Abstract
In the last few years, serological identification of tumour-associated antigens (TAAs) by recombinant cDNA expression cloning (SEREX) has enabled the mapping of humoral immune responses against TAAs in various types of cancer. The present paper describes the application of SEREX to Burkitt’s lymphoma (BL), a malignancy not previously characterized by SEREX. By using a cDNA library from a BL cell line that does not express IgG, technical difficulties related to background immunoglobulin clones were overcome. Screening with sera from three BL patients revealed immunoreactivity against seven different gene products, six of which represent known human genes. Five proteins had previously been characterized by SEREX in other malignancies or identified as targets of autoantibodies in autoimmune disease. Seroreactivity against ATF-2, a member of the AP-1 transcription factor family, was validated by enzyme-linked immunosorbent assay (ELISA) and Western blot analysis using recombinant ATF-2 protein. Autoantibody responses against ATF-2 were detected by ELISA in 6 of 8 BL patients, compared with 6 of 13 patients with T-cell non-Hodgkin’s lymphoma (T-NHL), 5 of 23 patients with follicular lymphoma and 2 of 27 diffuse large B-cell lymphoma patients. In contrast, reactivity was found in only 1 of 50 healthy volunteers. Next, we showed by immunohistochemistry that the activated form of ATF2 (ATF-2pp) was highly expressed in six different BL samples. We conclude that the SEREX approach with a B-cell cDNA source is applicable in NHL. Furthermore, we identified genes with possible involvement in the pathogenesis of BL using this technique.
Keywords: ATF-2, Burkitt’s lymphoma, Non-Hodgkin’s lymphoma, SEREX, Tumour immunology, Tumour-associated antigen
Introduction
Development of immunotherapeutic strategies for cancer is greatly aided by the identification of TAAs, capable of eliciting long-lasting immune response in patients. While most immunotherapies for cancer have focused on generating tumour-specific CD8+ cytotoxic T lymphocytes, a mounting body of evidence suggests that stimulation of antitumour CD4+ T cells may be required for highly effective therapy [37]. In that respect, there is a growing recognition that some tumour antigens elicit a strong integrated immune response involving both cellular and humoral immunity. Of particular interest are the cancer germline antigens (CGAs), a family of proteins expressed in a variable subset of patients with a given tumour entity and in testicular germ cells, but not, or at low levels, in other normal tissue [30, 45]. Humoral immune responses against TAAs have been mapped with the help of serological cDNA expression cloning (SEREX) in various types of cancer [11, 14, 31, 43, 44] including haematological neoplastic conditions such as leukaemia, Hodgkin’s lymphoma and non-Hodgkin’s lymphoma (NHL) [12, 21, 28, 40]. Some previous studies have reported technical difficulties applying SEREX to B-cell NHL [28]. Most B-NHL cells synthesize immunoglobulins, regularly of the IgG class, and together with antibodies from tumour-infiltrating normal B cells, this results in a high background of immunoglobulin clones during screening.
The present paper describes the application of SEREX to Burkitt’s lymphoma (BL), a malignancy not previously characterized by SEREX. BL is a highly aggressive B-NHL entity that includes endemic, sporadic and human immunodeficiency virus (HIV)–associated subtypes [4]. All subtypes are characterized by chromosomal rearrangements involving the c-myc proto-oncogene, leading to its deregulated overexpression. In endemic BL, tumour cells are latently infected with Epstein-Barr virus (EBV). In contrast, only a subset of sporadic and HIV-associated BLs are EBV-associated [22]. BL was chosen for SEREX analysis, since BL is quite common in immunosuppressed patients, and therefore immunosurveillance possibly plays a role in the pathogenesis of this disease. Furthermore, by using a cDNA library from a BL cell line that does not express IgG, but IgM, the described technical difficulties related to background immunoglobulin clones should be reduced.
By screening this library with pooled sera from three BL patients, seven different antigens were identified. Seroreactivity against the transcription factor ATF-2 was further validated, and its serological profile in patients with different NHL entities, as well as immunohistochemical studies, indicated a possibly important pathophysiological role in BL. EBV and v-myc are known to activate ATF-2 and the present study indicates a high expression of the activated ATF-2 in BL and demonstrates frequent autoantibody responses against this molecule in BL.
Material and methods
Sera, peripheral blood leukocytes, tumour and tonsil tissue and cell lines
Tumour biopsy samples, tonsil tissue and sera were obtained during routine clinical procedures with formal agreement by the patients and with approval by the regional ethics committee. Sera from three patients with histologically and cytogenetically verified BL were available for screening. Sera from 68 other patients with different NHL entities and 50 healthy volunteers were provided by the Central Laboratory, the Norwegian Radium Hospital (NRH), Oslo, Norway, and by the Blood Bank, Ullevål University Hospital. Formalin-fixed paraffin-embedded biopsy samples of six BL were obtained from the Department of Pathology, NRH. In all patients, the lymphoma diagnosis was established according to the WHO classification. The following cell lines from human lymphoid malignancies were used and maintained in RPMI 1640 supplemented with 10% foetal bovine serum, 100 U/ml penicillin G, and 100 U/ml of streptomycin sulphate: pre-B cell line Reh (ATCC CRL 8286), pro-B cell lines BV173 (DSZM ACC20) and Tom-1 [38], EBV-negative BL cell lines Ramos (ECACC 85030802) and Bjab (Dr G. Moldenhauer, University of Heidelberg, Heidelberg, Germany), EBV-positive BL cell lines Rael [27] and Namalva (ECACC 87060801) and human B-cell lymphoma U-698 (DSZM ACC 4).
