos. 3 and 21), respectively. These four patients had different HLA-DR haplotypes.
Fitting analyses for the 15-mer-long peptide, pp 32, inducing a T cell response in patient no. 6, could not be done as the actual HLA-DR type (DR6/DR9) is not included in SYFPEITHI.
Predictions were then made whether there were 9-mer peptide sequences within the 15-aa-long peptides that could fit to the MHC class I molecules of the respective patients. In all six patients, 9-aa-long peptides were identified that could fit to the actual HLA-ABC genotypes. T1/2 for binding (BIMAS) as well as the scores (SYFPEITHI) are shown in Table 1. These 9-mer peptides were however not synthesized and tested for functional activity.
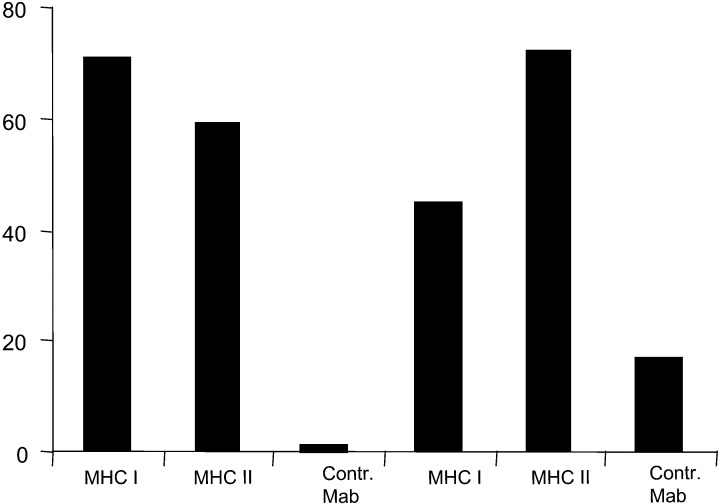
The proliferative response against the recombinant protein CEA as well as against 15-mer-long CEA-derived peptides could be blocked by MHC class I and class II monoclonal antibodies as exemplified in Fig. 1.
Fig. 1.
Inhibition of CEA peptide-specific proliferative T cell response by MHC class I and MHC class II monoclonal antibodies in patients nos. 6 and 7 vaccinated with rCEA and the adjuvant cytokine GM-CSF. PBMC were stimulated by the CEA-derived peptides 32 and 62, respectively, in the presence of anti-MHC class I, anti-MHC class II or a control mAb (see Materials and methods). Values are given as per cent inhibition
Discussion
Twenty-four colorectal carcinoma patients with stages II and III were immunized with a recombinant CEA protein. Details of the trial have been published recently [30]. Ten patients who developed a proliferative response against the CEA protein were selected for the present study with the aim to map HLA-DR T cell epitope regions. Sixty-four 15-aa-long overlapping peptides covering the external domain of the CEA molecule were used in a proliferation assay to identify T cell-recognizing regions. Six of the ten patients mounted an anti-peptide T cell response that could be demonstrated at two different time points (a repeatable response). These 15-aa-long peptides could be shown to fit to the HLA-DR genotype of the respective patients. In four patients a repeatable response was found against one peptide, in one patient against two peptides and in a further one patient against six peptides. Within these 15-mer-long peptides, 9-aa-long sequences could be identified that fitted to the actual MHC class I haplotype. The proliferative response against the 15-aa-long peptides could be inhibited by MHC class I and class II monoclonal antibodies, suggesting that MHC class I- as well as class II-restricted T cells recognized these 15-mer-long CEA-derived peptides. The T cell epitopes could be found spread over the entire external domain of the CEA molecule, but a higher frequency of HLA-DR epitopes seemed to be localized towards the C-terminal (Fig. 2).
Fig. 2.