cDNA library construction and immunoscreening
Total RNA was extracted from the cell line Ramos using the acid guanidine thiocyanate method and mRNA isolated with oligo-dT-Dynabeads (Dynal, Oslo, Norway) [15]. The cDNA library was constructed in the λZAP expression vector (Stratagene, LaJolla, CA, USA) with 1.5×106 primary recombinant clones amplified once before screening. Sera from three BL patients were used for immunoscreening as described previously [15]. In brief, sera were diluted 1:20 and extensively absorbed against Escherichia coli XL-1MRF’Blue cells transfected with wild-type λZAP phage and further diluted to a final titre of each serum of 1:200. Recombinant plaques (5×105) were screened with this dilution. After washing, filters were incubated with alkaline phosphatase–conjugated goat antihuman IgG, and immunoreactive plaques were visualised by incubation with 5-bromo-4-chloro-3-indolyl-phosphate and nitroblue tetrazolium. Positive plaques were subcloned until homogeneity, converted to plasmid clones in pBluescript using the Ex-Assist helper phage (Stratagene) and sequenced by means of BigDye Terminator Cycle Sequencing (Perkin Elmer Applied Biosystems, Warrington, UK) on an ABI Prism 310 Genetic Analyser (Perkin Elmer). DNA and deduced protein sequences were analysed using DNASIS for Windows 2.1 (Hitachi Software Engineering America, San Bruno, CA, USA) and compared with sequences in GeneBank and other public databases using BLAST, and with entries of the SEREX database (http://www.licr.org/SEREX.html). Searches within the SEREX database were done both for identical sequences and functionally related genes.
Expression analysis of ATF-2 in tissues and cell lines
Immunohistochemistry
Five-micrometre sections of formalin-fixed, paraffin-embedded tissue from six BL and five normal tonsils were analysed for ATF-2 expression. The deparaffinized sections were microwaved eight times in 0.01-M sodium citrate, pH 6.0, for 5 min to unmask epitopes. Immunostaining was performed using the EnVision+ Kit (DAKO, Glostrup, Denmark), according to the manufacturer’s protocol. Antibodies anti-ATF-2 (reacting with ATF-2 protein irrespective of the level of phosphorylation) and anti-phospho-ATF-2 (Thr69/71) (reacting only with ATF-2 phosphorylated at Thr69 and Thr71) (all, Cell Signalling Technology, Beverly, MA, USA) were diluted 1:25 and incubated overnight at 4°C. Dilutions of antibodies were made in phosphate-buffered saline (PBS), pH 7.4, 10 mg/ml BSA containing 1 mg/ml NaN3. Sections were counterstained with hematoxylin, dehydrated and mounted in Eukitt (Kindler, Freiburg, Germany).
Immunoblotting
Cells (3–5×106) from cell lines were lysed in sample buffer (glycerol 10%, β-mercaptoethanol 5%, 0.0625 M-Tris-HCl [pH 6.8], sodium dodecyl sulphate [SDS] 2.5% w/vol). Total protein (30 μg) from each sample was run through SDS/polyacrylamide gel electrophoresis (SDS/PAGE) and blotted onto nitrocellulose filters. Blocking, washing and incubation of the filters with antibodies anti-ATF-2 (Cell Signalling Technology) and anti-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were done according to the manufacturers’ protocols. After washing with PBS / 1 mg / ml Tween 20 (PBS-T), the filters were incubated with horseradish peroxidase (HRP) coupled to goat antirabbit IgG (DAKO) for 60 min at room temperature (RT). Enzyme activity was visualised by the enhanced chemiluminescence system ECL+ Plus (Amersham, Buckinghamshire, UK).
Northern analysis
Total RNA was isolated from cell lines as above. For Northern analysis of ATF-2 mRNA, 4-μg total RNA was separated by formaldehyde/agarose gel electrophoresis and transferred to nitrocellulose membranes. Hybridisation was performed with a 32P-labelled fragment covering the complete open reading frame (ORF) of the ATF-2 gene. The quality of RNA was confirmed by hybridisation to a 32P-labelled fragment of β-actin cDNA [15]. Quantification was performed on a Storm 840 Phosphorimager using ImageQuant 5.0 (Molecular Dynamics, Krefeld, Germany).
Serological analysis for anti-ATF-2 antibodies
An enzyme-linked immunosorbent assay (ELISA) was established to quantify IgG reactivity in serum against recombinant ATF-2. A PCR fragment equipped with appropriate restriction sites at the 5′ and 3′ ends and covering the complete ATF-2 ORF was cloned into the pQE30 vector (Qiagen, Hildesheim, Germany). The protein was produced in E.coli M15 cells (Qiagen) and purified using 8-M urea as the denaturing agent according to the manufacturer’s protocol, followed by dialysis in PBS. MaxiSorp plates (Nunc, Rochester, MN, USA) were coated overnight at 4°C with 50-μl ATF-2 protein in PBS (μg/ml). Plates were washed (Scan washer 400; Skatron Instruments, Norway) and blocked with PBS-T/50 mg/ml milk powder (MP). Sera were preincubated at 1:100 in PBS/10 mg/ml MP supplemented with 0.25 μg/μl E.coli M15 lysate for 2 h at RT, and 50 μl was subsequently added to each well for 2 h incubation at RT. Plates were washed again and incubated 1 h at RT with HRP-conjugated goat antihuman IgG (Sigma-Aldrich, Steinheim, Germany) diluted 1:5,000 in PBS-T/10 mg/ml MP. After washing, HRP reactivity was visualised using 1,2-phenylenediamine dihydrochloride (OPD) tablets (DAKO), and absorption at 492 nm read using a MultiscanEX photometer (Labsystems, Helsinki, Finland). All sera were tested in duplicate. Selected sera were retested in a competition assay, with preincubation of serum as above, but with addition of soluble recombinant ATF-2 or a similarly produced irrelevant protein (p352) [14].
Reduction of OD492 reading by 30% or more for ATF-2 competition compared with irrelevant protein was considered significant. Selected sera were tested by Western blot analysis, performed essentially as described above, but applying 0.2-μg recombinant ATF-2 and 0.2-μg p352 per lane in SDS/PAGE. Sera were diluted 1:100, and HRP-conjugated goat antihuman IgG (Sigma-Aldrich) diluted 1:5,000 in PBS-T/1% w/vol MP was used as secondary antibody.
Results
Immunoscreening of the Ramos cDNA library
Approximately 5×105 clones of the Ramos cDNA library were screened with pooled sera from three BL patients. Twelve independent clones were identified and sequenced. Sequence analysis and BLAST searches in publicly available databases revealed that these 12 clones were transcribed from seven different genomic loci, of which six are functionally characterized human genes (Table 1). Four different clones encoded the activation transcription factor ATF-2. All four clones covered at least 90% of the ORF of the ATF-2 gene, and the sequence of all clones was identical to that previously reported for ATF-2 (data not shown). ATF-2 is a member of the basic region leucine zipper (bZIP) transcription factor family, and is a component of the AP-1 complex [49].
Table 1.
Identity of clones isolated by serological screening
| Gene identified/accession no. | Positive clones (n) | Functional significance | Expression pattern | Identical and related entries in the SEREX database |
|---|---|---|---|---|
| Activation transcription factor 2/X15875 | 4 | Oncogenic AP1- transcription factor, interacts with c-myc and EBV | Ubiquitously expressed | One entry for ATF-3/NM_001674 (breast cancer) |
| Nm23-H2/NM_002512 | 3 | Nucleoside diphosphate kinase/transcription factor for c-myc | Overexpressed in tumours | Two entries for Nm23-H2 (glioma and colon cancer), two further entries for Nm23-H1(NM_000269) |
| S-adenosylhomocysteine hydrolase/M61831 | 1 | Hydrolase involved in transmethylation | Ubiquitously expressed | One entry for AHCYNM_000687 (cDNA from testis, unclassifiable patient) |
| Triosephosphate isomerase 1/NM_000365 | 1 | Enzyme of the glycolytic pathway | Overexpressed in tumours | Three entries for TPI1 (unclassifiable patient and breast cancer) |
| U2 snRNP-specific A′ protein/X13482 | 1 | Role in hnRNA splicing/known autoantigen | Ubiquitously expressed | Four entries for U2 small nuclear ribonucleo-protein auxiliary factor/NM_005089 (renal cancer, breast cancer, stomach cancer, and Hodgkin’s disease) |
| Ribosomal protein S2/NM_002952 | 1 | Ribosomal protein | Ubiquitously expressed | Two entries for ribosomal protein S2 (rps2) in prostate cancer |
| Unknown/AA195197 | 1 | Endogenous retroviral sequence? | No entry with similarity |
Three independent clones encoded nonmetastatic protein 23-H2 (Nm23-H2), a multifunctional protein with aberrant, and partly augmented expression, in human tumours [18, 32]. Furthermore, Nm23-H2 has been shown to be involved in the deregulation of the translocated c-myc allele in BL cells [24]. Proteins encoded by one clone each included a component of the small nuclear ribonucleoprotein (snRNP) U2, ribosomal protein S2, triosephosphate isomerase (TPI) and S-adenosinehomocysteine hydrolase (AHCY). TPI is a ubiquitously expressed enzyme of the glycolytic pathway, shown to be overexpressed in bladder, colon and lung carcinomas [6, 10, 35]. AHCY is a key enzyme of transmethylation, a mechanism also involved in controlling the replication of several viruses such as EBV [7, 16, 46]. The clone identical to EST AA195197 encoded a putative protein with significant homology to retroviral reverse transcriptases. The human genome contains a high number of elements with identical or near identical nucleotide sequences to this clone. This suggests that the clone is derived from a transcribed endogenous retroviral sequence within the human genome.