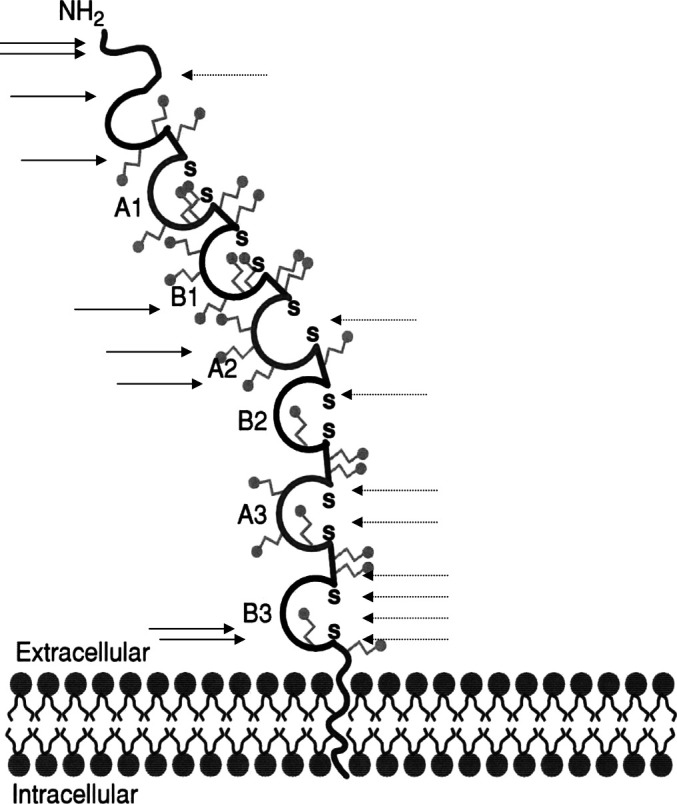
Location of MHC class I (filled arrows) and class II (dashed arrows) CEA epitopes reported in the literature (to the left) and in the present study (to the right). ≈glycosylation site, SS disulphide bridge between cysteins
This is the first study to our knowledge reporting on MHC class II binding functional epitopes of CEA. The amino-acid sequences and the HLA-DR restrictions are depicted in Table 2. A few peptides were promiscuous and could bind to more than one HLA-DR type. Promiscuous HLA-DR sequences have recently also been shown for the tumor antigen MAGE-3 [2]. The identification of promiscuous epitopes should be of benefit in a vaccination approach as such sequences might be utilized in a large number of patients regardless of their HLA-DR type.
Table 2.
MHC class II and class I T cell epitopes of CEA in the present study and in the literature
| Affinity for the T cell receptor (score/T1/2) | HLA type | Refr. | |
|---|---|---|---|
| Predicted functional HLA-DR T cell epitopes (present study*) | |||
| TYLWWVNSQSLPVSP | 19, 8, 10 | DR 1/DR 3/DR 7 | |
| GYVIGTQQATPGPAY | 10 | DR 3 | |
| LNLSCHSASNPSPQY | 12 | DR 4 | |
| GIPQQHTQVLFIAKI | 6, 16 | DR 4/DR 7 | |
| PKPSISSNNSKPVED | 12 | DR 7 | |
| YGPDTPIISPPDSSY | 14 | DR 7 | |
| GTYACFVSNLATGRN | 6 | DR 7 | |
| YECGIQNELSVDHSD | - | DR 7 | |
| TTVTTITVYAEPPKP | - | DR 6/DR 9 | |
| Predicted HLA-AB T cell epitopes (present study) | |||
| QNELSVDHS | 11/0.23 | A 1 | |
| IISPPDSSY | 20/5 | A 1 | |
| HTQVLFIAK | 13/- | A 1 | |
| GTYACFVSN | 9/- | A 1 | |
| TVTTITVYA | 11/2.4 | A 2 | |
| NLSCHSASN | 14/0.08 | A 2 | |
| QQATPGPAY | -/80,000, 17/- | B 62/A1 | |
| IPQQHTQVL | -/80,000 | B 7 | |
| GIPQQHTQV | 19/- | A 2 | |
| Published HLA-AB T cell epitopes | |||
| YLSGANLNL (CAP-1) | 25/98 | A 2 | 13 |
| HLFGYSWYK | 22/1,350 | A 3 | 22 |
| SYLSGANLNL | 22/300 | A 24 | X |
| QYSWFVNGTF | 20/140 | A 24 | 21 |
| TYACFVSNL | 22/200 | A 24 | 21 |
| HRWCIPWQR | 23/5,000 | B 27 | 23 |
| HRWCIPWQRL | -/10,000 | B 27 | 23 |
| FRVYPELPK | 23/2,000 | B 27 | 23 |
| IRSLVPSPRL | -/2,000 | B 27 | 23 |
*HLA-DR epitopes can only be predicted in SYFPEITHI and thus only the score for binding to the T cell receptor is depicted; – not available in the database
The relative binding affinities of these natural peptides were in the low to intermediate range [7] (scores 6–19). This might be an interesting observation as it has been shown in an autoimmune encephalitis mouse model that poorer binding sequences of the Golli protein (self antigen) region (target structure for immune recognition) induced a proliferative T cell response, which was not the case for those with the strongest affinity [15]. A similar observation was also made in the mouse lysozyme system [18]. In multiple myeloma, T cells recognizing MHC class II- as well as class I-restricted 15-aa- and 9-aa-long peptides, respectively, derived of the idiotypic tumor immunoglobulin (self antigen) had also low to intermediate scores [7]. Our data on the low to intermediate affinity for natural MHC class II binding 15-mer-long peptides are similar to those of natural MHC class I binding 9-mer peptides of other human tumor antigens derived of self structures. p53, WT1 and melanoma antigens had also a low to intermediate affinity for the T cell receptor [5, 9, 25, 29]. The findings might be compatible with the suggestion that T cells with a strong affinity for self peptides may be deleted clonally [14].