Searches for identical clones in the public part of the SEREX database
A search of SEREX (http://www.licr.org/SEREX.html) revealed that five of the identified antigens have been previously identified. Of note, most of the SEREX-defined antigens do not serve exclusively as TAAs. Many of these antigens frequently elicit antibody responses in autoimmune diseases or in apparently healthy humans. These antigens therefore also classify as autoantigens and their possible role in human malignancies remains to be clarified [37].
For Nm23-H2, the occurrence of autoantibodies has been described both in cancer patients and healthy volunteers [47]. Ribosomal protein S2 has been detected as an autoantigen in prostate cancer. TPI has been identified by SEREX in breast cancer and also as a mutated antigen recognized by CD4+ T cells in a patient with malignant melanoma [41]. In addition to these identical entries, molecules related to the snRNP U2 have been isolated by SEREX, and snRNPS, including components of U2, are frequently recognised by autoantibodies in patients with autoimmune disease [8, 29, 50]. AHCY is a key enzyme of transmethylation, a mechanism also involved in controlling the replication of several viruses such as EBV [7, 46]. Although no entries identical to the ATF-2 gene were found in the SEREX database, the related family member ATF-3 has been identified as an antigen eliciting humoral immune response in breast cancer patients.
Detection of anti-ATF-2 antibodies in sera
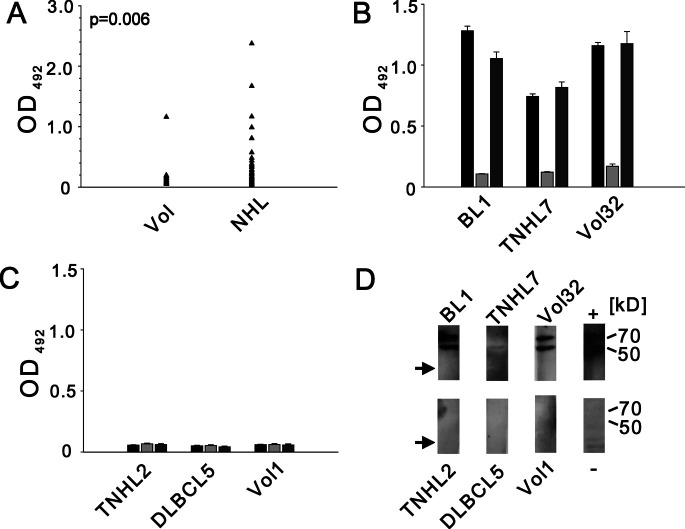
We chose to focus on ATF-2 in our further studies, because it has not been previously described as a SEREX antigen. Furthermore, ATF-2 may be linked to the development of BL, since it has previously been implicated to be involved in oncogenesis and to interact with c-myc and EBV [24, 34]. We performed an ELISA to reveal the occurrence of IgG autoantibodies against ATF-2. Sera from 50 healthy volunteers, 8 patients with BL (including the 3 patients used for screening) and 63 patients with other NHL entities were tested. Using an arbitrary OD492 of 0.2 as cutoff, 22 of 71 (31%) of patient sera displayed anti-ATF-2 reactivity, in contrast to 1 of 50 (2%) healthy volunteers (Fig. 1A). All sera with reactivity in ELISA and several negative cases were subjected to competition ELISA, where sera were preincubated with purified ATF-2 or irrelevant recombinant protein. Sera from 19 NHL patients and 1 healthy volunteer were confirmed as positive (Fig. 1B), whereas three patient sera could not be confirmed. Ten positive sera were further tested by Western blot analysis using purified recombinant ATF-2 as antigen and four sera with highly positive ELISA results showed reactivity against ATF-2 in immunoblotting (Fig. D). Sera with negative ELISA results were also negative by Western blot analysis.
Fig. 1A–D.
Analysis of anti-ATF-2 antibodies in sera from healthy volunteers and patients with non-Hodgkin’s lymphoma (NHL). A Results of enzyme-linked immunosorbent assay (ELISA) using recombinant ATF-2 as target antigen. Arrow depicts mean of duplicate experiments for each serum from healthy volunteers (Vol) and patients (NHL). OD 492, optical density at 492 nm. B, C Results of competition ELISA shown for individual positive (B) and negative (C) sera. Bars denote results in ELISA (black), after preincubation of serum with recombinant ATF-2 (light grey) or irrelevant protein p352 (dark grey) with error bars representing standard error of the mean of duplicate experiments. BL Burkitt’s lymphoma, T-NHL T-cell lymphoma, DLBCL diffuse large B-cell lymphoma. D Western blot analysis of individual sera from B and C. Expected protein bands corresponding to recombinant ATF-2 are indicated at 70 and 50 kDa. No reactivity to p352 (arrow) is seen. Lane titled “+” represents experiment using anti-ATF-2 primary antibody and appropriate secondary antibody (see text). Lane titled “−” shows reactivity of BL1 serum toward a lysate of E. coli M15 cells prepared in parallel with lane BL1
The frequency of anti-ATF-2 antibodies in patients with different NHL entities is shown in Table 2. Six of eight BL patients tested (75%) showed seroreactivity against ATF-2, as did 6 of 13 patients with T-NHL (46%). The frequency of ATF-2 autoantibodies appeared lower in DLBCL and FL, with 7 and 22% positive sera identified, respectively.
Table 2.
ATF-2 antibodies in sera from patients with NHL and healthy volunteers. ND not done
| DLBCL | Follicular lymphoma | Burkitt’s lymphoma | T-cell lymphoma | Normal volunteers | |
|---|---|---|---|---|---|
| ELISA | 2/27 | 5/23 | 6/8 | 6/13 | 1/50 |
| Immunoblotting | ND | ND | 1/4 | 2/5 | 1/1 |
Analysis of ATF-2 expression
Earlier reports have revealed ubiquitous expression of ATF-2 mRNA-transcripts in normal human tissues, with highest levels in brain, testis and thymus [52], and to our knowledge, no evidence for ATF-2 mRNA overexpression in B-cell lymphomas as compared to normal tissues has been reported [2]. Our own analysis of different lymphoma cell lines could not detect significant differences in ATF-2 mRNA levels or total ATF-2 protein in different NHL cell lines. In particular, we were unable to detect different expression levels when comparing cell lines form BL with other cell lines (data not shown).
ATF-2 is regulated at the post-translational level by phosphorylation of the amino acid residues threonine-69 and threonine-71, mainly mediated by stress-activated protein kinases (SAPKs) including Jun-NH2-terminal kinase (JNK) [19]. Therefore, to determine the level of activation of ATF-2 in BL, paraffin-embedded tissue from six patients with BL was analysed by immunohistochemistry for expression of total ATF-2 protein and the doubly phosphorylated isoform ATF-2pp (Thr69/71). All lymphomas expressed ATF-2pp in nearly 100% of viable tumour cells and at high levels, comparable to a subset of centroblasts in the normal germinal centre of tonsils. Most B cells in normal tonsils except for the subset of centroblasts expressed low levels of ATF-2pp (Fig. 2). In contrast, the total ATF-2 protein levels appeared similar in BL cells and most normal germinal centre B cells (data not shown).
Fig. 2A, B.
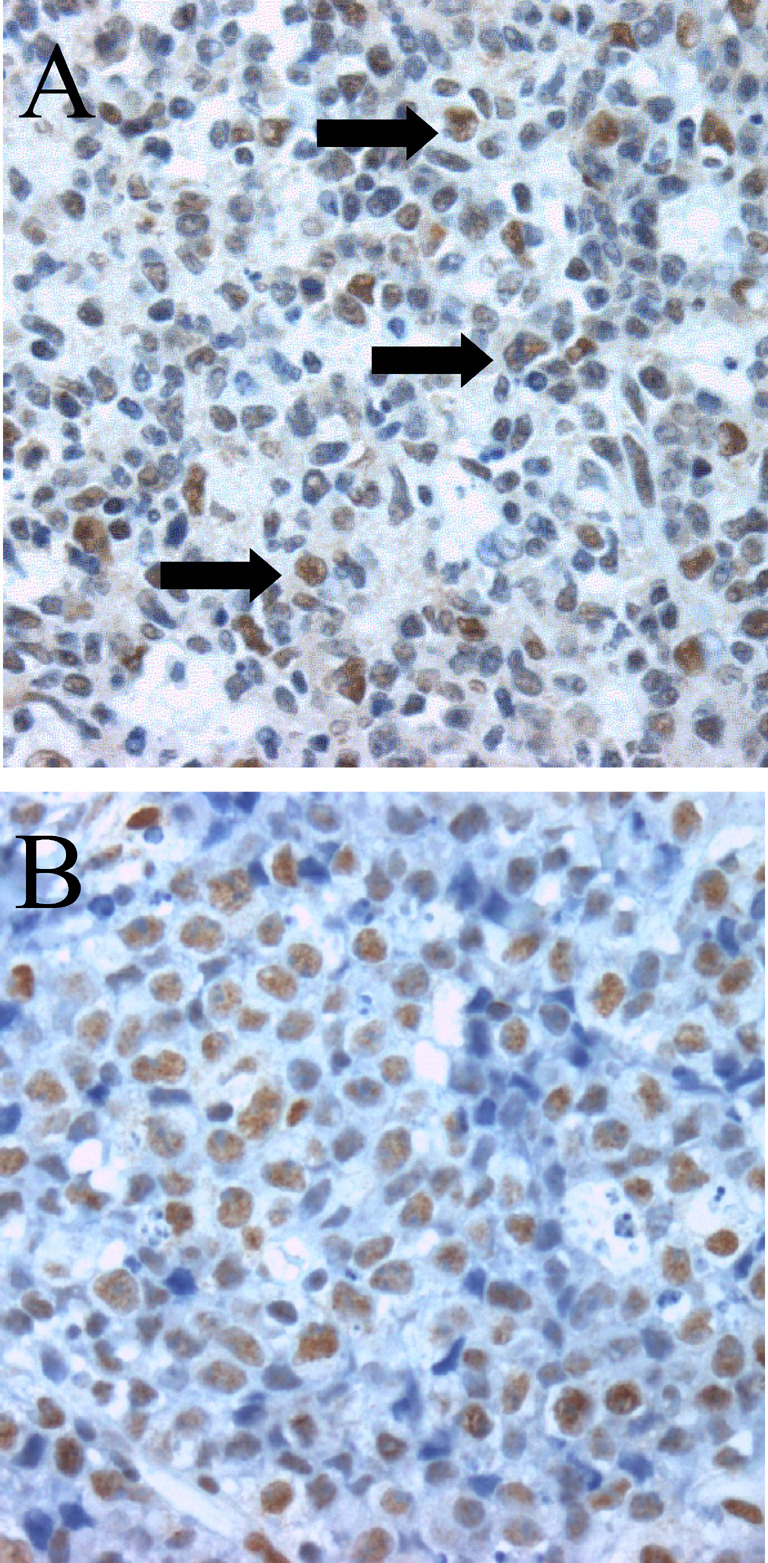
Immunohistochemical staining for doubly phosphorylated ATF-2pp (Thr69/71) in a tonsil biopsy sample (A) and a case of BL (B). A subpopulation of large centroblasts in germinal centres of tonsils (black arrows) expresses high levels of ATF-2pp in contrast to most of the other germinal centre cells that express only low levels of the protein. In BL, most of the tumour cells express high levels of ATF-2pp, with the exception of the apoptotic tumour cells