For the induction of an effective immune response both CD8 and CD4 T cells are required as well as cytotoxic antibodies [21]. It has become obvious that there is an emerging role for CD4 (Th) cells in an antitumor response, and consequently, it should be of importance to develop immunotherapeutic strategies evoking such a response in cancer patients [36]. Dual-specific peptides containing both cytotoxic and helper T cell epitopes may represent an attractive strategy for vaccine design with the aim to generate tumor-reactive CD4 as well as CD8 T cells. Both CD4 and CD8 T cells were efficiently generated from peripheral blood of melanoma patients after in vitro stimulation using a 14-mer peptide (NY-ESO-1:157–170) expressing helper as well as cytotoxic T cell epitopes [34]. An interesting finding in our study was that the functional 15-aa-long peptides contained 9-mer sequences fitting to the actual MHC class I haplotype. An indication that these 15-aa-long peptides might express functional class I as well as class II epitopes was that the proliferative response against these peptides could be inhibited by both MHC class I and class II monoclonal antibodies. However, no 9-mer peptides were produced and assayed in a functional test.
Previously, only class I-restricted CEA-derived peptides have been described. These peptides are shown in Table 2. All the peptides exhibited an intermediate affinity for the T cell receptor. Of our predicted class I epitopes, threeout of ten were in the intermediate range and the remaining of low affinity. The reason for this difference is not clear. However, those nine published 9-aa-long peptides were all selected for induction of CTL [10, 11, 12, 20, 22, 28], while we used a proliferative assay as a read-out system. The majority (8/9) of our identified 15-mer peptides were also in the low affinity range. There seemed to be a relation between the affinity of the 9-mer and the 15-mer peptides. One should, however, be aware that the prediction analyses in most cases could only be done for one of the MHC genotypes, as several HLA haplotypes are not present in the databases.
It was recently shown that the cytotoxic capability of idiotypic immunoglobulin-derived peptides could be increased by producing heteroclitic peptides, i.e., modification of conservative amino acids at the MHC binding residues, thereby increasing the affinity for the T cell receptor without changing the specificity [8]. Similarly, production of “heteroclitic” Ep-CAM-derived peptides allowed generation of cytotoxic T cells that demonstrated increased killing of target cells pulsed not only with the “heteroclitic” but also with the native peptide [27].
In summary, this is the first report on the identification of functional HLA-DR epitopes of CEA. The native peptides inducing a proliferative T cell response had a low to intermediate affinity for the T cell receptor. These 15-mer peptides also contained predicted class I-restricted T cell epitopes. The results might be of importance for the design of CEA-specific vaccines. Peptides with dual specificity may form a basis for production of subunit cancer vaccines, but measures should be taken to increase the affinity for the T cell receptor of the native peptides.
Acknowledgements
The skilful technical assistance of Ms. Anna-Lena Borg and Ms. Lena Virving is highly appreciated. For excellent secretarial help we thank Ms. Gunilla Burén. This study was supported by grants from the Swedish Cancer Society, the Cancer Society in Stockholm, the King Gustaf Vth Jubilee Fund, the Cancer and Allergy Foundation, the Gunnar Nilsson Cancer Foundation, the Swedish Medical Society, the Torsten and Ragnar Söderberg Foundation and the Research Fund of the Department of Oncology, Uppsala University Hospital.