Discussion
The present paper describes the use of the SEREX technology for identification of TAAs in BL. By screening a Ramos cDNA library with pooled sera of three BL patients, we detected humoral responses against seven antigens, showing that humoral immune responses to nonidiotypic antigens take place in BL.
The use of a IgM-expressing B cell line as a cDNA source reduced problems with false positive Ig clones, a problem frequently encountered when using SEREX in B-cell malignancies [28].To identify CGAs and to overcome the mentioned technical obstacles, others have used testis tissue as a cDNA source for SEREX, but no clear candidates for a possible NHL vaccination protocol were identified [23]. Since the routinely used immunotherapies for haematological malignancies use common lineage–specific haematological antigens [5] and since expression of CGAs in B-NHL has been shown to be limited [36], the appropriate cDNA source for identification of TAAs by SEREX in NHL appears to be lymphoma tissue [23]. We were not able to identify CGAs or haematological antigens in this study, which is in line with the findings of others [23].
Most of the previously identified SEREX-defined antigens are ubiquitously expressed [37]. This is consistent with our findings, where, except of the EST clone with unknown function from chromosome 6, all the detected antigens are known to be ubiquitously expressed in normal human tissues. Therefore none of these antigens appear to be obvious vaccine candidates for immunotherapy of cancer. However, it has been shown that coimmunisation with SEREX antigens and tumour antigens results in heightened resistance to tumour growth in mice [37]. Therefore, the repeated detection by SEREX of some of the antigens mentioned here might strengthen them as candidates for coimmunisation protocols in human [51]. It remains to be clarified, whether these coimmunisation antigens should be exclusive TAAs, which do not elicit humoral responses in healthy volunteers, or if it is possible to use autoantigens, as described [37]. It is in that respect of great interest that five of the seven antigens identified here, have been identified by the means of SEREX before.
To our knowledge, ATF-2 has not previously been detected by SEREX. It is a member of the basic region leucine zipper family of transcription factors, and together with the jun, fos and ATF subfamilies, it constitutes a component of the AP-1 complex [49, 53]. This transcription factor regulates gene expression in response to cell stress, growth factors [39], viral infections and inflammatory signals. Interestingly v-myc has been shown to activate and stabilize the phosphorylation of ATF-2 [34]. Despite of the known oncogenic potential of AP-1 transcription factors [53] and the known interaction of ATF-2 with both c-myc and EBV [1], little is known about the expression and phosphorylation of ATF-2 in NHL and leukaemia [33]. We therefore explored the expression pattern of ATF-2 and its activated, doubly phosphorylated isoform at the protein level in paraffin-embedded sections of six different BLs. Interestingly, most BL cells expressed high levels of the active isoform, with similar levels found only in subsets of centroblasts in reactive germinal centres. The total ATF-2 protein level was similar in BL cells and the majority of germinal centre B cells. The reason for enhanced phosphorylation of ATF-2 in BL cells and the possible effects of such changes remain to be determined. Recently, overexpression of c-jun and junB, to major components of AP-1, was found in Hodgkin’s disease and shown to contribute to proliferation of HD cell lines [33]. Similar overexpression was not seen in other B-NHL entities, and ATF-2 expression was not analysed. The fact that oncogenic viruses such as HTLV-1 and EBV exert some of their effects through activation of ATF-2, and ATF-2 contributes to c-jun–mediated transformation in experimental systems, may warrant further studies as to the contribution of ATF-2 to oncogenisis in BL [1, 13, 53, 54]. Interestingly, humoral immune responses against ATF-2 could be detected in patients with BL and T-NHL. The occurrence of anti-ATF-2 antibodies in six out of eight sera from patients with BL, but only in 1 out of 50 sera from the healthy controls in this analysis, underlines the potential pathophysiological relevance of this transcription factor, since only a fraction of the serologically defined TAAs do not elicit serological responses in apparently healthy humans. Others have argued that a cancer-related serological profile of an autoantigen might result from tumour-associated post-translational modifications [40]. The reason for autoantibody production in these patients is not known. The high level of ATF-2 phosphorylation in BL cells points to a post-translational change that possibly enhances immunogenicity of the protein. Three patient sera elicited high signals in ELISA, but the immunoreactivity failed to be verified in competition ELISA with the recombinant (unphosphorylated) ATF-2. Patient antibodies, which are specific against ATF-2pp, might be the reason for this. However, one of these patients elicited many apparently unspecific bands in Western analysis, and we conclude that we did not succeed in removing all anti-E. coli antibodies for this patient, despite intensive preabsorption, as indicated, and parallel treatment of the sera. This emphasises the need for eukaryotic expression cloning systems which would make the identification of post-translationally modified antigens more likely [23, 30] and specific.
In this respect, it is tempting to speculate that phosporylation itself may have created a new epitope recognized by phosphopeptide-specific T cells in the patients. Such T cells have been described to be a constituent of the normal T-cell repertoire [3, 25]. If this was found to be the case, the high level of expression of such an epitope in lymphomas compared with normal tissues, might provide a window of opportunity for a vaccination strategy. The high degree of apoptotic cells associated with BL may also contribute to immunogenicity, since apoptotic tumour cells have been shown to trigger autoantibody production, including autoantibodies to snRNP components, antigens identified along with ATF-2 in the present screening [17, 20].
Given the important pathophysiological role of c-myc in BL, it is remarkable that in addition to ATF-2 we identified two further molecules, which are related to c-myc, i.e. Nm23-H2 and AHCY. NM23-H2 has, besides activating transcription of c-myc [42], been shown to be a downstream target of c-myc itself [48]. Inhibitors of AHCY have been tested in vitro for their possible utilisation as an antiviral drug [9] and as a drug in the treatment of leukaemia and lymphoma. In these experiments, AHCY inhibitors were shown to induce apoptosis, by possibly down-regulating AP-1, nm23-H2 and c-myc [26].
In conclusion, we analysed the autoantibody repertoire of BL patients and revealed an intriguing serological profile against ATF-2 in patients with BL compared with healthy controls. Our findings indicate that SEREX, besides identifying potential tumour vaccine candidates, has the ability of detecting antigens of pathophysiological interest.
Acknowledgements
The authors would like to thank Inger Liv Nordli for excellent technical help and Rainer Siebert for verifying the BL diagnosis by FISH. This work was supported by the Norwegian Cancer Society and the Norwegian Research Council.
References