Abbreviations
- aa
amino acid
- CRC
colorectal carcinoma
- GM-CSF
granulocyte/monocyte colony stimulating factor
- CEA
carcino-embryonic antigen
- BCP
baculovirus control protein
- MHC
major histocompatibility complex
- pp
peptide
- TAA
tumor associated antigen
References
- 1.Benchimol Cell. 1989;57:327. doi: 10.1016/0092-8674(89)90970-7. [DOI] [PubMed] [Google Scholar]
- 2.Consogno Blood. 2003;101:1038. doi: 10.1182/blood-2002-03-0933. [DOI] [PubMed] [Google Scholar]
- 3.Fagerberg Proc Natl Acad Sci USA. 1995;92:4773. [Google Scholar]
- 4.Fields Int J Pept Protein Res. 1990;35:161. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 5.Graff-Dubois J Immunol. 2002;169:575. doi: 10.4049/jimmunol.169.1.575. [DOI] [PubMed] [Google Scholar]
- 6.Gulukota J Mol Biol. 1997;267:1258. doi: 10.1006/jmbi.1997.0937. [DOI] [PubMed] [Google Scholar]
- 7.Hansson L, Rabbani H, Fagerberg J, Österborg A, Mellstedt H (2003) T-cell epitopes within the complementarity-determining and framework regions of the tumor-derived immunoglobulin heavy chain in multiple myeloma. Blood [DOI] [PubMed]
- 8.Harig Blood. 2001;98:2999. doi: 10.1182/blood.V98.10.2999. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez J Immunol. 2000;164:596. [Google Scholar]
- 10.Horig H, Medina F, Conkright W, Kaufman H (2000) Strategies for cancer therapy using carcinoembryonic antigen vaccines. Expert reviews in molecular medicine [DOI] [PubMed]
- 11.Huarte Int J Cancer. 2002;97:58. doi: 10.1002/ijc.1579. [DOI] [PubMed] [Google Scholar]
- 12.Kawashima Cancer Res. 1999;59:431. [PubMed] [Google Scholar]
- 13.Marshall J Clin Oncol. 1999;17:332. [Google Scholar]
- 14.Matzinger Annu Rev Immunol. 1994;12:991. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 15.Maverakis J Autoimmun. 2000;15:315. doi: 10.1006/jaut.2000.0436. [DOI] [PubMed] [Google Scholar]
- 16.Morse Clin Cancer Res. 1999;5:1331. [PubMed] [Google Scholar]
- 17.Mosolits Cancer Immunol Immunother. 1999;47:315. doi: 10.1007/s002620050536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moudgil J Immunol. 1999;163:4232. [PubMed] [Google Scholar]
- 19.Nagorsen Cancer Res. 2000;60:4850. [PubMed] [Google Scholar]
- 20.Nukaya Int J Cancer. 1999;80:92. doi: 10.1002/(sici)1097-0215(19990105)80:1<92::aid-ijc18>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Pardoll Nat Med. 1998;4:525. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 22.Ras Hum Immunol. 1997;53:81. doi: 10.1016/S0198-8859(97)00032-3. [DOI] [PubMed] [Google Scholar]
- 23.Riethmuller J Clin Oncol. 1998;16:1788. doi: 10.1200/JCO.1998.16.5.1788. [DOI] [PubMed] [Google Scholar]
- 24.Samanci Cancer Immunol Immunother. 1998;47:131. doi: 10.1007/s002620050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theobald J Exp Med. 1997;185:833. doi: 10.1084/jem.185.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson J Clin Lab Anal. 1991;5:344. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- 27.Trojan Cancer Res. 2001;61:4761. [PubMed] [Google Scholar]
- 28.Tsang J Natl Cancer Inst. 1995;87:982. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 29.Tsuboi Cancer Immunol Immunother. 2002;51:614. doi: 10.1007/s00262-002-0328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ullenhag Cancer Res. 2002;62:1364. [PubMed] [Google Scholar]
- 31.van Cancer Immunol Immunother. 1997;45:207. doi: 10.1007/s002620050434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vermorken Lancet. 1999;353:345. doi: 10.1016/S0140-6736(98)07186-4. [DOI] [PubMed] [Google Scholar]
- 33.Zbar Br J Cancer. 1998;77:683. doi: 10.1038/bjc.1998.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng Cancer Res. 2002;62:3630. [PMC free article] [PubMed] [Google Scholar]
- 35.Zsigo J, Saneii H (1994) A facile, efficient method for the simultanous synthesis of high-quality individual peptides: Application of ACT Model 350 for rapid multiple synthesis of peptides by solid phase method. In: Hodges R, Smith JA (eds) Peptides, chemistry, structure and biology. Proceedings of the 13th American Peptide Symposium. Escom, Leiden, p 91
- 36.Zwaveling Cancer Res. 2002;62:6187. [PubMed] [Google Scholar]