- 1.Adamson J Virol. 2000;74:1224. doi: 10.1128/JVI.74.3.1224-1233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alizadeh Nature. 2000;403:503. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 3.Andersen J Immunol. 1999;163:3812. [PubMed] [Google Scholar]
- 4.Bellan J Clin Pathol. 2003;56:188. doi: 10.1136/jcp.56.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell Blood Rev. 2003;17:143. doi: 10.1016/S0268-960X(03)00005-5. [DOI] [PubMed] [Google Scholar]
- 6.Chen Proc Natl Acad Sci U S A. 2003;100:13537. doi: 10.1073/pnas.2233850100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang FASEB J. 1996;10:471. [Google Scholar]
- 8.De Trends Biochem Sci. 1999;24:281. doi: 10.1016/S0968-0004(99)01414-0. [DOI] [PubMed] [Google Scholar]
- 9.De Nat Rev Drug Discov. 2002;1:13. doi: 10.1038/nrd703. [DOI] [PubMed] [Google Scholar]
- 10.Duyndam Oncogene. 1999;18:2311. doi: 10.1038/sj.onc.1202584. [DOI] [PubMed] [Google Scholar]
- 11.Ehlken Int J Cancer. 2004;108:307. doi: 10.1002/ijc.11537. [DOI] [PubMed] [Google Scholar]
- 12.Eichmuller Proc Natl Acad Sci U S A. 2001;98:629. doi: 10.1073/pnas.021386498. [DOI] [Google Scholar]
- 13.Eliopoulos J Biol Chem. 1999;274:16085. doi: 10.1074/jbc.274.23.16085. [DOI] [PubMed] [Google Scholar]
- 14.Fossa Cancer Immunol Immunother. 2004;53:431. doi: 10.1007/s00262-003-0458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fossa Br J Cancer. 2000;83:743. doi: 10.1054/bjoc.2000.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs Mol Cell Biol. 1999;19:3289. doi: 10.1128/mcb.19.5.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gensler J Autoimmun. 2001;16:59. doi: 10.1006/jaut.2000.0464. [DOI] [PubMed] [Google Scholar]
- 18.Godfried Oncogene. 2002;21:2097. doi: 10.1038/sj.onc.1205259. [DOI] [PubMed] [Google Scholar]
- 19.Gupta Science. 1995;20:389. [Google Scholar]
- 20.Hansen Proc Natl Acad Sci U S A. 2001;98:12659. doi: 10.1073/pnas.171460798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartmann Br J Dermatol. 2004;150:252. doi: 10.1111/j.1365-2133.2004.05651.x. [DOI] [PubMed] [Google Scholar]
- 22.Hecht J Clin Oncol. 2000;18:3707. doi: 10.1200/JCO.2000.18.21.3707. [DOI] [PubMed] [Google Scholar]
- 23.Huang Cancer Immunol Immunother. 2002;51:655. doi: 10.1007/s00262-002-0320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji J Biol Chem. 1995;270:13392. doi: 10.1074/jbc.270.22.13392. [DOI] [PubMed] [Google Scholar]
- 25.Kastrup Adv Immunol. 2001;78:267. doi: 10.1016/S0065-2776(01)78006-6. [DOI] [PubMed] [Google Scholar]
- 26.Kim Exp Mol Med. 2000;32:197. doi: 10.1038/emm.2000.32. [DOI] [PubMed] [Google Scholar]
- 27.Klein Int J Cancer. 1972;10:44. doi: 10.1002/ijc.2910100108. [DOI] [PubMed] [Google Scholar]
- 28.Krackhardt Blood. 2002;100:2123. doi: 10.1182/blood-2002-02-0513. [DOI] [PubMed] [Google Scholar]
- 29.Lehmeier Proc Natl Acad Sci U S A. 1994;91:12317. doi: 10.1073/pnas.91.25.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Cancer Immunol Immunother. 2004;53:139. doi: 10.1007/s00262-003-0471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Line Cancer Immunol Immunother. 2002;51:574. doi: 10.1007/s00262-002-0322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez Gut. 1995;37:712. doi: 10.1136/gut.37.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathas EMBO J. 2002;21:4104. doi: 10.1093/emboj/cdf389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miethe Oncogene. 2001;20:8116. doi: 10.1038/sj.onc.1204966. [DOI] [PubMed] [Google Scholar]
- 35.Montgomerie Clin Biochem. 1997;30:613. doi: 10.1016/S0009-9120(97)00115-X. [DOI] [PubMed] [Google Scholar]
- 36.Nemecek Curr Opin Hematol. 2002;9:316. doi: 10.1097/00062752-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Nishikawa Proc Natl Acad Sci U S A. 2001;98:14571. doi: 10.1073/pnas.251547298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okabe Blood. 1987;69:990. [PubMed] [Google Scholar]
- 39.Ouwens EMBO J. 2002;21:3782. doi: 10.1093/emboj/cdf361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfreundschuh M. Exploitation of the B cell repertoire for the identification of human tumor antigens. Cancer Chemother Pharmacol. 2000;46(3–7):S3–S7. doi: 10.1007/pl00014046. [DOI] [PubMed] [Google Scholar]
- 41.Pieper J Exp Med. 1999;189:757. doi: 10.1084/jem.189.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Postel Science. 1993;261:478. doi: 10.1126/science.8392752. [DOI] [PubMed] [Google Scholar]
- 43.Preuss Immunol Rev. 2002;188:43. doi: 10.1034/j.1600-065X.2002.18805.x. [DOI] [PubMed] [Google Scholar]
- 44.Sahin Proc Natl Acad Sci U S A. 1995;92:11810. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scanlan Immunol Rev. 2002;188:22. doi: 10.1034/j.1600-065X.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 46.Schaade Med Microbiol. 2000;Immunol:13. doi: 10.1007/pl00008252. [DOI] [PubMed] [Google Scholar]
- 47.Schmits Int J Cancer. 2002;98:73. doi: 10.1002/ijc.10170. [DOI] [PubMed] [Google Scholar]
- 48.Schuldiner Gene. 2002;292:91. doi: 10.1016/S0378-1119(02)00668-6. [DOI] [PubMed] [Google Scholar]
- 49.Shaulian Nat Cell Biol. 2002;4:E131. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 50.Sillekens Nucleic Acids Res. 1989;17:1893. doi: 10.1093/nar/17.5.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stager Nat Med. 2003;9:1287. doi: 10.1038/nm933. [DOI] [PubMed] [Google Scholar]
- 52.Su Proc Natl Acad Sci U S A. 2002;99:4465. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Oncogene. 2001;20:2453. doi: 10.1038/sj.onc.1204239. [DOI] [PubMed] [Google Scholar]
- 54.Xu Oncogene. 1996;13:135. [Google Scholar]